Abstract
Shear-mediated platelet activation (SMPA) is central in thrombosis of implantable cardiovascular therapeutic devices. Despite the morbidity and mortality associated with thrombosis of these devices, our understanding of mechanisms operative in SMPA, particularly in free flowing blood, remains limited. Herein we present and discuss a range of emerging mechanisms for consideration for “free flow” activation under supraphysiologic shear. Further definition and manipulation of these mechanisms will afford opportunities for novel pharmacologic and mechanical strategies to limit SMPA and enhance overall implant device safety.
Keywords: Mechanotransduction, platelet activation, mechanical circulatory support, thrombosis, fluid shear stress
1. Introduction
Cardiovascular disease (CVD) remains the leading cause of morbidity and mortality in the Western world (Writing Group et al., 2016). While great advances have been made in the pharmacologic management of cardiovascular disease, for many CVD conditions, implanted medical devices, rather than drugs have emerged as the mainstay of therapy. In advanced atherosclerotic coronary artery disease, stents and endovascular scaffolds are the present standard-of-care for high-grade obstruction (Iqbal et al., 2013; Task Force et al., 2013). In patients with critical aortic stenosis, percutaneous heart valves in the form of transcatheter aortic valve replacement (TAVR) are being implanted at an increasing rate (Vahl et al., 2016). For patients with advanced and end-stage congestive heart failure, mechanical circulatory support devices in the form of ventricular assist devices (VADs) and the total artificial heart (TAH) are in widespread and increasing use (Kirklin et al., 2015). Despite the efficacy of these implants, they remain hampered and limited by a common adverse event – that of device-related thrombosis. Further, recent studies have demonstrated that present anti-coagulant and notably anti-platelet agents have limited efficacy as a means of limiting thrombosis (Sheriff et al., 2014; Valerio et al., 2016). The severity of this limitation covers a spectrum ranging from reduction of flow due to space occupation of the flow path, to possible thromboembolic events including stroke and most severely, complete cessation of flow leading to device failure with accompanying ischemia, infarction and possible death.
Central to device-related thrombosis is the initiation and propagation of thrombus formation as a result of platelet activation. When one considers mechanisms of platelet activation, prime drivers which always come to mind first are the numerous, redundant biochemical pathways involved, i.e. ADP, and collagen (Jennings, 2009). Additionally, thrombosis is often viewed from the perspective of Virchow’s triad – with contributions and a delicate balance between endothelial dysfunction or surface interactions, “inflammatory blood” (biochemical mediators), and altered flow (historically stagnation) (Kroll et al., 1996). However, in the realm of cardiovascular implant devices (CVIDs), and in the case of MCS devices in particular, many of these parameters are largely absent or minimally contributory. For example, VADs are devoid of endothelial cells in their endoluminal flow path and propel blood at high flows without large regions of stagnation. As such, for most CVIDs it is flow – notably in the form of high flow with accompanying elevated shear stress - that is the dominant activating element driving platelet activation and subsequent thrombosis. To provide perspective, while shear stresses in most arterial flows are in the range of 0–30 dynes/cm2 (Kroll et al., 1996), within present day continuous flow VADs, shear may exceed 1000 dynes/cm2 (Girdhar et al., 2012; Pirbodaghi et al., 2014).
In this paper, we highlight the increasingly expanding range of mechanisms and properties that appear to contribute to platelet activation under “hypershear” conditions, i.e. shear stress > 300 dynes/ cm2, commonly operative in CVIDs. We outline these largely mechanical, less conventional mechanisms, as they represent opportunities for novel pharmacologic and alternative therapeutic development to limit platelet activation.
2. Shear-Mediated Platelet Activation – The Traditional View
Shear-mediated platelet activation (SMPA), traditionally called shear-induced platelet activation (SIPA), has been studied for many years, with early work suggesting that a defined threshold exists for activation – below which platelets remain intact and above which they become activated (Hellums, 1994). Accompanying this concept has been the phenomenon of von Willebrand factor (vWF) – GPIb interaction leading to high shear flow platelet tethering, partitioning and loose adhesion to the vessel wall, allowing further integrin-mediated high affinity adhesion and biochemical mediator-facilitated activation (Chow et al., 1992; Moake et al., 1986). In recent years, our thinking as to SMPA has expanded. Specifically, it has become recognized that activation may occur directly in the “free flow” – within regions of a flowing blood column imparting high levels of intermittent or sustained shear exposure, without wall or conduit contact. Supporting this shift in perspective, are the fact that in many CVIDs the endothelium is absent, and as a result vWF tethering is non-operative, yet thrombosis occurs. Further, it has come to be recognized recently that under “hypershear” conditions, large molecular weight multimers of vWF are cleaved, rendering remnant vWF incapable of interacting effectively with platelets to initiate thrombosis (Meyer et al., 2010).
3. Potential Mechanisms of Hypershear Mediated Platelet Activation
How then can platelets be activated, independent of a GP1b-vWF mechanism, while rapidly flowing, rotating and spinning in free flowing blood under supraphyisologic shear (Soares et al., 2013)? Herein we outline a range of potential mechanisms (Fig. 1) including: 1) “mechano-destruction” – i.e. additive platelet (membrane) damage leading to a progressive increase in porogenisity and/or leakiness of the platelet with resultant influx of activating mediators with shape change and membrane fragmentation and inversion; 2) “mechano-activation” – i.e. shear-mediated activation of shear-sensitive channels and pores allowing influx of specific activators; and 3) “mechano-transduction” – that of “outside-in” signaling via a range of transducers – beyond previously described GP1b or platelet integrin (GPIIb/IIIa) pathways. These pathways include but are not limited to the a) cell membrane and b) defined biochemical-mechanical transmembrane and intracellular linkage elements leading to activation. Further, these mechanisms may be modulated via the intrinsic nature, or the modification thereof, of the material properties of the platelet – specifically overall platelet stiffness or platelet membrane fluidity. Below we provide evidence in support of these mechanisms.
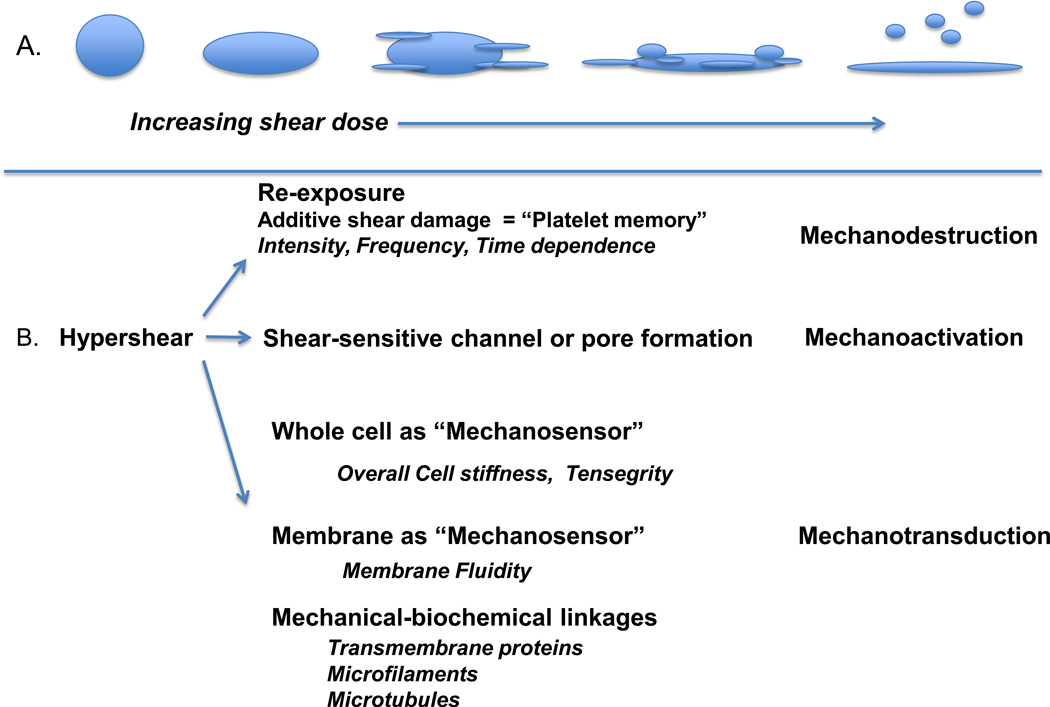
Fig. 1.
Proposed additional mechanisms of hypershear-mediated platelet activation. A.) With increasing shear dose = intensity × time, platelets undergo shape change, pseudopod extension, progressive additive damage with membrane rents and pore formation, fragmentation, membrane eversion, and ultimately microparticle generation. B.) Three mechanistic pathways are outlined: a mechanodestructive pathway in which repetitive shear damage accumulates, platelets being incapable of repair, eventually sustain irreversible damage – as illustrated in A above; a mechanoactivation pathway wherein shear-sensitive channels, pore and gates may open; and a mechanotransductive pathway in which the whole cell (based on stiffness), the cell membrane (based on fluidity), and mechanic-biochemical linkage pathways capture and convert shear to internal activating signals.
4. Additive Platelet Damage as a Mechanism of Activation – the Mechano-destructive Pathway
Early studies of SMPA largely utilized only constant shear stress exposures, with no analysis of subsequent platelet behavior or response during both low shear stress conditions (i.e. normal circulation) or repeated high shear stress exposure (i.e. recirculation in a CVID). The need to examine platelet behavior during and after dynamic, device-related flow conditions was highlighted by the observation of chronic platelet activation and thromboembolic events in mechanical heart valve patients despite antiplatelet therapy (Butchart et al., 2003). These complications still remain a challenge for current VAD recipients (Koliopoulou et al., 2016). Starting in the early 2000s, researchers began examining platelet activation in response to complex non-physiological fluid shear stress waveforms utilizing markers including thrombin and P-selectin (Zhang et al., 2003; Zhang et al., 2002). Our group expanded on these studies by subjecting gel-filtered platelets repeatedly to dynamic shear stress waveforms, both in a controlled fashion (Nobili et al., 2008; Sheriff et al., 2013) and to conditions extracted from computational fluid dynamics (CFD) simulations of CVIDs (Claiborne et al., 2013; Girdhar et al., 2012; Pelosi et al., 2014; Piatti et al., 2015), to mimic activation of platelets recirculating through devices. Both types of waveforms were selected based on their stress accumulation (SA), or product of the shear stress and exposure time dose, where simulation-extracted waveforms were representative of specific physical or high stress accumulation “hotspot” regions.
Three primary findings have emerged from our group’s work. First, repeated exposure to both constant and dynamic shear stress waveforms results in a corresponding increase in thrombin generation, dependent on the magnitude of shear stress and exposure time. As thrombin generation occurs with membrane damage and inversion, with exposure of negatively charged phospholipids, the findings support increasing damage as occurring with cumulative increasing shear. Thrombin generation is particularly prominent for higher shear magnitudes and longer exposure times (Sheriff et al., 2013). Second, we find that the frequency or dynamicity of the shear stress may be more damaging than the magnitude of the shear stress (Fig. 2) (Sheriff et al., 2013). This is particularly pertinent to perturbed flow regions in CVIDs and reflects earlier elongational shear stress observations. Third, and particularly relevant to issues of chronic platelet activation in CVID patients, is the subsequent activation of platelets after passing through these damaging shear stress conditions and circulating under physiological flow. This sensitization behavior is exacerbated under higher shear stress magnitudes (Sheriff et al., 2013) and is observed even after a single exposure to “hypershear” – millisecond-level exposure to shear stresses on the order of 1000 dyne/cm2 (Sheriff et al., 2016). These observations provide plausibility to the idea that platelets “remember” their past shear history accumulate damage leading to influx of activating ions, membrane inversion, fragmentation and eventual microparticle generation.
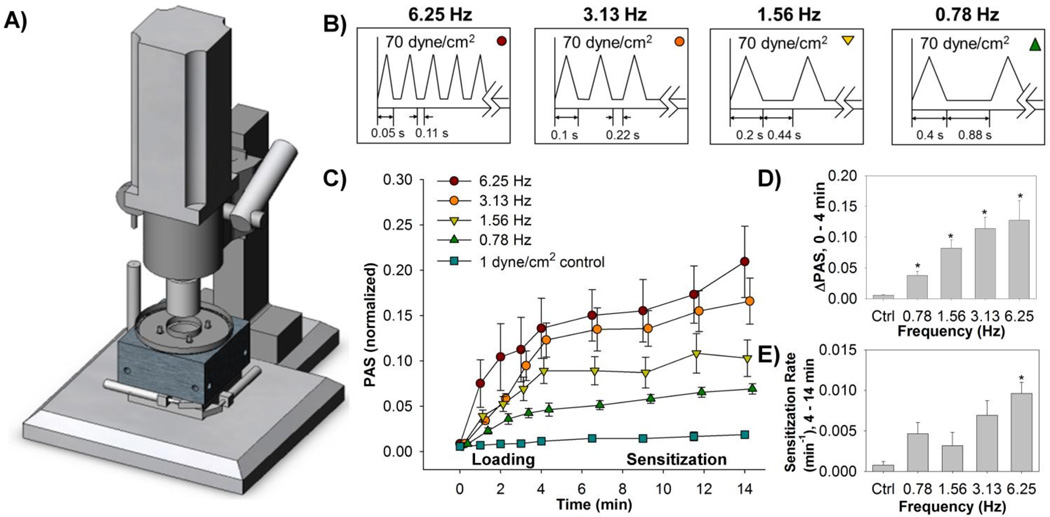
Fig. 2.
Gel-filtered platelets were exposed in a A) hemodynamic shearing device (HSD) to B) shear stress waveforms of varying frequencies for a 4 min "loading" phase. Subsequently, the platelets were exposed to 1 dyne/cm2 for an additional 10 min. C) Platelet activation state (PAS) measurements obtained at regular intervals during both the "loading" and "sensitization" phases show that the D) change in PAS and E) rate of sensitization are both dependent on the loading frequency (Adapted from Sheriff et al., 2013).
5. Mechano-activation – Activation of Shear-sensitive Channels
In a range of cell systems, specific shear-activatable channels, pores and gates have been identified. The prototype system in vascular biology has been the endothelial cell, with the recognition of shear-sensitive potassium channels (Gojova and Barakat, 2005; Ohno et al., 1993). Similarly, renal endothelial and epithelial cells have been shown to express shear stress-responsive sodium channels (Warnock et al., 2014). To date, limited data exists as to the presence of pre-existent or inducible channels or pores in platelets in response to shear. However, based on the growing recognition of these systems in a wide range of cell types, it is readily plausible that upon progressive and additive shear exposure, similar channels may exist in the platelet as well. Recent studies have identified a class of shear- stress sensitive channels – pannexins - in platelets (Taylor et al., 2015). Pannexins are a family with three member proteins, related to gap junctions, which form channels enhancing ATP release and Ca+2 uptake that are mechanically sensitive (Taylor et al., 2015). Recent studies have shown that activation of pannexin-1 enhances platelet activation (Taylor et al., 2014). Future studies are needed to further clarify the role of pannexins, and other potential shear-sensitive channels, as to their contribution to shear-mediated platelet activation.
6. Whole Cell Material Properties – Platelet Stiffness, Membrane Fluidity, and the Mechano-transductive Pathway
Beyond responding to a wide variety of biochemical autocrine, paracrine and endocrine mediators, all cells as a universal phenomenon respond to physical forces and cues in their environment (DuFort et al., 2011). To accomplish this, a range of sensing and transduction structures and mechanisms exist, including that of the cell membrane as well as a range of transmembrane biochemical-mechanical linkage pathways, to capture and convey extracellular physical stimuli intracellularly to effectuate a response (Orr et al., 2006). Beyond component sensors, the cell as a whole, based on its overall stiffness, may act as a sensor and a variable transducer/responder to exerted stress. This overall process of physical stimulus recognition and reaction is referred to as cell mechanotransduction (Hamill and Martinac, 2001; Ingber et al., 2014).
The cell membrane lipid bilayer itself may serve as a mechanotransducer (White and Frangos, 2007). Elements in this system include the exact membrane lipid composition, the presence of specialized lipids i.e. gangliosides, sphingolipids and cholesterol, specialized regions or lipid “rafts,” and transmembrane proteins (Bevers et al., 1998; Nishimura et al., 2006; Sun et al., 2007; Tocanne et al., 1994). A key variable modulating the membrane as a mechanosensor and transducer is the ability of these constitutive elements to spatially rearrange and laterally diffuse – i.e. the overall fluidity of the membrane. In other cell systems, changes in membrane fluidity has been shown to modulate shear stress mechano-chemical signal transduction (Los and Murata, 2004).
Transmembrane linkage systems are composed of a range of transmembrane “receptor” proteins which act as receivers and transducers of a physical force, e.g. shear stress, transmitting this stimulus intracellularly to effectuate a response. These include integrins, immunoglobulins, specific glycoproteins and over 50 other candidate molecules and complexes (Ross et al., 2013). These moieties typically are physically associated with submembrane assemblies which contain kinases which act via phosphorylation or other biochemical signaling pathways. Additionally, these proteins are often physically tethered to microtubules and microfilaments transmitting force and other physical cues within the cytoplasm. A generalized theory and system of organization, known as “tensegrity,” has been progressively developed and now well recognized, wherein these structural trans-cytoplasmic proteins and tubular constituents act on the whole of the cell, as well as regionally, via a continuum mechanics means (Ingber et al., 2014).
Our group has examined the impact of platelet stiffness as a bulk property modulating shear responsiveness, with the operative hypothesis being that exogenous shear stress will be more likely transmitted to or coupled with a more rigid body than a more elastomeric or deformable one. Recently we developed a methodology, utilizing dielectrophoresis, in which the stiffness of a single platelet could be measured (Leung et al., 2016). Utilizing this quantitative method, we have demonstrated that if the intrinsic resting stiffness of the platelet is increased, the overall responsiveness to supraphysiologic shear stress is heightened. Conversely, we have shown that if the overall stiffness is reduced, SMPA is reduced below normal. In support of this mechanism we have observed that increased platelet stiffness, associated with enhanced microtubule or actin skeleton organization, is associated with increased reactivity and conversely reduced stiffness, associated with microtubule or actin-myosin disorganization, results in decreased reactivity to shear. An example of this phenomenon is provided (Fig. 3).
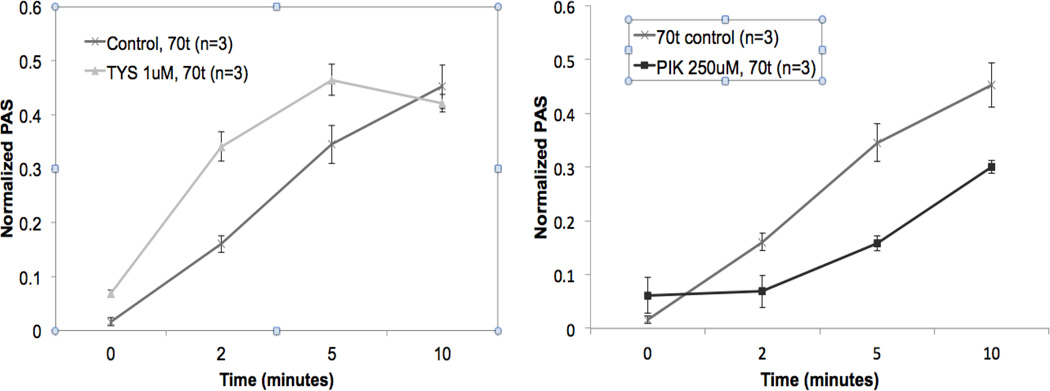
Fig. 3.
Effect of increasing platelet stiffness on shear-mediated platelet activation. Platelets stiffened via enhancing platelet actin polymerization and organization, via exposure to FTY720 S-phosphonate (Tysiponate, TYS, 1 µM, 10 min.) demonstrate increased responsiveness to 70 dyne/cm2 (70t) shear vs. non-TYS control (left). Platelets made less stiff, via inhibition of actinmyosin filaments and organization, after exposure to permanent inhibitor of myosin light chain kinase (PIK, 250 µM, 10 min.), demonstrate reduced responsiveness to shear vs. non-PIK control (right).
In recent studies, we have also examined the effect of modulating platelet membrane fluidity on SMPA. The operative hypothesis here is that an increase in fluidity will make the platelet more deformable under shear, less rigid and with greater likelihood to allow rapid diffusion and deflection of otherwise stiffly anchored transmembrane proteins. We observed that increasing membrane fluidity is clearly associated with a significant reduction of sensitivity to shear activation. Uniquely, mild alteration of platelet membrane fluidity was found to stabilize platelets over the range of shear stress experienced in VADs, i.e. up to 1000 dynes/cm2, while at the same time maintaining platelet reactivity to chemical agonists such as dimethyl sulfoxide (Fig. 4) (Tran et al., 2014).
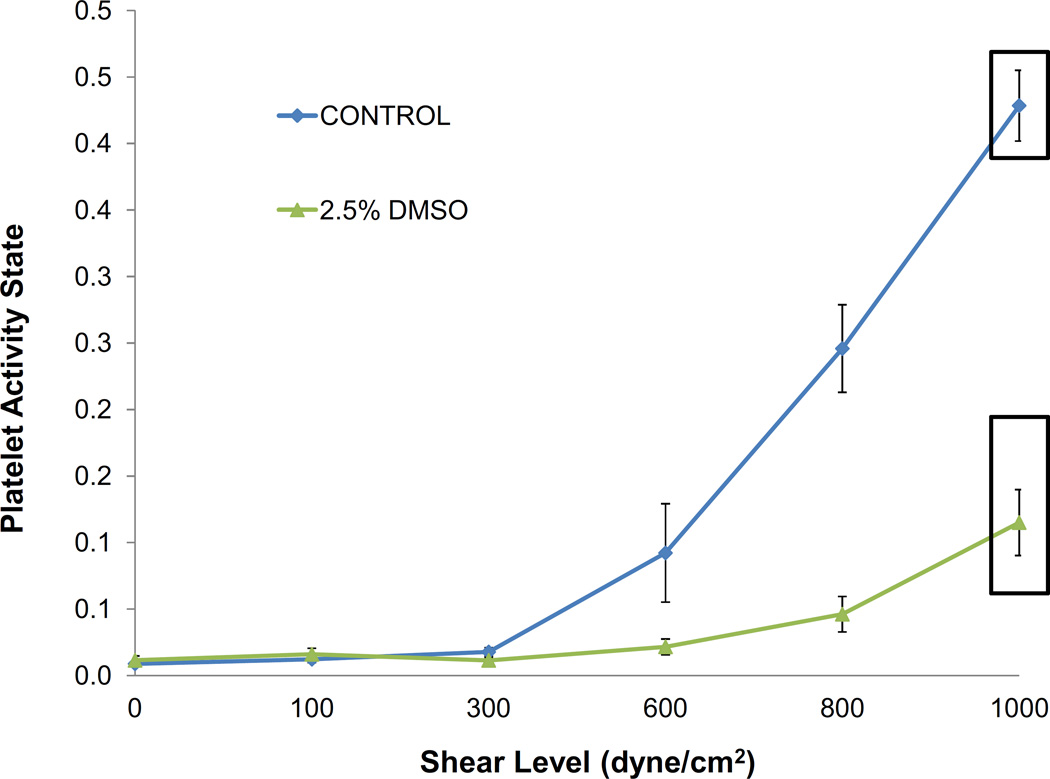
Fig. 4.
Effect of increasing membrane fluidity on shear-mediated platelet activation. Enhancing platelet membrane fluidity via exposure of platelets to dimethyl sulfoxide (DMSO) significantly limits reactivity to shear over a wide range up to 1000 dynes/cm2.
7. Conclusion
It is becoming increasingly clear that a range of mechanical mechanisms are vital, if not dominating, in platelet activation associated with hypershear exposure in CVIDs. With the persistence of adverse events experienced with CVIDs (Kirklin et al., 2015) coupled with the disturbing observation of a progressive rise in device thrombosis (Starling et al., 2014), new strategies are needed to limit SMPA. Pursuit of novel means of modulating the mechanisms and pathways outlined here will go far to impact a vital and increasing unmet clinical need and demand.
Acknowledgments
This manuscript was based in part on results generated from projects funded by a Quantum Grant from the National Institute of Biomedical Imaging and Bioengineering, National Institutes of Health (Award No. 5U01EB012487-05).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
References
- Bevers EM, Comfurius P, Dekkers DW, Harmsma M, Zwaal RF. Transmembrane phospholipid distribution in blood cells: control mechanisms and pathophysiological significance. Biological Chemistry. 1998;379:973–986. [PubMed] [Google Scholar]
- Butchart EG, Ionescu A, Payne N, Giddings J, Grunkemeier GL, Fraser AG. A new scoring system to determine thromboembolic risk after heart valve replacement. Circulation. 2003;108(Suppl 1):II68–II74. doi: 10.1161/01.cir.0000087383.62522.1e. [DOI] [PubMed] [Google Scholar]
- Chow TW, Hellums JD, Moake JL, Kroll MH. Shear stress-induced von Willebrand factor binding to platelet glycoprotein Ib initiates calcium influx associated with aggregation. Blood. 1992;80:113–120. [PubMed] [Google Scholar]
- Claiborne TE, Sheriff J, Kuetting M, Steinseifer U, Slepian MJ, Bluestein D. In vitro evaluation of a novel hemodynamically optimized trileaflet polymeric prosthetic heart valve. Journal of Biomechanical Engineering. 2013;135:021021. doi: 10.1115/1.4023235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuFort CC, Paszek MJ, Weaver VM. Balancing forces: architectural control of mechanotransduction. Nature Reviews Molecular Cell Biology. 2011;12:308–319. doi: 10.1038/nrm3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girdhar G, Xenos M, Alemu Y, Chiu WC, Lynch BE, Jesty J, Einav S, Slepian MJ, Bluestein D. Device thrombogenicity emulation: a novel method for optimizing mechanical circulatory support device thromboresistance. PLoS One. 2012;7:e32463. doi: 10.1371/journal.pone.0032463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gojova A, Barakat AI. Vascular endothelial wound closure under shear stress: role of membrane fluidity and flow-sensitive ion channels. Journal of Applied Physiology (1985) 2005;98:2355–2362. doi: 10.1152/japplphysiol.01136.2004. [DOI] [PubMed] [Google Scholar]
- Hamill OP, Martinac B. Molecular basis of mechanotransduction in living cells. Physiological Reviews. 2001;81:685–740. doi: 10.1152/physrev.2001.81.2.685. [DOI] [PubMed] [Google Scholar]
- Hellums JD. 1993 Whitaker Lecture: biorheology in thrombosis research. Annals if Biomedical Engineering. 1994;22:445–455. doi: 10.1007/BF02367081. [DOI] [PubMed] [Google Scholar]
- Ingber DE, Wang N, Stamenovic D. Tensegrity, cellular biophysics, and the mechanics of living systems. Reports on Progress in Physics. 2014;77:046603. doi: 10.1088/0034-4885/77/4/046603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iqbal J, Gunn J, Serruys PW. Coronary stents: historical development, current status and future directions. British Medical Bulletin. 2013;106:193–211. doi: 10.1093/bmb/ldt009. [DOI] [PubMed] [Google Scholar]
- Jennings LK. Mechanisms of platelet activation: need for new strategies to protect against platelet-mediated atherothrombosis. Thrombosis and Haemostasis. 2009;102:248–257. doi: 10.1160/TH09-03-0192. [DOI] [PubMed] [Google Scholar]
- Kirklin JK, Naftel DC, Pagani FD, Kormos RL, Stevenson LW, Blume ED, Myers SL, Miller MA, Baldwin JT, Young JB. Seventh INTERMACS annual report: 15,000 patients and counting. Journal of Heart and Lung Transplantation. 2015;34:1495–1504. doi: 10.1016/j.healun.2015.10.003. [DOI] [PubMed] [Google Scholar]
- Koliopoulou A, McKellar SH, Rondina M, Selzman CH. Bleeding and thrombosis in chronic ventricular assist device therapy: focus on platelets. Current Opinion in Cardiology. 2016;31:299–307. doi: 10.1097/HCO.0000000000000284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroll MH, Hellums JD, McIntire LV, Schafer AI, Moake JL. Platelets and shear stress. Blood. 1996;88:1525–1541. [PubMed] [Google Scholar]
- Leung SL, Lu Y, Bluestein D, Slepian MJ. Dielectrophoresis-Mediated Electrodeformation as a Means of Determining Individual Platelet Stiffness. Annals of Biomedical Engineering. 2016;44:903–913. doi: 10.1007/s10439-015-1383-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Los DA, Murata N. Membrane fluidity and its roles in the perception of environmental signals. Biochimica et Biophysica Acta. 2004;1666:142–157. doi: 10.1016/j.bbamem.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Meyer AL, Malehsa D, Bara C, Budde U, Slaughter MS, Haverich A, Strueber M. Acquired von Willebrand syndrome in patients with an axial flow left ventricular assist device. Circulation Heart Failure. 2010;3:675–681. doi: 10.1161/CIRCHEARTFAILURE.109.877597. [DOI] [PubMed] [Google Scholar]
- Moake JL, Turner NA, Stathopoulos NA, Nolasco LH, Hellums JD. Involvement of large plasma von Willebrand factor (vWF) multimers and unusually large vWF forms derived from endothelial cells in shear stress-induced platelet aggregation. Journal of Clinical Investigation. 1986;78:1456–1461. doi: 10.1172/JCI112736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura SY, Vrljic M, Klein LO, McConnell HM, Moerner WE. Cholesterol depletion induces solid-like regions in the plasma membrane. Biophysical Journal. 2006;90:927–938. doi: 10.1529/biophysj.105.070524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobili M, Sheriff J, Morbiducci U, Redaelli A, Bluestein D. Platelet activation due to hemodynamic shear stresses: damage accumulation model and comparison to in vitro measurements. ASAIO Journal. 2008;54:64–72. doi: 10.1097/MAT.0b013e31815d6898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno M, Gibbons GH, Dzau VJ, Cooke JP. Shear stress elevates endothelial cGMP. Role of a potassium channel and G protein coupling. Circulation. 1993;88:193–197. doi: 10.1161/01.cir.88.1.193. [DOI] [PubMed] [Google Scholar]
- Orr AW, Helmke BP, Blackman BR, Schwartz MA. Mechanisms of mechanotransduction. Developmental Cell. 2006;10:11–20. doi: 10.1016/j.devcel.2005.12.006. [DOI] [PubMed] [Google Scholar]
- Pelosi A, Sheriff J, Stevanella M, Fiore GB, Bluestein D, Redaelli A. Computational evaluation of the thrombogenic potential of a hollow-fiber oxygenator with integrated heat exchanger during extracorporeal circulation. Biomechanics and Modeling in Mechanobiology. 2014;13:349–361. doi: 10.1007/s10237-012-0445-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piatti F, Sturla F, Marom G, Sheriff J, Claiborne TE, Slepian MJ, Redaelli A, Bluestein D. Hemodynamic and thrombogenic analysis of a trileaflet polymeric valve using a fluid-structure interaction approach. Journal of Biomechics. 2015;48:3641–3649. doi: 10.1016/j.jbiomech.2015.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirbodaghi T, Asgari S, Cotter C, Bourque K. Physiologic and hematologic concerns of rotary blood pumps: what needs to be improved? Heart Failure Review. 2014;19:259–266. doi: 10.1007/s10741-013-9389-4. [DOI] [PubMed] [Google Scholar]
- Ross TD, Coon BG, Yun S, Baeyens N, Tanaka K, Ouyang M, Schwartz MA. Integrins in mechanotransduction. Current Opinion in Cell Biology. 2013;25:613–618. doi: 10.1016/j.ceb.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheriff J, Girdhar G, Chiu WC, Jesty J, Slepian MJ, Bluestein D. Comparative efficacy of in vitro and in vivo metabolized aspirin in the DeBakey ventricular assist device. Journal of Thrombosis and Thrombolysis. 2014;37:499–506. doi: 10.1007/s11239-013-0997-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheriff J, Soares JS, Xenos M, Jesty J, Bluestein D. Evaluation of Shear-Induced Platelet Activation Models Under Constant and Dynamic Shear Stress Loading Conditions Relevant to Devices. Annals of Biomedical Engineering. 2013 doi: 10.1007/s10439-013-0758-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheriff J, Tran PL, Hutchinson M, DeCook T, Slepian MJ, Bluestein D, Jesty J. Repetitive Hypershear Activates and Sensitizes Platelets in a Dose-Dependent Manner. Artificial Organs. 2016;40:586–595. doi: 10.1111/aor.12602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares JS, Gao C, Alemu Y, Slepian M, Bluestein D. Simulation of platelets suspension flowing through a stenosis model using a dissipative particle dynamics approach. Annals of Biomedical Engineering. 2013;41:2318–2333. doi: 10.1007/s10439-013-0829-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starling RC, Moazami N, Silvestry SC, Ewald G, Rogers JG, Milano CA, Rame JE, Acker MA, Blackstone EH, Ehrlinger J, Thuita L, Mountis MM, Soltesz EG, Lytle BW, Smedira NG. Unexpected abrupt increase in left ventricular assist device thrombosis. New England Journal of Medicine. 2014;370:33–40. doi: 10.1056/NEJMoa1313385. [DOI] [PubMed] [Google Scholar]
- Sun M, Northup N, Marga F, Huber T, Byfield FJ, Levitan I, Forgacs G. The effect of cellular cholesterol on membrane-cytoskeleton adhesion. Journal of Cell Science. 2007;120:2223–2231. doi: 10.1242/jcs.001370. [DOI] [PubMed] [Google Scholar]
- Task Force, M. Montalescot G, Sechtem U, Achenbach S, Andreotti F, Arden C, Budaj A, Bugiardini R, Crea F, Cuisset T, Di Mario C, Ferreira JR, Gersh BJ, Gitt AK, Hulot JS, Marx N, Opie LH, Pfisterer M, Prescott E, Ruschitzka F, Sabate M, Senior R, Taggart DP, van der Wall EE, Vrints CJ, Guidelines, E.S.C.C.f.P. Zamorano JL, Achenbach S, Baumgartner H, Bax JJ, Bueno H, Dean V, Deaton C, Erol C, Fagard R, Ferrari R, Hasdai D, Hoes AW, Kirchhof P, Knuuti J, Kolh P, Lancellotti P, Linhart A, Nihoyannopoulos P, Piepoli MF, Ponikowski P, Sirnes PA, Tamargo JL, Tendera M, Torbicki A, Wijns W, Windecker S, Document R, Knuuti J, Valgimigli M, Bueno H, Claeys MJ, Donner-Banzhoff N, Erol C, Frank H, Funck-Brentano C, Gaemperli O, Gonzalez-Juanatey JR, Hamilos M, Hasdai D, Husted S, James SK, Kervinen K, Kolh P, Kristensen SD, Lancellotti P, Maggioni AP, Piepoli MF, Pries AR, Romeo F, Ryden L, Simoons ML, Sirnes PA, Steg PG, Timmis A, Wijns W, Windecker S, Yildirir A, Zamorano JL. 2013 ESC guidelines on the management of stable coronary artery disease: the Task Force on the management of stable coronary artery disease of the European Society of Cardiology. European Heart Journal. 2013;34:2949–3003. doi: 10.1093/eurheartj/eht296. [DOI] [PubMed] [Google Scholar]
- Taylor KA, Wright JR, Mahaut-Smith MP. Regulation of Pannexin-1 channel activity. Biochemical Society Transactions. 2015;43:502–507. doi: 10.1042/BST20150042. [DOI] [PubMed] [Google Scholar]
- Taylor KA, Wright JR, Vial C, Evans RJ, Mahaut-Smith MP. Amplification of human platelet activation by surface pannexin-1 channels. Journal of Thrombosis and Haemostasis. 2014;12:987–998. doi: 10.1111/jth.12566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tocanne JF, Cezanne L, Lopez A, Piknova B, Schram V, Tournier JF, Welby M. Lipid domains and lipid/protein interactions in biological membranes. Chemistry and Physics of Lipids. 1994;73:139–158. doi: 10.1016/0009-3084(94)90179-1. [DOI] [PubMed] [Google Scholar]
- Tran PL, Valerio L, Yamaguchi J, Brengle W, DeCook T, Hutchinson M, Sen N, Bluestein D, Slepian MJ. Year Dimethyl Sulfoxide: A New Nemesis of Shear-Induced Platelet Activation. ASME 2014 3rd Global Congress on Nanoengineering for Medicine and Biology; San Francisco, CA. [Google Scholar]
- Vahl TP, Kodali SK, Leon MB. Transcatheter Aortic Valve Replacement 2016: A Modern-Day "Through the Looking-Glass" Adventure. Journal of the American College of Cardiology. 2016;67:1472–1487. doi: 10.1016/j.jacc.2015.12.059. [DOI] [PubMed] [Google Scholar]
- Valerio L, Tran PL, Sheriff J, Brengle W, Ghosh R, Chiu WC, Redaelli A, Fiore GB, Pappalardo F, Bluestein D, Slepian MJ. Aspirin has limited ability to modulate shear-mediated platelet activation associated with elevated shear stress of ventricular assist devices. Thrombosis Research. 2016;140:110–117. doi: 10.1016/j.thromres.2016.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warnock DG, Kusche-Vihrog K, Tarjus A, Sheng S, Oberleithner H, Kleyman TR, Jaisser F. Blood pressure and amiloride-sensitive sodium channels in vascular and renal cells. Nature Reviews Nephrology. 2014;10:146–157. doi: 10.1038/nrneph.2013.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White CR, Frangos JA. The shear stress of it all: the cell membrane and mechanochemical transduction. Philosophical Transactions of the Royal Society of London B: Biological Sciences. 2007;362:1459–1467. doi: 10.1098/rstb.2007.2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Writing Group, M; Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ, Huffman MD, Isasi CR, Jimenez MC, Judd SE, Kissela BM, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Magid DJ, McGuire DK, Mohler ER, 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Rosamond W, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Woo D, Yeh RW, Turner MB American Heart Association Statistics, C., Stroke Statistics, S. Heart Disease and Stroke Statistics-2016 Update: A Report From the American Heart Association. Circulation. 2016;133:e38–e60. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- Zhang JN, Bergeron AL, Yu Q, Sun C, McBride L, Bray PF, Dong JF. Duration of exposure to high fluid shear stress is critical in shear-induced platelet activation-aggregation. Thrombosis and Haemostasis. 2003;90:672–678. doi: 10.1160/TH03-03-0145. [DOI] [PubMed] [Google Scholar]
- Zhang JN, Bergeron AL, Yu Q, Sun C, McIntire LV, Lopez JA, Dong JF. Platelet aggregation and activation under complex patterns of shear stress. Thrombosis and Haemostasis. 2002;88:817–821. [PubMed] [Google Scholar]