Abstract
Key points
Chronic administration of the selective noradrenaline reuptake inhibitor (NRI) reboxetine (RBX) increased and prolonged the long‐term potentiation‐like plasticity induced by anodal transcranial direct current stimulation (tDCS) for over 24 h.
Chronic administration of RBX converted cathodal tDCS‐induced long‐term depression‐like plasticity into facilitation for 120 min.
Chronic noradrenergic activity enhancement on plasticity of the human brain might partially explain the delayed therapeutic impact of selective NRIs in depression and other neuropsychiatric diseases.
Abstract
Noradrenaline affects cognition and motor learning processes via its impact on long‐term potentiation (LTP) and depression (LTD). We aimed to explore the impact of single dose and chronic administration of the selective noradrenaline reuptake inhibitor (NRI) reboxetine (RBX) on plasticity induced by transcranial direct current stimulation (tDCS) in healthy humans via a double‐blinded, placebo‐controlled, randomized crossover study. Sixteen healthy volunteers received placebo or single dose RBX (8 mg) before anodal or cathodal tDCS of the primary motor cortex. Afterwards, the same subjects took RBX (8 mg day−1) consecutively for 21 days. During this period, two additional interventions were performed (RBX with anodal or cathodal tDCS), to explore the impact of chronic RBX treatment on plasticity. Plasticity was monitored by motor‐evoked potential amplitudes elicited by transcranial magnetic stimulation. Chronic administration of RBX increased and prolonged the LTP‐like plasticity induced by anodal tDCS for over 24 h. Chronic RBX significantly converted cathodal tDCS‐induced LTD‐like plasticity into facilitation, as compared to the single dose condition, for 120 min after stimulation. The results show a prominent impact of chronic noradrenergic enhancement on plasticity of the human brain that might partially explain the delayed therapeutic impact of selective NRIs in depression and other neuropsychiatric diseases.
Keywords: noradrenaline, neuroplasticity, transcranial direct current stimulation
Key points
Chronic administration of the selective noradrenaline reuptake inhibitor (NRI) reboxetine (RBX) increased and prolonged the long‐term potentiation‐like plasticity induced by anodal transcranial direct current stimulation (tDCS) for over 24 h.
Chronic administration of RBX converted cathodal tDCS‐induced long‐term depression‐like plasticity into facilitation for 120 min.
Chronic noradrenergic activity enhancement on plasticity of the human brain might partially explain the delayed therapeutic impact of selective NRIs in depression and other neuropsychiatric diseases.
Abbreviations
- ADM
abductor digiti minimi
- BL1
first baseline
- BL2
second baseline
- LTD
long‐term depression
- LTP
long‐term potentiation
- MEP
motor evoked potential
- NE
next evening
- NM
next morning
- NN
next noon
- NRI
noradrenaline reuptake inhibitor
- PLC
placebo
- RBX
reboxetine
- SE
same evening
- SSRI
selective serotonin reuptake inhibitor
- tDCS
transcranial direct current stimulation
- TMS
transcranial magnetic stimulation
Introduction
Noradrenaline affects learning and memory processes via modulating long‐term potentiation (LTP) and depression (LTD) (Tully et al. 2007; Wallings et al. 2016). The precise effect of noradrenaline on plasticity, as explored in animal models, is complex and depends on receptor subtype, concentration and site of action (Marzo et al. 2009). Specifically, noradrenaline affects the direction of LTP as well as LTD. Its specific impact depends on the activation of α‐ and β‐adrenoreceptors (Kemp & Manahan‐Vaughan, 2008; Marzo et al. 2009). Activation of adrenoreceptors affects various intracellular factors and modifications of ion channel opening (Marzo et al. 2009). Importantly, adrenoreceptors also impact on N‐methyl‐d‐aspartate (NMDA) and γ‐aminobutyric acid (GABA) receptors, and therefore influence the direction of LTP and LTD (Lei et al. 2007).
Non‐invasive brain stimulation techniques such as transcranial direct current stimulation (tDCS) have been established to induce LTP‐ and LTD‐like cortical excitability alterations in humans (Nitsche & Paulus, 2000). tDCS induces polarity‐dependent plasticity via its primary subthreshold effects on resting membrane potentials (Purpura & McMurtry, 1965; Nitsche et al. 2007). Anodal stimulation elicits neural depolarization, which enhances cortical excitability, whereas cathodal tDCS results in neural hyperpolarization, which diminishes cortical excitability. Stimulation for some minutes results in respective neuroplastic effects, which depend on glutamatergic mechanisms and are calcium‐dependent (Nitsche & Paulus, 2000, 2001; Nitsche et al. 2003 a, 2005). Nevertheless, tDCS‐induced plasticity differs from classical plasticity induction protocols, used primarily in vitro. The latter involve not tonic subthreshold, but pulsatile suprathreshold stimulation. Thus, mechanisms of plasticity might not be identical, although similarities like NMDA receptor dependency and calcium dependency are present (Liebtanz et al. 2002; Nitsche et al. 2003 b). A previous study has shown that acute administration of the monoamine reuptake inhibitor amphetamine enhances the duration of the aftereffects of anodal tDCS (Nitsche et al. 2004). Furthermore the aftereffects induced by both anodal and cathodal tDCS were reduced by a β‐adrenergic receptor blocker (Nitsche et al. 2004). The results of this study suggest that the adrenergic system significantly impacts on plasticity in humans, which is in accordance with the results from animal studies (Nakadate et al. 2006; Marzo et al. 2009).
Psychiatric diseases such as major depression are accompanied by compromised LTP, which can be restored by antidepressant treatment (Campell & Macqueen, 2004; Castren, 2004). Patients with major depression show reduced LTP‐like plasticity, as compared with healthy controls, and administration of antidepressant agents can increase LTP‐like plasticity (Normann et al. 2007). Furthermore, chronic administration of the selective noradrenaline reuptake inhibitor (NRI) reboxetine (RBX) restored spatial learning deficits and hippocampal synaptic plasticity in an animal model of depression (Bhagya et al. 2015). For studies in humans, acute administration of RBX improves cognition and motor performance in healthy and depressed subjects (Ferguson et al. 2003; Plewnia et al. 2004; Wang et al. 2009). In summary, selective NRIs might partially exert their treatment effects by enhancing LTP‐like plasticity in depression, and therefore improve learning and cognition. Thus selective NRIs might have a potential for treatment of psychiatric diseases accompanied by pathological alterations of plasticity. Knowledge about the impact of noradrenergic enhancement in humans on plasticity is, however, limited at present. Clinically, it usually takes weeks to obtain therapeutic effects through selective NRIs (Kasper et al. 2000), such that it might be relevant to learn about the impact of chronic noradrenergic activity enhancement on physiological processes.
Here, we explored the impact of single dose and chronic noradrenergic receptor activity enhancement via administration of the selective NRI RBX on tDCS‐induced motor cortical plasticity. We hypothesized that RBX increases LTP‐like plasticity induced by anodal tDCS, whereas cathodal tDCS‐induced LTD‐like plasticity should be abolished or converted into excitation. Additionally, a foregoing study found that chronic administration of the selective serotonin reuptake inhibitor (SSRI) citalopram increased and prolonged tDCS‐induced LTP‐like plasticity in healthy subjects as compared to placebo (Kuo et al. 2016). This might indicate more stable serotonergic enhancement or upregulation of respective receptors due to chronic administration (Pariente et al. 2001; Coppell et al. 2003). Since serotonin and noradrenaline are both neuromodulators and are effective for treating depression, they might show some similar patterns to the same plasticity‐induction protocol. We furthermore hypothesized that chronic administration of RBX should induce more prominent effects as compared to single dose administration.
Methods
Ethical approval
The study was approved by the ethics committee of the University Medical Center of Goettingen, and conformed to the standards set by the Declaration of Helsinki (2008 version). Written informed consent was obtained from all subjects who participated in the study before inclusion.
Subjects
Sixteen right‐handed, healthy subjects participated in the experiments (8 males, age 27.5 ± 4.01 (standard deviation) years). Subjects were all right‐handed, and between 18 and 50 years old. They had no history of chronic or acute neurological, psychiatric, or medical diseases, no family history of epilepsy, no present pregnancy, no cardiac pacemaker, no previous surgery involving implants to the head (cochlear implants, aneurysm clips, brain electrodes), and absent acute or chronic medication or drug intake, including nicotine consumption. Participants familiar with non‐invasive brain stimulation and pharmacological studies were preferred. However, responder or non‐responder status (i.e. to tDCS) did not serve as a criterion to include or exclude participants and we did not check for genetic polymorphisms (brain‐derived neurotrophic factor, catechol‐O‐methyl transferase, or others).
Transcranial direct current stimulation
Direct current was applied through a pair of saline‐soaked surface sponge electrodes (35 m²) and delivered by a battery‐driven constant current stimulator (neuroConn GmbH, Ilmenau, Germany) with a maximum output of 4.5 mA. The stimulating electrode was placed over the representational hotspot of the right abductor digiti minimi (ADM) muscle identified with transcranial magnetic stimulation (TMS), and the return electrode was placed contralaterally above the right orbit. A current strength of 1 mA was administered for 9 min for cathodal tDCS and 13 min for anodal tDCS. These stimulation durations induce cortical excitability alterations lasting approximately for 1 h after the end of stimulation (Nitsche & Paulus, 2001; Nitsche et al. 2003 a). In our study, we aimed to keep the classical protocol identical to other pharmacology‐tDCS studies in our lab to enhance comparability between studies (Kuo et al. 2008; Monte‐Silva et al. 2010; Fresnoza et al. 2014).
Pharmacological interventions
Reboxetine (RBX; 8 mg; Winthrop Arzneimittel GmbH, Frankfurt am Main, Germany) or equivalent placebo (PLC) medication (P‐tablet; Pfizer Italia S.r.I., Ascoli Piceno, Italy) was administered 2 h before the start of the experimental sessions. A sufficient plasma level of RBX is achieved 2 h after oral intake, and the respective dose is sufficient to elicit prominent effects in the central nervous system (Dostert et al. 1997). Steady state plasma concentrations are achieved after 5 days of drug intake (Pellizzoni et al. 1996). In clinical application, the majority of antidepressants have therapeutic effects after approximately 2 weeks of treatment (Dostert et al. 1997; Kasper et al. 2000). Therefore, we choose 3 weeks of administration of RBX for the chronic part of experiments, and started plasticity induction procedures after 2 weeks of administration in the chronic medication condition.
Monitoring of motor cortical excitability
TMS‐elicited motor evoked potentials (MEPs) were recorded to monitor excitability changes of the motor cortical representation of the right ADM muscle. Single‐pulse TMS was conducted by a Magstim 200 magnetic stimulator (Magstim, Whiteland, Dyfed, UK) with a figure‐of‐eight magnetic coil (diameter of one winding = 70 mm, peak magnetic field = 2.2 T). The coil was held tangentially to the skull, with the handle pointing backwards and laterally at an angle of 45 deg from midline, inducing a posterior–anterior current flow direction in the motor cortex. The optimal coil position (hotspot) was defined as the site where stimulation consistently resulted in the largest MEPs of the contralateral ADM. Surface EMG was recorded from the right ADM with Ag–AgCl electrodes in a belly‐tendon montage. The signals were filtered (2 Hz to 2 kHz, sampling rate 5 kHz), amplified, and then stored on computer via a Power 1401 data acquisition interface (Cambridge Electronic Design, Cambridge, UK).
Experimental procedures
The study was conducted in a double‐blinded, counter‐balanced and placebo‐controlled design. Each subject participated in six experimental sessions, including single dose RBX (the first 4 sessions) and chronic RBX (the last 2 sessions), or the respective placebo medication. For the single dose part, subjects received PLC or RBX combined with anodal or cathodal tDCS. Each experimental session was separated by 1 week to avoid cumulative effects. In the chronic RBX part, participants received RBX (8 mg day−1) consecutively for 21 days. The two sessions under chronic RBX application (RBX with anodal or cathodal tDCS) were conducted in counterbalanced order at the end of the second and third weeks after the start of chronic drug intake. At the beginning of each session, subjects were seated in a comfortable chair with head and arm rest. First the hotspot of the right ADM was determined over the left primary motor cortex, and 20 MEPs were recorded with the TMS intensity eliciting averaged 1 mV MEP as the first baseline (BL1). Two hours after administration of the medication, a second baseline (BL2) was obtained with the same intensity as BL1 to assess a possible influence of the medication on cortical excitability, and a third baseline (BL3) was recorded if necessary (if the BL2 MEP was outside the range of 80–120% of the BL1 MEP) with adjusted TMS intensity for ∼1 mV MEP amplitude. Afterwards, tDCS was performed between 12.00 and 13.00 h. Immediately after tDCS, 20 MEPs were recorded at the time points of 0, 5, 10, 15, 20, 25, 30, 60, 90 and 120 min, and also the same evening (SE; between 18.00 and 19.00 h), next morning (NM; between 09.00 and 10.00 h), next noon (NN; between 12.00 and 13.00 h), and next evening (NE; between 18.00 and 19.00 h) (Fig. 1).
Figure 1. Experimental course of the present study.
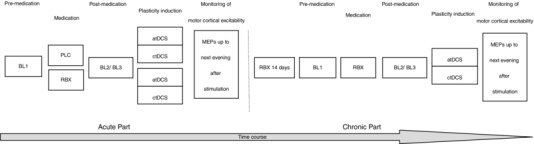
The study was conducted in two parts. For the single dose medication part, subjects received a single dosage of 8 mg reboxetine (RBX) or placebo (PLC) medication with anodal or cathodal tDCS. After these four sessions, which were separated by at least 1 week from each other, they took 8 mg RBX consecutively for 21 days. In the ‘chronic’ part of the experiments, the other two sessions (RBX with anodal or cathodal tDCS) were conducted at the end of the second and third week. For each session, first baseline MEPs (BL1) were recorded with TMS. Two hours after intake of the medication, a second baseline (BL2) was determined and adjusted if necessary (BL3). Afterwards, tDCS was applied and MEPs were recorded after stimulation at different time points until next evening.
Statistics
The individual MEP amplitude means of baselines 1, 2 and 3, and all time points after stimulation were calculated. After checking for normal distribution (Shapiro–Wilk test), a repeated‐measure analysis of variance (ANOVA) for the time bins up to the next evening after stimulation was calculated with the within‐subject factors time course, drug condition (placebo medication, single dose RBX and chronic RBX), stimulation type (anodal and cathodal) and the dependent variable raw MEP amplitude, including baseline 2, or, in case of adjustment of MEP amplitudes, baseline 3. The Mauchly test of sphericity was conducted, and the Greenhouse–Geisser correction was applied when necessary. In case of significant results of the ANOVA, post hoc comparisons were performed using Student's t test (paired samples, two tailed, critical P < 0.05, not corrected for multiple comparisons) to determine whether the MEP amplitudes before and after tDCS differed in each intervention condition and whether these differences depended on drug condition. Furthermore, we conducted an analysis of covariance (ANCOVA) with baseline raw MEP (baseline 2, or, in case of adjustment of MEP amplitudes, baseline 3) as co‐variate, and for the chronic medication condition with order as co‐variate to rule out systematic effects of order of conditions on the results.
To explore if baseline MEP and TMS intensity (percentage of maximal stimulator output, %MSO) needed to obtain baseline MEP differed between each session, the respective baselines were compared via Student's t test (paired samples, two tailed, P < 0.05, not corrected for multiple comparisons).
Results
All subjects tolerated tDCS and RBX well. None of them reported any side effects of either RBX or the stimulation upon request. RBX alone did not change baseline MEP amplitudes and stimulation intensity significantly, as revealed by Student's t tests comparing MEP between baseline 1 and baseline 2 (paired samples, two‐tailed, P > 0.05) (Table 1).
Table 1.
MEP amplitude and stimulation intensity before and after reboxetine (RBX) administration
| Stimulation | TMS parameter | Drug Condition | Baseline 1 | Baseline 2 | Baseline 3 | P |
|---|---|---|---|---|---|---|
| Anodal | MEP (mV) | PLC | 1.04 ± 0.12 | 0.98 ± 0.09 | 0.99 ± 0.06 | 0.396 |
| Acute RBX | 1.01 ± 0.03 | 0.95 ± 0.18 | 1.03 ± 0.16 | 0.153 | ||
| Chronic RBX | 1.04 ± 0.11 | 1.07 ± 0.08 | 1 ± 0.07 | 0.357 | ||
| % MSO | PLC | 52 ± 8.07 | 52 ± 8.07 | 50.3 ± 6.74 | 0.178 | |
| Acute RBX | 52.9 ± 7.11 | 52.9 ± 7.11 | 53.1 ± 7.56 | 0.445 | ||
| Chronic RBX | 52.1 ± 7.18 | 52.1 ± 7.18 | 51.4 ± 8.5 | 0.325 | ||
| Cathodal | MEP (mV) | PLC | 1.03 ± 0.14 | 0.99 ± 0.12 | 1.01 ± 0.09 | 0.517 |
| Acute RBX | 1.01 ± 0.13 | 0.97 ± 0.09 | 0.99 ± 0.08 | 0.946 | ||
| Chronic RBX | 0.94 ± 0.06 | 1.03 ± 0.12 | 1 ± 0.09 | 0.063 | ||
| % MSO | PLC | 52.9 ± 7.54 | 52.9 ± 7.54 | 53.3 ± 8.09 | 0.801 | |
| Acute RBX | 51.6 ± 6.19 | 51.6 ± 6.19 | 51.3 ± 7.43 | 0.163 | ||
| Chronic RBX | 51.9 ± 7.62 | 51.9 ± 7.62 | 51.3 ± 7.6 | 0.096 |
Shown are the mean MEP amplitudes ± SD and stimulation intensity (percentage of maximum stimulator output, %MSO) mean ± SD of baseline 1, 2 and 3. The intensity of TMS was determined to elicit MEPs with a peak to peak amplitude of ∼1 mV (baseline 1). A second baseline (baseline 2) was recorded 2 h after medication intake to determine the effect of the drug on cortical excitability and adjusted if necessary (baseline 3). Student's t tests revealed no significant differences between conditions (P > 0.05).
The Shapiro–Wilk test indicted that all data were normally distributed (all P > 0.05). The results of the ANOVA showed significant main effects of drug (F(2) = 7.843; P = 0.006), stimulation (F(1) = 19.852; P = 0.002), and significant drug × stimulation (F(2) = 10.159; P = 0.007), and stimulation × time (F(14) = 4.964; P = 0.005) interactions. In addition, the result of ANCOVA did not show a significant effect of baseline MEP (baseline 2, or, in case of adjustment of MEP amplitudes, baseline 3) (P = 0.723), and the chronic medication conditions did not result in a significant effect of order (P = 0.617) (Table 2).
Table 2.
Results of the repeated‐measures ANOVA
| Parameters | df | F | P |
|---|---|---|---|
| Drug | 2 | 7.843 | 0.006* |
| Stimulation | 1 | 19.852 | 0.002* |
| Time | 14 | 3.220 | 0.078 |
| Drug × Stimulation | 2 | 10.159 | 0.007* |
| Drug × Time | 28 | 2.034 | 0.118 |
| Stimulation × Time | 14 | 4.964 | 0.005* |
| Drug × Stimulation × Time | 28 | 1.356 | 0.125 |
*Significant results at P < 0.05. df, degrees of freedom.
As revealed by the respective post hoc test, under placebo medication, cathodal tDCS decreased MEP amplitudes for up to 60 min after stimulation, whereas anodal tDCS increased cortical excitability for 90 min. Single dose administration of RBX enhanced the MEP amplitudes significantly as compared to placebo medication at the time points of 10, 15 and 25 min after anodal tDCS, whereas it reversed cathodal tDCS‐induced inhibition into facilitation, which remained significant for up to 60 min after tDCS. Under chronic RBX, anodal tDCS resulted in larger MEP amplitudes compared to placebo medication at the time points of 10, 15, 20, 25, 30, 60 and 120 min and SE, NM, NN and NE after tDCS application. Furthermore, compared to the single dose condition, chronic RBX significantly increased MEP amplitudes at the time points of 20, 25, 30, 60, 120 min and SE, NM, NN and NE, which means the MEP amplitude enhancement was present for more than 24 h after tDCS until the next evening after stimulation. For cathodal tDCS, chronic RBX showed a similar effect to single dose administration compared to the placebo condition, which converted inhibition into facilitation with a more prominent excitability enhancement for 120 min after stimulation. In addition, chronic RBX resulted in a significant enhancement of MEP amplitudes compared to the single dose condition for 30 min and at the time point of 90 min after cathodal tDCS (Fig. 2).
Figure 2. Impact of single dose and chronic reboxetine (RBX) on transcranial direct current stimulation (tDCS)‐induced motor cortex plasticity.

Shown are raw MEP amplitudes after plasticity induction by anodal or cathodal tDCS under placebo (PLC), single dose RBX (sRBX), or chronic RBX (cRBX) conditions up to the next evening of the stimulation day. A, in the PLC condition (diamonds), anodal tDCS induced a significant excitability enhancement for up to 90 min after stimulation. Single dose RBX (squares) resulted in excitability enhancements for up to 120 min after stimulation. Chronic RBX (triangles) enhanced and prolonged these excitability enhancements until next evening. B, in the PLC condition (diamonds), cortical excitability was significantly reduced after cathodal tDCS for 60 min, whereas single dose (squares) and chronic RBX (triangles) converted the inhibitory effect into facilitation. Furthermore chronic RBX showed a significant enhancement of MEP amplitudes compared to the single dose condition for 30 min and at the time point of 90 min after stimulation. Error bars indicate SEM. The black symbols indicate significant differences of post‐stimulation MEP amplitudes from respective baseline values. Floating symbols (square: single dose RBX; triangle: chronic RBX) indicate a significant difference between respective RBX conditions and placebo medication at the same time points (Student's t test, two tailed, paired samples, P < 0.05). *Significant differences between acute and chronic RBX conditions at the same time points (Student's t test, two tailed, paired samples, P < 0.05). SE, same evening; NM, next morning; NN, next noon; NE, next evening.
Discussion
The results of this study show that single dose administration of the selective NRI RBX increased LTP‐like plasticity induced by anodal tDCS, whereas it turned cathodal tDCS‐induced LTD‐like plasticity into facilitation in healthy subjects. Moreover, under chronic administration, the LTP‐like effects of anodal tDCS were prolonged for more than 24 h after intervention, and thus lasted relatively longer than those under single dose administration or placebo medication. For cathodal tDCS, chronic RBX showed larger LTP‐like plasticity compared to single dose medication for 120 min after stimulation. The prolonged after‐effects of anodal tDCS achieved by chronic RBX might explain the relevance of long‐term administration of selective NRIs to exert optimal effects. Moreover, these findings support recent concepts that changes of neural plasticity are relevant for therapeutic effects of noradrenergic enhancement (Marzo et al. 2009; Bhagya et al. 2015).
Baseline MEP showed no significant differences after RBX intake, which differs from another study (Plewnia et al. 2002; Plewnia et al. 2004). This difference between study results might be due to the fact that we used a stimulation intensity which elicits single pulse MEPs with peak‐to‐peak amplitudes of on average 1 mV instead of 180% motor threshold in the foregoing one. The latter criterion will result in larger MEPs. The respective different effects of RBX on baseline MEP might be caused by different pharmacological mechanisms involved in low and high intensity parts of the recruitment curve. Thus high, but not low, amplitude MEPs are relevantly affected by the glutamatergic system (Paulus et al. 2008).
Mechanisms
Our results are in accordance with the findings of a previous experiment, in which the monoamine reuptake inhibitor amphetamine prolonged the duration of the LTP‐like after‐effects induced by anodal tDCS (Nitsche et al. 2004). They are also in line with results of animal slice experiments. Noradrenergic enhancement can enhance LTP and block LTD (Katsuki et al. 1997; Tully et al. 2007). The specific mechanism responsible for the effects of RBX on motor cortex plasticity in the human brain remains to be clarified in future studies. One candidate mechanism is the decrease of potassium conductance by the drug (Marzo et al. 2009). This would result in a depolarization of postsynaptic membranes and enhance calcium influx into the intraneuronal compartment through NMDA receptors and voltage‐dependent calcium channels (Gu, 2002). The direction of induced plasticity depends on the amount of intracellular calcium. High enhancement of intracellular calcium induces LTP, whereas low enhancement results in LTD (Lisman, 2001). Since the after‐effects of tDCS are NMDA receptor‐ and calcium‐dependent (Liebtanz et al. 2002; Nitsche et al. 2003 b), RBX might have strengthened the excitability enhancement induced by anodal tDCS through an enhancement of calcium influx, which might prolong the after‐effects of anodal tDCS. For cathodal tDCS, RBX might have shifted a small to large calcium increase through this mechanism and thus converted inhibition into facilitation. The calcium influx might be still lower for cathodal than for anodal tDCS, which would explain that the after‐effects of cathodal tDCS were shorter lasting. However, this suggested mechanism is speculative at present. Which specific noradrenaline receptor subtypes are involved in this mechanism is not clear. β‐Adrenoreceptors might be candidates, since in vivo and in vitro studies conducted in the dentate gyrus and in area CA1 of the hippocampus show that noradrenaline facilitates or induces LTP through β‐adrenoreceptors (Katsuki et al. 1997). Furthermore, since β‐adrenoreceptors decrease calcium activation‐dependent potassium conductance (Hass & Konnerth, 1983), they are relevant for the conversion from early to late LTP (Straube & Frey, 2003). Because noradrenaline also affects acetylcholine, serotonin and dopamine release and GABAergic activation (Page & Lucki, 2002), which have been shown to modulate tDCS‐induced plasticity, it cannot be ruled out that these modulators might have some impact on the effects (Kuo et al., 2007, 2008; Nitsche et al., 2009). Although the results of the present study are rather clear, it should be kept in mind that in this study we explored a specific dosage of RBX, which is not selective for a specific noradrenergic receptor, and explored plasticity of a specific cortical area. The effects of activation of adrenergic receptor subtypes on LTP and LTD might, however, differ (Katsuki et al. 1997; Kemp & Manahan‐Vaughan, 2008). Moreover, due to the neuromodulatory function of noradrenaline, noradrenergic activation might exert non‐linear dosage‐dependent effects, and specific effects might depend on receptor density of a specific area, which differs between regions (Katsuki et al. 1997; Marzo et al. 2009). These factors might explain at least partially conflicting results between studies. Future studies should thus consider the contribution of specific receptor subtypes, explore the impact of different dosages, and explore plasticity effects of noradrenaline in different cortical areas. These factors have been shown to contribute to the effects of other neuromodulators, such as dopamine, on plasticity (Kuo et al. 2008; Monte‐Silva et al. 2010).
Functional implications
Previous studies have shown that acute administration of RBX improves cognitive and motor performance in healthy subjects and depressed patients (Ferguson et al. 2003; Wang et al. 2009). Clinically, the majority of selective NRIs currently available have therapeutic effects only after approximately 2 weeks of treatment (Kasper et al. 2000), suggesting that in addition to the rapid inhibition of noradrenaline reuptake, other long‐term adaptive modifications are induced by chronic noradrenergic enhancement. Here, its impact on LTP‐like plasticity is a candidate mechanism. Indeed, deficient LTP was restituted in patients suffering from major depression after successful therapy (Player et al. 2014). In our study, repeated treatment with RBX significantly enhanced and prolonged LTP‐like plasticity induced by anodal tDCS for more than 24 h, suggesting induction of late phase LTP, whereas for single dose administration the after‐effects of anodal tDCS were only extended until the evening of the day of intervention. In accordance, animal models of depression suggest that repeated treatment with selective NRIs significantly restores hippocampal synaptic plasticity and reduces spatial learning deficits (Marzo et al. 2009; Bhagya et al. 2015). These findings indicate the possibility that the enhancement of synaptic plasticity may contribute to adaptive changes induced by long‐term antidepressant treatment. Interestingly, we found similar effects for the SSRI citalopram (Kuo et al. 2016). Whether this is a relevant mechanism which can explain the delayed effects of antidepressants on clinical symptoms remains to be shown directly. Beyond depression, compromised plasticity plays a role in various neurological and psychiatric diseases, and restitution by interventional approaches might be an important mechanism for reducing clinical symptoms. Noradrenergic enhancement might be relevant for diseases which are accompanied by deficient LTP, such as post‐stroke rehabilitation, and Parkinson´s disease, just to name a few. It was described that RBX can improve hand function in chronic stroke patients (Zittel et al. 2007). Moreover, tDCS has been introduced as a potential therapeutic tool for diverse neurological and psychiatric diseases (Flöel, 2014; Kuo et al. 2014). Given the strengthening effect of RBX on the aftereffects of tDCS, combining tDCS with selective NRIs might be a promising option to enhance the clinical impact of these interventions. Indeed, such synergistic effects have been demonstrated for the combination of a SSRI and tDCS as antidepressant therapy (Brunoni et al. 2013).
Limitations
Some potential limitations of the present study should be considered. First, due to the limited time frame, we explored only 3 weeks for chronic administration of RBX. Second, to rule out an interference effect of plasticity interventions, a 1 week intersession interval was required. Since we did not have the chance to explore behavioural effects of noradrenaline in the present study, presumed functional implications are speculative at present. Third, we did not obtain drug plasma levels, which would have enabled exploration of dosage‐dependent effects of the medication to some extent. Moreover, participants had not the exact identical duration of RBX intake in the respective chronic medication conditions (medication duration between 14 and 21 days). Participants received anodal stimulation after 2 weeks and cathodal stimulation after 3 weeks of RBX, or vice versa. To rule out systematic effects of order of conditions on the results, we conducted an ANCOVA for the chronic medication condition with order as co‐variate, which ruled out an order effect.
Future studies
Since animal experiments showed different effects of α‐ and β‐receptors on plasticity (Kemp & Manahan‐Vaughan, 2008; McElligott & Winder, 2008; Wojtowicz et al. 2010), future studies should consider the contribution of specific receptor subtypes (i.e. α1, α2 and β1, β2, β3 subtypes) in more detail. Moreover, we have found a ‘focusing effect’ of another neuromodulator, namely dopamine, on neuroplasticity, which strengthened focally induced but weakened or reversed non‐focally induced plasticity (Kuo et al. 2008; Fresnoza et al. 2014). In contrast, RBX might induce a de‐focusing effect on plasticity, which would be an interesting difference from other neuromodulators. Therefore, future studies should consider exploring the noradrenergic effects with different plasticity induction protocols, such as paired associative stimulation, which induce more focally restricted plasticity. Given the variability of neuromodulatory brain stimulation interventions (López‐Alonso et al. 2014; Wiethoff et al. 2014), replication of the results of this study is also warranted. Finally, the results of the study were obtained in healthy volunteers. In neuropsychiatric diseases, transmitter availability and other features of brain function might be different. Thus, the assessment of the current set‐up in patient groups suffering from depression, stroke and other neurological and psychiatric syndromes in which the adrenergic system or neuroplasticity is involved is required to explore transferability of results, and mechanisms.
Our results show modulatory effects of the selective NRI, RBX, on tDCS‐induced plasticity in the human motor cortex. RBX shifted tDCS‐induced plasticity in a facilitatory direction. This impact of noradrenaline on plasticity may be a relevant neurological foundation for the therapeutic effect of selective NRIs in depressed subjects. This finding also suggests that long‐term administration of selective NRIs might be a promising pathway to treat patients with neurological deficits associated with compromised LTP‐like plasticity. Furthermore, the results may help in understanding neuroplasticity processes in the human brain on the rational basis of pharmacological intervention.
Additional information
Competing interests
M‐F.K., H‐I.K., A.J. and G.B. received no financial support or compensation from any individual or corporate entity over the past three years for research or professional service and there are no personal financial holdings that could be perceived as constituting a potential conflict of interest. W.P. is member of Advisory Boards of GSK, UCB and Desitin. M.A.N. is member of the Advisory Board of Neuroelectrics.
Author contributions
The study was conducted in the laboratory of the Department of Clinical Neurophysiology, University Medical Center, Georg‐August‐University, Goettingen, Germany. H‐I.K., W.P., G.B., A.J., M.F.K. and M.A.N. contributed to the design of the work, acquisition, analysis and interpretation of data, and drafting the work or revising it critically for important intellectual content. All authors approved the final version of the manuscript and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All persons designated as authors qualify for authorship.
Funding
H‐I.K. is supported by a government scholarship, Taiwan. This study was supported by the BMBF‐project ‘Netzwerk psychische Erkrankungen’, grant 01EE1403C.
References
- Bhagya V, Srikumar B, Traju T & Rao B (2015). The selective noradrenergic reuptake inhibitor reboxetine restores spatial learning deficits, biochemical changes, and hippocampal synaptic plasticity in an animal model of depression. J Neurosci Res 93, 104–120. [DOI] [PubMed] [Google Scholar]
- Brunoni A, Valiengo L, Baccaro A, Zanao T, Oliveira JD & Goulart A (2013). The sertraline vs electrical current therapy for treating depression clinical study: results from a factorial, randomized, controlled trial. JAMA Psychiatry 70, 1–9. [DOI] [PubMed] [Google Scholar]
- Campell S & Macqueen G (2004). The role of the hippocampus in the pathophysiology of major depression. J Psychiatry Neurosci 29, 417–426. [PMC free article] [PubMed] [Google Scholar]
- Castren E (2004). Neurotrophic effects of antidepressant drugs. Curr Opin Pharmacol 4, 58–64. [DOI] [PubMed] [Google Scholar]
- Coppell A, Pei Q & Zetterstrom T (2003). Bi‐phasic change in BDNF gene expression following antidepressant drug treatment. Neuropharmacology 44, 903–910. [DOI] [PubMed] [Google Scholar]
- Dostert P, Benedetti M & Poggesi I (1997). Review of the pharmacokinetics and metabolism of reboxetine, a selective noradrenaline reuptake inhibitor. Eur Neuropsychopharmacol 1, 23–35. [DOI] [PubMed] [Google Scholar]
- Ferguson J, Wesnes K & Schwartz G (2003). Reboxetine versus paroxetine versus placebo: effects on cognitive functioning in depressed patients. Int Clin Psychopharmacol 18, 9–14. [DOI] [PubMed] [Google Scholar]
- Flöel A (2014). tDCS‐enhanced motor and cognitive function in neurological diseases. Neuroimage 85, 934–947. [DOI] [PubMed] [Google Scholar]
- Fresnoza S, Stiksrud E, Klinker F, Liebetanz D, Paulus W, Kuo MF & Nitsche MA (2014). Dosage‐dependent effect of dopamine D2 receptor activation on motor cortex plasticity in humans. J Neurosci 34, 10701–10709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Q (2002). Neuromodulatory transmitter systems in the cortex and their role cortical plasticity. Neuroscience 111, 815–835. [DOI] [PubMed] [Google Scholar]
- Hass H & Konnerth A (1983). Histamine and noradrenaline decrease calcium‐activated potassium conductance in hippocampal pyramidal cells. Nature 302, 432–434. [DOI] [PubMed] [Google Scholar]
- Kasper S, Giamal N & Hilger E (2000). Reboxetine: the first selective noradrenaline re‐uptake inhibitor. Exp Opin Pharmacother 1, 771–782. [DOI] [PubMed] [Google Scholar]
- Katsuki H, Izumi Y & Zorumski C (1997). Nordrenergic regulation of synaptic plasticity in the hippocampal CA1 region. J Neurophysiol 77, 3013–3020. [DOI] [PubMed] [Google Scholar]
- Kemp A & Manahan‐Vaughan D (2008). Beta‐adrenoreceptors comprise a critical element in learning‐facilitated long‐term plasticity. Cerebral Cortex 18, 1326–1334. [DOI] [PubMed] [Google Scholar]
- Kuo HI, Paulus W, Batsikadze G, Jamil A, Kuo M & Nitsche MA (2016). Chronic enhancement of serotonin facilitatory excitatory transcrnial direct current‐induced plasticity. Neuropsychopharmachology 41, 1223–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo MF, Grosch J, Fregni F, Paulus W & Nitshce MA (2007). Focusing effect of acetylcholine on neuroplasticity in the human motor cortex. J Neurosci 27, 1442–1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo MF, Paulus W & Nitsche MA (2008). Boosting focally‐induced brain plasticity by dopamine. Cereb Cortex 18, 648–651. [DOI] [PubMed] [Google Scholar]
- Kuo MF, Paulus W & Nitsche MA (2014). Therapeutic effects of non‐invasive brain stimulation with direct currents (tDCS) in neuropsychiatric diseases. Neuroimage 15, 948–960. [DOI] [PubMed] [Google Scholar]
- Lei S, Deng P, Porter J & Shin H (2007). Adrenergic facilitation of GABAergic transmission in rat entorhinal cortex. J Neurophysiol 98, 1868–1877. [DOI] [PubMed] [Google Scholar]
- Liebtanz D, Nitsche M, Tergau F & Paulus W (2002). Pharmacological approach to synaptic and membrane mechanisms of DC‐induced neuroplasticity in man. Brain 125, 2238–2247. [DOI] [PubMed] [Google Scholar]
- Lisman J (2001). Three Ca2+ levels affect plasticity differently: the LTP zone, the LTD zone and no man's land. J Physiol 532, 285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López‐Alonso V, Cheeran B, Río‐Rodríguez D & Fernández‐Del‐Olmo M (2014). Inter‐individual variability in response to non‐invasive brain stimulation paradigms. Brain Stimul 7, 327–380. [DOI] [PubMed] [Google Scholar]
- Marzo A, Bai J & Otani S (2009). Neuroplasticity regulation by noradrenaline in mammalian brain. Curr Neuropharmacol 7, 286–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElligott Z & Winder D (2008). Alpha1‐adrenergic receptor‐induced heterosynaptic long‐term depression in the bed nucleus of the stria terminalis is disrupted in mouse models of affective disorders. Neuropsychopharmachology 33, 2313–2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monte‐Silva K, Liebetanz D, Grundey J, Paulus W & Nitsche MA (2010). Dosage‐dependent non‐linear effect of L‐dopa on human motor cortex plasticity. J Physiol 588, 3415–3424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakadate K, Matsukawa M & Okado N (2006). Identification of adrenoreceptor subtype‐medisted changes in the density of synapses in the rat visual cortex. Neuroscience 138, 37–46. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Fricke K, Henschke U, Schlitterlau A, Liebtanz D, Lang N, Henning S, Tergau F & Paulus W (2003. b). Pharmacological modulation of cortical excitability shifts induced by transcrnial DC stimulation. J Physiol 533, 293–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsche MA, Grundey J, Liebetanz D, Lang N, Tergau F & Paulus W (2004). Catecholaminergic consolidation of motor cortical neuroplasticity in humans. Cereb Cortex 14, 1240–1245. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Klein C, Tergau F, Rothwell J & Paulus W (2003. a). Level of action of cathodal DC polarization induced inhibition of the human motor cortex. Clin Neurophysiol 144, 600–604. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Kuo M, Karrasch R, Warden B, Liebtanz D & Paulus W (2009). Serotonin affects transcranial direct current (tDCS)‐induced neuroplasticity in humans. Biol Psychiatry 66, 503–508. [DOI] [PubMed] [Google Scholar]
- Nitsche MA & Paulus W (2000). Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol 527, 633–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsche MA & Paulus W (2001). Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans. Neurology 57, 1899–1901. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Roth A, Kuo NF, Fischer AK, Liebetanz D & Lang N (2007). Timing‐dependent modulation of associative plasticity by general network excitability in the human motor cortex. J Neurosci 27, 3807–3812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsche MA, Seeber A, Frommann K, Klein CC, Rochford C, Nitsche MS, Fricke K, Liebetanz D, Lang N, Antal A, Paulus W & Tergau F (2005). Modulating parameters of excitability during and after transcranial direct current stimulation of the human motor cortex. J Phsyiol 568, 291–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Normann C, Schmitz D, Furmaier A, Doing C & Bach M (2007). Long‐term platsicity of visually evoked potentials in humans is altered in major depression. Biol Psychiatry 62, 373–380. [DOI] [PubMed] [Google Scholar]
- Page M & Lucki I (2002). Effects of acute and chronic reboxetine treatment on stress‐induced manoamine efflux in the rat frontal cortex. Neuropsychopharmachology 27, 238–247. [DOI] [PubMed] [Google Scholar]
- Pariente J, Loubinoux I, Carel C, Albucher J, Leger A, Manelfe C, Rascol O & Chollet F (2001). Flouxetine modulates motor performance and cerebral activation of patients recovering from stroke. Ann Neurol 50, 718–729. [DOI] [PubMed] [Google Scholar]
- Paulus W, Classen J, Cohen LG, Large C, Lazzaro VD, Nitsche MA, Pascual‐Leone A, Rosenow F, Rothwell JC & Ziemann U (2008). State of the art: Pharmacologic effects on cortical excitability measures tested by transcranial magnetic stimulation. Brain Stimul 1, 151–163. [DOI] [PubMed] [Google Scholar]
- Pellizzoni C, Poggesi I, Jørgensen N, Edwards D, Paus E & Benedetti M (1996). Pharmacokinetics of reboxetine in healthy volunteers. Single against repeated oral doses and lack of enzymatic alterations. Biopharm Drug Dispos 17, 623–633. [DOI] [PubMed] [Google Scholar]
- Player M, Taylor J, Weickert C, Sachder P, Martin D, Mitchell P, Rascol O & Chollet F (2014). Increase in PAS‐induced neuroplasticity after a treatment course of transcranial direct current stimulation for depression. J Affect Disord 160, 140–147. [DOI] [PubMed] [Google Scholar]
- Plewnia C, Hoppe J, Cohen L & Gerloff C (2004). Improved motor skill acquisition after selective stimulation of central norepinephrine. Neurology 62, 2124–2126. [DOI] [PubMed] [Google Scholar]
- Plewnia C, Hoppe J, Heimke C, Bartles M, Cohen L & Gerloff C (2002). Enhancement of human cortico‐motoneuronal excitability by the selective norepinephrine reuptake inhibitor reboxetine. Neurosci Lett 330, 231–234. [DOI] [PubMed] [Google Scholar]
- Purpura DP & McMurtry JG (1965). Intracellular activities and evoked potential changes during polarization of motor cortex. J Neurophysiol 28, 166–185. [DOI] [PubMed] [Google Scholar]
- Straube T & Frey J (2003). Involvement of beta‐adrenergic receptors in protein synthesis‐dependent late long‐term potentiation (LTP) in the dentate gyrus of freely moving rats: the critical role of the LTP induction strength. Neuroscience 119, 473–479. [DOI] [PubMed] [Google Scholar]
- Tully K, Y L, Tsvetkov E & Bolshakov V (2007). Norepinephrine enables the induction of associative long‐term potentiation at thalamo‐amygdala synapses. Pro Natl Acd Sci USA 104, 14146–14150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallings S, Milway J, Ingram M, Lau C, Morrison G & Martin G (2016). The effects of prolonged administration of norepinephrine reuptake inhibitors on long‐term potentiation in dentate gyrus, and on tests of spatial and object recognition memory in rats. Neurobiol Learn Mem 128, 92–102. [DOI] [PubMed] [Google Scholar]
- Wang L, Fink G, Dafotakis M & Grefkes C (2009). Noradrenergic stimulation and motor performance: different effects of reboxetine on movement kinematics and visuomotor abilities in healthy human subjects. Neuropsychologia 47, 1302–1312. [DOI] [PubMed] [Google Scholar]
- Wiethoff S, Hamada M & Rothwell J (2014). Variability in response to transcranial direct current stimulation of the motor cortex. Brain Stimul 7, 465–475. [DOI] [PubMed] [Google Scholar]
- Wojtowicz A, Fidzinski P, Heinemann U & Behr J (2010). Beta‐adrenergic receptor activation induces long‐lasting potentiation in burst‐spiking but not regular‐spiking cells at CA1‐aubiculum synapses. Neuroscience 171, 367–372. [DOI] [PubMed] [Google Scholar]
- Zittel S, Weiller C & Liepert J (2007). Reboxetine improves motor function in chronic stroke. A pilot study. J Neurol 254, 197–201. [DOI] [PubMed] [Google Scholar]


