Summary
Astrocytes extend highly branched processes that form functionally isolated microdomains, facilitating local homeostasis by redistributing ions, removing neurotransmitters and releasing factors to influence blood flow and neuronal activity. Microdomains exhibit spontaneous increases in calcium (Ca2+), but the mechanisms and functional significance of this localized signaling are unknown. By developing conditional, membrane anchored GCaMP3 mice, we found that microdomain activity that occurs in the absence of IP3-dependent release from endoplasmic reticulum arises through Ca2+ efflux from mitochondria during brief openings of the mitochondrial permeability transition pore. These microdomain Ca2+ transients were facilitated by the production of reactive oxygen species during oxidative phosphorylation and enhanced by expression of a mutant form of superoxide dismutase 1 (SOD1 G93A) that causes astrocyte dysfunction and neurodegeneration in amyotrophic lateral sclerosis (ALS). By localizing mitochondria to microdomains, astrocytes ensure local metabolic support for energetically demanding processes and enable coupling between metabolic demand and Ca2+ signaling events.
Introduction
Astrocytes are ubiquitous glial cells in the central nervous system that are highly conserved in both form and function from flies to mammals (Freeman and Rowitch, 2013). They are distributed in a grid-like manner, with cells spaced equidistant from one another, and extend highly ramified processes into the neuropil, forming a dense meshwork in which neurons, blood vessels and other glial cells are embedded (Kosaka and Hama, 1986). Astrocyte processes consist of thin lamellar sheets that are responsible for performing essential homeostatic processes that consume considerable energy, such as restoring ion gradients, removing neurotransmitters and refining neural networks. These membranous elaborations are often coupled to the rest of the cell by narrow cytoplasmic channels, creating functionally isolated “microdomains” that may restrict diffusion and allow astrocytes to adapt their physiology to local demands (Grosche et al., 1999). Despite the crucial importance of astrocyte microdomains in brain homeostasis, we know little about their functional characteristics or the dynamics of signaling in these restricted volumes, information that is essential to define how astrocytes contribute to brain function and dysfunction.
Because of their wide distribution and complex morphology, astrocytes are ideally situated to provide both global support of neural circuits and exert local control over active synapses (Araque et al., 2014). They express a variety of neurotransmitter receptors, in particular, metabotropic receptors that induce transient increases in intracellular Ca2+. In vivo studies in awake mice indicate that astrocytes throughout the brain experience Ca2+ increases when norepinephrine (NE) is released during periods of increased arousal, indicating that they are co-activated with neurons during state transitions (Paukert et al., 2014). In addition to these global changes in activity, astrocytes also exhibit spontaneous increases in Ca2+ within microdomains that appear to be cell autonomous (Khakh and McCarthy, 2015), as they occur in the absence of neuronal input and persist when astrocytes are maintained in isolation (Nett et al., 2002). Ca2+ changes in astrocytes can enhance glucose mobilization, alter hemodynamics, and influence the activity of surrounding neurons and glia by releasing neuroactive compounds such as ATP, D-serine, and glutamate (Haydon, 2001). In addition, astrocyte Ca2+ transients occur more frequently following CNS injury and are enhanced in regions of amyloid deposition in Alzheimer’s model mice (Kuchibhotla et al., 2009), demonstrating that this signaling is highly adaptable and dependent on the activity level of surrounding neurons. Nevertheless, the mechanisms that control Ca2+ signaling in astrocyte microdomains have remained uncertain and their relationship to receptor-mediated Ca2+ transients have not been defined.
To determine the mechanisms responsible for inducing localized Ca2+ signaling in astrocytes, we developed conditional transgenic mice in which a membrane anchored form of the genetically encoded Ca2+ sensor GCaMP3 could be expressed selectively within astrocytes, and software based on machine learning for unbiased selection and analysis of active domains. We show that spatially restricted Ca2+ transients in astrocyte processes that occur independent of release from ER stores are caused by Ca2+ efflux from mitochondria, in response to transient opening of the permeability transition pore (mPTP).
Results
Generation of conditional, membrane anchored GCaMP3 mice
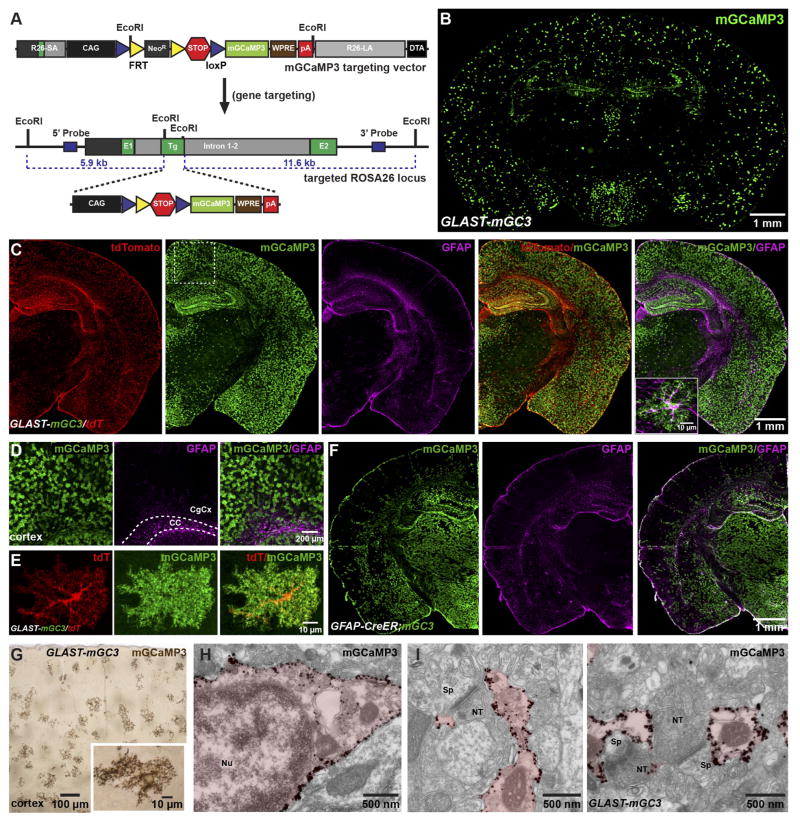
Astrocyte processes extend thin lamellar sheets that contain minimal cytoplasm presenting challenges for detection of Ca2+ changes with cytosolic indicators. To facilitate analysis of microdomain Ca2+ signaling in situ, we generated a transgenic mouse line in which a Cre-dependent allele encoding a membrane anchored variant of the genetically encoded calcium indicator GCaMP3 (Tian et al., 2009) (mGCaMP3) was knocked into the Rosa26 locus (Figure 1A) (see Methods). To express mGCaMP3 specifically in astrocytes, these Rosa26-lsl-mGCaMP3 mice were bred to GLAST-CreER BAC transgenic mice (Paukert et al., 2014) and mGCaMP3 expression was induced by injecting tamoxifen intraperitonealy (IP). Immunohistochemical analysis of GLAST-CreER;Rosa26-lsl-mGCaMP3 (GLAST-mGC3) mice several weeks after tamoxifen injection revealed that mGCaMP3 was expressed by astrocytes throughout the brain (Figure 1B, C); by lowering the dose of tamoxifen, sparse mGCaMP3 expression could be achieved, allowing unambiguous analysis of individual cells (Figure 1D, E). Although minimal recombination was observed in subcortical regions (Figure 1B, C), this pattern reflects low CreER expression by astrocytes, rather than differences in ability to express mGCaMP3, as mGCaMP3 was expressed by astrocytes in these regions when Rosa26-lsl-mGCaMP3 mice were bred to GFAP-CreER mice (Figure 1F). Postnatal induction of mGCaMP3 expression in these lines did not appear to induce reactive changes in astrocytes, as GFAP expression was not increased in the cortex (Figure 1C, D, F).
Figure 1. Conditional expression of mGCaMP3 in astrocytes.
(A) Gene trap strategy used to insert conditional mGCaMP3 transgene into murine Rosa26 gene. (Top) mGCaMP3 targeting vector which harbors 5′ short (R26-SA) and 3′ long (R26-LA) homology arms from Rosa26, the CMV enhancer chicken β-actin hybrid (CAG) promoter (black box), loxP (blue triangles) flanked neomycin resistance cassette (NeoR, gray box) flanked by two FRT sites (yellow triangles) and 3x SV40-polyA sequence (red hexagon), the mGCaMP3 cDNA (light green box), woodchuck post-transcriptional response element (WPRE, brown box), bovine growth hormone poly A sequence (red box) and negative selection cassette with diphtheria toxin fragment A (DTA, black box). (Bottom) Rosa26 allele with conditional mGCaMP3 cassette after homologous recombination. NeoR cassette was removed by in vivo site-specific recombination.
(B) Coronal section of brain from a GLAST-CreER;Rosa26-lsl-mGCaMP3 (GLAST-mGC3) mouse immunostained for mGCaMP3.
(C) Coronal hemi-section of brain from a GLAST-CreER;Rosa26-lsl-mGCaMP3;Rosa26-lsl-tdTomato (GLAST-mGC3/tdT) mouse immunostained for tdTomato, mGCaMP3 and GFAP. Inset shows one cortical astrocyte at higher magnification co-expressing mGCaMP3 and GFAP.
(D) High magnification images from boxed area in (C). CC: corpus callosum, CgCx: cingulate cortex.
(E) Images showing one cortical astrocyte from a GLAST-mGC3/tdT mouse (maximum intensity projected confocal z-stack) immunostained for mGCaMP3 and tdT.
(F) Coronal hemi-section of brain from a GFAP-CreER;Rosa26-lsl-mGCaMP3 (GFAP-CreER;mGC3) mouse immunostained for mGCaMP3 and GFAP.
(G) Coronal section of brain from a GLAST-mGC3 mouse showing silver intensified immunogold labeling of mGCaMP3. Inset shows one astrocyte at higher magnification.
(H, I) High magnification images of silver intensified immunogold labeling of mGCaMP3 in an astrocyte soma (H) and peri-synaptic processes (I) from GLAST-mGC3 mice. NT: nerve terminal, Nu: nucleus, Sp: spine.
To determine if mGCaMP3 is targeted to the plasma membrane and present in the fine processes of astrocytes, GLAST-mGC3 mice were crossed to Rosa26-lsl-tdTomato (tdT) (Ai14) mice, allowing visualization of the astrocyte cytosol (Figure 1C). Immunocytochemical analysis revealed a striking difference in the pattern of tdTomato (tdT) and EGFP expression within astrocytes: TdT was present in the soma and main branches of astrocyte processes, whereas mGCaMP3 outlined a larger volume of the cell and enhanced visualization of fine processes (Figure 1E). Electron microscopic (EM) analysis using silver-enhanced immunogold labeling (Figure 1G–I) revealed that mGCaMP3 was localized to the plasma membrane (Figure 1H) and present within thin lamellar extensions of astrocytes that ensheath synapses (Figure 1I), indicating that this genetic strategy can be used to localize a Ca2+ sensor within the finest processes of astrocytes.
Semi-automatic analysis of microdomain Ca2+ transients
Astrocytes exhibit global increases in intracellular Ca2+ in response to stimulation of metabotropic receptors, as well as spatially restricted, apparently spontaneous Ca2+ transients (Nett et al., 2002), the origin of which has not been determined. To assess whether mGCaMP3 is able to resolve these different signals, time lapse confocal imaging of individual cortical astrocytes was performed in acute brain slices from adult GLAST-mGC3 (Figure S1A, B) and GLAST-mGC3/tdT (Figure S1D–G) mice. Astrocytes exhibited spontaneous increases in mGCaMP3 fluorescence that were spatially restricted to discrete domains (Figure S1A–C and Movie S1), and exposure of slices to agonists for metabotropic glutamate (DHPG, 20 μM) or adrenergic receptors (norepinephrine, NE, 10 μM), applied in tetrodotoxin (TTX, 1 μM) to minimize neuronal activation, enhanced Ca2+ transients in astrocytes. This activity was qualitatively similar to that observed with Ca2+ indicator dyes or through viral delivery of GCaMPs (Di Castro et al., 2011; Shigetomi et al., 2013).
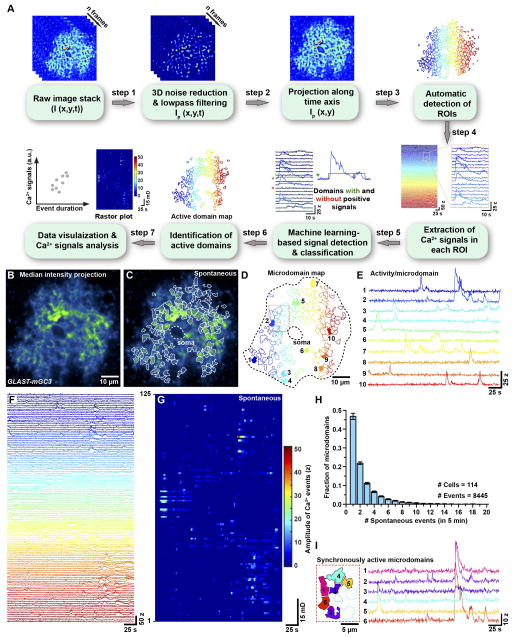
When imaging individual astrocytes with mGCaMP3, Ca2+ transients were observed in up to 100 distinct microdomains within the imaging volume, creating new challenges for unbiased analysis of cellular activity based on user-selected regions of interest. To define active regions and extract information about Ca2+ dynamics from each location, we developed custom software to perform semiautomated analysis of fluorescence changes (Figure 2A) (see Methods and Data S1). A key feature of this program involves identification of active regions using a machine-learning based algorithm. We named this software CaSCaDe (Ca2+ Signal Classification and Decoding), as each analysis step is dependent on the outcome of the previous step. The CaSCaDe analysis generates a spatial map of regions that exhibit dynamic changes in fluorescence (Figure 2B–D and Movie S2) and provides information about the number, frequency, amplitude (z-score, see Methods) and time course of events (Figure 2E–G and Figure S1D–P). Analysis of 74 cortical astrocytes revealed that microdomain Ca2+ transients occurred frequently throughout the cell (0.67 ± 0.38 Hz), encompassing many distinct sites during a five minute imaging period (average: 75 ± 30 microdomains). However, activity at each site was infrequent (0.5 ± 0.1 events/min/microdomain), with 69% of microdomains exhibiting only 1–2 events (Figure 2H), and often uncorrelated with activity at other sites (mean correlation coefficient (ρ), 0.02 ± 0.032), even when active regions were separated by < 10 μm (Figure 2I), consistent with the hypothesis that microdomains are functionally isolated (Grosche et al., 1999). Domains that exhibited independent activity could also exhibit highly correlated transients (Figure 2I), suggesting that there are multiple modes of elevating Ca2+ within astrocyte processes. Microdomain transients exhibited diverse waveforms, even at individual sites (Figure 2E–G), and slow kinetics (average duration: 8.5 ± 1.7 s). Although it has been reported that some Ca2+ transients in astrocytes are shorter in duration (650 – 750 ms) (Di Castro et al., 2011), we did not observe such rapid events, even when the sampling rate was increased to 15 Hz (Figure S2A–H).
Figure 2. Quantification of spontaneous microdomain Ca2+ transients in cortical astrocytes (See also Figure S1, Movie S1, S2 and Data S1).
(A) Image analysis based on CaSCaDe (Ca2+ Signal Classification and Decoding) algorithm.
(B) Image of one astrocyte from a GLAST-mGC3 mouse. Median intensity projection (pseudocolored) from 540 frames.
(C, D) Maps showing 125 spontaneously active microdomains that occurred in 260 s identified using the CaSCaDe algorithm. (C) All microdomains overlaid on median intensity projected image of astrocyte in (B). (D) Color-coded microdomain map used to uniquely identify each active region. Dashed line indicates cell border.
(E) Intensity versus time traces for 10 microdomains (from 125) (colors correspond to locations shown in D), showing characteristics of spontaneous Ca2+ transients in individual microdomains.
(F) Intensity versus time traces for 125 spontaneously active microdomains.
(G) Raster plot of microdomain activity, color-coded according to fluorescence change (z-score).
(H) Histogram illustrating the number of events that occurred per microdomain in five minutes. Data shown as mean ± SEM. 8445 events were analyzed from 114 cells.
(I) Spatial map and intensity versus time traces for six adjacent microdomains (area highlighted by red box in D) showing that they can exhibit both uncorrelated and correlated spontaneous activity.
Neuromodulators promote synchronous Ca2+ elevation in microdomains
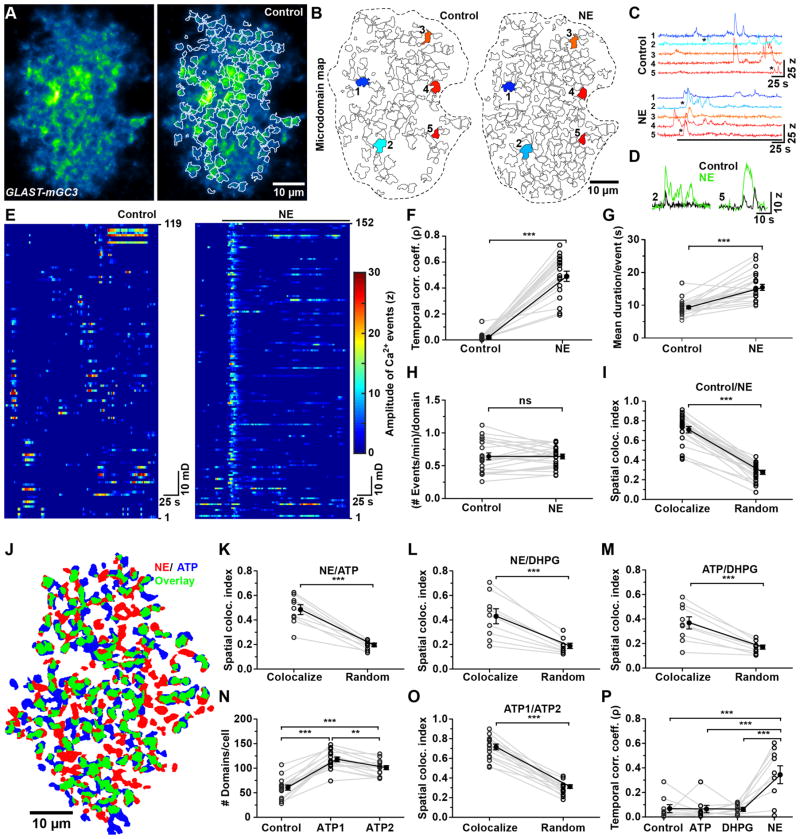
Neurotransmitters, such as NE that induce Ca2+ release from intracellular stores had a profound effect on Ca2+ levels in microdomains (Figure 3A, B and Movie S2). NE promoted a near-synchronous rise in Ca2+ in microdomains (mean correlation coefficient (ρ), 0.49 ± 0.16) (Figure 3C–F and Movie S2), increased the number of active microdomains per cell by 46% and enhanced the duration of events at each site by 65% (Figure 3G), but did not increase the frequency of transients within each domain (Figure 3H and Figure S2I). Regions experiencing Ca2+ elevations also changed their shape in response to adrenergic receptor stimulation (Figure 3B); nevertheless, for domains that were active during the control period, the site of maximal intensity (centroid) was the same in NE, suggesting that microdomain activity can be influenced by both cell surface receptor activation and cell intrinsic processes. Unexpectedly, not all spontaneously active microdomains were activated by NE (71 ± 15 % overlap, Figure 3I), and stimulation of metabotropic glutamate receptors (DHPG, 10 μM) or purinergic receptors (ATP, 100 μM) produced activity maps that were distinct from NE (Figure 3J–M). Indeed, only 50% of microdomains that were activated by various agonists overlapped. This could not be explained by trial-to-trial variability, as repetitive application of the same agonist (ATP) resulted in 71% overlap in active domains, despite the small rundown in ATP response to the second stimulus (Figure 3N, O). Moreover, only NE was able to induce strong temporal correlation among domains (Figure 3P), suggesting that microdomains are physiologically distinct, contain different combinations of receptors and can experience different modes of Ca2+ increases.
Figure 3. Neurotransmitters activate distinct microdomains in astrocyte processes (See also Figure S2 and Movie S2).
(A) Median intensity projection image (pseudocolored) of 540 frames from one astrocyte from a GLAST-mGC3 mouse (left). Map of spontaneously active microdomains (in TTX (1 μM)) overlaid on image (right).
(B) Map of microdomains recorded in control (119 domains, left) and after application of norepinephrine (0.5 μM NE, 152 domains, right). Dashed lines indicate cell border.
(C) Intensity versus time traces for five microdomains (corresponding to colors in B), showing characteristics of Ca2+ transients in control and following application of NE.
(D) Examples of microdomain Ca2+ transients from traces #2 and #5 (asterisks in C).
(E) Raster plots displaying timing and intensity of Ca2+ transients (119 microdomains in control, left, and 152 microdomains in NE, right).
(F) Graph of change in temporal correlation of Ca2+ transients among microdomains induced by NE (N = 24 cells, GLAST-mGC3 mice). Data shown as mean ± SEM. *** p < 0.0001 and * p = 0.01, paired two-tailed Student’s t-test.
(G – I) Graphs showing mean duration per event (G), frequency of events per domain (H) and spatial co-localization index (I) in control (+ TTX) and NE (+TTX). Data shown as mean ± SEM (n = 18 cells, GLAST-mGC3 mice). *** p < 0.0001, paired two-tailed Student’s t-test.
(J) Map showing distribution of active microdomains recorded after application of ATP (blue) and NE (red). Microdomains that exhibited activity in both conditions shown in green.
(K – M) Graphs comparing co-localization between microdomains recorded in NE (+ TTX) and ATP (+ TTX) (K), NE (+ TTX) and DHPG (+ TTX) (L), ATP (+ TTX) and DHPG (+ TTX) (M). After randomization, the spatial co-localization of microdomains between two conditions was significantly reduced. Data shown as mean ± SEM. N = 9 cells from GLAST-mGC3 mice. *** p < 0.0004, paired two-tailed Student’s t-test.
(N) Graph of number of active domains per cell in control and during two successive applications of ATP (ATP1, ATP2). Data shown as mean ± SEM. N = 15 cells from GLAST-mGC3 mice. *** p < 0.0001 and ** p < 0.001 repeated measure one-way ANOVA analysis with Tukey’s multiple comparisons post hoc test.
(O) Graph of spatial co-localization between active microdomains during two ATP applications. All experiments were done in the presence of TTX. After randomization, the spatial co-localization of microdomains between two conditions was significantly reduced. Data shown as mean ± SEM. N = 15 cells from GLAST-mGC3 mice. *** p < 0.0001, paired two-tailed Student’s t-test.
(P) Temporal correlation of Ca2+ transients among microdomains recorded during baseline (Control) and in the presence of metabotropic receptor agonists (ATP, DHPG, and NE; all in TTX). Data shown as mean ± SEM. N = 9 cells from GLAST-mGC3 mice. *** p = 0.0002, Repeated measures one-way ANOVA with Tukey’s Multiple Comparison Test.
Microdomain Ca2+ transients can arise through cell intrinsic mechanisms
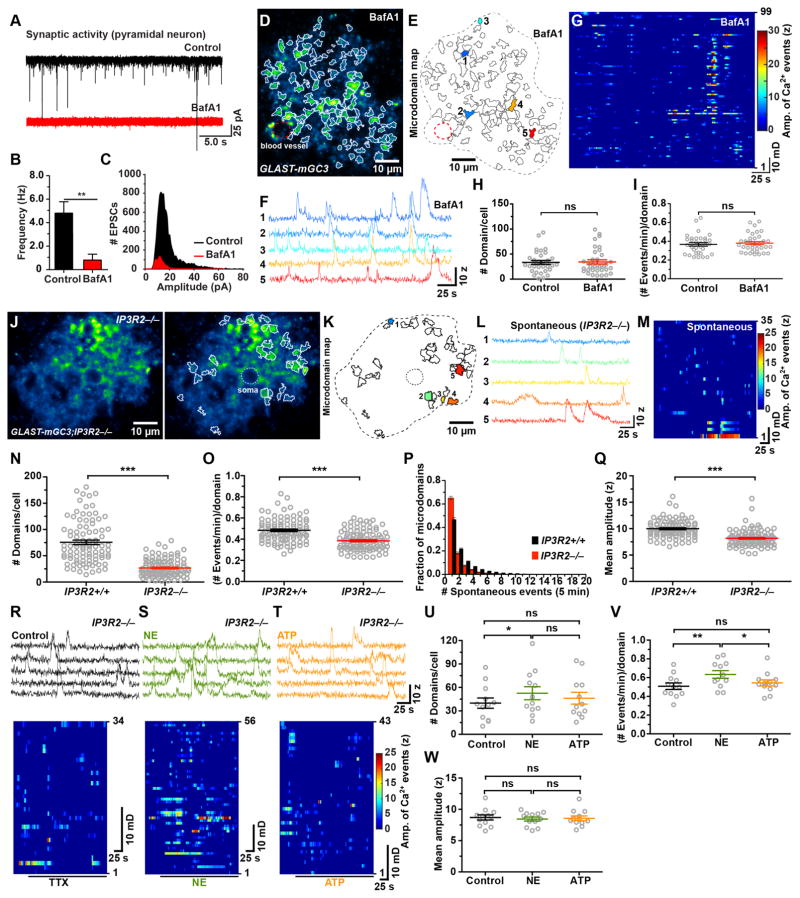
Microdomain Ca2+ transients persisted in TTX, but this does not exclude the possibility that they are induced by spontaneous fusion of synaptic vesicles. To address this possibility, we incubated slices in bafilomycin A1 (BafA1; 2 μM), which depletes vesicles of neurotransmitter, and veratridine (10 μM), which promotes opening of voltage-gated Na+ channels to enhance fusion of already-filled vesicles (Cavelier and Attwell, 2007). Although this manipulation dramatically reduced the frequency and amplitude of spontaneous EPSCs in cortical pyramidal neurons (Figure 4A–C), microdomain activity in astrocytes persisted (Figure 4D–I), with the number of active regions (Figure 4H), the frequency of events per microdomain (Figure 4I) and the average amplitude of Ca2+ transients (Control: 8.3 ± 0.3, n = 33; BafA1: 8.2 ± 0.2, n = 36; P = 0.71) unaltered. These findings support the hypothesis that spontaneous microdomain Ca2+ transients are intrinsically generated (Nett et al., 2002).
Figure 4. Spontaneous microdomain Ca2+ transients persist in the absence of neurotransmitter release and ER-dependent Ca2+ release (See also Figure S3 and Movie S3).
(A) Spontaneous EPSCs recorded from cortical pyramidal neurons in control conditions (black trace) and after treatment with veratidine (10 μM) and bafilomycin A1 (4 μM, BafA1, red trace).
(B) Histogram of the frequency of spontaneous EPSCs recorded in control conditions and after treatment with veratidine and BafA1. Data shown as mean ± SEM. N = 7 (untreated) N = 5 (BafA1) cells from control mice (GLAST-mGC3 or mGCaMP3/+). **p < 0.009, unpaired two-tailed Student’s t-test.
(C) Histogram of the amplitudes of spontaneous EPSCs recorded in control conditions and after treatment with veratidine and BafA1.
(D) Image of one astrocyte from a GLAST-mGC3 mouse showing median intensity projection (pseudocolored) from 540 frames (left) in an acute brain slice treated with veratridine and BafA1.
(E) Map of 99 spontaneously active microdomains recorded in veratridine and BafA1.
(F) Intensity versus time traces of 5 microdomains (corresponding to colors in E) showing characteristics of Ca2+ transients in veratridine and BafA1.
(G) Raster plot displaying Ca2+ transients from 99 microdomains in veratridine and BafA1.
(H, I) Graphs of number of microdomains per cell (H) and frequency of events per microdomain (I) recorded in control and in veratridine and BafA1. Data shown as mean ± SEM. N = 33 (untreated) and 36 (BafA1) cells from GLAST-mGC3 mice. ns: not significant, two-tailed Student’s t-test.
(J) Image of one astrocyte from a GLAST-mGC3;IP3R2−/−mouse showing median intensity projection (pseudocolored) from 260 s (left). Map of spontaneously active microdomains (in TTX (1μM)) overlaid on image (right).
(K) Map of all spontaneously active microdomains in 260 s (35 domains). Dashed line indicates cell border.
(L) Intensity versus time traces of five microdomains (corresponding to colors in B) showing characteristics of Ca2+ transients.
(M) Raster plot displaying Ca2+ transients from active regions in 260 s.
(N – Q) Graphs of number of microdomains per cell (N), frequency of events per domain (O), number of Ca2+ transients observed per microdomain (P) and mean amplitude (Q) in GLAST-mGC3 (Control, 94 cells) and GLAST-mGC3;IP3R2−/−(104 cells) mice. Data shown as mean ± SEM. *** p < 0.0001, unpaired two-tailed non-parametric Mann-Whitney test.
(R – T) Intensity versus time plots for 5 microdomains (top) and raster plots (bottom) displaying spontaneous Ca2+ transients (in control, black) and NE (10 μM, green) and ATP (100 μM, orange) from GLAST-mGC3;IP3R2−/−mice. All experiments were done in the presence of 1 μM TTX.
(U – W) Graphs of number of microdomains per cell (U), frequency of events per domain (V), and mean amplitude (W) of spontaneous Ca2+ transients (in TTX, black) and NE (10 μM, green) and ATP (100 μM, orange) from GLAST-mGC3;IP3R2−/− mice. Data shown as mean ± SEM. N = 12 cells for each condition. ns: not significant, p > 0.05, ** p < 0.01 and * p < 0.01 repeated measure one-way ANOVA analysis with Tukey’s multiple comparisons post hoc test.
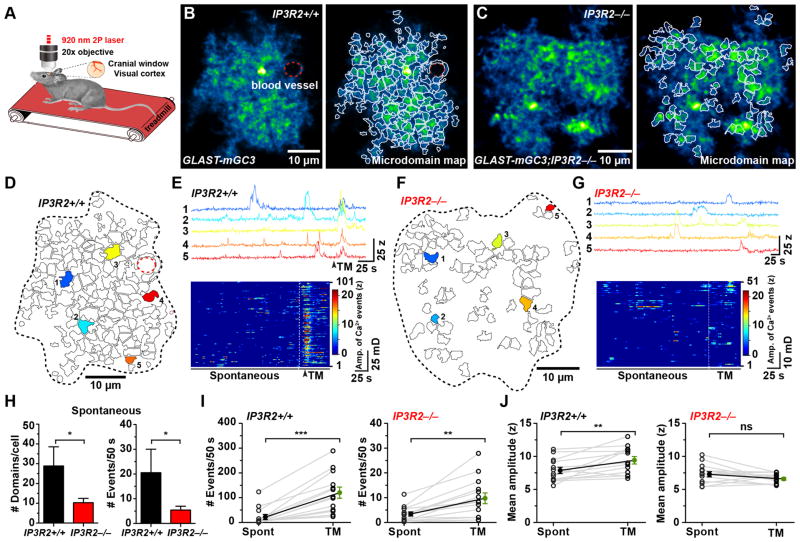
To determine if spontaneous events detected by mGCaMP3 arise from IP3-dependent Ca2+ release, we monitored spontaneous and evoked microdomain Ca2+ transients in mice that lack IP3R2 (Li et al., 2005), which is required for IP3-dependent Ca2+ release in astrocytes (Petravicz et al., 2008). The number of active domains was 65% lower in GLAST-mGC3;IP3R2−/− mice relative to controls (Figure 4J–N and Movie S3), and the number of events per domain was significantly reduced, with 83% of microdomains exhibiting only 1–2 events during the imaging period (Figure 4O, P). Moreover, the average event amplitude was significantly reduced in IP3R2−/− mice by 1.8 SD (z-score) (IP3R2+/+: 10.0 ± 0.2 z, n = 94; IP3R2−/− : 8.2 ± 0.2 z, n = 104; P < 0.0001) (Figure 4Q). Similar changes in microdomain activity were observed following depletion of ER Ca2+ stores in IP3R2+/+ mice with thapsigargin (Figure S3A–E), which prevented NE-induced synchronous microdomain activity (Figure S3F). These results indicate that the reduction of microdomain activity in IP3R2−/− mice does not result from developmental changes and that there are additional mechanisms that enable cytosolic Ca2+ changes in these domains.
If IP3R2 is required for enhancing Ca2+ transients following stimulation of Gq-coupled receptors, the response to metabotropic receptor agonists should be greatly attenuated. Indeed, no changes were observed in the number of microdomains (Figure 4R–U), the frequency of events per domain (Figure 4V), or the amplitude of events (Figure 4W) when ATP (100 μM) was applied to cortical slices from GLAST-mGC3;IP3R2−/− mice, supporting the conclusion that IP3R2 is the main IP3R receptor subtype in astrocytes and that other IP3Rs are not upregulated to compensate. Unexpectedly, NE (10 μM) elicited a small increase (24%) in the number of active microdomains (Figure 4S, U), as well as the frequency of events per domain (19%) (Figure 4V), without affecting the amplitudes of Ca2+ transients (Figure 4W).
To determine if IP3R2-independent microdomain Ca2+ transients also occur in astrocytes in vivo, we monitored spontaneous and NE-induced Ca2+ transients in control (GLAST-mGC3) and IP3R2−/− mice (GLAST-mGC3;IP3R2−/−) in primary visual cortex using two photon imaging through a cranial window without anesthesia (Figure 5A). During quiet resting periods, V1 astrocytes exhibited periodic microdomain Ca2+ transients that were unsynchronized (Figure 5B–E, and Movie S4), similar to those observed in acute slices. Microdomain activity in astrocytes in GLAST-mGC3;IP3R2−/− mice in vivo was also attenuated relative to controls (Figure 5F–G, and Movie S5), with fewer active domains (−64%) and fewer spontaneous events (−73%) observed during a comparable time period (Figure 5H; compare Figure 5D, E to Figure 5F, G). Astrocytes in primary visual cortex (V1) exhibit coordinated increases in cytosolic Ca2+ during locomotion, as NE is released when the state of arousal of the mice is increased (Paukert et al., 2014). In visual cortex, in vivo NE release led to a 4.5x increase in number of microdomain events (Figure 5I) and a 20% increase in mean amplitude of Ca2+ transients (z-score) (Figure 5J and Movie S4). In IP3R2−/− mice, enforced locomotion increased the frequency of microdomain events by 1.9x, but did not alter their amplitude (Figure 5G, I, J and Movie S5). These results indicate that periodic microdomain activity is an in vivo phenomenon that persists without IP3R2-mediated Ca2+ release.
Figure 5. Microdomain Ca2+ transients persist in vivo in IP3R2−/−mice (See also Movie S4, S5).
(A) In vivo two photon imaging configuration in which mice were allowed to walk on a linear treadmill.
(B) Median intensity projection image (pseudocolored) of one astrocyte in vivo from the primary visual cortex (V1) in a GLAST-mGC3;IP3R2+/+ mouse showing active regions during 260 s (left). Map of spontaneously active microdomains is overlaid on image (right). Dashed red line highlights blood vessel.
(C) Image of one astrocyte in vivo from visual cortex (V1) in a GLAST-mGC3;IP3R2−/−mouse, showing median intensity projection (pseudocolored) from 260 s (left). Map of all spontaneously active microdomains (101) that occurred in 286 s is overlaid on image (right).
(D) Map of all microdomains (101) that occurred in 286 s in astrocyte shown in (B) (GLAST-mGC3; IP3R2+/+) mouse.
(E) Intensity versus time plots for five microdomains (corresponding to colors in D) showing characteristics of Ca2+ transients in a GLAST-mGC3;IP3R2+/+ mouse (top). Raster plot displaying the activity of all microdomains (bottom). Timing of enforced locomotion highlighted by dashed line.
(F) Map of all microdomains (51) that occurred in 286 s in astrocyte shown in (C) (GLAST-mGC3;IP3R2−/−) mouse.
(G) Intensity versus time plots for five microdomains (corresponding to colors in F) showing characteristics of Ca2+ transients from cell in (F) (top). Raster plot displaying the activity of all microdomains (bottom). Timing of enforced locomotion highlighted by dashed line.
(H) Histograms of the average number of active microdomains per cell (left) and number of Ca2+ transients (Events) during 50 s of imaging (right), in IP3R2+/+ (GLAST-mGC3;IP3R2+/+) and IP3R2−/−(GLAST-mGC3;IP3R2−/−) mice during baseline activity. Data shown as mean ± SEM. N = 14 cells from GLAST-mGC3;IP3R2+/+ mice and 25 cells from GLAST-mGC3;IP3R2−/−mice were analyzed. ns: not significant, * p > 0.05, unpaired two-tailed Student’s t-test.
(I – J) Graphs showing the number of Ca2+ transients (Events) observed during 50 s of imaging (I) and mean amplitude (z-score) for microdomain Ca2+ transients (J) without stimulation (spontaneous, Spont) and during enforced locomotion on the treadmill (TM) for Control (IP3R2+/+) (left) and IP3R2−/−mice (right). Data shown as mean ± SEM. Note change in scale for the IP3R2−/−mice in (I). N = 13 cells from GLAST-mGC3;IP3R2+/+ mice and 14 cells from GLAST-mGC3;IP3R2−/−mice were analyzed. ns: not significant, p > 0.05, ** p < 0.009, *** p < 0.0001, paired two-tailed Student’s t-test.
Microdomain activity does not require TRPA1
Recent studies have implicated TRPA1 as a primary mediator of microdomain activity and a contributor to resting Ca2+ levels in astrocytes (Shigetomi et al., 2012). However, exposure to the TRPA1 antagonist HC-030031 (50 μM) did not alter microdomain Ca2+ transients in astrocytes from GLAST-mGC3;IP3R2−/− mice (Figure S3G), although it induced a slight drop in the resting Ca2+ level (Figure S3H). Moreover, inhibiting other plasma membrane proteins implicated in contributing to astrocyte Ca2+ transients, including CRAC channels (inhibited with GSK7975A), ryanodine receptors (inhibited with dantrolene), voltage dependent Ca2+ channels (inhibited with CdCl2), or Na+/Ca2+ exchangers (inhibited with Benzamil and CGP37157), also did not block microdomain Ca2+ transients in astrocytes (Figure S3I). These results suggest that localized Ca2+ transients in astrocytes arise from an intracellular source capable of transiently storing and periodically releasing Ca2+ into the cytosol.
Microdomain Ca2+transients co-localize with mitochondria
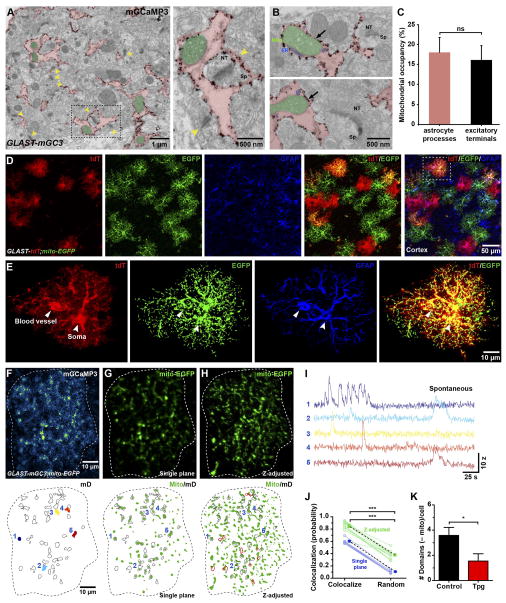
When analyzing the localization of mGCaMP3 in astrocytes by immuno-EM, we noticed that mitochondria were often located adjacent to the plasma membrane (distance: 11.3 ± 4.1 nm) in close proximity to mGCaMP3 (Figure 6A, B), and occupied a substantial portion of the area within fine processes (18 ± 4%) (Figure 6B, C), comparable to that occupied by mitochondria in excitatory nerve terminals (16 ± 4%) (Figure 6C). To visualize the distribution of mitochondria in astrocytes, we generated a Rosa26 targeted knock-in mouse line, in which the mitochondrial-targeting of cytochrome c oxidase VIIIa (C8a) was fused to the N-terminus of EGFP, allowing cell-specific labeling of mitochondria (Figure S4A, B) (see Methods). These Rosa26-lsl-mito-EGFP (mito-EGFP) mice were crossed to GLAST-CreER/+;Rosa26-lsl-tdTomato (Ai9) mice to also express cytosolic tdTomato (Figure S4C). Immunohistochemical analysis of these mice indicated that mitochondria were abundant in fine branches where microdomain Ca2+ transients occur (Figure 6D, E). Mitochondria have been shown to accumulate and release Ca2+ in a variety of cell types, including astrocytes (Rizzuto et al., 2012), raising the possibility that they contribute to cytosolic Ca2+ fluctuations within these compartments. To explore the spatial relationship between mitochondria and microdomain Ca2+ activity, we generated GLAST-mGC3;mito-EGFP triple transgenic mice and performed simultaneous time-lapse imaging of EGFP and mGCaMP3 (Figure 6F–H and Movie S6). Because signals arising from mito-EGFP exhibit minimal intensity fluctuations (Figure S4D–G) and were largely stationary during the imaging period (Movie S6), they could be extracted from time-lapse movies, preserving the dynamic changes in mGCaMP3 in the vicinity of mitochondria (Figure 6F–I and Figure S4H, I) (see Methods). In control conditions (ACSF + 1 μM TTX), 60% of spontaneous microdomain Ca2+ transients occurred at sites with mito-EGFP+ puncta, which increased to 85% when mitochondria 7 μm above and below the focal plane were included (z-adjusted), accounting for the ability of Ca2+ to diffuse beyond mitochondria (Figure 6J). When the location of mito-EGFP+ puncta was randomized within the astrocyte volume, co-localization was reduced to 12% (z-adjusted: 38%) (Figure 6J), suggesting that there is a specific association between mitochondria and regions where Ca2+ transients occur. Treatment with thapsigargin reduced the number of domains that were not co-localized with mitochondria (–mito domains) (Figure 6K), indicating that Ca2+ transients within these sites arise primarily from ER stores.
Figure 6. Microdomain Ca2+ transients co-localize with mitochondria (See also Figure S4 and Movie S6).
(A) Electron micrograph showing silver enhanced gold immunolabeling of mGCaMP3 in a GLAST-mGC3 mouse. Astrocyte processes are colored red and mitochondria in astrocyte processes are colored green. Yellow arrows highlight excitatory synapses. Image at right shows higher magnification image of area highlighted by boxed area at left.
(B) Electron micrographs showing spatial relationship between mitochondria and ER in astrocyte processes located in the vicinity of the excitatory nerve terminals.
(C) Histogram showing area within fine astrocyte processes and nerve terminals occupied by mitochondria. Data shown as mean ± SEM. ns: not significant, p > 0.05 unpaired two-tailed Student’s t-test.
(D) Images of cortical astrocytes from a GLAST-CreER;Rosa26-lsl-tdTomato;Rosa26-lsl-mito-EGFP (GLAST-tdT;mito-EGFP) mouse labeled with anti-GFAP (GFAP, blue), anti-mCherry (tdT, red) and anti-GFP antibodies.
(E) High magnification images of one astrocyte (highlighted by box in E) showing tdT, mGCaMP3 and GFAP localization.
(F) Image of one astrocyte in a GLAST-mGC3;mito-EGFP mouse showing median intensity projection (pseudocolored) from 260 s (top). Map of spontaneously active microdomains is overlaid on image (below). Dashed white line highlights cell border.
(G) Image of fluorescently tagged mitochondria from cell in (F) (top). Map of spontaneously active microdomains (mD, outlined in black) and mitochondria (Mito, green).
(H) Image of fluorescently tagged mitochondria in which z plane has been expanded to visualize mitochondria just above and below plane of imaging from cell in (F) (top). Map of spontaneously active microdomains (mD, outlined in black) and mitochondria (Mito, green). Additional co-localized areas highlighted in red.
(I) Intensity versus time plots for Ca2+ transients from five microdomains (colors correspond to locations shown in F, bottom), showing characteristics of spontaneous Ca2+ signals.
(J) Plot of co-localization between spontaneously active microdomains and mitochondria and co-localization after randomization of mitochondrial locations within the imaging plane (blue) and after z adjustment (green). Data shown as mean ± SEM. N = 7 cells (single plane) and N = 12 cells (z-plane corrected) from GLAST-mGC;mito-EGFP mice. *** p < 0.0001, paired two-tailed Student’s t-test.
(K) Histogram showing the decrease in active microdomains that did not co-localize with mitochondria after treatment with thapsigargin (Tpg, 1 μM, 60 minutes) to deplete ER Ca2+ stores. Data shown as mean ± SEM, N = 12 (untreated) and 11 (Tpg treated) cells. * p < 0.03, unpaired two-tailed Student’s t-test.
Transient opening of the mitochondrial permeability transition pore generates microdomain Ca2+ signals
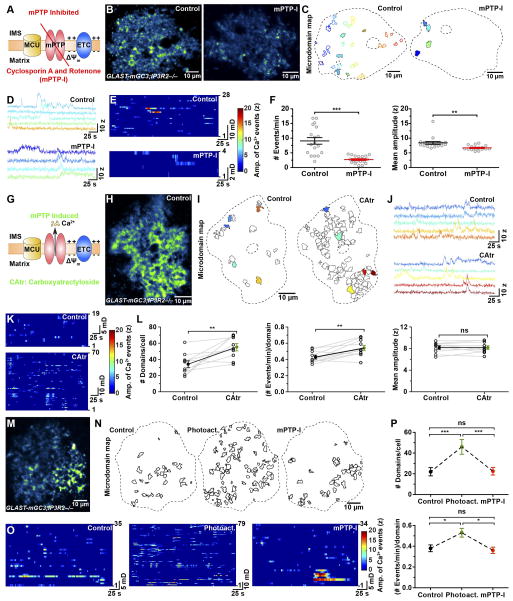
Ca2+ homeostasis in mitochondria is achieved through mitochondrial Na+/Ca2+ (mNCX) and H+/Ca2+ (mHCX) exchangers, the mitochondrial Ca2+ uniporter (MCU), and the mitochondrial permeability transition pore (mPTP) (Bernardi and Petronilli, 1996). To determine if mitochondria contribute to spontaneous microdomain Ca2+ transients, we inhibited mitochondrial Ca2+ influx and efflux pathways using CGP37157 (20 μM) (to inhibit mNCX and mHCX exchangers), cyclosporin A (20 μM) (to inhibit cyclophilin D, a component of the mPTP), and KB-R7943 (20 μM) (to inhibit MCU) (Rizzuto et al., 2012) in GLAST-mGC3;IP3R2−/− (Figure S5A–D), which EM analysis revealed had the same number and distribution of mitochondria in astrocyte processes as IP3R2+/+ mice (Figure S5E–G). Microdomain Ca2+ transients were virtually abolished in astrocytes from mice in the presence of these antagonists (Figure S5A, B), indicating that mitochondria participate in the generation of Ca2+ signals in astrocyte processes.
mPTP can act as a fast Ca2+ release channel (Bernardi and Petronilli, 1996), opening transiently to reset both Ca2+ and proton gradients across the mitochondrial inner membrane during periods of high respiration (Ichas et al., 1997). mPTP is formed from multiple components, including ATP synthesis machinery and other transport/carrier proteins, but the molecular identity of the pore has not been determined. Although there are no antagonists of the pore, mPTP can be inhibited by blocking the activity of its constituent proteins, such as cyclophilin D. Treatment of cortical slices from GLAST-mGC3;IP3R2−/− mice with cyclosporin A (CsA, 20 μM) reduced the number of microdomains by 33%, the frequency of microdomain Ca2+ transients by 43% and their amplitude by 17% (Figure S5H–J). However, cyclophilin D expression is much lower in astrocytes than neurons (Naga et al., 2007) (Figure S5K), which may explain partial inhibition by CsA. Thus, we applied CsA with rotenone (10 μM), a mitochondrial complex I blocker that has been used to inhibit mPTP activity in cells with reduced cyclophilin D (Li et al., 2012). Incubation of slices in these antagonists (mPTP-I solution) (Figure 7A) inhibited the frequency of microdomain Ca2+ transients by 70% (Figure 7B–F and Movie S7), and the few events recorded in these antagonists were shorter in duration (Control: 10.3 ± 0.9 s, n = 18; mPTP-I: 6.9 ± 0.5 s, n = 18; P = 0.002) and 20% smaller in amplitude (Figure 7F). As the CaSCaDe analysis has a false positive detection rate of about 5–10% (Figure S1K–P), these results suggest that mPTP is critically involved in generating microdomain Ca2+ transients. To further test mitochondrial involvement, we disrupted the mitochondrial potential, as agents that collapse this gradient block the transient opening of mPTP (Ichas et al., 1997) (Figure S5L). Acute application of the proton ionophore FCCP (5 μM) (Figure S5M–P) reduced the number of microdomains by 56%, as well as the frequency by 68%, and amplitude of Ca2+ transients by 23% (Figure S5Q–S). Conversely, acute application of carboxyatractyloside (CAtr), which stimulates mPTP opening by inhibiting adenine nucleotide translocase (ANT) (Wang et al., 2008) (Figure 7G), increased the number of active microdomains by 62% and the frequency of these events doubled, without affecting their amplitude (Figure 7H–L).
Figure 7. Mitochondrial membrane permeability transition pore (mPTP) regulates spontaneous Ca2+ transients (See also Figure S5–S7 and Movie S7, S8).
(A) Schematic showing configuration of membrane permeability transition pore (mPTP, red), mitochondrial Ca2+ uniporter (MCU, yellow) and the electron transport chain (ETC, blue). Pharmacological inhibition of mPTP (mPTP-I; cyclosporin A, 20 μM and rotenone, 10 μM) inhibits mPTP opening and prevents Ca2+ efflux from mitochondrial matrix into the cytosol.
(B) Images of astrocytes (median intensity projection of time-series image stack) showing active regions during 286 s in control (left) and after exposure to mPTP-I (right) (GLAST-mGC3;IP3R2−/−mouse).
(C) Maps of all spontaneously active microdomains in control (28) and after mPTP inhibition (4).
(D) Intensity versus time plots for five microdomains in control and four microdomains in mPTP-I treated slices (colors correspond to locations shown in C).
(E) Raster plot displaying Ca2+ transients from all active regions in 286 s in control (top) and mPTP-I (bottom) treated slices.
(F) Graphs showing relative frequency (left) and mean amplitude (z-score) of Ca2+ transients in control and mPTP-I treated slices. Data shown as mean ± SEM. N = 18 cells. *** p < 0.0001, ** p < 0.001, unpaired two-tailed Student’s t-test.
(G) Schematic showing enhancement of mPTP opening by carboxyatratyloside (CAtr, 20 μM), leading to enhanced Ca2+ efflux from the mitochondrial matrix to the cytosol.
(H) Image of an astrocyte (median intensity projection of time-series image stack) showing active regions during 286 s in a GLAST-mGC3;IP3R2−/−mouse.
(I) Maps of all spontaneously active microdomains during 260 s in control (19) and after mPTP activation (70) (CAtr treated).
(J) Intensity versus time plots of microdomain activity in control (left) and after CAtr treatment (right) (colors correspond to locations shown in I).
(K) Raster plots displaying Ca2+ transients in an astrocyte in control (top) and after CAtr treatment (bottom).
(L) Graphs showing changes in number of active microdomains per cell (left), event frequency per microdomain (middle), and mean amplitude of Ca2+ transients (right) recorded in control and after CAtr treatment. Data shown as mean ± SEM. For each condition 10 individual cells from GLAST-mGC3;IP3R2−/−mice were analyzed. ns: not significant, ** p < 0.006, paired two-tailed Student’s t-test.
(M) Image of an astrocyte (median intensity projection of time-series image stack) showing active regions during 286 s in a GLAST-mGC3;IP3R2−/−mouse.
(N) Maps of all spontaneously active microdomains during 260 s in control (left), after light exposure (Photoact., middle), and after mPTP-I treatment (right).
(O) Raster plots displaying Ca2+ transients in an astrocyte in control (left), after light exposure (Photoact., middle), and after mPTP-I treatment (right).
(P) Graphs showing changes in number of domains per cell (top) and event frequency/domain (bottom) in control, after light exposure (Photoact.), and after mPTP-I treatment. Data shown as mean ± SEM. N = 10 cells from GLAST-mGC3;IP3R2−/−mice. ns: not significant, *** p <0.0001, * p < 0.01 repeated measure one-way ANOVA analysis with Tukey’s multiple comparisons post hoc test.
To assess whether the effect of mPTP inhibitors on astrocytes was cell autonomous, we prepared pure cultures of cortical astrocytes from postnatal day 7 (P7) GLAST-mGC3;IP3R2−/− mice. Spatially-restricted spontaneous Ca2+ transients (micro events) were observed that were distinct from larger events that propagated over long distances (macro events) (Figure S6A, B), with approximately half of the activity mediated by spatially-restricted microdomains (size range: 5.5 – 22 μm2) (Figure S6F). mPTP-I reduced the frequency of all Ca2+ transients by 56%, without altering their amplitude (Figure S6C, D), and the duration of events were prolonged by 38% (Figure S6E), consistent with a reduction in mitochondrial Ca2+ buffering. The frequency of micro events was reduced by 93% by mPTP-I (Figure S6G), and the duration of the remaining 7% of the events were prolonged by 46% (Figure S6G). However, larger Ca2+ transients (macro events) were preserved in the presence of mPTP-I (Figure S6F), indicating that these events are independent of IP3R2 and transient opening of mPTP. Notably, such large events were not observed in astrocytes in brain slices. Astrocytes retained the ability to exhibit a Ca2+ rise in response to ATP after treatment with mPTP-I (Figure S6H), and ATP-evoked events were prolonged in duration, providing additional evidence that mitochondria were unable to sequester Ca2+ after mPTP inhibition (Jackson and Robinson, 2015; Stephen et al., 2015).
To define the contribution of mPTP to spontaneous microdomain Ca2+ transients when IP3R2 is present, we measured inhibition by CsA and rotenone (mPTP-I) in cortical slices from control mice (GLAST-mGC3). mPTP inhibition reduced the number of active microdomains by 35%, as well as the frequency (−42%) and amplitude (−14%) of these Ca2+ events, while the duration of events remained unaltered (Figure S7A–D). Similar to the behavior of astrocytes in control animals, NE (+ mPTP-I) promoted a near-synchronous rise in Ca2+ in microdomains (Figure S7E–F, compare to Figure 3E), increased the increase the frequency of events by 59%, and enhanced the duration of events at each site by 33% (Figure 3G), but did not increase the amplitude of Ca2+ transients (Figure S3G), indicating that acute disruption of mPTP did not impair cell viability or their ability to engage in receptor-induced Ca2+ signaling (Figure S7E–G).
To overcome limitations associated with pharmacological manipulations, we developed a genetic approach to selectively disrupt mitochondrial function in cortical astrocytes in vivo, by using the ability of the uncoupling protein 1 (UCP1), a component of mitochondria in brown fat cells, to create a proton (H+) leak across the inner mitochondrial membrane and reduce the rate of oxidative phosphorylation (Krauss et al., 2005). We generated an adeno-associated virus (AAV8) packaged with Δ9UCP1, a variant of UCP1 that does not require fatty acid for H+ transport (González-Barroso et al., 1997), and cytosolic mCherry under control of a human GFAP promoter (Figure S7H). Eight weeks after this virus was injected into the somatosensory cortex of GLAST-mGC3 mice, astrocytes expressing Δ9UCP1 could be identified by expression of mCherry (Figure S7I), and both mutant (mCherry+) and control (mCherry−) astrocytes exhibited spontaneous microdomain Ca2+ transients (Figure S7J, K). Expression Δ9UCP1 reduced the frequency of events per domain by 24%, the amplitude of Ca2+ transients by 21% (Figure S7L, M), prolonged their duration (compare traces in Figure S7J and K and raster plots in L) and increased the average domain size by 24% (Figure S7N), suggesting that mitochondrial Ca2+ buffering was impaired. This selective, genetic manipulation of astrocytes further supports the conclusion that mitochondria are an important contributor to microdomain Ca2+ transients in astrocyte processes.
Superoxide production enhances microdomain Ca2+transients
Mitochondria exhibit periodic bursts of superoxide production during periods of high respiration that induce opening of mPTP (Wang et al., 2008), suggesting that ROS may be a key regulator of microdomain activity. To determine if ROS production enhances microdomain Ca2+ transients by opening mPTP, we continuously illuminated astrocytes in slices from GLAST-mGC3;IP3R2−/− mice for five minutes with 488 nm laser light (Figure 7M, N and Movie S8), a manipulation that induces ROS (Kuga et al., 2011). This manipulation doubled the number of active microdomains per cell and enhanced the number of events per microdomain by 39% (Figure 7M–P), without changing their amplitudes (Control: 7.4 ± 0.3 z, n = 10; +Photoactivation: 7.7 ± 0.2 z, n = 10; P = 0.29). Subsequent exposure to mPTP-I markedly reduced this light-induced microdomain activity (Figure 7N–P, and Movie S8), indicating that ROS enhances mPTP-dependent Ca2+ efflux from mitochondria in astrocyte processes.
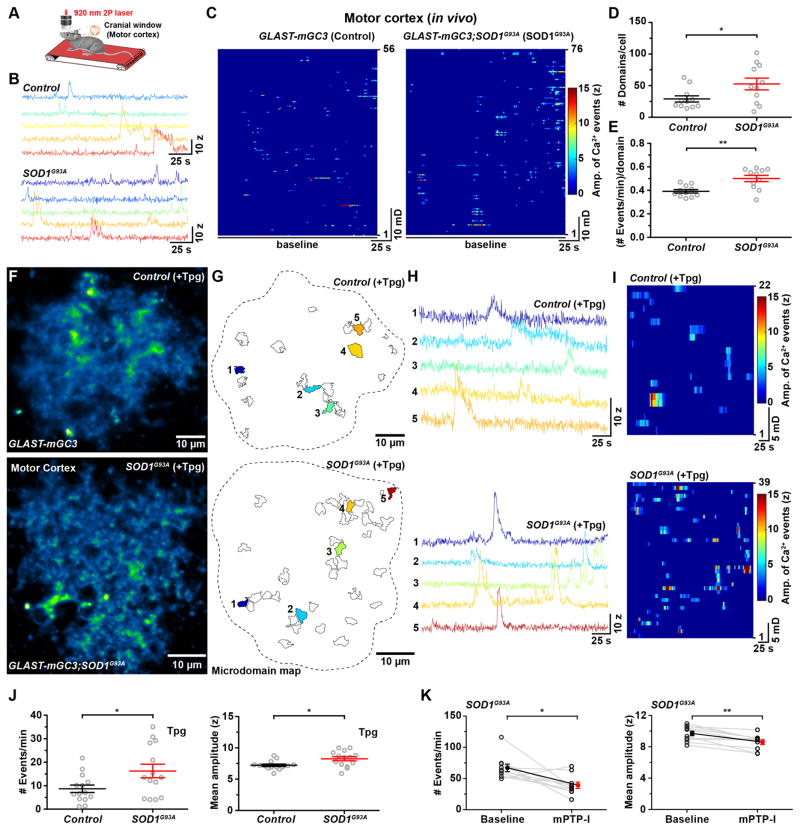
Mutations in the mitochondrial enzyme superoxide dismutase 1 (SOD1) in amyotrophic lateral sclerosis (ALS) induce mitochondrial stress and promote the dysfunction and eventual death of astrocytes and motor neurons (Liu et al., 2004). To determine if mitochondrial dysfunction induced by overexpression of mutant SOD1 also alters mPTP gating and microdomain Ca2+ signaling in astrocytes, we performed in vivo Ca2+ imaging in the motor cortex of GLAST-mGC3;SOD1G93A triple transgenic mice when animals became symptomatic (~110 days of age) (Figure 8A). Astrocytes in the motor cortex of ALS mice exhibited higher microdomain activity (Figure 8B), with more active microdomains per cell (Figure 8C, D), and more events within each microdomain (Figure 8E) relative to age-matched controls. To determine if mPTP hyperactivity contributes to this enhanced activity, we imaged mitochondria-induced microdomain Ca2+ transients in motor cortex slices of GLAST-mGC3;SOD1G93A mice (aged ~120 days) in which ER Ca2+ stores were depleted with thapsigargin (Figure 8F–I). Under these conditions, microdomain activity was enhanced by 87% in SOD1G93A mice with a slight increase (14%) in the amplitude of events (Figure 8F–J). Moreover, astrocyte activity in these animals exhibited greater inhibition (frequency: −42%; amplitude: −11%) by mPTP-I than controls (Figure 8K), suggesting that overexpression of mutant SOD1 enhances mPTP opening as a result of oxidative stress.
Figure 8. Enhanced mitochondrial Ca2+ efflux from astrocytes in ALS model (SOD1G93A) mice (See also Figure S8).
(A) Schematic showing the in vivo two photon imaging configuration in which mice were allowed to walk on a linear treadmill.
(B) Intensity versus time plots of microdomain activity in an astrocyte from a control mouse (top) and from a SOD1G93A mouse (bottom).
(C) Raster plots displaying Ca2+ transients in astrocyte microdomains from a control mouse (left) and from a SOD1G93A mouse (right).
(D, E) Graphs of the number of active microdomains per cell (D) and frequency of events per microdomain (E) in control and SOD1G93A mice. Data shown as mean ± SEM. N = 11 cells each from Control and SOD1G93A mice. * p < 0.01, ** p < 0.001 unpaired two-tailed Student’s t-test.
(F) Images of single astrocytes in acute slices of motor cortex from a GLAST-mGC3 (top) and a SOD1G93A mouse (bottom) showing median intensity projection (pseudocolored) from 260 s after treatment with thapsigargin (Tpg, 1 μM, 60 minutes).
(G) Maps of all active microdomains (Control: 22; SOD1G93A: 39) that occurred in 286 s in astrocytes shown in (F).
(H) Intensity versus time plots of microdomain activity in a control (top) and in a SOD1G93A mouse (bottom) (colors correspond to locations shown in corresponding maps in G).
(I) Raster plots displaying microdomain Ca2+ transients imaged over a period of 260 s recorded in Tpg in a control (top) and in a SOD1G93A mouse (bottom).
(J) Graphs comparing the frequency (left) and mean amplitude (z-score, right) of microdomain Ca2+ transients in Tpg treated cortical slices from aged matched control and SOD1G93A mice. Data shown as mean ± SEM. N = 14 cells each from Control and SOD1G93A mice. * p < 0.03, unpaired two-tailed Student’s t-test.
(K) Graphs comparing the frequency (left) and mean amplitude (z-score, right) of microdomain Ca2+ transients in SOD1G93A mice before (baseline) and after mPTP inhibition (mPTP-I, 30 min). Data shown as mean ± SEM. N = 10 cells each from Control and SOD1G93A mice. * p <0.02, ** p < 0.002, paired two-tailed Student’s t-test.
High extracellular glucose enhances microdomain Ca2+transients
Extracellular glucose levels may influence the degree to which mitochondria are mobilized to support ATP production and thereby alter mitochondrial Ca2+ efflux. Astrocytes in 1 mM glucose ACSF exhibited a comparable number of active microdomains and the average amplitude of events at these sites was similar (Figure S8A–C); however, the frequency of Ca2+ transients in each microdomain was reduced by 18% (Figure S8B), as expected if the rate of oxidative phosphorylation (and opening probability of mPTP) was reduced. The frequency of neuromodulator-evoked microdomain Ca2+ transients were similarly enhanced by NE (10 μM) in high and low glucose ACSF (Figure S8D), suggesting that fluctuations in extracellular glucose levels over this range do not alter the ability of this neuromodulator to stimulate mitochondrial activity.
Mitochondrial Ca2+ efflux is enhanced by neuronal activity
The close link between mitochondrial respiration rate and Ca2+ efflux through mPTP suggests that increases in metabolic demand, such as that induced by neuronal activity may promote microdomain activity in astrocytes. To explore this possibility, we imaged astrocytes in cortical slices from GLAST-mGC3;IP3R2−/− mice and enhanced neuronal activity by applying picrotoxin (100 μM) (Figure S8E–I). Enhancing neuronal activity increased the number of microdomains by 52%, and the frequency of Ca2+ transients by 62%, without affecting their amplitude (Figure S8J–L). This enhancement of microdomain activity was meditated by increased mPTP opening, as application of picrotoxin in the presence of mPTP inhibitors (Picro + mPTP-I) failed to enhance microdomain Ca2+ transients (Figure S8J–L). These results indicate that neuronal activity can promote cytosolic increases in Ca2+ in astrocytes independent of ER stores by promoting opening of mitochondrial mPTP, providing an explanation for ability of increases in arousal to enhance the frequency of microdomain activity in astrocytes in IP3R2−/− mice (Figure 5) (Kanemaru et al., 2014; Srinivasan et al., 2015).
Discussion
Mitochondria as a source of Ca2+ in astrocyte microdomains
Astrocytes extend highly ramified processes that make contact with blood vessels, nodes of Ranvier and synapses to facilitate ion redistribution, neurotransmitter uptake, and neuromodulation, activities that consume considerable energy (Bélanger et al., 2011). Regions where these events occur are often far removed from the cell body, as astrocyte processes consist of numerous thin lamellar extensions connected by narrow cytoplasmic bridges (Grosche et al., 1999). This complex geometry encourages functional isolation, but may also create local energetic demands. Although astrocytes are thought to use primarily glycolysis for ATP generation (Magistretti and Allaman, 2015), recent studies indicate that they contain numerous mitochondria and express enzymes necessary for oxidative phosphorylation (Hertz et al., 2007; Lovatt et al., 2007). By generating mice in which EGFP-tagged mitochondria were expressed only in astrocytes (Figure 6 and S4), we discovered that mitochondria are abundant in these thin processes, at a density comparable to that found in nerve terminals (Figure 6), where there is extraordinary metabolic demand.
In addition to producing ATP, mitochondria serve as a Ca2+ storage organelle that can profoundly influence intracellular signaling by buffering receptor-induced Ca2+ transients. During oxidative phosphorylation, the mitochondrial inner membrane periodically depolarizes due to reversible opening of mPTPs, non-selective pores that exhibit high Ca2+ permeability (Ichas et al., 1997). This periodic opening of mPTP dissipates pH gradients established during periods of high oxidative phosphorylation, enabling continued ATP production. mPTP opening is also associated with enhanced ROS production, events collectively referred to as “mitoflashes” (Wang et al., 2008). Mitoflashes have been observed in a variety of cell types, including neurons, cardiomyocytes, chondrocytes and fibroblasts, and are highly conserved from worms to mammals (Hou et al., 2014), suggesting that they are a critical component of mitochondrial function. Simultaneous imaging of intracellular Ca2+ and fluorescently tagged mitochondria in astrocytes revealed that microdomain Ca2+ transients were spatially correlated with mitochondria (Figure 6). Moreover, inhibiting mPTP function in astrocytes (with CsA and rotenone) or disrupting the mitochondrial membrane potential (with FCCP), manipulations that decrease mitoflashes in other cells, markedly reduced microdomain Ca2+ transients (Figure 7 and S5), while enhancing mPTP opening (with CAtr) increased these transients (Figure 7). Notably, volatile anesthetics that inhibit mPTP also block astrocyte Ca2+ transients in vivo (Nimmerjahn et al., 2009). These effects of mPTP modulation appear to be mediated primarily within astrocytes, as they were also effective in purified astrocyte cultures (Figure S6) and in brain slices from mice in which mitochondrial function was selectively disrupted in astrocytes by forcing expression of UCP1 (Figure S7).
The discovery that the low conductance “flickering” state of mPTP associated with mitoflashes also mediates Ca2+ efflux was enabled by the unique morphology of astrocytes. Due to membrane anchoring, mGCaMP3 was placed within 10 nm of mitochondria (Figure 6). Because of the close proximity of the Ca2+ sensor to mitochondria and the very small space in which Ca2+ can disperse, periodic Ca2+ efflux arising from brief openings of mPTP could be resolved. Such events would not be readily detected using cytosolic indicators, due to their low effective concentration in bulk solution and high endogenous Ca2+ buffering. Mitoflashes arising from individual mitochondria exhibit highly variable amplitudes and kinetics, and typically last for 10 – 20 s (Wang et al., 2008), comparable to the features of astrocyte microdomain Ca2+ transients (Figure 2), reflecting variations in the timing of mPTP opening and number of pores that are recruited within each mitochondrion. Together, these results suggest that the ‘cell intrinsic’ spontaneous Ca2+ transients that persist in astrocyte processes in the absence of IP3R-mediated Ca2+ release reflect stochastic opening of mPTP during periods of high oxidative phosphorylation. Notably, neuromodulators such as NE altered the duration of mPTP-elicited Ca2+ transients independent of ER mediated Ca2+ release, suggested that mPTP gating is modified during different behavioral states.
Functional coupling between mitochondria and ER Ca2+ stores in astrocytes
Spontaneous microdomain Ca2+ transients were markedly attenuated in IP3R2−/− mice (Figure 4 and 5), an effect that was mimicked by depletion of Ca2+ stores with thapsigargin (Figure S3), suggesting that ER stores also contribute to this intrinsic activity. Mitochondria and ER are often in close proximity (< 200 nm) and have been shown to form specialized junctions, “mitochondria-associated membranes” (MAMs), which facilitate direct exchange of ions and metabolites (Rizzuto et al., 1998). This close coupling may promote synergistic release of Ca2+. Indeed, interstitial cells of Cajal (ICC) in the gastrointestinal tract and submucosal cells in the colon exhibit rhythmic, spontaneous Ca2+ oscillations generated by Ca2+ exchange between ER Ca2+ stores and mitochondria (Ward et al., 2000; Yoneda et al., 2002) that are blocked when the electrochemical gradient across the inner mitochondrial membrane or IP3-dependent release of Ca2+ from ER stores are inhibited (Ward et al., 2000). Recent results suggest that activity in some astrocyte microdomains is dependent on extracellular Ca2+ influx (Rungta et al., 2016; Srinivasan et al., 2015), suggesting that mitochondrial Ca2+ is subject to homeostatic control or that there are also plasma membrane channels/transporters in these domains that enable Ca2+ influx independent of mitochondria.
Our results suggest that ROS promotes Ca2+ release in astrocyte microdomains, as this activity was dramatically enhanced by ROS, and chelating ROS or inhibiting mitoflash-associated ROS production attenuated microdomain Ca2+ transients (Figure 7). Mitochondria are the primary site of ROS production in cells, due to the continual loss of electrons from the electron transport chain during oxidative phosphorylation. As ROS production is proportional to the rate of oxidative phosphorylation (Mailloux and Harper, 2012), the frequency of microdomain Ca2+ transients, like mitoflashes, provides an indication of the rate of ATP production in astrocyte processes. IP3R2 contains more than 50 cysteine residues for potential thiol-mediated oxidation, and recent studies show that physiologically relevant levels of O2•– stimulate opening of IP3R2 (Bánsághi et al., 2014), raising the possibility that mitochondria-derived ROS may act directly on IP3R2. In addition, we observed that expression of a mutant form of SOD1 (G93A) that causes mitochondrial stress, enhanced mPTP opening and Ca2+ transients in astrocyte processes. Astrocytes exhibit reactive changes in SOD1G93A mice and have been shown to release factors that induce death of motor neurons (Nagai et al., 2007). This increase in Ca2+ signaling within astrocytes may contribute to secretion of neurotoxic molecules and accelerate neurodegeneration in ALS. Indeed, genetic deletion of cyclophilin D in SOD1G93A mice resulted in reduced astrocyte activation and enhanced motor neuron survival (Parone et al., 2013).
Functions of localized Ca2+ signaling in astrocytes
Astrocytes have long been considered to be crucial for metabolic support of the CNS, by virtue of their association with blood vessels, their accumulation of glycogen, and their expression of monocarboxylic acid transporters that can export lactate, pyruvate and ketone bodies to neurons for local production of ATP. Increasing neuronal activity reduces glycogen levels in astrocytes (Magistretti and Allaman, 2015), and inhibition of mitochondrial respiration with the gliotoxin fluoroacetate leads to swelling and fragmentation of ER, vacuolization, disruption of perivascular end-feet in astrocytes, and eventual neuronal degeneration (Paulsen et al., 1987), supporting the hypothesis that astrocytes play a key role in brain metabolism. However, the mechanisms that regulate ATP production within the highly ramified processes of astrocytes are not well understood. The ability to generate localized Ca2+ transients in these domains may facilitate ATP production by enhancing glycogenolysis (Ververken et al., 1982), as elevation of Ca2+ activates glycogen phosphorylase, which is responsible for extracting glucose from glycogen. In hepatocytes, binding of epinephrine to α- and β-adrenergic receptors stimulates glycogenolysis by triggering release of ER Ca2+ and generating cAMP (Hems and Whitton, 1980), and a similar NE-mediated control of glycogenolysis occurs in cultured astrocytes (Magistretti and Allaman, 2015).
In the absence of an external stimulus, Ca2+ transients in different microdomains were uncorrelated, suggesting that mitochondrial activity is regulated locally within astrocyte processes. Moreover, the frequency of Ca2+ transients varied by an order of magnitude at different sites (Figure 2H), suggesting that local rates of mitochondrial respiration, and thus metabolic demand, vary dramatically in different processes and can be modulated over a wide dynamic range. If mitoflash activity is a direct indication of mitochondrial respiration (Hou et al., 2014), then states of increased metabolic demand and cellular stress should enhance microdomain activity. Indeed, previous studies have shown that reactive astrocytes near amyloid plaques in mouse models of Alzheimer’s disease have higher rates of spontaneous Ca2+ transients (Kuchibhotla et al., 2009), and our findings show that microdomain activity is similarly enhanced in astrocytes in SOD1G93A mice (Figure 8). Furthermore, conditions that increase neuronal firing in vivo, such as increased arousal during locomotion (Figure 5) (Kanemaru et al., 2014; Srinivasan et al., 2015), and in vitro, such as enhanced synaptic excitation during GABA receptor blockade (Figure S8), increased microdomain activity in the absence of IP3R2 signaling, providing evidence of a direct link between metabolic rate and microdomain Ca2+ transients. Future studies involving selective manipulation of mitochondria within astrocytes will help define the roles of this ubiquitous form of Ca2+ signaling in the CNS in both health and disease.
STAR METHODS
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Both male and female mice were used for all experiments, and mice were randomly allocated to experimental groups. For ex vivo experiments, adult mice aged 7–12 weeks old were used and for in vivo experiments mice aged 15–20 weeks old were used, unless otherwise described. All mice (except end-stage SOD1G93A mice) were healthy with no obvious behavioral phenotype, and none of the experimental mice were immune compromised. Except for SOD1G93A animals, experimental mice were never involved in previous procedures or studies. For studies involving SOD1G93A mice, mutant and littermate control mice were first imaged in vivo through a cranial window and then their brains were used for ex vivo slice physiology experiments. Since we used inducible Cre-loxP system (GLAST-CreER;ROSA26-lsl-mGCaMP3) to express mGCaMP3 in astrocytes, all experimental mice were injected with tamoxifen (for details, see Tamoxifen injections section below). Mice were maintained on a 12 hour light/dark cycle, and food and water was provided ad libitum. All animal experiments were carried out in a strict compliance with protocols approved by the Animal Care and Use Committee at the Johns Hopkins University School of Medicine.
Transgenic Animal Models
The conditional Rosa26-lsl-mGCaMP3 and Rosa26-lsl-mito-EGFP mouse lines were generated by inserting a conditional allele into the ROSA26 locus (see Method details). Generation and genotyping of CreER driver lines GLAST-CreER (Paukert et al., 2014), GFAP-CreER (Ganat et al., 2006), tdTomato reporter mouse lines (Ai14, Allen Brain Institute) (Madisen et al., 2010), IP3R2−/−null mutants (Li et al., 2005) and SOD1G93A transgenic mice (Gurney et al., 1994) have been previously described.
METHOD DETAILS
Experimental Design
All ex vivo and in vivo experiments were replicated in more than nine cells derived from on an average 3 different mice (see figure legends for number of cells used for each experiments). In vitro experiments were repeated from at least two separate cohorts of mice, and more than ten coverslips were analyzed per condition. Most experiments were carried out in an unblended manner and a specific randomization strategy was not used. However, in experiments where agonists were applied sequentially (such as ATP, DHPG and NE), the sequence of the drug application was randomized. Statistical computations were not performed to determine the optimal sample-size for experiments. Cells located on the surface of the brain slice, those that had large blood vessels passing through them, or exhibited image registration artifacts were excluded from the data set.
Targeting vector for mGCaMP3 conditional allele
To localize GCaMP3 to the plasma membrane we fused the gene sequence encoding the first 8 amino acids of the modified MARCKS sequence (MGCCFSKT) to the first methionine (i.e. start ATG) of GCaMP3 sequence (termed mGCaMP3). To enhance expression of mGCaMP3, we used a strong ubiquitous CMV-βactin hybrid (CAG) promoter, which consists of three gene regulatory elements namely: 5′ cytomegalovirus early enhancer element, chicken β-actin promoter and rabbit b-globin intron. For inducible expression of mGCaMP3, a loxP flanked 3X SV40 polyA with FRT flanked Neomycin gene (loxP-STOP-loxP, “lsl”) “stopper” cassette was placed upstream of the coding sequence, preventing expression until cyclic recombinase (Cre) excises this gene sequence. To further enhance the expression of mGCaMP3, we sub-cloned the woodchuck hepatitis virus posttranscriptional regulatory element (WPRE) at the 3′ end of mGCaMP3 expression sequence (Figure 1A). The WPRE sequence assists in quick exit of mRNA from the nucleus, and increases the mRNA stability in the cytosol.
Targeting vector for mitoEGFP conditional allele
To localize EGFP to mitochondria, an N-terminal 25 amino acid targeting signal derived from mouse cytochrome c oxidase, subunit VIIIa (C8a) was fused to the N-terminus of EGFP (termed mito-EGFP) (Rizzuto et al., 1989). To facilitate assessments of mito-EGFP localization, a V5 epitope tag was fused to EGFP at the C-terminus. Similar to the strategy used for conditional expression of mGCaMP3, a stopper cassette containing a loxP flanked 3X SV40 polyA sequence was placed upstream of the mito-EGFP coding sequence, and the CAG promoter was used to control expression of mito-EGFP (Figure S4A).
Generation of mGCaMP3 & mitoEGFP knock-in mouse lines
To prevent gene-silencing effects and ensure consistent and long-term expression of these transgenes in all cell types, the CAG driven inducible mito-EGFP and mGCaMP3 transgenic constructs were targeted to the ubiquitously expressed ROSA26 locus. For homologous recombination in mouse embryonic stem (ES) cells, gene-targeting vectors for mito-EGFP and mGCaMP3 were assembled into a ROSA26 targeting plasmid containing a 2.3 kb 5′ homology arm, 4.3 kb 3′ homology arm, and PGK-DTA (Diphtheria toxin fragment A, downstream of 3′ homology arm) for negative selection. ES cells, derived from a SV129 mouse strain, were electroporated with the AsiSI linearized targeting vectors. A nested PCR screening strategy along the 5′ homology arm was used to identify ES cell clones harboring the correct genomic targeting event. To confirm proper targeting, Southern blot analysis was performed on EcoRI digested genomic DNA isolated from PCR positive ES cell clones and probed with a 494 bp long P32-labeled probe located 123 bp upstream 5′ homology arm of ROSA26 gene (Figure S4A). To confirm the integrity of the targeted ROSA26 locus, EcoRI digested ES cell genomic DNA was probed with P32-labeled 475 bp probe located 2.9 kb downstream of 3′ homology arm of ROSA26. After confirmation of the karyotypes, correctly targeted ES cell clones were used to generate chimeric mice by injection into blastocysts derived from SV129 females at the Johns Hopkins University Transgenic Core Laboratory. Germ line transmission was achieved by breeding male chimeric founders to C57Bl/6N wild-type female mice.
Genotyping of mGCaMP3 and mitoEGFP knock-in mouse lines
Routine genotyping of Rosa26-lsl-mGCaMP3 mice was performed by PCR using following primers: ROSA26-s (5′-ctctgctgcctcctggcttct-3′), ROSA26-as (5′-cgaggcggatcacaagcaata- 3′), CaM-s (5′-cacgtgatgacaaaccttgg-3′) and WPRE-as (5′-ggcattaaagcagcgtatcc-3′). These primers amplify a 327bp DNA fragment for the wildtype ROSA26 allele, 245 and 327 bp fragments for heterozygous mGCaMP3 mice, and a single 245 bp fragment for homozygous mGCaMP3 mice. Rosa26-lsl-mito-EGFP mice were genotyped using following primers: ROSA26-s, ROSA26-as and CMV-E-as (5′-tcaatgggcgggggtcgtt-3′). These primers amplify a 320bp DNA fragment for the wildtype ROSA26 allele, 250 and 320 bp fragments for heterozygous mito-EGFP mice, and a single 250 bp fragment for homozygous mito-EGFP mice. The Rosa26-lsl-mito-EGFP mouse line has been cryopreserved at the Jackson Laboratories (stock no: 021429).
Tamoxifen injections
The tamoxifen solution for injections (@ conc. 10 mg/mL) was freshly prepared by sonicating tamoxifen freebase (T5648, Sigma-Aldrich) in sunflower seed oil (S5007, Sigma-Aldrich) at room temperature for 10–12 min (with intermittent 20 s vortexing every 3–4 min). This solution was stored at 4°C for 5–7 days in a light protected condition or at −80°C for several months. Mice aged 3–4 weeks were intraperitoneally (i.p.) injected with tamoxifen at a dosage of 100 mg/kg body weight, once a day for two consecutive days, with each injection a minimum of 20 hours apart. All experiments were performed at least two weeks after the last tamoxifen injection.
Immunohistochemistry
6–8 weeks old mice were deeply anesthetized with pentobarbital and transcardially perfusion with 4 % paraformaldehyde (PFA) in 0.1 M sodium phosphate buffer (pH 7.4). Brains were isolated and postfixed in 4 % PFA for 16–18 h at 4° C. After post-fixation, brains were stored in phosphate-buffered saline (PBS) containing 0.2 % sodium azide at 4° C until further processed. 35 μm thick free-floating coronal brain sections were cut using a vibratome (VT1000S, Leica), and sections were collected in PBS with 0.2 % sodium azide and stored at 4° C. Before immunostaining sections were once rinsed in phosphate buffer saline (PBS), and then permeabilized for 10 min at room temperature (RT) in 0.3 % Triton-X100 in PBS. Then to prevent binding of antibodies to non-specific epitopes, sections were incubated for 1 hour at RT in a blocking buffer (0.3 % Triton-X100 and 5 % normal donkey serum in PBS). For immunolabeling, sections were incubated with primary antibodies diluted in blocking buffer for 16–18 hours at 4°C on an orbital shaker. After rinsing at RT with PBS 3x for 5 min each, sections were incubated on an orbital shaker with fluorescent dye-conjugated secondary antibodies diluted in blocking buffer for 3 hours at RT. After rinsing 3x in PBS, sections were mounted on charged glass-slides and coversliped in Aqua Ploy/Mount (Cat# 18606, Polysciences, Inc.). Primary antibodies used: goat anti-mCherry (1:5000, SICGEN), goat anti-GFP (1:5000, SICGEN), chicken anti-GFP (1:4000, Aves Lab, Inc.) and rabbit anti-GFAP (1:500, Dako), Secondary antibodies (raised in donkey): Alexa Fluor 488-, Alexa Fluor 546-, DyLight 650- or Cy2- conjugated secondary antibodies to rabbit, goat or chicken (1:500–1000, Thermo Fisher Scientific and Jackson ImmunoResearch). Images were acquired using a Zeiss LSM 510 Meta confocal microscope with a 25x (LD LCI Plan-Apochromat, Zeiss) or 63x (Plan-Apochromat, Zeiss) oil immersion objectives, and the pinhole set to 1 airy unit. Images represent maximum intensity projections of image stacks with a step size of 0.5 μm. Low magnification images were acquired as a multi-tiled array using an epifluorescence microscope (Cell Observer, Zeiss) equipped with a computer controlled stage and a 10x air objective, and were aligned in Zen software (Zeiss). Images were then processed with ImageJ.
Immuno-electron microscopy
For pre-embedding immuno-electron microscopy, GLAST-mGC3 and GLAST-mGC3;IP3R2−/− mice (16–17 weeks old) were transcardially perfused with 4 % paraformaldehyde/0.1 % glutaraldehyde in 0.1 M phosphate buffer (PB) under deep pentobarbital anesthesia. Brains, still within the skull, were postfixed in the same fixative solution for 4 h at 4° C. After post-fixation, brains were isolated and stored in 0.1 M PB with 0.05 % sodium azide at 4° C until further processed. After blocking with 5 % normal donkey serum in PBS, coronal sections (60 μm in thickness) were incubated overnight with rabbit anti-EGFP IgG (Frontier Institute) and then with anti-rabbit IgG conjugated to 1.4 nm gold particles (Thermo Fisher Scientific). Following silver enhancement (HQ silver, Nanoprobes), sections were osmificated, dehydrated and embedded in Epon 812 resin. Ultrathin sections (70 nm in thickness) were prepared with an ultramicrotome (Leica Ultracut UCT) and stained with 2 % uranyl acetate and 1 % lead citrate. Electron micrographs were taken with an H-7600 electron microscope (Hitachi, Tokyo, Japan).
Cortical astrocyte culture
Primary cortical astrocytes were cultured according to standard protocols, modified from (Schildge et al., 2013). Briefly, cortices of P4–7 GLAST-CreER;mGCaMP3/+;IP3R2−/−triple transgenic mouse pups were dissociated using a Neural Tissue Dissociation Kit (P) (Miltenyi Biotec). Isolated cells were plated on a T-75 flask coated with poly-l-lysine (Sigma-Aldrich) and fed with DMEM (Life Technologies) supplemented with 10 % heat-inactivated FBS (Life Technologies) and 1 % penicillin-streptomycin (Life Technologies). After 7 days, astrocytes formed a confluent layer at the bottom of the flask. These cells were then plated on 12 mm cover glass (Thermo Fisher Scientific) coated with poly-l-lysine (Sigma-Aldrich) and Natural Mouse Laminin (Thermo Fisher Scientific) at a density of 20,000 cells per cover glass. One day after plating, expression of mGCaMP3 was induced by applying 1 μM (Z)-4-hydroxytamoxifen (H7904, Sigma-Aldrich). At least 14 days later, astrocyte Ca2+ transients were imaged using a Zeiss LSM 710 microscope, as described below. To block mPTP activity, astrocyte cultures were incubated for 1 hour prior to Ca2+ imaging in mPTP inhibitors [Cyclosporin A (inhibits cyclophilin D, Tocris, 20 μM; takes long time to dissolve and needs filtration of remaining precipitates) and Rotenone (Mitochondrial complex I inhibitor, Tocris, 10 μM)].
Cloning and generation of UCP1 viral vector
To specifically express the Δ9 variant of the mouse uncoupling protein 1 (UPC1, genebank accession# NM_009463) (González-Barroso et al., 1997) in adult cortical astrocytes, this sequence was subcloned into a vector including a 2,178 bp human GFAP promoter fragment (exon1, genebank accession# M67446). To identify cells expressing Δ9UCP1, a cytosolic variant of red-fluorescent protein mCherry was fused to the Δ9UCP1 cDNA with the self-cleaving 2A peptide. To enhance the expression of mCherry and Δ9UCP1, the woodchuck hepatitis virus posttranscriptional regulatory element (WPRE) was sub-cloned at the 3′ end of the expression sequence (Figure S7H). The entire expression cassette (hGFAP-mCherry-2A- Δ9UCP1-WPRE) was cloned into an adeno-associated virus serotype 8 (AAV8) viral vector.
Viral injections
Adeno-associated virus was injected into adult mouse sensory cortex according to standard protocols. Briefly, 4 week old GLAST-CreER;mGCaMP3/+ double transgenic mice were injected with tamoxifen to induce expression of mGCaMP3. Mice 18 weeks post injection were anesthetized using isoflurane (Baxter), immobilized in a stereotaxic instrument (Leica Biosystems) and a burr hole made above the somatosensory cortex using a micro-drill (Harvard Apparatus). Virus was targeted to the somatosensory cortex at 1.0 mm posterior and 3.0 mm lateral to Bregma. 1×109 viral genomes of AAV8-hGFAP-mCherry-2A-Δ9UCP1-WPRE were injected 600 μm below the pial surface using a Nanoject (Drummond Scientific) at 23 nL per second. Ten weeks after the injection, acute brain slices were prepared for Ca2+ imaging.
Acute brain slice preparation
Mice were deeply anesthetized with isoflurane and decapitated using a guillotine. Their brains were dissected out and mounted on a vibratome (Leica VT100S) equipped with sapphire blade. Cortical slices 250 μm thick were cut in ice-cold N-methyl-D-glucamine (NMDG) based cutting solution, containing (in mM): 135 NMDG, 1 KCl, 1.2 KH2PO4, 1.5 MgCl2, 0.5 CaCl2, 10 Dextrose, 20 Choline Bicarbonate, (pH 7.4). Cortical slices were then transferred to artificial cerebral spinal fluid (ACSF) containing (in mM): 119 NaCl, 2.5 KCl, 2.5 CaCl2, 1.3 MgCl2, 1 NaH2PO4, 26.2 NaHCO3, and 11 Dextrose (292–298 mOsm/L) and were maintained at 37° C for 40 min, and at room temperature thereafter. Both NMDG solution and ACSF were bubbled continuously with 95 % O2/5 % CO2. All slice imaging experiments were carried out at room temperature.
Pharmacological manipulations (see KEY RESOURCES TABLE)
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Goat polyclonal anti-mCherry | SICGEN | Cat# AB0040-200; RRID:AB_2333092 |
| Goat polyclonal anti-GFP | SICGEN | Cat# AB0020-200; RRID:AB_2333099 |
| Chicken polyclonal anti-GFP | Aves Labs | Cat# GFP-1020; RRID:AB_10000240 |
| Rabbit polyclonal anti-GFAP | Dako | Cat# Z0334; RRID:AB_10013382 |
| Rabbit polyclonal anti-GFP (for immuno-EM) | Frontier Institute | Cat# GFP-Rb-Af2020; RRID:AB_2571573 |
| Donkey anti-Goat IgG (H+L), Alexa Fluor 488 | Thermo Fisher Scientific | Cat# A-11055; RRID:AB_2534102 |
| Donkey anti-Goat IgG (H+L), Alexa Fluor 546 | Thermo Fisher Scientific | Cat# A11056; RRID:AB_10584485 |
| Donkey anti-Rabbit IgG (H+L), DyLight 650 | Thermo Fisher Scientific | Cat# SA5-10049; RRID:AB_2556629 |
| Donkey anti-Chicken IgY (IgG) (H+L), Cy2 | Jackson ImmunoResearch Labs | Cat# 703-225-155; RRID:AB_2340370 |
| Goat anti-Rabbit Fab′ fragment IgG, NANOGOLD (for immuno-EM) | Thermo Fisher Scientific | Cat# N24916; RRID:AB_10104359 |
| Experimental Models: Mouse lines | ||
| Tg(Slc1a3-cre/ERT)1Nat/J | Dr. Jeremy Nathans, Johns Hopkins | RRID:IMSR_JAX:012586 |
| B6.Cg-Tg(GFAP-cre/ERT2)505Fmv/J | Jackson Laboratory | RRID:IMSR_JAX:012849 |
| Itpr2tm1Chen/Itpr2+ | Dr. Ju Chen, UC San Diego | RRID:MGI:3713675 |
| B6SJL-Tg(SOD1*G93A)1Gur/J | Jackson Laboratory | RRID:IMSR_JAX:002726 |
| B6;129-Gt(ROSA)26Sor(CAG-mGCaMP3) | This paper | N/A |
| B6;129-Gt(ROSA)26Sortm4(CAG-GFP*)Nat/J | This paper | RRID:IMSR_JAX:021429 |
| B6;129S6-Gt(ROSA)26Sortm14(CAG-tdTomato)Hze/J | Jackson Laboratory | RRID:IMSR_JAX:007908 |
| Chemicals (Pharmacological compounds) | ||
| (S)-3,5-Dihydroxyphenylglycine, DHPG | Tocris | Cat# 0805; CAS:162870-29-3 |
| Adenosine 5′-triphosphate magnesium salt | Sigma-Aldrich | Cat# A9187; CAS:74804-12-9 |
| Bafilomycin A1 | Enzo | Cat# BML-CM110-0100; CAS: 88899-55-2 |
| Benzamil | Tocris | Cat# 3380; CAS:161804-20-2 |
| Cadmium chloride | Sigma-Aldrich | Cat# 202908; CAS:10108-64-2 |
| Carbonyl cyanide 4 (trifluoromethoxy)phenylhydrazone (FCCP) | Tocris | Cat# 0453; CAS:370-86-5 |
| Carboxyatractyloside potassium salt | Calbiochem | Cat# 216201; CAS:35988-42-2 |
| CGP 37157 | Abcam | Cat# ab120012; CAS:75450-34-9 |
| Cyclosporin A | Tocris | Cat# 1101, CAS:59865-13-3 |
| Dantrolene, sodium salt | Tocris | Cat# 0507; CAS:14663-23-1 |
| DL-TBOA | Tocris | Cat# 1223; CAS:205309-81-5 |
| GSK-7975A | GlaxoSmithKline | N/A; CAS:1253186-56-9 |
| HC 030031 | Tocris | Cat# 2896; CAS:349085-38-7 |
| KB-R7943 mesylate | Tocris | Cat# 1244; CAS:182004-65-5 |
| L-(−)-Norepinephrine (+)-bitartrate salt monohydrate | Sigma-Aldrich | Cat# A9512; CAS:108341-18-0 |
| Picrotoxin | Sigma-Aldrich | Cat# P1675; CAS:124-87-8 |
| Rotenone | Tocris | Cat# 3616; CAS:83-79-4 |
| Tetrodotoxin citrate | Abcam | Cat# ab120055; CAS:18660-81-6 |
| Tamoxifen | Sigma-Aldrich | Cat# T5648; CAS:10540-29-1 |
| Thapsigargin | Sigma-Aldrich | Cat# T9033; CAS:67526-95-8 |
| Veratridine | Tocris | Cat# 2918; CAS:71-62-5 |
| Critical Commercial Assays | ||
| Neural Tissue Dissociation Kit (P) | Miltenyi Biotec | Cat# 130-092-628 |
| Recombinant DNA | ||
| AAV8-hGFAP-mCherry-2A-Δ9UCP1-WPRE | This paper | N/A |
| Sequence-Based Reagents | ||
| Primer: tcaatgggcgggggtcgtt (CMV-E-as) | (Paukert et al., 2014) | N/A |
| Primer: ctctgctgcctcctggcttct (ROSA26-s) | (Paukert et al., 2014) | N/A |
| Primer: cgaggcggatcacaagcaata (ROSA26-as) | (Paukert et al., 2014) | N/A |
| Primer: cacgtgatgacaaaccttgg (CaM-s) | This paper | N/A |
| Primer: ggcattaaagcagcgtatcc (WPRE-as) | This paper | N/A |
| Software and Algorithms | ||
| ZEN Blue/Black | Zeiss | RRID:SCR_013672 |
| ImageJ | https://imagej.nih.gov/ij/ | RRID:SCR_003070 |
| Fiji | http://fiji.sc | RRID:SCR_002285 |
| TurboReg | http://bigwww.epfl.ch/thevenaz/turboreg/ | RRID:SCR_014308 |
| MATLAB (R2014b) | MathWorks | RRID:SCR_001622 |
| LIBSVM | http://www.csie.ntu.edu.tw/~cjlin/libsvm/ | RRID:SCR_010243 |
| pClamp 9.2 | Molecular Devices | RRID:SCR_011323 |
| MiniAnalysis | Synaptosoft Inc. | RRID:SCR_014441 |
| Origin 8.0 | OriginLab Corp. | RRID:SCR_014212 |
| Prism 5.0 | GraphPad Software | RRID:SCR_002798 |
| Adobe Illustrator CS6 | Adobe | RRID:SCR_014198 |
| CaSCaDe | This paper | (see Data S1) |
For pharmacological manipulations involving thapsigargin (SERCA pump inhibitor, Sigma-Aldrich, 1 μM) and mitochondrial Ca2+ flux inhibitors [Cyclosporin A (inhibits cyclophilin D, Tocris, 20 μM; takes long time to dissolve in ACSF and requires filtration of remaining precipitates); Rotenone (mitochondrial complex I inhibitor, Tocris, 10 μM); KB-R7943 mesylate (inhibits mitochondrial Ca2+ uniporter, Tocris, 20 μM) and CGP37157 (inhibits mitochondrial Na+/Ca2+ exchanger, Abcam, 20 μM)], following recovery in ACSF at 37°C, brain sections were transferred to ACSF containing either drug or vehicle (DMSO) for 1 hour prior to Ca2+ imaging. To minimize the influence of neuronal activity on astrocyte Ca2+ signals, all pharmacological manipulations were performed in TTX (NaV antagonist, Abcam, 1 μM), unless otherwise noted. To activate metabotropic receptors, adrenergic, glutamatergic or purinergic receptor agonists L-(−)-norepinephrine (Sigma-Aldrich, 0.5 μM and 10 μM); (S)-DHPG (group I metabotropic glutamate receptor agonist, Tocris, 20 μM) or ATP (purinergic receptor agonist, Sigma-Aldrich, 100 μM) were dissolved in ASCF and applied through the superfusing solution. The following agents were acutely applied by addition to the superfusing ACSF without pre-incubation: Carboxyatractyloside (mitochondrial ADP/ATP translocase inhibitor, Calbiochem, 20 μM); Picrotoxin (GABAA receptor antagonist, Sigma-Aldrich, 100 μM); HC 030031 (TRPA1 channel antagonist, Tocris, 50 μM); Benzamil (plasma membrane Na+/Ca2+ exchanger inhibitor, Tocris, 100 μM); Dantrolene (ryanodine receptor antagonist, Tocris, 10 μM); Carbonyl cyanide 4-(trifluoromethoxy)phenylhydrazone (FCCP, uncoupler of oxidative phosphorylation, Tocris, 5 μM); GSK7975A (Orai/Icrac blocker, gift from GlaxoSmithKline, 10 μM).
Synaptic current recording and analysis
Cortical pyramidal neurons were visualized with an upright microscope (Zeiss Axioskop2 FS plus) equipped DIC optics and a CCD camera (Sony XC-73). Acute brain slices were prepared from either mGCaMP3/+ or GLAST-CreER/+;mGCaMP3/+ mice, as described above. Whole-cell recordings in voltage-clamp configuration were made under visual control using IR-DIC imaging, using pipettes filled with (in mM): 100 CsCH3SO3 [cesium methanesulfonate], 20 tetraethylammonium chloride, 20 HEPES, 1 MgCl2, 10 EGTA, 2 Na2ATP, and 0.2 NaGTP (pH 7.3, 295 mOsm). Pipette resistance was 3.8 – 4.2 MΩ and recordings were made without series resistance compensation. Baseline synaptic activity was assessed by recording spontaneous EPSCs (sEPSCs) at a holding potential of 80 mV (corrected for the junction potential) for 5 min in gap-free mode. To block vesicular release of neurotransmitters, following recovery in ACSF at 37°C for 40 min, acute brain slices were transferred to ACSF containing veratridine (voltage gated NaV opener, Tocris, 10 μM) for 5 minutes and then incubated in bafilomycin A1 (vesicular H+-ATPase inhibitor, Enzo, 4 μM) for at least 2.5 hours at room temperature (Cavelier and Attwell, 2007). While performing whole-cell voltage-clamp recordings of EPSCs (for 5 min), bafilomycin A1 (2 μM) and TTX (1μM) were applied throughout the experiment by recirculating continuously oxygenated ACSF containing these drugs using a peristaltic pump (Watson-Marlow Alitea, Sci-Q, 400 series).
Responses were recorded using a MultiClamp 700A amplifier (Molecular Devices), filtered at 3 kHz, digitized at 10 kHz and recorded to disk using pClamp9.2 software (Molecular Devices). Data were analyzed off-line using Clampfit (Molecular Devices), Origin (OriginLab) and Mini analysis (Synaptosoft) software. Input resistance and membrane capacitance were calculated from a 10 mV depolarizing step from a holding potential of −80 mV; experiments in which the input resistance changed by more than 15 % were excluded. Data are expressed as mean SEM throughout, and statistical significance was determined using the paired two-tailed Student’s test with a cutoff value of 0.05 and the Kolmogorov Smirnov (KS) test for cumulative distributions.
Time lapse fluorescence imaging in acute brain slices
Fluorescence changes arising from mGCaMP3, tdT or mito-EGFP were recorded from individual cortical astrocytes using a Zeiss LSM 710 microscope with 20x (NA 1.0) water immersion objective (Zeiss), using the 488 nm or 543 nm laser lines. Slices were continuously superfused with ACSF bubbled with 95 % O2/5 % CO2. Astrocytes are extremely photosensitive and exhibit increased Ca2+ transients when exposed to laser light (Kuga et al., 2011). Therefore, astrocytes were imaged at a low laser power of 29 μW, with no pixel averaging, high PMT gain (~ 800), and with a pinhole size of 2.69 airy units, corresponding to 3.6 μm of z-depth. For each experimental session, individual astrocytes (~ 3500–4900 μm2) were imaged at the pixel depth of 8 bit with a resolution of 512 X 512 pixels. Individual imaging sessions consisted of 600 frames at a frame scan rate of 2.1 Hz (0.484 s/frame). For laser induced photo-activation, cells were imaged using a 488 nm laser at 116 μW power for 5 minutes. To compensate for minor drift in the XY plane, image stacks were post hoc registered using TurboReg (plugin for ImageJ) for automatic alignment of images. During registration of 8-bit images TurboReg transforms images into a 32-bit format. After registration, images were reformatted to 16-bit, as reverting back to 8-bit blunted signal amplitudes. Surrounding background pixels that were not part of the cell (based on median-intensity projection of the image stack) were cropped to accelerate analysis. Astrocytes located on the surface of the slice, those that had large blood vessels passing through them, or exhibited image registration artifacts were excluded from the data set.
In vivo two photon microscopy
Mice aged 2–4 months were implanted with cranial windows, as described (Paukert et al., 2014). Mice were anesthetized with isoflurane (induction, 5 %; maintenance, 1.5–2.0 %, mixed with 0.5 l/min O2) and their body temperature was monitored and maintained at 37° C with a thermostat-controlled heating pad. An incision was made over the right cerebral hemisphere, the skin retracted, and the skull cleaned. A 2 × 2 mm region of skull over the visual cortex (centered at lambda and 3.5 mm lateral from the midline) was removed using a scalpel. A 2 × 2 mm piece of cover glass (VWR, No. 1) was placed within the craniotomy, sealed, and a 9 mm-wide aluminum head plate with a 5 mm circular opening was attached to the skull using dental cement (C&B Metabond; Parkell Bio-Materials Div.). In vivo imaging sessions began 3–4 hours after surgery, following complete recovery from anesthesia. During an imaging session, the head of the mouse was immobilized by attaching the head plate to a custom machined stage fitted with a freely-movable linear treadmill. Mice were kept on the stage for a maximum of two hours. Fluorescence images were collected using a Zeiss LSM 710 microscope equipped with a Zeiss 20x, 1.0 NA objective. Two photon excitation was achieved using a mode-locked Ti:sapphire laser (Coherent Ultra II) tuned to 920 nm. Each image frame was acquired at the rate of 0.484 s/frame (about 2.1 Hz) and was approximately 60 μm × 60 μm at 512 × 512 pixel resolution. The average power at the sample did not exceed 20 mW during the imaging session.
QUANTIFICATION AND STATISTICAL ANALYSIS
Automated extraction and analysis of Ca2+ transients
We developed an algorithm to identify and extract kinetic information about individual microdomain activity in MATLAB (Mathworks, Natick, MA). To remove background noise, 3D convolutions were performed using average and Gaussian filters of size 23 × 23 × 43 pixels (x,y,t) on time-series image stacks (I(x,y,t)). Then a noise filtered image stack (Ifil(x,y,t)) was obtained by subtracting the 3D convolution products of average and Gaussian filtering. To determine the locations of regions exhibiting dynamic changes in fluorescence, the mean intensity (Ibg) and standard deviation (σbg) of background pixels on sum-intensity projected noise filtered stacks (Ifil(x,y,t)) was calculated. Next, the sum-intensity projected image was binarized using a threshold value of Ibg + 2*σbg. After smoothing the image with a 43 × 43 pixels2 Gaussian filter and standard deviation of 3 pixel, regional maximas were detected on the sum-intensity projected image. To generate a mask of putative microdomains, all microdomains with more than two regional maxima using a marker-controlled watershed segmentation method were segmented, and microdomains with an area less than 5 × 5 pixels2 were considered at noise level and removed from the binarized image. Then based on this binarized mask of potential microdomains, the intensity level over time of individual microdomains from the raw time series image stack I(x,y,t)) was plotted. The intensity profile of each microdomain was normalized by subtracting the mean intensity value of its background and then to rescale, this value was divided by the standard deviation of the background. In this study, this value is represented as a modified z-score (z). Microdomain events were then automatically detected based on the following criteria: to consider an event to be positive it must consist of a regional maximum of more than 5.0 a.u. within 4 neighboring frames, the beginning and end time point of each event was identified based on the time point prior to or after the peak that had an intensity value larger than 0.5 a.u. Events that spanned less than 4 frames were excluded.
Machine learning-based signal classification
The Support Vector Machine (SVM) algorithm was used to detect active microdomains based on 75 parameters of each individual event. These parameters were extracted from a raw and two smoothed intensity profiles with different degree of smoothness. The smoothed intensity profiles were obtained from the difference between convolving raw intensity profiles with a Gaussian filter with unit standard deviation and an averaging filter of the same size. Two smoothed intensity profiles were obtained using filters of size 11 and 21 frames separately. Twenty-five parameters each were used to describe each individual event with these three intensity profiles. For initial training, the SVM algorithm was provided a set of 2500 fluorescence signals manually categorized as positive or negative, and various parameters of these events were normalized to a range between 0 and 1. These normalized parameters were used to generate a SVM model using libsvm. This SVM prediction model was applied to all events, and events classified as negative by libsvm were excluded. The overall accuracy from SVM prediction was >85 %, assessed by comparing the predicted results from an additional set of manually registered 2500 events, and ~95 % based on fluctuations in Ca2+-insensitive fluorescent protein tdTomato (Figure S1K–P).
Software Availability
The MATLAB code for CaSCaDe analysis routine (outlined above) is provided as supplemental text (see Data S1).
Statistical Analysis
Statistical analyses were performed using GraphPad Prism (version 5.0b) and Origin 8 (OriginLab Corp). All data sets were tested for Gaussian distribution with the D’Agostino-Pearson “omnibus K2″ normality test. If one of the two or both the datasets passed the normality test, a two-tailed unpaired t-test was used to compare the significance difference in means between the two groups, otherwise a nonparametric test (Mann-Whitney test) was chosen. For multiple group datasets, one-way ANOVA analysis was used, followed by Tukey’s multiple comparison tests for more than two groups. When two or more consecutive experiments were performed on the same cell, then a two-tailed paired t-test or a repeated measure ANOVA analysis followed by Bonferroni correction for multiple comparisons was carried out. Statistical tests used to measure significance are indicated in each figure legend along with the corresponding significance level (p value). For all ex vivo and in vivo experiments, N represents number of cells analyzed per experiment and the exact value of N is provided in the corresponding figure legends. For in vitro experiments (Figure S6), N represents number of 12 mm coverslips on which cells were grown and n represents number of cells analyzed per condition. In vitro experiments were repeated from at least two separate cohorts of mice. Data are reported as mean ± SEM and p < 0.05 was considered statistically significant.
CONTACT FOR REAGENT AND RESOURCE SHARING
Dr. Dwight Bergles (dbergles@jhmi.edu) is the Lead Contact for reagent and resource sharing. All published reagents will be shared on an unrestricted basis; reagent requests should be directed to the lead author.
Supplementary Material
Acknowledgments
We thank Dr. Jeremy Nathans (Johns Hopkins University) for assistance in generating mGCaMP3 and mito-EGFP mouse lines, and for providing GLAST-CreER BAC transgenic mice. We thank Drs. Loren Looger and Tianyi Mao at the (Janelia Research campus, HHMI) for the GCaMP3 construct, Dr. Ju Chen for providing IP3R2 null mutant mice (UCSD), Naiqing Ye for assistance with animal colony maintenance, and Holly Wellington and Chip Hawkins at the Johns Hopkins Transgenic Core for the assistance with generation of Rosa26 targeted mice. A.A. was supported in part by a Postdoctoral Fellowship from the National Multiple Sclerosis Society and 2015 NARSAD Young Investigator Grant from the Brain & Behavior Research Foundation. This work was supported by grants from the NIH MH084020 (Conte Center for Neuroscience), NS050274, and the Brain Science Institute at Johns Hopkins to D.E.B.
Footnotes
Author Contributions
A.A. and D.E.B. conceived the project, designed the experiments and wrote the manuscript with help from the other authors. A.A. generated and characterized the Rosa26-lsl-mGCaMP3 mice, conducted Ca2+ imaging and electrophysiology experiments, contributed to the development of CaSCaDe software and analyzed data. P-H.W. and D.W. designed and developed the CaSCaDe software. E.G.H. conducted in vivo Ca2+ imaging experiments. M.F. conducted immuno-electron microscopy and analyzed these data. M.A.T. generated the Rosa26-lsl-mito-EGFP mice. A.J.L. assisted with the experiments on cultured astrocytes and in vivo delivery of UCP1.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Araque A, Carmignoto G, Haydon PG, Oliet SHR, Robitaille R, Volterra A. Gliotransmitters travel in time and space. Neuron. 2014;81:728–739. doi: 10.1016/j.neuron.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bánsághi S, Golenár T, Madesh M, Csordás G, RamachandraRao S, Sharma K, Yule DI, Joseph SK, Hajnóczky G. Isoform- and species-specific control of inositol 1,4,5-trisphosphate (IP3) receptors by reactive oxygen species. J Biol Chem. 2014;289:8170–8181. doi: 10.1074/jbc.M113.504159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardi P, Petronilli V. The permeability transition pore as a mitochondrial calcium release channel: a critical appraisal. J Bioenerg Biomembr. 1996;28:131–138. doi: 10.1007/BF02110643. [DOI] [PubMed] [Google Scholar]
- Bélanger M, Allaman I, Magistretti PJ. Brain energy metabolism: focus on astrocyte-neuron metabolic cooperation. Cell Metabolism. 2011;14:724–738. doi: 10.1016/j.cmet.2011.08.016. [DOI] [PubMed] [Google Scholar]
- Cavelier P, Attwell D. Neurotransmitter depletion by bafilomycin is promoted by vesicle turnover. Neurosci Lett. 2007;412:95–100. doi: 10.1016/j.neulet.2006.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Castro MA, Chuquet J, Liaudet N, Bhaukaurally K, Santello M, Bouvier D, Tiret P, Volterra A. Local Ca2+ detection and modulation of synaptic release by astrocytes. Nat Neurosci. 2011;14:1276–1284. doi: 10.1038/nn.2929. [DOI] [PubMed] [Google Scholar]
- Freeman MR, Rowitch DH. Evolving concepts of gliogenesis: a look way back and ahead to the next 25 years. Neuron. 2013;80:613–623. doi: 10.1016/j.neuron.2013.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganat YM, Silbereis J, Cave C, Ngu H, Anderson GM, Ohkubo Y, Ment LR, Vaccarino FM. Early postnatal astroglial cells produce multilineage precursors and neural stem cells in vivo. J Neurosci. 2006;26:8609–8621. doi: 10.1523/JNEUROSCI.2532-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Barroso MM, Fleury C, Levi-Meyrueis C, Zaragoza P, Bouillaud F, Rial E. Deletion of amino acids 261–269 in the brown fat uncoupling protein converts the carrier into a pore. Biochemistry. 1997;36:10930–10935. doi: 10.1021/bi971104y. [DOI] [PubMed] [Google Scholar]
- Grosche J, Matyash V, Möller T, Verkhratsky A, Reichenbach A, Kettenmann H. Microdomains for neuron-glia interaction: parallel fiber signaling to Bergmann glial cells. Nat Neurosci. 1999;2:139–143. doi: 10.1038/5692. [DOI] [PubMed] [Google Scholar]
- Gurney ME, Pu HF, Chiu AY, Dal Canto MC, Polchow CY, Alexander DD, Caliendo J, Hentati A, Kwon YW, Deng HX, et al. Motor-Neuron Degeneration in Mice That Express a Human Cu, Zn Superoxide-Dismutase Mutation. Science. 1994;264:1772–1775. doi: 10.1126/science.8209258. [DOI] [PubMed] [Google Scholar]
- Haydon PG. GLIA: listening and talking to the synapse. Nat Rev Neurosci. 2001;2:185–193. doi: 10.1038/35058528. [DOI] [PubMed] [Google Scholar]
- Hems DA, Whitton PD. Control of hepatic glycogenolysis. Physiol Rev. 1980;60:1–50. doi: 10.1152/physrev.1980.60.1.1. [DOI] [PubMed] [Google Scholar]
- Hertz L, Peng L, Dienel GA. Energy metabolism in astrocytes: high rate of oxidative metabolism and spatiotemporal dependence on glycolysis/glycogenolysis. J Cereb Blood Flow Metab. 2007;27:219–249. doi: 10.1038/sj.jcbfm.9600343. [DOI] [PubMed] [Google Scholar]
- Hou T, Wang X, Ma Q, Cheng H. Mitochondrial flashes: new insights into mitochondrial ROS signaling and beyond. J Physiol (Lond) 2014;592:3703–3713. doi: 10.1113/jphysiol.2014.275735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichas F, Jouaville LS, Mazat JP. Mitochondria are excitable organelles capable of generating and conveying electrical and calcium signals. Cell. 1997;89:1145–1153. doi: 10.1016/s0092-8674(00)80301-3. [DOI] [PubMed] [Google Scholar]
- Jackson JG, Robinson MB. Reciprocal Regulation of Mitochondrial Dynamics and Calcium Signaling in Astrocyte Processes. Journal of Neuroscience. 2015;35:15199–15213. doi: 10.1523/JNEUROSCI.2049-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanemaru K, Sekiya H, Xu M, Satoh K, Kitajima N, Yoshida K, Okubo Y, Sasaki T, Moritoh S, Hasuwa H, et al. In vivo visualization of subtle, transient, and local activity of astrocytes using an ultrasensitive Ca(2+) indicator. CellReports. 2014;8:311–318. doi: 10.1016/j.celrep.2014.05.056. [DOI] [PubMed] [Google Scholar]
- Khakh BS, McCarthy KD. Astrocyte Calcium Signaling: From Observations to Functions and the Challenges Therein. Cold Spring Harb Perspect Biol. 2015:a020404. doi: 10.1101/cshperspect.a020404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosaka T, Hama K. Three-dimensional structure of astrocytes in the rat dentate gyrus. J Comp Neurol. 1986;249:242–260. doi: 10.1002/cne.902490209. [DOI] [PubMed] [Google Scholar]
- Krauss S, Zhang CY, Lowell BB. The mitochondrial uncoupling-protein homologues. Nat Rev Mol Cell Biol. 2005;6:248–261. doi: 10.1038/nrm1592. [DOI] [PubMed] [Google Scholar]
- Kuchibhotla KV, Lattarulo CR, Hyman BT, Bacskai BJ. Synchronous hyperactivity and intercellular calcium waves in astrocytes in Alzheimer mice. Science. 2009;323:1211–1215. doi: 10.1126/science.1169096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuga N, Sasaki T, Takahara Y, Matsuki N, Ikegaya Y. Large-scale calcium waves traveling through astrocytic networks in vivo. J Neurosci. 2011;31:2607–2614. doi: 10.1523/JNEUROSCI.5319-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Chauvin C, De Paulis D, De Oliveira F, Gharib A, Vial G, Lablanche S, Leverve X, Bernardi P, Ovize M, et al. Inhibition of complex I regulates the mitochondrial permeability transition through a phosphate-sensitive inhibitory site masked by cyclophilin D. Biochimica Et Biophysica Acta (BBA) - Bioenergetics. 2012;1817:1628–1634. doi: 10.1016/j.bbabio.2012.05.011. [DOI] [PubMed] [Google Scholar]
- Li X, Zima AV, Sheikh F, Blatter LA, Chen J. Endothelin-1-induced arrhythmogenic Ca2+ signaling is abolished in atrial myocytes of inositol-1,4,5-trisphosphate(IP3)-receptor type 2-deficient mice. Circ Res. 2005;96:1274–1281. doi: 10.1161/01.RES.0000172556.05576.4c. [DOI] [PubMed] [Google Scholar]
- Liu J, Lillo C, Jonsson PA, Vande Velde C, Ward CM, Miller TM, Subramaniam JR, Rothstein JD, Marklund S, Andersen PM, et al. Toxicity of familial ALS-linked SOD1 mutants from selective recruitment to spinal mitochondria. Neuron. 2004;43:5–17. doi: 10.1016/j.neuron.2004.06.016. [DOI] [PubMed] [Google Scholar]
- Lovatt D, Sonnewald U, Waagepetersen HS, Schousboe A, He W, Lin JHC, Han X, Takano T, Wang S, Sim FJ, et al. The transcriptome and metabolic gene signature of protoplasmic astrocytes in the adult murine cortex. J Neurosci. 2007;27:12255–12266. doi: 10.1523/JNEUROSCI.3404-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madisen L, Zwingman TA, Sunkin SM, Oh SW, Zariwala HA, Gu H, Ng LL, Palmiter RD, Hawrylycz MJ, Jones AR, et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci. 2010;13:133–140. doi: 10.1038/nn.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magistretti PJ, Allaman I. A cellular perspective on brain energy metabolism and functional imaging. Neuron. 2015;86:883–901. doi: 10.1016/j.neuron.2015.03.035. [DOI] [PubMed] [Google Scholar]
- Mailloux RJ, Harper ME. Mitochondrial proticity and ROS signaling: lessons from the uncoupling proteins. Trends Endocrinol Metab. 2012;23:451–458. doi: 10.1016/j.tem.2012.04.004. [DOI] [PubMed] [Google Scholar]
- Naga KK, Sullivan PG, Geddes JW. High cyclophilin D content of synaptic mitochondria results in increased vulnerability to permeability transition. J Neurosci. 2007;27:7469–7475. doi: 10.1523/JNEUROSCI.0646-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai M, Re DB, Nagata T, Chalazonitis A, Jessell TM, Wichterle H, Przedborski S. Astrocytes expressing ALS-linked mutated SOD1 release factors selectively toxic to motor neurons. Nat Neurosci. 2007;10:615–622. doi: 10.1038/nn1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nett WJ, Oloff SH, McCarthy KD. Hippocampal astrocytes in situ exhibit calcium oscillations that occur independent of neuronal activity. J Neurophysiol. 2002;87:528–537. doi: 10.1152/jn.00268.2001. [DOI] [PubMed] [Google Scholar]
- Nimmerjahn A, Mukamel EA, Schnitzer MJ. Motor behavior activates Bergmann glial networks. Neuron. 2009;62:400–412. doi: 10.1016/j.neuron.2009.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parone PA, Da Cruz S, Han JS, McAlonis-Downes M, Vetto AP, Lee SK, Tseng E, Cleveland DW. Enhancing mitochondrial calcium buffering capacity reduces aggregation of misfolded SOD1 and motor neuron cell death without extending survival in mouse models of inherited amyotrophic lateral sclerosis. J Neurosci. 2013;33:4657–4671. doi: 10.1523/JNEUROSCI.1119-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paukert M, Agarwal A, Cha J, Doze VA, Kang JU, Bergles DE. Norepinephrine controls astroglial responsiveness to local circuit activity. Neuron. 2014;82:1263–1270. doi: 10.1016/j.neuron.2014.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulsen RE, Contestabile A, Villani L, Fonnum F. An in vivo model for studying function of brain tissue temporarily devoid of glial cell metabolism: the use of fluorocitrate. J Neurochem. 1987;48:1377–1385. doi: 10.1111/j.1471-4159.1987.tb05674.x. [DOI] [PubMed] [Google Scholar]
- Petravicz J, Fiacco TA, McCarthy KD. Loss of IP3 receptor-dependent Ca2+ increases in hippocampal astrocytes does not affect baseline CA1 pyramidal neuron synaptic activity. J Neurosci. 2008;28:4967–4973. doi: 10.1523/JNEUROSCI.5572-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzuto R, Nakase H, Darras B, Francke U, Fabrizi GM, Mengel T, Walsh F, Kadenbach B, DiMauro S, Schon EA. A gene specifying subunit VIII of human cytochrome c oxidase is localized to chromosome 11 and is expressed in both muscle and non-muscle tissues. J Biol Chem. 1989;264:10595–10600. [PubMed] [Google Scholar]
- Rizzuto R, Pinton P, Carrington W, Fay FS, Fogarty KE, Lifshitz LM, Tuft RA, Pozzan T. Close contacts with the endoplasmic reticulum as determinants of mitochondrial Ca2+ responses. Science. 1998;280:1763–1766. doi: 10.1126/science.280.5370.1763. [DOI] [PubMed] [Google Scholar]
- Rizzuto R, De Stefani D, Raffaello A, Mammucari C. Mitochondria as sensors and regulators of calcium signalling. Nat Rev Mol Cell Biol. 2012;13:566–578. doi: 10.1038/nrm3412. [DOI] [PubMed] [Google Scholar]
- Rungta RL, Bernier L-P, Dissing-Olesen L, Groten CJ, LeDue JM, Ko R, Drissler S, MacVicar BA. Ca(2+) transients in astrocyte fine processes occur via Ca(2+) influx in the adult mouse hippocampus. Glia. 2016 doi: 10.1002/glia.23042. [DOI] [PubMed] [Google Scholar]
- Schildge S, Bohrer C, Beck K, Schachtrup C. Isolation and culture of mouse cortical astrocytes. J Vis Exp. 2013 doi: 10.3791/50079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigetomi E, Bushong EA, Haustein MD, Tong X, Jackson-Weaver O, Kracun S, Xu J, Sofroniew MV, Ellisman MH, Khakh BS. Imaging calcium microdomains within entire astrocyte territories and endfeet with GCaMPs expressed using adeno-associated viruses. J Gen Physiol. 2013;141:633–647. doi: 10.1085/jgp.201210949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigetomi E, Tong X, Kwan KY, Corey DP, Khakh BS. TRPA1 channels regulate astrocyte resting calcium and inhibitory synapse efficacy through GAT-3. Nat Neurosci. 2012;15:70–80. doi: 10.1038/nn.3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan R, Huang BS, Venugopal S, Johnston AD, Chai H, Zeng H, Golshani P, Khakh BS. Ca(2+) signaling in astrocytes from Ip3r2(−/−) mice in brain slices and during startle responses in vivo. Nat Neurosci. 2015;18:708–717. doi: 10.1038/nn.4001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephen TL, Higgs NF, Sheehan DF, Al Awabdh S, López-Doménech G, Arancibia-Carcamo IL, Kittler JT. Miro1 Regulates Activity-Driven Positioning of Mitochondria within Astrocytic Processes Apposed to Synapses to Regulate Intracellular Calcium Signaling. J Neurosci. 2015;35:15996–16011. doi: 10.1523/JNEUROSCI.2068-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian L, Hires SA, Mao T, Huber D, Chiappe ME, Chalasani SH, Petreanu L, Akerboom J, McKinney SA, Schreiter ER, et al. Imaging neural activity in worms, flies and mice with improved GCaMP calcium indicators. Nat Meth. 2009;6:875–881. doi: 10.1038/nmeth.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ververken D, Van Veldhoven P, Proost C, Carton H, De Wulf H. On the role of calcium ions in the regulation of glycogenolysis in mouse brain cortical slices. J Neurochem. 1982;38:1286–1295. doi: 10.1111/j.1471-4159.1982.tb07903.x. [DOI] [PubMed] [Google Scholar]
- Wang W, Fang H, Groom L, Cheng A, Zhang W, Liu J, Wang X, Li K, Han P, Zheng M, et al. Superoxide Flashes in Single Mitochondria. Cell. 2008;134:279–290. doi: 10.1016/j.cell.2008.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward SM, Ordog T, Koh SD, Baker SA, Jun JY, Amberg G, Monaghan K, Sanders KM. Pacemaking in interstitial cells of Cajal depends upon calcium handling by endoplasmic reticulum and mitochondria. J Physiol (Lond) 2000;525(Pt 2):355–361. doi: 10.1111/j.1469-7793.2000.t01-1-00355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneda S, Takano H, Takaki M, Suzuki H. Properties of spontaneously active cells distributed in the submucosal layer of mouse proximal colon. J Physiol (Lond) 2002;542:887–897. doi: 10.1113/jphysiol.2002.018705. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.