Abstract
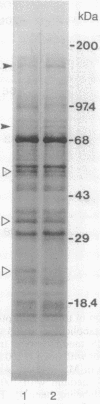
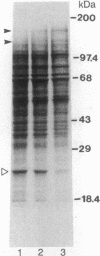
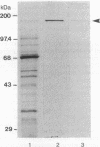
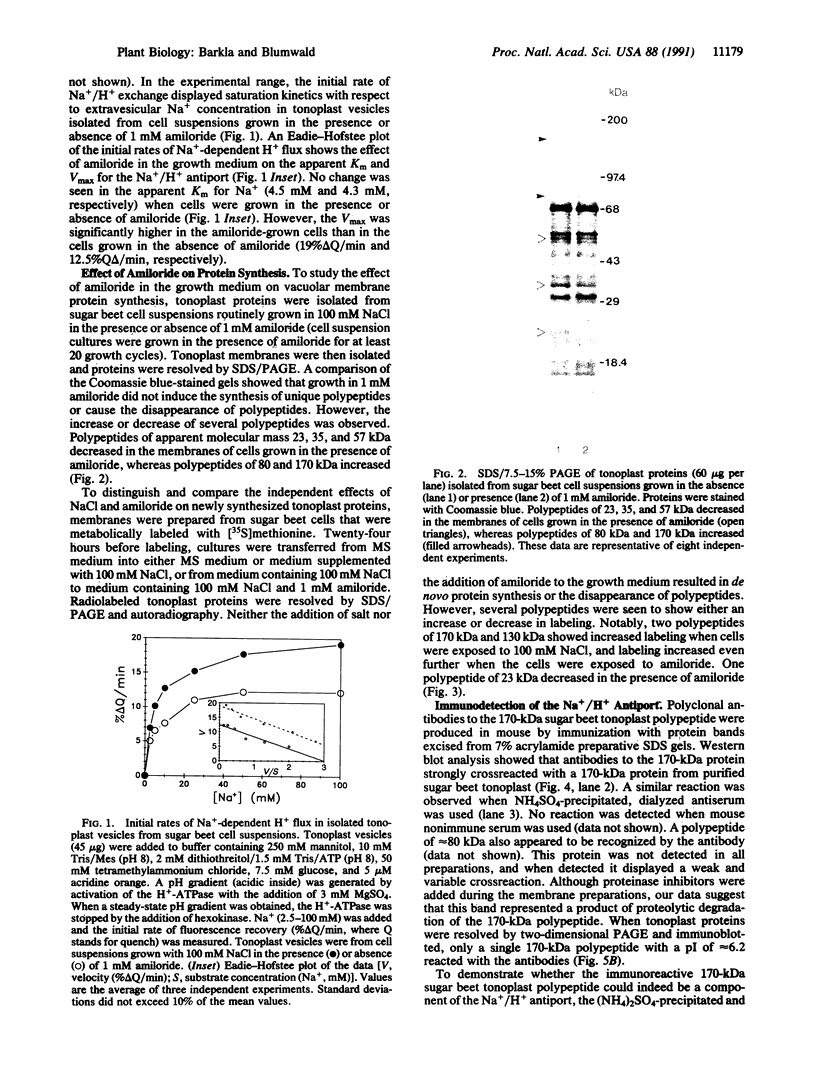
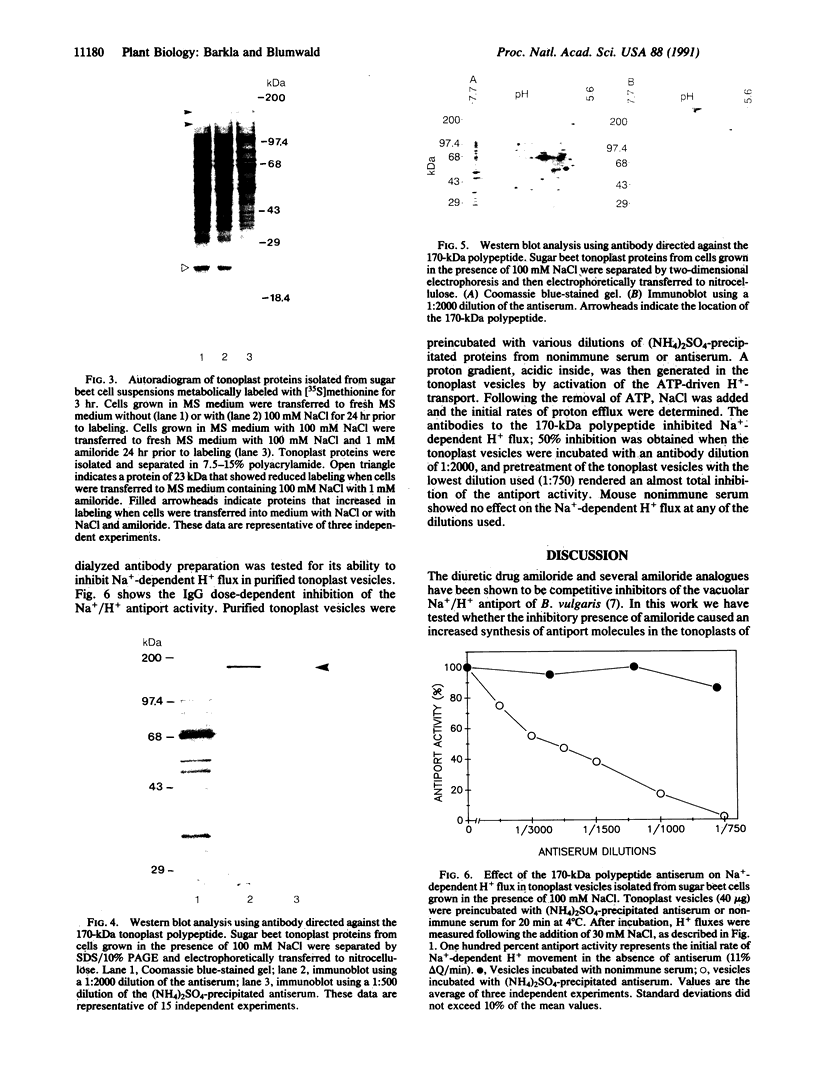
The effect of the addition of amiloride to the growth medium was tested on the Na+/H+ antiport activity of tonoplast vesicles isolated from sugar beet (beta vulgaris L.) cell suspensions. Cells grown in the presence of NaCl and amiloride displayed an increased antiport activity. Analysis of the kinetic data showed that while the affinity of the antiport for Na+ ions did not change, the maximal velocity of the Na+/H+ exchange increased markedly. These results suggest the addition of more antiport molecules to the tonoplast and/or an increase in the turnover rate of the Na+/H+ exchange. The increase in activity of the antiport by the presence of amiloride was correlated with the enhanced synthesis of a tonoplast 170-kDa polypeptide. The increased synthesis of this polypeptide was detected not only upon exposure of the cells to amiloride but also when the cells were exposed to high NaCl concentrations. Polyclonal antibodies against the 170-kDa polypeptide almost completely inhibited the antiport activity. These results suggest the association of the 170-kDa polypeptide with the vacuolar Na+/H+ antiport.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barkla B. J., Charuk J. H., Cragoe E. J., Blumwald E. Photolabeling of tonoplast from sugar beet cell suspensions by [h]5-(N-methyl-N-isobutyl)-amiloride, an inhibitor of the vacuolar na/h antiport. Plant Physiol. 1990 Jul;93(3):924–930. doi: 10.1104/pp.93.3.924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benos D. J. Amiloride: a molecular probe of sodium transport in tissues and cells. Am J Physiol. 1982 Mar;242(3):C131–C145. doi: 10.1152/ajpcell.1982.242.3.C131. [DOI] [PubMed] [Google Scholar]
- Blumwald E., Cragoe E. J., Poole R. J. Inhibition of na/h antiport activity in sugar beet tonoplast by analogs of amiloride. Plant Physiol. 1987 Sep;85(1):30–33. doi: 10.1104/pp.85.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumwald E., Poole R. J. Na/H Antiport in Isolated Tonoplast Vesicles from Storage Tissue of Beta vulgaris. Plant Physiol. 1985 May;78(1):163–167. doi: 10.1104/pp.78.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumwald E., Poole R. J. Salt tolerance in suspension cultures of sugar beet : induction of na/h antiport activity at the tonoplast by growth in salt. Plant Physiol. 1987 Apr;83(4):884–887. doi: 10.1104/pp.83.4.884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan T. W., Higashi R. M., Norlyn J., Epstein E. In vivo 23Na and 31P NMR measurement of a tonoplast Na+/H+ exchange process and its characteristics in two barley cultivars. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9856–9860. doi: 10.1073/pnas.86.24.9856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franchi A., Cragoe E., Jr, Pouysségur J. Isolation and properties of fibroblast mutants overexpressing an altered Na+/H+ antiporter. J Biol Chem. 1986 Nov 5;261(31):14614–14620. [PubMed] [Google Scholar]
- Garbarino J., Dupont F. M. NaCl Induces a Na/H Antiport in Tonoplast Vesicles from Barley Roots. Plant Physiol. 1988 Jan;86(1):231–236. doi: 10.1104/pp.86.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guern J., Mathieu Y., Kurkdjian A., Manigault P., Manigault J., Gillet B., Beloeil J. C., Lallemand J. Y. Regulation of Vacuolar pH of Plant Cells: II. A P NMR Study of the Modifications of Vacuolar pH in Isolated Vacuoles Induced by Proton Pumping and Cation/H Exchanges. Plant Physiol. 1989 Jan;89(1):27–36. doi: 10.1104/pp.89.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs E., Clad A. Electroelution of fixed and stained membrane proteins from preparative sodium dodecyl sulfate-polyacrylamide gels into a membrane trap. Anal Biochem. 1986 May 1;154(2):583–589. doi: 10.1016/0003-2697(86)90033-3. [DOI] [PubMed] [Google Scholar]
- L'Allemain G., Franchi A., Cragoe E., Jr, Pouysségur J. Blockade of the Na+/H+ antiport abolishes growth factor-induced DNA synthesis in fibroblasts. Structure-activity relationships in the amiloride series. J Biol Chem. 1984 Apr 10;259(7):4313–4319. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Pantoja O., Dainty J., Blumwald E. Tonoplast ion channels from sugar beet cell suspensions : inhibition by amiloride and its analogs. Plant Physiol. 1990 Dec;94(4):1788–1794. doi: 10.1104/pp.94.4.1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parry R. V., Turner J. C., Rea P. A. High purity preparations of higher plant vacuolar H+-ATPase reveal additional subunits. Revised subunit composition. J Biol Chem. 1989 Nov 25;264(33):20025–20032. [PubMed] [Google Scholar]
- Simchowitz L., Woltersdorf O. W., Jr, Cragoe E. J., Jr Intracellular accumulation of potent amiloride analogues by human neutrophils. J Biol Chem. 1987 Nov 25;262(33):15875–15885. [PubMed] [Google Scholar]