Abstract
Combining functionalization at a distant position from a reactive site with the creation of several consecutive stereogenic centres, including the formation of a quaternary carbon stereocentre, in acyclic system represents a pinnacle in organic synthesis. Here we report the regioselective Heck arylation of terminal olefins as a distant trigger for the ring-opening of cyclopropanes. This Pd-catalysed unfolding of the strained cycle, driving force of the chain-walking process, remarkably proved its efficiency and versatility, as the reaction proceeded regardless of the molecular distance between the initiation (double bond) and termination (alcohol) sites. Moreover, employing stereodefined polysubstituted cyclopropane vaults allowed to access sophisticated stereoenriched acyclic scaffolds in good yields. Conceptually, we demonstrated that merging catalytically a chain walking process with a selective C–C bond cleavage represents a powerful approach to construct linear skeleton possessing two stereogenic centres.
Functionalizing multiple, distant sites across a molecule is a challenge. Here the authors report a remote functionalization strategy, whereby an initial Heck reaction leads to chain-walking of palladium across a molecule, ring-opening cyclopropanes while retaining the stereochemistry in the product.
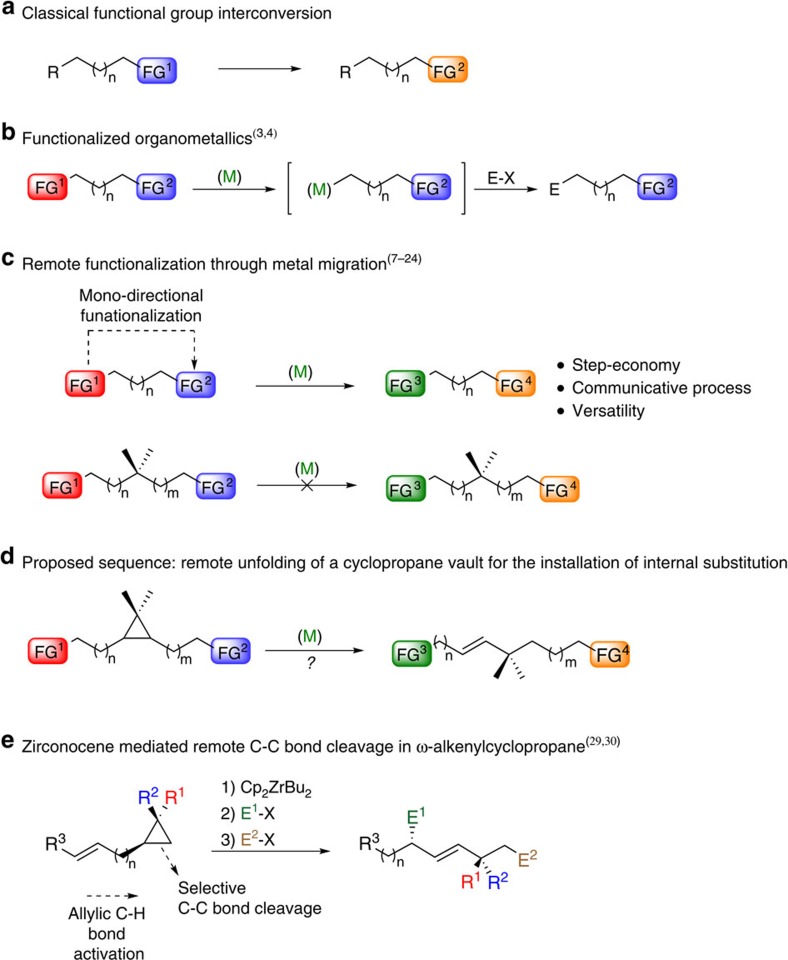
In the last few decades, the field of organic synthesis has witnessed tremendous evolutions. Traditionally, the organic chemistry community mainly focused its efforts on forming new covalent bonds at a single and generally most reactive functional group to interconvert functionalities (Fig. 1a)1,2. In recent years a paradigm shift occurred, deeply confronting our generally temporally linear retrosynthetic mindset to the benefit of more flexible, versatile and economical transformations. For instance in the field of organometallic chemistry, powerful strategies have been developed where specific metal-mediated reactions may be performed in the presence of other functional groups, thus drastically improving the integration of organometallic reagents in synthesis (Fig. 1b)3,4. Despite this impressive portfolio of synthetic strategies developed so far, it still appears challenging to functionalize at distant, unrelated and often unreactive positions from any functional group on a molecule (Fig. 1c). To address this issue, the concept of remote functionalization—where an initial interaction of a functional group leads to a selective reaction at a distal position—was introduced several decades ago5,6. Even though this strategy has rarely been considered in synthesis so far, recent reports have nonetheless contributed to substantially promote this powerful and singular approach7,8,9,10,11,12,13,14,15,16,17,18,19,20. In particular, a remote functionalization proceeding through transition metal-mediated chain-walking21 has the significant advantage of increasing the versatility and scope of a transformation through a communicative process associating two distant functional groups independently of their molecular distance (n) (ref. 22). An initial chemoselective interaction between a metal complex and a functional group (FG1, initiation site) may trigger the induction of a second functional group interconversion at a distant site (FG2, termination site), assuming an efficient and rapid metal complex migration along the molecule. This strategy potentially allows the interconversion of two functional groups at positions unrelated from each other in a single reaction vessel. However, chain-walking reactions are generally limited to saturated methylene connections between the functional groups. Although stereochemical information of tertiary centres might be conserved during the migration13, quaternary centres would prevent any metal migration process to occur until the desired termination site (Fig. 1c). To circumvent this latter problem, we hypothesized that one could develop a chain-walking/cyclopropane ring-opening/chain-walking sequence as depicted in Fig. 1d. The subsequent cyclopropane ‘vault' would, therefore, serve as a readily frangible platform to install internal substitutions between two distant functional groups on a same linear hydrocarbon chain. Merging metal-mediated alkene migration23,24 and selective C–C bond cleavage25,26,27,28, two cutting edge methods in organic synthesis, would represent a novel approach to mould strained cyclic molecules into sophisticated acyclic molecular skeletons. Over the last few years, our laboratory has been involved in the specific development of remote activation reactions29,30,31,32 using stoichiometric amount of low-valent zirconocene complexes. Among others, we disclosed the highly selective transformation of ω-ene cyclopropanes into acyclic bifunctionalized (E)-olefins (Fig. 1e)33,34. In light of this powerful one-pot entry to acyclic systems, we recently questioned the possibility of developing such sequence using a catalytic amount of a transition metal based complex enabling concomitantly C–C bond coupling, metal migration and a selective cleavage of a C–C bond.
Figure 1. Comparison between the different strategies for the functionalization of organic skeletons.
(a) Classical approach for the selective interconversion of a functional group. (b) Chemoselective interaction between a metal and a specific moiety allowing the creation of functionalized organometallic species, leading to a variety of electrophilic substitution reactions. (c) Remote functionalization through metal migration strategy enabling a transformation away from a functional group at a distant position (FG2); located further away from (FG1). Despite many inherent advantages, this approach does not allow the presence of the highly regarded quaternary carbon stereocentre. (d) Proposed sequence where a strategically stereodefined polysubstituted cyclopropane vault is used assuming that the ring-strain release may drive the chain-walking process towards the formation of acyclic products and allowing the introduction of an internal substitution on the carbon skeleton. (e) Illustration of this sequence was reported for the addition of stoichiometric amount of low-valent zirconocene derivatives to ω-alkenyl cyclopropanes generating a cascade of events composed by successive allylic C–H bond activations, selective C–C bond cleavage and finally reaction with two different electrophiles (E1X and E2X).
Results
Reaction design
For this purpose, one should consider that a catalytic long-range activation heavily relies on a rapid metal migration over the hydrocarbon chain and fast release of the metal from the termination site22. Following these requirements, we turned our attention to the Pd-catalysed Heck reactions33 that have been previously known to trigger an efficient migration of the organometallic species over hydrocarbon chains34. Based on the pioneering observation of Heck who reported the Pd-catalysed arylation of 4-penten-2-ol into 5-phenyl-pentan-2-one, although in low yield35, Larock consequently further developed this transformation with other non-allylic unsaturated alcohols36 (Fig. 2a). Ultimately, these works led to the most recent tour de force when Sigman successfully developed the enantioselective version of this reaction (Fig. 2b)13,37. Remarkably efficient and highly selective Heck arylation reactions over unactivated di- or trisubstituted alkenes and subsequent isomerization towards an alcohol or an electron-withdrawing group were successfully developed37,38,39,40. We, therefore, anticipated that the migration of a secondary organopalladium along the chain by fast β-hydride elimination and subsequent re-addition could eventually be driven by the ring-strain release from a PdII-catalysed β-cyclopropyl fragmentation41. In case of a selective C–C bond cleavage, a polysubstituted stereodefined cyclopropane—whose synthesis and reactivity with transition metals have been well established through diverse strategies42,43,44,45 would ultimately afford the acyclic internal olefin bearing the quaternary carbon stereocentre (Fig. 2c)46,47,48. Our proposed sequence stems from a detailed analysis of the potential factors driving the reaction to completion but also considers the ease of preparation of stereomerically enriched starting materials (1a–1s, dr≥92:08:0:0) (ref. 45). To start with, following the oxidative addition of an aryl halide to LnPd(0) complex, two regioisomers could result from the 1,2-aryl migratory insertion of the terminal olefin into the Pd(Ar) bond. Both resulting organopalladium species I and II could suffer β-hydride elimination and LnPdHX could either disengage from the molecule to afford the alkenyl derivative, or undergo readdition of the hydride on the isomerized olefin to trigger the chain-walking. Assuming that the palladium-walk would be an efficient process, it would reach at a certain point the sterically congested cyclopropane (III and IV, respectively) where a C–C bond cleavage might occur at its β-position. Both C–C bonds (C1–C2 or C1–C3) could presumably be cleaved, but intermediates V and VI resulting from a selective C1–C2 bond cleavage would enable further chain-walking towards the alcohol. One additional question would concern the configurational stability of the resulting tertiary organopalladium intermediate in V and VI as well as the stereofacial choice of the migration event. The continuation of the Pd-walk would ultimately end with the disengagement of LnPdHX to extrude the expected carbonyl derivative (2 and/or 3), and, regeneration of the active catalytic species LnPd(0) by reaction of the palladium hydride with a base. Therefore, the success of this rather convoluted intramolecular process would reckon on a complete control of all of these numerous elementary steps.
Figure 2. Examples of Pd-catalysed Heck arylation as an initiation step triggering a remote functionalization and mechanistic hypothesis.
(a) The first report of a Pd-intramolecular migration across a molecule was pioneered by Heck35 and later extended by Larock36 showing the versatility of the method across longer alkenyl chain. (b) Sigman reported the enantioselective and site-selective Heck reaction of diazonium salts, vinyl triflates, aryl boronic acids and indole derivatives with ω-alkenyl alcohols13,37,38,39,40. (c) Proposed catalytic sequence and mechanistic hypotheses for the transformation of cyclopropane 1 into regioisomers 2 and/or 3.
In lights of these elements, the selective conversion of 1 into 2 and/or 3 might appear strenuous, considering the possible pitfalls and potential by-products.
Arylation of allylcyclopropyl carbinol derivatives
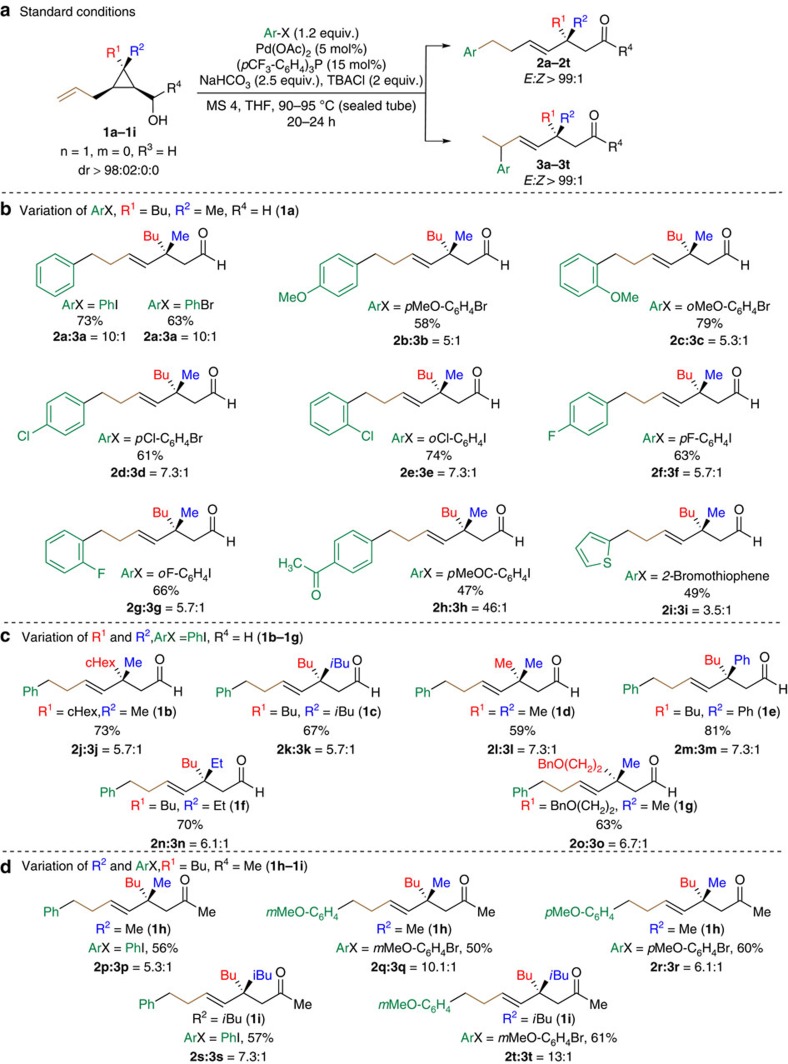
Using our model substrate, namely the syn allyl cyclopropyl carbinol (±)-1a (R1=Bu, R2=Me, R3=R4=H, n=m=0), synthesized diastereomerically enriched45, we first determined the optimal conditions for our proposed sequence using phenyl iodide for a Pd-based catalytic system. We found that in the presence of a catalytic amount of Pd(OAc)2 (5 mol%), tris(4-trifluoromethylphenyl)phosphine as ligand (15 mol%), NaHCO3 as inorganic base (2.5 equiv), tert-butylammonium chloride (2 equiv) and molecular sieves 4 Å (150 mg mmol−1 substrate), 1a and PhI (1.2 equiv) in tetrahydrofuran (THF) at 95 °C for 24 h afforded a mixture of aldehydes 2a and 3a in 73% combined yield with good regioselectivity (10:1) and as unique (E)-isomers (Fig. 3b, for the optimization table see Supplementary Table 1). No other positional alkene isomers could be detected suggesting that not only the final product was at no risk of further olefin isomerization but also that the chain-walking process was exclusively triggered by the initial Heck arylation. Following this optimization based on the coupling of phenyl iodide with 1a, other aryl halides were tested (Fig. 3b). To our delight, aryl bromides were also suitable partners as no significant difference were observed in the chemical outcome of the reaction (see Fig. 3b, formation of 2a:3a). This transformation was compatible with the presence of functional groups on the aryl moiety such as halides (2d–2g), electron-donating group (2b and 2c) and electron-withdrawing group including an interesting methyl ketone moiety (2h). Notably, 2-bromothiophene was also successfully incorporated onto 1a, albeit in lower yield and regioselectivity (2i). Not only the aryl moiety could be varied but the substitution pattern on the quaternary stereocentre (R1 and R2) may as well be easily modified through our previously described flexible approach for the synthesis of such cyclopropane rings43,45 (Fig. 3c). Indeed, compounds bearing the quaternary carbon stereocentre possessing linear (Me, Et, Bu, CH2CH2OBn), branched (iBu), cyclic (cHex) and aromatic (Ph) groups could be synthesized using similar strategy (1b–1g). We observed only few differences in the chemical outcome of the Heck coupling domino reaction, as the different starting materials all afforded the corresponding aldehydes (2j–2o) in good yields (59–81%) and regioselectivities (>5.7:1). It should be emphasized that our method could potentially generate carbon quaternary stereocentres of rather similar steric groups, such as butyl and iso-butyl chains, on an acyclic system (2k). Additionally, secondary alcohol derivatives (R4=Me, 1h and 1i) also underwent similar transformation to mainly yield the corresponding methyl ketones 2p–2t (Fig. 3d).
Figure 3. Scope of the reaction by varying ArX, R1, R2 and R4.
(a) Standard experiment conditions for the coupling of 1 with ArX yielding 2 and 3 using a Pd(0)-based catalytic system. (b) Scope of the aryl halide (ArX). (c) Scope of the substitution pattern on the cyclopropyl carbinol moiety (R1 and R2). (d) Examples involving the transformation of secondary alcohol derivatives (R4=Me) into ketones. Regioisomeric and E/Z ratios were determined by NMR and GC analyses of the crude reaction mixture. All represented yields are the combined yields of both regioisomers after purification by column chromatography.
Variation of chain-length in substrates
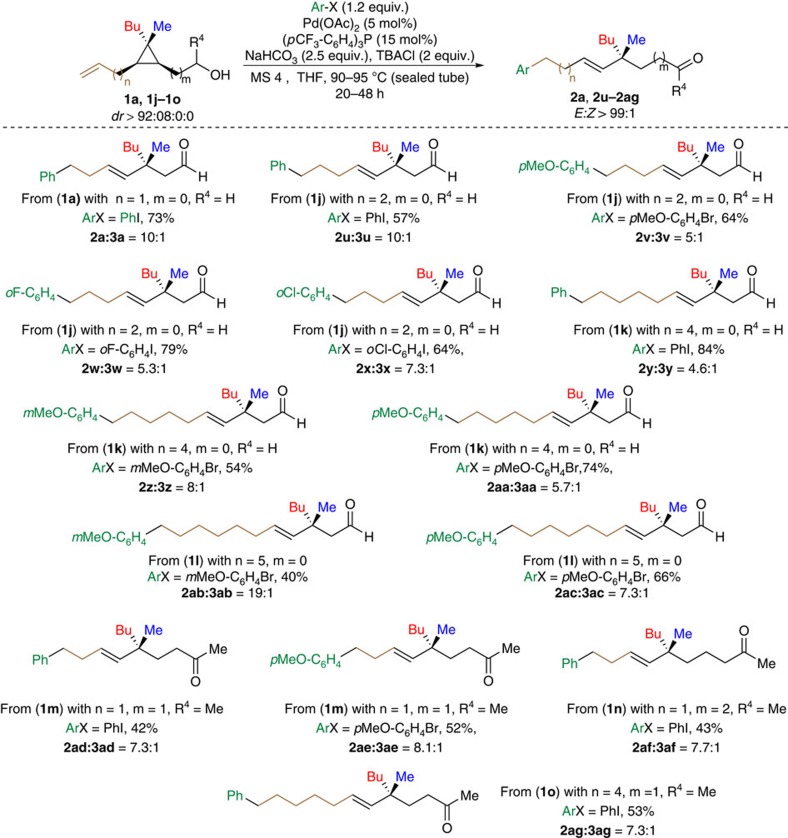
Most importantly, the exact same conditions still proceeded on analogous substrates with different chain-lengths (n and m), further demonstrating the efficient palladium chain-walking mechanism (Fig. 4, for the sake of clarity, all the following structures of the minor products 3u–3ap are omitted). The examples shown in Fig. 4, illustrates the case where 1 to 5 methylene units were present between the unsaturation and the cyclopropyl ring (2u–2ac). In parallel, increasing the molecular distance between the alcohol moiety and the cyclopropane (m) also led to acceptable yields of the corresponding carbonyl derivatives (2ad–2af, Fig. 4). Lengthening simultaneously both sides (1o, n=4, m=1) did not prevent the reaction to occur (2ag, Fig. 4), demonstrating the high versatility of the method. In all cases, as already previously mentioned, the E/Z ratio of the major 1,2-disubstituted olefin obtained in these products remained excellent (E/Z>99:01).
Figure 4. Scope of the chain lengths (n and m).
Increase of the molecular distance (n) between the double bond and cyclopropane moieties as well as the molecular distance (m) between the alcohol and cyclopropane and finally the situation where both chain length n and m are increased.
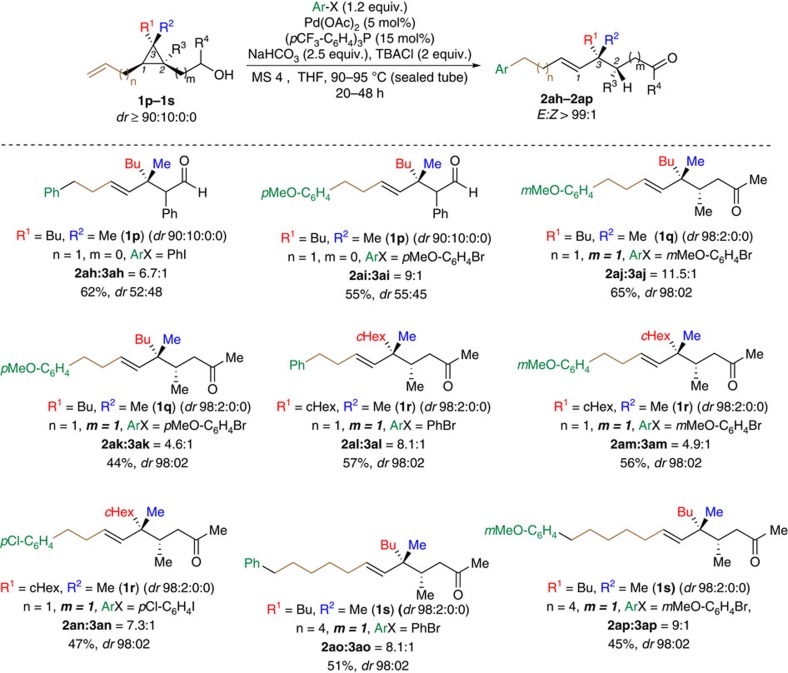
Encouraged by these positive results, we added one more level of complexity to this sequence with the unfolding of 1,2,2,3,3-pentasubstituted cyclopropanes as illustrated in Fig. 5. In this particular case, the selective C–C bond cleavage could be challenging as both substituted distal C–C bonds (C1–C2 versus C1–C3) have a similar substitution degree. To our delight, when 1p was treated in our optimized experimental conditions with two different ArX, only the products 2ah and 2ai resulting from a selective ring-cleavage were obtained in good yields as described in Fig. 5 suggesting the primordial role of the alcohol moiety in the selective C–C bond cleavage. However, the benzylic stereogenic centre was obtained as two diastereoisomers in an almost 1:1 ratio, presumably owing to an epimerization during the last step of the reaction sequence namely the keto-enol tautomerization as would be probed in our isotope labelling investigations discussed in Fig. 6. If our hypothesis that the keto-enol tautomerism induced epimerization of the stereocentre were true, the reaction on 1,1,2,2,3-pentasubstituted cyclopropanes possessing the alcohol moiety at least one carbon away of the tertiary stereocentre would be insensitive to the last chemical event (keto-enol tautomerism) and the stereochemistry of the tertiary stereocentre would, therefore, be preserved throughout the process.
Figure 5. Scope of the chain lengths (n and m) and substitution.
Remote Pd-catalysed Heck triggered ring-opening in 1,2,2,3,3-pentasubstituted cyclopropane derivatives (1p–1s). The relative configuration of 2aj was confirmed by X-ray analysis of the corresponding hydrazone 4 (see Supplementary Fig. 91). Relative configurations of other major products 2aj–2ap were assigned by analogy. The described yields are the combined yields of both regioisomers after purification by column chromatography. Regioisomeric, diasteromeric and E/Z ratios were determined by NMR and GC analyses of the crude mixture.
Figure 6. Control and isotope labelling experiments.
(a) Effect of the relative stereochemistry between the ω-alkenyl chain and the alcohol residue on the reaction outcome using standard reaction conditions (see Fig. 3a). (b) Isotopic labelling experiments. Deuterium incorporation was confirmed by NMR and mass spectrometry analyses of products 5–16 after purification over column chromatography.
Indeed, the remote arylation of ω-alkenyl cyclopropyl alcohols 1q–1t (m≥1) were tested with various ArX and we were delighted to see that compounds 2aj–2ap were obtained in satisfactory yields as a single set of diastereoisomers and as a single E-isomer as observed by NMR and GC analyses (dr=98:02) suggesting that the Pd does not disengage during the process and migrates on the same stereoface. The relative configuration was confirmed by X-ray analysis of the hydrazone derivative (4) of 2aj (see Supplementary Fig. 91). It should be emphasized that the formation of two contiguous stereocentres, including the formation of a quaternary carbon stereocentre, is still a challenging issue in synthesis and this approach proposes a highly stereoselective alternative route to such molecular backbones.
Mechanistic insights
To probe our mechanistic hypotheses, we conducted a series of control, including isotope labelling, experiments. We first studied the effect of the relative stereochemistry between the ω-alkenyl chain and the alcohol residue with PhI as coupling partner (Fig. 6a). When the anti diastereoisomer (±)-1aanti (dr=90:10:0:0) was treated under the same experimental condition as (±)-1asyn, the same final aldehyde 2a was obtained in similar yields and E/Z-selectivity albeit in lower 2a:3a isomeric ratio (3a not indicated in the Fig. 6). Isotope labelling experiments were also performed to map the metal-complex migration across the organic molecule (Fig. 6b). When two geminal deuterium atoms were located either at the terminal double bond or at the homoallylic positions (5 and 6, respectively), the expected products 11 and 12 were independently obtained confirming the well-established Pd-catalysed 1,2-hydrogen shift mechanism. Positioning tactically a deuterium atom on the cyclopropyl ring as illustrated in 7 enabled us to suggest that the ring-opening should proceed faster than a potential β-hydride elimination that would have afforded an alkylidenecyclopropane intermediate49. When a deuterium atom was strategically introduced at the other cyclopropyl carbon atom as in 8, the product 14 obtained after our cascade reaction was deuterium atom-free. A similar result was observed when 9 was treated under our Heck conditions as the monodeuterated aldehyde 15 was selectively obtained without traces of deuterium in α-position to the carbonyl group. These losses of deuterium atoms during these two keto-enol tautomerizations suggested a rapid exchange that was also accounted for the epimerization of the stereogenic centre in 2ah and 2ai as previously discussed (Fig. 5). However, when the keto-enol tautomerization took place one carbon further away owing to the introduction of one additional carbon centre as in 10, the reaction proceeded with preservation of the deuterium atom and 16 was obtained as a single diastereoisomer.
All these experiments enabled us to corroborate the proposed mechanism presented on Fig. 2 where the oxidative insertion of the aryl halide to LnPd(0) led to the LnPd(II)ArX species from which the migratory insertion on 1 occurred to deliver II. A facile and rapid migration of the metal over the alkyl chain through successive 1,2-hydrogen shifts (chain-walking) then leads to the cyclopropyl methyl palladium species III. At this stage, a selective ring-fragmentation proceeds into the unique formation of (E)-homoallyl organopalladium V. The highly selective C–C bond cleavage (when R3≠H) might be rationalized by the directionality of the second chain-walking event. Furthermore, the resulting tertiary organopalladium species (R3=Me) did not suffer any epimerization when m≥1 and continue to promote the chain walking on the same stereoface to finally yield the desired carbonyl derivative 2aj–2ap as the major products. When m=0 and, therefore, no additional chain-walking process can be performed, and the keto-enol tautomerization causes the epimerize of the stereocentre.
Discussion
In conclusion, we could report that a single transition metal based-catalytic system could successively mediate a (i) regioselective arylation of the terminal olefin, (ii) a chain-walking, (iii) a selective C–C bond cleavage of the cyclopropane, (iv) additional chain-walking and (v) release the carbonyl moiety to regenerate the active catalytic species. All these consecutive elementary steps proceeded in a one-pot operation with impressive levels of selectivities. During this study, we could uncover the highly selective C–C bond cleavage conditioned by the chain-walking directionality. As a result, the strategically positioning of the cyclopropane ‘vault' enabled the consecutive introduction of a tertiary and a quaternary stereocentre at unrelated positions between two distant functional groups into a linear hydrocarbon skeleton. This contrasts with catalytic chain-walking reactions that are generally perceived as proceeding exclusively across methylene chains. Additionally, the ease of synthesis of such diastereomerically pure ω-ene cyclopropyl derivatives (see Supplementary methods) associated with this proposed versatile and mild procedure allowed the downstream functionalization into further sophisticated acyclic hydrocarbon frameworks.
Methods
Materials
For NMR spectra of compounds in this manuscript, see Supplementary Figs 1–90. For the optimization of reaction conditions of compound 2a, see Supplementary Table 1. For the crystallographic data of compounds 4, see Supplementary Fig. 91, Supplementary Table 2 and Supplementary Note 1. For the experimental procedures and analytic data of compounds synthesized, see Supplementary Methods.
Preparation of compounds 2 and 11–16
A sealed vial (10 ml capacity) equipped with a magnetic stirring bar, was successively charged with an alcohol derivative 1a-1 s/5-10 (0.2 mmol), aryl bromide/iodide (0.24 mmol), Pd(OAc)2 (2.24 mg, 5 mol%), (pCF3-C6H4)3P (14 mg, 15 mol%), NaHCO3 (42 mg, 2.5 mmol), pre-activated molecular sieves (4 Å size, powdered, 30 mg) and tetrabutylammonium chloride (2 mmol) followed by addition of dry THF (0.4 ml). After sealing, the reaction mixture was stirred for 10 min at room temperature to obtain a homogeneous solution and was then placed on a pre-heated oil bath at 90–95 °C for 24–48 h. After completion of the reaction (as monitored by TLC), the reaction mixture was cooled down to room temperature, diluted with Et2O (5 ml) and filtered. The obtained residue was concentrated and subjected to a column chromatography using 3–8% Et2O in hexane as eluent to afford product 2a–2z, 2aa–2ap and 11–16 as a mixture with their corresponding inseparable regioisomers 3a–3z, 3aa–3ap and 11*-16*.
Data availability
The X-ray crystallographic coordinates for structure of compound 4 reported in this article have been deposited at the Cambridge Crystallographic Data Centre (CCDC) with the accession code CCDC 1500847. This data can be obtained free of charge from the Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif. The authors declare that all other data supporting the findings of this study are available within the article and Supplementary Information files, and also are available from the corresponding author upon reasonable request.
Additional information
How to cite this article: Singh, S. et al. A unique Pd-catalysed Heck arylation as a remote trigger for cyclopropane selective ring-opening. Nat. Commun. 8, 14200 doi: 10.1038/ncomms14200 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Supplementary figures, supplementary tables, supplementary note, supplementary methods and supplementary references.
Acknowledgments
This research was supported by the European Research Council under the Seventh Framework Program of the European Community (ERC grant agreement no. 338912). S.S. thanks the Planning and Budgeting Committee (PBC) of the Council for Higher Education for providing financial support. I.M. holds the Sir Michael and Lady Sobell Academic Chair.
Footnotes
The authors declare no competing financial interests.
Author contributions S.S., J.B. and A.V. planned, conducted and analysed experiments. I.M. designed and directed the project and wrote the manuscript with contributions by S.S., J.B. and A.V. All authors contributed to discussions.
References
- Carreira E. M. & Kvaerno L. Classics in Stereoselective Synthesis Wiley-VCH (2009). [Google Scholar]
- Corey E. J. & Lecture R. R. Retrosynthetic thinking-essentials and examples. Chem. Soc. Rev. 17, 111–133 (1988). [Google Scholar]
- Benischke A. D. et al. Polyfunctional zinc and magnesium organometallics for organic synthesis: some perspectives. Synthesis 48, 1101–1107 (2014). [Google Scholar]
- Boudier A. et al. New applications of polyfunctional organometallics in organic synthesis. Angew. Chem. Int. Ed. 39, 4414–4435 (2000). [PubMed] [Google Scholar]
- Schwarz H. Remote functionalization of C–H and C–C bonds by ‘naked' transition metal ions (Cosi Fan Tutte). Acc. Chem. Res. 22, 282–287 (1989). [Google Scholar]
- Breslow R. Biomimetic control of chemical selectivity. Acc. Chem. Res. 13, 170–177 (1980). [Google Scholar]
- Lin L., Romano C. & Mazet C. Palladium-catalyzed long-range deconjugative isomerization of highly substituted α,β-unsaturated carbonyl compounds. J. Am. Chem. Soc. 138, 10344–10350 (2016). [DOI] [PubMed] [Google Scholar]
- Topczewski J. J. et al. Palladium-catalysed transannular C–H functionalization of alicyclic amines. Nature 531, 220–224 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X.-C. et al. Ligand-enabled meta-C–H activation using a transient mediator. Nature 519, 334–338 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamasaki T. et al. Chain walking as a strategy for carbon–carbon bond formation at unreactive sites in organic synthesis: catalytic cycloisomerization of various 1,n-dienes. J. Am. Chem. Soc. 137, 16163–16171 (2015). [DOI] [PubMed] [Google Scholar]
- Dong Z., Wang J. & Dong G. Simple amine-directed meta-selective C–H arylation via Pd/norbornene catalysis. J. Am. Chem. Soc. 137, 5887–5890 (2015). [DOI] [PubMed] [Google Scholar]
- Tang R.-Y., Li G. & Yu J.-Q. Conformation-induced remote meta C–H activation of amines. Nature 507, 215–220 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei T.-S., Patel H. H. & Sigman M. S. Enantioselective construction of remote quaternary stereocentres. Nature 508, 340–344 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atienza C. C. H. et al. Bis(imino)pyridine cobalt-catalyzed dehydrogenative silylation of alkenes: scope, mechanism, and origins of selective allylsilane formation. J. Am. Chem. Soc. 136, 12108–12118 (2014). [DOI] [PubMed] [Google Scholar]
- Larionov E. et al. Scope and mechanism in palladium-catalyzed isomerizations of highly substituted allylic, homoallylic and alkenyl alcohols. J. Am. Chem. Soc. 136, 16882–16894 (2014). [DOI] [PubMed] [Google Scholar]
- Obligacion J. V. & Chirik P. J. Bis(imino)pyridine cobalt catalyzed alkene isomerization-hydroboration: a strategy for remote hydrofunctionalization with terminal selectivity. J. Am. Chem. Soc. 135, 19107–19110 (2013). [DOI] [PubMed] [Google Scholar]
- Wu L. et al. Ruthenium-catalyzed hydroformylation/reduction of olefins to alcohols: extending the scope to internal alkenes. J. Am. Chem. Soc. 135, 14306–14312 (2013). [DOI] [PubMed] [Google Scholar]
- Yuki Y. et al. Tandem isomerization/hydroformylation/hydrogenation of internal alkenes to n-alcohols using Rh/Ru dual- or ternary-catalyst systems. J. Am. Chem. Soc. 135, 17393–17400 (2013). [DOI] [PubMed] [Google Scholar]
- Kochi T. et al. Chain-walking strategy for organic synthesis: catalytic cycloisomerization of 1,n-dienes. J. Am. Chem. Soc. 134, 16544–16547 (2012). [DOI] [PubMed] [Google Scholar]
- Grotjahn D. B. et al. Extensive isomerization of alkenes using a bifunctional catalyst: an alkene zipper. J. Am. Chem. Soc. 129, 9592–9593 (2007). [DOI] [PubMed] [Google Scholar]
- Guan Z. et al. Chain-walking: a new strategy to control polymer topology. Science 283, 2059–2062 (1999). [DOI] [PubMed] [Google Scholar]
- Vasseur A., Bruffaerts J. & Marek I. Remote functionalization through alkene isomerization. Nat. Chem. 8, 209–219 (2016). [DOI] [PubMed] [Google Scholar]
- Larionov E., Li H. & Mazet C. Well-defined transition metal hydrides in catalytic isomerizations. Chem. Commun. 50, 9816–9826 (2014). [DOI] [PubMed] [Google Scholar]
- Hermann W. A. & Prinz M. in Applied Homogeneous Catalysis with Organometallic Compounds 2nd edn Wiley-VCH (2002). [Google Scholar]
- Souillart L. & Cramer N. Catalytic C–C bond activations via oxidative addition to transition metals. Chem. Rev. 115, 9410–9469 (2015). [DOI] [PubMed] [Google Scholar]
- Marek I. et al. Selective carbon-carbon bond cleavage for the stereoselective synthesis of acyclic systems. Angew. Chem. Int. Ed. 54, 414–429 (2015). [DOI] [PubMed] [Google Scholar]
- Murakami M. & Matsuda T. Metal-catalysed cleavage of carbon–carbon bonds. Chem. Commun. 47, 1100–1105 (2011). [DOI] [PubMed] [Google Scholar]
- Rybtchinski B. & Milstein D. Metal insertion into C–C bonds in solution. Angew. Chem. Int. Ed. 38, 870–883 (1999). [DOI] [PubMed] [Google Scholar]
- Vasseur A. et al. Remote functionalization of hydrocarbons with reversibility enhanced stereocontrol. Chem. Sci. 6, 2770–2776 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masarwa A. et al. Merging allylic carbon-hydrogen and selective carbon-carbon activation. Nature 505, 199–2013 (2014). [DOI] [PubMed] [Google Scholar]
- Chinkov N., Levin A. & Marek I. Unsaturated fatty alcohols derivatives as a source of substituted allylzirconocenes. Angew. Chem. Int. Ed. 45, 465–468 (2006). [DOI] [PubMed] [Google Scholar]
- Chinkov N., Majumdar S. & Marek I. Stereoselective preparation of dienyl zirconocene complexes via a tandem allylic C–H bond activation-elimination sequence. J. Am. Chem. Soc. 125, 13258–13264 (2003). [DOI] [PubMed] [Google Scholar]
- Beletskaya I. P. & Cheprakov A. V. The Heck reaction as a sharpening stone of palladium catalysis. Chem. Rev. 100, 3009–3066 (2000). [DOI] [PubMed] [Google Scholar]
- Franzoni I. & Mazet C. Recent trends in Pd-catalyzed remote functionalization of carbonyl compounds. Org. Biomol. Chem. 12, 233–241 (2012). [DOI] [PubMed] [Google Scholar]
- Melpolder J. B. & Heck R. F. A palladium-catalyzed arylation of allylic alcohols with aryl halides. J. Org. Chem. 41, 265–272 (1976). [Google Scholar]
- Larock R. C., Leung W.-Y. & Stoltz-Dunn S. Synthesis of aryl-substituted aldehydes and ketones via palladium-catalyzed coupling of aryl halides and non-allylic unsaturated alcohols. Tetrahedron Lett. 30, 6629–6632 (1989). [Google Scholar]
- Werner E. W. et al. Enantioselective Heck arylations of acyclic alkenyl alcohols using a redox-relay strategy. Science 338, 1455–1458 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C. et al. Enantioselective dehydrogenative Heck arylations of trisubstituted alkenes with indoles to construct quaternary stereocenters. J. Am. Chem. Soc. 137, 15668–15671 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel H. H. & Sigman M. S. Palladium-catalyzed enantioselective Heck arylation of acyclic alkenols using a redox-strategy. J. Am. Chem. Soc. 137, 3462–3465 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C. et al. Alkenyl carbonyl derivatives in enantioselective redox relay Heck reactions: accessing α,β-unsaturated systems. J. Am. Chem. Soc. 137, 7290–7293 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin M., Rubina M. & Gevorgyan V. Transition metal chemistry of cyclopropenes and cyclopropanes. Chem. Rev. 107, 3117–3179 (2007). [DOI] [PubMed] [Google Scholar]
- Lebel H. et al. Stereoselective cyclopropanation reactions. Chem. Rev. 103, 977–1050 (2003). [DOI] [PubMed] [Google Scholar]
- Müller S. D. & Marek I. Asymmetric copper-catalyzed carbozincation of cyclopropenes en route to the formation of diastereo- and enantiomerically enriched polysubstituted cyclopropanes. J. Am. Chem. Soc. 137, 15414–15417 (2015). [DOI] [PubMed] [Google Scholar]
- Marek I., Simaan S. & Masarwa A. Enantiomerically enriched cyclopropene derivatives: versatile building blocks in asymmetric catalysis. Angew. Chem. Int. Ed. 46, 7364–7376 (2007). [DOI] [PubMed] [Google Scholar]
- Didier D. et al. Modulable and highly diastereoselective carbometalation of cyclopropenes. Chem. Eur. J. 20, 1038–1048 (2014). [DOI] [PubMed] [Google Scholar]
- Quasdorf K. W. & Overman L. E. Catalytic enantioselective synthesis of quaternary carbon stereocentres. Nature 516, 181–191 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marek I. et al. All-carbon quaternary stereogenic centers in acyclic systems through the creation of several C–C bonds per chemical steps. J. Am. Chem. Soc. 136, 2682–2694 (2014). [DOI] [PubMed] [Google Scholar]
- Das J. P. & Marek I. Enantioselective synthesis of all-carbon quaternary stereogenic centers in acyclic systems. Chem. Commun. 47, 4593–4623 (2011). [DOI] [PubMed] [Google Scholar]
- Morandi B. & Carreira E. M. Rhodium-catalyzed cyclopropenation of alkynes: synthesis of trifluoromethyl-substituted cyclopropanes. Angew. Chem. Int. Ed. 49, 4294–4296 (2010). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary figures, supplementary tables, supplementary note, supplementary methods and supplementary references.
Data Availability Statement
The X-ray crystallographic coordinates for structure of compound 4 reported in this article have been deposited at the Cambridge Crystallographic Data Centre (CCDC) with the accession code CCDC 1500847. This data can be obtained free of charge from the Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif. The authors declare that all other data supporting the findings of this study are available within the article and Supplementary Information files, and also are available from the corresponding author upon reasonable request.