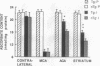Abstract
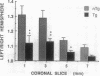
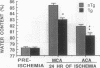
Oxygen-derived free radicals have been implicated in the pathogenesis of vasogenic edema and infarction caused by ischemia and reperfusion injury. In earlier studies, exogenously supplied liposome-entrapped CuZn superoxide dismutase (CuZn-SOD) ameliorated ischemic brain edema and infarction in rats following focal cerebral ischemia. To ascertain directly the role of SOD in the protection against superoxide radical-induced injury, we measured infarct size and water content 24 hr following focal cerebral ischemia in nontransgenic mice and in transgenic mice bearing the human SOD1 gene. These transgenic mice have 3.1-fold higher cellular CuZn-SOD activity in the brain than do their nontransgenic littermates. We also measured antioxidant levels (reduced glutathione and reduced ascorbate) of contralateral cortex, infarct cortex, surrounding cortex, and striatum. Infarct size and brain edema were significantly decreased in transgenic mice compared with nontransgenic mice. Reduced glutathione and reduced ascorbate levels decreased in the ischemic hemisphere, but levels in surrounding cortex and striatum were significantly higher in transgenic mice than in nontransgenic mice. These results indicate that increased endogenous SOD activity in brain reduces the level of ischemic damage and support the concept that superoxide radicals play an important role in the pathogenesis of infarction and edema following focal cerebral ischemia.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe K., Yoshida S., Watson B. D., Busto R., Kogure K., Ginsberg M. D. alpha-Tocopherol and ubiquinones in rat brain subjected to decapitation ischemia. Brain Res. 1983 Aug 22;273(1):166–169. doi: 10.1016/0006-8993(83)91107-1. [DOI] [PubMed] [Google Scholar]
- Adlard B. P., de Souza S. W., Moon S. The effect of age, growth retardation and asphyxia on ascorbic acid concentrations in developing brain. J Neurochem. 1973 Oct;21(4):877–881. doi: 10.1111/j.1471-4159.1973.tb07532.x. [DOI] [PubMed] [Google Scholar]
- Annerén K. G., Epstein C. J. Lipid peroxidation and superoxide dismutase-1 and glutathione peroxidase activities in trisomy 16 fetal mice and human trisomy 21 fibroblasts. Pediatr Res. 1987 Jan;21(1):88–92. doi: 10.1203/00006450-198701000-00019. [DOI] [PubMed] [Google Scholar]
- Astrup J., Siesjö B. K., Symon L. Thresholds in cerebral ischemia - the ischemic penumbra. Stroke. 1981 Nov-Dec;12(6):723–725. doi: 10.1161/01.str.12.6.723. [DOI] [PubMed] [Google Scholar]
- Baker M. Z., Badiello R., Tamba M., Quintiliani M., Gorin G. Pulse radiolytic study of hydrogen transfer from glutathione to organic radicals. Int J Radiat Biol Relat Stud Phys Chem Med. 1982 Jun;41(6):595–602. doi: 10.1080/09553008214550691. [DOI] [PubMed] [Google Scholar]
- Bederson J. B., Pitts L. H., Germano S. M., Nishimura M. C., Davis R. L., Bartkowski H. M. Evaluation of 2,3,5-triphenyltetrazolium chloride as a stain for detection and quantification of experimental cerebral infarction in rats. Stroke. 1986 Nov-Dec;17(6):1304–1308. doi: 10.1161/01.str.17.6.1304. [DOI] [PubMed] [Google Scholar]
- Bodannes R. S., Chan P. C. Ascorbic acid as a scavenger of singlet oxygen. FEBS Lett. 1979 Sep 15;105(2):195–196. doi: 10.1016/0014-5793(79)80609-2. [DOI] [PubMed] [Google Scholar]
- Chan P. H., Longar S., Fishman R. A. Protective effects of liposome-entrapped superoxide dismutase on posttraumatic brain edema. Ann Neurol. 1987 Jun;21(6):540–547. doi: 10.1002/ana.410210604. [DOI] [PubMed] [Google Scholar]
- Chan P. H., Yang G. Y., Chen S. F., Carlson E., Epstein C. J. Cold-induced brain edema and infarction are reduced in transgenic mice overexpressing CuZn-superoxide dismutase. Ann Neurol. 1991 May;29(5):482–486. doi: 10.1002/ana.410290506. [DOI] [PubMed] [Google Scholar]
- Chen S. T., Hsu C. Y., Hogan E. L., Maricq H., Balentine J. D. A model of focal ischemic stroke in the rat: reproducible extensive cortical infarction. Stroke. 1986 Jul-Aug;17(4):738–743. doi: 10.1161/01.str.17.4.738. [DOI] [PubMed] [Google Scholar]
- Cooper A. J., Pulsinelli W. A., Duffy T. E. Glutathione and ascorbate during ischemia and postischemic reperfusion in rat brain. J Neurochem. 1980 Nov;35(5):1242–1245. doi: 10.1111/j.1471-4159.1980.tb07882.x. [DOI] [PubMed] [Google Scholar]
- Dumuis A., Sebben M., Haynes L., Pin J. P., Bockaert J. NMDA receptors activate the arachidonic acid cascade system in striatal neurons. Nature. 1988 Nov 3;336(6194):68–70. doi: 10.1038/336068a0. [DOI] [PubMed] [Google Scholar]
- Dykens J. A., Stern A., Trenkner E. Mechanism of kainate toxicity to cerebellar neurons in vitro is analogous to reperfusion tissue injury. J Neurochem. 1987 Oct;49(4):1222–1228. doi: 10.1111/j.1471-4159.1987.tb10014.x. [DOI] [PubMed] [Google Scholar]
- ELLMAN G. L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959 May;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Epstein C. J., Avraham K. B., Lovett M., Smith S., Elroy-Stein O., Rotman G., Bry C., Groner Y. Transgenic mice with increased Cu/Zn-superoxide dismutase activity: animal model of dosage effects in Down syndrome. Proc Natl Acad Sci U S A. 1987 Nov;84(22):8044–8048. doi: 10.1073/pnas.84.22.8044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flamm E. S., Demopoulos H. B., Seligman M. L., Poser R. G., Ransohoff J. Free radicals in cerebral ischemia. Stroke. 1978 Sep-Oct;9(5):445–447. doi: 10.1161/01.str.9.5.445. [DOI] [PubMed] [Google Scholar]
- Frei B., England L., Ames B. N. Ascorbate is an outstanding antioxidant in human blood plasma. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6377–6381. doi: 10.1073/pnas.86.16.6377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall E. D., Braughler J. M. Central nervous system trauma and stroke. II. Physiological and pharmacological evidence for involvement of oxygen radicals and lipid peroxidation. Free Radic Biol Med. 1989;6(3):303–313. doi: 10.1016/0891-5849(89)90057-9. [DOI] [PubMed] [Google Scholar]
- Imaizumi S., Woolworth V., Fishman R. A., Chan P. H. Liposome-entrapped superoxide dismutase reduces cerebral infarction in cerebral ischemia in rats. Stroke. 1990 Sep;21(9):1312–1317. doi: 10.1161/01.str.21.9.1312. [DOI] [PubMed] [Google Scholar]
- Kinuta Y., Kikuchi H., Ishikawa M., Kimura M., Itokawa Y. Lipid peroxidation in focal cerebral ischemia. J Neurosurg. 1989 Sep;71(3):421–429. doi: 10.3171/jns.1989.71.3.0421. [DOI] [PubMed] [Google Scholar]
- Kitagawa K., Matsumoto M., Oda T., Niinobe M., Hata R., Handa N., Fukunaga R., Isaka Y., Kimura K., Maeda H. Free radical generation during brief period of cerebral ischemia may trigger delayed neuronal death. Neuroscience. 1990;35(3):551–558. doi: 10.1016/0306-4522(90)90328-2. [DOI] [PubMed] [Google Scholar]
- Kogure K., Watson B. D., Busto R., Abe K. Potentiation of lipid peroxides by ischemia in rat brain. Neurochem Res. 1982 Apr;7(4):437–454. doi: 10.1007/BF00965496. [DOI] [PubMed] [Google Scholar]
- Kontos H. A., Wei E. P. Superoxide production in experimental brain injury. J Neurosurg. 1986 May;64(5):803–807. doi: 10.3171/jns.1986.64.5.0803. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Liu T. H., Beckman J. S., Freeman B. A., Hogan E. L., Hsu C. Y. Polyethylene glycol-conjugated superoxide dismutase and catalase reduce ischemic brain injury. Am J Physiol. 1989 Feb;256(2 Pt 2):H589–H593. doi: 10.1152/ajpheart.1989.256.2.H589. [DOI] [PubMed] [Google Scholar]
- Lundy E. F., Solik B. S., Frank R. S., Lacy P. S., Combs D. J., Zelenock G. B., D'Alecy L. G. Morphometric evaluation of brain infarcts in rats and gerbils. J Pharmacol Methods. 1986 Nov;16(3):201–214. doi: 10.1016/0160-5402(86)90042-2. [DOI] [PubMed] [Google Scholar]
- Nishikimi M. Oxidation of ascorbic acid with superoxide anion generated by the xanthine-xanthine oxidase system. Biochem Biophys Res Commun. 1975 Mar 17;63(2):463–468. doi: 10.1016/0006-291x(75)90710-x. [DOI] [PubMed] [Google Scholar]
- Pellegrini-Giampietro D. E., Cherici G., Alesiani M., Carla V., Moroni F. Excitatory amino acid release and free radical formation may cooperate in the genesis of ischemia-induced neuronal damage. J Neurosci. 1990 Mar;10(3):1035–1041. doi: 10.1523/JNEUROSCI.10-03-01035.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravindranath V., Reed D. J. Glutathione depletion and formation of glutathione-protein mixed disulfide following exposure of brain mitochondria to oxidative stress. Biochem Biophys Res Commun. 1990 Jun 29;169(3):1075–1079. doi: 10.1016/0006-291x(90)92004-j. [DOI] [PubMed] [Google Scholar]
- Rehncrona S., Folbergrová J., Smith D. S., Siesjö B. K. Influence of complete and pronounced incomplete cerebral ischemia and subsequent recirculation on cortical concentrations of oxidized and reduced glutathione in the rat. J Neurochem. 1980 Mar;34(3):477–486. doi: 10.1111/j.1471-4159.1980.tb11170.x. [DOI] [PubMed] [Google Scholar]
- Sadrzadeh S. M., Eaton J. W. Hemoglobin-mediated oxidant damage to the central nervous system requires endogenous ascorbate. J Clin Invest. 1988 Nov;82(5):1510–1515. doi: 10.1172/JCI113759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siesjö B. K., Agardh C. D., Bengtsson F. Free radicals and brain damage. Cerebrovasc Brain Metab Rev. 1989 Fall;1(3):165–211. [PubMed] [Google Scholar]
- Simon R., Shiraishi K. N-methyl-D-aspartate antagonist reduces stroke size and regional glucose metabolism. Ann Neurol. 1990 Jun;27(6):606–611. doi: 10.1002/ana.410270604. [DOI] [PubMed] [Google Scholar]
- Slivka A., Mytilineou C., Cohen G. Histochemical evaluation of glutathione in brain. Brain Res. 1987 Apr 21;409(2):275–284. doi: 10.1016/0006-8993(87)90712-8. [DOI] [PubMed] [Google Scholar]
- Swanson R. A., Morton M. T., Tsao-Wu G., Savalos R. A., Davidson C., Sharp F. R. A semiautomated method for measuring brain infarct volume. J Cereb Blood Flow Metab. 1990 Mar;10(2):290–293. doi: 10.1038/jcbfm.1990.47. [DOI] [PubMed] [Google Scholar]
- Turrens J. F., Crapo J. D., Freeman B. A. Protection against oxygen toxicity by intravenous injection of liposome-entrapped catalase and superoxide dismutase. J Clin Invest. 1984 Jan;73(1):87–95. doi: 10.1172/JCI111210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson B. D., Busto R., Goldberg W. J., Santiso M., Yoshida S., Ginsberg M. D. Lipid peroxidation in vivo induced by reversible global ischemia in rat brain. J Neurochem. 1984 Jan;42(1):268–274. doi: 10.1111/j.1471-4159.1984.tb09728.x. [DOI] [PubMed] [Google Scholar]