Abstract
The relationship between outer hair cell (OHC) loss and cochlear sensitivity is still unclear, because in many animal models there exist surviving but dysfunctional OHCs and also injured/dead inner hair cells (IHC). Styrene is an ototoxic agent, which targets and destroys OHCs starting from the third row to the second and first rows depending on the exposure level. The remaining cells may be less affected. In this experiment, rats were exposed to styrene by gavage at different doses (200–800 mg/kg/day) for varying periods (5 days/week for 3–12 weeks). An interesting finding was that the cochlear sensitivity was not affected in a few rats with all OHCs in the third row being destroyed by styrene. A further loss of OHCs was usually accompanied with a linear input/output (I/O) function of cochlear compound action potentials (CAP), indicating the loss of cochlear amplification. However, normal CAP amplitudes at the highest stimulation level of 90 dB SPL were often observed when all OHCs were destroyed, indicating normal function of the remaining IHCs. The OHC-loss/hearing-loss relation appeared to be a sigmoid-type function. Initially, styrene-induced OHC losses (<33%) did not result in a significant threshold shift. Then CAP threshold shift increased dramatically with OHC loss from 33% to 66%. Then, CAP threshold changed less with OHC loss. The data suggest a tri-modal relationship between OHC loss and cochlear amplification. That is, under the condition that all surviving OHCs are ideally functioning, the cochlear amplifier is not affected until 33% of OHCs are absent, then the gain of the amplifier decreases proportionally with the OHC loss, and at last the amplifier may fail completely when more than 67% of OHCs are lost.
Keywords: outer hair cell, cochlear amplification, hearing loss, styrene ototoxicity, rat
1. Introduction
Outer hair cells (OHCs) are known to play an essential role in the cochlear active process or cochlear amplification. The cells contribute to the cochlear amplifier via their length changes which is termed electromotility (Ashmore, 1987; Brownell et al., 1985; Dallos, 1992; Kachar et al., 1986; Zhao and Santos-Sacchi, 1999). Cochlear amplification increases cochlear sensitivity by about 40 dB as reflected in compound action potential (CAP) input/output (I/O) functions in rats and guinea pigs (Chen, 2006; Chen and Liu, 2005; Chen and Zhao, 2007). Loss of OHC electromotility due to the absence of the OHC motor protein, prestin, was also reported to result in a greater than 40 dB cochlear sensitivity loss in mice (Liberman et al., 2002). However, the relationship between OHC loss and hearing loss is inconsistent, in part because of the potential injuries to surviving OHCs or the death of inner hair cells (IHCs). For example, a noise-induced permanent threshold shift (PTS) up to about 40 dB has been observed in many experimental animals, including rats, mice, gerbils, guinea pigs, and rabbits, without significant hair cell loss in the low to middle frequency region (Ahn et al., 2005; Borg, 1987; Borg and Engstrom, 1989; Borg et al., 1995; Chen, 2002, 2006; Chen et al., 1999, 2001; Chen and Fechter, 2003; Chen and Zhao, 2007; Lataye et al., 2000; Murashita et al., 2006; Ohlemiller et al., 2000; Vicente-Torres and Schacht, 2006; White et al., 1998 Yamasoba et al., 1999). It has been shown that the first 40-dB noise-induced PTS mainly results from loss of cochlear amplification (Chen, 2006; Chen and Liu, 2005; Chen and Zhao, 2007). The loss of cochlear amplification without OHC loss suggests that the remaining OHCs may be injured and/or dysfunctional. In contrast, noise-induced OHC loss was observed in the chinchilla with less than 5 dB PTS (Hamernik et al., 1989). However, noise-induced PTS, starting from less than 10 dB, was associated with not only OHC loss but also IHC loss (Hamernik et al., 1989).
Styrene is one of the most ototoxic industrial solvents (Gagnaire and Langlais, 2005). It targets OHCs as well as Deiters cells (DCs) starting from the third row (Campo et al., 2001; Chen et al., 2007; Gagnaire and Langlais, 2005; Lataye et al., 2000, 2001, 2003; Loquet et al., 1999, 2000; Makitie et al., 2003). IHCs are relatively insensitive to styrene exposure. Previously, we have observed that, even in cochleae in which all OHCs were missing, the maximal CAP amplitude was still at the normal level, indicating that the remaining IHCs were still functioning after the loss of all OHCs. Interestingly, in some rats, styrene-induced OHC loss up to a certain level (1/3) did not affect cochlear function, indicating that the remaining OHCs were not affected by the death of their neighbors and still functioned normally. Thus, the styrene-injured ear may be an appropriate model with which to study the relation between OHC loss and cochlear amplification. This study examined the relation between styrene-induced OHC loss and CAP threshold shift. The data suggest a tri-modal relation between OHC loss and cochlear amplification.
2. Methods
2.1. Subjects
Fifty Long Evans pigmented rats (male, 300–350 g) were acquired from Harlan Sprague Dawley Inc. and housed in the State University of New York (SUNY) at Buffalo animal facility after delivery. All animal facilities are registered with the US Department of Agriculture and are inspected semiannually by the members of the Institutional Animal Care and Use Committee (IACUC) serving the Research Foundation of SUNY. Background noise level in the colony room was 45 dBA. Temperature was maintained at 71°F. Lights were on from 6:00 am to 6:00 pm. All procedures regarding the use and handling of animals and styrene exposures were reviewed and approved by the IACUC.
2.2. Styrene exposure
To obtain different degrees of styrene-induced cochlear damage, thirty-five rats were exposed to styrene by oral gavage once a day for 5 days per week for varying periods and doses: 200 mg/kg/d for 12 wks (n=6), 300 mg/kg/d for 3 wks (n=6), 400 mg/kg/d for 3 (n=6) or 6 wks (n=6), and 800 mg/kg/d for 3 wks (n=11). Exposure to styrene at doses of 200, 400, and 800 mg/kg was shown to produce blood levels of 6.0, 9.8, and 20.7 µg/ml respectively (Chen et al., 2007). Six rats, treated with styrene at a dosage of 100 mg/kg for 24 weeks, were not included in this analysis because there was no hearing loss or hair cell loss. Nine rats were treated with olive oil as controls for styrene-induced threshold shift determination.
2.3. Cochlear functional assessment
Styrene-induced auditory threshold shifts were determined by CAP recording 5 days after the last day of styrene exposure. The styrene-induced hearing loss was permanent, since it has been observed that hearing loss developed by styrene exposure longer than 2 weeks did not continue to change with time, worsen or recover (Campo et al., 2001; Chen et al., 2007). The shortest treatment period in this experiment was 3 weeks.
Rats were deeply anesthetized with ketamine (50 mg/kg, i.m.) and xylazine (6 mg/kg, i.m.). The right cochlea was surgically exposed using a ventro-lateral approach and a silver wire electrode was carefully placed on the round window for recording of cochlear responses. A silver chloride reference electrode was placed in the neck muscles. Tone bursts at different frequencies (2, 6, 8, 12, 16, 20, 24, 30, and 40 kHz) were generated in a real time processor (Tucker-Davis Technologies (TDT) RP2.1, system-3, TDT, Alachua, FL). The signals with 10-ms duration and 1-ms rise/fall times were attenuated by a TDT PA5 programmable attenuator and then amplified using a high voltage amplifier (HVA-1,Wilsonics, Inc.) and transduced by the 1/2" microphone (ACO 7013, ACO Pacific, Inc., Belmont, CA) placed within a speculum that opened to the eardrum. Sound levels at the position of the eardrum were calibrated at all the test frequencies using a 1/8" microphone (4138 B&K) using an artificial chamber. The cochlear potentials were amplified with a DAM50 Bio-amplifier (World Precision Instruments, Sarasota, FL). The gain of the preamplifier was set at 1000, and the band of the filter was set from 0.1 Hz to 3 kHz. The cochlear responses were averaged 50 times using the real time processor, stored in a computer, and analyzed using MATLAB 6.1 (The Mathworks Inc., Natick, MA). Amplitudes of CAP (N1) were measured and plotted as a function of stimulation level, known as the I/O function. CAP thresholds were obtained from the CAP I/O functions at 5µV level (i.e., the stimulation level which induced a 5-µV response). Threshold shift in each animal was obtained by subtracting the mean CAP threshold in the control animals from the measured animal.
2.4. Hair cell counting and examination of the organ of Corti
2.4.1. Hair cell counting
The deeply anesthetized animals were decapitated after CAP recording and the cochleae were removed immediately. The round and oval windows and the apex of the cochlea were opened to facilitate the perfusion. The cochleae were perfused with an incubation solution containing 0.05% tetranitro blue tetrazolium (TNBTZ), 0.05M sodium succinate, and 0.05M phosphate buffer and then incubated in the solution for 1 h (37°C). TNBTZ is an electron acceptor that, on reduction, precipitates as an insoluble and highly colored formazan. Succinate dehydrogenese (SDH) in the cell oxidizes sodium succinate and provides electrons for the reduction of the electron acceptor. Thus, the cell is colored. The stained cochleae were fixed in 10% buffered formalin for 2 days. After fixation, the cochleae were decalcified in 7% ethylenediamine tetraacetic acid (EDTA) solution for 3 days or longer as needed. Cochlear micro-dissection was accomplished under a light microscope. Hair cells within each section of 250µ length on the basilar membrane were counted under a light microscope (DMBA300 Digital Microscope, Microscope World). The counted cell numbers were compared to the normal numbers obtained in a group of control rats and cell losses were expressed as %. Cell losses were plotted as a function of % distance from the cochlear apex (cochleograms). Representative photomicrographs were captured using the microscope’s build-in camera (DMBA300 Digital Microscope, 400×) with the provided software (Motic Image Plus 2.0 ML).
2.4.2. Examination of the organ of Corti
Some of the cochleae were directly fixed in the 10% buffered formalin for 12 h before examining the organ of Corti for damage to its cellular components. The fixed cochleae were transferred into phosphate buffered saline (PBS). The organ of Corti was dissected out from the bony shell of the cochlea, and stained with fluorescein isothiocyanate (FITC) labeled phalloidin (Sigma) at a concentration of 5 µg / 1 ml PBS containing 0.25% Triton-X-100 and 1% bovine serum albumin for 30 min at room temperature. The tissue was then co-labeled with propidium iodide (PI, Sigma) at a concentration of 5 µg / 1 ml PBS containing 0.25% Triton-X-100 and 1% bovine serum albumin for 10 minutes. The stained specimen was mounted on a slide with ProLong Gold antifade reagent (Invitrogen Molecular Probes) and examined using the Carl Zeiss Laser Scanning Systems LSM 510. Images were captured and analyzed using Zeiss LSM Image Examiner (v. 4,0,0,91).
2.5. Statistical analysis
For analysis of the relation between hearing loss and OHC loss, nonlinear regression (fifth order polynomial) and linear regression analyses were performed using GraphPad Prism software (v. 5.01). For comparisons of hair cell loss or hearing loss between groups a two-way ANOVA was performed using the above-noted commercial software package. A p-value < 0.05 was considered to be statistically significant.
3. Results
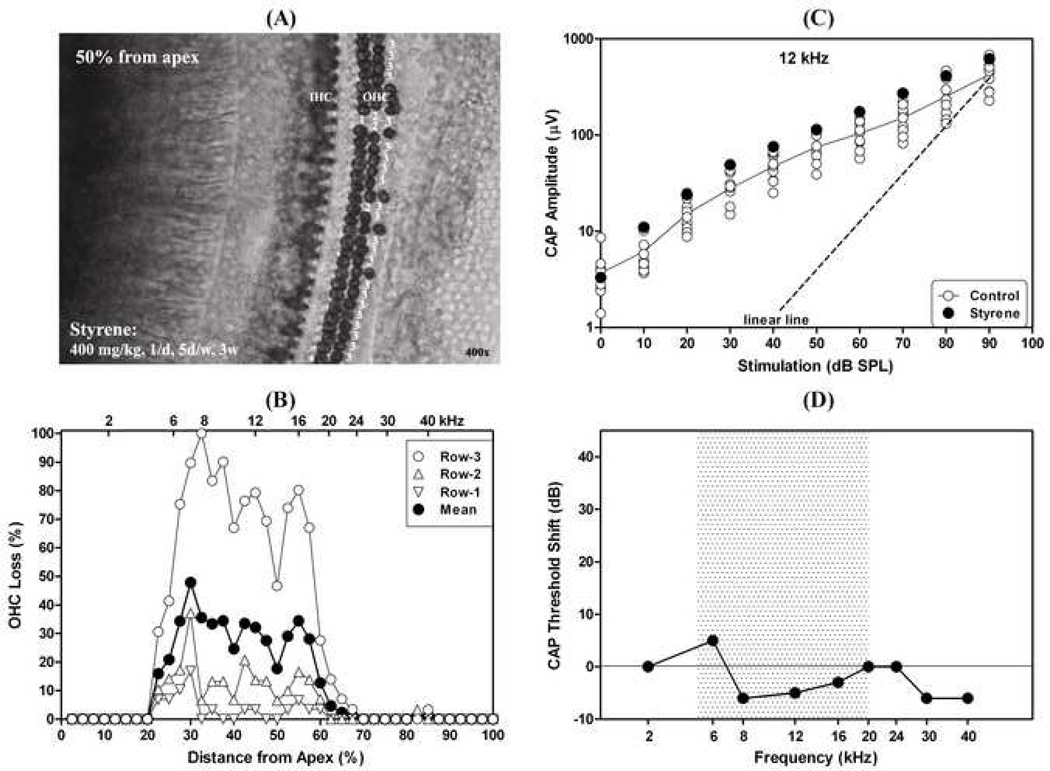
Figure 1 presents an example showing styrene-induced OHC loss, but without hearing loss. The rat was exposed to styrene by gavage for 3 weeks at a dose of 400 mg/kg (1/day; 5 days/week). Cochlear function and hair cells were examined 5 days after the last day of the styrene treatment. The styrene exposure destroyed almost all of the OHCs in the third row and a few OHCs in the second and first rows (Fig. 1A, right cochlea). The lesion occurred in the mid-frequency region (about 20–60% distance from the apex, Fig. 1B). Although the third-row OHCs were missing, the CAP I/O function at 12 kHz (filled circles in Fig. 1C), related to the location in the center of the lesion area (45% from the apex (Muller, 1991)), was at the control levels (opened circles, Fig. 1C). CAP I/O functions at other frequencies were also at the control levels (not shown). Figure 1D presents CAP threshold shifts at different frequencies compared to the control group. There was no CAP threshold shift at any frequency. The normal cochlear responses (Fig. 1C and D) indicate that the remaining OHCs in this animal were functioning and sufficient to maintain normal cochlear amplification.
Fig. 1.
An example showing styrene-induced OHC loss in the third row without effect on cochlear sensitivity. (A): Surface preparation from the middle turn (about 50% distance from the apex) in the cochlea (right). Missing OHCs in the third row are marked by “3”, in the second row by “2”, and in the first row by “1”; (B): Missing OHCs by row as a function of % distance from the cochlear apex; (C): CAP I/O function at 12 kHz (filled circles), which is related to the center of the injured region (45% from the apex). Opened circles are controls. The dashed line at linear I/O (i.e., 1-dB increase of stimulation yields 1-dB increase in CAP amplitude); (D): CAP threshold shift as a function of frequency. The shaded area indicates the injured region in this cochlea. Styrene exposure: by gavage at a dose of 400 mg/kg, 1/day for 5 days per week for 3 weeks.
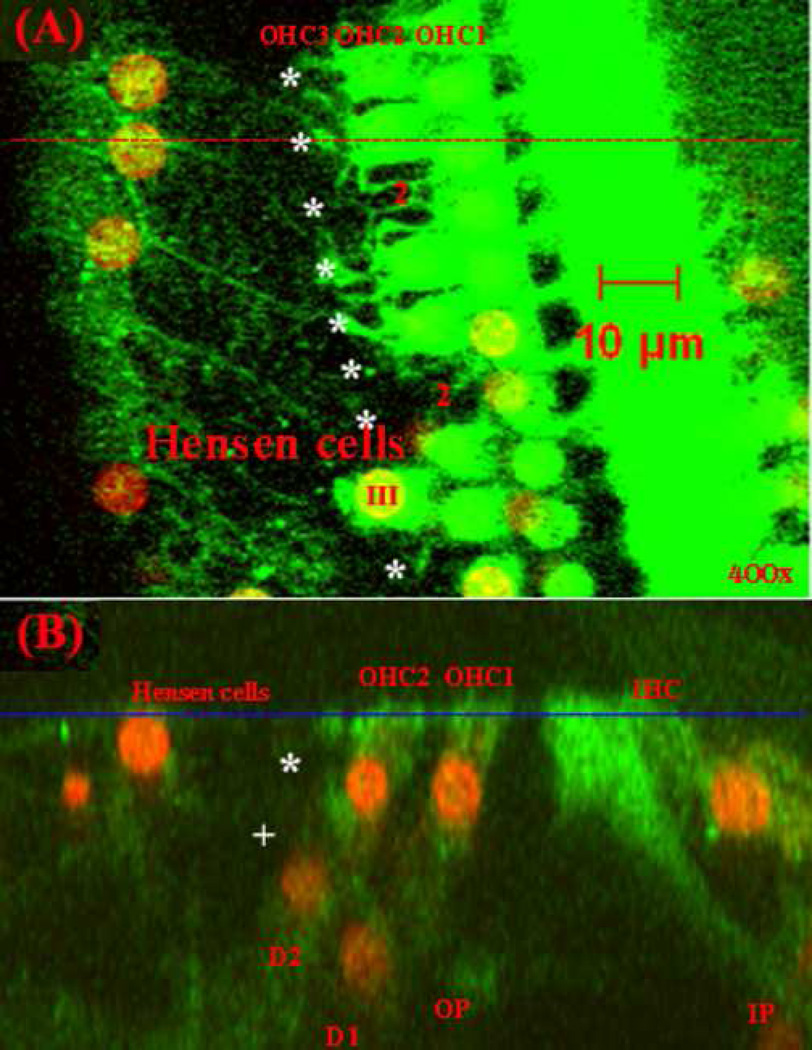
The left cochlea of this rat was double-stained with FITC-labeled phalloidin (green, showing F-actin) and PI (red, showing nuclei) for further examination of the styrene-induced injuries in the organ of Corti. Figure 2 presents confocal images captured at the center of the lesion area in the cochlea (about 50% from the apex). A similar number of OHCs were absent as in the right cochlea. The majority of OHCs in the third row were destroyed (see asterisks in Fig. 2A). Two OHCs in the second row were also missing (marked by “2”). The damaged tissue appeared to be covered with expanded Hensen cells. Figure 2B is a sectional image of the organ of Corti at the position indicated by the red dashed line in Figure 2A. The OHCs and the related DCs in the third row were missing (indicated with asterisk and plus, respectively). Although the OHCs and DCs in the third row were destroyed, the remaining cells in the organ of Corti, including OHCs (OHC1 and OHC2), DCs (D1 and D2), outer pillar cells (OPs), inner pillar cells (IPs), and IHCs, all remained in their appropriate positions. Thus, the system appeared to still be working, although the third row was absent.
Fig. 2.
Images of the organ of Corti from the middle turn (50% from the apex) in the left cochlea of the same animal as in Figure 1, showing missing OHCs and Deiters cells (DCs) and expanded Hensen cells covering the injured tissue. (A): The confocal image at the level of the reticular lamina (indicated by the blue line in B); (B): The sectional image at the position indicated by the red line in (A).
Green: labeled actin; Red: labeled DNA; Missing OHCs in the third row are marked with “*” and the remaining OHC is marked with “III”; Missing OHCs in the second row are marked with “2”; The missing DC is marked with “+”; OHC1, OHC2, OHC3: OHCs in the first, second, and third row; D1 and D2: Deiters cells in the first and second row; OP: outer pillar cell; IP: inner pillar cell.
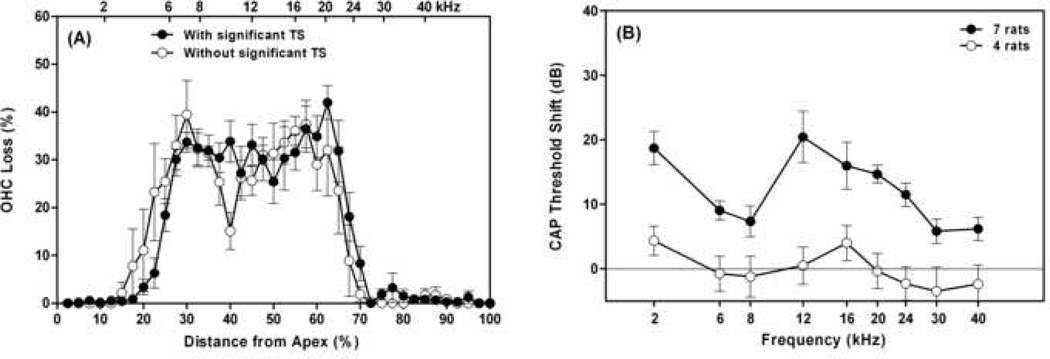
Eleven subjects from the 35 rats in this report showed styrene-induced OHC loss in the third row (33% loss) in the middle turn similar to that shown in Figure 1. Four of the eleven did not show significant functional loss (comparing open circles in Fig. 3A and B). The mean OHC loss in these animals in the lesion area (25–65% from the apex) was 29.9% (SE=1.4%) and the mean PTS in the related frequency-region of 6–20 kHz was only 0.4 dB (SE=0.9 dB). It appeared that the remaining 67% of OHCs in these animals were functioning and sufficient to maintain normal cochlear amplification. Seven of the eleven rats, however, showed threshold shifts (comparing filled circles in Fig. 3A and B), which were significantly higher than that of the other four rats (p<0.0001). The mean OHC loss in these 7 animals in the lesion area (25–65% from the apex) was 31.4% (SE=1.2%) and the mean PTS in the related frequency-region (6–20 kHz) was 13.5 dB (SE=2.4 dB). These differences may be due to subtle and unrecognized injuries to the remaining OHCs.
Fig. 3.
Different effects of styrene-induced OHC loss in the third row on cochlear sensitivity: without reduction of cochlear sensitivity in some rats (opened circles), but with threshold loss in some other rats (filled circles). (A) OHC losses as a function of % distance from the cochlear apex; (B) CAP threshold shifts as a function of frequency. Vertical bars are standard errors (SEs).
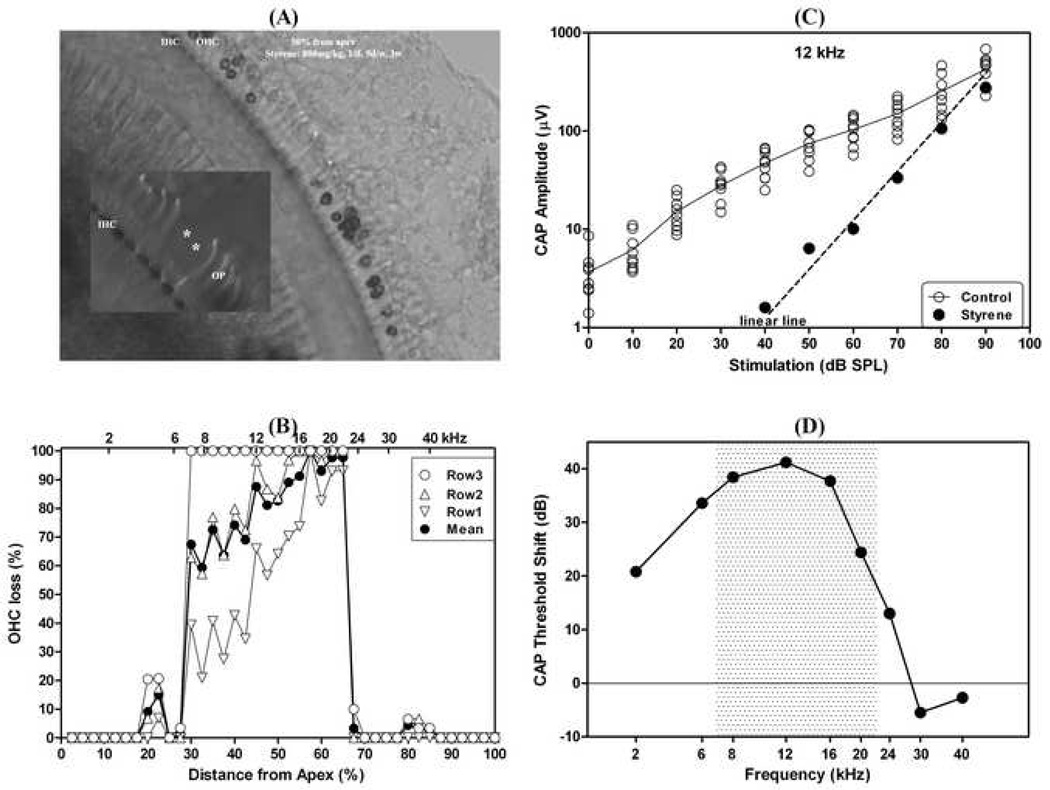
Figure 4 presents an example showing severe OHC losses (60–100%, see Fig. 4A and B), resulting in threshold shifts up to 40 dB. However, there was only a slight CAP reduction at the highest stimulation level of 90 dB SPL (Fig. 4C). This rat was exposed to styrene by gavage for 3 weeks at a dose of 800 mg/kg (1/day; 5 days/week). The styrene exposure in this rat destroyed all OHCs in the third row, 60–100% of the OHCs in the second row, and 30–100% of the OHCs in the first row in different locations within the region of about 30–70% from the apex (Fig. 4A and B). A detailed examination in this cochlea showed that the related DCs and some of the OPs were also destroyed, but the IHCs had normal looking nuclei (not shown). The IHCs had heavy SDH staining even in the severely damaged region where all OHCs and OPs were destroyed (see asterisks in the insert in Fig. 4A). Figure 4C presents the CAP I/O function of this ear at 12 kHz (filled circles), which is related to the cochlear location in the center of the lesion area (45% from the apex (Muller, 1991)). The CAP amplitudes are coincident with the linear line (i.e., 1-dB increase of stimulation yields a 1-dB increase of CAP amplitude), indicating a complete loss of cochlear amplification. The CAP amplitude at the highest stimulation level of 90 dB SPL was in the control range (see open circles). The unchanged maximal CAP amplitude indicated that the IHCs and the related nerve fibers were functioning well, although all OHCs, DCs, and even some of the OPs were destroyed. Figure 4D presents CAP threshold shifts as a function of frequency.
Fig. 4.
An example showing 60–100% OHC loss after styrene exposure with an up to 40 dB threshold shift, but without a significant reduction in maximal CAP amplitude at the highest stimulation level of 90 dB SPL. (A): Surface preparation from the middle turn (about 50% from the apex) with less than 20 OHCs remained. The insert shows IHCs in the region with losses of outer pillar cells (marked by asterisks); (B): OHC losses as a function of % distance from the cochlear apex; (C): CAP I/O function at 12 kHz (filled circles), which is related to the center of the injured area. The CAP amplitudes are consistent with the linear I/O line (dashed line), with an about 40-dB threshold shift, but without a significant reduction in the maximal CAP amplitude at the high stimulation level (90 dB SPL). Opened circles are controls; (D): CAP threshold shifts as a function of frequency. The shaded area indicates the injured region in this cochlea. Styrene exposure: 800 mg/kg by gavage, 1/day, 5 days/week, for 3 weeks.
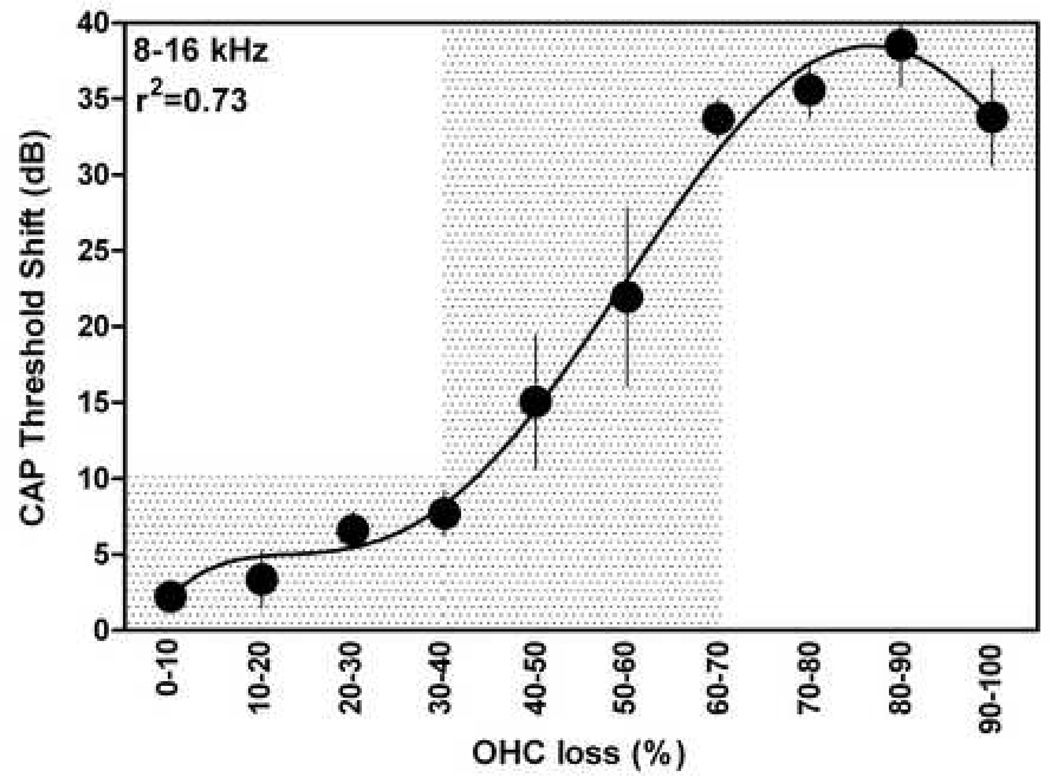
In order to understand the relationship between styrene-induced hearing loss and hair cell loss, CAP threshold shifts at different frequencies were plotted as a function of OHC losses in the related regions. Since styrene-induced OHC loss was restricted to the mid-frequency region, only CAP threshold shifts at frequencies ranging from 8 to 16 kHz were included in the data analysis for showing the OHC-loss/hearing-loss relationship. Other frequencies were not included because they were related to the regions on the edges of the lesion area (6 and 20 kHz) or out of the area (2, 24, 30, and 40 kHz, see example in Fig. 3A). CAP threshold shifts were grouped based on OHC loss in their related cochlear regions, averaged, and plotted as a function of OHC loss in Figure 5. The threshold shifts as a function of OHC loss are analyzed using the fifth order polynomial nonlinear curve fitting (Prism, version 5.01). It appeared that CAP threshold shift increases slightly when OHC loss is < 30–40%. Then the threshold shift increases dramatically to about 40 dB with the increases in OHC loss. Finally, exceeding a certain level (60–70%), OHC loss had less effect on the cochlear sensitivity.
Fig. 5.
Styrene-induced TSs as a function of OHC loss over three rows. Only CAP TSs at 8, 10, 12, and 16 kHz are included in the analysis. TS: threshold shift. Vertical bars are standard errors (SEs).
4. Discussion
It is well known that OHCs play an essential role in the cochlear active process via their electromotility, which enhances responses via phenomenon known as cochlear amplification (Ashmore, 1987; Brownell et al., 1985; Dallos, 1992; Kachar et al., 1986; Zhao and Santos-Sacchi, 1999). Based on CAP I/O functions in rats and guinea pigs, the cochlear amplifier provides a stimulation level dependent gain of up to about 40 dB (Chen and Liu, 2005; Chen, 2006; Chen and Zhao, 2007). It has been reported that absence of OHC electromotility due to a prestin gene knockout in mice resulted in a loss of 40–60 dB in cochlear sensitivity (Liberman et al., 2002). However, the relationship between OHC loss and cochlear amplification is still unclear. How many OHCs does the normal cochlear amplifier require? Is the cochlear amplifier an all-or-none amplifier? This is an important knowledge for planning hearing therapy in those patients with a certain number of remaining but dysfunctional OHCs.
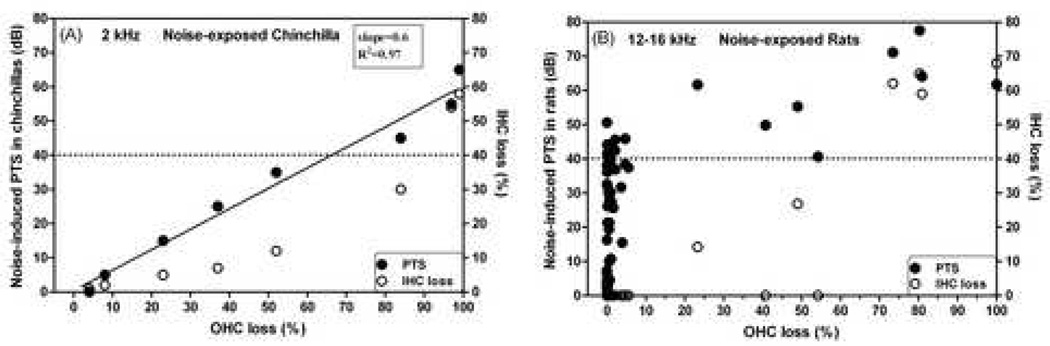
In the chinchilla, noise-induced hearing loss (NIHL) increases with OHC loss (see filled circles and solid line in Fig. 6A, modified from Hamernik et al. (1989)). When all OHCs were missing, a threshold shift of about 60 dB was observed. The linear OHC-loss/hearing-loss relationship suggests that the cochlear amplifier is not an all-or-none amplifier. The gain of the cochlear amplifier appeared to be proportional to the number of remaining OHC. However, IHC loss was also observed (see open circles in Fig. 6A), which would affect cochlear sensitivity as well.
Fig. 6.
Noise-induced PTS as a function of OHC loss. (A): in the chinchilla modified from Hamernik et al. (1989); (B): in the rat modified from Chen and Fechter (2003). Filled circles: PTS; Open circles: IHC loss.
In contrast, as shown by the solid circles in Fig 6B, which is modified from Chen and Fechter (2003), NIHL in the rat up to 40 dB was not related to OHC loss. Significant OHC loss (>5%) or IHC loss (open circles) was observed only when PTS exceeded 40 dB. However, the noise-induced PTS of 40 dB was shown to mainly result from loss of cochlear amplification (Chen and Liu, 2005; Chen, 2006; Chen and Zhao, 2007). The hearing loss was proposed to result from OHC dysfunction. Uncoupling of hair-cell stereocilia from the tectorial membrane may also be a possible mechanism for the loss of cochlear amplification (Flock et al, 1999; Nordmann et al, 2000). The noise-injured and dysfunctional OHCs in rats may survive. However, the styrene-induced injuries in OHCs usually lead to cell death, because the injuries trigger apoptosis (Chen et al., 2007).
OHCs are known to be much more susceptible than IHCs to ototoxic industrial solvents, such as styrene (Campo et al., 2001; Chen et al., 2007; Gagnaire and Langlais, 2005; Lataye et al., 2000, 2001, 2003; Loquet et al., 1999, 2000; Makitie et al., 2003). Our previous study showed that styrene exposure targeted OHCs and the related DCs and caused apoptosis, programmed cell death, which is believed to minimize the harmful effect of the cell death on neighboring cells (Chen et al., 2007). Furthermore, the styrene exposure targeted OHCs and DCs starting from the third row (Chen et al., 2007). Hensen cells appeared to be less sensitive to styrene and played an important role in tissue healing in the styrene-damaged organ of Corti. After an OHC was destroyed, Hensen cells appeared to expand to cover the wounded tissue (see Fig. 2A,B), which probably provided protection for the remaining cells against harmful secondary effects. The programmed cell death pathway (apoptosis) and the ordered OHC death from the third row to the first row and with an immediate tissue healing mechanism may prevent damage to the remaining OHCs and the IHCs. In a few cases, losses of OHCs and DCs in the third row were observed without an effect on the cochlear amplification (see Fig. 1). Although it is unclear whether or not all of the remaining 67% of OHCs in the cochlea were normally functioning, it is certain that 67% of functioning OHCs are sufficient to maintain normal cochlear amplification. In some other cases, OHC loss in the third row was observed with hearing loss (see filled circles in Fig. 3). We believe that in those cases the styrene exposure caused injuries in some of the remaining OHCs leading to the functional loss. OHC loss exceeding 1/3 appeared to affect cochlear amplification. The gain appeared to decline with OHC loss. A complete loss of cochlear amplification occurred when 2/3 of OHCs were missing. We propose a tri-modal relation between OHC loss and cochlear amplification. That is, under the condition that all surviving OHCs are ideally functioning, the cochlear amplifier is not affected until 1/3 of OHCs are missing, then the gain of the amplifier decreases proportionally with the OHC loss, and at last the amplifier may fail completely when more than 2/3 of OHCs are lost. It is still unclear whether or not 1/3 of healthy OHCs provide some cochlear amplification, because we do not know whether or not the remaining 1/3 of OHCs in the styreneinjured cochlea are functioning.
Acknowledgments
This study is supported by NIOSH grant 1R01OH008113-01A1. The authors thank Eric Bielefeld for his comments and editorial help.
Abbreviations
- CAP
compound action potential
- DC
Deiters cell
- FITC
fluorescein isothiocyanate
- NIHL
noise-induced hearing loss
- I/O
input/output
- IHC
inner hair cell
- IP
inner pillar cell
- OHC
outer hair cell
- OP
outer pillar cell
- PBS
phosphate buffer saline
- PTS
permanent threshold shift
- SDH
succinate dehydrogenese
- TNBTZ
tetranitro blue tetrazolium
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahn JH, Kang HH, Kim YJ, Chung JW. Anti-apoptotic role of retinoic acid in the inner ear of noise-exposed mice. Biochem. Biophys. Res. Commun. 2005;335:485–490. doi: 10.1016/j.bbrc.2005.07.114. [DOI] [PubMed] [Google Scholar]
- Ashmore JF. A fast motile response in guinea pig outer hair cells: the cellular basis of the cochlear amplifier. J. Physiol. (Lond) 1987;388:323–347. doi: 10.1113/jphysiol.1987.sp016617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borg E. Loss of hair cells and threshold sensitivity during prolonged noise exposure in normotensive albino rats. Hear. Res. 1987;30:119–126. doi: 10.1016/0378-5955(87)90129-8. [DOI] [PubMed] [Google Scholar]
- Borg E, Engstrom B. Noise level, inner hair cell damage, audiometric features, and equal-energy hypothesis. J. Acoust. Soc. Am. 1989;86:1776–1782. doi: 10.1121/1.398609. [DOI] [PubMed] [Google Scholar]
- Borg E, Canlon B, Engstrom B. Noise-induced hearing loss: Literature review and experiments in rabbits. Scand. Audiol. 1995;24(40):1–147. [PubMed] [Google Scholar]
- Brownell WE, Bader CR, Bertrand D, de Ribaupierre Y. Evoked mechanical responses of isolated cochlear outer hair cells. Science. 1985;227:194–196. doi: 10.1126/science.3966153. [DOI] [PubMed] [Google Scholar]
- Campo P, Lataye R, Loquet G, Bonnet P. Styrene-induced hearing loss: a membrane insult. Hear. Res. 2001;154:170–180. doi: 10.1016/s0378-5955(01)00218-0. [DOI] [PubMed] [Google Scholar]
- Chen GD. Effect of hypoxia on noise-induced auditory impairment. Hear. Res. 2002;172:186–195. doi: 10.1016/s0378-5955(02)00582-8. [DOI] [PubMed] [Google Scholar]
- Chen GD. Prestin gene expression in the rat cochlea following intense noise exposure. Hear. Res. 2006;222:54–61. doi: 10.1016/j.heares.2006.08.011. [DOI] [PubMed] [Google Scholar]
- Chen GD, Fechter LD. The relationship between noise-induced hearing loss and hair cell loss in rats. Hear. Res. 2003;177:81–90. doi: 10.1016/s0378-5955(02)00802-x. [DOI] [PubMed] [Google Scholar]
- Chen GD, Liu Y. Mechanisms of noise-induced hearing loss potentiation by hypoxia. Hear. Res. 2005;200:1–9. doi: 10.1016/j.heares.2004.08.016. [DOI] [PubMed] [Google Scholar]
- Chen GD, Zhao HB. Effects of intense noise exposure on the outer hair cell plasma membrane fluidity. Hear. Res. 2007;226:14–21. doi: 10.1016/j.heares.2006.06.007. [DOI] [PubMed] [Google Scholar]
- Chen GD, McWilliams ML, Fechter LD. Intermittent noise induced hearing loss and the influence of carbon monoxide. Hear. Res. 1999;138:181–191. doi: 10.1016/s0378-5955(99)00157-4. [DOI] [PubMed] [Google Scholar]
- Chen GD, Chi LH, Kostyniak PJ, Henderson D. Styrene induced alterations in biomarkers of exposure and effects in the cochlea: Mechanisms of hearing loss. Toxicol. Sci. 2007;98:167–177. doi: 10.1093/toxsci/kfm078. [DOI] [PubMed] [Google Scholar]
- Chen GD, Kong J, Reinhard K, Fechter LD. NMDA-receptor blocker protects against permanent NIHL but not its potentiation by CO. Hear. Res. 2001;154:108–115. doi: 10.1016/s0378-5955(01)00228-3. [DOI] [PubMed] [Google Scholar]
- Dallos P. The active cochlea. J. Neurosci. 1992;12:4575–4585. doi: 10.1523/JNEUROSCI.12-12-04575.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flock A, Flock B, Fridberger A, Scarfone E, Ulfendahl M. Supporting cells contribute to control of hearing sensitivity. J. Neurosci. 1999;19:4498–4507. doi: 10.1523/JNEUROSCI.19-11-04498.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagnaire F, Langlais C. Relative ototoxicity of 21 aromatic solvents. Arch. Toxicol. 2005;79:346–354. doi: 10.1007/s00204-004-0636-2. [DOI] [PubMed] [Google Scholar]
- Hamernik RP, Patterson JH, Turrentine GA, Ahroon WA. The quantitative relation between sensory cell loss and hearing thresholds. Hear. Res. 1989;38:199–212. doi: 10.1016/0378-5955(89)90065-8. [DOI] [PubMed] [Google Scholar]
- Kachar B, Brownell WE, Altschuler R, Fex J. Electrokinetic shape changes of cochlear outer hair cells. Nature. 1986;322:365–368. doi: 10.1038/322365a0. [DOI] [PubMed] [Google Scholar]
- Lataye R, Campo P, Loquet G. Combined effects of noise and styrene exposure on hearing function in the rat. Hear. Res. 2000;139:86–96. doi: 10.1016/s0378-5955(99)00174-4. [DOI] [PubMed] [Google Scholar]
- Lataye R, Campo P, Barthelemy C, Loquet G, Bonnet P. Cochlear pathology induced by styrene. Neurotoxicol. Teratol. 2001;23:71–79. doi: 10.1016/s0892-0362(00)00114-8. [DOI] [PubMed] [Google Scholar]
- Lataye R, Campo P, Pouyatos B, Cossec B, Blachere V, Morel G. Solvent ototoxicity in the rat and guinea pig. Neurotoxicol. Teratol. 2003;25:39–50. doi: 10.1016/s0892-0362(02)00326-4. [DOI] [PubMed] [Google Scholar]
- Liberman MC, Gao JG, He DZZ, Wu XD, Jia SP, Zuo J. Prestin is required for electromotility of the outer hair cell and for the cochlear amplifier. Nature. 2002;419:300–304. doi: 10.1038/nature01059. [DOI] [PubMed] [Google Scholar]
- Loquet G, Campo P, Lataye R. Comparison of toluene-induced and styrene-induced hearing losses. Neurotoxicol. Teratol. 1999;21:689–697. doi: 10.1016/s0892-0362(99)00030-6. [DOI] [PubMed] [Google Scholar]
- Loquet G, Campo P, Lataye R, Cossec B, Bonnet P. Combined effects of exposure to styrene and ethanol on the auditory function in the rat. Hear. Res. 2000;148:173–180. doi: 10.1016/s0378-5955(00)00151-9. [DOI] [PubMed] [Google Scholar]
- Makitie A, Pirvola U, Pyykko I, Sakakibara H, Riihimaki V, Ylikoski J. The ototoxic interaction of styrene and noise. Hear. Res. 2003;179:9–20. doi: 10.1016/s0378-5955(03)00066-2. [DOI] [PubMed] [Google Scholar]
- Muller M. Frequency representation in the rat cochlea. Hear. Res. 1991;51:247–254. doi: 10.1016/0378-5955(91)90041-7. [DOI] [PubMed] [Google Scholar]
- Murashita H, Tabuchi K, Hoshino T, Tsuji S, Hara A. The effects of tempol, 3-aminobenzamide and nitric oxide synthase inhibitors on acoustic injury of the mouse cochlea. Hear. Res. 2006;214:1–6. doi: 10.1016/j.heares.2005.12.008. [DOI] [PubMed] [Google Scholar]
- Nordmann AS, Bohne BA, Harding GW. Histopathological differences between temporary and permanent threshold shift. Hear. Res. 2000;139:13–30. doi: 10.1016/s0378-5955(99)00163-x. [DOI] [PubMed] [Google Scholar]
- Ohlemiller KK, McFadden SL, Ding DL, Lear PM, Ho YS. Targeted mutation of the gene for cellular glutathione peroxidase (Gpx1) increases noise-induced hearing loss in mice. JARO. 2000;1:243–254. doi: 10.1007/s101620010043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicente-Torres MA, Schacht J. A BAD link to mitochondrial cell death in the cochlea of mice with noise-induced hearing loss. J. Neurosci. Res. 2006;83:1564–1572. doi: 10.1002/jnr.20832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White DR, Boettcher FA, Miles LR, Gratton MA. Effectiveness of intermittent and continuous acoustic stimulation in preventing noise-induced hearing and hair cell loss. J. Acoust. Soc. Am. 1998;103:1566–1572. doi: 10.1121/1.421303. [DOI] [PubMed] [Google Scholar]
- Yamasoba T, Schacht J, Shoji F, Miller JM. Attenuation of cochlear damage from noise trauma by an iron chelator, a free radical scavenger and glial cell line-derived neurotrophic factor in vivo. Brain Res. 1999;815:317–325. doi: 10.1016/s0006-8993(98)01100-7. [DOI] [PubMed] [Google Scholar]
- Zhao HB, Santos-Sacchi J. Auditory collusion and a coupled couple of outer hair cells. Nature. 1999;399:359–362. doi: 10.1038/20686. [DOI] [PubMed] [Google Scholar]