Abstract
Prostate cancer (PCA) is the leading malignancy in men and the second leading cause of cancer-related deaths. Hypoxia (low O2 condition) is considered an early event in prostate carcinogenesis associated with an aggressive phenotype. In fact, clinically, hypoxia and hypoxia-related biomarkers are associated with treatment failure and disease progression. Hypoxia-inducible factor 1 (HIF-1) is the key factor that is activated under hypoxia, and mediates adaptation of cells to hypoxic conditions through regulating the expression of genes associated with angiogenesis, epithelial-to-mesenchymal transition (EMT), metastasis, survival, proliferation, metabolism, stemness, hormone-refractory progression, and therapeutic resistance. Besides HIF-1, several other signaling pathways including PI3K/Akt/mTOR, NADPH oxidase (NOX), Wnt/β-catenin, and Hedgehog are activated in cancer cells under hypoxic conditions, and also contribute in hypoxia-induced biological effects in HIF-1-dependent and -independent manners. Hypoxic cancer cells cause extensive changes in the tumor microenvironment both local and distant, and recent studies have provided ample evidence supporting the crucial role of nanosized vesicles “exosomes” in mediating hypoxia-induced tumor microenvironment remodeling. Exosomes’ role has been reported in hypoxia-induced angiogenesis, stemness, activation of cancer-associated fibroblasts (CAFs), and EMT. Together, existing literature suggests that hypoxia plays a predominant role in PCA growth and progression, and PCA could be effectively prevented and treated via targeting hypoxia/hypoxia-related signaling pathways.
Keywords: hypoxia, hypoxia-inducible factor 1, prostate cancer, exosomes, biomarkers
I. INTRODUCTION
Prostate cancer (PCA) is the most common non-cutaneous cancer in men, and according to the American Cancer Society reports, 220,800 new cases and 27,540 deaths from PCA are estimated in the United States in 2015.1 To reduce PCA mortality, we need a better understanding of biological events that play a fundamental role in prostate carcinogenesis. In this regard, hypoxia (low oxygen conditions) is considered an early event during prostate carcinogenesis associated with aggressive phenotype, and plays an important role in PCA growth, promotion, metastasis, and hormone-refractory progression.2–4 Hypoxia induces genetic, metabolic, and proteome-related changes leading to increased glycolysis, angiogenesis, stemness, and selection of resistant clones.2,5,6 In fact, tumor hypoxia status and hypoxia-related biomarkers are associated with poor prognosis and the major reason for treatment failure and disease relapse.7–10 Therefore, PCA could be effectively prevented and treated through targeting hypoxia-induced signaling pathways and biological effects.
Under hypoxic conditions, several signaling pathways are activated in cancer cells to adjust to the harsh and inhospitable environment. Although signaling mediated by HIF1 (hypoxia-inducible factor 1) constitutes a potent hypoxic response mechanism, there are several instances of hypoxia responses that are HIF independent, and could be executed by other pathways such as phosphatidylinositol 3-kinases (PI3K)-Akt-mammalian target of rapamycin (mTOR), NADPH oxidase (NOX), Wnt/β-catenin etc.3,6,11–14 Together, these signaling pathways are instrumental in the adaptation of cancer cells to hypoxic conditions through promoting angiogenesis, anaerobic metabolism, invasiveness, motility, survival, stemness, drug resistance, etc. Besides the genetic/epigenetic changes, hypoxic cancer cells enforce extensive tumor microenvironment remodeling to further improve their survival chances. For example, hypoxic cancer cells promote the growth of endothelial cells in neighboring blood vessels via releasing pro-angiogenic factors. Similarly, hypoxic cancer cells promote the activation of cancer-associated fibroblasts (CAFs) that further supports angiogenesis as well as cancer cells invasiveness, stemness, and immune cells recruitment in the tumor microenvironment. Another interesting feature emerging in the hypoxic tumor environment is a metabolic symbiosis between different cellular components, where hypoxic cells use glucose via glycolysis, generating lactic acid that is consumed by normoxic cells (cancer cells, endothelial cells, fibroblasts, macrophages, etc.) via monocarboxylate transporter 1 (MCT1).15–18 The use of lactate as primary source of energy by normoxic cells spared the glucose for hypoxic cells in the deeper tissues. Besides, hypoxia in the primary tumor also modulates the environment at distant sites to support metastasis (these sites are known as pre-metastatic niches).19,20 Recent studies have confirmed that “exosomes” (endosome-derived vesicles of ~30–150 nm in diameter) serve important roles in intercellular communication through transport of bioactive molecules between cells, and that hypoxic cancer cells secrete more and distinct exosomes compared to healthy cells.21–24 Exosomes are loaded with unique proteins, lipids, nucleic acid (mRNA, miRNA, and DNA, etc.), metabolites, and sugars, and are considered important players in tumor microenvironment remodeling, both local and distant.19,21,23,24 It is believed that a better molecular understanding of exosomes biogenesis in hypoxic cancer cells as well as their effect on receipt cells could offer new avenues for cancer control.
In this review, we describe various signaling pathways that are activated in PCA cells under hypoxic conditions. Even though, we have primarily focused on signaling pathways related to PCA cells, but wherever possible we have also described studies in other cancer cells. We have also discussed the role of exosomes as messenger of hypoxic response in the tumor microenvironment. In the last, we have discussed the possibility of using hypoxia/hypoxia-related signaling as potential biomarker for disease prognosis as well as drug target in PCA prevention and treatment.
II. HYPOXIA-INDUCED SIGNALING PATHWAYS
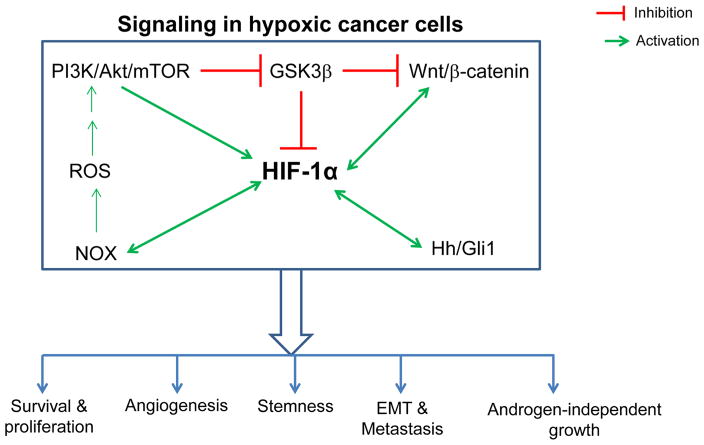
Several signaling pathways are activated in cancer cells under hypoxic conditions including HIF, PI3K/Akt/mTOR, NOX, Wnt/β-catenin, and Hedgehog (Fig. 1). These pathways are briefly discussed below in this section.
FIG. 1.
Activation of signaling pathways in hypoxic cancer cells. In hypoxic cancer cells, several signaling pathways are activated including HIF-1α, PI3K/Akt/mTOR, Wnt/β-catenin, NOX, and Hedgehog. These signaling pathways have significant cross talk, and together they contribute to hypoxia-induced cancer cells survival, proliferation, angiogenesis, stemness, EMT, metastasis, and androgen-independent growth.
A. HIF Signaling
HIF belongs to the large family of basic helix-loop-helix proteins and is a heterodimer of a stable HIF-1β subunit (known as the aryl hydrocarbon receptor nuclear translocator), and one of three oxygen-regulated HIF-α subunits (HIF-1α, HIF-2α, and HIF-3α). Depending on the cell type, HIF-1 regulates more than 2% of all human genes controlling energy metabolism, neovascularization, survival, stemness, invasiveness, etc. In fact, HIF is the major mediator of cellular adaptation to hypoxic conditions.25–27 HIF activation is a multistep process involving HIF-α stabilization, nuclear translocation, hetero-dimerization, transcriptional activation, and interaction with other proteins.28,29 HIF-1α overexpression is linked with the increased mortality in patients with various malignancies.7,30–33 This association is primarily based on the HIF-1-mediated regulation of genes that play pivotal roles in the central features of cancer pathogenesis such as angiogenesis, metastasis, invasion, stemness, metabolism, and anti-apoptosis.27,28,34,35
Under normoxic conditions, prolyl hydroxylases (PHD1, 2, and 3) hydroxylate the site-specific proline residues of HIF-1α in the presence of oxygen, Fe2+, oxoglutarate (2-OG), and ascorbate. Hydroxylated HIF-1α is recognized by von Hippel-Lindau (VHL), which serves as the targeting subunit of an E3 ubiquitin ligase complex, and tags HIF-1α with polyubiquitin, which is then recognized by proteasome for degradation. Under hypoxic condition, PHDs activity is decreased resulting in lesser HIF-1α prolyl hydroxylation, which in turn leads to decreased rates of HIF-1α polyubiquitination and proteolysis. Therefore, under hypoxia, HIF-1α is stabilized, translocates to nucleus, dimerizes with HIF-1β, and binds to hypoxia response element (HRE) within the promoters of target genes.4,6,27 HIF-1α also binds with co-activator CBP/p300 and activates the expression of target genes including glucose transporters, glycolytic enzymes, angiogenesis, proliferation, survival, metastasis, stemness, and drug resistance.2,6,33 HIF-1α and CBP/p300 interaction is also dependent on oxygen level since factor-inhibiting HIF-1α (FIH) is known to hydroxylate HIF-1α at the Asn-803 site in the presence of O2 preventing the binding of HIF-1α to CBP/p300. HIF-1α can also be regulated through oxygen-independent mechanisms in a cell type-specific manner. For example, HIF-1α can be activated by oncogenic mutations of PTEN (phosphatase and tensin homolog), VHL, succinate dehydrogenase (SDH), and fumarate dehydrogenase (FH).36 Also, it could also be activated by the increased activity of ERBB2, SRC, RAS/MAPK, and PI3K/Akt/mTOR pathways.36 Furthermore, HIF-1α is also stabilized by reactive oxygen species (ROS), which block PHD activities and also activate MAPKs.36. Similar to HIF-1α, HIF-2α is also regulated by oxygen-dependent hydroxylation and compared with HIF-1α, it has several overlapping as well as distinct targets. On the contrary, HIF-3α is considered a negative regulator of hypoxia-inducible gene expression.
Several studies have reported that HIF-1α activation regulates several key biological events in hypoxic PCA cells. Ghafar et al. reported that exposure to acute hypoxia even for 24 h increased the aggressive characteristics and survival properties in human PCA LNCaP cells through upregulation of HIF-1α expression.37 Hypoxia-induced HIF-1α also promoted survival in PCA cells through upregulation of IL8 receptors (CXCR1 and CXCR2).38 Besides, HIF-1α enhanced the choline kinase expression in hypoxia prostate cancer cell resulting in higher phosphocholine and total choline levels. 39 Horii et al. reported that in PCA cells, HIF-1α interacts with androgen receptor (AR) to activate PSA expression under hypoxic condition.40 Mitani et al. also reported that under low androgen condition, hypoxia enhances transcriptional activity of AR through HIF-1α.41 Together, these two studies supported that hypoxia/HIF-1α promotes the advanced hormone-refractory progression of PCA.
It is well established that hypoxia/HIF-1α status of the tumor is associated with radio resistance and poor prognosis following radiotherapy. However, exposures to hypoxia even postirradiation activated the HIF-1α and contributed to aggressive phenotype and radio resistance.42 Ma et al. reported that PCA cells under hypoxia exhibited higher expression of HIF-1α, HIF-2α, and stem cell biomarkers (Oct3/4 and Nanog), as well as increased stemlike properties.43 Importantly, S100A8 and S100A9 are overexpressed in several neoplasms including PCA and play an important role in proliferation, inflammation, and metastasis. And the expression of these two molecules was increased in hypoxic PCA cells through a direct regulation by HIF-1α.44 Similarly, CX3CR1 expression was strongly increased in androgen-independent PCA cells following exposure to hypoxia associated with higher motility and invasiveness.45 Importantly, attenuation of HIF-1α abrogated hypoxia-induced upregulation of CX3CR1, and also prevented their motility and invasiveness under hypoxic environment.45 Hypoxia also increased the invasiveness of PCA cells through HIF-1α- and TNFα-mediated stabilization of EMT promoter Snail.46 We have also observed an increase in HIF-1α expression in hypoxic PCA cells associated with activation of several lipogenesis-related molecules and lipid accumulation.23 Huss et al. have reported that angiogenic switch in TRAMP (transgenic adenocarcinoma mouse prostate transgenic model) mice is driven in part by HIF-1α early in PCA progression.47 We have also observed that HIF-1α expression increases with the disease progression in TRAMP mice associated with higher levels of several angiogenesis-related biomarkers (VEGF, VEGFR1, VEGFR2, and microvessel density) as well as increased metastasis to distant organs. 48. Overall, hypoxia-induced HIF-1α signaling plays an important role in the angiogenesis, stemness, lipogenesis, EMT, metastasis, and hormone-refractory progression in PCA cells (Fig. 1).
B. PI3K/Akt/mTOR Signaling
In addition to its role in normal cell, PI3K/Akt/mTOR pathway is also important in the development of several cancers.49–51 This pathway plays a key role in various cellular functions including proliferation, survival, migration, adhesion, invasion, metabolism, and angiogenesis.49–51 PI3K is a family of enzymes that phosphorylates the 3′-OH of the inositol ring of phosphatidyl inositol. PI3K generates an important lipid second messenger called phosphatidylinositol 3,4,5-triphosphate (PIP3), which plays a crucial role in several signaling pathways.52 Furthermore, PIP3 activates the serine/threonine kinases PDK1 and Akt. On the contrary, the PTEN gene encodes a phosphatase that opposes the action of PI3K, which in turn reduces the level of activated Akt. Akt is known to control protein synthesis and cell growth by leading to the phosphorylation of mTOR. Under hypoxic conditions, the PI3K/Akt/mTOR pathway is activated in cancer cells (including PCA cells), and plays important role in adaptation of cells to low O2 conditions.53–57 Several studies have suggested that the PI3K/Akt/mTOR pathway mainly acts through promoting HIF-1α expression under hypoxic conditions.56–58 In this regard, Wang et al. reported that both Akt and mTOR are activated in multiple myeloma U266 cells and their inhibition by LY294002 and rapamycin, respectively, resulted in inhibition of hypoxia-induced HIF-1α expression.57 Blancher et al. reported the role of PI3K in regulating the HIF-1α but not HIF-2α expression in breast and renal cancer cells.54 Chen et al. reported that blocking PI3K/Akt or mTOR activity strongly diminished the hypoxia-induced HIF-1α expression and VEGF secretion in human bladder cancer cells.59 Interestingly, Manohar et al. identified and tested the efficacy of a novel HIF-1α inhibitor P31555 in PCA cells, and concluded that P3155 also inhibits hypoxia-induced HIF-1α expression through inhibiting the PI3K/Akt/mTOR pathway.60 Yan et al. reported that Akt and its downstream signaling is activated in hepatocellular carcinoma cells (HCC) and controls the hypoxia-induced EMT in these cells.55 Importantly, Akt is also activated in PCA cells under chronic hypoxic conditions and plays an important role in the hormone-refractory progression of the disease.53
Several studies have aimed at understating the detailed mechanism underlying the PI3K/Akt/mTOR activation and their role in increasing HIF-1α expression in hypoxic cancer cells.12,61 In an in-depth study, Joshi et al. clearly showed that hypoxia alone might not be sufficient to render HIF-1α resistant to proteasomal cleavage and degradation, and in fact requires PTEN inactivation or activation of PI3K/Akt.12 This study showed that PI3K inhibitors and PTEN could promote HIF-1α degradation in hypoxic glioma cells through regulating the cytoplasmic localization of MDM2 (murine double mutant 2).12 Hudson et al. suggested the role of mTOR beyond translational control of HIF-1α, and suggested that mTOR could regulate HIF-1α expression through affecting the oxygen-dependent degradation (ODD) domain in HIF-1α.13 In another study, Shen et al. reported that under severe hypoxic conditions, gastric cancer cells secrete a higher amount of TGF-β, which activates Akt along with Smad2/3 via autocrine loop.62 Additionally, ROS role has been suggested in the activation of the PI3K/Akt/mTOR pathway under hypoxic condition, which in turn increases HIF-1α expression.61 Ader et al. also showed that under hypoxic conditions, sphinogosine kinase 1 (SphK1) is activated in ROS-dependent manner in human PCA PC3 cells and increases the HIF-1α expression dependent on Akt activation and glycogen synthase kinase 3β (GSK3β) inactivation.63 Therefore, ROS could directly/indirectly activate the PI3K/Akt/mTOR pathway, which could then increase HIF-1α expression (Fig. 1). Overall, it is clear that PI3K/Akt/mTOR activation not only controls HIF-1α expression in normoxic cancer cells but also under hypoxic conditions (Fig. 1). It is also expected that activated PI3K/Akt/mTOR pathway would enhance the expression of their other target genes involved in cell proliferation, survival, motility, etc., and therefore play an important role in hypoxia-induced biological effects in cancer cells (Fig. 1). However, more studies are warranted to clearly establish the molecular mechanisms involved in the activation of PI3K/Akt/mTOR in hypoxic cancer cell.
C. NOX Signaling
The NOX system consists of catalytic subunits [gp91phox (NOX1-5) and p22phox] on the membrane and regulatory subunits (p40phox, p47phox, p67phox, and Rac1/2) toward the cytoplasmic side. The NOX system passes an electron to oxygen and generates superoxide that is rapidly converted to hydrogen peroxide and causes oxidation of redox-sensitive cysteine residues in target molecules. NOX-generated reactive ROS acts as a secondary messenger and is involved in the regulation of several survival and mitogenic signaling pathways.64–67 Importantly, dysregulated NOX-dependent ROS generation is associated with several chronic diseases including cancer.68,69 NOX-mediated ROS generation activates several oncogenes (e.g., receptor tyrosine kinases, Src, and Ras), inactivates several tumor suppressor genes (e.g., PTEN, p53 and TSC2),70 and is considered critical in the transformation as well as maintenance of malignant and resistant phenotype in cancer cells.64,65,69–73 There are abundant reports suggesting that the NOX system plays an important role in PCA growth and progression.64–67,73,74 Lim et al. reported that NOX1 is overexpressed in a high percentage of human prostate tumors associated with elevated ROS generation and increased tumorigenicity.66
Hypoxia is one of the key elements that promote NOX activity.75,76 Cycling hypoxic glioblastoma cells showed higher NOX4 expression associated with high ROS levels and radio resistance.77 Diebold et al. reported that hypoxia rapidly increased NOX4 expression in pulmonary artery smooth-muscle cells (PASMCs).76 Chromatin immunoprecipitation (CHIP) analysis identified HIF-1α binding to NOX4 gene regulating its expression, and activated NOX4 maintained the ROS level and supported the proliferation of PASMCs.76 In response to intermittent hypoxia, HIF-1α increased the NOX2 expression in the central and peripheral nervous system of mice as well as in cultured cells.75 Contrary to these reports, Block et al. reported that NOX (1 and 4) and p22phox regulate HIF-2α expression in VHL-deficient renal cell carcinoma.78 Nanduri et al. also reported that HIF-1α activation by intermittent hypoxia requires NOX stimulation by xanthine oxidase in rat pheochromocytoma PC12 cells.14 J Moon et al. reported that NOX-mediated ROS production plays an important role in HIF-1α activation following hyperthermia treatment in cancer cells.79 Overall, it seems that in hypoxic cells, HIF-1α, and NOX promote each other’s expression in a feed-forward manner and execute hypoxic response (Fig. 1).
D. Wnt/β-Catenin Signaling
The Wnt/β-catenin signaling pathway contributes to the pathogenesis of several diseases including cancer.80–84 In the absence of Wnts, β-catenin is phosphorylated by the “destruction complex,” which includes adenomatous polyposis coli (APC), axin 2, casein kinase 1, and GSK3. The phosphorylated β-catenin is subsequently ubiquitinated and degraded via the proteasomal-mediated pathway. Wnts binding to the Frizzled family of receptors and the LDL-receptor-related proteins (LRP) 5 and 6 corepressors inhibits the destruction complex allowing β-catenin to accumulate, translocate to the nucleus, and activate Wnt target genes such as cyclin D1, c-myc, etc.80–82 Importantly, GSK3β could also regulate the stability of HIF-1α as inhibition of GSK3β increased HIF-1α levels, whereas its overexpression reduced HIF-1α levels.85,86
β-catenin expression is reported to be higher in PCA compared with normal prostate tissue and associated with disease progression.11,87 Mitani et al. reported the role of β-catenin along with HIF-1α in AR activation under low androgen conditions leading to hormone-refractory PCA progression.11 This study showed that under hypoxic conditions, HIF-1α binds to β-catenin and promotes its nuclear translocation, and together they enhance AR transactivation by accelerating N-terminal and C-terminal interaction.11 HIF-1α also modulates Wnt/βcatenin signaling in hypoxic embryonic stem cells by enhancing βcatenin activation and the expression of the downstream effectors LEF-1 and TCF-1.88 Wnt/β-catenin pathway also plays an important role in HIF-1α-mediated EMT in human prostate carcinoma cells.83 To et al. reported that hypoxia activated β-catenin levels independent of classical APC and p53 pathways but stimulated by PI3K/Akt signaling in a Nurr77-dependent manner in colon cancer cells.89 Furthermore, the β-catenin-Nurr77 feed-forward loop promoted the migration, invasion, and EMT in these cells.89 Liu et al. also reported that hypoxia-induced β-catenin activation increases the invasiveness and metastasis of hepatocellular carcinoma cells (HCCs) through promoting EMT and MMP2 (matrix metalloproteinase 2) expression.84 More importantly, positive expression of β-catenin in HCC tissue microarray was associated with HIF-1α expression, and co-expression of β-catenin and HIF-1α was correlated with shorter overall patient survival and time to disease recurrence. Zhang et al. further confirmed that Wnt/βcatenin signaling enhances hypoxia-induced EMT and survival in HCC by increasing HIF-1α activity.90 Similarly, Liu et al. reported that β-catenin activation under hypoxic conditions plays an important role in regulating the invasiveness of gastric cancer cells through activating the urokinase plasminogen activator (uPA) and MMP7 expression.91 Interestingly, this study found that β-catenin could regulate HIF-1α expression in hypoxic cancer cells. In another study, Scholten II et al. reported that Wnt/βcatenin signaling is downregulated by hypoxia in osteosarcoma cells (OS), which appears to rely on both HIF-independent and -dependent mechanisms.81 This study suggested that an inverse association between Wnt/βcatenin and HIF, but interestingly further downregulation of Wnt/βcatenin, mitigated the hypoxia-induced chemoresistance in these cells.81 Santoyo-Ramos et al. suggested even complicated interaction between Wnt/βcatenin and HIF-1.82 PIn hypoxic colon cancer cells, HIF-1α promoted Wnt/βcatenin signaling, EMT, and stemness, while HIF-2α seems to negatively regulate Wnt/βcatenin signaling.82 Furthermore, Lim et al. reported that HIF1α could obstruct Wnt/βcatenin signaling pathway by inhibiting the hARD1-mediated activation of βcatenin.92 Verras et al. also reported that tumor hypoxia could block Wnt/βcatenin signaling through the induction of endoplasmic stress in cancer cells; however, tumor cells harboring common βcatenin pathway mutations were insensitive to this hypoxic effect.93 These outcomes were considered as hypoxia-induced selective pressure for the growth of cancer cells with mutations in the βcatenin pathway.93 Overall, Wnt/βcatenin signaling seems to have significant cross talk with the HIF-1 pathway and plays an important role in inducing the growth, EMT, metastasis, and androgen-independent growth in hypoxic cancer cells (Fig. 1).
E. Hedgehog Signaling
The Hedgehog (Hh) signaling pathway plays a critical role in numerous processes during embryonic development including cell growth, differentiation patterning, and organogenesis. In normal adult tissue, the Hh pathway regulates stem cell population maintenance, tissue repair, and regeneration. Aberrant activation of Hh signaling has been reported in several malignancies including PCA.94–97 Gli1 is the transcriptional target of the Hh pathway and an activator of target genes including cyclin D1/D2, N-myc, bcl2, VEGF, MMP9, oct4, sox2, etc.96 In the absence of Hh ligands, patched 1 (PTCH1) holds Smoothened (SMO), a seven-transmembrane spanning protein, in an inactive state and further downstream signaling is inhibited. On binding of Hh ligands to PTCH1, SMO dissociates from PTCH1, signaling is transduced, and Gli1 is activated, which then activates the expression of target genes. Onishi et al. reported that hypoxia activates the Hh signaling pathway in pancreatic ductal adenocarcinoma cells (PDACs) by increasing the transcription of SMO in a ligand-independent manner and increases the invasiveness of PDAC.97 Importantly, this study showed that silencing of HIF1α did not affect the transcription of Hh-related genes under hypoxia as well as silencing of SMO or Gli1 did not affect HIF1α expression under hypoxia, suggesting that Hh and HIF1α pathways act independent of each other.97 Lei et al. also reported that Hh signaling plays an important role in hypoxia-induced EMT and invasiveness of pancreatic cancer cells, but surprisingly this study suggested that activation of Hh signaling under hypoxic conditions is dependent on HIF-1α activation.98 However, these two studies employed different pancreatic cancer cell lines, and that could be the possible reason for HIF1α dependent or independent activation of Hh signaling. Lei et al. reported a similar role of Gli1 in hypoxia-induced EMT and invasiveness of breast cancer cells.99 In another study, Zhou et al. reported that SHH-Gli1 activities are increased under hypoxia in renal cell carcinoma (RCC) cells mediated by HIF2α.100 Interestingly, this study also showed that SHH-Gli1 signaling positively regulates HIF2α expression in normoxic RCC cells.100 There is increasing evidence suggesting the active role of Hh signaling in the development and progression of PCA94–96 but to our knowledge role of Hh signaling has not been investigated in hypoxic PCA cells.
III. EXOSOMES AS MESSENGER OF HYPOXIC RESPONSE
Exosomes are small vesicles originating from late endosomes and secreted into the extracellular milieu after the fusion of multivesicular bodies (MVBs) with the plasma membrane. Exosomes have been implicated in the pathogenesis of several diseases including cancer.21,101 Recent literature has established a critical role for these nanosized vesicles in intercellular communication, primary tumor growth, angiogenesis, pre-metastatic niches preparation, metastasis, drug resistance, immunosuppression, and disease relapse.21,102–105 Importantly, hypoxic cancer cells secrete a higher amount of exosomes loaded with unique cargo proteins, miRs and lipids, and promote tumor growth and progression.22,104 King et al. showed that breast cancer cells secrete a higher amount of exosomes under hypoxic condition in an HIF-1α-dependent manner.22 This study also found increased level of miR-210 in exosomes released by hypoxic breast cancer cells. Umezu et al. reported that hypoxia-resistant multiple myeloma (HR-MM) cells produced more exosomes than the parental cells under normoxia or acute hypoxia conditions with significantly higher miR-135 expression.102 Moreover, exosomal miR-135 from HR-MM cells directly suppressed the FIH-1 and enhanced the tube formation in endothelial cells. Kucharzewska et al. reported that exosomes mediate hypoxia-dependent intercellular signaling in highly malignant brain tumor glioblastoma multiforme (GBM) cells.101 The molecular profile of hypoxic exosomes reflected the hypoxic response of GBM donor cells and GBM patient’s tumors and were enriched in MMP9, pentraxin 3, IL8, platelet-derived growth factor (PDGF), and plasminogen activator inhibitor 1 (PAI1).101 This study also showed that exosomes derived from hypoxic GBM cells strongly induced angiogenesis in vitro, ex vivo, and in vivo through phenotypic modulation of endothelial cells. Tadokoro et al. also reported that exosomes derived from hypoxic leukemia cells enhanced tube formation in human umbilical vein endothelial cells via miR-210.103 Parolini et al. demonstrated that exosome release and uptake is increased in an acidic pH, suggesting that acidic microenvironment in hypoxic tumors could contribute to the increased rate of exosomal release and uptake by cancer cells.106 In an earlier study, Park et al. also showed that under hypoxic conditions tumor cells secrete proteins and exosomes responsible for increased angiogenesis and metastatic potential.107 Aga et al. reported the presence of transcriptionally active HIF-1α in the exosomes of nasopharyngeal carcinoma cells that played a role in the induction of receipt cells.108 Under hypoxic conditions, adipocyte-released exosomes promoted lipogenesis in 3T3-L1 recipient cells.109 Besides exosomes, hypoxia significantly enhanced the microvesicles (formed by direct budding from the membranes; ~ size 100–≥1000 nm) biogenesis in a HIF1α- and RAB22A GTPase-dependent manner, and promoted the formation of focal adhesions, invasiveness, and metastasis in naïve breast cancer cells.104
We have recently reported that exosomes secreted by PCA cells under hypoxic conditions induce EMT, invasiveness, and stemness in naïve PCA cells as well as promote CAF phenotype in naïve normal prostate fibroblasts.24 We have also reported that hypoxic PCA exosomes are loaded with unique proteins with higher expression of canonical markers, MMP2/9, and signaling molecules compared to normoxic PCA exosomes.24 In another study, we reported that hypoxic PCA exosomes are loaded with a significantly higher amount of triglycerides, and hypoxic PCA exosomes-induced invasiveness could be inhibited by celecoxib.23 Furthermore, this study suggested the important role of lipids in the biogenesis of exosomes under both normoxic and hypoxic conditions.23 In a recent review, Sceneay et al. summarized the role of exosomes in the preparation of pre-metastatic niches and metastasis, suggesting that exosomes secreted by hypoxic cancer cells could have systemic effects and could promote cancer metastasis.19 It is also suggested that by using the advanced mass spectrometry based proteomics analysis of tumor-derived exosomes, it is possible to estimate the oxygenation status of the patient’s tumors.110 Therefore, exosomes could be a useful biomarker in making appropriate treatment decisions in the clinic. Overall, there is ample evidence suggesting that exosomes could mediate the biological effects of hypoxic cancer cells in the tumor microenvironment (Fig. 2).
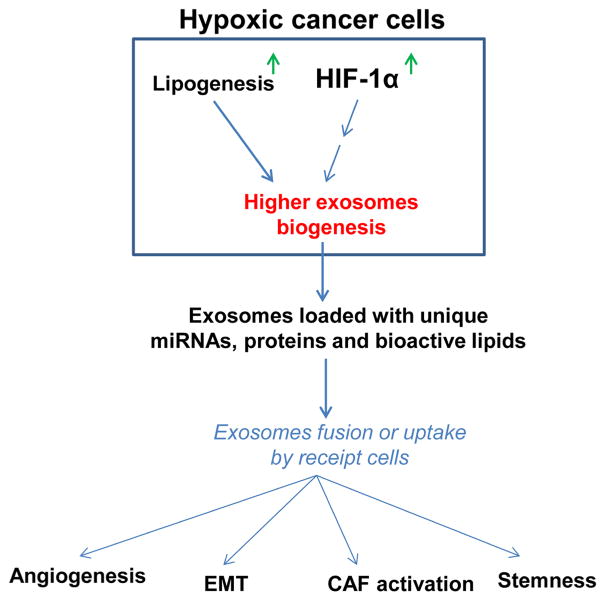
FIG 2.
Exosomes act as messenger of hypoxic response in cancer cells. Biogenesis of exosomes is increased in hypoxic cancer cells dependent on HIF-1α and cellular lipid level, and in turn hypoxic cancer cells secrete higher amounts of exosomes loaded with unique cargo (miRNAs, proteins, and bioactive lipids). Hypoxic cancer cells exosomes uptake by receipt cells in the tumor microenvironment results in higher angiogenesis, EMT, CAF activation, and increased stemness.
IV. HYPOXIA/HYPOXIA-INDUCED SIGNALING MOLECULES AS BIOMARKER FOR PCA DIAGNOSIS AND PROGNOSIS: TRANSLATIONAL RELEVANCE
There are several reports supporting that hypoxia in prostate tumor is an early event and also an independent risk factor for disease progression and treatment failure.7–10,111 Hypoxia in tumor is measured by analyzing tissues for hypoxia-related biomarkers (mostly HIF-1α and HIF-1α regulated genes) or by using pimonidazole dye (a 2-nitro-imidazole compound that forms a covalent bond with cellular macromolecules at oxygen levels below 1.3%) or pO2 is directly measured using Eppendorf microelectrodes. There are several studies suggesting that HIF-1α could be a good biomarker to predict PCA progression as well as treatment outcomes.7,112 HIF-1α is overexpressed in primary prostate cancers as compared with normal prostate epithelium.113 Du et al. confirmed the high levels of HIF-1α in prostate cancer as compared with benign prostate hyperplasia (BPH) and normal tissue.114 Pipinikas et al. suggested that HIF-1α mRNA levels in blood could be useful in improving the diagnosis of early stages of PCA.115 Therefore, HIF-1α overexpression appears to be an early event in PCA pathogenesis.
Ranasinghe et al. measured HIF-1α expression by immunohistochemistry (IHC) in 100 human prostate tumors, which were collected following radical prostectomy or transurethral resection of the prostate (TURP).7 They found that HIF-1α expression in tumors is an independent risk factor for disease progression associated with significantly reduced metastasis-free survival and CRPC (castrate-resistant prostate cancer)-free survival.7 More importantly, this study also found that prostate tumors lacking HIF-1α expression did not metastasize or developed CRPC.7 Yasuda et al. also characterized the role of HIF-1α (by IHC) in predicting the success of neo-adjuvant hormone therapy in patients with adenocarcinoma.112 This study also found that strong HIF-1α expression after neoadjuvant hormone therapy suggests a relatively high risk of disease recurrence and treatment failure. Stewart et al. showed that the transcript levels of hypoxia-associated genes LOX (lysyl oxidase) and GLUT-1 (glucose transporter-1) were significantly higher in PCA compared to BPH tissue and correlated with Gleason score.116 In another study, Vergis et al. concluded that for PCA patients treated with radiotherapy, higher HIF-1α and VEGF (one of the HIF-1α regulated gene) expression is significant predictor of a shorter time to biochemical failure independent of clinical tumor stage, Gleason Score, serum PSA concentration, and dose of radiotherapy.10 Similarly, for patients treated with surgery, the higher expression of HIF-1α, VEGF, and osteopontin (also regulated by HIF-1α) was associated with a shorter time to biochemical failure independent of pathological tumor stage, Gleason score, serum PSA concertation, and margin status.10 This study clearly suggested that HIF-1α expression or expression of HIF-1α regulated genes could be a predictor of the biochemical failure independent of treatment modality.
Similar findings, as above for HIF-1α, were reported when hypoxia was measured through pimonidazole or microelectrodes. Ragnum et al. characterized the hypoxia area in primary tumors by using pimonidazole.8 PCA patients were injected pimonidazole (IV) the day before robot-assisted laparoscopic radical prostatectomy (RALP). This study found that pimonidazole immunoscore is significantly higher for tumors at a high clinical stage and with lymph node metastasis.8 Pimonidazole gene signature also reflected an aggressive hypoxic PCA phenotype characterized by upregulation of proliferation, DNA repair, and hypoxia-response gene.8 Carnell et al. also reported that increased pimonidazole staining was observed in tumors with high Gleason score.117 Milosevic et al. conducted one of the largest clinical studies of PCA hypoxia in 247 patients, with localized PCA before radiotherapy with or without hormonal therapy, with direct measurement of tumor oxygen levels by using ultrasound-guided transrectal needle-electrode.9 This study found that tumor hypoxia is associated with early biochemical relapse after radiotherapy and predicts local recurrence.9 Earlier, Turaka et al. reported that hypoxia in prostate cancer (low mean hypoxic prostate/muscle pO2 ratio) significantly predicts for poor long-term biochemical outcome.118
Therefore, it is clear that hypoxia and/or the activation of hypoxia-related signaling in prostate tumors could determine treatment success as well as disease progression. These results also suggest that PCA growth and progression could be effectively prevented via targeting hypoxia/hypoxia-related signaling. This rationale is strongly supported by recent studies suggesting the efficacy of HIF-1α inhibitors to prevent the disease progression and treatment failure.4,34,119,120 Platz et al. identified that nonspecific HIF-1α inhibitor digoxin, a cardiac glycoside derived from foxglove used to treat congestive heart failure and arrhythmia, has strong efficacy to inhibit the proliferation of androgen-dependent and -independent PCA cells in cell culture.119 Furthermore, this study based on the clinical data from the Health Professional Follow-Up study (47,884 men followed up from 1986 through 2006) concluded that compared with nonusers, men who regularly used digoxin had a 25% lower risk of PCA, including disease that was potentially lethal.119 This study strongly suggests the potential usefulness of digoxin in reducing the risk of developing PCA or to treat undetected PCA. In a retrospective review of prospectively collected medical records, Ranasinghe et al. concluded that nonspecific HIF-1α inhibitors (digoxin, metformin, and angiotensin-2 receptor blocker) increase the progression-free survival and decrease the risk of developing CRPC and metastasis in PCA patients on continuous androgen deprivation therapy.120 Extensive efforts are now being put into targeting HIF-1 and associated pathways by small molecules inhibitors (e.g., YC-1 and PX-478), HIF-1 DNA activity (e.g., polyamides and echinomycin), and HIF-1 transcriptional activity (e.g., chetomin, proteasome inhibitors, and amphotericin B).4,121
V. CONCLUSIONS
Hypoxia is one of the hallmarks of solid tumors, endowing them with malignant, aggressive, and treatment-refractory characteristics. HIF-1α is the major signaling pathway that is activated under hypoxic conditions and promotes angiogenesis, survival, EMT, metastasis, etc. However, several other signaling pathways such as PI3K/Akt/mTOR, NOX, Wnt/catenin, and Hedgehog are also activated under hypoxic conditions, and play a vital role in hypoxia-induced biological effects. It is also clear now that exosomes play an important role in transferring message from hypoxic cancer cells to normoxic cells in the tumor microenvironment, thereby contributing toward cancer growth, progression, and metastasis. Clinical studies clearly suggest the potential of hypoxia/hypoxia signaling biomarkers in the prognosis of the disease as well as in making key treatment decisions. However, we need to further develop reliable noninvasive methods to longitudinally measure hypoxia status in the tumors. In this regard, exosomes could be potentially useful in determining the hypoxia status of the tumors, but we need to establish biomarkers (proteins, miRNA, and lipids) that are distinctly present in the exosomes released by hypoxic cancer cells. Overall, further research in this area could be potentially beneficial in the effective management and control of cancer.
Acknowledgments
This work was supported by R21 Grant No. CA199628 and DOD Award No. W81XWH-15-1-0188 to GD.
ABBREVIATIONS
- APC
adenomatous polyposis coli
- AR
androgen receptor
- BPH
benign prostate hyperplasia
- CAFs
cancer-associated fibroblasts
- CXCR
CXC chemokine receptor
- EMT
epithelial-to-mesenchymal transition
- HIF-1
hypoxia-inducible factor 1
- FH
fumarate dehydrogenase
- FIH
factor-inhibiting HIF-1α
- Hh
Hedgehog
- IL8
interleukin 8
- MAPK
mitogen-activated protein kinase
- MDM2
murine double mutant 2
- mTOR
mammalian target of rapamycin
- PAI1
plasminogen activator inhibitor 1
- PCA
prostate cancer
- PDGF
platelet-derived growth factor
- PHD
prolyl hydroxylase
- PI3K
phosphatidylinositol 3-kinases
- SDH
succinate dehydrogenase
- VEGF
vascular endothelial growth factor
- VEGFR
vascular endothelial growth factor receptor
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. Cancer J Clinicians. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Fraga A, Ribeiro R, Principe P, Lopes C, Medeiros R. Hypoxia and prostate cancer aggressiveness: a tale with many endings. Clin Genitourinary Cancer. 2015;13:295–301. doi: 10.1016/j.clgc.2015.03.006. [DOI] [PubMed] [Google Scholar]
- 3.Butterworth KT, McCarthy HO, Devlin A, Ming L, Robson T, McKeown SR, Worthington J. Hypoxia selects for androgen independent LNCaP cells with a more malignant geno- and phenotype. Int J Cancer. 2008;123:760–8. doi: 10.1002/ijc.23418. [DOI] [PubMed] [Google Scholar]
- 4.Stewart GD, Ross JA, McLaren DB, Parker CC, Habib FK, Riddick AC. The relevance of a hypoxic tumour microenvironment in prostate cancer. BJU Int. 2010;105:8– 13. doi: 10.1111/j.1464-410X.2009.08921.x. [DOI] [PubMed] [Google Scholar]
- 5.Jain RK. Antiangiogenesis strategies revisited: from starving tumors to alleviating hypoxia. Cancer Cell. 2014;26:605–22. doi: 10.1016/j.ccell.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harris AL. Hypoxia--a key regulatory factor in tumour growth. Nat Rev Cancer. 2002;2:38–47. doi: 10.1038/nrc704. [DOI] [PubMed] [Google Scholar]
- 7.Ranasinghe WK, Xiao L, Kovac S, Chang M, Michiels C, Bolton D, Shulkes A, Baldwin GS, Patel O. The role of hypoxia-inducible factor 1alpha in determining the properties of castrate-resistant prostate cancers. PloS One. 2013;8:e54251. doi: 10.1371/journal.pone.0054251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ragnum HB, Vlatkovic L, Lie AK, Axcrona K, Julin CH, Frikstad KM, Hole KH, Seierstad T, Lyng H. The tumour hypoxia marker pimonidazole reflects a transcriptional programme associated with aggressive prostate cancer. Br J Cancer. 2015;112:382–90. doi: 10.1038/bjc.2014.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Milosevic M, Warde P, Menard C, Chung P, Toi A, Ishkanian A, McLean M, Pintilie M, Sykes J, Gospodarowicz M, Catton C, Hill RP, Bristow R. Tumor hypoxia predicts biochemical failure following radiotherapy for clinically localized prostate cancer. Clin Cancer Res. 2012;18:2108–14. doi: 10.1158/1078-0432.CCR-11-2711. [DOI] [PubMed] [Google Scholar]
- 10.Vergis R, Corbishley CM, Norman AR, Bartlett J, Jhavar S, Borre M, Heeboll S, Horwich A, Huddart R, Khoo V, Eeles R, Cooper C, Sydes M, Dearnaley D, Parker C. Intrinsic markers of tumour hypoxia and angiogenesis in localised prostate cancer and outcome of radical treatment: a retrospective analysis of two randomised radiotherapy trials and one surgical cohort study. Lancet Oncol. 2008;9:342–51. doi: 10.1016/S1470-2045(08)70076-7. [DOI] [PubMed] [Google Scholar]
- 11.Mitani T, Harada N, Nakano Y, Inui H, Yamaji R. Coordinated action of hypoxia-inducible factor-1alpha and beta- catenin in androgen receptor signaling. J Biol Chem. 2012;287:33594–606. doi: 10.1074/jbc.M112.388298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Joshi S, Singh AR, Durden DL. MDM2 regulates hypoxic hypoxia-inducible factor 1alpha stability in an E3 ligase, proteasome, and PTEN-phosphatidylinositol 3-kinase-AKT-dependent manner. J Biol Chem. 2014;289:22785–97. doi: 10.1074/jbc.M114.587493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hudson CC, Liu M, Chiang GG, Otterness DM, Loomis DC, Kaper F, Giaccia AJ, Abraham RT. Regulation of hypoxia-inducible factor 1alpha expression and function by the mammalian target of rapamycin. Mol Cell Biol. 2002;22:7004–14. doi: 10.1128/MCB.22.20.7004-7014.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nanduri J, Vaddi DR, Khan SA, Wang N, Makarenko V, Semenza GL, Prabhakar NR. HIF-1alpha activation by intermittent hypoxia requires NADPH oxidase stimulation by xanthine oxidase. PloS One. 2015;10:e0119762. doi: 10.1371/journal.pone.0119762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rattigan YI, Patel BB, Ackerstaff E, Sukenick G, Koutcher JA, Glod JW, Banerjee D. Lactate is a mediator of metabolic cooperation between stromal carcinoma associated fibroblasts and glycolytic tumor cells in the tumor microenvironment. Exp Cell Res. 2012;318:326–35. doi: 10.1016/j.yexcr.2011.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Polet F, Feron O. Endothelial cell metabolism and tumour angiogenesis: glucose and glutamine as essential fuels and lactate as the driving force. J Internal Med. 2013;273:156–65. doi: 10.1111/joim.12016. [DOI] [PubMed] [Google Scholar]
- 17.1Sonveaux P, Copetti T, De Saedeleer CJ, Vegran F, Verrax J, Kennedy KM, Moon EJ, Dhup S, Danhier P, Frerart F, Gallez B, Ribeiro A, Michiels C, Dewhirst MW, Feron O. Targeting the lactate transporter MCT1 in endothelial cells inhibits lactate-induced HIF-1 activation and tumor angiogenesis. PloS One. 2012;7:e33418. doi: 10.1371/journal.pone.0033418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Colegio OR, Chu NQ, Szabo AL, Chu T, Rhebergen AM, Jairam V, Cyrus N, Brokowski CE, Eisenbarth SC, Phillips GM, Cline GW, Phillips AJ, Medzhitov R. Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature. 2014;513:559–63. doi: 10.1038/nature13490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sceneay J, Smyth MJ, Moller A. The pre-metastatic niche: finding common ground. Cancer Metast Rev. 2013;32:449–64. doi: 10.1007/s10555-013-9420-1. [DOI] [PubMed] [Google Scholar]
- 20.Sceneay J, Chow MT, Chen A, Halse HM, Wong CS, Andrews DM, Sloan EK, Parker BS, Bowtell DD, Smyth MJ, Moller A. Primary tumor hypoxia recruits CD11b+/Ly6Cmed/Ly6G+ immune suppressor cells and compromises NK cell cytotoxicity in the premetastatic niche. Cancer Res. 2012;72:3906–11. doi: 10.1158/0008-5472.CAN-11-3873. [DOI] [PubMed] [Google Scholar]
- 21.Milane L, Singh A, Mattheolabakis G, Suresh M, Amiji MM. Exosome mediated communication within the tumor microenvironment. J Control Release. 2015 doi: 10.1016/j.jconrel.2015.06.029. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 22.King HW, Michael MZ, Gleadle JM. Hypoxic enhancement of exosome release by breast cancer cells. BMC Cancer. 2012;12:421. doi: 10.1186/1471-2407-12-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schlaepfer IR, Nambiar DK, Ramteke A, Kumar R, Dhar D, Agarwal C, Bergman B, Graner M, Maroni P, Singh RP, Agarwal R, Deep G. Hypoxia induces triglycerides accumulation in prostate cancer cells and extracellular vesicles supporting growth and invasiveness following reoxygenation. Oncotarget. 2015;6:22836–56. doi: 10.18632/oncotarget.4479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ramteke A, Ting H, Agarwal C, Mateen S, Somasagara R, Hussain A, Graner M, Frederick B, Agarwal R, Deep G. Exosomes secreted under hypoxia enhance invasiveness and stemness of prostate cancer cells by targeting adherens junction molecules. Mol Carcinogen. 2015;54:554–65. doi: 10.1002/mc.22124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prabhakar NR, Semenza GL. Adaptive and maladaptive cardiorespiratory responses to continuous and intermittent hypoxia mediated by hypoxia-inducible factors 1 and 2. Physiol Rev. 2012;92:967–1003. doi: 10.1152/physrev.00030.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yuan G, Nanduri J, Khan S, Semenza GL, Prabhakar NR. Induction of HIF-1alpha expression by intermittent hypoxia: involvement of NADPH oxidase, Ca2+ signaling, prolyl hydroxylases, and mTOR. Journal of Cellular Physiology. 2008;217:674–85. doi: 10.1002/jcp.21537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Semenza GL. HIF-1 and tumor progression: pathophysiology and therapeutics. Trends Mol Med. 2002;8:S62–7. doi: 10.1016/s1471-4914(02)02317-1. [DOI] [PubMed] [Google Scholar]
- 28.Pouyssegur J, Dayan F, Mazure NM. Hypoxia signalling in cancer and approaches to enforce tumour regression. Nature. 2006;441:437–43. doi: 10.1038/nature04871. [DOI] [PubMed] [Google Scholar]
- 29.Mazure NM, Brahimi-Horn MC, Berta MA, Benizri E, Bilton RL, Dayan F, Ginouves A, Berra E, Pouyssegur J. HIF-1: master and commander of the hypoxic world. A pharmacological approach to its regulation by siRNAs. Biochem Pharmacol. 2004;68:971–80. doi: 10.1016/j.bcp.2004.04.022. [DOI] [PubMed] [Google Scholar]
- 30.Huang M, Chen Q, Xiao J, Yao T, Bian L, Liu C, Lin Z. Overexpression of hypoxia-inducible factor-1alpha is a predictor of poor prognosis in cervical cancer: a clinicopathologic study and a meta-analysis. Int J Gynecol Cancer. 2014;24:1054–64. doi: 10.1097/IGC.0000000000000162. [DOI] [PubMed] [Google Scholar]
- 31.Wang Q, Hu DF, Rui Y, Jiang AB, Liu ZL, Huang LN. Prognosis value of HIF-1alpha expression in patients with non-small cell lung cancer. Gene. 2014;541:69–74. doi: 10.1016/j.gene.2014.03.025. [DOI] [PubMed] [Google Scholar]
- 32.Chen Z, He X, Xia W, Huang Q, Zhang Z, Ye J, Ni C, Wu P, Wu D, Xu J, Qiu F, Huang J. Prognostic value and clinicopathological differences of HIFs in colorectal cancer: evidence from meta-analysis. PloS One. 2013;8:e80337. doi: 10.1371/journal.pone.0080337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Semenza GL. Hypoxia-inducible factors: mediators of cancer progression and targets for cancer therapy. Trends Pharmacol Sci. 2012;33:207–14. doi: 10.1016/j.tips.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;3:721–732. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- 35.Kitajima Y, Miyazaki K. The critical impact of HIF-1a on gastric cancer biology. Cancers. 2013;5:15–26. doi: 10.3390/cancers5010015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lu X, Kang Y. Hypoxia and hypoxia-inducible factors: master regulators of metastasis. Clin Cancer Res. doi: 10.1158/1078-0432.CCR-10-1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ghafar MA, Anastasiadis AG, Chen MW, Burchardt M, Olsson LE, Xie H, Benson MC, Buttyan R. Acute hypoxia increases the aggressive characteristics and survival properties of prostate cancer cells. Prostate. 2003;54:58– 67. doi: 10.1002/pros.10162. [DOI] [PubMed] [Google Scholar]
- 38.Maxwell PJ, Gallagher R, Seaton A, Wilson C, Scullin P, Pettigrew J, Stratford IJ, Williams KJ, Johnston PG, Waugh DJ. HIF-1 and NF-kappaB-mediated upregulation of CXCR1 and CXCR2 expression promotes cell survival in hypoxic prostate cancer cells. Oncogene. 2007;26:7333–45. doi: 10.1038/sj.onc.1210536. [DOI] [PubMed] [Google Scholar]
- 39.Glunde K, Shah T, Winnard PT, Jr, Raman V, Takagi T, Vesuna F, Artemov D, Bhujwalla ZM. Hypoxia regulates choline kinase expression through hypoxia-inducible factor-1 alpha signaling in a human prostate cancer model. Cancer Res. 2008;68:172–80. doi: 10.1158/0008-5472.CAN-07-2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Horii K, Suzuki Y, Kondo Y, Akimoto M, Nishimura T, Yamabe Y, Sakaue M, Sano T, Kitagawa T, Himeno S, Imura N, Hara S. Androgen-dependent gene expression of prostate-specific antigen is enhanced synergistically by hypoxia in human prostate cancer cells. Mol Cancer Research. 2007;5:383–91. doi: 10.1158/1541-7786.MCR-06-0226. [DOI] [PubMed] [Google Scholar]
- 41.Mitani T, Yamaji R, Higashimura Y, Harada N, Nakano Y, Inui H. Hypoxia enhances transcriptional activity of androgen receptor through hypoxia-inducible factor-1 alpha in a low androgen environment. J Steroid Biochem Mol Biol. 2011;123:58–64. doi: 10.1016/j.jsbmb.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 42.Hennessey D, Martin LM, Atzberger A, Lynch TH, Hollywood D, Marignol L. Exposure to hypoxia following irradiation increases radioresistance in prostate cancer cells. Urol Oncol. 2013;31:1106–6. doi: 10.1016/j.urolonc.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 43.Ma Y, Liang D, Liu J, Axcrona K, Kvalheim G, Stokke T, Nesland JM, Suo Z. Prostate cancer cell lines under hypoxia exhibit greater stem-like properties. PloS One. 2011;6:e29170. doi: 10.1371/journal.pone.0029170. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 44.Grebhardt S, Veltkamp C, Strobel P, Mayer D. Hypoxia and HIF-1 increase S100A8 and S100A9 expression in prostate cancer. Int J Cancer. 2012;131:2785–94. doi: 10.1002/ijc.27591. [DOI] [PubMed] [Google Scholar]
- 45.Xiao LJ, Chen YY, Lin P, Zou HF, Lin F, Zhao LN, Li D, Guo L, Tang JB, Zheng XL, Yu XG. Hypoxia increases CX3CR1 expression via HIF-1 and NFkappaB in androgen-independent prostate cancer cells. Int Jgy. 2012;41:1827–36. doi: 10.3892/ijo.2012.1610. [DOI] [PubMed] [Google Scholar]
- 46.Lv L, Yuan J, Huang T, Zhang C, Zhu Z, Wang L, Jiang G, Zeng F. Stabilization of Snail by HIF-1alpha and TNF-alpha is required for hypoxia-induced invasion in prostate cancer PC3 cells. Mol Biol Rep. 2014;41:4573–82. doi: 10.1007/s11033-014-3328-x. [DOI] [PubMed] [Google Scholar]
- 47.Huss WJ, Hanrahan CF, Barrios RJ, Simons JW, Greenberg NM. Angiogenesis and prostate cancer: identification of a molecular progression switch. Cancer Res. 2001;61:2736–43. [PubMed] [Google Scholar]
- 48.Raina K, Rajamanickam S, Singh RP, Deep G, Chittezhath M, Agarwal R. Stage-specific inhibitory effects and associated mechanisms of silibinin on tumor progression and metastasis in transgenic adenocarcinoma of the mouse prostate model. Cancer Res. 2008;68:6822–30. doi: 10.1158/0008-5472.CAN-08-1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Karar J, Maity A. PI3K/AKT/mTOR Pathway in Angiogenesis. Front Mol Neuroscie. 2011;4:51. doi: 10.3389/fnmol.2011.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bader AG, Kang S, Zhao L, Vogt PK. Oncogenic PI3K deregulates transcription and translation. Nat Rev Cancer. 2005;5:921–9. doi: 10.1038/nrc1753. [DOI] [PubMed] [Google Scholar]
- 51.Edlind MP, Hsieh AC. PI3K-AKT-mTOR signaling in prostate cancer progression and androgen deprivation therapy resistance. Asian J Androl. 2014;16:378–86. doi: 10.4103/1008-682X.122876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Toker A, Cantley LC. Signalling through the lipid products of phosphoinositide-3-OH kinase. Nature. 1997;387:673–6. doi: 10.1038/42648. [DOI] [PubMed] [Google Scholar]
- 53.Yamasaki M, Nomura T, Sato F, Mimata H. Chronic hypoxia induces androgen-independent and invasive behavior in LNCaP human prostate cancer cells. Urol Oncol. 2013;31:1124–31. doi: 10.1016/j.urolonc.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 54.Blancher C, Moore JW, Robertson N, Harris AL. Effects of ras and von Hippel-Lindau (VHL) gene mutations on hypoxia-inducible factor (HIF)-1alpha, HIF-2alpha, and vascular endothelial growth factor expression and their regulation by the phosphatidylinositol 3′-kinase/Akt signaling pathway. Cancer Res. 2001;61:7349–55. [PubMed] [Google Scholar]
- 55.Yan W, Fu Y, Tian D, Liao J, Liu M, Wang B, Xia L, Zhu Q, Luo M. PI3 kinase/Akt signaling mediates epithelial- mesenchymal transition in hypoxic hepatocellular carcinoma cells. Biochem Biophys Res Commun. 2009;382:631–6. doi: 10.1016/j.bbrc.2009.03.088. [DOI] [PubMed] [Google Scholar]
- 56.Befani CD, Vlachostergios PJ, Hatzidaki E, Patrikidou A, Bonanou S, Simos G, Papandreou CN, Liakos P. Bortezomib represses HIF-1alpha protein expression and nuclear accumulation by inhibiting both PI3K/Akt/TOR and MAPK pathways in prostate cancer cells. J Mol Med (Berl) 2012;90:45–54. doi: 10.1007/s00109-011-0805-8. [DOI] [PubMed] [Google Scholar]
- 57.Wang F, Zhang W, Guo L, Bao W, Jin N, Liu R, Liu P, Wang Y, Guo Q, Chen B. Gambogic acid suppresses hypoxia-induced hypoxia-inducible factor-1alpha/vascular endothelial growth factor expression via inhibiting phosphatidylinositol 3-kinase/Akt/mammalian target protein of rapamycin pathway in multiple myeloma cells. Cancer Sci. 2014;105:1063–70. doi: 10.1111/cas.12458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lee SH, Jee JG, Bae JS, Liu KH, Lee YM. A group of novel HIF-1alpha inhibitors, glyceollins, blocks HIF-1alpha synthesis and decreases its stability via inhibition of the PI3K/AKT/mTOR pathway and Hsp90 binding. J Cell Physiol. 2015;230:853–62. doi: 10.1002/jcp.24813. [DOI] [PubMed] [Google Scholar]
- 59.Chen MC, Hsu WL, Hwang PA, Chou TC. Low molecular weight fucoidan inhibits tumor angiogenesis through downregulation of HIF-1/VEGF signaling under hypoxia. Marine Drugs. 2015;13:4436–51. doi: 10.3390/md13074436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Manohar SM, Padgaonkar AA, Jalota-Badhwar A, Sonawane V, Rathos MJ, Kumar S, Joshi KS. A novel inhibitor of hypoxia-inducible factor-1alpha P3155 also modulates PI3K pathway and inhibits growth of prostate cancer cells. BMC Cancer. 2011;11:338. doi: 10.1186/1471-2407-11-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Movafagh S, Crook S, Vo K. Regulation of hypoxia-inducible factor-1a by reactive oxygen species: new developments in an old debate. J Cell Biochem. 2015;116:696–703. doi: 10.1002/jcb.25074. [DOI] [PubMed] [Google Scholar]
- 62.Shen X, Xue Y, Si Y, Wang Q, Wang Z, Yuan J, Zhang X. The unfolded protein response potentiates epithelial-to-mesenchymal transition (EMT) of gastric cancer cells under severe hypoxic conditions. Med Oncol. 2015;32:447. doi: 10.1007/s12032-014-0447-0. [DOI] [PubMed] [Google Scholar]
- 63.Ader I, Brizuela L, Bouquerel P, Malavaud B, Cuvillier O. Sphingosine kinase 1: a new modulator of hypoxia inducible factor 1alpha during hypoxia in human cancer cells. Cancer Research. 2008;68:8635–42. doi: 10.1158/0008-5472.CAN-08-0917. [DOI] [PubMed] [Google Scholar]
- 64.Khandrika L, Kumar B, Koul S, Maroni P, Koul HK. Oxidative stress in prostate cancer. Cancer Lett. 2009;282:125–36. doi: 10.1016/j.canlet.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kumar B, Koul S, Khandrika L, Meacham RB, Koul HK. Oxidative stress is inherent in prostate cancer cells and is required for aggressive phenotype. Cancer Res. 2008;68:1777–85. doi: 10.1158/0008-5472.CAN-07-5259. [DOI] [PubMed] [Google Scholar]
- 66.Lim SD, Sun C, Lambeth JD, Marshall F, Amin M, Chung L, Petros JA, Arnold RS. Increased Nox1 and hydrogen peroxide in prostate cancer. Prostate. 2005;62:200–7. doi: 10.1002/pros.20137. [DOI] [PubMed] [Google Scholar]
- 67.Li Q, Fu GB, Zheng JT, He J, Niu XB, Chen QD, Yin Y, Qian X, Xu Q, Wang M, Sun AF, Shu Y, Rui H, Liu LZ, Jiang BH. NADPH oxidase subunit p22(phox)-mediated reactive oxygen species contribute to angiogenesis and tumor growth through AKT and ERK1/2 signaling pathways in prostate cancer. Biochim Biophys Acta. 2013;1833:3375–85. doi: 10.1016/j.bbamcr.2013.09.018. [DOI] [PubMed] [Google Scholar]
- 68.Lambeth JD. Nox enzymes, ROS, and chronic disease: an example of antagonistic pleiotropy. Free Radical Biol Med. 2007;43:332–47. doi: 10.1016/j.freeradbiomed.2007.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.6Kamata T. Roles of Nox1 and other Nox isoforms in cancer development. Cancer Sci. 2009;100:1382–8. doi: 10.1111/j.1349-7006.2009.01207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Block K, Gorin Y. Aiding and abetting roles of NOX oxidases in cellular transformation. Nat Rev Cancer. 2012;12:627–37. doi: 10.1038/nrc3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Suh YA, Arnold RS, Lassegue B, Shi J, Xu X, Sorescu D, Chung AB, Griendling KK, Lambeth JD. Cell transformation by the superoxide-generating oxidase Mox1. Nature. 1999;401:79–82. doi: 10.1038/43459. [DOI] [PubMed] [Google Scholar]
- 72.Itoh T, Terazawa R, Kojima K, Nakane K, Deguchi T, Ando M, Tsukamasa Y, Ito M, Nozawa Y. Cisplatin induces production of reactive oxygen species via NADPH oxidase activation in human prostate cancer cells. Free Radical Res. 2011;45:1033–9. doi: 10.3109/10715762.2011.591391. [DOI] [PubMed] [Google Scholar]
- 73.Arbiser JL, Petros J, Klafter R, Govindajaran B, McLaughlin ER, Brown LF, Cohen C, Moses M, Kilroy S, Arnold RS, Lambeth JD. Reactive oxygen generated by Nox1 triggers the angiogenic switch. Proc Natl Acad Sci U S A. 2002;99:715–20. doi: 10.1073/pnas.022630199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Brar SS, Corbin Z, Kennedy TP, Hemendinger R, Thornton L, Bommarius B, Arnold RS, Whorton AR, Sturrock AB, Huecksteadt TP, Quinn MT, Krenitsky K, Ardie KG, Lambeth JD, Hoidal JR. NOX5 NAD(P)H oxidase regulates growth and apoptosis in DU 145 prostate cancer cells. Am J Physiol Cell Physiol. 2003;285:C353–69. doi: 10.1152/ajpcell.00525.2002. [DOI] [PubMed] [Google Scholar]
- 75.Yuan G, Khan SA, Luo W, Nanduri J, Semenza GL, Prabhakar NR. Hypoxia-inducible factor 1 mediates increased expression of NADPH oxidase-2 in response to intermittent hypoxia. J Cell Physiol. 2011;226:2925–33. doi: 10.1002/jcp.22640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Diebold I, Petry A, Hess J, Gorlach A. The NADPH oxidase subunit NOX4 is a new target gene of the hypoxia-inducible factor-1. MoleBiol Cell. 2010;21:2087–96. doi: 10.1091/mbc.E09-12-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hsieh CH, Wu CP, Lee HT, Liang JA, Yu CY, Lin YJ. NADPH oxidase subunit 4 mediates cycling hypoxia-promoted radiation resistance in glioblastoma multiforme. Free Radical Biol Med. 2012;53:649–58. doi: 10.1016/j.freeradbiomed.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 78.Block K, Gorin Y, Hoover P, Williams P, Chelmicki T, Clark RA, Yoneda T, Abboud HE. NAD(P)H oxidases regulate HIF-2alpha protein expression. J Biol Chem. 2007;282:8019–26. doi: 10.1074/jbc.M611569200. [DOI] [PubMed] [Google Scholar]
- 79.Moon EJ, Sonveaux P, Porporato PE, Danhier P, Gallez B, Batinic-Haberle I, Nien YC, Schroeder T, Dewhirst MW. NADPH oxidase-mediated reactive oxygen species production activates hypoxia-inducible factor-1 (HIF-1) via the ERK pathway after hyperthermia treatment. Proc Natl Acad Sci U S A. 2010;107:20477–82. doi: 10.1073/pnas.1006646107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Moon RT, Kohn AD, De Ferrari GV, Kaykas A. WNT and beta-catenin signalling: diseases and therapies. Nature reviews Genetics. 2004;5:691–701. doi: 10.1038/nrg1427. [DOI] [PubMed] [Google Scholar]
- 81.Scholten DJ, 2nd, Timmer CM, Peacock JD, Pelle DW, Williams BO, Steensma MR. Down regulation of Wnt signaling mitigates hypoxia-induced chemoresistance in human osteosarcoma cells. PloS One. 2014;9:e111431. doi: 10.1371/journal.pone.0111431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Santoyo-Ramos P, Likhatcheva M, Garcia-Zepeda EA, Castaneda-Patlan MC, Robles-Flores M. Hypoxia-inducible factors modulate the stemness and malignancy of colon cancer cells by playing opposite roles in canonical Wnt signaling. PloS One. 2014;9:e112580. doi: 10.1371/journal.pone.0112580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhao JH, Luo Y, Jiang YG, He DL, Wu CT. Knockdown of beta-Catenin through shRNA cause a reversal of EMT and metastatic phenotypes induced by HIF-1alpha. Cancer Invest. 2011;29:377–82. doi: 10.3109/07357907.2010.512595. [DOI] [PubMed] [Google Scholar]
- 84.Liu L, Zhu XD, Wang WQ, Shen Y, Qin Y, Ren ZG, Sun HC, Tang ZY. Activation of beta-catenin by hypoxia in hepatocellular carcinoma contributes to enhanced metastatic potential and poor prognosis. Clin Cancer Res. 2010;16:2740–50. doi: 10.1158/1078-0432.CCR-09-2610. [DOI] [PubMed] [Google Scholar]
- 85.Flugel D, Gorlach A, Michiels C, Kietzmann T. Glycogen synthase kinase 3 phosphorylates hypoxia-inducible factor 1alpha and mediates its destabilization in a VHL-independent manner. Mol Cell Biol. 2007;27:3253–65. doi: 10.1128/MCB.00015-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Schnitzer SE, Schmid T, Zhou J, Eisenbrand G, Brune B. Inhibition of GSK3beta by indirubins restores HIF-1alpha accumulation under prolonged periods of hypoxia/anoxia. FEBS Lett. 2005;579:529–33. doi: 10.1016/j.febslet.2004.12.023. [DOI] [PubMed] [Google Scholar]
- 87.Chesire DR, Ewing CM, Sauvageot J, Bova GS, Isaacs WB. Detection and analysis of beta-catenin mutations in prostate cancer. Prostate. 2000;45:323–34. doi: 10.1002/1097-0045(20001201)45:4<323::aid-pros7>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 88.Mazumdar J, O’Brien WT, Johnson RS, LaManna JC, Chavez JC, Klein PS, Simon MC. O2 regulates stem cells through Wnt/beta-catenin signalling. Nat Cell Biol. 2010;12:1007–13. doi: 10.1038/ncb2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.To SK, Zeng WJ, Zeng JZ, Wong AS. Hypoxia triggers a Nur77-beta-catenin feed-forward loop to promote the invasive growth of colon cancer cells. Br J Cancer. 2014;110:935–45. doi: 10.1038/bjc.2013.816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhang Q, Bai X, Chen W, Ma T, Hu Q, Liang C, Xie S, Chen C, Hu L, Xu S, Liang T. Wnt/beta-catenin signaling enhances hypoxia-induced epithelial-mesenchymal transition in hepatocellular carcinoma via crosstalk with hif-1alpha signaling. Carcinogenesis. 2013;34:962–73. doi: 10.1093/carcin/bgt027. [DOI] [PubMed] [Google Scholar]
- 91.Liu HL, Liu D, Ding GR, Liao PF, Zhang JW. Hypoxia-inducible factor-1alpha and Wnt/beta-catenin signaling pathways promote the invasion of hypoxic gastric cancer cells. Mol Med Rep. 2015;12:3365–73. doi: 10.3892/mmr.2015.3812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lim JH, Chun YS, Park JW. Hypoxia-inducible factor-1alpha obstructs a Wnt signaling pathway by inhibiting the hARD1-mediated activation of beta-catenin. Cancer Res. 2008;68:5177–84. doi: 10.1158/0008-5472.CAN-07-6234. [DOI] [PubMed] [Google Scholar]
- 93.Verras M, Papandreou I, Lim AL, Denko NC. Tumor hypoxia blocks Wnt processing and secretion through the induction of endoplasmic reticulum stress. Mol Cell Biol. 2008;28:7212–24. doi: 10.1128/MCB.00947-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Karhadkar SS, Bova GS, Abdallah N, Dhara S, Gardner D, Maitra A, Isaacs JT, Berman DM, Beachy PA. Hedgehog signalling in prostate regeneration, neoplasia and metastasis. Nature. 2004;431:707–12. doi: 10.1038/nature02962. [DOI] [PubMed] [Google Scholar]
- 95.Sanchez P, Hernandez AM, Stecca B, Kahler AJ, DeGueme AM, Barrett A, Beyna M, Datta MW, Datta S, Ruiz i Altaba A. Inhibition of prostate cancer proliferation by interference with SONIC HEDGEHOG-GLI1 signaling. Proc Natl Acad Sci U S A. 2004;101:12561–6. doi: 10.1073/pnas.0404956101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gonnissen A, Isebaert S, Haustermans K. Hedgehog signaling in prostate cancer and its therapeutic implication. Int J Mol Sci. 2013;14:13979–4007. doi: 10.3390/ijms140713979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Onishi H, Kai M, Odate S, Iwasaki H, Morifuji Y, Ogino T, Morisaki T, Nakashima Y, Katano M. Hypoxia activates the hedgehog signaling pathway in a ligand-independent manner by upregulation of Smo transcription in pancreatic cancer. Cancer Sci. 2011;102:1144–50. doi: 10.1111/j.1349-7006.2011.01912.x. [DOI] [PubMed] [Google Scholar]
- 98.Lei J, Ma J, Ma Q, Li X, Liu H, Xu Q, Duan W, Sun Q, Xu J, Wu Z, Wu E. Hedgehog signaling regulates hypoxia induced epithelial to mesenchymal transition and invasion in pancreatic cancer cells via a ligand-independent manner. Mol Cancer. 2013;12:66. doi: 10.1186/1476-4598-12-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lei J, Fan L, Wei G, Chen X, Duan W, Xu Q, Sheng W, Wang K, Li X. Gli-1 is crucial for hypoxia-induced epithelial-mesenchymal transition and invasion of breast cancer. Tumour Biol. 2015;36:3119–26. doi: 10.1007/s13277-014-2948-z. [DOI] [PubMed] [Google Scholar]
- 100.Zhou J, Wu K, Gao D, Zhu G, Wu D, Wang X, Chen Y, Du Y, Song W, Ma Z, Authement C, Saha D, Hsieh JT, He D. Reciprocal regulation of hypoxia-inducible factor 2alpha and GLI1 expression associated with the radioresistance of renal cell carcinoma. Int J Radiat Oncol Biol Phys. 2014;90:942–51. doi: 10.1016/j.ijrobp.2014.06.065. [DOI] [PubMed] [Google Scholar]
- 101.Kucharzewska P, Christianson HC, Welch JE, Svensson KJ, Fredlund E, Ringner M, Morgelin M, Bourseau-Guilmain E, Bengzon J, Belting M. Exosomes reflect the hypoxic status of glioma cells and mediate hypoxia-dependent activation of vascular cells during tumor development. Proc Natl Acad Sci U S A. 2013;110:7312–7. doi: 10.1073/pnas.1220998110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Umezu T, Tadokoro H, Azuma K, Yoshizawa S, Ohyashiki K, Ohyashiki JH. Exosomal miR-135b shed from hypoxic multiple myeloma cells enhances angiogenesis by targeting factor-inhibiting HIF-1. Blood. 2014;124:3748–57. doi: 10.1182/blood-2014-05-576116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tadokoro H, Umezu T, Ohyashiki K, Hirano T, Ohyashiki JH. Exosomes derived from hypoxic leukemia cells enhance tube formation in endothelial cells. J Biol Chem. 2013;288:34343–51. doi: 10.1074/jbc.M113.480822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wang T, Gilkes DM, Takano N, Xiang L, Luo W, Bishop CJ, Chaturvedi P, Green JJ, Semenza GL. Hypoxia-inducible factors and RAB22A mediate formation of microvesicles that stimulate breast cancer invasion and metastasis. Proc Natl Acad Sci U S A. 2014;111:E3234–42. doi: 10.1073/pnas.1410041111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Azmi AS, Bao B, Sarkar FH. Exosomes in cancer development, metastasis, and drug resistance: a comprehensive review. Cancer Metast Rev. 2013;32:623–42. doi: 10.1007/s10555-013-9441-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Parolini I, Federici C, Raggi C, Lugini L, Palleschi S, De Milito A, Coscia C, Iessi E, Logozzi M, Molinari A, Colone M, Tatti M, Sargiacomo M, Fais S. Microenvironmental pH is a key factor for exosome traffic in tumor cells. J Biol Chem. 2009;284:34211–22. doi: 10.1074/jbc.M109.041152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Park JE, Tan HS, Datta A, Lai RC, Zhang H, Meng W, Lim SK, Sze SK. Hypoxic tumor cell modulates its microenvironment to enhance angiogenic and metastatic potential by secretion of proteins and exosomes. Mol Cell Proteomics. 2010;9:1085–99. doi: 10.1074/mcp.M900381-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Aga M, Bentz GL, Raffa S, Torrisi MR, Kondo S, Wakisaka N, Yoshizaki T, Pagano JS, Shackelford J. Exosomal HIF1alpha supports invasive potential of nasopharyngeal carcinoma-associated LMP1-positive exosomes. Oncogene. 2014;33:4613–22. doi: 10.1038/onc.2014.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sano S, Izumi Y, Yamaguchi T, Yamazaki T, Tanaka M, Shiota M, Osada-Oka M, Nakamura Y, Wei M, Wanibuchi H, Iwao H, Yoshiyama M. Lipid synthesis is promoted by hypoxic adipocyte-derived exosomes in 3T3-L1 cells. Biochem Biophys Res Commun. 2014;445:327–33. doi: 10.1016/j.bbrc.2014.01.183. [DOI] [PubMed] [Google Scholar]
- 110.Thomas SN, Liao Z, Clark D, Chen Y, Samadani R, Mao L, Ann DK, Baulch JE, Shapiro P, Yang AJ. Exosomal Proteome Profiling: A Potential Multi-Marker Cellular Phenotyping Tool to Characterize Hypoxia-Induced Radiation Resistance in Breast Cancer. Proteomes. 2013;1:87–108. doi: 10.3390/proteomes1020087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhong H, Semenza GL, Simons JW, De Marzo AM. Upregulation of hypoxia-inducible factor 1alpha is an early event in prostate carcinogenesis. Cancer Detect Prevent. 2004;28:88–93. doi: 10.1016/j.cdp.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 112.Yasuda M, Shimizu M, Fujita M, Miyazawa M, Tang X, Kajiwara H, Osamura RY, Shoji S, Tokunaga M, Terachi T. Usefulness of hypoxia inducible factor-1 alpha in evaluating the prostatic adenocarcinoma viability following neoadjuvant hormone therapy. Cancer Detect Prevent. 2007;31:396–401. doi: 10.1016/j.cdp.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 113.Zhong H, De Marzo AM, Laughner E, Lim M, Hilton DA, Zagzag D, Buechler P, Isaacs WB, Semenza GL, Simons JW. Overexpression of hypoxia-inducible factor 1alpha in common human cancers and their metastases. Cancer Res. 1999;59:5830–5. [PubMed] [Google Scholar]
- 114.Du Z, Fujiyama C, Chen Y, Masaki Z. Expression of hypoxia-inducible factor 1alpha in human normal, benign, and malignant prostate tissue. Chin Med J. 2003;116:1936–9. [PubMed] [Google Scholar]
- 115.Pipinikas CP, Carter ND, Corbishley CM, Fenske CD. HIF-1alpha mRNA gene expression levels in improved diagnosis of early stages of prostate cancer. Biomarkers. 2008;13:680–91. doi: 10.1080/13547500802591992. [DOI] [PubMed] [Google Scholar]
- 116.Stewart GD, Gray K, Pennington CJ, Edwards DR, Riddick AC, Ross JA, Habib FK. Analysis of hypoxia-associated gene expression in prostate cancer: lysyl oxidase and glucose transporter-1 expression correlate with Gleason score. Oncol Rep. 2008;20:1561–7. [PubMed] [Google Scholar]
- 117.Carnell DM, Smith RE, Daley FM, Saunders MI, Bentzen SM, Hoskin PJ. An immunohistochemical assessment of hypoxia in prostate carcinoma using pimonidazole: implications for radioresistance. Int J Radiat Oncol Biol Phys. 2006;65:91–9. doi: 10.1016/j.ijrobp.2005.11.044. [DOI] [PubMed] [Google Scholar]
- 118.Turaka A, Buyyounouski MK, Hanlon AL, Horwitz EM, Greenberg RE, Movsas B. Hypoxic prostate/muscle PO2 ratio predicts for outcome in patients with localized prostate cancer: long-term results. Int J Radiat Oncol Biol Phys. 2012;82:e433–9. doi: 10.1016/j.ijrobp.2011.05.037. [DOI] [PubMed] [Google Scholar]
- 119.Platz EA, Yegnasubramanian S, Liu JO, Chong CR, Shim JS, Kenfield SA, Stampfer MJ, Willett WC, Giovannucci E, Nelson WG. A novel two-stage, transdisciplinary study identifies digoxin as a possible drug for prostate cancer treatment. Cancer Discov. 2011;1:68–77. doi: 10.1158/2159-8274.CD-10-0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ranasinghe WK, Sengupta S, Williams S, Chang M, Shulkes A, Bolton DM, Baldwin G, Patel O. The effects of nonspecific HIF1alpha inhibitors on development of castrate resistance and metastases in prostate cancer. Cancer Med. 2014;3:245–51. doi: 10.1002/cam4.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Melillo G. Targeting hypoxia cell signaling for cancer therapy. Cancer Metast Rev. 2007;26:341–52. doi: 10.1007/s10555-007-9059-x. [DOI] [PubMed] [Google Scholar]