Abstract
Latent tuberculosis infection (LTBI) is evidence of immunological control of tuberculosis. Dormancy survival regulator (DosR) regulon-encoded proteins may have a role in the maintenance of LTBI. T cell responses to Rv1733c, Rv0081, Rv1735c, and Rv1737c DosR regulon-encoded proteins were found to be most frequent among household contacts of TB cases from Uganda compared to other DosR proteins, but antibody responses were not described. We characterized antibody responses to these proteins in individuals from Uganda. Antibodies to Rv1733c, Rv0081, Rv1735c, and Rv1737c DosR regulon-encoded proteins were measured in 68 uninfected individuals, 62 with LTBI, and 107 with active pulmonary tuberculosis (APTB) cases. There were no differences in the concentrations of antibodies to Rv0081, Rv1735c, and Rv1737c DosR regulon-encoded proteins between individuals with LTBI and APTB and those who were uninfected. LTBI was associated with higher concentrations of antibodies to Rv1733c in female participants [adjusted geometric mean ratio: 1.812, 95% confidence interval (CI): 1.105 2.973, and p = 0.019] but not in males (p value for interaction = 0.060). Antibodies to the four DosR regulon-encoded proteins investigated may not serve as good biomarkers of LTBI in the general population. More of the M.tb proteome needs to be screened to identify proteins that induce strong antibody responses in LTBI.
1. Introduction
Mycobacterium tuberculosis (M.tb) is the causative agent of tuberculosis (TB), a disease that affects millions of people worldwide. In 2015, there were approximately 10.4 million cases of TB and 1.5 million deaths from it [1]. This disease is transmitted via inhalation of aerosolized bacilli and replicates within alveolar macrophages in lung tissue [2]. Once infected, the majority of healthy individuals are able to recruit immune cells to the affected sites and encase the invading mycobacteria in a structure known as a granuloma. This rich cellular environment favours activation of macrophages and killing of phagocytosed M.tb through the release of reactive oxygen species and nitrogen intermediates [3]. Despite these adverse conditions, tubercle bacilli may sometimes persist and remain in a nonreplicative state called latency [4]. In this state they remain dormant but can reactivate and cause disease when immune suppression occurs [5]. Only 5–10% of immunocompetent individuals with latent tuberculosis infection (LTBI) ever progress to active disease in their lifetime [6]. This suggests an ability of the majority of people with LTBI to effectively control M.tb infection. Current knowledge suggests that bacterial suppression principally involves T cell mediated immunity [5]. Little is known about humoral immunity in LTBI because B cells and antibodies are thought to have a negligible role in the control of TB infection [7]. However, antibody responses are observed in LTBI [8, 9]. Recent findings by Lu et al. show that antibodies from individuals with LTBI improve macrophage killing of engulfed bacilli [10]. In addition to these functional properties, antibodies could serve as important biomarkers of M.tb infection.
During latency, tubercle bacilli live in a microenvironment of reduced nutrients, hypoxia [11, 12], and low concentrations of nitric oxide. Simulation of this state in in vitro experiments has shown increased Mycobacterium expression of 48 proteins encoded in the dormancy survival regulator (DosR) regulon [3]. These findings suggest that DosR regulon-encoded proteins may have a role in the maintenance of latency. Studies with human subjects have demonstrated strong cell mediated responses to DosR regulon-encoded proteins in individuals with LTBI that exceed those from TB cases and uninfected controls [12, 13]. This suggests that immune responses to DosR regulon-encoded proteins might be used as specific biomarkers of LTBI.
The currently used tests for the diagnosis of LTBI are the tuberculin skin test (TST) and the interferon gamma release assays [5]. Despite their wide use, both of these tests are unable to differentiate active TB from LTBI [14]. The use of anti-DosR immune responses for the diagnosis of LTBI could help overcome this limitation. Since these proteins are able to induce cell mediated responses in the latent state of M.tb infection, they may also induce humoral immune responses. If shown to be the case, antibodies against DosR regulon-encoded proteins could have a potential use as biomarkers of LTBI. Without the need for the isolation of cells or a period of cell stimulation, the measurement of these antibodies in serum or plasma may present a cheaper, easier, and faster means of diagnosing LTBI compared to the measurement of cell mediated responses.
Several studies have reported the use of M.tb proteins, such as the 38-kDa [15–18] protein as well as early secretory antigen target- (ESAT-) 6 [15, 17, 19], culture filtrate protein- (CFP-) 10 [17, 19], and MTB48 proteins [18, 19] for serodiagnosis of active pulmonary tuberculosis (APTB). However, very few studies have identified proteins that can be used for the serodiagnosis of LTBI. A study by Davidow et al. [15] reported a lack of antibodies in LTBI agreeing with the widely held notion that humoral responses are only induced during active TB and not during latency. However, there are studies that contradict this. Research by Eleftheriadis et al. [20] described the use of antibodies to lipoarabinomannan (LAM) in the diagnosis of latent TB in haemodialysis patients as well as healthy volunteers with no clinical evidence of active TB. The authors went on to show that LTBI positivity by anti-LAM antibodies correlated well with TST positivity in all these individuals. Furthermore, a study Chen et al. showed that individuals with LTBI had higher antibody responses to Rv1985c compared to BCG vaccinated individuals who had no known contact with TB cases [9]. Additionally, research from our own group has described increased levels of antimycobacterial specific plasmablasts and memory B cells among healthy individuals with likely latent TB infection [8]. Although the results of all these studies have not been reproduced in other populations, they do give us reason to suggest that LTBI could induce antibody responses to mycobacterial antigens. The search for M.tb proteins with possible serodiagnostic value for LTBI is therefore still warranted.
A study by Black et al. [21] was able to describe interferon gamma responses to 51 DosR regulon-encoded proteins in LTBI positive, human immunodeficiency virus- (HIV-) negative adult household contacts of APTB cases from The Gambia, Uganda, and South Africa. It showed four DosR regulon-encoded proteins, Rv1733c, Rv0081, Rv1735c, and Rv1737c were most frequently recognized in latent TB infected HHCs from Uganda compared to other DosR proteins, with percentages of 79%, 70%, 61%, and 50%, respectively. The DosR protein, Rv1733c, has also been reported to be frequently recognized by T cell responses in individuals with LTBI from Germany [13] and Netherlands [12]. A more recent multicentre study of individuals from sub-Saharan Africa showed Rv1733c, Rv1735c, and Rv1737c were among the proteins that induced T cell responses in individuals with LTBI that were higher than those induced in active TB [22]. One could suppose that these findings reflect increased expression of these proteins in LTBI.
Although all of the studies mentioned above characterized cell mediated responses to these proteins, they did not look at humoral responses. The T cells induced by these DosR proteins may provide help to B cells and promote antibody responses. The detection of such antibodies in peripheral blood could help in serodiagnosis of LTBI. In this present study, we investigated IgG antibody responses against M.tb H37Rv proteins, Rv1733c, Rv0081, Rv1735c, and Rv1737c in a Ugandan cohort comprising healthy uninfected individuals, those with LTBI and APTB cases. Our main objective was to determine whether these antibodies could be used as biomarkers of LTBI.
2. Materials and Methods
2.1. Study Design and Population
This was a cross-sectional study nested in a TB household contact study based in Kampala, Uganda [23]. It was exploratory in nature and the number of individuals investigated was limited to those whose samples were available from the larger study. A total of 237 study participants were included in our investigations. They came from Kisenyi and Kitebi municipalities of Kampala district. This number is comprised of 68 uninfected individuals, 62 with LTBI, and 107 APTB cases. They were defined as having LTBI if they had both a positive QuantiFERON®-TB Gold In-Tube (QFT) test and TST result with no signs or symptoms of active TB and were defined as being uninfected if they had both a negative QFT and TST result. Individuals with APTB had a positive acid-fast bacilli result after performing sputum smear microscopy and had either just began treatment or had been on treatment for less than 4 weeks. Stored samples collected between 2010 and 2011 were used for these investigations. These included serum samples from the uninfected individuals and those with LTBI and QFT nil control supernatants from the APTB cases. The nil control QFT supernatants were used because of a lack of stored sera or plasma from these individuals.
2.2. Ethical Clearance
All samples were obtained from study participants following written informed consent. This study obtained ethical clearance to carry out this research from the Makerere University School of Medicine Research & Ethics Committee and the Uganda National Council for Science & Technology.
2.3. Antigens
Antibody responses to Rv1733c, Rv0081, Rv1735c, and Rv1737c had not been studied before in any similar population and for this reason we also chose to look at antibodies against a well-characterized M.tb protein, CFP-10/ESAT-6 fusion protein [18, 19], in the same individuals as a control.
Recombinant DosR regulon-encoded proteins, Rv1733c, Rv0081, Rv1735c, and Rv1737c, and CFP-10/ESAT-6 fusion protein were kindly provided by Professor Tom H. M. Ottenhoff, Leiden University Medical Centre. These proteins were expressed in Escherichia coli BL21(DE3) and purified, using methods previously described by the Ottenhoff group [21]. They were then freeze-dried and shipped to Uganda where they were reconstituted in phosphate-buffered saline (PBS) containing 1% dimethyl sulfoxide for use in antibody assays.
2.4. Procedure of IgG Antibody ELISA
Immunolon® 4 HBX microtiter plates (Thermoscientific, USA) were coated with 5 μg/well of antigen or 0.1% skimmed milk (control for nonspecific binding) in carbonate-bicarbonate buffer (pH 9.6) at 4°C overnight. Additionally, each plate was coated with eight, twofold serial dilutions of human IgG reference standard (Genscript) at a top concentration of 625 ng/ml. Following overnight incubation, the plates were washed four times with PBS (pH 7.4) containing 0.05% Tween 20 (PBS-T). The plates were then blocked with 1% skimmed milk in PBS-T for 2 hours at room temperature. A dilution of 1 in 50 was made of each serum/supernatant sample in 0.1% skimmed milk PBS-T (assay buffer) and then 50 μl was added to antigen-coated and control wells. A pooled sample of nil control QFT supernatants from 40 active TB cases was included on each plate as a positive control for the assay. All samples and positive controls were assayed in duplicate to minimize effects of random variations in volumes due to pipetting errors. After an incubation of 2 hours at room temperature, the wells were washed as before and incubated with 50 μl/well polyclonal rabbit anti-human IgG conjugated with horseradish peroxidase (Dako, Denmark) at 0.5 μg/ml for another hour at the room temperature. Plates were then washed and enzyme activity detected by incubation with 100 μl/well o-phenylenediamine (Sigma) containing hydrogen peroxide for 15 minutes. The reaction was stopped by addition of 25 μl/well 2 mol/litre sulfuric acid and thereafter the optical density (OD) measured at a test wavelength of 490 nm and a reference wavelength of 630 nm in an ELISA plate reader (Biotek). The ODs from the human IgG standard concentrations on every plate were used to generate standard reference curves for use in conversion of sample ODs to antibody concentrations in ng/ml. The concentrations of the control wells were subtracted from the test antigen wells to eliminate background antibody levels. We then multiplied the result by the sample dilution factor of 50.
2.5. Data Analysis
Data was analysed using Stata release 14.0 statistical package (Statacorp LP, College Station, TX, USA). Our main exposure was M.tb infection status while our outcomes were antibody responses. Other exposures or potential confounders considered were age, gender, HIV infection status, and socioeconomic status (SES). The latter was defined by the quality of household external structure and roofing as well as the kind of lighting employed [23]. Age was not stratified but was left as a continuous variable during the analyses.
The crude analysis involved the use of the chi-squared test to check for associations between potential confounders and M.tb infection. Further crude analysis involved the use of the Kruskal Wallis tests to check for differences in the distribution of IgG antibodies across groups of uninfected individuals, those with LTBI and APTB cases. The antibody data was then transformed to natural log (concentration +1) and linear regression analysis with bootstrap estimated confidence intervals was used to test for associations between M.tb infection status and the antibody responses [24] while adjusting for the confounders, age, gender, and HIV infection status. The results were then back-transformed to provide geometric mean ratios. We used this approach because all of our antibody data did not follow the normal distribution and there was still evidence of nonnormality even after log transformation.
3. Results
3.1. Demographic Characteristics
The study participants included individuals of varied age, gender, HIV infection status, and SES. These characteristics, along with the results of chi-square tests of associations with M.tb infection state, are shown in Table 1. Uninfected individuals were younger than those with LTBI and APTB. This may be explained in part by the selection criteria for APTB cases, as these individuals were only enrolled into the study if they were beyond the age of 18 years and may therefore have biased this group of individuals. The same criterion was not imposed upon those who were uninfected or had LTBI. The observation that individuals with LTBI were older than those who were uninfected may reflect increasing risk of exposure to M.tb with age [25]. There were more males than females with APTB and more females than males with LTBI, reflecting the disparity in TB infection rates across gender [26]. There were more HIV positive individuals among the APTB cases in comparison to the other groups. This is likely due to the fact that HIV coinfection is a known risk factor for acquisition of TB disease [6]. We found no association between SES and M.tb infection status.
Table 1.
Study participant characteristics.
| Characteristic | Uninfected (n = 68) | LTBI (n = 62) | Active PTB (n = 107) | p value1 |
|---|---|---|---|---|
| Mean age & range (years) | 16 (1, 66) | 24 (1, 66) | 30 (18, 53) | <0.0001 |
| Females | 39 (57.4%) | 41 (65%) | 44 (41.1%) | 0.006 |
| HIV positive | 6 (8.8%) | 5 (7.9%) | 42 (39.3%) | <0.0001 |
| Low SES2 | 34 (50.8%) | 32 (51%) | 58 (61%) | 0.309 |
LTBI = latent tuberculosis infection, SES = socioeconomic status, and APTB = active pulmonary tuberculosis.
1 p values are from chi-square tests of associations.
2Individuals were either of low or medium SES.
3.2. Factors Associated with Concentrations of IgG Antibodies to DosR Proteins and CFP-10/ESAT-6
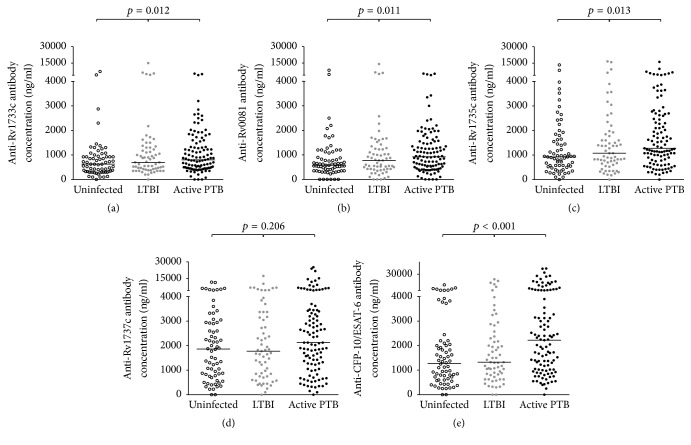
We made crude comparisons of the concentrations of IgG antibodies to the different proteins across groups of uninfected individuals, those with LTBI and APTB cases using the Kruskal–Wallis test. With the exception of anti-Rv1737c antibodies, there was evidence of differences in the concentrations of all the antibodies across the three groups (Figure 1).
Figure 1.
Scatter dot plots showing IgG antibody concentrations. The tuberculin skin test (TST) and the QuantiFERON TB Gold In-Tube (QFT) were used to determine if individuals were uninfected or had latent tuberculosis infection (LTBI). Sputum smear microscopy was used to define APTB cases. The horizontal bars shown are median concentrations of IgG antibodies in each group. The p values shown correspond to results from Kruskal–Wallis test.
Linear regression analysis was used to test for associations between M.tb infection status and antibody responses to the DosR proteins (Table 2). The uninfected individuals were the baseline comparison group. Our hypothesis was that LTBI would be associated with higher concentrations of antibodies to the four DosR regulon-encoded proteins compared to the uninfected individuals. Results from unadjusted analysis showed no associations between LTBI and antibodies to the four DosR regulon-encoded proteins. APTB appeared to be associated with higher anti-Rv0081 and anti-Rv1735c antibodies. There was a similar trend with anti-Rv1737c antibodies but there was only marginal evidence to this effect. After adjusting for the effects of age, gender, and HIV infections status, we still did not see any associations between LTBI and the antibodies to the DosR proteins. There was no longer an association between APTB and anti-Rv1735c antibodies and the evidence for a positive association between APTB and antibodies to Rv0081 was not as strong. As expected from prior literature [18, 19], we found that APTB was associated with higher concentrations of antibodies to CFP-10/ESAT-6 compared to uninfected individuals in both unadjusted and adjusted analyses. There was no evidence of any difference in anti-CFP-10/ESAT-6 antibodies between uninfected individuals and those with LTBI which is also consistent with prior literature [15].
Table 2.
Associations between Mycobacterium tuberculosis infection status and concentrations of IgG antibodies to DosR proteins and CFP-10/ESAT-6 in study participants.
| Antibody/factor | Crude geometric mean ratio (95% CI) | p value | Adjusted geometric mean ratio (95% CI)1 | p value |
|---|---|---|---|---|
| Anti-Rv1733c antibodies | ||||
| Uninfected | 1 | 1 | ||
| LTBI | 1.374 (0.915–2.063) | 0.125 | 1.240 (0.792–1.942) | 0.346 |
| APTB | 1.346 (0.916–1.974) | 0.131 | 1.370 (0.904–2.078) | 0.138 |
| Anti-Rv0081 antibodies | ||||
| Uninfected | 1 | 1 | ||
| LTBI | 1.437 (0.761–2.714) | 0.264 | 1.382 (0.700–2.727) | 0.343 |
| APTB | 1.790 (1.007–3.184) | 0.047 | 1.707 (0.956–3.046) | 0.071 |
| Anti-Rv1735c antibodies | ||||
| Uninfected | 1 | 1 | ||
| LTBI | 1.203 (0.774–1.869) | 0.411 | 1.059 (0.651–1.722) | 0.818 |
| APTB | 1.529 (1.058–2.210) | 0.024 | 1.333 (0.912–1.949) | 0.138 |
| Anti-Rv1737c antibodies | ||||
| Uninfected | 1 | 1 | ||
| LTBI | 1.147 (0.704–1.868) | 0.581 | 1.122 (0.650–1.938) | 0.680 |
| APTB | 1.479 (0.966–2.264) | 0.072 | 1.352 (0.801–2.282) | 0.259 |
| Anti-CFP-10/ESAT-6 antibodies | ||||
| Uninfected | 1 | 1 | ||
| LTBI | 1.162 (0.682–1.981) | 0.580 | 1.019 (0.567–1.829) | 0.951 |
| APTB | 2.199 (1.415–3.418) | <0.001 | 2.020 (1.279–3.190) | 0.003 |
LTBI = latent tuberculosis infection; APTB = active pulmonary tuberculosis.
1Regression analyses were performed with bootstrap confidence intervals generated from 1000 replicate samples of data. The data was from 68 individuals who were uninfected, 62 with LTBI, and 105 with APTB. Adjusting was done for age, gender, and HIV infection status. Analysis was performed on log transformed values and back-transformed to provide geometric mean ratios.
There was a higher concentration of antibodies to Rv1733c [adjusted geometric mean ratio (adjusted GMR): 1.013, 95% confidence interval (CI): 1.000–1.027, and p = 0.05], Rv1735c (adjusted GMR: 1.016, 95% CI: 1.002–1.031, and p = 0.022), and CFP-10/ESAT-6 (adjusted GMR): 1.015, 95% CI: 1.002–1.028, and p = 0.027) with increasing age. This observation may be a result of increasing risk of exposure to M.tb with age [25]. We found that HIV infection was associated with lower concentrations of antibodies to Rv1733c (adjusted GMR: 0.498, 95% CI: 0.286–0.870, and p = 0.014], Rv1735c (adjusted GMR: 0.688, 95% CI: 0.477–0.993, and p = 0.046), and CFP-10/ESAT-6 (adjusted GMR: 0.496, 95% CI: 0.327–0.754, and p = 0.001). This result may reflect the suppressive effects of HIV infection on B cell immunity [27]. As discussed previously, HIV is a known risk factor for TB disease progression [6]. We performed a test of interaction to see if HIV infection could be modifying the effect of M.tb infection on the antibody responses to the DosR proteins but found no evidence in support of this.
Females had lower concentrations of antibodies to the CFP-10/ESAT-6 (adjusted GMR: 0.496, 95% CI: 0.453–0.988, and p = 0.043) compared to males. This finding may reflect gender related differences in the immune response to pathogens [28]. Gender is known to influence rates of M.tb infection as well as the rate of progression to TB disease [29]. According to the WHO, more males than females are diagnosed with TB [1]. Based on this information, we performed a test of interaction to see whether gender could be modifying the effect of M.tb infection on antibody responses. We found evidence to show that this was the case for antibody responses to Rv0081 (p value for interaction = 0.040) and marginal evidence of a similar interaction with antibody responses to Rv1733c (p value for interaction = 0.060). We stratified the analysis of these two antibodies according to gender and found that LTBI was associated with significantly higher concentrations of antibodies to Rv1733c among female participants (Table 3) but not in their male counterparts. We found marginal evidence of a similar trend with the antibodies to Rv0081. We found no association between APTB and antibodies to both these antigens in either gender.
Table 3.
Associations between Mycobacterium tuberculosis infection status and concentrations of IgG antibodies to Rv1733c and Rv0081 DosR proteins in male and female study participants, after adjusting for potential confounders.
| Antibody/factor | Adjusted geometric mean ratio (95% CI)1 | p value |
|---|---|---|
| Anti-Rv1733c antibodies in males | ||
| Uninfected | 1 | |
| LTBI | 0.670 (0.292–1.538) | 0.345 |
| APTB | 1.041 (0.636–1.704) | 0.873 |
| Anti-Rv1733c antibodies in females | ||
| Uninfected | 1 | |
| LTBI | 1.812 (1.105–2.973) | 0.019 |
| APTB | 1.718 (0.877–3.369) | 0.115 |
| Anti-Rv0081 antibodies in males | ||
| Uninfected | 1 | |
| LTBI | 0.631 (0.300–1.331) | 0.227 |
| APTB | 1.183 (0.674–2.075) | 0.558 |
| Anti-Rv0081 antibodies in females | ||
| Uninfected | 1 | |
| LTBI | 2.229 (0.833–5.966) | 0.111 |
| APTB | 2.484 (0.948–6.505) | 0.064 |
LTBI = latent tuberculosis infection and APTB = active pulmonary tuberculosis.
1Regression analyses were performed with bootstrap confidence intervals generated from 1000 replicate samples of data. The data was from 29 uninfected males, 21 males with LTBI, 62 males with active PTB, 39 uninfected females, 41 females with LTBI, and 43 females with APTB. The p value for interaction was 0.06 for Rv1733c and 0.04 for Rv0081. Adjusting was done for age and HIV infection status. Analysis was performed on log transformed values and back-transformed to provide geometric mean ratios.
4. Discussion
This study described antibody responses to Rv1733c, Rv0081, Rv1735c, and Rv1737c DosR encoded proteins in Ugandan individuals with LTBI and APTB and those who were uninfected. The main finding was that LTBI was associated with higher anti-Rv1733c antibodies in female participants. Anti-Rv0081 antibodies were also elevated in these individuals; however the evidence to show that this was a true increase was not as strong. There were no associations between M.tb infection status and the antibody responses to Rv1735c and Rv1737c proteins.
Increased expression of Rv1733c in latency may have been able to stimulate a specific antibody response in some individuals with LTBI that could be detected in peripheral blood. This finding was not entirely surprising because cell mediated immune responses to Rv1733c, in particular, were reported to be the most frequent among individuals with LTBI from four African countries, including Uganda [21]. A study by Leyten et al. [12] in Netherlands also reported that the majority of individuals with LTBI were shown to produce strong cell mediated responses to Rv1733c. The ability of Rv1733c to induce strong cell mediated and antibody responses in some individuals with LTBI may also be linked to its immunogenicity. This may equally explain the lack of significant antibody responses to Rv0081, Rv1735c, and Rv1737c in LTBI. A study of immune responses to vaccinia virus showed that promotion of antibody responses by T cells is antigen specific [30]. This same research showed that proteins that induce strong T cell responses are associated with better antibody responses compared to those that do not. Although Rv0081, Rv1735c, and Rv1737c DosR regulon-encoded proteins have been shown to induce T cell responses [21] they may not be sufficiently strong enough to promote good B cell responses.
The adjusted analyses showed no differences in antibody responses to any of the DosR regulon-encoded proteins between APTB cases and uninfected individuals. This contrasts with findings from a study by Mattos et al. in Brazil that reported higher levels of IgG1 antibodies against Rv1733c in TB cases compared to uninfected controls [31]. Our participants were from a TB household contact study and as a result the uninfected individuals may have had exposure to M.tb. Uganda also has a high burden of TB [1] and this may translate into higher exposure to TB and higher anti-TB immune responses at a population level compared to regions of lower TB prevalence. This may have contributed to the lack of significant differences in anti-DosR antibodies between our APTB cases and uninfected individuals.
The initial comparisons made using graphs and the Kruskal Wallis test showed evidence of differences in anti-Rv1733c, anti-Rv0081, and anti-Rv1735c, antibodies across the three groups with individuals with LTBI and APTB appearing to have higher concentrations of these antibodies compared to the uninfected controls. The unadjusted linear regression analysis also showed evidence of higher anti-Rv0081 and anti-Rv1735c antibodies in APTB cases compared to uninfected controls. The APTB cases and individuals with LTBI were older than the uninfected controls and so these initial observations may have been influenced by age and degree of exposure to M.tb. This is plausible because after age was included in the regression model, the evidence for these associations was lost.
This study highlighted gender related difference in the antibody responses to mycobacterial antigens with females having lower anti-CFP-10/ESAT-6 antibodies compared to males. There has been a report of lower PPD specific cell mediated responses in women compared to men [32] but we are not aware of any other studies that have reported a similar difference in the antibody response. We also showed that gender influences the relationship between LTBI status and antibody responses to the DosR proteins, Rv1733c and Rv0081. Some have suggested that the sexual differences in TB may be related to immune response [26]. More study may be required to decipher the implications of these disparities and also to discover what their true causes are.
The observation that anti-CFP-10/ESAT-6 antibodies were higher in APTB cases compared to uninfected controls concurred with previous literature [18, 19] and helped to validate our antibody assay. The fact that these antibody responses were not elevated in individuals with LTBI may point to a possible use of anti-CFP-10/ESAT-6 antibodies as markers of disease progression. Longitudinal studies could be used to investigate this further.
There were some limitations of this study. The fact that we used QFT nil control supernatants from APTB cases instead of serum means that comparisons between antibody concentrations from these individuals and the other groups where serum samples were used should be interpreted with caution. These supernatants were harvested from undiluted whole blood following overnight culture. This could be sufficient time to allow secretion of antibodies into supernatants by M.tb specific antibody secreting cells [33] and could consequently affect antibody concentration. However, there was no difference in the concentrations of antibodies to the DosR proteins between APTB cases for which only QFT nil control supernatants were tested and uninfected individuals for which serum samples were used, in the adjusted analysis. This may imply that even if there was an increase in DosR specific antibodies in the APTB supernatant samples, this may not be large enough to lead to a significant difference in the antibody concentration between the two sample types. Furthermore, we have observed strong and statistically significant correlation between concentrations of anti-mycobacterial antibodies obtained from testing QFT supernatant and serum sample pairs from TB household contacts (Supplementary Figure S1 in Supplementary Material available online at https://doi.org/10.1155/2017/1593143).
On the whole, these results showed that antibodies against Rv1733c, Rv0081, Rv1735c, and Rv1737c DosR regulon-encoded proteins may not serve as good biomarkers of LTBI. The fact that the antibody responses to Rv1733c were only associated with LTBI among females reduces its utility in the general population. However, Mycobacterium specific antibody [9, 20] and B cell responses [8] have been detected in TB latency. There is therefore still a need to identify M.tb proteins that induce strong antibody responses in LTBI.
Supplementary Material
Correlation of concentrations of IgG antibodies to Rv0081 and Rv1733c antigens between paired QFT supernatant and serum samples. Scatter plots were created by plotting each antibody concentration obtained from analysis of supernatant against antibody concentration obtained from analysis of serum from the same individual. Spearman's correlation was used to generate the r and p values shown.
Acknowledgments
This research was supported by a Wellcome Trust Uganda PhD Fellowship in Infection and Immunity held by SK, funded by a Wellcome Trust Strategic Award Grant no. 084344. Simon G. Kimuda also received support from a Commonwealth Scholarship Commission Split Site PhD Fellowship and through the DELTAS Africa Initiative (Grant no. 107743). The DELTAS Africa Initiative is an independent funding scheme of the African Academy of Sciences (AAS), Alliance for Accelerating Excellence in Science in Africa (AESA), and supported by the New Partnership for Africa's Development Planning and Coordinating Agency (NEPAD Agency) with funding from the Wellcome Trust (Grant no. 107743) and the UK Government. The authors thank Moses Egesa and the Kampala Tuberculosis (KTB) study staff for collecting and storing the samples. The authors also thank the Coinfection Studies Programme laboratory team at the MRC/UVRI Uganda Research Unit on AIDS.
Abbreviation
- APTB:
Active pulmonary tuberculosis
- CFP-10/ESAT-6:
Culture filtrate protein-10/early secretory antigen target-6
- DosR:
Dormancy survival regulator
- LTBI:
Latent tuberculosis infection
- QFT:
QuantiFERON®-TB Gold In-Tube
- SES:
Socioeconomic status.
Disclosure
The current address for Jonathan Levin is Division of Epidemiology and Biostatistics, School of Public Health, University of the Witwatersrand, Private Bag 3, Wits, Johannesburg 2050, South Africa. The views expressed in this publication are those of the authors and not necessarily those of AESA, NEPAD Agency, Wellcome Trust, or the UK Government.
Competing Interests
The authors declare no conflict of interests.
References
- 1.WHO. Global Tuberculosis Report. Geneva, Switzerland: WHO; 2016. http://www.who.int/tb/publications/global_report/en/ [Google Scholar]
- 2.Ahmad S. Pathogenesis, immunology, and diagnosis of latent Mycobacterium tuberculosis infection. Clinical and Developmental Immunology. 2011;2011:17. doi: 10.1155/2011/814943.814943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Voskuil M. I., Schnappinger D., Visconti K. C., et al. Inhibition of respiration by nitric oxide induces a Mycobacterium tuberculosis dormancy program. Journal of Experimental Medicine. 2003;198(5):705–713. doi: 10.1084/jem.20030205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leistikow R. L., Morton R. A., Bartek I. L., Frimpong I., Wagner K., Voskuil M. I. The Mycobacterium tuberculosis DosR regulon assists in metabolic homeostasis and enables rapid recovery from nonrespiring dormancy. Journal of Bacteriology. 2010;192(6):1662–1670. doi: 10.1128/jb.00926-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mack U., Migliori G. B., Sester M., et al. LTBI: latent tuberculosis infection or lasting immune responses to M. tuberculosis? A TBNET consensus statement. European Respiratory Journal. 2009;33(5):956–973. doi: 10.1183/09031936.00120908. [DOI] [PubMed] [Google Scholar]
- 6.Pawlowski A., Jansson M., Sköld M., Rottenberg M. E., Källenius G. Tuberculosis and HIV co-infection. PLoS Pathogens. 2012;8(2) doi: 10.1371/journal.ppat.1002464.e1002464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Phuah J. Y., Mattila J. T., Lin P. L., Flynn J. L. Activated B cells in the granulomas of nonhuman primates infected with Mycobacterium tuberculosis . American Journal of Pathology. 2012;181(2):508–514. doi: 10.1016/j.ajpath.2012.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sebina I., Biraro I. A., Dockrell H. M., Elliott A. M., Cose S. Circulating B-lymphocytes as potential biomarkers of tuberculosis infection activity. PLoS ONE. 2014;9(9) doi: 10.1371/journal.pone.0106796.e106796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen J., Wang S., Zhang Y., et al. Rv1985c, a promising novel antigen for diagnosis of tuberculosis infection from BCG-vaccinated controls. BMC Infectious Diseases. 2010;10, article 273 doi: 10.1186/1471-2334-10-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lu L. L., Chung A. W., Rosebrock T. R., et al. A functional role for antibodies in tuberculosis. Cell. 2016;167(2):433.e14–443.e14. doi: 10.1016/j.cell.2016.08.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Betts J. C., Lukey P. T., Robb L. C., McAdam R. A., Duncan K. Evaluation of a nutrient starvation model of Mycobacterium tuberculosis persistence by gene and protein expression profiling. Molecular Microbiology. 2002;43(3):717–731. doi: 10.1046/j.1365-2958.2002.02779.x. [DOI] [PubMed] [Google Scholar]
- 12.Leyten E. M. S., Lin M. Y., Franken K. L. M. C., et al. Human T-cell responses to 25 novel antigens encoded by genes of the dormancy regulon of Mycobacterium tuberculosis . Microbes and Infection. 2006;8(8):2052–2060. doi: 10.1016/j.micinf.2006.03.018. [DOI] [PubMed] [Google Scholar]
- 13.Schuck S. D., Mueller H., Kunitz F., et al. Identification of T-cell antigens specific for latent Mycobacterium tuberculosis infection. PLoS ONE. 2009;4(5) doi: 10.1371/journal.pone.0005590.e5590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.WHO. Global Tuberculosis Report. Geneva, Switzerland: World Health Organization; 2013. http://apps.who.int/iris/bitstream/10665/91355/1/9789241564656_eng.pdf. [Google Scholar]
- 15.Davidow A., Kanaujia G. V., Shi L., et al. Antibody profiles characteristic of Mycobacterium tuberculosis infection state. Infection and Immunity. 2005;73(10):6846–6851. doi: 10.1128/iai.73.10.6846-6851.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Siev M., Wilson D., Kainth S., et al. Antibodies against mycobacterial proteins as biomarkers for HIV-associated smear-negative tuberculosis. Clinical and Vaccine Immunology. 2014;21(6):791–798. doi: 10.1128/CVI.00805-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gennaro M. L., Affouf M., Kanaujia G. V., Brusasca P. N., Mangura B., Reichman L. Antibody markers of incident tuberculosis among HIV-infected adults in the USA: A Historical Prospective Study. International Journal of Tuberculosis and Lung Disease. 2007;11(6):624–631. [PubMed] [Google Scholar]
- 18.Wu X., Yang Y., Zhang J., et al. Humoral immune responses against the Mycobacterium tuberculosis 38-kilodalton, MTB48, and CFP-10/ESAT-6 antigens in tuberculosis. Clinical and Vaccine Immunology. 2010;17(3):372–375. doi: 10.1128/cvi.00287-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu X., Yang Y., Zhang J., et al. Comparison of antibody responses to seventeen antigens from Mycobacterium tuberculosis . Clinica Chimica Acta. 2010;411(19-20):1520–1528. doi: 10.1016/j.cca.2010.06.014. [DOI] [PubMed] [Google Scholar]
- 20.Eleftheriadis T., Tsiaga P., Antoniadi G., et al. The value of serum antilipoarabinomannan antibody detection in the diagnosis of latent tuberculosis in hemodialysis patients. American Journal of Kidney Diseases. 2005;46(4):706–712. doi: 10.1053/j.ajkd.2005.06.021. [DOI] [PubMed] [Google Scholar]
- 21.Black G. F., Thiel B. A., Ota M. O., et al. Immunogenicity of novel DosR regulon-encoded candidate antigens of Mycobacterium tuberculosis in three high-burden populations in Africa. Clinical and Vaccine Immunology. 2009;16(8):1203–1212. doi: 10.1128/cvi.00111-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sutherland J. S., Lalor M. K., Black G. F., et al. Analysis of host responses to Mycobacterium tuberculosis antigens in a multi-site study of subjects with different TB and HIV infection states in Sub-Saharan Africa. PLoS ONE. 2013;8(9) doi: 10.1371/journal.pone.0074080.e74080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Biraro I. A., Egesa M., Toulza F., et al. Impact of co-infections and BCG immunisation on immune responses among household contacts of tuberculosis patients in a Ugandan cohort. PLoS ONE. 2014;9(11) doi: 10.1371/journal.pone.0111517.e111517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lule S. A., Mawa P. A., Nkurunungi G., et al. Factors associated with tuberculosis infection, and with anti-mycobacterial immune responses, among five year olds BCG-immunised at birth in Entebbe, Uganda. Vaccine. 2015;33(6):796–804. doi: 10.1016/j.vaccine.2014.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wood R., Liang H., Wu H., et al. Changing prevalence of tuberculosis infection with increasing age in high-burden townships in South Africa. International Journal of Tuberculosis and Lung Disease. 2010;14(4):406–412. [PMC free article] [PubMed] [Google Scholar]
- 26.Neyrolles O., Quintana-Murci L. Sexual inequality in tuberculosis. PLoS Medicine. 2009;6(12) doi: 10.1371/journal.pmed.1000199.e1000199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moir S., Fauci A. S. B cells in HIV infection and disease. Nature Reviews Immunology. 2009;9(4):235–245. doi: 10.1038/nri2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oertelt-Prigione S. The influence of sex and gender on the immune response. Autoimmunity Reviews. 2012;11(6-7):A479–A485. doi: 10.1016/j.autrev.2011.11.022. [DOI] [PubMed] [Google Scholar]
- 29.Diwan V. K., Thorson A. Sex, gender, and tuberculosis. The Lancet. 1999;353(9157):1000–1001. doi: 10.1016/s0140-6736(99)01318-5. [DOI] [PubMed] [Google Scholar]
- 30.Sette A., Moutaftsi M., Moyron-Quiroz J., et al. Selective CD4+ T cell help for antibody responses to a large viral pathogen: deterministic linkage of specificities. Immunity. 2008;28(6):847–858. doi: 10.1016/j.immuni.2008.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mattos A. M. M., Chaves A. S., Franken K. L. M. C., et al. Detection of IgG1 antibodies against Mycobacterium tuberculosis DosR and Rpf antigens in tuberculosis patients before and after chemotherapy. Tuberculosis. 2016;96:65–70. doi: 10.1016/j.tube.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 32.Nielsen N. O., Soborg B., Børresen M., Andersson M., Koch A. Cytokine responses in relation to age, gender, body mass index, Mycobacterium tuberculosis infection, and otitis media among inuit in greenland. American Journal of Human Biology. 2013;25(1):20–28. doi: 10.1002/ajhb.22332. [DOI] [PubMed] [Google Scholar]
- 33.Lalvani A., Connell D. W. Tuberculosis immunodiagnosis: delving below the surface. Thorax. 2013;68(3):204–206. doi: 10.1136/thoraxjnl-2012-202481. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Correlation of concentrations of IgG antibodies to Rv0081 and Rv1733c antigens between paired QFT supernatant and serum samples. Scatter plots were created by plotting each antibody concentration obtained from analysis of supernatant against antibody concentration obtained from analysis of serum from the same individual. Spearman's correlation was used to generate the r and p values shown.