INTRODUCTION
Elevated blood pressure (BP) is a causal risk factor cardiovascular disease (CVD). Epidemiological analyses have established the graded and continuous association between higher BP and CVD. Moreover, randomized clinical trials among individuals with hypertension have demonstrated, in aggregate, a reduction in CVD events by 20%, coronary heart disease (CHD) by 17%, stroke by 27%, and heart failure by 28% for every 10-mmHg systolic BP-lowering with medical therapy.1 Therefore, prevention, detection, treatment, and control of elevated BP, and its clinical correlate “hypertension,” is an important public health priority and a primary target for CVD prevention.
Whereas national health surveys have demonstrated improvements in hypertension awareness and treatment, strategic opportunities to improve the efficiency and efficacy of applying BP-lowering therapy in patients most likely to benefit remain.2 Global CVD risk assessment, using multivariable prediction models that estimate absolute CVD risk from routinely-measured clinical variables, has been promulgated in several CVD prevention guidelines for cholesterol management to target the most intensive preventive therapies to those at highest absolute CVD risk.3–5 Hypertension guidelines, however, have instead relied solely on isolated BP thresholds and BP goals to guide treatment initiation and intensity. In this review, we provide a rationale for incorporating global CVD risk assessment in BP treatment decision-making and propose a framework to guide its implementation in future CVD prevention guidelines.
The role of global CVD risk assessment in CVD prevention guidelines
Epidemiologic analyses have firmly established that CVD is a multifactorial condition and that modest increases of several CVD risk factors can often lead to greater overall risk than a severe elevation of a single risk factor.6–9 Therefore, multivariable risk prediction tools have been developed to estimate CVD risk from multiple risk factors, and guideline developers have advocated for their incorporation into clinical decision making.10
The notion of matching the intensity of risk factor management to the absolute risk of CVD has been proposed for decades.11–13 In the United States, this framework was first widely disseminated during the 27th Bethesda Conference in 1996,12 and was operationalized in the 2001 Third Adult Treatment Panel cholesterol guidelines (ATP-III).14 Based in large part on the: 1) continuous association between cholesterol and vascular risk and 2) heterogeneity of this risk at any given cholesterol level based on the associated risk factor burden, ATP-III recommended the use of a multivariable risk prediction assessment tool to guide cholesterol-lowering treatment.14 This paradigm has also been embraced by guidelines worldwide for cholesterol management3–5 and has even been used to guide aspirin management.15
CVD risk-based cholesterol guidelines
In 2013, the American College of Cardiology (ACC) and American Heart Association (AHA) guidelines on cholesterol treatment further advanced this paradigm by eliminating the use of low-density lipoprotein (LDL) cholesterol thresholds to guide statin initiation. Instead, these guidelines identified 4 statin benefit groups where net benefit from statin therapy had been observed in clinical trials among individuals with: clinical atherosclerotic CVD or ASCVD; LDL-cholesterol ≥190 mg/dL suggestive of familiar hypercholesterolemia; diabetes mellitus; or a 10-year predicted ASCVD risk ≥7.5% based on a combination of CVD risk factors. The final group applied to the general adult population without prevalent disease and the 7.5% risk threshold identified a risk level where clinical trial evidence suggested net benefit from statin therapy.3 In addition to reaffirming the importance of risk stratification, these guidelines also emphasized the importance of shared decision-making between the clinician and patient to contextualize ASCVD risk assessment with the patient’s potential for benefit or harm from treatment, individual factors (like family history, subclinical disease markers, or long-term risk) that could up- or down-classify risk, and the patient’s values and preferences.3 This risk-based approach to CVD prevention has been shown to prevent more events while requiring treatment of fewer individuals than prior guidelines that focused on single risk factor thresholds and goals.16, 17 Moreover, large meta-analyses using individual participant data from randomized clinical trials have provided empiric support for risk-based approaches to cholesterol reduction by demonstrating that the relative risk reduction from statins is similar across different risk strata, and therefore, absolute risk reduction is greater in those with higher pretreatment CVD risk.18 The risk-based approach adopted by cholesterol treatment guidelines stands in stark contrast to BP treatment guidelines that instead prioritize single risk factor thresholds and goals.19
Epidemiologic data supportive of CVD risk-based BP management
Driven by the specific inclusion criteria used in landmark clinical trials of BP-lowering therapies, management of elevated BP has traditionally been anchored to discrete BP levels used to define hypertension (BP ≥140/≥90 mmHg). However, the notion that a single threshold might distinguish “hypertension” from “normotension” has been challenged from the beginning.20
The Multiple Risk Factor Intervention Trial (MRFIT), a primary prevention trial testing the effect of a multifactor intervention program on coronary heart disease mortality in men was one of the first studies with sufficient power to demonstrate the risk for heart disease mortality associated with modest systolic and diastolic BP elevations.6 Data on 316,099 white men aged 35–57 years who were screened and examined as part of the study were followed for 12 years for cause-specific mortality. Analyses demonstrated a graded positive association with coronary heart disease mortality across all levels of systolic BP ≥ 110 mmHg and diastolic blood pressure ≥ 70 mmHg.6 These data were later definitively confirmed by the Prospective Studies Collaboration, which included data from 61 observational studies with individual participant data from 1 million adults.21 These analyses also demonstrated the continuous, log-linear association of systolic and diastolic BP with vascular death. For every 20/10 mmHg higher BP, there was an associated doubling of the risk for death from heart disease, stroke, or death from other vascular causes, a relationship that continued down to a BP of 115/75 mmHg without evidence of a threshold.21
In addition to the continuous risk from elevated BP, observational studies have also demonstrated the marked variation in CVD risk at any given BP level based on the presence of associated risk factors. Among MRFIT screenees at the highest quintile of systolic BP (i.e. SBP ≥ 142 mmHg), age-adjusted heart disease death rates varied nearly 6-fold among men based on smoking status and cholesterol level (62.6 per 10,000 person-years among smokers with the highest quintile of cholesterol level compared with 13.7 per 10,000 person-years among nonsmokers with the lowest quintile of cholesterol level).6 Similarly, data from the Framingham Heart Study demonstrate how the relationship between BP category and 10-year CHD risk can vary not just by BP level but also by the presence of additional CVD risk factors such as elevated total cholesterol, low high-density lipoprotein cholesterol, presence of diabetes, and presence smoking.7 Thus, for a 60 year old man with a systolic BP between 130–139 mmHg or diastolic BP between 85–89 mmHg, 10-year CHD risk could vary between 46% and 12% based on the associated risk factor burden (Figure 1).
Figure 1.
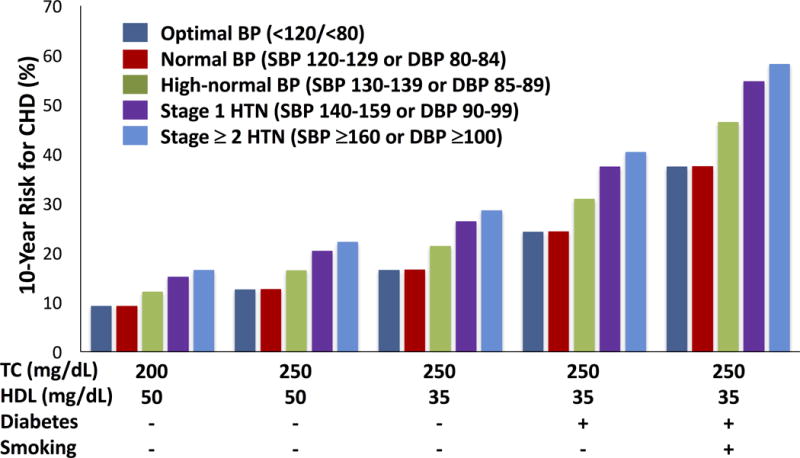
10-year coronary heart disease risk for a 60 year old man based on blood pressure level and risk factor burden. Risk factor burden increases left to right with the addition of high total cholesterol level (≥ 250 mg/dL), low HDL cholesterol (≤ 35 mg/dL), presence of diabetes, and presence of smoking. (Abbreviations: CHD = coronary heart disease, BP = blood pressure, HTN = hypertension, TC = total cholesterol, HDL = high-density lipoprotein cholesterol). Data adapted from Wilson et al.7
The importance of accounting for the presence of additional CVD risk factors has particular relevance for BP given the well-documented risk factor clustering that is seen in hypertensive individuals. In the Framingham Heart Study, for example, more than 80% of hypertensive individuals had 1 or more co-existing risk factor and 55% had 2 or more risk factors.22 Additional analyses from Framingham that cross-classified BP stages with risk categories used in the Sixth Report of the Joint National Committee on the Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC-VI) demonstrated that only 2.4% of individuals with high-normal or hypertensive BP’s were stratified to risk group A, the lowest risk group without any CVD risk factors or target organ damage (Table 1).23 Similarly, more recent analyses from the Kaiser Permanente managed care organization demonstrated that 56% of hypertensive patients had at least one additional risk factor such as diabetes, hyperlipidemia, or obesity.24 Further, cumulative medical care costs increased with each additional risk factor. Thus, these analyses highlight how frequently CVD risk factors travel with elevated BP and support the notion of adopting a multifactorial framework for guiding BP management.
Table 1.
Proportion of adults in the Framingham Heart Study classified into each JNC-VI risk group by blood pressure stage.
| Blood pressure stage | Risk group A n (%) |
Risk group B n (%) |
Risk group C n (%) |
Total n (%) |
|---|---|---|---|---|
| High-normal (130–139/85–89 mmHg) |
23 (3.4) | 441 (66.1) | 203 (30.4) | 667 (100) |
| Stage 1 (140–159/90–99 mmHg) |
24 (3.8) | 410 (64.1) | 206 (32.2) | 640 (100) |
| Stage 2 and 3 (≥ 160/≥100 mmHg or treated) |
21 (1.4) | 807 (54.3) | 659 (44.3) | 1,487 (100) |
| Total | 68 (2.4) | 1,658 (59.3) | 1,068 (38.2) | 2,794 (100) |
Risk groups as defined by JNC-VI: Risk group A = no cardiovascular disease (CVD) risk factors, clinical CVD, or target organ damage; Risk group B = at least 1 CVD risk factor (excluding diabetes mellitus); Risk group C = diabetes mellitus, CVD, or evidence of target organ damage. Data from Lloyd-Jones et al.23
Unaddressed CVD risk in the “hypertension” paradigm
Clinical practice guidelines have broadly adopted the “hypertension” paradigm to guide BP-related treatment decisions. In this paradigm, individuals who have hypertension (defined by BP ≥140 mmHg systolic or ≥90 mmHg diastolic) are eligible for risk-reducing therapies whereas those with lower levels are not. However, the categorization of elevated BP into “hypertension” and “normotension,” without incorporation of coexisting CVD risk factors can lead to substantial unaddressed CVD risk.
In 2008, Lawes et al. estimated that 54% of stroke and 47% of ischemic heart disease worldwide were attributable to high BP (defined as systolic BP ≥ 115 mmHg) but only half of this burden occurred in people meeting criteria for hypertension.25 Data from 6,859 participants in the Framingham Heart Study who were free of hypertension and CVD also demonstrated the CVD risk associated with high-normal BP’s (BP 130–139/80–85 mmHg).26 Compared to participants with optimal BP (BP <120/<80 mmHg), participants with high-normal BP had a risk factor-adjusted hazard ratio for CVD of 1.6 (95% CI 1.1–2.2) in men and 2.5 (95% CI 1.6–4.1) in women. Moreover, 80% of participants with high-normal BP had at least one additional CVD risk factor.27
More recent analyses among middle-aged participants from the Framingham Offspring and Atherosclerosis Risk in Communities studies demonstrate similar findings and suggest a potential role for multivariable risk assessment in identifying at-risk individuals.28 At 10 years of follow-up, approximately half of excess ASCVD events attributable to non-optimal systolic BP (defined as systolic BP ≥ 120 mmHg) occurred at levels not currently eligible for BP-lowering treatment initiation or treatment intensification (Table 2). However, multivariable risk estimation with the ACC/AHA Pooled Cohort equations provided a strategy for identifying persons likely to benefit from risk-reducing therapies across the full spectrum of systolic BP, particularly among those who were not already treated with BP-lowering medications (Figure 2).28 These findings have even greater relevance for future strategies to reduce BP-related disease with national trends from 2003 to 2012 demonstrating an increase in the prevalence of prehypertension from 27% to 33%.29
Table 2.
Excess atherosclerotic cardiovascular disease (ASCVD) events at 10 years by baseline systolic blood pressure category
|
Untreated (n=14,856) | ||||
|---|---|---|---|---|
| Baseline SBP (mmHg) | ASCVD events (n) | Expected ASCVD events (n) | Excess ASCVD events (n) | % of Excess ASCVD events |
|
| ||||
| <120 | 135 | – | – | – |
| 120–129 | 114 | 52 | 62 | 31.6 |
| 130–139 | 77 | 30 | 47 | 24.1 |
| 140–149 | 38 | 15 | 23 | 11.8 |
| 150–159 | 33 | 7 | 26 | 13.2 |
| ≥160 | 45 | 6 | 39 | 19.6 |
|
| ||||
| Total | 442 | 110 | 197 | 100.0 |
|
Treated (n=4,042) | ||||
| Baseline SBP (mmHg) | ASCVD events (n) | Expected ASCVD events (n) | Excess ASCVD events (n) | % of Excess ASCVD events |
|
| ||||
| <120 | 59 | 20 | 39 | 16.9 |
| 120–129 | 61 | 16 | 45 | 19.5 |
| 130–139 | 43 | 12 | 31 | 13.4 |
| 140–149 | 41 | 8 | 33 | 14.2 |
| 150–159 | 35 | 5 | 30 | 13.1 |
| ≥160 | 58 | 5 | 53 | 22.9 |
|
| ||||
| Total | 297 | 67 | 230 | 100.0 |
ASCVD event defined as nonfatal myocardial infarction, coronary heart disease death, nonfatal stroke, and fatal stroke. Excess ASCVD events are calculated as the difference between observed and expected ASCVD events, using the ASCVD event rate in the group with untreated SBP <120 mmHg as the reference (Expected in stratum = N in stratum × event rate in untreated SBP <120 mmHg. Excess = Observed in stratum – Expected in stratum). Data from Karmali et al.28
Figure 2.
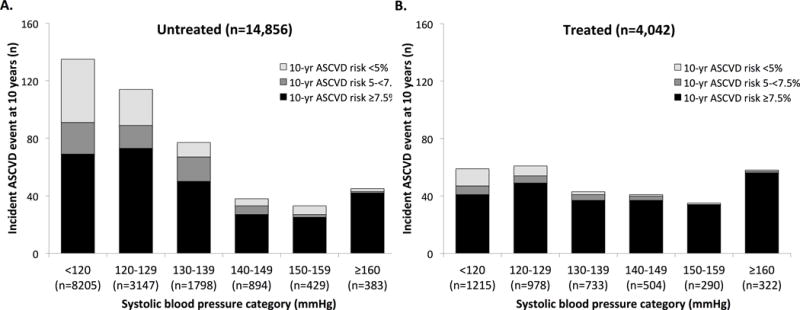
Incident atherosclerotic cardiovascular disease events at 10 years by baseline systolic blood pressure group for participants who are untreated (panel A) and treated (panel B) with antihypertensive therapy at baseline. Events at each systolic blood pressure group are categorized by 10-year predicted risk at baseline. (Abbreviations: ASCVD = atherosclerotic cardiovascular disease). Data from Karmali et al.28
Analyses from clinical trials supportive of risk-based blood pressure-lowering
While epidemiologic analyses have provided a rationale for adopting a risk-based framework to manage elevated BP, secondary analyses of BP-lowering clinical trials and prospective clinical trials have been instrumental in demonstrating the benefits of intensive BP-lowering therapy in high-risk groups.
The Systolic Hypertension in the Elderly Program (SHEP) was one of the first studies to suggest a role for pretreatment risk stratification to maximize treatment benefit. In a post-hoc analysis, Ferrucci et al. used the AHA Multiple Risk Factor Assessment equation to stratify 4,189 SHEP participants without cardiovascular disease into 4 risk quartiles.30 Relative risk reduction from stepped BP-lowering therapy with chlorthalidone was similar across the four risk quartiles; however, incidence rates for CVD events were greater in each successive risk quartile, and consequently, numbers needed to treat for 4.5 years to prevent 1 CVD event were progressively smaller, decreasing from 160, 100, 48, and 37.30 A principal limitation of this analysis, however, was that mean age among trial participants was 72 years and average BP was 172/77 mmHg, limiting the generalizability of these findings to the broader population.
To account for these limitations and extend the evidence base to lower-risk individuals with a broader range of BPs, the Blood Pressure Lowering Treatment Tralists’ Collaboration (BPLTTC) recently completed an individual participant data meta-analysis among 51,917 participants from 11 randomized clinical trials who received active/more-intensive BP-lowering treatment or placebo/less-intensive treatment.31 Trials included those that used standard BP thresholds to determine study eligibility as well as trials like the Heart Outcomes Prevention Evaluation (HOPE) and the Action in Diabetes and Vascular Disease (ADVANCE) studies that used high-risk status independent of specific BP entry criteria. This meta-analysis demonstrated that BP-lowering therapy provided similar relative risk reductions across patient groups with markedly different levels of baseline CVD risk, but progressively greater absolute risk reductions at higher levels of baseline risk (Table 3).31 This same pattern was seen in a range of secondary analyses for specific CVD outcome subtypes and after adjusting for differences in baseline and achieved BP levels. In other words, the BPLTTC meta-analysis provided empiric evidence that the absolute benefits achieved with BP-lowering therapy were driven by the combination of CVD risk factors determining risk of a CVD event rather than just the initial BP level in isolation. The clinical implications of these findings are demonstrated in Figure 3, and provide support for the notion that the most intensive BP-lowering therapies should be directed to those at highest CVD risk.
Table 3.
Relative and absolute effects of blood pressure reduction on cardiovascular disease (CVD) events by different levels of CVD risk.
| 5-year CVD risk | Risk ratio (95% CI) | Risk difference (95% CI) | CVD events prevented per 1000 treated (95% CI) |
|---|---|---|---|
| < 11% | 0.82 (0.73, 0.93) | −1.41 (−2.05, −0.77) | 14 (8, 21) |
| 11 to < 15% | 0.85 (0.75, 0.96) | −1.95 (−3.09, −0.82) | 20 (8, 31) |
| 15 to < 21% | 0.87 (0.78, 0.98) | −2.41 (−4.04, −0.77) | 24 (8, 40) |
| ≥ 21% | 0.85 (0.76, 0.95) | −3.84 (−6.06, −1.61) | 38 (16, 61) |
| p for trend = 0.30 | p for trend = 0.04 |
CVD events defined as myocardial infarction, stroke, heart failure, and CVD death. Data from Sundström et al.31
Figure 3.
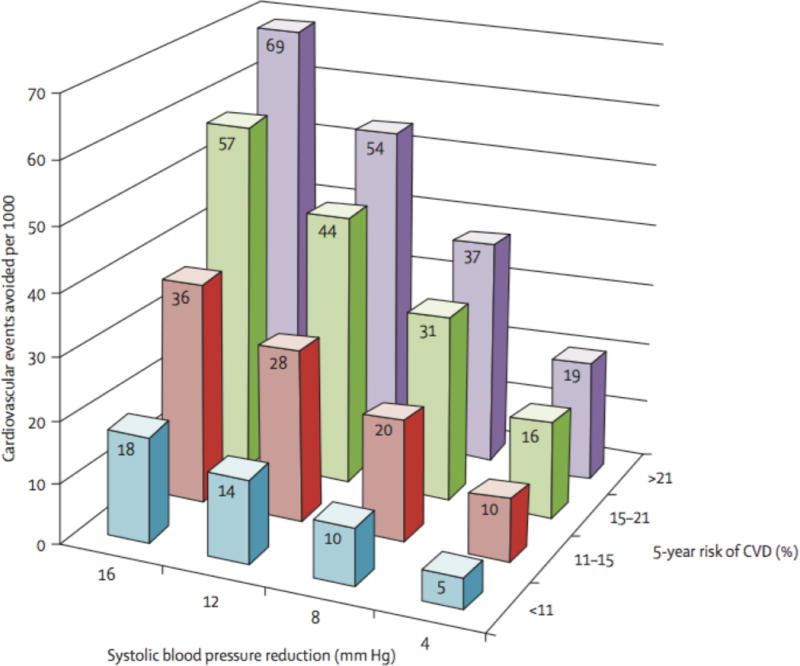
Cardiovascular disease (CVD) events avoided by baseline risk and magnitude of systolic blood pressure lowering. Data from Sundström et al.31
This principle was recently tested prospectively by the Systolic Blood Pressure Intervention Trial (SPRINT), which randomized 9,361 high-risk, non-diabetic participants to intensive systolic BP reduction below 120 mmHg compared with conventional systolic BP reduction below 140 mmHg.32 For participants who were at high-risk due to age ≥ 75 years, chronic kidney disease, clinical or subclinical CVD, or global 10-year CVD risk ≥ 15% by Framingham risk score, intensive systolic BP reduction of approximately 15 mmHg was associated with a 25% reduction in CVD events and a 27% reduction in all-cause mortality.32 This resulted in numbers needed to treat of 61 and 90 over 3.3 years to prevent 1 CVD event and 1 all-cause death, respectively. Importantly, benefits of BP reduction were not observed in Heart Outcomes Evaluation-3 (HOPE-3), a clinical trial testing a fixed-dose combination therapy with candesartan and hydrochlorothiazide in 12,705 intermediate risk participants (10-year CVD risk ~10%) who achieved only a 6 mmHg systolic BP reduction.33 Taken together with SPRINT, HOPE-3 highlights the need both for high CVD risk status and intensive systolic BP reduction to avoid CVD events.
Implications of a CVD risk reduction treatment paradigm
The potential merits of a risk-based paradigm for BP management have been demonstrated by several simulation studies and cost-effectiveness analyses.34–38 One such study proposed that use of risk prediction tools to guide risk factor management provided a mechanism for “individualized guidelines” that had the potential to improve outcomes while at the same time reducing healthcare costs.35
More recent modeling by Sussman et al. directly compared a conventional “treat-to-target” BP treatment approach with a multivariable risk-based strategy called a “benefit-based tailored” approach.37 In the treat-to-target approach, BP-lowering therapy was initiated and titrated toward a fixed BP target of 140/90 mmHg (or 130/85 mmHg for participants with diabetes mellitus). In contrast, for the benefit-based tailored approach, the Framingham risk score was used to estimate 10-year absolute risk of a CVD event and expected net benefit from treatment was calculated using expected relative risk reductions and treatment harms from BP-lowering therapy (both obtained from randomized trial evidence). In Markov modeling of 5 years of treatment, the benefit-based tailored approach prevented 900,000 more CVD events, saved 2.8 million more quality-adjusted life-years, and used 6% fewer medications compared with the treat-to-target approach in a nationally-representative sample of American adults without prevalent CVD.37 Furthermore, among those individuals treated differently by each strategy, those treated by the benefit-based tailored approach had twice the benefit compared with the treat-to-target approach (saving 159 quality-adjusted life-years per 1000 treated versus 74 quality-adjusted life years per 1000 treated).
Limitations of CVD risk in BP management
Any proposal for greater use of absolute CVD risk assessment in BP management must also account for potential limitations of this approach. First, unlike cholesterol levels where there are no clear health risks or symptoms from intensive LDL-cholesterol reduction in higher risk individuals, this is not the case with BP. Given the importance of BP for tissue perfusion and known risks of excessively low BPs like symptomatic hypotension, treatment-related falls, and renal dysfunction, there is, not surprisingly, a “floor” to BP reduction in high-risk individuals, which may also vary from individual to individual. Second, given the strong effect of age on short-term CVD risk estimation, CVD prediction algorithms that utilize 5- or 10-year time horizons often shift treatment eligibility to elderly individuals at the expense of younger ones. Thus, younger adults with markedly elevated BPs may be unlikely to reach risk-based treatment thresholds in spite of higher lifetime risk for CVD related to their elevated BP. Conversely, older adults with relatively normal BP levels may reach treatment thresholds due to their age in spite of little BP-related CVD risk.39 The risks of modestly elevated BP levels during young adulthood have been shown in longitudinal cohort studies like the Coronary Artery Risk Development in Young Adults. This study has shown greater amount of coronary artery calcification and worsened left ventricular mass and cardiac mechanical dysfunction in middle-aged adults who had higher cumulative exposure to non-optimal BP levels during young adulthood.40–42 Third, although multiple analyses have demonstrated that the absolute risk reductions from BP-lowering therapy may increase with increasing CVD risk, other meta-analyses have shown that residual risk (i.e. risk of a vascular event that occurs in spite of BP-lowering treatment) also increases with baseline CVD risk.43 These findings highlight the limitations of BP-lowering therapies to completely reverse CVD risk that has been accrued over time and has led some to propose earlier treatment in low-risk individuals with mild BP elevations to maximize longer term benefits from treatment.43, 44
A potential framework for future BP treatment guidelines
We propose that future clinical practice guidelines for BP management should move away from sole consideration of BP thresholds and goals to guide treatment decisions. Instead, future BP guidelines should adopt a more holistic view of elevated BP, one that accounts for the presence of concomitant CVD risk factors that are routinely measured in clinical practice. These multiple risk factors modify an individual’s absolute CVD risk and, therefore, his or her potential for treatment benefit. However, rather than adopting an entirely risk-based approach, we also believe there is a continued role for BP levels to guide treatment thresholds for individuals at low short-term risk and treatment floors for individuals at high short-term risk.
Long-term follow-up from epidemiologic studies have consistently shown that many young and middle-aged adults with systolic BP levels ≥ 160 mmHg may have low short-term CVD risk due to their younger age but high lifetime CVD risk during 20 or more years of follow-up.45, 46 Such an observation provides guidance for a potential systolic BP treatment threshold, above which young individuals might receive BP-lowering treatment due to high lifetime CVD risk regardless of their low short-term risk status. Among these younger individuals, there is also a need for additional research to identify interventions that may blunt the vascular and myocardial alterations that occur from cumulative exposure to elevated BP, even in the normotensive range. Conversely, the known health risks from excessive BP reduction and limited clinical trial experience with systolic BP’s < 120 mmHg provide guidance for a potential floor for systolic BP reduction.
Thus, for individuals with systolic BP levels in the broad middle-range (i.e. systolic BP 120–159 mmHg), we believe that future BP treatment guidelines might incorporate multivariable absolute CVD risk assessment and risk-based treatment thresholds that are defined by observed treatment benefits in clinical trials to guide BP-lowering treatment decisions. In a manner similar to the ACC/AHA cholesterol guidelines, these guidelines should also emphasize the importance of shared clinician-patient decision-making to contextualize a patient’s CVD risk information with expected benefit from BP-lowering therapies, potential for adverse effects, and individual preferences.3 In this paradigm, a clinician provides expert guidance and modifies the strength of recommendation based on a patient’s clinical profile, but treatment decisions for primary prevention are made jointly, respecting the autonomy of an informed patient to make a choice that aligns with his or her values and preferences.47
In an era of “precision medicine,” advances in functional genomics, transcriptomics, proteomics, and metabolomics provide novel opportunities to further characterize individuals beyond simple risk factor measurements.48 While, at present, such data has lacked the statistical power to be incorporated into CVD risk prediction equations, they may hold promise to not only refine risk assessment but also personalize BP-lowering treatment strategies to maximize therapeutic response and minimize the potential for adverse effects.49 Importantly, risk-based treatment strategies using traditional risk factors and novel biomarkers at the individual level must be complemented by broad-based, public-health strategies to promote primordial prevention of elevated BP to improve overall cardiovascular health in the population.50
Conclusions
In conclusion, the current “hypertension” paradigm does not account for the continuous risk associated with elevated BP or the multifactorial nature of CVD, the primary consequence of elevated BP. Adopting a risk-based framework to enrich BP-lowering treatment decisions would not only provide a strategy to account for these risk factors but also align BP treatment guidelines more closely with cholesterol treatment guidelines in primary prevention. Additionally, using CVD risk assessment to guide treatment thresholds and intensity would help move BP management away from arbitrary cut-points to a personalized treatment strategy geared toward the CVD risk and potential for benefit of the individual.
Supplementary Material
Acknowledgments
Sources of Funding
Dr. Karmali was supported by an NLHBI training grant in cardiovascular epidemiology and prevention during the preparation of this manuscript (T32 HL069771).
Footnotes
Disclosures
Dr. Karmali and Dr. Lloyd-Jones have no disclosures.
References
- 1.Ettehad D, Emdin CA, Kiran A, Anderson SG, Callender T, Emberson J, Chalmers J, Rodgers A, Rahimi K. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet. 2016;387:957–967. doi: 10.1016/S0140-6736(15)01225-8. [DOI] [PubMed] [Google Scholar]
- 2.Guo F, He D, Zhang W, Walton RG. Trends in prevalence, awareness, management, and control of hypertension among United States adults, 1999 to 2010. J Am Coll Cardiol. 2012;60:599–606. doi: 10.1016/j.jacc.2012.04.026. [DOI] [PubMed] [Google Scholar]
- 3.Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S1–45. doi: 10.1161/01.cir.0000437738.63853.7a. [DOI] [PubMed] [Google Scholar]
- 4.National Institute for Health and Care Excellence (NICE) clinical guideline CG181: Cardiovascular disease: risk assessment and reduction, including lipid modification. National Clinical Guideline Centre; Jul, 2014. https://nice.org.uk/guidance/cg181 (accessed 15 September 2016) [Google Scholar]
- 5.Piepoli MF, Hoes AW, Agewall S, et al. 2016 European guidelines on cardiovascular disease prevention in clinical practice: the Sixth Joint Task Force of the European Society of Cardiology and other societies on cardiovascular disease prevention in clinical practice (constituted by representatives of 10 societies and by invited experts) developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR) Eur Heart J. 2016;37:2315–2381. doi: 10.1093/eurheartj/ehw106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Neaton JD, Wentworth D. Serum cholesterol, blood pressure, cigarette smoking, and death from coronary heart disease. Overall findings and differences by age for 316,099 white men. Multiple Risk Factor Intervention Trial research group. Arch Intern Med. 1992;152:56–64. [PubMed] [Google Scholar]
- 7.Wilson PW, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–1847. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 8.Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A, Lanas F, McQueen M, Budaj A, Pais P, Varigos J, Lisheng L, INTERHEART Study Investigators Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364:937–952. doi: 10.1016/S0140-6736(04)17018-9. [DOI] [PubMed] [Google Scholar]
- 9.O’Donnell MJ, Xavier D, Liu L, et al. INTERSTROKE Investigators Risk factors for ischaemic and intracerebral haemorrhagic stroke in 22 countries (the INTERSTROKE study): a case-control study. Lancet. 2010;376:112–123. doi: 10.1016/S0140-6736(10)60834-3. [DOI] [PubMed] [Google Scholar]
- 10.Smith SC, Jr, Jackson R, Pearson TA, Fuster V, Yusuf S, Faergeman O, Wood DA, Alderman M, Horgan J, Home P, Hunn M, Grundy SM. Principles for national and regional guidelines on cardiovascular disease prevention: a scientific statement from the World Heart and Stroke Forum. Circulation. 2004;109:3112–3121. doi: 10.1161/01.CIR.0000133427.35111.67. [DOI] [PubMed] [Google Scholar]
- 11.Jackson R, Barham P, Bills J, Birch T, McLennan L, MacMahon S, Maling T. Management of raised blood pressure in New Zealand: a discussion document. BMJ. 1993;307:107–110. doi: 10.1136/bmj.307.6896.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.27th Bethesda conference. Matching the intensity of risk factor management with the hazard for coronary disease events. J Am Coll Cardiol. 1996;27:957–1047. [PubMed] [Google Scholar]
- 13.Jackson R. Updated New Zealand cardiovascular disease risk-benefit prediction guide. BMJ. 2000;320:709–710. doi: 10.1136/bmj.320.7236.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 15.Bibbins-Domingo K. Aspirin use for the primary prevention of cardiovascular disease and colorectal cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2016;164:836–845. doi: 10.7326/M16-0577. [DOI] [PubMed] [Google Scholar]
- 16.Hayward RA, Krumholz HM, Zulman DM, Timbie JW, Vijan S. Optimizing statin treatment for primary prevention of coronary artery disease. Ann Intern Med. 2010;152:69–77. doi: 10.7326/0003-4819-152-2-201001190-00004. [DOI] [PubMed] [Google Scholar]
- 17.Pandya A, Sy S, Cho S, Weinstein MC, Gaziano TA. Cost-effectiveness of 10-year risk thresholds for initiation of statin therapy for primary prevention of cardiovascular disease. JAMA. 2015;314:142–150. doi: 10.1001/jama.2015.6822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mihaylova B, Emberson J, Blackwell L, Keech A, Simes J, Barnes EH, Voysey M, Gray A, Collins R, Baigent C. The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: meta-analysis of individual data from 27 randomised trials. Lancet. 2012;380:581–590. doi: 10.1016/S0140-6736(12)60367-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8) JAMA. 2014;311:507–520. doi: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- 20.Pickering G. Normotension and hypertension: The mysterious viability of the false. Am J Med. 1978;65:561–563. doi: 10.1016/0002-9343(78)90839-2. [DOI] [PubMed] [Google Scholar]
- 21.Lewington S, Clarke R, Qizilbash N, Peto R, Collins R. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–1913. doi: 10.1016/s0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- 22.Kannel WB. Risk stratification in hypertension: new insights from the Framingham study. Am J Hypertens. 2000;13:3s–10s. doi: 10.1016/s0895-7061(99)00252-6. [DOI] [PubMed] [Google Scholar]
- 23.Lloyd-Jones DM, Evans JC, Larson MG, O’Donnell CJ, Wilson PW, Levy D. Cross-classification of JNC VI blood pressure stages and risk groups in the Framingham Heart study. Arch Intern Med. 1999;159:2206–2212. doi: 10.1001/archinte.159.18.2206. [DOI] [PubMed] [Google Scholar]
- 24.Weycker D, Nichols GA, O’Keeffe-Rosetti M, Edelsberg J, Khan ZM, Kaura S, Oster G. Risk-factor clustering and cardiovascular disease risk in hypertensive patients. Am J Hypertens. 2007;20:599–607. doi: 10.1016/j.amjhyper.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 25.Lawes CM, Vander Hoorn S, Rodgers A. Global burden of blood-pressure-related disease, 2001. Lancet. 2008;371:1513–1518. doi: 10.1016/S0140-6736(08)60655-8. [DOI] [PubMed] [Google Scholar]
- 26.Vasan RS, Larson MG, Leip EP, Evans JC, O’Donnell CJ, Kannel WB, Levy D. Impact of high-normal blood pressure on the risk of cardiovascular disease. N Engl J Med. 2001;345:1291–1297. doi: 10.1056/NEJMoa003417. [DOI] [PubMed] [Google Scholar]
- 27.Vasan RS, Larson MG, Leip EP, Kannel WB, Levy D. Assessment of frequency of progression to hypertension in non-hypertensive participants in the Framingham Heart study: a cohort study. Lancet. 2001;358:1682–1686. doi: 10.1016/S0140-6736(01)06710-1. [DOI] [PubMed] [Google Scholar]
- 28.Karmali KN, Ning H, Goff DC, Lloyd-Jones DM. Identifying individuals at risk for cardiovascular events across the spectrum of blood pressure levels. J Am Heart Assoc. 2015;4:e002126. doi: 10.1161/JAHA.115.002126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yoon SS, Gu Q, Nwankwo T, Wright JD, Hong Y, Burt V. Trends in blood pressure among adults with hypertension: United States, 2003 to 2012. Hypertension. 2015;65:54–61. doi: 10.1161/HYPERTENSIONAHA.114.04012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ferrucci L, Furberg CD, Penninx BW, DiBari M, Williamson JD, Guralnik JM, Chen JG, Applegate WB, Pahor M. Treatment of isolated systolic hypertension is most effective in older patients with high-risk profile. Circulation. 2001;104:1923–1926. doi: 10.1161/hc4101.097520. [DOI] [PubMed] [Google Scholar]
- 31.Sundström J, Arima H, Woodward M, Jackson R, Karmali K, Lloyd-Jones D, Baigent C, Emberson J, Rahimi K, MacMahon S, Patel A, Perkovic V, Turnbull F, Neal B. Blood pressure-lowering treatment based on cardiovascular risk: a meta-analysis of individual patient data. Lancet. 2014;384:591–598. doi: 10.1016/S0140-6736(14)61212-5. [DOI] [PubMed] [Google Scholar]
- 32.Wright JT, Jr, Williamson JD, Whelton PK, et al. SPRINT Research Group A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373:2103–2116. doi: 10.1056/NEJMoa1511939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lonn EM, Bosch J, Lopez-Jaramillo P, et al. HOPE-3 Investigators Blood-pressure lowering in intermediate-risk persons without cardiovascular disease. N Engl J Med. 2016;374:2009–2020. doi: 10.1056/NEJMoa1600175. [DOI] [PubMed] [Google Scholar]
- 34.Gaziano TA, Steyn K, Cohen DJ, Weinstein MC, Opie LH. Cost-effectiveness analysis of hypertension guidelines in South Africa: absolute risk versus blood pressure level. Circulation. 2005;112:3569–3576. doi: 10.1161/CIRCULATIONAHA.105.535922. [DOI] [PubMed] [Google Scholar]
- 35.Eddy DM, Adler J, Patterson B, Lucas D, Smith KA, Morris M. Individualized guidelines: the potential for increasing quality and reducing costs. Ann Intern Med. 2011;154:627–634. doi: 10.7326/0003-4819-154-9-201105030-00008. [DOI] [PubMed] [Google Scholar]
- 36.Cobiac LJ, Magnus A, Barendregt JJ, Carter R, Vos T. Improving the cost-effectiveness of cardiovascular disease prevention in Australia: a modelling study. BMC Public Health. 2012;12:398. doi: 10.1186/1471-2458-12-398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sussman J, Vijan S, Hayward R. Using benefit-based tailored treatment to improve the use of antihypertensive medications. Circulation. 2013;128:2309–2317. doi: 10.1161/CIRCULATIONAHA.113.002290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Basu S, Yudkin JS, Sussman JB, Millett C, Hayward RA. Alternative strategies to achieve cardiovascular mortality goals in China and India: a microsimulation of target- versus risk-based blood pressure treatment. Circulation. 2016;133:840–848. doi: 10.1161/CIRCULATIONAHA.115.019985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jackson R, Lawes CM, Bennett DA, Milne RJ, Rodgers A. Treatment with drugs to lower blood pressure and blood cholesterol based on an individual’s absolute cardiovascular risk. Lancet. 2005;365:434–441. doi: 10.1016/S0140-6736(05)17833-7. [DOI] [PubMed] [Google Scholar]
- 40.Kishi S, Teixido-Tura G, Ning H, Venkatesh BA, Wu C, Almeida A, Choi EY, Gjesdal O, Jacobs DR, Jr, Schreiner PJ, Gidding SS, Liu K, Lima JA. Cumulative blood pressure in early adulthood and cardiac dysfunction in middle age: the CARDIA study. J Am Coll Cardiol. 2015;65:2679–2687. doi: 10.1016/j.jacc.2015.04.042. [DOI] [PubMed] [Google Scholar]
- 41.Liu K, Colangelo LA, Daviglus ML, Goff DC, Pletcher M, Schreiner PJ, Sibley CT, Burke GL, Post WS, Michos ED, Lloyd-Jones DM. Can antihypertensive treatment restore the risk of cardiovascular disease to ideal levels?: the coronary artery risk development in young adults (CARDIA) study and the multi-ethnic study of atherosclerosis (MESA) J Am Heart Assoc. 2015;4:e002275. doi: 10.1161/JAHA.115.002275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pletcher MJ, Bibbins-Domingo K, Lewis CE, Wei GS, Sidney S, Carr JJ, Vittinghoff E, McCulloch CE, Hulley SB. Prehypertension during young adulthood and coronary calcium later in life. Ann Intern Med. 2008;149:91–99. doi: 10.7326/0003-4819-149-2-200807150-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thomopoulos C, Parati G, Zanchetti A. Effects of blood pressure lowering on outcome incidence in hypertension: 3. Effects in patients at different levels of cardiovascular risk–overview and meta-analyses of randomized trials. J Hypertens. 2014;32:2305–2314. doi: 10.1097/HJH.0000000000000380. [DOI] [PubMed] [Google Scholar]
- 44.Zanchetti A. Bottom blood pressure or bottom cardiovascular risk? How far can cardiovascular risk be reduced? J Hypertens. 2009;27:1509–1520. doi: 10.1097/HJH.0b013e32832e9500. [DOI] [PubMed] [Google Scholar]
- 45.Allen N, Berry JD, Ning H, Van Horn L, Dyer A, Lloyd-Jones DM. Impact of blood pressure and blood pressure change during middle age on the remaining lifetime risk for cardiovascular disease: the Cardiovascular Lifetime Risk Pooling Project. Circulation. 2012;125:37–44. doi: 10.1161/CIRCULATIONAHA.110.002774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lloyd-Jones DM, Leip EP, Larson MG, D’Agostino RB, Beiser A, Wilson PW, Wolf PA, Levy D. Prediction of lifetime risk for cardiovascular disease by risk factor burden at 50 years of age. Circulation. 2006;113:791–798. doi: 10.1161/CIRCULATIONAHA.105.548206. [DOI] [PubMed] [Google Scholar]
- 47.Martin SS, Sperling LS, Blaha MJ, Wilson PW, Gluckman TJ, Blumenthal RS, Stone NJ. Clinician-patient risk discussion for atherosclerotic cardiovascular disease prevention: Importance to implementation of the 2013 ACC/AHA guidelines. J Am Coll Cardiol. 2015;65:1361–1368. doi: 10.1016/j.jacc.2015.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Collins FS, Varmus H. A new initiative on precision medicine. N Engl J Med. 2015;372:793–795. doi: 10.1056/NEJMp1500523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vasan RS. Biomarkers of cardiovascular disease: Molecular basis and practical considerations. Circulation. 2006;113:2335–2362. doi: 10.1161/CIRCULATIONAHA.104.482570. [DOI] [PubMed] [Google Scholar]
- 50.Lloyd-Jones DM, Hong Y, Labarthe D, et al. American Heart Association Strategic Planning Task Force and Statistics Committee Defining and setting national goals for cardiovascular health promotion and disease reduction: The american heart association’s strategic impact goal through 2020 and beyond. Circulation. 2010;121:586–613. doi: 10.1161/CIRCULATIONAHA.109.192703. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


