Abstract
It has been widely recognized that inflammation, particularly chronic inflammation, can increase the risk of cancer and that the simultaneous treatment of inflammation and cancer may produce excellent therapeutic effects. Berberine, an alkaloid isolated from Rhizoma coptidis, has broad applications, particularly as an antibacterial agent in the clinic with a long history. Over the past decade, many reports have demonstrated that this natural product and its derivatives have high activity against both cancer and inflammation. In this review, we summarize the advances in studing berberine and its derivatives as anti-inflammatory and anti-tumor agents in the digestive system; we also discuss their structure-activity relationship. These data should be useful for the development of this natural product as novel anticancer drugs with anti-inflammation activity.
Keywords: berberine, natural product, anti-inflammatory drug, anticancer drug, digestive system, structure-activity relationship
Introduction
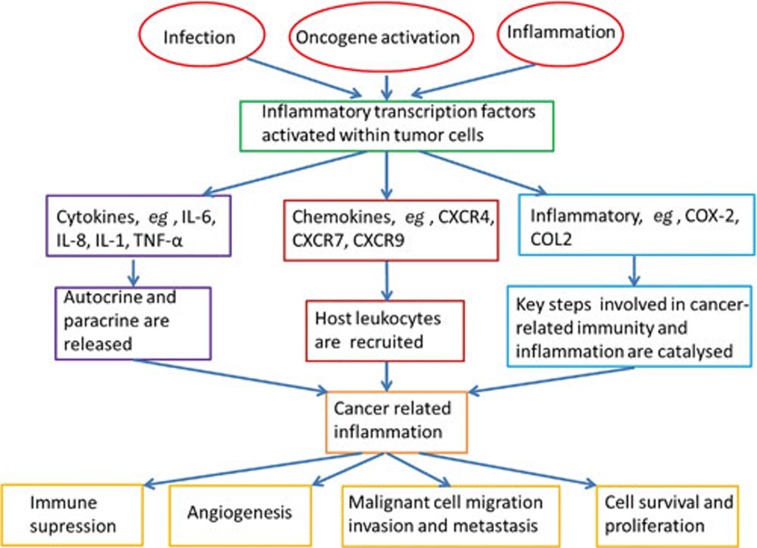
In 1863, Rudolf Virchow hypothesized that cancer originated from chronic inflammation1. Modern research has revealed that approximately 20% of human cancers are related to chronic inflammation caused by infections, exposure to irritants or autoimmune diseases2. Epidemiological studies have indicated that the occurrence and development of up to 15% of tumors are related to infections and that chronic inflammation can especially increase the risk of cancer3,4. Inflammation is induced in complex ways by microbial and viral infections and can also result from allergens and autoimmune diseases. In general, chronic inflammation is thought to be particularly harmful and related to cell carcinogenesis2. The carcinogenic mechanisms caused by chronic inflammation are complicated and include genetic mutation induction, angiogenesis promotion and cell proliferation. Carcinogenesis caused by inflammation is mainly related to the abnormal expression of inflammatory cytokines, including TNF-α, IL-6, IL-8, IL-1, colony stimulating factor (CSF), and macrophage migration inhibitory factor (MIF) (Figure 1)5. These inflammatory cytokines have a paradoxical effect on cancers by both promoting and inhibiting tumor growth. These cytokines have both local and systemic tumorigenic actions in experimental models of cancer and in patients with cancer2,6. However, they also induce the hemorrhagic necrosis of tumors7. These results show that the relationship between inflammation and cancer is extremely complicated (Figure 1). Nevertheless, studies have revealed that the simultaneous treatment of both inflammation and cancer may have excellent therapeutic effects, and this strategy has thus become a new research area5,6.
Figure 1.
The molecular basis of cancer-related inflammation.
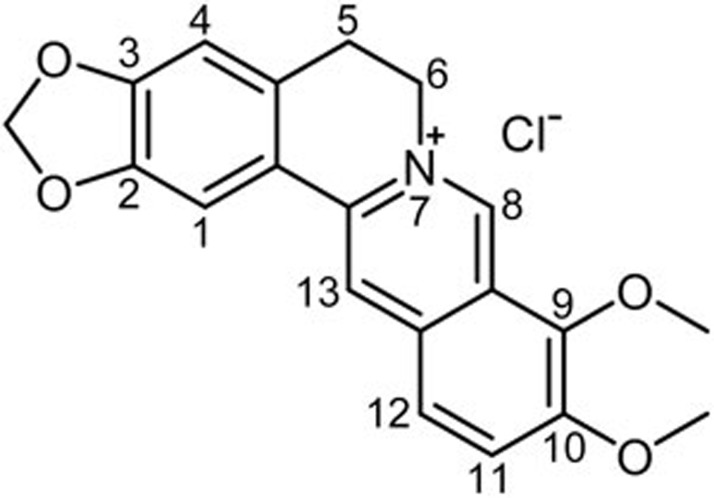
Rhizoma coptidis has been used for centuries in China as an ingredient of many prescriptions to treat dysentery, and its main active component is berberine (Figure 2)8. Impressively, recent studies have shown that berberine, a small molecule alkaloid isolated from the plant, exerts anti-inflammatory and anticancer effects8,9,10,11,12. Berberine possesses anti-inflammatory activity and suppresses proinflammatory responses by inhibiting mitogen-activated protein kinase signaling and cellular reactive oxygen species production9. Berberine is also able to inhibit the growth of various types of cancer cells, promote the apoptosis of tumor cells, induce the differentiation of tumor cells, and suppress the metastasis of tumor cells10. Berberine also possesses the ability to form strong complexes with either DNA or RNA to induce DNA damage and promote telomerase inhibition and topoisomerase poisoning13. Moreover, it can also alter mitochondrial membrane potential, regulate the expression of Bcl-2 family members and the level of cell cyclin and related proteins, and inhibit some cell signaling pathways10. Overall, these effects of berberine may lead to cell cycle arrest, induce cell death via apoptosis and activate autophagy.
Figure 2.
Structure of berberine.
Several derivatives of berberine have been synthesized to improve bioactivity and bioavailability. Structural modifications have mainly focused on the C-8, C-9, C-10, C-12 and C-13 positions of berberine14, and some new skeleton analogs have been synthesized. Modification at C-8 or C-13 mainly increases its antimicrobial activity, which is also closely related to the length of the substituent chain. The substituent at the C-9 position of berberine may increase anti-tumor activity8.
It is well known that berberine exerts its activity mainly in the gastrointestinal system, which is due to its low bioavailability15. Therefore, special attention should be paid to the bioactivity of this natural product in the gastrointestinal system. To the best of our knowledge, there is no systematic review of the pharmacological effects of berberine and its derivatives in the digestive system. Here, we review the main advances of berberine and its derivatives as anti-inflammatory and anti-tumor agents in the digestive system to provide useful information for anticancer drug development based on berberine.
Anti-inflammatory activity in the digestive system
The anti-gastroenteritis effect and its mechanism
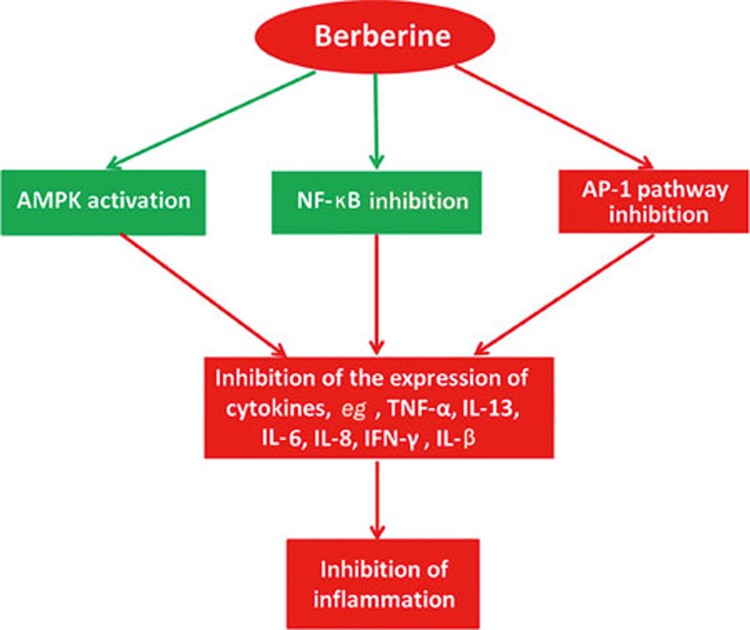
Berberine has been used to treat inflammatory bowel disease (IBD), eg, dysentery, for many years. Numerous investigations on berberine have revealed its anti-inflammatory activities in the digestive system8,11,12, which mainly contribute to the protection of the intestinal epithelial barrier and the regulation of intestinal inflammatory cytokines and transcription (Figure 3). Microbiology and genetics studies have shown that IBD results from an excessive immune response of the intestinal flora induced by a mucosal immune system disorder or a change in the intestinal flora composition.
Figure 3.
Summary of the anti-inflammatory effect of berberine.
The anti-inflammatory activity of berberine was detected by the reduction of proinflammatory cytokines such as TNF-α, IL-13, IL-6, IL-8 and IFN-γ (Figure 3). It has been reported that berberine is able to completely antagonize the TNF-α-mediated barrier defects in cell models, which is related to tyrosine kinase, pAkt and NF-κB pathways16. One in vitro study demonstrated that berberine has the ability to ameliorate proinflammatory cytokine-induced intestinal epithelia tight junction damage17,18. This process is regulated by cytokines15 such as the Th2 cytokine interleukin-13 (IL-13), as well as TNF-α and IFN-γ19. IL-8 is an important cytokine for the recruitment and activation of polymorph nuclear neutrophil cells that are abundant in the intestinal lesions of IBD. Berberine is beneficial to the mucosal healing process, possibly by inhibiting IL-8 production. For example, IL-8 production in rectal mucosa is inhibited by berberine at a concentration of 10.0 nmol/L in trinitrobenzene sulfonic acid (TNB)-induced colitis in rats20. These effectors not only play important roles in inflammation but are also involved in the process of tumor development and dissemination17,18,19,20.
The transcription factor activator protein 1 (AP-1) plays a critical role in inflammation and carcinogenesis. Berberine can significantly inhibit the binding activity of NF-κB and AP-1 at concentrations of 10-4 mol/L or higher. Further study revealed that berberine inhibited LPS-induced MCP-1/CCL2 production in vitro via an AP-1 and NF-κB-dependent pathway21,22,23,24,25. It has been reported that berberine repressed proinflammatory responses through AMP-activated protein kinase (AMPK) activation in macrophages, significantly down-regulating the expression of proinflammatory genes such as TNF-α, IL-1β, IL-6, monocyte chemo-attractant protein-1 (MCP-1), inducible nitric oxide synthase (iNOS), and cyclooxygenase-2 (COX-2). Moreover, these inhibitory effects of berberine on proinflammatory responses were abrogated by an AMPK inhibitor, or dominant-negative AMPK, which indicated that berberine would down-regulate proinflammatory responses in macrophages via AMPK stimulation26,27. In inflammatory responses, COX-2 plays an important role in the synthesis of prostaglandins (PGs) from arachidonic acid. An investigation revealed that berberine could inhibit COX-2 expression and prostaglandin E2 (PGE2) levels28.
The anti-hepatic inflammatory effect and its mechanism
Berberine can down-regulate several hepatic proinflammatory genes, including TNF-α, IL-6 and serum amyloid A3 (SAA3), which are proposed to play important roles in the development of steatohepatitis29. This effect of berberine may occur via the activation of AMPK and inhibition of NF-κB30. The anti-inflammatory effect of berberine in hepatic cells has been observed in different animal models. Some in vitro and in vivo studies carried out in male albino rats have shown that berberine decreased the expression of both TNF-α and COX-2 in a hepatotoxicity rat model induced by cyclophosphamide (CP)31. Berberine is able to effectively inhibit the production of IL-6 and TNF-α in HepG2 cells. Its mechanism of action for anti-inflammation could be attributed to the inhibition of ERK1/2 activation32. This anti-inflammatory activity was similar to the data in a recent study reporting that berberine inhibited the LPS-induced inflammatory response in macrophages26. Other researchers studied the mechanism of the anti-hepatitis effect of berberine and found that berberine significantly decreased the expression of pro-/anti-inflammatory and/or Th1/Th2 cytokines, suggesting that berberine alleviated spontaneous inflammation in non-obese diabetic mice33.
The anti-tumor activity of berberine in the digestive system
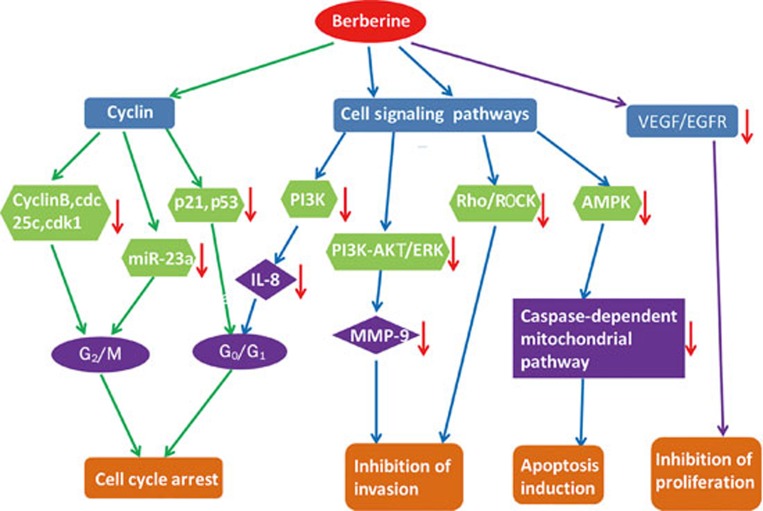
The anti-tumor activity of berberine mainly includes inhibiting the growth of tumor cells, promoting tumor cell apoptosis, inducing the differentiation of tumor cells and inhibiting the expression and metastasis of tumor cells. As summarized in Figure 4, the mechanism of these effects is the down-regulation of the level of cyclin and growth factor receptors and the inhibition of some signaling pathways9. Moreover, berberine has a preventive effect on tumors via the regulation of inflammatory cytokines.
Figure 4.
Summary of the anti-tumor effect of berberine. “→” stands for inhibition or down-regulation.
With its wide spectrum of anti-tumor properties, berberine has potential applications as a complementary medicine for the prevention and treatment of human cancers. Studies have already shown that berberine has potential anti-tumor activities in the digestive system including nasopharyngeal carcinoma (NPC), gastric cancer, liver cancer and intestinal cancer9,34,35 (Table 1).
Table 1. Effects of berberine on various cancer cell lines.
| Cell line | Origin | Effect |
|---|---|---|
| NCE-1, NCE-2, NPC5-8F, C666-1 | Nasopharyngeal carcinoma | Telomerase, Ezrin and STAT3 inhibition |
| MGC803 | Gastric carcinoma | Down-regulation of Bcl-2 and up-regulation of Bax and p53; cycle arrest and cell apoptosis |
| HCC, HepG2, SMMC-7721, Bel7402 | Hepatoma | Cytochrome c release; Bcl-2/Bcl-xL decrease; activation of the AMPK-mediated caspase-dependent mitochondrial pathway; down-regulation of the Rho/ROCK signaling pathway |
| HT29, SW480 | Colorectal cancer | Cell cycle arrest; loss of mitochondrial membrane potential; induction of Bcl-2 family proteins; COX-2 regulation |
Berberine has also been used in combination with drugs or radiotherapy, which enhances the effect of other drugs and radiotherapy or reduces their side effects. The administration of berberine with cisplatin and evodiamine increased their cytotoxic effects on many cancer cell types34. The toxicity of vincristine towards hepatoma cells is reduced by the combinatorial effects of berberine, and cell resistance to drugs is decreased by combination treatment with berberine35. Moreover, the combination of radiotherapy and berberine exerts a synergistic cytotoxic effect on different tumor cell lines36.
The anti-nasopharyngeal carcinoma effect and its mechanism
NPC is endemic in Southern China and Southeast Asia, where radiotherapy has been the primary treatment; however, radiotherapy is not effective for advanced–stage NPC. Therefore, it is of importance to find effective and safe drugs to treat patients with NPC37. Studies have demonstrated that berberine induced apoptosis and impaired the migration and invasion of the human NPC CNE-1 cell line by reducing the expression of the epithelial-mesenchymal transition-related twist38. The inhibition of proliferation and telomerase activity may improve the therapeutic effect against NPC39. In addition, berberine has an inhibitory effect on the growth of CNE-2 cell lines, with IC50 values of 49.5±5.8 (48 h) and 13.3±2.0 (72 h) μmol/L. The activity might be related to its inhibition of cyclin B, cdc25c, and CDK1 proteins40.
It has been reported that berberine can suppress the invasive properties of NPC cell lines through the inhibition of Ezrin phosphorylation41. Berberine can suppress metastasis by enhancing the expression of the metastasis suppression gene NM23-H1 or by targeting Rho kinase-mediated Ezrin phosphorylation in the NPC 5-8F cell line42. Ezrin is highly expressed in metastatic tumors and is involved in filopodia formation as well as in the promotion of tumor metastasis. Thus, Ezrin may serve as a potential target for anti-metastatic therapy.
Current research has revealed that the signal transducer and activator of transcription 3 (STAT3) plays a pivotal role in NPC development43. The activation of STAT3 may contribute to both the development and progression of NPC44,45. Thus, targeting aberrant STAT3 signaling may provide an effective and novel strategy for the treatment of NPC. It has been reported that berberine suppressed the tumorigenicity and growth of NPC cells by inhibiting STAT3 activation in vivo and in vitro. In one study, C666-1 cells were injected subcutaneously into the flank of nude mice. The treatment mice were injected intraperitoneally with low or high doses of berberine (5 and 10 mg/kg, respectively) every two days. Berberine could suppress STAT3 activation in NPC grown as a xenografted tumor in nude mice. The researchers examined whether berberine could suppress STAT3 in NPC cell growth in vitro. The data indicated that berberine could suppress the constitutive activation of STAT3 in NPC cells and inhibit their survival ability46. Mcl-1 is an anti-apoptotic member of the Bcl-2 family and promotes cell growth, survival and angiogenesis through the transcriptional up-regulation of STAT347. It has been reported that berberine can suppress the constitutive activation of STAT3 in human NPC cells by down-regulating the activity of Mcl-1. Moreover, the berberine-mediated down-regulation of Mcl-1 expression is accompanied by the down-regulation of c-FLIP, allowing the induction of TRAIL-mediated apoptosis46,47,48,49.
The anti-gastric cancer effect and its mechanism
Berberine is able to inhibit the proliferation and induce the apoptosis of gastric cancer cells. It has been reported that berberine (10 μg/mL) can cause G0/G1 cell arrest and cell apoptosis. The underlying mechanism may be attributed to the down-regulation of Bcl-2 and the up-regulation of Bax and p53 in a time- and dose-dependent manner50. Other studies have demonstrated that the mechanism might be related to the inhibition of the PI3K signaling pathway51. IL-8 is a type of autocrine growth factor that induces tumor blood vessel growth and promotes the growth of a wide variety of tumor invasion, metastasis and proliferation52,53. Berberine reduces the level of IL-8, which is related to its inhibition of PI3K signaling pathways54,55. A synergistic anti-gastric cancer effect was also observed when berberine was used in combination in vivo and in vitro models, such as the combination of berberine and d-limonene. The combination exerted synergistic anticancer effects on MGC803 cells by inducing cell cycle arrest, inhibiting reactive oxygen species (ROS) production, and inducing apoptosis through the mitochondria-mediated intrinsic pathway56.
Berberine exhibits the ability to induce apoptosis in human gastric cancer cells by interacting with nucleic acids, especially DNA, in vitro57. Studies have shown that berberine can cause G0/G1 cell cycle arrest and cell apoptosis49 and that the mechanism is related to cell cycle-related proteins such as p21, Cdk2, Cdk4, and cyclins D1 and E56,57. Some studies have reported that berberine can down-regulate the expression of mutant p53 and p21 and block human gastric carcinoma cell entrance to the cell cycle in G0/G1 phase58. In addition, other studies have reported that berberine enables tumor cells to be blocked in the G2/M phase59,60.
The anti-hepatoma effect and its mechanism
Previous studies have confirmed the anti-tumor effects of berberine on the human hepatocellular carcinoma (HCC) cell line by inhibiting proliferation and inducing apoptosis in HCC cells61. CD147 is highly expressed in HCC cells, which can promote tumor invasion and metastasis, inhibit apoptosis and anoikis, promote tumor angiogenesis, and confer resistance to some chemotherapeutic drugs62,63. It has been reported that berberine induces both apoptosis and cell death in HepG2 cells, which correlates with the down-regulation of CD14764. AMPK is a metabolic sensing protein kinase. Its activation is accompanied by an apoptotic effect that occurs in a caspase-dependent manner through the mitochondrial pathway. Studies have demonstrated that the activation of AMPK leads to the induction of apoptosis in numerous human cancer cell types65. Studies have also demonstrated that berberine selectively inhibited the growth of human hepatocellular cancer cells by inducing AMPK-mediated caspase-dependent mitochondrial pathway cell apoptosis66. Synergistic anti-tumor effects were also observed when berberine was used in combination to treat hepatoma. The combined use of berberine and evodiamine could significantly enhance the apoptosis of SMMC-7721 cells, which was related to the up-regulation of the level of TNF-α67. In addition, the use of berberine in combination with the microtubule poison vincristine has been proven to be efficient against hepatoma cell lines by potentiating the pro-apoptotic effect of the single drug68. Other studies have shown that the interstitial implantation of radioactive seed 125I induced hepatoma cell apoptosis. This effect could be enhanced when 125I was combined with berberine, which induces apoptosis, cell degeneration and necrosis69. Studies have also indicated that the anti-tumor activity of gamma radiation is significantly enhanced by berberine via the activation of the p38 MAPK pathway and ROS generation in human hepatoma cells36,70.
Berberine inhibits the migration of hepatoma cells, as confirmed by several reports. Berberine has gradually entered the limelight for its potentially therapeutic effect against the invasion and metastasis of various cancer cell lines. It has been reported that berberine inhibited the migration of hepatocellular carcinoma cells by down-regulating the Rho/ROCK signaling pathway71. Some researchers have observed that berberine exhibits significant cytotoxicity in HepG2 cells, mainly through the up-regulation of ROS production, but is ineffective in normal liver cells72. Further study has indicated that berberine may be a potential alternative therapy against invasive hepatoma cells through PI3K-AKT and ERK pathway-dependent down-regulation of metalloproteinase-9 expression.
Berberine can block the cell cycle of hepatoma cells. The mechanism mostly occurs through its downstream-regulated genes or proteins. Researchers used a computational pipeline based on a ligand-protein inverse docking program and mining of the 'Connectivity MAP' data to explore potential target proteins for berberine. Their computational and experimental results showed that CaM might be a potential target of berberine. A biological assay indicated that berberine induced G1 cell cycle arrest in Bel7402 cells partially by interacting with CaM and blocking subsequent signal cascades. These results provided new hints for the study of the mechanism of the anticancer action of berberine on tumor cells73. The tumor suppressor p53 was reported to play a key role in the anti-tumor action of berberine. A recent study has revealed that berberine may up-regulate p53 expression by suppressing its inner inhibitor MDM2 at the post-transcriptional level74,75. It has been reported that berberine may increase the expression of the primary precursor, precursor and mature forms of miR-23a, which could enhance berberine-induced G2/M cell cycle arrest76.
The anti-colorectal cancer effect and its mechanism
Berberine can inhibit the growth of human colorectal adenocarcinoma in vivo and in vitro. The inhibition is related to the induction of G1/S and G2/M cell cycle arrest, which depends on the regulation of checkpoint protein expression. It has been reported that berberine can inhibit colorectal adenocarcinoma growth by inducing G2/M phase arrest and down-regulating the expression of related cyclins, such as cyclins B1, cdc2 and cdc25c. The IC50 value is 40.79±4.11 μmol/L (72 h)77. Other studies indicated that a combination of berberine and evodiamine could inhibit cell proliferation by down-regulating miR-17–92 and E2F1 protein expression in colorectal cancer HT29 cells78. Berberine is combined with irinotecan to potentiate the cytotoxicity of colon cancer cells, which might be attributed to an increased rate of apoptosis, possibly mediated by the inhibition of NF-κB activation35. Research has indicated that berberine can inhibit the growth of colon carcinoma lovo cells and induce their apoptosis; the mechanism is associated with inhibition of COX-2 expression79. EGFR is a tyrosine kinase that participates in the regulation of cellular homeostasis, which influences cell proliferation, apoptosis, migration, survival and complex processes, including angiogenesis and tumorigenesis80. Berberine was able to decrease proliferation and EGFR expression levels in colon epithelial cells, which correlated with the enhancement of Cbl activity81.
The current study has demonstrated that berberine possesses the ability to cause cell cycle arrest, induce apoptosis and inhibit inflammation in colon cancer cells. Studies have shown that berberine arrested the SW480 cell cycle at the G2/M phase. Some biochemical events were observed, including the loss of mitochondrial membrane potential, the release of cytochrome-c into the cytosol, the induction of Bcl-2 family proteins and caspases, and the cleavage of poly (ADP-ribose) polymerase (PARP). Furthermore, berberine inhibited caspase-8-mediated angiogenesis, as confirmed through the inhibition of the expression of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL), vascular endothelial growth factor (VEGF) and survivin82. Apoptosis-inducing factor (AIF), a mitochondrial oxidoreductase, is one of the best-studied mediators stimulating caspase-independent cell death. Berberine induces the ROS-mediated stimulation of AIF activation through cathepsin B release and PARP activation, which leads to caspase-independent cell death in colon tumor cells83.
Berberine not only exhibits growth suppression effects and induces apoptosis but can also prevent the formation of colorectal carcinoma, which depends on the regulation of the related protein expression of berberine. COX-2 plays a key role in the formation of cancers, and berberine possesses a down-regulatory effect on the mRNA and protein expression of COX-284. Numerous studies have demonstrated that PPARγ prevents the formation of and induces apoptosis in colon cancer85,86. Thus, PPARγ is likely to become a new target for the prevention and treatment of colon cancer. It has been reported that berberine exhibited inhibitory effects on the proliferation of lovo cells but decreased the expression level of PPARγ mRNA in lovo cells87. Berberine can also prevent the appearance of malignant morphology and ultrastructural changes of azoxymethane (AOM)-induced cancer by producing apoptosis-like changes. Thus, berberine inhibits neoplastic transformation by inducing the antioxidant defense system88.
Structure-activity relationships of the derivatives of berberine
Anti-inflammation activity
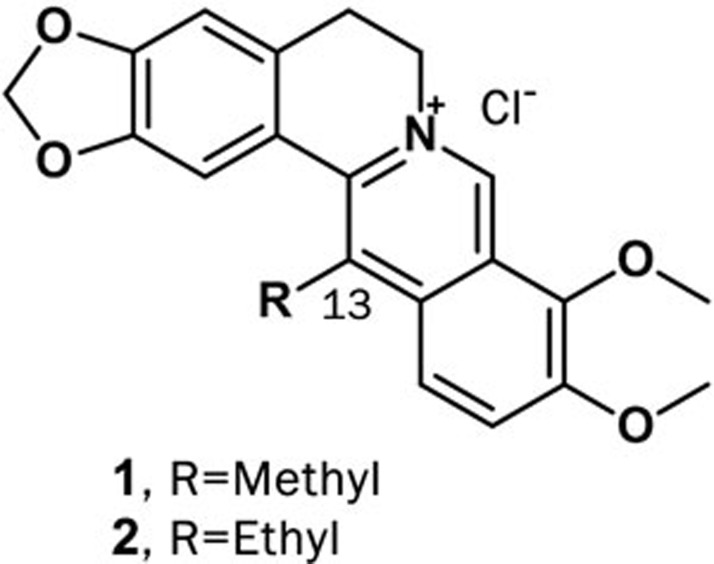
Berberine exhibits anti-inflammatory effects via inhibiting the activation of related inflammatory factors such as iNOS, COX-2, IL-1, IL-6 and NF-κB. However, research on the anti-inflammatory effects of berberine derivatives in the digestive system is rare. Recently, 13-alkyl-substituted berberines have been synthesized and shown to be more active than berberine against certain bacterial species and human cancer cell lines. The concentrations of the 50% inhibition of NO production (IC50) of compounds 1 and 2 were 11.64 and 9.32 μmol/L, respectively (Figure 5). Neither the mRNA expression of iNOS, COX-2 or TNF-α nor the protein expression of COX-2 or TNF-α was affected by the compounds, but the production of PGE2 in LPS-stimulated RAW 264.7 cells was significantly reduced. Strikingly, compounds 1 and 2 increased the production of IL-12 in LPS-treated splenic macrophages89.
Figure 5.
Structures of compounds 1 and 2.
Anti-tumor activity
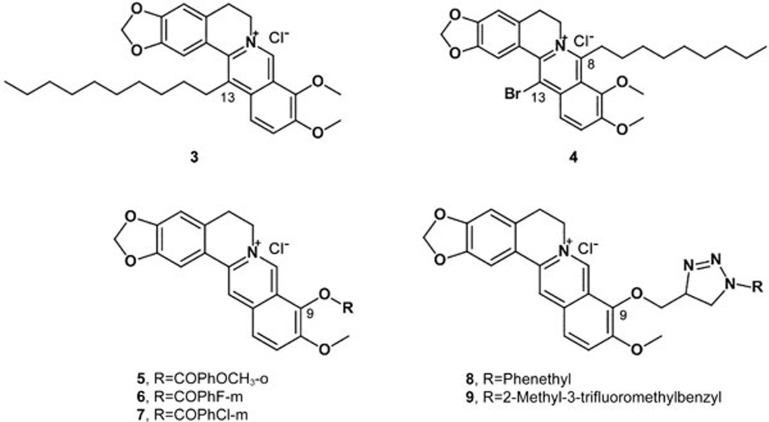
Structural modifications have been carried out by several research groups to investigate the structure-activity relationship of anti-tumor activity and the corresponding mechanism of berberine derivatives. These works have mainly focused on the C-8, C-9, and C-13 positions for various pharmaceutical purposes. Fourteen compounds of C-13-substituted berberine derivatives were designed and synthesized; seven of these were novel compounds, and all target compounds showed higher anti-tumor activities than berberine. In particular, compound 3 exhibited potential anticancer activities on HepG2 cells, with an IC50 value of 0.08 μg/mL (Figure 6). The primary structure-activity relationship demonstrated that 13-alkyl berberine derivatives showed obviously higher anti-tumor activities than 13-benzyl berberine derivatives, and a moderate alkyl chain length was beneficial for anti-tumor activity90.
Figure 6.
Structures of compounds 3–9.
Ding synthesized a series of 8-alkyl-13-bromo-berberine derivatives to study their anti-tumor activities. The results showed that the carbon chain length of the derivatives was highly correlated with tumor cell sensitivity. Compound 4 (8-octyl-13-bromo-berberine) showed remarkable anti-tumor activity, and its inhibitory rate was 96.82% at 32 μg/mL. The IC50 of compound 4 was 3.33 μg/mL (Figure 6)91. Li reported a novel berberine–bile acid analog that exhibited greater cytotoxicity than berberine in several human cancer cell lines. The study indicated that berberine induces ROS-triggered caspase-dependent and caspase-independent apoptosis pathways in SMMC-7721 cells92.
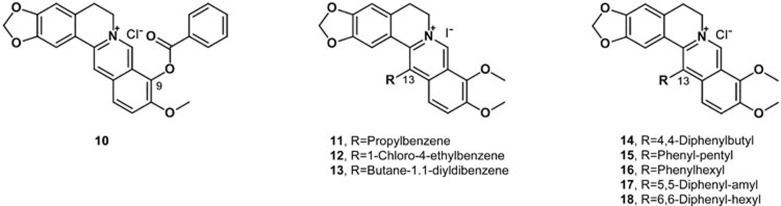
Bi synthesized a series of cycloberberine derivatives and evaluated their anticancer activity. Compounds 5, 6 and 7 showed strong inhibition on human HepG2 cells, with IC50 values of 176, 123 and 91 nmol/L, respectively (Figure 6). In particular, compound 7 significantly inhibited the activity of DNA TopI at 0.1 mg/mL, indicating that the mechanism might involve cell cycle arrest at the G2/M phase of HepG2 cells by berberine93.
Bhowmik investigated the structural effects and thermodynamics of the DNA binding of six berberine analogs with alkyl chains of varying length and a terminal phenyl group at the C-13 position. Phenylalkyl substitution at the C-13 position considerably enhanced DNA binding, especially when the analog has a -(CH2)3-linker94. Jin designed and synthesized a series of new triazolyl berberine derivatives to search for novel anticancer agents; most of the synthesized compounds displayed stronger anticancer activity against SMMC-7721 than berberine. Among these derivatives, compounds 8 and 9 exhibited the most potent inhibitory activities against the SMMC-7721 cell lines, with IC50 values of 14.861±2.4 μmol/L and 16.798±3.4 μmol/L, respectively (Figure 6)95. Li designed and synthesized a series of new 1,13-cycloprotoberberine derivatives defined by variations at the 9-position and evaluated their cytotoxicity in HCT116 (a human colorectal cancer cell line) cells. The result revealed that the replacement of 9-methoxyl with an ester moiety significantly enhanced antiproliferative activity in vitro. Further study showed that compound 10 (Figure 7) significantly inhibited the activity of DNA topoisomerase I (Top I) and led to Top II G2/M phase arrest, resulting in tumor cell growth reduction96. To explore the anticancer activity of the derivatives of berberine, Luis synthesized three berberine derivatives, compounds 11, 12 and 13 (Figure 7). A bioassay of these compounds on two human colon carcinoma cell lines showed that the derivatives induced cell cycle arrest and cell death through apoptosis, and the effect of the derivatives was stronger than that of the lead compound97. To improve its efficacy and bioavailability, these researchers designed and synthesized several derivatives of 9-substituted berberine derivatives. Among the derivatives, five compounds (14–18) significantly affected the proliferation of human colon carcinoma cell lines. Remarkably, these active compounds exhibited the ability to induce autophagy98.
Figure 7.
Structures of compounds 10–18.
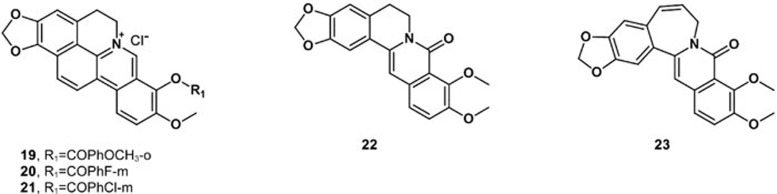
A series of cycloberberine derivatives were synthesized and evaluated for their anti-tumor activity in vitro. Among these analogs, compounds 19–21 showed strong inhibition on human HepG2 cells by blocking cells at the G2/M phase induced by the significant inhibition of DNA Top I99. Oxyberberine (22) belongs to the protoberberine alkaloids isolated mainly from Acangelisia gusanlung, Cocculus orbiculatus and Phellodendron amurense. Oxyberberine showed significant cytotoxicity against HCT116 cells, with an ED50 value of 3.0 μmol/L100. Synthetic studies of these alkaloids were performed, and various analogs were synthesized. The novel benzoazepinoisoquinolone 23, a seven membered B-ring of oxyberberine, was reported with anti-tumor activity against cell lines originating from human tumors. The IC50 value of compound 23 was 2.80 μmol/L for HCT116 cells (Figure 8)100,101.
Figure 8.
Structures of compounds 19–23.
Conclusions and perspective
In conclusion, inflammation, especially chronic inflammation, is an indispensable participant in the neoplastic process, fostering the proliferation, survival and migration of tumor cells. In addition, some anti-inflammatory drugs, such as chemotactic factor antagonists, glucocorticoids and non-steroidal anti-inflammatory drugs, have been used as auxiliary anticancer treatments in the clinic. As a natural compound with both anti-inflammatory and anti-tumor activities, berberine shows great potency in cancer treatment. Furthermore, as the anti-inflammatory and anti-tumor activities of berberine are not particularly strong, the structural modification of berberine is necessary and has good prospects. Previous reports of structural modifications to berberine have mainly focused on a single site, such as the C-8, C-9, C-12, C-13 position. However, this study is the first to systematically evaluate the structural modifications of berberine for the treatment of inflammation and cancer in the digestive system.
For developing berberine-based novel anticancer drugs, the activity and balance of both anti-inflammation and anti-tumor properties should be more closely monitored while investigating the structure-activity relationship of berberine derivatives in the digestive system. The structure of berberine should be modified to produce more active compounds with high bioavailability and metabolic stability. Working in this way, we believe that novel anticancer drugs based on the natural product berberine with both anti-inflammation and anti-tumor activities could be developed in the near future.
Acknowledgments
This work was supported by the Natural Science Foundation of Shanghai, China (No 14ZR1447800, 15ZR1441200) and the State Key Laboratory of Bioactive Substance and Function of Natural Medicines (No GTZK201606).
References
- Coussens LM, Werb Z. Inflammation and cancer. Nature 2002; 420: 860–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crusz SM, Balkwill FR. Inflammation and cancer: advance and new agents. Nat Rev Clin Oncol 2015; 12: 584–96. [DOI] [PubMed] [Google Scholar]
- Mbeunkui F. Johannn DJ Jr. Cancer and the tumor microenvironment: a review of an essential relationship. Cancer Chemother Pharmacol 2009; 63: 571–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutcliffe S, Platz EA. Inflammation and prostate cancer: a focus on infection. Curr Urol Rep 2008; 9: 243–9. [DOI] [PubMed] [Google Scholar]
- Liu Z, Xiao B, Mao XH, Zou QM. Research progress on relationship between inflammation and tumor. Prog Mod Biomed 2009; 9: 591–4. [Google Scholar]
- Sziosarek PW, Grimshaw MJ, Kulbe H, Wlison JL, Wilbanks GD, Burke F, et al. Expression and regulation of tumor necrosis factor alpha in normal and malignant ovarian epithelium. Mol Cancer Ther 2006; 5: 382–90. [DOI] [PubMed] [Google Scholar]
- Balkwill F. Tumour necrosis factor and cancer. Nat Rev Cancer 2009; 9: 361–71. [DOI] [PubMed] [Google Scholar]
- Jin X, Song X, Cao YB, Jiang YY, Sun QY. Research progress in structural modification and pharmacological activities of berberine. J Pharma Prac 2014; 32: 171–5. [Google Scholar]
- Jabbarzadeh Kaboli P, Rahmat A, Ismail P, Ling KH. Targets and mechanisms of berberine, a natural drug with potential to treat cancer with special focus on breast cancer. Eur J Pharmacol 2014; 740: 584–95. [DOI] [PubMed] [Google Scholar]
- Ortiz LM, Lombardi P, Tillhon M, Scovassi Al. Berberine, an epiphany against cancer. Molecules 2014; 19: 12349–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Xun K, Wang Y, Chen X. A systematic review of the anticancer properties of berberine: a natural product from Chinese herbs. Anti-cancer Drugs 2009; 13: 757–69. [DOI] [PubMed] [Google Scholar]
- Tang J, Feng Y, Tsao S, Wang N, Curtain R, Wang Y. Berberine and Coptidis rhizoma as novel antineoplastic agents: a review of traditional use and biomedical investigations. J Ethnopharmacol 2009; 13: 5–17. [DOI] [PubMed] [Google Scholar]
- Wang Y, Kheir MM, Chai Y, Hu J, Xing D, Lei F, et al. Comprehensive study in the inhibitory effect of berberine on gene transcription, including TATA box. PLoS One 2011; 6: e23495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang ZJ, Zeng Y, Lan P, Sun PH, Chen WM. Advances in structural modifications and biological activities of berberine: an active compound in traditional Chinese medicine. Mini Rev Med Chem 2011; 11: 1122–9. [DOI] [PubMed] [Google Scholar]
- Li B, Zhu WL, Chen KX. Advances in the study of berberine and its derivatives. Acta Pharm Sin 2008; 43: 773–87. [PubMed] [Google Scholar]
- Amasheh M, Fromm A, Krug SM, Amasheh S, Andress S, Zeitz M, et al. TNF alpha-induced and berberine-antagonized tight junction barrier impairment via tyrosine kinase, Akt and NF kappa B singaling. J Cell Sci 2010; 123: 4145–55. [DOI] [PubMed] [Google Scholar]
- Li N, Gu L, Qu l, Gong J, Li Q, Zhu W, et al. Berberine attenuates pro-inflammatory cytokine-induced tight junction disruption in an in vitro model of intestinal epithelial cell. Eur J Pharm Sci 2010; 40: 1–8. [DOI] [PubMed] [Google Scholar]
- Gu L, Li N, Gong J, Li Q, Zhu W, Li J. Berberine ameliorates intestinal epithelial tight-junction damage and down-regulates myosin light chain kinase pathways in a mouse model of endotoxinemia. J Infect Dis 2011; 203: 1602–12. [DOI] [PubMed] [Google Scholar]
- Amasheh M, Grotjohann I, Amasheh S, Fromm A, Soderholm JD, Zeitz M, et al. Regulation of mucosal structure and barrier function in rat colon exposed to tumor necrosis factor alpha and interferon gamma in vitro: a novel model for studying the pathomechanisms of inflammatory bowel disease cytokines. Scand J Gastroenterol 2009; 44: 1226–35. [DOI] [PubMed] [Google Scholar]
- Zhou H, Mineshhita S. The effect of berberine chloride on experiment colitis in rat in vivo and in vitro. J Pharmacol Exp Ther 2000; 294: 822–9. [PubMed] [Google Scholar]
- De Plean IG, Han XB, Liu X, Hsueh W, Ghosh S, May MJ. Lipopolysaccharide induces CXCL2/macrophage inflammatory protein-2 gene expression in enterocytes via NF-kappaB activation: independence from endogenous TNF-alpha and platelet-activating factor. Immunology 2006; 118: 153–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remppis A, Bea F, Greten HJ, Buttler A, Wang H, Zhou Q, et al. Rhizoma Coptidis inhibits LPS-induced MCP-1/CCL2 production in murine macrophages via an AP-1 and NF-kappaB-dependent pathway. Mediators Inflamm 2010; 2010: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Wang HD, Lu DX, Wang YP, Qi RB, Fu YM, et al. Neutral sulfate berberine modulates cytokine secretion and increases survival in endotoxemic mice. Acta Pharmacol Sin 2006; 27: 1199–205. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Piao XL, Piao XS, Lu T, Wang D, Kim SW. Preventive effect of Coptis chinensis and berberine on intestinal injury in rats challenged with lipopolysaccharides. Food Chem Toxicol 2010; 49: 61–9. [DOI] [PubMed] [Google Scholar]
- Li HM, Wang YY, Wang HD, Cao WJ, Yu XH, Lu DX, et al. Berberine protects against lipopolysaccharide-induced intestinal injury in mice via alpha 2 adrenoceptor-independent mechanisns. Acta Pharmacol Sin 2011; 32: 1362–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong HW, Hsu KC, Lee JW, Ham M, Huh JY, Shin HJ, et al. Berberine suppresses pro-inflammatory responses through AMPK activation in macrophages. Am J Physiol Endocrinol Metab 2009; 296: E955–64. [DOI] [PubMed] [Google Scholar]
- Yan F, Wang L, Shi Y, Cao H, Liu L, Washington MK, et al. Berberine promotes recovery of colitis and inhibits inflammatory responses in colonic macrophages and epithelial cells in DSS-treated mice. Am J Physiol Gastrointest Liver Physiol 2012; 302: G504–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo CL, Chi CW, Liu TY. The anti-inflammatory potential of berberine in vitro and in vivo. Cancer Lett 2004; 203: 127–37. [DOI] [PubMed] [Google Scholar]
- Jeong HW, Hsu KC, Lee JW, Ham M, Huh JY, Kim WS, et al. Berberine suppresses proinflammatory responses through AMPK activation in macrophages. Am J Physiol Endocrinol Metab 2009; 296: E955–64. [DOI] [PubMed] [Google Scholar]
- Kim WS, Lee YS, Cha SH, Jeong HW, Choe SS, Lee MR, et al. Berberine improves lipid dysregulation in obesity by controlling central and peripheral AMPK activity. Am J Physiol Endocrinol Metab 2009; 296: E812–9. [DOI] [PubMed] [Google Scholar]
- Germoush MO, Mahmoud AM. Berberine mitigates cyclophosphamide-induced hepatotoxicity by modulating antioxidant status and inflammatory cytokines. J Cancer Res Clin Oncol 2014; 140: 1103–9. [DOI] [PubMed] [Google Scholar]
- Lou TJ, Zhang ZA, Xi ZL. Berberine inhibits inflammatory response and ameliorates insulin resistance in hepatocytes. Inflammation 2011; 34: 659–67. [DOI] [PubMed] [Google Scholar]
- Chueh WH, Lin JY. Protective effect of isoquinoline alkaloid berberine on spontaneous inflammation in the spleen, liver and kidney of non-obese diabetic mice through down-regulating gene expression ratios of pro-/anti-inflammatory and Th1/Th2 cytokines. Food Chem 2012; 131: 1263–71. [Google Scholar]
- Youn MJ, So HS, Cho HJ, Kim HJ, Kim Y, Lee JH, et al. Berberine, a natural product, combined with cisplatin enhanced apoptosis through a mitochondria/caspase-mediated pathway in HeLa cells. Biol Pharm Bull 2008; 31: 789–95. [DOI] [PubMed] [Google Scholar]
- Yu M, Tong X, Qi B, Qu H, Dong S, Yu S, et al. Berberine enhances chemosensitivity to irinotecan in colon cancer via inhibition of NF-κB. Mol Med Rep 2014; 9: 249–54. [DOI] [PubMed] [Google Scholar]
- Peng PL, Kuo WH, Tseng HC, Chou FP. Synergistic tumor-killing effect of radiation and berberine combined treatment in lung cancer: the contribution of autophagic cell death. Int J Radiat Oncol Biol Phys 2008; 70: 529–42. [DOI] [PubMed] [Google Scholar]
- Lo KW, To KF, Huang DP. Focus on nasopharyngeal carcinoma. Cancer Cell 2004; 5: 423–8. [DOI] [PubMed] [Google Scholar]
- Li CH, Wu DF, Ding Y, Zhao Y, Zhou KY, Xu DF. Berberine hydrochloride impact on physiological processes and modulation of Twist levels in nasopharyngeal carcinoma CNE-1 cells. Asian Pac J Cancer Prev 2014; 15: 1851–7. [DOI] [PubMed] [Google Scholar]
- Li CH, Peng G, Li JY, Wang XJ, Xu DF, Zhou KY. Antitumor effect of berberine hydrochloride in the serum and plasma on nasopharyngeal carcinoma CNE-1 cell: A comparative study. J Med Postgra 2013; 26: 676–9. [Google Scholar]
- Cai YC, Xian LJ. Inhibition of berberine on growth of human nasopharyngeal carcinoma cells CNE-2 in vivo and in vitro. Zhongcaoyao 2006; 37: 1521–6. [Google Scholar]
- Tang FQ, Wang DS, Duan CJ, Huang DM, Wu Y, Chen Y, et al. Berberine inhibits metastasis of nasopharyngeal carcinoma 5-8F cells by targeting Rho kinase-mediated Ezrin phosphorylation at threonine 567. J Biol Chem 2009; 284: 27456–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu SJ, Sun YM, Tian DF, He YC, Zeng L, He Y, et al. Down-regulated NM23-H1 expression is associated with intracranial invasion of nasopharyngeal carcinoma. Br J Cancer 2008; 98: 363–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho Y, Tsao SW, Zeng M, Lui VW. STAT3 as a therapeutic target for Epstein-Barr virus (EBV)-associated nasopharyngeal carcinoma. Cancer Lett 2013; 330: 141–9. [DOI] [PubMed] [Google Scholar]
- Lui VW, Wang EY, Ho Y, Hong B, Wong SC, Tao Q, et al. STAT3 activation contributes directly to Epstein-Barr virus-mediated invasiveness of nasopharyngeal cancer cells in vitro. Int J Cancer 2009; 125: 1884–93. [DOI] [PubMed] [Google Scholar]
- Liu YP, Tan YN, Wang ZL, Zeng L, Lu ZX, Li LL, et al. Phosphorylation and nuclear translocation of STAT3 regulated by the Epstein-Barr virus latent membrane protein 1 in nasopharyngeal carcinoma. Int J Mol Med 2008; 21: 153–62. [PubMed] [Google Scholar]
- Tsang CM, Cheung YC, Lui VW. Berberine suppresses tumorigenicity and growth of nasopharyngeal carcinoma cells by inhibiting STAT3 activation induced by tumor associated fibroblasts. BMC Cancer 2013; 13: 619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo CL, Chi CW, Liu TY. Modulation of apoptosis by berberine through inhibition of cyclooxygenase-2 and Mcl-1 expression in oral cancer cells. In Vivo 2005; 19: 247–52. [PubMed] [Google Scholar]
- Refaat A, Abd-Rabou A, Reda A. TRAIL combinations: The new “trail” for cancer therapy. Oncol Lett 2014; 7: 1327–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Refaat A, Abdelhamed S, Yagita H, Inoue H, Yokoyama S, Hayakawa Y, et al. Berberine enhances tumor necrosis factor-related apoptosis-inducing ligand-mediated apoptosis in breast cancer. Oncol Lett 2013; 6: 840–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sha SM, Zhang YG, Xu B, Wang HH, Kong XY, Wu KC. Effect of berberine on cell proliferation and apoptosis in gastric carcinoma cells. Modern Oncol 2011; 19: 629–33. [Google Scholar]
- Lou JL, Qiu QY, Lin HS, Qi X, Pei YX, He XJ, et al. The effect of berberine on cell proliferation, cell cycle and CD44V6 expression in gastric cancer cell. Chin J Immunol 2004; 20: 315–7. [Google Scholar]
- Lai Y, Shen Y, Liu XH, Zhang Y, Zeng Y, Liu YF. Interleukin-8 induces the endothelial cell migration through the activation of phosphoinositide 3-kinase-Rac1/RhoA pathway. Int J Biol Sci 2011; 7: 782–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ning Y, Manegold PC, Hong YK, Zhang W, Pohl A, Lurje G, et al. Interleukin-8 is associated with proliferation, migration, angiogenesis and chemosensitivity in vitro and in vivo in colon cancer cell line models. Int J Cancer 2011; 128: 2038–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantena SK, Sharma SD, Katiyar SK. Berberine, a natural product, induces G1-phase cell cycle arrest and caspase-3-dependent apoptosis in human prostate carcinoma cells. Mol Cancer Ther 2006; 5: 296–308. [DOI] [PubMed] [Google Scholar]
- Eom KS, Hong JM, Youn MJ, Youn MJ, So HS, Park R, et al. Berberine induces G1 arrest and apoptosis in human glioblastoma T98G cells through mitochondrial/caspases pathway. Biol Pharm Bull 2008; 31: 558–62. [DOI] [PubMed] [Google Scholar]
- Zhang XZ, Wang N, Liu DW, Tang GY, Zhang HY. Synergistic inhibitory effect of berberine and d-linmonene on human gastric carcinoma cell line MGC803. J Med Food 2014; 17: 955–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li GY, Yang LX, Wang YP. The study apoptosis of human gastric carcinoma BGC-823 cell induced by berberine. Pharmacol Clin Chin 2005; 21: 16–8. [Google Scholar]
- Chen G, Ke SD, Wang QG, Hu SM. Effect of berberine on proliferation and apoptosis of gastric adenocarcinoma cells AGS. Res Integr Tradit West Med 2011; 3: 4–7. [Google Scholar]
- Lin CC, Lin SY, Chung JG, Lin JP, Chen GY, Kao ST. Down-regulation of cyclin B1 and up-regulation of Wee1 by berberine promotes entry of leukemia cells into the G2/M-phase of the cell cycle. Anticancer Res 2006; 26: 1097–104. [PubMed] [Google Scholar]
- Serafim TL, Oliveira PJ, Sardao VA, Perkins E, Parke D, Holy J. Different concentrations of berberine result in distinct cellular localization patterns and cell cycle effects in a melanoma cell line. Cancer Chemother Pharmacol 2008; 61: 1007–18. [DOI] [PubMed] [Google Scholar]
- Wang N, Feng Y, Zhu M, Tsang CM, Man K, Tong Y, et al. Berberine induces autophagic cell death and mitochondrial apoptosis in liver cancer cells: the cellular mechanism. J Cell Biochem 2010; 111: 1426–36. [DOI] [PubMed] [Google Scholar]
- Dai JY, Dou KF, Wang CH, Zhao P, Lau WB, Tao L, et al. The interaction of HAb18G/CD147 with integrin alpha6beta1 and its implications for the invasion potential of human hepatoma cells. BMC Cancer 2009; 9: 337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuang YH, Chen X, Su J, Wu LS, Liao LQ, Li D, et al. RNA interference targeting the CD147 induces apoptosis of multi-drug resistant cancer cells related to XIAP depletion. Cancer Lett 2009; 276: 189–95. [DOI] [PubMed] [Google Scholar]
- Hou Q, Tang X, Liu HQ, Tang J, Yang Y, Jing X, et al. Berberine induced cell death in human hepatoma cells in vitro by downregulating CD147. Cancer Sci 2011; 102: 1287–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji C, Yang B, Yang YL, He SH, Miao DS, He L, et al. Exogenous cell-permeable C6 ceramide sensitizes multiple cancer cell lines to doxorubicin-induced apoptosis by promoting AMPK activation and Mtorc1 inhibition. Oncogene 2010; 29: 6557–68. [DOI] [PubMed] [Google Scholar]
- Yang XL, Huang N. Berberine induces selective apoptosis through the AMPK-mediated mitochondrial/caspase pathway in hepatocellular. Mol Med Rep 2013; 8: 505–10. [DOI] [PubMed] [Google Scholar]
- Wang XN, Han X, Xu LN, Yin LH, Xu YW, Qi Y, et al. Enhancement of apoptosis of human hepatocellular carcinoma SMMC-7721 cells through synergy of berberine and evodiamine. Phytomedicine 2008; 15: 106–8. [DOI] [PubMed] [Google Scholar]
- Wang L, Wei D, Han X, Zhang W, Fan C, Zhang J, et al. The combinational effect of vincristine and berberine on growth inhibition and apoptosis induction in hepatoma cells. J Cell Biochem 2014; 115: 721–30. [DOI] [PubMed] [Google Scholar]
- Wang RH. Pathological change of HEP-G2 cells induced by radioactive particle 125I combined with berberine. Chin J Curr Adv Gen Surg 2012; 15: 841–4. [Google Scholar]
- Hur JM, Hyun MS, Lim SY, Lee WY, Kim D. The combination of berberine and irradiation enhances anti-cancer effects via activation of p38 MAPK pathway and ROS generation in human hepatoma cells. J Cell Biochem 2009; 107: 955–64. [DOI] [PubMed] [Google Scholar]
- Wang N, Feng Y, Lau EP, Tsang C, Ching Y, Man K, et al. F-actin reorganization and inactivation of rho signaling pathway involved in the inhibitory effect of Coptidis Rhizoma on hepatoma cell migration. Integr Cancer Ther 2010; 9: 354–64. [DOI] [PubMed] [Google Scholar]
- Liu B, Wang GS, Yang J, Pan X, Yang Z, Zang L. Berberine inhibits human hepatoma cell invasion without cytotoxicity in healthy hepatocytes. PloS One 2011; 6: e21416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma C, Tang KL, Liu Q, Zhu R, Cao Z. Calmodulin as a potential target by which berberine induces cell cycle arrest in human hepatoma Bel7402 cells. Chem Biol Drug Des 2013; 81: 775–83. [DOI] [PubMed] [Google Scholar]
- Choi MS, Yuk DY, Oh JH, Jung HY, Han SB, Moon DC, et al. Berberine inhibits human neuroblastoma cell growth through induction of p53-dependent apoptosis. Anticancer Res 2008; 28: 3777–84. [PubMed] [Google Scholar]
- Choi MS, Oh JH, Kim SM, Jung HY, Yoo HS, Lee YM, et al. Berberine inhibits p53-dependent cell growth through induction of apoptosis of prostate cancer cells. Int J Oncol 2009; 34: 1221–30. [PubMed] [Google Scholar]
- Wang N, Zhu MF, Wang XB, Tan HY, Tsao SW, Feng Y. Berberine-induced tumor suppressor p53 up-regulation gets involved in the regulatory network of MIR-23a in hepatocellular carcinoma. Biochim Biophys Acta 2014; 1839: 849–57. [DOI] [PubMed] [Google Scholar]
- Cai YC, Xia Q, Luo RZ, Huang P, Sun Y, Shi Y, et al. Berberine inhibits the growth of human colorectal adenocarcinoma in vitro and in vivo. J Nat Med 2014; 68: 53–62. [DOI] [PubMed] [Google Scholar]
- Chang JR, Wang JH, Kuang ZY, Deng RD, Gui SH. Regulation mechanism of berberine and evodiamine on cell cycle by miR-17-92 cluster in colorectal cancer HT29 cells. Pharmacol Clin Chin Mater Med 2014; 30: 19–22. [Google Scholar]
- Wu K, Yang JX, Zhou QX. Study on the effects of berberine on colon carcinoma in vitro. Chin Pharm 2010; 21: 1360–1. [Google Scholar]
- Wheeler DL, Dunn EF, Harari PM. Understanding resistance to EGFR inhibitors-impact on future treatment strategies. Nat Rev Clin Oncol 2010; 7: 493–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LH, Cao HL, Lu N, Liu L, Wang B, Hu T, et al. Berberine inhibits proliferation and down-regulates epidermal growth factor receptor through activation of Cb1 in colon tumor cells. PLoS One 2013; 8: e56666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chidambara Murthy KN, Jayaprakasha GK, Patil BS. The natural alkaloid berberine targets multiple pathways to induce cell death in cultured human colon cancer cells. Eur J Pharmacol 2012; 688: 14–21. [DOI] [PubMed] [Google Scholar]
- Wang LH, Liu LP, Shi Y, Cao H, Chaturvedi R, Calcutt MW, et al. Berberine induces caspase-independent cell death in colon tumor cells through activation of apoptosis-inducing factor. PLoS One 2012; 7: e36418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu K, Yang JX, Zhou QX. Preventive effect of berberine on experimental colon cancer and relationship with cyclooxygenase-2 expression. Chin Chin Mat Med 2010; 35: 2768–72. [PubMed] [Google Scholar]
- Kaur J, Sanyal SN. Modulation of inflammatory changes in early stages of colon cancer through activation of PPARgamma by diclofenac. Eur J Cancer Prev 2010; 19: 319–27. [DOI] [PubMed] [Google Scholar]
- Dionne S, Levy E, Levesque D, Seidman EG. PPARgamma ligand 15-deoxy-delta 12,14-prostaglandin J2 sensitizes human colon carcinoma cells to TWEAK-induced apoptosis. Anticancer Res 2010; 30: 157–66. [PubMed] [Google Scholar]
- Wu K, He BC, Zhou QX. Preventive and curative effects of berberine on experimental colon cancer in rats and its relationship to expression of peroxisome proliferator-activated receptor γ. Chin J Biologicals 2011; 24: 952–6. [Google Scholar]
- Thirupurasundari CJ, Padmini R, Devaraj SN. Effect of berberine on the antioxidant status, ultrastructural modifications and protein bound carbohydrates in azoxymethane-induced colon cancer in rats. Chem Biol Interact 2008; 177: 190–5. [DOI] [PubMed] [Google Scholar]
- Lee DU, Kang YJ, Park MK, Lee YS, Seo HG, Kim TS, et al. Effects of 13-alkyl-substituted berberine alkaloids on the expression of COX-II, TNF-alpha, iNOS, and IL-12 production in LPS-stimulated macrophages. Life Sci 2003; 73: 1401–12. [DOI] [PubMed] [Google Scholar]
- Zhao WL, Li YB, Wang YX, Wang YX, Bi CW, Shao RG, et al. Synthesis and structure-activity relationship of 13-substituted berberine derivatives as anti-cancer agents. Chin Med Herald 2013; 10: 17–20. [Google Scholar]
- Ding YP, Ye XL, Zhu JY, Zhu XK, Li XG. Synthesis of 8-alkyl-13-bromo-berberine hydrochloride derivatives and their effect on proliferation of human HepG2 cell line. Chin Tradit Herb Drugs 2010; 41: 1765–70. [Google Scholar]
- Li Q, Zhang L, Zu Y, Liu T, Zhang B, He W. Generation of reactive oxygen species by a novel berberine-bile acid analog mediates apoptosis in hepatocarcinoma SMMC-7721 cells. Biochem Biophys Res Commun 2013; 433: 432–7. [DOI] [PubMed] [Google Scholar]
- Bi CW, Zhang CX, Li YB, Zhao WL, Shao RG, Mei L, et al. Synthesis and structure-activity relationship of cycloberberine as anti-cancer agent. Acta Pharm Sin 2013; 48: 1800–6. [PubMed] [Google Scholar]
- Bhowmik D, Hossain M, Buzzetti F, D'Auria R, Lombardi P, Kumar GS. Biophysical studies on the effect of the 13 position substitution of the anticancer alkaloid berberine on its DNA binding. J Phys Chem 2012; 116: 2314–24. [DOI] [PubMed] [Google Scholar]
- Jin X, Yan L, Li HJ, Wang RL, Hu ZL, Jiang YY, et al. Novel triazolyl berberine derivatives prepared via CuAAC click chemistry: synthesis, anticancer activity and structure-activity relationships. Anticancer Agents Med Chem 2015; 15: 89–98. [PubMed] [Google Scholar]
- Li YB, Zhao WL, Wang YX, Zhang CX, Jiang JD, Bi CW, et al. Discovery, synthesis and biological evaluation of cycloprotoberberine derivatives as potential antitumor agents. Eur J Med Chem 2013; 68: 463–72. [DOI] [PubMed] [Google Scholar]
- Guamán Ortiz LM, Tillhon M, Parks M, Dutto I, Prosperi E, Savio M, et al. Multiple effects of berberine derivatives on colon cancer cells. BioMed Res Int 2014; 2014: 924585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guamán Ortiz LM, Croce AL, Aredia F, Sapienza S, Fiorillo G, Syeda TM, et al. Effect of new berberine derivatives on colon cancer cells. Acta Biochim Biophys Sin 2015; 47: 824–33. [DOI] [PubMed] [Google Scholar]
- Min YD, Kwon HC, Yang MC, Lee KH, Choi SU, Lee KR. Isolation of limonoids and alkaloids from Phellodendron amurense and their multidrug resistance (MDR) reversal activity. Acta Pharm Sin 2007; 30: 58–63. [DOI] [PubMed] [Google Scholar]
- Li B, Wang GH, Yang M, Xu ZJ, Zeng BB, Wang HY, et al. Overman rearrangement and PomeranzeFritsch reaction for the synthesis of benzoazepinoisoquinolones to discover novel antitumor agents. Eur J Med Chem 2013; 70: 677–84. [DOI] [PubMed] [Google Scholar]
- Li B, Wang GH, Xu ZJ, Zhang Y, Haung XG, Zeng BB, et al. Discovery of N-substituted 3-arylisoquinolone derivatives as antitumor agents originating from O-substituted 3-arylisoquinolines via [2,3] or [3,3] rearrangement. Eur J Med Chem 2014; 77: 204–10. [DOI] [PubMed] [Google Scholar]