Abstract
Background
A clear understanding of the neural basis of consciousness is fundamental to research in clinical and basic neuroscience disciplines, and anesthesia. Recently, decreased efficiency of information integration was suggested as a core network feature of propofol-induced unconsciousness. However, it is unclear whether this finding can be generalized to dexmedetomidine, which has a different molecular target.
Methods
Dexmedetomidine was administered as a 1 μg/kg bolus over 10 minutes, followed by a 0.7 μg/kg/hr infusion to healthy human volunteers, 18–36 years of age (n = 15). Resting state functional magnetic resonance (rsfMRI) data were acquired during baseline, dexmedetomidine-induced altered arousal, and recovery states. Zero-lag correlations between rsfMRI signals extracted from 131 brain parcellations were used to construct weighted brain networks. Network efficiency, degree distribution, and node strength were computed using graph analysis. Parcellated brain regions were also mapped to known resting state networks to study functional connectivity changes.
Results
Dexmedetomidine significantly reduced local and global efficiency of graph theory derived networks. Dexmedetomidine also reduced the average brain connectivity strength without impairing the degree distribution. Functional connectivity within and between all resting state networks was modulated by dexmedetomidine.
Conclusions
Dexmedetomidine is associated with a significant drop in the capacity for efficient information transmission at both local and global levels. These changes result from reductions in the strength of connectivity, and also manifest as reduced within and between resting state network connectivity. These findings strengthen the hypothesis that conscious processing relies on an efficient system of information transfer in the brain.
Introduction
Understanding the neural basis of consciousness is fundamental to research in clinical and basic neuroscience disciplines, and anesthesia.1 However, we are yet to clearly decipher how the human brain mediates consciousness. This is because the brain is a complex biological system composed of components that interact dynamically to give rise to higher brain functions.2,3 For example, the sensory experience of a painfully hot object comprises of various composite experiences4 (i.e. intensity, duration, quality, emotional distress) that are integrated by the brain and represented as a unified experience of pain.2,3,5-9
Synchronization of resting state functional MRI (rsfMRI) slow signals is a proxy for the putative neural syntax that integrates various brain regions into networks mediating higher brain functions.10-13 Numerous investigations of anesthesia-induced changes to rsfMRI networks have been conducted. For a review, see Hudetz, 2012.14 However, less studied is how anesthetics alter the capacity of information transfer in rsfMRI brain networks, and how this relates to the brain state in question. This graph-theoretic approach, which is distinct from standard functional connectivity analyses, is based on the fundamental premise that the brain is topologically organized to maximize information transfer within and between networks.15,16 This approach parallels theories of consciousness that stress integrated information processing.8,9
Network efficiency is a graph theoretical measure of information exchange. A recent study of the gamma amino-butyric acid receptor agonist propofol described decreased efficiency of information transfer as a key differentiating feature of conscious and unconscious brain states.17 Similarly, decreased efficiency of information transfer has also been suggested to account for decreased consciousness during non-rapid eye movement sleep.18,19 Thus, altered states of arousal may be associated with network reconfigurations that favor decreased efficiency of communication. However, the generalizability of this finding is unclear. Thus, studies of anesthetics from different drug classes are necessary to help refine our knowledge of information processing correlates of unconsciousness.
Using a graph theoretical approach, we studied network changes associated with dexmedetomidine, an alpha-2 adrenergic agonist that activates endogenous sleep pathways.20-24 We hypothesized that dexmedetomidine would result in decreased capacity for information integration as represented by surrogate markers of local and global network communication (local and global efficiency). Furthermore, we hypothesized that this decrease in information integration results from reduced synchronization strength between brain regions. In previous work we found that dexmedetomidine preferentially decreased blood flow and metabolism in the thalamus, Default Mode Network, and bilateral Frontoparietal Networks.1 Therefore, we also hypothesized that between-network disruption in these and other RSNs are associated with dexmedetomidine.
To explore these hypotheses, blood oxygen level dependent (BOLD) signals obtained during baseline, dexmedetomidine-induced altered arousal, and recovery states were analyzed (n = 15, 18 to 36 years of age). Zero-lag correlations between rsfMRI signals extracted from 131 brain parcellations were used to construct weighted brain networks. Network efficiency and node strength were computed using graph analysis. Parcellated brain regions were also mapped to known RSNs (Table 1). This dataset was previously reported in a within-network analysis of the Default Mode and Frontoparietal Networks.1
Table 1.
Regions spanning the right and left hemispheres were separated to create hemisphere specific regions. The complete parcellation scheme consisted of a total of 131 regions (Table 1). A list of these regions, their co-ordinates, as well as a description of the anatomical landmarks used for the subdivision of the Harvard-Oxford labels in smaller parcellates have previously been published.
| Networks | Regions | Abbreviations |
MNI Co-
ordinates |
||
|---|---|---|---|---|---|
| x | y | z | |||
| 1 | Brain stem | BrStem | 0 | −26 | −28 |
| 1 | Amygdala Right | Amyg_R | 24 | −4 | −18 |
| 1 | Caudate Right | Caud_R | 12 | 12 | 10 |
| 1 | Globus Pallidus Right | GP_R | 16 | −2 | −2 |
| 1 | Hippocampus Right | Hipp_R | 28 | −22 | −16 |
| 1 | Nucleus Accumbens Right | NAc_R | 10 | 10 | −8 |
| 1 | Putamen Right | Put_R | 20 | −4 | 0 |
| 1 | Thalamus Right | Thal_R | 10 | −18 | 8 |
| 1 | Thalamus Left | Thal_L | 10 | −18 | 8 |
| 1 | Putamen Left | Put_L | −20 | −4 | 0 |
| 1 | Nucleus Accumbens Left | NAc_L | −10 | 10 | −8 |
| 1 | Hippocampus Left | Hipp_L | −28 | −22 | −16 |
| 1 | Globus Pallidus Left | GP_L | −16 | −2 | −2 |
| 1 | Caudate Left | Caud_L | −12 | 14 | 8 |
| 1 | Amygdala Left | Amyg_L | −24 | −4 | −18 |
| 2 | Central Opercular Cortex Right | Cop_R | 48 | −4 | 8 |
| 2 | Dorsal Anterior Insula Right | dINSa_R | 32 | 20 | 0 |
| 2 | Middle Insula Right | INSm_R | 40 | −2 | −2 |
| 2 | Posterior Insula Right | INSp_R | 38 | −14 | 8 |
| 2 | Postcentral Gyrus Right | PostC_R | 54 | −20 | 46 |
| 2 | Precentral Gyrus Right | PreC_R | 44 | −8 | 52 |
| 2 | Supra Marginal Gyrus Right | SMGa_R | 58 | 32 | 40 |
| 2 | Ventral Anterior Insula Right | vINSa_R | 36 | 10 | −14 |
| 2 | Temporal Occipital Fusiform Cortex Right | TOF_R | 34 | −54 | −16 |
| 2 | Temporal Fusiform Cortex, posterior division Right |
TFCp_R | 36 | −16 | −32 |
| 2 | Planum Polare Right | PlP_R | 48 | −4 | −6 |
| 2 | Planum Temporale Right | PlT_R | 60 | −22 | 8 |
| 2 | Heschls Gyrus (includes H1 and H2) Right | He_R | 48 | −18 | −6 |
| 2 | Cuneal Cortex Right | Cun_R | 4 | −82 | 30 |
| 2 | Intracalcarine Cortex Right | IC_R | 6 | −68 | 12 |
| 2 | Lateral Occipital Cortex, inferior division Right |
LOcci_R | 48 | −78 | −2 |
| 2 | Lateral Occipital Cortex, superior division Right |
LOccs_R | 40 | −78 | 34 |
| 2 | Occipital Fusiform Gyrus Right | OccFG_R | 28 | −76 | −14 |
| 2 | Occipital Pole Right | OccP_R | 8 | −100 | 6 |
| 2 | Precuneous Cortex Right | pCun_R | 4 | −64 | 38 |
| 2 | Supracalcarine Cortex Right | Sc_R | 2 | −84 | 12 |
| 2 | Supracalcarine Cortex Left | Sc_L | −2 | −84 | 12 |
| 2 | Precuneous Cortex Left | pCun_L | −4 | −82 | 30 |
| 2 | Occipital Pole Left | OccP_L | −8 | −100 | 6 |
| 2 | Occipital Fusiform Gyrus Left | OccFG_L | −28 | −76 | −14 |
| 2 | Lateral Occipital Cortex, superior division Left |
LOccs_L | −40 | −78 | 34 |
| 2 | Lateral Occipital Cortex, inferior division Left |
LOcci_L | −48 | −78 | −2 |
| 2 | Intracalcarine Cortex Left | IC_L | −28 | −76 | −14 |
| 2 | Cuneal Cortex Left | Cun_L | −6 | −74 | 12 |
| 2 | Heschls Gyrus (includes H1 and H2) Left | He_L | −48 | −18 | 6 |
| 2 | Planum Temporale Left | PlT_L | −60 | −22 | 8 |
| 2 | Planum Polare Left | PlP_L | −48 | −4 | −6 |
| 2 | Temporal Fusiform Cortex, posterior division Left |
TFCp_L | −36 | −16 | −32 |
| 2 | Temporal Occipital Fusiform Cortex Left | TOF_L | −34 | −54 | −16 |
| 2 | Ventral Anterior Insula Left | vINSa_L | −36 | 10 | −14 |
| 2 | Supra Marginal Gyrus Left | SMGa_L | −54 | −56 | 26 |
| 2 | Precentral Gyrus Left | PreC_L | −44 | −8 | 52 |
| 2 | Postcentral Gyrus Left | PostC_L | −58 | 32 | 40 |
| 2 | Posterior Insula Left | INSp_L | −38 | −14 | 8 |
| 2 | Middle Insula Left | INSm_L | −40 | −2 | −2 |
| 2 | Dorsal Anterior Insula Left | dINSa_L | −32 | 20 | 0 |
| 2 | Central Opercular Cortex Left | Cop_L | −48 | −4 | 8 |
| 3 | Caudal Anterior Cingulate Right | ACCc_R | 4 | 40 | −2 |
| 3 | Rostral Anterior Cingulate mid posterior Right |
ACCrm_R | −6 | 60 | 8 |
| 3 | Rostral Anterior Cingulate posterior Right | ACCrp_R | −6 | −2 | 42 |
| 3 | Rostral Anterior Cingulate Right | ACCr_R | 2 | 28 | 18 |
| 3 | Subgenual Anterior Cingulate Right | ACCsg_R | 4 | 16 | −14 |
| 3 | Dorsal Medial Prefrontal Cortex, anterior division Right |
dMPFCa_R | 4 | 50 | 28 |
| 3 | Frontal Orbital Cortex Right | FO_R | 40 | 20 | 4 |
| 3 | Medial Prefrontal Cortex Right | MPFC_R | 6 | 60 | 8 |
| 3 | Ventral Medial Prefrontal Cortex Right | vMPFC_R | 4 | 50 | −20 |
| 3 | Angular Gyrus Right | Ang_R | 54 | −56 | 26 |
| 3 | Cingulate Gyrus, posterior division Right | Cingp_R | 4 | −38 | 32 |
| 3 | Cingulate Gyrus, posterior division Left | Cingp_L | −4 | −38 | 32 |
| 3 | Angular Gyrus Left | Ang_L | −48 | −4 | 8 |
| 3 | Ventral Medial Prefrontal Cortex Left | vMPFC_L | −4 | 50 | −20 |
| 3 | Medial Prefrontal Cortex Left | MPFC_L | −6 | 60 | 8 |
| 3 | Frontal Orbital Cortex Left | FO_L | −40 | 30 | −14 |
| 3 | Dorsal Medial Prefrontal Cortex, anterior division Left |
dMPFCa_L | −4 | 50 | 28 |
| 3 | Subgenual Anterior Cingulate Left | ACCsg_L | −2 | 28 | 18 |
| 3 | Rostral Anterior Cingulate posterior Left | ACCrp_L | 6 | 18 | 34 |
| 3 | Rostral Anterior Cingulate mid posterior Left |
ACCrm_L | 20 | 28 | 18 |
| 3 | Rostral Anterior Cingulate Left | ACCr_L | −6 | 18 | 34 |
| 3 | Caudal Anterior Cingulate Left | ACCc_L | −4 | 40 | −2 |
| 4 | Frontal Pole Right | FP_R | 30 | 54 | 20 |
| 4 | Orbito Frontal Pole Right | OFP_R | 32 | 58 | −6 |
| 4 | Superior Frontal Gyrus Right | SFG_R | 20 | 18 | 62 |
| 4 | Middle Frontal Gyrus Right | MFG_R | 40 | 20 | 44 |
| 4 | Supramarginal Gyrus, posterior division Right |
SMGp_R | 60 | −48 | 32 |
| 4 | Superior Parietal Lobule Right | SPL_R | 32 | −50 | 60 |
| 4 | Mid Anterior Cingulate Right | ACCm_R | 6 | −2 | 42 |
| 4 | Dorsal Medial Prefrontal Cortex, posterior division Right |
dMPFCp_R | 4 | 26 | 48 |
| 4 | Frontal Operculum Cortex Right | Fop_R | −48 | −32 | 20 |
| 4 | Inferior Frontal Gyrus, pars opercularis Right |
IFGpo_R | 54 | 14 | 16 |
| 4 | Inferior Frontal Gyrus, pars triangularis Right |
IFGpt_R | −50 | 30 | 16 |
| 4 | Parietal Operculum Cortex Right | Pop_R | 48 | −32 | 20 |
| 4 | Supplementary Motor Area Right | SMA_R | 4 | −2 | 58 |
| 4 | Supplementary Motor Area Left | SMA_L | −4 | −2 | 58 |
| 4 | Parietal Operculum Cortex Left | Pop_L | −38 | 4 | 0 |
| 4 | Inferior Frontal Gyrus, pars triangularis Left | IFGpt_L | −50 | 30 | 16 |
| 4 | Inferior Frontal Gyrus, pars opercularis Left | IFGpo_L | −54 | −20 | 46 |
| 4 | Inferior Frontal Gyrus, pars opercularis Left | Fop_L | 40 | 30 | −14 |
| 4 | Dorsal Medial Prefrontal Cortex, posterior division Left |
dMPFCp_L | −4 | 26 | 48 |
| 4 | Mid Anterior Cingulate Left | ACCm_L | −4 | 50 | −20 |
| 4 | Superior Parietal Lobule Left | SPL_L | −60 | −48 | 32 |
| 4 | Supramarginal Gyrus, posterior division Left |
SMGp_L | −40 | 20 | 4 |
| 4 | Middle Frontal Gyrus Left | MFG_L | −40 | 20 | 44 |
| 4 | Superior Frontal Gyrus Left | SFG_L | −22 | 22 | 54 |
| 4 | Orbito Frontal Pole Left | OFP_L | −32 | 58 | −6 |
| 4 | Frontal Pole Left | FP_L | −30 | 54 | 20 |
| 5 | Temporal Pole Right | TP_R | 40 | 16 | −30 |
| 5 | Superior Temporal Gyrus, anterior division Right |
STGa_R | 58 | −4 | −6 |
| 5 | Superior Temporal Gyrus, posterior division Right |
STGp_R | 66 | −26 | 6 |
| 5 | Temporal Fusiform Cortex, anterior division Right |
TFCa_R | 32 | −6 | −42 |
| 5 | Parahippocampal Gyrus, anterior division Right |
pHippa_R | 34 | −6 | −34 |
| 5 | Parahippocampal Gyrus, posterior division Right |
pHippp_R | 34 | −32 | −18 |
| 5 | Inferior Temporal Gyrus, anterior division Right |
ITGa_R | 50 | −6 | −40 |
| 5 | Inferior Temporal Gyrus, posterior division Right |
ITGp_R | 56 | −32 | −24 |
| 5 | Inferior Temporal Gyrus, temporooccipital part Right |
ITGtp_R | 56 | −54 | −18 |
| 5 | Lingual Gyrus Right | Ling_R | 10 | −68 | −2 |
| 5 | Middle Temporal Gyrus, anterior division Right |
MTGa_R | 58 | −2 | −22 |
| 5 | Middle Temporal Gyrus, posterior division Right |
MTGp_R | 66 | −22 | 12 |
| 5 | Middle Temporal Gyrus, temporooccipital part Right |
MTGto_R | 60 | −52 | 0 |
| 5 | Middle Temporal Gyrus, temporooccipital part Left |
MTGto_L | −60 | −52 | 0 |
| 5 | Middle Temporal Gyrus, posterior division Left |
MTGp_L | −66 | −22 | 12 |
| 5 | Middle Temporal Gyrus, anterior division Left |
MTGa_L | −58 | −2 | −22 |
| 5 | Lingual Gyrus Left | Ling_L | −10 | −68 | −2 |
| 5 | Inferior Temporal Gyrus, temporooccipital part Left |
ITGtp_L | −56 | −54 | −18 |
| 5 | Inferior Temporal Gyrus, posterior division Left |
ITGp_L | −56 | −32 | −24 |
| 5 | Inferior Temporal Gyrus, anterior division Left |
ITGa_L | −50 | −6 | −40 |
| 5 | Parahippocampal Gyrus, posterior division Left |
pHippp_L | −34 | −32 | −18 |
| 5 | Parahippocampal Gyrus, anterior division Left |
pHippa_L | −34 | −6 | −34 |
| 5 | Temporal Fusiform Cortex, anterior division Left |
TFCa_L | −32 | −6 | −42 |
| 5 | Superior Temporal Gyrus, posterior division Left |
STGp_L | −66 | −26 | 6 |
| 5 | Superior Temporal Gyrus, anterior division Left |
STGa_L | −58 | −4 | −6 |
| 5 | Temporal Pole Left | TP_L | −40 | 16 | −30 |
MNI, Montreal Neurological Institute
Materials and Methods
Imaging Visit
The Human Research Committee at the Massachusetts General Hospital approved this study (Protocol #: 2011P002333; NCT01485380). Written informed consent was obtained after the nature and possible study consequences of the study were explained to each healthy volunteer, 18–36 years of age (n=15). All volunteers were required to be American Society of Anesthesiologists Physical Status I. Brain imaging was performed with the Biograph mMR scanner (Siemens Healthcare, Erlangen, Germany), which allows simultaneous acquisition of whole-body PET and 3 Tesla MRI data. At the beginning of the imaging visit, structural MRI (MPRAGE volume, TR/TE = 2100/3.24ms, flip angle = 7°, voxel size = 1mm isotropic) was acquired for the purpose of anatomical localization, and spatial normalization of the imaging data. BOLD fMRI data were collected using a whole brain T2*-weighted gradient echo BOLD echo planar imaging pulse sequence was used (TR/TE =3000/35ms, flip angle=90°, voxel size=2.3×2.3×3.8mm, number of slices=35).
Dexmedetomidine was administered as a 1mcg/kg loading bolus over 10 minutes, followed by a 0.7mcg/kg/hr infusion. During the infusion period, an anesthesiologist monitored cuff blood pressure, capnography, electrocardiogram and pulse-oximetry. Volunteers were instructed to keep their eyes open during the course of the study. During start of the dexmedetomidine infusion (i.e. bolus), a 20-minute “induction” pulsed arterial spin label (pASL) scan was acquired. Altered arousal was defined as the onset of sustained eye closure/lack of response to a verbal request to open the eyes at 1-minute intervals during the induction pASL scan. Sustained eye closure and lack or response to verbal stimuli was confirmed in all subjects during the induction pASL. The dexmedetomidine-induced altered arousal BOLD rsfMRI scans were acquired after the induction pASL scan. At the conclusion of the dexmedetomidine infusion, a 20-minute “recovery” pulsed arterial spin label (pASL) scan was also acquired. During this period, arousal was also periodically assessed verbally at 1-minute intervals. Spontaneous eye opening and a positive response to give a thumbs-up signal were used to determine recovery. Arousability to the verbal stimulus was confirmed in all subjects during the 20-minute recovery pASL scan. The recovery BOLD rsfMRI scan was acquired after the pASL scan. The resting state nature of our data acquisition precluded continuous assessments of arousal during rsfMRI BOLD data acquisition.
Data Preprocessing
Both FMRIB Software Library v5.0 (FSL) and Analysis of Functional Neuro-Images software were used to preprocess data in line with procedures adapted for the 1000 Functional Connectomes project.25 Data were slice time corrected for interleaved acquisitions using Fourier interpolation, motion corrected using least squares alignment of each volume to the eighth image using Fourier interpolation, despiked of extreme time series outliers using a continuous transformation function, temporal band-pass filtered between 0.009–0.3 Hz using Fourier transformation, and further filtered to remove linear and quadratic trends using AFNI. In addition, FSL was used for spatially smoothing the images (Gaussian kernel FWHM = 6 mm), and for normalizing mean-based intensity by the same factor (10,000). Next, eight nuisance signals (time courses of white matter and cerebrospinal fluid, and six motion parameters) were used as regressors of no interest. White matter and cerebrospinal fluid timeseries were extracted from masks obtained by segmenting each individual's high-resolution structural image using FMRIB's Automated Segmentation Tool, thresholded at 80% tissue type probability. The six motion parameters were generated in the FSL based motion-correction step. These six vectors included rotational movement around three axes (pitch, yaw, and roll) and movement in each of the three cardinal directions (X, Y, and Z). All of these steps were conducted in native functional space.
For registration, FMRIB's Linear and Non LINEAR Image Registration Tools were used for transformations from native functional and structural space to the Montreal Neurological Institute MNI152 template with 2×2×2 mm resolution. First, the high-resolution structural image was registered to the MNI152 2mm template with a 12-degree-of-freedom linear affine transformation. The transformation was further refined using FMRIB's Non LINEAR Image Registration Tool. Next, each participant's functional data were registered to their high-resolution structural image using a linear transformation with 6 degrees of freedom. The structural-to-standard nonlinear transformation matrix was used to register the functional volume to MNI152 standard space.
Brain parcellation and time course extraction
The brain was anatomically parcellated using a previously published parcellation scheme.26 Briefly, the Harvard Oxford Atlas was refined by increasing the anatomical partitioning of the cingulate, medial and lateral prefrontal cortices. In addition, anatomical partitioning of the insular label was also performed. Thus, instead of the single ROI spanning the entire insula in the Harvard Oxford Atlas, the insula was subdivided into posterior, middle, dorsal anterior, and ventral anterior regions based on a previously published scheme.27 Regions spanning the right and left hemispheres were separated to create hemisphere specific regions. The complete parcellation scheme consisted of a total of 131 regions (Table 1). A list of these regions, their co-ordinates, as well as a description of the anatomical landmarks used for the subdivision of the Harvard-Oxford labels in smaller parcellates have previously been published.26 Each region of interest was designated as a node. Thus from each node, the BOLD time series were extracted and averaged to generate a 131 time series for each subject.
RSN Network construction
Each parcellated brain region or node was classified as belonging to a designated RSN based on spatial overlap with a specific RSN map. The RSN maps were identified using functional connectivity maps in the neurosynth framework (last accessed 2/15/16).28 Each region was represented in only one RSN, based on maximum overlap.
Graph Analysis
Whole brain networks were constructed and network measures were assessed using the Brain Connectivity Toolbox. Formulae used for calculating network measures has been previously described.29 For each patient, the BOLD time series in each region was correlated with every other region to create a 131 × 131 weighted connectivity or adjacency matrix. The adjacency matrices were thresholded at connectivity densities of 0.05, 0.1, 0.15, 0.2, 0.25 and 0.3.
The following graph functions were computed from each matrix:
Strength
This function measures the strength of connections in the graph. Node strength is the sum of weights of links connected to the node.
where CIJ is the undirected weighted connection matrix. Mean connectivity strength of the graph was measured by averaging str from all nodes.
Global and local efficiencies
Efficiency is a measure of the network’s capacity for parallel information transfer between nodes through multiple series of edges. The average global efficiency of information transfer in graph having n nodes can be calculated from the inverse of the edge distances di,j
The quantity above is a measure of the global efficiency of information transfer for the whole graph . There is also a local efficiency for each vertex vi measuring how efficiently its neighbors can communicate when vertex vi is removed. If the subgraph of all neighbors of vi is denoted by , then its local efficiency is approximately equivalent to the clustering coefficient Ci.30
Statistical comparisons
Comparison between local and global efficiency and mean connectivity strength between the three states was first conducted using repeated measures ANOVA (Table 2). Post-hoc comparisons were conducted on significant ANOVA findings using the paired t-test. Significance was set at p < 0.05 and a Bonferroni correction for multiple comparisons was employed. Statistics on graph results and graphical presentations of networks were performed with custom code written in MATLAB.
Table 2.
Comparison between local and global efficiency and mean connectivity strength between the three states was first conducted using repeated measures ANOVA
| Threshold | Mean | STD | F-value | P-value | |
|---|---|---|---|---|---|
| Mean local efficiency | |||||
|
| |||||
| 0.05 | Awake | 0.501 | 0.040 | 0.499 | 0.611 |
| Dexmedetomidine | 0.485 | 0.052 | |||
| Recovery | 0.491 | 0.032 | |||
| 0.1 | Awake | 0.604 | 0.056 | 2.960 | 0.064 |
| Dexmedetomidine | 0.557 | 0.064 | |||
| Recovery | 0.577 | 0.029 | |||
| 0.15 | Awake | 0.620 | 0.074 | 3.703 | 0.034 |
| Dexmedetomidine | 0.554 | 0.071 | |||
| Recovery | 0.593 | 0.045 | |||
| 0.2 | Awake | 0.607 | 0.084 | 3.561 | 0.038 |
| Dexmedetomidine | 0.533 | 0.079 | |||
| Recovery | 0.580 | 0.054 | |||
| 0.25 | Awake | 0.607 | 0.084 | 3.702 | 0.034 |
| Dexmedetomidine | 0.533 | 0.079 | |||
| Recovery | 0.580 | 0.054 | |||
| 0.3 | Awake | 0.577 | 0.107 | 3.766 | 0.032 |
| Dexmedetomidine | 0.484 | 0.086 | |||
| Recovery | 0.537 | 0.072 | |||
|
| |||||
| Global efficiency | |||||
|
| |||||
| 0.05 | Awake | 0.176 | 0.032 | 0.870 | 0.428 |
| Dexmedetomidine | 0.164 | 0.020 | |||
| Recovery | 0.170 | 0.014 | |||
| 0.1 | Awake | 0.283 | 0.029 | 4.150 | 0.023 |
| Dexmedetomidine | 0.259 | 0.021 | |||
| Recovery | 0.276 | 0.016 | |||
| 0.15 | Awake | 0.333 | 0.039 | 5.210 | 0.010 |
| Dexmedetomidine | 0.295 | 0.029 | |||
| Recovery | 0.319 | 0.022 | |||
| 0.2 | Awake | 0.360 | 0.050 | 4.660 | 0.015 |
| Dexmedetomidine | 0.314 | 0.037 | |||
| Recovery | 0.342 | 0.031 | |||
| 0.25 | Awake | 0.377 | 0.058 | 4.440 | 0.018 |
| Dexmedetomidine | 0.324 | 0.042 | |||
| Recovery | 0.356 | 0.039 | |||
| 0.3 | Awake | 0.388 | 0.065 | 4.180 | 0.023 |
| Dexmedetomidine | 0.330 | 0.047 | |||
| Recovery | 0.363 | 0.044 | |||
|
| |||||
| Mean node strength | |||||
|
| |||||
| 0.05 | Awake | 5.351 | 0.377 | 3.126 | 0.055 |
| Dexmedetomidine | 5.033 | 0.393 | |||
| Recovery | 5.238 | 0.231 | |||
| 0.1 | Awake | 9.916 | 1.002 | 3.510 | 0.040 |
| Dexmedetomidine | 9.025 | 0.984 | |||
| Recovery | 9.564 | 0.659 | |||
| 0.15 | Awake | 14.031 | 1.768 | 3.589 | 0.037 |
| Dexmedetomidine | 12.470 | 1.633 | |||
| Recovery | 13.380 | 1.185 | |||
| 0.2 | Awake | 17.792 | 2.632 | 3.602 | 0.037 |
| Dexmedetomidine | 15.500 | 2.306 | |||
| Recovery | 16.801 | 1.776 | |||
| 0.25 | Awake | 21.257 | 3.573 | 3.603 | 0.037 |
| Dexmedetomidine | 18.192 | 2.982 | |||
| Recovery | 19.893 | 2.415 | |||
| 0.3 | Awake | 24.449 | 4.577 | 3.587 | 0.037 |
| Dexmedetomidine | 20.586 | 3.649 | |||
| Recovery | 22.690 | 3.089 | |||
STD, standard deviation
RSN functional connectivity analyses were also conducted using custom code written in MATLAB. First we present mean networks for the three conditions (awake, dexmedetomidine and recovery). To assess statistical differences, each network was held at a threshold proportional to 50% of strongest connections and these matrices were compared using a paired t-test. Each test was corrected for multiple comparisons setting the false discovery rate to p < 0.05. The regions that showed significant changes in connectivity are shown as binarized maps where nodes are sorted based on membership to RSN groupings. To calculate the percentage of modulated connections within and between RSN networks as a discrete value, the total number of significantly modulated connections that survived correction for multiple comparison were summed for within-RSN network and between-network connections. This value was then divided by the number of total possible connections for any given combination and multiplied by hundred to calculate the percentage of modulated connections. The final values were represented as a heat map to qualitatively assess network modulations.
Results
Dexmedetomidine disrupts local and global efficiency of brain networks
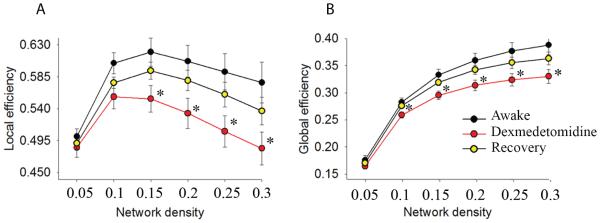
The local efficiency of weighted brain networks was significantly disrupted during the dexmedetomidine-induced altered arousal state compared to the awake state (Fig 1A; 0.15: p = 0.003, 0.2: p = 0.004, 0.25: p = 0.004, 0.3: p = 0.004). During the recovery state, the local efficiency reverted to higher values compared to the dexmedetomidine-induced state. However, these findings did not meet our conservative Bonferroni-adjusted threshold for significance (Fig 1A; 0.15: p = 0.03, 0.2: p = 0.029, 0.25: p = 0.029, 0.3: p = 0.038). There were no statistically significant differences between awake and recovery state comparisons (Fig 1A; p > 0.025).
Figure 1. Local and Global efficiency of neural information transfer is disrupted by dexmedetomidine.
A,B. Local and global efficiency are significantly decreased during the dexmedetomidine-induced altered arousal state and reverted to higher values during the recovery state. This observation was consistent at a range of network densities. *, p < 0.025 for awake vs. dexmedetomidine, error bars represent ±SEM.
The global efficiency of weighted brain networks was significantly disrupted during the dexmedetomidine-induced altered arousal state compared to the awake state (Fig 1B; 0.1: p = 0.006, 0.15: p = 0.001, 0.2: p=0.002, 0.25: p=0.002, and 0.3: p=0.002). During the recovery state, the global efficiency was significantly increased for most, but not all network thresholds (Fig 1B; 0.1: p = 0.004, 0.15: p = 0.005, 0.2: p = 0.01, 0.25: p = 0.017, 0.3: p = 0.026). There were no statistically significant differences between awake and recovery state comparisons (Fig 1B; p > 0.025)
Dexmedetomidine reduces the strength of synchronizations in brain networks
Next, using a graph-theoretic approach, a significant reduction in the mean strength of nodal connectivity was found during the dexmedetomidine-induced altered arousal state compared to the awake state (Fig. 2A; 0.1: p = 0.006, 0.15: p = 0.005, 0.2: p = 0.005, 0.25: p = 0.005, 0.3: p = 0.005). The recovery state was associated with increased mean strength of nodal connectivity compared to the dexmedetomidine-induced altered arousal state. However, this change in strength was not significant (Fig 2A; 0.05: p = 0.052, 0.1: p = 0.051, 0.15: p = 0.053, 0.2: p = 0.055, 0.25: = 0.055, 0.3: p = 0.056). There were no statistically significant differences between awake and recovery state comparisons (Fig 2A; p > 0.025)
Figure 2. Dexmedetomidine modulates strength of connectivity in brain networks.
A. Mean strength of nodal connectivity in weighted networks reported for different network sparsities (*, p < 0.025 for awake vs. dexmedetomidine, error bars represent ±SEM). B. Mean strength of connection presented for each node. Nodes are ordered in ascending order of strength of connections to show that the most strongly connected nodes were the most disrupted by dexmedetomidine. C. Mean degree (total number of connections) of each node presented in ascending order. D-F. Representations of large-scale network topology illustrating how connectivity strength (weights) modulated by dexmedetomidine contribute to alterations in network architecture. The positioning of nodes is topological rather than anatomical. The algorithm positions network nodes based on strength of connections so that regions with common connections are placed in a group and the physical distance between the nodes is adjusted based on the weight of the connection. The awake state shows an ordered modular structure with a large number of within module connections. The modules are held together by global connections. In the dexmedetomidine-induced state, the strengths of connection (weights) are reduced at both local (within module) and global (between module) levels. In the recovery state, the strength of connections is increased, but does not fully revert to the awake state. All three representative networks were constructed at a threshold proportional to 0.25 connections.
To study the nodes that principally contributed to the changes in mean strength of connectivity, nodes were first sorted (lowest to highest strength of connectivity during the awake state) and then plotted by the strength of connectivity (Fig. 2B). The largest decreases in connectivity strength were most evident in strongly connected nodes. During the recovery state, the mean strength of connectivity reverted to higher values compared to the dexmedetomidine-induced altered arousal state. The degree distribution (number of connection at each node) was studied to understand whether the changes in strength of connectivity at the nodal level primarily resulted from a reduction in a number of connections versus a reduction in the strength of connection. Dexmedetomidine did not strongly modulate the number of connections at the nodal level (Fig. 2C).
To graphically illustrate our results, plots of network graphs where nodes with common connections are plotted as clusters and the distances between nodes represent strength of connectivity were made (Fig 2D-F). This network representation more clearly depicts that changes in network strength of connections is a significant driver of topological changes during the dexmedetomidine-induced altered arousal state.
Dexmedetomidine alters connection within and between resting state networks
Parcellated brain regions were sorted based on composition to RSNs. During the dexmedetomidine-induced altered arousal state, the strength of connectivity within and between networks held at the same link density appeared different compared to the baseline and recovery states (Fig. 3A-C). Contrasts between the states were analyzed to characterize connectivity changes associated with dexmedetomidine (Awake vs. Dexmedetomidine; contrast 1; Fig 3D), and recovery (Recovery vs. Dexmedetomidine; contrast 2; Fig 3E). Although volunteers were responsive to verbal commands during the recovery period, they remained mildly sedated due to residual drug effects. Therefore, contrasts between the awake and recovery states (Awake vs. Recovery; contrast 3; Fig 3F) were also analyzed. Only connectivity changes in contrast 1 were significant after correction for multiple comparisons. Therefore corrected maps for contrast 1, and uncorrected maps (p < 0.01) for contrast 2 and 3 are shown (Fig. 3D-F).
Figure 3. Dexmedetomidine modulates within-network and between-network connections when networks are organized based on meta-analytic resting state networks.
A-C. Adjacency matrices representing mean connectivity pattern in A. awake, B. dexmedetomidine and C. recovery states. The adjacency matrices consist of parcellation nodes ordered based on their membership in 5 resting state networks listed in boxed legend. Resting state networks were identified based on node overlap with connectivity maps in the neurosynth metaanalytic framework. D-F. Contrast maps showing regions that significantly changed in connectivity within and between the 5 listed networks. Results are based on t-tests, where contrast shown in D is FDR corrected at p < 0.05. E and F are uncorrected at p < 0.01. G-I Matrices showing percentage of nodes modulated in D-F. The percent values in G-I are based on sum of significantly modulated nodes observed in D-F respectively, scaled by total number of possible connections.
FDR, false discovery rate.
The percentages of modulated connections scaled by the total number of possible connections are presented for qualitative assessment (Fig. 3, G-I). The dexmedetomidine-induced altered arousal state was associated with significant reductions in functional connectivity between regions that comprise all RSNs (Fig. 3D; Awake > Dexmedetomidine, false discovery rate corrected p < 0.05). Recovery was associated with partial restoration of connectivity within the Default Mode Network, and increased functional connectivity between subcortical regions and all other networks (Fig. 3E; Recovery > Dexmedetomidine, uncorrected p < 0.01). Functional connectivity changes associated with dexmedetomidine did not fully revert to baseline values during the recovery state (Fig. 3F; Awake > Recovery, uncorrected p < 0.01). The awake state, relative to the recovery state had more within-network connectivity in Attention/Executive Networks (Fig. 3F,I). Also, the Language/Memory Networks had more between-network connectivity with subcortical regions, Sensory Network, Default Mode Network, and Attention/Executive (Fig. 3F,I).
Discussion
We previously reported that impaired thalamic information processing – loss of functional connectivity between the Default Mode Network and the thalamus and bidirectional changes between the Frontoparietal Networks and cerebellar clusters – is a neural correlate of dexmedetomidine-induced altered arousal.1 In this investigation, we applied network metrics of information processing to study differences between baseline, dexmedetomidine-induced altered arousal and recovery states. First, we found that dexmedetomidine reversibly reduced the local and global efficiency of brain networks. Second, dexmedetomidine was associated with a reduction in the mean strength of nodal connectivity but did not alter the relative distribution of connections between nodes. Third, by using a global network approach, we show that these changes are not specific to any given RSN. Taken together, these findings parallel the decreased efficiency of information transfer within the brain that has been reported for propofol-induced unconsciousness17,31 and non-rapid eye movement sleep,18,19 and strengthen the hypothesis that conscious processing in the brain relies on an efficient system of information transfer.
Local and global efficiency are both measures of information integration that are derived from the characteristic path length (the average shortest path length between all possible pairs of nodes).32 Because the paths in this investigation represent statistical dependency or functional connectivity between nodes, our efficiency and mean node strength findings are consistent with our previous report of dexmedetomidine-induced functional connectivity changes.1 Although our approach of representing each brain region in only one RSN based on maximum overlap was different from the independent component analysis method we previously employed, our finding that functional connectivity was reduced between the DMN and subcortical regions is also consistent with our previous report.1 However, our present results extend upon those previously reported findings by showing that functional connectivity changes between subcortical regions are not specific to any RSN. Since sleep slow-delta (0.1 – 4 Hz) and spindle oscillations (13-16 Hz) reflect altered sensory information processing in the brainstem and thalamus,33 it follows that dexmedetomidine-induced altered arousal which is also associated with slow-delta and spindle oscillations34-36 should manifest with altered subcortical-cortical functional connectivity. We speculate that other anesthesia-induced slow-delta oscillations (propofol, sevoflurane, nitrous oxide),35,37-41 theta oscillations (4-8 Hz; ketamine, sevoflurane),37,38,42 frontal alpha oscillations (8-12 Hz; propofol, sevoflurane)35,37-41 and gamma oscillations (< 40 Hz; ketamine)42 may also manifest as altered subcortical-cortical and cortico-cortical fMRI bold network connectivity. A likely mechanism for this speculated finding is the disruption of “normal sensory processing” gamma oscillations (>40 Hz) that have been related to fMRI BOLD signals.43
Recovery from the dexmedetomidine-induced altered arousal state was associated with partially recovered connectivity between brain regions that comprise the Default Mode Network. Notably, within network alterations in Default Mode Network connectivity have been reported for dexmedetomidine,1 sevoflurane,44 propofol45 and ketamine.46 Thus, this RSN, which is associated with stimulus-independent thought and self-consciousness,47-49 may play a significant role in recovery from altered states of arousal. Our findings also suggest that key differences between the baseline and recovery states were increased within-network connectivity in Attention/Executive and Language/Memory Networks. Thus, recovery from altered states of arousal may follow a graded pathway where within-network restoration of Default Mode functional connectivity precedes within-network connectivity in Attention/Executive and Language/Memory Networks. This finding which suggests gradations in the level of arousal is consistent with an information integration theory of consciousness9 may result from specific time-varying disruption (altered arousal) or reintegration (recovery) of hub nodes.
Hubs are crucial for efficient information transmission in brain networks.50 Although a detailed analysis of network hub dynamics was beyond the scope of this investigation, our finding of reduced strength of connectivity in highly connected nodes suggests that dexmedetomidine disrupts hub nodes. Importantly, a consistent finding from graph theoretical studies of electroencephalogram51 and rsfMRI17,31 data during propofol-induced unconsciousness is the disruption of hubs nodes. Further confirming the role of hubs in information transfer, a simulation of hub disruption using various anesthetics (propofol, sevoflurane, ketamine) resulted in disrupted surrogates of information transfer.52 This suggests that even though anesthetics have distinct pharmacological targets – reflected by differences in behavioral states and oscillatory dynamics – preferential disruption of information flow at network hubs is a common “macrocircuit” dynamic. An open question is whether unique patterns of hub disruption may explain the pharmacological and behavioral diversity inherent to these anesthetics.
A limitation of this study is that the level of arousal was not experimentally manipulated in a graded manner. Therefore, studies of dexmedetomidine and other anesthetics with graded manipulations of level of arousal levels are needed to more clearly delineate how the efficiency measures described in this manuscript covary with the level of arousal. Another limitation of the present study is that the present results cannot be directly translated to molecular or neurophysiological function at the neuronal level. We note that although statistically significant, our effect sizes were small in spite of clinically significant alterations to the level of arousal. However, this finding is likely a reflection of the mathematical construct underlying efficiency measures (i.e. global and local efficiency are not exponential functions).10 Finally, it is important to note that the use of graph theoretical metrics as a proxy for higher cognitive processes is not definitive.
Our findings demonstrate that dexmedetomidine is associated with a significant drop in the capacity for efficient information transfer in functional networks at both local and global levels. These findings strengthen the hypothesis that conscious processing relies on an efficient system of information transfer in the brain.
Acknowledgments
Funding: This work was supported by the National Institutes of Health (Bethesda, MD) R01 AG053582 (to OA) and TR01 GM104948 (to ENB); and by the Department of Anesthesia, Critical Care and Pain Medicine, Massachusetts General Hospital, Boston, Massachusetts.
Footnotes
Conflict of interest: The authors do not have any conflicts of interest to declare.
References
- 1.Akeju O, Loggia ML, Catana C, Pavone KJ, Vazquez R, Rhee J, Contreras Ramirez V, Chonde DB, Izquierdo-Garcia D, Arabasz G, Hsu S, Habeeb K, Hooker JM, Napadow V, Brown EN, Purdon PL. Disruption of thalamic functional connectivity is a neural correlate of dexmedetomidine-induced unconsciousness. Elife. 2014;3:e04499. doi: 10.7554/eLife.04499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Crick F, Koch C. A framework for consciousness. Nat Neurosci. 2003;6:119–26. doi: 10.1038/nn0203-119. [DOI] [PubMed] [Google Scholar]
- 3.Tononi G, Edelman GM, Sporns O. Complexity and coherency: integrating information in the brain. Trends Cogn Sci. 1998;2:474–84. doi: 10.1016/s1364-6613(98)01259-5. [DOI] [PubMed] [Google Scholar]
- 4.Hashmi JA, Davis KD. Effect of static and dynamic heat pain stimulus profiles on the temporal dynamics and interdependence of pain qualities, intensity, and affect. J Neurophysiol. 2008;100:1706–15. doi: 10.1152/jn.90500.2008. [DOI] [PubMed] [Google Scholar]
- 5.Hipp JF, Hawellek DJ, Corbetta M, Siegel M, Engel AK. Large-scale cortical correlation structure of spontaneous oscillatory activity. Nat Neurosci. 2012;15:884–90. doi: 10.1038/nn.3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baars BJ, Gage NM. Cognition, brain, and consciousness: Introduction to cognitive neuroscience. Academic Press; 2010. [Google Scholar]
- 7.Buzsáki G, Draguhn A. Neuronal oscillations in cortical networks. science. 2004;304:1926–1929. doi: 10.1126/science.1099745. [DOI] [PubMed] [Google Scholar]
- 8.Dehaene S, Changeux JP. Experimental and theoretical approaches to conscious processing. Neuron. 2011;70:200–27. doi: 10.1016/j.neuron.2011.03.018. [DOI] [PubMed] [Google Scholar]
- 9.Tononi G. An information integration theory of consciousness. BMC Neurosci. 2004;5:42. doi: 10.1186/1471-2202-5-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Achard S, Bullmore E. Efficiency and cost of economical brain functional networks. PLoS Comput Biol. 2007;3:e17. doi: 10.1371/journal.pcbi.0030017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van den Heuvel MP, Stam CJ, Kahn RS, Hulshoff Pol HE. Efficiency of functional brain networks and intellectual performance. J Neurosci. 2009;29:7619–24. doi: 10.1523/JNEUROSCI.1443-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buckner RL, Andrews-Hanna JR, Schacter DL. The brain's default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- 13.Loggia ML, Kim J, Gollub RL, Vangel MG, Kirsch I, Kong J, Wasan AD, Napadow V. Default mode network connectivity encodes clinical pain: an arterial spin labeling study. Pain. 2013;154:24–33. doi: 10.1016/j.pain.2012.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hudetz AG. General anesthesia and human brain connectivity. Brain Connect. 2012;2:291–302. doi: 10.1089/brain.2012.0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bullmore E, Sporns O. The economy of brain network organization. Nat Rev Neurosci. 2012;13:336–49. doi: 10.1038/nrn3214. [DOI] [PubMed] [Google Scholar]
- 16.Bullmore E, Sporns O. Complex brain networks: graph theoretical analysis of structural and functional systems. Nat Rev Neurosci. 2009;10:186–98. doi: 10.1038/nrn2575. [DOI] [PubMed] [Google Scholar]
- 17.Monti MM, Lutkenhoff ES, Rubinov M, Boveroux P, Vanhaudenhuyse A, Gosseries O, Bruno MA, Noirhomme Q, Boly M, Laureys S. Dynamic change of global and local information processing in propofol-induced loss and recovery of consciousness. PLoS Comput Biol. 2013;9:e1003271. doi: 10.1371/journal.pcbi.1003271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Uehara T, Yamasaki T, Okamoto T, Koike T, Kan S, Miyauchi S, Kira J, Tobimatsu S. Efficiency of a "small-world" brain network depends on consciousness level: a resting-state FMRI study. Cereb Cortex. 2014;24:1529–39. doi: 10.1093/cercor/bht004. [DOI] [PubMed] [Google Scholar]
- 19.Boly M, Perlbarg V, Marrelec G, Schabus M, Laureys S, Doyon J, Pelegrini-Issac M, Maquet P, Benali H. Hierarchical clustering of brain activity during human nonrapid eye movement sleep. Proc Natl Acad Sci U S A. 2012;109:5856–61. doi: 10.1073/pnas.1111133109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Correa-Sales C, Rabin BC, Maze M. A hypnotic response to dexmedetomidine, an alpha 2 agonist, is mediated in the locus coeruleus in rats. Anesthesiology. 1992;76:948–52. doi: 10.1097/00000542-199206000-00013. [DOI] [PubMed] [Google Scholar]
- 21.Nacif-Coelho C, Correa-Sales C, Chang LL, Maze M. Perturbation of ion channel conductance alters the hypnotic response to the alpha 2-adrenergic agonist dexmedetomidine in the locus coeruleus of the rat. Anesthesiology. 1994;81:1527–34. doi: 10.1097/00000542-199412000-00029. [DOI] [PubMed] [Google Scholar]
- 22.Nelson LE, Lu J, Guo T, Saper CB, Franks NP, Maze M. The alpha2-adrenoceptor agonist dexmedetomidine converges on an endogenous sleep-promoting pathway to exert its sedative effects. Anesthesiology. 2003;98:428–36. doi: 10.1097/00000542-200302000-00024. [DOI] [PubMed] [Google Scholar]
- 23.Jorm CM, Stamford JA. Actions of the hypnotic anaesthetic, dexmedetomidine, on noradrenaline release and cell firing in rat locus coeruleus slices. Br J Anaesth. 1993;71:447–9. doi: 10.1093/bja/71.3.447. [DOI] [PubMed] [Google Scholar]
- 24.Chiu TH, Chen MJ, Yang YR, Yang JJ, Tang FI. Action of dexmedetomidine on rat locus coeruleus neurones: intracellular recording in vitro. Eur J Pharmacol. 1995;285:261–8. doi: 10.1016/0014-2999(95)00417-j. [DOI] [PubMed] [Google Scholar]
- 25.Biswal BB, Mennes M, Zuo X-N, Gohel S, Kelly C, Smith SM, Beckmann CF, Adelstein JS, Buckner RL, Colcombe S, Dogonowski A-M, Ernst M, Fair D, Hampson M, Hoptman MJ, Hyde JS, Kiviniemi VJ, Kötter R, Li S-J, Lin C-P, Lowe MJ, Mackay C, Madden DJ, Madsen KH, Margulies DS, Mayberg HS, McMahon K, Monk CS, Mostofsky SH, Nagel BJ, Pekar JJ, Peltier SJ, Petersen SE, Riedl V, Rombouts SARB, Rypma B, Schlaggar BL, Schmidt S, Seidler RD, Siegle GJ, Sorg C, Teng G-J, Veijola J, Villringer A, Walter M, Wang L, Weng X-C, Whitfield-Gabrieli S, Williamson P, Windischberger C, Zang Y-F, Zhang H-Y, Castellanos FX, Milham MP. Toward discovery science of human brain function. Proceedings of the National Academy of Sciences. 2010;107:4734–4739. doi: 10.1073/pnas.0911855107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hashmi JA, Kong J, Spaeth R, Khan S, Kaptchuk TJ, Gollub RL. Functional network architecture predicts psychologically mediated analgesia related to treatment in chronic knee pain patients. J Neurosci. 2014;34:3924–36. doi: 10.1523/JNEUROSCI.3155-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kelly C, Toro R, Di Martino A, Cox CL, Bellec P, Castellanos FX, Milham MP. A convergent functional architecture of the insula emerges across imaging modalities. Neuroimage. 2012;61:1129–42. doi: 10.1016/j.neuroimage.2012.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yarkoni T, Poldrack RA, Nichols TE, Van Essen DC, Wager TD. Large-scale automated synthesis of human functional neuroimaging data. Nat Methods. 2011;8:665–70. doi: 10.1038/nmeth.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rubinov M, Sporns O. Complex network measures of brain connectivity: uses and interpretations. Neuroimage. 2010;52:1059–69. doi: 10.1016/j.neuroimage.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 30.Achard S, Bullmore E. Efficiency and cost of economical brain functional networks. PLoS Comput Biol. 2007;3:e17–e17. doi: 10.1371/journal.pcbi.0030017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schroter MS, Spoormaker VI, Schorer A, Wohlschlager A, Czisch M, Kochs EF, Zimmer C, Hemmer B, Schneider G, Jordan D, Ilg R. Spatiotemporal reconfiguration of large-scale brain functional networks during propofol-induced loss of consciousness. J Neurosci. 2012;32:12832–40. doi: 10.1523/JNEUROSCI.6046-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Latora V, Marchiori M. Efficient behavior of small-world networks. Phys Rev Lett. 2001;87:198701. doi: 10.1103/PhysRevLett.87.198701. [DOI] [PubMed] [Google Scholar]
- 33.Steriade M, Contreras D, Amzica F. Synchronized sleep oscillations and their paroxysmal developments. Trends Neurosci. 1994;17:199–208. doi: 10.1016/0166-2236(94)90105-8. [DOI] [PubMed] [Google Scholar]
- 34.Akeju O, Kim SE, Vazquez R, Rhee J, Pavone KJ, Hobbs LE, Purdon PL, Brown EN. Spatiotemporal Dynamics of Dexmedetomidine-Induced Electroencephalogram Oscillations. PLoS One. 2016;11:e0163431. doi: 10.1371/journal.pone.0163431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Akeju O, Pavone KJ, Westover MB, Vazquez R, Prerau MJ, Harrell PG, Hartnack KE, Rhee J, Sampson AL, Habeeb K, Gao L, Pierce ET, Walsh JL, Brown EN, Purdon PL. A comparison of propofol- and dexmedetomidine-induced electroencephalogram dynamics using spectral and coherence analysis. Anesthesiology. 2014;121:978–89. doi: 10.1097/ALN.0000000000000419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huupponen E, Maksimow A, Lapinlampi P, Sarkela M, Saastamoinen A, Snapir A, Scheinin H, Scheinin M, Merilainen P, Himanen SL, Jaaskelainen S. Electroencephalogram spindle activity during dexmedetomidine sedation and physiological sleep. Acta Anaesthesiol Scand. 2008;52:289–94. doi: 10.1111/j.1399-6576.2007.01537.x. [DOI] [PubMed] [Google Scholar]
- 37.Akeju O, Hamilos AE, Song AH, Pavone KJ, Purdon PL, Brown EN. GABAA circuit mechanisms are associated with ether anesthesia-induced unconsciousness. Clin Neurophysiol. 2016;127:2472–81. doi: 10.1016/j.clinph.2016.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Akeju O, Westover MB, Pavone KJ, Sampson AL, Hartnack KE, Brown EN, Purdon PL. Effects of sevoflurane and propofol on frontal electroencephalogram power and coherence. Anesthesiology. 2014;121:990–8. doi: 10.1097/ALN.0000000000000436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lewis LD, Weiner VS, Mukamel EA, Donoghue JA, Eskandar EN, Madsen JR, Anderson WS, Hochberg LR, Cash SS, Brown EN, Purdon PL. Rapid fragmentation of neuronal networks at the onset of propofol-induced unconsciousness. Proc Natl Acad Sci U S A. 2012;109:E3377–86. doi: 10.1073/pnas.1210907109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pavone KJ, Akeju O, Sampson AL, Ling K, Purdon PL, Brown EN. Nitrous oxide-induced slow and delta oscillations. Clin Neurophysiol. 2016;127:556–64. doi: 10.1016/j.clinph.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Purdon PL, Pierce ET, Mukamel EA, Prerau MJ, Walsh JL, Wong KF, Salazar-Gomez AF, Harrell PG, Sampson AL, Cimenser A, Ching S, Kopell NJ, Tavares-Stoeckel C, Habeeb K, Merhar R, Brown EN. Electroencephalogram signatures of loss and recovery of consciousness from propofol. Proc Natl Acad Sci U S A. 2013;110:E1142–51. doi: 10.1073/pnas.1221180110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Akeju O, Song AH, Hamilos AE, Pavone KJ, Flores FJ, Brown EN, Purdon PL. Electroencephalogram signatures of ketamine anesthesia-induced unconsciousness. Clin Neurophysiol. 2016;127:2414–22. doi: 10.1016/j.clinph.2016.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nir Y, Fisch L, Mukamel R, Gelbard-Sagiv H, Arieli A, Fried I, Malach R. Coupling between neuronal firing rate, gamma LFP, and BOLD fMRI is related to interneuronal correlations. Curr Biol. 2007;17:1275–85. doi: 10.1016/j.cub.2007.06.066. [DOI] [PubMed] [Google Scholar]
- 44.Palanca BJ, Mitra A, Larson-Prior L, Snyder AZ, Avidan MS, Raichle ME. Resting-state Functional Magnetic Resonance Imaging Correlates of Sevoflurane-induced Unconsciousness. Anesthesiology. 2015;123:346–56. doi: 10.1097/ALN.0000000000000731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Boveroux P, Vanhaudenhuyse A, Bruno MA, Noirhomme Q, Lauwick S, Luxen A, Degueldre C, Plenevaux A, Schnakers C, Phillips C, Brichant JF, Bonhomme V, Maquet P, Greicius MD, Laureys S, Boly M. Breakdown of within- and between-network resting state functional magnetic resonance imaging connectivity during propofol-induced loss of consciousness. Anesthesiology. 2010;113:1038–53. doi: 10.1097/ALN.0b013e3181f697f5. [DOI] [PubMed] [Google Scholar]
- 46.Bonhomme V, Vanhaudenhuyse A, Demertzi A, Bruno MA, Jaquet O, Bahri MA, Plenevaux A, Boly M, Boveroux P, Soddu A, Brichant JF, Maquet P, Laureys S. Resting-state Network-specific Breakdown of Functional Connectivity during Ketamine Alteration of Consciousness in Volunteers. Anesthesiology. 2016;125:873–888. doi: 10.1097/ALN.0000000000001275. [DOI] [PubMed] [Google Scholar]
- 47.Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98:676–82. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mason MF, Norton MI, Van Horn JD, Wegner DM, Grafton ST, Macrae CN. Wandering minds: the default network and stimulus-independent thought. Science. 2007;315:393–5. doi: 10.1126/science.1131295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vincent JL, Kahn I, Snyder AZ, Raichle ME, Buckner RL. Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. J Neurophysiol. 2008;100:3328–42. doi: 10.1152/jn.90355.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.van den Heuvel MP, Sporns O. Network hubs in the human brain. Trends Cogn Sci. 2013;17:683–96. doi: 10.1016/j.tics.2013.09.012. [DOI] [PubMed] [Google Scholar]
- 51.Lee H, Mashour GA, Noh GJ, Kim S, Lee U. Reconfiguration of network hub structure after propofol-induced unconsciousness. Anesthesiology. 2013;119:1347–59. doi: 10.1097/ALN.0b013e3182a8ec8c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moon JY, Lee U, Blain-Moraes S, Mashour GA. General relationship of global topology, local dynamics, and directionality in large-scale brain networks. PLoS Comput Biol. 2015;11:e1004225. doi: 10.1371/journal.pcbi.1004225. [DOI] [PMC free article] [PubMed] [Google Scholar]