Abstract
Rationale and Objective
Evaluation of nodal involvement in early-stage breast cancers (T1 or T2) changed following the Z11 trial; however, not all patients meet the Z11 inclusion criteria. Hence, the relevance of ultrasound imaging of the axilla and fine-needle aspiration biopsy (FNA) in early-stage breast cancers was investigated.
Materials and Methods
In this single-center, retrospective study, 758 subjects had pathology-verified breast cancer diagnosis over a 3-year period, of which 128 subjects with T1/T2 breast tumors had abnormal axillary lymph nodes on ultrasound, had FNA, and proceeded to axillary surgery. Ultrasound images were reviewed and analyzed using multivariable logistic regression to identify the features predictive of positive FNA. Accuracy of FNA was quantified as the area under the receiver operating characteristic curve with axillary surgery as reference standard.
Results
Of 128 subjects, 61 and 65 were positive on FNA and axillary surgery, respectively. Sensitivity, specificity, positive- and negative-predictive values of FNA were 52/65 (80%), 54/63 (85.7%), 52/61(85.2%) and 54/67 (80.5%), respectively. After adjusting for neoadjuvant chemotherapy between FNA and surgery, a positive FNA was associated with higher likelihood for positive axillary surgery (odds ratio: 22.7; 95% CI: 7.2–71.3, p<0.0001), and the accuracy of FNA was 0.801 (95% CI: 0.727–0.876). Among ultrasound imaging features, cortical thickness and abnormal hilum were predictive (p<0.017) of positive FNA with accuracy of 0.817 (95% CI: 0.741–0.893).
Conclusion
Ultrasound imaging and FNA can play an important role in the management of early breast cancers even in the post-Z11 era. Higher weightage can be accorded to cortical thickness and hilum during ultrasound evaluation.
Keywords: Breast cancer, Ultrasound, Lymph node, Axilla, Fine-needle aspiration, Sentinel node biopsy, axillary lymph node dissection
Introduction
Management of breast cancer has been evolving over the last few decades. Less invasive procedures with lower morbidity have been shown to be equally effective with no adverse impact on outcomes [1]. Metastatic involvement of the axilla is an important prognostic indicator on the outcome in breast cancer patients. In stage I-II breast cancers, tumor grade, size, receptor status, and lymphovascular invasion of the tumor increase the odds for involvement of the axilla [2–6]. The imaging modality of choice for evaluation of the axilla is ultrasound (US). When suspicious lymph nodes are identified on US, fine-needle aspiration biopsy (FNA) or core-needle biopsy typically follows, both to establish a histological diagnosis and to guide further management. A number of studies have outlined the benefits of ultrasound and FNA in the evaluation of axillary lymph nodes [7–16].
The publication of the American College of Surgeons Oncology Group (ACOSOG) Z11 trial, however resulted in a paradigm shift in the way early T1 and T2 breast cancers are managed at most institutions [17, 18]. The study showed no benefit for axillary lymph node dissection (ALND) over surgical sentinel node biopsy (SNB) for patients with low burden of axillary disease who underwent breast conserving therapy and received whole breast irradiation. It established SNB as the standard-of-care [1, 17–19]. As a consequence, the value of imaging and interventional procedures of axillary lymph nodes has been questioned [3, 19, 20]. In order to remain relevant and continue to contribute meaningfully to patient care it is important for breast imagers to adapt to these changes.
The number of patients for whom the results from the Z11 trial are applicable is unclear. Some studies estimate that less than 7% of T1 and T2 breast cancers meet the inclusion criteria stated in the Z11 trial [3, 6, 21]. Patients being treated with neoadjuvant chemotherapy (NCT), opting for mastectomy, patients undergoing partial breast radiation, patients with clinically involved nodes, three or more positive sentinel nodes, or nodes with extranodal extension on SNB fall outside the scope of the Z11 eligibility [20].
It is therefore important to quantify and explore means to improve the sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) of US imaging of the axillary lymph nodes. It is equally important to ensure that the gross disease in the axilla is not missed in a clinically negative patient. We therefore undertook a retrospective analysis of our early breast cancer patients who had suspicious lymph node morphology on US imaging, had a FNA, and proceeded to have either SNB or ALND, with the aim of identifying morphological features in the suspicious lymph node that could be given higher weightage. In addition this would assist breast-imaging radiologists to better select cases meriting percutaneous biopsy while avoiding overlap with the Z11 trial’s inclusion criteria.
Methods and Materials
This single-institution, retrospective, study was conducted in adherence to a protocol approved by the institutional review board (IRB) and in compliance with Hospital Insurance Portability and Accountability Act (HIPAA). Our IRB waived the requirement for informed consent for this retrospective study. Female subjects who had pathology-verified diagnosis of breast cancer during the year range 2007 to 2010, and had undergone ultrasound-guided FNA of the axillary lymph node, and who subsequently underwent either SNB or ALND, were eligible for inclusion in the study.
Human subjects
The institutional cancer registry identified 758 subjects with diagnosis of breast cancer over the time period considered. Among these subjects, 353/758 (46.6%) had ultrasound imaging of the axilla and 178/353 (50.4%) of these patients had undergone ultrasound-guided FNA. Of these 178 subjects, 28 were excluded; surgical pathology report was missing in 17, 4 were intra-mammary lymph nodes, 3 FNAs were in males, clinical staging of primary tumor was missing in electronic medical records of 2 subjects, 1 FNA was performed while the subject was on chemotherapy, and 1 FNA was performed post-excisional biopsy with positive margins. Thus, the remaining 150/178 (84.3%) subjects had complete data for analysis. Among these subjects, 128/150 (85.3%) were clinically assessed to be stage T2 or lower. This group of 128 study subjects with T1 and T2 breast cancers formed the basis for our analysis. Medical records were reviewed to determine if a subject underwent neoadjuvant chemotherapy (NCT) after ultrasound-guided FNA and prior to SNB or ALND. The review indicated that 49/150 (32.7%) of subjects and 35/128 (25%) of subjects with clinical staging of T2 or lower, had undergone neoadjuvant chemotherapy (NCT) between the time of ultrasound-guided FNA and the surgical procedure (SNB or ALND). Table 1 summarizes the distribution of tumor stage, grade and the surgical procedure following FNA in subjects with T2 or lower cancers. There were 59/128 (46%) subjects with T2 cancers, 53/128 (41%) were high-grade tumors and 74/128 (58%) underwent ALND.
Table 1.
Distribution of tumor stage, grade and the axillary surgery following FNA in subjects with stage T2 or lower cancers
| n | |
|---|---|
| Tumor stage | |
| T1a | 7 |
| T1b | 14 |
| T1c | 48 |
| T2 | 59 |
| Tumor grade | |
| I | 17 |
| II | 58 |
| III | 53 |
| Axillary surgery | |
| SNB | 54 |
| ALND | 65 |
| SNB followed by ALND | 9 |
Ultrasound imaging and FNA
Ultrasound imaging (IU-22, Philips Healthcare, Bothell, WA, USA) was performed by any one of the 5 board-certified breast imaging radiologists (2–21 years of experience) using a 17-5 MHz or a 12-5 MHZ linear array transducer. If the ultrasound imaging features were deemed by the radiologist based on their individual subjective criteria to be indicative of an atypical lymph node, then the radiologist proceeded with the FNA using a 22-gauge (G) hypodermic needle, 10 cc syringe and mild suction. We generally obtain two samples on every patient and the specimens were handed to cytopathologist on site, to verify sample adequacy. On occasion, when the cytopathologist was unavailable, two samples per patient were obtained and placed in cytorich (Thermo Fisher Scientific, Inc., Kalamazoo, MI) for subsequent touch-prep.
Retrospective review
Ultrasound imaging features of each case were independently reviewed by 1 of 3 study radiologists blinded to prior imaging, biopsy and surgical results. The 150 cases were equally divided among the 3 radiologists. The study radiologist reviewing the imaging features measured and recorded the node length and width in mm, cortical thickness along the long axis of the lymph node in mm, and when the thickness exceeded 3 mm categorized it as focal or diffuse, categorized abnormal vascularity as hilar or non-hilar blood flow, categorized nodal margin as distinct or ill-defined, and, categorized hilum status as normal or abnormal with fatty replacement or eccentric displacement. Additionally, for focal bulge and thickening, our criteria was to document in a single lymph node, a clear contour abnormality on the long axis, as on occasion adjacent matted lymph nodes can give the appearance of a focal bulge in one view.
Reference standard
The pathology results from ultrasound-guided FNA were considered the reference standard or the truth for the analysis determining the association between ultrasound imaging features and the pathology results from ultrasound-guided FNA. For the analysis determining the association between ultrasound-guided FNA and the surgical procedure (SNB or ALND), pathology results from the surgical procedure were considered the reference standard or the truth. If a subject underwent both SNB and ALND, then result from ALND was considered the truth for the above analysis.
Statistical analysis
Multivariable logistic regression models (SAS® 9.3, SAS Institute Inc., Cary, NC) were used for analysis. Categorical imaging features from ultrasound were numerically coded. Pathology results from ultrasound-guided FNA and from the surgical procedure (SNB or ALND) were binary coded (0: negative, 1: positive). For the analysis determining the association between the ultrasound imaging features and the pathology results from ultrasound-guided FNA, all of the ultrasound imaging features and measurements were eligible for entry into the model as predictors, and the pathology results from ultrasound-guided FNA served as outcome variable. Effects associated with p<0.05 were considered statistically significant. For the analysis determining the association between ultrasound-guided FNA and the surgical procedure (SNB or ALND), the pathology results from the surgical procedure served as the outcome variable. Pathology results from ultrasound-guided FNA, and whether the subject underwent NCT or not (binary coded), served as predictors for the above analysis. Additional analysis was performed using univariate logistic regression for the cohort of subjects who did not undergo NCT, with results from FNA as predictor and that from axillary surgery as the outcome variable. The above analyses were performed to odds ratio and to generate receiver operating characteristic (ROC) curves and area under the curve (AUC) for all subjects (n=150), and for subjects with clinical staging of T2 or lower (n=128).
Results
The pathology results from ultrasound-guided FNA of the axillary lymph node were positive in 78/150 (52%) of subjects and the pathology results from the surgical procedure (SNB or ALND) were positive in 79/150 (52.7%) of subjects. For the core group of 128 subjects staged T2 or lower, the pathology results from ultrasound-guided FNA of the lymph node were positive in 61/128 (47.7%) subjects and the pathology results from the surgical procedure (SNB or ALND) were positive in 65/128 (50.8%) subjects. Of the 61 positive lymph nodes on FNA, 52 were positive at surgery (PPV: 85.2%). Nine patients were negative at ALND, and all nine patients had interim neoadjuvant chemotherapy. Fifty two of 65 (sensitivity: 80%) patients who had positive results on axillary surgery (SNB or ALND) had a positive FNA. Of the 67 who had negative FNA’s (includes 1 non-diagnostic sample), discordant results were observed in 13 patients. Fifty four of 67 (NPV: 80.5%) patients with a negative FNA were negative at SNB. Six of the 13 patients who had positive results at SNB had microscopic metastasis in a single lymph node and a seventh patient had micro metastasis in two lymph nodes. The number of lymph nodes biopsied at surgery in these 7 patients varied from 1–8 and 2/7 patients proceeded to have a negative ALND. Another 5 patients with negative FNA results had macroscopic disease at ALND (4/5 of these patients had macroscopic disease on SNB and 1/5 had a negative SNB). The one with non-diagnostic sample on FNA, had a positive SNB, but was negative at ALND. This patient had interim neoadjuvant chemotherapy. In the 63 patients with negative nodes at surgery, 54 had negative FNA’s (specificity: 85.7%).
Ultrasound imaging features
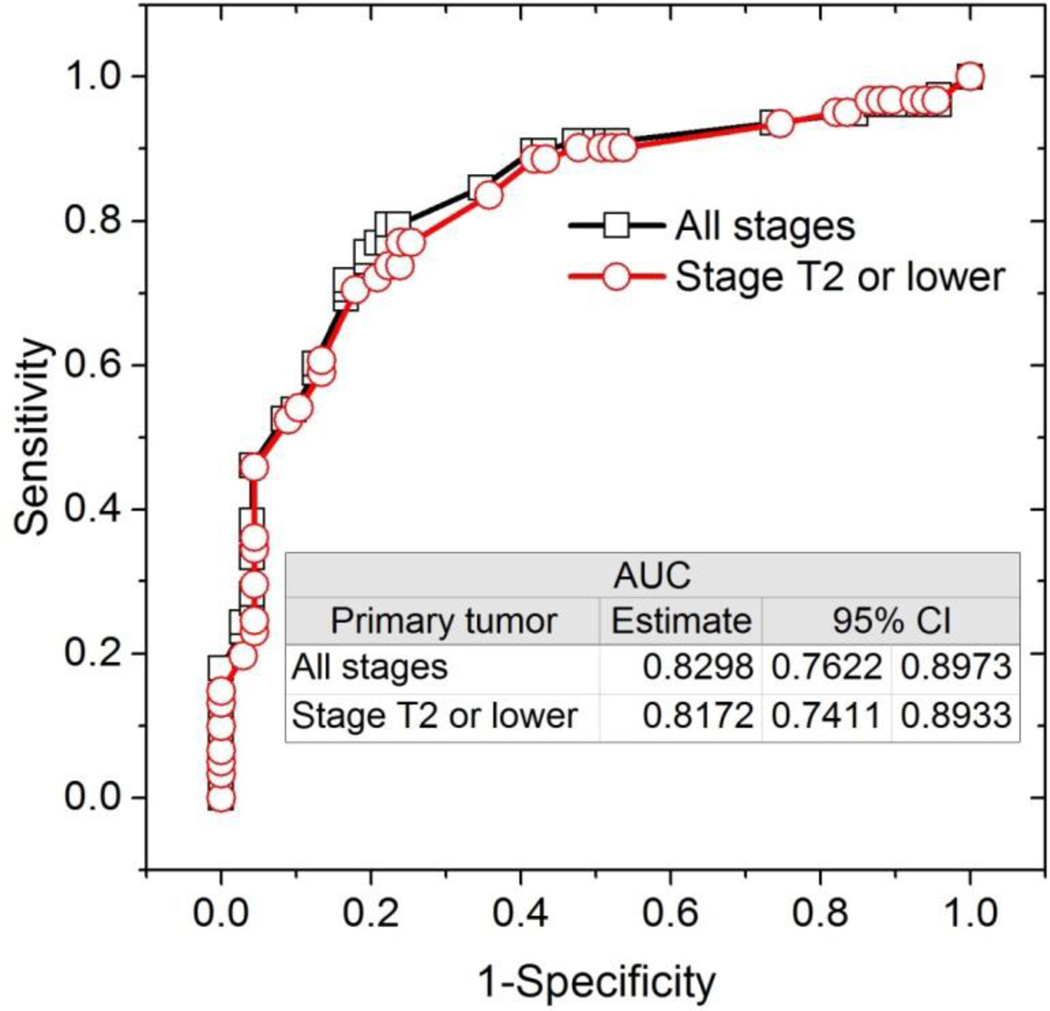
Among the ultrasound imaging features and measurements of the axillary lymph node considered, stepwise selection of the predictors by the multivariable logistic regression model identified cortical thickness in mm (p=0.0048) and hilum status (p=0.0002) as significant predictors of a positive result from ultrasound-guided FNA. None of the other ultrasound imaging features and measurements were statistically significant (p>0.3769) for entry into the model. For all patients and for patients with clinical staging of T2 or lower, the likelihood of obtaining positive results from pathology following ultrasound-guided FNA increased with increasing cortical thickness and when the hilum was abnormal. Table 2 summarizes the odds ratio and 95% confidence interval (CI). With cortical thickness and abnormal hilum as predictors, the generated ROC plots along with the AUCs and 95% CI are shown in Figure 1. The model estimates of the AUCs were similar between the analyses with all patients and with patients clinically staged to be T2 or lower.
Table 2.
Odds ratio and 95% confidence intervals of ultrasound imaging features predictive of a positive result from ultrasound-guided fine-needle aspiration biopsy (FNA). Other than cortical thickness in mm and hilum status categorized as normal or abnormal, none of the other ultrasound imaging features and measurements was significantly (p>0.3769) associated with a positive FNA.
| Ultrasound imaging feature | Odds Ratio Estimate | 95% Confidence Intervals | p-value | |
|---|---|---|---|---|
| All breast cancer stages (n=150) | ||||
| Cortical thickness in mm | 1.268 | 1.075 | 1.496 | 0.0048 |
| Hilum (abnormal vs. normal) | 5.271 | 2.217 | 12.530 | 0.0002 |
| Stage T2 or lower (n=128) | ||||
| Cortical thickness in mm | 1.258 | 1.042 | 1.520 | 0.0172 |
| Hilum (abnormal vs. normal) | 4.509 | 1.735 | 11.718 | 0.0020 |
Figure 1.
ROC curve indicating that cortical thickness in mm and hilum status were predictive of a positive result from ultrasound-guided FNA.
Axillary surgery
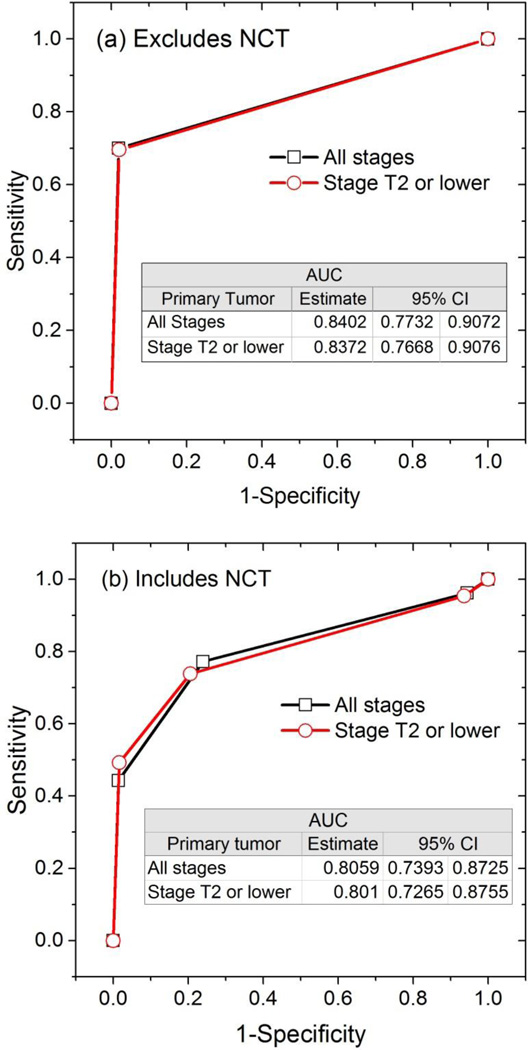
As noted earlier, some of the subjects had undergone interim neoadjuvant chemotherapy (NCT) between the time of ultrasound-guided FNA and the surgical procedure (SNB or ALND). Hence, the data were analyzed for the cohort of subjects who did not undergo NCT using a univariate model, and the data for the entire study sample that includes subjects who underwent NCT were analyzed using multivariable models. For the cohort that did not undergo NCT, ultrasound-guided FNA was a significant predictor of axillary lymph node metastasis (p<0.0001) with a positive FNA associated with higher likelihood for axillary lymph node metastasis (Table 3). For the entire study sample that included subjects who underwent interim NCT, the results from multivariable model that included FNA results and whether a subject underwent interim NCT as predictors indicate that ultrasound-guided FNA was a significant predictor (p<0.0001) of axillary metastasis for breast cancers of stage T2 or lower and for all stages (Table 3). After adjusting for whether a subject underwent NCT, a positive ultrasound-guided FNA was associated with higher likelihood for axillary lymph node metastasis for breast cancers of all stages and for stage II or lower cancers. Table 3 summarizes the odds ratio and 95% CI. The generated ROC plots along with the AUCs and 95% CI are shown in Figure 2a for the cohort that excluded subjects who underwent interim NCT and are shown in Figure 2b for the entire study sample that included subjects who underwent interim NCT. In both figures, the model estimates of the AUCs were similar between the analyses with all tumor stages and with subjects clinically staged to be T2 or lower.
Table 3.
Odds ratio and 95% confidence intervals of the predictor(s) associated with axillary lymph node metastasis from pathology analysis of sentinel node biopsy (SNB) or axillary lymph node dissection (ALND). Some of the subjects underwent interim neoadjuvant chemotherapy (NCT) between the time of ultrasound-guided fine-needle aspiration biopsy (FNA) and the surgical procedure and was included in the model. Hence, the results are presented for the cohort of subjects who did not undergo NCT, and for the entire study sample that includes subjects who underwent NCT.
| Predictor | Odds Ratio Estimate | 95% Confidence Intervals | p-value | |
|---|---|---|---|---|
| Cohort: Excludes subjects who underwent NCT (univariate analysis) | ||||
| All breast cancer stages (n=101) | ||||
| FNA (positive vs. negative) | 116.646 | 14.724 | 924.084 | <0.0001 |
| Stage T2 or lower (n=93) | ||||
| FNA (positive vs. negative) | 105.133 | 13.158 | 840.046 | <0.0001 |
| Cohort: Includes subjects who underwent NCT (multivariable analysis) | ||||
| All breast cancer stages (n=150) | ||||
| FNA (positive vs. negative) | 25.533 | 8.209 | 79.418 | <0.0001 |
| NCT (yes vs. no) | 0.224 | 0.07 | 0.722 | 0.0122 |
| Stage T2 or lower (n=128) | ||||
| FNA (positive vs. negative) | 22.672 | 7.205 | 71.342 | <0.0001 |
| NCT (yes vs. no) | 0.226 | 0.066 | 0.768 | 0.0172 |
Figure 2.
ROC curves showing that a positive FNAB is predictive of axillary lymph node metastasis from surgical procedure (a) for the cohort of subjects who did not undergo interim NCT, and (b) for the entire study sample that included subjects who underwent NCT.
Discussion
Following the publication of the Z11 trial [17, 18], it is no longer standard clinical practice to routinely image the axilla in clinically negative T1 and T2 breast cancers at many institutions. It is therefore imperative for breast imagers to adapt to changing practices of the surgeons. Some practices [4, 8] continue to routinely use axillary US imaging in the clinically negative breast cancer patients. At our center and post Z11 trial, we perform US of the axilla for lymph node evaluation only if there is a question of a palpable concern on clinical evaluation, or a suspicious lymph node is observed on imaging (mammogram, breast magnetic resonance imaging or other incidental investigations such as computerized tomography of the chest or a positron emission tomographic scan), or select triple-negative tumors, or when reconstruction is planned synchronously with mastectomy. It is important that clinically occult gross disease in the axilla is not missed. Post Z11, surgeons at our institution want us to only biopsy lymph nodes of T1 and T2 breast cancer patients with a high index of suspicion. It is therefore relevant for the breast imager to be aware which of the US imaging features of axillary lymph nodes in T1 and T2 breast cancer patients merit higher weightage to improve the diagnostic accuracy. This study addresses this need.
A number of morphological features, such as cortical thickness exceeding 3 mm, effacement, eccentric displacement or absence of the fatty hilum, non-hilar blood flow, loss of the reniform outline, indistinctness of the lymph node margins, and increasing lymph node size have been described as predictors of abnormality in the literature [12, 22–24]. At our institution, a lymph node exhibiting any one or a combination of these features was considered suspicious and subjected to a biopsy at the discretion of the interpreting radiologist (Figures 3 and 4). We placed less emphasis on the size of the lymph node alone. Distinguishing a metastatic lymph node from a reactive lymph node (Figure 5) on imaging is difficult [3]. In our analysis, cortical thickness (p =0.0048) and an abnormal hilar morphology [fatty replacement or eccentric displacement] (p=0.0002) were statistically significant predictors of a positive FNA with diagnostic accuracy (AUC) of 82% for T1 and T2 cancers, and should be in our estimate given higher weightage. Zhu et al [25] observed a cortical thickness of >3.5 mm as the single most important predictor of three or more axillary lymph node involvement. Saffar et al [26] noted a cortical thickness of >3mm increases the likelihood of axillary lymph node metastasis. Amonkar et al [27] in their grading system noted hilar fatty replacement correlated best with positive FNA. Our study reaffirms these findings and found both image features were important.
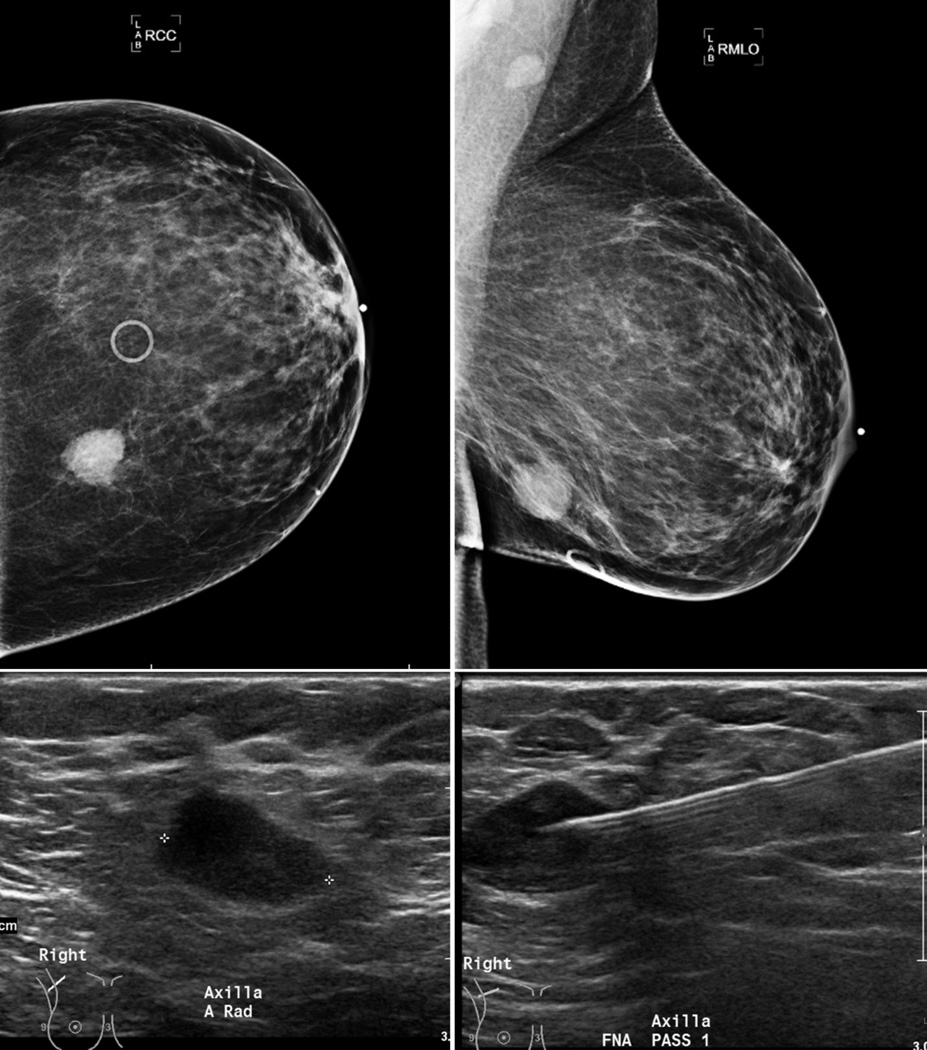
Figure 3.
48 year old female with a lower inner quadrant invasive ductal carcinoma (T2 tumor–2.1 × 1.6 cm mass, grade-3) (top row). Right axilla (RMLO view) demonstrates an oval heterogeneous anechoic lymph node (left bottom), without fatty hila, cortical thickening and abnormal vascularity (image not shown). FNA (needle in appropriate position, bottom right) was positive.
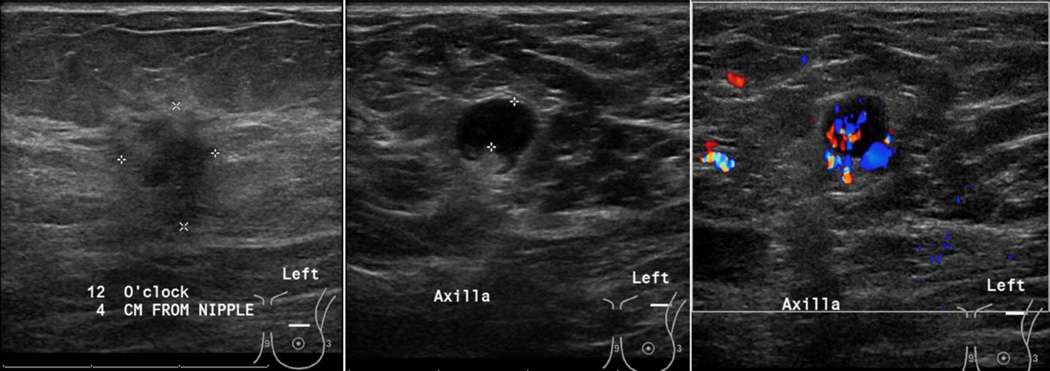
Figure 4.
75 year old female, with a left breast primary tumor (T1 – 1.4 × 1 cm at 12:00 clock) that was pathology-verified as invasive ductal carcinoma. Evaluation of the axilla demonstrated a round LN, with eccentric displacement of central fatty hila, focal cortical thickness of 5mm and non-hilar blood flow. FNA was positive for malignant cells of ductal origin.
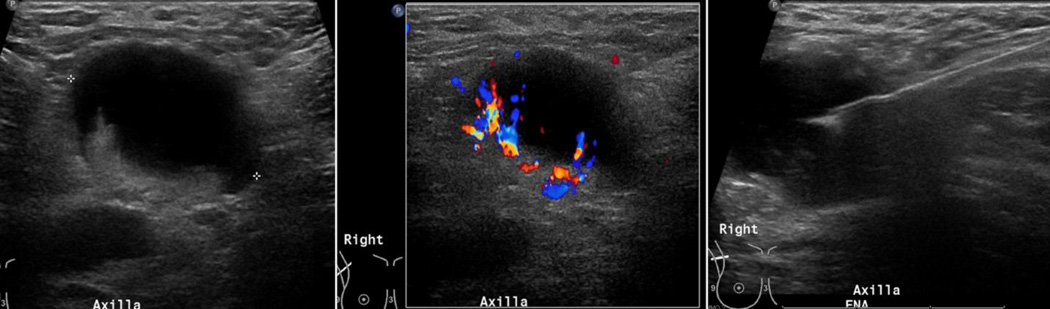
Figure 5.
31 year old female presenting with a palpable right axillary mass. Baseline mammogram was negative (not shown). US evaluation of the right axilla demonstrated an enlarged lymph node with eccentric displacement of fatty hila, diffuse cortical thickness exceeding 3mm and non-hilar abnormal blood flow on color imaging (left and middle). FNA showing the needle in the abnormal cortex (right). Histology: Focal necrosis and inflammation, no evidence of malignancy (Kikuchi disease was a suggested possibility). Distinction between a reactive, inflamed node from any cause and metastatic disease is difficult on imaging.
The reported sensitivity, specificity, PPV, and NPV of percutaneous tissue sampling vary widely [3–5, 8–16]. Both FNA and core biopsy have been described [14, 28]. Core biopsy obtains a better tissue sample, but the presence of large vessels and delicate nerve plexus increases the risk for complications [28]. Rautiainen et al [28] recommend routine use of core biopsy over FNA and considered it a safe procedure. However, Rao et al [14] found no additional benefit for the core biopsy and considered the less invasive FNA as the better option. At our institution and during the study period, all lymph node biopsies were fine-needle aspiration cytology. On FNA, non-diagnostic samples contribute to lower sensitivity [8]. We had only one non-diagnostic sample in our cohort, as we typically obtain at least two samples. Generally, a cytopathologist was on site to confirm adequacy and additional samples were obtained, if requested. On the rare occasion when a cytopathologist was unavailable, we usually obtained two samples and were sent in cytorich for touch-prep.
SNB is currently the established standard-of-care over the more morbid ALND particularly in early breast cancer [1, 17, 18, 29]. However, this entails a two-stage surgical procedure – a SNB, followed by lumpectomy with or without ALND. SNB is not free from complications. Seroma, limitation of shoulder movement, nerve injury and lymphedema have been described [30]. A 5–15% false-negative rate for SNB has been reported [20, 31]. Hieken et al. [3], Park et al [8] and Baruah et al [16] have noted that following US evaluation and FNA of the suspicious lymph node, 10–30% of potential SNB could have been averted in their respective series. Boughey et al [32] discuss the additional cost burden associated with SNB procedure.
The percentage of suspicious lymph nodes 61/128 (47.7%) that were positive on FNA in our study was slightly higher than that quoted by others. Hiekin et al [3] observed 33% of suspicious lymph nodes were positive at biopsy. Yip et al [33] observed 38% of nodes involved in T1 and T2 breast cancers. Tumor size, grade, size of the metastasis, receptor status and lymphovascular invasion all contributed to positive lymph node biopsy [2–5, 11–13, 33, 34]. We did not specifically analyze our data along those lines. In our study, there were 59/128 (46%) subjects with T2 cancers, 53/128 (41%) were high-grade tumors. Higher tumor grade and larger size may have possibly accounted for the higher nodal involvement in our series.
We also analyzed our false-positives and false-negatives, with pathology following surgical procedure as the truth, and provide estimates of sensitivity (80%), specificity (85.7%), PPV (85.2%) and NPV (80.5%). Our sensitivity rates were higher than that quoted in most studies [3–5, 8–10, 12, 15, 16] and is related to our very low non-diagnostic sample rate. Higher tumor grade, larger size, obtaining at least 2 samples per patient, the availability of on-site pathologist to verify sample adequacy, and a potential bias associated with selecting subjects with a higher index of suspicion for FNA could have all contributed to the higher sensitivity in our study. Our PPV rates compare well with those by MacNeil et al [11], but is lower than that reported by Hieken et al [3] and Leenders et al [7]. All 9 false-positive cases with FNA had interim neoadjuvant chemotherapy and response to chemotherapy may explain their negative histology at surgery. Micrometastasis in a single lymph node in 6 instances and in two lymph nodes in the seventh case accounted for 53.8% (7/13) of our false negatives. MacNeil et al [11] observed that micrometastasis contributed to only 16% of their false-negatives. Regards the other 6 patients, all had SNB. Five of 6 patients had macrometastasis in the axillary lymph nodes and 1 had a negative SNB. Four of 5 patients had macrometastasis on ALND. US combined with FNA missed macroscopic disease in 5/67 (7.5%) that could have potentially impacted care. Others such as Leenders et al [7] and MacNeil et al [11] have reported higher false-negative rates. We continue to recommend patients with negative cytology should proceed to SNB for better evaluation of the axillary status.
Our study was based on abnormality in a single lymph node. A Mayo study [3] suggested documenting the number of suspicious lymph nodes on scanning prior to biopsy. They observed that patients, who had multiple suspicious nodes on scanning and a positive node on biopsy, predicted for 3 or more lymph node involvement and extranodal extension on SNB necessitating axillary clearance. In their opinion, in this subset of patients, US imaging and biopsy was a good indication for proceeding to ALND directly. These findings were also validated by Boland et al [35], but Cools-Lartigue et al [4] did not observe greater nodal involvement or extranodal extension in their set of patients with positive nodes on FNA.
Our study had a number of limitations. This was a retrospective review. Not all early breast cancer patients had an US. Patients with a negative US were not biopsied and therefore not included in the study. The decision to proceed with FNA was made by one of 5 breastimaging radiologists based on their individual subjective criteria, with experience varying from 2 to 21 years.
Conclusion
In summary, it is important to recognize that the results of the Z11 trial may not be applicable to all early-stage breast cancer patients as a number of such patients fall outside that trial’s inclusion criteria. US evaluation followed by FNA is a safe, simple and widely available procedure that is relatively free from complications. Our results show that FNA is predictive of axillary node involvement and has the potential to avoid the intermediate step of SNB, a surgical procedure in select cases. Among the ultrasound imaging morphological features, cortical thickness and hilar abnormality in lymph nodes predict for a positive outcome at pathology with 82% accuracy and could help in selecting cases that merit FNA or core biopsy. Hence, ultrasound imaging and FNA can continue to play an important role in the management of early breast cancers even in the post-Z11 era.
Acknowledgments
This work was supported in part by the National Cancer Institute (NCI) of the National Institutes of Health (NIH) grants R01 CA195512 and R01 CA199044. The contents are solely the responsibility of the authors and do not necessarily reflect the official views of the NIH or the NCI.
List of Abbreviations
- US
Ultrasound
- SNB
Sentinel node biopsy
- FNA
Fine-needle aspiration biopsy
- ALND
Axillary lymph node dissection
- NCT
Neoadjuvant chemotherapy
- PPV
Positive predictive value
- NPV
Negative predictive value
- ROC
Receiver operating characteristic
- AUC
Area under ROC
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Gopal R. Vijayaraghavan, Department of Radiology, University of Massachusetts Medical School, Worcester, MA 01655..
Srinivasan Vedantham, Department of Radiology, University of Massachusetts Medical School, Worcester, MA 01655..
Milliam Kataoka, Department of Radiology, University of Massachusetts Medical School, Worcester, MA 01655..
Carolynn DeBenedectis, Department of Radiology, University of Massachusetts Medical School, Worcester, MA 01655..
Robert Quinlan, Department of Surgery, University of Massachusetts Medical School, Worcester, MA 01655..
References
- 1.Black DM, Mittendorf EA. Landmark trials affecting the surgical management of invasive breast cancer. Surg Clin North Am. 2013;93(2):501–518. doi: 10.1016/j.suc.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rivadeneira DE, Simmons RM, Christos PJ, Hanna K, Daly JM, Osborne MP. Predictive factors associated with axillary lymph node metastases in T1a and T1b breast carcinomas: analysis in more than 900 patients. J Am Coll Surg. 2000;191(1):1–6. doi: 10.1016/s1072-7515(00)00310-0. discussion -8. [DOI] [PubMed] [Google Scholar]
- 3.Hieken TJ, Trull BC, Boughey JC, et al. Preoperative axillary imaging with percutaneous lymph node biopsy is valuable in the contemporary management of patients with breast cancer. Surgery. 2013;154(4):831–838. doi: 10.1016/j.surg.2013.07.017. discussion 8–40. [DOI] [PubMed] [Google Scholar]
- 4.Cools-Lartigue J, Sinclair A, Trabulsi N, et al. Preoperative axillary ultrasound and fine-needle aspiration biopsy in the diagnosis of axillary metastases in patients with breast cancer: predictors of accuracy and future implications. Ann Surg Oncol. 2013;20(3):819–827. doi: 10.1245/s10434-012-2609-7. [DOI] [PubMed] [Google Scholar]
- 5.Koelliker SL, Chung MA, Mainiero MB, Steinhoff MM, Cady B. Axillary lymph nodes: US-guided fine-needle aspiration for initial staging of breast cancer--correlation with primary tumor size. Radiology. 2008;246(1):81–89. doi: 10.1148/radiol.2463061463. [DOI] [PubMed] [Google Scholar]
- 6.Joh JE, Han G, Kiluk JV, Laronga C, Khakpour N, Lee MC. Indications for axillary ultrasound use in breast cancer patients. Clin Breast Cancer. 2012;12(6):433–437. doi: 10.1016/j.clbc.2012.09.009. [DOI] [PubMed] [Google Scholar]
- 7.Leenders MW, Broeders M, Croese C, et al. Ultrasound and fine needle aspiration cytology of axillary lymph nodes in breast cancer. To do or not to do? Breast. 2012;21(4):578–583. doi: 10.1016/j.breast.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 8.Park SH, Kim MJ, Park BW, Moon HJ, Kwak JY, Kim EK. Impact of preoperative ultrasonography and fine-needle aspiration of axillary lymph nodes on surgical management of primary breast cancer. Ann Surg Oncol. 2011;18(3):738–744. doi: 10.1245/s10434-010-1347-y. [DOI] [PubMed] [Google Scholar]
- 9.Jung J, Park H, Park J, Kim H. Accuracy of preoperative ultrasound and ultrasound-guided fine needle aspiration cytology for axillary staging in breast cancer. ANZ J Surg. 2010;80(4):271–275. doi: 10.1111/j.1445-2197.2009.05090.x. [DOI] [PubMed] [Google Scholar]
- 10.Sauer T, Karesen R. The value of preoperative ultrasound guided fine-needle aspiration cytology of radiologically suspicious axillary lymph nodes in breast cancer. Cytojournal. 2014;11:26. doi: 10.4103/1742-6413.141820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.MacNeill M, Arnott I, Thomas J. Fine needle aspiration cytology is a valuable adjunct to axillary ultrasound in the preoperative staging of breast cancer. J Clin Pathol. 2011;64(1):42–46. doi: 10.1136/jcp.2010.083063. [DOI] [PubMed] [Google Scholar]
- 12.Mainiero MB, Cinelli CM, Koelliker SL, Graves TA, Chung MA. Axillary ultrasound and fine-needle aspiration in the preoperative evaluation of the breast cancer patient: an algorithm based on tumor size and lymph node appearance. AJR Am J Roentgenol. 2010;195(5):1261–1267. doi: 10.2214/AJR.10.4414. [DOI] [PubMed] [Google Scholar]
- 13.Chang MC, Crystal P, Colgan TJ. The evolving role of axillary lymph node fine-needle aspiration in the management of carcinoma of the breast. Cancer Cytopathol. 2011;119(5):328–334. doi: 10.1002/cncy.20152. [DOI] [PubMed] [Google Scholar]
- 14.Rao R, Lilley L, Andrews V, Radford L, Ulissey M. Axillary staging by percutaneous biopsy: sensitivity of fine-needle aspiration versus core needle biopsy. Ann Surg Oncol. 2009;16(5):1170–1175. doi: 10.1245/s10434-009-0421-9. [DOI] [PubMed] [Google Scholar]
- 15.Krishnamurthy S, Sneige N, Bedi DG, et al. Role of ultrasound-guided fine-needle aspiration of indeterminate and suspicious axillary lymph nodes in the initial staging of breast carcinoma. Cancer. 2002;95(5):982–988. doi: 10.1002/cncr.10786. [DOI] [PubMed] [Google Scholar]
- 16.Baruah BP, Goyal A, Young P, Douglas-Jones AG, Mansel RE. Axillary node staging by ultrasonography and fine-needle aspiration cytology in patients with breast cancer. Br J Surg. 2010;97(5):680–683. doi: 10.1002/bjs.6964. [DOI] [PubMed] [Google Scholar]
- 17.Giuliano AE, Hunt KK, Ballman KV, et al. Axillary dissection vs no axillary dissection in women with invasive breast cancer and sentinel node metastasis: a randomized clinical trial. Jama. 2011;305(6):569–575. doi: 10.1001/jama.2011.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Giuliano AE, McCall L, Beitsch P, et al. Locoregional recurrence after sentinel lymph node dissection with or without axillary dissection in patients with sentinel lymph node metastases: the American College of Surgeons Oncology Group Z11 randomized trial. Ann Surg. 2010;252(3):426–432. doi: 10.1097/SLA.0b013e3181f08f32. discussion 32–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cody HS, 3rd, Houssami N. Axillary management in breast cancer: what's new for 2012? Breast. 2012;21(3):411–415. doi: 10.1016/j.breast.2012.01.011. [DOI] [PubMed] [Google Scholar]
- 20.Humphrey KL, Saksena MA, Freer PE, Smith BL, Rafferty EA. To do or not to do: axillary nodal evaluation after ACOSOG Z11 Trial. Radiographics. 2014;34(7):1807–1816. doi: 10.1148/rg.347130141. [DOI] [PubMed] [Google Scholar]
- 21.Ainsworth RK, Kollias J, Le Blanc A, De Silva P. The clinical impact of the American College of Surgeons Oncology Group Z-0011 trial--results from the BreastSurgANZ National Breast Cancer Audit. Breast. 2013;22(5):733–735. doi: 10.1016/j.breast.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 22.Ecanow JS, Abe H, Newstead GM, Ecanow DB, Jeske JM. Axillary staging of breast cancer: what the radiologist should know. Radiographics. 2013;33(6):1589–1612. doi: 10.1148/rg.336125060. [DOI] [PubMed] [Google Scholar]
- 23.Abe H, Schmidt RA, Sennett CA, Shimauchi A, Newstead GM. US-guided core needle biopsy of axillary lymph nodes in patients with breast cancer: why and how to do it. Radiographics. 2007;27(Suppl 1):S91–S99. doi: 10.1148/rg.27si075502. [DOI] [PubMed] [Google Scholar]
- 24.Lee B, Lim AK, Krell J, et al. The efficacy of axillary ultrasound in the detection of nodal metastasis in breast cancer. AJR Am J Roentgenol. 2013;200(3):W314–W320. doi: 10.2214/AJR.12.9032. [DOI] [PubMed] [Google Scholar]
- 25.Zhu Y, Zhou W, Zhou JQ, et al. Axillary Staging of Early-Stage Invasive Breast Cancer by Ultrasound-Guided Fine-Needle Aspiration Cytology: Which Ultrasound Criteria for Classifying Abnormal Lymph Nodes Should Be Adopted in the Post-ACOSOG Z11 Trial Era? J Ultrasound Med. 2016;35(5):885–893. doi: 10.7863/ultra.15.06019. [DOI] [PubMed] [Google Scholar]
- 26.Saffar B, Bennett M, Metcalf C, Burrows S. Retrospective preoperative assessment of the axillary lymph nodes in patients with breast cancer and literature review. Clin Radiol. 2015;70(9):954–959. doi: 10.1016/j.crad.2015.04.019. [DOI] [PubMed] [Google Scholar]
- 27.Amonkar SJ, Oates E, McLean L, Nicholson S. Pre-operative staging of the axilla in primary breast cancer. By redefining the abnormal appearing node can we reduce investigations without affecting overall treatment? Breast. 2013;22(6):1114–1118. doi: 10.1016/j.breast.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 28.Rautiainen S, Masarwah A, Sudah M, et al. Axillary lymph node biopsy in newly diagnosed invasive breast cancer: comparative accuracy of fine-needle aspiration biopsy versus core-needle biopsy. Radiology. 2013;269(1):54–60. doi: 10.1148/radiol.13122637. [DOI] [PubMed] [Google Scholar]
- 29.Fleissig A, Fallowfield LJ, Langridge CI, et al. Post-operative arm morbidity and quality of life. Results of the ALMANAC randomised trial comparing sentinel node biopsy with standard axillary treatment in the management of patients with early breast cancer. Breast Cancer Res Treat. 2006;95(3):279–293. doi: 10.1007/s10549-005-9025-7. [DOI] [PubMed] [Google Scholar]
- 30.Purushotham AD, Upponi S, Klevesath MB, et al. Morbidity after sentinel lymph node biopsy in primary breast cancer: results from a randomized controlled trial. J Clin Oncol. 2005;23(19):4312–4321. doi: 10.1200/JCO.2005.03.228. [DOI] [PubMed] [Google Scholar]
- 31.Hindie E, Groheux D, Brenot-Rossi I, Rubello D, Moretti JL, Espie M. The sentinel node procedure in breast cancer: nuclear medicine as the starting point. J Nucl Med. 2011;52(3):405–414. doi: 10.2967/jnumed.110.081711. [DOI] [PubMed] [Google Scholar]
- 32.Boughey JC, Moriarty JP, Degnim AC, Gregg MS, Egginton JS, Long KH. Cost modeling of preoperative axillary ultrasound and fine-needle aspiration to guide surgery for invasive breast cancer. Ann Surg Oncol. 2010;17(4):953–958. doi: 10.1245/s10434-010-0919-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yip CH, Taib NA, Tan GH, Ng KL, Yoong BK, Choo WY. Predictors of axillary lymph node metastases in breast cancer: is there a role for minimal axillary surgery? World J Surg. 2009;33(1):54–57. doi: 10.1007/s00268-008-9782-7. [DOI] [PubMed] [Google Scholar]
- 34.Wada N, Imoto S, Yamauchi C, Hasebe T, Ochiai A. Predictors of tumour involvement in remaining axillary lymph nodes of breast cancer patients with positive sentinel lymph node. Eur J Surg Oncol. 2006;32(1):29–33. doi: 10.1016/j.ejso.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 35.Boland MR, Prichard RS, Daskalova I, et al. Axillary nodal burden in primary breast cancer patients with positive pre-operative ultrasound guided fine needle aspiration cytology: management in the era of ACOSOG Z011. Eur J Surg Oncol. 2015;41(4):559–565. doi: 10.1016/j.ejso.2015.01.011. [DOI] [PubMed] [Google Scholar]