Abstract
Calcium homeostasis modulator 1 (CALHM1), formerly known as FAM26C, was recently identified as a physiologically important plasma membrane ion channel. CALHM1 and its Caenorhabditis elegans homolog, CLHM-1, are regulated by membrane voltage and extracellular Ca2+ concentration ([Ca2+]o). In the presence of physiological [Ca2+]o (~1.5 mM), CALHM1 and CLHM-1 are closed at resting membrane potentials but can be opened by strong de-polarizations. Reducing [Ca2+]o increases channel open probability, enabling channel activation at negative membrane potentials. Together, voltage and Ca2+o allosterically regulate CALHM channel gating. Through convergent evolution, CALHM has structural features that are reminiscent of connexins and pannexins/innexins/LRRC8 (volume-regulated anion channel (VRAC)) gene families, including four trans-membrane helices with cytoplasmic amino and carboxyl termini. A CALHM1 channel is a hexamer of CALHM1 monomers with a functional pore diameter of ~14 Å. CALHM channels discriminate poorly among cations and anions, with signaling molecules including Ca2+ and ATP able to permeate through its pore. CALHM1 is expressed in the brain where it plays an important role in cortical neuron excitability induced by low [Ca2+]o and in type II taste bud cells in the tongue that sense sweet, bitter, and umami tastes where it functions as an essential ATP release channel to mediate nonsynaptic neuro-transmitter release. CLHM-1 is expressed in C. elegans sensory neurons and body wall muscles, and its genetic deletion causes locomotion defects. Thus, CALHM is a voltage- and Ca2+o-gated ion channel, permeable to large cations and anions, that plays important roles in physiology.
Keywords: Connexin, Pannexin, ATP, Taste, Extracellular calcium, Voltage gated
Introduction
Calcium homeostasis modulator 1 (CALHM1), previously known as FAM26C, was discovered in a search for human genes with enriched expression in the hippocampus in a region of chromosome 10 linked to enhanced risk for late-onset Alzheimer’s disease [8]. A nonsynonymous polymorphism, Pro86Leu, was identified as a possible modifier of the age of onset of Alzheimer’s disease [8, 17]. In humans, five homologs of CALHM1 were found by sequence database searches [8]. The CALHM gene family, CALHM1, and its homologs were collectively identified as the FAM26 gene family, where six genes are in two clusters on two chromosomes. CALHM1/ FAM26C is clustered on chromosome 10 with FAM26A and FAM26B genes, which were designated as CALHM3 and CALHM2, respectively. FAM26D, FAM26E, and FAM26F genes, to which CALHM names have not been assigned, are located in a cluster on chromosome 6. All CALHM/FAM26 genes are present throughout vertebrates, but they lack significant sequence homology to other known genes. Outside of vertebrates, CALHM1 homologs are absent in yeast and Drosophila, but Caenorhabditis elegans (C. elegans) possesses a single homolog, clhm-1. The fact that CALHM1 is conserved across >20 species, including C. elegans, mouse, and human, suggested its fundamental importance in biological processes. Until recently, however, no physiological functions of the CALHM proteins were known.
Human CALHM1 is predicted to be a membrane protein with 346 amino acids. Based on membrane topology prediction algorithms, it was originally suggested to contain four transmembrane (TM) spanning helices. Although FAM26 proteins lack sequence similarities with other known proteins, it was speculated that CALHM1 might be related to NMDA receptors, because it possesses an amino acid sequence at the carboxyl terminal end of predicted TM2 that is similar to one in the ion selectivity filter of Ca2+-permeable NMDA receptors [8]. Although immunolocalization of expressed recombinant CALHM1 in mammalian culture cells revealed a predominantly intracellular labeling, whole-cell patch clamp electrophysiology of CALHM1-expressing CHO cells revealed the presence of a new Gd3+-sensitive, Ca2+-permeable outwardly rectifying current [8]. Furthermore, when extracellular Ca2+ was re-introduced to cells that had been exposed for several minutes to a medium lacking Ca2+, a sustained rise in cytoplasmic Ca2+ concentration ([Ca2+]i) was observed specifically in CALHM1-transfected cells [8]. Similar Ca2+-add back responses have been observed in subsequent studies including in single cells, where the possibility of artifactual responses due to leaked extracellular Ca2+ indicator dye was eliminated [11, 20, 36]. The apparent enhanced Ca2+ permeability in response to Ca2+ removal and add-back is insensitive to pharmacologic and genetic inhibition of store-operated Ca2+ entry (SOCE). In addition, the magnitude of CALHM1-dependent and SOCE [Ca2+]i signals is additive [8, 20].
Exogenous expression of CALHM1 or P86L-CALHM1 elevated basal [Ca2+]i [11, 26, 36]. In contrast, the magnitude of the Ca2+ add-back response measured in cell populations was reduced in cells transiently expressing P86L-CALHM1 [8, 9, 26, 36]. Nevertheless, when measured at the single-cell level, the magnitude of the response was not different in some studies [11, 36]. Similarly, W114A-CALHM1-expressing cells have a reduced Ca2+ add-back response compared with cells expressing wild-type CALHM1, whereas the biophysical properties of the CALHM1 channel in Xenopus oocytes are unaffected by the mutation [44]. Fewer P86L-CALHM1-expressing cells that wild-type CALHM1-expressing cells responded with a Ca2+ add-back response, suggesting that this polymorphism as well as the W114A mutation may confer less plasma membrane Ca2+ permeability than wild-type CALHM1 due to trafficking deficits [36].
Together, these results suggested that CALHM1 expression conferred a novel plasma membrane Ca2+ permeability. However, it remained unclear if exposure of the cells to a medium depleted of Ca2+ was required to observe Ca2+ permeability. Furthermore, it remained unknown whether CALHM1 was an ion channel or ion channel regulator. Finally, if CALHM1 was an ion channel, its structural and functional features, regulatory properties, and gating mechanisms were unknown. Here, we summarize recent advances in our understanding of the structural and biophysical properties as well as physiological roles of CALHM ion channels.
Structural features of CALHM1
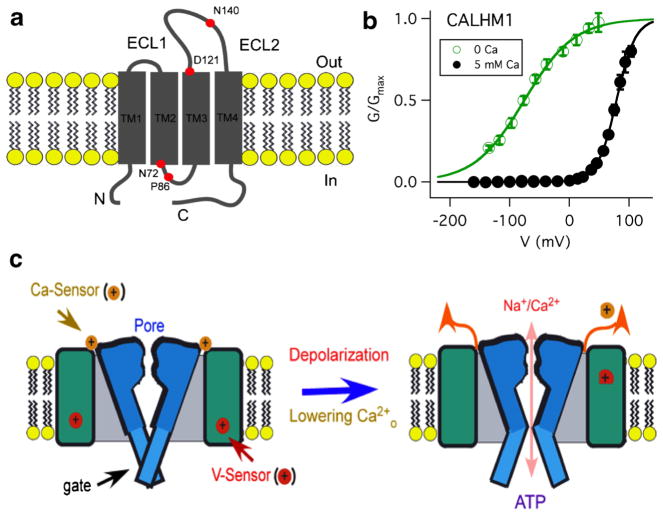
CALHM1 monomers homo-multimerize to form a functional ion channel [8] [20, 36]. To date, no high-resolution structure of a CALHM1 is available. Secondary structural analysis and TM domain prediction algorithms suggested that a CALHM1 monomer has four transmembrane (TM) helices. The topology of the CALHM1 monomer was experimentally determined by demonstrating that Asn140 is glycosylated [8, 36], establishing an extracellular localization of the second extracellular loop (ECL) between putative TM3 and TM4 (Fig. 1a), and that a carboxyl terminal antibody only accessed its epitope from the cytoplasm [36]. These results indicated that a CALHM1 monomer has four TM domains with both the amino and carboxyl termini located in the cytoplasm.
Fig. 1.
Voltage- and Ca2+o-gated CALHM channels. a Transmembrane topology of CALHM channel. Asn72, Pro86, Asp121, and Asn140 are indicated for human CALHM1. ECL1 and ECL2 are extracellular loops 1 and 2, respectively. N amino terminus, C carboxyl terminus, TM transmembrane domain. b Normalized conductance-voltage (G-V) relations for human CALHM1 in 5 mM Ca2+ and 0 Ca2+. Solid lines are Boltzmann function fits to the experimental data. c CALHM1 channel contains a voltage sensor and a Ca2+ sensor that detects extracellular Ca2+ concentration. The CALHM channel gate is closed by physiological [Ca2+]o and hyperpolarized voltages (left). Depolarization and/or lowering [Ca2+]o can open the gate, enabling Na+ and Ca2+ influx and ATP efflux (right)
The CALHM1 channel was established to be a hexamer of CALHM1 monomers [36]. Under nonreducing conditions, two CALHM1 bands were present in SDS-PAGE of lysates from transiently transfected N2A cells, with apparent molecular weights of 80 and 250 kDa, which correspond to two and six times the predicted mass of a CALHM1 monomer. In nondenaturing blue native PAGE, one CALHM1 band was observed at approximately 240 kDa [36]. These results suggested that CALHM1 is a hexamer. Single-molecule subunit counting of a functional carboxyl terminally enhanced green fluorescent protein (EGFP)-tagged CALHM1 (CALHM1-EGFP) expressed in Xenopus oocytes was employed to confirm this biochemical indication. CALHM1-EGFP generated ionic currents similar to untagged CALHM1. Two independent methods were used to detect bleaching steps. Most immobile fluorescent spots bleached in five steps, with many bleaching in six steps, and none bleaching in more than six steps. The distribution of bleaching steps was best fitted with a binomial distribution with six subunits. Together, biophysical measurements and biochemistry suggest that a CALHM1 channel is a hexamer of CALHM1 monomers [36].
This four-TM hexameric structure is shared with connexins [21], pannexins and innexins [47], volume-regulated anion channel (VRAC, aka LRRC8, SWELL1) [31, 45], and Orai1 channels [14]. CALHMs, connexins, and pannexins/innexins/ VRAC also share other secondary structural features. All three families possess an amino terminal α-helix, a beta-sheet in extracellular loop 2 (ECL2), cysteines in both ECLs, and, except for connexins, α-helical regions in the carboxyl termini that align well [36]. Nevertheless, CALHM1 lacks homology with any of these channel families, and it does not have any sequence similarity to other known ion channels, suggesting that it belongs to a novel ion channel family [36].
CALHM1 is the pore-forming subunit of an ion channel
To explore the possible ion channel function of CALHM1, recombinant human CALHM1 (hCALHM1) was expressed in Xenopus oocytes. In solutions containing 2 mM Ca2+ and 1 mM Mg2+, depolarizing the membrane voltage generated large outward currents that activated with slow kinetics (τ~ 3 s at +60 mV) and deactivated at hyperpolarized voltages (τ= 0.2 s at −80 mV) specifically in CALHM1-expressing oocytes [20]. Similar currents were observed in oocytes co-injected with Xenopus connexin-38 antisense oligonucleotide to inhibit endogenous Cx38 currents and with 1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid (BAPTA) to inhibit Ca2+-activated Cl− currents [5]. An EGFP-tagged hCALHM1 had similar gating properties and localized strongly to the plasma membrane [20]. Although currents were observed in oocytes expressing CALHM channels, CALHM1 could either be an ion channel or activate an endogenous conductance. To distinguish between these possibilities, potential pore-lining residues were mutated and ion selectivity was determined. Site-directed mutagenesis of the asparagine at position 72 (Fig. 1a), within the sequence reminiscent of that of the NMDA receptor ion selectivity filter, was without effect on the conductance or ion selectivity [20], suggesting that if CALHM1 was indeed an ion channel, it was unlikely to have structural similarity with the NMDA receptor pore region. CALHM1 is predicted to have four TM helices, similar to Ca2+-selective Orai1 channels, which have acidic residues near the end of TM domains that play key roles in ion permeation [24, 48]. Mutation of aspartic acid at position 121, predicted to reside at the extracellular end of putative TM3 (Fig. 1a), to either alanine, cysteine or arginine changed CALHM1 ion selectivity, whereas mutation to glutamic acid to preserve the negative charge was without effect [20]. These results suggest that CALHM1 is likely a pore-forming subunit of a novel ion channel [20].
To determine whether CALHM1 is the founding member of an ion channel family, the C. elegans CALHM homolog CLHM-1 was expressed in Xenopus oocytes and electrophysiological studies were performed. Although CLHM-1 and hCALHM1 have only ~16 % sequence identity, CLHM-1 localized to the plasma membrane and exhibited channel properties very similar to CALHM1 [37]. Mutation of aspartic acid at position 125 at the extracellular end of putative TM3 altered ion selectivity as observed for the homologous mutation in CALHM1 [37]. It remains to be determined whether TM3 lines the ion permeation pathway in CALHM channels, and how this conserved aspartic acid (Asp121/Asp125) contributes to CALHM channel ion selectivity and conductance. Nevertheless, these results indicate that CALHM proteins are an evolutionarily conserved family of ion channels. Whether other vertebrate CALHM homologs function as ion channels remains unknown.
CALHM channels have weak ion selectivity
Analyses of the ion permeability properties of hCALHM1 and CLHM-1 revealed that they have weak ion selectivities [20, 36, 37]. The relative permeabilities were estimated as PNa/PCa/ PK/PCl = 1:11:1.2:0.6 for hCALHM1 and 1:3.6:1.1:0.5 for CLHM-1, respectively. Similar results were obtained with bath Na+ replaced by K+ in either 0 or 2 mM Ca2+o. Thus, the permeability properties of both channels are quite similar, with a notable anion permeability. It is possible that the relative permeability of Ca2+ may be overestimated, because the changes of the reversal potentials in response to [Ca2+]o were measured at a low NaCl concentration due to difficulty in measuring changes in Ca2+ reversal potentials in normal ex-tracellular NaCl concentration using two-electrode voltage clamp in Xenopus oocytes.
The weak relative ion selectivity of CALHM channels suggests that either the ion selectivity filter is nondiscriminatory or the ion conduction pore is wide. To distinguish these possibilities, reversal potentials were measured with various tetraalkylammonium monovalent cations with different sizes, including tetramethylammonium (TMA+, ionic radius 3.47 Å), tetraethylammonium (TEA+, 4.00 Å), and tetrabutylammonium (TBA+, 4.94 Å) as permeant ions. Relative to Na+, PTMA/PTEA/PTBA is equal to 0.31:0.21:0.07 [36]. A plot of the molecular masses of each amine as well as small monovalent cations against their permeabilities was well fitted with an exponential relationship, suggesting that the size of the cation, rather than binding within the pore, is the major determinant of its permeation [36]. Incorporating viscous drag of each ion in an excluded volume model was used to estimate the functional diameter of the CALHM1 pore to be 14.2 Å. This estimated pore diameter was independently confirmed by optical analyses of the permeation of fluorescent dyes of different sizes [36]. Thus, a wide pore of CALHM channels likely accounts for its weak ion selectivity. The relative ion permeabilities and pore size of CALHM channels are similar to those of connexin hemichannels [1, 21, 46]. However, CALHM1 does not form gap junction channels [36].
CALHM channels are regulated by extracellular [Ca2+]
Removal and subsequent add-back of Ca2+o strongly elevated [Ca2+]i in CALHM1-transfected HT-22 [8] and N2A cells [20]. The relationship between the currents recorded from CALHM1-expressing cells and the Ca2+ add-back response was explored by exposing CALHM1-expressing oocytes to solutions with different [Ca2+]o [20]. Reductions of [Ca2+]o induced large inward currents at −80 mV in oocytes expressing CALHM1 in a reversible and concentration- and time-dependent fashion with half-maximal inhibitory [Ca2+]o of ~220 μM with a Hill coefficient ~2. Importantly, simple surface charge effects could not account for this [Ca2+]o regulation [20]. Similar Ca2+o-dependent CALHM1 gating was also observed in mammalian cells, demonstrating that CALHM1 channel gating is activated by reduced [Ca2+]o as well as de-polarization [20]. This Ca2+o-dependent gating of CALHM1 is the biophysical basis for the Ca2+ add-back responses in CALHM1-expressing cells. The dose-response relation for Mg2+ is similar in shape to that of Ca2+ at the same holding potential, but with an apparent affinity over tenfold lower than that for Ca2+ (IC50 of Mg2+=3.3 mM) [20]. CLHM-1 is similarly regulated by [Ca2+]o and [Mg2+]o [37].
CALHM1 currents exhibit outward rectification in the presence of 2 mM Ca2+o. The rectification could be caused either by voltage-dependent pore block by extracellular divalent cations or by modulation of an intrinsic voltage-dependent gating mechanism. Ca2+o did not alter the linear instantaneous current-voltage (I–V) relation or the single-channel current amplitude and conductance [20, 36, 37], indicating that Ca2+o is unlikely a voltage-dependent pore blocker. Like CALHM channels, connexin hemichannels are activated by a reduction of [Ca2+o] [19, 32]. Neutralization of an aspartic acid residue at position 50 (D50N) in the first ECL of Cx26 strongly altered Ca2+o regulation [19, 35]; however, the mechanism remains poorly defined [12]. Furthermore, as CALHM channels have a much shorter ECL1 (<10 amino acids) compared to connexin hemichannels (~30 amino acids), the relevance of specific ECL1 residues in the regulation of Ca2+o-dependent gating is unclear. Thus, the location of the Ca2+o-binding sites as well as the Ca2+ o regulation mechanism remains unknown for CALHM channels.
CALHM1 and CLHM-1 are weakly voltage-gated ion channels
Since Ca2+o is unlikely to modulate CALHM gating by an extracellular divalent cation pore block mechanism, CALHM channels should either possess intrinsic voltage-dependent gating or pore block by intracellular divalent cations. Injection into Xenopus oocytes of 50 nl of 10 mM BAPTA or a mixture of 10 mM EGTA and 10 mM EDTA to chelate intracellular divalent cations did not alter the weakly voltage-dependent conductance-voltage (G-V) relation in the absence of extracellular divalent cations [20, 37]. Furthermore, in excised inside-out macropatches with pipette and bath solutions lacking divalent cations, the open probability Po of hCALHM1 channels was greater at +80 mV than at −80 mV [20]. These results demonstrated that CALHM1 is a weakly voltage-gated channel that possesses an intrinsic voltage sensor coupled to a gate to open and close it.
The molecular basis for weakly voltage-dependent gating of CALHM channels is unknown. CALHM channels do not have canonical voltage sensors as found in traditional voltage-gated ion channels, which have several basic residues in the S4 segment (or TM4). Structurally analogous connexin hemi-channels are also weakly voltage-dependent and, like CALHM channels, lack canonical voltage sensors. In connexins, negatively charged residues at the second position in the amino terminus and in the interface of TM1 and ECL1 form a charge complex that has been suggested to contribute as integral parts of the voltage sensor [28, 30, 42]. However, the relevance of insights in connexins for CALHM channels remains unclear. hCALHM1 and mouse CALHM1 (mCALHM1) each possess one negatively charged residue at the third and second positions in the amino terminus, respectively, but CLHM-1, which has a similar voltage dependence, lacks charged residues in its amino terminus. Neutralization of the negatively charged residue in hCALHM1 did not alter voltage-dependent gating in either the absence or the presence of Ca2+o (unpublished results). Furthermore, the negatively charged residues in the connexin ECL1 are not conserved in CALHM1 and CLHM-1 channels, and CALHM channels have a much shorter ECL1 compared to those of connexin hemichannels. It was proposed that Ca2+ acts as an intra-pore gating particle in human Cx37 channels [29]. It is possible that the permeant ions contribute to voltage-dependent gating in CALHM channels. In summary, insights into the mechanisms of the intrinsic voltage-dependent gating in CALHM channels remain to be elucidated, and the nature of the voltage sensor in CALHM channels and its motions in response to changes in the membrane potential remain to be identified.
Ca2+o and membrane voltage are allosterically coupled to regulate gating of CALHM channels
Both membrane voltage and [Ca2+]o regulate CALHM channel gating, with depolarization and low [Ca2+]o resulting in activation (Fig. 1c). To understand the relationship between these two modes of gating regulation, the effects of membrane voltage on the [Ca2+]o sensitivity of channel gating were explored. Hyperpolarization of the membrane potential significantly increased the apparent Ca2+ affinity, indicating that Ca2+o regulation is strongly voltage-dependent with membrane hyperpolarization enhancing the efficacy of extracellular Ca2+ to close the channels. It is possible that this coupling arises because the Ca2+-binding sites are located in the membrane electric field or because conformations of the Ca2+-binding sites are voltage-dependent. On the other hand, Ca2+o not only right-shifted the conductance-voltage relation to stabilize channels at closed states without reducing the apparent maximum conductance, but also significantly increased the voltage dependence of CALHM1 gating (Fig. 1b). These results indicate that Ca2+o and membrane voltage are allosterically coupled to open and close the gate of the CALHM1 channel [20]. However, the lack of knowledge regarding identities of the gate, Ca2+-binding sites, and voltage sensor precludes our understanding of this allosteric coupling mechanism.
Pharmacology of CALHM ion channels
The pharmacology of CALHM channels has been explored by measuring recombinant hCALHM currents in Xenopus oocytes [20, 37]. Blockers of voltage-gated K+ channels (10 mM TEA), Na+ channels (10 μM tetrodotoxin), or Ca2+ channels (1 mM verapamil, 100 μM nifedipine); NMDA receptor inhibitors (100 μM MK-801, 100 μM memantine); or pannexin and connexin channel inhibitors (200 μM carbenoxolone, 1 mM probenecid, 30 μM mefloquine, 200 μM quinine) were without effect on CALHM1 currents. Flufenamic acid (200 μM), a Cl− channel blocker, was also without effect, while niflumic acid (200 μM) activated the channel currents weakly. CALHM1 currents are inhibited by relatively nonselective ion channel blockers including Gd3+ (100 μM), ruthenium red (20 μM), and Zn2+ (20 μM), while they are partially blocked by the nonspecific inositol trisphosphate (InsP3) receptor and Orai channel inhibitor 2-APB (1 mM).
Pharmacological properties of the CLHM-1 channel have also been examined [37]. Gd3+ (100 μM) and ruthenium red (80 μM) inhibit CLHM-1 currents, while the currents were partially blocked by Zn2+. CLHM-1 currents were insensitive to MK801 (100 μM), L-type voltage-gated Ca2+ channel blocker nimodipine (10 μM), TRP channel blocker SKF96365 (50 μM), SERCA blocker thapsigargin (100 nM), and niflumic acid (200 μM). Notably, carbenoloxone (200 μM) and octanol (1 mM) augmented CLHM-1 currents. Thus, although specific CALHM channel inhibitors have yet to be found, CALHM1 and CLHM-1 share an appreciably similar pharmacological sensitivity profile, which is distinct from other previously characterized ion channels, including connexin hemichannels. An important goal is to identify specific pharmacologic inhibitors and activators of CALHM channels that can be used in physiological studies to elucidate their functional roles and as probes to provide insights into structure-function relations in the channel.
Polymorphisms in human CALHM1 possibly associate with Alzheimer’s disease
CALHM1 was originally identified in a search for genes linked to late-onset Alzheimer’s disease. Human genetic studies suggest that one polymorphism in hCALHM1 (proline at residue 86 replaced by leucine (P86L)) is a possible risk factor for late-onset Alzheimer’s disease (LOAD) [8, 17], although existing genetic data supporting a role for CALHM1 in LOAD pathogenesis remain controversial [2, 3, 25]. A meta-analysis suggested that the hCALHM1-P86L polymorphism is not an independent risk factor for LOAD but is associated with the age of onset [17]. Studies in mammalian cells showed that the P86L variant caused a partial inhibition of the effect of CALHM1 on Ca2+ influx and amyloid-beta (Aβ) metabolism [9, 26, 34, 43]. However, no differences were observed in the gating and permeation properties between wild type (WT) and P86L-CALHM1 expressed in Xenopus oocytes or N2A cells, suggesting that the P86L polymorphism does not affect the intrinsic biophysical properties of hCALHM1 channels [20]. Two other hCALHM1 variants, G330D and R154H, were identified in early-onset Alzheimer’s disease (EOAD) patients [34]. The R154H variant affected CALHM1-dependent Ca2+ uptake and Aβ accumulation in mammalian cells [44]. However, both G330D and R154H channels have similar channel gating and permeation properties as hCALHM1-WT expressed in Xenopus oocytes [44]. Irrespective of the Alzheimer’s disease association, recent studies have also focused on other physiologically significant roles of CALHM channels in vivo by characterizing Calhm1 and clhm-1 knockout (KO) animals.
Physiological roles of CALHM ion channels
Neuronal excitability in response to reduced [Ca2+]o
Although endogenous CALHM1 protein has not yet been localized in the brain, CALHM1 messenger RNA (mRNA) has been be detected in cerebral neuronal cells [8, 9] including in mouse cortical and hippocampal pyramidal neurons using single-cell mRNA amplification [20]. Due to the slow gating properties of CALHM1 and the lack of specific inhibitors, it has not yet been possible to distinguish CALHM1 currents from large endogenous currents in neurons. Nevertheless, removal and subsequent add-back of Ca2+o elevate [Ca2+]i much more robustly in cortical neurons from WT vs. Calhm1 KO mice, indicating that endogenous CALHM1 may function in neuronal plasma membranes as it does in heterologous systems [20].
Decreases in [Ca2+]o to <0.1 mM have been measured in confined spaces in the brain such as synaptic clefts, especially during conditions that cause strong glutamate release and activation of postsynaptic Ca2+ influx pathways, for example during seizures, spreading depression, anoxia, and traumatic injury (see [20]). Such [Ca2+]o decreases increase neuronal excitability, but the mechanisms are poorly understood. Because CALHM1 was discovered in the brain and its gating is regulated by [Ca2+]o, a possible role for CALHM1 in mediating low [Ca2+]o enhancement of neuronal excitability was examined by comparing the electrophysiological responses of primary cultured cortical neurons from WT and Calhm1 KO mice to reduction of [Ca2+]o. Reducing [Ca2+]o from 1.5 to 0.2 mM reduced the input resistance by ~50 % and increased excitability in neurons from WT mice. In contrast, reductions of [Ca2+]o failed to alter input resistance or to enhance excitability of cortical neurons from Calhm1 KO mice [20]. These results suggest that CALHM1 contributes to enhanced neuronal excitability in response to low [Ca2+]o [20].
CALHM1 plays an essential role in taste perception as an ATP release channel
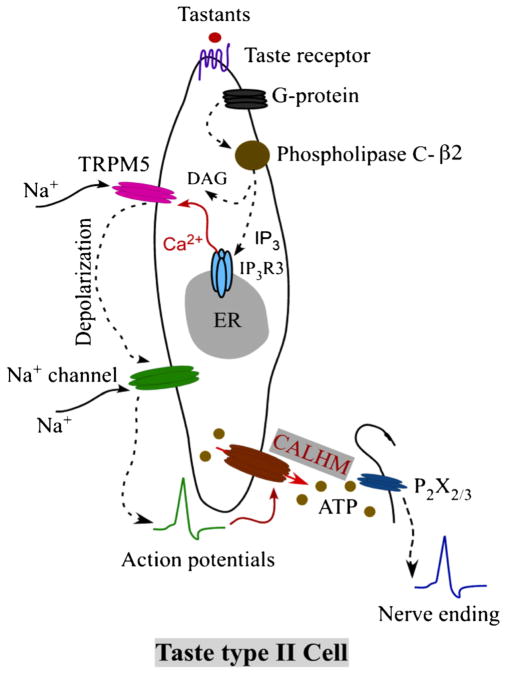
CALHM1 was also found to be expressed in primate taste buds [27]. Embedded in the tongue epithelium, taste buds sense molecules in foods and drinks and transmit taste information to afferent gustatory nerves. Each taste bud contains a heterogeneous population of approximately 100 taste cells [5, 38]. Each polarized taste cell is dedicated to one of the five basic taste qualities: sweet, bitter, salt, sour, and umami [4, 22, 49]. Taste cells are categorized into types I, II, and III cells based on ultrastructural features and gene expression profiles. Type I cells have a glial-like support function, with a subset mediating amiloride-sensitive salt taste. Type III cells sense sour taste and are the only type of taste cell to form conventional synapses with gustatory nerve processes, although the primary neurotransmitter is unknown. Type II cells sense either sweet-, umami- or bitter-tasting molecules, depending upon their expression of G protein-coupled taste receptor genes (TAS1R and TAS2R gene families). Notably, the expression of CALHM1 in taste buds is specific for type II cells [27, 39]. Type II cells share a common intracellular signal transduction cascade. Activation of taste receptors in apical microvilli engages heterotrimeric G proteins to activate phospholipase C-β2 (PLCβ2) which generates inositol trisphosphate to trigger Ca2+ release from the endoplasmic reticulum through type 3 inositol trisphosphate receptors (IP3R3). The rise of [Ca2+]i activates plasma membrane monovalent cation- selective TRPM5 channels at the basolateral membrane, which causes membrane depolarization that triggers Na+ action potentials and release of ATP as the neurotransmitter through a non-exocytotic mechanism [13], as depicted in Fig. 2.
Fig. 2.
Transduction cascade of taste signals for sweet, umami, and bitter. Tastants bind to taste receptors in the apical membranes of type II taste cells and activate G protein and phospholipase C-β2 (PLC-β2), resulting in the production of InsP3 (IP3) and diacylglycerol (DAG). IP3 binds to InsP3 receptor type 3 (IP3R3), triggering Ca2+ release from the endoplas- mic reticulum (ER). Elevated cytoplasmic Ca2+ concentration activates TRPM5, which depolarizes the membrane, triggering Na+ action potentials that activate CALHM channels to release ATP. ATP binds to P2X2/3 receptors on afferent gustatory nerves to transmit taste information to the brain
The molecular mechanism of ATP release from type II cells lacking conventional synapses to the afferent gustatory nerves has been a long-standing enigma since the discovery of ATP as the primary neurotransmitter [10, 16, 38]. Connexin hemichannels and pannexin1 channels are involved in physiological extracellular ATP signaling as ATP release channels [6, 7, 18]. Ion channel-mediated mechanisms including connexin hemichannels and pannexin 1 had been proposed to mediate ATP release in type II cells [15, 33], yet their involvement remained controversial [38]. Recently, two laboratories [40, 41] reported that pannxin1 was not required for taste-evoked release of ATP or neural and behavioral responses to taste stimuli. CALHM1 was discovered as an ion channel with structural similarities to connexin and pannexin channels [20, 36], and transcriptome analysis revealed the expression of CALHM genes in primate taste buds, exclusively in type II cells [27]. The apparent structural and functional similarities to those channels, the anion permeability, and the wide pore of CALHM1 raised the possibility that CALHM1 might also be an ATP release channel [39]. Reduction of [Ca2+]o or membrane depolarization, mechanisms that activate CALHM1 channels, induced ATP release specifically in CALHM1-expressing cells, including HeLa and COS-1 cells and Xenopus oocytes [39]. ATP release was sensitive to ruthenium red, a CALHM1 channel blocker, but not to inhibitors of other cellular ATP release mechanisms including brefeldin A (vesicular release), DCPIB (volume-sensitive Cl− channels), A438079 (P2X7 receptor channels), heptanol (connexin hemichannels), and carbenoloxone (pannexins and connexins). Similar ruthenium red-sensitive, membrane depolarization-and low [Ca2+]o-induced ATP release was observed in cells expressing mCALHM1, suggesting that mCALHM1 also functions as an ion channel with properties similar to hCALHM1. These results demonstrated that the CALHM1 channel is a conduit for ATP release from CALHM1-expressing cells and is a novel voltage-gated ATP release channel [39].
Single-cell RT-PCR and double-labeling in situ hybridization approaches demonstrated that Calhm1 mRNA expression is confined to taste cells expressing marker genes for type II cells, Plcβ2 or Trpm5. The selective Calhm1 expression in type II cells was further confirmed by the absence of Calhm1 expression in taste buds of type II cell-null Skn-1a KO mice [23]. Whole-cell patch clamp recordings in freshly isolated mouse type II taste cells revealed slowly activating voltage-gated currents that were reminiscent of CALHM1 currents. Similar currents were not observed in type I or III cells [39]. The CALHM1-like currents were Gd3+ sensitive and, importantly, strongly diminished in type II cells from Calhm1 KO mice. Notably, taste-evoked ATP release from taste buds observed in WT mice was absent in the Calhm1 KO mice [39]. Calhm1 KO mice lacked behavioral and gustatory nerve responses specifically to sweet, umami, and bitter tastes. Genetic deletion of Calhm1 eliminated the ability to release ATP in response to bitter stimuli without affecting the upstream components, including Ca2+ responses to taste stimuli, TRPM5 expression, activity of voltage-gated Na+ channels, and the ATP content of taste buds. These physiological, behavioral, biochemical, and genetic data provide compelling evidence for an essential role for CALHM1 in sweet, umami, and bitter taste perception by mediating ATP release from type II taste cells [39].
CLHM-1 is a conditionally toxic channel that regulates C. elegans locomotion
C. elegans CLHM-1 was found to be expressed in the plasma membrane of sensory neurons and body wall muscles [37]. Since the body wall muscles control motility, a fluid mechanics approach was used to quantitate the swimming gait of clhm-1 mutants. Compared to WT animals, clhm-1 mutants exhibited defects in locomotory kinematics and biomechanics, suggesting that CLHM-1 regulates locomotion in C. elegans. The motility defects observed in clhm-1 mutant animals could be rescued by muscle-specific expression of either CLHM-1 or hCALHM1, suggesting that in addition to shared biophysical properties, the function of these proteins is conserved in vivo [37].
Overexpression of CLHM-1 or CALHM1 in the body wall muscles caused embryonic lethality in a dose-dependent manner. Overexpression of CLHM-1 or hCALHM1 in C. elegans touch neurons, a model utilized to study toxic ion channels, caused necrotic-like neurodegeneration and loss of touch sensitivity, establishing that CALHM family channels are conditionally neurotoxic [37]. The toxicity associated with CLHM-1overexpression observed in the touch neurons and body wall muscles was partially suppressed by chemical reagents and genetic mutations that altered Ca2+ levels and plasma membrane expression of CLHM-1 [37], suggesting that membrane depolarization and/or Ca2+ overload might be the mechanism of cytotoxicity of CALHM channels.
Summary
Voltage- and Ca2+o-gated CALHM channels constitute a novel ion channel family that is widely expressed in the brain and taste buds throughout vertebrates and in sensory neurons and body wall muscles in C. elegans. Here, we have reviewed the recent advances in our understanding of CALHM channel biological roles, structural features, and gating properties. CALHM channels play important roles in neuronal excitability, neurotransmission of tastes, and muscle cell function. CALHM has intriguing structural and gating properties, including intrinsic voltage-dependent gating without a canonical voltage sensor and a wide pore permeable to Ca2+ and ATP. The voltage- and Ca2+o-dependent gating mechanisms, structural features that define the gate and ion permeation pathway and additional physiological roles, remain to be discovered in the future.
Acknowledgments
This work was supported by National Institutes of Health (NIH) Grants R01DC012538 (J.K.F. and Z.M.) and R03DC014328 (J.E.T.), an American Heart Postdoctoral fellowship 12POST11940054 (J.E.T.), JSPS KAKENHI Grants (25893201 and 26713008) (A.T.), The Salt Science Research Foundation (1429 and 1542) (A.T.), Society for Research on Umami Taste, Nestlé Nutrition Council, Japan (A.T.), and Kyoto Prefectural Public University Corporation (A.T.).
References
- 1.Beblo DA, Veenstra RD. Monovalent cation permeation through the connexin40 gap junction channel. Cs, Rb, K, Na, Li, TEA, TMA, TBA, and effects of anions Br, Cl, F, acetate, aspartate, glutamate, and NO3. J Gen Physiol. 1997;109:509–522. doi: 10.1085/jgp.109.4.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beecham GW, Schnetz-Boutaud N, Haines JL, Pericak-Vance MA. CALHM1 polymorphism is not associated with late-onset Alzheimer disease. Ann Hum Genet. 2009;73:379–381. doi: 10.1111/j.1469-1809.2009.00509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bertram L, Schjeide BM, Hooli B, Mullin K, Hiltunen M, Soininen H, Ingelsson M, Lannfelt L, Blacker D, Tanzi RE. No association between CALHM1 and Alzheimer’s disease risk. Cell. 2008;135:993–994. doi: 10.1016/j.cell.2008.11.030. author reply 994-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chandrashekar J, Kuhn C, Oka Y, Yarmolinsky DA, Hummler E, Ryba NJ, Zuker CS. The cells and peripheral representation of sodium taste in mice. Nature. 2010;464:297–301. doi: 10.1038/nature08783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chaudhari N, Roper SD. The cell biology of taste. J Cell Biol. 2010;190:285–296. doi: 10.1083/jcb.201003144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cotrina ML, Lin JH, Lopez-Garcia JC, Naus CC, Nedergaard M. ATP-mediated glia signaling. J Neurosci. 2000;20:2835–2844. doi: 10.1523/JNEUROSCI.20-08-02835.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cotrina ML, Lin JH, Nedergaard M. Cytoskeletal assembly and ATP release regulate astrocytic calcium signaling. J Neurosci. 1998;18:8794–8804. doi: 10.1523/JNEUROSCI.18-21-08794.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dreses-Werringloer U, Lambert JC, Vingtdeux V, Zhao H, Vais H, Siebert A, Jain A, Koppel J, Rovelet-Lecrux A, Hannequin D, Pasquier F, Galimberti D, Scarpini E, Mann D, Lendon C, Campion D, Amouyel P, Davies P, Foskett JK, Campagne F, Marambaud P. A polymorphism in CALHM1 influences Ca2+ homeostasis, Abeta levels, and Alzheimer’s disease risk. Cell. 2008;133:1149–1161. doi: 10.1016/j.cell.2008.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dreses-Werringloer U, Vingtdeux V, Zhao H, Chandakkar P, Davies P, Marambaud P. CALHM1 controls the Ca2+-dependent MEK, ERK, RSK and MSK signaling cascade in neurons. J Cell Sci. 2013;126:1199–1206. doi: 10.1242/jcs.117135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Finger TE, Danilova V, Barrows J, Bartel DL, Vigers AJ, Stone L, Hellekant G, Kinnamon SC. ATP signaling is crucial for communication from taste buds to gustatory nerves. Science. 2005;310:1495–1499. doi: 10.1126/science.1118435. [DOI] [PubMed] [Google Scholar]
- 11.Gallego-Sandin S, Alonso MT, Garcia-Sancho J. Calcium homoeostasis modulator 1 (CALHM1) reduces the calcium content of the endoplasmic reticulum (ER) and triggers ER stress. Biochem J. 2011;437:469–475. doi: 10.1042/BJ20110479. [DOI] [PubMed] [Google Scholar]
- 12.Harris AL, Contreras JE. Motifs in the permeation pathway of connexin channels mediate voltage and Ca2+ sensing. Front Physiol. 2014;5:113. doi: 10.3389/fphys.2014.00113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hisatsune C, Yasumatsu K, Takahashi-Iwanaga H, Ogawa N, Kuroda Y, Yoshida R, Ninomiya Y, Mikoshiba K. Abnormal taste perception in mice lacking the type 3 inositol 1,4, 5-trisphosphate receptor. J Biol Chem. 2007;282:37225–37231. doi: 10.1074/jbc.M705641200. [DOI] [PubMed] [Google Scholar]
- 14.Hou X, Pedi L, Diver MM, Long SB. Crystal structure of the calcium release-activated calcium channel Orai. Science. 2012;338:1308–1313. doi: 10.1126/science.1228757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang YJ, Maruyama Y, Dvoryanchikov G, Pereira E, Chaudhari N, Roper SD. The role of pannexin 1 hemichannels in ATP release and cell-cell communication in mouse taste buds. Proc Natl Acad Sci U S A. 2007;104:6436–6441. doi: 10.1073/pnas.0611280104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kinnamon SC, Finger TE. A taste for ATP: neurotransmission in taste buds. Front Cell Neurosci. 2013;7:264. doi: 10.3389/fncel.2013.00264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lambert JC, Sleegers K, Gonzalez-Perez A, Ingelsson M, Beecham GW, Hiltunen M, Combarros O, Bullido MJ, Brouwers N, Bettens K, Berr C, Pasquier F, Richard F, Dekosky ST, Hannequin D, Haines JL, Tognoni G, Fievet N, Dartigues JF, Tzourio C, Engelborghs S, Arosio B, Coto E, De Deyn P, Del Zompo M, Mateo I, Boada M, Antunez C, Lopez-Arrieta J, Epelbaum J, Schjeide BM, Frank-Garcia A, Giedraitis V, Helisalmi S, Porcellini E, Pilotto A, Forti P, Ferri R, Delepine M, Zelenika D, Lathrop M, Scarpini E, Siciliano G, Solfrizzi V, Sorbi S, Spalletta G, Ravaglia G, Valdivieso F, Vepsalainen S, Alvarez V, Bosco P, Mancuso M, Panza F, Nacmias B, Bossu P, Hanon O, Piccardi P, Annoni G, Mann D, Marambaud P, Seripa D, Galimberti D, Tanzi RE, Bertram L, Lendon C, Lannfelt L, Licastro F, Campion D, Pericak-Vance MA, Soininen H, Van Broeckhoven C, Alperovitch A, Ruiz A, Kamboh MI, Amouyel P. The CALHM1 P86L polymorphism is a genetic modifier of age at onset in Alzheimer’s disease: a meta-analysis study. J Alzheimers Dis. 2010;22:247–255. doi: 10.3233/JAD-2010-100933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Locovei S, Bao L, Dahl G. Pannexin 1 in erythrocytes: function without a gap. Proc Natl Acad Sci U S A. 2006;103:7655–7659. doi: 10.1073/pnas.0601037103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lopez W, Gonzalez J, Liu Y, Harris AL, Contreras JE. Insights on the mechanisms of Ca2+ regulation of connexin26 hemichannels revealed by human pathogenic mutations (D50N/Y) J Gen Physiol. 2013;142:23–35. doi: 10.1085/jgp.201210893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ma Z, Siebert AP, Cheung KH, Lee RJ, Johnson B, Cohen AS, Vingtdeux V, Marambaud P, Foskett JK. Calcium homeostasis modulator 1 (CALHM1) is the pore-forming subunit of an ion channel that mediates extracellular Ca2+ regulation of neuronal excitability. Proc Natl Acad Sci U S A. 2012;109:E1963–E1971. doi: 10.1073/pnas.1204023109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maeda S, Nakagawa S, Suga M, Yamashita E, Oshima A, Fujiyoshi Y, Tsukihara T. Structure of the connexin 26 gap junction channel at 3.5 A resolution. Nature. 2009;458:597–602. doi: 10.1038/nature07869. [DOI] [PubMed] [Google Scholar]
- 22.Matsumoto I, Ohmoto M, Abe K. Functional diversification of taste cells in vertebrates. Semin Cell Dev Biol. 2013;24:210–214. doi: 10.1016/j.semcdb.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matsumoto I, Ohmoto M, Narukawa M, Yoshihara Y, Abe K. Skn-1a (Pou2f3) specifies taste receptor cell lineage. Nat Neurosci. 2011;14:685–687. doi: 10.1038/nn.2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McNally BA, Yamashita M, Engh A, Prakriya M. Structural determinants of ion permeation in CRAC channels. Proc Natl Acad Sci U S A. 2009;106:22516–22521. doi: 10.1073/pnas.0909574106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Minster RL, Demirci FY, DeKosky ST, Kamboh MI. No association between CALHM1 variation and risk of Alzheimer disease. Hum Mutat. 2009;30:E566–E569. doi: 10.1002/humu.20989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moreno-Ortega AJ, Buendia I, Mouhid L, Egea J, Lucea S, Ruiz-Nuno A, Lopez MG, Cano-Abad MF. CALHM1 and its polymorphism P86L differentially control Ca homeostasis, mitogen-activated protein kinase signaling, and cell vulnerability upon exposure to amyloid beta. Aging Cell. 2015 doi: 10.1111/acel.12403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moyer BD, Hevezi P, Gao N, Lu M, Kalabat D, Soto H, Echeverri F, Laita B, Yeh SA, Zoller M, Zlotnik A. Expression of genes encoding multi-transmembrane proteins in specific primate taste cell populations. PLoS One. 2009;4:e7682. doi: 10.1371/journal.pone.0007682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oh S, Rivkin S, Tang Q, Verselis VK, Bargiello TA. Determinants of gating polarity of a connexin 32 hemichannel. Biophys J. 2004;87:912–928. doi: 10.1529/biophysj.103.038448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Puljung MC, Berthoud VM, Beyer EC, Hanck DA. Polyvalent cations constitute the voltage gating particle in human connexin37 hemichannels. J Gen Physiol. 2004;124:587–603. doi: 10.1085/jgp.200409023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Purnick PE, Oh S, Abrams CK, Verselis VK, Bargiello TA. Reversal of the gating polarity of gap junctions by negative charge substitutions in the N-terminus of connexin 32. Biophys J. 2000;79:2403–2415. doi: 10.1016/S0006-3495(00)76485-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qiu Z, Dubin AE, Mathur J, Tu B, Reddy K, Miraglia LJ, Reinhardt J, Orth AP, Patapoutian A. SWELL1, a plasma membrane protein, is an essential component of volume-regulated anion channel. Cell. 2014;157:447–458. doi: 10.1016/j.cell.2014.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ripps H, Qian H, Zakevicius J. Properties of connexin26 hemichannels expressed in Xenopus oocytes. Cell Mol Neurobiol. 2004;24:647–665. doi: 10.1023/B:CEMN.0000036403.43484.3d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Romanov RA, Kolesnikov SS. Electrophysiologically identified subpopulations of taste bud cells. Neurosci Lett. 2006;395:249–254. doi: 10.1016/j.neulet.2005.10.085. [DOI] [PubMed] [Google Scholar]
- 34.Rubio-Moscardo F, Seto-Salvia N, Pera M, Bosch-Morato M, Plata C, Belbin O, Gene G, Dols-Icardo O, Ingelsson M, Helisalmi S, Soininen H, Hiltunen M, Giedraitis V, Lannfelt L, Frank A, Bullido MJ, Combarros O, Sanchez-Juan P, Boada M, Tarraga L, Pastor P, Perez-Tur J, Baquero M, Molinuevo JL, Sanchez-Valle R, Fuentes-Prior P, Fortea J, Blesa R, Munoz FJ, Lleo A, Valverde MA, Clarimon J. Rare variants in calcium homeostasis modulator 1 (CALHM1) found in early onset Alzheimer’s disease patients alter calcium homeostasis. PLoS One. 2013;8:e74203. doi: 10.1371/journal.pone.0074203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sanchez HA, Villone K, Srinivas M, Verselis VK. The D50N mutation and syndromic deafness: altered Cx26 hemichannel properties caused by effects on the pore and intersubunit interactions. J Gen Physiol. 2013;142:3–22. doi: 10.1085/jgp.201310962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Siebert AP, Ma Z, Grevet JD, Demuro A, Parker I, Foskett JK. Structural and functional similarities of calcium homeostasis modulator 1 (CALHM1) ion channel with connexins, pannexins, and innexins. J Biol Chem. 2013;288:6140–6153. doi: 10.1074/jbc.M112.409789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tanis JE, Ma Z, Krajacic P, He L, Foskett JK, Lamitina T. CLHM-1 is a functionally conserved and conditionally toxic Ca2+-permeable ion channel in Caenorhabditis elegans. J Neurosci. 2013;33:12275–12286. doi: 10.1523/JNEUROSCI.5919-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Taruno A, Matsumoto I, Ma Z, Marambaud P, Foskett JK. How do taste cells lacking synapses mediate neurotransmission? CALHM1, a voltage-gated ATP channel. Bioessays. 2013;35:1111–1118. doi: 10.1002/bies.201300077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Taruno A, Vingtdeux V, Ohmoto M, Ma Z, Dvoryanchikov G, Li A, Adrien L, Zhao H, Leung S, Abernethy M, Koppel J, Davies P, Civan MM, Chaudhari N, Matsumoto I, Hellekant G, Tordoff MG, Marambaud P, Foskett JK. CALHM1 ion channel mediates purinergic neurotransmission of sweet, bitter and umami tastes. Nature. 2013;495:223–226. doi: 10.1038/nature11906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tordoff MG, Aleman TR, Ellis HT, Ohmoto M, Matsumoto I, Shestopalov VI, Mitchell CH, Foskett JK, Poole RL. Normal taste acceptance and preference of PANX1 knockout mice. Chem Senses. 2015;40:453–459. doi: 10.1093/chemse/bjv025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vandenbeuch A, Anderson CB, Kinnamon SC. Mice lacking pannexin 1 release ATP and respond normally to all taste qualities. Chem Senses. 2015;40:461–467. doi: 10.1093/chemse/bjv034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Verselis VK, Ginter CS, Bargiello TA. Opposite voltage gating polarities of two closely related connexins. Nature. 1994;368:348–351. doi: 10.1038/368348a0. [DOI] [PubMed] [Google Scholar]
- 43.Vingtdeux V, Chandakkar P, Zhao H, Blanc L, Ruiz S, Marambaud P. CALHM1 ion channel elicits amyloid-beta clearance by insulin-degrading enzyme in cell lines and in vivo in the mouse brain. J Cell Sci. 2015;128:2330–2338. doi: 10.1242/jcs.167270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vingtdeux V, Tanis JE, Chandakkar P, Zhao H, Dreses-Werringloer U, Campagne F, Foskett JK, Marambaud P. Effect of the CALHM1 G330D and R154H human variants on the control of cytosolic Ca2+ and Abeta levels. PLoS One. 2014;9:e112484. doi: 10.1371/journal.pone.0112484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Voss FK, Ullrich F, Munch J, Lazarow K, Lutter D, Mah N, Andrade-Navarro MA, von Kries JP, Stauber T, Jentsch TJ. Identification of LRRC8 heteromers as an essential component of the volume-regulated anion channel VRAC. Science. 2014;344:634–638. doi: 10.1126/science.1252826. [DOI] [PubMed] [Google Scholar]
- 46.Wang HZ, Veenstra RD. Monovalent ion selectivity sequences of the rat connexin43 gap junction channel. J Gen Physiol. 1997;109:491–507. doi: 10.1085/jgp.109.4.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang J, Dahl G. SCAM analysis of Panx1 suggests a peculiar pore structure. J Gen Physiol. 2010;136:515–527. doi: 10.1085/jgp.201010440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yamashita M, Navarro-Borelly L, McNally BA, Prakriya M. Orai1 mutations alter ion permeation and Ca2+-dependent fast inactivation of CRAC channels: evidence for coupling of permeation and gating. J Gen Physiol. 2007;130:525–540. doi: 10.1085/jgp.200709872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yarmolinsky DA, Zuker CS, Ryba NJ. Common sense about taste: from mammals to insects. Cell. 2009;139:234–244. doi: 10.1016/j.cell.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]