Abstract
Background
Health systems may play an important role in identification of patients at-risk of opioid medication overdose. However, standard measures for identifying overdose risk in administrative data do not exist.
Objective
Examine the association between opioid medication overdose and 2 validated measures of non-medical use of prescription opioids within claims data.
Research Design
A longitudinal retrospective cohort study that estimated associations between overdose and non-medical use.
Subjects
Adult Pennsylvania Medicaid program 2007-2012 patients initiating opioid treatment who were: non-dual eligible, without cancer diagnosis, and not in long-term care facilities or receiving hospice.
Measures
Overdose (International Classification of Disease, 9th edition, prescription opioid poisonings codes), opioid abuse (opioid use disorder diagnosis while possessing an opioid prescription), opioid misuse (a composite indicator of number of opioid prescribers, number of pharmacies, and days supplied), and dose exposure during opioid treatment episodes.
Results
A total of 372,347 Medicaid enrollees with 583,013 new opioid treatment episodes were included in the cohort. Opioid overdose was higher among those with abuse (1.5%) compared to those without (0.2%, p<0.001). Overdose was higher among those with probable (1.8%) and possible (0.9%) misuse compared to those without (0.2%, p<0.001). Abuse (adjusted rate ratio [ARR]=1.52, 95% CI=1.10-2.10), probable misuse (ARR=1.98, 95% CI=1.46-2.67), and possible misuse (ARR=1.76, 95% CI=1.48-2.09) were associated with significantly more events of opioid medication overdose compared to those without.
Conclusions
Claims-based measures can be employed by health systems to identify individuals at-risk of overdose who can be targeted for restrictions on opioid prescribing, dispensing, or referral to treatment.
Keywords: Opioid medication overdose, health claims, opioid medication misuse and abuse
Introduction
Prescription opioid overdose (poisonings) and mortality have risen at alarming rates since 2000,1 with fatal overdoses now at 44 per day.2 This epidemic has spurred a national call to action, with the White House urging health systems to invest in surveillance, prevention, and treatment.3,4 Opioid surveillance and overdose prevention efforts, such as prescription monitoring and lock-in programs, rely on the ability of payers and healthcare systems to accurately identify patients at-risk of opioid overdose. Commonly used measures in opioid medication use and overdose surveillance include indicators of exposure, such as morphine milligram equivalents (MME).5-12 Yet, MME does not differentiate patient from prescribing behavior. There are, unfortunately, no standard indicators for measuring overdose risk in administrative data routinely available to these stakeholders.13
Researchers and health systems have employed various conceptual and operational definitions to measure non-medical use of prescription opioids13-15 that could be beneficial for identifying overdose risk. The definition of non-medical use often hinges on motivation for use, whether the drug was prescribed for those using it, or opioid use disorder symptoms.14 One promising operational definition identifies non-medical use based on addiction diagnoses. White et al.16 and Rice et al.17 developed and demonstrated criterion validity13 for a diagnosis-basedmeasure of what they labeled ‘abuse’ constituted by individuals receiving an opioid prescription while simultaneously having an opioid use disorder or poisoning diagnosis. Other researchers have identified opioid utilization patterns consistent with what is labeled ‘misuse.’ Sullivan et al.18 developed and demonstrated criterion validity13 for a utilization-based measure of misuse that combines numbers of prescribers from which patients received opioid prescriptions, numbers of pharmacies where prescriptions were filled, and days-supplied of short- and long-acting opioid medications.
It is unknown whether these tools designed for defining non-medical use predict risk of overdose. We sought to determine whether these measures, which rely on diagnoses and prescription fill behaviors, were associated with overdose. Using Pennsylvania (PA) Medicaid data, we constructed a heterogeneous cohort of patients initiating prescription opioid treatment. While claims data do not permit us to capture care for which cash was paid16-18 medication diversion outside of prescriber/pharmacy reimbursement systems19 and likely underestimate the prevalence of non-medical use of opioids—our findings provide an important advancement to the field by testing these relationships and informing data-driven health system efforts to target prevention and intervention for individuals with overdose risk. Moreover, some systems have recently implemented policies restricting access to opioids based on dosage or number of prescription fills.20,21 The measures we examine could offer valuable information that may augment existing approaches.
Methods
Design and Cohort
This investigation was a longitudinal retrospective cohort study using PA Medicaid data from 2007-2012, a state with the 8th highest overdose rate in the US and opioid prescribing above national averages.1,22 We obtained study data from the PA Department of Human Services. Based on Medicaid enrollment data, we excluded individuals from our cohort who were non-PA residents, were <18, or were dual-eligible for Medicare (due to age >64 or disability) because of inability to capture medication use. Using pharmacy claims, we then identified all individuals with a new prescription fill for ≥1 oral, transdermal, or submucosal opioid medication. Using medical claims, we excluded patients with any cancer diagnosis and individuals receiving long-term care for ≥90 days and/or hospice services (online-only Figure 1).
Given our goal was to identify whether the diagnosis-based abuse measure and/or utilization-based misuse measure predicted overdose, we constructed opioid treatment episodes based on initiation of opioid use rather than including all prevalent users. The index event was an opioid fill preceded by a 6-month period wherein patients had continuous enrollment in Medicaid. We also excluded those having any medical claims for opioid medication fills, diagnoses of opioid use disorders, or overdose during the 6 months prior to the index opioid prescription. We determined episodes would end when there was a 6-month gap between fills. A 6-month gap in fills to end episodes was selected given this timing follows previous conventions in behavioral health literature.23 Furthermore, because our key independent variables (described below) require at least 6 months to accrue necessary opioid-related behaviors to begin to construct, a 6-month gap demarcates a reasonable end of the opioid exposure that could be used to identify these risks of interest.24 Some enrollees had multiple opioid treatment episodes, and we included all episodes in our analyses meeting criteria (online-only Figure 2). This study was designated exempt by the University of Pittsburgh Institutional Review Board.
Variables
Our overdose outcome followed previously published methods for identifying prescription opioid overdose using International Classification of Disease, 9th edition (ICD-9)25 codes in health claims data.26 We included: 965.00 (opium poisoning), 965.02 (methadone poisoning), 965.09 (opiate poisoning-not elsewhere classified), E.850.1 (accidental methadone poisoning), and E.850.2 (accidental opioid poisoning-not elsewhere classified. See online-only Table 1 for ICD-10 crosswalk).26 These codes were dichotomized, indicating patients had experienced an overdose or not (no overdose=0, overdose=1) during opioid treatment. Within the Medicaid claims data analyzed for this project, these codes capture: 1) non-fatal overdoses resulting in emergency department visits, hospitalization, or other medical care and 2) fatal overdoses resulting in emergency department visits or hospitalization. We do not, however, capture overdoses occurring outside of hospitals or other healthcare settings. Although evidence suggests for every fatal overdose there are 20-30 non-fatal events,27 we cannot assess what proportion of overdose events are captured in our data.
Our indicators of opioid overdose risk followed previously published conceptualizations.16-18 The diagnosis-based indicator of abuse, called abuse hereafter, followed the White et al.16 and Rice et al.17 operationalization in that patients with an opioid use disorder (ICD-9 304.0, 304.00, 304.01, 304.02, 304.03, 304.7, 304.70, 304.71, 304.72, 304.73, 305.5, 305.50, 305.51, 305.52, 305.53. See online-only Table 1 for ICD-10 crosswalk) while possessing a prescription opioid medication were classified as positive for abuse (no abuse=0, abuse=1). Our use of the term “abuse” is not necessarily consistent with a clinical diagnosis using established criteria in the Diagnostic and Statistical Manual of Mental Disorders (DSM) but rather a construct used in the literature that can be discretely measured in administrative data. This current operationalization of abuse was modified slightly from the previously published in that poisoning codes were not included given their presence in the overdose outcome indicator (i.e., ICD-9 965.00, 965.02, 965.09). To avoid including diagnoses of opioid use disorder recorded during an overdose event, we required opioid use disorder claims to be present ≥7 days preceding overdose events (mean days between first abuse event and first overdose event was 297, SD=293.2, results not shown).
The utilization-based misuse indicator, called misuse hereafter, followed Sullivan et al.'s18 operationalization. This indicator is calculated by coding number of opioid prescribers (≤2 prescribers=0, 3-4 prescribers=1, ≥5 prescribers=2), number of pharmacies used for medication filling (≤2 pharmacies=0, 3-4 pharmacies=1, ≥5 pharmacies=2), days supplied of short-acting opioids, and days of supply of long-acting opioids (≤185 days=0, 186-240 days=1, >240 days=2) over 6-month periods. Six-month periods were subsequently summated into 1 year periods, as in the previous study.18 Total scores were divided into 3 categories: no misuse (0-1), possible misuse (2-4), and probable misuse (≥5). Our longitudinal design contains some episodes spanning multiple years and some patients having varying levels of no, possible, and probable misuse within a single continuous opioid treatment episode. Therefore, we assigned patients’ highest individual misuse score within analyses for those with multiple misuse categorizations within-episode. In addition, a quarter (n=147,057) of patient records in our dataset had missing prescriber identification numbers for pharmacy claims. Therefore, we could not calculate the misuse indicator for these individuals (see Analysis section for methods of handling missing data).
Categorical daily MME was included to compare its association with overdose to abuse and misuse given its prevalent use within the opioid medication overdose and non-medical use literature.5-12 MME was constructed by converting total within-episode opioid supply into morphine equivalents, dividing by days supplied, and coding into 4 levels (≥100 MME/day, 50-<100 MME/day, 20-<50 MME/day, <20 MME/day).28 Level of MME/day varied by individual across episodes.
Covariates were measured in each person-episode's baseline period (i.e., 6 months prior to the index opioid fill) and included age (18-29, 30-39, 40-49, 50-64 years), sex, race/ethnicity (white, minority), initial Medicaid eligibility category (General Assistance, Supplemental Security Income, Temporary Assistance for Needy Families, Waiver) with some enrollees changing type within episode, Medicaid plan type (fee-for-service, managed care organization), and urban/rural living location (Rural-Urban Continuum Codes29,30). We included measures of comorbidity as covariates (online-only Table 2), including alcohol use disorders (abuse/dependence), non-opioid drug use disorders (abuse/dependence), individual mental health disorders (adjustment, anxiety, mood, personality, miscellaneous), individual pain diagnoses (back, neck, arthritis/joint, headache/migraine), and HIV/AIDS.31 We likewise included a modified Elixhauser comorbidity index with conditions removed that were included as separate covariates. We also included methadone maintenance (procedure codes H0020/J1230), buprenorphine use (forms approved for opioid use disorder), benzodiazepine use, and muscle relaxant use. Methadone maintenance and buprenorphine use were captured in the periods following the index fill but before the overdose. We included having ≥1 emergency department visits as a health services indicator. All covariates were categorical except the comorbidity index, which was a count measure, and age and length of episodes were ordinal.
Analyses
To summarize individual demographic and health indicators within our cohort, we calculated unduplicated patient-level univariable statistics. To report overdose, abuse, misuse, and MME/day, we calculated episode-level univariable statistics. To assess the relationship between overdose and (a) abuse, (b) misuse, and (c) MME/day, we estimated an episode-level generalized linear model with general estimating equation (GEE) using log link function and Poisson distribution where follow-up time was treated as an offset term.32 Overdose events were modeled as the dependent variable across time and abuse, misuse, and MME/day were included as independent variables—each occurring in time order prior to overdose. The GEE allowed us to cluster episodes within patient, accounting for within person correlation. The exchangeable correlation matrix was employed to account for standard error correlation. To ease interpretation, we also report adjusted predicted rate of overdose events per person days for abuse, misuse, and MME/day from the model. Analyses were conducted using SAS 9.4.33 Mentioned above, prescriber identification number was missing for some cohort members not allowing us to create the misuse variable for these individuals. To account for this missing information and reduce bias associated therewith, we employed the inverse probability weighting (IPW) method. In the IPW method, we first built a logistic regression model of nonmissing/missing prescriber identification number as the outcome variable, and abuse, misuse, and all other demographic/health indicators (listed above) as predicting covariates. For each patient with nonmissing prescriber identification number, a weight was then calculated as the inverse of the estimated probability from the fitted logistic regression. These weights were incorporated into the main overdose model. A nonweighted main overdose model (complete case analysis) was also fit for comparison. The results using these approaches were consistent.
Results
Descriptive and Univariable Results
Table 1 displays cohort demographics measured at the patient-level (online-only Table 3 for comorbid health condition summary statistics). A total of 372,347 Medicaid enrollees were included in our cohort. Individuals in the cohort had a mean of 1.6 (SD=0.9) episodes, and the average follow up days for individuals within episode was 488.5 (SD=388.8. Online-only Table 3).
Table 1.
Patient-level Cohort Characteristics 2007-2012 (N=372,347)
| Characteristics | n (%) |
|---|---|
| Age, yearsa | 31.4 (11.5) |
| Female | 259,154 (69.6) |
| Race | |
| White | 203,985 (54.8) |
| Black | 108,529 (29.2) |
| Hispanic | 45,241 (12.1) |
| Other | 14,592 (3.9) |
| Urban living area | 317,197 (85.2) |
| Type of eligibility | |
| General Assistance | 42,802 (11.5) |
| Supplemental Security Income | 94,488 (25.4) |
| Temporary Assistance to Needy Families | 220,650 (59.2) |
| Waiver | 14,407 (3.9) |
| Type of health plan | |
| Managed care | 287,399 (77.2) |
| Fee-for-service | 84,948 (22.8) |
Mean (standard deviation)
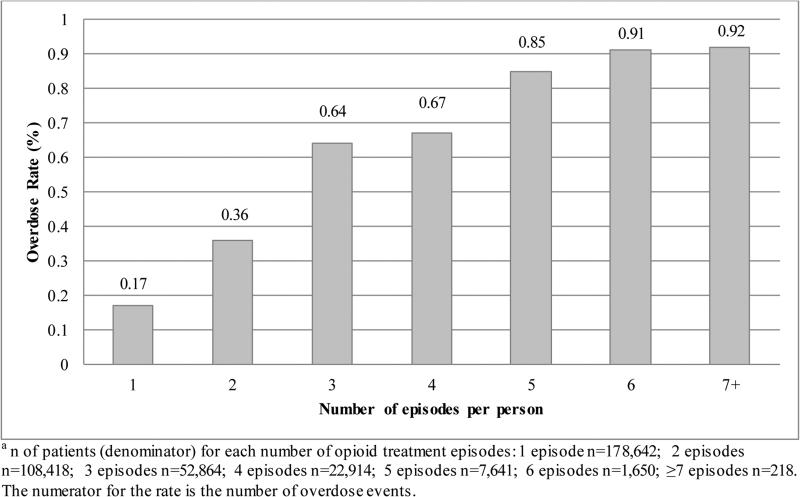
Table 2 displays episode-level results (N=583,013) and unadjusted overdose prevalence. We detected 1,270 (0.2%) total opioid overdose events across all opioid treatment episodes. Figure 1 displays the rate of overdose among patients by number of opioid treatment episodes, which ranged from 0.17% for patients with 1 episode up to 0.92% for those with ≥7 episodes.
Table 2.
Potential Risk Factors for Opioid Medication Overdose (N=583,013)
| Overdose, n (%) |
|||
|---|---|---|---|
| Potential Risk Factors | Yes | No | p |
| Totala | 1,270 (0.2) | 581,743 (99.8) | |
| Abuse | |||
| Yes | 278 (1.5) | 18,813 (98.5) | <.001 |
| No | 992 (0.2) | 562,930 (99.8) | |
| Categorical misuse | |||
| Probable misuse | 51 (1.8) | 2,829 (98.2) | |
| Possible misuse | 180 (0.9) | 20,713 (99.1) | <.001 |
| Missing misuseb | 236 (0.2) | 145,406 (99.8) | |
| No misuse | 803 (0.2) | 412,795 (99.8) | |
| Categorical MEM, MEM/day | |||
| <20 | 200 (0.2) | 91,150 (99.8) | |
| 20-49.9 | 810 (0.2) | 390,772 (99.8) | <.001 |
| 50-99.9 | 190 (0.2) | 84,337 (99.8) | |
| ≥100 | 70 (0.5) | 15,484 (99.5) | |
Counted for all eligible episodes.
145,642 (25.0%) records missing provider ID missing in the claim files.
Figure 1. Overdose Rate Based on Number of Opioid Treatment Episodes per Person (N=372,347) a.
This figure contains overdose rates for patients within the cohort by the number of individual opioid treatment episodes they have.
Table 2 also displays unadjusted frequencies of abuse, misuse, and MME/day by overdose. We detected a total of 19,019 (3.3%) instances of abuse prior to overdose. The rate of opioid overdose was significantly higher among those with abuse (1.5%) compared to those without (0.2%, p<0.001). A relatively small share of opioid treatment episodes were marked by misuse before overdose, with 2,880 (0.5%) having probable misuse and 20,893 (3.6%) with possible misuse (results not shown). Overdose was significantly higher among those with probable (1.8%) and possible (0.9%) misuse compared to those without (0.2%, p<0.001).
Multivariable Results
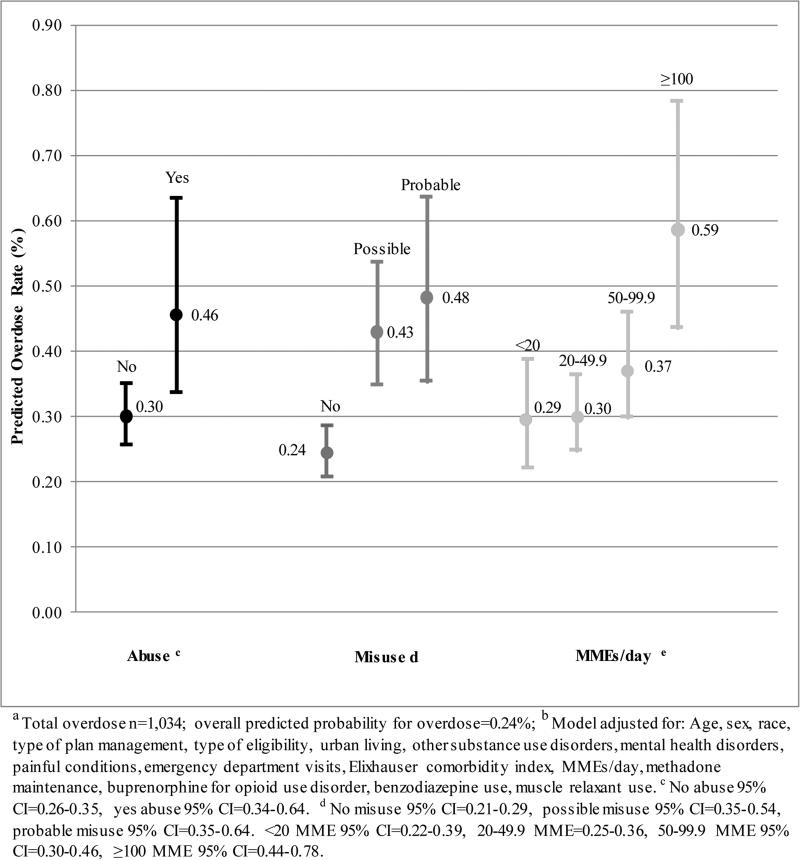
In the multivariable model (Table 3), the strongest opioid consumption overdose indicators were probable misuse (adjusted rate ratio [ARR]=1.98, 95% CI=1.46-2.67) and possible misuse (ARR=1.76, 95% CI=1.48-2.09), compared to no misuse. Individuals who had opioid medication abuse had a 52% higher rate overdose (95% CI=1.10-2.10) compared to those without. Consuming ≥100 MME/day was associated with higher a rate overdose compared to those with <20 MME/day (ARR=1.96, 95% CI=1.36-2.83). Figure 2 reports adjusted rates for overdose per average person days (where 488.5 was the average number of follow-up days for subjects in the cohort). The predicted number of overdose events based on the multivariable results was 0.46 per average person days among those with abuse compared to 0.30 with no abuse. The rate of overdose was 0.48 among those with probable misuse and 0.43 among those with possible misuse, compared to 0.24 among those with no misuse. Individuals with ≥100 MME/day had a predicted overdose rate of 0.59 compared to 0.29-0.37 for those at lower doses.
Table 3.
Generalized Linear Model Estimates and Adjusted Rate Ratios for Overdosea
| Predictors | Estimate (SE) | ARR (95% CI) | P Value |
|---|---|---|---|
| Opioid use risk factors | |||
| Diagnosis-based abuse | 0.42 (0.17 ) | 1.52 (1.10-2.10 ) | .01 |
| Utilization-based misuse (reference=No misuse) | |||
| Possible misuse | 0.56 (0.09 ) | 1.76 (1.48-2.09 ) | <.001 |
| Probable misuse | 0.68 (0.15 ) | 1.98 (1.46-2.67 ) | <.001 |
| Morphine milligram equivalents/day (reference=<20MMEs/day) | |||
| 20-49.9 | 0.02 (0.10 ) | 1.02 (0.85-1.23 ) | .83 |
| 50-99.9 | 0.23 (0.13 ) | 1.26 (0.97-1.63 ) | .08 |
| ≥100 | 0.67 (0.19 ) | 1.96 (1.36-2.83 ) | <.001 |
|
Demographics | |||
| Age, y | −0.16 (0.04 ) | 0.86 (0.79-0.93 ) | <.001 |
| Female | −0.46 (0.08 ) | 0.63 (0.54-0.74 ) | <.001 |
| White | 0.44 (0.12 ) | 1.55 (1.22-1.96 ) | <.001 |
| Urban living area | 0.57 (0.12 ) | 1.78 (1.39-2.27 ) | <.001 |
|
Medicaid enrollment and eligibility | |||
| Fee-for-service | −0.60 (0.25 ) | 0.55 (0.34-0.90 ) | .02 |
| Type of eligibility (reference=TANFb) | |||
| General assistance | 0.06 (0.11 ) | 1.06 (0.85-1.32 ) | .62 |
| Supplemental Security Income | 0.08 (0.08 ) | 1.08 (0.92-1.27 ) | .33 |
| Waiver | −0.56 (0.28 ) | 0.57 (0.33-0.98 ) | .04 |
|
Comorbidities and health service use | |||
| Alcohol abuse/dependence | 0.43 (0.12 ) | 1.54 (1.22-1.94 ) | <.001 |
| Drug abuse/dependence | 0.42 (0.11 ) | 1.52 (1.22-1.89 ) | <.001 |
| Adjustment disorders | 0.18 (0.18 ) | 1.20 (0.84-1.71 ) | .32 |
| Anxiety disorders | 0.25 (0.09 ) | 1.28 (1.07-1.54 ) | .01 |
| Mood disorders | 0.27 (0.08 ) | 1.31 (1.11-1.54 ) | .001 |
| Personality disorders | 0.23 (0.24 ) | 1.26 (0.79-2.01 ) | .33 |
| Miscellaneous mental health disorders | −0.18 (0.16 ) | 0.83 (0.61-1.15 ) | .26 |
| Back pain | 0.04 (0.10 ) | 1.04 (0.86-1.26 ) | .70 |
| Neck pain | −0.09 (0.13 ) | 0.91 (0.71-1.17 ) | .46 |
| Arthritis /joint pain | −0.10 (0.08 ) | 0.90 (0.77-1.06 ) | .22 |
| Headache/migraine pain | −0.22 (0.17 ) | 0.81 (0.58-1.12 ) | .20 |
| HIV/AIDS | 0.06 (0.29 ) | 1.06 (0.60-1.87 ) | .84 |
| Emergency department visits | 0.49 (0.08 ) | 1.64 (1.40-1.92 ) | <.001 |
| Elixhauser comorbidity index | −0.05 (0.04 ) | 0.95 (0.88-1.03 ) | .23 |
|
Other medication use | |||
| Any muscle relaxant use | 0.22 (0.10 ) | 1.25 (1.02-1.53 ) | .03 |
| Any benzodiazepine use | 0.35 (0.10 ) | 1.42 (1.17-1.73 ) | <.001 |
| Methadone maintenance | 0.64 (0.15 ) | 1.89 (1.40-2.56 ) | <.001 |
| Buprenorphine for opioid use disorder | −0.41 (0.17 ) | 0.66 (0.47-0.93 ) | .02 |
Poisson regression with log link function is used. Model is offset by log days of follow-up to account for individual exposure to opioid treatment within episode.
Temporary Assistance to Needy Families.
Figure 2. Predicted Overdose Rate with 95% Confidence Intervals by Abuse, Misuse, and Daily Morphine Milligram Equivalents (MMEs/day) Adjusted for All Covariates and Offset by Log Length of Episode a,b.
This figure contains the adjusted predicted overdose rates for opioid abuse, misuse, and daily morphine milligram equivalents.
Additionally, methadone agonist therapy claims (ARR=1.89, 95% CI=1.40-2.56) and having ≥1 emergency department visits were associated with higher rates for overdose (ARR=1.64, 95% CI=1.40-1.92). Alcohol use disorders (ARR=1.54, 95% CI=1.22-1.94) and non-opioid drug use disorders (ARR=1.52, 95% CI=1.22-1.89) were associated with higher rates of overdose. Any benzodiazepine use (ARR=1.42, 95% CI=1.17-1.73), mood disorders (ARR=1.31, 95% CI=1.11-1.54), anxiety disorders (ARR=1.28, 95% CI=1.07-1.54), and any muscle relaxant use (ARR=1.25, 95% CI=1.02-1.53) were also associated with overdose.
Discussion
Our results show these claims-based measures of non-medical use of prescription opioids, abuse and misuse, were associated with some of the highest rates of overdose—even when adjusted for exposure of daily opioid dosages (MME/day). No previous studies, to our knowledge, have examined abuse and misuse indicators as predictors of overdose, particularly in a longitudinal cohort. These potential risk factors constructed with claims data can be measured by payers to identify individuals at-risk for opioid overdose and potentially better differentiate patient from prescribing behavior compared to other measures, such as daily dosages.
Diagnosis-based Abuse and Utilization-based Misuse
One of the strongest indicators associated with overdose was abuse. Active management of opioid prescriptions among patients with opioid use disorders must be a continual priority for practitioners.34-36 Health systems should actively identify these patients for intervention and referral. Furthermore, this measure requires less data to construct and is conceptually simpler than the misuse indicator and could be amenable to plan/programs seeking to employ more easily implemented validated metrics of overdose risk.
Misuse was also associated with overdose and has the potential to be valuable for health systems to monitor potentially modifiable behaviors and/or filling patterns. This indicator encompasses patient prescription seeking behaviors (e.g., “shopping”), including number of prescribers and pharmacies where opioids were filled and long- and short-acting opioids. Prescription claims do not necessarily lead to consumption, and certainly, absence of a fill does not necessarily reflect lack of use.19 Nevertheless, health systems (such as payers, prescription monitoring programs, which are active in nearly all US states) and pharmacy benefits managers could direct interventions at the components of the misuse indicator to reduce overdose risk. For example, systems could reduce or cap numbers of prescribers from whom patients receive medications or limit patients to filling prescriptions at a particular pharmacy through ‘lock-in’ programs.37 Promising data show success for these programs in reducing volume of medication prescribed, lowering costs, and increasing medication adherence.38 Lock-in programs are recommended by the Centers for Medicare and Medicaid Services as possible tools for improving monitoring and reducing diversion.39 Despite concerns in the field regarding restricting access to opioids for pain management,40 recent research has documented most heroin users engaged in non-medical use of prescription opioids prior to initiating heroin.41-42 This fact underscores the importance of better managing access to these medications to mitigate risk for transitioning from prescription opioids to heroin.
It is important to note that despite the relationships evident between overdose and abuse and misuse, many opioid treatment episodes with overdose events did not have these conditions present. On a population level, these indicators can be important tools for health systems to identify overdose risk. Nevertheless, some individuals will not be identified using these metrics because, for instance, they obtain opioids outside the healthcare system, they have not been recognized as suffering from an opioid use disorder, or other reasons. To complement claims-based measures such as those we examine, additional data sources from electronic health record systems and other sources could be explored as this research continues. Given lags in claims submission (greater than 6 months for some healthcare providers), more “real-time” data from electronic health records and other sources could be examined in order to facilitate more rapid risk identification.
Other Risk Factors
Additional risk factors were identified in our analyses. The larger the number of opioid treatment episodes was associated with higher overdose rates. Health systems have the capability to monitor patients regularly going on and off of opioid medications. Moreover, concomitant prescriptions of other psychoactive/high abuse potential medications were associated with some of the highest odds for overdose compared to other covariates. Such prescribing practices should be the target of formulary management tools that, if triggered, would initiate system warnings, prior authorization, and/or medication review. We recognize, however, our design intentionally excluded previous overdose in order to create a clean baseline period. Given evidence showing that non-fatal overdose is a risk factor for subsequent overdose,43 health systems may consider incorporating this information into surveillance systems.
A number of other mental, behavioral, and physical health conditions were related to increased risk for overdose. The relationship between co-occurring health conditions and overdose has been documented previously8,44,45 and calls for multidimensional monitoring by health systems.
Limitations
Our study has some important limitations. Despite advantages in our study, which include PA having high opioid use and overdose rates,1,22 the PA Medicaid program being among the largest in the country, and these analyses containing both fee-for-service and managed care claims—46-48 the results herein represent one state in the northeastern US. Furthermore, Medicaid populations tend to predominantly consist of Temporary Assistance to Needy Families recipients. Thus, our findings may not generalize to other regions or populations. We note specifically that we lack prescription data on the elderly dually enrolled in Medicare. Furthermore, our overdose measure was based on ICD-9 codes from inpatient or emergency department claims. Therefore, we do not capture overdoses occurring outside of hospital. We also may both underestimate and overestimate misuse of prescription opioids using claims data because we are unable to measure diversion of opioid medications. Yet, the constellation of factors constituting the abuse and misuse indicators have been generally documented to be associated with unhealthy and problematic consumption patterns5,7,8,16-18 and warrant observation and possible scrutiny. We also recognize opioid use disorders are likely underdiagnosed and under-coded within claims.49 Our claims-based measures of medication use and diagnoses may have poor sensitivity and specificity due to the nature of substance abuse coding and prescription opioid diversion. Nevertheless, our findings show strong associations between several claims-based risk factors and overdose—a first step in establishing a paramount line of scientific inquiry. Prospective research should seek to further develop and refine overdose risk prediction tools. Finally, we acknowledge the association between overdose and methadone maintenance therapy in our findings (ARR: 1.89, 95% CI=1.40-2.56). To examine the contribution methadone maintenance makes within the overdose outcome model, we removed this indicator and re-estimated the model. The only substantive difference was the ARR between abuse and overdose increased from 1.52 (95% CI=1.10-2.10) to 1.86 (95% CI=1.38-2.50), suggesting some variance accounted for by abuse is shared by methadone maintenance. These results do not indicate engaging in methadone treatment is in itself a risk for overdose but may contribute to some degree. Our future research will seek to better understand this relationship in order to provide recommendations to the field on how to maximize safety and benefit for these individuals.
Conclusion
Health systems possess rich data resources with the potential to be employed for enhancing national efforts in monitoring and targeting interventions against opioid overdose. Our findings indicate the importance of systems identifying those at-risk of overdose and working to implement policies and programs to protect patient health. Our findings should be replicated within other Medicaid and commercial plans, as well as they should be prospectively examined. Confirming these results would provide added support for systems to respond to urging from the White House to work and invest in integrating surveillance infrastructure into claims management systems. Indeed, the same technologies that pharmacists utilize to verify insurance benefits could be programmed with abuse and misuse indicators that would alert pharmacists at point of service. If patients’ cumulative behaviors triggered misuse alerts, for instance, these could be followed with patient-focused medication therapy management interventions50 and naloxone referrals. Payers similarly could regularly run abuse and misuse algorithms that once triggered would initiate system-level interventions (e.g., lock-in, prior authorization). Such efforts could help curb these behaviors and safeguard patient health.
Supplementary Material
Acknowledgments
Funding
Drs. Cochran, Donohue, Gordon, and Gellad are supported by a grant from the Centers for Disease Control and Prevention (U01CE002496). This work was also supported in part by an intergovernmental agreement between the Pennsylvania Department of Human Services and the University of Pittsburgh.
Footnotes
Conflicts
None
Contributor Information
Gerald Cochran, University of Pittsburgh, School of Social Work, University of Pittsburgh, School of Medicine, Department of Psychiatry, University of Pittsburgh, Center for Pharmaceutical Policy and Prescribing, 4200 Forbes Ave., 2117 CL, Pittsburgh, PA 15260.
Adam J. Gordon, VA Pittsburgh Healthcare System, University of Pittsburgh, School of Medicine, Division of General Internal Medicine, University of Pittsburgh, Center for Pharmaceutical Policy and Prescribing, University Drive C (151-C), Pittsburgh, PA 15240, Phone: 412-360-2214, adam.gordon@va.gov.
Wei-Hsuan Lo-Ciganic, University of Arizona College of Pharmacy, 1295 N Martin Ave, Tucson, AZ 85721, Phone: 520-626-9535, lociganic@pharmacy.arizona.edu.
Walid F. Gellad, VA Pittsburgh Healthcare System, University of Pittsburgh, School of Medicine, Division of General Internal Medicine, University of Pittsburgh, Center for Pharmaceutical Policy and Prescribing, University Drive C (151-C), Pittsburgh, PA 15240, Phone: 412-360-2267, walid.gellad@pitt.edu.
Winfred Frazier, University of Pittsburgh, School of Medicine, Division of General Internal Medicine, 5475 Penn Ave, Pittsburgh, PA 15206, wtf8@pitt.edu.
Carroline Lobo, University of Pittsburgh, Graduate School of Public Health, 130 De Soto St, Pittsburgh, PA 15261, Phone: 412-624-9141, cpl13@pitt.edu.
Joyce Chang, University of Pittsburgh School of Medicine, University of Pittsburgh Graduate School of Public Health, 200 Meyran Avenue, Pittsburgh, PA 15213, Phone: 412-692-4868, changjh@upmc.edu.
Ping Zheng, University of Pittsburgh, Graduate School of Public Health, 130 De Soto St, Pittsburgh, PA 15261, Phone: 412-624-3086, piz3@pitt.edu.
Julie M. Donohue, University of Pittsburgh, Graduate School of Public Health, University of Pittsburgh, Center for Pharmaceutical Policy and Prescribing, 130 De Soto St, Pittsburgh, PA 15261, 412-624-4562, jdonohue@pitt.edu.
References
- 1.Rudd R, Aleshire N, Zibbell J, Gladden M. Increases in Drug and Opioid Overdose Deaths — United States, 2000–2014. Centers for Disease Control and Prevention; Atlanta, GA: 2015. [Google Scholar]
- 2.Centers for Disease Control and Prevention [July 16, 2015];Prescription Drug Overdose Data: Deaths from Prescription Opioid Overdose. 2015 http://www.cdc.gov/drugoverdose/data/overdose.html.
- 3.White House . Obama Administration Announces Public and Private Sector Efforts to Address Prescription Drug Abuse and Heroin Use [press release] Office of the Press Secretary; Washington DC: 2015. [Google Scholar]
- 4.Haegerich TM, Paulozzi LJ, Manns BJ, Jones CM. What we know, and don’t know, about the impact of state policy and systems-level interventions on prescription drug overdose. Drug Alcohol Depend. 2014;145:34–47. doi: 10.1016/j.drugalcdep.2014.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Logan J, Liu Y, Paulozzi L, Zhang K, Jones C. Opioid prescribing in emergency departments: the prevalence of potentially inappropriate prescribing and misuse. Med Care. 2013;51:646–653. doi: 10.1097/MLR.0b013e318293c2c0. [DOI] [PubMed] [Google Scholar]
- 6.Mack KA, Zhang K, Paulozzi L, Jones C. Prescription practices involving opioid analgesics among Americans with Medicaid, 2010. J Health Care Poor Underserved. 2015;26:182–198. doi: 10.1353/hpu.2015.0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu Y, Logan JE, Paulozzi LJ, Zhang K, Jones CM. Potential misuse and inappropriate prescription practices involving opioid analgesics. Am J Manag Care. 2013;19:648–665. [PubMed] [Google Scholar]
- 8.Bohnert ASB, Valenstein M, Bair MJ, et al. Association between opioid prescribing patterns and opioid overdose-related deaths. JAMA. 2011;305:1315–1321. doi: 10.1001/jama.2011.370. [DOI] [PubMed] [Google Scholar]
- 9.Paulozzi LJ, Strickler GK, Kreiner PW, Koris CM. Controlled Substance Prescribing Patterns--Prescription Behavior Surveillance System, Eight States, 2013. Morbidity and mortality weekly report. Surveillance summaries. 2015;64:1–14. doi: 10.15585/mmwr.ss6409a1. [DOI] [PubMed] [Google Scholar]
- 10.Dasgupta N, Funk MJ, Proescholdbell S, Hirsch A, Ribisl KM, Marshall S. Cohort Study of the Impact of High-dose Opioid Analgesics on Overdose Mortality. Pain Med. 2015 doi: 10.1111/pme.12907. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 11.Gwira Baumblatt JA, Wiedeman C, Dunn JR, Schaffner W, Paulozzi LJ, Jones TF. High-risk use by patients prescribed opioids for pain and its role in overdose deaths. JAMA Intern Med. 2014;174:796–801. doi: 10.1001/jamainternmed.2013.12711. [DOI] [PubMed] [Google Scholar]
- 12.Dunn KM, Saunders KW, Rutter CM, et al. Opioid prescriptions for chronic pain and overdose: a cohort study. Ann Intern Med. 2010;152:85–92. doi: 10.1059/0003-4819-152-2-201001190-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cochran G, Woo B, Lo-Ciganic W-H, Gordon AJ, Donohue JM, Gellad WF. Defining nonmedical use of prescription opioids within health care claims: a systematic review. Subst Abus. 2015;36:192–202. doi: 10.1080/08897077.2014.993491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barrett SP, Meisner JR, Stewart SH. What constitutes prescription drug misuse? Problems and pitfalls of current conceptualizations. Curr Drug Abuse Rev. 2008;1:255–262. doi: 10.2174/1874473710801030255. [DOI] [PubMed] [Google Scholar]
- 15.Compton WM, Volkow ND. Abuse of prescription drugs and the risk of addiction. Drug Alcohol Depend. 2006;83(Suppl 1):S4–7. doi: 10.1016/j.drugalcdep.2005.10.020. [DOI] [PubMed] [Google Scholar]
- 16.White AG, Birnbaum HG, Schiller M, Tang J, Katz NP. Analytic models to identify patients at risk for prescription opioid abuse. Am J Manag Care. 2009;15:897–906. [PubMed] [Google Scholar]
- 17.Rice JB, White AG, Birnbaum HG, Schiller M, Brown DA, Roland CL. A Model to Identify Patients at Risk for Prescription Opioid Abuse, Dependence, and Misuse. Pain Medicine. 2012;13:1162–1173. doi: 10.1111/j.1526-4637.2012.01450.x. [DOI] [PubMed] [Google Scholar]
- 18.Sullivan MD, Edlund MJ, Fan M-Y, Devries A, Brennan Braden J, Martin BC. Risks for possible and probable opioid misuse among recipients of chronic opioid therapy in commercial and Medicaid insurance plans. The TROUP Study Pain. 2010;150:332–339. doi: 10.1016/j.pain.2010.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Substance Abuse and Mental Health Services Administration . Results from the 2013 National Survey on Drug Use and Health: Summary of National Findings. Substance Abuse and Mental Health Services Administration; Rockville, MD: 2014. [Google Scholar]
- 20.Morden NE, Zerzan JT, Rue TC, et al. Medicaid prior authorization and controlled-release oxycodone. Med Care. 2008;46(6):573–580. doi: 10.1097/MLR.0b013e31816493fb. [DOI] [PubMed] [Google Scholar]
- 21.Centers for Medicare and Medicaid Services . Best Practices for Addressing Prescription Opioid Overdoses, Misuse and Addiction. Centers for Medicare and Medicaid Services; Baltimore, MD: 2016. [Google Scholar]
- 22.Centers for Disease Control . Prevention Status Report 2013: Prescription Drug Overdose. Centers for Disease Control; Pennsylvania. Atlanta, GA: 2013. [Google Scholar]
- 23.Melfi CA, Croghan TW. Use of claims data for research on treatment and outcomes of depression care. Med Care. 1999;37(4 Suppl Lilly):As77–80. doi: 10.1097/00005650-199904001-00010. [DOI] [PubMed] [Google Scholar]
- 24.Hornbrook MC, Hurtado AV, Johnson RE. Health Care Episodes: Definition, Measurement and Use. Med Care Res Rev. 1985;42(2):163–218. doi: 10.1177/107755878504200202. [DOI] [PubMed] [Google Scholar]
- 25.World Health Organization . International Statistical Classification of Diseases and Related Health Problems, 9th Edition (ICD-9) World Health Organization; Geneva: 2011. [Google Scholar]
- 26.Unick GJ, Rosenblum D, Mars S, Ciccarone D. Intertwined epidemics: national demographic trends in hospitalizations for heroin- and opioid-related overdoses, 1993-2009. Plos One. 2013;8(2):e54496–e54496. doi: 10.1371/journal.pone.0054496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Darke S, Mattick RP, Degenhardt L. The ratio of non-fatal to fatal heroin overdose. Addiction. 2003;98(8):1169–1171. doi: 10.1046/j.1360-0443.2003.00474.x. [DOI] [PubMed] [Google Scholar]
- 28.Prescription Drug Monitoring Program training and Technical Assistance Center . Technical Assistance Guide No. 01-13, Calculating Daily Morphine, Milligram Equivalents. Brandeis University, The Heller School for Policy and Management; Boston, MA: 2013. [Google Scholar]
- 29.United States Department of Agriculture [October 3, 2014];Rural-Urban Continuum Codes. 2013 http://www.ers.usda.gov/data-products/rural-urban-continuum-codes/documentation.aspx#.VC61zGddUrA.
- 30.Baldwin LM, Andrilla CH, Porter MP, Rosenblatt RA, Patel S, Doescher MP. Treatment of early-stage prostate cancer among rural and urban patients. Cancer. 2013;119:3067–3075. doi: 10.1002/cncr.28037. [DOI] [PubMed] [Google Scholar]
- 31.Sullivan MD, Edlund MJ, Fan M-Y, DeVries A, Brennan Braden J, Martin BC. Trends in use of opioids for non-cancer pain conditions 2000–2005 in Commercial and Medicaid insurance plans. The TROUP study Pain. 2008;138:440–449. doi: 10.1016/j.pain.2008.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.SAS Institute Inc. SAS/STAT(R) 9.22 Users Guide . The GENMOD Procedure. SAS Institute Inc; Cary, NC: 2010. [Google Scholar]
- 33.SAS 9.4 [computer program] Cary; p. NC2013. [Google Scholar]
- 34.Chang Y-P, Compton P. Management of chronic pain with chronic opioid therapy in patients with substance use disorders. Addict Sci Clin Pract. 2013;8:21. doi: 10.1186/1940-0640-8-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Savage SR, Kirsh KL, Passik SD. Challenges in Using Opioids to Treat Pain in Persons With Substance Use Disorders. Addict Sci Clin Pract. 2008;4:4–25. doi: 10.1151/ascp08424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nuckols TK, Anderson L, Popescu I, et al. Opioid prescribing: a systematic review and critical appraisal of guidelines for chronic pain. Ann Intern Med. 2014;160:38–47. doi: 10.7326/0003-4819-160-1-201401070-00732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roberts AW, Skinner AC. Assessing the present state and potential of Medicaid controlled substance lock-in programs. J Manag Care Spec Pharm. 2014;20:439–446c. doi: 10.18553/jmcp.2014.20.5.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Centers for Disease Control and Prevention . Patient Review & Restriction Programs Lessons learned from state Medicaid programs. Centers for Disease Control and Prevention; Atlanta, GA: 2012. [Google Scholar]
- 39.Centers for Medicare and Medicaid Services . Drug Diversion in the Medicaid Program: State Strategies for Reducing Prescription Drug Diversion in Medicaid. Department of Health and Human Services, Centers for Medicare and Medicaid Services, Center for Program Integrity; Baltimore, MD: 2012. [Google Scholar]
- 40.Alford DP. Opioid Prescribing for Chronic Pain — Achieving the Right Balance through Education. N Engl J Med. 2016;374:301–303. doi: 10.1056/NEJMp1512932. [DOI] [PubMed] [Google Scholar]
- 41.Cicero TJ, Ellis MS, Surratt HL, Kurtz SP. The changing face of heroin use in the United States: a retrospective analysis of the past 50 years. JAMA Psychiatry. 2014;71:821–826. doi: 10.1001/jamapsychiatry.2014.366. [DOI] [PubMed] [Google Scholar]
- 42.Cicero TJ, Ellis MS. Abuse-Deterrent Formulations and the Prescription Opioid Abuse Epidemic in the United States: Lessons Learned From OxyContin. JAMA Psychiatry. 2015;72:424–430. doi: 10.1001/jamapsychiatry.2014.3043. [DOI] [PubMed] [Google Scholar]
- 43.Hall AJ, Logan JE, Toblin RL, et al. Patterns of abuse among unintentional pharmaceutical overdose fatalities. JAMA. 2008;300(22):2613–2620. doi: 10.1001/jama.2008.802. [DOI] [PubMed] [Google Scholar]
- 44.Webster LR, Cochella S, Dasgupta N, et al. An analysis of the root causes for opioid-related overdose deaths in the United States. Pain Med. 2011;12(Suppl 2):S26–S35. doi: 10.1111/j.1526-4637.2011.01134.x. [DOI] [PubMed] [Google Scholar]
- 45.Centers for Disease Control and Prevention Overdose deaths involving prescription opioids among Medicaid enrollees- Washington, 2004-2007. MMWR. Morbidity And Mortality Weekly Report. 2009;58:1171–1175. [PubMed] [Google Scholar]
- 46.Centers for Disease Control and Prevention . Health, United States 2012: With Special Feature on Emergency Care. Centers for Disease Control; Hyattsville, MD: 2013. [Google Scholar]
- 47.Dartmouth [March 8, 2014];The Dartmouth Atlas of Health Care. 2014 http://www.dartmouthatlas.org/.
- 48.Pating DR, Miller MM, Goplerud E, Martin J, Ziedonis DM. New systems of care for substance use disorders: treatment, finance, and technology under health care reform. Psychiatr Clin North Am. 2012;35:327–356. doi: 10.1016/j.psc.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 49.Kim HM, Smith EG, Stano CM, et al. Validation of key behaviourally based mental health diagnoses in administrative data: suicide attempt, alcohol abuse, illicit drug abuse and tobacco use. BMC Health Serv Res. 2012;12(1):1–9. doi: 10.1186/1472-6963-12-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Viswanathan M, Kahwati LC, Golin CE, et al. Medication therapy management interventions in outpatient settings: A systematic review and meta-analysis. JAMA Intern Med. 2015;175(1):76–87. doi: 10.1001/jamainternmed.2014.5841. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.