Abstract
Rationale
The extent to which non-α4β2 versus α4β2* nAChRs contribute to the behavioral effects of varenicline and other nAChR agonists is unclear.
Objectives
The purpose of this study was to characterize the discriminative stimulus effects of varenicline and nicotine using various nAChR agonists and antagonists to elucidate possible non-α4β2 nAChR mechanisms.
Methods
Separate groups of male C57BL/6J mice were trained to discriminate varenicline (3.2 mg/kg) or nicotine (1 mg/kg). Test drugs included mecamylamine, the nAChR agonists epibatidine, nicotine, cytisine, varenicline, RTI-102, the β2-containing nAChR antagonist dihydro-β-erythroidine (DHβE), the α7 nAChR agonist PNU-282987, the α7 antagonist methyllycaconitine (MLA), the α3β4 antagonist 18-methoxycoronaridine (18-MC), and the non-nAChR drugs midazolam and cocaine.
Results
In nicotine-trained mice, maximum nicotine-appropriate responding was: 95% nicotine, 94% epibatidine; 63% varenicline; 58% cytisine; and less than 50% for RTI-102, PNU-282987, midazolam, and cocaine. In varenicline-trained mice, maximum varenicline-appropriate responding was: 90% varenicline, 86% epibatidine; 74% cytisine; 80% RTI-102; 50% cocaine; and 50% or less for nicotine, PNU-282987, and midazolam. Drugs were studied to doses that abolished operant responding. Mecamylamine antagonized the discriminative stimulus effects, but not the rate-decreasing effects, of nicotine and varenicline. DHβE antagonized the discriminative stimulus and rate-decreasing effects of nicotine, but not varenicline in either the nicotine or varenicline discrimination assays. The discriminative stimulus, but not rate-decreasing, effects of epibatidine were antagonized by DHβE regardless of the training drug.
Conclusions
These results suggest that α4β2* nAChRs differentially mediate the discriminative stimulus effects of nicotine and varenicline, and suggest that varenicline has substantial non-α4β2 nAChR activity.
Keywords: nicotine, varenicline, drug discrimination, DHβE, mecamylamine, β2 nAChRs
Introduction
Varenicline (Chantix) is a tobacco dependence pharmacotherapy that is often significantly more effective than nicotine (Hays et al. 2008). In vitro, varenicline has greatest affinity for α4β2* nicotinic acetylcholine receptors (nAChRs) (* denotes the possible presence of an additional subunit), where it acts as a partial agonist (Rollema et al. 2007a). Partial agonism at α4β2* nAChRs is believed to be the mechanism by which varenicline is clinically effective (Rollema et al. 2007b). However, varenicline has recently been demonstrated to be a full agonist at α7 and α3β4 nAChRs, and is only ~8-fold and ~25-fold less potent at α7 and α3β4 nAChRs, respectively, than at α4β2* nAChRs (Mihalak et al. 2006; Grady et al. 2010). Varenicline is more potent for α7 and α3β4 nAChRs than nicotine, and while having greater affinity for α4β2* nAChRs, is less selective than nicotine for α4β2* nAChRs compared to α7 and α3β4 nAChRs (Grady et al. 2010). Furthermore, varenicline has been shown to be a potent full agonist at 5HT3 receptors (Lummis et al. 2011).
Drug discrimination is a pharmacologically selective procedure that has utility in examining the in vivo receptor mechanisms that mediate the effects of CNS drugs (Colpaert 1999). In other words, drugs that share discriminative stimulus effects may have similar mechanisms of action in vivo. Similarly, ligands with selectivity for α4β2* nAChRs over α3β4 and α7 nAChRs share discriminative stimulus effects with nicotine when nicotine is used as a training drug (Smith et al. 2007). This would suggest that α4β2* nAChRs mediate the discriminative stimulus effects of nicotine, and drugs that produce qualitatively similar effects to nicotine are α4β2* nAChR agonists. Varenicline has been shown to fully and partially substitute for nicotine, depending on the training conditions (Rollema et al. 2007a; Jutkiewicz et al. 2011; Cunningham and McMahon 2013). This would suggest some overlap in the receptors that mediate the effects of varenicline and nicotine, and those receptors are presumed to be α4β2* nAChRs. However, it has also been demonstrated that the β2 selective nAChR antagonist dihydro-β-erythroidine (DHβE; Papke et al. 2008) will not antagonize the discriminative stimulus effects of varenicline in nicotine-trained animals (Cunningham and McMahon 2013). In another study, the rate-decreasing effects and hypothermic effects of nicotine and varenicline were differentially antagonized by DHβE, and cross-tolerance to these effects of varenicline from nicotine treatment was significantly less than tolerance to nicotine (de Moura and McMahon, 2016). These results suggested that varenicline and nicotine differ in their pharmacology to produce in vivo effects. While studies investigating varenicline in vitro have identified its full agonist effects at α7 and α3β4 nAChRs, there is a paucity in literature examining the extent to which the behavioral effects of varenicline are due to actions at non-α4β2 nAChRs.
The current study examined the relative contribution of α4β2*- versus non-α4β2 nAChRs to the discriminative stimulus effects of varenicline. Whereas nicotine has been used frequently as a training drug, to our knowledge, varenicline has not yet been characterized as a training drug. Because drug discrimination has utility in elucidating receptor mechanisms, training varenicline as a discriminative stimulus would be the ideal method to identify receptors that mediate its in vivo effects. Separate groups of mice were trained to discriminate either nicotine or varenicline; the effects of various nAChR agonists were systematically compared between training drugs to identify underlying neuropharmacological mechanisms. Test drugs included the low efficacy α4β2* nAChR agonists RTI-102 and cytisine, the high efficacy α4β2* nAChR agonist epibatidine, and the α7 nAChR agonist PNU-282987 (Hajós et al. 2005; Abdrakhmanova et al. 2006; Grady et al. 2010). Assuming functionally equivalent training doses, if nicotine and varenicline only differed in efficacy at a common receptor, drugs that only partially substitute for nicotine should be more likely to substitute for varenicline (Colpaert 1988). Furthermore, it might be predicted that nAChR agonists would be more potent in the varenicline discrimination than in the nicotine discrimination (Colpaert 1988). Receptor theory would predict that if partial substitution of varenicline for a nicotine discriminative stimulus is a function of low efficacy, nicotine should be dose-dependently antagonized by varenicline (Kenakin 1997; Kenakin 2002).
If the discriminative stimulus effects of nicotine and varenicline are differentially mediated by multiple nAChR subtypes, the substitution profiles of other nAChR agonists would be dependent on shared subtype selectivity with the training drugs. Furthermore, if nicotine and varenicline differed in their in vivo selectivity for various nAChR subtypes, they should be differentially antagonized by nAChR selective antagonists. Therefore, nicotine and varenicline were combined with mecamylamine, DHβE, the α7 nAChR antagonist methyllycaconitine (MLA; Ward et al. 1990), and the α3β4* antagonist 18-methoxycoronaridine (18-MC; Grady et al. 2010; Kuehne et al. 2003). The overall nAChR selectivity of the varenicline and nicotine discriminations was examined by testing the monoamine transporter blocker cocaine and the benzodiazepine midazolam.
The decision to use mice for this experiment was as follows. First, much of the rationale was based on a previous study conducted in mice by Cunningham and McMahon (2013) in which changing the nicotine training dose did not impact the potency of varenicline to substitute for nicotine as would have been predicted. This suggested that the discriminative stimulus effects of varenicline could not be attributed to only α4β2* nAChRs. Furthermore, DHβE unexpectedly failed to antagonize the discriminative stimulus effects of varenicline in nicotine-trained mice (Cunningham and McMahon 2013). Because this result was novel and the interpretations of that result were contrary to the view that varenicline and nicotine only differ in efficacy at α4β2 nACHRs (Rollema et al. 2007a), it was important to replicate Cunningham and McMahon (2013). Finally, C57BL/6J mice have been used extensively in drug discrimination experiments to examine the pharmacology of nicotine and related compounds (Stolerman et al. 1999; Cunningham and McMahon 2011; Rodriguez et al. 2014; Moerke et al. 2016).
Materials and Methods
Subjects
Sixteen male C57BL/6J mice (The Jackson Laboratory, Bar Harbor, ME) were purchased at 8 weeks of age and were individually housed under a 14/10-h light/dark cycle. Mice were fed 2.5 g of food (Dustless Precision Pellets 500 mg, Rodent Grain-Based Diet, Bio-Serv, Frenchtown, NJ) immediately after experimental sessions to maintain body weights at 85% of the normal growth curve provided by the vendor. Water was continuously available in the home cage. Operant conditioning sessions were conducted 7 days per week. These studies were approved by the Institutional Animal Care and Use Committee of The University of Texas Health Science Center at San Antonio and were conducted in accordance with the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals (Institute for Laboratory Animal Research 2011).
Drugs
Drugs were nicotine hydrogen tartrate salt, with a pH adjusted to 7 (Sigma-Aldrich, St. Louis, MO), varenicline dihydrochloride (National Institute on Drug Abuse, Rockville, MD), ±epibatidine dihydrochloride hydrate (Sigma-Aldrich), cytisine (Atomole Scientific, Hubei, China), 2-fluoro-3-(4-nitro-phenyl)deschloroepibatidine (RTI-102; Dr. F. Ivy Carroll, Research Triangle Institute, NC), N-[(3R)-1-azabicyclo[2.2.2]oct-3-yl]-4-chlorobenzamide hydrochloride (PNU-282987; National Institute on Drug Abuse), ±mecamylamine hydrochloride (Waterstone Technology, LLC, Carmel, IN), dihydro-β-erythroidine hydrobromide (DHβE; Tocris Biosciences, Bristol, UK), methyllycaconitine citrate salt (MLA; National Institute on Drug Abuse), 18-methoxycoronaridine hydrochloride (18-MC; Obiter Research, Champaign, IL), midazolam hydrochloride (Ben Venue Labs, Bedford, OH), and cocaine hydrochloride (Sigma-Aldrich). Nicotine, varenicline, epibatidine, cytisine, RTI-102, midazolam, and DHβE were administered subcutaneously. PNU-282987, MLA, mecamylamine, 18-MC, and cocaine were administered intraperitoneally. All drugs were administered in a volume of saline equivalent to 10 ml/kg. Drug doses were expressed as the weight of the forms listed above except for nicotine dose, which was expressed as the base weight.
Apparatus
Operant conditioning chambers (ENV-307A-CT, MedAssociates, St. Albans, VT) were kept in ventilated, sound-attenuating boxes. Each operant conditioning chamber contained a house light, a recessed 2.2 cm-diameter hole for reinforcer presentation on one wall, and three identical holes horizontally arranged and spaced 5.5 cm apart on the opposite wall. The center of each hole was 1.6 cm from the floor. When the leftmost and rightmost holes were illuminated, disruption of a photobeam in a hole resulted in access to 0.01 cc of 50% v/v unsweetened condensed milk/water through the single hole on the opposite wall. The operant conditioning chambers were connected to a computer through an interface (MED-SYST-8, MedAssociates). Med-PC software (MedAssociates) controlled experimental events and provided a record of responses. A white-noise generator was used to mask external sounds.
Discrimination Training
The operant conditioning procedure in this study has previously been used for other nicotine discrimination studies (Cunningham and McMahon 2013; Rodriguez et al. 2014; Moerke et al. 2016). After 7 days of habituation to the animal housing room, operant conditioning sessions were conducted during the light period at the same time each day, 7 days per week. Mice were placed in the operant conditioning chamber for 60 min with two holes illuminated. Disruption of a photobeam in either the right or left hole resulted in 10 s access to milk. While milk was available for 10 s a light on the ceiling of the chamber was illuminated and lights in the holes containing the photobeams were extinguished. Photobeam disruptions during milk presentation had no programmed consequence. After mice earned 100 reinforcers per session for four consecutive sessions, the response requirement was systematically increased to a fixed ratio 10 (FR10) and sessions were shortened to 25 min in length. Sessions were divided into a 10-min pretreatment, during which any disruption of the photobeams had no programmed consequence, followed by 15 min of milk availability under the FR10 schedule.
One group of 8 mice was trained to discriminate nicotine (1 mg/kg) while a second group of 8 mice was trained to discriminate varenicline (3.2 mg/kg). This dose of varenicline was selected because increasing the dose by a quarter log unit markedly disrupts operant conditioning responding (Cunningham and McMahon 2013). Because there are currently no published papers in which varenicline has been trained as a discriminative stimulus, a relatively large dose of varenicline that did not decrease operant responding was selected to increase the likelihood that varenicline would exert stimulus control over choice behavior. Training sessions consisted of administration of either saline or the training dose 10 min prior to milk being available. During the pretreatment period, all lights in the operant conditioning chamber were extinguished. Following the 10 min pretreatment, lights in the left and right holes in which responses could be made were illuminated signaling the beginning of the operant conditioning session. During saline-training sessions, 10 responses in the correct hole (left for half of the mice and right for the other half) resulted in presentation of milk. During drug-training sessions, 10 responses in the opposite hole were required to obtain milk delivery. Correct holes remained the same for each mouse throughout the study. The training sequence included two consecutive days of drug training followed by two consecutive days of saline training. To pass a training session, two criteria had to be satisfied. First, a minimum of 80% of the total responses during the 15-min response period needed to be correct. Second, any incorrect responses made prior to delivery of the first reinforcer needed to be less than 10 (i.e., the first reinforcer had to be obtained after less than 19 responses total in both holes). If the mouse only partially completed the FR requirement in the correct hole, responses in the incorrect hole did not reset the ratio requirement in the correct hole. The first test was conducted once five consecutive or six out of seven training sessions had satisfied the criteria. Test criteria were determined on an individual basis, i.e., as soon as a mouse met the criteria, testing commenced in that mouse. After the first test, training alternated between one saline- and one drug-training session. If a mouse failed, the training condition was repeated. However, the next training session was switched to the alternative so that there were no more than two consecutive days of either training condition. Subsequent test sessions were conducted after the mice passed three consecutive training sessions.
Discrimination Testing
Test sessions were identical to training sessions, except that 10 responses in either hole resulted in presentation of milk. Dose-response tests were conducted with the respective training drugs, followed by dose-response tests with the nAChR agonists varenicline (in the nicotine discrimination), nicotine (in the varenicline discrimination), epibatidine, cytisine, RTI-102, PNU-282987, cocaine, and midazolam. The dose-response function for one drug was completed before initiating tests with another. The order in which the drugs were tested was not systematic, but the doses of each drug were studied in ascending order. Dose-response functions were generated in ascending order. To minimize order effects, only one dose or dose combination was studied per session, and another dose or dose combination was studied only after a minimum of 3 training sessions. This potential limitation could be addressed by using non-systematically ordered dosing sequences in future experiments. Drugs were administered from doses that were ineffective (i.e, produced less than 20% drug-appropriate responding) up to doses that produced greater than or equal to 80% drug-appropriate responding, antagonized drug-appropriate responding produced by a second drug, decreased response rate to less than 20% of the saline control, or were deemed potentially toxic. Dose-response functions for all the nAChR agonists were conducted prior to testing with antagonists. Cocaine and midazolam were studied after all the nAChR agonists. For previously studied drugs (Cunningham and McMahon 2013; Rodriguez et al. 2014), doses were selected based on their capacity to substitute for, or antagonize, a 1 mg/kg nicotine discriminative stimulus. Additional doses were examined as needed so that a full dose-response function was established. DHβE (1 mg/kg and 3.2 mg/kg), MLA (10 and 17.8 mg/kg), mecamylamine (3.2 mg/kg), and 18-MC (17.8 and 32 mg/kg) were combined with various doses of nicotine and varenicline. To examine whether partial substitution of varenicline for nicotine was due to differences in efficacy at a receptor subtype, varenicline (1.78 and 3.2 mg/kg) was combined with the training dose (1 mg/kg) of nicotine. Varenicline and epibatidine were studied in combination with DHβE (3.2 mg/kg) in the nicotine discrimination, and nicotine and epibatidine were studied in combination with DHβE (3.2 mg/kg) in the varenicline discrimination. Dose-response functions for the training drugs were determined twice: once before and again after tests with other drugs. All drugs were administered immediately prior to placing mice in the box for the 10-min pretreatment with the exception of DHβE (administered 5 min before placement in the operant conditioning chamber) and mecamylamine, MLA, and 18-MC (administered 10 min before placement in the operant conditioning chamber). When the antagonists were studied in combination with an agonist, the agonist was administered after the antagonist, immediately before placement into the operant conditioning chamber.
Data analyses
Discrimination and response rate data are expressed as the mean ± standard error of the mean (S.E.M.). Discrimination data are expressed as the percentage of drug-appropriate responses out of the total number of responses. Response rate is expressed as a percentage of control for each mouse, defined as the mean response rate from the five saline training sessions immediately preceding the test. When the response rate is less than 20% of control for an individual mouse, discrimination data for that dose in that mouse are not plotted or analyzed. Discrimination data at a particular dose were plotted and analyzed only when all 8 mice responded greater than 20% of saline control. All response rate data are plotted and analyzed.
All statistics were conducted using GraphPad Prism version 5.0 for Windows (San Diego, CA). Dose-response data were fitted with straight lines using linear regression. The linear portion of a dose-response function, as determined per individual mouse, included not more than one dose producing less than 20% effect. Larger doses of nicotine, varenicline, epibatidine, cytisine, RTI-102, PNU-282987, midazolam, and cocaine were included up to the dose that increased drug-appropriate responding to greater than 80% or decreased response rate to less than 20% of control, or were deemed potentially toxic. The slopes and intercepts of different functions were compared with an F-ratio test. If the F-ratio value was significant, then the dose-response functions were considered significantly different from each other. When the mean effect of drug-appropriate responding was greater than 50%, the ED50 values and corresponding 95% confidence limits were calculated according to Tallarida (2000). If the 95% confidence limits of the ED50 values did not overlap, the drugs were considered to have significantly different potencies. The effects of antagonists alone were examined with ANOVA or t-test. Antagonism of nicotine and varenicline with mecamylamine and DHβE was examined with F-ratio tests, while antagonism of nicotine with varenicline, and nicotine and varenicline with MLA and 18-MC were examined using a two-way Student’s t-test. Drugs were considered to fully substitute for the training drug if they produced ≥80% drug-appropriate responding, and to partially substitute if they produced between 41–79% drug-appropriate responding. A one-way ANOVA with a Tukey’s Multiple Comparisons Test analyzed the rate decreasing effects of DHβE alone to the response rate of saline.
Results
Substitution Tests
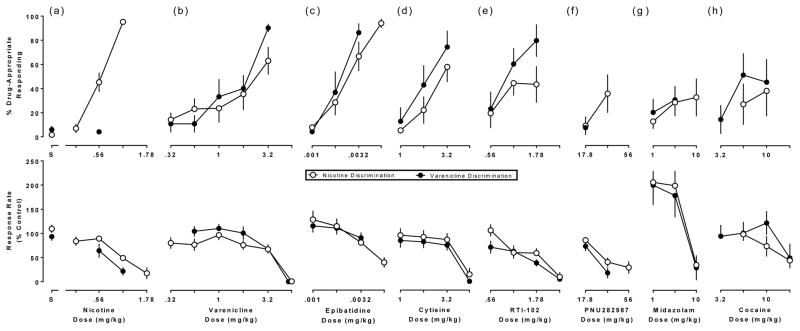
An average of two drug tests were conducted each week, for a total of two years. The first determination of the nicotine dose-response function was not significantly different from the subsequent dose-response determination (p>0.05), and the first determination of the varenicline dose-response function was not significantly different from the second. The determinations were averaged for further analyses. Saline produced a maximum of 2% and 6% drug-appropriate responding in the nicotine and varenicline discriminations, respectively (Fig. 1a, top). In varenicline-trained mice, nicotine did not substitute for the varenicline discriminative stimulus (Fig. 1a, top, filled circle). In nicotine-trained mice, varenicline only partially substituted for the nicotine discriminative stimulus (Fig. 1b, top, open circle). Epibatidine fully substituted for nicotine at a dose of 0.0056 mg/kg (Fig. 1c, top, open circle), while cytisine and RTI-102 partially substituted for the nicotine discriminative stimulus (Fig. 1d,e, top, open circles). PNU-282987, midazolam, and cocaine did not substitute for nicotine (Fig. 1f,g,h, top, open circles). In varenicline-trained mice, epibatidine and RTI-102 fully substituted for the varenicline discriminative stimulus (Fig. 1c and 1e, top, filled circles) while cytisine and cocaine partially substituted (Fig. 1d,h, top, filled circles). PNU-282987 and midazolam did not substitute for the varenicline discriminative stimulus (Fig.1f,g, top, filled circles). Table 1 summarizes maximum drug-appropriate responding, with ED50 values for discriminative stimulus effects and response rates.
Fig. 1. Effects of (a) nicotine, (b) varenicline, (c) epibatidine, (d) cytisine, (e) RTI-102, (f) PNU-282987, (g) midazolam, and (h) cocaine in separate groups of mice trained to discriminate 1 mg/kg of nicotine (open circles) or 3.2 mg/kg of varenicline (filled circles).
Top panels show drug-appropriate responding on the ordinate and drug dose on the abscissae, while bottom panels show response rate normalized to the saline control on the ordinate as a function of dose on the abscissae.
Table 1.
Percentage of drug-appropriate responding, and ED50 values for discriminative stimulus and rate-decreasing effects produced by test drugs in the nicotine (a) and varenicline (b) discriminations.
| (a) Nicotine Discrimination- | |||
|---|---|---|---|
| Test drug | % Drug-appropriate responding | Discriminative stimulus ED50 (95% CL) | Response rate ED50 (95% CL) |
| Saline | 1.8 | NA | NA |
| Nicotine | 95 | 0.57 (0.52–0.62) | 0.98 (0.76–1.3) |
| Varenicline | 63 | 1.9 (0.76–4.8) | 4.3 (1.6–11) |
| Epibatidine | 94 | 0.0025 (0.0019–0.0031) | 0.0051 (0.0033–0.0079) |
| Cytisine | 58 | 2.3 (1.8–2.9) | 4.3 (3.4–5.4) |
| RTI-102 | 44 | NA | 1.7 (1.2–2.4) |
| PNU-282987 | 36 | NA | 34 (25–46) |
| Midazolam | 33 | NA | 9.2 (6.3–13) |
| Cocaine | 39 | NA | 16 (5.9–44) |
| (b) Varenicline Discrimination- | |||
|---|---|---|---|
| Test drug | % Drug-appropriate responding | Discriminative stimulus ED50 (95% CL) | Response rate ED50 (95% CL) |
| Saline | 6.0 | NA | NA |
| Nicotine | 4.2 | NA | 0.69 (0.51–0.92) |
| Varenicline | 90 | 1.3 (0.79–2.3) | 3.5 (2.3–5.1) |
| Epibatidine | 86 | 0.0020 (0.0015–0.0027) | NA |
| Cytisine | 74 | 1.9 (1.3–2.8) | 3.8 (3.2–4.5) |
| RTI-102 | 80 | 0.92 (0.65–1.3) | 1.1 (0.74–1.8) |
| PNU-282987 | 7.7 | NA | 22 (19–27) |
| Midazolam | 31 | NA | 6.8 (2.6–17) |
| Cocaine | 51 | 7.4 (4.5–12) | 14 3.1–63) |
Combined Effects of Varenicline and Nicotine
In mice discriminating nicotine, varenicline (1.78 mg/kg) produced 35% drug-appropriate responding; this dose of varenicline combined with the training dose (1 mg/kg) of nicotine resulted in 98% nicotine-appropriate responding, which was not significantly different from that produced by the training dose in combination with saline (95%) (Table 2). A larger dose (3.2 mg/kg) of varenicline combined with the training dose of nicotine markedly decreased response rate (i.e., to 2% of control).
Table 2.
Percentage of drug-appropriate responding and response rate expressed as a percentage of the saline control for the training dose of nicotine (top) and varenicline (bottom) in combination with saline or other test drugs. t-tests compare the %drug-appropriate responding produced by drug combinations to the respective training doses alone.
| Nicotine Discrimination Assay | |||
|---|---|---|---|
| Test drug(s) (mg/kg) | Drug-appropriate responding (%) | Response rate (% control) | |
| Nicotine (1) | |||
| + Saline | 95% | 49% | |
| + Varenicline (1.78) | 98% | t3=0.76, p=0.50 | 21% |
| + Varenicline (3.2) | NA | 2% | |
| + MLA (10) | 94% | t5=0.59, p=0.58 | 41% |
| + MLA (17.8) | NA | 0% | |
| + 18-MC (17.8) | 82% | t5=1.60, p=0.17 | 26% |
| + 18-MC (32) | NA | 0% | |
| Varenicline Discrimination Assay | |||
|---|---|---|---|
| Test drug(s) (mg/kg) | Drug-appropriate responding (%) | Response rate (% control) | |
| Varenicline (3.2) | |||
| + Saline | 90% | 68% | |
| + MLA (10) | 98% | t4=1.77, p=0.15 | 41% |
| + MLA (17.8) | NA | 0% | |
| + 18-MC (17.8) | 97% | t4=1.22, p=0.29 | 26% |
| + 18-MC (32) | NA | 0% | |
NA, not applicable; response decreased to less than 20% of control
Mecamylamine, MLA, DHβE, and 18-MC in Combination with Saline, Nicotine, or Varenicline
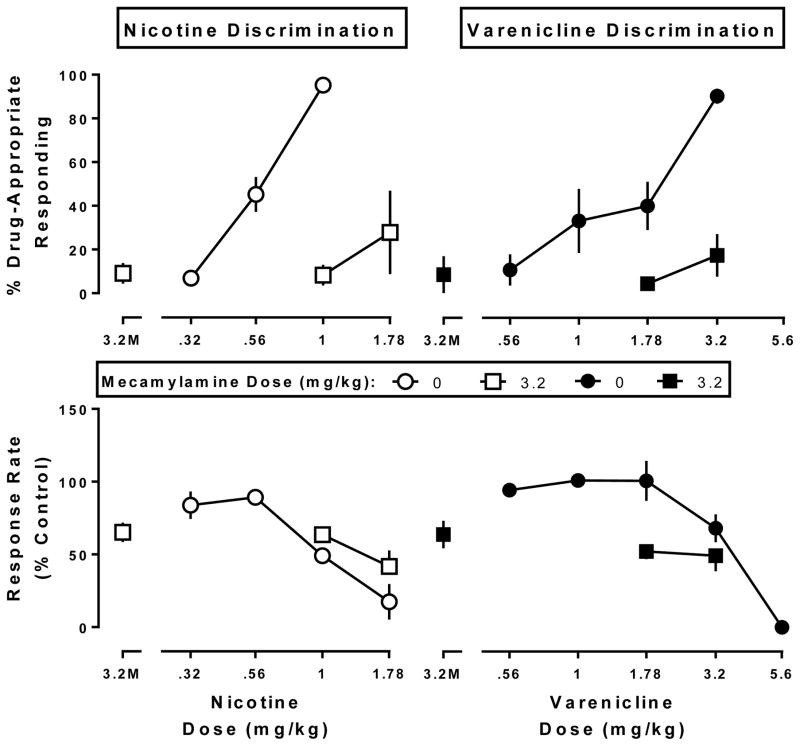
Mecamylamine (3.2 mg/kg) in combination with saline produced 9% drug-appropriate responding in both the nicotine and varenicline discrimination assays and significantly decreased response rate to 65% of control in the nicotine discrimination assay (t7=3.61, p<0.01) and 64% of control in the varenicline discrimination assay (t5=2.17, p<0.05) (Fig. 2, squares above 3.2M). Mecamylamine (3.2 mg/kg) antagonized the discriminative stimulus effects of nicotine (F2,28=32.5, p<0.0001), but did not significantly modify the rate-decreasing effects of nicotine (Fig. 2 left). Mecamylamine antagonized the discriminative stimulus effects of varenicline (F2,27=10.0, p=0.0006), but did not significantly modify the rate-decreasing effects of varenicline (Fig. 2 right).
Fig. 2. Antagonism of the nicotine discriminative stimulus (left panels) and the varenicline discriminative stimulus (right panels) by mecamylamine (3.2 mg/kg).
Top panels show drug-appropriate responding (ordinate) as a function of dose (abscissae), while the bottom panels display response rate normalized to saline control (ordinate) measured as a function of dose (abscissae). 3.2M, effects of mecamylamine alone (3.2 mg/kg).
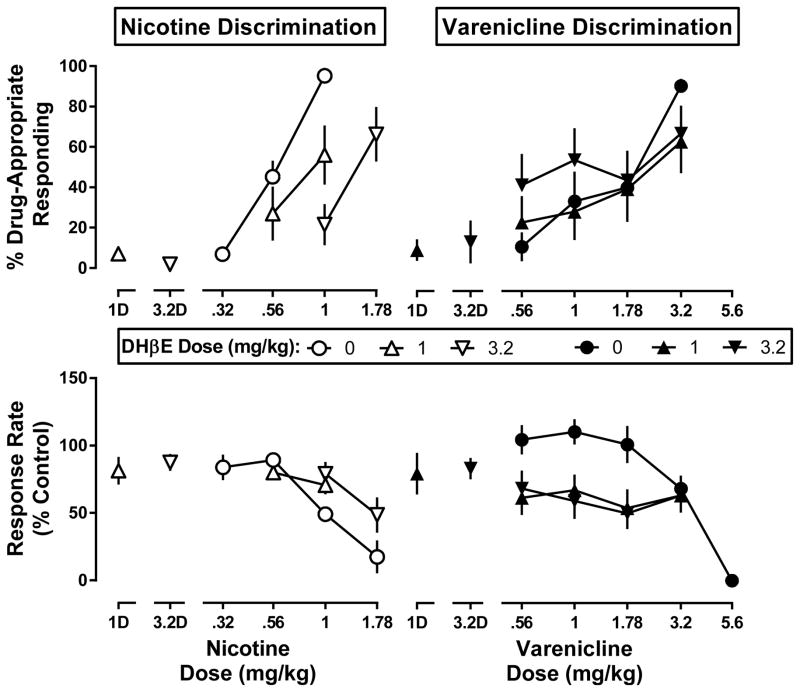
DHβE (1 and 3.2 mg/kg) in combination with saline produced no more than 13% drug-appropriate responding in the nicotine and varenicline discrimination assays and did not significantly decrease response rate (Fig. 3, symbols above 1D and 3.2D). DHβE produced significant rightward shifts in the nicotine discrimination dose-response functions (F2,30=8.28, p=0.0014 at 1 mg/kg, and F2,32=14.4, p<0.0001 at 3.2 mg/kg; Fig. 3 top left). DHβE did not significantly antagonize the effects of nicotine to decrease response rate at a dose of 1 mg/kg DHβE (Fig. 3 bottom left), but did antagonize the rate-decreasing effects of nicotine at a dose of 3.2 mg/kg DHβE (F2,35=5.06, p=0.0117; Fig. 3 bottom left). DHβE (1 mg/kg and 3.2 mg/kg) did not significantly modify the varenicline discrimination dose-response curve, but DHβE did significantly increase the potency of varenicline to produce rate-decreasing effects (F2,41=7.0, p=0.0025 at 1 mg/kg and F2,42=5.1, p=0.011 at 3.2 mg/kg) (Fig. 3 right).
Fig. 3. Effects of DHβE (1 and 3.2 mg/kg) in combination with the nicotine discriminative stimulus (left panels) and the varenicline discriminative stimulus (right panels).
Top panels show drug-appropriate responding (ordinate) as a function of dose (abscissa), while the bottom panels display response rate normalized to saline control (ordinate) measured as a function of dose (abscissa). 1D and 3.2D, effects of DHβE alone (1 and 3.2 mg/kg).
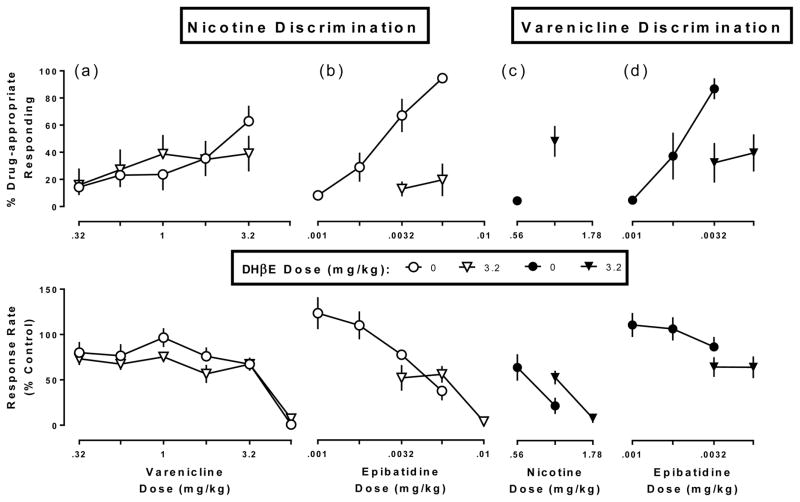
In mice trained to discriminate nicotine, DHβE (3.2 mg/kg) did not antagonize either the discriminative stimulus effects of varenicline (Fig. 4a top), or the rate-decreasing effects of varenicline (Fig. 4a bottom). In mice trained to discriminate nicotine, 3.2 mg/kg DHβE significantly antagonized the discriminative stimulus effects of epibatidine (Fig. 4b top; F2,29=14.0, p<0.0001) but not the rate-decreasing effects of epibatidine (Fig. 4b bottom). In mice trained to discriminate varenicline, DHβE did not significantly modify the nicotine dose-response function to produce rate-decreasing effects (Fig. 4c), yet when 1 mg/kg nicotine was tested in combination with 3.2 mg/kg DHβE, all 8 mice were able to respond above 20% of the control response rate, and varenicline-appropriate responding was 48%. Furthermore, in varenicline-trained mice, DHβE antagonized the discriminative stimulus effects of epibatidine (Fig. 4d top; F2,24=4.55, p=0.0211), but not the rate-decreasing effects of epibatidine (Fig. 4d bottom).
Fig. 4. Effects of DHβE (3.2 mg/kg) in combination with varenicline or epibatidine in the nicotine discrimination assay (leftmost and middle left panels), and in combination with nicotine and epibatidine in the varenicline discrimination assay (middle right and rightmost panels).
Top panels show drug-appropriate responding (ordinate) as a function of dose (abscissae), while the bottom panels display response rate normalized to saline control (ordinate) measured as a function of dose (abscissae).
MLA (10 mg/kg) did not significantly antagonize either the discriminative stimulus or rate-decreasing effects of the training doses in either the nicotine or varenicline discrimination assays (Table 2). A larger dose of MLA (17.8 mg/kg) in combination with the training doses suppressed responding. 18-MC (17.8 mg/kg) did not significantly antagonize the discriminative stimulus effects or rate-decreasing effects of either training dose (Table 2). Operant responding was suppressed when a larger dose of 18-MC (32 mg/kg) was combined with the training doses.
Discussion
Varenicline was successfully trained as a discriminative stimulus in mice. In mice trained to discriminate nicotine, varenicline only partially substituted for nicotine. However, in mice trained to discriminate varenicline, nicotine did not substitute for varenicline. Furthermore, the α4β2 nAChR antagonist DHβE dose-dependently antagonized the discriminative stimulus effects of nicotine, but not those of varenicline. While mecamylamine antagonized the discriminative stimulus effects of both nicotine and varenicline, nicotine and varenicline failed to cross-substitute for one another, suggesting the involvement of multiple receptors. Furthermore, it appears that greater tolerance to the rate-decreasing effects of 1 mg/kg nicotine occurred in mice trained to discriminate nicotine than in the varenicline discrimination. This was evidenced by all of the mice being able to respond at a sufficient rate at 1 mg/kg nicotine in the nicotine discrimination, but not in the varenicline discrimination. It is possible that the rate-decreasing effects of nicotine in the varenicline discrimination prevented nicotine from substituting for varenicline.
It might be predicted that drugs that partially substituted for nicotine would be more likely to substitute for varenicline, and that the potency of drugs to produce discriminative stimuli would be greater in the varenicline discrimination than in the nicotine discrimination if either: (a) the nicotine and varenicline training doses are functionally equivalent and varenicline had lower efficacy than nicotine, or (b) if the nicotine training dose was functionally larger than that of the varenicline training dose (Colpaert 1988). However, only the α4β2* nAChR partial agonist RTI-102 (Abdrakhmanova et al. 2006) differentially substituted for nicotine and varenicline. Cytisine, another low efficacy nAChR agonist (Grady et al. 2010), did not substitute in either assay and did not significantly differ in potency to produce drug-appropriate responding or decrease response-rate, as evidenced by overlapping 95% confidence limits for the respective ED50 values. Similarly, epibatidine fully substituted for both training drugs and did not differ in potency to produce drug-appropriate responding, suggesting some overlap in the receptors that mediate the discriminative stimulus effects of nicotine, varenicline, and epibatidine.
The nicotine and varenicline discrimination assays were pharmacologically selective inasmuch as both PNU-282987, cocaine, and midazolam did not fully substitute for either training drug. Failure of PNU-282987 to substitute for the nicotine discriminative stimulus was consistent with PNU-282987 being a selective α7 nAChR agonist (Brioni et al. 1996; Stolerman et al. 2004; Smith et al. 2007). PNU-282987 did not substitute for varenicline either, suggesting limited involvement of α7 nAChRs in both discrimination assays. The benzodiazepine midazolam and monoamine-transporter blocker cocaine did not produce high levels of nicotine- or varenicline-appropriate responding, suggesting that these assays are pharmacologically selective for nAChRs.
The nonselective nAChR antagonist mecamylamine (3.2 mg/kg) attenuated the effects of both nicotine and varenicline, suggesting that both drugs exert discriminative stimulus effects through nAChRs. Mecamylamine failed to antagonize the rate-decreasing effects of nicotine and varenicline, which contradicts previous studies demonstrating that the rate-decreasing effects of nicotine and varenicline were antagonized by mecamylamine (de Moura and McMahon 2016). However, this current study and the de Moura and McMahon (2016) study differed in the amount of nicotine and varenicline exposure the mice received. Repeated exposure to nicotine and varenicline in the current study, which was greater than in the de Moura and McMahon (2016) experiment prior to chronic nicotine treatment, may have diminished the capacity of mecamylamine to antagonize the rate-decreasing effects of nicotine and varenicline. Furthermore, 3.2 mg/kg mecamylamine has significant rate-decreasing effects on its own which may have limited its ability to antagonize the rate-decreasing effects of nicotine and varenicline. As demonstrated previously, the β2-containing nAChR antagonist DHβE attenuated the discriminative stimulus effects of nicotine (Stolerman et al. 1997; Cunningham and McMahon 2013). Contrary to Stolerman et al. (1997), DHβE (3.2 mg/kg) antagonized the rate-decreasing effects of nicotine, but this could be a function of different species (rats vs. mice), training dose (0.1–0.4 vs. 1 mg/kg nicotine), or schedule of reinforcement (tandem variable interval 1:FR10 vs FR10). DHβE did not antagonize the effects of varenicline in either the nicotine or varenicline discrimination assays. Failure of DHβE to antagonize varenicline in nicotine-trained mice has been demonstrated previously (Cunningham and McMahon 2013), but failure to antagonize varenicline in the varenicline discrimination is novel. It is possible that DHβE may not be a very effective nAChR antagonist, however, the most parsimonious explanation would be that α4β2* nAChRs are not the primary mechanism of action of varenicline. Consistent with this interpretation, varenicline failed to antagonize the discriminative stimulus effects of nicotine in the nicotine discrimination. If partial substitution of varenicline for nicotine was due to lower efficacy than nicotine, then varenicline would be expected to antagonize the discriminative stimulus effects of nicotine (Kenakin 1997; Kenakin 2002). Antagonism of a high efficacy agonist by a lower efficacy agonist has been demonstrated in rats discriminating fentanyl (Colpaert and Janssen 1984); cyclazocine and nalorphine partially substituted for and both dose-dependently antagonized the discriminative stimulus effects of fentanyl. This dual agonist/antagonist action of cyclazocine and nalorphine was an example of a competitive interaction at a common receptor of drugs with lower intrinsic activity (e.g. cyclazocine and nalorphine) than a training drug (e.g. fentanyl). Differential antagonism of nicotine and varenicline with DHβE was also replicated using other dependent measures (i.e., rate-decreasing and hypothermic effects), where DHβE antagonized nicotine but not varenicline (de Moura and McMahon 2016). However, other results suggest that α4β2* nAChRs play a role in the discriminative stimulus effects of varenicline: epibatidine substituted for the varenicline discriminative stimulus, and this was antagonized by DHβE in the varenicline discrimination. Substitution for varenicline by epibatidine, and antagonism of epibatidine by DHβE, as well as lack of antagonism of varenicline by DHβE imply an important role of α4β2* nAChRs and at the same time, a role for non-α4β2 nAChRs. However, failure of any of the other studied antagonists (e.g., MLA and 18-MC) to antagonize varenicline suggests that further study is needed to determine what the other nAChR subtypes may be.
DHβE has been shown to antagonize the discriminative stimulus effects of nicotine (Stolerman et al. 1997; Cunningham and McMahon 2013; Rodriguez et al. 2013) as well the effects of nicotine on simple schedule-controlled responding (Cunningham and McMahon 2011; de Moura and McMahon 2016) and body temperature (Rodriguez et al. 2013; de Moura and McMahon 2016; Moerke et al. 2016). It is interesting to note that in the varenicline discrimination, 1 mg/kg nicotine produced 4% varenicline-appropriate responding when studied alone, but in the presence of 3.2 mg/kg DHβE, 1 mg/kg nicotine produced 48% varenicline-appropriate responding. This result is consistent with the interpretation that the α4β2* nAChR mediated rate-decreasing effects of nicotine prevented it from substituting for varenicline. However, when the rate-decreasing effects of 1 mg/kg nicotine are antagonized, nicotine still does not fully substitute for the varenicline discriminative stimulus. Furthermore, results from this study and others demonstrate that epibatidine is consistently antagonized by DHβE, but varenicline is not (de Moura and McMahon 2016). Because this differential antagonism appeared consistent between different studies and dependent measures, the question arose as to how in an assay with a strong α4β2* nAChR component (e.g., the nicotine discrimination) does DHβE antagonize epibatidine but not varenicline, and in an assay in which DHβE fails to antagonize the training drug (e.g., the varenicline discrimination), how does DHβE antagonize the effects of epibatidine?
Both pharmacological (Smith et al. 2007; Desai and Bergman 2010) and transgenic approaches (Picciotto et al. 1998; Shoaib et al. 2002) strongly implicate the involvement of α4β2* nAChRs in the behavioral effects of nAChR agonists. However, the current findings highlight the importance of nAChRs besides α4β2* in the behavioral effects of varenicline, which is the most effective smoking cessation aid on the market today (Hays et al. 2008). Previous studies using either multiple nicotine training doses (Jutkiewicz et al. 2011; Cunningham and McMahon 2013) or cytisine as a training drug (Chandler and Stolerman 1997) have strongly implicated a role for multiple nAChR subtypes in the discriminative stimulus effects of nicotine, and failure of DHβE to antagonize varenicline in the nicotine discrimination is consistent with a possible contribution of non-α4β2* nAChRs to the discriminative stimulus effects of nicotine.
Previous studies with nicotine have demonstrated that training dose impacts the capacity of DHβE to antagonize the discriminative stimulus of nicotine, where DHβE is more likely to antagonize nicotine when a low training dose is used versus a high training dose (Jutkiewicz et al. 2011). A training dose of 3.2 mg/kg varenicline could be so high of a training dose that DHβE is limited in its ability to antagonize varenicline. This warrants further investigation by attempting to train a lower dose of varenicline as a discriminative stimulus.
Another possible explanation for the differential antagonism of nicotine and varenicline by DHβE is that varenicline has effects at other receptor types that overshadow antagonism by a subtype selective antagonist. Some drugs acting at more than one receptor subtype (e.g., nicotine) are postulated to have discriminative stimulus effects that can be divided into multiple, discrete components based on the differential contributions of the individual receptor subtypes. Stolerman and colleagues studied mixtures of pharmacologically distinct drugs (i.e., midazolam and nicotine) to better understand multi-component discriminations. When the potencies of the individual drugs to produce discriminative stimulus effects were taken into account, mixtures containing more effective doses of nicotine than midazolam increased the likelihood that nicotine alone would substitute and decreased the likelihood that midazolam alone would substitute (Garcha and Stolerman 1989). Furthermore, while both nicotine and midazolam would generalize to the discriminative stimulus effects of the mixture, mecamylamine, but not flumazenil, would antagonize the discriminative stimulus effects of the large dose nicotine, small dose midazolam mixture (White and Stolerman 1994). The effects of varenicline were not antagonized by an α7 nAChR antagonist (MLA) or an α3β4 nAChR antagonist (18-MC). These results may be an indication of the limitations of MLA and 18-MC as antagonists in vivo, which are drugs with the greatest affinity for α7 and α3β4 nAChRs, respectively (Grady et al. 2010). MLA, for instance, while having greatest affinity for α7 nAChRs (~100 fold greater than at β2 nAChRs), has similar potency to antagonize nAChR agonists at both α7 and α3β4 nAChRs (Grady et al., 2010). However, it is unlikely that the lack of antagonism of nicotine and varenicline by MLA is due to nonselective binding. If multiple nAChR subtypes mediate the varenicline discriminative stimulus, then actions at one or more of these nAChR subtypes may overshadow the actions at another, resulting in different probabilities of antagonism by subtype-selective antagonists (White and Stolerman 1994; Green and Grant 1998). This interpretation is speculative and would require further investigation with the conditional discrimination methods similar to those used to elucidate ethanol receptor pharmacology (Grant 1999).
The major finding of this study is that DHβE differentially antagonized the discriminative stimulus effects of nicotine and varenicline. Regardless of the training conditions (i.e., nicotine or varenicline), DHβE did not antagonize the effects of varenicline. This study does not discount the importance of α4β2* nAChRs to the in vivo pharmacology of varenicline, but instead suggests that the discriminative stimulus effects are due to agonist actions at a combination of α4β2* and non-α4β2 nAChRs. From a clinical perspective, these results indicate that varenicline may be having effects at receptors that are not α4β2* nAChRs. The extent to which actions at non-α4β2 nAChRs limit the clinical effectiveness of varenicline is unclear, or whether non-α4β2* nAChRs contribute to any adverse effects. This study highlights the need to elucidate the receptor pharmacology of varenicline and characterize how the various nAChRs may contribute to the in vivo effects produced by varenicline.
Acknowledgments
Funding
This work was supported by the National Institutes of Health National Institute on Drug Abuse [Grant DA25267].
The authors thank Dr. F. Ivy Carroll for generously providing RTI-102, and Ursula Villarreal-Moura for editorial assistance.
Footnotes
Disclosures
Authors have no conflicts of interest.
References
- Abdrakhmanova GR, Damaj MI, Carroll FI, Martin BR. 2-fluoro-3-(4-nitro-phenyl)deschloroepibatidine is a novel potent competitive antagonist of human neuronal α4β2 nAChRs. Mol Pharmacol. 2006;69:1945–1952. doi: 10.1124/mol.105.021782. [DOI] [PubMed] [Google Scholar]
- Brioni JD, Kim DJ, O’Neill AB. Nicotine cue: lack of effect of the α7 niconic receptor antagonist methyllycaconitine. Eur J Pharmacol. 1996;301:1–5. doi: 10.1016/0014-2999(96)00010-6. [DOI] [PubMed] [Google Scholar]
- Chandler CJ, Stolerman IP. Discriminative stimulus properties of the nicotinic agonist cytisine. Psychopharmacology (Berl) 1997;129:257–264. doi: 10.1007/s002130050188. [DOI] [PubMed] [Google Scholar]
- Colpaert FC, Janssen PA. Agonist and antagonist effects of prototype opiate drugs in rats discriminating fentanyl from saline: characteristics of partial generalization. J Pharmacol Exp Ther. 1984;230:193–199. [PubMed] [Google Scholar]
- Colpaert FC. Intrinsic activity and discriminative effects of drugs. In: Colpaert FC, Balster RL, editors. Transduction mechanisms of drug stimuli. Springer; Berlin Heidelberg, Berlin: 1988. pp. 154–160. [DOI] [PubMed] [Google Scholar]
- Colpaert FC. Drug discrimination in neurobiology. Pharmcol Biochem Behav. 1999;64:337–345. doi: 10.1016/s0091-3057(99)00047-7. [DOI] [PubMed] [Google Scholar]
- Cunningham CS, McMahon LR. The effects of nicotine, varenicline, and cytisine on schedule- controlled responding in mice: differences in α4β2 nicotinic receptor activation. Eur J Pharmacol. 2011;654:47–52. doi: 10.1016/j.ejphar.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham CS, McMahon LR. Multiple nicotine training doses in mice as a basis for differentiating the effects of smoking cessation aids. Psychopharmacology (Berl) 2013;228:321–333. doi: 10.1007/s00213-013-3037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Moura FB, McMahon LR. Differential antagonism and tolerance/cross-tolerance among nicotinic acetylcholine receptor agonists: scheduled-controlled responding and hypothermia in C57BL/6J mice. Behav Pharmacol. 2016;27:240–248. doi: 10.1097/FBP.0000000000000233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai RI, Bergman J. Drug discrimination in methamphetamine-trained rats: effects of cholinergic nicotinic compounds. J Pharmacol Exp Ther. 2010;335:807–816. doi: 10.1124/jpet.110.173773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Food and Drug Administration. Public health advisory: FDA requires new boxed warnings for smoking cessations drugs Chantix and Zyban. [Accessed 9/30/2016];FDA.gov. 1999 http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm169988.htm.
- Garcha HS, Stolerman IP. Discrimination of a drug mixture in rats: role of training dose, and specificity. Behav Pharmacol. 1989;1:25–31. doi: 10.1097/00008877-198900110-00004. [DOI] [PubMed] [Google Scholar]
- Grady SR, Drenan RM, Breining SR, Yohannes D, Wageman CR, Fedorov NB, McKinney S, Whiteaker P, Bencherif M, Lester HA, Marks MJ. Structural differences determine the relative selectivity of nicotinic compounds for native α4β2*-, α6β2*-, α3β4*- and α7-nicotinic acetylcholine receptors. Neuropharmacology. 2010;58:1054–1066. doi: 10.1016/j.neuropharm.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant K. Strategies for understanding the pharmacological effects of ethanol with drug discrimination procedures. Pharmacol Biochem Behav. 1999;64:261–267. doi: 10.1016/s0091-3057(99)00075-1. [DOI] [PubMed] [Google Scholar]
- Green KL, Grant KA. Evidence for overshadowing by components of the heterogeneous discriminative stimulus effects of ethanol. Drug Alcohol Depend. 1998;52:149–159. doi: 10.1016/s0376-8716(98)00086-6. [DOI] [PubMed] [Google Scholar]
- Hajós M, Hurst RS, Hoffman WE, Krause K, Wall TM, Higdon NR, Groppi VE. The selective α7 nicotinic acetylcholine receptor agonist PNU-282978 [N-[(3R)-1-azabicyclo[2.2.2]octy-3-yl]-4-chlorobenzamine hydrochloride] enhances GABAergic synaptic activity in brain slices and restores auditory gating deficits in anesthetized rats. J Pharmacol Exp Ther. 2005;312:1213–1222. doi: 10.1124/jpet.104.076968. [DOI] [PubMed] [Google Scholar]
- Hays JT, Ebbert JO, Sood A. Efficacy and safety of varenicline for smoking cessation. Am J Med. 2008;121:S32–S42. doi: 10.1016/j.amjmed.2008.01.017. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Shiffman S, Callas P, Zhang J. A meta-analysis of the efficacy of over-the-counter nicotine replacement. Tob Control. 2003;12:21–27. doi: 10.1136/tc.12.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute for Laboratory Animal Research. Guide for the Care and Use of Laboratory Animals. 8. Institute for Laboratory Animal Research, Division of Earth and Life Sciences, National Research Council; Washington, DC: 2011. [Google Scholar]
- Jutkiewicz EM, Brooks EA, Kynaston AD, Rice KC, Woods JH. Patterns of nicotinic receptor antagonism: nicotine discrimination studies. J Pharmacol Exp Ther. 2011;339:194–202. doi: 10.1124/jpet.111.182170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenakin TP. Pharmacologic analysis of drug-receptor interaction. Philadelphia: Lippincott-Raven; 1997. [Google Scholar]
- Kenakin T. Drug efficacy at G protein-coupled receptors. Annu Rev Pharmacol Toxicol. 2002;42:349–379. doi: 10.1146/annurev.pharmtox.42.091401.113012. [DOI] [PubMed] [Google Scholar]
- Kuehne ME, He L, Jokiel PA, Pace CJ, Fleck MW, Maisonneuve IM, Glick SD, Bidlack JM. Synthesis and biological evaluation of 18-methoxycoronaridine congeners. Potential antiaddiction agents. J Med Chem. 2003;46:2716–2730. doi: 10.1021/jm020562o. [DOI] [PubMed] [Google Scholar]
- LeSage MG, Shelley D, Ross JT, Carroll FI, Corrigall WA. Effects of the nicotinic receptor partial agonists varenicline and cytisine on the discriminative stimulus effects of nicotine in rats. Pharmacol Biochem Behav. 2009;91:461–467. doi: 10.1016/j.pbb.2008.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lummis SC, Thompson AJ, Bencherif M, Lester HA. Varenicline is a potent agonist of the human 5- hydroxytryptamine3 receptor. J Phamacol Exp Ther. 2011;339:125–131. doi: 10.1124/jpet.111.185306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihalak KB, Carroll FI, Luetje CW. Varenicline is a partial agonist at alpha4beta2 and a full agonist at alpha7 neuronal nicotinic receptors. Mol Pharmacol. 2006;70:801–805. doi: 10.1124/mol.106.025130. [DOI] [PubMed] [Google Scholar]
- Papke RL, Dwoskin LP, Crooks PA, Zheng G, Zhang Z, McIntosh JM, Stokes C. Extending the analysis of nicotinic receptor antagonists with the study of alpha6 nicotinic receptor subunit chimeras. Neuropharmacology. 2008;54:1189–1200. doi: 10.1016/j.neuropharm.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picciotto MR, Zoli M, Rimondini R, Léna C, Marubio LM, Pich EM, Fuxe K, Changeux JP. Acetylcholine receptors containing the β2 subunit are involved in the reinforcing properties of nicotine. Nature. 1998;391:173–177. doi: 10.1038/34413. [DOI] [PubMed] [Google Scholar]
- Rollema H, Chambers LK, Coe JW, Glowa J, Hurst RS, Lebel LA, Lu Y, Mansbach RS, Mather RJ, Rovetti CC, Sands SB, Schaeffer E, Schulz DW, Tingley FD, III, Williams KE. Pharmacological profile of the α4β2 nicotinic acetylcholine receptor partial agonist varenicline, an effective smoking cessation aid. Neuropharmacology. 2007a;52:985–994. doi: 10.1016/j.neuropharm.2006.10.016. [DOI] [PubMed] [Google Scholar]
- Rollema H, Coe JW, Chambers LK, Hurst RS, Stahl SM, Williams KE. Rationale, pharmacology and clinical efficacy of partial agonists of α4β2 nACh receptors for smoking cessation. Trends Pharmacol Sci. 2007b;28:316–325. doi: 10.1016/j.tips.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Shoaib M, Gommans J, Morley A, Stolerman IP, Grailhe R, Changeux JP. The role of nicotinic receptor beta-2 subunits in nicotine discrimination and conditioned taste aversion. Neuropharmacology. 2002;42:530–539. doi: 10.1016/s0028-3908(01)00194-0. [DOI] [PubMed] [Google Scholar]
- Smith JW, Mogg A, Tafi E, Peacey E, Pullar IA, Szekeres P, Tricklebank M. Ligands selective for α4β2 but not α3β4 or α7 nicotinic receptors generalise to the nicotine discriminative stimulus in the rat. Psychopharmacology (Berl) 2007;190:157–170. doi: 10.1007/s00213-006-0596-8. [DOI] [PubMed] [Google Scholar]
- Stolerman IP, Chandler CJ, Garcha HS, Newton JM. Selective antagonism of behavioural effects of nicotine by dihydro-β-erythroidine in rats. Psychopharmacology (Berl) 1997;129:390–397. doi: 10.1007/s002130050205. [DOI] [PubMed] [Google Scholar]
- Stolerman IP, Naylor C, Elmer GI, Goldberg SR. Discrimination and self-administration of nicotine by inbred strains of mice. Psychopharmacology (Berl) 1999;141:297–306. doi: 10.1007/s002130050837. [DOI] [PubMed] [Google Scholar]
- Stolerman IP, Chamberlain S, Bizarro L, Fernandes C, Schalkwyk L. The role of nicotinic receptor α7 subunits in nicotine discrimination. Neuropharmacology. 2004;46:363–371. doi: 10.1016/j.neuropharm.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Tallarida RJ. Drug Synergism and Dose-Effect Data Analysis. Chapman Hall/CRC Press; Boca Raton: 2000. [Google Scholar]
- Ward JM, Cockcroft VB, Lunt GG, Smillie FS, Wonnacott S. Methyllycaconitine: a selective probe for neuronal alpha-bungarotoxin binding sites. FEBS Lett. 1990;270:45–48. doi: 10.1016/0014-5793(90)81231-c. [DOI] [PubMed] [Google Scholar]
- White JA, Stolerman IP. Antagonism of a nicotine plus midazolam discriminative cue in rats. Behav Pharmacol. 1994;5:351–355. doi: 10.1097/00008877-199406000-00013. [DOI] [PubMed] [Google Scholar]