Abstract
Social attachments are ubiquitous among humans and integral to human health. Although great efforts have been made to elucidate the neural underpinnings regulating social attachments, we still know relatively little about the neuronal and neurochemical regulation of social attachments. As a laboratory animal research model, the socially monogamous prairie vole (Microtus ochrogaster) displays behaviors paralleling human social attachments and thus has provided unique insights into the neural regulation of social behaviors. Research in prairie voles has particularly highlighted the significance of neuropeptidergic regulation of social behaviors, especially of the roles of oxytocin (OT) and vasopressin (AVP). This article aims to review these findings. We begin by discussing the role of the OT and AVP systems in regulating social behaviors relevant to social attachments, and thereafter restrict our discussion to studies in prairie voles. Specifically, we discuss the role of OT and AVP in adult mate attachments, biparental care, social isolation, and social buffering as informed by studies utilizing the prairie vole model. Not only do these studies offer insight into social attachments in humans, but they also point to dysregulated mechanisms in several mental disorders. We conclude by discussing these implications for human health.
Introduction
Social behaviors are evident in a large number of animal species and range from simple to complex. Enduring selective social bonds are particularly interesting due to their complexity, rarity in the animal kingdom, prevalence in human societies, and profound effects on human health. A social bond is a hypothetical construct of adaptive, relatively enduring processes characterized by proximity seeking between partners, a preference for the partner, stress upon separation from the partner, and the cessation of the stress response at reunion (46, 176). The terms “bond” and “attachment” may have different meanings across fields and therefore it is important to clarify the definition in the context of this review. In attachment theory in psychology, “attachment” is primarily used to describe the bond between a mother and child, while other fields use the terms attachment and bonding interchangeably, as in this text, to describe enduring relationships more generally (i.e., partner or parent) (4,37,46). The most common types of social bonds in human societies include selective attachments between adult mates (pair bonds) and attachments between parents and their offspring (parent-offspring attachments). Although pair bonds are most evident in industrialized societies that primarily adopt monogamous life strategies, they occur across all human societies, as do parent-offspring attachments. Data from both human and animal studies indicate that the social attachments associated with monogamous life strategies are beneficial for all members involved. For example, paired individuals in stable marital relationships live longer than unpaired individuals (133). Paired individuals also benefit from decreased stress as well as better immune and cardiovascular health (15, 150, 266). Additionally, children benefit from biparental care which co-occurs with pair bonding (213,234). Studies in humans have provided some insights into brain regions that are potentially involved in regulating social attachments, both between members of the opposite sex and between parents and offspring. Notably, studies using functional magnetic resonance imaging (fMRI) have implicated a largely common set of brain regions involved in both types of attachments (151, 185, 192, 256). However, despite the importance of pair bonds in human welfare, we still know surprisingly little about the neurobiological and neurochemical mechanisms underlying social attachment.
While the depiction of social attachments may differ across species, most mammals display certain, similar forms of social relationships. As a result, non-human animal models have provided opportunities for scientists to systematically investigate the neurobiological underpinning of social attachment. In particular, several non-human primate species, such as marmosets (Callithrix jacchus) (83) and tamarins (Callicebus) (299), form family groups consisting of paired adults and their offspring and display social behaviors associated with a monogamous life strategy, including pair bonding and biparental care. These animals can serve as excellent models to research the neurobiology of social attachment, although such an approach is not practical for many labs. On the other hand, rodents display attachment behaviors, primarily mother-offspring attachments, and thus have been utilized extensively in the study of the neuronal and neurochemical regulation of maternal behavior (160). Although research in rodent models has greatly increased our understanding of the neurobiology underlying maternal behavior, most rodents do not display pair bonds nor father-offspring attachments and, therefore, cannot serve as appropriate animal models for the study of bonding behavior observed in humans.
Recently, the prairie vole (Microtus ochrogaster)—a rodent species that displays strong attachments between adult mates and biparental care toward offspring—has emerged as an excellent rodent model for studying social attachments and the underlying neurochemical mechanisms (231, 296). In prairie voles, pair bonding between mates, parental (both maternal and paternal) care toward offspring, and the influences of the social environment on those behaviors have been well described (47, 100, 288). In addition, the neurochemical regulation of pair bonding, particularly the roles of the neuropeptides oxytocin (OT) and vasopressin (AVP), has been extensively examined in prairie voles (39, 288). These data have greatly enhanced our understanding of the neurobiology of the formation and maintenance of adult mate attachments. In this review, we primarily focus on the neuropeptides OT and AVP. First, we provide some background information of these neuropeptides and their involvement in the brain in regulating specific social behaviors including social recognition as well as sexual and maternal behavior. Thereafter, we focus on research from the prairie vole model regarding OT and AVP regulation of social attachments and responses to stress and social buffering.
Neuropeptides Oxytocin and Vasopressin
OT and AVP systems in the brain
OT and AVP are two nonapeptides that have received considerable attention due to their critical roles in a variety of physiological and behavioral functions. These two peptides are evolutionarily conserved and differ from each other by only two amino acids (1,184). OT and AVP producing neurons are found in the densest clusters in the paraventricular nucleus (PVN) and the supraoptic nucleus (SON) of the hypothalamus, and consist of large magnocellular neurons and relatively smaller parvocellular neurons (98, 161). Magnocellular neurons project primarily onto the posterior pituitary gland by which OT and AVP are released into the blood stream where they regulate bodily functions such as osmoregulation in the kidney by AVP and uterine contractions during childbirth as well as milk letdown from mammary tissue during lactation by OT. Magnocellular neurons also innervate the spinal cord, midbrain, and multiple forebrain regions, such as the nucleus accumbens (NAcc) and central amygdala (156,231,257,262). Magnocellular neurons can release neuropeptides via axonal, somatic, and dendritic release, resulting in passive diffusion and potentially into the third ventricle (107, 217, 262, 300). Parvocellular OT and AVP neurons project onto magnocellular neurons within the PVN as well as to the spinal cord, brain stem, forebrain, and median eminence, where release influences anterior pituitary function (13,82,161,262). In addition to the PVN and SON, OT and AVP synthesizing neurons are found in smaller densities in the amygdala (273), bed nucleus of the stria terminalis (BNST) (88), anterior hypothalamus (AH) and medial preoptic area (MPOA) (189,278), and project to each other as well as to the NAcc, lateral septum (LS), and ventral tegmental area (VTA) (161), among other brain regions (Fig. 1).
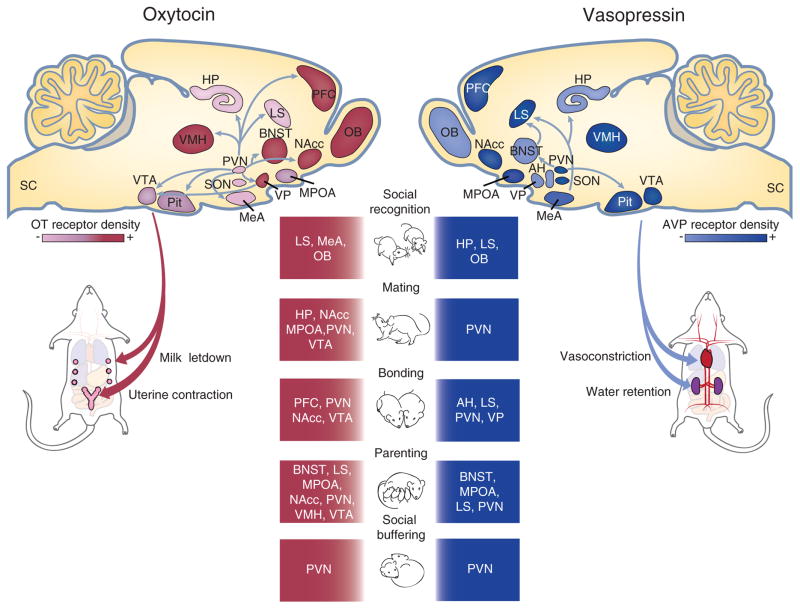
Figure 1.
Schematic drawings of sagittal brain sections illustrating OT (right) and AVP (left) neurons and their projections to selected brain regions important in social behaviors. Colored brain regions also indicate the distribution and regional density of OT receptors (red) and AVP receptors (blue) in the brain. OT and AVP are released from the pituitary gland into the blood circulation to regulate peripheral functions such as the milk letdown reflex and uterine contractions in females as well as vasoconstriction and water retention in both males and females. In addition, OT and AVP are released throughout the brain to regulate a variety of complex social behaviors including social recognition, mating, bonding, parenting, and social buffering. AH, anterior hypothalamus; BNST, bed nucleus of the stria terminalis; HP, hippocampus; LS, lateral septum; MeA medial amygdala. MPOA, medial preoptic area of the hypothalamus; NAcc, nucleus accumbens; OB, olfactory bulb; PFC, prefrontal cortex; Pit, pituitary gland; SON, supraoptic nucleus; VMH, ventromedial hypothalamus; VP, ventral pallidum; VTA, ventral tegmental area.
The receptors for OT and AVP are G protein-coupled receptors. OT acts on the OT receptors (OTR), while there are three types of AVP receptors, two of which (V1aR and V1bR) are primarily located in the central nervous system (CNS) and another (V2R) in the peripheral nervous system (252). While OT and AVP cells and their innervations are highly conserved across mammalian species, receptor distribution patterns and regional densities in the brain can vary greatly between species (6,139). A notable example is the difference in OTR and V1aR density between brain regions involved in visual processing in primates and brain regions involved in olfactory processing in rodents (91). These receptor patterns are thought to reflect the differences in primary sensory modalities between species. There is also substantial overlap in OTR and V1aR distribution in other forebrain and limbic regions that regulate conserved behaviors between primates and rodents, such as in the NAcc, BNST, and ventromedial nucleus of the hypothalamus (VMH) (89,90). The differences in OT and AVP systems across and even within species can be mediated by variation in gene sequences and differences in the regulation of gene expression (132). In contrast to classical neurotransmitters, OT and AVP as well as their receptors are directly encoded in the genome (161) and their expression can therefore be modulated through hormones, experience, and environment (80). Additionally, recent reports indicate that OT and AVP can act on each other’s receptors to regulate behaviors due to the similarity of their molecular structure, and the high degree of conservation between the OT and AVP peptides, although the molecular signaling pathway activated by their receptors differs (249). Therefore, it cannot be excluded that the effects of OT/AVP on behavior and physiology, as informed through studies utilizing pharmacological injections, may be due to cross talk between receptors, and future studies can aim to clarify this discrepancy by identifying the activated signaling pathways since these differ between receptor types (6). Finally, it is relevant to mention that sex differences exist between OT and AVP producing neurons, fibers, and receptors in mammals, which will be discussed in more detail in later sections in the context of the prairie vole literature (79).
The roles of OT and AVP in cognitive and behavioral functions
There is a substantial amount of evidence from animal models indicating that OT and AVP act across an interconnected neural network to regulate social behaviors including social recognition, maternal care, reproduction, and aggression. For rodents, social communication begins with the olfactory system, as odorants are the primary form by which conspecifics distinguish each other. Odors containing an individual signature are initially detected by the olfactory epithelium and vomeronasal organ which project onto the olfactory bulbs (211), where OT and AVP neurons as well as their receptors reside and are involved in social recognition (75, 260). Both the main and accessory olfactory bulbs project to the medial amygdala (MeA) where OTR is necessary for social recognition in mice (85). AVP/OT projections between the MeA, LS, and hippocampus additionally regulate social recognition and behaviors where the ability to recognize conspecifics is important (28, 63, 158, 263, 264).
In addition to social recognition, OT and AVP have been shown to regulate sexual and maternal behavior. During mating, OT neurons in the PVN are activated and OT is released locally in both male and female rats (87, 193, 265). In males, PVN OT regulates penile erections via projections to the spinal cord, hippocampus, VTA, and cortical amygdala and through interactions with dopamine, glutamate, and nitric oxide (12, 181, 254). PVN OT release during mating has also been implicated in mediating the anxiolytic effects of sex in both male and female rats (193,265). On the other hand, AVP acts in an opposing manner, decreasing sexual behavior, and facilitating aggression. ICV injections of AVP in female rats decreases lordosis behavior, the reflexive mating posture in female rodents, as well as proceptive hop and darting behaviors and increases aggression toward males (207). In the context of maternal behavior, OT plays a critical role in regulating maternal behavior particularly during the onset, when OT is released centrally before, during, and directly after parturition (162,186,191,206). OTR has also been implicated in high maternal responsiveness to pups (49,50), with female rats that display high levels of maternal responsiveness demonstrating higher OTR binding in brain regions such as the MPOA, VTA, and PVN compared to females that display low levels of maternal care (49). The AVP system has also been implicated in maternal behavior particularly by its actions within the MPOA and BNST (33–36). Notably, virally upregulating V1aRs in the MPOA increases maternal behavior (33). These data highlight the role of OT and AVP in modulating multiple types of social behaviors in a common set of brain regions. Figure 1 shows schematic drawings of rodent sagittal brain sections illustrating OT and AVP projections and their receptors in the brain, as well as their involvements in a variety of social behaviors.
While studies in traditional laboratory rodent species have provided insights into the neural regulation of some aspects of social behaviors, they are limited by species-specific behaviors and context. Unfortunately, most rodents do not display features of monogamy characteristic of human behaviors, such as adult mate attachments and biparental care, thereby restricting the research into these behaviors important for human health. In recent years, emergence of the prairie vole model has provided an excellent opportunity to study the neurobiology of social bonds. Great efforts have been made to examine the roles of neurochemicals, especially the neuropeptides OT and AVP, and their interactions in regulating social behaviors and processes associated with the monogamous life strategy in prairie voles. These data have significantly enhanced our understanding of the neurochemical mechanisms regulating the full repertoire of social attachment behaviors in humans.
The Prairie Vole Model
Monogamous life strategy and social behaviors
Voles are small rodent species belonging to the genus Microtus that exhibit remarkable variations in life strategies and social behaviors (48,271,293,296,297). Of particular interest is the prairie vole (Microtus ochrogaster), a species that exhibits features of social monogamy including long term, selective pair bonds between adult males and females and biparental care toward offspring (Fig. 2). The prairie vole has provided a useful model to investigate the neurobiology of social attachments especially for their pair bonding behavior that is easily inducible in a laboratory setting and is paralleled to the male-female bonding observed in human societies. Moreover, other species of voles, such as the meadow vole (Microtus pennsylvanicus), display a promiscuous life strategy, tolerating conspecifics seasonally, with pair bonds and paternal investment depending on fertility status and winter-like day-lengths (27, 117, 202, 203). Comparative studies between monogamous and promiscuous vole species have provided some of the first insights into the neural mechanisms regulating pair bonds, to be reviewed further later (293, 296).
Figure 2.
Socially monogamous prairie voles display several types of social behaviors that have been studied in laboratory conditions. (A) Photograph of a pair of male and female prairie voles with their pups in the nest. (B) Pair bonding behavior is measured using a 3-h partner preference test. The testing apparatus consists of three chambers connected by hollow tubes. At the beginning of the test, the subject is placed in the center cage and allowed to freely explore the other two cages containing either the partner or a conspecific stranger. (C) In both male and female prairie voles, 24-h cohabitation with mating reliably induces an increase in side-by-side contact with the partner versus a stranger, and this partner preference is not observed following 6-h cohabitation. (D) Selective aggression is another indicator of pair bonding. While sexually naïve males are not aggressive, pair-bonded males display aggression selectively toward stranger males and females, but not toward their partners. (E) Upon litter birth, both male and female prairie voles share the natal nest and engage in parental care. Data are shown as mean ± SEM. *P < 0.05. Alphabetic letters indicate the results from a post-hoc test following an ANOVA. Bars labeled with different letters differ significantly from each other. Data adapted, with permission, from (11, 102, 274, 283).
Early field studies utilized live-traps and tracking technology to characterize the natural social structure of the prairie vole. Live-trapping data revealed that pairs of males and females were repeatedly caught together during breeding and nonbreeding seasons (96). Data from subsequent studies using radiotelemetry demonstrated that male-female pairs exhibited overlapping home ranges, inhabited the same nest, and often remained together in the nest especially during the breeding seasons (130). These discoveries were followed by laboratory experiments which confirmed male-female pair bonding in prairie voles and provided further details regarding the behaviors associated with a monogamous life strategy (93). Virgin male and female prairie voles are socially affiliative and nonaggressive toward each other. However, mating induces drastic changes in their behaviors—paired males and females remain affiliative toward each other, but no longer display affiliative behavior toward other conspecifics. Instead, the pair bonded prairie voles avoid and even attack conspecific strangers if they come too close (97). Moreover, the pair bonded male and female share a nest and display biparental behaviors toward their offspring (117, 178, 199, 259).
In prairie voles, pair bonding behavior has been examined in the laboratory primarily by using two behavioral tests. The partner preference test was initially developed by Dr. Sue Carter’s lab and is conducted using an apparatus consisting of three chambers connected by tubes (281, 283) (Fig. 2B). During the test, the subject is free to roam the apparatus while the familiar (partner) and unfamiliar (stranger) conspecifics are confined in their own cages. During the 3-h test, the duration and frequency that the subject spends in each cage and interacts with the partner or stranger are recorded. Partner preference formation is defined if the subject spends significantly more time in side-by-side contact with the partner versus a stranger. It has been shown that sexually naïve male and female prairie voles usually spend approximately equal times with the partner and stranger, and 18 to 24 h of ad lib mating reliably induces partner preferences in prairie voles (105, 281) (Fig. 2C).
Another behavioral index of pair bonding is selective aggression. As aforementioned, sexually naïve prairie voles are highly affiliative toward conspecifics. However, after mating, both the male and female become aggressive toward conspecific strangers but not the partner, and this “selective” aggression can be assessed by a resident intruder test (RIT) (278,283). In this paradigm, the intruder (partner or stranger) is introduced into the home cage of the subject for 5-min behavioral interactions. The duration and frequency of offensive (e.g., biting and chasing) and defensive (e.g., defensive upright postures) aggression as well as nonsocial behaviors (e.g., self-grooming and locomotion) displayed by the subject are recorded. Data have shown that sexually naïve prairie voles are not aggressive (241). However, mating induces aggression selectively toward conspecific strangers but not the partner (102, 136, 283) (Fig. 2D). This selective aggression is displayed by both male and female prairie voles, but more robust in the former than the latter (97, 281).
Prairie voles also display biparental care, another unique behavioral characteristic associated with their monogamous life strategy. Male prairie voles stay in the natal nest after birth of their offspring (Fig. 2E) and display paternal behavior, similar to maternal behavior except for nursing, toward their offspring (199). Male prairie voles contribute to nest building and display direct parental behaviors including licking, grooming, huddling, and retrieving the pups (95). Interestingly, sexually naïve male prairie voles can display this spontaneous paternal behavior when they are exposed to unrelated pups, and this behavior can be enhanced by mating and pair bonding experience with females and by the birth of males’ own offspring (22). Prairie voles therefore provide a unique opportunity for the study of the neurobiology of paternal behavior, paralleling and in comparison to the neurobiology of maternal behavior.
It is worth mentioning that the life strategy and associated behaviors of other vole species have also been studied. For example, the meadow vole is a promiscuous species. Sexually naïve meadow voles are largely solitary, depending on season, and aggressive toward conspecifics (94,117). Mating does not induce partner preference nor selective aggression in meadow voles (117, 199). As Microtine rodent species are phylogenetically similar but show remarkable differences in their life strategy and social behaviors, they have provided a unique comparative model for studying social behaviors and their underlying neurochemical mechanisms.
OT and AVP systems in the vole brains
Early comparative studies took advantage of the species differences in life strategies and social behaviors and compared the OT and AVP systems in the brains between monogamous and promiscuous vole species. These studies uncovered striking species differences in the OTR and V1aR distribution patterns (137, 138) (Fig. 3). For example, monogamous vole species, such as the prairie voles and pine voles (Microtus pinetorum), have higher densities of OTR binding in the prefrontal cortex (PFC) and NAcc as well as the BNST and lateral amygdala, compared to the promiscuous meadow and montane (Microtus montanus) voles (137). Conversely, lower densities of OTR binding are found in the LS, ventromedial nucleus of the hypothalamus, and cortical amygdala in the former than the latter vole species. Similarly, differences are also found for V1aR binding in the brain between vole species with different life strategies and social behaviors. Specifically, monogamous vole species have higher densities of V1aR binding in the ventral pallidum (VP), BNST, and central and lateral amygdala, among other regions, as well as lower densities of V1aR binding in the LS and PFC compared to promiscuous vole species (138,244,278,294). These species differences in protein receptors were confirmed by subsequent studies on OTR and V1aR mRNA labeling using in situ hybridization (292,298). It is important to note that such differences in the distribution patterns and regional quantities of the OTR and V1aR are not just species specific, but closely related to the different life strategy and social behaviors of the vole species, implicating their potential roles in different social behaviors (123, 169, 290).
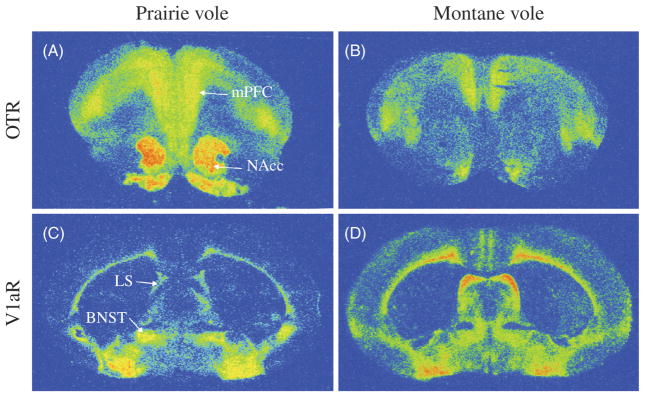
Figure 3.
Autoradiograms showing the distribution of the OTR and vasopressin 1a receptor (V1aR) in the brain of the monogamous prairie voles and nonmonogamous montane voles. The densities of OTR binding in the nucleus accumbens (NAcc) and medial prefrontal cortex (mPFC) are higher in the prairie vole (A) than in the montane vole (B). Additionally, the density of V1aR binding is higher in the BNST and lower in the lateral septum (LS) in the prairie vole (C) compared to the montane vole (D). Data adapted, with permission, from (137,138,279).
OT and AVP producing cells and fibers have also been examined in vole brains. Cells that contain and are immunoreactive (-ir) for OT are found in the PVN, SON, MPOA, amygdala, and BNST while AVP-ir cells are also found in the PVN, SON, and MPOA as well as the suprachiasmatic nucleus of the hypothalamus (SCN), BNST, MeA, and AH (21,270,279). OT-ir fibers are found in the NAcc, while dense clusters of AVP-ir fibers are found in the PVN, SON, LS, lateral habenula, and MPOA (229). Some scattered AVP-ir fibers are aslo found in the BNST and MeA (21, 279). Similar distribution patterns of OT-ir and AVP-ir cells and fibers are found in the brains of monogamous and promiscuous voles with some subtle species differences (270, 279). In general, the patterns of OT and AVP cells and fibers in the vole brains are similar to that found in other species of rodents including rats and mice (229), indicating that such systems are evolutionarily conserved. Most interestingly, the different patterns of OTR and V1aR in the vole brains indicate their roles in regulating social behaviors associated with species-specific life strategies (123, 138).
The role of OT and AVP in pair bonding
The functional role of OT in partner preference formation was first identified in a pharmacological study in female prairie voles (282). In this study, ICV injections of OT potentiated partner preference formation in sexually naive female prairie voles briefly exposed to a male (for 6 h) compared to females injected with a vehicle, and this effect was blocked by an OTR antagonist (282). Furthermore, ICV injections of an OT antagonist disrupted partner preference in female prairie voles (135). These effects were further replicated in female prairie voles and expanded into male prairie voles (52, 144). Subsequent studies have been focused on identifying the specific brain areas in which OT facilitates partner preferences. As OTR densities in the NAcc and PFC are significantly higher in prairie voles than in meadow and montane voles (137), these two areas have been the focus. Indeed, infusions of an OTR antagonist directly into either the NAcc or PFC disrupts partner preference formation in female prairie voles, indicating the necessity of the OTR in these brain regions for pair bonding (290, 293). OT is also released in the NAcc of female prairie voles during interactions with a male (229), and OT injections directly into the NAcc facilitate partner preference formation (172). Furthermore, upregulation of OTRs in the NAcc facilitates partner preference formation in female prairie voles, and this effect is blocked by injections of an OTR antagonist (147,230,269). Moreover, drug induced decreases in the OTR in the PFC disrupts partner preference formation in female prairie voles, but this deficit is rescued via OT administration directly into the PFC, which also results in altered NAcc activity (288).
As OTR signaling plays a critical role in mediating OT effects on partner preferences, efforts have also been made to alter OTR densities in selected brain areas and then examine the effects on behavior. Increasing OTR expression in the NAcc via viral vector-mediated gene transfer accelerates partner preference formation in adult female prairie voles (230). In addition, virally upregulating OTR in the NAcc of juvenile female prairie voles also results in accelerated partner preference formation as adults (147). Conversely, decreasing OTR density in the NAcc disrupts partner preference formation. Injections of a viral vector into the NAcc containing a short hairpin RNA (shRNA) designed to interfere with OTR mRNA results in OTR knockdown by approximately 45% in female prairie voles (146). This selective and incomplete knockdown is sufficient to disrupt partner preference formation (146). Prairie voles naturally display remarkable variation in the density of OTRs in brain regions, such as the NAcc, and this variation has been implicated in mediating individual differences in affiliative behaviors in this species (197, 230). Furthermore, drugs of abuse alter central OTR densities and impair pair bonding in prairie voles (289). For example, amphetamine treatment decreases OTR density in the PFC and disrupts partner preference formation in female prairie voles (289). Injections of OT into the PFC of amphetamine treated subjects rescues partner preference formation and alters NAcc activity via OTR activation (289). Interestingly, data from a recent study show that epigenetic events associated with mating and cohabitation with a male partner can enhance OTR expression in the NAcc of female prairie voles to facilitate partner preferences and suggests a mechanism by which experience with a mate can facilitate pair bond formation (269). Pharmacologically blocking histone deacetylation in female prairie voles during cohabitation with a male increases OTR gene expression in the NAcc as well as histone acetylation levels at the OTR gene promotor region. These changes are associated with a facilitation of partner preference, which can be blocked by subsequent injections of an OTR antagonist into the NAcc. Overall, data indicate that OT in the brain, particularly in the NAcc and PFC, plays an important role in partner preference formation (Fig. 4A and B). These data also implicate the rewarding aspects of mating and affiliation with a partner and possible interactions between OT and other “reward” neurochemicals, such as dopamine, in regulating partner preferences in prairie voles (289, 293).
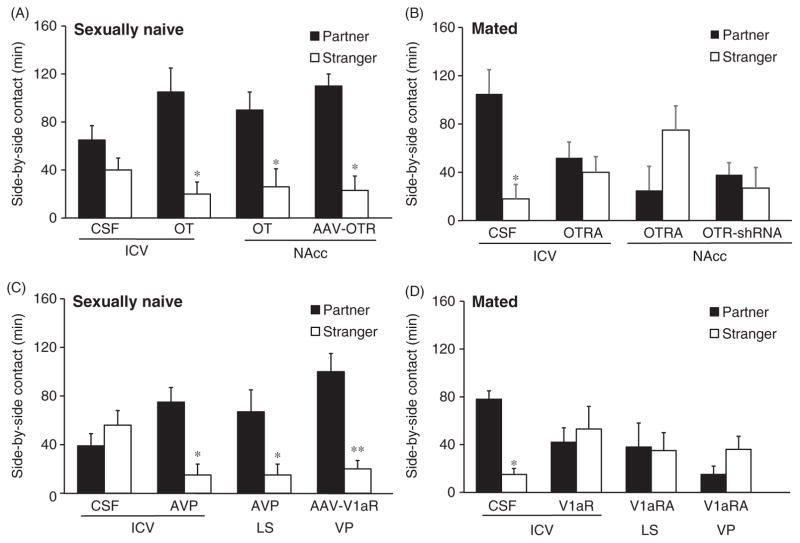
Figure 4.
The effects of OT and AVP on partner preference behavior in prairie voles. (A) Control females that receive ICV injections of CSF do not display partner preferences after 6 h of cohabitation with a male, whereas females receiving OT injections into the ventricle (OT ICV), nucleus accumbens (OT NAcc), or viral vector injections (AAV-OTR) for OTR over expression in the NAcc, do display partner preferences. (B) 24 h of mating and cohabitation with a male reliably induces partner preferences in control females (CSF), but this behavior is prevented by ICV or intra-NAcc injections of an OTR antagonist (OTRA) or downregulation of OXTR by injections of interfering short hairpin RNA (OTR-shRNA). (C) In male prairie voles, brief cohabitation does not induce partner preferences. However, AVP injections into the ventricle (ICV) and lateral septum (LS) as well as upregulation of the V1aR in the ventral pallidum (VP) via viral vector mediated gene transfer (AAV-V1aR) facilitate partner preference formation. (D) 24-h mating and cohabitation with a female induces partner preference in male prairie voles but this behavior is blocked by injections of the V1aR antagonist (V1aRA) into the ventricle (ICV), LS, or VP. Although OT and AVP effects are illustrated here by data from females and males, respectively, both neuropeptides have been shown to affect partner preference behavior in male and female prairie voles. Data are shown as mean ± SEM. *P < 0.05, **P < 0.01. Data adapted, with permission, from (135,171,172,215,231,282,283).
AVP is also involved in pair bonding behaviors and the V1aR is of particular importance (Fig. 4C and D). ICV injections of AVP facilitate, while V1aR antagonists inhibit partner preference formation in both male and female prairie voles (52,283,295). Specifically, V1aR activation is necessary for partner preference formation and expression (78). ICV V1aR antagonism prior to mating blocks partner preference formation while central V1aR antagonism after mating but before a partner preference test blocks the expression of partner preference (78). In the nonmonogamous montane vole, AVP administration does not alter affiliative behaviors (295). Subsequent studies aimed to identify specific brain regions where V1aRs act to regulate partner preference formation in prairie voles. As AVP in the LS was recognized for its role in social recognition (29, 62) and an increase in AVP release in the LS had been reported in male prairie voles that have mated and cohabited with a female (22), V1aR in the LS was tested for a role in partner preference formation (171). Injections of AVP directly into the LS induced partner preference formation in the absence of mating while a V1aR antagonist blocked mating-induced partner preference formation in male prairie voles (171). Therefore, AVP in the LS plays a critical role in the neurobiology of pair bond formation in prairie voles.
In addition to the LS, V1aR activation in the VP regulates partner preference formation in prairie voles. V1aR in the VP was initially implicated in pair bonding due to comparative studies demonstrating the higher densities of V1aR in the monogamous voles compared to promiscuous voles and due to the neuroanatomical location of the VP in the brain’s reward circuitry; the VP is the main output center of the NAcc and has been implicated in drug reward and reinforcement (177). Mating has additionally been shown to increase neural activity in the VP of male prairie voles, and this is dependent on pallidal V1aRs (170). Injections of a V1aR antagonist into the VP blocks partner preference formation in male prairie voles (170), while overexpressing the V1aR in the VP induces partner preference formation in the absence of mating (215). Conversely, decreasing V1aR in the VP disrupts partner preference formation in mated male prairie voles (23). Considering these results and the fact that V1aR densities vary considerably within prairie voles, natural variation in V1aR distribution in the VP may account for intraspecies variation in pair bonding behaviors (23, 214). In support of this interpretation, artificially increasing V1aR density in the VP of promiscuous meadow voles by using viral vector V1aR gene transfer can induce a partner preference in this species (169).
Comparison of the V1aR gene between monogamous and nonmonogamous vole species has revealed differences in gene structure (295). Specifically, there is an expanded repetitive microsatellite in the 5′ regulatory region of the V1aR gene in monogamous vole species compared to nonmonogamous vole species which may account for differences in receptor distribution (124). To determine if these differences in gene structure translated to differences in social behaviors, Young and colleagues created transgenic mice containing the prairie vole V1aR gene (295). Transgenic mice carrying the prairie vole V1aR genetic sequence demonstrated a V1aR binding pattern in the brain that was more comparable to a prairie vole than a wild-type mouse (295). Furthermore, these transgenic mice showed increased affiliative behaviors after central injections of AVP (295). The significance of differences in v1ar gene structure and transcription were later tested in cell cultures and shown to be cell-type specific (124). Altogether, data indicate that species differences in gene structure may give rise to species-specific differences in central V1aR densities and distribution patterns (120, 124).
In addition to an involvement in regulating partner preference formation, AVP is also both necessary and sufficient for selective aggression in prairie voles. ICV injections of AVP facilitate whereas a V1aR antagonist blocks selective aggression in male prairie voles, respectively (283). In particular, AVP actions in the anterior hypothalamus (AH) have been shown to regulate selective aggression in prairie voles (102, 103). Selective aggression in pair bonded male prairie voles is associated with an increase in neuronal activation of AVP cells in the AH as well as increased V1aR binding in the same brain region (102). Furthermore, this is associated with an increase in AVP release in the AH as measured via brain microdialysis during a RIT test in pair-bonded males (103). In sexually naïve male prairie voles, selective aggression toward a conspecific can be induced via pharmacological activation of V1aRs in the AH (101,103). Viral vector mediated V1aR over-expression in the AH also facilitates aggression toward novel females in sexually naive males (103). Interestingly, treatment with amphetamine also results in increased V1aRs in the AH and induces aggression toward conspecifics, although the selectivity is lost as these males also attack their partners (103). This amphetamine induced aggression is blocked via administration of a V1aR antagonist in the AH (103).
Although the current review is focused on the neuropeptides AVP and OT, it is worth mentioning that several other neurochemicals and hormones have also been implicated in pair bonding behavior in prairie voles. For example, the neurotransmitter dopamine (DA) has been implicated in regulating the salience of social cues via interactions with OT, and is necessary for the formation and maintenance of pair bonds (10, 172, 239). Activating DA receptors in the NAcc results in a receptor-specific effect—D2R activation facilitates partner preference formation whereas D1R activation enhances selective aggression associated with pair bonding in prairie voles (10). Concurrent activation of both OTR and D2R in the NAcc is essential for partner preference formation, implicating DA and OT interactions in social bonding (172). The D1R and D2R are located on cell specific populations containing opioids and regulate the motivational aspects of pair bond formation and maintenance. D2Rs in the NAcc shell are located on enkephalin containing neurons which bind to the μ-opioid receptor to regulate the hedonic properties of mating and is necessary for partner preference formation in prairie voles (220–222). In contrast, D1Rs in the NAcc shell are located on dynoprhin containing neurons which regulate aversive motivation and are necessary for the expression of selective aggression in prairie voles (220, 222).
Hormones, such as estrogen and corticosterone (CORT), also play roles in regulating affiliative behavior in prairie voles. In female rodents, the effects of OT on adult behaviors, including sexual (12) and maternal (26, 49, 204, 208) behaviors, are largely dependent on estrogen. Interestingly, OT also has organizational effects on ERα expression in prairie voles, and ERα can modulate changes in social behavior (58,60,159,286). For example, in male prairie voles, ERα expression in the MeA and BNST is inversely correlated with prosocial behavior (60, 163). It has also been demonstrated that pair bond formation in prairie voles are partly mediated by the HPA axis and CORT responses (68). For example, exposure to an opposite sex, but not same sex, conspecific reduces circulating CORT levels in prairie voles and CORT, in turn, influences the formation of pair bonds (69,72). Interestingly, increases in circulating CORT show sexually dimorphic effects on pair bonding—it facilitates partner preference in male, but inhibits the same behavior in female prairie voles (68, 69, 72). Both AVP and OT can influence CORT release via interactions with the HPA axis. For example, ICV injections of OT in male and female prairie voles decrease CORT (72). OT largely inhibits HPA activity while AVP activates it. Therefore, the HPA axis is sensitive to social cues and can influence the regulation of social behavior via interactions with OT and AVP (68).
The role of OT and AVP in parental behavior
Prairie voles belong to 3% to 4% of mammalian species that display male-female bonding and biparental care toward offspring—behaviors associated (155). After mating and pair bond formation, both male and female prairie voles stay in the same nest where females become pregnant and gestation occurs in approximately 21 days following mating. Upon litter birth, mother voles display the full range of maternal behaviors, including pup nursing, huddling, licking/grooming, and retrieving, as observed in other rodent species (117). Prairie vole fathers also display high levels of parental behaviors like their female partners except for pup nursing (117). Prairie vole pups raised with both parents receive significantly higher levels of licking/grooming and have a faster rate of physical development (e.g., eat solid food and move out of the nest) compared to pups raised in the absence of their fathers, indicating the importance of paternal behavior on pup development in this species (2, 3, 275). Parental behavior, such as pup licking and grooming, appears to be coordinated between mother and father prairie voles (2). Mothers lick and groom pups more than the father when both parents are present. However, when the mother leaves the nest, the father increases pup licking and grooming (2). It also appears that prairie vole mothers and fathers can coordinate their nesting time, so that one parent is almost always in the nest and pups are left alone very rarely (2). Interestingly, prairie voles are among few rodent species where sexually naïve individuals can display spontaneous parental behaviors toward conspecific pups (199). In prairie voles, the instance and levels of spontaneous parental behaviors varies among individuals and between sexes as more males display spontaneous parental behavior than females (173). In addition, the levels of spontaneous parental behavior displayed by sexually naïve prairie voles can be enhanced by the animal’s sexual and social experience with opposite sex conspecifics (22, 142, 227). Finally, juvenile voles that stay in the parent’s nest also display parental behavior toward their younger offspring (alloparental behavior), except for nursing (117, 199, 276).
One interesting aspect from the study of parental behavior in prairie voles is their individual differences in parental and other social behaviors as well as the heritability of their behavioral traits over generations. Prairie voles display remarkable individual variation in the levels of parental care toward their offspring and these levels are consistent among individuals (209). Differences in parental behavior received by pups (i.e., high contact vs. low contact parenting styles) are associated with altered social and alloparental behaviors displayed by those pups during their adolescence (209). Furthermore, offspring raised by single mothers demonstrate lower levels of spontaneous parental behavior, delayed partner preference formation, and altered anxiety-like behaviors in adulthood, compared to those reared by both parents, further demonstrating the importance of biparental environment for the normal development of the offspring (2, 3).
In an early effort in studying neural mechanisms of parental behavior in voles, c-Fos, a protein product of the immediate early gene c-fos, was used as a neural activation marker to map brain areas activated by pup exposure. Increased neuronal activation was found by pup exposure in several limbic brain areas including the MeA, LS, AOB, MPOA, and medial BNST (153, 279). The involvement of these brain areas in parental behavior has been further supported by data from subsequent studies. For example, lesions of the MeA have been found to affect parental behavior in male and female prairie voles, albeit differently. In virgin females, lesions of the MeA facilitate maternal behavior and this effect is dependent on gonadal steroid hormones as ovariectomy eliminates this effect (190). Conversely, MeA lesions in adult, pair bonded male prairie voles decrease paternal behavior toward a pup (152). The MeA receives direct projections from the main and accessory olfactory bulbs (167, 183), and these upstream structures have also been implicated in parental behavior in prairie voles. For example, a significantly higher number of male prairie voles receiving bilateral lesions of the olfactory bulbs attacked pups compared to control males (154). These data suggest that the olfactory system and subsequent downstream brain regions (i.e., MeA) are necessary for normal paternal responding in males and females but in a sexually dimorphic manner.
Several pieces of evidence have laid out a foundation for studying neuropeptide, especially OT and AVP, regulation of parental behavior in prairie voles. First, neuropeptides, such as OT and AVP, have been well documented in regulating maternal behavior in other mammalian species (30, 35, 192). Second, many of the brain areas that are activated by pup exposure/interaction in prairie voles contain OT and/or AVP producing neurons, projections, or receptors (153, 192, 195). Third, monogamous and promiscuous vole species show remarkable differences in the OTR and V1aR expression in the brain, indicating the potential role of OT and AVP systems in regulating species-specific social behaviors including parental behavior (137, 138). Fourth, OT and AVP have been shown to play important roles in regulating pair bonding behavior in prairie voles (99, 192). It is hypothesized that the same neurochemical systems and circuitry are involved in the regulation of a suit of social behaviors, such as pair bonding and biparental care, associated with a monogamous life strategy (104,144). It should be noted that although the neurochemical regulation of maternal behavior has been extensively studied using other rodent models such as rats, mice, and even primates (196, 226, 251), we know virtually nothing about the neurochemical regulation of paternal behavior. Therefore, the prairie vole model provides an excellent opportunity to examine endogenous (e.g., neurochemicals and hormones) and exogenous (e.g., mating and/or social experience with the partner) factors that regulate/influence male parental care (192,226). Indeed, the majority of the efforts in studying the neurobiology of parental behavior in voles has been focused on paternal behavior, although maternal behavior has also been examined.
In an early study comparing monogamous prairie voles with promiscuous montane voles (M. montanus), males and females at 1 or 6 days following the birth of their offspring were compared with their conspecific, sexually naïve counterparts for OT and AVP gene expression and receptor binding in the hypothalamus (280). OT mRNA expression in the PVN and SON as well as OTR binding in the VMH were significantly increased in females of both species following parturition and maternal experience, but these changes were not found in males of either species. These data are consistent with the demonstrated role of hypothalamic OT in maternal behavior found in other rodent species (35). Most interestingly, AVP gene expression in the PVN and SON were significantly increased in mother and father prairie voles, but not in promiscuous montane voles, compared to their sexually naïve counterparts, indicating a species-specific role of brain AVP in regulating monogamous social behaviors (280). In a separate study, it was found that compared to virgin males, father prairie voles displayed higher levels of OT labeled neurons in the PVN (149). Additionally, pup exposure increases the percentage of c-Fos labeled neurons that coexpress OT or AVP staining in the PVN and increases peripheral OT in virgin males (148). These data further implicate a role for OT and AVP in the transition and regulation of parental behaviors in male prairie voles.
Additional data further indicate the role of OT and AVP in mediating parental behaviors in both male and female prairie voles. In male prairie voles, ICV injections of an OTR antagonist or a V1aR antagonist have no effects on paternal behaviors in virgin males. However, combined ICV injections of the OTR and V1aR antagonist at a high dose decreases paternal behavior, indicating that OT and AVP may coordinate with each other in regulating paternal behavior in prairie voles (17). In female prairie voles, alloparental responsiveness (specifically huddling) is correlated with OTR binding in the shell subdivision of the NAcc (197). Adult virgin females that display maternal behavior also have higher OTR binding in the NAcc compared to females that have either attacked pups or showed no maternal behavior (198) (Fig. 5A). These data indicate that OTR in the NAcc may be important for maternal behavior in prairie voles. Indeed, injections of an OTR antagonist directly into the NAcc of adult virgin female prairie voles impairs maternal behavior (198) (Fig. 5B). In a recent study, RNAi knockdown of OTR in the NAcc blocked alloparental behavior in female prairie voles (146) (Fig. 5C and D). Additionally, virally upregulating OTRs in the NAcc of juvenile female prairie voles increases adult alloparental responding (147). As of yet, no investigations have looked at the functional role of OTR in the NAcc in paternal behavior in prairie voles. However, a recent study has found that in the monogamous mandarin vole (Microtus mandarinus), fathers have higher levels of OTR mRNA in the NAcc compared to both virgin and pair bonded males (268). Additionally, pup exposed prairie vole fathers have higher levels of OT-ir staining in the PVN, compared to virgin males, and OT neurons in the PVN project to the NAcc, among other brain regions (149). Further support for OT action within the NAcc in facilitating male parental behavior comes from manipulations of OT release in mice. Although mice display low levels of OTRs in the NAcc and male mice are generally not paternal, male mice still displayed more paternal behaviors toward pups after OT activity was facilitated in the NAcc (5). These data implicate OT regulation of parental behavior in the NAcc although future studies need to address this specificity in male prairie voles.
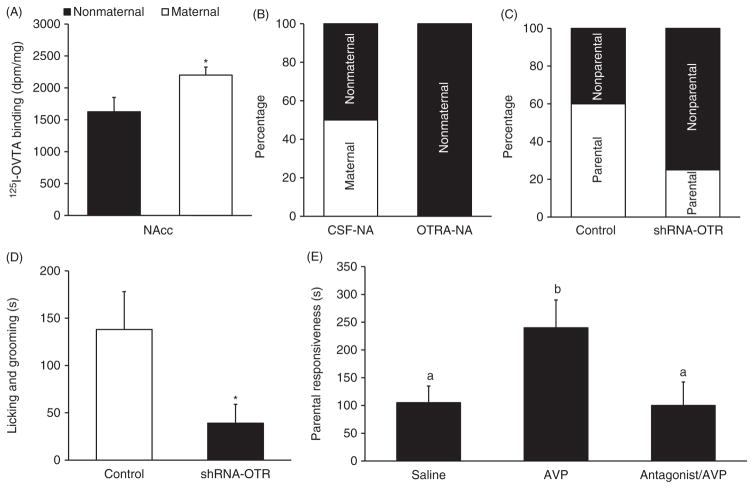
Figure 5.
The role of brain OT and AVP in the regulation of parental behaviors in prairie voles. (A) Oxytocin receptor (OTR) binding in the nucleus accumbens (NAcc) is higher in spontaneously maternal than in nonmaternal females. (B) Intra-NAcc injections of an OTR antagonist (OTR-NA) result in more female voles that do not display spontaneous maternal behavior compared to controls (CSF-NA). (C) Downregulating OTR expression in the NAcc via injections of a short hairpin OTR interfering RNA (shRNA-OTR) decreases the number of juvenile female voles displaying alloparental behavior, compared to control females (control) injected with a scrambled sequence. (D) Female voles receiving shRNA-OTR injections into the NAcc spend less time licking and grooming pups, compared to control females. (E) Male prairie voles receiving ICV injections of AVP display a higher level of spontaneous paternal responsiveness to pups compared to males receiving control injections (Saline) or injections of AVP with an AVP receptor antagonist (antagonist/AVP). Data are shown as mean ± SEM. *P < 0.05. Data adapted, with permission, from (24, 198, 272).
Brain AVP has been particularly implicated in parental behavior in prairie voles. Early studies mapping AVP system in the vole brain found that male voles have more AVP-ir mRNA labeled cells in the BNST and MeA and a higher density of AVP-ir fibers in the LS, compared to females (21, 277). This sexually dimorphic AVP pathway is similar to what has been reported in other species of rodents (66). However, an interesting species difference was also found: male prairie voles have a higher density of AVP-ir fibers in the LS than male meadow voles (270). In studies comparing prairie voles at different stages during reproduction, it was found that after 3 days of mating/cohabitation with a female, male prairie voles showed a significant increase in the number of AVP mRNA labeled cells in the BNST but a decrease in the density of AVP-ir fibers in the LS, compared to their sexually naïve counterparts (21,22,277). As AVP neurons in the BNST project to the LS (65), the decreased AVP-ir staining in the LS has been interpreted as an increase in AVP release associated with fatherhood (21). Importantly, such changes in AVP activity in the BNST-LS pathway were not found in promiscuous meadow voles nor in female prairie voles, indicating a species- and sex-specific effect in male prairie voles (21, 22, 277). This change in AVP activity could be associated with the increase in paternal responsiveness postpairing (22). In fact, the functional significance of AVP action in the LS on regulating paternal behavior has been demonstrated in a pharmacological study in virgin male prairie voles. AVP injections into the LS increased paternal behavior, whereas this effect was blocked by preinjections of a V1aR antagonist (272) (Fig. 5E). It is interesting to note that intra-LS AVP injections also facilitate pair bonding behavior in male prairie voles (171). AVP’s role in maternal behavior has been less studied compared to paternal behavior, but it has been shown that intra-LS AVP injections induce persistent parental behavior in female rats (205). Additionally, in a study in female prairie voles, the density of V1aR in the VP and PVN positively correlated with the length of female’s pregnancy (200) raising the intriguing possibility that pregnancy may facilitate V1aR upregulation in these brain regions in preparation for increasing maternal approach to pup stimuli.
The literature on the neural regulation of paternal behavior is severely lacking compared to maternal and adult mate bonding behaviors. Due to the paternal nature of male prairie voles, future studies can benefit from utilizing the prairie vole model to further investigate the brain regions and neurochemicals involved in the onset and maintenance of paternal care.
Stress and social buffering
It is well known that in humans, attachment with partners, relatives, or even friends act as a protective buffer against many negative consequences of life stress, whereas disruption or lack of social attachments can lead to pathologies via dysregulation of the HPA axis and downstream effects (55, 99, 247). For example, divorce is associated with increased reports of distress and depressive symptoms, but increased social support ameliorates some of these effects (174). Loss of partners or close relatives leads to grief and associated symptoms including dysphoria, anxiety, depression, sleep disturbance, cardiovascular problems, and immune system deficits (242). Loneliness has been used to describe real or perceived social isolation in the human literature (45). Feelings of loneliness include distressing feelings of social isolation and increase the risk for depression as well as other chronic diseases (40, 42, 43, 45, 56, 126, 127). Partner loss often leads to increased loneliness which has also been linked to an increased mortality risk (125, 131, 210). Although several hormones (e.g., cortisol and estrogen) and neurochemical systems (e.g., OT, AVP, and DA) have been implicated in mediating loneliness, the consequences of social attachment disruption, and social buffering on stress responses in humans (41, 42, 44, 92, 250), we still know little about the neurobiology of attachment disruption and social buffering. In traditional laboratory rodent species and primates, studies have shown that social isolation leads to negative responses. Acute and chronic social isolation, in general, induce anxiety-like and depression-like behaviors, enhance the response of the HPA axis, and increase activities of several neurochemical systems, including OT and AVP, compared to socially housed counterparts (247). Conversely, reunion with conspecifics can ameliorate the behavioral, hormonal, and neurochemical responses associated with social isolation and stress experience (132, 219, 248). Recent emergence of the prairie vole model has provided an excellent opportunity to study both the benefits and consequences of social relationships, especially the relationship with a bonding partner, on the brain and behaviors (99, 247). Data have shown that OT and AVP are involved not only in the formation of pair bonds (as reviewed earlier), but also in the response to social isolation/ partner separation as well as social buffering of stress responses in prairie voles (38, 99, 247).
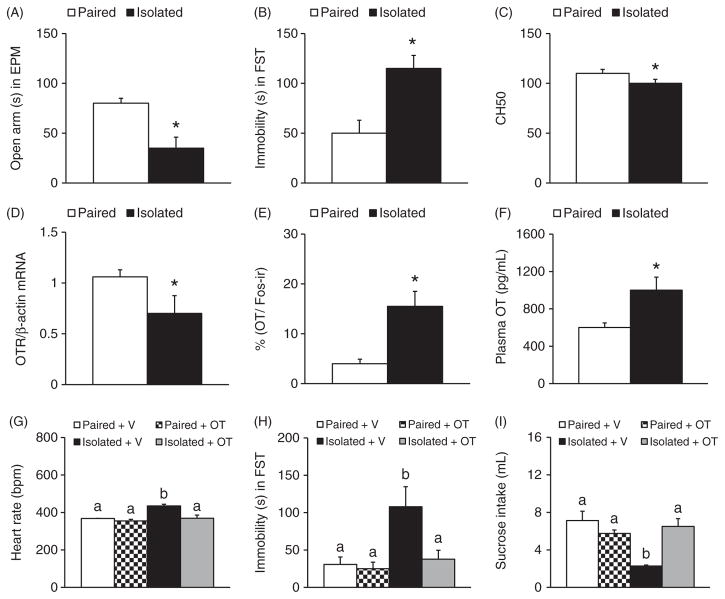
Social isolation paradigms have been utilized to study the effects of social isolation on behavior and bodily functions in prairie voles. In general, sexually naïve voles that are singly housed (socially isolated) are compared with ones that are housed together with same-sex cage mates. In adult female prairie voles, 4-weeks social isolation increases heart rate, decreases heart rate variability, and enhances anxiety-like and depression-like behaviors as well as pup-directed aggression (108, 109, 111, 112, 115). Four weeks of social isolation also induces endothelial dysfunction—an implication for depression and cardiovascular diseases (212)—as well as enhances agnostic behavior in females and disrupts immune responses in both male and female prairie voles (236). Thus, social isolation induces behavioral, autonomic, and immune dysregulations in prairie voles similar to those induced by other stressors (109) (Fig. 6A–C). Further, such chronic social isolation has been found to affect neurochemical systems in the brain: it significantly decreases OTR mRNA in the hypothalamus in both male and female prairie voles, and increases plasma OT in females (216) (Fig. 6D). Another study found that plasma OT is increased in both socially isolated males and females in addition to increasing the number of c-Fos/OT double labeled cells in the PVN after experiencing the RIT, suggesting that OT is involved in the neuroendocrine responses to acute behavioral stressors in socially isolated prairie voles (109) (Fig. 6E and F). Most interestingly, chronic (14 days), peripheral OT treatment prevents the autonomic and behavioral consequences of social isolation in female prairie voles (Fig. 6G–I). Specifically, OT treatment prevents the autonomic changes in response to acute behavioral stressors (e.g., EPM and RIT) and prevents isolation-induced increases in depression-like behaviors (e.g., reduced sucrose intake and increased immobility in the forced swim test) (114) (Fig. 6G–I). Therefore, the OT system is activated when animals experience social isolation, and OT may compensate for the autonomic response and its subsequent effects on behaviors (113, 114).
Figure 6.
The effects of social isolation on behaviors, brain OT, and immune responses in sexually naive female prairie voles. Compared to the pair-housed controls (paired), females that are socially isolated from cage mates for 4 weeks (isolated) spend less time in the open arms during an elevated plus maze test (EPM) (A) and more time immobile during a forced swim test (FST) (B). Isolation experience also decreases CH50, which measures the activity of the immune system’s classical complement pathway (C). In addition, Isolated females show a decrease in OTR mRNA expression in the hypothalamus (D), an increase in the percentage of c-Fos labeled OT-immunoreactive neurons in the PVN following a 5-min RIT (E), and an elevation in circulating OT levels (F), compared to the Paired controls. Such social isolation-induced increases in heart rate (G) and immobile duration during the FST (H) as well as the decrease in sucrose intake (I) are prevented by daily OT administration. Data are shown as mean ± SEM. *P < 0.05. Alphabetic letters indicate the results from a post-hoc test following an ANOVA. Bars labeled with different letters differ significantly from each other. Data adapted, with permission, from (109, 110, 114, 216, 236).
Social isolation during development has also been shown to affect behaviors and neurochemical markers in the brain. In male prairie voles, social isolation for 6 weeks following weaning significantly increases anxiety-like behavior and enhances mRNA expression in the PVN of OT, AVP, and corticotrophin-releasing hormone (CRH), a hormone and neurotransmitter involved in stress responses (201). In addition to increasing anxiety and depressive-like behaviors, 6 weeks of social isolation reduces cell proliferation, survival or neuronal differentiation in the amygdala, MPOA, and VMH in a brain region-specific manner in female prairie voles (168). In female prairie voles, social isolation for either 4 or 21 days following weaning also increases CRH-ir staining in the PVN and AVP-ir in the SON, compared to pair housed controls (232). Further, chronic social isolation at longer durations (e.g., 60 days) also increases depression-like behavior, plasma levels of OT, AVP, and the stress hormone CORT, as well as OT-ir and CRH-ir staining in the PVN of female prairie voles (109). In another study, repeated social isolation (one hour each day for 4 weeks) increased plasma AVP in both male and female prairie voles (216).
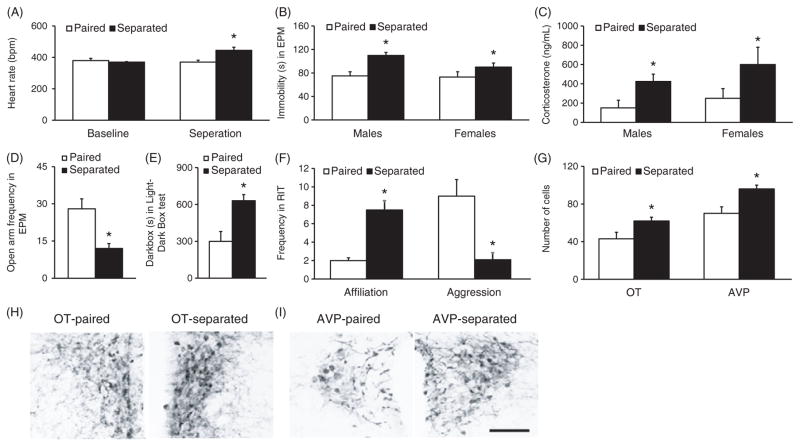
Separation from a paired partner has also been utilized to examine the consequences of breaking pair bonds in prairie voles (Fig. 7). In the first study to investigate the effects of partner separation on prairie voles, pair bonded male prairie voles were separated from their bonded partner for 5 days and compared with pair-housed controls for their behavioral and hormonal responses to an acute behavioral stressor (32). Male voles separated from the female partner had increased circulating levels of CORT and displayed increased depression-like behavior in the forced swim test and tail-suspension test (32). Interestingly, administration of a CRHR antagonist into the ventricles prevented the increase in depression-like behaviors in male prairie voles separated from their female partner. A subsequent study also found that in addition to increasing depression-like behavior and circulating stress hormones, 5 days of partner separation also leads to autonomic imbalance characterized by increased sympathetic and decreased parasympathetic tones in both male and female prairie voles (179) (Fig. 7A–C). A more recent study investigated longer-term effects of partner separation on emotional and social behaviors, stress hormones, and neurochemistry (255). Partner loss for 2 weeks significantly increased anxiety-like as well as depression-like behaviors, and increased the density of OT-ir, AVP-ir, and CRH-ir staining in the PVN of male voles (Fig. 7D–I). At 4 weeks following partner loss, males also failed to display partner preferences as well as selective aggression and had elevated levels of plasma CORT. Thus, partner loss elicits anxiety-like and depression-like behaviors, disrupts social bonding, and alters OT and AVP as well as CRH, systems that are initially involved in the regulation of pair bond formation. These data indicate the utility of the prairie vole model to study the neurobiology underlying partner loss and grief (243).
Figure 7.
The effects of partner separation on the physiology, behavior, and neurochemical staining in the brain of pair-bonded prairie voles. Compared to the paired controls (paired), 5 days of separation from the mating partner (separated) resulted in increases in heat rate (A), immobile time during a forced swim test (FST) (B), and circulating levels of CORT following the FST (C) in both male and female prairie voles. In male prairie voles, 2 weeks of separation from their female partners led to a decreased entry to the open arms during an elevated plus maze test (EPM) (D) and an increased duration in the dark box during a light-dark box test (E), in comparison to the Paired controls. Furthermore, these Separated males also showed an increase in social affiliation and a decrease in aggression during an RIT (F) as well as increases in the number of neurons stained for OT and AVP in the paraventricular nucleus of the hippocampus (PVN) (G, H, and I), compared to Paired controls. Data are shown as mean ± SEM. *P < 0.05. Scale bar = 100 μm. Data adapted, with permission, from (179, 255).
A most recent study has shed light on potential mechanisms of action underlying separation induced depression-like behaviors in pair bonded male prairie voles and found an interaction between the OT and CRH system in the NAcc. Depression-like behavior in pair bonded males separated from their female partner for 5 days is abolished with administration of a CRHR2 antagonist directly into the NAcc (31). Notably, OT administration in the NAcc also prevents depression-like behaviors in separated males compared to paired males (31). Furthermore, partner separation decreases OT mRNA in the PVN and OTR binding in the NAcc of pair bonded male prairie voles (31). The major source of OT in the NAcc is from the PVN and as the majority of OT projection neurons in the PVN contain somatic and dendritic CRHR2, these data suggest that CRHR2 activation may decrease PVN to NAcc OT transmission in male prairie voles separated from the female partner resulting in depression-like behaviors (31). In fact, brain microdialysis reveals that CRHR2 antagonist or agonist injections into the ventricle increase and decrease OT release within the NAcc, respectively (31). Moreover, CRHR2 in the NAcc is majorly restricted to OT fibers suggesting that CRHR2 manipulations in the NAcc alter OT activity (31). Electrophysiological recordings of PVN OT neurons after administration of a CRHR2 agonist indicate that CRHR2 activation indirectly decreases the activity of OT neurotransmission, most likely by reducing glutamatergic drive to OT neurons (31). Therefore, partner loss increases CRHR2 and decreases OT activity in the NAcc, respectively, and CRHR2 may act on OT neurons in the PVN that project to the NAcc to suppress NAcc OT release and increase depression-like behavior.
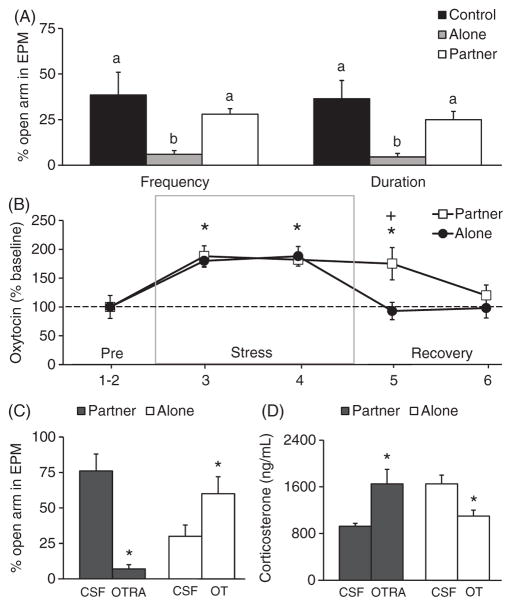
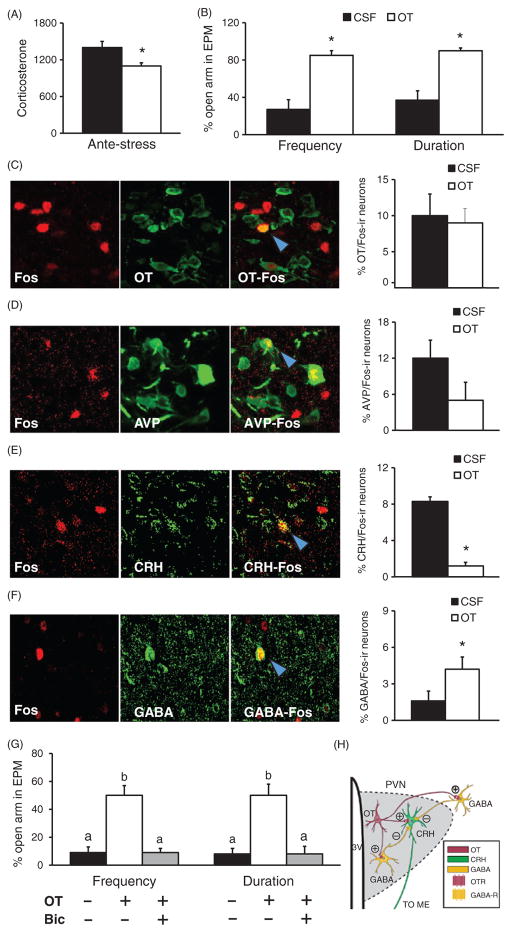
It is well documented that OT is released during social interactions and can act as an anxiolytic, such as during sex (265) and in response to pup suckling (188). The aforementioned data in prairie voles, altogether, indicate that the OT system in the brain is activated during mating, pair bonding, and responses to social isolation and separation from the bonded partner, suggesting that OT may play biphasic roles not only in facilitating the cognitive and behavioral functions associated with pair bond formation, but also in mediating the consequences of breaking a pair bond. In addition, OT release during social interactions may alleviate some of the negative consequences of stress. Data from a recent study in female prairie voles clearly illustrates the effects of social buffering from a mating partner on stress responses and the role of brain OT in mediating such effects (248) (Fig. 8). For example, 1 h of immobilization (IMO) is an established stressor for both sexually naive and pair bonded prairie voles as it induces anxiety-like behavior and increases plasma levels of CORT (245, 248). For pair-bonded female prairie voles, however, such behavioral and hormonal stress responses are only found in ones recovering alone but not in ones recovering with the male partner following the stress (248) (Fig. 8A). Brain micro-dialysis revealed that OT release within the PVN increased during IMO and this increase was sustained when female voles were recovering with the male partner, but not alone (Fig. 8B). These data indicate that the bonded partner may have social buffering effects in ameliorating stress responses to IMO, probably due to the augmented social interactions initiated from the male partner during recovery (248). Intra-PVN administration of an OTR antagonist blocked the effects of social buffering whereas administration of OT reduced behavioral and CORT responses to IMO in females that recovered alone (Fig. 8C and D). Together, these data indicate that PVN OT is both necessary and sufficient to regulate the ameliorating effects of social support from a male partner on the stress response in female prairie voles (248). Potential mechanisms of action were further explored in a subsequent study. In this study (246), intra-PVN OT administration diminished behavioral and hormonal responses to an acute behavioral stressor [elevated platform stress (EPS)], again demonstrating anxiolysis (Fig. 9A and B). Most interestingly, such OT treatment increased activation of GABAergic neurons in the PVN after stress, as indicated by increased GABA/Fos double labeling of neurons, and deactivated the CRH system, indicated by decreased CRH/Fos double labeling, compared to the cerebrospinal fluid (CSF) treated controls (Fig. 9C–F). As in the PVN many synapses are GABAergic (67,129) and more than half of CRH neurons express GABAA receptors (57), it has been hypothesized that PVN OT activates GABA which, in turn, diminishes CRH activity, leading to a decrease in HPA activity and subsequent changes in behavioral responses to stress. This hypothesis is further supported by data showing that GABA receptor antagonism impairs the anxiolytic effects of OT (246) (Fig. 9G). Together, these data suggest that OT in the brain can interact with other neurochemicals to mediate social buffering effects on stress responses in a brain region-specific manner in prairie voles (Fig. 9H).
Figure 8.
The effects of IMO stress and social buffering on anxiety-like behaviors and brain OT activity in female prairie voles. Subjects experience 1-h IMO stress, recover alone (alone) or with the male partner (partner) for 30 min, and receive a 5-min EPM test. (A) Females that recover alone (alone) enter the open arms less frequently and spend less time there in the EPM test, compared to handled females (control) and females recovering with a partner (partner). (B) Data from in vivo brain microdialysis show that OT release in the PVN increases during IMO stress, and this increased OT release is sustained during the recovery if the subject recovers with a partner but not alone. (C and D) Intra-PVN injections of an OTRA in the subjects recovering with the partner block social buffering effects on anxiety-like behaviors and plasma CORT. Conversely, OT injections into the PVN of the subjects recovering alone mimic the effects of a partner by reducing anxiety like behaviors and circulating levels of CORT (D). Data are shown as mean ± SEM. *P < 0.05. Alphabetic letters indicate the results from a post-hoc test following an ANOVA. Bars labeled with different letters differ significantly from each other. Data adapted, with permission, from (248).
Figure 9.
The effects of OT treatment in the PVN on stress induced changes in circulating CORT, anxiety-like behaviors, and neural activity in female prairie voles. Intra-PVN injections of OT before an EPS inhibit the stress-induced rise in plasma CORT (A) and anxiety-like behavior during the EPM (B). Although such OT injection does not alter the percentage of OT (C) or AVP (D) neurons in the PVN that are double-labeled with c-Fos, it decreases the number of CRH/Fos neurons (E) and increases the number of GABA/Fos (F) neurons in the PVN. Blue arrows point to double labeled cells. Furthermore, intra-PVN injections of the GABAA receptor antagonist, bicuculline (Bic), prevent OT effects in reducing anxiety-like behavior following the EPS stress (G). (H) A hypothetical model suggesting that OT release in the PVN may activate GABAergic interneurons in the PVN and GABAergic projecting neurons to the PVN, which, in turn, can inhibit CRH neurons and potentially decrease activity of the hypothalamic-pituitary-adrenal axis. Data are shown as mean ± SEM. *P < 0.05. Data adapted, with permission, from (246).
Social buffering in prairie voles has been further documented in a recent study as well as evidence of consoling behavior by this species (38). Similar to the previous study, prairie voles that experience a stressor demonstrate decreased anxiety-like behaviors after recovering with a familiar cage-mate versus recovering alone. Moreover, the nonstressed cagemate increased affiliative behavior toward their stressed cagemate, but not toward the stranger, indicating consoling behavior in this species. Interestingly, meadow voles did not show consoling behaviors toward stressed conspecifics. Administration of an OT antagonist into the anterior cingulate cortex blocked consoling behavior indicating a role for OT in consoling behavior and empathy in prairie voles. The anterior cingulate cortex may be one brain region involved in regulating empathy directed behaviors in prairie voles and has been implicated previously in empathy in humans (38).
Research in humans has demonstrated parallels between the neural circuitry involved in regulating effects of subjective social isolation in humans (loneliness) and social isolation in rodent models like the prairie vole (45). Therefore, the prairie vole can be used as a model to study both the benefits and consequences of social relationships on the brain and behavior. Data indicate that in addition to being sensitive to the social environment, both OT and AVP are also integrally involved in regulating the HPA axis and social buffering (128). Considering the ubiquity of social attachments and impact on human health, it will be beneficial to understand the mechanisms underlying the adaptive aspects of social buffering as well as the maladaptive consequences associated with loneliness and partner loss (bereavement) to combat stress induced negative symptomology. While partner loss, especially through death, is associated with grieving in humans, complicated grief represents a prolonged state of grief resulting in maladaptive consequences (242). It may in fact be adaptive to terminate a bond after partner loss versus maintaining this bond in the absence of the partner. Therefore, identifying the mechanisms associated with disintegration of a pair bond can help inform treatment for individuals suffering from prolonged bereavement. The prairie vole can be used as a model for partner loss induced depression and anxiety (32, 255).
Sex differences
Sex differences have been amply demonstrated in behavior and in the neurochemical and hormonal systems that underline the behavior. Males and females are exposed to different concentrations of endogenous hormones which may differentially affect the expression of neurochemicals in some brain regions in a sex-specific manner, such as AVP expression in the extended amygdala (6). Such sex differences have also been demonstrated in the neurochemical regulation of social behaviors in prairie voles. For example, early studies on the neuropeptide regulation of pair bonding were primarily focused on OT in females and AVP in males, implicating potential sex-specific effects of OT and AVP on pair bonding (48, 135, 281, 283). Subsequent studies have shown that OT and AVP are likely involved in regulating pair bonding in both male and female prairie voles (52,144,170). For example, ICV administrations of either OT or AVP were shown to mediate partner preference formation in both male and female prairie voles (52). However, male voles seem to be more sensitive than female voles to AVP in displaying partner preferences, still indicating potential sex differences in the neuropeptide regulation of pair bonding (52). Furthermore, sex differences in developmental exposure to neuropeptides may exist independently from adult exposure (16, 18, 19). In addition, sex differences may also exist in the target brain regions where AVP and OT regulate pair-bonding behavior. The literature suggests that OT in the NAcc seems to be more important in females (293), whereas AVP in the VP (215, 293), LS (171), and AH (103) plays a critical role in males.
The differences in behavior between pair-bonded male and female prairie voles can be at least partly accounted for by differences in the regulation of AVP and OT synthesis by hormones. Synthesis of AVP is dependent on androgens (73, 182), and androgen-dependent vasopressin synthesis is critical, particularly during development, for partner preference formation in adult male prairie voles (59). Additionally, OTR expression can be regulated by estrogen and estrogen levels differ between males and females across the lifespan (98, 223). In turn, OT can also regulate estrogen receptor expression differently between male and female prairie voles (159). AVP and OT signaling during development also affects the expression of adult AVP and OT systems, probably via interactions with sex hormones, thereby further influencing sex specific behaviors. For example, OT manipulations on PND1 affect later expression of OT and AVP cells in the PVN in a sexually dimorphic manner (287). OT manipulations early in development also affect later V1aR expression differently between male and female prairie voles (20). An OT injection on PND1 increases V1aR binding in the VP of adult males while an OTR antagonist injection decreases binding (20). Conversely, OT treatment on PND1 decreases V1aR binding in the VP of adult females. Furthermore, an OTR antagonist injection on PND1 differentially affects male and female prairie vole alloparental behavior, implicating the functional significance of receptor differences in behavior (19).
Stressors also affect pair bonding differently between male and female prairie voles and this is mediated by CORT. Increased CORT release, either after stress or CORT injections, potentiates partner preference formation in male prairie voles (71), but inhibits the same behavior in females (69,70). In unstressed sexually naive females, CORT levels decrease upon interaction with a novel male but increase if the female is already pair bonded, thereby influencing the facilitation or blockade of partner preference depending on the social history of the female (69). Furthermore, in males, partner loss increases CORT as well as depressive and anxiety-like behaviors, effects which are blocked with a central CRHR antagonist injection (32). This suggests that partner loss is stressful and therefore CORT as well as other stress hormones, are mediating factors of pair bond maintenance.
Alcohol can also influence pair bonding behavior in prairie voles in a sexually dimorphic manner (8). When given access to both water and alcohol, prairie voles show a preference for the alcohol over the water. This alcohol preference is further influenced by social housing status (8). Pair-housed siblings will drink more alcohol than singly housed subjects (8), suggesting that social interactions promote alcohol drinking. Interestingly, voluntary alcohol consumption in male prairie voles inhibits partner preference formation but facilitates the same behavior in females (7). It has been hypothesized that alcohol acts as an anxiolytic resulting in decreased CORT levels and thereby facilitating pair bonding in females but inhibiting it in males, although the relationship between alcohol, anxiety, and the CORT response in prairie voles needs to be investigated in more detail (7).
Conclusions and Implications
Research in lower order taxa reveals a role for nonapeptides in regulating social behaviors, such as reproduction and courtship, while research in rats and mice largely informs of a role in regulating maternal behavior. The main evidence from the large body of research now in prairie voles strongly implicates that OT and AVP play critical roles in mediating multiple facets of social bonds that are characteristic of monogamy and akin to human attachment. These findings have translated to humans with high predictive validity, suggesting evolutionarily conserved neuronal mechanisms. For example, intranasal OT treatment in humans has been shown to increase the recognition of emotion, the memory of human faces, gaze and fixation to the eye region of faces, the attractiveness of faces, altruism, and positive communicative behavior (14, 74, 77, 119, 224, 225, 237, 253). Intranasal AVP treatment has also been found to increase facial recognition memory, altruism, and empathy (118,224,225,258). A growing number of human studies have also found correlations between differences in the OT and AVP receptor genes and social behaviors (141). For example, single nucleotide polymorphisms in the OTR gene have been implicated in general emotional facial recognition, (180), memory (243), empathy (61, 228, 284), and infant to caregiver attachment style (51). Moreover, sequence variations in the v1ar and v1br genes have been associated with social functioning including aggression, emotion, and empathy (141, 166, 175, 285). Interestingly, sequence variations of the genes for V1aR and OTR between the prairie vole and other vole species have also been implicated in deferentially regulating social behaviors by regulating the distribution patterns of V1aR and OTR (124,140,194), indicating the utility of the vole model in specifically examining genetic mechanisms and their potential roles in social behaviors.
It is important to note that the effects of OT and AVP on human behavior are largely studied by intranasal administration. The extent to which intranasal administration activates central receptors is not entirely clear and sometimes even controversial (165). For instance, in a recent fMRI study in rats, the blood-oxygen-level dependent (BOLD) response to peripheral administration of OT did not match the BOLD response to ICV OT administration (86). Therefore, the findings from human studies utilizing intranasal OT/AVP should be interpreted with caution. Additionally, the effects of OT in social behavior in humans, such as in increasing trust (157), are not always replicated and sometimes contradicting effects are reported in the human literature (25,240). This may be at least in part due to several factors that are not always accounted for in human studies including differences in testing contexts, baseline OT levels, prior early life experiences of the participants, and sex differences (224,239,261). These data indicate the necessity for further use of appropriate animal models under more controlled laboratory conditions to study the role of OT and AVP and interactions with genes and environment in the regulation of social behavior and cognitive functions.
Nevertheless, the large volume of data now existing on the effects of OT across species have informed conceptualizations on the role of OT in social behavior (53, 64, 84, 107, 134,187,218,239). Updated conceptualizations posit that OT mediates salience assignment and regulates attentional selection to social signals via interactions with the dopaminergic system (239). During development, OT signaling interacts with the early environment to shape these processes and to generate internal working models, based off sensory input, to influence self-referential processing and contextual behavior (134, 164, 218). Genetic or environmental insults that affect early OT signaling or influence sensory processing, therefore, can have long-term downstream consequences on OT regulation of adult behavior (218). Data from both human and animal studies lend support to these perspectives. For instance, in humans, intranasal OT facilitates social stress, indicating enhanced awareness of social stimuli and valence appraisal (81). Intranasal OT also increases attention toward the eye region of faces (119) and social stimulus-induced pupil dilation (164), suggesting a role for OT in social attention. In rodents, OT not only facilitates maternal care, but it also facilitates maternal aggression during the presence of an intruder indicating context dependent effects (35). Furthermore, OTRs are located throughout the mesolimbic dopaminergic system and central injections of OT increase DA release in brain reward regions (107,233). OT and DA interactions also regulate maternal behavior (238) and are necessary for the formation of a pair bond in prairie voles (172). Notably, in humans, intranasal OT increases neural responses to images of female partners but not unfamiliar females in brain reward regions such as the VTA and NAcc in male participants (235), implicating OT and DA interaction effects in human pair bonding as well. Intranasal OT in humans has also been reported to increase VTA activity in response to both positive and negative cues, further supporting the role of OT in salience assignment, regardless of the direction (116). Within this conceptual framework, OT can regulate prosocial or antisocial behavior depending on context as well as individual differences and early life experience. However, relatively less is known about the role of OT and AVP signaling during early development in organizing the socioemotional brain and adult behavior. The expression of OTR and V1aR during development is transient in a species specific manner suggesting that OT and AVP may play particularly significant roles at specific time periods to shape adult behavior (122, 145). Significantly, recent data from mice indicates a novel role for OT signaling in regulating cross-modal and experience-dependent cortical plasticity during a developmental time period that coincides with peak cortical OTR expression (122, 300). These data implicate a sensitive time period during development where neuropeptides and experience may interact to organize the neural circuitry regulating social behavior, but further research into this area is necessary (106,121,122,300). Further, considering the role of OT in regulating all aspects of maternal-offspring interactions including birth, lactation, and the suckling response as well as OTR distribution of the offspring, it would be interesting to further explore the impact of the mother on regulating infant OT during sensitive developmental time periods and subsequent adult behavior (49, 106, 121, 143).
Finally, data from research in humans implicates the OT and AVP systems in disorders, including neurodevelopmental disorders, such as autism spectrum disorder (ASD) and schizophrenia, as well as social anxiety disorder (SAD). OT has been proposed as a therapeutic for treating individuals with ASDs (9, 267, 291) as well as SAD (54, 76), although further basic science is necessary to fully understand OT and AVP neurobiology (267). Furthermore, loneliness is associated with various health risks such as cardiovascular disease, anxiety, and depression in humans (127). As OT is involved in mediating the negative effects of social isolation and stress responses, it would be interesting to explore the potential role of OT in preventing these deficits. The therapeutic potential of neuropeptides in improving social function is very exciting. However, the mechanistic details remain to be further understood in humans, including interactions of the OT and AVP systems with genetic predispositions and early environment. The unique social behavior displayed by prairie voles combined with the rich data set of neuropeptidergic regulation of social behavior make the prairie vole a useful animal model in research significant for human health.
Acknowledgments
The authors thank Ms. Meghan Donovan for critical reading of the manuscript and Mr. Charles Badland for his advice for the figures. This work was supported by the National Institutes of Health grants R01 MH058616 and R01 MH089852 to Z.X.W. M.T. was supported by the NIH program training grant (T32 MH093311, P.K. Keel and L.A. Eckel).
References
- 1.Acher R, Chauvet J, Chauvet MT. Man and the chimaera. Selective versus neutral oxytocin evolution. Adv Exp Med Biol. 1995;395:615–627. [PubMed] [Google Scholar]
- 2.Ahern TH, Hammock EAD, Young LJ. Parental division of labor, coordination, and the effects of family structure on parenting in monogamous prairie voles (Microtus ochrogaster) Dev Psychobiol. 2011;53(2):118–131. doi: 10.1002/dev.20498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahern TH, Young LJ. The impact of early life family structure on adult social attachment, alloparental behavior, and the neuropeptide systems regulating affiliative behaviors in the monogamous prairie vole (Microtus ochrogaster) Front Behav Neurosci. 2009;3:17. doi: 10.3389/neuro.08.017.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ainsworth MD. Object relations, dependency, and attachment: A theoretical review of the infant-mother relationship. Child Dev. 1969;40(4):969–1025. [PubMed] [Google Scholar]
- 5.Akther S, Korshnova N, Zhong J, Liang M, Cherepanov SM, Lopatina O, Komleva YK, Salmina AB, Nishimura T, Fakhrul AA, Hirai H, Kato I, Yamamoto Y, Takasawa S, Okamoto H, Higashida H. Cd38 in the nucleus accumbens and oxytocin are related to paternal behavior in mice. Mol Brain. 2013;6:41. doi: 10.1186/1756-6606-6-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Albers HE. Species, sex and individual differences in the vasotocin/ vasopressin system: Relationship to neurochemical signaling in the social behavior neural network. Front Neuroendocrinol. 2015;36:49–71. doi: 10.1016/j.yfrne.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anacker AMJ, Ahern TH, Hostetler CM, Dufour BD, Smith ML, Cocking DL, Li J, Young LJ, Loftis JM, Ryabinin AE. Drinking alcohol has sex-dependent effects on pair bond formation in prairie voles. Proc Natl Acad Sci U S A. 2014;111(16):6052–6057. doi: 10.1073/pnas.1320879111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anacker AMJ, Loftis JM, Kaur S, Ryabinin AE. Prairie voles as a novel model of socially facilitated excessive drinking. Addict Biol. 2011;16(1):92–107. doi: 10.1111/j.1369-1600.2010.00234.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anagnostou E, Soorya L, Brian J, Dupuis A, Mankad D, Smile S, Jacob S. Intranasal oxytocin in the treatment of autism spectrum disorders: A review of literature and early safety and efficacy data in youth. Brain Res. 2014;1580:188–198. doi: 10.1016/j.brainres.2014.01.049. [DOI] [PubMed] [Google Scholar]
- 10.Aragona BJ, Liu Y, Yu YJ, Curtis JT, Detwiler JM, Insel TR, Wang Z. Nucleus accumbens dopamine differentially mediates the formation and maintenance of monogamous pair bonds. Nat Neurosci. 2006;9(1):133–139. doi: 10.1038/nn1613. [DOI] [PubMed] [Google Scholar]
- 11.Aragona BJ, Wang Z. Dopamine regulation of social choice in a monogamous rodent species. Front Behav Neurosci. 2009;3:15. doi: 10.3389/neuro.08.015.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Argiolas A, Melis MR. Neuropeptides and central control of sexual behaviour from the past to the present: A review. Prog Neurobiol. 2013;108:80–107. doi: 10.1016/j.pneurobio.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 13.Armstrong WE, Warach S, Hatton GI, McNeill TH. Subnuclei in the rat hypothalamic paraventricular nucleus: A cytoarchitectural, horseradish peroxidase and immunocytochemical analysis. Neuroscience. 1980;5(11):1931–1958. doi: 10.1016/0306-4522(80)90040-8. [DOI] [PubMed] [Google Scholar]
- 14.Auyeung B, Lombardo MV, Heinrichs M, Chakrabarti B, Sule A, Deakin JB, Bethlehem RAI, Dickens L, Mooney N, Sipple JAN, Thiemann P, Baron-Cohen S. Oxytocin increases eye contact during a real-time, naturalistic social interaction in males with and without autism. Transl Psychiatry. 2015;5:e507. doi: 10.1038/tp.2014.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Badura B. Social support and community health care. Soz- Präventivmedizin. 1988;33(2):79–85. doi: 10.1007/BF02098308. [DOI] [PubMed] [Google Scholar]
- 16.Bales KL, Carter CS. Sex differences and developmental effects of oxytocin on aggression and social behavior in prairie voles (Microtus ochrogaster) Horm Behav. 2003;44(3):178–184. doi: 10.1016/s0018-506x(03)00154-5. [DOI] [PubMed] [Google Scholar]
- 17.Bales KL, Kim AJ, Lewis-Reese AD, Sue Carter C. Both oxytocin and vasopressin may influence alloparental behavior in male prairie voles. Horm Behav. 2004;45(5):354–361. doi: 10.1016/j.yhbeh.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 18.Bales KL, Perkeybile AM, Conley OG, Lee MH, Guoynes CD, Downing GM, Yun CR, Solomon M, Jacob S, Mendoza SP. Chronic intranasal oxytocin causes long-term impairments in partner preference formation in male prairie voles. Biol Psychiatry. 2013;74(3):180–188. doi: 10.1016/j.biopsych.2012.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bales KL, Pfeifer LA, Carter CS. Sex differences and developmental effects of manipulations of oxytocin on alloparenting and anxiety in prairie voles. Dev Psychobiol. 2004;44(2):123–131. doi: 10.1002/dev.10165. [DOI] [PubMed] [Google Scholar]
- 20.Bales KL, Plotsky PM, Young LJ, Lim MM, Grotte N, Ferrer E, Carter CS. Neonatal oxytocin manipulations have long-lasting, sexually dimorphic effects on vasopressin receptors. Neuroscience. 2007;144(1):38–45. doi: 10.1016/j.neuroscience.2006.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bamshad M, Novak MA, De Vries GJ. Sex and species differences in the vasopressin innervation of sexually naive and parental prairie voles, Microtus ochrogaster and meadow voles, Microtus pennsylvanicus. J Neuroendocrinol. 1993;5(3):247–255. doi: 10.1111/j.1365-2826.1993.tb00480.x. [DOI] [PubMed] [Google Scholar]
- 22.Bamshad M, Novak MA, de Vries GJ. Cohabitation alters vasopressin innervation and paternal behavior in prairie voles (Microtus ochrogaster) Physiol Behav. 1994;56(4):751–758. doi: 10.1016/0031-9384(94)90238-0. [DOI] [PubMed] [Google Scholar]
- 23.Barrett CE, Keebaugh AC, Ahern TH, Bass CE, Terwilliger EF, Young LJ. Variation in vasopressin receptor (AVPR1a) expression creates diversity in behaviors related to monogamy in prairie voles. Horm Behav. 2013;63(3):518–526. doi: 10.1016/j.yhbeh.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barrett CE, Keebaugh AC, Laprairie JL, Jenkins JJ, Young LJ. RNAi knockdown of oxytocin receptor in the nucleus accumbens inhibits social attachment and parental care in monogamous female prairie voles. Soc Neurosci. 2015;10:561–570. doi: 10.1080/17470919.2015.1040893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bartz J, Simeon D, Hamilton H, Kim S, Crystal S, Braun A, Vicens V, Hollander E. Oxytocin can hinder trust and cooperation in borderline personality disorder. Soc Cogn Affect Neurosci. 2011;6(5):556–563. doi: 10.1093/scan/nsq085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bealer SL, Lipschitz DL, Ramoz G, Crowley WR. Oxytocin receptor binding in the hypothalamus during gestation in rats. Am J Physiol Regul Integr Comp Physiol. 2006;291(1):R53–R58. doi: 10.1152/ajpregu.00766.2005. [DOI] [PubMed] [Google Scholar]
- 27.Beery AK, Routman DM, Zucker I. Same-sex social behavior in meadow voles: Multiple and rapid formation of attachments. Physiol Behav. 2009;97(1):52–57. doi: 10.1016/j.physbeh.2009.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bielsky IF, Hu S-B, Ren X, Terwilliger EF, Young LJ. The V1a vasopressin receptor is necessary and sufficient for normal social recognition: A gene replacement study. Neuron. 2005;47(4):503–513. doi: 10.1016/j.neuron.2005.06.031. [DOI] [PubMed] [Google Scholar]
- 29.Bluthe RM, Schoenen J, Dantzer R. Androgen-dependent vasopressinergic neurons are involved in social recognition in rats. Brain Res. 1990;519(1–2):150–157. doi: 10.1016/0006-8993(90)90073-k. [DOI] [PubMed] [Google Scholar]
- 30.Bosch OJ. Maternal aggression in rodents: Brain oxytocin and vasopressin mediate pup defence. Philos Trans R Soc Lond B Biol Sci. 2013;368(1631):20130085. doi: 10.1098/rstb.2013.0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bosch OJ, Dabrowska J, Modi ME, Johnson ZV, Keebaugh AC, Barrett CE, Ahern TH, Guo J, Grinevich V, Rainnie DG, Neumann ID, Young LJ. Oxytocin in the nucleus accumbens shell reverses CRFR2-evoked passive stress-coping after partner loss in monogamous male prairie voles. Psychoneuroendocrinology. 2016;64:66–78. doi: 10.1016/j.psyneuen.2015.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bosch OJ, Nair HP, Ahern TH, Neumann ID, Young LJ. The CRF system mediates increased passive stress-coping behavior following the loss of a bonded partner in a monogamous rodent. Neuropsychopharmacol. 2009;34(6):1406–1415. doi: 10.1038/npp.2008.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bosch OJ, Neumann ID. Brain vasopressin is an important regulator of maternal behavior independent of dams’ trait anxiety. Proc Natl Acad Sci U S A. 2008;105(44):17139–17144. doi: 10.1073/pnas.0807412105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bosch OJ, Neumann ID. Vasopressin released within the central amygdala promotes maternal aggression. Eur J Neurosci. 2010;31(5):883–891. doi: 10.1111/j.1460-9568.2010.07115.x. [DOI] [PubMed] [Google Scholar]
- 35.Bosch OJ, Neumann ID. Both oxytocin and vasopressin are mediators of maternal care and aggression in rodents: From central release to sites of action. Horm Behav. 2012;61(3):293–303. doi: 10.1016/j.yhbeh.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 36.Bosch OJ, Pförtsch J, Beiderbeck DI, Landgraf R, Neumann ID. Maternal behaviour is associated with vasopressin release in the medial pre-optic area and bed nucleus of the stria terminalis in the rat. J Neuroendocrinol. 2010;22(5):420–429. doi: 10.1111/j.1365-2826.2010.01984.x. [DOI] [PubMed] [Google Scholar]
- 37.Bowlby J. Attachment and Loss. New York: Basic Books; 1969. [Google Scholar]
- 38.Burkett JP, Andari E, Johnson ZV, Curry DC, de Waal FBM, Young LJ. Oxytocin-dependent consolation behavior in rodents. Science. 2016;351(6271):375–378. doi: 10.1126/science.aac4785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Burkett JP, Young LJ. The behavioral, anatomical and pharmacological parallels between social attachment, love and addiction. Psychopharmacology (Berl) 2012;224(1):1–26. doi: 10.1007/s00213-012-2794-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cacioppo JT, Cacioppo S. The phenotype of loneliness. Eur J Dev Psychol. 2012;9(4):446–452. doi: 10.1080/17405629.2012.690510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cacioppo JT, Cacioppo S, Capitanio JP, Cole SW. The neuroendocrinology of social isolation. Annu Rev Psychol. 2015;66:733–767. doi: 10.1146/annurev-psych-010814-015240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cacioppo JT, Cacioppo S, Cole SW, Capitanio JP, Goossens L, Boomsma DI. Loneliness across phylogeny and a call for comparative studies and animal models. Perspect Psychol Sci. 2015;10(2):202–212. doi: 10.1177/1745691614564876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cacioppo JT, Hawkley LC, Thisted RA. Perceived social isolation makes me sad: 5-year cross-lagged analyses of loneliness and depressive symptomatology in the chicago health, aging, and social relations study. Psychol Aging. 2010;25(2):453–463. doi: 10.1037/a0017216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cacioppo S, Capitanio JP, Cacioppo JT. Toward a neurology of loneliness. Psychol Bull. 2014;140(6):1464–1504. doi: 10.1037/a0037618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cacioppo S, Grippo AJ, London S, Goossens L, Cacioppo JT. Loneliness: Clinical import and interventions. Perspect Psychol Sci. 2015;10(2):238–249. doi: 10.1177/1745691615570616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Carter CS. Attachment and Bonding: A New Synthesis. Cambridge, Mass: MIT Press; 2005. p. 509. [Google Scholar]
- 47.Carter CS, Boone EM, Pournajafi-Nazarloo H, Bales KL. Consequences of early experiences and exposure to oxytocin and vasopressin are sexually dimorphic. Dev Neurosci. 2009;31(4):332–341. doi: 10.1159/000216544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Carter CS, DeVries AC, Getz LL. Physiological substrates of mammalian monogamy: The prairie vole model. Neurosci Biobehav Rev. 1995;19(2):303–314. doi: 10.1016/0149-7634(94)00070-h. [DOI] [PubMed] [Google Scholar]
- 49.Champagne F, Diorio J, Sharma S, Meaney MJ. Naturally occurring variations in maternal behavior in the rat are associated with differences in estrogen-inducible central oxytocin receptors. Proc Natl Acad Sci U S A. 2001;98(22):12736–12741. doi: 10.1073/pnas.221224598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Champagne FA, Weaver ICG, Diorio J, Sharma S, Meaney MJ. Natural variations in maternal care are associated with estrogen receptor alpha expression and estrogen sensitivity in the medial preoptic area. Endocrinology. 2003;144(11):4720–4724. doi: 10.1210/en.2003-0564. [DOI] [PubMed] [Google Scholar]
- 51.Chen FS, Barth ME, Johnson SL, Gotlib IH, Johnson SC. Oxytocin receptor (OXTR) polymorphisms and attachment in human infants. Front Psychol. 2011;2:200. doi: 10.3389/fpsyg.2011.00200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cho MM, DeVries AC, Williams JR, Carter CS. The effects of oxytocin and vasopressin on partner preferences in male and female prairie voles (Microtus ochrogaster) Behav Neurosci. 1999;113(5):1071–1079. doi: 10.1037//0735-7044.113.5.1071. [DOI] [PubMed] [Google Scholar]
- 53.Churchland PS, Winkielman P. Modulating social behavior with oxytocin: How does it work? What does it mean? Horm Behav. 2012;61(3):392–399. doi: 10.1016/j.yhbeh.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Clark-Elford R, Nathan PJ, Auyeung B, Mogg K, Bradley BP, Sule A, Müller U, Dudas RB, Sahakian BJ, Baron-Cohen S. Effects of oxytocin on attention to emotional faces in healthy volunteers and highly socially anxious males. Int J Neuropsychopharmacol. 2015;18(2) doi: 10.1093/ijnp/pyu012. n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cohen S, Wills TA. Stress, social support, and the buffering hypothesis. Psychol Bull. 1985;98(2):310–357. [PubMed] [Google Scholar]
- 56.Cole SW, Capitanio JP, Chun K, Arevalo JMG, Ma J, Cacioppo JT. Myeloid differentiation architecture of leukocyte transcriptome dynamics in perceived social isolation. Proc Natl Acad Sci U S A. 2015;112:15142–15147. doi: 10.1073/pnas.1514249112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cullinan WE. GABA(a) receptor subunit expression within hypophysiotropic CRH neurons: A dual hybridization histochemical study. J Comp Neurol. 2000;419(3):344–351. doi: 10.1002/(sici)1096-9861(20000410)419:3<344::aid-cne6>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 58.Cushing BS. Neonatal Effects of Oxytocin: Development and Organizational. Hauppauge: Nova Science Publishers, Inc; 2009. [Google Scholar]
- 59.Cushing BS, Okorie U, Young LJ. The effects of neonatal castration on the subsequent behavioural response to centrally administered arginine vasopressin and the expression of V1a receptors in adult male prairie voles. J Neuroendocrinol. 2003;15(11):1021–1026. doi: 10.1046/j.1365-2826.2003.01097.x. [DOI] [PubMed] [Google Scholar]
- 60.Cushing BS, Razzoli M, Murphy AZ, Epperson PM, Le W-W, Hoffman GE. Intraspecific variation in estrogen receptor alpha and the expression of male sociosexual behavior in two populations of prairie voles. Brain Res. 2004;1016(2):247–254. doi: 10.1016/j.brainres.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 61.Dadds MR, Moul C, Cauchi A, Dobson-Stone C, Hawes DJ, Brennan J, Urwin R, Ebstein RE. Polymorphisms in the oxytocin receptor gene are associated with the development of psychopathy. Dev Psychopathol. 2014;26(1):21–31. doi: 10.1017/S0954579413000485. [DOI] [PubMed] [Google Scholar]
- 62.Dantzer R, Bluthe RM, Koob GF, Le Moal M. Modulation of social memory in male rats by neurohypophyseal peptides. Psychopharmacology (Berl) 1987;91(3):363–368. doi: 10.1007/BF00518192. [DOI] [PubMed] [Google Scholar]
- 63.Dantzer R, Koob GF, Bluthé RM, Le Moal M. Septal vasopressin modulates social memory in male rats. Brain Res. 1988;457(1):143–147. doi: 10.1016/0006-8993(88)90066-2. [DOI] [PubMed] [Google Scholar]
- 64.De Dreu CKW, Kret ME. Oxytocin conditions intergroup relations through upregulated in-group empathy, cooperation, conformity, and defense. Biol Psychiatry. 2016;79(3):165–173. doi: 10.1016/j.biopsych.2015.03.020. [DOI] [PubMed] [Google Scholar]
- 65.De Vries GJ, Best W, Sluiter AA. The influence of androgens on the development of a sex difference in the vasopressinergic innervation of the rat lateral septum. Brain Res. 1983;284(2–3):377–380. doi: 10.1016/0165-3806(83)90019-6. [DOI] [PubMed] [Google Scholar]
- 66.De Vries GJ, Panzica GC. Sexual differentiation of central vasopressin and vasotocin systems in vertebrates: Different mechanisms, similar endpoints. Neuroscience. 2006;138(3):947–955. doi: 10.1016/j.neuroscience.2005.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Decavel C, Van den Pol AN. GABA: A dominant neurotransmitter in the hypothalamus. J Comp Neurol. 1990;302(4):1019–1037. doi: 10.1002/cne.903020423. [DOI] [PubMed] [Google Scholar]
- 68.DeVries AC. Interaction among social environment, the hypothalamic-pituitary-adrenal axis, and behavior. Horm Behav. 2002;41(4):405–413. doi: 10.1006/hbeh.2002.1780. [DOI] [PubMed] [Google Scholar]
- 69.DeVries AC, DeVries MB, Taymans S, Carter CS. Modulation of pair bonding in female prairie voles (Microtus ochrogaster) by corticosterone. Proc Natl Acad Sci U S A. 1995;92(17):7744–7748. doi: 10.1073/pnas.92.17.7744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.DeVries AC, DeVries MB, Taymans SE, Carter CS. The effects of stress on social preferences are sexually dimorphic in prairie voles. Proc Natl Acad Sci U S A. 1996;93(21):11980–11984. doi: 10.1073/pnas.93.21.11980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.DeVries AC, Guptaa T, Cardillo S, Cho M, Carter CS. Corticotropin-releasing factor induces social preferences in male prairie voles. Psychoneuroendocrinology. 2002;27(6):705–714. doi: 10.1016/s0306-4530(01)00073-7. [DOI] [PubMed] [Google Scholar]
- 72.DeVries AC, Taymans SE, Carter CS. Social modulation of corticosteroid responses in male prairie voles. Ann N Y Acad Sci. 1997;807:494–497. doi: 10.1111/j.1749-6632.1997.tb51949.x. [DOI] [PubMed] [Google Scholar]
- 73.DeVries GJ, Buijs RM, Van Leeuwen FW, Caffé AR, Swaab DF. The vasopressinergic innervation of the brain in normal and castrated rats. J Comp Neurol. 1985;233(2):236–254. doi: 10.1002/cne.902330206. [DOI] [PubMed] [Google Scholar]
- 74.Ditzen B, Schaer M, Gabriel B, Bodenmann G, Ehlert U, Heinrichs M. Intranasal oxytocin increases positive communication and reduces cortisol levels during couple conflict. Biol Psychiatry. 2009;65(9):728–731. doi: 10.1016/j.biopsych.2008.10.011. [DOI] [PubMed] [Google Scholar]
- 75.Dluzen DE, Muraoka S, Engelmann M, Landgraf R. The effects of infusion of arginine vasopressin, oxytocin, or their antagonists into the olfactory bulb upon social recognition responses in male rats. Peptides. 1998;19(6):999–1005. doi: 10.1016/s0196-9781(98)00047-3. [DOI] [PubMed] [Google Scholar]
- 76.Dodhia S, Hosanagar A, Fitzgerald DA, Labuschagne I, Wood AG, Nathan PJ, Phan KL. Modulation of resting-state amygdala-frontal functional connectivity by oxytocin in generalized social anxiety disorder. Neuropsychopharmacol. 2014;39(9):2061–2069. doi: 10.1038/npp.2014.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Domes G, Kumbier E, Heinrichs M, Herpertz SC. Oxytocin promotes facial emotion recognition and amygdala reactivity in adults with asperger syndrome. Neuropsychopharmacol. 2014;39(3):698–706. doi: 10.1038/npp.2013.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Donaldson ZR, Spiegel L, Young LJ. Central vasopressin V1a receptor activation is independently necessary for both partner preference formation and expression in socially monogamous male prairie voles. Behav Neurosci. 2010;124(1):159–163. doi: 10.1037/a0018094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dumais KM, Veenema AH. Vasopressin and oxytocin receptor systems in the brain: Sex differences and sex-specific regulation of social behavior. Front Neuroendocrinol. 2015;40:1–23. doi: 10.1016/j.yfrne.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ebstein RP, Knafo A, Mankuta D, Chew SH, Lai PS. The contributions of oxytocin and vasopressin pathway genes to human behavior. Horm Behav. 2012;61(3):359–379. doi: 10.1016/j.yhbeh.2011.12.014. [DOI] [PubMed] [Google Scholar]
- 81.Eckstein M, Scheele D, Weber K, Stoffel-Wagner B, Maier W, Hurlemann R. Oxytocin facilitates the sensation of social stress. Hum Brain Mapp. 2014;35(9):4741–4750. doi: 10.1002/hbm.22508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Eliava M, Melchior M, Knobloch-Bollmann HS, Wahis J, da Silva Gouveia M, Tang Y, Ciobanu AC, Triana Del Rio R, Roth LC, Althammer F, Chavant V, Goumon Y, Gruber T, Petit-Demoulière N, Busnelli M, Chini B, Tan LL, Mitre M, Froemke RC, Chao MV, Giese G, Sprengel R, Kuner R, Poisbeau P, Seeburg PH, Stoop R, Charlet A, Grinevich V. A new population of parvocellular oxytocin neurons controlling magnocellular neuron activity and inflammatory pain processing. Neuron. 2016;89(6):1291–1304. doi: 10.1016/j.neuron.2016.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Evans S. The pair-bond of the common marmoset, Callithrix jacchus jacchus: An experimental investigation. Anim Behav. 1983;31(3):651–658. [Google Scholar]
- 84.Feldman R, Monakhov M, Pratt M, Ebstein RP. Oxytocin pathway genes: Evolutionary ancient system impacting on human affiliation, sociality, and psychopathology. Biol Psychiatry. 2016;79(3):174–184. doi: 10.1016/j.biopsych.2015.08.008. [DOI] [PubMed] [Google Scholar]
- 85.Ferguson JN, Aldag JM, Insel TR, Young LJ. Oxytocin in the medial amygdala is essential for social recognition in the mouse. J Neurosci. 2001;21(20):8278–8285. doi: 10.1523/JNEUROSCI.21-20-08278.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ferris CF, Yee JR, Kenkel WM, Dumais KM, Moore K, Veenema AH, Kulkarni P, Perkybile AM, Carter CS. Distinct bold activation profiles following central and peripheral oxytocin administration in awake rats. Front Behav Neurosci. 2015;9:245. doi: 10.3389/fnbeh.2015.00245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Flanagan LM, Pfaus JG, Pfaff DW, McEwen BS. Induction of FOS immunoreactivity in oxytocin neurons after sexual activity in female rats. Neuroendocrinology. 1993;58(3):352–358. doi: 10.1159/000126562. [DOI] [PubMed] [Google Scholar]
- 88.Fliers E, Guldenaar SE, van de Wal N, Swaab DF. Extrahypothalamic vasopressin and oxytocin in the human brain; presence of vasopressin cells in the bed nucleus of the stria terminalis. Brain Res. 1986;375(2):363–367. doi: 10.1016/0006-8993(86)90759-6. [DOI] [PubMed] [Google Scholar]
- 89.Freeman SM, Inoue K, Smith AL, Goodman MM, Young LJ. The neuroanatomical distribution of oxytocin receptor binding and mRNA in the male rhesus macaque (Macaca mulatta) Psychoneuroendocrinology. 2014;45:128–141. doi: 10.1016/j.psyneuen.2014.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Freeman SM, Walum H, Inoue K, Smith AL, Goodman MM, Bales KL, Young LJ. Neuroanatomical distribution of oxytocin and vasopressin 1a receptors in the socially monogamous coppery titi monkey (Callicebus cupreus) Neuroscience. 2014;273:12–23. doi: 10.1016/j.neuroscience.2014.04.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Freeman SM, Young LJ. Comparative perspectives on oxytocin and vasopressin receptor research in rodents and primates: Translational implications. J Neuroendocrinol. 2016;28(4) doi: 10.1111/jne.12382. n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fujisawa TX, Nishitani S, Obara T, Shinohara K. Loneliness depends on salivary estradiol levels in adolescent females. Neuro Endocrinol Lett. 2012;33(5):525–529. [PubMed] [Google Scholar]
- 93.Gavish L, Carter CS, Getz LL. Further evidences for monogamy in the prairie vole. Anim Behav. 1981;29(3):955–957. [Google Scholar]
- 94.Getz LL. Aggressive behavior of the meadow and prairie voles. J Mammal. 1962;43(3):351–358. [Google Scholar]
- 95.Getz LL, Carter CS. Prairie-vole partnerships. Am Sci. 1996;84(1):56–62. [Google Scholar]
- 96.Getz LL, Carter CS, Gavish L. The mating system of the prairie vole, Microtus ochrogaster: Field and laboratory evidence for pair-bonding. Behav Ecol Sociobiol. 1981;8(3):189–194. [Google Scholar]
- 97.Getz LL, McGuire B, Pizzuto T, Hofmann JE, Frase B. Social organization of the prairie vole (Microtus ochrogaster) J Mammal. 1993;74(1):44–58. [Google Scholar]
- 98.Gimpl G, Fahrenholz F. The oxytocin receptor system: Structure, function, and regulation. Physiol Rev. 2001;81(2):629–683. doi: 10.1152/physrev.2001.81.2.629. [DOI] [PubMed] [Google Scholar]
- 99.Gobrogge K, Wang Z. Neuropeptidergic regulation of pair-bonding and stress buffering: Lessons from voles. Horm Behav. 2015;76:91–105. doi: 10.1016/j.yhbeh.2015.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gobrogge KL. Sex, drugs, and violence: Neuromodulation of attachment and conflict in voles. Curr Top Behav Neurosci. 2014;17:229–264. doi: 10.1007/7854_2013_264. [DOI] [PubMed] [Google Scholar]
- 101.Gobrogge KL, Jia X, Liu Y, Wang Z. Neurochemical mediation of affiliation and aggression associated with pair-bonding. Biol Psychiatry. 2016 doi: 10.1016/j.biopsych.2016.02.013. n/a (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gobrogge KL, Liu Y, Jia X, Wang Z. Anterior hypothalamic neural activation and neurochemical associations with aggression in pair-bonded male prairie voles. J Comp Neurol. 2007;502(6):1109–1122. doi: 10.1002/cne.21364. [DOI] [PubMed] [Google Scholar]
- 103.Gobrogge KL, Liu Y, Young LJ, Wang Z. Anterior hypothalamic vasopressin regulates pair-bonding and drug-induced aggression in a monogamous rodent. Proc Natl Acad Sci USA. 2009;106(45):19144–19149. doi: 10.1073/pnas.0908620106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Goodson JL, Kabelik D. Dynamic limbic networks and social diversity in vertebrates: From neural context to neuromodulatory patterning. Front Neuroendocrinol. 2009;30(4):429–441. doi: 10.1016/j.yfrne.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gray GD, Dewsbury DA. A quantitative description of copulatory behavior in prairie voles (Microtus ochrogaster) Brain Behav Evol. 1973;8(6):426–452. [PubMed] [Google Scholar]
- 106.Grinevich V, Desarménien MG, Chini B, Tauber M, Muscatelli F. Ontogenesis of oxytocin pathways in the mammalian brain: Late maturation and psychosocial disorders. Front Neuroanat. 2014;8:164. doi: 10.3389/fnana.2014.00164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Grinevich V, Knobloch-Bollmann HS, Eliava M, Busnelli M, Chini B. Assembling the puzzle: Pathways of oxytocin signaling in the brain. Biol Psychiatry. 2016;79(3):155–164. doi: 10.1016/j.biopsych.2015.04.013. [DOI] [PubMed] [Google Scholar]
- 108.Grippo AJ, Carter CS, McNeal N, Chandler DL, Larocca MA, Bates SL, Porges SW. 24-hour autonomic dysfunction and depressive behaviors in an animal model of social isolation: Implications for the study of depression and cardiovascular disease. Psychosom Med. 2011;73(1):59–66. doi: 10.1097/PSY.0b013e31820019e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Grippo AJ, Gerena D, Huang J, Kumar N, Shah M, Ughreja R, Carter CS. Social isolation induces behavioral and neuroendocrine disturbances relevant to depression in female and male prairie voles. Psychoneuroendocrinology. 2007;32(8–10):966–980. doi: 10.1016/j.psyneuen.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Grippo AJ, Ihm E, Wardwell J, McNeal N, Scotti M-AL, Moenk DA, Chandler DL, LaRocca MA, Preihs K. The effects of environmental enrichment on depressive and anxiety-relevant behaviors in socially isolated prairie voles. Psychosom Med. 2014;76(4):277–284. doi: 10.1097/PSY.0000000000000052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Grippo AJ, Lamb DG, Carter CS, Porges SW. Social isolation disrupts autonomic regulation of the heart and influences negative affective behaviors. Biol Psychiatry. 2007;62(10):1162–1170. doi: 10.1016/j.biopsych.2007.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Grippo AJ, Moffitt JA, Sgoifo A, Jepson AJ, Bates SL, Chandler DL, McNeal N, Preihs K. The integration of depressive behaviors and cardiac dysfunction during an operational measure of depression: Investigating the role of negative social experiences in an animal model. Psychosom Med. 2012;74(6):612–619. doi: 10.1097/PSY.0b013e31825ca8e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Grippo AJ, Pournajafi-Nazarloo H, Sanzenbacher L, Trahanas DM, McNeal N, Clarke DA, Porges SW, Sue Carter C. Peripheral oxytocin administration buffers autonomic but not behavioral responses to environmental stressors in isolated prairie voles. Stress Amst Neth. 2012;15(2):149–161. doi: 10.3109/10253890.2011.605486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Grippo AJ, Trahanas DM, Zimmerman RR, Porges SW, Carter CS. Oxytocin protects against negative behavioral and autonomic consequences of long-term social isolation. Psychoneuroendocrinology. 2009;34(10):1542–1553. doi: 10.1016/j.psyneuen.2009.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Grippo AJ, Wu KD, Hassan I, Carter CS. Social isolation in prairie voles induces behaviors relevant to negative affect: Toward the development of a rodent model focused on co-occurring depression and anxiety. Depress Anxiety. 2008;25(6):E17–E26. doi: 10.1002/da.20375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Groppe SE, Gossen A, Rademacher L, Hahn A, Westphal L, Gründer G, Spreckelmeyer KN. Oxytocin influences processing of socially relevant cues in the ventral tegmental area of the human brain. Biol Psychiatry. 2013;74(3):172–179. doi: 10.1016/j.biopsych.2012.12.023. [DOI] [PubMed] [Google Scholar]
- 117.Gruder-Adams S, Getz LL. Comparison of the mating system and paternal behavior in Microtus ochrogaster and M pennsylvanicus. J Mammal. 1985;66(1):165–167. [Google Scholar]
- 118.Guastella AJ, Kenyon AR, Alvares GA, Carson DS, Hickie IB. Intranasal arginine vasopressin enhances the encoding of happy and angry faces in humans. Biol Psychiatry. 2010;67(12):1220–1222. doi: 10.1016/j.biopsych.2010.03.014. [DOI] [PubMed] [Google Scholar]
- 119.Guastella AJ, Mitchell PB, Dadds MR. Oxytocin increases gaze to the eye region of human faces. Biol Psychiatry. 2008;63(1):3–5. doi: 10.1016/j.biopsych.2007.06.026. [DOI] [PubMed] [Google Scholar]
- 120.Hammock EAD, Lim MM, Nair HP, Young LJ. Association of vasopressin 1a receptor levels with a regulatory microsatellite and behavior. Genes Brain Behav. 2005;4(5):289–301. doi: 10.1111/j.1601-183X.2005.00119.x. [DOI] [PubMed] [Google Scholar]
- 121.Hammock EAD. Developmental perspectives on oxytocin and vasopressin. Neuropsychopharmacol. 2015;40(1):24–42. doi: 10.1038/npp.2014.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hammock EAD, Levitt P. Oxytocin receptor ligand binding in embryonic tissue and postnatal brain development of the c57bl/6j mouse. Front Behav Neurosci. 2013;7:195. doi: 10.3389/fnbeh.2013.00195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hammock EAD, Young LJ. Variation in the vasopressin V1a receptor promoter and expression: implications for inter- and intraspecific variation in social behaviour. Eur J Neurosci. 2002;16(3):399–402. doi: 10.1046/j.1460-9568.2002.02083.x. [DOI] [PubMed] [Google Scholar]
- 124.Hammock EAD, Young LJ. Functional microsatellite polymorphism associated with divergent social structure in vole species. Mol Biol Evol. 2004;21(6):1057–1063. doi: 10.1093/molbev/msh104. [DOI] [PubMed] [Google Scholar]
- 125.Hawkley LC, Browne MW, Cacioppo JT. How can i connect with thee? let me count the ways. Psychol Sci. 2005;16(10):798–804. doi: 10.1111/j.1467-9280.2005.01617.x. [DOI] [PubMed] [Google Scholar]
- 126.Hawkley LC, Cacioppo JT. Loneliness matters: A theoretical and empirical review of consequences and mechanisms. Ann Behav Med. 2010;40(2):218–227. doi: 10.1007/s12160-010-9210-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Heinrich LM, Gullone E. The clinical significance of loneliness: A literature review. Clin Psychol Rev. 2006;26(6):695–718. doi: 10.1016/j.cpr.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 128.Heinrichs M, Baumgartner T, Kirschbaum C, Ehlert U. Social support and oxytocin interact to suppress cortisol and subjective responses to psychosocial stress. Biol Psychiatry. 2003;54(12):1389–1398. doi: 10.1016/s0006-3223(03)00465-7. [DOI] [PubMed] [Google Scholar]
- 129.Herman JP, Tasker JG, Ziegler DR, Cullinan WE. Local circuit regulation of paraventricular nucleus stress integration: Glutamate-GABA connections. Pharmacol Biochem Behav. 2002;71(3):457–468. doi: 10.1016/s0091-3057(01)00681-5. [DOI] [PubMed] [Google Scholar]
- 130.Hofmann JE, Getz LL, Gavish L. Home range overlap and nest cohabitation of male and female prairie voles. Am Midl Nat. 1984;112(2):314–319. [Google Scholar]
- 131.Holt-Lunstad J, Smith TB, Baker M, Harris T, Stephenson D. Loneliness and social isolation as risk factors for mortality: A meta-analytic review. Perspect Psychol Sci. 2015;10(2):227–237. doi: 10.1177/1745691614568352. [DOI] [PubMed] [Google Scholar]
- 132.Hostinar CE, Sullivan RM, Gunnar MR. Psychobiological mechanisms underlying the social buffering of the hypothalamic-pituitary-adrenocortical axis: a review of animal models and human studies across development. Psychol Bull. 2014;140(1):256–282. doi: 10.1037/a0032671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.House JS, Landis KR, Umberson D. Social relationships and health. Science. 1988;241(4865):540–545. doi: 10.1126/science.3399889. [DOI] [PubMed] [Google Scholar]
- 134.Hurlemann R, Scheele D. Dissecting the role of oxytocin in the formation and loss of social relationships. Biol Psychiatry. 2016;79(3):185–193. doi: 10.1016/j.biopsych.2015.05.013. [DOI] [PubMed] [Google Scholar]
- 135.Insel TR, Hulihan TJ. A gender-specific mechanism for pair bonding: Oxytocin and partner preference formation in monogamous voles. Behav Neurosci. 1995;109(4):782–789. doi: 10.1037//0735-7044.109.4.782. [DOI] [PubMed] [Google Scholar]
- 136.Insel TR, Preston S, Winslow JT. Mating in the monogamous male: Behavioral consequences. Physiol Behav. 1995;57(4):615–627. doi: 10.1016/0031-9384(94)00362-9. [DOI] [PubMed] [Google Scholar]
- 137.Insel TR, Shapiro LE. Oxytocin receptor distribution reflects social organization in monogamous and polygamous voles. Proc Natl Acad Sci. 1992;89(13):5981–5985. doi: 10.1073/pnas.89.13.5981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Insel TR, Wang ZX, Ferris CF. Patterns of brain vasopressin receptor distribution associated with social organization in microtine rodents. J Neurosci. 1994;14(9):5381–5392. doi: 10.1523/JNEUROSCI.14-09-05381.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Insel TR, Winslow JT, Williams JR, Hastings N, Shapiro LE, Carter CS. The role of neurohypophyseal peptides in the central mediation of complex social processes–evidence from comparative studies. Regul Pept. 1993;45(1–2):127–131. doi: 10.1016/0167-0115(93)90194-d. [DOI] [PubMed] [Google Scholar]
- 140.Insel TR, Young L, Wang Z. Molecular aspects of monogamy. Ann N Y Acad Sci. 1997;807(1):302–316. doi: 10.1111/j.1749-6632.1997.tb51928.x. [DOI] [PubMed] [Google Scholar]
- 141.Israel S, Lerer E, Shalev I, Uzefovsky F, Reibold M, Bachner-Melman R, Granot R, Bornstein G, Knafo A, Yirmiya N, Ebstein RP. Molecular genetic studies of the arginine vasopressin 1a receptor (AVPR1a) and the oxytocin receptor (OXTR) in human behaviour: From autism to altruism with some notes in between. Prog Brain Res. 2008;170:435–449. doi: 10.1016/S0079-6123(08)00434-2. [DOI] [PubMed] [Google Scholar]
- 142.Jean-Baptiste N, Terleph TA, Bamshad M. Changes in paternal responsiveness of prairie voles (Microtus ochrogaster) in response to olfactory cues and continuous physical contact with a female. Ethology. 2008;114(12):1239–1246. [Google Scholar]
- 143.Jensen Peña C, Champagne FA. Implications of temporal variation in maternal care for the prediction of neurobiological and behavioral outcomes in offspring. Behav Neurosci. 2013;127(1):33–46. doi: 10.1037/a0031219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Johnson ZV, Walum H, Jamal YA, Xiao Y, Keebaugh AC, Inoue K, Young LJ. Central oxytocin receptors mediate mating-induced partner preferences and enhance correlated activation across forebrain nuclei in male prairie voles. Horm Behav. 2016;79:8–17. doi: 10.1016/j.yhbeh.2015.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kang HJ, Kawasawa YI, Cheng F, Zhu Y, Xu X, Li M, Sousa AMM, Pletikos M, Meyer KA, Sedmak G, Guennel T, Shin Y, Johnson MB, Krsnik Z, Mayer S, Fertuzinhos S, Umlauf S, Lisgo SN, Vortmeyer A, Weinberger DR, Mane S, Hyde TM, Huttner A, Reimers M, Kleinman JE, Sestan N. Spatiotemporal transcriptome of the human brain. Nature. 2011;478(7370):483–489. doi: 10.1038/nature10523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Keebaugh AC, Barrett CE, Laprairie JL, Jenkins JJ, Young LJ. RNAi knockdown of oxytocin receptor in the nucleus accumbens inhibits social attachment and parental care in monogamous female prairie voles. Soc Neurosci. 2015;10(5):561–570. doi: 10.1080/17470919.2015.1040893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Keebaugh AC, Young LJ. Increasing oxytocin receptor expression in the nucleus accumbens of pre-pubertal female prairie voles enhances alloparental responsiveness and partner preference formation as adults. Horm Behav. 2011;60(5):498–504. doi: 10.1016/j.yhbeh.2011.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kenkel WM, Paredes J, Yee JR, Pournajafi-Nazarloo H, Bales KL, Carter CS. Neuroendocrine and behavioural responses to exposure to an infant in male prairie voles. J Neuroendocrinol. 2012;24(6):874–886. doi: 10.1111/j.1365-2826.2012.02301.x. [DOI] [PubMed] [Google Scholar]
- 149.Kenkel WM, Suboc G, Carter CS. Autonomic, behavioral and neuroendocrine correlates of paternal behavior in male prairie voles. Physiol Behav. 2014;128:252–259. doi: 10.1016/j.physbeh.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kiecolt-Glaser JK, Newton TL. Marriage and health: his and hers. Psychol Bull. 2001;127(4):472–503. doi: 10.1037/0033-2909.127.4.472. [DOI] [PubMed] [Google Scholar]
- 151.Kim P, Rigo P, Mayes LC, Feldman R, Leckman JF, Swain JE. Neural plasticity in fathers of human infants. Soc Neurosci. 2014;9(5):522–535. doi: 10.1080/17470919.2014.933713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kirkpatrick B, Carter CS, Newman SW, Insel TR. Axon-sparing lesions of the medial nucleus of the amygdala decrease affiliative behaviors in the prairie vole (Microtus ochrogaster): Behavioral and anatomical specificity. Behav Neurosci. 1994;108(3):501–513. doi: 10.1037//0735-7044.108.3.501. [DOI] [PubMed] [Google Scholar]
- 153.Kirkpatrick B, Kim JW, Insel TR. Limbic system fos expression associated with paternal behavior. Brain Res. 1994;658(1–2):112–118. doi: 10.1016/s0006-8993(09)90016-6. [DOI] [PubMed] [Google Scholar]
- 154.Kirkpatrick B, Williams JR, Slotnick BM, Carter CS. Olfactory bulbectomy decreases social behavior in male prairie voles (M. ochrogaster) Physiol Behav. 1994;55(5):885–889. doi: 10.1016/0031-9384(94)90075-2. [DOI] [PubMed] [Google Scholar]
- 155.Kleiman DG. Monogamy in mammals. Q Rev Biol. 1977;52(1):39–69. doi: 10.1086/409721. [DOI] [PubMed] [Google Scholar]
- 156.Knobloch HS, Charlet A, Hoffmann LC, Eliava M, Khrulev S, Cetin AH, Osten P, Schwarz MK, Seeburg PH, Stoop R, Grinevich V. Evoked axonal oxytocin release in the central amygdala attenuates fear response. Neuron. 2012;73(3):553–566. doi: 10.1016/j.neuron.2011.11.030. [DOI] [PubMed] [Google Scholar]
- 157.Kosfeld M, Heinrichs M, Zak PJ, Fischbacher U, Fehr E. Oxytocin increases trust in humans. Nature. 2005;435(7042):673–676. doi: 10.1038/nature03701. [DOI] [PubMed] [Google Scholar]
- 158.Kovács GL, Buijs RM, Bohus B, van Wimersma Greidanus TB. Microinjection of arginine8-vasopressin antiserum into the dorsal hippocampus attenuates passive avoidance behavior in rats. Physiol Behav. 1982;28(1):45–48. doi: 10.1016/0031-9384(82)90099-3. [DOI] [PubMed] [Google Scholar]
- 159.Kramer KM, Yoshida S, Papademetriou E, Cushing BS. The organizational effects of oxytocin on the central expression of estrogen receptor alpha and oxytocin in adulthood. BMC Neurosci. 2007;8:71. doi: 10.1186/1471-2202-8-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Kuroda KO, Tachikawa K, Yoshida S, Tsuneoka Y, Numan M. Neuromolecular basis of parental behavior in laboratory mice and rats: With special emphasis on technical issues of using mouse genetics. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35(5):1205–1231. doi: 10.1016/j.pnpbp.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 161.Landgraf R, Neumann ID. Vasopressin and oxytocin release within the brain: A dynamic concept of multiple and variable modes of neuropeptide communication. Front Neuroendocrinol. 2004;25(3–4):150–176. doi: 10.1016/j.yfrne.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 162.Landgraf R, Neumann I, Pittman QJ. Septal and hippocampal release of vasopressin and oxytocin during late pregnancy and parturition in the rat. Neuroendocrinology. 1991;54(4):378–383. doi: 10.1159/000125917. [DOI] [PubMed] [Google Scholar]
- 163.Lei K, Cushing BS, Musatov S, Ogawa S, Kramer KM. Estrogen receptor-alpha in the bed nucleus of the stria terminalis regulates social affiliation in male prairie voles (Microtus ochrogaster) Plos One. 2010;5(1):e8931. doi: 10.1371/journal.pone.0008931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Leknes S, Wessberg J, Ellingsen D-M, Chelnokova O, Olausson H, Laeng B. Oxytocin enhances pupil dilation and sensitivity to “hidden” emotional expressions. Soc Cogn Affect Neurosci. 2013;8(7):741–749. doi: 10.1093/scan/nss062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Leng G, Ludwig M. Intranasal oxytocin: Myths and delusions. Biol Psychiatry. 2016;79(3):243–250. doi: 10.1016/j.biopsych.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 166.Levin R, Heresco-Levy U, Bachner-Melman R, Israel S, Shalev I, Ebstein RP. Association between arginine vasopressin 1a receptor (AVPR1a) promoter region polymorphisms and prepulse inhibition. Psychoneuroendocrinology. 2009;34(6):901–908. doi: 10.1016/j.psyneuen.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 167.Licht G, Meredith M. Convergence of main and accessory olfactory pathways onto single neurons in the hamster amygdala. Exp Brain Res. 1987;69(1):7–18. doi: 10.1007/BF00247024. [DOI] [PubMed] [Google Scholar]
- 168.Lieberwirth C, Liu Y, Jia X, Wang Z. Social isolation impairs adult neurogenesis in the limbic system and alters behaviors in female prairie voles. Horm Behav. 2012;62(4):357–366. doi: 10.1016/j.yhbeh.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Lim MM, Wang Z, Olazábal DE, Ren X, Terwilliger EF, Young LJ. Enhanced partner preference in a promiscuous species by manipulating the expression of a single gene. Nature. 2004;429(6993):754–757. doi: 10.1038/nature02539. [DOI] [PubMed] [Google Scholar]
- 170.Lim MM, Young LJ. Vasopressin-dependent neural circuits underlying pair bond formation in the monogamous prairie vole. Neuroscience. 2004;125(1):35–45. doi: 10.1016/j.neuroscience.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 171.Liu Y, Curtis JT, Wang Z. Vasopressin in the lateral septum regulates pair bond formation in male prairie voles (Microtus ochrogaster) Behav Neurosci. 2001;115(4):910–919. doi: 10.1037//0735-7044.115.4.910. [DOI] [PubMed] [Google Scholar]
- 172.Liu Y, Wang ZX. Nucleus accumbens oxytocin and dopamine interact to regulate pair bond formation in female prairie voles. Neuroscience. 2003;121(3):537–544. doi: 10.1016/s0306-4522(03)00555-4. [DOI] [PubMed] [Google Scholar]
- 173.Lonstein JS, De Vries GJ. Comparison of the parental behavior of pair-bonded female and male prairie voles (Microtus ochrogaster) Physiol Behav. 1999;66(1):33–40. doi: 10.1016/s0031-9384(98)00270-4. [DOI] [PubMed] [Google Scholar]
- 174.Lorenz FO, Wickrama KAS, Conger RD, Elder GH. The short-term and decade-long effects of divorce on women’s midlife health. J Health Soc Behav. 2006;47(2):111–125. doi: 10.1177/002214650604700202. [DOI] [PubMed] [Google Scholar]
- 175.Luppino D, Moul C, Hawes DJ, Brennan J, Dadds MR. Association between a polymorphism of the vasopressin 1b receptor gene and aggression in children. Psychiatr Genet. 2014;24(5):185–190. doi: 10.1097/YPG.0000000000000036. [DOI] [PubMed] [Google Scholar]
- 176.Mason WA, Mendoza SP. Generic aspects of primate attachments: Parents, offspring and mates. Psychoneuroendocrinology. 1998;23(8):765–778. doi: 10.1016/s0306-4530(98)00054-7. [DOI] [PubMed] [Google Scholar]
- 177.McBride WJ, Murphy JM, Ikemoto S. Localization of brain reinforcement mechanisms: Intracranial self-administration and intracranial place-conditioning studies. Behav Brain Res. 1999;101(2):129–152. doi: 10.1016/s0166-4328(99)00022-4. [DOI] [PubMed] [Google Scholar]
- 178.McGuire B, Novak M. A comparison of maternal behaviour in the meadow vole (Microtus pennsylvanicus), prairie vole (M. ochrogaster) and pine vole (M. pinetorum) Anim Behav. 1984;32(4):1132–1141. [Google Scholar]
- 179.McNeal N, Scotti M-AL, Wardwell J, Chandler DL, Bates SL, Larocca M, Trahanas DM, Grippo AJ. Disruption of social bonds induces behavioral and physiological dysregulation in male and female prairie voles. Auton Neurosci Basic Clin. 2014;180:9–16. doi: 10.1016/j.autneu.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Melchers M, Montag C, Markett S, Reuter M. Relationship between oxytocin receptor genotype and recognition of facial emotion. Behav Neurosci. 2013;127(5):780–787. doi: 10.1037/a0033748. [DOI] [PubMed] [Google Scholar]
- 181.Melis MR, Argiolas A. Central control of penile erection: A re-visitation of the role of oxytocin and its interaction with dopamine and glutamic acid in male rats. Neurosci Biobehav Rev. 2011;35(3):939–955. doi: 10.1016/j.neubiorev.2010.10.014. [DOI] [PubMed] [Google Scholar]
- 182.Miller MA, Urban JH, Dorsa DM. Steroid dependency of vasopressin neurons in the bed nucleus of the stria terminalis by in situ hybridization. Endocrinology. 1989;125(5):2335–2340. doi: 10.1210/endo-125-5-2335. [DOI] [PubMed] [Google Scholar]
- 183.Mohedano-Moriano A, de la Rosa-Prieto C, Saiz-Sanchez D, Ubeda-Bañon I, Pro-Sistiaga P, de Moya-Pinilla M, Martinez-Marcos A. Centrifugal telencephalic afferent connections to the main and accessory olfactory bulbs. Front Neuroanat. 2012;6:19. doi: 10.3389/fnana.2012.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Mohr E, Meyerhof W, Richter D. Vasopressin and oxytocin: Molecular biology and evolution of the peptide hormones and their receptors. Vitam Horm. 1995;51:235–266. doi: 10.1016/s0083-6729(08)61040-7. [DOI] [PubMed] [Google Scholar]
- 185.Moll J, Bado P, de Oliveira-Souza R, Bramati IE, Lima DO, Paiva FF, Sato JR, Tovar-Moll F, Zahn R. A neural signature of affiliative emotion in the human septohypothalamic area. J Neurosci. 2012;32(36):12499–12505. doi: 10.1523/JNEUROSCI.6508-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Neumann I, Russell JA, Landgraf R. Oxytocin and vasopressin release within the supraoptic and paraventricular nuclei of pregnant, parturient and lactating rats: A microdialysis study. Neuroscience. 1993;53(1):65–75. doi: 10.1016/0306-4522(93)90285-n. [DOI] [PubMed] [Google Scholar]
- 187.Neumann ID, Slattery DA. Oxytocin in general anxiety and social fear: A translational approach. Biol Psychiatry. 2016;79(3):213–221. doi: 10.1016/j.biopsych.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 188.Neumann ID, Wigger A, Torner L, Holsboer F, Landgraf R. Brain oxytocin inhibits basal and stress-induced activity of the hypothalamopituitary-adrenal axis in male and female rats: Partial action within the paraventricular nucleus. J Neuroendocrinol. 2000;12(3):235–243. doi: 10.1046/j.1365-2826.2000.00442.x. [DOI] [PubMed] [Google Scholar]
- 189.Ni R-J, Shu Y-M, Wang J, Yin J-C, Xu L, Zhou J-N. Distribution of vasopressin, oxytocin and vasoactive intestinal polypeptide in the hypothalamus and extrahypothalamic regions of tree shrews. Neuroscience. 2014;265:124–136. doi: 10.1016/j.neuroscience.2014.01.034. [DOI] [PubMed] [Google Scholar]
- 190.Numan M, Numan MJ, English JB. Excitotoxic amino acid injections into the medial amygdala facilitate maternal behavior in virgin female rats. Horm Behav. 1993;27(1):56–81. doi: 10.1006/hbeh.1993.1005. [DOI] [PubMed] [Google Scholar]
- 191.Numan M, Stolzenberg DS. Medial preoptic area interactions with dopamine neural systems in the control of the onset and maintenance of maternal behavior in rats. Front Neuroendocrinol. 2009;30(1):46–64. doi: 10.1016/j.yfrne.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 192.Numan M, Young LJ. Neural mechanisms of mother-infant bonding and pair bonding: Similarities, differences, and broader implications. Horm Behav. 2016;77:98–112. doi: 10.1016/j.yhbeh.2015.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Nyuyki KD, Waldherr M, Baeuml S, Neumann ID. Yes, i am ready now: Differential effects of paced versus unpaced mating on anxiety and central oxytocin release in female rats. PloS One. 2011;6(8):e23599. doi: 10.1371/journal.pone.0023599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Okhovat M, Berrio A, Wallace G, Ophir AG, Phelps SM. Sexual fidelity trade-offs promote regulatory variation in the prairie vole brain. Science. 2015;350(6266):1371–1374. doi: 10.1126/science.aac5791. [DOI] [PubMed] [Google Scholar]
- 195.Olazábal DE. Comparative analysis of oxytocin receptor density in the nucleus accumbens: An adaptation for female and male alloparental care? J Physiol Paris. 2014;108(2–3):213–220. doi: 10.1016/j.jphysparis.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 196.Olazábal DE, Pereira M, Agrati D, Ferreira A, Fleming AS, González-Mariscal G, Lévy F, Lucion AB, Morrell JI, Numan M, Uriarte N. Flexibility and adaptation of the neural substrate that supports maternal behavior in mammals. Neurosci Biobehav Rev. 2013;37(8):1875–1892. doi: 10.1016/j.neubiorev.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 197.Olazábal DE, Young LJ. Species and individual differences in juvenile female alloparental care are associated with oxytocin receptor density in the striatum and the lateral septum. Horm Behav. 2006;49(5):681–687. doi: 10.1016/j.yhbeh.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 198.Olazábal DE, Young LJ. Oxytocin receptors in the nucleus accumbens facilitate “spontaneous” maternal behavior in adult female prairie voles. Neuroscience. 2006;141(2):559–568. doi: 10.1016/j.neuroscience.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 199.Oliveras D, Novak M. A comparison of paternal behaviour in the meadow vole Microtus pennsylvanicus, the pine vole m. pinetorum and the prairie vole m. cchrogaster. Anim Behav. 1986;34(2):519–526. [Google Scholar]
- 200.Ophir AG, Sorochman G, Evans BL, Prounis GS. Stability and dynamics of forebrain vasopressin receptor and oxytocin receptor during pregnancy in prairie voles. J Neuroendocrinol. 2013;25(8):719–728. doi: 10.1111/jne.12049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Pan Y, Liu Y, Young KA, Zhang Z, Wang Z. Post-weaning social isolation alters anxiety-related behavior and neurochemical gene expression in the brain of male prairie voles. Neurosci Lett. 2009;454(1):67–71. doi: 10.1016/j.neulet.2009.02.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Parker KJ, Lee TM. Interaction of photoperiod and testes development is associated with paternal care in Microtus pennsylvanicus (meadow voles) Physiol Behav. 2002;75(1–2):91–95. doi: 10.1016/s0031-9384(01)00636-9. [DOI] [PubMed] [Google Scholar]
- 203.Parker KJ, Lee TM. Female meadow voles (Microtus pennsylvanicus) demonstrate same-sex partner preferences. J Comp Psychol. 2003;117(3):283–289. doi: 10.1037/0735-7036.117.3.283. [DOI] [PubMed] [Google Scholar]
- 204.Pedersen CA. Oxytocin control of maternal behavior regulation by sex steroids and offspring stimuli. Ann N Y Acad Sci. 1997;807:126–145. doi: 10.1111/j.1749-6632.1997.tb51916.x. [DOI] [PubMed] [Google Scholar]
- 205.Pedersen CA, Ascher JA, Monroe YL, Prange AJ. Oxytocin induces maternal behavior in virgin female rats. Science. 1982;216(4546):648–650. doi: 10.1126/science.7071605. [DOI] [PubMed] [Google Scholar]
- 206.Pedersen CA, Boccia ML. Oxytocin antagonism alters rat dams’ oral grooming and upright posturing over pups. Physiol Behav. 2003;80(2–3):233–241. doi: 10.1016/j.physbeh.2003.07.011. [DOI] [PubMed] [Google Scholar]
- 207.Pedersen CA, Boccia ML. Vasopressin interactions with oxytocin in the control of female sexual behavior. Neuroscience. 2006;139(3):843–851. doi: 10.1016/j.neuroscience.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 208.Pedersen CA, Caldwell JD, Walker C, Ayers G, Mason GA. Oxytocin activates the postpartum onset of rat maternal behavior in the ventral tegmental and medial preoptic areas. Behav Neurosci. 1994;108(6):1163–1171. doi: 10.1037//0735-7044.108.6.1163. [DOI] [PubMed] [Google Scholar]
- 209.Perkeybile AM, Griffin LL, Bales KL. Natural variation in early parental care correlates with social behaviors in adolescent prairie voles (Microtus ochrogaster) Front Behav Neurosci. 2013;7:21. doi: 10.3389/fnbeh.2013.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Petitte T, Mallow J, Barnes E, Petrone A, Barr T, Theeke L. A systematic review of loneliness and common chronic physical conditions in adults. Open Psychol J. 2015;8(Suppl 2):113–132. doi: 10.2174/1874350101508010113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Petrulis A. Chemosignals, hormones and mammalian reproduction. Horm Behav. 2013;63(5):723–741. doi: 10.1016/j.yhbeh.2013.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Peuler JD, Scotti M-AL, Phelps LE, McNeal N, Grippo AJ. Chronic social isolation in the prairie vole induces endothelial dysfunction: Implications for depression and cardiovascular disease. Physiol Behav. 2012;106(4):476–484. doi: 10.1016/j.physbeh.2012.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Phares V, Compas BE. The role of fathers in child and adolescent psychopathology: Make room for daddy. Psychol Bull. 1992;111(3):387–412. doi: 10.1037/0033-2909.111.3.387. [DOI] [PubMed] [Google Scholar]
- 214.Phelps SM, Young LJ. Extraordinary diversity in vasopressin (V1a) receptor distributions among wild prairie voles (Microtus ochrogaster): Patterns of variation and covariation. J Comp Neurol. 2003;466(4):564–576. doi: 10.1002/cne.10902. [DOI] [PubMed] [Google Scholar]
- 215.Pitkow LJ, Sharer CA, Ren X, Insel TR, Terwilliger EF, Young LJ. Facilitation of affiliation and pair-bond formation by vasopressin receptor gene transfer into the ventral forebrain of a monogamous vole. J Neurosci. 2001;21(18):7392–7396. doi: 10.1523/JNEUROSCI.21-18-07392.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Pournajafi-Nazarloo H, Kenkel W, Mohsenpour SR, Sanzenbacher L, Saadat H, Partoo L, Yee J, Azizi F, Carter CS. Exposure to chronic isolation modulates receptors mRNAs for oxytocin and vasopressin in the hypothalamus and heart. Peptides. 2013;43:20–26. doi: 10.1016/j.peptides.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 217.Pow DV, Morris JF. Dendrites of hypothalamic magnocellular neurons release neurohypophysial peptides by exocytosis. Neuroscience. 1989;32(2):435–439. doi: 10.1016/0306-4522(89)90091-2. [DOI] [PubMed] [Google Scholar]
- 218.Quattrocki E, Friston K. Autism, oxytocin and interoception. Neurosci Biobehav Rev. 2014;47:410–430. doi: 10.1016/j.neubiorev.2014.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Remage-Healey L, Adkins-Regan E, Romero LM. Behavioral and adrenocortical responses to mate separation and reunion in the zebra finch. Horm Behav. 2003;43(1):108–114. doi: 10.1016/s0018-506x(02)00012-0. [DOI] [PubMed] [Google Scholar]
- 220.Resendez SL, Aragona BJ. Aversive motivation and the maintenance of monogamous pair bonding. Rev Neurosci. 2013;24(1):51–60. doi: 10.1515/revneuro-2012-0068. [DOI] [PubMed] [Google Scholar]
- 221.Resendez SL, Dome M, Gormley G, Franco D, Nevárez N, Hamid AA, Aragona BJ. M-opioid receptors within subregions of the striatum mediate pair bond formation through parallel yet distinct reward mechanisms. J Neurosci. 2013;33(21):9140–9149. doi: 10.1523/JNEUROSCI.4123-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Resendez SL, Kuhnmuench M, Krzywosinski T, Aragona BJ. K-opioid receptors within the nucleus accumbens shell mediate pair bond maintenance. J Neurosci. 2012;32(20):6771–6784. doi: 10.1523/JNEUROSCI.5779-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Richard S, Zingg HH. The human oxytocin gene promoter is regulated by estrogens. J Biol Chem. 1990;265(11):6098–6103. [PubMed] [Google Scholar]
- 224.Rilling JK, Demarco AC, Hackett PD, Chen X, Gautam P, Stair S, Haroon E, Thompson R, Ditzen B, Patel R, Pagnoni G. Sex differences in the neural and behavioral response to intranasal oxytocin and vasopressin during human social interaction. Psychoneuroendocrinology. 2014;39:237–248. doi: 10.1016/j.psyneuen.2013.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Rilling JK, DeMarco AC, Hackett PD, Thompson R, Ditzen B, Patel R, Pagnoni G. Effects of intranasal oxytocin and vasopressin on cooperative behavior and associated brain activity in men. Psychoneuroendocrinology. 2012;37(4):447–461. doi: 10.1016/j.psyneuen.2011.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Rilling JK, Young LJ. The biology of mammalian parenting and its effect on offspring social development. Science. 2014;345(6198):771–776. doi: 10.1126/science.1252723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Roberts RL, Miller AK, Taymans SE, Carter CS. Role of social and endocrine factors in alloparental behavior of prairie voles (Microtus ochrogaster) Can J Zool. 1998;76(10):1862–1868. [Google Scholar]
- 228.Rodrigues SM, Saslow LR, Garcia N, John OP, Keltner D. Oxytocin receptor genetic variation relates to empathy and stress reactivity in humans. Proc Natl Acad Sci. 2009;106(50):21437–21441. doi: 10.1073/pnas.0909579106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Ross HE, Cole CD, Smith Y, Neumann ID, Landgraf R, Murphy AZ, Young LJ. Characterization of the oxytocin system regulating affiliative behavior in female prairie voles. Neuroscience. 2009;162(4):892–903. doi: 10.1016/j.neuroscience.2009.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Ross HE, Freeman SM, Spiegel LL, Ren X, Terwilliger EF, Young LJ. Variation in oxytocin receptor density in the nucleus accumbens has differential effects on affiliative behaviors in monogamous and polygamous voles. J Neurosci. 2009;29(5):1312–1318. doi: 10.1523/JNEUROSCI.5039-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Ross HE, Young LJ. Oxytocin and the neural mechanisms regulating social cognition and affiliative behavior. Front Neuroendocrinol. 2009;30(4):534–547. doi: 10.1016/j.yfrne.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Ruscio MG, Sweeny T, Hazelton J, Suppatkul P, Sue Carter C. Social environment regulates corticotropin releasing factor, corticosterone and vasopressin in juvenile prairie voles. Horm Behav. 2007;51(1):54–61. doi: 10.1016/j.yhbeh.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 233.Sanna F, Argiolas A, Melis MR. Oxytocin-induced yawning: Sites of action in the brain and interaction with mesolimbic/mesocortical and incertohypothalamic dopaminergic neurons in male rats. Horm Behav. 2012;62(4):505–514. doi: 10.1016/j.yhbeh.2012.08.010. [DOI] [PubMed] [Google Scholar]
- 234.Sarkadi A, Kristiansson R, Oberklaid F, Bremberg S. Fathers’ involvement and children’s developmental outcomes: A systematic review of longitudinal studies. Acta Pædiatrica. 2008;97(2):153–158. doi: 10.1111/j.1651-2227.2007.00572.x. [DOI] [PubMed] [Google Scholar]
- 235.Scheele D, Wille A, Kendrick KM, Stoffel-Wagner B, Becker B, Güntürkün O, Maier W, Hurlemann R. Oxytocin enhances brain reward system responses in men viewing the face of their female partner. Proc Natl Acad Sci U S A. 2013;110(50):20308–20313. doi: 10.1073/pnas.1314190110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Scotti M-AL, Carlton ED, Demas GE, Grippo AJ. Social isolation disrupts innate immune responses in both male and female prairie voles and enhances agonistic behavior in female prairie voles (Microtus ochrogaster) Horm Behav. 2015;70:7–13. doi: 10.1016/j.yhbeh.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Shahrestani S, Kemp AH, Guastella AJ. The impact of a single administration of intranasal oxytocin on the recognition of basic emotions in humans: a meta-analysis. Neuropsychopharmacol. 2013;38(10):1929–1936. doi: 10.1038/npp.2013.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Shahrokh DK, Zhang T-Y, Diorio J, Gratton A, Meaney MJ. Oxytocin-dopamine interactions mediate variations in maternal behavior in the rat. Endocrinology. 2010;151(5):2276–2286. doi: 10.1210/en.2009-1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Shamay-Tsoory SG, Abu-Akel A. The social salience hypothesis of oxytocin. Biol Psychiatry. 2016;79(3):194–202. doi: 10.1016/j.biopsych.2015.07.020. [DOI] [PubMed] [Google Scholar]
- 240.Shamay-Tsoory SG, Fischer M, Dvash J, Harari H, Perach-Bloom N, Levkovitz Y. Intranasal administration of oxytocin increases envy and schadenfreude (gloating) Biol Psychiatry. 2009;66(9):864–870. doi: 10.1016/j.biopsych.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 241.Shapiro LE, Dewsbury DA. Differences in affiliative behavior, pair bonding, and vaginal cytology in two species of vole (Microtus ochrogaster and M. montanus) J Comp Psychol. 1990;104(3):268–274. doi: 10.1037/0735-7036.104.3.268. [DOI] [PubMed] [Google Scholar]
- 242.Shear MK. Complicated grief. N Engl J Med. 2015;372(2):153–160. doi: 10.1056/NEJMcp1315618. [DOI] [PubMed] [Google Scholar]
- 243.Skuse DH, Lori A, Cubells JF, Lee I, Conneely KN, Puura K, Lehtimäki T, Binder EB, Young LJ. Common polymorphism in the oxytocin receptor gene (OXTR) is associated with human social recognition skills. Proc Natl Acad Sci U S A. 2014;111(5):1987–1992. doi: 10.1073/pnas.1302985111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Smeltzer MD, Curtis JT, Aragona BJ, Wang Z. Dopamine, oxytocin, and vasopressin receptor binding in the medial prefrontal cortex of monogamous and promiscuous voles. Neurosci Lett. 2006;394(2):146–151. doi: 10.1016/j.neulet.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 245.Smith AS, Lieberwirth C, Wang Z. Behavioral and physiological responses of female prairie voles (Microtus ochrogaster) to various stressful conditions. Stress Amst Neth. 2013;16(5):531–539. doi: 10.3109/10253890.2013.794449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Smith AS, Tabbaa M, Lei K, Eastham P, Butler MJ, Linton L, Altshuler R, Liu Y, Wang Z. Local oxytocin tempers anxiety by activating GABAa receptors in the hypothalamic paraventricular nucleus. Psychoneuroendocrinology. 2015;63:50–58. doi: 10.1016/j.psyneuen.2015.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Smith AS, Wang Z. Salubrious effects of oxytocin on social stress-induced deficits. Horm Behav. 2012;61(3):320–330. doi: 10.1016/j.yhbeh.2011.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Smith AS, Wang Z. Hypothalamic oxytocin mediates social buffering of the stress response. Biol Psychiatry. 2014;76(4):281–288. doi: 10.1016/j.biopsych.2013.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Song Z, McCann KE, McNeill JK, Larkin TE, Huhman KL, Albers HE. Oxytocin induces social communication by activating arginine-vasopressin V1a receptors and not oxytocin receptors. Psychoneuroendocrinology. 2014;50:14–19. doi: 10.1016/j.psyneuen.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Steptoe A, Owen N, Kunz-Ebrecht SR, Brydon L. Loneliness and neuroendocrine, cardiovascular, and inflammatory stress responses in middle-aged men and women. Psychoneuroendocrinology. 2004;29(5):593–611. doi: 10.1016/S0306-4530(03)00086-6. [DOI] [PubMed] [Google Scholar]
- 251.Stolzenberg DS, Champagne FA. Hormonal and non-hormonal bases of maternal behavior: The role of experience and epigenetic mechanisms. Horm Behav. 2015;77:204–210. doi: 10.1016/j.yhbeh.2015.07.005. [DOI] [PubMed] [Google Scholar]
- 252.Stoop R. Neuromodulation by oxytocin and vasopressin in the central nervous system as a basis for their rapid behavioral effects. Curr Opin Neurobiol. 2014;29:187–193. doi: 10.1016/j.conb.2014.09.012. [DOI] [PubMed] [Google Scholar]
- 253.Striepens N, Matusch A, Kendrick KM, Mihov Y, Elmenhorst D, Becker B, Lang M, Coenen HH, Maier W, Hurlemann R, Bauer A. Oxytocin enhances attractiveness of unfamiliar female faces independent of the dopamine reward system. Psychoneuroendocrinology. 2014;39:74–87. doi: 10.1016/j.psyneuen.2013.09.026. [DOI] [PubMed] [Google Scholar]
- 254.Succu S, Sanna F, Cocco C, Melis T, Boi A, Ferri G-L, Argiolas A, Melis MR. Oxytocin induces penile erection when injected into the ventral tegmental area of male rats: Role of nitric oxide and cyclic gmp. Eur J Neurosci. 2008;28(4):813–821. doi: 10.1111/j.1460-9568.2008.06385.x. [DOI] [PubMed] [Google Scholar]
- 255.Sun P, Smith AS, Lei K, Liu Y, Wang Z. Breaking bonds in male prairie vole: Long-term effects on emotional and social behavior, physiology, and neurochemistry. Behav Brain Res. 2014;265:22–31. doi: 10.1016/j.bbr.2014.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Swain JE, Kim P, Spicer J, Ho SS, Dayton CJ, Elmadih A, Abel KM. Approaching the biology of human parental attachment: Brain imaging, oxytocin and coordinated assessments of mothers and fathers. Brain Res. 2014;1580:78–101. doi: 10.1016/j.brainres.2014.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Swanson LW, Sawchenko PE. Hypothalamic integration: Organization of the paraventricular and supraoptic nuclei. Annu Rev Neurosci. 1983;6:269–324. doi: 10.1146/annurev.ne.06.030183.001413. [DOI] [PubMed] [Google Scholar]
- 258.Tabak BA, Meyer ML, Castle E, Dutcher JM, Irwin MR, Han JH, Lieberman MD, Eisenberger NI. Vasopressin, but not oxytocin, increases empathic concern among individuals who received higher levels of paternal warmth: A randomized controlled trial. Psychoneuroendocrinology. 2015;51:253–261. doi: 10.1016/j.psyneuen.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Thomas JA, Birney EC. Parental care and mating system of the prairie vole. Microtus ochrogaster Behav Ecol Sociobiol. 1979;5(2):171–186. [Google Scholar]
- 260.Tobin VA, Hashimoto H, Wacker DW, Takayanagi Y, Langnaese K, Caquineau C, Noack J, Landgraf R, Onaka T, Leng G, Meddle SL, Engelmann M, Ludwig M. An intrinsic vasopressin system in the olfactory bulb is involved in social recognition. Nature. 2010;464(7287):413–417. doi: 10.1038/nature08826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Tops M, van Peer JM, Korf J, Wijers AA, Tucker DM. Anxiety, cortisol, and attachment predict plasma oxytocin. Psychophysiology. 2007;44(3):444–449. doi: 10.1111/j.1469-8986.2007.00510.x. [DOI] [PubMed] [Google Scholar]
- 262.van den Pol AN. The magnocellular and parvocellular paraventricular nucleus of rat: Intrinsic organization. J Comp Neurol. 1982;206(4):317–345. doi: 10.1002/cne.902060402. [DOI] [PubMed] [Google Scholar]
- 263.van Wimersma Greidanus TB, Maigret C. The role of limbic vasopressin and oxytocin in social recognition. Brain Res. 1996;713(1–2):153–159. doi: 10.1016/0006-8993(95)01505-1. [DOI] [PubMed] [Google Scholar]
- 264.Veenema AH, Beiderbeck DI, Lukas M, Neumann ID. Distinct correlations of vasopressin release within the lateral septum and the bed nucleus of the stria terminalis with the display of intermale aggression. Horm Behav. 2010;58(2):273–281. doi: 10.1016/j.yhbeh.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 265.Waldherr M, Neumann ID. Centrally released oxytocin mediates mating-induced anxiolysis in male rats. Proc Natl Acad Sci U S A. 2007;104(42):16681–16684. doi: 10.1073/pnas.0705860104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Waltz M, Badura B, Pfaff H, Schott T. Marriage and the psychological consequences of a heart attack: A longitudinal study of adaptation to chronic illness after 3 years. Soc Sci Med. 1988;27(2):149–158. doi: 10.1016/0277-9536(88)90323-1. [DOI] [PubMed] [Google Scholar]
- 267.Walum H, Waldman ID, Young LJ. Statistical and methodological considerations for the interpretation of intranasal oxytocin studies. Biol Psychiatry. 2016;79(3):251–257. doi: 10.1016/j.biopsych.2015.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Wang B, Li Y, Wu R, Zhang S, Tai F. Behavioral responses to pups in males with different reproductive experiences are associated with changes in central ot, th and otr, d1r, d2r mRNA expression in mandarin voles. Horm Behav. 2015;67:73–82. doi: 10.1016/j.yhbeh.2014.11.013. [DOI] [PubMed] [Google Scholar]
- 269.Wang H, Duclot F, Liu Y, Wang Z, Kabbaj M. Histone deacetylase inhibitors facilitate partner preference formation in female prairie voles. Nat Neurosci. 2013;16(7):919–924. doi: 10.1038/nn.3420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Wang Z. Species differences in the vasopressin-immunoreactive pathways in the bed nucleus of the stria terminalis and medial amygdaloid nucleus in prairie voles (Microtus ochrogaster) and meadow voles (Microtus pennsylvanicus) Behav Neurosci. 1995;109(2):305–311. doi: 10.1037//0735-7044.109.2.305. [DOI] [PubMed] [Google Scholar]
- 271.Wang Z, Aragona BJ. Neurochemical regulation of pair bonding in male prairie voles. Physiol Behav. 2004;83(2):319–328. doi: 10.1016/j.physbeh.2004.08.024. [DOI] [PubMed] [Google Scholar]
- 272.Wang Z, Ferris CF, De Vries GJ. Role of septal vasopressin innervation in paternal behavior in prairie voles (Microtus ochrogaster) Proc Natl Acad Sci U S A. 1994;91(1):400–404. doi: 10.1073/pnas.91.1.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Wang Z, Hulihan TJ, Insel TR. Sexual and social experience is associated with different patterns of behavior and neural activation in male prairie voles. Brain Res. 1997;767(2):321–332. doi: 10.1016/s0006-8993(97)00617-3. [DOI] [PubMed] [Google Scholar]
- 274.Wang Z, Insel T. Parental Care: Evolution, Mechanisms, And Adaptive Significance. San Diego, CA: Academic Press; 1996. p. 737. [Google Scholar]
- 275.Wang Z, Novak MA. Influence of the social environment on parental behavior and pup development of meadow voles (Microtus pennsylvanicus) and prairie voles (M. ochrogaster) J Comp Psychol. 1992;106(2):163–171. [Google Scholar]
- 276.Wang Z, Novak MA. Alloparental care and the influence of father presence on juvenile prairie voles, Microtus ochrogaster. Anim Behav. 1994;47(2):281–288. [Google Scholar]
- 277.Wang Z, Smith W, Major DE, De Vries GJ. Sex and species differences in the effects of cohabitation on vasopressin messenger RNA expression in the bed nucleus of the stria terminalis in prairie voles (Microtus ochrogaster) and meadow voles (Microtus pennsylvanicus) Brain Res. 1994;650(2):212–218. doi: 10.1016/0006-8993(94)91784-1. [DOI] [PubMed] [Google Scholar]
- 278.Wang Z, Young LJ, Liu Y, Insel TR. Species differences in vasopressin receptor binding are evident early in development: Comparative anatomic studies in prairie and montane voles. J Comp Neurol. 1997;378(4):535–546. [PubMed] [Google Scholar]
- 279.Wang Z, Zhou L, Hulihan TJ, Insel TR. Immunoreactivity of central vasopressin and oxytocin pathways in microtine rodents: A quantitative comparative study. J Comp Neurol. 1996;366(4):726–737. doi: 10.1002/(SICI)1096-9861(19960318)366:4<726::AID-CNE11>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 280.Wang ZX, Liu Y, Young LJ, Insel TR. Hypothalamic vasopressin gene expression increases in both males and females postpartum in a biparental rodent. J Neuroendocrinol. 2000;12(2):111–120. doi: 10.1046/j.1365-2826.2000.00435.x. [DOI] [PubMed] [Google Scholar]
- 281.Williams JR, Catania KC, Carter CS. Development of partner preferences in female prairie voles (Microtus ochrogaster): The role of social and sexual experience. Horm Behav. 1992;26(3):339–349. doi: 10.1016/0018-506x(92)90004-f. [DOI] [PubMed] [Google Scholar]
- 282.Williams JR, Insel TR, Harbaugh CR, Carter CS. Oxytocin administered centrally facilitates formation of a partner preference in female prairie voles (Microtus ochrogaster) J Neuroendocrinol. 1994;6(3):247–250. doi: 10.1111/j.1365-2826.1994.tb00579.x. [DOI] [PubMed] [Google Scholar]
- 283.Winslow JT, Hastings N, Carter CS, Harbaugh CR, Insel TR. A role for central vasopressin in pair bonding in monogamous prairie voles. Nature. 1993;365(6446):545–548. doi: 10.1038/365545a0. [DOI] [PubMed] [Google Scholar]
- 284.Wu N, Li Z, Su Y. The association between oxytocin receptor gene polymorphism (OXTR) and trait empathy. J Affect Disord. 2012;138(3):468–472. doi: 10.1016/j.jad.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 285.Wu N, Shang S, Su Y. The arginine vasopressin v1b receptor gene and prosociality: Mediation role of emotional empathy. PsyCh J. 2015;4(3):160–165. doi: 10.1002/pchj.102. [DOI] [PubMed] [Google Scholar]
- 286.Yamamoto Y, Carter CS, Cushing BS. Neonatal manipulation of oxytocin affects expression of estrogen receptor alpha. Neuroscience. 2006;137(1):157–164. doi: 10.1016/j.neuroscience.2005.08.065. [DOI] [PubMed] [Google Scholar]
- 287.Yamamoto Y, Cushing BS, Kramer KM, Epperson PD, Hoffman GE, Carter CS. Neonatal manipulations of oxytocin alter expression of oxytocin and vasopressin immunoreactive cells in the paraventricular nucleus of the hypothalamus in a gender-specific manner. Neuroscience. 2004;125(4):947–955. doi: 10.1016/j.neuroscience.2004.02.028. [DOI] [PubMed] [Google Scholar]
- 288.Young KA, Gobrogge KL, Liu Y, Wang Z. The neurobiology of pair bonding: Insights from a socially monogamous rodent. Front Neuroendocrinol. 2011;32(1):53–69. doi: 10.1016/j.yfrne.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Young KA, Liu Y, Gobrogge KL, Wang H, Wang Z. Oxytocin reverses amphetamine-induced deficits in social bonding: Evidence for an interaction with nucleus accumbens dopamine. J Neurosci. 2014;34(25):8499–8506. doi: 10.1523/JNEUROSCI.4275-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Young LJ. Oxytocin and vasopressin receptors and species-typical social behaviors. Horm Behav. 1999;36(3):212–221. doi: 10.1006/hbeh.1999.1548. [DOI] [PubMed] [Google Scholar]
- 291.Young LJ, Barrett CE. Can oxytocin treat autism? Science. 2015;347(6224):825–826. doi: 10.1126/science.aaa8120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Young LJ, Huot B, Nilsen R, Wang Z, Insel TR. Species differences in central oxytocin receptor gene expression: Comparative analysis of promoter sequences. J Neuroendocrinol. 1996;8(10):777–783. doi: 10.1046/j.1365-2826.1996.05188.x. [DOI] [PubMed] [Google Scholar]
- 293.Young LJ, Lim MM, Gingrich B, Insel TR. Cellular mechanisms of social attachment. Horm Behav. 2001;40(2):133–138. doi: 10.1006/hbeh.2001.1691. [DOI] [PubMed] [Google Scholar]
- 294.Young LJ, Murphy Young AZ, Hammock EAD. Anatomy and neurochemistry of the pair bond. J Comp Neurol. 2005;493(1):51–57. doi: 10.1002/cne.20771. [DOI] [PubMed] [Google Scholar]
- 295.Young LJ, Nilsen R, Waymire KG, MacGregor GR, Insel TR. Increased affiliative response to vasopressin in mice expressing the V1a receptor from a monogamous vole. Nature. 1999;400(6746):766–768. doi: 10.1038/23475. [DOI] [PubMed] [Google Scholar]
- 296.Young LJ, Wang Z. The neurobiology of pair bonding. Nat Neurosci. 2004;7(10):1048–1054. doi: 10.1038/nn1327. [DOI] [PubMed] [Google Scholar]
- 297.Young LJ, Wang Z, Insel TR. Neuroendocrine bases of monogamy. Trends Neurosci. 1998;21(2):71–75. doi: 10.1016/s0166-2236(97)01167-3. [DOI] [PubMed] [Google Scholar]
- 298.Young LJ, Winslow JT, Nilsen R, Insel TR. Species differences in V1a receptor gene expression in monogamous and nonmonogamous voles: Behavioral consequences. Behav Neurosci. 1997;111(3):599–605. doi: 10.1037//0735-7044.111.3.599. [DOI] [PubMed] [Google Scholar]
- 299.Zahed SR, Kurian AV, Snowdon CT. Social dynamics and individual plasticity of infant care behavior in cooperatively breeding cotton-top tamarins. Am J Primatol. 2010;72(4):296–306. doi: 10.1002/ajp.20782. [DOI] [PubMed] [Google Scholar]
- 300.Zheng J-J, Li S-J, Zhang X-D, Miao W-Y, Zhang D, Yao H, Yu X. Oxytocin mediates early experience-dependent cross-modal plasticity in the sensory cortices. Nat Neurosci. 2014;17(3):391–399. doi: 10.1038/nn.3634. [DOI] [PubMed] [Google Scholar]