Abstract
Background
Acute chorioamnionitis contributes to premature birth, and is associated with post-birth complications. How chorioamnionitis impacts neonate’s developing immune system has not been well defined.
Methods
Blood from extremely preterm infants (≤28 weeks gestation) was drawn at the 1st, 2nd, and 4th week of life. Blood was either left unstimulated or stimulated for 4 hours with PMA/ionomycin. mRNA expression of transcription factors in unstimulated cells (RORC, TBET, GATA3, FOXP3) and inflammatory cytokines (IFN-γ, TNF-α, IL-2, IL-4, IL-5, and IL-6) in unstimulated and stimulated cells were analyzed. Data were analyzed based on the diagnosis of chorioamnionitis, funisitis and bronchopulmonary dysplasia (BPD).
Results
At 1 week of life, exposure to funisitis, but not maternal chorioamnionitis was associated with an increased expression of RORC and RORC/FOXP3 ratio. These increases in RORC and RORC/FOXP3 ration were sustained over the 4 weeks of follow-up. Leukocytes from infants who developed BPD had increased stimulated and unstimulated IL-4 at the 1st week of life, but these increases were not sustained over time. In contrast, infants with mild BPD had a sustained decrease in stimulated IL-2.
Conclusion
Chorioamnionitis exposure, in particular to funisitis, lead to enhanced Th17-like responses that persist for 4 weeks after birth. Infants who later developed BPD did not exhibit a strikingly distinct immune profile.
INTRODUCTION
Neonatal T cell responses historically were thought to be deficient due to immaturity(1), although the supporting evidence came mainly from murine studies(2). However, more recent studies have demonstrated that human fetal development is a unique developmental stage in which fetal innate and adaptive responses are highly plastic and are influenced by in utero exposure to antigens or inflammatory mediators(3). Fetal T cells first appear in the human thymus at ~10 weeks of gestational age (GA)(4), and the fetal CD4+ T cells are biased towards differentiation into regulatory T cells (Treg)(5). However, a subset of T cells with a memory-like phenotype develops in the human fetus and displays inflammatory effector functions at birth(6). Other human fetal immune cells also appear to be functional. Notably, innate lymphoid cells (ILC) of different types have been identified in human fetuses(7). Fetal organs also contain KIR-expressing differentiated natural killer (NK) cells as early as 18 weeks GA(8). Mucosa-associated invariant T cells, which recognize microbial metabolites to produce pro-inflammatory cytokines such as IFN-γ and IL-17, have also been identified in the fetal small intestine, liver and lung(9).
The development of the immune system of extremely low gestational age neonates (ELGANS) born before 28 weeks of gestation, is of particular interest. Many of these infants now survive, but they have increased prevalence of long-term inflammatory morbidities, notably bronchopulmonary dysplasia (BPD) and necrotizing enterocolitis (NEC)(10). In particular, BPD is a progressive lung injury syndrome with lung inflammation occurring prior to preterm birth and/or following birth and mechanical ventilation. Importantly, an estimated 25–40% of preterm births are associated with placental inflammation, which is often subclinical(11–13). This inflammation may be localized to the maternal placenta or membrane (chorioamnionitis) or progress to a fetal inflammatory response, as evidenced by cord inflammation (funisitis) and elevated cord plasma levels of IL-6(14). This pattern has been reproduced experimentally in animal models; notably, fetal sheep and rhesus macaques respond to intra-amniotic LPS with skin, gut and lung inflammation as well as systemic changes in immune cell phenotype(15–18). Although the fetus can be exposed to cytokines and microbial products through the amniotic fluid, the consequences of this in utero exposure to chorioamnionitis or funisitis on the developing infant immune system are poorly understood(18).
Most studies of human neonate immune responses, including those of preterm infants, are cross-sectional analyses using cord blood, due to blood sampling limitations in this population. Our objectives were to define the immune profile of infants at-risk to develop BPD, and determine whether in-utero exposure to chorioamnionitis or funisitis influenced the neonate’s immune responses. We thus chose a longitudinal approach using blood samples collected within the 1st 2nd, and 4th week of life. We measured the mRNA expression of a panel of proteins associated with immune responses. We included specific transcription factors associated with IL-17, IFN-γ, IL-4 and IL-5 producing immune cells (T cells, NK or ILC), namely RORC (the human ortholog of RORγT), TBET and GATA3. We also determined the expression of the transcription factor Forkhead box protein 3 (FOXP3), which is associated with Treg development and is a critical mediator of Treg suppressive activity. We also measured the induction of selected cytokine mRNAs (IFN-γ, IL-2, IL-4, IL-5, TNF-α, and IL-6) at baseline and after a short polyclonal stimulation. We used a quantitative RT-PCR approach on unseparated blood, since methods are now available to extract RNA for RT-PCR analysis from whole blood, without the need for separating mononuclear cells before stimulation(19,20). Because quantitative RT-PCR is such a sensitive technique, a broad immune profile can be evaluated within the constraints of the extremely limited blood volumes that are available from preterm infants.
RESULTS
Demographic and clinical characteristics of the preterm infants
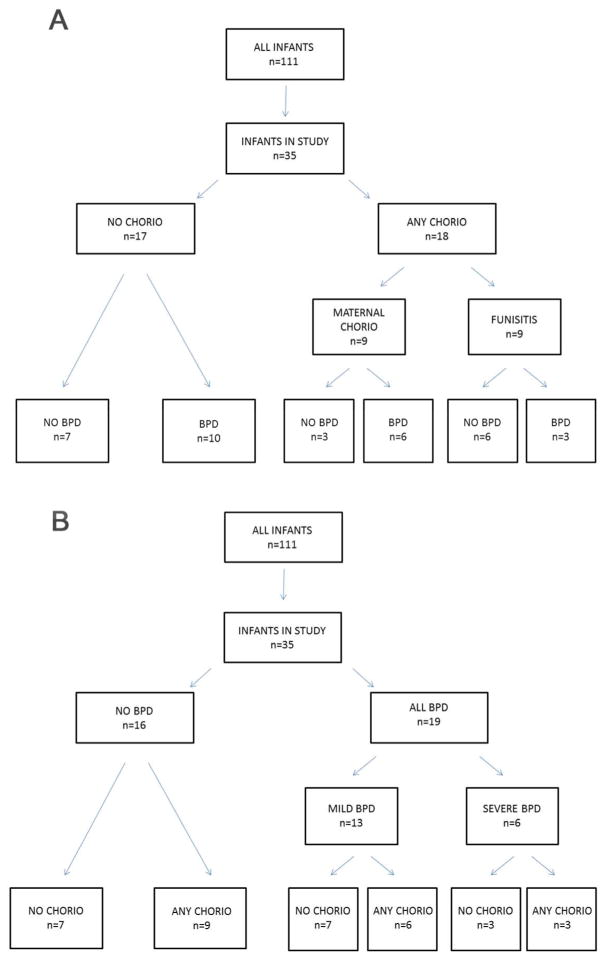
35 infants who were part of the NHLBI funded Prematurity Respiratory Outcomes Program(21) were included in this study based on availability of blood samples at Good Samaritan Hospital, University of Cincinnati Medical Center, and Cincinnati Children’s Hospital Medical Center (Cincinnati, OH). They were characterized according to the diagnosis of histological chorioamnionitis (maternal chorio), or chorio plus funisitis (any chorio) (Table 1). All infants diagnosed with funisitis were also exposed to maternal chorio (Figure 1, panel A). Infants were also classified according to BPD diagnosis and its severity (Figure 1, panel B). Blood samples were available from 32 infants in the first week of life, 26 infants at the second week of life, and 14 infants in the fourth week of life. The majority of the infants were exposed to antenatal steroids prior to delivery, with no difference in treatment prevalence among the groups, p>0.05 (Table 1).
Table 1.
Clinical characteristics of preterm infants selected for the study.
| ALL INFANTS |
DEVELOPED BPD
|
EXPOSED TO CHORIO
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| (n=35) | NO BPD (n=16) |
ALL BPD (n=19) |
MILD BPD (n=13) |
SEVERE BPD (n=6) |
NO CHORIO (n=17) |
ANY CHORIO (n=18) |
MATERNAL CHORIO (n=9) |
FUNISITIS (n=9) |
|
|
|
|
|
|||||||
| Gestational age (Weeks ± SEM) | 26.5±0.2 | 26.7±0.2 | 26.2±0.3 | 26.8±0.3 | 25.1±0.4 | 26.6±0.3 | 26.4±0.3 | 26.4±0.5 | 26.32±0.4 |
| Birth Weight (grams ± SEM) | 860±32 | 903±43 | 823±43 | 873±56 | 716±31 | 888±48 | 833±41 | 865±65 | 802±47 |
| Sex (% Male) | 66 | 75 | 58 | 54 | 66 | 59 | 72 | 78 | 67 |
| Race (%Caucasian/African American/Asian) | 63/31/3 | 63/31/6 | 63/37/0 | 85/15/0 | 17/83/0 | 76/24/0 | 50/44/6 | 56/33/11 | 44/56/0 |
| Delivery mode (%Vaginal) | 31 | 50 | 16 | 15 | 17 | 24 | 39 | 44 | 33 |
| Use of antenatal steroids (%) | 88 | 81 | 95 | 100 | 83 | 88 | 89 | 100 | 78 |
Figure 1. Tree diagrams depicting categorization of infants.
Infants were partitioned by A) chorio and funisitis diagnosis and B) BPD diagnosis. Mild BPD is oxygen use of <30% at 36 weeks and severe BPD is oxygen use >30% and other ventilator support.
Immune profile for the first week of life
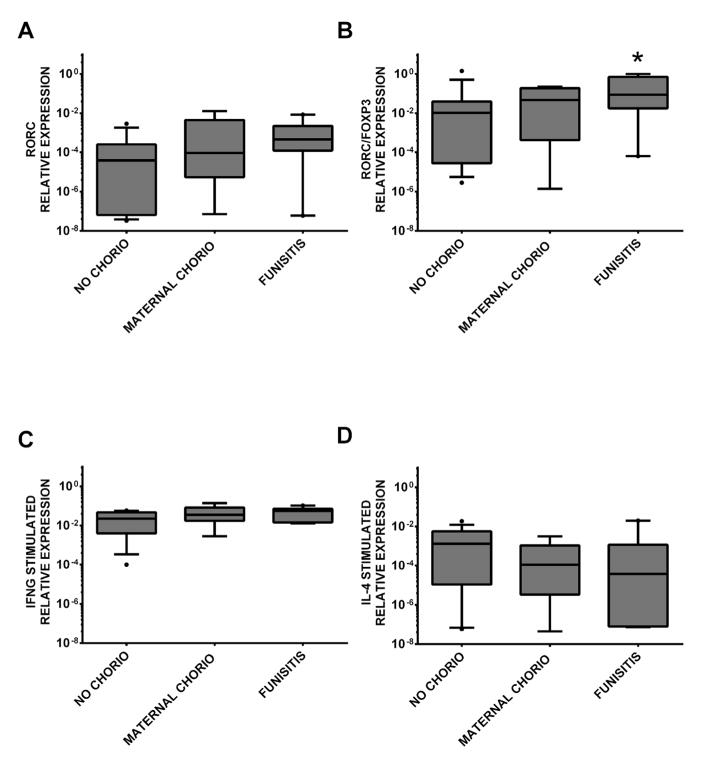
All comparisons of chorio and funisitis groups were made to the no chorio group, and all comparisons of infants with BPD were made with the no BPD group. Additionally, the inflammatory markers were normalized to ubiquitin ligase (UBE2D2). As shown in Supplemental Figure S1A,B, CD3 expression was similar in all the groups (p>0.05). Infants exposed to any chorio had a trend towards higher expression of RORC mRNA (no chorio vs. any chorio, Mann Whitney U-test, Bonferroni-Holm adjusted p-value=0.05). Although FOXP3 mRNA expression was not significantly affected by chorio exposure (data not shown), the ratio of RORC to FOXP3 expression was higher in infants exposed to any chorio (no chorio vs. any chorio, adjusted p=0.07). Because chorio is a progressive condition that varies in severity, we next evaluated whether its severity influences neonatal immune responses. When RORC expression and RORC/FOXP3 ratio were analyzed comparing no chorio, maternal chorio and funisitis infants respectively, only the subgroup of infants diagnosed with funisitis was different from the no chorio group whereas infants diagnosed with maternal chorio were not different (no chorio vs. funisitis, adjusted p=0.08, p=0.05; Figure 2, panel A and B). Furthermore, exposure to any chorio resulted in a trend toward higher stimulated IFN-γ mRNA expression (no chorio vs. any chorio, p=0.09). However, when we determined the contribution of maternal chorio and funisitis, the groups were not significantly different (Figure 2, panel C). Similarly, infants with any chorio had a trend toward lower expression of IL-4 mRNA upon stimulation than the infants without any chorio (p=0.10); but analysis of funisitis vs. maternal chorio did not show evidence of significance (Figure 2, panel D). No significant differences with maternal chorio or funisitis exposure in the first week of life were found for any of the other markers (GATA3, TBET, IL-2, IL-5, IL-6 or TNF-α mRNA with or without stimulation).
Figure 2. Gene expression levels of immune targets in preterm infants with or without chorio and/or funisitis in the first week of life.
Relative gene expression of A) RORC p=0.08 (no chorio v. funisitis), B) RORC/FOXP3 ratio p=0.05 (no chorio v. funisitis), C) IFNG stimulated and D) IL4 stimulated in no chorio (n=17), maternal chorio (n=9) and funisitis (n=9) groups. Groups were compared using unpaired t tests when data were normally distributed, otherwise Mann Whitney U-test was used. When there were significant differences in the initial 2-group comparisons (no chorio v. any chorio), Bonferroni-Holm corrections were used to account for the multiple comparisons (no chorio v. maternal chorio, no chorio v. funisitis), *=p≤0.05.
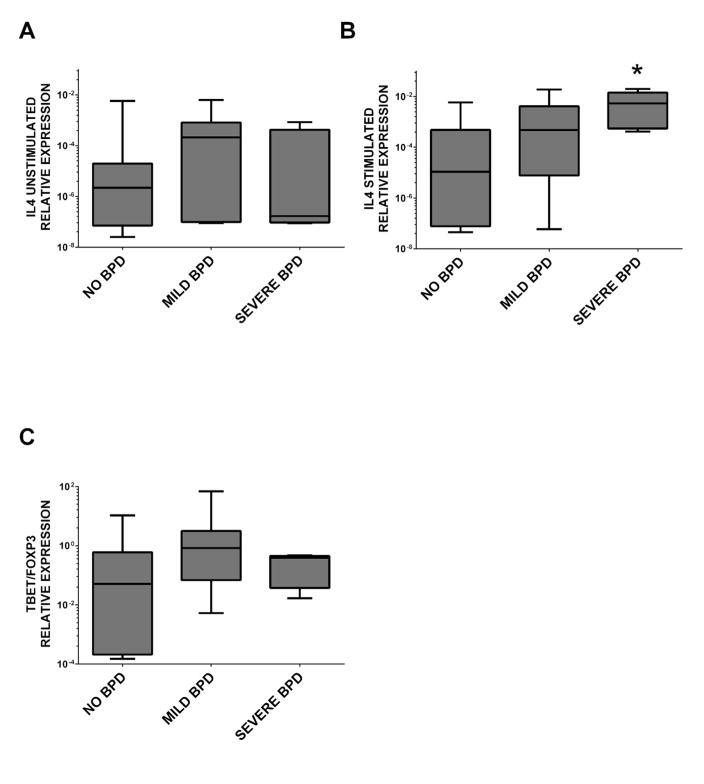
In the first week of life, infants who were later diagnosed as having BPD and infants with mild BPD had a trend towards increased expression of IL-4 mRNA in absence of stimulation compared to those who did not (no BPD v. all BPD, adjusted p=0.10, no BPD v. mild BPD adjusted p=0.09). However, there was no difference with severe BPD infants (Figure 3, panel A). In addition, stimulated IL-4 mRNA expression was significantly increased in BPD infants (no BPD vs. all BPD, adjusted p=0.02), and this increase was particularly found in the severe BPD group (adjusted p=0.02, Figure 3, panel B). There was a trend toward an increased TBET/FOXP3 ratio with BPD (no BPD vs. all BPD adjusted p=0.10), but severe and mild BPD groups were similar (Figure 3, panel D). No other significant differences associated with BPD were found for any of the other markers (GATA3, FOXP3, IL-5, IL-6 or TNF-α mRNA with or without stimulation).
Figure 3. Gene expression levels of immune targets in preterm infants with or without BPD in the first week of life.
Relative gene expression of A) IL-4 unstimulated, B) IL-4 stimulated p=0.02 (no BPD v. severe BPD), and C) TBET/FOXP3 ratio in no BPD (n=16), mild BPD (n=13) and severe BPD (n=6) groups. Groups were compared using unpaired t tests when data were normally distributed, otherwise Mann Whitney U-test was used. When there were significant differences in the initial 2-group comparisons (no BPD v. all BPD), Bonferroni-Holm corrections were used to account for the multiple comparisons (no BPD v. mild BPD, no BPD v. severe BPD), *=p≤0.05.
To eliminate the possibility that chorio may be a confounding factor in the analysis of BPD infants, we next excluded infants with chorio or funisitis from the analysis of BPD. Stimulated expression of IFN-γ was increased in infants who were later diagnosed with BPD (p=0.06, Supplemental Figure S2A online). Higher expression of stimulated IL-4 mRNA and TBET/FOXP3 ratio were still found in this secondary analysis (Supplemental Figure S2B,C online).
Change in immune profile over age of infants
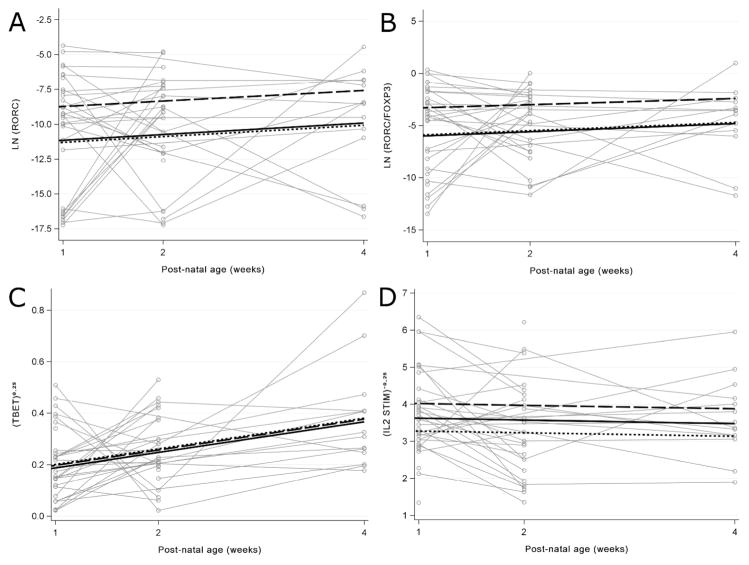
To analyze differences between groups over the first four weeks of life, repeated measures models were constructed to test for the association of cytokine and transcription factor expression with chorio, funisitis and BPD. Regression coefficients (betas) estimated from generalized estimating equation (GEE) models were used to measure the average change in immune profile associated with the diagnosis. There was a robust and sustained increase in the expression of RORC mRNA and the RORC/FOXP3 ratio associated with funisitis over the 4-week period (beta=2.50, p=0.004, Figure 4, panel A; and beta=2.51, p=0.001, Figure 4, panel B, respectively). Again, this increase was only seen with funisitis and not with maternal chorio (Figure 4, panel A and B). FOXP3, TBET/FOXP3, TBET, GATA3, GATA3/FOXP3, IFN-γ, TNF-α, IL-2, IL-4, IL-6 or IL-5 were not significantly associated with funisitis or maternal chorio in this longitudinal analysis.
Figure 4. Changes in gene expression levels of immune targets over infant age for the first 4 weeks of life in preterm infants with chorio, funisitis, or BPD using Generalized Estimating Equations (GEE) models.
Changes in relative gene expression were determined for A) natural logarithm of RORC (beta=2.50, p=0.04) and B) the natural logarithm of the RORC/FOXP3 ratio (beta=2.51, p=0.001) in no chorio (n=17) (dotted line), maternal chorio (n=9) (solid line) and funisitis (n=9) (long dashed line) groups. Changes in relative gene expression were determined for C) transformed TBET (mild BPD, beta for transformed (TBET)0.25=−0.004, p=0.09; severe BPD, beta for transformed (TBET)0.25=−0.01, p=0.73 and D) transformed stimulated IL-2 (beta for transformed IL-2−0.25=0.62, p=0.04 in no BPD (n=16) (dotted line), mild BPD (n=13) (long dashed line) and severe BPD (n=6) (solid line).
The development of BPD was characterized by a significant decrease in the expression of stimulated IL-2 mRNA in BPD infants compared to infants without BPD (beta for transformed IL-2−0.25=0.62, p=0.04, Figure 4, panel D). Compared to no BPD, slightly decreased levels of TBET was evident but not significantly associated with mild BPD (beta for transformed TBET0.25=−0.004, p=0.09) or severe BPD (beta for transformed TBET0.25 = −0.01, p=0.73, Figure 4, panel C). No significant associations were found for FOXP3, RORC, RORC/FOXP3, TBET/FOXP3, GATA3, GATA3/FOXP3, IFN-γ, TNF-α, IL-2, IL-5, IL-4, or IL-6 in this longitudinal analysis.
DISCUSSION
We evaluated the fetal immune response to chorioamnionitis or funisitis, because fetal inflammation is very common and has been associated with brain, lung, and intestinal injury(22). Chorioamnionitis is defined as inflammation of the membranes shared between the mother and the developing fetus; whereas funisitis is inflammation within the umbilical cord. Previous studies that have examined immune responses of neonates to inflammation have focused on chorio and did not distinguish between funisitis and chorio(23).
In our study, funisitis, not maternal chorio, was associated with the inc reased expression of RORC mRNA, indicating that funisitis acts on the population of cord blood RORC+ cells that have been described in neonates born from normal pregnancies(6,24). In the cord blood, a small subset of CCR6+ or CD161+ effector memory T cells (TEM) has a profile of pro-TH17 cells, expressing RORC, IL-1R and IL-23R(6,24). These cells produce IL-17 but only if they are activated in the presence of IL-1β and IL-23(6,24). It is thus possible that the pro-inflammatory cytokine profile created by severe chorio as evidenced by elevated serum IL-1β, IL-6, and IL-8 levels(25), could activate or expand these pro-Th17 cells. Of note, our recent study of fetal inflammation in a rhesus macaque model supports this hypothesis, as we observed a transient increase in IL-17+ cells, mainly in the fetal spleen and lymph nodes(26).
In addition to increased RORC expression, we also found a trend towards increased stimulated IFN-γ mRNA expression in infants exposed to any chorio. However, the reduced number of infants in each group likely did not provide enough power to definitively determine the contribution of maternal chorio or funisitis. However, this increase suggests the existence of a subset of cells that can develop a Th1 phenotype early in life as described by Zhang et. al, and this response may be exacerbated in the presence of inflammation(6,27,28).
Changes over time may identify potential biomarkers for BPD since BPD develops postnatally. An objective of our study was thus to determine whether a specific immune profile existed in infants that were developing BPD. Our results do not support such concept, as there were no striking differences in the immune profile of infants who developed BPD, including those who developed severe BPD versus those who did not. This finding is in agreement with the fact that the CD4+ T cells from neonates who did or did not develop BPD were found to express similar levels of many cytokines, including IL-6(23). In addition, there was a trend of increased TBET expression at the first week; but over time TBET appears to be similar among groups. Stimulated IL-4 appears to be increased in severe BPD infants. Although IL-4 may not play a major role in BPD pathogenesis, because low levels are found in the tracheal aspirates of BPD infants (29). Elevated levels may be associated with lung pathologies such as asthma, through IL-4 actions on lung fibroblast development(30). We did find that stimulated IL-2 mRNA levels were decreased in BPD infants over the 4 weeks of evaluation. This decrease in IL-2 corresponds to the increase in transformed stimulated (IL-2)−0.25 from the GEE model in neonates with mild BPD displayed in Figure 4 panel D. The small sample number of samples with severe BPD may have limited the ability to detect this trend in this subgroup. This result is in concordance with reports of a decrease in circulating CD4+ cells in BPD infants(23,31), suggesting activation of the adaptive immune system and possible mobilization into the lung. On the other hand, there has been a report of a higher proportion of activated circulating CD4+ T cells observed in BPD infants(31). However, we did not find changes in CD3 mRNA expression with BPD (Supplementary Figure S1B).
While a strength of the study was the longitudinal analysis of neonatal samples, there were missing samples particularly at the four week timepoint. Available samples were similarly distributed across chorioamnionitis, funisitis, and BPD subgroups. GEE models incorporating the missing samples were similar to models using samples we had available
We chose to analyze neonatal blood, as compared to other studies that used cord blood, because it allowed us to examine the developing immune profile of neonates over the first weeks of life, something that had not yet been done. However, this strategy has limitations because we were only able to get a limited volume of blood, preventing us from determining the cellular sources of RORC and IFN-γ. Likewise, we were unable to measure IL-17 mRNA levels in our samples, as we found that whole blood profiling was too insensitive to detect IL-17 mRNA in both neonates and adult blood. Increased expression of RORC at the very least in our study denotes the possibility of an emerging TH17-like cell population since RORC is a crucial mediator of its differentiation(32). However, no conclusion about the absence or presence of IL-17 can be drawn strictly based off increased RORC mRNA. Another acknowledged limitation is the fact that the blood may not fully reflect what is happening in fetal organs. Indeed, we have shown in animal models of chorioamnionitis that the pro-inflammatory responses measured in the blood were blunted compared to those developing in lymphoid organs or lungs(18). This important caveat has to be kept in mind for all studies of neonatal responses using blood.
Overall, our findings demonstrate that ELGANS have the capacity to respond to in utero inflammation with a pro-inflammatory TH1/TH17 like phenotype. While these responses may be protective against pathogens, they may also contribute to the fetal inflammatory response syndrome, which involves multi-organ inflammation and injury(14). Moreover, our studies emphasize the important concept that funisitis and localized maternal chorioamnionitis do not have the same impact on the developing fetal immune system, and future studies of fetal inflammation need to clearly distinguish the two pathologies.
METHODS
Patient Recruitment
Mothers delivering ≤28 weeks gestation provided informed consent for collection of pregnancy related information and peripheral blood from their infants with a protocol approved by the IRB of Good Samaritan Hospital (Cincinnati, OH), University of Cincinnati Medical Center (Cincinnati, OH) and Cincinnati Children’s Hospital Medical Center (Cincinnati, OH). From 111 ELGANS, 35 were selected for this study because of availability of blood samples; the other 76 infants were not consented for blood. Mother and infant demographics were also collected.
Diagnosis of Bronchopulmonary dysplasia (BPD) and chorioamnionitis (chorio)
The infants were diagnosed with BPD as defined by the need for oxygen at 36 weeks gestational age(33). This group was then divided into infants who were on <30% oxygen and low gas flows (mild BPD) and those on oxygen with gas flows ≥2 liters/min or on oxygen and continuous positive airway pressure (severe BPD). No infant required mechanical ventilation at 36 weeks.
The chorioamnion, placenta and umbilical cord were scored for antenatal inflammation using a standardized procedure with histologic sections of chorioamnion, umbilical cord and placenta stained with hematoxylin and eosin according to Redline’s criteria(34). Fetal inflammation was defined by funisitis generating neutrophilic infiltration surrounding the umbilical cord vessels and Wharton’s jelly. 3 groups of infants were thus identified: no chorio, maternal chorio, and funisitis.
Collection and Processing of Infant Blood
Fresh blood from infants was collected in Microtainer tubes with dipotassium EDTA (BD Diagnostics, Sparks Glencoe, MD) during the first, second and fourth week of age, at the same time a clinical sample was needed. Within 4 hours of collection, blood was diluted with an equal volume of complete medium (RPMI 1640, supplemented with 10% heat-inactivated fetal calf-serum, HEPES, Glutamine) and divided into two equal aliquots: one left untreated and the other stimulated with PMA (50ng/ml) (Sigma-Aldrich, St. Louis, MO) and ionomycin (1ug/ml) (EMD Millipore, Billerica, MA). The cultures were incubated for 4h at 37°C. Red blood cells were lysed using 1X RBC Lysis Buffer according to the manufacturer’s instructions (eBioscience, San Diego, CA) and the leukocytes were pelleted and stored at −80°C until the extraction of mRNA.
mRNA Amplification and quantitative RT-PCR (qPCR) analysis
mRNA was isolated from unstimulated and stimulated leukocytes using the μMACS mRNA Isolation kit (Miltenyi Biotec, Auburn, CA) and then amplified using the Ovation PicoSL WTA System V2 kit according to the manufacturer’s instructions (NuGEN Technologies, Inc, San Carlos, CA). Amplified cDNA was purified with the MinElute Reaction Cleanup kit (Qiagen) and quality and quantity analyzed using a Bioanalyzer 2100 system (Qiagen, Valencia, CA; Agilent, Wilmington, DE). cDNA from unstimulated and stimulated samples was quantified for 12 target genes in 8 separate panels by qPCR using TaqMan probes (TIB Molbiol LLC, Adelphia, NJ) and the LightCycler 480 Probes Master kit on the LightCycler 480 Instrument II (Roche Diagnostics, Indianapolis, IN). Transcription factor mRNA (RORC, TBET, GATA3 and FOXP3) were analyzed only in the unstimulated fraction, while the remaining target mRNA (CD3, IFN-γ, IL-5, IL-2, IL-4, IL-6 and TNF-α) were analyzed for both unstimulated and stimulated fractions to measure activation. Ubiquitin ligase (UBE2D2) mRNA expression was used for housekeeping. Primer and TaqMan probe information is given in Supplementary Table S1 online. TaqMan probes were used in either monoplex or duplex reactions; for duplex reactions, color compensation was performed using LightCycler 480 II software according to the manufacturer’s instructions (Roche Diagnostics, Indianapolis, IN).
Statistical Analysis
Demographic data for the preterm infants are reported as percent of the population or mean ± SEM. Differences in steroid treatment prevalence between groups were tested using Fisher’s Exact test. Statistical analysis of qPCR data was performed using Prism 6.0 software for Windows (Graphpad, La Jolla, CA). For analyses of data for the first week of life, normality was checked for comparisons between groups. When the data were normally distributed, unpaired t-tests were used for comparisons of no chorio versus any chorio and no BPD versus all BPD, the Mann Whitney U-test was used. When there were significant differences in the initial 2-group comparisons, Bonferroni-Holm adjustments were used to account for the multiple comparisons between no chorio, maternal chorio, and funisitis groups and no BPD, mild BPD, severe BPD groups. To examine the repeated measures over time using linear models, Box-cox transformations were used to determine the optimal power transformation for each cytokine or transcription factor. GEE models were constructed on the transformed data [natural logarithm: RORC, FOXP3, IFN-γ, IL-5 and TNF-α or power: (TBET)0.25, (GATA3)0.25, (IL-2)−0.25] to test for association between cytokine and transcription factor levels and the medical diagnoses of chorio, funisitis, and BPD. GEE models account for the within-subject correlation of repeated observations on individuals. Separate models were tested for funisitis, chorioamnionitis without funisitis, and BPD (mild vs no BPD and severe BPD vs. mild BPD vs no BPD). We examined characteristics of missing data and tested for association with severity of chorioamnionitis, funisitis, and BPD. Multiple imputation procedures were used to examnine potential effects of missing data. A data augmentation algorthim was used to generate 50 copies of the data set, each of which contains different estimates of the missing values. GEE models were estimated on the filled-in data. Parameter estimates and standard errors were pooled and compared to the analytic model results. SAS version 9.3 was used to conduct the analysis (SAS Institute Inc., Cary, NC). Statistical significance was accepted at p<0.05 and statistical trends at p<0.1.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health (Bethesda, MD) grant U01 HL101800 (to CAC and AHJ) and Eunice Kennedy Shriver National Institute of Child Health and Human Development (Bethesda, MD) diversity supplement RO1HD078127-03 (to CMJ).
Footnotes
There is no conflict of interest to be reported.
References
- 1.Maródi L. Innate cellular immune responses in newborns. Clinical Immunol. 2006;118:137–44. doi: 10.1016/j.clim.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 2.Adkins B, Leclerc C, Marshall-Clarke S. Neonatal adaptive immunity comes of age. Nat Rev Immunol. 2004;4:553–64. doi: 10.1038/nri1394. [DOI] [PubMed] [Google Scholar]
- 3.Mold JE, McCune JM. Immunological tolerance during fetal development: from mouse to man. Adv Immunol. 2012;115:73–111. doi: 10.1016/B978-0-12-394299-9.00003-5. [DOI] [PubMed] [Google Scholar]
- 4.Miller LH, Haynes JD, McAuliffe FM, Shiroishi T, Durocher JR, McGinniss MH. Evidence for differences in erythrocyte surface receptors for the malarial parasites, Plasmodium falciparum and Plasmodium knowlesi. J Exp Med. 1977;146:277–81. doi: 10.1084/jem.146.1.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mold JE, Venkatasubrahmanyam S, Burt TD, et al. Fetal and adult hematopoietic stem cells give rise to distinct T cell lineages in humans. Science. 2010;330:1695–9. doi: 10.1126/science.1196509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang X, Mozeleski B, Lemoine S, et al. CD4 T Cells with Effector Memory Phenotype and Function Develop in the Sterile Environment of the Fetus. Sci Transl Med. 2014;6:238ra72–ra72. doi: 10.1126/scitranslmed.3008748. [DOI] [PubMed] [Google Scholar]
- 7.Hazenberg MD, Spits H. Human innate lymphoid cells. Blood. 2014;124:700–9. doi: 10.1182/blood-2013-11-427781. [DOI] [PubMed] [Google Scholar]
- 8.Ivarsson MA, Loh L, Marquardt N, et al. Differentiation and functional regulation of human fetal NK cells. J Clin Invest. 2013;123:3889–901. doi: 10.1172/JCI68989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leeansyah E, Loh L, Nixon DF, Sandberg JK. Acquisition of innate-like microbial reactivity in mucosal tissues during human fetal MAIT-cell development. Nat Commun. 2014;5:3143. doi: 10.1038/ncomms4143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eichenwald EC, Stark AR. Management and Outcomes of Very Low Birth Weight. N Engl J Med. 2008;358:1700–11. doi: 10.1056/NEJMra0707601. [DOI] [PubMed] [Google Scholar]
- 11.Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;371:75–84. doi: 10.1016/S0140-6736(08)60074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Romero R, Gotsch F, Pineles B, Kusanovic JP. Inflammation in pregnancy: its roles in reproductive physiology, obstetrical complications, and fetal injury. Nutr Rev. 2007;65:S194–202. doi: 10.1111/j.1753-4887.2007.tb00362.x. [DOI] [PubMed] [Google Scholar]
- 13.Stoll BJ, Hansen NI, Higgins RD, et al. Very low birth weight preterm infants with early onset neonatal sepsis: the predominance of gram-negative infections continues in the National Institute of Child Health and Human Development Neonatal Research Network, 2002–2003. The Pediatr Infect Dis J. 2005;24:635–9. doi: 10.1097/01.inf.0000168749.82105.64. [DOI] [PubMed] [Google Scholar]
- 14.Gomez R, Romero R, Ghezzi F, Yoon BH, Mazor M, Berry SM. The fetal inflammatory response syndrome. Am J Obstet Gynecol. 1998;179:194–202. doi: 10.1016/s0002-9378(98)70272-8. [DOI] [PubMed] [Google Scholar]
- 15.Kallapur SG, Nitsos I, Moss TJM, et al. IL–1 Mediates Pulmonary and Systemic Inflammatory Responses to Chorioamnionitis Induced by Lipopolysaccharide. Am J Respir Crit Care Med. 2009;179:955–61. doi: 10.1164/rccm.200811-1728OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wolfs TGAM, Buurman WA, Zoer B, et al. Endotoxin Induced Chorioamnionitis Prevents Intestinal Development during Gestation in Fetal Sheep. PLoS ONE. 2009;4:e5837. doi: 10.1371/journal.pone.0005837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kemp MW, Saito M, Nitsos I, Jobe AH, Kallapur SG, Newnham JP. Exposure to In Utero Lipopolysaccharide Induces Inflammation in the Fetal Ovine Skin. Reprod Sci. 2011;18:88–98. doi: 10.1177/1933719110380470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kallapur SG, Presicce P, Rueda CM, Jobe AH, Chougnet CA. Fetal Immune Response to Chorioamnionitis. Semin Reprod Med. 2014;32:056–67. doi: 10.1055/s-0033-1361823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Daegelmann C, Herberth G, Roder S, et al. Association between suppressors of cytokine signalling, T-helper type 1/T-helper type 2 balance and allergic sensitization in children. Clin Exp Allergy. 2008;38:438–48. doi: 10.1111/j.1365-2222.2007.02913.x. [DOI] [PubMed] [Google Scholar]
- 20.Herberth G, Daegelmann C, Weber A, et al. Association of neuropeptides with Th1/Th2 balance and allergic sensitization in children. Clin Exp Allergy. 2006;36:1408–16. doi: 10.1111/j.1365-2222.2006.02576.x. [DOI] [PubMed] [Google Scholar]
- 21.Pryhuber GS, Maitre NL, Ballard RA, et al. Prematurity and respiratory outcomes program (PROP): study protocol of a prospective multicenter study of respiratory outcomes of preterm infants in the United States. BMC Pediatr. 2015;15:37. doi: 10.1186/s12887-015-0346-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gantert M, Been JV, Gavilanes AWD, Garnier Y, Zimmermann LJI, Kramer BW. Chorioamnionitis: a multiorgan disease of the fetus[quest] J Perinatol. 2010;30:S21–S30. doi: 10.1038/jp.2010.96. [DOI] [PubMed] [Google Scholar]
- 23.Misra RS, Shah S, Fowell DJ, et al. Preterm cord blood CD4+ T cells exhibit increased IL-6 production in chorioamnionitis and decreased CD4+ T cells in bronchopulmonary dysplasia. Hum Immunol. 2015;76:329–38. doi: 10.1016/j.humimm.2015.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cosmi L, De Palma R, Santarlasci V, et al. Human interleukin 17–producing cells originate from a CD161(+)CD4(+) T cell precursor. J Exp Med. 2008;205:1903–16. doi: 10.1084/jem.20080397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nishimaki S, Shima Y, Sato M, An H, Kadota K, Yokota S. Postnatal changes of cytokines in premature infants with or without funisitis. J Matern Fetal Neonatal Med. 2014;27:1545–9. doi: 10.3109/14767058.2013.867321. [DOI] [PubMed] [Google Scholar]
- 26.Kallapur SG, Presicce P, Senthamaraikannan P, et al. Intra-Amniotic IL-1β Induces Fetal Inflammation in Rhesus Monkeys and Alters the Regulatory T Cell/IL-17 Balance. J Immunol. 2013;191:1102–9. doi: 10.4049/jimmunol.1300270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matsuoka T, Matsubara T, Katayama K, Takeda K, Koga M, Furukawa S. Increase of cord blood cytokine-producing T cells in intrauterine infection. Pediatr Int. 2001;43:453–7. doi: 10.1046/j.1442-200x.2001.01445.x. [DOI] [PubMed] [Google Scholar]
- 28.Romero R, Chaemsaithong P, Korzeniewski Steven J, et al. Clinical chorioamnionitis at term II: the intra-amniotic inflammatory response. J Perinat Med. 2015;44:5–22. doi: 10.1515/jpm-2015-0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baier RJ, Loggins J, Kruger TE. Interleukin-4 and 13 concentrations in infants at risk to develop Bronchopulmonary Dysplasia. BMC Pediatr. 2003;3:8. doi: 10.1186/1471-2431-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sempowski GD, Beckmann MP, Derdak S, Phipps RP. Subsets of murine lung fibroblasts express membrane-bound and soluble IL-4 receptors. Role of IL-4 in enhancing fibroblast proliferation and collagen synthesis. J Immunol. 1994;152:3606–14. [PubMed] [Google Scholar]
- 31.Turunen R, Vaarala O, Nupponen I, et al. Activation of T Cells in Preterm Infants with Respiratory Distress Syndrome. Neonatology. 2009;96:248–58. doi: 10.1159/000220764. [DOI] [PubMed] [Google Scholar]
- 32.Ivanov II, McKenzie BS, Zhou L, et al. The Orphan Nuclear Receptor RORγt Directs the Differentiation Program of Proinflammatory IL-17+ T Helper Cells. Cell. 126:1121–33. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 33.Shennan AT, Dunn MS, Ohlsson A, Lennox K, Hoskins EM. Abnormal Pulmonary Outcomes in Premature Infants: Prediction From Oxygen Requirement in the Neonatal Period. Pediatrics. 1988;82:527–32. [PubMed] [Google Scholar]
- 34.Redline RW, Faye-Petersen O, Heller D, et al. Amniotic infection syndrome: nosology and reproducibility of placental reaction patterns. Pediatr Dev Pathol. 2003;6:435–48. doi: 10.1007/s10024-003-7070-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.