Abstract
Reactive oxygen species (ROS) and reactive nitrogen species (RNS) play important roles in regulating cell survival and death. Silibinin is a natural polyphenolic flavonoid isolated from milk thistle with anti-tumor activities, but it was found to induce cytoprotective ROS/RNS in human breast cancer MCF-7 cells. Furthermore, treatment with silibinin down-regulates ERα expression in MCF-7 cells, and inducing both autophagy and apoptosis. In this study we explored the relationship between ER-associated pathways and RNS/ROS in MCF-7 cells. We also investigated the molecular mechanisms underlying the reciprocal regulation between ROS/RNS levels and autophagy in the death signaling pathways in silibinin-treated MCF-7 cells. Silibinin (100–300 μmol/L) dose-dependently increased ROS/RNS generation in MCF-7 cells (with high expression of ERα and low expression of ERβ) and MDA-MB-231 cells (with low expression of ERα and high expression of ERβ). Scavenging ROS/RNS significantly enhanced silibinin-induced death of MCF-7 cells, but not MDA-MB231 cells. Pharmacological activation or blockade of ERα in MCF-7 cells significantly enhanced or decreased, respectively, silibinin-induced ROS/RNS generation, whereas activation or block of ERβ had no effect. In silibinin-treated MCF-7 cells, exposure to the ROS/RNS donators decreased the autophagic levels, whereas inhibition of autophagy with 3-MA significantly increased ROS/RNS levels. We further showed that increases in ROS/RNS generation, ERα activation or autophagy down-regulation had protective roles in silibinin-treated MCF-7 cells. Under a condition of ERα activation, scavenging ROS/RNS or stimulating autophagy enhanced the cytotoxicity of silibinin. These results demonstrate the existence of two conflicting pathways in silibinin-induced death of MCF-7 cells: one involves the down-regulation of ERα and thereby augmenting the pro-apoptotic autophagy downstream, leading to cell death; the other involves the up-regulation of pro-survival ROS/RNS; and that the generation of ROS/RNS and autophagy form a negative feedback loop whose balance is regulated by ERα.
Keywords: human breast cancer, MCF-7 cells, MDA-MB-231 cells, silibinin, ERα, reactive oxygen species (ROS), reactive nitrogen species (RNS), autophagy, apoptosis
Introduction
Breast cancer is one of the most common malignancies and remains the second leading cause of cancer death among women1. The estrogen receptors (ERs), ERα and ERβ, exert opposing effects on cellular processes, including proliferation, apoptosis, cell migration and cancer development. Reactive oxygen species (ROS) and reactive nitrogen species (RNS) play important roles in the regulation of cell survival. Although more research has concentrated on the role of ROS/RNS in MCF-7 breast cancer cells2,3,4,5, the exact molecular mechanisms involving ROS and RNS are far from clear6,7. Under physiological conditions, cells maintain redox balance through the generation and elimination of ROS/RNS; moderate levels of ROS/RNS function as signals promoting cell proliferation and survival, whereas severe increases of ROS/RNS induce cell death8. It has been proposed that, in MCF-7 cells, ROS/RNS induced by cytotoxic concentrations of silibinin are protective and are augmented by inter-regulation between RNS and ROS2,3. Autophagy is an intracellular catabolic process that plays a critical role in the degradation of cytoplasmic proteins9. Accumulating evidence indicates that the induction of autophagy may promote apoptosis10,11. Our previous study has shown that treatment with silibinin down-regulates ERα expression in MCF-7 cells, thus resulting in the induction of both autophagy and apoptosis12. Moreover, silibinin induces apoptosis through the mitochondrial pathway by up-regulating ERβ activity, which is not involved in the modulation of autophagy in MCF-7 cells13. There are very few studies in the literature exploring the relationship between ER-associated pathways and RNS/ROS in cancer cells. Therefore, the aim of the present study wais to test the hypothesis that the generation of pro-survival ROS/RNS is regulated by ERs.
Materials and methods
Reagents
Silibinin with a purity of 99% was purchased from Jurong Best Medicine Material (Zhenjiang, Jiangsu, China) and was dissolved in dimethylsulfoxide (DMSO) to make a stock solution. MEM complete medium was used to dilute the stock solution, and the final concentration of DMSO was kept below 0.1% in cell culture to minimize any cytotoxic effects on cells. The reagents 4,5-diaminofluorescein diacetate (DAF-2 DA) and rapamycin were obtained from Cayman Chemical (Ann Arbor, MI, USA). 2',7'-dichlorofluorescein diacetate (DCF-DA), N-acetylcysteine (NAC), SOD, tert-butylhydroperoxide (tBHP), N-nitro-L-arginine methyl ester (L-NAME), N-(3-(aminomethyl) benzyl) acetamidine (1400W), sodium nitroprusside (SNP), 3-methyladenine (3-MA), methylthiazolyldiphenyl-tetrazolium bromide (MTT), acridine orange (AO), propidium iodide (PI), RNase A, monodansyl cadaverine (MDC), estrogen receptor alpha (ERα)-specific antagonist 1,3-bis(4-hydroxyphenyl)-4-methyl-5-[4-(2-piperidinylethoxy)phenol]-1H-pyrazole dihydrochloride (MPP dihydrochloride), estrogen receptor β (ERβ)-selective agonist 2,3-bis(4-hydroxyphenyl)-propionitrile (diarylpropionitrile, DPN), ERβ full antagonist 2-phenyl-3-(4-hydroxyphenyl)-5,7-bis(trifluoromethyl)-pyrazolo[1,5-a]pyrimidine (PHTPP), primary antibody against LC3 (L8918), trypan blue and (±)-6-hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid (Trolox) were purchased from Sigma Chemical (St Louis, MO, USA). The ERα receptor agonist 4,4',4''-(4-propyl-[1H]-pyrazole-1,3,5-triyl)trisphenol (PPT) was purchased from Tocris Bioscience (Bristol, UK). Primary antibodies against iNOS (sc-651), p62 (sc-25575), caspase-6 (sc-1232), inhibitor of caspase-activated DNase (ICAD) (sc-9066), poly-ADP-ribose polymerase (PARP) (sc-7150) and β-actin (sc-47778), as well as horseradish peroxidase-conjugated secondary antibodies, were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). The SuperSignal West Pico Chemiluminescent Substrate®, used in conjunction with the horseradish peroxidase (HRP) enzyme, was purchased from Thermo Scientific (Rockford, IL, USA). The GFP-LC3 plasmid was purchased from Wuhan Miaolingbio Bioscience & Technology (Wuhan, Hubei, China). Lipofectamine 2000 Transfection Reagent was purchased from Thermo Fisher Scientific (Carlsbad, CA, USA). Hoechst 33258 was purchased from Wanleibio (Shenyang, Liaoning, China).
Cell culture
Two human breast cancer cells, MCF-7 and MDA-MB-231, were obtained from the American Type Culture Collection (ATCC) (Manassas, VA, USA). MCF-7 cells were cultured in Minimum Essential Medium (MEM, Gibco, Grand Island, NY, USA). MDA-MB-231 cells were cultured in Dulbecco's Modified Eagle's Medium (DMEM, Gibco, Grand Island, NY, USA). All media were supplemented with 10% fetal bovine serum (FBS) (Beijing Yuanheng Shengma Research Institution of Biotechnology, Beijing, China), penicillin (100 U/mL) and streptomycin (100 mg/mL). Cells were incubated at 37 °C with 5% CO2 in a humidified atmosphere. All experiments were performed on logarithmically growing cells.
Morphological changes
MCF-7 cells were seeded on culture plates and incubated with silibinin or other tested agents for 24 h. Then, cellular morphology was observed by using phase contrast microscopy (Leica, Nussloch, Germany).
Nuclear damage observed by acridine orange (AO) staining
Cells were labeled with AO (a fluorescent, selective DNA- and RNA-binding dye), and then changes in nuclear morphology of apoptotic cells were examined.
After incubation with the treatments for the indicated time periods, the cells were stained with 20 μg/mL AO at 37 °C for 15 min, and the morphology was observed by fluorescence microscopy (Olympus, Tokyo, Japan).
Observation of nuclear damage by Hoechst 33258 staining
MCF-7 cells (5×104/well) were cultured in 24-well culture plates. After 24 h of incubation, the cells were treated with the indicated treatments for 24 h. The cells were then incubated with 100 μL of Hoechst 33258 at 37 °C for 30 min, and the nuclear changes in fluorescence were observed with a fluorescence inverted microscope (Olympus, Tokyo, Japan).
GFP-LC3 plasmid transfection
Transient transfection was performed with Lipofectamine 2000 Transfection Reagent, peraccording to the manufacturer's protocol. Briefly, the cells were seeded at 2.5×104/well in 0.5 mL of growth medium in a 24-well plate. After 24 h, the cells were incubated with 0.4 μg of GFP-LC3 plasmid and 1 μL of Lipofectamine 2000 Transfection Reagent per well. Six hours later, fresh growth medium was added. After 24 h, the cells were subjected to the indicated treatments for 24 h. Photomicrographs of GFP-LC3 were obtained by fluorescence microscopy.
Flow cytometric analysis
Propidium iodide (PI), a fluorescent dye that specifically binds to cellular DNA, was used to quantify DNA content. MCF-7 cells were seeded into 6-well cell culture plates (Corning, NY, USA) at a density of 3×105 cells/well and cultured for 24 h. MCF-7 cells were incubated with the treatments for the indicated time periods, then harvested and rinsed with cold PBS. The cells were fixed in 70% ethanol at 4 °C for at least 18 h. The cell pellets were stained with the fluorescent probe solution containing 50 μg/mL PI and 1 mg/mL DNase-free RNaseA in PBS on ice in the dark for 1 h. The DNA fluorescence of PI-stained cells was evaluated with a FACScan flow cytometer (Becton Dickinson, Franklin Lakes, NJ, USA).
The fluorescent compound MDC has been used as a tracer for autophagic vacuoles. Cells subjected to the indicated treatments were collected, incubated with 0.05 mmol/L MDC solution in the dark at 37 °C for 30 min and analyzed by FACScan flow cytometry.
For measuring intracellular ROS and RNS generation, the MCF-7 cells were incubated with 10 mmol/L DCF-DA and DAF-2 DA, respectively, at 37 °C for 30 min. The cells were harvested, and the pellets were suspended in 1 mL PBS. The samples were analyzed by FACScan flow cytometry.
Cytotoxicity assay
The cells were subjected to the indicated treatments for 24 h. Then, the cells were rinsed twice with ice-cold PBS and incubated with 100 μL of 0.5 mg/mL MTT solution at 37 °C for 3 h. The supernatant was discarded, and the residual cell layer was dissolved with 150 μL DMSO. Thereafter, the optical absorbance (A value) was measured at the 490 nm wavelength by using a microplate reader (Thermo Scientific Multiskan MK3, Shanghai, China). The cell growth inhibitory ratio was calculated using the following equation:
Cell growth inhibitory ratio (%)=100×(A490, control−A490, sample)/(A490, control−A490, blank)
Trypan blue staining
MCF-7 cells (3×105/well) were cultured in 6-well culture plates. After 24 h of incubation, the cells were subjected to the indicated treatments for 24 h. The cells were then harvested and stained with 4 μg/mL trypan blue. Dead cells were counted using a hemocytometer. The cell death ratio was calculated as
Western blot analysis
After the indicated treatments, both adherent and floating cells were collected at the predetermined time points and lysed with RIPA lysis buffer (Beyotime, Haimen, Jiangsu, China) supplemented with PMSF (1 mmol/L) for 30 min. After centrifugation at 12 000×g for 10 min, the supernatant was collected, and the protein concentration was determined with Bio-Rad protein assay reagent (Bio-Rad, Hercules, CA, USA). The lysates, were adjusted to contain equal amounts of total protein, and separated on 10%–13% SDS-PAGE gels. The protein bands were transferred to Millipore Immobilon®-P Transfer Membranes (Millipore Corporation, Billerica, MA, USA). After being blocked with 5% skim milk at room temperature for 2 h, the membranes were incubated with primary antibodies at 4 °C overnight and then with the corresponding HRP-conjugated secondary antibodies at room temperature for 2 h. The blots were visualized using SuperSignal West Pico Chemiluminescent Substrate® purchased from Thermo Scientific (Rockford, IL, USA).
Statistical analysis
All the data and results obtained by at least three independent experiments are expressed as the means±SD. Comparisons between groups were determined using Student's t-test. One-tailed P-values were deemed to be significant when P<0.05.
Results
Silibinin induces ROS/RNS production in MCF-7 and MDA-MB-231 human breast cancer cells; scavenging ROS/RNS enhances silibinin-induced cell death in MCF-7 but not MDA-MB-231 cells
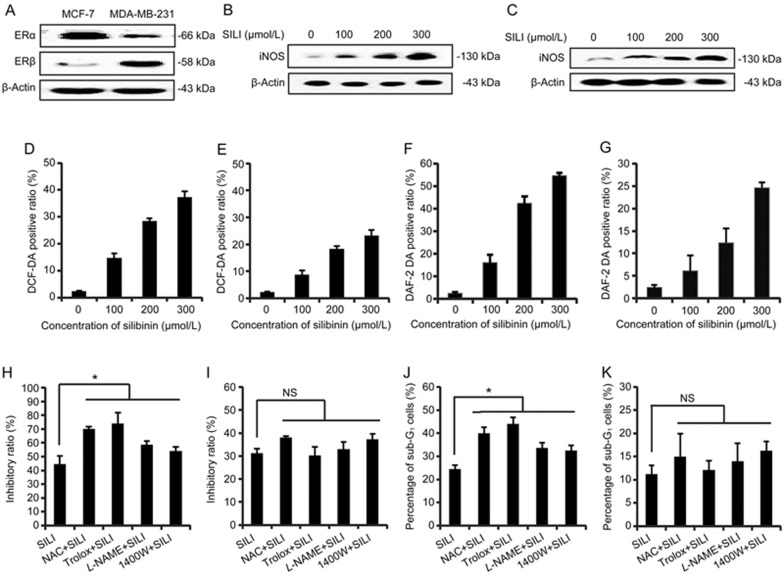
The ROS/RNS-specific fluorescent probes DCF-DA/DAF-2 DA were used to investigate silibinin-induced ROS/RNS production14. To elucidate the relationship between silibinin-induced ROS/RNS and estrogen receptors, we examined the generation of ROS/RNS in two different strains of human breast cancers: the MCF-7 cell line, which has inherently high expression of ERα and low expression of ERβ, and the MDA-MB-231 cell line, which has low expression of ERα and high expression of ERβ. Western blot results of the ERα and ERβ levels of MCF-7 cells and MDA-MB-231 cells were consistent with the results from previous reports15,16 (Figure 1A). The flow cytometric results showed that silibinin induced ROS/RNS generation in a dose-dependent manner in both human breast cancer cell lines (Figure 1D–1G). The treatment with silibinin increased the expression of inducible nitric oxide synthase (iNOS) in a dose-dependent manner (Figure 1B and 1C). As shown in Figure 1H, the growth inhibition by silibinin in MCF-7 cells was enhanced by the non-enzymatic antioxidant NAC as well as another ROS scavenger, Trolox. Furthermore, the inhibitor of nitric oxide synthase L-NAME and the iNOS inhibitor 1400W both enhanced silibinin-induced MCF-7 cell death. Flow cytometric analysis after PI staining showed that NAC and Trolox pre-treatment significantly increased the ratio of sub-G1 cells compared with silibinin-treated cells (Figure 1J). However, unlike the MCF-7 cells, the ROS/RNS scavengers did not affect the viability of the MDA-MB-231 cells (Figure 1I and 1K). These results suggested that the down-regulation of ERα and/or up-regulation of ERβ in silibinin-treated MCF-7 cells12,13 might decrease the levels of ROS/RNS.
Figure 1.
Comparison between the two human breast cancer cell lines; silibinin induces ROS/RNS generation, which in turn represses silibinin-induced MCF-7 cell death. (A) The differential expression of ERα and ERβ levels were compared between two human breast cancer cell lines. iNOS levels in (B) MCF-7 cells and (C) MDA-MB-231 cells incubated with 0, 100, 200, or 300 μmol/L of silibinin for 24 h. β-Actin was used as a loading control. (D) MCF-7 cells and (E) MDA-MB-231 cells were incubated with different concentrations of silibinin for 24 h, then subjected to flow cytometric analysis of ROS levels after DCF-DA staining. The positive ratios are presented in the histogram. (F) MCF-7 cells and (G) MDA-MB-231 cells were incubated with different concentrations of silibinin for 24 h, then subjected to flow cytometric analysis of RNS levels after DAF-2 DA staining. The positive ratios are presented in the histogram. (H) MCF-7 cells and (I) MDA-MB-231 cells were treated with 200 μmol/L silibinin in the presence/absence of NAC (2.5 mmol/L), Trolox (100 μmol/L), L-NAME (0.5 mmol/L) and 1400W (500 μmol/L) for 24 h, and then relative cell numbers were measured by MTT assays. *P<0.05, vs silibinin-only treatment group. n=3. Mean±SD. NS: not significant, compared with the silibinin-only treatment group. The apoptotic (J) MCF-7 cells and (K) MDA-MB-231 cells stained with PI (Sub-G1 fraction) were measured by flow cytometric analysis. Analysis of the percentage of sub-G1 was measured in each group. *P<0.05 vs silibinin only treatment group. n=3. Mean±SD. NS: not significant, compared with the silibinin-only treatment group.
ROS/RNS generation was enhanced by the activation of ERα but not ERβ
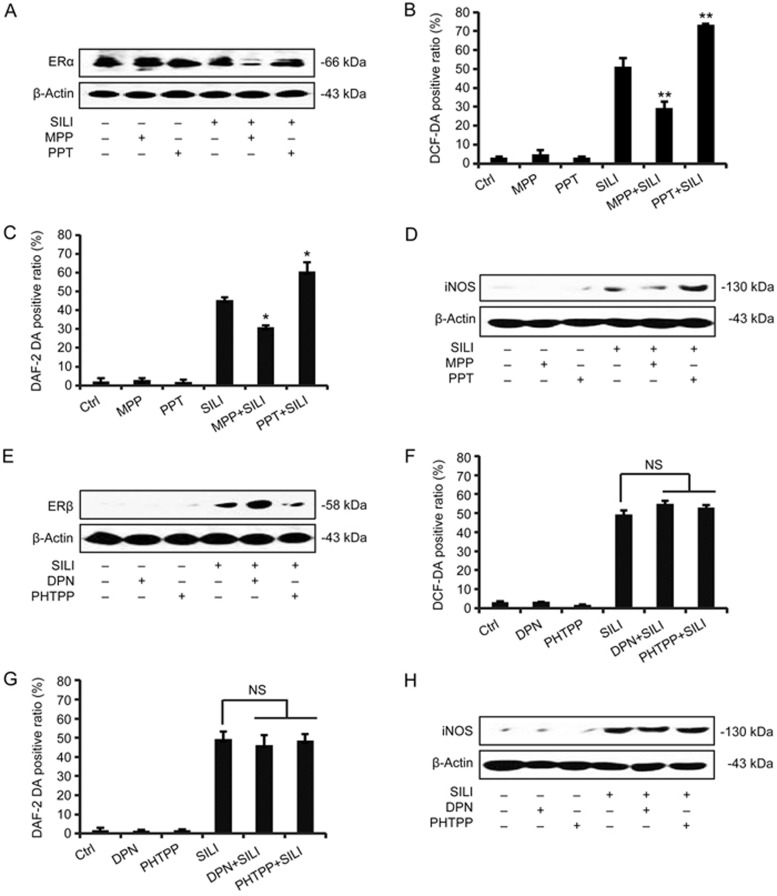
The cytotoxicity of silibinin in MCF-7 cells is correlated with the down-regulation of ERα and up-regulation of ERβ12,13. In this study, we found that silibinin induced the production of ROS/RNS in two different breast cancer cell lines exhibiting different ERα and ERβ expression. Because the scavenging of ROS/RNS enhanced silibinin-induced cell death in MCF-7 cells, but not in MDA-MB-231 cells, as shown above (Figure 1H–1K), we focused on MCF-7 cells to further address the role of estrogen receptor α in regulating the levels of ROS and RNS. The ERα antagonists MPP or agonist PPT were applied at a concentration of 10 μmol/L or 0.25 μmol/L, respectively17, and the effects were evaluated by Western blot analysis (Figure 2A and 2D). As shown in Figure 2B and 2C, compared with the silibinin alone group, the PPT-treated group had significantly increased levels of ROS/RNS. Moreover, the increase in ROS/RNS by silibinin was partially reversed by MPP pre-treatment. In contrast, pre-treatment with the ERβ agonists DPN or antagonist PHTPP had no influence on the production of ROS/RNS (Figure 2F and 2G). Western blot analysis of iNOS expression confirmed these effects on RNS levels (Figure 2D and 2H). These results demonstrated that the decreased ERα expression caused by MPP treatment suppressed the levels of protective ROS/RNS.
Figure 2.
Differential effects of ERα and ERβ on the generation of ROS/RNS induced by silibinin. (A–D) MCF-7 cells were treated with MPP (10 μmol/L) or PPT (0.25 μmol/L) for 3 h, and then the cells were co-incubated with 200 μmol/L silibinin for another 24 h. (A) Western blotting analysis detecting ERα levels. β-Actin was used as a loading control. The analytic diagrams of flow cytometric changes of ROS and RNS levels were analyzed by flow cytometry with DCF-DA (B) and DAF-2 DA staining (C). The results are expressed as the mean±SD of three independent experiments. *P<0.05, **P<0.01 vs silibinin-only treatment group. (D) The protein levels of iNOS were determined by Western blot analysis. β-Actin was used as a loading control. (E–H) After the treatment with DPN (10 μmol/L) or PHTPP (1 μmol/L) for 3 h, the cells were co-incubated with 200 μmol/L silibinin for another 24 h. (E) The ERβ levels were examined by Western blot analysis. β-Actin was used as a loading control. The DCF-DA positive ratio (F) and DAF-2 DA positive ratio (G) were measured by flow cytometric analysis. NS: not significant, compared with the silibinin and DPN/PHTPP-treated group. (H) The protein levels of iNOS were measured by Western blot analysis.
ROS/RNS induced by silibinin down-regulated the autophagic level
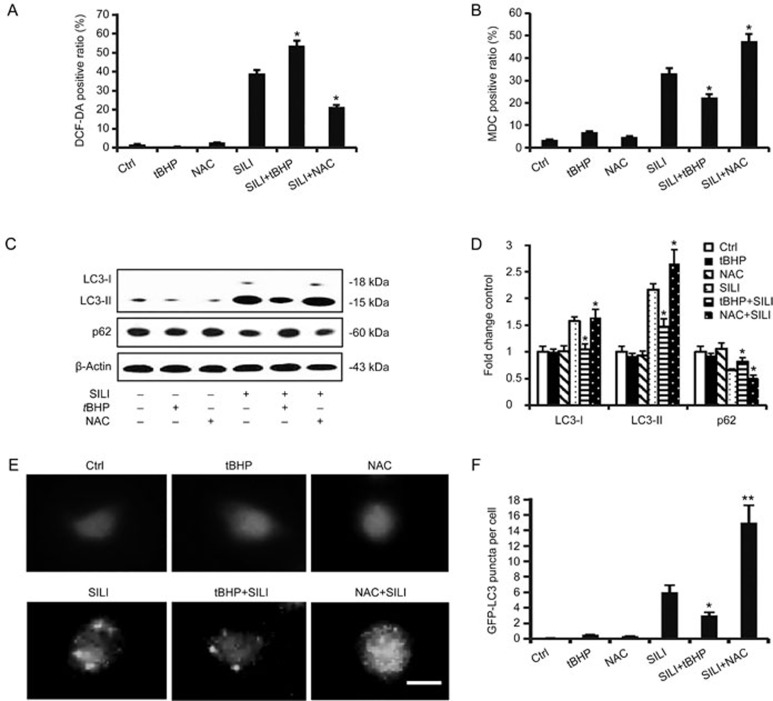
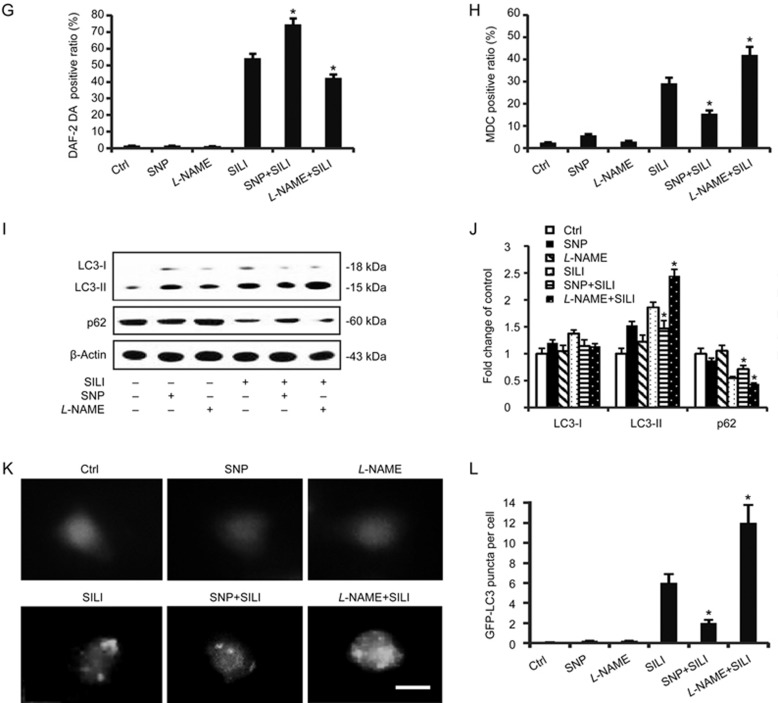
Previous reports have demonstrated that the inhibition of ERα augments autophagy, which promotes silibinin-induced MCF-7 cell apoptosis, but ERβ does not participate in this process12,13. The generation of ROS/RNS is partly due to the activation of ERα but not ERβ. In view of the above results, we investigated the relationship between ROS/RNS and autophagy. NAC/L-NAME was applied to eliminate ROS/RNS production, and tBHP/SNP was used to donate ROS/RNS (Figure 3A and 3G). The MDC positive ratio was alleviated by treatment with tBHP or SNP, compared with silibinin treatment alone, but was enhanced by the treatment with NAC/L-NAME (Figure 3B and 3H). Furthermore, the treatment with tBHP or SNP up-regulated the expression of autophagy-associated protein p62 and decreased the level of autophagy marker LC3-II18 (Figure 3C and 3I), thus indicating a decrease in autophagy. The photomicrographs of GFP-LC3 by fluorescence microscopy confirmed these results (Figure 3E and 3K). In contrast, NAC and L-NAME promoted autophagy. These results suggest that ROS/RNS negatively regulate silibinin-induced autophagy in MCF-7 cells.
Figure 3A–3F.
ROS/RNS negatively controlled autophagy induced by silibinin. (A–F) The cells were pre-treated with tBHP (20 μmol/L) or NAC (2.5 mmol/L) for 1 h before treatment with 200 μmol/L of silibinin for 24 h. (A) The fluorescence of DCF-DA dye, produced through reaction with ROS, was measured by flow cytometric analysis. The data are presented as the means±SD of three independent experiments. *P<0.05 vs silibinin control group. (B) Relative quantification of positive MDC staining detected by flow cytometric analysis. The data are presented as the mean±SD of three independent experiments. *P<0.05 vs silibinin control group. (C) Western blotting analysis of p62 and LC3 levels. (D) Band density of the specific proteins were analyzed with Quantity One Image Software and the results are expressed as average density to β-actin. *P<0.05 vs silibinin-only treatment group. n=3. Mean±SD. (E) Cells transfected with GFP-LC3 plasmid were observed through fluorescence microscopy. Scale bar 10 μm. (F) The GFP-LC3 puncta per cell were calculated, and quantitative results are presented as the means±SD. *P<0.05, **P<0.01 vs the silibinin-treated group.
Figure 3G–3L.
ROS/RNS negatively controlled autophagy induced by silibinin. (G–L) Before treatment with silibinin, cells were exposed to SNP (0.5 μmol/L) or L-NAME (0.5 mmol/L) for 1 h. (G) Analysis of RNS levels by flow cytometry after staining with DAF-2 DA. The data are presented as the means±SD of three independent experiments. *P<0.05 vs silibinin control group. (H) Flow cytometric analyses of autophagic cell ratios after MDC staining. The data are presented as the means±SD of three independent experiments. *P<0.05 vs silibinin control group. (I) Western blotting analysis for the detection of p62 and LC3 levels. β-Actin was used as an equal loading control. (J) Band density of the specific protein was analyzed with Quantity One image software, and the results are expressed as average density relative to β-actin. (K) Cells transfected with GFP-LC3 plasmid were observed by fluorescence microscopy. Scale bar 10 μm. (L) The quantitative results are presented as the mean±SD. *P<0.05 vs the silibinin-treated group.
Autophagy attenuated levels of cytoprotective ROS/RNS in silibinin-treated MCF-7 cells
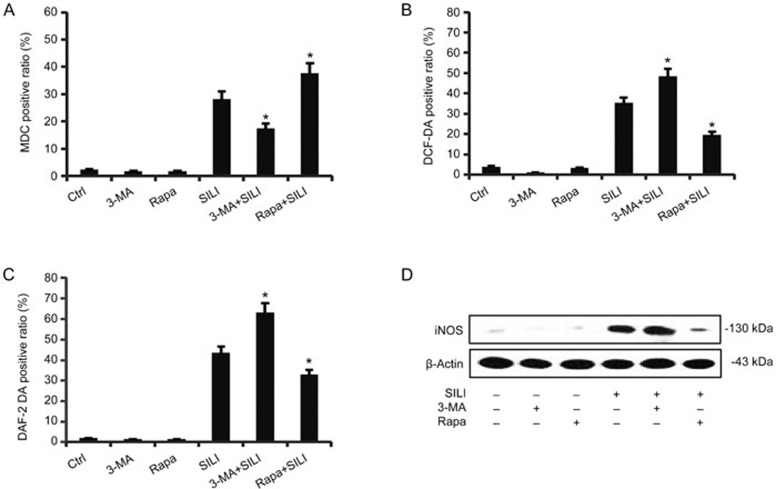
RNS acted together with ROS, increasing cell survival, whereas autophagy resulted in cell death. Therefore, we questioned whether there was any crosstalk between ROS/RNS and autophagy. Rapamycin, a canonical mTOR (mammalian target of rapamycin) inhibitor, was applied to determine the effect of autophagy on the generation of ROS/RNS19. Interestingly, rapamycin effectively inhibited silibinin-induced ROS/RNS generation (Figure 4B and 4C). On the other hand, DCF-DA/DAF-2 DA staining showed that autophagic inhibitor 3-MA significantly enhanced ROS/RNS generation in silibinin-treated cells. These data indicated that autophagy partially inhibits the generation of cytoprotective ROS/RNS. Consistently with the above data, the iNOS expression in MCF-7 cells was also enhanced by 3-MA, but inhibited by rapamycin (Figure 4D).
Figure 4.
ROS/RNS were negatively regulated by autophagy in silibinin-treated MCF-7 cells. The cells were incubated with 200 μmol/L of silibinin in the presence or absence of 3-MA (2 mmol/L) or rapamycin (10 nmol/L) for 24 h. The positive ratios of DCF-DA (B) and DAF-2 DA (C) were measured by flow cytometric analysis. The data are presented as the mean±SD of three independent experiments. *P<0.05 vs silibinin control group. (D) The iNOS levels were examined by Western blot analysis. β-Actin was used as a loading control.
Modulation of ROS/RNS and ERα-associated autophagy was involved in silibinin-induced cytotoxicity
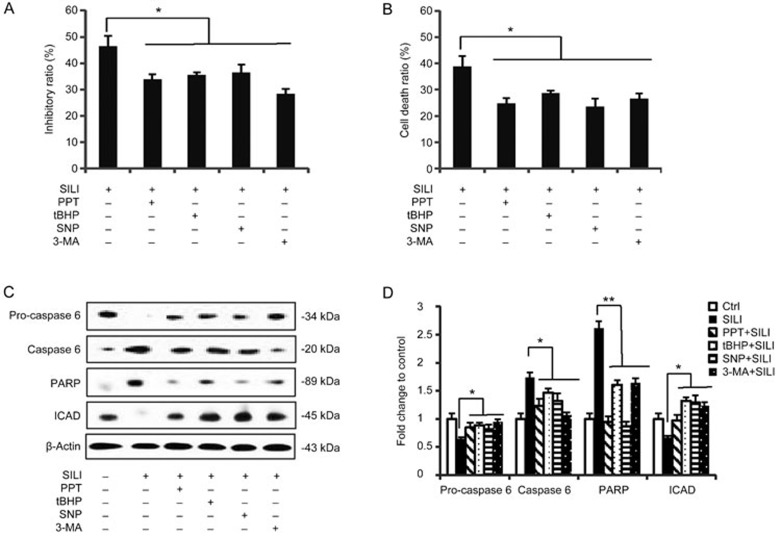
To further clarify the action of ERα in proliferation, we measured the cell survival rate during ROS/RNS up-regulation, ERα activation or autophagy inhibition. The results from MTT assays demonstrated that treatments with ROS/RNS donor tBHP/SNP, ERα agonist PPT and specific autophagic inhibitor 3-MA all decreased cell death caused by silibinin treatment in MCF-7 cells (Figure 5A). To further illustrate cell death rates, trypan blue staining was performed (Figure 5B). At the protein level, apoptosis is characterized by the sequential activation of caspase cascades; however, MCF-7 cells do not express caspase-320,21. Among the remaining caspases, caspase-6 plays a major role as the death executioner in MCF-7 cells12,13, catalyzing the specific cleavage of many key cellular proteins such as ICAD and PARP, thus inducing apoptotic cell death. Western blot analysis showed that the silibinin-induced activation of caspase-6 as well as the caspase substrates PARP and ICAD were all reversed by co-treatment with PPT, tBHP/SNP or 3-MA (Figure 5C and 5D). These findings indicate that increases in ROS/RNS production, as well as ERα activation or autophagy down-regulation, have protective roles in apoptosis of silibinin-treated MCF-7 cells.
Figure 5.
Activation of ERα, up-regulation of ROS/RNS and down-regulation of autophagy attenuated the cytotoxicity of silibinin. Cells were incubated with 200 μmol/L of silibinin for 24 h in the presence or absence of PPT (0.25 μmol/L), tBHP (20 μmol/L), SNP (0.5 μmol/L) or 3-MA (2 mmol/L) for 3 h. (A) Cell viabilities were measured by MTT assays. *P<0.05 vs silibinin-only treatment group. n=3. Mean±SD. (B) Staining MCF-7 cells with trypan blue after corresponding treatments. The quantitative cell death ratios are presented as mean±SD. *P<0.05 vs the silibinin-treated group. (C) Western blot analysis detecting the levels of pro-caspase 6, caspase 6, PARP and ICAD. β-Actin was used as a loading control. (D) Band density of the specific protein was analyzed with Quantity One image software, and the results are expressed as average density to β-actin. The data are presented as the mean±SD of three independent experiments. *P<0.05, **P<0.01 vs silibinin control group.
Scavenging ROS/RNS or stimulating autophagy enhanced the cytotoxicity of silibinin in a background of ERα activation
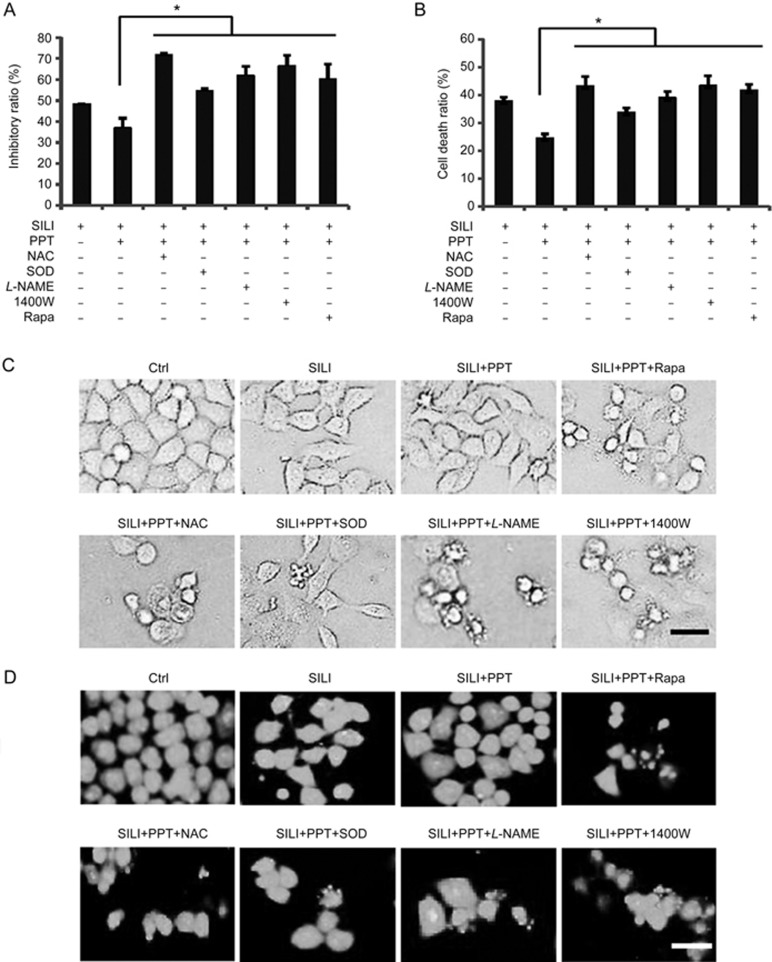
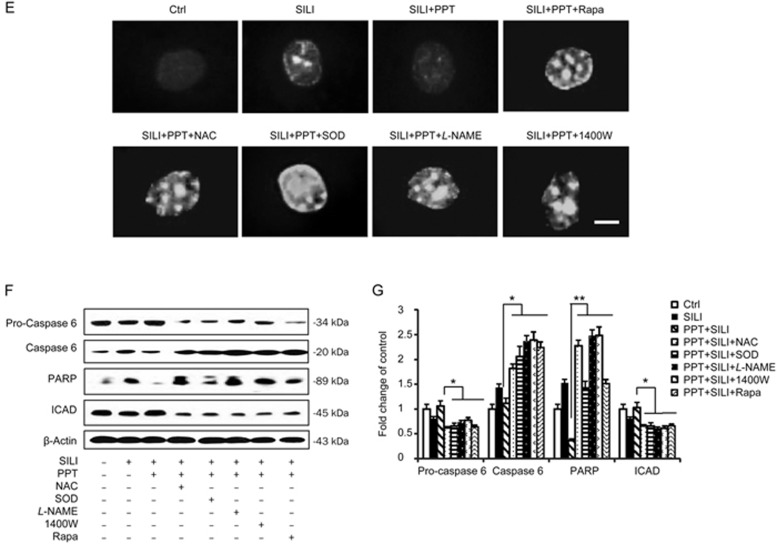
As shown in Figure 6A and 6B, we used MTT assays and trypan blue staining to determine the effects of scavenging ROS/RNS and the up-regulation of autophagy on the growth of MCF-7 cells. MCF-7 cell death was attenuated following the activation of ERα by PPT administration, whereas this effect was not observed in cells treated with silibinin alone. However, this cytoprotective effect was blocked by pre-treatment with ROS scavengers, NAC/SOD, RNS scavengers, L-NAME/1400W or the autophagy inducer rapamycin. The morphological changes were observed by phase contrast microscopy or fluorescence microscopy with AO staining. The apoptotic features such as membrane blebbing and granular apoptotic bodies in the presence of NAC/SOD, L-NAME/1400W or rapamycin were all significantly increased compared with the observations in the silibinin plus PPT-treated group (Figure 6C and 6D). The nuclear morphological changes were observed with Hoechst 33258 staining (Figure 6E). Western blot analysis showed that the activation of caspase-6 and the caspase substrates PARP was augmented by co-treatment with NAC/SOD, L-NAME/1400W or rapamycin, compared with the silibinin plus PPT-treatment (Figure 6F and 6G). All these results indicated that scavenging of ROS/RNS or stimulation of autophagy enhances the cytotoxicity of silibinin in a background of ERα activation (Figure 7).
Figure 6A–6D.
Scavenging ROS/RNS and stimulating autophagy enhanced the cytotoxicity of silibinin under a background of ERα activation. The cells were treated with 200 μmol/L of silibinin for 24 h in the presence or absence of 0.25 μmol/L PPT, 2.5 mmol/L NAC, 200 U/L SOD, 0.5 mmol/L L-NAME, 500 μmol/L 1400W or 10 nmol/L rapamycin. (A) Cell viabilities were measured by MTT assays. *P<0.05 vs silibinin and PPT treatment group. n=3. Mean±SD. (B) Cell death ratios were estimated by the trypan blue exclusion method. The quantitative cell death ratios are presented as means±SD. *P<0.05 vs the silibinin and PPT-treated group. The morphologic changes were observed by phase contrast microscopy (C), or fluorescence microscopy with AO staining (D), scale bar=20 μm.
Figure 6E–6G.
Scavenging ROS/RNS and stimulating autophagy enhanced the cytotoxicity of silibinin under a background of ERα activation. The cells were treated with 200 μmol/L of silibinin for 24 h in the presence or absence of 0.25 μmol/L PPT, 2.5 mmol/L NAC, 200 U/L SOD, 0.5 mmol/L L-NAME, 500 μmol/L 1400W or 10 nmol/L rapamycin. (E) Morphological changes of nuclei and DNA fragmentation in MCF-7 cells with Hoechst 33258 staining, scale bar=10 μm. (F) Western blot analysis of pro-caspase 6, caspase 6, PARP and ICAD levels. β-Actin was used as a loading control. (G) Band density of the specific protein was analyzed with Quantity One image software, and the results are expressed as an average density to β-actin. The data are presented as the mean±SD of three independent experiments. *P<0.05 vs silibinin control group.
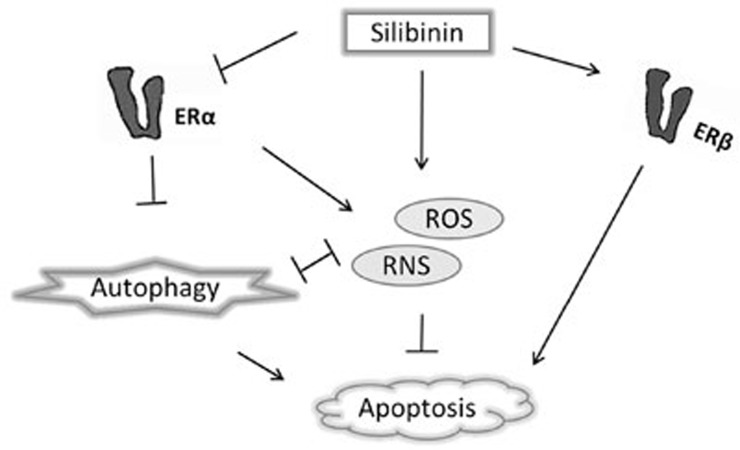
Figure 7.
Schematic representation of the relationship between ROS/RNS and autophagy mediated by ERα in silibinin-induced human breast cancer MCF-7 cell death. This diagram illustrates that, unlike ERβ activation, which induces apoptosis without the involvement of ROS/RNS and autophagy, ERα activation increases the generation of protective ROS/RNS and inhibits pro-apoptotic autophagy, thus leading to MCF-7 cell survival. Silibinin induces cell apoptosis by increasing ERβ expression and decreasing ERα expression.
Discussion
This study was conducted on the basis of previous findings that silibinin induces protective ROS/RNS generation in MCF-7 cells2,3 and inhibits ERα, thereby promoting downstream autophagic flux12. In the present study, we found that ERα had a role in the modulation of ROS/RNS; however, ERβ had no effect on this modulation. Furthermore, we found that both ROS and RNS acted against autophagy. However, autophagy also restricted the production of ROS and RNS. The complicated crosstalk between the pro-survival ROS/RNS and ERα-regulated autophagy signaling pathway was found to determine the fate of silibinin-treated MCF-7 cells. The potential anti-tumor drug silibinin is a natural polyphenolic flavonoid compound isolated from milk thistle, and several studies in recent years have shown that silibinin functions as either a pro-oxidant or anti-oxidant both in vivo and in vitro2,22,23. Classically, ROS/RNS are considered to be deleterious agents, causing a vast range of pathological conditions; however, their protective effects have more recently been appreciated. Many studies have suggested that ROS/RNS may function as a part of signal-transduction pathways regulating the transformation and uncontrolled growth potential of tumor cells24,25. Their protective effects have been found to contribute to the initiation of cancer through accelerating pro-tumorigenic signaling pathways and changing the activity of the tyrosine phosphatases superfamily26. For instance, the inhibition of PTEN by ROS promotes the PI3K/Akt signaling pathway, which may be the most frequently activated signaling pathway in cancer cells27,28,29. In this study, we observed that cytotoxic doses of silibinin induced protective ROS/RNS generation in MCF-7 cells. This finding is consistent with results reported by Wang et al2,3 Previously, we have reported that silibinin exhibits a cytotoxic effect against MCF-7 cells through the down-regulation of ERα and up-regulation of ERβ signaling pathways12,13. Accumulating evidence demonstrates that ERα stimulates ROS generation and modulates the intracellular redox state in a variety of cancer cells30,31,32,33. Thus, we speculated that the estrogen receptor might be a necessary component in regulating the generation of ROS/RNS in silibinin-treated MCF-7 cells.
To test this hypothesis, we modulated the activity of the estrogen receptor by adding an ERα-specific agonist/antagonist PPT/MPP and ERβ-specific agonist/antagonist DPN/PHTPP and compared the changes in ROS/RNS with those treated with silibinin alone. Our results showed that the generation of pro-survival ROS/RNS was partly controlled by the activated ERα but not ERβ. These results were consistent with those of other studies that have been performed34,35, suggesting that modulation of the estrogen receptor is involved in ROS production. It is likely that ERα plays a greater role than that of ERβ in the activation of pro-survival ROS/RNS in silibinin-treated MCF-7 cells. ERα binds in a ligand-dependent manner to the p85α regulatory subunit of phosphatidylinositol-3-OH kinase (PI3K), thus leading to the activation of Akt and endothelial nitric oxide synthase (eNOS)36. As shown in our previous papers, ERα mediates the survival of MCF-7 cells through activation of the PI3K/Akt signaling pathway, but ERβ has no influence on this process12,13. In addition, a large number of published reports have indicated that the expression of antioxidant enzymes is due to regulation of the ERα/ERβ ratio34,35,37. We conclude that the effects of estrogen receptors on pro-survival free radicals may be due, at least partly to the down-regulation of ERα, but may also be due to the down-regulation of the ERα/ERβ ratio caused by the silibinin treatment.
Autophagy is a catabolic process involved in the degradation and recycling of damaged components within cells38. Many studies have proposed a role of autophagy as a mechanism to carry out cell death, owing to the excessive degradation of essential cellular components that are required for normal cell function39,40,41. Our previous research suggests that co-treatment with silibinin and MPP results in autophagy and consequently promotes apoptosis in MCF-7 cells12. Unlike ERα, ERβ does not affect the autophagic process13. Autophagy and the autophagy machinery regulate both redox balance and ROS formation under distinct circumstances. Autolysosomes have been proposed to be sources for ROS38. Our results demonstrate a negative feedback loop between autophagy and ROS production in silibinin-treated MCF-7 cells, adding new evidence of their interactions.
ROS/RNS have a variety of effects on autophagy, depending on the cell type29,42. In MCF-7 cells, silibinin- and ERα antagonist MPP-induced cell growth inhibition is further augmented by rapamycin treatment, but partially reversed by 3-MA12. In this study, eliminating the pro-survival ROS by NAC or scavenging RNS by L-NAME elevated the expression of LC3-II, a typical marker of autophagy. Inhibition of ROS/RNS production caused an increase in the autophagic ratio. However, suppression of autophagy by 3-MA caused an increase in ROS/RNS release. Therefore, ROS/RNS and autophagy play a negative feedback role in silibinin-induced human breast cancer MCF-7 cell death. However, it has also been reported that ROS stimulates autophagy in PC-3 cells9 and MCF-7 cells43. It appears that the regulation between ROS and autophagy is cell type dependent. Moreover, the differences may be due to the differential expression of estrogen receptors in cells. Some other factors, such as the drug used or the medium and serum supplemented, might influence the results as well.
Consistently with the work of Wang2, our results have suggested that the estrogen receptor promotes protective ROS production under stress against cell death30. We speculate that the production of ROS/RNS is regulated by several pathways, including ERα activation. By down-regulating ERα, silibinin decreases ROS/RNS; however, because there were some other undefined pathways by which ROS/RNS were up-regulated, silibinin increased the level of ROS/RNS in MCF-7 cells overall. We tried unsuccessfully to identify the ROS/RNS-up-regulating pathways in silibinin-treated MCF-7 cells.
In summary, our results validated the existence of two conflicting pathways in silibinin-induced MCF-7 cell death: one pathway involves the down-regulation of ERα and thereby augments the pro-apoptotic autophagy downstream, leading to cell death; the other pathway involves the up-regulation of pro-survival ROS/RNS.
These findings at the molecular level significantly contribute to understanding of the mechanisms by which the estrogen receptor, oxidative stress and autophagy regulate human diseases such as cancer, and provide a starting point for the design of novel therapeutic approaches.
Author contribution
Nan ZHENG, Wei-wei LIU, and Takashi IKEJIMA designed the research; Nan ZHENG, Lu LIU, and Fei LI performed the research; Shin-Ichi TASHIRO and Satoshi ONODERA contributed reagents; Nan ZHENG analyzed the data and wrote the paper; Toshihiko HAYASHI and Takashi IKEJIMA revised the paper.
Abbreviations
ER, estrogen receptor; ROS, reactive oxygen species; RNS, reactive nitrogen species; 3-MA, 3-methyladenine; DMSO, dimethyl sulfoxide; DAF-2 DA, 4,5-diaminofluorescein diacetate; DCF-DA, 2',7'-dichlorofluorescein diacetate; NAC, N-acetylcysteine; SOD, superoxide dismutase; tBHP, tert-butylhydroperoxide; L-NAME, N-nitro-L-arginine methyl ester; 1400W, N-(3-(aminomethyl) benzyl) acetamidine; SNP, sodium nitroprusside; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; AO, acridine orange; MDC, monodansyl cadaverine; MPP, 1,3-bis(4-hydroxyphenyl)-4-methyl-5-[4-(2-piperidinylethoxy)phenol]-1H-pyrazole dihydrochloride; DPN, diarylpropionitrile; ICAD, inhibitor of caspase-activated DNase; PARP, poly-ADP-ribose polymerase; PHTPP, 2-phenyl-3-(4-hydroxyphenyl)-5,7-bis(trifluoromethyl)-pyrazolo[1,5-a]-pyrimidine; PI, propidium iodide; iNOS, inducible nitric oxide synthase; LC3, microtubule-associated protein light chain 3; mTOR, mammalian target of rapamycin; PI3K/Akt, phosphatidylinositol 3 kinase (PI3K)/protein kinase B (Akt); SILI, silibinin.
Acknowledgments
This research was supported by National Natural Science Foundation of China (No 81273517).
References
- Althuis MD, Dozier JM, Anderson WF, Devesa SS, Brinton LA. Global trends in breast cancer incidence and mortality 1973–1997. Int J Epidemiol 2005; 34: 405–12. [DOI] [PubMed] [Google Scholar]
- Wang HJ, Jiang YY, Wei XF, Huang H, Tashiro S, Onodera S, et al. Silibinin induces protective superoxide generation in human breast cancer MCF-7 cells. Free Radic Res 2010; 44: 90–100. [DOI] [PubMed] [Google Scholar]
- Wang HJ, Wei XF, Jiang YY, Huang H, Yang Y, Fan SM, et al. Silibinin induces the generation of nitric oxide in human breast cancer MCF-7 cells. Free Radic Res 2010; 44: 577–84. [DOI] [PubMed] [Google Scholar]
- Singh R, Shankar BS, Sainis KB, Singh R. TGF-beta1-ROS-ATM-CREB signaling axis in macrophage mediated migration of human breast cancer MCF7 cells. Cell Signal 2014; 26: 1604–15. [DOI] [PubMed] [Google Scholar]
- Lu C, Heldt JM, Guille-Collignon M, Lemaitre F, Jaouen G, Vessieres A, et al. Quantitative analyses of ROS and RNS production in breast cancer cell lines incubated with ferrocifens. ChemMedChem 2014; 9: 1286–93. [DOI] [PubMed] [Google Scholar]
- Thomas C, Gustafsson JA. The different roles of ER subtypes in cancer biology and therapy. Nat Rev Cancer 2011; 11: 597–608. [DOI] [PubMed] [Google Scholar]
- Chen GG, Vlantis AC, Zeng Q, van Hasselt CA, Chen GG. Regulation of cell growth by estrogen signaling and potential targets in thyroid cancer. Curr Cancer Drug Targets 2008; 8: 367–77. [DOI] [PubMed] [Google Scholar]
- Trachootham D, Lu W, Ogasawara MA, Nilsa RD, Huang P, Trachootham D. Redox regulation of cell survival. Antioxid Redox Signal 2008; 10: 1343–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Kim KY, Yu SN, Park SK, Choi HD, Ji JH, et al. Autophagy inhibition enhances silibinin-induced apoptosis by regulating reactive oxygen species production in human prostate cancer PC-3 cells. Biochem Biophys Res Commun 2015; 468: 151–6. [DOI] [PubMed] [Google Scholar]
- Kundu M, Thompson CB. Autophagy: basic principles and relevance to disease. Annu Rev Pathol 2008; 3: 427–55. [DOI] [PubMed] [Google Scholar]
- Gozuacik D, Kimchi A. Autophagy and cell death. Curr Top Dev Biol 2007; 78: 217–45. [DOI] [PubMed] [Google Scholar]
- Zheng N, Zhang P, Huang H, Liu W, Hayashi T, Zang L, et al. ERalpha down-regulation plays a key role in silibinin-induced autophagy and apoptosis in human breast cancer MCF-7 cells. J Pharmacol Sci 2015; 128: 97–107. [DOI] [PubMed] [Google Scholar]
- Zheng N, Liu L, Liu W, Zhang P, Huang H, Zang L, et al. ERbeta up-regulation was involved in silibinin-induced growth inhibition of human breast cancer MCF-7 cells. Arch Biochem Biophys 2016; 591: 141–9. [DOI] [PubMed] [Google Scholar]
- Eruslanov E, Kusmartsev S. Identification of ROS using oxidized DCFDA and flow-cytometry. Methods Mol Biol 2010; 594: 57–72. [DOI] [PubMed] [Google Scholar]
- Lee SO, Nadiminty N, Wu XX, Lou W, Dong Y, Ip C, et al. Selenium disrupts estrogen signaling by altering estrogen receptor expression and ligand binding in human breast cancer cells. Cancer Res 2005; 65: 3487–92. [DOI] [PubMed] [Google Scholar]
- Chan KK, Leung TH, Chan DW, Wei N, Lau GT, Liu SS, et al. Targeting estrogen receptor subtypes (ERalpha and ERbeta) with selective ER modulators in ovarian cancer. J Endocrinol 2014; 221: 325–36. [DOI] [PubMed] [Google Scholar]
- Harris HA, Katzenellenbogen JA, Katzenellenbogen BS. Characterization of the biological roles of the estrogen receptors, ERalpha and ERbeta, in estrogen target tissues in vivo through the use of an ERalpha-selective ligand. Endocrinology 2002; 143: 4172–7. [DOI] [PubMed] [Google Scholar]
- Park SY, Park MY, Park HG, Lee KJ. Kook, Kim WJ, et al. Nitric oxide-induced autophagy and the activation of AMPK pathway protect against apoptosis in human dental pulp cells. Int Endod J 2016. . [DOI] [PubMed]
- Duan WJ, Li YF, Liu FL, Deng J, Wu YP, Yuan WL, et al. A SIRT3/AMPK/autophagy network orchestrates the protective effects of trans-resveratrol in stressed peritoneal macrophages and RAW 264.7 macrophages. Free Radic Biol Med 2016; 95: 230–42. [DOI] [PubMed] [Google Scholar]
- Janicke RU, Sprengart ML, Wati MR, Porter AG. Caspase-3 is required for DNA fragmentation and morphological changes associated with apoptosis. J Biol Chem 1998; 273: 9357–60. [DOI] [PubMed] [Google Scholar]
- Janicke RU. MCF-7 breast carcinoma cells do not express caspase-3. Breast Cancer Res Treat 2009; 117: 219–21. [DOI] [PubMed] [Google Scholar]
- Fan S, Yu Y, Qi M, Sun Z, Li L, Yao G, et al. P53-mediated GSH depletion enhanced the cytotoxicity of NO in silibinin-treated human cervical carcinoma HeLa cells. Free Radic Res 2012; 46: 1082–92. [DOI] [PubMed] [Google Scholar]
- Fan S, Li L, Chen S, Yu Y, Qi M, Tashiro S, et al. Silibinin induced-autophagic and apoptotic death is associated with an increase in reactive oxygen and nitrogen species in HeLa cells. Free Radic Res 2011; 45: 1307–24. [DOI] [PubMed] [Google Scholar]
- Xia C, Meng Q, Liu LZ, Rojanasakul Y, Wang XR, Jiang BH, et al. Reactive oxygen species regulate angiogenesis and tumor growth through vascular endothelial growth factor. Cancer Res 2007; 67: 10823–30. [DOI] [PubMed] [Google Scholar]
- Klaunig JE, Kamendulis LM. The role of oxidative stress in carcinogenesis. Annu Rev Pharmacol Toxicol 2004; 44: 239–67. [DOI] [PubMed] [Google Scholar]
- Tonks NK. Protein tyrosine phosphatases: from genes, to function, to disease. Nat Rev Mol Cell Biol 2006; 7: 833–46. [DOI] [PubMed] [Google Scholar]
- Hay N. The Akt-mTOR tango and its relevance to cancer. Cancer Cell 2005; 8: 179–83. [DOI] [PubMed] [Google Scholar]
- Gupta SC, Singh R, Pochampally R, Watabe K, Mo YY. Acidosis promotes invasiveness of breast cancer cells through ROS-AKT-NF-kappaB pathway. Oncotarget 2014; 5: 12070–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Shi QF, Ye YC, Tashiro S, Onodera S, Ikejima T. Activated O2(*-) and H2O2 mediated cell survival in SU11274-treated non-small-cell lung cancer A549 cells via c-Met-PI3K-Akt and c-Met-Grb2/SOS-Ras-p38 pathways. J Pharmacol Sci 2012; 119: 150–9. [DOI] [PubMed] [Google Scholar]
- Fan D, Liu SY, van Hasselt CA, Vlantis AC, Ng EK, Zhang H, et al. Estrogen receptor alpha induces prosurvival autophagy in papillary thyroid cancer via stimulating reactive oxygen species and extracellular signal regulated kinases. J Clin Endocrinol Metab 2015; 100: 561–71. [DOI] [PubMed] [Google Scholar]
- Mobley JA, Brueggemeier RW. Estrogen receptor-mediated regulation of oxidative stress and DNA damage in breast cancer. Carcinogenesis 2004; 25: 3–9. [DOI] [PubMed] [Google Scholar]
- Okoh V, Deoraj A, Roy D. Estrogen-induced reactive oxygen species-mediated signalings contribute to breast cancer. Biochim Biophys Acta- Rev Cancer 2011; 1815: 115–33. [DOI] [PubMed] [Google Scholar]
- Wang ZC, Wijewickrama GT, Peng KW, Dietz BM, Yuan L. Estrogen receptor alpha enhances the rate of oxidative DNA damage by targeting an equine estrogen catechol metabolite to the nucleus. J Biol Chem 2009; 284: 8624–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miro AM, Sastre-Serra J, Pons DG, Valle A, Roca P, Oliver J. 17beta-Estradiol regulates oxidative stress in prostate cancer cell lines according to ERalpha/ERbeta ratio. J Steroid Biochem 2011; 123: 133–9. [DOI] [PubMed] [Google Scholar]
- Nadal-Serrano M, Sastre-Serra J, Pons DG, Miro AM, Oliver J, Roca P, et al. The ERalpha/ERbeta ratio determines oxidative stress in breast cancer cell lines in response to 17beta-estradiol. J Cell Biochem 2012; 113: 3178–85. [DOI] [PubMed] [Google Scholar]
- Simoncini T, Hafezi-Moghadam A, Brazil DP, Ley K, Chin WW, Liao JK. Interaction of oestrogen receptor with the regulatory subunit of phosphatidylinositol-3-OH kinase. Nature 2000; 407: 538–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadal-Serrano M, Pons DG, Sastre-Serra J, Blanquer-Rossello M, Roca P, Oliver J, et al. Genistein modulates oxidative stress in breast cancer cell lines according to ERalpha/ERbeta ratio: effects on mitochondrial functionality, sirtuins, uncoupling protein 2 and antioxidant enzymes. Int J Biochem Cell Biol 2013; 45: 2045–51. [DOI] [PubMed] [Google Scholar]
- Navarro-Yepes J, Burns M, Anandhan A, Khalimonchuk O, del Razo LM, Quintanilla-Vega B, et al. Oxidative stress, redox signaling, and autophagy: cell death versus survival. Antioxid Redox Signal 2014; 21: 66–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu S, Kanaseki T, Mizushima N, Mizuta T, Arakawa-Kobayashi S, Thompson CB, et al. Role of Bcl-2 family proteins in a non-apoptotic programmed cell death dependent on autophagy genes. Nat Cell Biol 2004; 6: 1221–8. [DOI] [PubMed] [Google Scholar]
- Denton D, Shravage B, Simin R, Mills K, Berry DL, Baehrecke EH, et al. Autophagy, not apoptosis, is essential for midgut cell death in Drosophila. Curr Biol 2009; 19: 1741–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong JL, Hill DS, McKee CS, Hernandez-Tiedra S, Lorente M. Exploiting cannabinoid-induced cytotoxic autophagy to drive melanoma cell death. J Invest Dermatol 2015; 135: 1629–37. [DOI] [PubMed] [Google Scholar]
- Rabkin SW. Nitric oxide-induced cell death in the heart: the role of autophagy. Autophagy 2007; 3: 347–9. [DOI] [PubMed] [Google Scholar]
- Jiang K, Wang W, Jin X, Wang Z, Ji Z, Meng G. Silibinin, a natural flavonoid, induces autophagy via ROS-dependent mitochondrial dysfunction and loss of ATP involving BNIP3 in human MCF7 breast cancer cells. Oncol Rep 2015; 33: 2711–8. [DOI] [PMC free article] [PubMed] [Google Scholar]