Abstract
Male pattern baldness can have substantial psychosocial effects, and it has been phenotypically linked to adverse health outcomes such as prostate cancer and cardiovascular disease. We explored the genetic architecture of the trait using data from over 52,000 male participants of UK Biobank, aged 40–69 years. We identified over 250 independent genetic loci associated with severe hair loss (P<5x10-8). By splitting the cohort into a discovery sample of 40,000 and target sample of 12,000, we developed a prediction algorithm based entirely on common genetic variants that discriminated (AUC = 0.78, sensitivity = 0.74, specificity = 0.69, PPV = 59%, NPV = 82%) those with no hair loss from those with severe hair loss. The results of this study might help identify those at greatest risk of hair loss, and also potential genetic targets for intervention.
Author summary
Living with male pattern baldness can be stressful and embarrassing. Previous studies have shown baldness to have a complex genetic architecture, with particularly strong signals on the X chromosome. However, these studies have been limited by small sample sizes. Here, we present the largest genome-wide study of baldness to date, using data from over 52,000 male participants in the UK Biobank study. We identify over 200 novel findings. We also split our dataset in two to build and apply a genetic predictor of baldness. Of those with a polygenic score below the median, 14% had severe hair loss and 39% no hair loss. By contrast, of those with a polygenic score in the top 10%, 58% reported moderate-to-severe hair loss.
Introduction
Male pattern baldness affects around 80% of men by the age of 80 years [1], and it can have substantial psychosocial impacts via changes in self-consciousness and social perceptions [2, 3]. In addition to alterations in physical appearance, some, but not all, studies have identified negative health outcomes associated with baldness including increased risk of prostate cancer [4–6] and cardiovascular disease [7–9]. Baldness is known to be substantially heritable [10]. Here, we use a large, population-based dataset to identify many of the genes linked to variation in baldness, and build a genetic score to improve the prediction of severe hair loss.
The total proportion of variance in male pattern baldness that can be attributed to genetic factors has been estimated in twin studies to be approximately 80% for both early- and late-onset hair loss [11, 12]. Newer molecular-genetic methods have estimated the single-nucleotide polymorphism (SNP)-based, common-variant heritability of baldness at around 50% [13]. Molecular methods also indicate a degree of overlap between genetic variants linked to baldness and those linked to phenotypes such as height, waist-hip ratio, age at voice drop in males, age at menarche in females, and presence of a unibrow [14].
A number of studies have identified specific genetic variants linked to variations in baldness, usually with the AR gene showing the strongest association. The largest published genome-wide association study (GWAS) to date highlighted eight independent genetic loci that were linked to baldness; the top AR SNP yielded an odds ratio of 2.2 in a case-control meta-analysis of 12,806 individuals of European ancestry [15]. One of the autosomal hits identified in that study was found to be in a gene linked to Parkinson’s disease. More recently, a review paper highlighted fifteen loci from six studies that have been associated at genome-wide significance (P<5x10-8) with baldness; two of these were located on the X chromosome [16].
Several attempts have been made to build predictors of male pattern baldness using polygenic risk scores. Heilmann et al. found, using a case-control design with ~600 per arm, that a predictor based on 34,186 SNPs explained 4.5% of the variance on the liability scale [17]. Marcińska et al. used candidate genes to build 5-SNP and 20-SNP polygenic predictors, which performed well when considering prediction of early-onset male pattern baldness, but poorly when considering those with no baldness versus those with severe baldness across all ages [18]. Most recently, a 20-SNP predictor was assessed in three European studies [13]. It achieved a maximum Area Under the Curve (AUC) prediction of 0.74 in an early-onset cohort, but weaker estimates in the other two, late-onset cohorts (AUC = 0.69 and 0.71). These values correspond to poor-to-fair predictions of baldness. In addition, in that study, age was included in the predictor, explaining the bulk of the differences. A meta-analysis of the three cohorts’ GWAS studies identified a novel locus on chromosome 6. The study also estimated the SNP-based heritability of early-onset (56% (SE 22%) from the autosomes, 23% (SE 1.1%) from the X chromosome) and late-onset baldness (42% (SE 23%) from the autosomes, 10% (SE 5%) from the X chromosome).
The present study
The UK Biobank study [19] (http://www.ukbiobank.ac.uk) is a large, population-based genetic epidemiology cohort. At its baseline assessment (2006–2010), around 500,000 individuals aged between 40 and 70 years and living in the UK completed health and lifestyle questionnaires and provided biological samples for research.
The present study reports a GWAS of male pattern baldness in the UK Biobank cohort, which is over four times the size of the previously-largest meta-analytic study [15]. After completing the GWAS, we split the cohort into a large ‘discovery’ sample of 40,000 participants in which the GWAS was re-run. The regression weights from this GWAS were used to perform a prediction analysis in the sub-sample of 12,000 participants who did not contribute to the GWAS. We determined the accuracy of the polygenic profile score by discriminating between those with severe hair loss and those with no hair loss.
Results
The mean age of the 52,874 men was 57.2 years (SD 8.0). 16,724 (31.6%) reported no hair loss, 12,135 (23.0%) had slight hair loss, 14,234 (26.9%) had moderate hair loss, and 9,781 (18.5%) had severe hair loss.
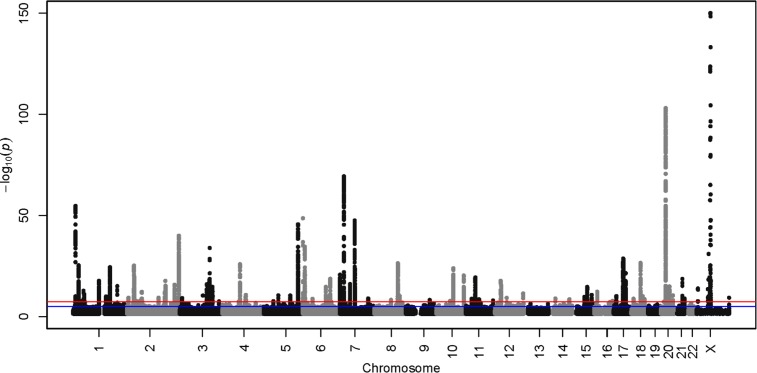
The genome-wide association study of the four-category self-reported baldness measure in 52,874 White British men from UK Biobank yielded 13,029 autosomal hits from the imputed data (P<5x10-8), in addition to 117 hits (out of 14,350 genotyped SNPs) on the X chromosome (Fig 1). The QQ plot for the autosomal GWAS is shown in S1 Fig. An LD clumping analysis indicated that these hits can be attributed to 247 independent autosomal regions. All previously reported autosomal hits [10, 13–16] that mapped to SNPs in our study (62 out of 68 SNPs) replicated with a maximum P-value of 0.006 (54 out of 62 lookups had P<5x10-8, S1 Table). The previously reported X chromosome variant from Li et al. [15] and the variant from Richards et al. [10] also replicated with P-values that were effectively zero (S1 Table). The chromosome 6 hit (rs4959410) from Liu et al. [13], which was not supported by additional SNPs in the region, failed to replicate (P = 0.37). All other hits from Liu et al. [13] had been previously reported in the literature. A list of the top 20 independent autosomal hits are presented in Table 1. The top 10 independent X chromosome hits are presented in Table 2; rs140488081 and rs7061504 are intronic SNPs in the OPHN1 gene. After conditioning on the top SNP (rs73221556), 47 SNPs (including the two lead X chromosome SNPs from the literature: rs2497938 and rs6625163) remained significant at P<5x10-8. In the UK Biobank data, the two lead SNPs from the literature were in very high LD (R2 = 0.98). Summary output for all of the SNPs is available at the following URL: http://www.ccace.ed.ac.uk/node/335. A list of the 287 independent loci are reported in S2 Table.
Fig 1. Manhattan Plot of imputed autosomal GWAS and genotyped X chromosome of male pattern baldness (p-values truncated at 1x10-150).
Table 1. Top 20 independent autosomal GWAS hits.
| Chr | Position | SNP ID | Effect allele | MAF | Beta | SE | P | SNPs in Clump | SNPs with P<0.0001 | Gene | Function |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 11040385 | rs7542354 | A | 0.22 | -0.12 | 0.007 | 5.74 x10-55 | 86 | 80 | C1orf127 | intronic |
| 1 | 25467880 | rs12745121 | A | 0.31 | -0.07 | 0.007 | 5.51 x10-26 | 134 | 56 | NA | NA |
| 1 | 170361164 | rs10919382 | G | 0.38 | 0.07 | 0.006 | 4.54 x10-25 | 514 | 463 | NA | NA |
| 2 | 32181424 | rs13021718 | A | 0.14 | -0.09 | 0.009 | 7.26 x10-26 | 541 | 396 | MEMO1/DPY30 | intronic |
| 2 | 239695893 | rs11684254 | G | 0.35 | 0.09 | 0.006 | 1.10 x10-40 | 140 | 134 | AC144525.1 | 3downstream |
| 3 | 139032333 | rs7642536 | C | 0.14 | 0.11 | 0.009 | 1.28 x10-34 | 49 | 40 | MRPS22 | intronic |
| 4 | 81197949 | rs7680591 | A | 0.42 | 0.07 | 0.006 | 1.44 x10-26 | 107 | 103 | FGF5 | intronic |
| 5 | 158320877 | rs1422798 | G | 0.38 | -0.09 | 0.006 | 2.84 x10-46 | 261 | 176 | EBF1 | intronic |
| 6 | 396321 | rs12203592 | T | 0.22 | 0.11 | 0.007 | 2.64 x10-49 | 8 | 8 | IRF4 | intronic/5upsteam |
| 6 | 9327556 | rs9357047 | C | 0.44 | 0.08 | 0.006 | 3.07 x10-35 | 229 | 194 | NA | NA |
| 7 | 18896988 | rs71530654 | G | 0.40 | 0.11 | 0.006 | 4.68 x10-70 | 98 | 98 | HDAC9 | intronic |
| 7 | 68587797 | rs939963 | C | 0.45 | -0.09 | 0.006 | 3.58 x10-48 | 104 | 94 | NA | NA |
| 8 | 109145555 | rs79206101 | T | 0.01 | 0.31 | 0.028 | 4.91 x10-27 | 25 | 24 | AP001331.1 | 5upstream |
| 17 | 44066172 | rs112385572 | G | 0.24 | -0.08 | 0.007 | 8.45 x10-29 | 376 | 366 | MAPT | intronic |
| 17 | 44787313 | rs538628 | C | 0.22 | -0.08 | 0.007 | 3.60 x10-27 | 81 | 77 | NSF | intronic |
| 18 | 42814156 | rs8085664 | A | 0.28 | -0.07 | 0.007 | 3.47 x10-27 | 338 | 306 | SLC14A2 | intronic |
| 20 | 21894764 | rs6035986 | T | 0.43 | 0.12 | 0.006 | 1.20 x10-87 | 0 | 0 | NA | NA |
| 20 | 22033819 | rs201593 | A | 0.43 | 0.13 | 0.006 | 1.06 x10-103 | 662 | 662 | LOC100270679/ RP11-125P18.1 | 5upstream |
| 20 | 22100070 | rs7362397 | T | 0.30 | -0.11 | 0.007 | 3.02 x10-65 | 0 | 0 | NA | NA |
| 20 | 22100072 | rs7362398 | T | 0.30 | -0.11 | 0.007 | 3.02 x10-65 | 0 | 0 | NA | NA |
Table 2. List of top 10 genotyped male pattern baldness GWAS hits for the X chromosome.
| Chr | BP | SNP ID | Effect Allele | Beta | SE | P | SNPs in clump | SNPs with P<0.0001 |
|---|---|---|---|---|---|---|---|---|
| X | 65933285 | rs73221556 | A | -0.53 | 0.02 | <5.1 x10-178 | 19 | 19 |
| X | 66481800 | rs12558842 | C | -0.54 | 0.03 | <5.1 x10-178 | 8 | 8 |
| X | 67003584 | rs5919427 | C | -0.35 | 0.02 | 5.1 x10-178 | 11 | 11 |
| X | 67496002 | rs140488081 | T | -0.40 | 0.01 | 4.6 x10-61 | 9 | 4 |
| X | 65083247 | rs147154263 | T | -0.43 | 0.02 | 3.6 x10-58 | 2 | 2 |
| X | 67139063 | rs148652266 | A | -0.51 | 0.01 | 7.7 x10-45 | 1 | 1 |
| X | 65541956 | rs145867342 | T | -0.32 | 0.01 | 1.1 x10-44 | 2 | 2 |
| X | 67363801 | rs7061504 | G | 0.19 | 0.04 | 1.7 x10-38 | 5 | 4 |
| X | 58005480 | rs147829649 | G | -0.28 | 0.01 | 1.3 x10-31 | 1 | 0 |
| X | 66337545 | rs17216820 | T | 0.19 | 0.02 | 5.8 x10-26 | 2 | 2 |
The gene-based analysis identified 112 autosomal genes and 13 X chromosome genes that were associated with baldness after a Bonferroni correction (P<0.05/18,061 and P<0.05/567, respectively). The top gene-based hit was, as expected, the androgen receptor on the X chromosome (P = 2.0x10-269). A full list of the autosomal significant gene-based hits is provided in S3 Table and significant genes on the X chromosome are shown in S4 Table. A significant enrichment (FDR <0.05) was found for 143 gene sets; the full results are presented in S5 Table.
Using common genetic variants with a minor allele frequency of at least 1%, GCTA-GREML analysis found that 47.3% (SE 1.3%) of the variance in baldness can be explained by common autosomal genetic variants, while 4.6% (SE 0.3%) can be explained by common X chromosome variants.
Genetic correlations were examined between male pattern baldness and 24 cognitive, health, and anthropometric traits using LD Score regression. No significant associations were found; all estimates were close to zero (S6 Table).
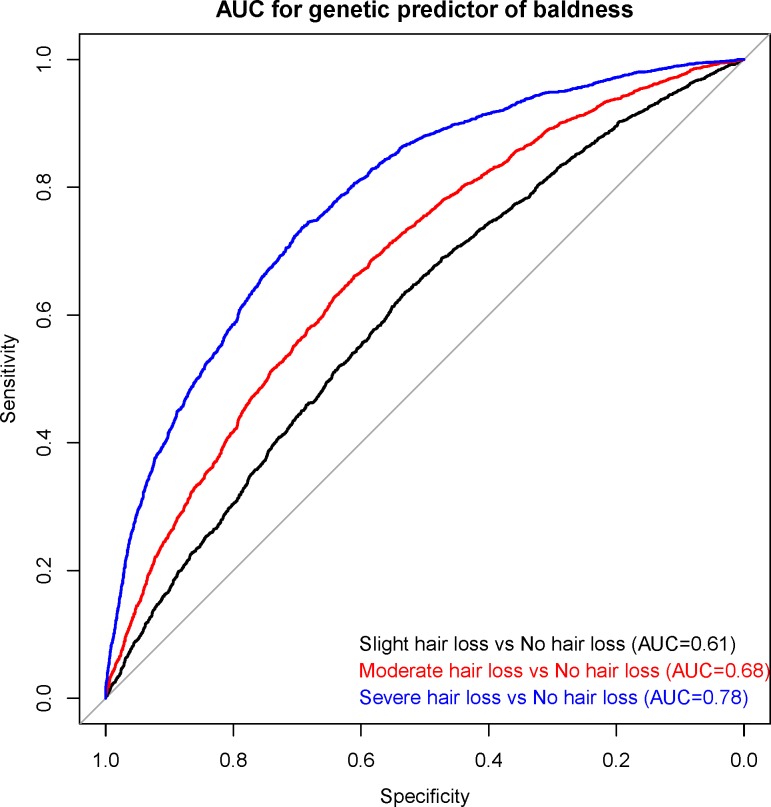
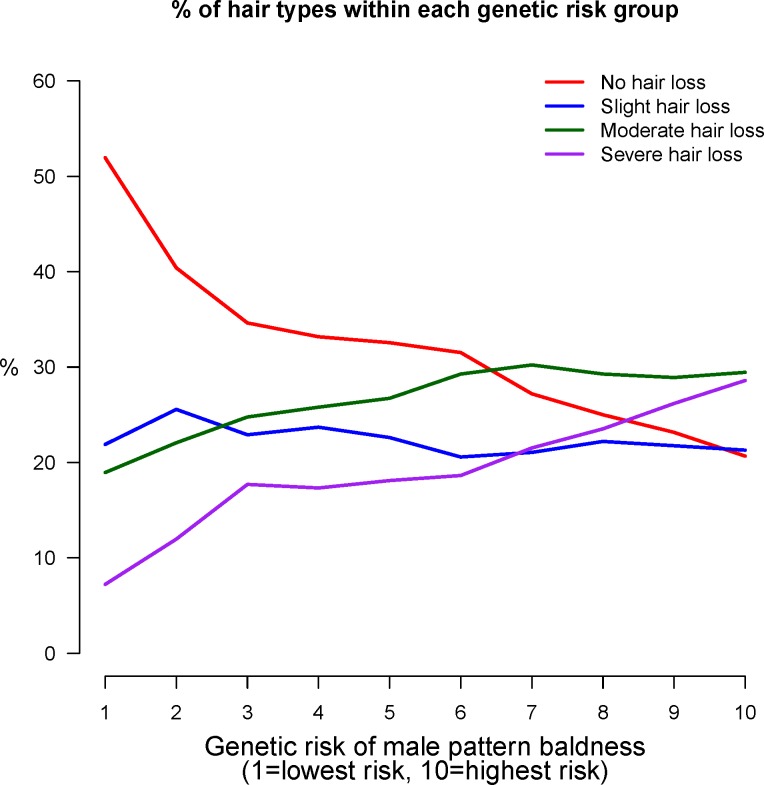
The GWAS for self-reported baldness was re-run on a sub-sample of 40,000 individuals—retaining an equal proportion of each of the four baldness patterns as observed in the full cohort—to allow a polygenic prediction score to be built and applied to the remaining, independent sample of 12,874 individuals. The most powerful predictions from comparing the extreme phenotype groups were observed at the P<1x10-5 threshold for both the autosomal and X chromosome polygenic scores (Table 3). The optimal autosomal polygenic score yielded an AUC of 0.75 for discriminating between those with no hair loss (n = 4,123) and those with severe hair loss (n = 2,456). The corresponding AUC for the optimal X chromosome polygenic score was 0.62. An additive combination of the autosomal and X chromosome polygenic scores gave an AUC of 0.78 (sensitivity = 0.74, specificity = 0.69, PPV = 0.59, NPV = 0.82) for severe hair loss, 0.68 (sensitivity = 0.66, specificity = 0.61, PPV = 0.58, NPV = 0.68) for moderate hair loss, and 0.61 (sensitivity = 0.64, specificity = 0.53, PPV = 0.49, NPV = 0.68) for slight hair loss (Fig 2). Adding age as a covariate boosted the AUC to 0.79 for severe hair loss (P<2x10-16), 0.70 for moderate hair loss (P<2x10-16), and 0.61 for slight hair loss (P = 0.019). Fig 3 shows the proportion of participants in the four baldness groups for each polygenic risk decile of male pattern baldness. Of those with a baldness polygenic score below the median, 14% reported severe hair loss and 39% no hair loss. By contrast, of those with a polygenic score in the top 10%, 58% reported moderate-to-severe hair loss.
Table 3. AUC results for severe hair loss versus no hair loss for all autosomal and X chromosome polygenic thresholds.
| Genomic region | P value threshold | n SNPs | AUC |
|---|---|---|---|
| Autosomal | <1x10-20 | 11 | 0.648 |
| <5x10-8 | 107 | 0.725 | |
| <1x10-5 | 261 | 0.748 | |
| <0.01 | 7365 | 0.725 | |
| <0.05 | 28097 | 0.701 | |
| <0.10 | 51178 | 0.687 | |
| <0.50 | 205679 | 0.663 | |
| <1 | 346958 | 0.662 | |
| X chromosome | <1x10-20 | 16 | 0.606 |
| <5x10-8 | 44 | 0.612 | |
| <1x10-5 | 70 | 0.621 | |
| <0.01 | 284 | 0.619 | |
| <0.05 | 785 | 0.618 | |
| <0.10 | 1329 | 0.615 | |
| <0.50 | 4746 | 0.611 | |
| <1 | 7989 | 0.611 |
Fig 2. Area under the curve plot for discriminating those with hair loss from those with no loss.
Fig 3. Distribution of hair loss by male pattern baldness polygenic score decile in the independent sample.
The results of the partitioned heritability analysis indicated that 27 of the functional annotations from the baseline model were statistically significant (S2 Fig and S7 Table). These significant annotations included a broad array of functional elements including histone marks, enhancer regions, conserved regions, and DNaseI hypersensitivity sites (DHS). The ten tissue types were then tested for significance after controlling for the baseline model. Following correction for multiple testing, all ten of the tissue groups showed significant enrichment (S3 Fig and S7 Table).
Discussion
In this large GWAS study of male pattern baldness, we identified 287 independent genetic signals that were linked to differences in the trait, a substantial advance over the previous largest GWAS meta-analysis, which identified eight independent signals [15]. We showed—in line with a previous study [13], but with much greater precision—that a substantial proportion of individual differences in hair loss patterns can be explained by common genetic variants on the autosomes as well as on the X chromosome. However, the variance explained by X chromosome variants is much lower for late-onset compared to early-onset male pattern baldness [13]. Finally, by splitting our cohort into a discovery and a prediction sample, we showed a predictive discrimination (AUC = 0.78) between those with no hair loss and those with severe hair loss.
Despite there being genetic overlap for SNP hits associated with baldness and Parkinson’s Disease—first noted in Li et al. [15] and replicated here—we observed no statistically significant genetic correlations after correcting for multiple testing between baldness and any of the health, cognitive, or anthropometric outcomes we studied. There were very small (maximum absolute genetic correlation of 0.13) but nominally-significant associations with height, bipolar disorder, number of children born, and age at menarche, such that the genes associated with more hair loss were linked to shorter stature, younger age at menarche, fewer offspring, and a lower risk of bipolar disorder (all P<0.05). The local but not global overlap of SNPs associated with baldness and other traits, such as Parkinson's Disease, might be explained by chance due to the large number of hits for baldness, or pleiotropy at single sites with no systemic overlap. The point estimates for the genetic correlations were all near zero, suggesting true null associations as opposed to a lack of statistical power to detect modest-sized correlations.
As mentioned above, the GWAS identified 247 independent autosomal loci and 40 independent X chromosome loci. The top 20 hits from the autosomes were located in or near to genes that have been associated with, for example, hair growth/length in mice (FGF5) [20], grey hair (IRF4) [21], cancer (breast: MEMO1 [22], bladder: SLC14A2 [23]), histone acetylation (HDAC9), and frontotemporal dementia (MAPT) [24]. A previous GWAS showed an association of IRF4 with both hair colour and hair greying, but not with male pattern baldness [21]. HDAC9 has been identified as a baldness susceptibility gene in a previous study [25]. Two of the top 10 X chromosome SNPs were located in OPHN1, a gene previously associated with X-linked mental retardation [26].
Of the top autosomal gene-based findings (maximum P = 3.1x10-15), RSPO2 has been linked to hair growth in dogs. PGDFA has been linked to hair follicle development [27]; EBF1 is expressed in dermal papillae in mature hair follicles [28]; PRR23B is proximal to a GWAS hit for eyebrow thickness [21]; and WNT10A has been linked to both straight hair [29] and dry hair [30]. The WNT signaling pathway is involved in the activation of β-catenin, which regulates the differentiation of follicular keratinocytes, which form the hair follicle [31].
The top X chromosome gene-based findings included the androgen receptor (AR), which has been well established as a baldness associated gene [32], along with its upstream (EDA2R) and downstream (OPHN1) genes. EDA2R plays a role in the maintenance of hair and teeth as part of the tumor necrosis factor receptor. Onset of male pattern baldness could be influenced by EDA2R via activation of nuclear proto-oncoprotein c-Jun, which is linked to transcription activation of AR [33]. Two other genes included in the gene-based findings, OPHN1 and ZC4H2, have previously been associated with X-linked mental retardation [26, 34]. One limitation of our X chromosome analysis was that it contained genotyped SNPs only. The imputed X chromosome SNP data for UK Biobank have not yet been released but will likely provide further clues about the genetic architecture of male pattern baldness.
Many of the genes identified are associated with hair structure and development, which may be critical for the process of hair loss. For example, animal models indicate that FGF5 is critical for the inhibition of hair growth and mutations in FGF5 are associated with excessively long eyelashes in humans [35]. It is possible that genetic variants leading to higher levels of expression of this gene result in greater inhibition of hair growth, leading to male pattern baldness. As a second example, the RSPO2 gene is associated with hair growth in dogs [36]. It is part of the Wnt signalling pathway needed for the establishment of hair follicles [37]. Variation in the activity of this pathway caused by genetic variants within PSPO2 may lead to differences in levels of hair growth in men and may contribute to male pattern baldness. The inclusion of hits on the X chromosome, specifically the Androgen Receptor, suggests that hormonal mechanisms are also involved in hair loss. It is possible that the hair structure proteins interact biologically with sex hormones, leading to a higher prevalence of baldness.
The results of the gene set analysis indicated that the genomic regions with the greatest evidence for association with male pattern baldness are united by a shared biological theme. In particular, these associated regions appear to converge on the transcription factor complex, and transcription factor binding gene sets.
The most significant gene set, GO:0005667, corresponded to the transcription factor complex gene set, which includes the gene ALX4. ALX4 was found to be mutated in a patient with frontonasal dysplasia, presenting with alopecia [38]. Of the other genome-wide significant gene sets, ENSG00000141027 (NCOR1 subnetwork), includes members of the histone deacetylase (HDAC) family [39]. HDAC9 is associated with male pattern baldness (the present paper and Li et al. [15]). GO:0003682 (transcription factor binding), includes the murine gene Cux1 that is important for, amongst other things, hair growth [40]. GO:0003712 (transcription cofactor activity), includes the gene AIRE, which is associated with alopecia [41]. MP:0000097, (short maxilla), and GO:0044212 (transcription regulatory region DNA binding), both include the murine gene Grhl1. Grhl1-null mice suffer from a delay in coat growth and later hair loss [42]. It is important to note that, as with all pathway analyses, the results are dependent on the gene sets defined in the databases used. These rely on accurate functional annotations, which are continually updated.
The main strength of this study is its large sample size and phenotypic homogeneity. Many meta-analytic studies of complex traits are weakened by different cohorts collecting data at different time-points, under different protocols, in different populations. The present study replicated all of the previously identified autosomal hits for baldness from Li et al. [15] and Heilmann-Heimbach et al.,[16] suggesting a degree of robustness in phenotypic measurement, which was briefer here than in previous studies of male pattern baldness. Whereas the genomic inflation factor from the GWAS was large (1.09, Q-Q plot in S1 Fig), this is likely to be a result of genuine polygenic effects. We have used identical analysis protocols for other traits with far lower SNP-based heritabilities in the same UK Biobank cohort and observed no evidence of inflation [43].
Conclusion
We identified over two hundred independent, novel genetic correlates of male pattern baldness—an order of magnitude greater than the list of previous genome-wide hits. Our top SNP and gene-based hits were in genes that have previously been associated with hair growth and development. We also generated a polygenic predictor that discriminated between those with no hair loss and those with severe hair loss. Whereas accurate predictions for an individual are still relatively crude, of those with a genetic score in the top 10% of the distribution, 58% reported moderate-to-severe hair loss. The release of genetic data on the full UK Biobank cohort will further refine these predictions and increase our understanding of the genetic architecture of male pattern baldness.
Methods
Data
Data came from the first release of genetic data of the UK Biobank study and analyses were performed under the data application 10279. Ethical approval for UK Biobank was granted by the Research Ethics Committee (11/NW/0382).
Genotyping information
Genotyping details including quality control steps have been reported previously [43]. Briefly, from the sample with genetic data available as of June 2015, 112,151 participants remained after the following exclusion criteria were applied: SNP missingness, relatedness, gender mismatch, non-British ancestry, and failed quality control for the UK BiLEVE study [43]. For the current analysis, an imputed dataset was used for the autosomes (reference set panel combination of the UK10K haplotype and 1000 Genomes Phase 3 panels: http://biobank.ctsu.ox.ac.uk/crystal/refer.cgi?id=157020). Imputed data were not available for the X chromosome, hence only genotyped variants were considered. X chromosome quality control steps included a minor allele frequency cut-off of 1% and a genotyping call rate cut-off of 98% [44]. For the imputed autosomal data, we restricted the analyses to variants with a minor allele frequency >0.1% and an imputation quality score >0.1.
Male pattern baldness phenotype
From the sample of 112,151 unrelated White British participants with genetic data, we identified 52,874 men with a self-reported response to UK Biobank question 2395, which was adapted from the Hamilton-Norwood scale [45, 46]. These men were asked to choose, from four patterns (no loss; slight loss; moderate loss; severe loss), the one that matched their hair coverage most closely. Fig 4 shows a screenshot of the four options.
Fig 4. Screenshot of UK Biobank question 2395 on male pattern baldness, adapted with permission.

GWAS of male pattern baldness on the whole sample
A genome-wide association study was conducted using baldness pattern residuals as the dependent variable. The residuals were obtained from a linear regression model of baldness pattern on age, assessment centre, genotyping batch and array, and 10 principal components to correct for population stratification.
The GWAS for the imputed autosomal dataset was performed in SNPTest v2.5.1 [47] via an additive model, using genotype probability scores. The GWAS for the X chromosome was performed in Plink [48, 49].
Identification of independent GWAS signals
The number of independent signals from the GWAS was determined using LD-clumping [48, 49] based on the LD structure annotated in the 1000 genomes project [50]. Index SNPs were identified (P<5x10-8) and clumps were formed for SNPs with P<1x10-5 that were in LD (R2>0.1) and within 500kb of the index SNP. SNPs were assigned to no more than one clump.
Lookup of published male pattern baldness hits
GWAS lookups were performed for the top hits reported in Richards et al. [10], Li et al. [15], Heilmann-Heimbach et al. [16], Liu et al. [13] and Pickrell et al. [14].
Gene-based correlates of male pattern baldness
Gene-based analyses were performed using MAGMA [51]. SNPs were mapped to genes according to their position in the NCBI 37.3 build map. No additional boundary was added beyond the genes start and stop site. For the autosomal genes the summary statistics from the imputed GWAS were used to derive gene-based statistics using the 1000 genomes (phase 1, release 3) to model linkage disequilibrium. For genes on the X chromosome the genotype data from UK Biobank was used and the gene-based statistic was derived using each participant’s phenotype score. Gene-set pathway analyses were carried out in DEPICT [52] using the genome-wide significant autosomal SNPs as input.
GWAS of male pattern baldness on a sub-sample of 40,000 and trait prediction in the residual sample of 12,874 participants
For the prediction analysis, the GWAS was re-run on a randomly selected cohort of 40,000 individuals to give regression weights for prediction, leaving an independent cohort of 12,874 in which to test the polygenic predictor. The methods for the GWAS were identical to those reported for the full sample. The regression weights from the GWAS on the 40,000 cohort were used to construct polygenic scores in the target dataset at P value thresholds of <1x10-20, <5x10-8, <1x10-5, <0.01, <0.05, <0.1, <0.2, <0.5, <1 using PRSice software [53]. PRSice creates polygenic scores by calculating the sum of alleles associated with male pattern baldness across many genetic loci, weighted by their effect sizes estimated from the male pattern baldness GWAS. Prior to calculating the scores, SNPs in the prediction dataset were clumped across 250kb sliding windows at an R2>0.25. Thereafter, each threshold was used to discriminate between those with no hair loss and those with severe hair loss via logistic regression with results being reported for the optimal predictor only. A predictor for both the autosomes and X chromosome were built and assessed independently and additively. Receiver operator characteristic (ROC) curves were plotted and areas under the curve (AUC) were calculated using the pROC package in R [54, 55].
Heritability of male pattern baldness
SNP-based heritability of baldness was estimated using GCTA-GREML [56] after applying a relatedness cut-off of >0.025 in the generation of the autosomal (but not X chromosome) genetic relationship matrix.
Genetic correlations with male pattern baldness
Linkage disequilibrium score (LDS) regression analyses [57] were used to generate genetic correlations between baldness and 24 cognitive, anthropometric, and health outcomes, where phenotypic correlations or evidence of shared genetic architecture have been found (S7 Table). Due to the large effects in the APOE region for Alzheimer's disease, 500kb was removed from around each side of this region and the analysis was repeated for the Alzheimer's—male pattern baldness analysis. The Alzheimer’s data set without this region is referred to as 'Alzheimer’s 500kb'. In total, we carried out 25 hypothesis tests. Multiple testing was controlled for using a false discovery rate (FDR) correction [58]. An overview of the GWAS summary data for the anthropometric and health outcomes is provided in S1 Appendix.
Partitioned heritability of male pattern baldness
Stratified linkage disequilibrium score (SLDS) regression [59] was used to determine if a specific group of SNPs made a greater contribution to the heritability of male pattern baldness than would be expected by the size of the SNP set. Firstly, a baseline model was derived using 52 overlapping, functional categories. Secondly, a cell-specific model was constructed by adding each of the 10 cell-specific functional groups to the baseline model and the level of enrichment was obtained. Multiple testing was controlled for using FDR correction [58] in both the functional category and cell-specific analysis.
Supporting information
(XLSX)
(XLSX)
NSNPS is the number of SNPs in the gene.
(XLSX)
NSNPS is the number of SNPs in the gene.
(XLSX)
(XLSX)
The heritability Z-score and the mean χ2 indicate the level of power to detect association where a heritability Z-score of >4 and a mean χ2 >1.02 being considered well powered [57]. None of the 25 tests performed survived FDR control for multiple comparisons. Nominally significant genetic correlations highlighted in bold. ADHD, attention deficit hyperactivity disorder; MDD, major depressive disorder.
(XLSX)
Prop._SNPs refers to the proportion of SNPs from the data set that were a part of the corresponding functional annotation. Statistical significance indicated in bold. Tissue groups are listed in the first ten rows followed by the functional annotation groups.
(XLSX)
Verbal-numerical reasoning and childhood intelligence were examined as educational attainment (genetic association with baldness reported by Pickrell et al. 2016 [14]) can be used as a proxy phenotype for general cognitive ability. Metabolic traits were included as metabolic disease has been associated with baldness (references noted in the review paper by Heilmann-Heimbach et al. 2016 [16]). Psychiatric disorders were included due to the association between baldness and neurological conditions such as Parkinson’s disease. Genetic correlations have been observed between baldness and the listed anthropometric and developmental traits [14]. Fertility traits [60] were selected due to the published associations between baldness and the androgen receptor.
(XLSX)
(DOCX)
(PDF)
The enrichment statistic is the proportion of heritability found in each functional group divided by the proportion of SNPs in each group (Pr(h2)/Pr(SNPs)). Error bars are jackknife standard errors around the estimate of enrichment. The dashed line indicates no enrichment found when Pr(h2)/Pr(SNPs) = 1. FDR correction indicated significance at P = 0.011 indicated by asterisk
(TIF)
In each functional group divided by the proportion of SNPs in each group (Pr(h2)/Pr(SNPs). Error bars are jackknife standard errors around the estimate of enrichment. The dashed line indicates no enrichment found when Pr(h2)/Pr(SNPs) = 1. FDR correction indicated significance at P = 0.037 indicated by asterisk.
(TIF)
Data availability
Summary data from this paper can be downloaded from the Centre for Cognitive Ageing and Cognitive Epidemiology website (http://www.ccace.ed.ac.uk/node/335).
Funding Statement
This research was conducted, using the UK Biobank Resource, in The University of Edinburgh Centre for Cognitive Ageing and Cognitive Epidemiology, part of the cross-council Lifelong Health and Wellbeing Initiative (MR/K026992/1). Funding from the Biotechnology and Biological Sciences Research Council (BBSRC) and Medical Research Council (MRC) is gratefully acknowledged. WDH is supported by a grant from Age UK (Disconnected Mind Project). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Hamilton JB. Patterned loos of hair in man: types and incidence. Annals of the New York Academy of Sciences. 1951;53(3):708–28. [DOI] [PubMed] [Google Scholar]
- 2.Alfonso M, Richter-Appelt H, Tosti A, Viera MS, García M. The psychosocial impact of hair loss among men: a multinational European study. Current Medical Research and Opinion. 2005;21(11):1829–36. 10.1185/030079905X61820 [DOI] [PubMed] [Google Scholar]
- 3.Cash TF. The psychosocial consequences of androgenetic alopecia: a review of the research literature. British Journal of Dermatology. 1999;141(3):398–405. [DOI] [PubMed] [Google Scholar]
- 4.Cremers RG, Aben KK, Vermeulen SH, den Heijer M, van Oort IM, Kiemeney LA. Androgenic alopecia is not useful as an indicator of men at high risk of prostate cancer. European Journal of Cancer. 2010;46(18):3294–9. 10.1016/j.ejca.2010.05.020 [DOI] [PubMed] [Google Scholar]
- 5.Zhou CK, Levine PH, Cleary SD, Hoffman HJ, Graubard BI, Cook MB. Male Pattern Baldness in Relation to Prostate Cancer–Specific Mortality: A Prospective Analysis in the NHANES I Epidemiologic Follow-up Study. American Journal of Epidemiology. 2016;183(3):210–7. 10.1093/aje/kwv190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou CK, Pfeiffer RM, Cleary SD, Hoffman HJ, Levine PH, Chu LW, et al. Relationship Between Male Pattern Baldness and the Risk of Aggressive Prostate Cancer: An Analysis of the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial. Journal of Clinical Oncology. 2015;33(5):419–25. 10.1200/JCO.2014.55.4279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Christoffersen M, Frikke-Schmidt R, Schnohr P, Jensen GB, Nordestgaard BG, Tybjaerg-Hansen A. Visible Age-Related Signs and Risk of Ischemic Heart Disease in the General Population: A Prospective Cohort Study. Circulation. 2013. [DOI] [PubMed] [Google Scholar]
- 8.Shahar E, Heiss G, Rosamond WD, Szklo M. Baldness and Myocardial Infarction in Men: The Atherosclerosis Risk in Communities Study. American Journal of Epidemiology. 2008;167(6):676–83. 10.1093/aje/kwm365 [DOI] [PubMed] [Google Scholar]
- 9.Trieu N, Eslick GD. Alopecia and its association with coronary heart disease and cardiovascular risk factors: A meta-analysis. International Journal of Cardiology. 2014;176(3):687–95. 10.1016/j.ijcard.2014.07.079 [DOI] [PubMed] [Google Scholar]
- 10.Richards JB, Yuan X, Geller F, Waterworth D, Bataille V, Glass D, et al. Male-pattern baldness susceptibility locus at 20p11. Nat Genet. 2008;40(11):1282–4. 10.1038/ng.255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nyholt DR, Gillespie NA, Heath AC, Martin NG. Genetic Basis of Male Pattern Baldness. Journal of Investigative Dermatology. 2003;121(6):1561–4. 10.1111/j.1523-1747.2003.12615.x [DOI] [PubMed] [Google Scholar]
- 12.Rexbye H, Petersen I, Iachina M, Mortensen J, McGue M, Vaupel JW, et al. Hair Loss Among Elderly Men: Etiology and Impact on Perceived Age. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2005;60(8):1077–82. [DOI] [PubMed] [Google Scholar]
- 13.Liu F, Hamer MA, Heilmann S, Herold C, Moebus S, Hofman A, et al. Prediction of male-pattern baldness from genotypes. Eur J Hum Genet. 2016;24(6):895–902. 10.1038/ejhg.2015.220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pickrell JK, Berisa T, Liu JZ, Segurel L, Tung JY, Hinds DA. Detection and interpretation of shared genetic influences on 42 human traits. Nat Genet. 2016;48(7):709–17. 10.1038/ng.3570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li R, Brockschmidt FF, Kiefer AK, Stefansson H, Nyholt DR, Song K, et al. Six Novel Susceptibility Loci for Early-Onset Androgenetic Alopecia and Their Unexpected Association with Common Diseases. PLoS Genet. 2012;8(5):e1002746 10.1371/journal.pgen.1002746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heilmann-Heimbach S, Hochfeld LM, Paus R, Nöthen MM. Hunting the genes in male-pattern alopecia: how important are they, how close are we and what will they tell us? Experimental Dermatology. 2016;25(4):251–7. 10.1111/exd.12965 [DOI] [PubMed] [Google Scholar]
- 17.Heilmann S, Brockschmidt FF, Hillmer AM, Hanneken S, Eigelshoven S, Ludwig KU, et al. Evidence for a polygenic contribution to androgenetic alopecia. British Journal of Dermatology. 2013;169(4):927–30. 10.1111/bjd.12443 [DOI] [PubMed] [Google Scholar]
- 18.Marcińska M, Pośpiech E, Abidi S, Andersen JD, van den Berge M, Carracedo Á, et al. Evaluation of DNA Variants Associated with Androgenetic Alopecia and Their Potential to Predict Male Pattern Baldness. PLoS ONE. 2015;10(5):e0127852 10.1371/journal.pone.0127852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sudlow C, Gallacher J, Allen N, Beral V, Burton P, Danesh J, et al. UK Biobank: An Open Access Resource for Identifying the Causes of a Wide Range of Complex Diseases of Middle and Old Age. PLoS Med. 2015;12(3):e1001779 10.1371/journal.pmed.1001779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hébert JM, Rosenquist T, Götz J, Martin GR. FGF5 as a regulator of the hair growth cycle: Evidence from targeted and spontaneous mutations. Cell. 1994;78(6):1017–25. [DOI] [PubMed] [Google Scholar]
- 21.Adhikari K, Fontanil T, Cal S, Mendoza-Revilla J, Fuentes-Guajardo M, Chacón-Duque J-C, et al. A genome-wide association scan in admixed Latin Americans identifies loci influencing facial and scalp hair features. Nature Communications. 2016;7:10815 10.1038/ncomms10815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sorokin AV, Chen J. MEMO1, a new IRS1-interacting protein, induces epithelial-mesenchymal transition in mammary epithelial cells. Oncogene. 2013;32(26):3130–8. 10.1038/onc.2012.327 [DOI] [PubMed] [Google Scholar]
- 23.Rafnar T, Vermeulen SH, Sulem P, Thorleifsson G, Aben KK, Witjes JA, et al. European genome-wide association study identifies SLC14A1 as a new urinary bladder cancer susceptibility gene. Human Molecular Genetics. 2011;20(21):4268–81. 10.1093/hmg/ddr303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rademakers R, Cruts M, van Broeckhoven C. The role of tau (MAPT) in frontotemporal dementia and related tauopathies. Human Mutation. 2004;24(4):277–95. 10.1002/humu.20086 [DOI] [PubMed] [Google Scholar]
- 25.Brockschmidt FF, Heilmann S, Ellis JA, Eigelshoven S, Hanneken S, Herold C, et al. Susceptibility variants on chromosome 7p21.1 suggest HDAC9 as a new candidate gene for male-pattern baldness. British Journal of Dermatology. 2011;165(6):1293–302. 10.1111/j.1365-2133.2011.10708.x [DOI] [PubMed] [Google Scholar]
- 26.Billuart P, Bienvenu T, Ronce N, des Portes V, Vinet MC, Zemni R, et al. Oligophrenin-1 encodes a rhoGAP protein involved in X-linked mental retardation. Nature. 1998;392(6679):923–6. 10.1038/31940 [DOI] [PubMed] [Google Scholar]
- 27.Karlsson L, Bondjers C, Betsholtz C. Roles for PDGF-A and sonic hedgehog in development of mesenchymal components of the hair follicle. Development. 1999;126(12):2611–21. [DOI] [PubMed] [Google Scholar]
- 28.Rendl M, Lewis L, Fuchs E. Molecular Dissection of Mesenchymal–Epithelial Interactions in the Hair Follicle. PLoS Biol. 2005;3(11):e331 10.1371/journal.pbio.0030331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Medland SE, Nyholt DR, Painter JN, McEvoy BP, McRae AF, Zhu G, et al. Common Variants in the Trichohyalin Gene Are Associated with Straight Hair in Europeans. The American Journal of Human Genetics. 2009;85(5):750–5. 10.1016/j.ajhg.2009.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Adaimy L, Chouery E, Mégarbané H, Mroueh S, Delague V, Nicolas E, et al. Mutation in WNT10A Is Associated with an Autosomal Recessive Ectodermal Dysplasia: The Odonto-onycho-dermal Dysplasia. The American Journal of Human Genetics. 2007;81(4):821–8. 10.1086/520064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huelsken J, Vogel R, Erdmann B, Cotsarelis G, Birchmeier W. β-Catenin Controls Hair Follicle Morphogenesis and Stem Cell Differentiation in the Skin. Cell. 2001;105(4):533–45. [DOI] [PubMed] [Google Scholar]
- 32.Hillmer AM, Hanneken S, Ritzmann S, Becker T, Freudenberg J, Brockschmidt FF, et al. Genetic Variation in the Human Androgen Receptor Gene Is the Major Determinant of Common Early-Onset Androgenetic Alopecia. The American Journal of Human Genetics. 2005;77(1):140–8. 10.1086/431425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Prodi DA, Pirastu N, Maninchedda G, Sassu A, Picciau A, Palmas MA, et al. EDA2R Is Associated with Androgenetic Alopecia. Journal of Investigative Dermatology. 2008;128(9):2268–70. 10.1038/jid.2008.60 [DOI] [PubMed] [Google Scholar]
- 34.Hirata H, Nanda I, van Riesen A, McMichael G, Hu H, Hambrock M, et al. ZC4H2 Mutations Are Associated with Arthrogryposis Multiplex Congenita and Intellectual Disability through Impairment of Central and Peripheral Synaptic Plasticity. The American Journal of Human Genetics. 2013;92(5):681–95. 10.1016/j.ajhg.2013.03.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Higgins CA, Petukhova L, Harel S, Ho YY, Drill E, Shapiro L, et al. FGF5 is a crucial regulator of hair length in humans. Proceedings of the National Academy of Sciences. 2014;111(29):10648–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cadieu E, Neff MW, Quignon P, Walsh K, Chase K, Parker HG, et al. Coat Variation in the Domestic Dog Is Governed by Variants in Three Genes. Science. 2009;326(5949):150–3. 10.1126/science.1177808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Andl T, Reddy ST, Gaddapara T, Millar SE. WNT Signals Are Required for the Initiation of Hair Follicle Development. Developmental Cell. 2002;2(5):643–53. [DOI] [PubMed] [Google Scholar]
- 38.Ferrarini A, Gaillard M, Guerry F, Ramelli G, Heidi F, Keddache CV, et al. Potocki–shaffer deletion encompassing ALX4 in a patient with frontonasal dysplasia phenotype. American Journal of Medical Genetics Part A. 2014;164(2):346–52. [DOI] [PubMed] [Google Scholar]
- 39.Orii N, Ganapathiraju MK. Wiki-Pi: A Web-Server of Annotated Human Protein-Protein Interactions to Aid in Discovery of Protein Function. PLoS ONE. 2012;7(11):e49029 10.1371/journal.pone.0049029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alcalay NI, Heuvel GBV. Regulation of cell proliferation and differentiation in the kidney. Frontiers in bioscience (Landmark edition). 2009;14:4978–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alzolibani AA, Zari S, Ahmed AA. Epidemiologic and genetic characteristics of alopecia areata (part 2). Acta Dermatovenerol Alp Pannonica Adriat. 2012;21(1):15–9. [PubMed] [Google Scholar]
- 42.Wilanowski T, Caddy J, Ting SB, Hislop NR, Cerruti L, Auden A, et al. Perturbed desmosomal cadherin expression in grainy head‐like 1‐null mice. The EMBO Journal. 2008;27(6):886–97. 10.1038/emboj.2008.24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Davies G, Marioni RE, Liewald DC, Hill WD, Hagenaars SP, Harris SE, et al. Genome-wide association study of cognitive functions and educational attainment in UK Biobank (N = 112 151). Mol Psychiatry. 2016;21(6):758–67. 10.1038/mp.2016.45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.König IR, Loley C, Erdmann J, Ziegler A. How to Include Chromosome X in Your Genome-Wide Association Study. Genetic Epidemiology. 2014;38(2):97–103. 10.1002/gepi.21782 [DOI] [PubMed] [Google Scholar]
- 45.Giles GG, Severi G, Sinclair R, English DR, McCredie MRE, Johnson W, et al. Androgenetic Alopecia and Prostate Cancer: Findings from an Australian Case-Control Study. Cancer Epidemiology Biomarkers & Prevention. 2002;11(6):549–53. [PubMed] [Google Scholar]
- 46.Norwood OT. Male pattern baldness: classification and incidence. South Med J. 1975;68(11):1359–65. [DOI] [PubMed] [Google Scholar]
- 47.Marchini J, Howie B, Myers S, McVean G, Donnelly P. A new multipoint method for genome-wide association studies by imputation of genotypes. Nat Genet. 2007;39(7):906–13. 10.1038/ng2088 [DOI] [PubMed] [Google Scholar]
- 48.Chang CC, Chow CC, Tellier LC, Vattikuti S, Purcell SM, Lee JJ. Second-generation PLINK: rising to the challenge of larger and richer datasets. GigaScience. 2015;4(1):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Purcell S, Chang CC. PLINK v1.90b3i. Available from: https://www.cog-genomics.org/plink2.
- 50.The 1000 Genomes Project Consortium. An integrated map of genetic variation from 1,092 human genomes. Nature. 2012;491(7422):56–65. 10.1038/nature11632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.de Leeuw CA, Mooij JM, Heskes T, Posthuma D. MAGMA: Generalized Gene-Set Analysis of GWAS Data. PLoS Comput Biol. 2015;11(4):e1004219 10.1371/journal.pcbi.1004219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pers TH, Karjalainen JM, Chan Y, Westra H-J, Wood AR, Yang J, et al. Biological interpretation of genome-wide association studies using predicted gene functions. Nature Communications. 2015;6:5890 10.1038/ncomms6890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Euesden J, Lewis CM, O’Reilly PF. PRSice: Polygenic Risk Score software. Bioinformatics. 2015;31(9):1466–8. 10.1093/bioinformatics/btu848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.R Core Team. R: A language and environment for statistical computing. 2013.
- 55.Robin X, Turck N, Hainard A, Tiberti N, Lisacek F, Sanchez J-C, et al. pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinformatics. 2011;12(1):77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yang J, Benyamin B, McEvoy BP, Gordon S, Henders AK, Nyholt DR, et al. Common SNPs explain a large proportion of the heritability for human height. Nat Genet. 2010;42(7):565–9. 10.1038/ng.608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bulik-Sullivan BK, Loh P-R, Finucane HK, Ripke S, Yang J, Schizophrenia Working Group of the Psychiatric Genomics C, et al. LD Score regression distinguishes confounding from polygenicity in genome-wide association studies. Nat Genet. 2015;47(3):291–5. 10.1038/ng.3211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society Series B (Methodological). 1995;57(1):289–300. [Google Scholar]
- 59.Finucane HK, Bulik-Sullivan B, Gusev A, Trynka G, Reshef Y, Loh P-R, et al. Partitioning heritability by functional annotation using genome-wide association summary statistics. Nat Genet. 2015;47(11):1228–35. 10.1038/ng.3404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Barban N, Jansen R, de Vlaming R, Vaez A, Mandemakers JJ, Tropf FC, et al. Genome-wide analysis identifies 12 loci influencing human reproductive behavior. Nat Genet. 2016;advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
(XLSX)
NSNPS is the number of SNPs in the gene.
(XLSX)
NSNPS is the number of SNPs in the gene.
(XLSX)
(XLSX)
The heritability Z-score and the mean χ2 indicate the level of power to detect association where a heritability Z-score of >4 and a mean χ2 >1.02 being considered well powered [57]. None of the 25 tests performed survived FDR control for multiple comparisons. Nominally significant genetic correlations highlighted in bold. ADHD, attention deficit hyperactivity disorder; MDD, major depressive disorder.
(XLSX)
Prop._SNPs refers to the proportion of SNPs from the data set that were a part of the corresponding functional annotation. Statistical significance indicated in bold. Tissue groups are listed in the first ten rows followed by the functional annotation groups.
(XLSX)
Verbal-numerical reasoning and childhood intelligence were examined as educational attainment (genetic association with baldness reported by Pickrell et al. 2016 [14]) can be used as a proxy phenotype for general cognitive ability. Metabolic traits were included as metabolic disease has been associated with baldness (references noted in the review paper by Heilmann-Heimbach et al. 2016 [16]). Psychiatric disorders were included due to the association between baldness and neurological conditions such as Parkinson’s disease. Genetic correlations have been observed between baldness and the listed anthropometric and developmental traits [14]. Fertility traits [60] were selected due to the published associations between baldness and the androgen receptor.
(XLSX)
(DOCX)
(PDF)
The enrichment statistic is the proportion of heritability found in each functional group divided by the proportion of SNPs in each group (Pr(h2)/Pr(SNPs)). Error bars are jackknife standard errors around the estimate of enrichment. The dashed line indicates no enrichment found when Pr(h2)/Pr(SNPs) = 1. FDR correction indicated significance at P = 0.011 indicated by asterisk
(TIF)
In each functional group divided by the proportion of SNPs in each group (Pr(h2)/Pr(SNPs). Error bars are jackknife standard errors around the estimate of enrichment. The dashed line indicates no enrichment found when Pr(h2)/Pr(SNPs) = 1. FDR correction indicated significance at P = 0.037 indicated by asterisk.
(TIF)
Data Availability Statement
Summary data from this paper can be downloaded from the Centre for Cognitive Ageing and Cognitive Epidemiology website (http://www.ccace.ed.ac.uk/node/335).