Abstract
Background
Single ventricle patients experience a high rate of brain injury and adverse neurodevelopmental outcome, however, the incidence of brain abnormalities throughout surgical reconstruction and its relationship with cerebral blood flow, oxygen delivery and carbon dioxide reactivity remains unknown.
Methods
Single ventricle patients were studied with MRI scans immediately prior to bidirectional Glenn (pre-BDG), prior to Fontan and then 3–9 months after Fontan reconstruction.
Results
One hundred and sixty eight consecutive subjects recruited into the project underwent 235 scans: 63 pre-BDG (mean age 4.8+1.7 months), 118 BDG (2.9+1.4 years) and 54 after Fontan (2.4+1.0 years). Non-acute ischemic white matter changes on T2 weighted imaging, focal tissue loss, and ventriculomegaly were all more commonly detected in BDG and Fontans compared to pre-BDG (P<0.05). BDG patients has significantly higher CBF than Fontan patients. The odds of discovering brain injury adjusting for surgical stage as well as 2 or more co-existing lesions within a patient all decreased (63–75% and 44% respectively) with increasing amount of cerebral blood flow (P<0.05). In general, there was no association of oxygen delivery (with the exception of ventriculomegaly in the BDG group) or carbon dioxide reactivity with neurological injury.
Conclusion
Significant brain abnormalities are commonly present in single ventricle patients and detection of these lesions increase as children progress through staged surgical reconstruction with multiple co-existing lesions more common earlier than later. In addition, this study demonstrated that BDG patients had greater CBF than Fontan patients and that there exists an inverse association of various indices of CBF with these brain lesions, however, CO2 reactivity, oxygen delivery (with one exception) were not associated with brain lesion development.
Keywords: Heart defects-congenital, Cerebrovascular disorders, Magnetic resonance imaging, Pediatrics, Fontan procedure
Besides a high risk of mortality and adverse outcomes,1 single ventricle patients undergoing staged surgical reconstruction culminating in the Fontan operation face a well-known concern for poor neurologic outcome due to both brain injury and delayed maturation.2,3,4,5,6,7 This has presumably led to the findings of poor neurodevelopmental outcome in this patient population.8,9,10,11 The etiology of these brain lesions is multifactorial and includes genetics, cyanosis and numerous surgeries which involve cardiopulmonary bypass and deep hypothermic circulatory arrest. In addition, the multiple surgeries these children undergo change their physiology, pulmonary and systemic blood flow patterns and hemodynamics over the course of their vulnerable first years of life which may also contribute to the genesis of this issue.
The purpose of this study was to determine the prevalence of brain abnormalities in single ventricle patients as they progress through staged surgical reconstruction and if a link exists between cerebral blood flow (CBF) and oxygen delivery (O2D) with cerebral abnormalities. Patients were assessed by cardiac magnetic resonance (CMR) and brain magnetic resonance imaging (MRI) immediately prior to hemiFontan or bidirectional Glenn (pre-BDG), immediately prior to Fontan (BDG) and 3–9 months after Fontan. We speculated that the CBF, O2D and CO2 reactivity (i.e., hypercarbia increases CBF) at various stages would correlate with brain abnormalities. The importance of these findings are not only to determine the etiology of such lesions but may lead to important changes in the approach to surgical and medical management of these patients in order to preserve maximal neurologic function.
Material and Methods
Patients
This was a single center, NIH sponsored, prospective study of single ventricle patients throughout staged surgical reconstruction; all were enrolled from April 2009 – June 2014. The inclusion criteria included any patient < 10 years of age with single ventricle physiology undergoing elective cardiac reconstructive surgery at our institution. The patient needed to be stable enough to undergo an approximately 1-hour CMR and MRI scan under general anesthesia. Exclusion criteria included contraindication to CMR and MRI imaging (e.g., pacemakers or any contraindicated ferromagnetic material). Demographics obtained included age, gender, body surface area, diagnosis and stage of surgical reconstruction. Informed consent for participation was obtained from all participants’ families. The hospital’s Institutional Review Board approved the prospective study and all patients gave informed consent.
Study procedure
Patients underwent a CMR and MRI immediately prior to heart surgery for pre-BDG and BDG and 3–9 months after Fontan completion. In those prior to surgery, the patient was prepared in the operating room with intravenous and arterial line placement; all participants were administered general anesthesia of nitrous oxide and sevoflourane of < 1 MAC, were paralyzed, endotracheally or nasotracheally intubated and mechanically ventilated using a minute ventilation to achieve a PaCO2 of 40+2 mm Hg. The patient was then transported via stretcher to the adjacent scanner (Siemens Avanto 1.5 Tesla whole body MRI system, Siemens Medical Solutions, Malvern, PA). The patient was placed in the supine position, head first into the scanner utilizing the 6-channel head coil and 8-channel body array coil; all imaging was performed at isocenter. Baseline CBF and CMR imaging was performed before 3–7 % CO2 was instilled into the inhaled gas to create hypercarbic conditions, increasing CBF; this lasted 15–20 minutes during which time cerebral anatomy imaging was performed. At the end of the 20 minutes of hypercarbia measurement of flows were repeated. Hypercarbic conditions aimed for a PaCO2 in the 60s mm Hg on arterial blood gas. In those who were 3–9 months after Fontan, only room air conditions were studied since an arterial line was not present to measure arterial blood gases. Studies lasted approximately one hour; afterwards, the patient was immediately removed from the scan room and transported either to the operative suite where surgery was performed or to the recovery area. On completion of the MRI, the study was read by a staff neuroradiologist and a determination made to proceed to surgery or to wake child in discussions with the neurologist and surgeon.12
CMR and MRI Protocol
To measure CBF and oxygen delivery, two methodologies were used; a) phase contrast magnetic resonance (PCMR) and b) arterial spin labeling (ASL).
- PCMR: After localizers, a stack of static steady state free precession images of the thorax were obtained to assess cardiovascular anatomy and to obtain the exact slice positions and orientations for retrospective, ECG-gated, through plane PCMR which was performed across: a) right and left jugular veins, b) superior (SVC) and c) the aorta or neoaorta. Velocity encodings (VENC) across the veins were initially performed at 60 cm/sec and 150 cm/sec across the aorta; if blood flow exceeded that, a higher VENC was then used. Multiple excitations were used to offset respiratory motion. The sum of the jugular veins was considered CBF and O2D was calculated from CBF and arterial blood gas using the following equation:
- O2D = CBF * ([0.003 * PO2] + [1.34 * O2sat * Hgb])
ASL: This perfusion technique uses magnetically labeled arterial blood water as a nominally diffusible flow tracer. The pseudocontinuous “pCASL” sequence is a modified version of the flow-sensitive alternating inversion recovery (FAIR) technique.13,14 For optimal labeling, a hyperbolic secant (HS) inversion pulse is generated using the MATPULSE software15 with 15.36ms duration, 17uT RF amplitude and 95% tagging efficiency. A gradient of 0.7mT/m is applied along with the HS pulse during tag, while the HS pulse is applied in the absence of gradient during control. The slab of the slice-selective inversion is 10cm thick. The saturation pulse is applied to a 10 cm slab adjacent and inferior to the selective inversion slab. A delay time (w) is inserted between the saturation and excitation pulses. Imaging parameters were: matrix size = 64×64, TR/TE = 3000/29ms, slice thickness = 8mm with 2mm gap. Seven slices were be acquired sequentially from inferior to superior using a gradient echo EPI sequence, and each slice acquisition took about 80ms. The FOV was 20–22 cm.
To assess cerebral anatomy, gradient localizers were used to locate the brain and were used as a basis to perform the following anatomic brain imaging:
3D volumetric T1-weighted MPRAGE (Magnetization Prepared Rapid Acquisition Gradient Echo: TR/TE/TI=1980/2.65/1100, flip angle=150, slice thickness 1.5 mm, matrix 256×256)
3D volumetric T2-weighted SPACE (Sampling Perfection with Application optimized Contrasts using different flip angle Evolution): TR/TE=3200/453, slice thickness 2 mm, matrix 256×254)
3D susceptibility-weighted imaging (SWI: TR/TE=49/40, slice thickness 2 mm, matrix 256×177)
Diffusion-weighted imaging (TR/TE=2903/86 ms, slice thickness=4 mm, three b values of 0, 500, and 100 mm/s2, matrix 128×128)
2D T2-weighted coronal imaging (TR/TE=6000/112, slice thickness 4 mm, no gap, matrix 448×336).
All images in native form and multiplanar reformat were reviewed by a pediatric neuroradiologist (AV) who was blinded to the results of CBF and O2D. Abnormalities identified included non-acute ischemic changes on T2 weighted imaging, periventricular leukomalacia (PVL), focal tissue loss and atrophy (indicating sub-acute and chronic stroke), ventriculomegaly, and finally, susceptibility weighted imaging (SWI) evaluation of cerebral venous prominence (due to high deoxyhemoglobin levels), choroid plexus susceptibility (due to bleeds or high deoxyhemoglobin levels), and cerebral parenchymal microbleeds. Definitions of these abnormalities are in table 1.
Table 1.
Definitions of brain abnormalities
| Brain Abnormality* | Definition |
|---|---|
| Acute Ischemic Changes | Any acute ischemia and infract seen in the brain based on diffusion weighted imaging trace maps and apparent diffusion coefficient maps |
| PVL | Lesions with typical appearance and location of periventricular leukomalacia, including punctate white matter injury |
| Nonacute Ischemic T2 Changes | Focal lesions without acute ischemic findings that are likely to be result of prior ischemia based on imaging appearance and which follow vascular territories |
| Delayed Myelination | Assessment of myelination based on well established norms of myelination progression on the basis of T1-weighted and T2-weighted images |
| Developmental Defect (Malformation) | Any congenital malformation of the brain |
| Generalized Atrophy | Diffuse volume loss in the brain |
| Focal Tissue Loss & Atrophy | Any non-diffuse focal loss and atrophy of brain parenchymal tissue |
| Ventriculomegaly | Enlargement of the ventricles due to any cause; may or may not be associated with generalized volume loss |
| Intracranial hemorrhage – gross (acute or chronic) |
Detection of gross brain parenchymal or extra-axial hemorrhage, acute or chronic, based on any combination of imaging sequences |
| Intraventricular Hemorrhage | Presence of any new or old intraventricular hemorrhage |
| Operculum | Whether the Sylvian operculum remains widely open or has normally closed |
| SWI Veins | Presence or absence of abnormal prominence of the cortical and medullary veins based on susceptibility weighted imaging maximum intensity projection images |
| Choroid Plexus Susceptibility | Presence or absence of susceptibility in either choroids plexus on susceptibility weighted imaging |
Note that while these classifiers are distinct, there may often be overlap in some of the features for a particular lesion or patient.
Statistics
Descriptive statistics were used and recorded as mean + standard deviation. CBF measured using PCMR was indexed to BSA as well as to aortic flow and brain volume (as a percent). Predictors of brain lesions included CBF, CBF/brain volume (grams), O2D, O2D/brain volume as well as CBFASL (ml O2/100 grams of brain tissue/minute). CBF reactivity was calculated by dividing the difference between CBF in room air and CBF in hypercarbia with the difference in PCO2 between the 2 conditions; the same was performed with O2D. Logistic or ordinal regression models within each surgery stage (pre-BDG, BDG, and Fontan) separately were utilized as an exploratory analysis for the relationships of CBF, O2D, CBF measured using PCMR indexed to BSA, and CBFASL with brain abnormalities. As multiple tests were performed, we used a P-value of 0.003 as a threshold for the results from these models. Mixed-effects linear regression models with random intercepts (MLRM) were applied to examine the change in the CBF outcomes across the three surgery stages. Logistic regression models using generalized estimating equations (GEE) were used to compare stages of surgery as well as to assess the relationships of CBF, O2D, CBF measured using PCMR indexed to BSA, and CBFASL with brain abnormalities after adjusting for stage of surgery. Random intercept Poisson regression models (PRM) were used for count data. A P-value < 0.05 was considered significant. MLRM, logistic regression models using GEE, and PRM account for correlations arising from the repeated measures. Stata 14.2 (Stata corp, College Station, TX) software was used to conduct statistical analyses. We applied Benjamini and Hochberg false discovery rate (FDR) method16 to derive corrected P-values to address the issue of multiple testing. We used Bonferroni procedure for the post hoc comparisons among 3 groups for CBF.
Results
Study Population
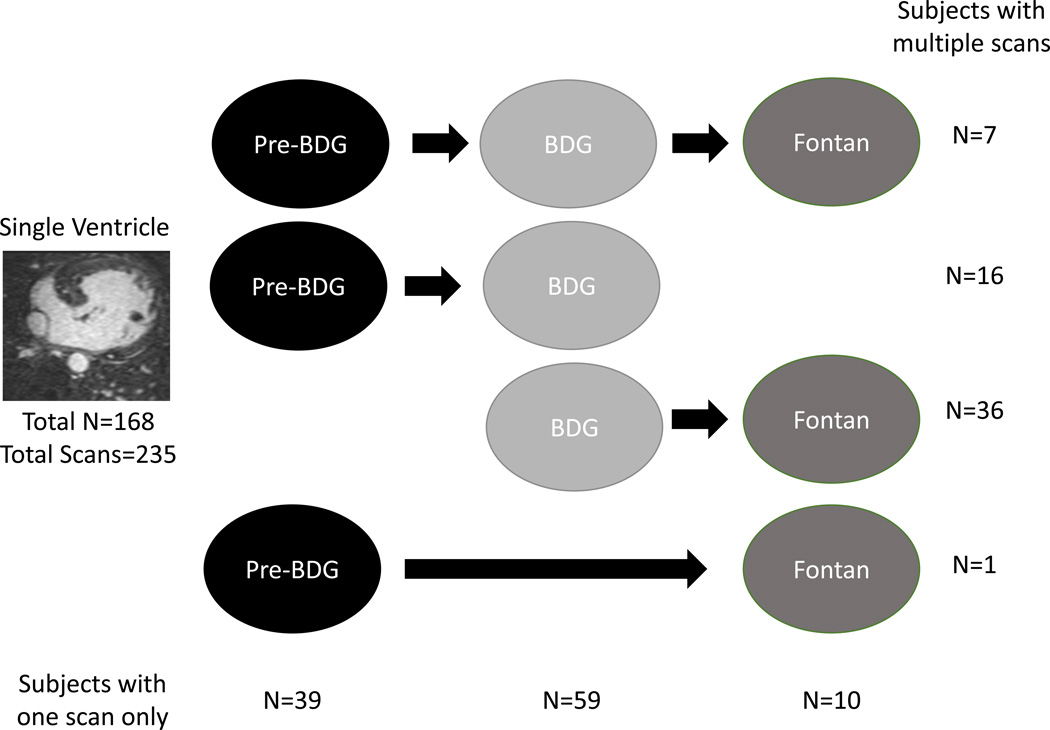
One hundred and sixty eight single ventricle patients who underwent 235 MRI scans comprise the study population; 60 subjects underwent 127 scans at different surgical stages. Figure 1 is a flow diagram of the distribution of scans at each stage as well as how many subjects had multiple scans; demographics of the patients at each stage are listed in table 2.
Figure 1. Breakdown of single ventricle patients studied by stage of surgery.
Numbers on the right represent the number of patients with multiple scans and those at the bottom represent those with a single scan only.
Table 2.
Demographics of patients at each imaging instance
| Pre-BDG N=63 |
BDG N=118 |
Fontan N=54 |
Total N=235 |
|
|---|---|---|---|---|
| Male | 40 (64%) | 70 (60%) | 37 (69%) | 147 (64%) |
| Female | 23 (37%) | 48 (41%) | 17 (32%) | 88 (37%) |
| Age in years* (range) |
0.45 ± 0.13 (0.27 – 0.78) |
3.25 ± 0.96 (2.08 – 7.64) |
3.92 ± 1.22 (2.13 – 8.36) |
2.64 ± 1.63 (0.27 – 8.36) |
| Height (cm)* | 63.2 ± 6.20 | 91.7 ± 14.04 | 97.3 ± 11.79 | 85.7 ± 17.93 |
| Weight (kg)* | 6.30 ± 1.12 | 13.4 ± 2.96 | 15.7 ± 3.02 | 12.1 ± 4.41 |
| BSA (m2)* | 0.33 ± 0.04 | 0.58 ± 0.10 | 0.64 ± 0.09 | 0.53 ± 0.15 |
| Time from prior surgery to MRI (months)* |
4.9 + 1.5 | 29 + 14.6 | 8.6 + 5.3 | 18.1 + 15.7 |
| Heart rate (BPM) † | 136 ± 25 | 116 ± 20 | 115± 20 | 121 ± 23 |
| Systolic BP (mm Hg) ‡ |
107 ± 26 | 110 ± 23 | 99 ± 16 | 107 ± 23 |
| Diastolic BP (mm Hg)§ |
54 ± 16 | 64 ± 20 | 53 ± 17 | 59 ± 19 |
| Hemoglobin (mg/dl) | 13.5 ± 1.72 | 15.5 ± 1.83 | N/A | 14.8 ± 2.02 |
| PO2 in RA/PO2 in hypercarbia |
45.8 ± 4.05/ 51.5 ± 6.13 |
51.7 ± 7.19/ 60.3 ± 8.94 |
N/A | - |
| O2 sat in RA/O2 sat in hypercarbia |
79.6 ± 4.77/ 76.5 ± 7.32 |
83.9 ± 6.73/ 83.1 ± 6.24 |
N/A | - |
| Cardiopulmonary bypass time (minutes) |
84.6 ± 27.39 | 61.1 ± 27.77 | 70.0 ± 18.21 | 68.5 ± 27.20 |
| Circulatory arrest Time (minutes) |
43.1 ± 14.69 | 28.0 ± 18.66 | 26.5 ± 9.81 | 31.3 ± 16.89 |
| Morphology | ||||
| HLHS | 40 | 62 | 28 | 130 |
| Tricuspid Atresia | 7 | 8 | 3 | 18 |
| DORV | 6 | 12 | 6 | 24 |
| Pulmonary Atresia | 4 | 11 | 8 | 23 |
| Unbalanced AVC | 3 | 13 | 4 | 20 |
| TGA | 0 | 4 | 0 | 4 |
| DILV | 0 | 4 | 4 | 8 |
| Other | 3 | 4 | 1 | 8 |
P<0.05 for comparison between all 3 groups,
P<0.05 for comparison between all 3 groups except BDG to Fontan,
P<0.05 for comparison between BDG to Fontan.
P<0.05 for comparison between all 3 groups except pre-BDG vs Fontan.
AVC=atrioventricular canal, BP=blood pressure, BPM=beats per minute, BSA (m2)=body surface area in meters squared cm=centimeters, DILV=double inlet left ventricle, DORV=double outlet right ventricle, HLHS=hypoplastic left heart syndrome, Kg=kilograms, mg/dl=milligrams/deciliter, mm Hg=millimeters of mercury, TGA=transposition of the great arteries.
Brain lesions and stage of surgical reconstruction
Stage of surgical reconstruction had a significant effect on the types of lesions observed. Table 3 lists all the brain lesions present as raw data on the MRI scans of the entire cohort while table 4 lists the significant differences noted by surgical stage (statistically non-significant differences not shown). Periventricular leukomalacia (PVL) was more common in BDG than either pre-BDG or Fontans. SWI venous prominence was more common in pre-BDG than in BDG or Fontan (prominent vs normal or prominent vs borderline); these were more common in BDG than Fontan for borderline vs normal and prominent vs normal. Table 5 lists what was felt to be the 3 most important brain lesions and grouped for biologic plausibility (atrophy=generalized atrophy+focal tissue loss and atrophy, developmental malformations=developmental defects+delayed myelination and hemorrhage=interventricular hemorrhage+ gross intracranial hemorrhage; acute ischemic changes and periventricular leukomalacia (PVL), also thought to be important, are listed in table 4).
Table 3.
Descriptive data of brain abnormalities divided by surgical stage
| Brain Abnormality, N (%) | Pre-BDG | BDG | Fontan | Total |
|---|---|---|---|---|
| Nonacute Ischemic T2 Changes | ||||
| No | 57 (93) | 70 (62) | 37 (70) | 164 (72) |
| Yes | 4 (7) | 43 (38) | 16 (30) | 63 (29) |
|
Acute Ischemic Changes DWI/ADC |
||||
| No | 60 (99) | 112 (99) | 53 (100) | 225 (99) |
| Yes | 2 (3) | 1 (1) | 0 (0) | 3 (1) |
| PVL | ||||
| No | 52 (84) | 65 (59) | 39 (72) | 156 (69) |
| Yes | 10 (16) | 46 (41) | 15 (28) | 71 (31) |
| Generalized Atrophy | ||||
| No | 61 (98) | 102 (90) | 49 (91) | 212 (92) |
| Borderline | 1 (2) | 6 (5) | 3 (6) | 10 (4) |
| Yes | 0 (0) | 6 (5) | 2 (4) | 8 (4) |
| Focal Tissue Loss & Atrophy | ||||
| No | 58 (94) | 87 (78) | 44 (82) | 189 (83) |
| Yes | 4 (7) | 24 (22) | 10 (19) | 38 (17) |
| Ventriculomegaly | ||||
| No | 47 (76) | 73 (64) | 33 (62) | 153 (67) |
| Borderline | 12 (19) | 22 (19) | 13 (25) | 47 (21) |
| Yes | 3 (5) | 19 (17) | 7 (13) | 29 (13) |
| SWI veins | ||||
| Normal | 1 (2) | 25 (22) | 29 (54) | 55 (24) |
| Borderline | 5 (8) | 58 (51) | 17 (32) | 80 (35) |
| Prominent | 55 (90) | 30 (27) | 8 (15) | 93 (41) |
| Choroid Plexus Susceptibility | ||||
| No | 5 (8) | 43 (38) | 44 (81) | 92 (40) |
| Yes | 56 (92) | 70 (62) | 10 (19) | 136 (60) |
| Delayed Myelination | ||||
| No | 60 (97) | 110 (99) | 53 (98) | 223 (98) |
| Yes | 2 (3) | 1 (1) | 1 (2) | 4 (2) |
|
Intracranial Hemorrhage – Gross (Acute or Chronic) |
||||
| No | 54 (87) | 107 (95) | 49 (93) | 210 (92) |
| Yes | 8 (13) | 6 (5) | 4 (8) | 18 (8) |
|
Developmental Defect (Malformation) |
||||
| No | 59 (95) | 107 (95) | 51 (94) | 217 (95) |
| Yes | 3 (5) | 6 (5) | 3 (6) | 12 (5) |
| Intraventricular Hemorrhage | ||||
| No | 58 (97) | 112 (98) | 53 (98) | 223 (98) |
| Yes | 2 (3) | 2 (2) | 1 (2) | 5 (2) |
| Operculum | ||||
| Closed | 30 (49) | 107 (95) | 51 (94) | 188 (83) |
| Open | 31 (51) | 6 (5) | 3 (6) | 40 (18) |
Table 4.
Logistic Regression Analysis with Generalized Estimating Equations of Brain Abnormalities (Only significant differences are shown)
| Endpoints | Odds ratio (95% CI) | P value |
|---|---|---|
| Non-acute Ischemic T2 Changes | 0.0001 | |
| BDG vs. pre-BDG | 8.75 (3.173, 24.150) | <0.0001 |
| Fontan vs. pre-BDG | 6.16 (2.007, 18.916) | 0.0015 |
| Fontan vs. BDG | 0.70 (0.418, 1.185) | 0.1866 |
| PVL | 0.0002 | |
| BDG vs. pre- BDG | 3.68 (1.834, 7.383) | 0.0002 |
| Fontan vs. pre- BDG | 2.00 (0.896, 4.462) | 0.0905 |
| Fontan vs. BDG | 0.54 (0.329, 0.899) | 0.0175 |
| Ventriculomegaly | ||
| Yes vs. No | 0.0237 | |
| BDG vs. pre- BDG | 2.84 (1.342, 6.028) | 0.0064 |
| Fontan vs. pre- BDG | 2.53 (1.205, 5.319) | 0.0142 |
| Fontan vs. BDG | 0.89 (0.667, 1.187) | 0.4269 |
| SWI veins | ||
| Borderline versus Normal | 0.0008 | |
| BDG vs. pre- BDG | 0.48 (0.055, 4.229) | 0.5099 |
| Fontan vs. pre- BDG | 0.12 (0.014, 1.077) | 0.0583 |
| Fontan vs. BDG | 0.25 (0.119, 0.543) | 0.0004 |
| Prominent versus Normal | <0.0001 | |
| BDG vs. pre- BDG | 0.02 (0.003, 0.179) | 0.0004 |
| Fontan vs. pre- BDG | 0.01 (0.001, 0.043) | <0.0001 |
| Fontan vs. BDG | 0.23 (0.096, 0.557) | 0.0011 |
| Prominent versus Borderline | <0.0001 | |
| BDG vs. pre-BDG | 0.05 (0.016, 0.127) | <0.0001 |
| Fontan vs. pre-BDG | 0.04 (0.011, 0.142) | <0.0001 |
| Fontan vs. BDG | 0.88 (0.351, 2.221) | 0.7911 |
Table 5.
Descriptive data of grouped brain abnormalities divided by surgical stage
| Brain Abnormality, N (%) | Pre-BDG | BDG | Fontan | Total |
|---|---|---|---|---|
| Atrophy | ||||
| No | 57 (92) | 82 (73) | 40 (74) | 179 (78) |
| Yes | 5 (8) | 31 (27) | 14 (26) | 50 (22) |
| Developmental Malformations | ||||
| No | 57 (92) | 104 (95) | 50 (93) | 211 (93) |
| Yes | 5 (8) | 6 (6) | 4 (7) | 15 (7) |
| Hemorrhage | ||||
| No | 51 (85) | 105 (93) | 48 (91) | 204 (90) |
| Yes | 9 (15) | 8 (7) | 5 (9) | 22 (10) |
Because multiple brain abnormalities may co-exist in the same patient, the entire cohort was divided into those with > 2 brain lesions and those with less. Stage of surgery had a significant effect on the number of brain abnormalities (P=0.0001). Pre-BDG and BDG had more patients with > 2 (N=59, 97% and N=102, 90% respectively) than those in the Fontan group (N=37, 70%) with odds ratios of 13.6 and 4.1 respectively (P=0.01 and 0.0005 respectively). Table 6 lists the number of brain abnormalities according to surgical stage for those who underwent multiple scans. Pre-BDG had 45% and BDG had 29% more brain abnormalities than those after Fontan (P=0.01 and 0.03 respectively).
Table 6.
Number of brain lesions in individual patients by surgery
| # of brain abnormalities (%) |
Pre-BDG | BDG | Fontan | Total |
|---|---|---|---|---|
| 0 (None) | 1 (4) | 4 (7) | 9 (21) | 14 (11) |
| 1 | 0 (0) | 10 (18) | 11 (26) | 21 (17) |
| 2 | 5 (21) | 15 (26) | 7 (16) | 27 (22) |
| 3 | 9 (38) | 10 (18) | 6 (14) | 25 (20) |
| 4 | 3 (13) | 6 (11) | 7 (16) | 16 (13) |
| 5 | 3 (13) | 8 (14) | 1 (2) | 12 (10) |
| 6 | 2 (8) | 2 (4) | 0 (0) | 4 (3) |
| 7 | 1 (4) | 2 (4) | 2 (5) | 5 (4) |
| Total | 24 | 57 | 43 | 124* |
Data are presented as frequency count and percentage.
3 uninterpretable data sets leaving 124 instead of 127 scans
Examples of brain lesions observed are demonstrated in figures 2–5.
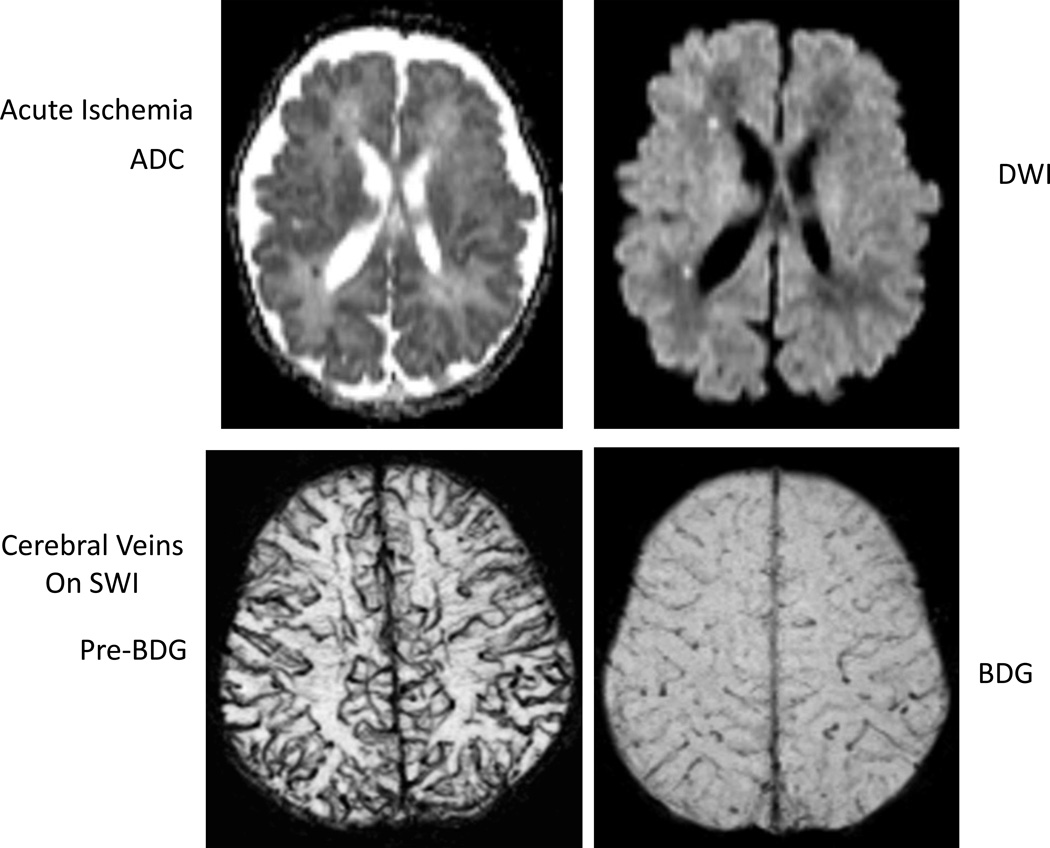
Figure 2. Example of acute ischemia on diffusion imaging and cerebral veins on susceptibility weighted images (SWI).
ADC=apparent diffusion coefficient, DWI=diffusion weighted imaging.
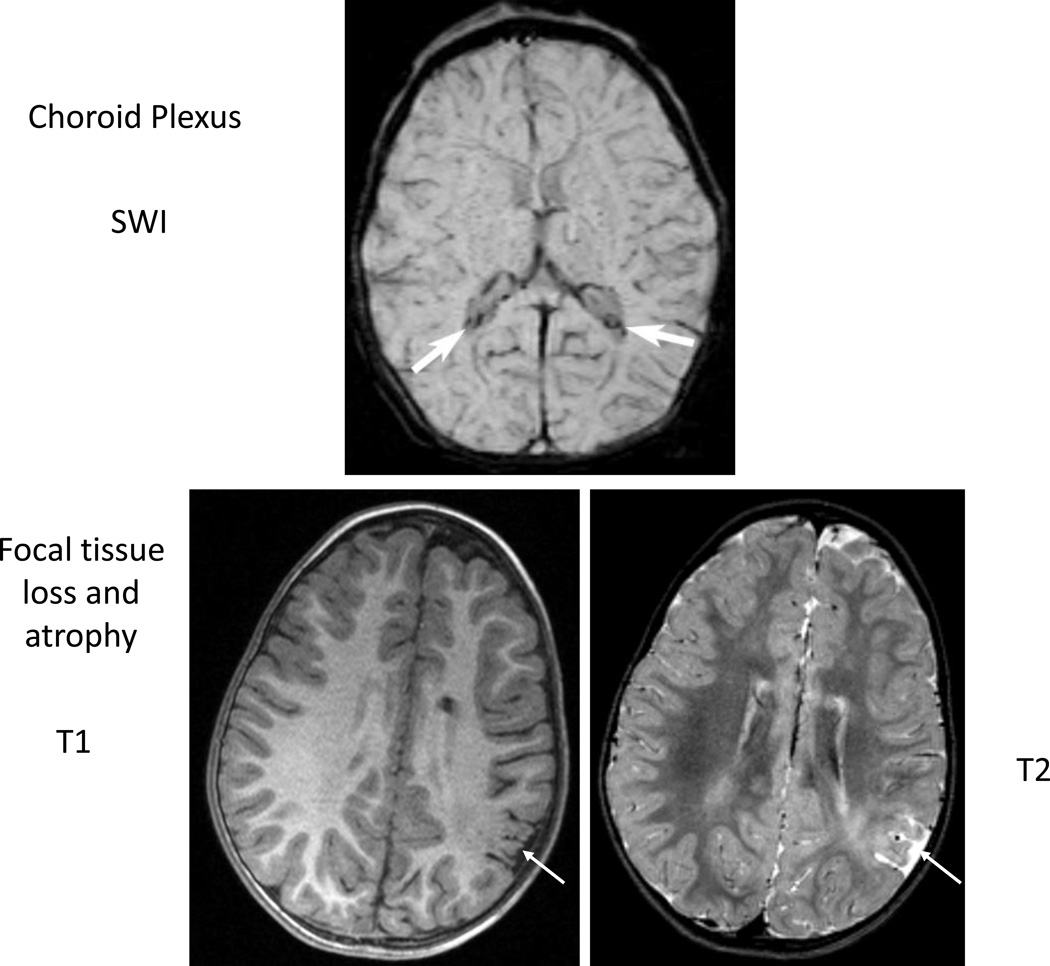
Figure 5. Example of choroid plexus susceptibility and focal tissue loss and atrophy.
Association of brain lesions with CBF
For the entire group, CBF indexed to aortic flow, brain volume and CBFASL was not associated with brain lesions in the pre-BDG group. In Fontan, with one percent increase in CBF the odds of non-acute ischemic changes on T2 decrease by 7.8% (P=0.0003) and the odds of prominent SWI venous prominence compared to combined normal and borderline SWI venous prominence decreases by 4.2% (P=0.0025).
Results from the logistic regression analysis using GEE for the relationships of brain abnormalities with CBF/BSA and CBFASL after adjusting for stage effect were presented in table 7 and table 8, respectively. Correcting for multiple testing, the odds of non-acute ischemic changes on T2, focal tissue loss and atrophy, and SWI veins (borderline vs normal) all decrease (by 63, 75, and 73% respectively) with 1% increasing amount of CBF by PCMR. For CBF by pCASL, with one unit increase, the odds of PVL and focal tissue loss and atrophy all decreased (by 33 and 9% respectively).
Table 7.
Logistic Regression Analysis with Generalized Estimating Equations of Brain Abnormalities using CBF as measured by PCMR indexed to BSA as a covariate
| Endpoints | Odds ratio (95% CI) | P value |
|---|---|---|
| Non-acute Ischemic Changes T2 | ||
| Yes versus No | 0.61 (0.43, 0.87) | 0.0061 |
| Periventricular Leukomalacia | ||
| Yes versus No | 0.88 (0.71, 1.10) | 0.27 |
| Focal Tissue Loss & Atrophy | ||
| Yes versus No | 0.50 (0.32, 0.77) | 0.002 |
| Ventriculomegaly | ||
| Borderline versus No | 1.00 (0.95, 1.04) | 0.89 |
| Yes versus No | 0.58 (0.32, 1.05) | 0.0704 |
| Yes versus Borderline | 0.57 (0.32, 1.00) | 0.0496 |
| SWI veins | ||
| Borderline versus Normal | 0.52 (0.34, 0.79) | 0.0023 |
| Prominent versus Normal | 0.74 (0.51, 1.09) | 0.13 |
| Prominent versus Borderline | 1.11 (0.73, 1.69) | 0.61 |
CI, Confidence Interval.
The odds ratio is reported for 0.5 units (∼ 1 standard deviation) increase in CBF as measured by PCMR indexed to BSA.
Table 8.
Logistic Regression Analysis with Generalized Estimating Equations of Brain Abnormalities using CBF as measured by PCASL as a covariate
| Endpoints | Odds ratio (95% CI) | P value |
|---|---|---|
| Non-acute Ischemic Changes T2 | ||
| Yes versus No | 0.90 (0.82, 0.99) | 0.0299 |
| Periventricular Leukomalacia | ||
| Yes versus No | 0.67 (0.49, 0.90) | 0.0071 |
| Focal Tissue Loss & Atrophy | ||
| Yes versus No | 0.91 (0.84, 0.97) | 0.0082 |
| Ventriculomegaly | ||
| Borderline versus No | 0.94 (0.76, 1.16) | 0.58 |
| Yes versus No | 0.92 (0.84, 1.01) | 0.0717 |
| Yes versus Borderline | 0.93 (0.84, 1.04) | 0.19 |
| SWI veins | ||
| Borderline versus Normal | 1.79 (0.77, 4.17) | 0.18 |
| Prominent versus Normal | 1.41 (0.79, 2.53) | 0.25 |
| Prominent versus Borderline | 0.84 (0.59, 1.18) | 0.31 |
CI, Confidence Interval.
The odds ratio is reported for 1 unit (∼ 1 standard deviation) increase in CBF as measured by PCASL.
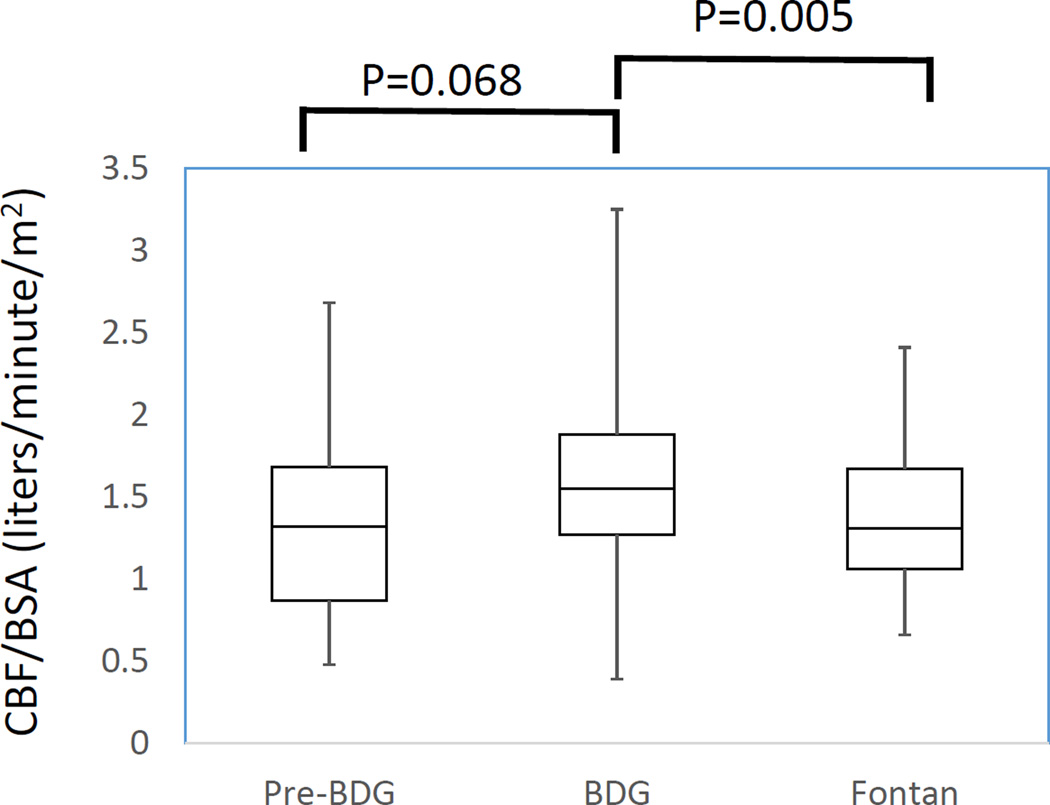
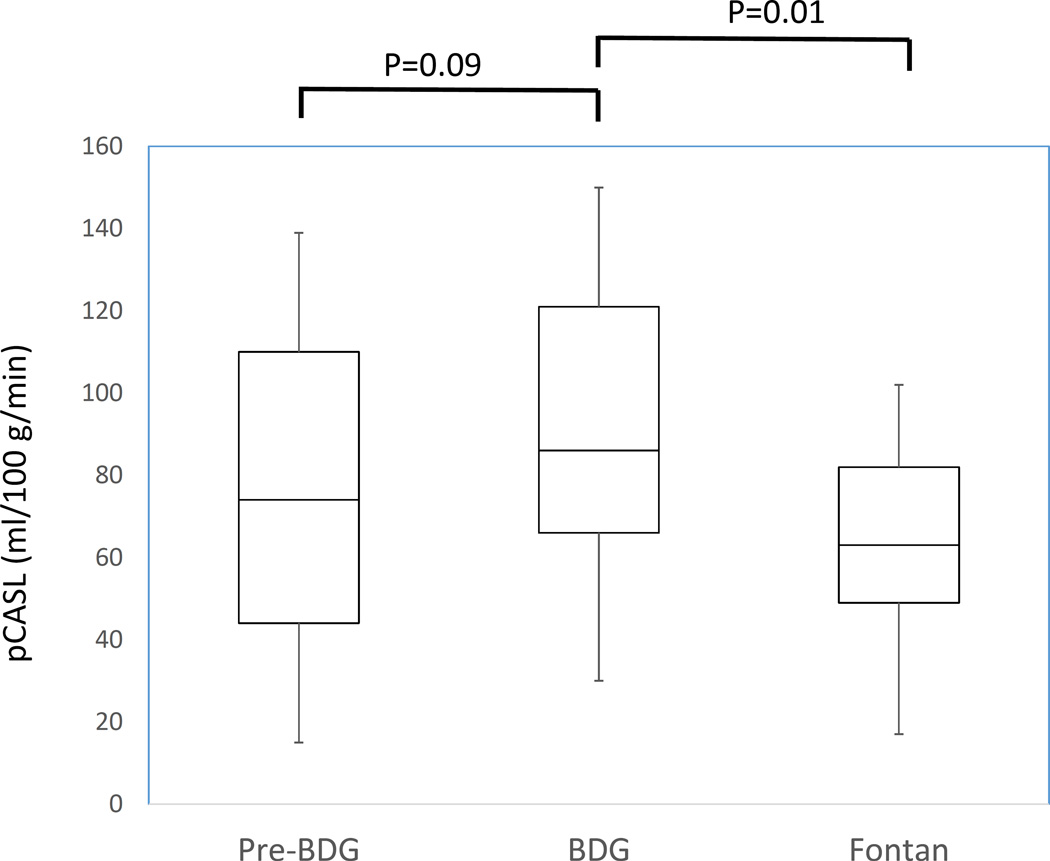
For those patients with serial MRI scans, figure 6 demonstrates the change in CBF/BSA and pCASL across stages. Results from the MLRM reveal that the BDG patients had significantly higher CBF/BSA and CBF as measured by pCASL than Fontan patients (P=0.005 and P=0.01 respectively).
Figure 6. Plot of cerebral blood flow / body surface area (CBF/BSA) (A) and pCASL (B) in patients with serial MRIs. There were 24, 57 and 43 scans in patients at the pre-BDG, BDG and Fontan stage respectively for a total of 124 scans in 60 patients. See figure 1 and table 6 for further delineation of patients with serial scans.
In analyzing the number of co-existent brain lesions, for the entire cohort, the odds > 2 brain lesions decreases by 34% with one unit increase in CBF/BSA (P=0.01) and this result stayed significant after adjusting for surgery (a decrease of 44% with one unit increase in CBF/BSA, P=0.0061). For those with serial MRIs, a one unit increase in CBF/BSA within a subject was associated with 20% decrease in the number of brain abnormalities after adjusting for surgery (P=0.01).
Association of Brain lesions with O2D
There was no significant effect of O2D on brain abnormalities across all 3 stages of surgical reconstruction with the exception of ventriculomegaly in the BDG stage, where a 1 unit increase in CBF decreased the odds of definite ventriculomegaly compared to combined no and borderline ventriculomegaly by 1% (P=0.05).
Association of brain lesions with reactivity
No significant effect was noted on the reactivity on the odds of observing brain abnormalities.
Discussion
This study is the first to investigate brain abnormalities throughout staged surgical reconstruction in single ventricle patients both as a cross section and with serial imaging. Our data demonstrated that significant brain injuries are commonly present in single ventricle patients and that there is an accumulation of the number of patients with injury (such as non-acute ischemic changes, atrophy, and ventriculomegaly) as the children progress to Fontan; while this may be expected, it is the first time that this has been documented. Exceptions to this generalization include PVL (more commonly seen at the BDG stage), SWI venous prominence, choroid plexus susceptibility, and open operculum which were all more common in pre-BDG. PVL is easily detectable in the neonate and young infant, but small foci of punctate white matter PVL injury typically fade over time and hence may no longer be detectable by standard MRI. Similarly, the operculum gradually closes as the brain matures. Prominence of the cerebral veins on susceptibility weighted imaging is closely related to excessive relative level of deoxyhemoglobin in the cerebral vessels, which was shown to decrease after the pre-BDG stage. Similarly, choroid plexus susceptibility is due to relative deoxyhemoglobin levels and also small choroid plexus bleeds. This appearance also decreased after the pre-BDG stage.
A greater number of patients pre-BDG and BDG groups demonstrated more co-existing brain abnormalities than Fontan patients. This would be expected as brain abnormalities in this study are a function of being congenital (eg developmental malformations, operculum), acquired (eg stroke), healing and detectability. For example, an infant may have an operculum and PVL, both of which may not be evident on magnetic resonance because of closure of the operculum and decreasing the detectability of PVL as the patient ages.
This study also demonstrated an inverse association of various indices of CBF with these brain abnormalities (i.e., higher CBF, the lower the incidence of abnormalities), either as a single lesion or multiple co-existent abnormalities. CO2 reactivity and oxygen delivery (with the exception of ventriculomegaly in the BDG group), however, were not associated with brain lesion development. Note that there may be some overlap between the brain injury variables evaluated in this study, such as between atrophy and ventriculomegaly, or between non-acute ischemic T2 changes and focal tissue loss which were accounted for in the 3–5 main groups we chose. However, we chose all these variables to capture a larger number of features.
Neonates and infants with congenital heart disease undergoing magnetic resonance have been found to have significant brain lesions before and after cardiac surgery,2,7,17,18,19 the incidence increasing with brain immaturity.20 In the case of single ventricle lesions, reasons for preoperative neurological injury may include genetic syndromes, low cardiac output, events such as cardiac arrest, fetal and neonatal ischemia as well as cyanosis causing hypoxia. Postoperatively, cardiopulmonary bypass and deep hypothermic circulatory arrest as well as cerebral emboli may also contribute to the development of cerebral lesions in addition to the preoperative causes. Although cardiopulmonary bypass and hypothermic circulatory arrest could cause expansion and progression of cerebral hemorrhage by heparinization of an already damaged brain,21 there is debate in the literature as to whether these procedures do4,18,19 or do not22,23 put patients at risk. Our study clearly demonstrated that many neurologic lesions become greater in the number of patients affected as they undergo an increased number of surgeries; whether that has to do with surgical factors alone or other factors such as the natural course of the disease remains a question. It also should be noted that white matter injury is easier to visualize in the myelinated vs unmyelinated brain which would make it more likely to be identified in the older versus the younger brain. Stroke risk increases with time in single ventricle24 and chronic hypoxia may increase risk for white matter injury and loss of cortical volume.
The frequency of abnormalities consistent with focal stroke in the pre-BDG stage in our study was ∼15% (acute and non-acute ischemic changes + focal tissue loss). There are a number of reports of pre-operative stroke in the literature in single ventricle patients undergoing neonatal surgery ranging from Miller et al with 8%17 to Andropolous et al20 with 14% and Block et al23 with 17% of patients. Our number is consistent with their studies.
The inverse association of CBF with the various neurological injuries seen in single ventricle patients, adjusting for stage of surgery, although suggesting a link between these cerebral hemodynamics and anatomy, does not parse out cause and effect. Decreased CBF may be an etiology for or may be caused by anatomic brain abnormalities. Other studies have found this same inverse association; for example, Licht et al demonstrated the inverse link between CBF and periventricular leukomalacia in a study of 25 term infants with congenital heart disease5 while Fukuda et al found this in two studies, one with 3625 and one with 67 low birth weight infants.26 Reila et al. showed that regional CBF was decreased in the region of the lesions in Sturge-Weber syndrome in children,27 however, this has been also demonstrated in adults; for example, Doi et al28 found microbleeds inversely associated with CBF in Alzheimer’s disease.
This study utilized both PCMR and PCASL to measure CBF; each technique has its own advantages and disadvantages including differences in accuracy, the wider availability of PCMR and its ability to yield velocity as well as the anatomic and regional delineation of CBF that PCASL can acquire. In addition, PCASL generally has lower signal-to-noise than PCMR although jugular PCMR will not account for smaller posterior cerebral draining vessels. Finally, PCASL measures blood in the brain while PCMR measures blood draining from the brain. All these factors may be the reason why PCMR and PCASL both correlated with focal tissue loss and atrophy but PCMR correlated with SWI veins and non-acute ischemic changes (PCASL did not) while PCASL correlated with PVL (PCMR did not)
There was no association detected between O2D and CO2 reactivity with cerebral injury with one exception. As O2D was a global measurement, the lesions found may not have impacted or be caused by a “global measurement” as opposed to a “regional” measurement. As for CO2 reactivity, injury may either increase or decrease CBF or be variable because of loss of regulatory centers. If it is random or based on other physiologic factors such as total cardiac index or amount of aortic to pulmonary collateral flow,29 then it is no surprise that there is no systematic correlation either way. Similarly, CO2 reactivity was a global measurement and the same reasoning may apply. It is true that CBF was also a global measurement, however, the total amount of blood flow appears to be more important than O2D.
One significance of this study is that various lesions on brain MRIs in children30,31,32,33 as well as decreased CBF34,35,36,37,38,39 have been correlated with adverse neurodevelopmental outcome. The mechanism for neurological injury alone impairing outcome is clear. In addition, the brain requires a certain amount of CBF to function independent of neurological injury of which a recent review of 25 studies bears this out.40 In a study by Koide et al,41 CBF and cognitive function was measured in 14 bradycardic adults before and after pacemaker implantation. Prior to implantation, verbal cognitive function was lower in bradycardic patients than in age-matched control subjects, however, after pacemaker implantation, both CBF and verbal intelligence improved. Combining the concepts of neurodevelopment, anatomic brain lesions and CBF with poor neurodevelopmental outcomes in single ventricle patients8,9,10,11 presents a plausible mechanism for these less than optimal results. In our study of patients with serial MRIs, those in the BDG stage had higher CBF/BSA than Fontan patients. We speculate that techniques to improve cerebral preservation as well as increase CBF (for example, increasing cardiac output) or possibly even leaving patients in the BDG stage for as long as hemodynamically possible may lead to optimizing neurological outcome. In the least, measurement of CBF and identification of brain abnormalities may enhance recognition of single ventricle patients at risk for poor outcome and facilitate early intervention if appropriate. Studies are currently underway to address this speculation.
Limitations
Our study cannot make a recommendation on the routine use of preoperative brain MRI or CBF throughout staged surgical reconstruction because of a lack of knowledge on whether it might contribute to poor outcomes in patients who do not receive preoperative brain MRIs although this study suggests this strategy may be useful and have clinical implications; further investigations are underway to make a definitive statement.
As previously noted, this investigation makes an association between CBF and neurologic injury and does not tease out cause and effect. A longer range, more complicated study design would be needed to determine this.
Due to the lack of painful stimuli patients did not require deep levels of anesthesia and therefore anesthetic levels were kept as light as possible (< 1 MAC) while measuring CBF; this may have had an effect on CBF. Additionally, sevoflourane was chosen as the anesthetic which has the least effect on CBF.
Conclusion
Significant brain abnormalities are commonly present in single ventricle patients and detection of these lesions increase as children progress through staged surgical reconstruction with multiple co-existing lesions more common earlier than later. In addition, this study has demonstrated an inverse association of various indices of CBF with these brain lesions, however, CO2 reactivity and oxygen delivery (with the exception of ventriculomegaly in the BDG group) were not associated with brain lesion development. We speculate that techniques to improve cerebral preservation as well as increase CBF may lead to optimizing neurological outcome and that measurement of CBF and identifying brain abnormalities may be able to identify single ventricle patients at risk for poor outcome; studies are currently underway to address this speculation.
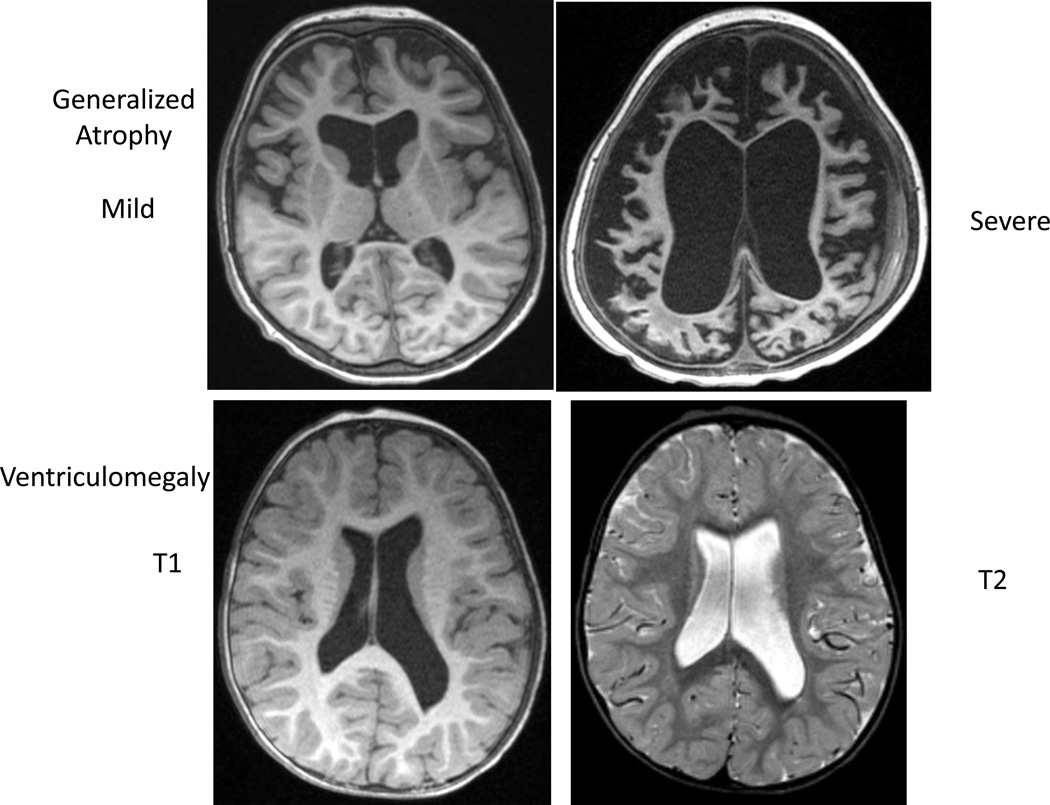
Figure 3. Example of generalized atrophy and ventriculomegaly.
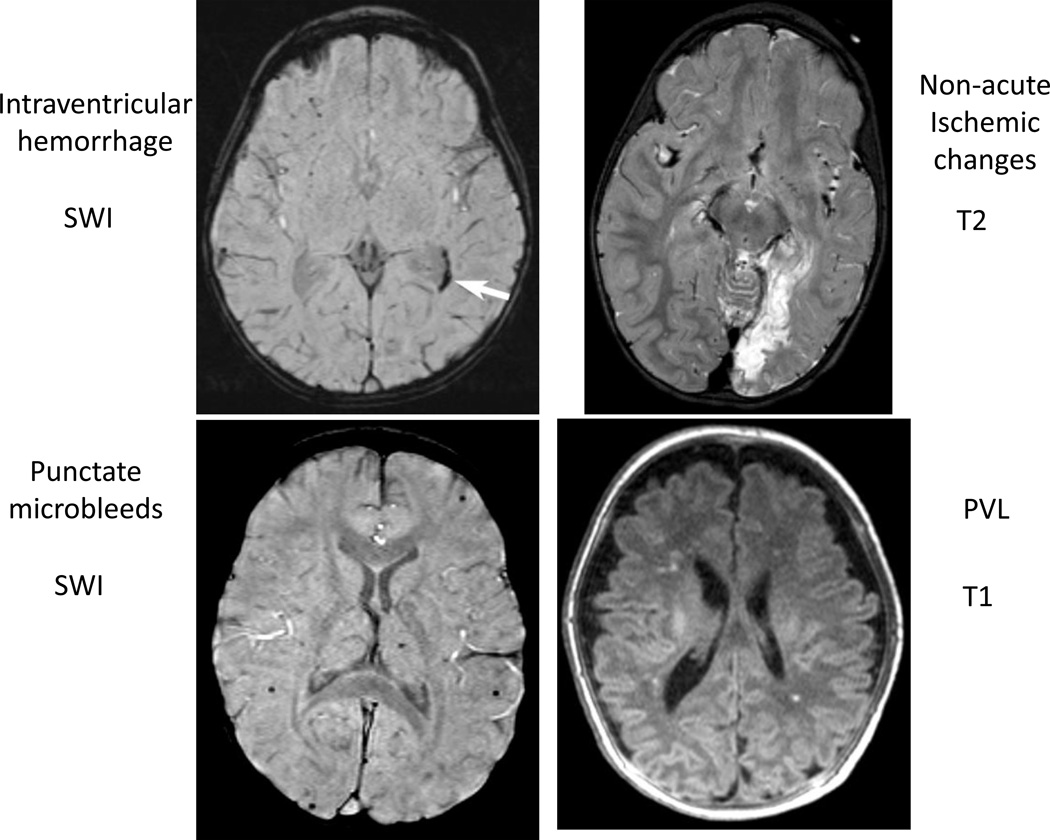
Figure 4. Example of intraventricular hemorrhage on susceptibility weighted images (SWI), non-acute ischemic changes, punctate microbleeds on SWI and punctate periventricular leukomalacia (PVL).
Clinical Perspective.
What is new
Significant brain abnormalities are commonly present in single ventricle patients with detection of these lesions increasing as children progress through staged surgical reconstruction.
In addition, there exists an inverse association of various indices of cerebral blood flow with these brain lesions.
What are the clinical implications
Cerebral blood flow and neurologic injury are potentially modifiable factors that may impact neurodevelopmental outcomes and quality of life
In addition, measurement of cerebral blood flow and identification of brain abnormalities may enhance recognition of single ventricle patients at risk for poor outcome and facilitate early intervention if appropriate.
Acknowledgments
Funding Sources
This study was funded by National Heart Lung and Blood Institute grant 1R01HL090615-01 (PI Fogel) and National Institute of Neurological Disorders and Stroke 1RO1NS072338, RO1NS060653, U01HD087180 (Licht) and the June and Steve Wolfson Family Foundation (Licht).
Footnotes
Clinical Trial Registration: www.clinicaltrials.gov. Identifier: NCT02135081
Disclosures
Dr. Fogel receives grants from Edwards Life Sciences and AMAG.
References
- 1.Ohye RG, Sleeper LA, Mahony L, Newburger JW, Pearson GD, Goldberg CS, Tabbutt S, Frommelt PC, Ghanayem NS, Laussen PC, Rhodes JF, Lewis AB, Mital S, Ravishankar C, Williams IA, Dunbar-Masterson C, Atz AM, Colan S, Minich LL, Pizzaro C, Kanter KR, Jaggers J, Jacobs JP, Krawczeski CD, Pike N, McCrindle BW, Virzi L, Gaynor JW Pedatric Heart Network Investigators. Comparison of Shunt Types in the Norwood Procedure for Single-Ventricle Lesions. N Engl J Med. 2010;362:1980–1992. doi: 10.1056/NEJMoa0912461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mahle WT, Tavani F, Zimmerman RA, Nicolson SC, Galli KK, Gaynor JW, Clancy RR, Montenegro LM, Spray TL, Chiavacci RM, Wernovsky G, Kerth CD. An MRI study of neurological injury before and after congenital heart surgery. Circulation. 2002;106(suppl I):I109–I114. [PubMed] [Google Scholar]
- 3.Licht DJ, Shera DM, Clancy RR, Wernovsky G, Montenegro LM, Nicolson SC, Zimmerman RA, Spray TL, Gaynor JW, Vossough A. Brain maturation is delayed in infants with complex congenital heart defects. J Thorac Cardiovasc Surg. 2009;137:529–536. doi: 10.1016/j.jtcvs.2008.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tavani F, Zimmerman RA, Clancy RR, Wernovsky G, Montenegro LM, Nicolson SC, Zimmerman RA, Spray TL, Gaynor JW, Vossough A. Incidental intracranial hemorrhage after uncomplicated birth: MRI before and after neonatal heart surgery. Neuroradiology. 2003;45:253–258. doi: 10.1007/s00234-003-0946-8. [DOI] [PubMed] [Google Scholar]
- 5.Licht DJ, Wang J, Silvestre DW, Nicolson SC, Montenegro LM, Wernovsky G, Tabbutt S, Durning SM, Shera DM, Gaynor JW, Spray TL, Clancy RR, Zimmerman RA, Detre JA. Preoperative cerebral blood flow is diminished in neonates with severe congenital heart defects. J Thorac Cardiovasc Surg. 2004;128:841–849. doi: 10.1016/j.jtcvs.2004.07.022. [DOI] [PubMed] [Google Scholar]
- 6.Galli KK, Zimmerman RA, Jarvik GP, Wernovsky G, Kuypers MK, Clancy RR, Montenegro LM, Mahle WT, Newman MF, Saunders AM, Nicolson SC, Spray TL, Gaynor JW. Periventricular leukomalacia is common after neonatal cardiac surgery. J Thorac Cardiovasc Surg. 2004;127:692–704. doi: 10.1016/j.jtcvs.2003.09.053. [DOI] [PubMed] [Google Scholar]
- 7.Goff DA, Shera DM, Tang S, Lavin NA, Durning SM, Nicolson SC, Montenegro LM, Rome JJ, Gaynor JW, Spray TL, Vossough A, Licht DJ. Risk factors for preoperative periventricular leukomalacia in term neonates with hypoplastic left heart syndrome are patient related. J Thorac Cardiovasc Surgery. 2014;147:1312–1318. doi: 10.1016/j.jtcvs.2013.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ravishankar C, Zak V, Williams IA, Bellinger DC, Gaynor JW, Ghanayem NS, Krawczeski CD, Licht DJ, Mahony L, Newburger JW, Pemberton VL, Williams RV, Sananes R, Cook AL, Atz T, Khaikin S, Hsu DT for the Pediatric Heart Network Investigators. Association of impaired linear growth and worse neurodevelopmental outcome in infants with single ventricle physiology: a report from the pediatric heart network infant single ventricle trial. J Pediatr. 2013;162:250–256.e2. doi: 10.1016/j.jpeds.2012.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wernovsky G, Stiles KM, Gauvreau K, Gentles TL, duPlessis AJ, Bellinger DC, Walsh AZ, Burnett J, Jonas RA, Meyer JE, Newberger JW. Cognitive development after the Fontan operation. Circulation. 2000;102:883–889. doi: 10.1161/01.cir.102.8.883. [DOI] [PubMed] [Google Scholar]
- 10.Mahle WT, Clancy RR, Moss EM, Gerdes M, Jobes DR, Wernovsky G. Neurodevelopmental outcome and lifestyle assessment in school-aged and adolescent children with hypoplastic left heart syndrome. Pediatrics. 2000;105:1082–1089. doi: 10.1542/peds.105.5.1082. [DOI] [PubMed] [Google Scholar]
- 11.Forbess JM, Visconti KJ, Hancock-Friesen C, Howe RC, Bellinger DC, Jonas RA. Neurodevelopmental outcome after congenital heart surgery: Results from an institutional registry. Circulation. 2002;106:I-95–I-102. [PubMed] [Google Scholar]
- 12.Fogel MA, Pawlowski T, Schwab PJ, Nicolson SC, Montenegro LM, Diaz Berenstein L, Spray TL, Gaynor JW, Fuller S, Keller MS, Harris MA, Whitehead KK, Vossough A, Licht DJ. Brain Magnetic Resonance Immediately Prior To Surgery In Single Ventricles and Surgical Postponement. Ann Thorac Surg. 2014;98:1693–1698. doi: 10.1016/j.athoracsur.2014.05.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim SG. Quantification of relative cerebral blood flow change by flow-sensitive alternating inversion recovery (FAIR) technique: application to functional mapping. Magn Reson Med. 1995;34:293–301. doi: 10.1002/mrm.1910340303. [DOI] [PubMed] [Google Scholar]
- 14.Wang J, Alsop DC, Li L. Comparison of quantitative perfusion imaging using arterial spin labeling at 1.5 and 4 Tesla. Magn Reson Med. 2002;48:242–254. doi: 10.1002/mrm.10211. [DOI] [PubMed] [Google Scholar]
- 15.Matson GB. An integrated program for amplitude-modulated RF pulse generation and re-mapping with shaped gradients. Magn Reson Imaging. 1994;12:1205–1225. doi: 10.1016/0730-725x(94)90086-7. [DOI] [PubMed] [Google Scholar]
- 16.Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J Royal Statistical Society. Series B. 1995;57:289–300. [Google Scholar]
- 17.Miller SP, McQuillen PS, Hamrick S, Xu D, Glidden DV, Charlton N, Karl T, Azakie A, Ferriero DM, Barkovich AJ, Vigneron DB. Abnormal brain development in newborns with congenital heart disease. N Engl J Med. 2007;357:1928–1938. doi: 10.1056/NEJMoa067393. [DOI] [PubMed] [Google Scholar]
- 18.Dent CL, Spaeth JP, Jones BV, Schwartz SM, Glauser TA, Hallinan B, Pearl JM, Khoury PR, Kurth CD. Brain magnetic resonance imaging abnormalities after the Norwood procedure using regional cerebral perfusion. J Thorac Cardiovasc Surg. 2006;131:190–197. doi: 10.1016/j.jtcvs.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 19.McQuillen PS, Barkovich AJ, Hamrick SEG, Perez M, Ward P, Glidden DV, Azakie A, Karl T, Miller SP. Temporal and anatomic risk profile of brain injury with neonatal repair of congenital heart defects. Stroke. 2007;38:736–741. doi: 10.1161/01.STR.0000247941.41234.90. [part 2] [DOI] [PubMed] [Google Scholar]
- 20.Andropoulos DB, Hunter JV, Nelson DP, Stayer SA, Stark AR, McKenzie ED, Heinle JS, Graves DE, Fraser CD. Brain immaturity is associated with brain injury before and after neonatal cardiac surgery with high-flow bypass and cerebral oxygenation monitoring. J Thorac Cardiovasc Surg. 2010;139:543–556. doi: 10.1016/j.jtcvs.2009.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Woodman RC, Harker LA. Bleeding complications associated with cardiopulmonary bypass. Blood. 1990;76:1680–1697. [PubMed] [Google Scholar]
- 22.Monagle P, Chalmers E, Chan A, DeVeber G, Kirkham F, Massicotte P, Michelson AD for the American College of Chest Physicians. Antithrombotic therapy in neonates and children: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. (8th Edition) 2008;133(6 Suppl):887S–968S. doi: 10.1378/chest.08-0762. [DOI] [PubMed] [Google Scholar]
- 23.Beca K, Gunn JK, Coleman L, Hope A, Reed PW, Hunt RW, Finucane K, Brizard C, Dance B, Shekerdemian LS. New White Matter Brain Injury After Infant Heart Surgery Is Associated With Diagnostic Group and the Use of Circulatory Arrest. Circulation. 2013;127:971–979. doi: 10.1161/CIRCULATIONAHA.112.001089. [DOI] [PubMed] [Google Scholar]
- 24.Block AJ, McQuillen PS, Chau V, Glass H, Poskitt KJ, Barkovich AJ, Esch M, Soulikias W, Azakie A, Campbell A, Miller SP. Clinically silent preoperative brain injuries do not worsen with surgery in neonates with congenital heart disease. J Thorac Cardiovasc Surg. 2010;140:550–557. doi: 10.1016/j.jtcvs.2010.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khairy P, Harris L, Landzberg MJ, Fernandes SM, Barlow A, Mercier LA, Viswanathan S, Chetaille P, Gordon E, Dore A, Cecchin F. Long-term survival, modes of death, and predictors of mortality in patients with Fontan surgery. Circulation. 2008;117:85–92. doi: 10.1161/CIRCULATIONAHA.107.738559. [DOI] [PubMed] [Google Scholar]
- 26.Fukuda S, Mizuno K, Kakita H, Kato T, Hussein MH, Ito T, Daoud GA, Kato I, Suzuki S, Togari H. Late circulatory dysfunction and decreased cerebral blood flow volume in infants with periventricular leukomalacia. Brain Dev. 2008;30:589–594. doi: 10.1016/j.braindev.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 27.Fukuda S, Kato T, Kakita H, Yamada Y, Hussein MH, Kato I, Suzuki S, Togari H. Hemodynamics of the cerebral arteries of infants with periventricular leukomalacia. Pediatrics. 2006;117:1–8. doi: 10.1542/peds.2004-1719. [DOI] [PubMed] [Google Scholar]
- 28.Reila AR, Stump DA, Roach ES, Mclean WT, Garcia JC. Regional cerebral blood flow characteristics of the Sturge-Weber syndrome. Pediatr Neurol. 1985;1:85–90. doi: 10.1016/0887-8994(85)90042-6. [DOI] [PubMed] [Google Scholar]
- 29.Doi H, Inamizu S, Saito BY, Murai H, Araki T, Kira JI. Analysis of cerebral lobar microbleeds and a decreased cerebral blood flow in a memory clinic setting. Intern Med. 2015;54:1027–1033. doi: 10.2169/internalmedicine.54.3747. [DOI] [PubMed] [Google Scholar]
- 30.Fogel MA, Li C, Wilson F, Pawlowski T, Nicolson SC, Montenegro LM, Diaz-Berenstein L, Spray TL, Gaynor JW, Fuller S, Keller MS, Harris MA, Whitehead KK, Clancy R, Elci O, Bethel J, Vossough A, Licht DJ. Relationship of Cerebral Blood Flow to Aortic to Pulmonary Collateral/Shunt Flow In Single Ventricles. Heart. 2015;101:1325–1331. doi: 10.1136/heartjnl-2014-307311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gäddlin PO, Finnström O, Samuelsson S, Wadsby M, Wang C, Leijon I. Academic achievement, behavioural outcomes, and MRI findings at 15 years of age in very low birthweight children. Acta Paediatr. 2008;84:343–349. doi: 10.1111/j.1651-2227.2008.00925.x. [DOI] [PubMed] [Google Scholar]
- 32.Pierrat V, Haouari N, Liska A, Thomas D, Subtil D, Truffert P Groupe d’Etudes en Epidemiologie Perinatale. Prevalence, causes, and outcome at 2 years of age of newborn encephalopathy: population based study. Arch Dis Child Fetal Neonatal Ed. 2005;90:F257–F261. doi: 10.1136/adc.2003.047985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Limperopoulos C, Bassan H, Gauvreau K, Robertson RL, Sullivan NR, Benson CB, Avery L, Stewart J, Soul JS, Ringer SA, Volpe JJ, duPlessis AJ. Does cerebellar injury in premature infants contribute to the high prevalence of long-term cognitive, learning, and behavioral disability in survivors? Pediatrics. 2007;120:584–593. doi: 10.1542/peds.2007-1041. [DOI] [PubMed] [Google Scholar]
- 34.Badr LK, Bookheimer S, Purdy I, Deeb M. Predictors of neurodevelopmental outcome for preterm infants with brain injury: MRI, medical and environmental factors. Early Human Development. 2009;85:279–284. doi: 10.1016/j.earlhumdev.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cheng HH, Wypij D, Laussen PC, Bellinger DC, Stopp CD, Soul JS, Newburger JW, Kussman BD. Cerebral Blood Flow Velocity and Neurodevelopmental Outcome in Infants Undergoing Surgery for Congenital Heart Disease. Ann Thoracic Surg. 2014;98:125–132. doi: 10.1016/j.athoracsur.2014.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hunt RW, Evans N, Rieger I, Kluckow M. Low superior vena cava flow and neurodevelopment at 3 years in very preterm infants. J Peds. 2004;145:588–592. doi: 10.1016/j.jpeds.2004.06.056. [DOI] [PubMed] [Google Scholar]
- 37.Eixarch E, Meler E, Iraola A, Illa M, Crispi F, Hernandez-Andrade E, Gratacos E, Figueras F. Neurodevelopmental outcome in 2-year-old infants who were small-for-gestational age term fetuses with cerebral blood flow redistribution. Ultrasound Obstet Gynecol. 2008;32:894–899. doi: 10.1002/uog.6249. [DOI] [PubMed] [Google Scholar]
- 38.Leppanen M, Ekholm E, Palo P, Maunu J, Munck P, Parkkola R, Matomaki J, Lapinleimu H, Haataja L, Lehtonen L, Rautava P PIPARI Study Group. Abnormal antenatal Doppler velocimetry and cognitive outcome in very-low-birth-weight infants at 2 years of age. Ultrasound Obstet Gynecology. 2010;36:178–185. doi: 10.1002/uog.7694. [DOI] [PubMed] [Google Scholar]
- 39.Butler RW, Dickinson WA, Katholi C, Halsey JH., Jr The comparative effects of organic brain disease on cerebral blood flow and measured intelligence. Annals of Neurology. 1983;13:155–159. doi: 10.1002/ana.410130208. [DOI] [PubMed] [Google Scholar]
- 40.Lou HC, Skov H, Henriksen L. Intellectual impairment with regional cerebral dysfunction after low neonatal cerebral blood flow. Acta Paedoatrica Scandinavica. 1989;360:72–82. doi: 10.1111/j.1651-2227.1989.tb11285.x. [DOI] [PubMed] [Google Scholar]
- 41.Bakker MJ, Hofmann J, Churches OF, Badcock NA, Kohler M, Keage HA. Cerebrovascular function and cognition in childhood: a systematic review of transcranial Doppler studies. BMC Neurology. 2014;14:43–62. doi: 10.1186/1471-2377-14-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Koide H, Kobayashi S, Kitani M, Tsunematsu T, Nakazawa Y. Improvement of cerebral blood flow and cognitive function following pacemaker implantation in patients with bradycardia. Gerontology. 1994;40:279–85. doi: 10.1159/000213597. [DOI] [PubMed] [Google Scholar]