Abstract
Background
We had previously demonstrated that guanfacine, an α2a-adrenergic agonist, attenuated the effect of stress on smoking-lapse behavior in regular daily smokers. Heart rate variability (HRV), a measure of vagal activity, may be a potential mechanism underlying the relationship between stress, smoking, and relapse.
Methods
We examined whether guanfacine (0mg vs. 3mg/day; n=26) altered changes in high-frequency heart rate variability (HF-HRV) following stress and ad-lib smoking using a validated laboratory analogue of smoking-lapse behavior. All participants completed a parent study evaluating the effects of guanfacine on stress-precipitated smoking. Each subject completed two laboratory sessions assessing the effects of guanfacine on HF-HRV following stress imagery (vs. neutral imagery; order counterbalanced) and smoking.
Results
Results demonstrated that guanfacine did not increase tonic levels of HF-HRV relative to placebo. Following the stress vs neutral imagery manipulation (prior to ad-lib smoking), there were no significant changes in HF-HRV in the placebo group. In contrast, guanfacine increased phasic HF-HRV following stress imagery and decreased HF-HRV following neutral imagery. Ad-libitum smoking following both the stress and neutral conditions decreased HF-HRV in the placebo group across both imagery conditions. In contrast, guanfacine attenuated stress- and smoking-related decreases in phasic HF-HRV relative to the neutral imagery condition.
Conclusions
This is the first demonstration that a noradrenergic target altered dynamic changes in HF-HRV in response to stress and smoking, suggesting that guanfacine alters HF-HRV response to stress. Findings support current theories which suggest that phasic changes in HRV is an important marker of the stress response.
Keywords: heart rate variability, HF-HRV, smoking, stress, guanfacine, noradrenergic
INTRODUCTION
Tobacco use is a global epidemic and the leading cause of preventable morbidity and mortality worldwide (World Health Organization 2014), and current cigarette smoking prevalence among U.S. adults was approximately 16.8% in 2014 (Centers for Disease Control and Prevention 2015). Despite the extensive medical and financial consequences, smokers often fail to maintain long-term abstinence and relapse to tobacco use remains high, such that approximately 70 % of cigarette users relapse to smoking within one year, even with the most efficacious treatments (Jorenby et al. 2006). One of the principal mechanisms associated with the initiation, maintenance of, and relapse to smoking is stress (al’Absi 2006; McKee et al. 2003). Cigarette users (35 – 100%) self-report stress and negative affect as causal factors when recounting relapse episodes (Borland 1990; Brandon 1994; Shiffman 1982), and measures of smoking lapse behavior indicate that quick increases in negative affect were predictive of relapse to cigarette smoking (Ferguson and Shiffman 2014). Acute stress has also been shown to reduce the ability to resist smoking, increase the intensity of smoking, and increase craving and physiologic activity in daily smokers (McKee et al. 2011). Thus, targeting stress-related mechanisms underlying relapse to cigarette smoking may be of therapeutic benefit.
Heart rate variability (HRV), changes in time between beat-to-beat intervals, can be used as a measure of cardiovascular sympathetic autonomic activity, and is thought to reflect vagal reactivity and autonomic nervous system flexibility (Berntson et al. 1997). The high frequency range (HF-HRV; 0.15 – 0.4 Hz), mediated by the release of acetylcholine from the vagus nerve and possibly influenced by both cholinergic and adrenergic activity (Ahmed et al. 1994; Yeragani et al. 1993), reflects beat-to-beat vagal activity as well as variation in vagal effects on respiration and heart rate during a breathing cycle (Akselrod et al. 1981; Berntson et al. 1997). HF-HRV is often used as an index of parasympathetic functioning, reflecting the ability of the body to downregulate sympathetic activation, and may be a potential marker underlying the relationship between stress and smoking. Dysregulated sympathetic autonomic activity is thought to reflect pathological states (Thayer et al. 2010). Specifically, lower tonic HRV has been associated with higher susceptibility to morbidity and mortality. A recent meta-analysis confirmed findings that there are substantial reductions in HRV across psychiatric disorders, including substance dependence (Alvares et al. 2016). Conversely, higher tonic HRV has been associated with healthier physiological profiles (Berntson et al. 2008; Friedman 2007; Thayer et al. 2010; Udo et al. 2014).
Consequently, theories posited by Thayer and colleagues (Thayer et al. 2012) suggest that appropriate context- and goal-oriented emotionality and self-regulation is reflective of tonic, or resting, HRV. Higher tonic HRV is correlated with an ability to better adapt to stress (Park et al. 2014). The role of phasic HRV, changes in HRV in response to a stimulus, is less clear, but there is an indication that phasic HRV serves as a protective function in response to stressors (Park et al. 2014). Along with higher tonic HRV, higher phasic HRV is related to successful regulatory control (Park et al. 2014). For example, recovering alcoholics exhibit increased phasic HRV when exposed to alcohol cues only if they later reported an increased ability to resist drinking (Ingjaldsson et al. 2003). With regard to tobacco use, acute and chronic cigarette smoking has been shown to attenuate phasic HF-HRV (Dinas et al. 2013; Hayano et al. 1990). Likewise, laboratory-induced stress and cigarette smoking have been shown to additively decrease phasic HF-HRV and this decrease was associated with less time to initiate smoking (Ashare et al. 2012), indicating that HRV may be a key target to understanding the physiological basis for the relationship between stress and substance use, particularly smoking.
Guanfacine, an α2-adrenergic receptor agonist that reduces noradrenergic activity, has been shown to block drug-seeking behaviors in both animals and humans (Fox et al. 2012; Le et al. 2011; McKee 2013; McKee et al. 2014). In the parent study to the present investigation, guanfacine attenuated the effect of stress on smoking-lapse behavior, decreased ad-libitum smoking and tobacco craving, and normalized cortisol responding in smokers (McKee et al. 2014). Guanfacine has also been shown to reduce the number of cigarettes per day following a quit attempt during a 4-week-proof-of-concept treatment period in smokers (McKee 2013) and attenuate stress- and cue-induced nicotine craving in early abstinent cocaine-dependent individuals (Fox et al. 2012). Taken together, these findings suggest that targeting the noradrenergic system for stress-induced relapse to smoking could be of therapeutic value, potentially elucidating the underlying mechanisms associated with stress-induced smoking behavior. Given the influence of the noradrenergic system on the autonomic nervous system (Sawchenko and Swanson 1981; Svensson 1987), one mechanism through which guanfacine may reduce stress-precipitated smoking is by reducing sympathetic autonomic activity. Guanfacine acts centrally and has been shown to reduce sympathetic outflow (Van Zwieten et al. 1984). Thus, guanfacine may reverse or normalize the effect of stress and cigarette smoking on HRV. This mechanism, linking the noradrenergic agent guanfacine to reduced smoking behavior through its influence on sympathetic autonomic activity, has yet to be empirically tested.
Using a well-established laboratory paradigm of stress-precipitated smoking-lapse behavior (McKee et al. 2014; McKee et al. 2011), we assessed whether guanfacine would alter HRV in smokers as a secondary analysis to our primary study (McKee et al. 2014). To our knowledge, this is the first study to examine the effects of a noradrenergic agent on HRV during stress-precipitated smoking. It was hypothesized that guanfacine would increase tonic levels of HF-HRV in smokers and increase phasic response of HF-HRV following stress versus neutral imagery, elucidating a potential mechanism underlying the effects of guanfacine on stress-precipitated smoking. We based our hypothesis on previous findings from our laboratory that HF-HRV is additively decreased by stress and ad-libitum smoking (Ashare et al. 2012) and that guanfacine improves physiologic reactivity and smoking-lapse behavior in response to stress in daily smokers (McKee et al. 2014).
MATERIALS AND METHODS
Participants
Participants were consented for the parent study (McKee et al. 2014) and the current protocol at the same time. Of the n=33 subjects who completed the parent study, n=26 consented to the collection of HRV during the laboratory sessions. Participants were 18 – 60 years of age, smoked at least 10 cigarettes/day, had urine cotinine levels ≥ 150 ng/ml, and were normotensive (sitting BP > 90/60 and < 160/100 mmHG). Exclusion criteria included past 6-month Axis-I psychiatric disorders (except nicotine dependence), illicit drug use, treatment for smoking-cessation within the past 6-months, and significant medical conditions or concurrent medicine that would contraindicate smoking behavior, guanfacine administration, or HRV assessment. Participants were not taking any psychotropic medications and did not have any significant cardiac, pulmonary, or endocrine disorders. Eligibility screening included a physical examination, an EKG, and basic blood chemistries. For a detailed description of eligibility screening, see McKee et al. (2014).
Guanfacine treatment
The medication condition was double-blind and placebo-controlled. Randomization to guanfacine (3 mg/day) or a matching placebo (0 mg/day) was stratified by gender. 3 mg/day immediate-release guanfacine was used in the parent study due to previous findings that this dose attenuates nicotine craving in cocaine-dependent smokers, with minimal side effects (Fox et al. 2012). Guanfacine was administered twice daily and titrated to steady-state levels over 21 days (0.5 mg days 1–3, 1.5 mg days 4–7, 2 mg days 8–12, 2.5 mg days 13–15, 3 mg days 16–21). Thereafter, subjects were maintained at 3 mg/day guanfacine or placebo through the completion of both laboratory sessions. The laboratory sessions were conducted 1–7 days apart.
Procedures
Intake Sessions
This study was approved by the Yale Human Investigations Committee, and written informed consent was obtained from all the participants at the start of the intake session. The Structured Clinical Interview for DSM-IV (First et al. 1995) was used to assess current psychiatric disorders (except nicotine dependence). The Timeline Followback (Toll et al. 2005) was used to assess past 30-day smoking behavior. The Fagerström Nicotine Dependence Test (FTND) (Heatherton et al. 1991), expired breath carbon monoxide (CO) levels, and urine pregnancy and urine drug screens were also assessed at intake.
Script Development Session
A personalized guided imagery procedure was used to induce the stress and neutral conditions (McKee et al. 2011; Sinha 2009). The stress imagery script was developed by having participants provide a detailed description of a personal stressful experience that occurred in the last 6 months that they perceived as ‘most stressful’. Perceived stress was rated on a 10-point Likert scale where 1 = ‘not at all stressful’ and 10 = ‘the most stress they recently felt in their life.’ Only situations rated as 8 or higher were accepted as appropriate for script development. A neutral-relaxing script was developed from participants’ description of a personal relaxing situation. Scripts were developed by a PhD-level clinician, narrated and audiotaped for presentation during the laboratory sessions, and were approximately 5 min in length.
Laboratory Sessions
Participants individually completed two 6.5-hour laboratory sessions (stress vs. neutral imagery; order counterbalanced). The current laboratory paradigm has been validated using various relapse precipitants, including alcohol (McKee et al. 2006) and stress (McKee et al. 2011). Additional details of the study design and results from the effects of guanfacine on smoking behavior have been presented previously in the parent study, which included the 26 participants reported here (McKee et al. 2014).
Baseline assessment period
Laboratory sessions started at 9:00am. Participants were asked to refrain from smoking beginning at 10:00pm the previous night, which was confirmed with a CO reading of less than 50% of their baseline CO level (Kahler et al. 2012; Odum et al. 2002). Guanfacine (1.5 mg) or placebo was administered at 10:00am, and participants were provided with a standardized lunch at 11:15am to control for time since last food consumption. From 10:00am to 12:30pm, subjects were able to watch television or read.
Personalized imagery procedure
During the laboratory session at 12:55pm (~15 h after their last cigarette), participants were given the following instructions for guided imagery in each of the stress and neutral-relaxing conditions: ‘You will soon hear a situation being described to you. Your task is to close your eyes and imagine yourself in the situation being described, as if it were happening right now. Allow yourself to become completely involved in the situation, by involving your mind and body in actually doing what is being described. Continue imagining until you are asking to stop.’ The participant then listened to the script (stress or neutral-relaxing) via headphones.
Delay and Smoking self-administration period
At 1:10pm, participants were presented with a tray containing 8 cigarettes of their preferred brand, a lighter, and an ashtray. Participants were told that they could smoke at any time over the next 50 minutes. However, for each 5-minute block of time in which they delayed or ‘resisted’ smoking, they would earn $1 for a maximum of $10. Time when subjects announced they wanted to smoke (range 0 – 50 minutes) was recorded. Once the participant self-terminated the delay period (or reached 50 minutes), the 60-minute ad-lib smoking session started. Participants were instructed to ‘smoke as much or as little as they wished’. Subjects were discharged at 3:15pm.
Timing of assessments
HRV was recorded continuously beginning at 10:00am until participants were discharged at 3:15pm. The length of the delay period (i.e., time to initiate smoking), number of cigarettes smoked during the ad-lib period, and tobacco craving were collected and reported elsewhere (McKee et al. 2014).
Measures
Heart rate variability
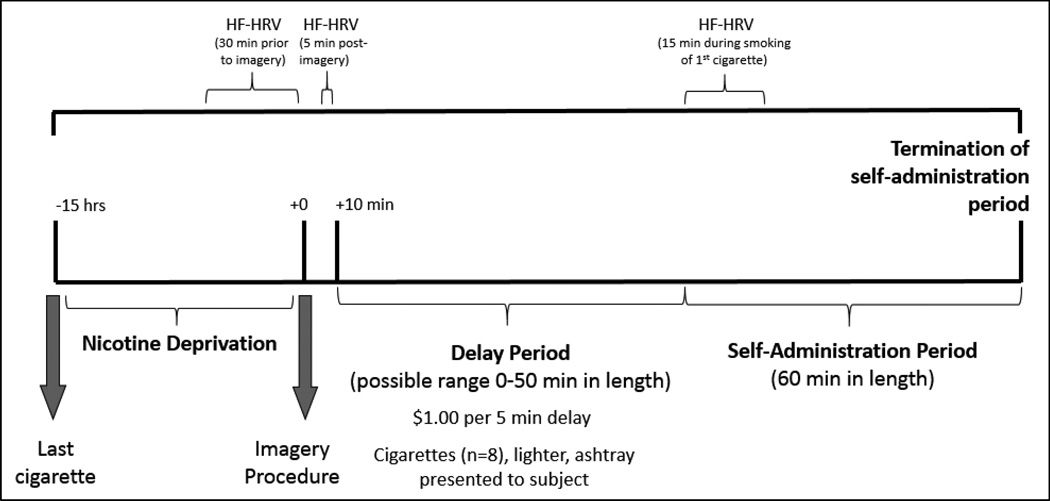
An ambulatory electrocardiography (ECG) monitor (Holter; GE Marquette SEER digital system) was used to record heart rhythm during the session. Recordings were digitally sampled and analyzed using a GE-Marquette system. Tapes were manually reviewed to identify R-R intervals with annotations denoting normal beats, types of ectopics, and noise, and then processed and analyzed with customized software as in prior work (Bigger et al. 1992; Lampert et al. 2005). Recordings with greater than 20% interpolated segments were excluded from further analysis. The R-R interval time series were sampled using a box-car window (Berger et al. 1986) to obtain 1,024 samples per 5 min (3.41333 Hz). The power spectra was calculated using Fourier analysis with a Parzen window on 4 min segments with a 1 min sliding window, corrected for attenuation due to windowing and sampling (Hamming 1973), and integrated over five standard frequency bands (Bigger et al. 1992). High-frequency power (HF; 0.15 to 0.40 Hz), a marker of parasympathetic activity (Akselrod et al. 1981; Pagani et al. 1986), was compared between guanfacine and placebo groups. Following the imagery presentation, we calculated the average HF power band for a 5-minute period (before cigarettes were presented). During the ad-libitum smoking period, we calculated the average HF power band for a 15-minute period during the smoking of the first cigarette for smokers only. The natural log of the average value of HF-HRV were the primary dependent variables. Baseline values were calculated as the natural log of the average value of HF-HRV over the 30 min prior to the imagery condition (Berntson et al. 1997). See Fig. 1 for a timeline of laboratory procedures to evaluate HF-HRV following stress (or neutral) imagery and smoking self-administration.
Figure 1.
Timeline of laboratory procedures to evaluate HF-HRV following stress (or neutral) imagery and smoking self-administration. Note: Baseline HF-HRV was collected over the 30 min prior to the start of the imagery procedure. Following the imagery procedure, HF-HRV was collected for a 5 min period (prior to cigarette presentation). During the self-administration period, HF-HRV was collected for a 15 min period during the smoking of the first cigarette.
Statistical analysis
Multivariate analyses of variance were used to examine change scores by imagery condition (stress, neutral-relaxing) and medication condition (guanfacine, placebo) on HF-HRV following the imagery condition (vs. pre-imagery baseline), and following the ad-lib smoking period (vs. post-imagery levels). Age was controlled in the analyses as it is known to affect HRV (Ryan et al. 1994), and substantially reduce residuals. Sex, FTND, and cigarettes per day were also evaluated as covariates, and were retained if they reduced residual variance, or were otherwise excluded. We conducted exploratory analysis to evaluate whether change in HF-HRV from pre- to post-imagery was predictive of the number of cigarettes smoked during the ad-lib period and time to initial cigarette. Separate regression analysis assessed interactions between change in HF-HRV and medication on the number of cigarettes smoked following stress and neutral-relaxing imagery. Significant interactions were described using correlations.
RESULTS
Baseline Characteristics
Guanfacine (3 mg/day) and placebo groups were well-matched for baseline demographic variables and smoking behavior (see Table 1). Participants were primarily Caucasian, high school educated, and moderately nicotine-dependent (FTND). Mean CO levels at the start of the laboratory sessions (9:00am) were 13.12 (SE=1.392) and 12.09 (SE=1.095) for the stress and neutral-relaxing conditions, respectively, and values did not differ by medication.
Table 1.
Baseline Characteristics by Medication Condition, Mean (SD) or n (%)
| Placebo (n=12) |
3 mg/day Guanfacine (n=14) |
p | ||
|---|---|---|---|---|
| Age | 36.8 (14.5) | 35.1 (11.3) | 0.73 | |
| Sex | 0.54 | |||
| Female | 5 (41.7) | 5 (35.7) | ||
| Male | 7 (58.3) | 9 (64.3) | ||
| Race | 0.83 | |||
| White | 9 (75) | 11 (78.6) | ||
| Other | 3 (25) | 3 (21.4) | ||
| Education | 0.95 | |||
| ≤ High school | 7 (58.3) | 8 (57.1) | ||
| ≥ College | 5 (41.7) | 6 (42.9) | ||
| Marital Status | 0.84 | |||
| Not married | 9 (75) | 10 (71.4) | ||
| Married | 3 (25) | 4 (28.6) | ||
| Tobacco Use | ||||
| Cigarettes per day | 16.8 (8.2) | 20.0 (8.5) | 0.33 | |
| FTND score*a | 5.2 (2.1) | 5.9 (2.5) | 0.46 | |
| CO level at intake | 27.5 (14.2) | 26.9 (17.8) | 0.92 | |
FTND, Fagerström Test for Nicotine Dependence
Range: scores ≥ 4 for nicotine dependence.
Note: no difference between groups using chi-square or ANOVA where appropriate.
Heart Rate Variability
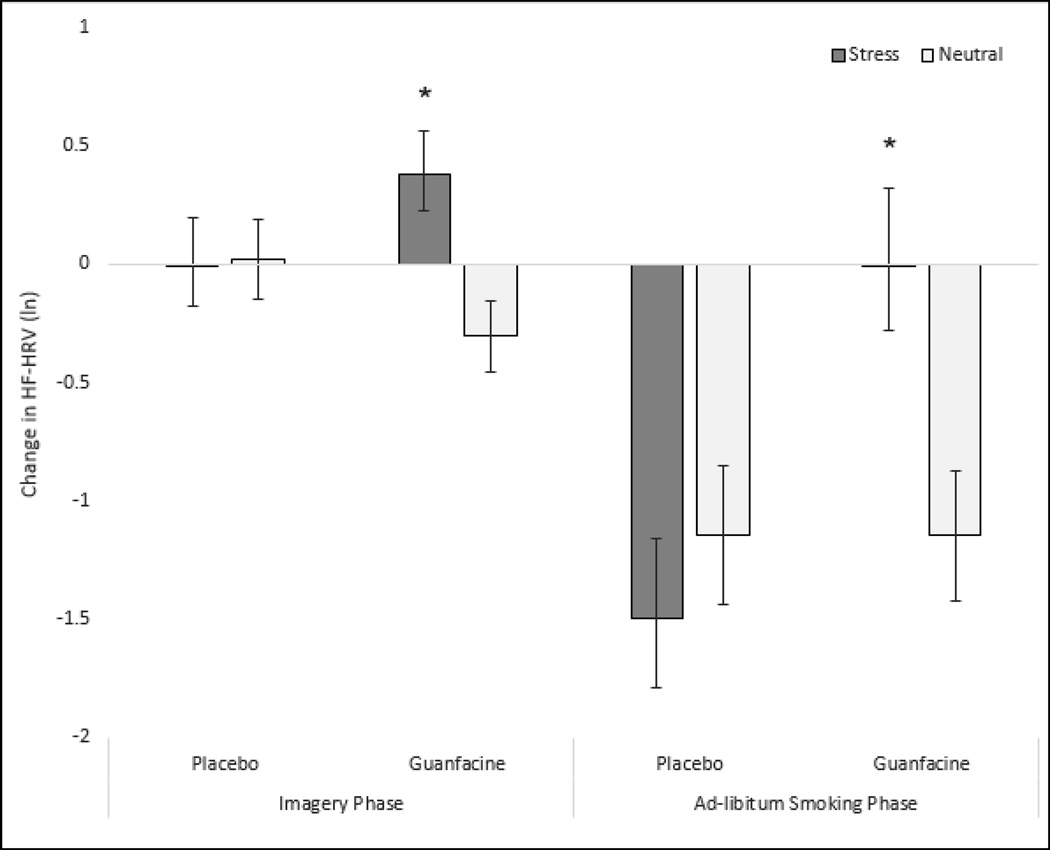
At baseline and preceding personalized imagery exposure, tonic HF-HRV did not differ by medication randomization group (guanfacine mean=6.86 (SE=0.3), placebo mean=6.51, (SE=0.32); F1,24=0.65, p=0.43). Following personalized imagery exposure, HRV demonstrated a significant condition by medication interaction (F1,23=4.3, p=0.049; Cohen’s d=0.86; see Fig. 2). In the placebo group, there were no differences in phasic HF-HRV across stress and neutral imagery conditions. In contrast, guanfacine increased phasic HF-HRV following stress imagery, and decreased HF-HRV following neutral imagery. Following personalized imagery exposure and ad-libitum smoking, HF-HRV demonstrated a significant condition by medication interaction (F1,15=25.42, p<0.0001; Cohen’s d=2.60; see Fig. 2). In the placebo group, stress and ad-libitum smoking additively decreased phasic HF-HRV across imagery conditions. In contrast, guanfacine attenuated stress- and smoking-related decreases in phasic HF-HRV, and this effect was specific to stress relative to neutral imagery.
Figure 2.
Mean(±SE) change in values of the natural log (ln) of HF-HRV following imagery and ad-lib smoking. *Guanfacine significantly different versus placebo.
Secondary Outcomes
On average, individuals who decided to smoke smoked within 1 minute of the start of the ad-libitum smoking self-administration period. The number of cigarettes smoked ranged from 0 to 4 for both the stress and neutral-relaxing sessions (stress mean=1.70 (SE=0.21), neutral-relaxing mean=1.45 (SE=0.21)).
Prior to smoking, HF-HRV demonstrated a significant condition by medication interaction (t=−2.38, p=0.03), such that greater increases in phasic HF-HRV following stress imagery were predictive of less cigarettes smoked in the ad-libitum smoking phase in the guanfacine-treated group only (r=−0.56, p<.05). In contrast, there was no relationship between change in HRV and cigarettes smoked in the placebo group. There was no relationship between change in HRV and time to initial cigarette.
DISCUSSION
To our knowledge, this is the first examination of the effects of the noradrenergic agent guanfacine on HF-HRV during stress-precipitated smoking. Overall, findings demonstrated that guanfacine produced phasic changes in HF-HRV in response to stress, but did not change baseline or tonic levels. Using a validated human laboratory analogue of smoking-lapse behavior (McKee et al. 2012), we demonstrated that smokers in the placebo group exhibited blunted HF-HRV in response to stress, whereas guanfacine increased HF-HRV following stress, supporting our hypothesis. Prior work on HF-HRV as an index of stress indicates that the greater change in state or phasic HF-HRV following stress is indicative of the successful regulation of an emotional or stress response (Thayer et al. 2012). Thus, increases in phasic HF-HRV following stress may predict better smoking outcomes in regular daily smokers. Indeed, the present study demonstrated that guanfacine increased HF-HRV following stress versus the neutral imagery condition. Further, this increase in HF-HRV was associated with later decreases in the number of cigarettes smoked during the ad-libitum smoking period. Whereas some previous work has suggested that decreases rather than increases in HF-HRV in response to stress are indicative of better regulatory functioning, our findings suggest that increases in HF-HRV may result in more favorable regulatory functioning in response to stress, especially given the observed relation between increased HF-HRV and fewer cigarettes smoked in the guanfacine-treated group. We did not find a significant relationship between change in HF-HRV from pre- to post-imagery and time to initial cigarette. However, previous findings from our laboratory indicate that decreased phasic HF-HRV following stress and cigarette smoking was associated with less time to initiate smoking (Ashare et al., 2012). Guanfacine also reduced HF-HRV following neutral-relaxing imagery, indicating a reduction in phasic HF-HRV during a relaxing state. It is unclear why guanfacine decreased HF-HRV following neutral-relaxing imagery. Future work is needed to address the effect of guanfacine on HF-HRV during neutral-relaxing states and to elucidate the relationship between HF-HRV and time to initiate smoking. Overall, daily smokers treated with guanfacine may have more physiological flexibility to respond to stress leading to reduced vulnerability to smoking relapse. The parent investigation to the present study demonstrated that guanfacine, which attenuates noradrenergic activity and stress reactivity, increased the ability to resist smoking and decreased ad-libitum smoking (McKee et al. 2014). This is in agreement with other substance abuse work demonstrating that recovering alcoholics who had an increased ability to resist drinking also exhibited increases in phasic HRV (Ingjaldsson et al. 2003).
In contrast, placebo findings in our sample of smokers demonstrated blunted HF-HRV levels in response to stress, consistent with our prior findings (Ashare et al. 2012). In addition to blunted HF-HRV, smokers also demonstrate dysregulated, or blunted, hypothalamic pituitary adrenal (HPA)-axis activation after chronic nicotine exposure, which may reflect a reduced ability to respond to stress and increased risk for relapse (al’Absi 2006; al’Absi et al. 2003; al’Absi et al. 2005; al’Absi et al. 2015; Baron et al. 1995; Childs and de Wit 2009). Preclinical and clinical findings support this notion, indicating that dysregulated autonomic function is predictive of relapse to drug use (Junghanns et al. 2003; Koob 2008). It is likely that chronic nicotine exposure may contribute to chronic autonomic dysregulation across stress systems, reducing the physiological capacity to respond to stress. Our findings suggest that reduced HF-HRV in the placebo group may be reflective of blunted vagal reactivity, and that this reduction in autonomic nervous system flexibility may be related to poorer smoking outcomes following stress exposure.
With regard to HF-HRV following stress and ad-libitum smoking, we demonstrated that stress and ad-libitum smoking additively decreased HF-HRV in the placebo group, and that decreases in HF-HRV were demonstrated across imagery conditions. In contrast, guanfacine attenuated decreases in HF-HRV following stress and ad-libitum smoking, suggesting that guanfacine improves autonomic flexibility. This effect of guanfacine was specific to the stress imagery condition versus neutral imagery. This is consistent with, and extends, previous findings that stress and ad-libitum smoking additively decreased HF-HRV in nicotine-dependence smokers (Ashare et al. 2012), and that low HRV is associated with mortality and may be a marker for disease (Thayer et al. 2012; Thayer et al. 2010). Further, the ability of guanfacine to alter the effect of stress and cigarette smoking on HF-HRV may be one mechanism by which guanfacine reduces stress-precipitated smoking behavior, as demonstrated in the parent study to the present investigation (McKee et al. 2014). Alternatively, because HF-HRV was measured following stress rather than during, it is possible that decreases in HF-HRV following stress and ad-libitum smoking in the placebo group may be reflective of a dysregulated ability to regulate stress. Thus, the attenuation of decreases in HF-HRV following stress and smoking in the guanfacine group may be reflective of a recovery of these regulatory processes. It is also possible that the observed effects of guanfacine on HF-HRV in response to stress are likely indirect considering that guanfacine reduces centrally-mediated sympathetic nervous system outflow and that parasympathetic nervous system outflow does not necessarily correspondingly change (Berntson et al. 1991).
Thayer et al. (2012) also posits that tonic (resting) HF-HRV may be a marker for dynamic flexibility of sympathetic autonomic activity. Higher tonic HRV has been associated with an increased ability to make context-appropriate emotional responses (Ruiz-Padial et al. 2003), whereas low tonic HRV has been associated with a delayed response to stress (Thayer et al. 2012; Weber et al. 2010). In the present investigation, we found no differences in tonic HF-HRV between medication groups at baseline and preceding imagery exposure, indicating that tonic HF-HRV in smokers may not be altered by guanfacine following 21 days of treatment, counter to our hypothesis. Individuals who were post-myocardial infarction optimized to β-adrenergic antagonists displayed increased HRV following 12-months of treatment (Jokinen et al. 2003). Similarly, four to six months of treatment with sertraline, a selective serotonin reuptake inhibitor (SSRI), for depression increases HRV (Glassman et al. 2007; McFarlane et al. 2001). Both cardiac events and depression are known to suppress HRV, and longer-term medication treatment, 16 weeks and up to 12 months of treatment, for those conditions improved tonic HRV. It is possible that longer-term guanfacine treatment, versus the three-week treatment period used in the current study, may also improve tonic HRV levels. Future work is needed to investigate the effects of extended guanfacine treatment on stress and HRV in a sample of regular daily smokers.
There are several limitations of the present investigation that warrant mention. As this was a secondary investigation, the sample size was relatively modest. However, the sample size is comparable to other laboratory studies of stress in smokers (McKee et al. 2014), and medication effects on HF-HRV were significant. Nonetheless, it will be important to replicate findings with a larger sample size. Another possible limitation is that respiration was not controlled. However, the rate of respiration may not have a strong impact on HF-HRV reactivity to experimental manipulation in the laboratory. Subjects were relatively sedentary throughout each laboratory session and were not subjected to postural changes, which may alter respiration (Cacioppo et al. 1994). Previous work has demonstrated that slow-paced breathing and simple mental activities that cause changes in respiration frequency may affect HF-HRV (Bernardi et al. 2000; Song and Lehrer 2003), but that stress-induced HF-HRV does not differ between controlled and spontaneous breathing (Bernardi et al. 2000; Madden and Savard 1995; Pagani et al. 1991). Third, the manner in which cigarettes were smoked, including manner of inhalation, may have affected HF-HRV. Future work is needed to investigate the effects of smoking inhalation and respiration frequency on HF-HRV in the human laboratory. Fourth, while we demonstrated that guanfacine may have no effect on baseline (or tonic) levels of HF-HRV during the laboratory sessions, we did not have a pre-medication treatment baseline. Further, the use of guanfacine to alter or normalize the effect of stress and smoking on HF-HRV may not be invariably adaptive since decreases in HF-HRV in response to stress are thought to promote an allocation of attentional resources to adapt to or ‘solve’ a stressful situation, and preventing such a response may inhibit appropriate ‘problem-solving’ in response to stress and, ultimately, may not be beneficial. Lastly, recordings with greater than 20% interpolated segments were excluded from further analysis. While this threshold may be high, it is incorporated into the software package that was used to process and analyze the HF-HRV data, and we have previously used this threshold to assess HF-HRV in our laboratory (Ashare et al., 2012; Udo et al., 2014).
Overall, results indicate that guanfacine may be of potential pharmacotherapeutic benefit for smoking cessation. Using a human laboratory paradigm of smoking-lapse behavior, we demonstrated for the first time that guanfacine significantly increased phasic HF-HRV following stress in regular daily smokers, and may improve autonomic flexibility in response to stress and smoking. Our findings extend clinical results demonstrating that stress and smoking additively decrease HF-HRV in nicotine-dependent individuals, and support the notion that phasic changes in HRV may be an important marker of the stress response and smoking. These findings support further development of the use of guanfacine for stress-precipitated smoking behavior.
Acknowledgments
Funding: Supported by National Institutes of Health (NIH) P50DA033945, R01DA035001, R01AA022285, RL1DA024857; and the Yale CTSA-UL1RR024139 and National Institute on Drug Abuse (NIDA) T32 DA007238
SAM has consulting to Cerecor and Embera, has received research support for investigator-initiated studies from Pfizer, Inc., and has ownership in Lumme, Inc.
Footnotes
CONFLICT OF INTEREST
All other authors declare that they have no conflict of interest.
COMPLIANCE WITH ETHICAL STANDARDS
This study was approved by the Yale Human Investigations Committee. Informed consent was obtained from all participants in accordance with the Declaration of Helsinki.
REFERENCES
- Ahmed MW, Kadish AH, Parker MA, Goldberger JJ. Effect of physiologic and pharmacologic adrenergic stimulation on heart rate variability. Journal of the American College of Cardiology. 1994;24:1082–1090. doi: 10.1016/0735-1097(94)90874-5. [DOI] [PubMed] [Google Scholar]
- Akselrod S, Gordon D, Ubel FA, Shannon DC, Berger AC, Cohen RJ. Power spectrum analysis of heart rate fluctuation: a quantitative probe of beat-to-beat cardiovascular control. Science (New York, NY) 1981;213:220–222. doi: 10.1126/science.6166045. [DOI] [PubMed] [Google Scholar]
- al’Absi M. Hypothalamic-pituitary-adrenocortical responses to psychological stress and risk for smoking relapse. International Journal of Psychophysiology. 2006;59:218–227. doi: 10.1016/j.ijpsycho.2005.10.010. [DOI] [PubMed] [Google Scholar]
- al’Absi M, Wittmers LE, Erickson J, Hatsukami D, Crouse B. Attenuated adrenocortical and blood pressure responses to psychological stress in ad libitum and abstinent smokers. Pharmacology Biochemistry and Behavior. 2003;74:401–410. doi: 10.1016/s0091-3057(02)01011-0. [DOI] [PubMed] [Google Scholar]
- al’Absi M, Hatsukami D, Davis GL. Attenuated adrenocorticotropic responses to psychological stress are associated with early smoking relapse. Psychopharmacology. 2005;181:107–117. doi: 10.1007/s00213-005-2225-3. [DOI] [PubMed] [Google Scholar]
- al’Absi M, Nakajima M, Allen S, Lemieux A, Hatsukami D. Sex differences in hormonal responses to stress and smoking relapse: a prospective examination. Nicotine & Tobacco Research. 2015;17:382–389. doi: 10.1093/ntr/ntu340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvares GA, Quintana DS, Hickie IB, Guastella AJ. Autonomic nervous system dysfunction in psychiatric disorders and the impact of psychotropic medications: a systematic review and meta-analysis. Journal of psychiatry & neuroscience: JPN. 2016;41:89. doi: 10.1503/jpn.140217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashare RL, Sinha R, Lampert R, Weinberger AH, Anderson GM, Lavery ME, Yanagisawa K, McKee SA. Blunted vagal reactivity predicts stress-precipitated tobacco smoking. Psychopharmacology. 2012;220:259–268. doi: 10.1007/s00213-011-2473-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron JA, Comi RJ, Cryns V, Brinck-Johnsen T, Mercer NG. The effect of cigarette smoking on adrenal cortical hormones. Journal of Pharmacology and Experimental Therapeutics. 1995;272:151–155. [PubMed] [Google Scholar]
- Berger RD, Akselrod S, Gordon D, Cohen RJ. An efficient algorithm for spectral analysis of heart rate variability. IEEE transactions on bio-medical engineering. 1986;33:900–904. doi: 10.1109/TBME.1986.325789. [DOI] [PubMed] [Google Scholar]
- Bernardi L, Wdowczyk-Szulc J, Valenti C, Castoldi S, Passino C, Spadacini G, Sleight P. Effects of controlled breathing, mental activity and mental stress with or without verbalization on heart rate variability. Journal of the American College of Cardiology. 2000;35:1462–1469. doi: 10.1016/s0735-1097(00)00595-7. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Bigger JT, Jr, Eckberg DL, Grossman P, Kaufmann PG, Malik M, Nagaraja HN, Porges SW, Saul JP, Stone PH, van der Molen MW. Heart rate variability: origins, methods, and interpretive caveats. Psychophysiology. 1997;34:623–648. doi: 10.1111/j.1469-8986.1997.tb02140.x. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Cacioppo JT, Quigley KS. Autonomic determinism: the modes of autonomic control, the doctrine of autonomic space, and the laws of autonomic constraint. Psychological review. 1991;98:459. doi: 10.1037/0033-295x.98.4.459. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Norman GJ, Hawkley LC, Cacioppo JT. Cardiac autonomic balance versus cardiac regulatory capacity. Psychophysiology. 2008;45:643–652. doi: 10.1111/j.1469-8986.2008.00652.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigger JT, Jr, Fleiss JL, Steinman RC, Rolnitzky LM, Kleiger RE, Rottman JN. Frequency domain measures of heart period variability and mortality after myocardial infarction. Circulation. 1992;85:164–171. doi: 10.1161/01.cir.85.1.164. [DOI] [PubMed] [Google Scholar]
- Borland R. Slip-ups and relapse in attempts to quit smoking. Addictive Behaviors. 1990;15:235–245. doi: 10.1016/0306-4603(90)90066-7. [DOI] [PubMed] [Google Scholar]
- Brandon TH. Negative Affect as Motivation to Smoke. Current Directions in Psychological Science. 1994;3:33–37. [Google Scholar]
- Cacioppo JT, Uchino BN, Berntson GG. Individual differences in the autonomic origins of heart rate reactivity: The psychometrics of respiratory sinus arrhythmia and preejection period. Psychophysiology. 1994;31:412–419. doi: 10.1111/j.1469-8986.1994.tb02449.x. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Current Cigarette Smoking Among Adults-United States 2005–2014. Morbidity and Mortality Weekly Report. 2015;64:1233–1240. doi: 10.15585/mmwr.mm6444a2. [DOI] [PubMed] [Google Scholar]
- Childs E, de Wit H. Hormonal, cardiovascular, and subjective responses to acute stress in smokers. Psychopharmacology. 2009;203:1–12. doi: 10.1007/s00213-008-1359-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinas PC, Koutedakis Y, Flouris AD. Effects of active and passive tobacco cigarette smoking on heart rate variability. International journal of cardiology. 2013;163:109–115. doi: 10.1016/j.ijcard.2011.10.140. [DOI] [PubMed] [Google Scholar]
- Ferguson SG, Shiffman S. Effect of high-dose nicotine patch on craving and negative affect leading up to lapse episodes. Psychopharmacology. 2014;231:2595–2602. doi: 10.1007/s00213-013-3429-6. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB. Structured Clinical Interview for DSM-IV Axis I Disorders, Patient Edition, January 1995 FINAL. SCID-I/P Version 2.0) New York, NY: Biometrics Research Department, New York State Psychiatric Institute; 1995. [Google Scholar]
- Fox HC, Seo D, Tuit K, Hansen J, Kimmerling A, Morgan PT, Sinha R. Guanfacine effects on stress, drug craving and prefrontal activation in cocaine dependent individuals: preliminary findings. Journal of psychopharmacology (Oxford, England) 2012;26:958–972. doi: 10.1177/0269881111430746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman BH. An autonomic flexibility-neurovisceral integration model of anxiety and cardiac vagal tone. Biological psychology. 2007;74:185–199. doi: 10.1016/j.biopsycho.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Glassman AH, Bigger JT, Gaffney M, Van Zyl LT. Heart rate variability in acute coronary syndrome patients with major depression: influence of sertraline and mood improvement. Archives of general psychiatry. 2007;64:1025–1031. doi: 10.1001/archpsyc.64.9.1025. [DOI] [PubMed] [Google Scholar]
- Hamming R. Numerical methods for scientists and engineers. 2nd. New York: McGraw-Hill; 1973. [Google Scholar]
- Hayano J, Yamada M, Sakakibara Y, Fujinami T, Yokoyama K, Watanabe Y, Takata K. Short- and long-term effects of cigarette smoking on heart rate variability. The American journal of cardiology. 1990;65:84–88. doi: 10.1016/0002-9149(90)90030-5. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. British journal of addiction. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Ingjaldsson JT, Laberg JC, Thayer JF. Reduced heart rate variability in chronic alcohol abuse: relationship with negative mood, chronic thought suppression, and compulsive drinking. Biological psychiatry. 2003;54:1427–1436. doi: 10.1016/s0006-3223(02)01926-1. [DOI] [PubMed] [Google Scholar]
- Jokinen V, Tapanainen JM, Seppanen T, Huikuri HV. Temporal changes and prognostic significance of measures of heart rate dynamics after acute myocardial infarction in the beta-blocking era. The American journal of cardiology. 2003;92:907–912. doi: 10.1016/s0002-9149(03)00968-8. [DOI] [PubMed] [Google Scholar]
- Jorenby DE, Hays JT, Rigotti NA, Azoulay S, Watsky EJ, Williams KE, Billing CB, Gong J, Reeves KR, Group VPS. Efficacy of varenicline, an α4β2 nicotinic acetylcholine receptor partial agonist, vs placebo or sustained-release bupropion for smoking cessation: a randomized controlled trial. Jama. 2006;296:56–63. doi: 10.1001/jama.296.1.56. [DOI] [PubMed] [Google Scholar]
- Junghanns K, Backhaus J, Tietz U, Lange W, Bernzen J, Wetterling T, Rink L, Driessen M. Impaired serum cortisol stress response is a predictor of early relapse. Alcohol and Alcoholism. 2003;38:189–193. doi: 10.1093/alcalc/agg052. [DOI] [PubMed] [Google Scholar]
- Kahler CW, Metrik J, Spillane NS, Leventhal AM, McKee SA, Tidey JW, McGeary JE, Knopik VS, Rohsenow DJ. Sex differences in stimulus expectancy and pharmacologic effects of a moderate dose of alcohol on smoking lapse risk in a laboratory analogue study. Psychopharmacology. 2012;222:71–80. doi: 10.1007/s00213-011-2624-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. A role for brain stress systems in addiction. Neuron. 2008;59:11–34. doi: 10.1016/j.neuron.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampert R, Ickovics J, Horwitz R, Lee F. Depressed autonomic nervous system function in African Americans and individuals of lower social class: a potential mechanism of race- and class-related disparities in health outcomes. American heart journal. 2005;150:153–160. doi: 10.1016/j.ahj.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Le A, Funk D, Juzytsch W, Coen K, Navarre BM, Cifani C, Shaham Y. Effect of prazosin and guanfacine on stress-induced reinstatement of alcohol and food seeking in rats. Psychopharmacology. 2011;218:89–99. doi: 10.1007/s00213-011-2178-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden K, Savard G. Effects of mental state on heart rate and blood pressure variability in men and women. Clinical Physiology. 1995;15:557–569. doi: 10.1111/j.1475-097x.1995.tb00544.x. [DOI] [PubMed] [Google Scholar]
- McFarlane A, Kamath MV, Fallen EL, Malcolm V, Cherian F, Norman G. Effect of sertraline on the recovery rate of cardiac autonomic function in depressed patients after acute myocardial infarction. American heart journal. 2001;142:617–623. doi: 10.1067/mhj.2001.116766. [DOI] [PubMed] [Google Scholar]
- McKee SA. Why is it more difficult for women to quit smoking? Translating knowledge into practice American Psychological Association Annual Convention. Honolulu, HI: 2013. [Google Scholar]
- McKee SA, Krishnan-Sarin S, Shi J, Mase T, O’Malley SS. Modeling the effect of alcohol on smoking lapse behavior. Psychopharmacology. 2006;189:201–210. doi: 10.1007/s00213-006-0551-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee SA, Maciejewski PK, Falba T, Mazure CM. Sex differences in the effects of stressful life events on changes in smoking status. Addiction. 2003;98:847–855. doi: 10.1046/j.1360-0443.2003.00408.x. [DOI] [PubMed] [Google Scholar]
- McKee SA, Potenza M, Kober H, Sofuoglu M, Arnsten A, Picciotto MR, Weinberger AH, Ashare RL, Sinha R. A translational investigation targeting stress-reactivity and pre-frontal cognitive control with guanfacine for smoking cessation. Journal of Psychopharmacology. 2014 doi: 10.1177/0269881114562091. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee SA, Sinha R, Weinberger AH, Sofuoglu M, Harrison EL, Lavery M, Wanzer J. Stress decreases the ability to resist smoking and potentiates smoking intensity and reward. Journal of psychopharmacology. 2011;25:490–502. doi: 10.1177/0269881110376694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee SA, Weinberger AH, Shi J, Tetrault J, Coppola S. Developing and validating a human laboratory model to screen medications for smoking cessation. Nicotine Tob Res. 2012;14:1362–1371. doi: 10.1093/ntr/nts090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odum AL, Madden GJ, Bickel WK. Discounting of delayed health gains and losses by current, never-and ex-smokers of cigarettes. Nicotine & Tobacco Research. 2002;4:295–303. doi: 10.1080/14622200210141257. [DOI] [PubMed] [Google Scholar]
- Pagani M, Lombardi F, Guzzetti S, Rimoldi O, Furlan R, Pizzinelli P, Sandrone G, Malfatto G, Dell’Orto S, Piccaluga E, et al. Power spectral analysis of heart rate and arterial pressure variabilities as a marker of sympatho-vagal interaction in man and conscious dog. Circulation research. 1986;59:178–193. doi: 10.1161/01.res.59.2.178. [DOI] [PubMed] [Google Scholar]
- Pagani M, Mazzuero G, Ferrari A, Liberati D, Cerutti S, Vaitl D, Tavazzi L, Malliani A. Sympathovagal interaction during mental stress. A study using spectral analysis of heart rate variability in healthy control subjects and patients with a prior myocardial infarction. Circulation. 1991;83:43–51. [PubMed] [Google Scholar]
- Park G, Vasey MW, Van Bavel JJ, Thayer JF. When tonic cardiac vagal tone predicts changes in phasic vagal tone: the role of fear and perceptual load. Psychophysiology. 2014;51:419–426. doi: 10.1111/psyp.12186. [DOI] [PubMed] [Google Scholar]
- Ruiz-Padial E, Sollers JJ, 3rd, Vila J, Thayer JF. The rhythm of the heart in the blink of an eye: emotion-modulated startle magnitude covaries with heart rate variability. Psychophysiology. 2003;40:306–313. doi: 10.1111/1469-8986.00032. [DOI] [PubMed] [Google Scholar]
- Ryan SM, Goldberger AL, Pincus SM, Mietus J, Lipsitz LA. Gender- and age-related differences in heart rate dynamics: are women more complex than men? Journal of the American College of Cardiology. 1994;24:1700–1707. doi: 10.1016/0735-1097(94)90177-5. [DOI] [PubMed] [Google Scholar]
- Sawchenko P, Swanson L. Central noradrenergic pathways for the integration of hypothalamic neuroendocrine and autonomic responses. Science (New York, NY) 1981;214:685–687. doi: 10.1126/science.7292008. [DOI] [PubMed] [Google Scholar]
- Shiffman S. Relapse following smoking cessation: a situational analysis. Journal of consulting and clinical psychology. 1982;50:71–86. doi: 10.1037//0022-006x.50.1.71. [DOI] [PubMed] [Google Scholar]
- Sinha R. Modeling stress and drug craving in the laboratory: implications for addiction treatment development. Addiction biology. 2009;14:84–98. doi: 10.1111/j.1369-1600.2008.00134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song H-S, Lehrer PM. The effects of specific respiratory rates on heart rate and heart rate variability. Applied psychophysiology and biofeedback. 2003;28:13–23. doi: 10.1023/a:1022312815649. [DOI] [PubMed] [Google Scholar]
- Svensson TH. Peripheral, autonomic regulation of locus coeruleus noradrenergic neurons in brain: putative implications for psychiatry and psychopharmacology. Psychopharmacology. 1987;92:1–7. doi: 10.1007/BF00215471. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Ahs F, Fredrikson M, Sollers JJ, 3rd, Wager TD. A meta-analysis of heart rate variability and neuroimaging studies: implications for heart rate variability as a marker of stress and health. Neuroscience and biobehavioral reviews. 2012;36:747–756. doi: 10.1016/j.neubiorev.2011.11.009. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Yamamoto SS, Brosschot JF. The relationship of autonomic imbalance, heart rate variability and cardiovascular disease risk factors. International journal of cardiology. 2010;141:122–131. doi: 10.1016/j.ijcard.2009.09.543. [DOI] [PubMed] [Google Scholar]
- Toll BA, Cooney NL, McKee SA, O’malley SS. Do daily interactive voice response reports of smoking behavior correspond with retrospective reports? Psychology of Addictive Behaviors. 2005;19:291. doi: 10.1037/0893-164X.19.3.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udo T, Weinberger AH, Grilo CM, Brownell KD, DiLeone RJ, Lampert R, Matlin SL, Yanagisawa K, McKee SA. Heightened vagal activity during high-calorie food presentation in obese compared with non-obese individuals--results of a pilot study. Obesity research & clinical practice. 2014;8:e201–e298. doi: 10.1016/j.orcp.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Zwieten P, Thoolen M, Timmermans PB. The hypotensive activity and side effects of methyldopa, clonidine, and guanfacine. Hypertension. 1984;6:28. doi: 10.1161/01.hyp.6.5_pt_2.ii28. [DOI] [PubMed] [Google Scholar]
- Weber CS, Thayer JF, Rudat M, Wirtz PH, Zimmermann-Viehoff F, Thomas A, Perschel FH, Arck PC, Deter HC. Low vagal tone is associated with impaired post stress recovery of cardiovascular, endocrine, and immune markers. Eur J Appl Physiol. 2010;109:201–211. doi: 10.1007/s00421-009-1341-x. [DOI] [PubMed] [Google Scholar]
- World Health Organization. 2014 http://www.who.int/campaigns/no-tobacco-day/2014/event/en/
- Yeragani VK, Pohl R, Berger R, Balon R, Ramesh C, Glitz D, Srinivasan K, Weinberg P. Decreased heart rate variability in panic disorder patients: a study of power-spectral analysis of heart rate. Psychiatry research. 1993;46:89–103. doi: 10.1016/0165-1781(93)90011-5. [DOI] [PubMed] [Google Scholar]