Abstract
Purpose
We sought to determine the presence of germ cells in the gonads of patients with disorders of sex development to establish whether preservation of germ cells for future fertility potential is possible. We hypothesized that germ cells are present but vary by age and diagnosis.
Materials and Methods
We reviewed histology from patients with disorders of sex development who underwent gonadectomy/biopsy from 2002 to 2014 at a single institution for pathological classification of the gonad, composition of gonadal stroma and germ cell presence.
Results
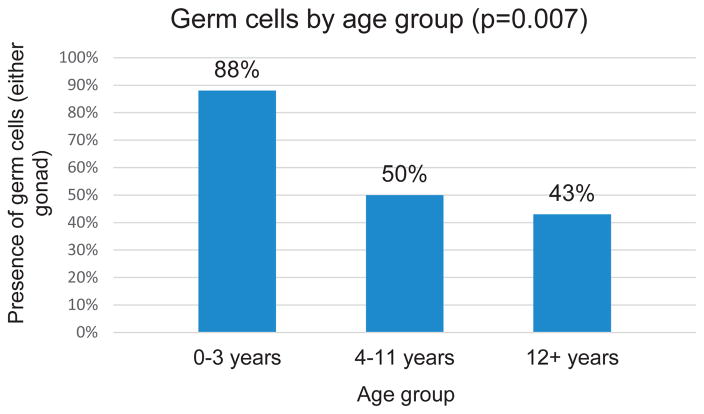
A total of 44 patients were identified and germ cells were present in 68%. The presence and average number of germ cells per mm2 were analyzed by gonad type and diagnosis. By gonad type all ovotestes, most testes, ovaries and dysgenetic testes, and 15% of streak gonads had germ cells present. By diagnosis germ cells were present in all patients with complete androgen insensitivity syndrome, Denys-Drash syndrome, SRY mutation, mixed gonadal dysgenesis, ovotesticular conditions and StAR (steroid acute regulatory protein) deficiency, in some patients with persistent müllerian duct syndrome, XO/XY Turner syndrome and disorders of sex development not otherwise specified, and in none with complete or partial gonadal dysgenesis. Germ cells were present in the gonads of 88% of patients 0 to 3 years old, 50% of those 4 to 11 years old and 43% of those older than 12 years.
Conclusions
Germ cells were present in the majority of our cohort and the presence decreased with age. This novel, fertility driven evaluation of germ cell quantity in a variety of disorders of sex development suggests that fertility potential may be greater than previously thought. Further studies must be done to evaluate a larger population and examine germ cell quality to determine the viability of these germ cells.
Keywords: testis, ovary, disorders of sex development, fertility, germ cells
Differences (disorders) of sex development are conditions in which there is incongruence among chromosomal, gonadal and phenotypic sex of an individual.1 The 2006 International Consensus Conference on intersex disorders acknowledged controversial issues and made management recommendations.2 The 2016 update addressed continuing change, including that the risk of GC neoplasia can be stratified by diagnosis and immunohistochemical findings in the biopsy.3 Also new to this document was a paragraph on fertility but with recognition that data about the best FP options remain inadequate.4,5
Progress in FP has been spurred through oncofertility, a field established to preserve fertility for patients with cancer undergoing treatments leading to infertility.6,7 Use of these FP techniques could be expanded to individuals with DSD. Given the progress in risk stratification for neoplasia and the availability of emerging fertility techniques, the conventional gonadectomy approach in the DSD population must be rethought. We may be able to offer fertility to the next generation of patients with DSD.
However, unlike the oncology population, individuals with DSD often have inherent subfertility. Their infertility risks are due to 1) abnormal gonadal development with progressive gonadal failure, 2) gonadectomy for malignancy risk, 3) abnormal hormone production, resulting in impaired gamete production, 4) discordance between gonadal type and gender identity, leading to an assumption of infertility, and 5) anatomical barriers. Furthermore, there is a risk of genetic transmission of the DSD phenotype to offspring, which may be addressed with pre-implantation genetic screening but must be carefully considered.
If FP is to be used to enable genetic offspring in the DSD population, fertility potential must be established. Fertility potential in this context refers to having cellular precursors to functional gametes, which may allow for fertility in the individual in the future if the cells mature in vivo, or if scientific techniques develop to mature them in vitro. It should not be considered guaranteed fertility.
Previous studies evaluated GCs in select populations8–12 but the goal of this pilot study was to determine the presence of GCs in the gonads of individuals with a broad range of DSDs as a possible indicator of fertility potential. Additionally, we examined the relationship between age and the presence of GCs in this population.
MATERIALS AND METHODS
Following institutional review board approval, patients 0 to 18 years old with DSD who underwent gonadectomy or biopsy at our institution from 2002 to 2014 were identified and included in study. Control gonads were obtained from patients who underwent gonadectomy for benign processes and from autopsy specimens (table 1). Patients were identified via a computer database search of surgical and pathology records. The hematoxylin and eosin stained slides were reviewed by a single pathologist (MKF) for pathological gonad classification and quantification of GCs.
Table 1.
Control population with no exogenous hormone exposure
| Control Gonads (Tanner stage) | No. Pts (%) | Gonadectomy Reason | No. Pts (%) |
|---|---|---|---|
| Ovary (20 ovaries): | 14 | ||
| Prepubertal | 11 (69) | Ovarian cryopreservation | 7 (50) |
| Tanner 2 | 2 (13) | Autopsy | 6 (42) |
| Tanner 3 | 1 (6) | Ovarian hemorrhagic cyst | 1 (7) |
| Tanner 5 | 5 (31) | Unknown | 1 (7) |
| Testis (22 testes ): | 16 | ||
| Prepubertal | 13 (81) | Autopsy | 6 (38) |
| Tanner 2 | 1 (6) | Orchiectomy for mass/tumor | 6 (25) |
| Tanner 3 | 1 (6) | Cryopreservation | 2 (13) |
| Unknown | 1 (6) | Biopsy of lesion/exploration | 2 (13) |
Pathological Gonad Classification
The overall morphological classification of the gonads was divided into 6 categories, including testis, ovary, dysgenetic testis, streak gonad, ovotestis and no gonad present. Dysgenetic testes were defined by the presence of seminiferous tubules or cords containing SCs with or without GCs. The tubules/cords are usually separated by a loose stroma. They appear disorganized, show complex branching patterns and variable sizes, and can extend directly to the often thin or absent tunica albuginea. Streak gonads were defined as those composed predominantly of fibrous and/or ovarian stroma with diminished or absent GCs. Ovotestes were defined as gonads composed of testicular and ovarian tissue. The gonads of patients with CAIS have a testicular morphology, often with Sertoli cell adenomas and/or hamartomatous nodules distorting the histological architecture.8,11,13 In this study GC assessment was performed on the compressed testicular tissue found between these nodules.
Germ Cell Quantification
GC morphology and cell counts were derived from hematoxylin and eosin stained sections. The average number of GCs per mm2 was calculated using the highest and lowest mm2 fields and 8 additional random fields for a total of 10 fields. In some small specimens with fewer than 10 fields the average of available fields was calculated.
In the ovaries GCs were more easily defined as they were usually surrounded by follicular cells. In some DSD cases primordial GCs were seen without surrounding follicular cells. In the testes definitive GC identification was more difficult. GCs located at the base of the seminiferous tubules were more easily distinguished from SCs due to the distinct morphology. However, GCs found away from the tubule base had a gonocyte appearance with some resembling SCs. In this study we did not distinguish between gonocytes and mature GCs in the testes. Atypical appearing GCs with pyknotic nuclei or “smudgy” chromatin with or without hypereosinophilic cytoplasm were also identified in all types of gonads, including controls.
Unless clearly apoptotic or necrotic, definitive GCs with atypical nuclear or cytoplasmic features were included. If multiple gonads were available, each gonad from the same patient was independently scored and classified. The gonad with the higher average GC count was used in the analysis for all continuous variables.
Statistical Analysis
Bivariate tests of association were performed using the Fisher exact test and Student t-test. The relationship between age and the average number of GCs per mm2 in each gonad was assessed using a linear regression model. We used the logarithm of the average GC count per mm2 to normalize the residuals. The assumptions of the model were checked by plotting the residuals. All statistical analysis was performed using SAS® Enterprise Guide®, version 7.1 with 2-tailed p <0.05 considered statistically significant.
RESULTS
General
Of the 44 patients with DSD gonadal biopsy, biopsy and excision, excision and autopsy were done in 11, 4, 28 and 1, respectively. Indications for surgery were diagnostic biopsy, gonadectomy for neoplasia, gonadectomy for the risk of neoplasia or unknown. Two patients underwent gonadal procedures at 2 discrete time points. At surgery the average age was 76.4 months (median 34, range 0 to 216).
Seven patients had a total of 8 diagnoses of neoplasia (table 2). Of the 44 patients the Tanner stage of breast or testicular development at surgery was stage 1 in 32, stage 2 in 3, stage 3 in 4, stage 4 in 1, stage 5 in 2 and unknown in 24. Three patients had exposure to exogenous estrogen, including 1 with Tanner stage 1 who was on Premarin® for 3 months and 2 with Tanner stage 2 who were on estradiol for 12 and 14 months, respectively. No patients had exposure to exogenous testosterone.
Table 2.
Gonadal neoplasia
| DSD Diagnosis (neoplasia type) | Age at Neoplasia Diagnosis (mos) |
|---|---|
| XO/XY Turner syndrome: | |
| Bilat gonadoblastoma | 145 |
| Gonadoblastoma + dysgerminoma in lt streak ovary | 192 |
| Partial gonadal dysgenesis (dysgerminoma in gonadoblastoma) | 200 |
| Mixed gonadal dysgenesis: | |
| Gonadoblastoma in streak gonad | 9 |
| Gonadoblastoma in testicular tissue with ovarian stroma | 191 |
| Ambiguous genitalia (yolk sac tumor, mature teratoma) | 146 |
| Diagnosis unknown (mixed intratubular germ cell neoplasia + gonadoblastoma in dysgenetic testis) | 215 |
GC Analysis
Overall, GCs were present in 30 of the 44 patients (68%).
By Gonad Type
GCs were present in 6 of 6 ovotestis cases (100%), 28 of 37 testes (75%), 9 of 11 ovaries (81%), 11 of 15 dysgenetic testes (73%) and 7 of 42 streak gonads (15%) (table 3).
Table 3.
Germ cells by gonad type
| Gonad Type | % GC Presence (No./total No.) | Mean ± SD % Atypia (range) | Mean ± SD No. GCs/mm2
|
|
|---|---|---|---|---|
| Ovarian | Testicular | |||
| Testis | 75 (28/37) | 14.8 ± 9.8 (2.5–45) | Not applicable | 79 ± 101 (1–286) |
| Ovary | 81 (9/11) | 19 ± 18.2 (3–53) | 70 ± 74 (3–190) | Not applicable |
| Dysgenetic testis | 73 (11/15) | 38.3 ± 19.1 (14–71) | Not applicable | 23 ± 15 (5–52) |
| Streak gonad | 15 (7/42) | 38.3 ± 18.8 (19–71) | 96 ± 185 (1–420) | 2 ± 0 |
| Ovotestis | 100 (6/6) | 23.5 ± 5.0 (18–30) | 190 ± 37 (150–230) | 24 ± 38 (5–100) |
Finding no gonad on pathology evaluation was not applicable.
By Diagnosis
All 6 patients with CAIS, the 1 with Denys-Drash syndrome, all 6 with MGD, the 1 with StAR deficiency, the 1 with urogenital sinus abnormality and the 2 with ovotesticular DSD had GCs present (table 4). One of the 2 patients with PMDS, 1 of the 9 with XO/XY Turner syndrome and 10 of the 12 with DSD not otherwise specified had GCs. The single patient with complete gonadal dysgenesis and neither of the 2 with partial gonadal dysgenesis had GCs. In CAIS gonads Sertoli cell nodules and areas of hyperplasia contained no GCs but all 6 of these patients had GCs present in areas between nodules.
Table 4.
Germ cells by diagnosis
| DSD Diagnosis | Median Mos Age at Gonadectomy/Biopsy (range) | No. GCs /Total No. | GC Type | Mean ± SD No. GCs/mm2 (range) |
|---|---|---|---|---|
| Complete androgen insensitivity syndrome | 171 (1–205) | 6/6 | Testis | 62 ± 111 (2–286) |
| Complete gonadal dysgenesis | 209 | 0/1 | Not applicable | |
| Denys-Drash syndrome | 15 | 1/1 | Testis | 31 |
| SRY mutation | 15 | 1/1 | Testis | 280 |
| Mixed gonadal dysgenesis | 15 (3–191) | 6/6 | Ovary, testis | 19 ± 14 (10–35), 22 ± 21 (2–43) |
| Ovotesticular DSD | 4 (3–5) | 2/2 | Ovary, testis | 190 ± 57 (21–230), 62 ± 54 (23–100) |
| Partial gonadal dysgenesis | 120 (39–200) | 0/2 | Not applicable | |
| Persistent müllerian duct syndrome | 63 (10–79) | 1/2 | Testis | 38 |
| StAR protein deficiency | 12 | 1/1 | Testis | 38 |
| XO/XY Turner syndrome | 145 (39–216) | 1/9 | Ovary | 1 |
| Urogenital sinus/cloacal abnormality | 0 | 1/1 | Ovary | 190 |
| DSD not otherwise specified* | 11 (0–215) | 10/12 | Ovary, testis | 156 ± 152 (2.6–420), 47 ± 51 (5–142) |
In 12 patients exact DSD diagnosis was unclear from available records so they were classified with DSD not otherwise specified.
By Age
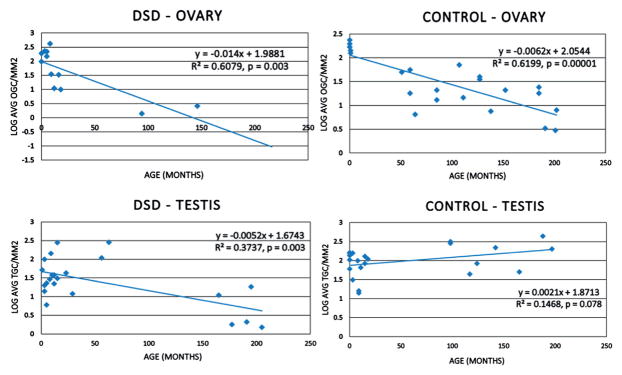
We examined the data using 3 distinct age groups chosen to reflect infancy and early childhood, childhood and adolescence. A comparison of the presence of GCs in either gonad between the groups (ages 0 to 3, 4 to 11 and 12 years or greater) was significant for a decreased likelihood of GCs by higher age group (Fisher exact test p = 0.007, fig. 1). Figure 2 illustrates the relationship between the average number of ovarian and testicular GCs per mm2 in patients with DSD and controls. In the DSD group the average number of ovarian GCs per mm2 showed a strong inverse correlation dependent on age (p = 0.003). A similar negative correlation was observed in control patients (p = 0.00001). The average number of testicular GCs per mm2 in patients with DSD also showed a strong inverse correlation with age (p = 0.003). However, in the testicular control group there was a trend toward a positive correlation between age and the average number of testicular GCs per mm2 (p = 0.078).
Figure 1.
Presence of germ cells by age group of cases
Figure 2.
Relationship between age and germ cell counts. OGC, ovary germ cells. TGC, testis germ cells.
The mean ± SD percentage of atypical GCs found in each control gonad type was 19.4% ± 9.4% (range 0% to 38%) for the testis and 9.1% ± 3.9% (range 4% to 16%) for the ovary. Table 3 shows the percentage of atypical GCs in each gonad type in patients with DSD. The rate of atypia in DSD ovaries was greater than in controls (p = 0.026) whereas in DSD testes it was similar to that in controls (p = 0.15).
DISCUSSION
Infertility has been assumed in many DSD diagnoses. In this pilot study we assessed the presence of GCs as an indicator of fertility potential and found that 68% of pediatric patients with DSD in our cohort had GCs present. GCs were more likely to be present at a younger age. This fertility potential, as shown in the majority of our young patients with DSD, should cause us to reconsider the management of this population. Specifically, our finding of declining GC counts with increasing age suggest that successful preservation of fertility potential may be best achieved at younger ages.
Reframing Fertility Potential
Guercio et al reviewed fertility issues in DSD.4 They stated that 1) for women with testicular dysgenesis motherhood may be possible with hormone replacement and oocyte donation but in those with disorders of androgen synthesis or action the absence of the uterus or a fallopian tube impairs motherhood and 2) many males with DSD have oligospermia or azoospermia, leading to infertility. While noting that assisted reproductive techniques may be helpful, this review focused on traditional views of fertility and currently available technology. However, by reframing these traditional views of fertility potential and incorporating emerging technology, FP options may be greater than previously thought.14 In fact, our data revealed fertility potential in all patients with CAIS, MGD and ovotesticular DSD.
Current technology allows for successful FP in reproductively mature individuals via embryo, oocyte or sperm banking and ovarian tissue cryopreservation with future autotransplantation. Reproductive maturity depends on pubertal development with maturation of primary oocytes and spermatogonia in mid puberty.5 FP techniques for prepubertal individuals remain experimental. Gonadal tissue may be harvested and cryopreserved but in vitro follicle growth of ovarian tissue or maturation of sperm remains investigational.15,16
Experimental protocols are currently in place for cryopreservation of immature gonadal tissue in the pediatric oncology population, which should be expanded to the DSD population if our findings are confirmed in a multicenter study, and by immunohistochemical analysis of GC quality. Gametes incongruent with gender identity should not be considered definitive of infertility as expanding assisted reproductive techniques and societal attitudes toward nontraditional family structures could allow for genetic offspring from this biological material.
Fertility Preservation Counseling
Our pilot findings of fertility potential in the majority of young patients with DSD necessitate further study and confirmation. However, if substantiated, they would require a shift in counseling for families and patients with DSD. This counseling must begin early in the discussion of a diagnosis. Ethical concerns must also be addressed, particularly that this is a discussion about “cryopotential” rather than techniques proven to be successful.17 Counsel should include discussion of the possibility of few or no GCs present due to the underlying condition, patient age or the concurrent presence of neoplasia in the gonad18 along with the risk of passing a heritable DSD to offspring and the availability of a pre-implantation genetic diagnosis.
Tumor Risk, and Biopsy, Gonadectomy and Fertility Preservation Timing
A major controversy in DSD management involves the risk of GC neoplasia with the subsequent debate over the timing of biopsy or gonadectomy. While it was not the focus of this study, our data show that 7 of 44 patients had neoplasia, of whom 6 had gonadoblastoma. In another 4 patients other types of GC neoplasia developed in the second decade of life, as is typically observed.3 Providers, families and patients weigh the risks of neoplasia, the possibilities for endogenous hormone production, future fertility and the importance of autonomous medical decision making.
The fertility potential data in this study require that a discussion of the optimal timing of FP must also be considered. While the shift has been toward delaying gonadectomy to allow for autonomous decisions, earlier preservation of gonadal tissue may provide greater fertility potential. Delaying gonadectomy in some conditions, such as CAIS, may actually decrease fertility potential. Only 1 of the 9 patients with XO/XY Turner syndrome had GCs but the median age at evaluation was 145 months. However, in the similar MGD population all 6 patients had GCs but the median age was 15 months. Given that these diagnoses fall on a spectrum, this suggests that the XO/XY Turner population may have had GCs present if gonadal tissue were obtained earlier.
However, options are not strictly limited to FP via gonadectomy. Future management could include biopsy at a young age to evaluate the risk of neoplasia with concurrent harvest of gonadal tissue for cryopreservation. Further evaluation is needed of the implications of a biopsy on the likelihood of spontaneous gonadal function and the effect of decreasing the pool of cells for in vivo maturation.
Pubertal status or hormone exposure could also affect GC presence and optimal timing of FP but this was not assessed in our study.
Limitations and Future Directions
This study had limitations in methods and small sample size. Normal gonads show temporal and spatial variation during maturation. In the ovary the number of GCs decreases beginning prenatally and throughout childhood,19–21 such that by menarche virtually all GCs (follicles) are restricted to a narrow strip of cortex. Few studies have assessed testicular GC variability with age12,13,22 but they demonstrate a prepubertal rise in testicular GC numbers, which is maintained into adulthood.
In our study patients with DSD and controls showed large variations in the number of GCs per mm2, which was related to age, spatial distribution (in patients with DSD only) and diagnosis, accounting for the large SDs of the average number of GCs per mm2. As noted, many CAIS gonads contained GCs in compressed normal tissue between hamartomatous nodules of Sertoli-cell only tubules and without GCs present. Therefore, we did not include the surface area of the hamartomatous nodules when counting the average number of GCs per mm2. Finally, although we used standard morphological methods to identify what we considered to be definitive GCs, the morphology varied in DSD ovaries.
Another limitation of this study is the relatively small number of each DSD diagnosis, limiting conclusions. A multicenter study is planned to confirm these findings and address questions about the optimum timing of FP for specific DSD diagnoses, and the effect of pubertal development and exogenous hormone exposure on GC presence.
Finally, while this study revealed the presence of GCs in most patients and the fact that the quantity varied with age and diagnosis, we did not assess quality, that is the ability to use these GCs successfully via in vitro fertilization methods. Further immunohistochemical analysis of the GCs will be helpful to assess this question.
CONCLUSIONS
To our knowledge this is the first study to assess the presence of GCs in patients with a broad variety of DSD diagnoses as a marker of fertility potential. It shows that the majority of these patients have fertility potential but this potential declines with age. These findings shift the paradigm of fertility potential and preservation in the DSD population and may require us to reexamine our approach to DSD management. Further evaluation is needed to confirm the findings of this study in a larger cohort of individuals with DSD to determine the potential for each specific diagnosis, the quality of GCs present and the optimal timing for fertility preservation.
Acknowledgments
Supported by the Stanley Manne Research Institute Clinical and Translational Award (CF) and Center for Reproductive Health After Disease P50HD076188 from the National Center for Translational Research in Reproduction and Infertility, National Institutes of Health.
Abbreviations and Acronyms
- CAIS
complete androgen insensitivity syndrome
- DSD
disorder (difference) of sex development
- FP
fertility preservation
- GC
germ cell
- MGD
mixed gonadal dysgenesis
Footnotes
No direct or indirect commercial incentive associated with publishing this article.
The corresponding author certifies that, when applicable, a statement(s) has been included in the manuscript documenting institutional review board, ethics committee or ethical review board study approval; principles of Helsinki Declaration were followed in lieu of formal ethics committee approval; institutional animal care and use committee approval; all human subjects provided written informed consent with guarantees of confidentiality; IRB approved protocol number; animal approved project number.
References
- 1.Ohnesorg T, Vilain E, Sinclair AH. The genetics of disorders of sex development in humans. Sex Dev. 2014;8:262. doi: 10.1159/000357956. [DOI] [PubMed] [Google Scholar]
- 2.Houk CP, Hughes IA, Ahmed SF, et al. Summary of consensus statement on intersex disorders and their management. International Intersex Consensus Conference Pediatrics. 2006;118:753. doi: 10.1542/peds.2006-0737. [DOI] [PubMed] [Google Scholar]
- 3.Lee PA, Nordenstrom A, Houk CP, et al. Global disorders of sex development update since 2006: perceptions, approach and care. Horm Res Paediatr. 2016;85:158. doi: 10.1159/000442975. [DOI] [PubMed] [Google Scholar]
- 4.Guercio G, Costanzo M, Grinspon RP, et al. Fertility issues in disorders of sex development. Endocrinol Metab Clin North Am. 2015;44:867. doi: 10.1016/j.ecl.2015.07.012. [DOI] [PubMed] [Google Scholar]
- 5.Lee PA, Rogol A, Houk CP. Optimizing potential for fertility: fertility preservation considerations for the pediatric endocrinologist. Endocrinol Metab Clin North Am. 2009;38:761. doi: 10.1016/j.ecl.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 6.Woodruff TK. The emergence of a new inter-discipline: oncofertility. Cancer Treat Res. 2007;138:3. doi: 10.1007/978-0-387-72293-1_1. [DOI] [PubMed] [Google Scholar]
- 7.De Vos M, Smitz J, Woodruff TK. Fertility preservation in women with cancer. Lancet. 2014;384:1302. doi: 10.1016/S0140-6736(14)60834-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Regadera J, Martinez-Garcia F, Paniagua R, et al. Androgen insensitivity syndrome: an immunohistochemical, ultrastructural, and morphometric study. Arch Pathol Lab Med. 1999;123:225. doi: 10.5858/1999-123-0225-AIS. [DOI] [PubMed] [Google Scholar]
- 9.Reynaud K, Cortvrindt R, Verlinde F, et al. Number of ovarian follicles in human fetuses with the 45, X karyotype. Fertil Steril. 2004;81:1112. doi: 10.1016/j.fertnstert.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 10.Nakhal RS, Hall-Craggs M, Freeman A, et al. Evaluation of retained testes in adolescent girls and women with complete androgen insensitivity syndrome. Radiology. 2013;268:153. doi: 10.1148/radiol.13121068. [DOI] [PubMed] [Google Scholar]
- 11.Kaprova-Pleskacova J, Stoop H, Bruggenwirth H, et al. Complete androgen insensitivity syndrome: factors influencing gonadal histology including germ cell pathology. Mod Pathol. 2014;27:721. doi: 10.1038/modpathol.2013.193. [DOI] [PubMed] [Google Scholar]
- 12.Ribeiro Scolfaro M, Aparecida Cardinalli I, Gabas Stuchi-Perez E, et al. Morphometry and histology of gonads from 13 children with dysgenetic male pseudohermaphroditism. Arch Pathol Lab Med. 2001;125:652. doi: 10.5858/2001-125-0652-MAHOGF. [DOI] [PubMed] [Google Scholar]
- 13.Nistal M, Paniagua R, Gonzalez-Peramato P, et al. Perspectives in Pediatric Pathology, Chapter 5. Gonadal Dysgenesis Pediatr Dev Pathol. 2015;18:259. doi: 10.2350/14-04-1471-PB.1. [DOI] [PubMed] [Google Scholar]
- 14.Johnson EK, Finlayson C. Preservation of fertility potential for gender and sex diverse individuals. Transgender Health. 2016;1:41. doi: 10.1089/trgh.2015.0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Valli H, Phillips BT, Shetty G, et al. Germline stem cells: toward the regeneration of spermatogenesis. Fertil Steril. 2014;101:3. doi: 10.1016/j.fertnstert.2013.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xiao S, Zhang J, Romero MM, et al. In vitro follicle growth supports human oocyte meiotic maturation. Sci Rep. 2015;5:17323. doi: 10.1038/srep17323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wallace WH, Kelsey TW, Anderson RA. Fertility preservation in pre-pubertal girls with cancer: the role of ovarian tissue cryopreservation. Fertil Steril. 2016;105:6. doi: 10.1016/j.fertnstert.2015.11.041. [DOI] [PubMed] [Google Scholar]
- 18.Pavone ME, Hirshfeld-Cytron J, Lawson AK, et al. Fertility preservation outcomes may differ by cancer diagnosis. J Hum Reprod Sci. 2014;7:111. doi: 10.4103/0974-1208.138869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anderson RA, McLaughlin M, Wallace WH, et al. The immature human ovary shows loss of abnormal follicles and increasing follicle developmental competence through childhood and adolescence. Hum Reprod. 2014;29:97. doi: 10.1093/humrep/det388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Poirot CJ, Martelli H, Genestie C, et al. Feasibility of ovarian tissue cryopreservation for prepubertal females with cancer. Pediatr Blood Cancer. 2007;49:74. doi: 10.1002/pbc.21027. [DOI] [PubMed] [Google Scholar]
- 21.Kelsey TW, Anderson RA, Wright P, et al. Data-driven assessment of the human ovarian reserve. Mol Hum Reprod. 2012;18:79. doi: 10.1093/molehr/gar059. [DOI] [PubMed] [Google Scholar]
- 22.Paniagua R, Nistal M. Morphological and histometric study of human spermatogonia from birth to the onset of puberty. J Anat. 1984;139:535. [PMC free article] [PubMed] [Google Scholar]