Abstract
Background
Intraductal tubulopapillary neoplasm (ITPN) is a relatively recently described member of the pancreatic intraductal neoplasm family. Thus, the literature on its histologic and immunohistochemical features, clinical behavior, and its similarities and differences from other pancreatic neoplasms is limited.
Design
Thirty-three cases of ITPN, the largest series to date, were identified. Immunohistochemical labeling for cytokeratins, glycoproteins, pancreatic enzymes, markers for intestinal and neuroendocrine differentiation, and antibodies associated with genetic alterations previously described in pancreatic neoplasms were performed. Clinicopathologic features and survival was assessed.
Results
Seventeen patients were female, fourteen were male. Mean age was 55 years (range, 25–79). Median overall tumor size was 4.5 cm (range, 0.5–15). Forty-five percent of the tumors occurred in the head, 32% in the body/tail, and 23% showed diffuse involvement. Microscopically, the tumors were characterized by intraductal nodules composed of tightly packed small tubular glands lined by cuboidal cells lacking apparent mucin. Although it was often challenging to determine its extent, invasion was present in 71%. Almost all tumors labeled for CAM5.2, CK7 and CK19; most expressed CA19.9, MUC1 and MUC6. CDX2, MUC2, trypsin, chymotrypsin, chromogranin and synaptophysin were not expressed. SMAD4 expression was retained in 100%, p16 expression and p53 overexpression was seen in 33% and 27%. Follow-up information was available for twenty-two patients (median follow-up, 45 months; range, 11–173). Two patients with invasive carcinoma died of disease at 23 and 41 months. One patient died of unrelated causes at 49 months. Twelve patients were alive with disease. Seven patients were alive with no evidence of disease. The overall 1-, 3- and 5-year survival rates were 100% in patients without an invasive component and 100%, 91% and 71% in patients with an invasive component (p=0.7).
Conclusions
ITPN is a distinct clinicopathologic entity in the pancreas. Despite the difficulties of determining the extent of invasive carcinoma in many cases, the overall outcome appears relatively favorable and substantially better than that of conventional ductal adenocarcinoma, even when only the cases with invasive carcinoma are considered.
INTRODUCTION
Since the first description more than three decades ago1, intraductal papillary mucinous neoplasm (IPMN) of the pancreas has become widely recognized as one of the most common cyst-forming pancreatic neoplasms, and several variants of intraductal neoplasms have been described including the gastric, intestinal, or pancreatobiliary subtypes2–9. All IPMNs have variable papilla formation and produce mucin, but each subtype has certain distinctive histologic, immunohistochemical and genetic features. These tumors also exhibit a variable degree of cytoarchitectural atypia (low-grade and high-grade)6 and may be associated with different types of invasive carcinoma (tubular or colloid)2, 10–17. The current (2010) World Health Organization designate “intraductal oncocytic papillary neoplasm (IOPN)18” neoplasm as one of the subtypes of IPMN, as well8. However, there are many differences between these entities arguing against this classification including their distinct molecular features19, and biologic behavior20, 21 compared to other subtypes of IPMN.
Recently, another intraductal neoplasm with a distinctive pattern of growth has been described8: Intraductal tubulopapillary neoplasm (ITPN) has minimal papilla formation, instead filling the ducts with back-to-back tubular glands, and is not associated with extensive luminal or intracellular mucin accumulation8. However, the literature on ITPN is still very limited, due to the rarity of this disease. Our current understanding of this neoplasm is mainly based on individual case reports, analyses of small series or a metaanalysis of the literature22–36. The diagnostic criteria have also been poorly defined, leading to inconsistent pathologic classification and overlap with the pancreatobiliary subtype of IPMN.
Herein, we present the largest clinicopathologic studies of ITPN in an effort to more fully define the histologic and immunohistochemical features, clinical behavior, and similarities and differences from IPMNs as well as other pancreatic neoplasms.
MATERIALS AND METHODS
The surgical pathology and consultation files of Memorial Sloan Kettering Cancer Center (New York, NY), Emory University School of Medicine (Atlanta, GA), University of Verona and Negrar Hospital (Verona, Italy), IPATIMUP (Porto, Portugal), and Technical University (Munich, Germany) were searched for cases of grossly visible intraductal pancreatic neoplasms with a predominantly tubular growth pattern and minimal mucin production, with or without an associated invasive adenocarcinoma component. Available gross photographs and descriptions as well as all histologic sections were re-evaluated to confirm the diagnosis and further characterize the spectrum of histology findings. Available medical records, including imaging study reports, were reviewed to obtain clinical data including age, gender, presenting symptoms, treatment, and outcome; for the consultation cases, contributing physicians were contacted.
Representative formalin-fixed paraffin-embedded tissue sections of each case, for which a paraffin block or unstained sections were available, were immunolabeled using the standard avidin-biotin peroxidase method. The following general groups of immunohistochemical stains were performed: keratins; glycoprotein markers; pancreatic enzymes, intestinal, hepatocellular, and neuroendocrine differentiation markers; and antibodies associated with genetic alterations previously described for other pancreatic neoplasms. The antibodies used along with their sources, dilutions, and pretreatment conditions are listed in Supplemental Table 1. The controls used were as follows: benign and neoplastic pancreatic tissue for CAM5.2, CK7, CK19, B72.3, CA125, CA19.9, monoclonal CEA, trypsin, chymotrypsin, chromogranin, synaptophysin, SMAD4, β-Catenin and E-Cadherin; benign and neoplastic colon tissue for MUC2 and CDX2; benign gastric mucosa for MUC5AC and MUC6, benign liver for HepPar-1; and colonic adenocarcinoma for p53.
For all antibodies, labeling in at least 10% of cells was considered to be expression (labeling in 10–25% of cells was considered to be focal). For p53 and CDX2, only nuclear labeling was regarded as expression. For SMAD4, loss of labeling in the face of retained labeling in non-neoplastic nuclei was regarded to be abnormal. For E-cadherin, loss of the normal cell membrane pattern and for β-catenin, an alteration from the normal cell membrane pattern to nuclear labeling was considered abnormal.
Statistical Analysis
Mean, median and ranges were used to describe quantitative variables. The 1-, 3-, and 5-year survivals were retrieved from Life Tables. Kaplan-Meier survival curves and the log-rank test were used for survival analyses. For all analyses, the IBM-SPSS version 20.0 was used, and the threshold for statistical significance was set at P<0.05.
RESULTS
We identified a total of thirty-three cases that met the criteria for ITPN described above. The clinicopathological features of the cases analyzed are summarized in Tables 1 and 2.
Table 1.
Clinicopathologic features of the cases analyzed
| n (%) | |
|---|---|
| Mean age (range) (years) | 55 (25–79) |
| Female/Male | 1.2 |
| Specimen type | |
| Total pancreatectomy | 5 (21) |
| Pancreatoduodenectomy | 9 (38) |
| Distal pancreatectomy | 8 (33) |
| Biopsy | 2 (8) |
| Unknown | 9 |
| Tumor location | |
| Head | 10 (45) |
| Body | 1 (5) |
| Tail | 6 (27) |
| Diffuse | 5 (23) |
| Unknown | 11 |
| Median overall tumor size | 4.5 cm |
| Invasive component | |
| Present | 22 (71) |
| <10% | 12 |
| 10–50% | 7 |
| >50% | 3 |
| Absent | 9 (29) |
| Unknown (Biopsy cases) | 2 |
| Papilla formation | |
| Present | 12 (36) |
| Absent | 21 (64) |
| Necrosis | |
| Present | 21 (64) |
| Absent | 12 (36) |
| Unknown | |
| Follow-up | |
| Mean follow-up (range) (months) | 60 (11–173) |
| Median follow-up (range) (months) | 45 (11–173) |
| No evidence of disease | 7 (32) |
| Alive with disease | 12 (55) |
| Died of disease | 2 (9) |
| Died of other causes | 1 (4) |
| Unknown | 11 |
Table 2.
The clinicopathological features of the cases analyzed
| Case | Age (year) | Sex | Symptom | Site | Size (cm) | Invasion (%) | Survival (month) | Outcome |
|---|---|---|---|---|---|---|---|---|
| 1 | 51 | F | Abdominal pain | Head | 2.5 | Yes (<10%) | 41 | DOD |
| 2 | 63 | F | Abdominal pain, steatorrhea | Diffuse | 4.5 | Yes (<10%) | 49 | DOC |
| 3 | 53 | F | Abdominal discomfort | Head | 15 | Yes (10–50%) | 23 | DOD |
| 4 | 36 | F | None | Diffuse | N/A | Yes (<10%) | 134 | AWD |
| 5 | 65 | F | Abdominal pain | Diffuse | N/A | No | 16 | AWD |
| 6 | 25 | F | None | Tail | 10 | Yes (<10%) | 18 | NED |
| 7 | 61 | M | Abdominal pain, weight loss | Head | 8 | Yes (<10%) | 12 | AWD |
| 8 | 58 | F | None | Tail | 9 | Yes (>50%) | 173 | AWD |
| 9 | 72 | F | None | Head | 6 | Yes (<10%) | 87 | AWD |
| 10 | 38 | M | None | Pancreatic duct | 4 | Biopsy only | 51 | AWD |
| 11 | 53 | M | Abdominal pain | Head | 5.4 | Yes (<10%) | 164 | AWD |
| 12 | 45 | M | Abdominal pain | Diffuse | 0.5 | Yes (<10%) | N/A | N/A |
| 13 | 60 | M | None | N/A | N/A | Yes (10–50%) | N/A | N/A |
| 14 | 49 | M | None | N/A | N/A | Yes (10–50%) | N/A | N/A |
| 15 | 53 | F | None | Head | 5 | Yes (<10%) | N/A | N/A |
| 16 | 62 | F | None | Diffuse | N/A | Yes (<10%) | N/A | N/A |
| 17 | N/A | N/A | None | N/A | N/A | No | N/A | N/A |
| 18 | 56 | F | Abdominal pain | Body | 2.5 | No | 72 | AWD |
| 19 | 36 | M | None | N/A | N/A | No | 11 | NED |
| 20 | 71 | F | None | Tail | 5 | No | 120 | AWD |
| 21 | 53 | M | None | Tail | 2 | Yes (10–50%) | 95 | NED |
| 22 | 53 | M | Abdominal pain | Pancreatic duct | N/A | Biopsy only | N/A | N/A |
| 23 | 50 | M | Abdominal pain | N/A | N/A | Yes (10–50%) | 77 | NED |
| 24 | 73 | F | Epigastric pain, nausea, vomiting | Tail | 3.7 | Yes (>50%) | 64 | NED |
| 25 | 79 | F | Abdominal pain, weight loss | Head | 9 | Yes (>50%) | 37 | AWD |
| 26 | 75 | M | N/A | Tail | 1.5 | No | 20 | AWD |
| 27 | 64 | M | Epigastric pain | Head | 3 | Yes (<10%) | 19 | NED |
| 28 | 67 | M | Abdominal pain | Head | 3.5 | Yes (10–50%) | 13 | NED |
| 29 | 40 | F | None | Head | 3 | No | N/A | N/A |
| 30 | 57 | F | None | N/A | 1 | No | 16 | AWD |
| 31 | N/A | N/A | None | N/A | N/A | No | N/A | N/A |
| 32 | 53 | F | None | N/A | 6 | Yes (<10%) | N/A | N/A |
| 33 | 46 | F | None | N/A | 10 | Yes (10–50%) | N/A | N/A |
AWD: Alive with disease, NED: No evidence of disease, DOD: Died of disease, DOC: Died of other causes
N/A: Not available
Clinical Findings
The patients included seventeen females and fourteen males; the gender was unknown for two cases. Patients’ ages ranged from 25 to 79 years (mean=55 years). Presenting symptoms included abdominal pain, nausea, vomiting, steatorrhea, and weight loss. None of the patients presented with jaundice. Five patients had experienced prior episodes of acute pancreatitis. Two patients had diabetes mellitus. One patient had been treated for cholangiocarcinoma, diagnosed three years prior to ITPN. Another patient had a family history of pancreas cancer, although detailed family history data were not known for most of the cases.
The neoplasms were distributed throughout the gland, 45% were located in the head, 32% in the body/tail, and 23% diffusely involved the gland. Both solid and cystic regions within each case were seen on cross-sectional imaging. In five cases (mostly those involving the head of the gland), the intraductal nature of the lesion was specifically recognized on pre-operative imaging as duct of Wirsung dilatation, with a stated differential diagnosis of IPMN. Other cases were reported as partially cystic masses.
All but two patients were treated primarily by surgical resection; two patients only underwent biopsy of an intraductal polypoid mass due to co-morbid illnesses. None of the patients received neoadjuvant chemotherapy and two patient with recurrences received chemotherapy.
Pathologic Findings
Grossly, the tumors ranged from 0.5 to 15 cm (median=4.5 cm) in greatest dimension. Of the fifteen cases for which detailed gross information was available, the intraductal nature of the tumor was specifically documented in only 60% of the cases. Fifty-three percent of the tumors were described as predominantly solid or polypoid lesions, some within the dilated ducts or cysts. One of these tumors consisted of a grossly circumscribed solid mass in the tail of the gland, with a finger-like projection extending into the main pancreatic duct (Figure 1). The remaining 47% were described as cystic or multicystic lesions. None of the cases was described to exhibit luminal mucin accumulation grossly. The stroma between the nodules and cysts was densely sclerotic.
Figure 1.
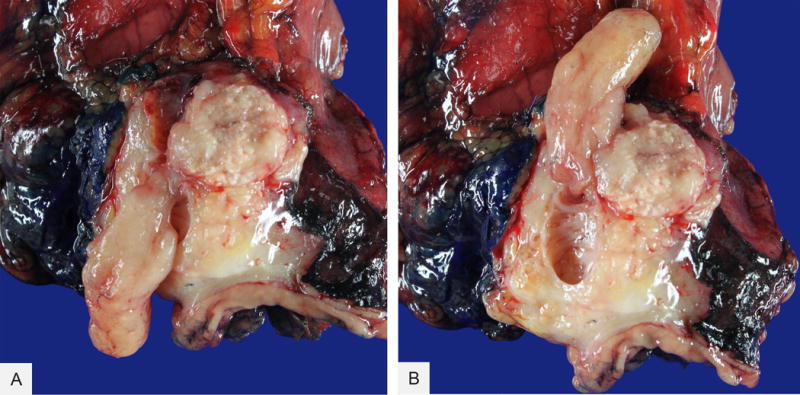
Intraductal tubulopapillary neoplasm, gross appearance. Note the polypoid component (A) within the main pancreatic duct (B)
Microscopically, the tumors consisted of variably sized circumscribed nodules of back-to-back tubular glands, resulting in large cribriform structures surrounded by fibrotic stroma (Figure 2). Papilla formation was seen only focally in 36% of the cases. The intraductal location was confirmed in every case by demonstrating focal continuity of the neoplastic epithelium with histologically normal-appearing ductal epithelium that either surrounded the tumor nodules or partially lined the cystic spaces into which the polypoid tumor masses projected (Figure 3). However, the majority of intraductal neoplastic proliferations expanded the ducts such that no residual non-neoplastic ductal epithelium remained along the lumen and some of these nodular tumor growths showed irregular contours with strands of cells extending into the surrounding pancreatic parenchyma with an associated stromal desmoplastic reaction. The size of the individual nodules ranged from microscopic (corresponding to the diameter of medium-sized interlobular ducts) to one case in which an individual cyst measured 10 cm. Thus, in many cases, the intraductal proliferation appeared to extend from the major pancreatic ducts into smaller secondary ducts, with each involved ductal profile appearing as a separate tumor nodule in cross section. In 58% of the cases, multiple prominent lymphoid aggregates were identified around the tumor.
Figure 2.
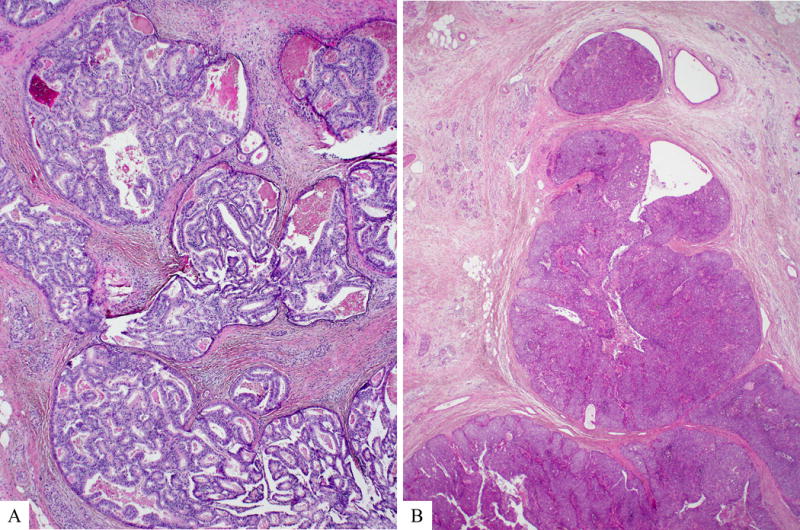
Microscopically, intraductal tubulopapillary neoplasm is characterized by nodules of back to back tubular glands, resulting in large cribriform structures within dilated pancreatic ducts (A). Solid areas with scattered abortive glandular arrangements may also be seen (B).
Figure 3.
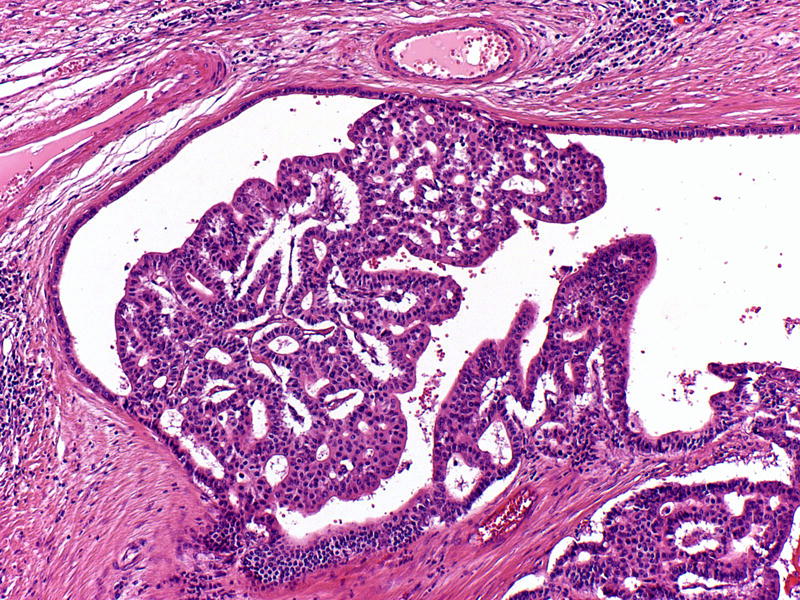
Continuity of the neoplastic epithelium with histologically normal-appearing ductal epithelium may be identified in some tumor nodules.
Within the tumor nodules, there were tightly packed small glands lined by predominantly cuboidal cells with minimal to modest amounts of eosinophilic to amphophilic cytoplasm (Figure 4). Four cases revealed clear cell morphology, three focally, one extensively (Figure 5). Foci with amorphous acidophilic secretions were present in three cases (Figure 6). However, in all but cases, there was no or only minimal intracellular mucin; only one case with more columnar tumor cells revealed some intracellular mucin (this case revealed all the other characteristic features of ITPN). The nuclei were round to oval and moderately to markedly atypical. Some cases had centrally located, single, prominent nucleoli. Mitotic figures were readily identifiable. Also, the majority (64%) of the cases showed necrosis within the tumor nodules, often with comedo-like pattern (Figure 7). Some tumors also had hypercellular, desmoplastic stroma within the center of the tumor nodules, although the nodule periphery remained circumscribed. Of note, one tumor revealed calcifications with ossification.
Figure 4.
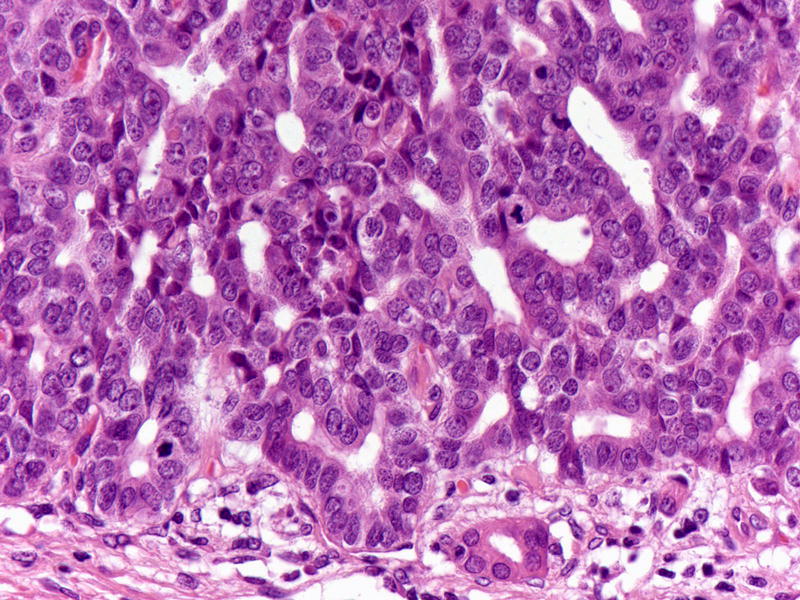
Tumor cells are cuboidal with modest amount of eosinophilic to amphophilic cytoplasm and round to oval atypical nucleus. Mitotic figures are easily identified.
Figure 5.
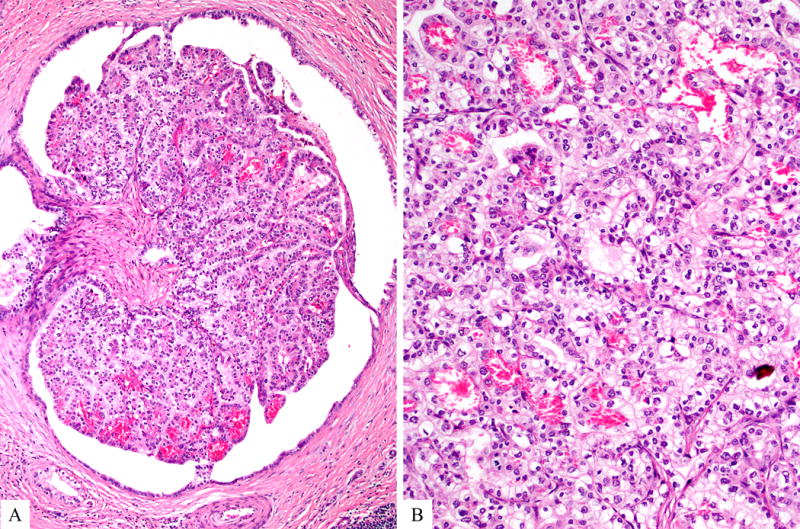
Rare cases may reveal focal or extensive clear cell morphology.
Figure 6.
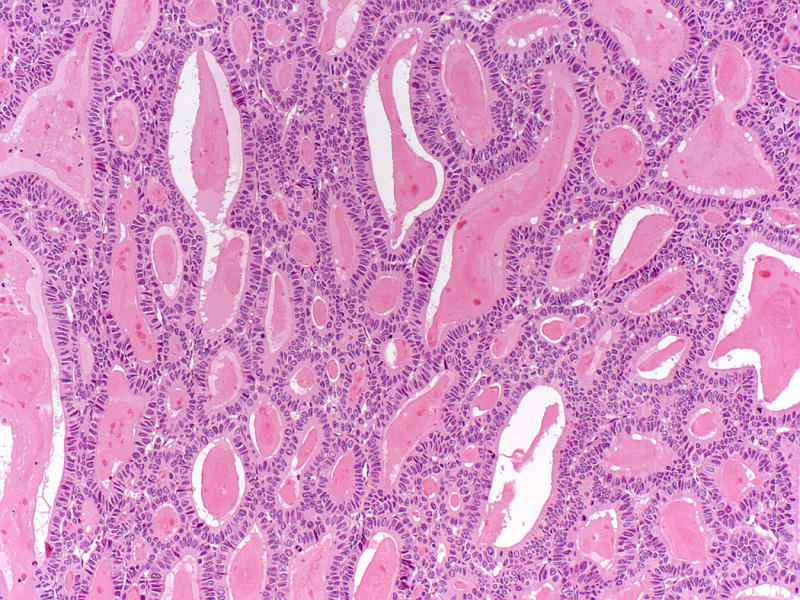
Intraluminal eosinophilic secretions may be seen but intracellular mucin is minimal/non-existent in intraductal tubulopapillary neoplasms.
Figure 7.
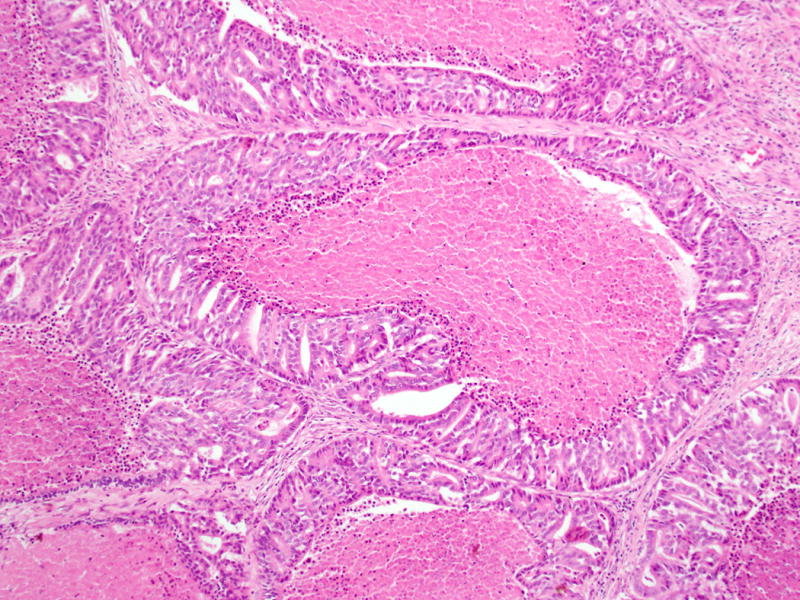
Necrosis within the tumor nodules, some with comedo-like pattern, is common.
Because many of the tumor nodules lacked a peripheral rim of non-neoplastic ductal epithelium, it was often very difficult to determine whether invasive carcinoma was present, and even for cases with established invasive carcinoma, it was challenging to determine its extent. Foci in which there were thin strands of cells extending away from the edges of the nodules were interpreted to represent stromal invasion (Figure 8A). In a few cases there were individual malignant glands clearly infiltrating into the stroma (Figure 8B). In two cases, the presence of invasion could not be assessed due to the biopsy nature of the specimens. Of thirty-one resections, nine (29%) cases had no definite invasive carcinoma in the slides available for review. Twenty-two (71%) cases had invasive carcinoma components that were ranged from minute (representing less than 10% of the tumor, n=12) to substantial (10%–50% of the tumor, n=7) to extensive (more than 50% of the tumor, n=3). Three cases had lymphovascular invasion, and three had perineural invasion. Of known sixteen cases, none had lymph node metastases.
Figure 8.
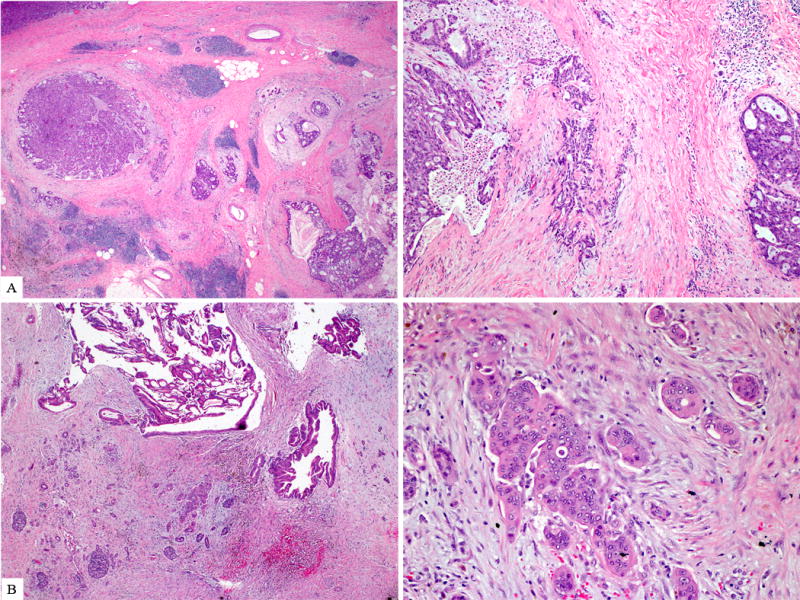
Invasion, either in the form of individual/thin strands of cells extending away from the periphery of the involved ducts (A) or individual malignant glands clearly infiltrating (B) into the surrounding desmoplastic stroma, were identified in two third of the tumors.
Adjacent uninvolved pancreatic parenchyma was either normal or atrophic with islet aggregation. Features of chronic pancreatitis were not identified. There was infrequent pancreatic intraepithelial neoplasia (PanIN), and in every instance, the PanIN was low-grade. One case also revealed an incidental neuroendocrine microadenoma.
Immunohistochemical Findings
The results of the immunohistochemical studies are summarized in Table 3.
Table 3.
Results of the immunohistochemical studies
| Positive or Normal (%) | Negative or Abnormal (%) | |
|---|---|---|
| Keratins | ||
| Cam 5.2 | 12/12 (100) | 0/12 (0) |
| CK 7 | 16/16 (100) | 0/12 (0) |
| CK 19 | 16/18 (89) | 0/18 (0) |
| CK 20 | 3/16 (19)* | 13/16 (81) |
| Glycoproteins | ||
| B72.3 | 2/7 (29) | 5/7 (71) |
| CA125 | 2/14 (14)& | 12/14 (86) |
| CA19.9 | 13/14 (93) | 1/14 (7) |
| mCEA | 6/12 (50) | 6/12 (50) |
| MUC1 | 15/17 (88) | 2/17 (12) |
| MUC2 | 0/17(0) | 17/17 (100) |
| MUC5AC | 1/24 (4)& | 23/24 (96) |
| MUC6 | 17/25 (68) | 8/25 (32) |
| Lineage Markers | ||
| CDX2 | 1/14 (7)* | 13/14 (93) |
| HepPar-1 | 1/6 (17)& | 5/6 (83) |
| Chromogranin | 1/27 (4)* | 26/27 (96) |
| Synaptophysin | 1/27 (4)* | 26/27 (96) |
| Chymotrypsin | 0/24 (0) | 24/24 (100) |
| Trypsin | 0/26 (0) | 26/26 (100) |
| Molecular Markers | ||
| β-Catenin | 14/15 (93) – membranous | 1/15 (7) – nuclear |
| E-Cadherin | 16/16 (100) – membranous | 0/16 (0) – nuclear |
| p16 | 4/12 (33) | 8/12 (67) |
| Nuclear p53 accumulation | 4/15 (27) | 11/15 (73) |
| SMAD4 | 16/16 (100) | 0/16 (0) |
Focal (10–25% of cells) labeling
Only rare, scattered cells
All tested tumors labeled with CAM5.2, and CK7; 89% labeled with CK19. In contrast, only 19% of the tumors contained rare, scattered cells that labeled with CK20, and in one of these tumors, rare scattered cells also labeled with CDX2. Ninety-three percent of the tumors labeled with CA19.9, which is typically expressed in ductal epithelial cells and ductal neoplasms. Monoclonal CEA was also expressed in half of the cases. Tumor-specific glycoproteins were less consistently expressed, ranging from 14% of the tumors focally labeling with CA125 to 29% diffusely labeling with B72.3. The MUC family of proteins showed a fairly consistent pattern of labeling, with 88% and 68% of the tumors expressing MUC1 and MUC6 (Figure 9), respectively but none expressing MUC2. Similarly, ITPNs were consistently negative for MUC5AC, except one tumor with focal MUC5AC expression. Of note, this case was otherwise a typical ITPN; therefore it was not excluded from the study.
Figure 9.
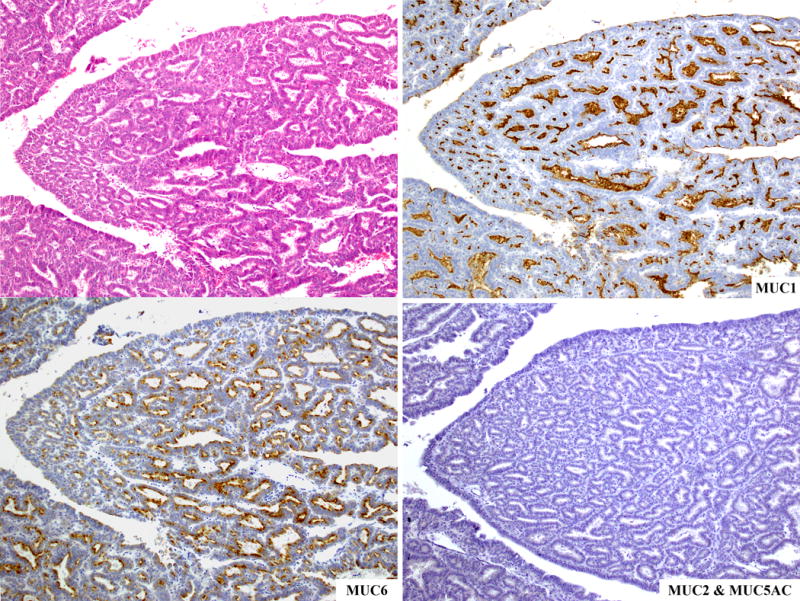
The MUC family of proteins shows a fairly consistent pattern of labeling, with most cases staining for MUC1 and MUC6 but no cases expressing neither MUC2 nor MUC5AC.
There was no expression of the pancreatic enzymes, trypsin and chymotrypsin in any of the tumors and only one tumor focally expressed HepPar-1. In contrast to other ductal neoplasms of the pancreas, scattered chromogranin and synaptophysin positive cells were identified very rarely (4%). Among antibodies associated with genetic alterations previously described in other pancreatic neoplasms, SMAD4 loss occurred in none of the ITPNs, and p16 expression and nuclear p53 accumulation were found in only 33% and 27%, respectively. Similarly, E-Cadherin expression was normal (membranous) in all and only one revealed abnormal (nuclear) β-Catenin reactivity.
Clinical Outcome
Clinical follow-up information was available for twenty-two cases, with a median follow-up of 45 months (range, 11–173). Only two patients with invasive carcinoma died of disease at 23 and 41 months, respectively. Twelve patients were alive with disease, with a median follow-up of 61.5 months (range, 12–173). Of these, four patients had histologically proven local recurrences (one also had liver metastasis), and another two had liver metastases after 5 to 72 months. At the time of last follow-up, seven patients were alive with no evidence of disease, with a median follow-up of 19 months (range, 11–95). One of these patients, treated initially by pancreatoduodenectomy, was found to have dilated ducts in the pancreatic remnant, radiographically suspicious for recurrence; however, completion pancreatectomy showed only ductal ectasia without evidence of the neoplasm. One patient died of unrelated causes at 49 months.
The overall 1, 3- and 5-year survival rates were 100, 93 and 77%, respectively. The overall 5-year survival rate was 100% in patients without an invasive component and 1, 3- and 5-year survival rates were 100%, 91% and 71%, respectively in patients with an invasive component (p=0.7) (Figure 10). Attempts to correlate the clinical outcome with the extent of invasive carcinoma were failed as the patient with the greatest volume of invasive carcinoma (representing more than 50% of the tumor) was free of disease at 173 months, whereas one of the two patients who died of disease had a minute invasive carcinoma (representing less than 10% of the tumor). More confusing was the fact that two patients without identifiable invasive carcinoma had recurrence of disease, although only limited slides from the primary presentation were available for review in these cases.
Figure 10.
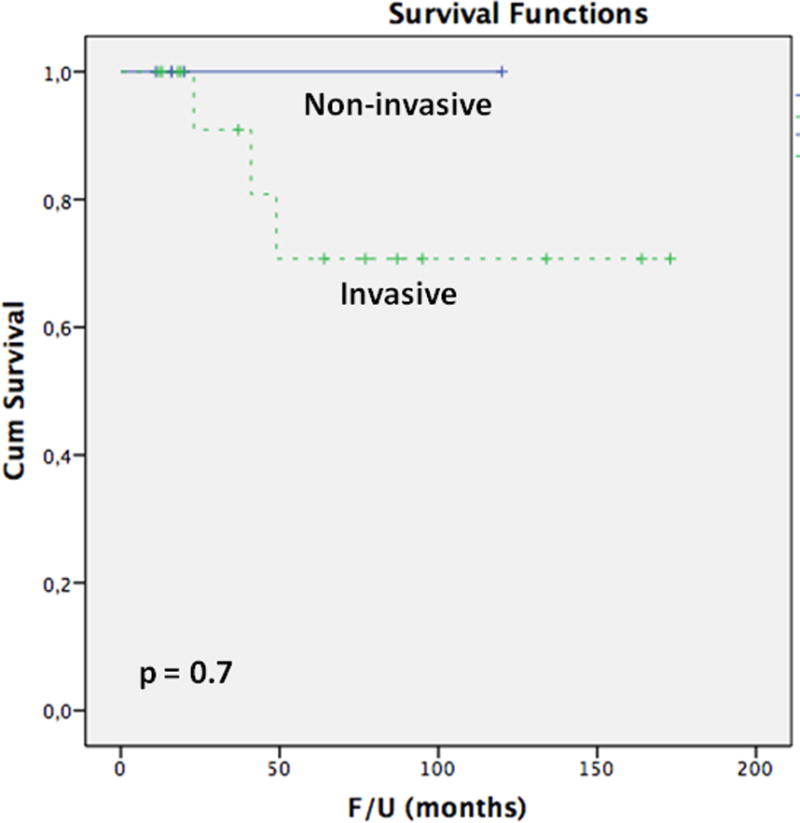
The Kaplan-Meier analysis comparing the overall disease-specific survivals of patients without an invasive component and patients with an invasive component.
DISCUSSION
With intraductal papillary mucinous neoplasms (IPMNs) as the prototype, the family of intraductal neoplasms of the pancreas includes a variety of architectural patterns and cytologic variants7, 8. Some of these are simply regarded as subtypes of IPMNs2–6. IOPN is more distinctive18–21, 37–39; however, the current (2010) WHO designated this neoplasm to be a subtype of IPMN (“oncocytic type”) as well8. In the last couple of decades, IPMNs have been subjected to detailed studies, results of which have enormously added to our knowledge of IPMNs2–6, 10–14, 16, 17. However, experience with ITPN is limited.
In this study, we analyzed thirty-three cases. All of our patients are adults (mean age=55 years) and there is a slight female predominance (F:M=1.2). Like IPMNs, ITPNs most commonly involve the head of the pancreas but may also arise in the tail or involve the entire gland. Recently a case of multifocal ITPN has also been reported33. The degree of ductal dilation varies somewhat, with some cases showing only minimally dilated ducts due to the extensive obliteration of the ductal lumina. Most patients presented with symptoms of pancreatitis, abdominal pain or sense of fullness. Jaundice is distinctly unusual and lacking in all patients in our series. Similarly, in their metaanalysis of publications on surgically resected ITPN, Date et al. identified only 3 cases with jaundice36.
Microscopically, most ITPNs show a predominant or exclusive tubular growth pattern, and despite the entity’s name, true papilla formation is seen only focally in 36% of the cases. Some regions of the intraductal tumor grow as solid sheets of cells with minimal lumen formation. This pattern has also been observed in IOPN18, 21. In contrast to the abundant mucinous cytoplasm that characterizes many cases of IPMN3, 7, 8, there is usually no obvious intracellular mucin in ITPNs. Predominantly cuboidal tumor cells reveal minimal to modest amounts of eosinophilic to amphophilic and rarely clear cytoplasm (12% of our cases). Recently, Ahls et al. also reported an ITPN with clear cell phenotype29. Rare cases of ITPN with extensive calcification35 or stromal osseous and cartilaginous metaplasia28 have also been described, similar to one of our cases with calcification and bone formation. Nuclear atypia varies from moderate to marked, and mitotic figures are easily identified. Some cases have necrosis within the intraductal component and others demonstrate foci of stromal desmoplasia. The presence of these features of high grade dysplasia, along with the absence of residual benign ductal epithelium surrounding many of the tumor nodules, conspire to make the recognition of the intraductal nature of these neoplasms very difficult. In fact, on high power examination where the circumscribed contours of the tumor nodules are not apparent, it is easy to mistake the intraductal foci for highly cellular invasive carcinoma, although the architectural and cytologic differences from most cases of conventional pancreatic ductal adenocarcinoma are striking.
Invasive carcinomas arising in association with ITPNs typically consist of individual cells or small, angulated non-mucinous glands extending away from the periphery of the involved ducts into the surrounding desmoplastic stroma (Figure 8). The extent of invasive carcinoma can be very difficult to assess, but in approximately one-third of our cases, there was substantial to extensive invasive carcinoma that was relatively easy to recognize. In 39% of the cases there were irregularities in some of the tumor nodules that we interpreted as focal invasive carcinoma. The remaining cases showed no evidence of invasion.
Despite the presence of invasive carcinoma in approximately two-thirds of the cases, the overall outcome appears to be quite favorable. Previously, lymph node and liver metastases were rarely reported34. In our series, of twenty-two patients with available follow up information, only two patients (9%) died of disease and six others (27%) suffered from recurrences or metastases and among the recurrences were a few patients with minute invasive carcinoma. Thus, it appears that there exists a potential for progression even in the absence of obvious widespread invasive carcinoma or, more likely, the presence of invasion can be morphologically so subtle that it is difficult to accurately quantify.
Immunohistochemically, ITPNs share ductal differentiation with IPMNs, and with most other pancreatic neoplasms, by labeling with wide-spectrum (CAM5.2) and specific (CK7 and CK19) cytokeratin antibodies as well as expressing certain glycoproteins including B72.3, CA19.9, and monoclonal CEA. However, most ITPNs do not label with CA125. Similarly, nuclear p53 accumulation, which is found in 60% of pancreatic ductal adenocarcinomas and in 0–38% (weighted mean, 21%) of IPMNs, – usually with high-grade dysplasia or invasive carcinoma40–42 – is only seen in 27% of ITPNs. SMAD4 is also normally expressed (retained) in ITPNs. This contrasts with loss of expression in 55% of ductal adenocarcinomas, although non-invasive IPMNs also typically have normal patterns of SMAD4 expression7, 43.
The expression patterns for MUCs have been correlated with the direction of differentiation of pancreatic neoplasms towards normal cell counterparts of the gastrointestinal tract4, 12, 44–47. MUC1 is expressed in almost all examples of pancreatobiliary type neoplasms, including ductal adenocarcinomas and pancreatobiliary subtype IPMNs, and its expression has been associated with increased metastatic ability of pancreatic cancer cell lines48–50. MUC6 is expressed in ductal adenocarcinomas, pancreatobiliary type IPMNs, IOPNs and serous cystadenomas51, 52. MUC5AC is widely expressed in ductal adenocarcinomas, all subtypes of IPMNs (gastric, intestinal, and pancreatobiliary), and IOPNs4. Finally, expression of MUC2 (and CDX2) defines the intestinal pathway of differentiation, characteristic of intestinal subtype of IPMNs and associated colloid carcinomas4, 12. In our series of ITPN, MUC1 and MUC6 were expressed, whereas MUC2 and MUC5AC were not. Previous studies analyzing single cases or small series have also reported similar results22–36, except one case in which MUC5AC is reported to be positive53.
The expression of MUC1 and MUC6 suggests that ITPN has pancreatic (ductal) and possibly also gastric pyloric gland differentiation. The lack of MUC5AC points to an absence of gastric foveolar differentiation, which contrasts with the common expression of this marker in IPMNs4, 11, 54. Finally, the lack of intestinal differentiation in ITPNs is also reflected in the absence of MUC2 (and for CDX2) expression. Thus, it appears that ITPN lacks the gastroenteric differentiation seen in IPMN, and instead shows pancreatic duct differentiation morphologically and immunohistochemically.
A detailed study we performed recently on the genetic features of ITPNs has shown that ITPN is genetically distinct from IPMN and ductal adenocarcinoma, as it does not harbor the vast majority of the previously reported IPMN or ductal adenocarcinoma-related mutations55. The most important genotypic difference from IPMN and ductal adenocarcinoma is the absence of KRAS and BRAF mutations in ITPNs55 (The vast majority of ductal adenocarcinomas harbor mutations of KRAS7. The adenocarcinomas without mutations of KRAS often have mutations of BRAF56. Pancreatic intraepithelial neoplasia and IPMN also frequently harbor mutations of KRAS7). Emerging studies analyzing a small number of cases for selected gene mutations have also reported predominantly similar results25, 26, 29, 31, 34, 53, 57–60. These findings provide support not only for the proposition that ITPN is a distinct pancreatic intraductal neoplasm with different morphologic features and biologic behavior, but also for the hypothesis that distinct genetic pathways of tumor development correspond to the characteristic morphologic patterns of these different neoplasms. Of note, Schlitter et al.’s recent molecular analyses of biliary counterparts of ITPN61 have highlighted the low prevalence of alterations of the common oncogenic signaling pathways in these neoplasms as well62.
The differential diagnosis of ITPN includes other intraductal tumors such as IPMNs, IOPNs, as well as other pancreatic neoplasms that may rarely grow within the ducts (acinar cell carcinoma, pancreatoblastoma, and well differentiated pancreatic neuroendocrine tumors). Most other intraductal neoplasms have a fundamentally papillary architecture, allowing relatively easy separation of IPMNs from ITPNs13, 14, 38. IPMNs may have some limited tubular growth, especially at the periphery of the involved ducts, but complete expansion and obliteration of the ductal profiles by a tubular proliferation is unusual. Similarly, IOPN can exhibit a solid or tubular growth pattern but ITPN lacks the abundant granular eosinophilic cytoplasm of IOPN. The back-to-back tubules of ITPN, each of which has a relatively small lumen, closely simulate the pattern of acinar neoplasms63–67. Most acinar cell carcinomas are large solid lesions and demonstrate no involvement of the native pancreatic ducts. However, rare cases have been described in which an intraductal growth pattern is present either focally or extensively64, 68–70. Fortunately, immunohistochemistry is very helpful. Acinar cell carcinomas consistently express pancreatic enzymes such as trypsin, chymotrypsin, and lipase and generally lack expression of CK1963–67. ITPNs consistently fail to express pancreatic enzymes. Pancreatoblastomas, which occur predominantly in infants, are also fundamentally acinar neoplasms of the pancreas that rarely have an intraductal growth pattern66, 71. Again, the presence of acinar differentiation can be detected immunohistochemically, and pancreatoblastomas additionally have squamous nest that are helpful in their separation from ITPNs. Finally, well differentiated pancreatic neuroendocrine tumors can rarely show an intraductal growth pattern72–75 but the characteristic diffuse staining for neuroendocrine markers can separate these from ITPNs72–75.
In summary, ITPN is a distinct clinicopathologic entity in the pancreas. By its intraductal nature, it resembles IPMNs and by its acinar pattern, mimics acinar cell carcinoma; however, it has several distinguishing characteristics. (1) no visible secreted mucin, (2) highly cellular complex intracellular tumor nodules showing predominantly tubular or cribriform patterns, (3) absence of intracytoplasmic mucin, (4) atypical nuclei, (5) absence of acinar and neuroendocrine differentiation, and (6) despite the histologic indicators of a high-grade malignancy and even an invasive carcinoma component, a relatively favorable overall outcome.
Supplementary Material
Acknowledgments
The authors thank and Ms. Tanisha Daniel and Ms. Dana Haviland for their assistance during manuscript preparation and Ms. Allyne Manzo and Ms. Lorraine Corsale for their assistance with the figures.
FUNDING
This work has been supported by the Cancer Center Support Grant (CCSG) / Core Grant / P30 CA008748.
References
- 1.Ohhashi K, Murakami Y, Maruyama M. Four cases of mucous secreting pancreatic cancer. Prog Dig Endosc. 1982;20:348–351. (in Japanese, abstract in English) [Google Scholar]
- 2.Adsay NV, Merati K, Basturk O, et al. Pathologically and biologically distinct types of epithelium in intraductal papillary mucinous neoplasms: delineation of an “intestinal” pathway of carcinogenesis in the pancreas. Am J Surg Pathol. 2004;28:839–48. doi: 10.1097/00000478-200407000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Hruban RH, Takaori K, Klimstra DS, et al. An illustrated consensus on the classification of pancreatic intraepithelial neoplasia and intraductal papillary mucinous neoplasms. Am J Surg Pathol. 2004;28:977–87. doi: 10.1097/01.pas.0000126675.59108.80. [DOI] [PubMed] [Google Scholar]
- 4.Furukawa T, Kloppel G, Volkan Adsay N, et al. Classification of types of intraductal papillary-mucinous neoplasm of the pancreas: a consensus study. Virchows Arch. 2005;447:794–9. doi: 10.1007/s00428-005-0039-7. [DOI] [PubMed] [Google Scholar]
- 5.Adsay V, Mino-Kenudson M, Furukawa T, et al. Pathologic Evaluation and Reporting of Intraductal Papillary Mucinous Neoplasms of the Pancreas and Other Tumoral Intraepithelial Neoplasms of Pancreatobiliary Tract: Recommendations of Verona Consensus Meeting. Ann Surg. 2016;263:162–77. doi: 10.1097/SLA.0000000000001173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Basturk O, Hong SM, Wood LD, et al. A Revised Classification System and Recommendations From the Baltimore Consensus Meeting for Neoplastic Precursor Lesions in the Pancreas. Am J Surg Pathol. 2015;39:1730–41. doi: 10.1097/PAS.0000000000000533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hruban R, Pitman MB, Klimstra DS. AFIP Atlas of Tumor Pathology. Washington, DC: American Registry of Pathology; 2007. Tumors of the Pancreas. [Google Scholar]
- 8.Adsay NV, Kloppel G, Fukushima N, et al. Intraductal neoplasms of the pancreas. In: Bosman FT, Carneiro F, Hruban RH, et al., editors. WHO classification of tumors. WHO Press; Lyon: 2010. pp. 304–313. [Google Scholar]
- 9.Kloppel G, Basturk O, Schlitter AM, et al. Intraductal neoplasms of the pancreas. Semin Diagn Pathol. 2014;31:452–66. doi: 10.1053/j.semdp.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 10.Adsay NV, Pierson C, Sarkar F, et al. Colloid (mucinous noncystic) carcinoma of the pancreas. Am J Surg Pathol. 2001;25:26–42. doi: 10.1097/00000478-200101000-00003. [DOI] [PubMed] [Google Scholar]
- 11.Luttges J, Zamboni G, Longnecker D, et al. The immunohistochemical mucin expression pattern distinguishes different types of intraductal papillary mucinous neoplasms of the pancreas and determines their relationship to mucinous noncystic carcinoma and ductal adenocarcinoma. Am J Surg Pathol. 2001;25:942–8. doi: 10.1097/00000478-200107000-00014. [DOI] [PubMed] [Google Scholar]
- 12.Adsay NV, Merati K, Andea A, et al. The dichotomy in the preinvasive neoplasia to invasive carcinoma sequence in the pancreas: differential expression of MUC1 and MUC2 supports the existence of two separate pathways of carcinogenesis. Mod Pathol. 2002;15:1087–95. doi: 10.1097/01.MP.0000028647.98725.8B. [DOI] [PubMed] [Google Scholar]
- 13.Adsay NV, Conlon KC, Zee SY, et al. Intraductal papillary-mucinous neoplasms of the pancreas: an analysis of in situ and invasive carcinomas in 28 patients. Cancer. 2002;94:62–77. doi: 10.1002/cncr.10203. [DOI] [PubMed] [Google Scholar]
- 14.D’Angelica M, Brennan MF, Suriawinata AA, et al. Intraductal papillary mucinous neoplasms of the pancreas: an analysis of clinicopathologic features and outcome. Ann Surg. 2004;239:400–8. doi: 10.1097/01.sla.0000114132.47816.dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ishida M, Egawa S, Aoki T, et al. Characteristic clinicopathological features of the types of intraductal papillary-mucinous neoplasms of the pancreas. Pancreas. 2007;35:348–52. doi: 10.1097/mpa.0b013e31806da090. [DOI] [PubMed] [Google Scholar]
- 16.Yopp AC, Allen PJ. Prognosis of invasive intraductal papillary mucinous neoplasms of the pancreas. World J Gastrointest Surg. 2010;2:359–62. doi: 10.4240/wjgs.v2.i10.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yopp AC, Katabi N, Janakos M, et al. Invasive carcinoma arising in intraductal papillary mucinous neoplasms of the pancreas: a matched control study with conventional pancreatic ductal adenocarcinoma. Ann Surg. 2011;253:968–74. doi: 10.1097/SLA.0b013e318214bcb4. [DOI] [PubMed] [Google Scholar]
- 18.Adsay NV, Adair CF, Heffess CS, et al. Intraductal oncocytic papillary neoplasms of the pancreas. Am J Surg Pathol. 1996;20:980–94. doi: 10.1097/00000478-199608000-00007. [DOI] [PubMed] [Google Scholar]
- 19.Basturk O, Tan M, Bhanot U, et al. The oncocytic subtype is genetically distinct from other pancreatic intraductal papillary mucinous neoplasm subtypes. Mod Pathol. 2016 doi: 10.1038/modpathol.2016.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marchegiani G, Mino-Kenudson M, Ferrone CR, et al. Oncocytic-type intraductal papillary mucinous neoplasms: a unique malignant pancreatic tumor with good long-term prognosis. J Am Coll Surg. 2015;220:839–44. doi: 10.1016/j.jamcollsurg.2015.01.051. [DOI] [PubMed] [Google Scholar]
- 21.Askan G, Klimstra DS, Adsay V, et al. “Oncocytic-type” of Intraductal Papillary Mucinous Neoplasm (IPMN): An Analysis of 25 Cases [abstract] Mod Pathol. 2016 doi: 10.1038/modpathol.2016.98. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tajiri T, Tate G, Kunimura T, et al. Histologic and immunohistochemical comparison of intraductal tubular carcinoma, intraductal papillary-mucinous carcinoma, and ductal adenocarcinoma of the pancreas. Pancreas. 2004;29:116–22. doi: 10.1097/00006676-200408000-00006. [DOI] [PubMed] [Google Scholar]
- 23.Tajiri T, Tate G, Inagaki T, et al. Intraductal tubular neoplasms of the pancreas: histogenesis and differentiation. Pancreas. 2005;30:115–21. doi: 10.1097/01.mpa.0000148513.69873.4b. [DOI] [PubMed] [Google Scholar]
- 24.Hisa T, Nobukawa B, Suda K, et al. Intraductal carcinoma with complex fusion of tubular glands without macroscopic mucus in main pancreatic duct: dilemma in classification. Pathol Int. 2007;57:741–5. doi: 10.1111/j.1440-1827.2007.02163.x. [DOI] [PubMed] [Google Scholar]
- 25.Furukawa T, Hatori T, Fukuda A, et al. Intraductal tubular carcinoma of the pancreas: case report with molecular analysis. Pancreas. 2009;38:235–7. doi: 10.1097/MPA.0b013e318175e3e0. [DOI] [PubMed] [Google Scholar]
- 26.Yamaguchi H, Shimizu M, Ban S, et al. Intraductal tubulopapillary neoplasms of the pancreas distinct from pancreatic intraepithelial neoplasia and intraductal papillary mucinous neoplasms. Am J Surg Pathol. 2009;33:1164–72. doi: 10.1097/PAS.0b013e3181a162e5. [DOI] [PubMed] [Google Scholar]
- 27.Hioki M, Nakagohri T, Ikumoto T, et al. Intraductal tubular carcinoma of the pancreas: case report with review of literature. Anticancer Res. 2010;30:4435–41. [PubMed] [Google Scholar]
- 28.Jokoji R, Tsuji H, Tsujimoto M, et al. Intraductal tubulopapillary neoplasm of pancreas with stromal osseous and cartilaginous metaplasia; a case report. Pathol Int. 2012;62:339–43. doi: 10.1111/j.1440-1827.2012.02791.x. [DOI] [PubMed] [Google Scholar]
- 29.Ahls MG, Niedergethmann M, Dinter D, et al. Case report: Intraductal tubulopapillary neoplasm of the pancreas with unique clear cell phenotype. Diagn Pathol. 2014;9:11. doi: 10.1186/1746-1596-9-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Furuhata A, Minamiguchi S, Mikami Y, et al. Intraductal tubulopapillary neoplasm with expansile invasive carcinoma of the pancreas diagnosed by endoscopic ultrasonography-guided fine needle aspiration: a case report. Diagn Cytopathol. 2014;42:314–20. doi: 10.1002/dc.23083. [DOI] [PubMed] [Google Scholar]
- 31.Guan H, Gurda G, Lennon AM, et al. Intraductal tubulopapillary neoplasm of the pancreas on fine needle aspiration: case report with differential diagnosis. Diagn Cytopathol. 2014;42:156–60. doi: 10.1002/dc.22890. [DOI] [PubMed] [Google Scholar]
- 32.Del Chiaro M, Mucelli RP, Blomberg J, et al. Is intraductal tubulopapillary neoplasia a new entity in the spectrum of familial pancreatic cancer syndrome? Fam Cancer. 2014;13:227–9. doi: 10.1007/s10689-013-9696-x. [DOI] [PubMed] [Google Scholar]
- 33.Kolby D, Thilen J, Andersson R, et al. Multifocal intraductal tubulopapillary neoplasm of the pancreas with total pancreatectomy: report of a case and review of literature. Int J Clin Exp Pathol. 2015;8:9672–80. [PMC free article] [PubMed] [Google Scholar]
- 34.Matthews Y, McKenzie C, Byrne C, et al. Intraductal tubulopapillary neoplasm of pancreas with associated invasive carcinoma, lymph node, rectal and hepatic metastases. Pathology. 2015;47:169–71. doi: 10.1097/PAT.0000000000000228. [DOI] [PubMed] [Google Scholar]
- 35.Takayama S, Maeda T, Nishihara M, et al. A case of intraductal tubulopapillary neoplasm of pancreas with severe calcification, a potential pitfall in diagnostic imaging. Pathol Int. 2015;65:501–6. doi: 10.1111/pin.12322. [DOI] [PubMed] [Google Scholar]
- 36.Date K, Okabayashi T, Shima Y, et al. Clinicopathological features and surgical outcomes of intraductal tubulopapillary neoplasm of the pancreas: a systematic review. Langenbecks Arch Surg. 2016;401:439–47. doi: 10.1007/s00423-016-1391-6. [DOI] [PubMed] [Google Scholar]
- 37.Jyotheeswaran S, Zotalis G, Penmetsa P, et al. A newly recognized entity: intraductal “oncocytic” papillary neoplasm of the pancreas. Am J Gastroenterol. 1998;93:2539–43. doi: 10.1111/j.1572-0241.1998.00714.x. [DOI] [PubMed] [Google Scholar]
- 38.Adsay NV, Longnecker DS, Klimstra DS. Pancreatic tumors with cystic dilatation of the ducts: intraductal papillary mucinous neoplasms and intraductal oncocytic papillary neoplasms. Semin Diagn Pathol. 2000;17:16–30. [PubMed] [Google Scholar]
- 39.Patel SA, Adams R, Goldstein M, et al. Genetic analysis of invasive carcinoma arising in intraductal oncocytic papillary neoplasm of the pancreas. Am J Surg Pathol. 2002;26:1071–7. doi: 10.1097/00000478-200208000-00014. [DOI] [PubMed] [Google Scholar]
- 40.Sessa F, Solcia E, Capella C, et al. Intraductal papillary-mucinous tumours represent a distinct group of pancreatic neoplasms: an investigation of tumour cell differentiation and K-ras, p53 and c-erbB-2 abnormalities in 26 patients. Virchows Arch. 1994;425:357–67. doi: 10.1007/BF00189573. [DOI] [PubMed] [Google Scholar]
- 41.Yamaguchi K, Chijiiwa K, Noshiro H, et al. Ki-ras codon 12 point mutation and p53 mutation in pancreatic diseases. Hepatogastroenterology. 1999;46:2575–81. [PubMed] [Google Scholar]
- 42.Sasaki S, Yamamoto H, Kaneto H, et al. Differential roles of alterations of p53, p16, and SMAD4 expression in the progression of intraductal papillary-mucinous tumors of the pancreas. Oncol Rep. 2003;10:21–5. [PubMed] [Google Scholar]
- 43.Furukawa T, Fujisaki R, Yoshida Y, et al. Distinct progression pathways involving the dysfunction of DUSP6/MKP-3 in pancreatic intraepithelial neoplasia and intraductal papillary-mucinous neoplasms of the pancreas. Mod Pathol. 2005;18:1034–42. doi: 10.1038/modpathol.3800383. [DOI] [PubMed] [Google Scholar]
- 44.Matsuzawa K, Akamatsu T, Katsuyama T. Mucin histochemistry of pancreatic duct cell carcinoma, with special reference to organoid differentiation simulating gastric pyloric mucosa. Hum Pathol. 1992;23:925–33. doi: 10.1016/0046-8177(92)90407-t. [DOI] [PubMed] [Google Scholar]
- 45.Nakamura A, Horinouchi M, Goto M, et al. New classification of pancreatic intraductal papillary-mucinous tumour by mucin expression: its relationship with potential for malignancy. J Pathol. 2002;197:201–10. doi: 10.1002/path.1109. [DOI] [PubMed] [Google Scholar]
- 46.Terris B, Dubois S, Buisine MP, et al. Mucin gene expression in intraductal papillary-mucinous pancreatic tumours and related lesions. J Pathol. 2002;197:632–7. doi: 10.1002/path.1146. [DOI] [PubMed] [Google Scholar]
- 47.Nagata K, Horinouchi M, Saitou M, et al. Mucin expression profile in pancreatic cancer and the precursor lesions. J Hepatobiliary Pancreat Surg. 2007;14:243–54. doi: 10.1007/s00534-006-1169-2. [DOI] [PubMed] [Google Scholar]
- 48.Yonezawa S, Nakamura A, Horinouchi M, et al. The expression of several types of mucin is related to the biological behavior of pancreatic neoplasms. J Hepatobiliary Pancreat Surg. 2002;9:328–41. doi: 10.1007/s005340200037. [DOI] [PubMed] [Google Scholar]
- 49.Yonezawa S, Goto M, Yamada N, et al. Expression profiles of MUC1, MUC2, and MUC4 mucins in human neoplasms and their relationship with biological behavior. Proteomics. 2008;8:3329–41. doi: 10.1002/pmic.200800040. [DOI] [PubMed] [Google Scholar]
- 50.Satoh S, Hinoda Y, Hayashi T, et al. Enhancement of metastatic properties of pancreatic cancer cells by MUC1 gene encoding an anti-adhesion molecule. Int J Cancer. 2000;88:507–18. doi: 10.1002/1097-0215(20001115)88:4<507::aid-ijc1>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 51.Kosmahl M, Wagner J, Peters K, et al. Serous cystic neoplasms of the pancreas: an immunohistochemical analysis revealing alpha-inhibin, neuron-specific enolase, and MUC6 as new markers. Am J Surg Pathol. 2004;28:339–46. doi: 10.1097/00000478-200403000-00006. [DOI] [PubMed] [Google Scholar]
- 52.Basturk O, Khayyata S, Klimstra DS, et al. Preferential expression of MUC6 in oncocytic and pancreatobiliary types of intraductal papillary neoplasms highlights a pyloropancreatic pathway, distinct from the intestinal pathway, in pancreatic carcinogenesis. Am J Surg Pathol. 2010;34:364–70. doi: 10.1097/PAS.0b013e3181cf8bb6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Muraki T, Uehara T, Sano K, et al. A case of MUC5AC-positive intraductal neoplasm of the pancreas classified as an intraductal tubulopapillary neoplasm? Pathol Res Pract. 2015;211:1034–9. doi: 10.1016/j.prp.2015.10.009. [DOI] [PubMed] [Google Scholar]
- 54.Zen Y, Fujii T, Itatsu K, et al. Biliary papillary tumors share pathological features with intraductal papillary mucinous neoplasm of the pancreas. Hepatology. 2006;44:1333–43. doi: 10.1002/hep.21387. [DOI] [PubMed] [Google Scholar]
- 55.Bhanot U, Basturk O, Berger M, et al. Molecular Characteristics of the Pancreatic Intraductal Tubulopapillary Neoplasm (Abstract) Modern Pathology. 2015;28:1761A. [Google Scholar]
- 56.Calhoun ES, Jones JB, Ashfaq R, et al. BRAF and FBXW7 (CDC4, FBW7, AGO, SEL10) mutations in distinct subsets of pancreatic cancer: potential therapeutic targets. Am J Pathol. 2003;163:1255–60. doi: 10.1016/S0002-9440(10)63485-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Esposito I, Bauer A, Hoheisel JD, et al. Microcystic tubulopapillary carcinoma of the pancreas: a new tumor entity? Virchows Arch. 2004;444:447–53. doi: 10.1007/s00428-004-0984-6. [DOI] [PubMed] [Google Scholar]
- 58.Yamaguchi H, Kuboki Y, Hatori T, et al. Somatic mutations in PIK3CA and activation of AKT in intraductal tubulopapillary neoplasms of the pancreas. Am J Surg Pathol. 2011;35:1812–7. doi: 10.1097/PAS.0b013e31822769a0. [DOI] [PubMed] [Google Scholar]
- 59.Yamaguchi H, Kuboki Y, Hatori T, et al. The discrete nature and distinguishing molecular features of pancreatic intraductal tubulopapillary neoplasms and intraductal papillary mucinous neoplasms of the gastric type, pyloric gland variant. J Pathol. 2013;231:335–41. doi: 10.1002/path.4242. [DOI] [PubMed] [Google Scholar]
- 60.Amato E, Molin MD, Mafficini A, et al. Targeted next-generation sequencing of cancer genes dissects the molecular profiles of intraductal papillary neoplasms of the pancreas. J Pathol. 2014;233:217–27. doi: 10.1002/path.4344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Katabi N, Torres J, Klimstra DS. Intraductal tubular neoplasms of the bile ducts. Am J Surg Pathol. 2012;36:1647–1655. doi: 10.1097/PAS.0b013e3182684d4f. [DOI] [PubMed] [Google Scholar]
- 62.Schlitter AM, Jang KT, Kloppel G, et al. Intraductal tubulopapillary neoplasms of the bile ducts: clinicopathologic, immunohistochemical, and molecular analysis of 20 cases. Mod Pathol. 2016;29:93. doi: 10.1038/modpathol.2015.106. [DOI] [PubMed] [Google Scholar]
- 63.Klimstra DS, Heffess CS, Oertel JE, et al. Acinar cell carcinoma of the pancreas. A clinicopathologic study of 28 cases. Am J Surg Pathol. 1992;16:815–37. doi: 10.1097/00000478-199209000-00001. [DOI] [PubMed] [Google Scholar]
- 64.Basturk O, Zamboni G, Klimstra DS, et al. Intraductal and papillary variants of acinar cell carcinomas: a new addition to the challenging differential diagnosis of intraductal neoplasms. Am J Surg Pathol. 2007;31:363–70. doi: 10.1097/01.pas.0000213376.09795.9f. [DOI] [PubMed] [Google Scholar]
- 65.La Rosa S, Adsay V, Albarello L, et al. Clinicopathologic study of 62 acinar cell carcinomas of the pancreas: insights into the morphology and immunophenotype and search for prognostic markers. Am J Surg Pathol. 2012;36:1782–95. doi: 10.1097/PAS.0b013e318263209d. [DOI] [PubMed] [Google Scholar]
- 66.Wood LD, Klimstra DS. Pathology and genetics of pancreatic neoplasms with acinar differentiation. Semin Diagn Pathol. 2014;31:491–7. doi: 10.1053/j.semdp.2014.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Klimstra DS, Adsay V. Acinar neoplasms of the pancreas-A summary of 25 years of research. Semin Diagn Pathol. 2016 doi: 10.1053/j.semdp.2016.05.009. [DOI] [PubMed] [Google Scholar]
- 68.Fabre A, Sauvanet A, Flejou JF, et al. Intraductal acinar cell carcinoma of the pancreas. Virchows Arch. 2001;438:312–5. doi: 10.1007/s004280000342. [DOI] [PubMed] [Google Scholar]
- 69.Hashimoto M, Matsuda M, Watanabe G, et al. Acinar cell carcinoma of the pancreas with intraductal growth: report of a case. Pancreas. 2003;26:306–8. doi: 10.1097/00006676-200304000-00016. [DOI] [PubMed] [Google Scholar]
- 70.Toll AD, Mitchell D, Yeo CJ, et al. Acinar cell carcinoma with prominent intraductal growth pattern: case report and review of the literature. Int J Surg Pathol. 2011;19:795–9. doi: 10.1177/1066896909339912. [DOI] [PubMed] [Google Scholar]
- 71.Klimstra DS, Wenig BM, Adair CF, et al. Pancreatoblastoma. A clinicopathologic study and review of the literature. Am J Surg Pathol. 1995;19:1371–89. doi: 10.1097/00000478-199512000-00005. [DOI] [PubMed] [Google Scholar]
- 72.Akatsu T, Wakabayashi G, Aiura K, et al. Intraductal growth of a nonfunctioning endocrine tumor of the pancreas. J Gastroenterol. 2004;39:584–8. doi: 10.1007/s00535-004-1347-4. [DOI] [PubMed] [Google Scholar]
- 73.Chetty R, El-Shinnawy I. Intraductal pancreatic neuroendocrine tumor. Endocr Pathol. 2009;20:262–6. doi: 10.1007/s12022-009-9093-z. [DOI] [PubMed] [Google Scholar]
- 74.Hechtman JF, Franssen B, Labow DM, et al. Intraductal polypoid lipid-rich neuroendocrine tumor of the pancreas with entrapped ductules: case report and review of the literature. Endocr Pathol. 2013;24:30–5. doi: 10.1007/s12022-012-9231-x. [DOI] [PubMed] [Google Scholar]
- 75.Kiyonaga M, Matsumoto S, Mori H, et al. Pancreatic neuroendocrine tumor with extensive intraductal invasion of the main pancreatic duct: a case report. JOP. 2014;15:497–500. doi: 10.6092/1590-8577/2741. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


