Abstract
Traumatic brain injury (TBI) is a major cause of disability and death in patients who experience a traumatic injury. Mitochondrial dysfunction is one of the main factors contributing to secondary injury in TBI-associated brain damage. Evidence of compromised mitochondrial function after TBI has been, but the molecular mechanisms underlying the pathogenesis of TBI are not well understood. Silent information regulator family protein 1 (SIRT1), a member of the NAD+-dependent protein deacetylases, has been shown to exhibit neuroprotective activities in animal models of various pathologies, including ischemic brain injury, subarachnoid hemorrhage and several neurodegenerative diseases. In this study, we investigated whether SIRT1 also exert neuroprotective effect post-TBI, and further explored the possible regulatory mechanisms involved in TBI pathogenesis. A lateral fluid-percussion (LFP) brain injury model was established in rats to mimic the insults of TBI. The expression levels of SIRT1, p-p38, cleaved caspase-9 and cleaved caspase-3 were all markedly increased and reached a maximum at 12 h post-TBI. In addition, mitochondrial function was impaired, evidenced by the presence of swollen and irregularly shaped mitochondria with disrupted and poorly defined cristae, a relative increase of the percentage of neurons with low ΔΨm, the opening of mPTP, and a decrease in neuronal ATP content, especially at 12 h post-TBI. Pretreatment with the SIRT1 inhibitor sirtinol (10 mg/kg, ip) induced p-p38 activation, exacerbated mitochondrial damage, and promoted the activation of the mitochondrial apoptosis pathway. In contrast, pretreatment with the p38 inhibitor SB203580 (200 μg/kg, ip) significantly attenuated post-TBI-induced expression of both cleaved caspase-9 and cleaved caspase-3 and mitochondrial damage, whereas it had no effects on SIRT1 expression. Together, these results reveal that the 12 h after TBI may be a crucial time at which secondary damage occurs; the activation of SIRT1 expression and inhibition of the p38 MAPK pathway may play a neuroprotective role in preventing secondary damage post-TBI. For this reason, both SIRT1 and p38 are likely to be important targets to prevent secondary damage post-TBI.
Keywords: traumatic brain injury, lateral fluid-percussion, SIRT1, p38, mitochondria, sirtinol, SB203580, neuroprotection
Introduction
Traumatic brain injury (TBI) is one of the leading causes of morbidity and mortality worldwide, and its incidence is dramatically increasing1,2,3. In recent decades, great progress has been made in clarifying some of the pathophysiological mechanisms associated with TBI1,4,5,6 . TBI consists of a primary insult, which is the mechanical displacement of tissue that occurs at the time of injury, and a secondary insult, which occurs gradually and involves a number of cellular processes1,4,5,6,7,8. Because the primary insult is not amenable to treatment, the secondary brain insult has been the major focus of most studies to identify potential targets for treating TBI4. However, despite extensive research to develop new neuroprotective therapies, surgery and neurocritical care remain the only treatment options available for TBI patients.
Mechanisms underlying the pathophysiology of secondary brain insults include excitotoxicity, neuroinflammation, vascular abnormalities, mitochondrial dysfunction, free radical formation, and neuronal and glial cell death9,10,11,12,13. In particular, mitochondria play a crucial role in the negative downstream effects of TBI2,4,7. Normal mitochondrial function is essential for maintaining neuronal metabolic homeostasis but is disturbed in TBI2,14. Studies have reported that the impairment of energy production caused by mitochondrial dysfunction is strongly associated with the pathogenesis of ischemia and TBI1,7,15. Similarly, patients with profound mitochondrial impairment after TBI have poor prognoses1,2. Silent information regulator family protein 1 (SIRT1), a nicotinamide adenine dinucleotide (NAD+)-dependent histone deacetylase, has been shown to improve mitochondrial biogenesis in animal models and cultured neurons16,17,18. Furthermore, several studies have suggested that activation of SIRT1 may rescue mitochondrial function and block apoptosis in cerebral ischemia and neurodegenerative diseases16,19,20. Thus, we propose that SIRT1 provides a new therapeutic target for TBI.
Importantly, some signaling molecules downstream of TBI have already been characterized. For example, mitogen-activated protein kinase (MAPK) cascades, such as the p38, extracellular signal-regulated kinase (ERK) and c-Jun N-terminal kinase (JNK) cascades, have been implicated in neuronal apoptosis after brain ischemia21,22,23. p38 MAPK has also been shown to be involved in mitochondrial dynamics in cerebral ischemic injury, thus suggesting a potential neuroprotective benefit of p38 inhibition21. In recent years, in both myocardial ischemia-reperfusion and acute lung injury, the effect of SIRT1 activation in correlation with the JNK, ERK and p38 MAPK pathways has been investigated24,25. It has been shown that SIRT1 synergistically interacts with p-ERK in mediating neuronal apoptosis induced by mechanical injury both in vitro and in vivo, thus indicating that SIRT1 may interact with the MAPK pathway and consequently promote neuronal survival26.
Therefore, we hypothesized that both SIRT1 and the MAPK pathway are activated post-TBI and that these factors may interact and together mediate the downstream effects of TBI. To this end, we established a lateral fluid-percussion (LFP) brain injury rat model to mimic the primary and secondary insults of TBI. We then analyzed expression of SIRT1, p-p38, p-JNK, p-ERK1/2, cleaved caspase-9 and cleaved caspase-3 post-induced brain injury. We also observed mitochondrial injury-related indicators, such as the opening of the mitochondrial permeability transition pores (mPTP) and changes in the low mitochondrial membrane potential (ΔΨm) and ATP. We further characterized the interaction between SIRT1 and the p38 MAPK pathway by specifically inhibiting either SIRT1 or p38 to determine whether SIRT1 has a neuroprotective effect via the p38 MAPK pathway in a model of TBI.
Materials and methods
Animals
Male Sprague-Dawley rats (body weight 220–250 grams) were housed individually under controlled environmental conditions with a 12-h light/dark cycle and were given unrestricted access to pellet food and water throughout the study. The animals were purchased from the Experimental Animal Center of the Southern Medical University in Guangzhou, China (Certification: SCXK (Guangzhou) 2011-0015). All surgical interventions were performed under anesthesia with a mixture of 13.3% urethane and 0.5% chloralose (0.65 mL/100 g body weight, ip) using a standardized protocol established in our laboratory. All efforts were made to reduce the number of animals used and to minimize animal discomfort. The experimental protocols were approved by the Animal Care and Use Committee of the Southern Medical University, Guangzhou, China. None of the authors are members of this committee. The care of animals was in accordance with the guidelines of the US National Institutes of Health and the Chinese National Institute of Health.
Experimental groups and drug administration
The first experiment to analyze the expression of relevant proteins and mitochondrial injury-related indicators in injured-side cortices at different time points was divided into six subgroups including a sham-treated control group, as well as groups sacrificed at 6, 12, 24, 48, and 72 h post-TBI (n=12 for each group). The second experiment was performed to study the effects of the SIRT1 inhibitor 12 h post-TBI. Rats were arranged in four groups, including the sham-treated control group, TBI group, TBI vehicle group [dimethyl sulfoxide (DMSO; Sigma St Louis, MO); intraperitoneal (30 μg/kg) 30 min before TBI], and the TBI+sirtinol group (sirtinol, Selleck, USA, intraperitoneal, 10 mg/kg27,28, 30 min before TBI, n=12 for each group). The third experiment was conducted to explore the effects of the p38 inhibitor 12 h post-TBI. Rats were randomized into four groups, including the sham-treated control group, TBI group, TBI vehicle group [dimethyl sulfoxide (DMSO; Sigma St Louis, MO); intraperitoneal, 30 μg/kg, 30 min before TBI], and the TBI+SB203580 group [(SB203580, Life Technologies, USA), intraperitoneal, 200 μg/kg29, 30 min before TBI, n=9 for each group]. Sham-treated animals underwent identical preparatory procedures, including craniotomy, but were not injured.
Lateral fluid-percussion (LFP) brain injury
Surgical procedures were performed as previously described30,31,32,33,34. Briefly, anesthetized rats were placed in a stereotaxic frame. After incision of the scalp, the temporal muscles were reflected, and a 4.8 mm craniotomy was drilled (2.5 mm lateral to the sagittal sinus and centered between the bregma and lambda). A hollow female Luer-Lok fitting was placed directly over the dura and rigidly fixed using dental cement. Before the induction of trauma, the female Luer-Lok was connected to the fluid percussion injury device via a transducer (Biomedical Engineering Facility, Medical College of Virginia, USA). For the infliction of TBI, a metal pendulum was released from a pre-selected height, thus leading to a rapid injection of normal saline into the closed cranial cavity. A pulse of increased intracranial pressure of 21–23 ms duration was elicited, controlled, and recorded by an oscilloscope (Agilent 54622D, MEGAZoom, Germany). The severity of the injury was altered by adjusting the amount of force generated by the pendulum. For the present experiment, a severe injury was induced (3.5±0.2 atmospheres20,21). Sham-treated animals underwent identical preparatory procedures, including craniotomy, but were not injured.
Isolation of rat cortical neurons
Neurons are sensitive to hypoxic ischemia and require constant immersion in Dulbecco's modified Eagle's minimal essential medium (DMEM) throughout the process of neuron isolation. To rapidly isolate cortical neurons from the injured-side of the rat brain, we followed a previously described method with some minor modifications35,36,37. After completion of the above TBI procedures, the injured-side cortex was cut into fragments, and cells were dissociated by incubation for 30 min at 37 °C with 2 mg/mL papain in DMEM. To produce a purified population of cells, we used the immune adherence method. Cell suspensions were poured into anti-neural cell adhesion molecule (NCAM)-coated petri dishes (Millipore, USA) and placed on a shaker for 1 h, after which adhered cells were collected. Trypan blue was used to exclude non-viable cells.
Western blotting
Protein was extracted from the injured-side cortexes of animals from all treatment groups by using a Total Protein Extraction Kit (BestBio, Shanghai, China), according to the manufacturer's protocols. The protein concentrations of extracts were determined using an Enhanced BCA Protein Assay Kit (Beyotime Institute of Biotechnology, China). Western blot analysis was performed as previously described26,38 using primary antibodies against the following proteins: SIRT1, cleaved-caspase-9, cleaved-caspase-3, p-p38, p-ERK1/2, p-JNK, p38, ERK1/2, and JNK (all used at 1:1000; Abcam, Cambridge, UK) and β-actin (Beyotime Institute of Biotechnology, China). An HRP-conjugated anti-rabbit/mouse IgG antibody was used as the secondary antibody (Beyotime Institute of Biotechnology, China), and the signal was visualized using ECL substrate (Pierce, Rockford, IL, USA).
Morphological observation
The morphological changes in neuronal mitochondria were observed using transmission electron microscopy. The injured-side cortex tissue was fixed with 2.5% glutaraldehyde and stained with cacodylate-buffered osmium tetroxide (OsO4). Sections were prepared and examined under an electron microscope (Philips CM10; Philips, Eindhoven, The Netherlands)37,39,40.
Immunohistochemical staining
Immunohistochemical staining was performed as previously described38,41. The injured-side cortexes from animals in all treatment groups was removed and immediately immersed in 4% paraformaldehyde for over 24 h at 4 °C. Cortex sections (5 μm thick) were blocked in 3% H2O2 and 3% normal goat serum and incubated overnight with anti-mouse SIRT1 polyclonal antibodies (1:200, Abcam, England). The secondary antibodies, secondary biotinylated conjugates and diaminobenzidine were from the GTVisionTM III SP rabbit/mouse HRP kit (DAB) (Dako, Denmark). An examiner blinded to the experimental groups randomly counted the cells labeled with SIRT1 throughout five lesioned regions in the injured-side cortex under a 400× light microscope.
Measurement of mitochondrial membrane potential
The mitochondrial membrane potential (ΔΨm) was measured using the fluorescent probe JC-1 (Invitrogen, CA, USA). In mitochondria with normal membrane potentials, JC-1 forms aggregates that fluoresce red, whereas in damaged, depolarized mitochondria, JC-1 forms monomers that fluoresce green. Isolated neurons were incubated in DMEM containing 5 μmol/L JC-1 for 15 min at 37 °C. Relative fluorescence was subsequently measured by flow cytometry (BD FACSVerseTM; BD, USA). Data were analyzed using BD FAC Suite software37,42.
Determination of mPTP
The mitochondrial permeability transition pore (mPTP) opening was measured by incubating isolated neurons at room temperature for 15 min in the dark in DMEM containing 1 μmol/L calcein-AM (Invitrogen, CA, USA) and 2 mmol/L CoCl2. Cells were then analyzed using flow cytometry (BD FACSVerseTM; BD, USA) to quantify green fluorescence, and resulting mPTP values were determined using BD FAC Suite software37,42.
Measurement of cellular ATP
Neuronal ATP content was measured using a luciferase-based assay (CellTiter-Glo, Madison, WI, USA). This assay measures ATP through the energy-dependent luciferase/luciferin reaction and provides information on cell viability. The test was performed according to the manufacturer's instructions. Cells were counted in a hemocytometer by using the trypan blue exclusion method, and 100 μL CellTiter-Glo reagent was added to 100 μL cell suspension containing 10 000 cells in each well of a standard opaque-walled 96-well plate. Plates were allowed to incubate at room temperature for 10 min, and the luminescence was recorded in an automatic microplate reader (SpectraMax M5; Molecular Devices, Sunnyvale, CA, USA)37,42.
Statistical analysis
All data were analyzed for statistical significance using the SPSS 13.0 software (SPSS, Chicago, IL, USA). Data are expressed as the mean±SD. All data were analyzed by one-way analysis of variance combined with Tukey's multiple-comparisons test. P values <0.05 were considered to be statistically significant.
Results
SIRT1 expression is significantly elevated in injured cortex post-TBI
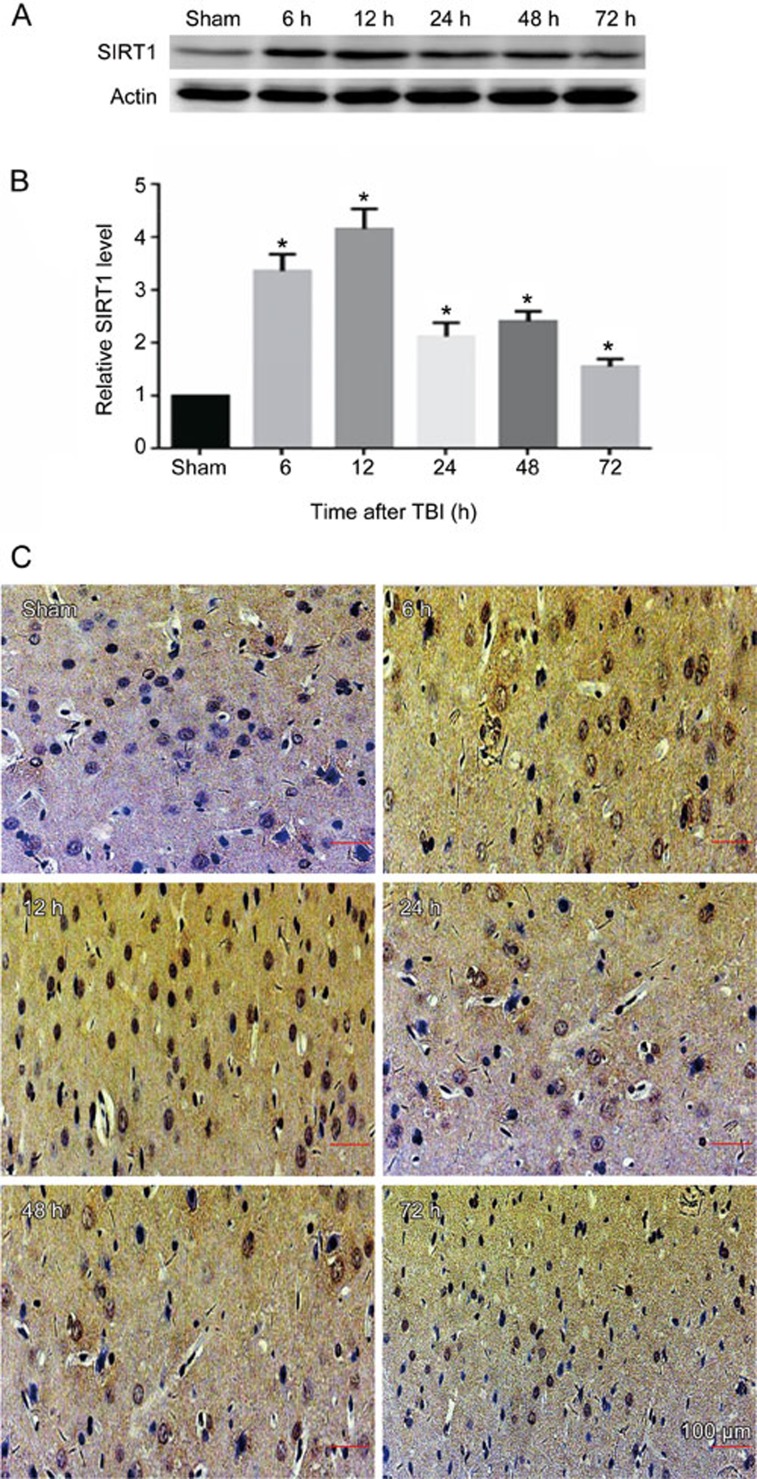
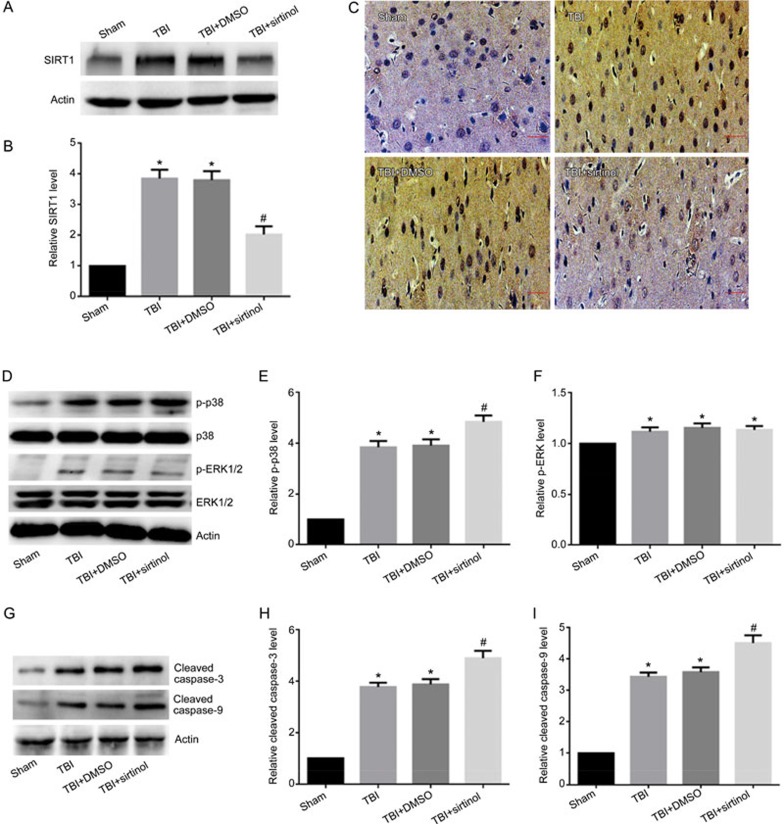
We analyzed the expression of SIRT1 protein in the cortexes of injured-side rat brains exposed to TBI induced by LFP at various time points by Western blotting. We determined that the level of SIRT1 expression in the sham-treated group was relatively low. SIRT1 expression increased 6 h post-TBI, peaked after 12 h, and remained high 24, 48, and 72 h post-TBI (Figure 1A, 1B). The expression of SIRT1 was also analyzed by immunohistochemical staining. We similarly found that SIRT1 was highly expressed in the cortex in the 12-h post-TBI group, a result consistent with the results from the Western blot analysis (Figure 1C).
Figure 1.
SIRT1 protein expression is increased in the injured cortex of rats exposed to TBI induced by LFP. Injured-side cortices were isolated at 6, 12, 24, 48, and 72 h post-TBI. (A) The expression of SIRT1 and β-actin. (B) Quantification of Western blots for SIRT1. (C) The expression of SIRT1 was determined by immunohistochemical staining post-TBI. β-Actin was used as loading control. *P<0.05 vs sham group. n=6/group.
MAPK pathway is activated post-TBI
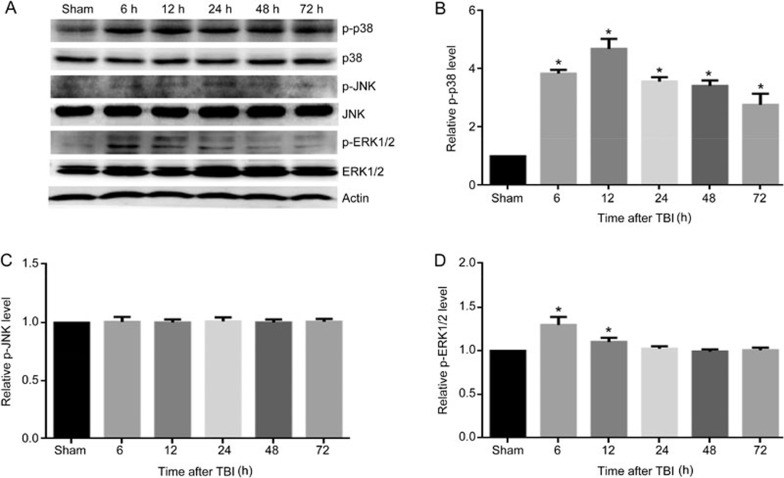
To explore whether mitogen-activated protein (MAP) kinases, a family of proteins involved in multiple phosphorylation/dephosphorylation signaling cascades, were involved in the pathogenesis of TBI, three well characterized MAPK family members, p38, ERK and JNK, were examined by Western blotting. The results showed that the protein level of phosphorylated p38 (p-p38) was significantly elevated at 6 h, peaked at 12 h, and remained elevated for 72 h post-TBI (Figure 2A, 2B). Although TBI induced an upregulation of phosphorylated ERK1/2 (p-ERK1/2) 6 and 12 h post-TBI, the expression levels increased slightly and declined quickly (Figure 2A, 2D). We also found that TBI elicited no effects on phosphorylated JNK (p-JNK) expression (Figure 2A, 2C). Together, these results suggest that the p38 MAPK pathway may play an important role in the immediate injury response post-TBI.
Figure 2.
The MAPK pathway is activated post-TBI. The injured-side cortex of the rat brain was isolated at 6, 12, 24, 48, and 72 h post-TBI. (A) The expression of p-p38, p38, p-ERK1/2, ERK1/2, p-JNK, JNK, and β-actin. (B–D) Quantification of Western blots for p-p38, p-JNK, and p-ERK1/2. The expression of p-p38 (A, B) and p-ERK1/2 (A, D) were significantly elevated in rats post-TBI, whereas there was no change in expression of p-JNK (A, C). β-Actin was used as loading control. n=6/group. Mean±SD. *P<0.05 vs sham group.
TBI induces severe mitochondrial damage in neurons of injured cortices and leads to apoptosis by triggering the mitochondrial pathway
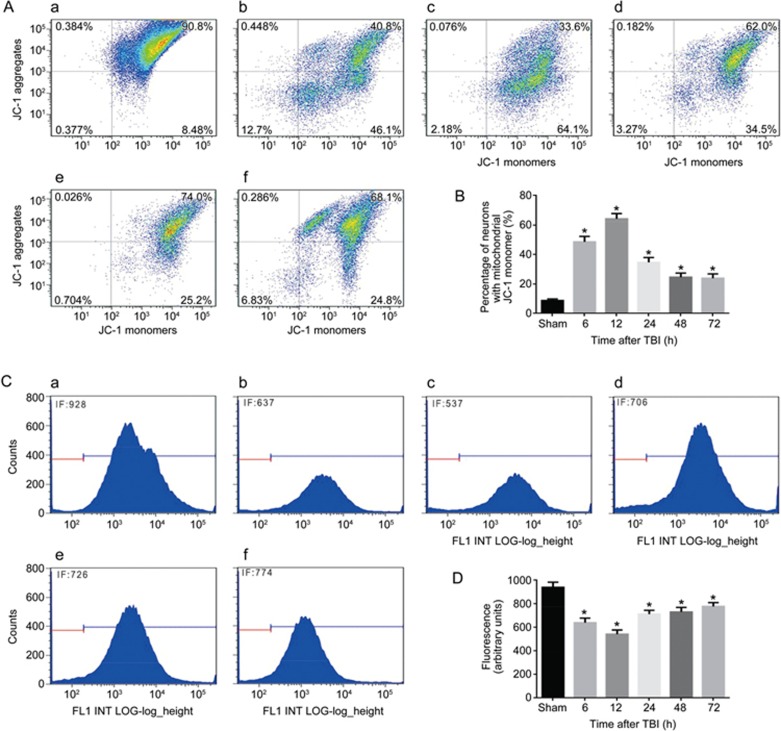
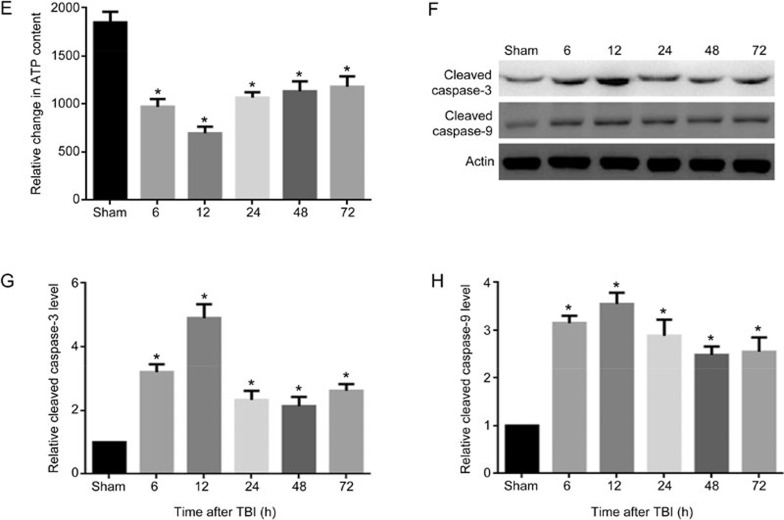
To assess the mitochondrial membrane potential, we measured ΔΨm of neuronal mitochondria that were isolated from the injured-side cortexes of the rat brains at 6, 12, 24, 48, and 72 h after TBI. To investigate mitochondrial function, neuronal mitochondrial depolarization (low ΔΨm) was assayed and expressed as the change in JC-1 fluorescence from red to green. We found that the percentage of neurons with low ΔΨm increased from 8.4% in the sham-treated group to 46% at 6 h post-TBI, further increased to 64% at 12 h, and then decreased to 34%, 25%, and 24% at 24, 48, and 72 h after TBI, respectively (Figure 3A, 3B). To further confirm whether TBI induces mitochondrial damage, the opening of mPTP was analyzed by flow cytometry. Normal neuronal mitochondrial fluorescence intensity was observed in the sham-treated group, whereas significantly less mitochondrial fluorescence intensity was detected in the TBI groups. In particular, the lowest fluorescence intensity was observed 12 h post-TBI (Figure 3C, 3D). To evaluate the function of neuronal mitochondria after TBI, neuronal ATP content was determined using the CellTiter-Glo luciferase bioluminescence method. The neuronal ATP content post-TBI also decreased significantly compared with that of the sham-treated group (Figure 3E). Then, we detected the level of cleaved caspase-3, the active form of caspase-3, to determine whether apoptosis occurred in post-TBI neurons. The results showed that the protein levels of cleaved caspase-3 were significantly increased at 6 h, peaked at 12 h, and then slightly decreased at 24, 48, and 72 h post-TBI (Figure 3F, 3G). We also analyzed the change in cleaved caspase-9, which is the key activation protein in the mitochondrial apoptosis pathway. We found that cleaved caspase-9 was also activated at 6 h and lasted for 72 h post-TBI (Figure 3F, 3H). These results showed that the mitochondria in rat neurons were damaged after TBI induced by LFP, especially 12 h after TBI, and that TBI probably leads to activation of the mitochondrial apoptosis pathway by triggering caspase-9/caspase-3 activation.
Figure 3A-3D.
TBI induces severe mitochondrial damage in neurons of injured cortices and leads to apoptosis by triggering the mitochondrial pathway. Neurons were isolated from the injured-side cortex of rat brains at 6, 12, 24, 48, and 72 h post-TBI. (A) The loss of ΔΨm was measured by JC-1 and analyzed by flow cytometry. (a–f) represent the sham group and 6, 12, 24, 48. and 72 h post-TBI, respectively. (B) Quantification of mitochondrial depolarization expressed as JC-1 monomer (green fluorescence) at different times post-TBI. (C) The mPTP opening was measured by staining with calcein-AM and CoCl2 and analyzed by flow cytometry for green fluorescence. (a–f) represent the sham group and 6, 12, 24, 48, and 72 h post-TBI, respectively. (D) Quantification of mitochondrial green fluorescence intensity post-TBI at different times as indicated. Neurons with low ΔΨm (A, B) and the mPTP opening (C, D) were increased. n=6/group. Mean±SD. *P<0.05 vs sham group.
Figure 3E-3H.
(E) Quantification of the change in neuronal ATP levels following TBI at different times as indicated. (F) The expression levels of cleaved caspase-3, cleaved caspase-9 and β-actin were detected by Western blotting, β-Actin was used as a loading control. (G) Quantification of Western blots for cleaved caspase-3. (H) Quantification of Western blots for cleaved caspase-9. ATP level in neurons was decreased (E). The expression of cleaved caspase-3 and cleaved caspase-9 were significantly elevated in rats post-TBI (F–H). n=6/group. Mean±SD. *P<0.05 vs sham group.
The SIRT1 inhibitor exacerbates TBI-induced mitochondrial damage, promotes neuronal apoptosis and activates p38 MAPK signaling
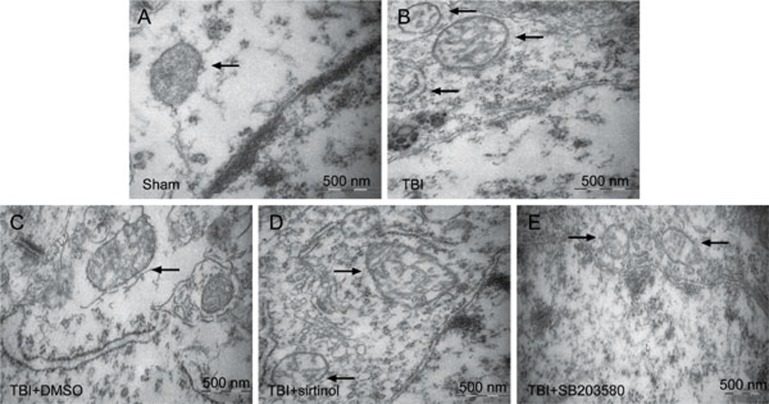
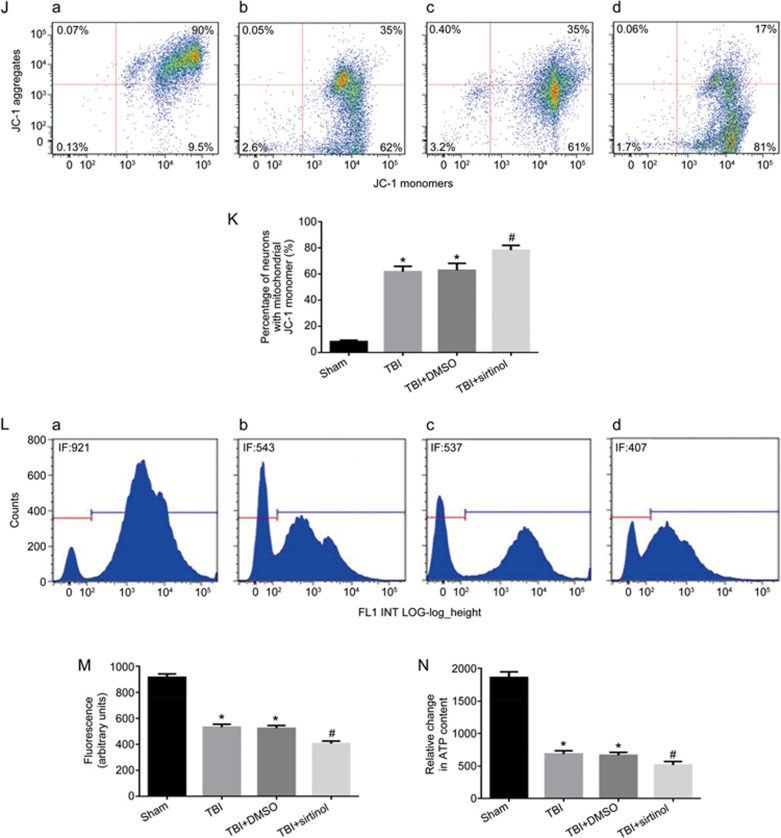
To demonstrate the effect of endogenous SIRT1 on neuronal survival, the SIRT1 inhibitor sirtinol (10 mg/kg) was injected into the intraperitoneal cavity 30 min before LFP-induced TBI27,28. Western blotting and immunohistochemical staining results demonstrated that the expression of SIRT1 12 h post-TBI was inhibited by sirtinol (Figure 4A–4C). In rats at 12 h post-TBI, compared with the TBI group, inhibition of SIRT1 resulted in a significant increase in p-p38, cleaved caspase-9 and cleaved caspase-3 (Figure 4D, 4E, 4G–4I), whereas the expression of p-ERK1/2 was not significantly changed (Figure 4D, 4F). Transmission electron microscopy was used to examine the mitochondrial morphology. Cells from the sham-treated group showed normal mitochondria with preserved membranes and cristae (Figure 5A). In contrast, the mitochondria appeared swollen and irregularly shaped with disrupted and poorly defined cristae in the 12 h post-TBI group and 12 h post-TBI+DMSO group (Figure 5B, 5C), and the SIRT1 inhibitor exacerbated these effects (Figure 5D). As shown in Figure 4, SIRT1 inhibition, compared with the TBI group, exacerbated mitochondrial damage post-TBI, including an increase in low ΔΨm (Figure 4J, 4K) and the opening of mPTP (Figure 4L, 4M) and a relative decrease in neuronal ATP 12 h post-TBI (Figure 4N). Together, these results suggest that SIRT1 may be involved in the mitochondrial apoptosis pathway post-TBI through the regulation of p38 MAPK signaling.
Figure 4A-AI.
SIRT1 inhibitor exacerbates TBI-induced mitochondrial damage, promotes neuronal apoptosis and activates p38 MAPK signaling. Rats were injected intraperitoneally with the SIRT1 inhibitor sirtinol (10 mg/kg) 30 min before LFP-induced TBI. After 12 h, rats were sacrificed. (A) The expression of SIRT1 and β-actin were detected by Western blot. β-Actin was used as loading control. (B) Quantification of Western blots for SIRT1. (C) The expression of SIRT1 was analyzed by immunohistochemical staining post-TBI. (D) The expression levels of p-p38, p38, p-ERK1/2, ERK1/2 and β-actin were detected by Western blot. β-Actin was used as loading control (n=3/group). (E) Quantification of Western blots for p-p38. (F) Quantification of Western blots for p-ERK1/2. (G) The expression of cleaved caspase-3, cleaved caspase-9 and β-actin were detected by Western blot. β-Actin was used as loading control. (H) Quantification of Western blots for cleaved caspase-3. (I) Quantification of Western blots for cleaved caspase-9. SIRT1 inhibitor decreased the expression of SIRT1 (A–C), and increased the expression of p-p38 (D, E), cleaved caspase-3 (G, H) and cleaved caspase-9 (G, I), and it have no effect on the expression of p-ERK1/2 (D, F). There was no difference between the TBI and the TBI+DMSO groups. n=6/group. Mean±SD. *P<0.05 vs sham group. #P<0.05 vs TBI group.
Figure 5.
Mitochondrial morphology was observed by transmission electron microscopy. Rats were injected intraperitoneally with the SIRT1 inhibitor sirtinol (10 mg/kg) 30 min or the p38 inhibitor SB203580 (200 μg/kg) 30 min before LFP-induced TBI. After 12 h, rats were sacrificed. (A–E) represent the sham group, TBI group, TBI+DMSO group, TBI+sirtinol group and TBI+SB203580, respectively. There was no difference between the TBI and the TBI+DMSO groups. Mitochondria are with poorly defined cristae in the TBI and TBI+DMSO group (B, C), the SIRT1 inhibitor exacerbated these alterations (C), while the p-38 inhibitor could partially alleviate mitochondria damage. n=3/group.
Figure 4J-4N.
SIRT1 inhibitor exacerbates TBI-induced mitochondrial damage, promotes neuronal apoptosis and activates p38 MAPK signaling. Rats were injected intraperitoneally with the SIRT1 inhibitor sirtinol (10 mg/kg) 30 min before LFP-induced TBI. After 12 h, rats were sacrificed. (J) The loss of ΔΨm was measured by JC-1 and analyzed by flow cytometry. (a–d) represent the sham group, TBI group, TBI+DMSO group and TBI+sirtinol group, respectively. (K) Quantification of mitochondrial depolarization expressed as JC-1 monomer (green fluorescence) in all treatment groups post-TBI. (L) mPTP opening was measured by staining with calcein-AM and CoCl2 and analyzed by flow cytometry for green fluorescence. (a–d) represent the sham group, TBI group, TBI+DMSO group and TBI+sirtinol group, respectively. (M) Quantification of mitochondrial green fluorescence intensity post-TBI in all treatment groups. (N) Quantification of the change in neuronal ATP levels following TBI in all treatment groups. SIRT1 inhibitor also increased the low ΔΨm (J, K) and the opening of mPTP (L, M), and decreased neuronal ATP levels (N). There was no difference between the TBI and the TBI+DMSO groups. n=6/group. Mean±SD. *P<0.05 vs sham group. #P<0.05 vsTBI group.
Inhibition of p-p38 alleviates mitochondrial damage and neuronal apoptosis
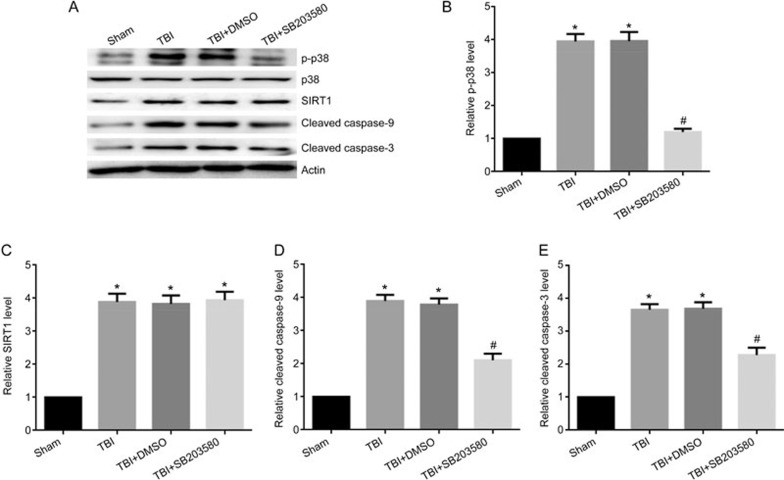
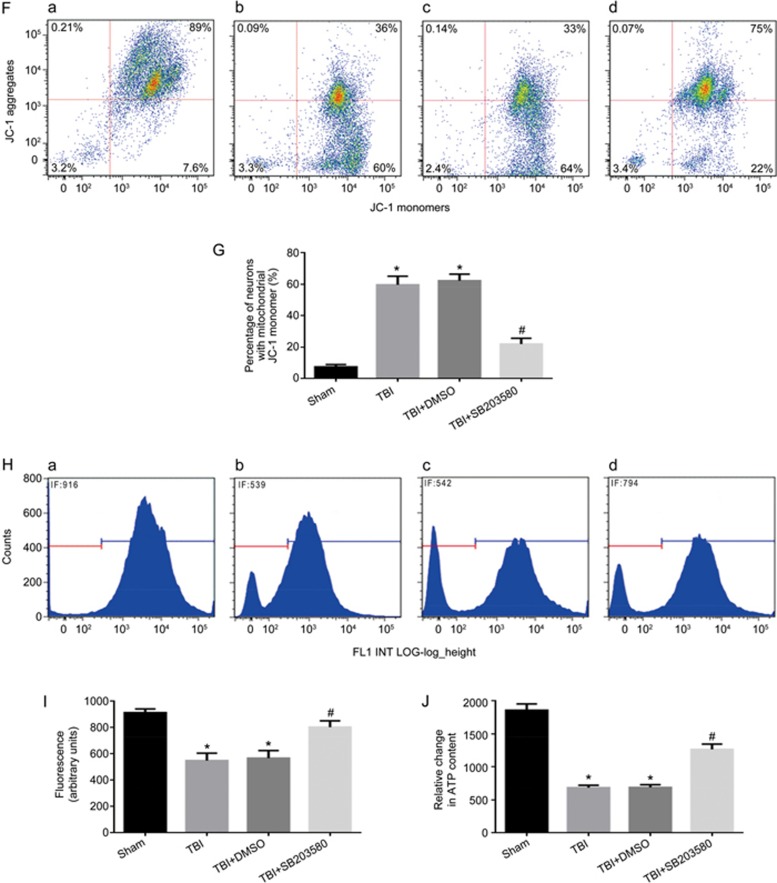
To determine whether the activation of the p38 MAPK pathway could regulate the level of SIRT1 by mediating the mitochondrial apoptosis pathway in vivo, rats were injected intraperitoneally with the p38 MAPK inhibitor SB203580 (200 μg/kg) 30 min before LFP-induced TBI29. After 12 h, rats were sacrificed. p38 inhibition, compared with that in the TBI group, significantly decreased the expression of p-p38 (Figure 6A, 6B), whereas it had no significant effect on the level of SIRT1 (Figure 6A, 6C). When p-p38 expression was inhibited, the activation of cleaved caspase-9 and cleaved caspase-3 was significantly decreased (Figure 6A, 6D, and 6E). The p38 inhibitor also alleviated the TBI-induced damage of neuronal mitochondria that resulted in swollen and irregularly shaped mitochondria with disrupted and poorly defined cristae 12 h post-TBI (Figure 5E). Furthermore, the low ΔΨm was significantly decreased (Figure 6F, 6G), the mitochondrial fluorescence intensity representing the opening of mPTP was significantly increased (Figure 6H, 6I) and the amount of neuronal ATP was significantly increased 12 h post-TBI (Figure 6J). Together, these results suggest that inhibition of p-p38 leads to a decrease in mitochondrial apoptosis post-TBI and has no significant effects on the level of SIRT1.
Figure 6A-6E.
Inhibition of p-p38 can alleviate mitochondrial damage and neuronal apoptosis. Rats were injected ip with the p38 inhibitor SB203580 (200 μg/kg) 30 min before TBI induced by LFP. After 12 h, rats were sacrificed. (A) The expression of p-p38, p38, SIRT1, cleaved caspase-9, cleaved caspase-3 and β-actin were detected by Western blot. β-Actin was used as loading control. (B) Quantification of Western blots for p-p38. (C) Quantification of Western blots for SIRT1. (D) Quantification of Western blots for cleaved caspase-9. (E) Quantification of Western blots for cleaved caspase-3. There was no difference between the TBI and the TBI+DMSO groups. P-38 inhibitor decreased the expression of p-p38 (A, B), cleaved caspase-9 (A, D) and cleaved caspase-3 (A, E). n=6/group. Mean±SD. *P<0.05 vs sham group. #P<0.05 vs TBI group.
Figure 6F-6J.
Inhibition of p-p38 can alleviate mitochondrial damage and neuronal apoptosis. Rats were injected ip with the p38 inhibitor SB203580 (200 μg/kg) 30 min before TBI induced by LFP. After 12 h, rats were sacrificed. (F) The loss of ΔΨm was measured by JC-1 and analyzed by flow cytometry. (a–d) represent the sham group, TBI group, TBI+DMSO group and TBI+SB203580 group, respectively. (J) Quantification of mitochondrial depolarization expressed as JC-1 monomer (green fluorescence) of all treatment groups post-TBI. (H) mPTP opening was measured by staining with calcein-AM and CoCl2 and analyzed by flow cytometry for green fluorescence. (a–d) represent the sham group, TBI group, TBI+DMSO group and TBI+ SB203580 group, respectively. (I) Quantification of mitochondrial green fluorescence intensity post-TBI of all treatment groups. (G) Quantification of the change in neuronal ATP levels following TBI of all treatment groups. There was no difference between the TBI and the TBI+DMSO groups. P-38 inhibitor decreased the low ΔΨm (F, G) and the opening of mPTP (H, I), and increased neuronal ATP levels (J). n=6/group. Mean±SD. *P<0.05 vs sham group. #P<0.05 vs TBI group.
Discussion
TBI is considered to be the leading cause of morbidity and mortality in young adults worldwide1,2. Although there are various degrees of severity of TBI, all forms of this injury have been shown to affect the function of mitochondria1. Mitochondrial dysfunction is recognized as the main contributing factor to secondary injury, which is the major cause of TBI-associated brain damage1. Thus, mitochondria may play a crucial role in the pathophysiological process of TBI1. Under normal conditions, mitochondria provide an essential function in maintaining metabolic homeostasis of neurons. Therefore, mitochondrial damage can lead to oxidative stress, subsequent apoptosis and decreased cellular energy production, as has been reported in multiple animal model studies1,4,7. In the present study, our results confirmed that mitochondrial function was impaired post-TBI, as indicated by the swollen and irregularly shaped appearance of the mitochondria with disrupted and poorly defined cristae, an increase in low ΔΨm and the opening of mPTP, and a decrease in relative ATP content post-TBI. Mitochondrial damage may initiate a cascade of events leading to further damage post-TBI. Increasing evidence suggests that mitochondrial dysfunction and increased apoptosis play important roles in the pathogenesis of brain damage. Caspase-9 has been shown to be a critical factor in the mitochondrial apoptosis pathway, and caspase-3 plays a key role in apoptotic cell death10,24. Our analysis revealed a significant increase in both cleaved caspase-9 and cleaved caspase-3 post-TBI, thus suggesting an increase in apoptosis in the brain post-TBI. In addition, our studies indicated that both mitochondrial damage and the occurrence of apoptosis peaked 12 h post-TBI; therefore, we speculated that the secondary injury might occur in the 12 h post-TBI. However, the mechanism by which this occurs remains to be elucidated.
Mitogen-activated protein kinases (MAPKs) are serine/threonine protein kinases that are involved in a number of cellular processes including stress response, apoptosis, and cell survival. MAPKs are relatively highly expressed in the central nervous system26. Activated MAPKs play an essential role in neural cell fate, specifically in the survival/death of neurons after ischemic brain injury21,23. One of the well-characterized MAPK family members, p38 MAPK, has been shown to be preferentially activated by various environmental stresses, such as heat shock, X-rays, and ultraviolet irradiation. After activation, p38 MAPK subsequently regulates apoptosis/autophagy, cell survival/death, and the inflammatory response23,43,44,45,46,47. Potential roles of p38 MAPK in neuronal apoptosis are receiving increasing attention. It has been shown in several recent studies that increased p38 MAPK activity plays a crucial role in neuronal death in response to stress stimuli48,49,50, whereas inhibition of p38 MAPK decreases cerebral ischemic injury and results in neuroprotection in vitro and in vivo48,51,52. In the present study, TBI caused a significant increase in p-p38 activity, neuronal apoptosis and mitochondrial damage, whereas the p38 MAPK inhibitor reduced this relative increase in p-p38, neuronal apoptosis and mitochondrial damage. This finding suggests that the p38 MAPK pathway is involved in the regulation of neuronal apoptosis and that inhibition of p38 activation may have a neuroprotective effect in the post-TBI brain.
Sirtuins, a family of NAD+-dependent deacetylases, have been implicated in longevity, aging, metabolism and resistance to oxidative stress. Specifically, SIRT1 has recently become a target for drug development, because it is involved in processes such as cancer, inflammation, cardiovascular disease, and diabetes and has crucial roles in stress-responsive signaling pathways27,28,53,54. In the brain, previous studies have shown that SIRT1 plays a role in neurodegenerative disorders, differentiation of stem cells, normal cognitive function and synaptic plasticity55,56,57. Furthermore, neuroprotective properties of SIRT1 have been described in ischemic stroke and subarachnoid hemorrhage27,28,38. Using Western blotting and immunohistochemical analyses, we demonstrated that SIRT1 levels were significantly increased in the brain post-TBI, with a peak at 12 h, a finding consistent with previous reports in models of subarachnoid hemorrhage and cerebral ischemia. This result suggests that SIRT1 upregulation might be involved in the damage response of TBI. Here, to explore the role of SIRT1 post-TBI, we pretreated rats with the SITR1 inhibitor sirtinol before initiation of TBI. We observed that sirtinol downregulated the protein level of SIRT1, which in turn further exacerbated mitochondrial damage and apoptosis 12 h post-TBI, thus indicating that SIRT1 may play a neuroprotective role and may prevent the occurrence of secondary damage post-TBI.
On the molecular level, several signaling pathways may be involved in the neuroprotection conferred by SIRT1 activation. SIRT1 over-expression has been reported to block LPS- and nicotine-induced phosphatidylinositol 3-kinase (PI3K), p38, JNK, ERK, protein kinase C (PKC) and nuclear factor kappa B (NF-κB) activation24,25,58,59. Moreover, the flavonoid icariin enhances neuronal survival after oxygen and glucose deprivation by increasing SIRT1 expression. Furthermore, the p38 inhibitor SB203580 suppresses the expression of SIRT1 induced by icariin, thus suggesting a potential relationship between SIRT1 and the p38 MAPK pathway in promoting neuronal survival60. Another study has recently reported that SIRT1 may protect lung tissue against burn-induced acute lung injury by attenuating apoptosis of pulmonary microvascular endothelial cells via p38 MAPK signaling24. To probe the potential relationship between SIRT1 and p38-induced mitochondrial damage and apoptosis of neurons post-TBI, we first analyzed the expression of cleaved caspase-3, the change in ΔΨm, the opening of mPTP, the amount of ATP and the activation of p38 MAPK in the presence of a SIRT1 inhibitor. We found that the SIRT1 inhibitor sirtinol triggered an increase in the expression of cleaved caspase-9 and cleaved caspase-3, exacerbated mitochondrial damage, and promoted the activation of p-p38. We subsequently used SB203580, an inhibitor of p38 phosphorylation, in a similar set of experiments. We found that p38 inhibition had no significant effects on the expression of SIRT1, thus suggesting that p38 is downstream of SIRT1. Furthermore, p38 inhibition also resulted in a significant reduction in mitochondrial apoptosis induced by TBI. Some studies have indicated that inhibition of p38 MAPK signaling attenuates apoptosis in cerebral ischemic injury, thus suggesting a prominent role of p38 MAPK in the protective effects of SIRT1 in preventing neuronal apoptosis. Here, we speculate that p38 may be modified by both phosphorylation and acetylation or that the phosphorylation of p38 can be affected by an acetylated protein; therefore, an increase in the expression of SIRT1 may result in an increase in acetylation of p38 or the upstream signal of p38, thus leading to an increase in the substrates of p38. At this point, when the tissue or cells are stressed, the phosphorylation of p38 may be enhanced. Therefore, the precise mechanism by which SIRT1 modulates p38 activity remain to be elucidated in future studies.
In conclusion, our study demonstrated that both mitochondrial damage and the occurrence of apoptosis peaked at 12 h post-TBI, thus leading to secondary brain injury. p38 MAPK was activated 6–12 h after the TBI stress process, which was involved in the genesis of mitochondrial damage and apoptosis during secondary brain injury. Meanwhile, SIRT1 was also activated at the same time points that p38 was activated. Therefore, activation of SIRT1 expression and inhibition of the p38 MAPK pathway may play a neuroprotective role in preventing the occurrence of secondary damage post-TBI. Therefore, both stimulation of SIRT1 and inhibition of p38 are likely to be important targets for preventing secondary brain damage.
Author contribution
Ke-sen ZHAO and Ming ZHAO participated in the research design; Hong YANG contributed to the writing of the manuscript; Li LI and Bi-ying ZHOU conducted the experiments; Zheng-tao GU and Feng LI performed the data analysis; and Mac MAEGELE contributed to developing the fluid percussion injury device.
References
- Hiebert JB, Shen Q, Thimmesch AR, Pierce JD. Traumatic brain injury and mitochondrial dysfunction. Am J Med Sci 2015; 350: 132–8. [DOI] [PubMed] [Google Scholar]
- Wang WX, Visavadiya NP, Pandya JD, Nelson PT, Sullivan PG, Springer JE. Mitochondria-associated microRNAs in rat hippocampus following traumatic brain injury. Exp Neurol 2015; 265: 84–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez SL, Labombarda F, Gonzalez DM, Mougel A, Guennoun R, Schumacher M, et al. Progesterone neuroprotection in spinal cord trauma involves up-regulation of brain-derived neurotrophic factor in motoneurons. J Steroid Biochem Mol Biol 2005; 94: 143–9. [DOI] [PubMed] [Google Scholar]
- Gajavelli S, Sinha VK, Mazzeo AT, Spurlock MS, Lee SW, Ahmed AI, et al. Evidence to support mitochondrial neuroprotection, in severe traumatic brain injury. J Bioenerg Biomembr 2015; 47: 133–48. [DOI] [PubMed] [Google Scholar]
- Katz DI, White DK, Alexander MP, Klein RB. Recovery of ambulation after traumatic brain injury. Arch Phys Med Rehabil 2004; 85: 865–9. [DOI] [PubMed] [Google Scholar]
- Ucar T, Tanriover G, Gurer I, Onal MZ, Kazan S. Modified experimental mild traumatic brain injury model. J Trauma 2006; 60: 558–65. [DOI] [PubMed] [Google Scholar]
- Boeck CR, Carbonera LS, Milioli ME, Constantino LC, Garcez ML, Rezin GT, et al. Mitochondrial respiratory chain and creatine kinase activities following trauma brain injury in brain of mice preconditioned with N-methyl-D-aspartate]. Mol Cell Biochem 2013; 384: 129–37. [DOI] [PubMed] [Google Scholar]
- Fujimoto S, Katsuki H, Kume T, Kaneko S, Akaike A. Mechanisms of oxygen glucose deprivation-induced glutamate release from cerebrocortical slice cultures. Neurosci Res 2004; 50: 179–87. [DOI] [PubMed] [Google Scholar]
- Rink A, Fung KM, Trojanowski JQ, Lee VM, Neugebauer E, McIntosh TK. Evidence of apoptotic cell death after experimental traumatic brain injury in the rat. Am J Pathol 1995; 147: 1575–83. [PMC free article] [PubMed] [Google Scholar]
- Clark RS, Kochanek PM, Watkins SC, Chen M, Dixon CE, Seidberg NA, et al. Caspase-3 mediated neuronal death after traumatic brain injury in rats. J Neurochem 2000; 74: 740–53. [DOI] [PubMed] [Google Scholar]
- Sullivan PG, Keller JN, Bussen WL, Scheff SW. Cytochrome c release and caspase activation after traumatic brain injury. Brain Res 2002; 949: 88–96. [DOI] [PubMed] [Google Scholar]
- Lifshitz J, Sullivan PG, Hovda DA, Wieloch T, McIntosh TK. Mitochondrial damage and dysfunction in traumatic brain injury. Mitochondrion 2004; 4: 705–13. [DOI] [PubMed] [Google Scholar]
- Ziebell JM, Morganti-Kossmann MC. Involvement of pro- and anti-inflammatory cytokines and chemokines in the pathophysiology of traumatic brain injury. Neurotherapeutics 2010; 7: 22–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraste M. Oxidative phosphorylation at the fin de siecle. Science 1999; 283: 1488–93. [DOI] [PubMed] [Google Scholar]
- Yang Y, Jiang S, Dong Y, Fan C, Zhao L, Yang X, et al. Melatonin prevents cell death and mitochondrial dysfunction via a SIRT1-dependent mechanism during ischemic-stroke in mice. J Pineal Res 2015: 61–70. [DOI] [PubMed]
- Sun Q, Hu H, Wang W, Jin H, Feng G, Jia N. Taurine attenuates amyloid beta 1-42-induced mitochondrial dysfunction by activating of SIRT1 in SK-N-SH cells. Biochem Biophys Res Commun 2014; 447: 485–9. [DOI] [PubMed] [Google Scholar]
- Khan RS, Dine K, Das SJ, Shindler KS. SIRT1 activating compounds reduce oxidative stress mediated neuronal loss in viral induced CNS demyelinating disease. Acta Neuropathol Commun 2014; 2: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan RS, Fonseca-Kelly Z, Callinan C, Zuo L, Sachdeva MM, Shindler KS. SIRT1 activating compounds reduce oxidative stress and prevent cell death in neuronal cells. Front Cell Neurosci 2012; 6: 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalla R, Donmez G. The role of sirtuins in Alzheimer's disease. Front Aging Neurosci 2013; 5: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayasena T, Poljak A, Smythe G, Braidy N, Munch G, Sachdev P. The role of polyphenols in the modulation of sirtuins and other pathways involved in Alzheimer's disease. Ageing Res Rev 2013; 12: 867–83. [DOI] [PubMed] [Google Scholar]
- Zhang XM, Zhang L, Wang G, Niu W, He Z, Ding L, et al. Suppression of mitochondrial fission in experimental cerebral ischemia: The potential neuroprotective target of p38 MAPK inhibition. Neurochem Int 2015; 90: 1–8. [DOI] [PubMed] [Google Scholar]
- Sugino T, Nozaki K, Takagi Y, Hattori I, Hashimoto N, Moriguchi T, et al. Activation of mitogen-activated protein kinases after transient forebrain ischemia in gerbil hippocampus. J Neurosci 2000; 20: 4506–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nozaki K, Nishimura M, Hashimoto N. Mitogen-activated protein kinases and cerebral ischemia. Mol Neurobiol 2001; 23: 1–19. [DOI] [PubMed] [Google Scholar]
- Bai X, Fan L, He T, Jia W, Yang L, Zhang J, et al. SIRT1 protects rat lung tissue against severe burn-induced remote ALI by attenuating the apoptosis of PMVECs via p38 MAPK signaling. Sci Rep 2015; 5: 10277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becatti M, Taddei N, Cecchi C, Nassi N, Nassi PA, Fiorillo C. SIRT1 modulates MAPK pathways in ischemic-reperfused cardiomyocytes. Cell Mol Life Sci 2012; 69: 2245–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Luo P, Guo Q, Li S, Zhang L, Zhao M, et al. Interactions between SIRT1 and MAPK/ERK regulate neuronal apoptosis induced by traumatic brain injury in vitro and in vivo. Exp Neurol 2012; 237: 489–98. [DOI] [PubMed] [Google Scholar]
- Hernandez-Jimenez M, Hurtado O, Cuartero MI, Ballesteros I, Moraga A, Pradillo JM, et al. Silent information regulator 1 protects the brain against cerebral ischemic damage. Stroke 2013; 44: 2333–7. [DOI] [PubMed] [Google Scholar]
- Hurtado O, Hernandez-Jimenez M, Zarruk JG, Cuartero MI, Ballesteros I, Camarero G, et al. Citicoline (CDP-choline) increases Sirtuin1 expression concomitant to neuroprotection in experimental stroke. J Neurochem 2013; 126: 819–26. [DOI] [PubMed] [Google Scholar]
- Yamashita S, Hirata T, Mizukami Y, Cui YJ, Fukuda S, Ishida K, et al. Repeated preconditioning with hyperbaric oxygen induces neuroprotection against forebrain ischemia via suppression of p38 mitogen activated protein kinase. Brain Res 2009; 1301: 171–9. [DOI] [PubMed] [Google Scholar]
- Dixon CE, Lyeth BG, Povlishock JT, Findling RL, Hamm RJ, Marmarou A, et al. A fluid percussion model of experimental brain injury in the rat. J Neurosurg 1987; 67: 110–9. [DOI] [PubMed] [Google Scholar]
- Hicks R, Soares H, Smith D, McIntosh T. Temporal and spatial characterization of neuronal injury following lateral fluid-percussion brain injury in the rat. Acta Neuropathol 1996; 91: 236–46. [DOI] [PubMed] [Google Scholar]
- Laurer HL, McIntosh TK. Experimental models of brain trauma. Curr Opin Neurol 1999; 12: 715–21. [DOI] [PubMed] [Google Scholar]
- Rolfe A, Sun D. Stem cell therapy in brain trauma: implications for repair and regeneration of injured brain in experimental TBI models 2015. In: Kobeissy FH, editor. Brain neurotrauma: molecular, neuropsychological, and rehabilitation aspects. Boca Raton (FL): CRC Press/Taylor & Francis; 2015. Chapter 42. Frontiers in Neuroengineering. [PubMed]
- Chen B, Mutschler M, Yuan Y, Neugebauer E, Huang Q, Maegele M. Superimposed traumatic brain injury modulates vasomotor responses in third-order vessels after hemorrhagic shock. Scand J Trauma Resusc Emerg Med 2013; 21: 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer GJ, Torricelli JR. Isolation and culture of adult neurons and neurospheres. Nat Protoc 2007; 2: 1490–8. [DOI] [PubMed] [Google Scholar]
- Ray B, Bailey JA, Sarkar S, Lahiri DK. Molecular and immunocytochemical characterization of primary neuronal cultures from adult rat brain: differential expression of neuronal and glial protein markers. J Neurosci Methods 2009; 184: 294–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Song R, Chen Y, Zhao M, Zhao KS. Polydatin — a new mitochondria protector for acute severe hemorrhagic shock treatment. Expert Opin Investig Drugs 2013; 22: 169–79. [DOI] [PubMed] [Google Scholar]
- Zhou XM, Zhang X, Zhang XS, Zhuang Z, Li W, Sun Q, et al. SIRT1 inhibition by sirtinol aggravates brain edema after experimental subarachnoid hemorrhage. J Neurosci Res 2014; 92: 714–22. [DOI] [PubMed] [Google Scholar]
- Barrientos SA, Martinez NW, Yoo S, Jara JS, Zamorano S, Hetz C, et al. Axonal degeneration is mediated by the mitochondrial permeability transition pore. J Neurosci 2011; 31: 966–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crouser ED, Julian MW, Joshi MS, Bauer JA, Wewers MD, Hart JM, et al. Cyclosporin A ameliorates mitochondrial ultrastructural injury in the ileum during acute endotoxemia. Crit Care Med 2002; 30: 2722–8. [DOI] [PubMed] [Google Scholar]
- Fu B, Zhang J, Zhang X, Zhang C, Li Y, Zhang Y, et al. Alpha-lipoic acid upregulates SIRT1-dependent PGC-1alpha expression and protects mouse brain against focal ischemia. Neuroscience 2014; 281C: 251–7. [DOI] [PubMed] [Google Scholar]
- Wang X, Song R, Bian HN, Brunk UT, Zhao M, Zhao KS. Polydatin, a natural polyphenol, protects arterial smooth muscle cells against mitochondrial dysfunction and lysosomal destabilization following hemorrhagic shock. Am J Physiol Regul Integr Comp Physiol 2012; 302: R805–14. [DOI] [PubMed] [Google Scholar]
- Harada J, Sugimoto M. An inhibitor of p38 and JNK MAP kinases prevents activation of caspase and apoptosis of cultured cerebellar granule neurons. Jpn J Pharmacol 1999; 79: 369–78. [DOI] [PubMed] [Google Scholar]
- Irving EA, Bamford M. Role of mitogen- and stress-activated kinases in ischemic injury. J Cereb Blood Flow Metab 2002; 22: 631–47. [DOI] [PubMed] [Google Scholar]
- Sridharan S, Jain K, Basu A. Regulation of autophagy by kinases. Cancers (Basel) 2011; 3: 2630–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui X, Kong N, Ye L, Han W, Zhou J, Zhang Q, et al. p38 and JNK MAPK pathways control the balance of apoptosis and autophagy in response to chemotherapeutic agents. Cancer Lett 2014; 344: 174–9. [DOI] [PubMed] [Google Scholar]
- Xia Z, Dickens M, Raingeaud J, Davis RJ, Greenberg ME. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science 1995; 270: 1326–31. [DOI] [PubMed] [Google Scholar]
- Li H, Zhou S, Wu L, Liu K, Zhang Y, Ma G, et al. The role of p38MAPK signal pathway in the neuroprotective mechanism of limb postconditioning against rat cerebral ischemia/reperfusion injury. J Neurol Sci 2015; 357: 270–5. [DOI] [PubMed] [Google Scholar]
- Wang W, Tang L, Li Y, Wang Y. Biochanin A protects against focal cerebral ischemia/reperfusion in rats via inhibition of p38-mediated inflammatory responses. J Neurol Sci 2015; 348: 121–5. [DOI] [PubMed] [Google Scholar]
- Takeda K, Ichijo H. Neuronal p38 MAPK signalling: an emerging regulator of cell fate and function in the nervous system. Genes Cells 2002; 7: 1099–111. [DOI] [PubMed] [Google Scholar]
- Liu XW, Ji EF, He P, Xing RX, Tian BX, Li XD. Protective effects of the p38 MAPK inhibitor SB203580 on NMDA-induced injury in primary cerebral cortical neurons. Mol Med Rep 2014; 10: 1942–8. [DOI] [PubMed] [Google Scholar]
- Strassburger M, Braun H, Reymann KG. Anti-inflammatory treatment with the p38 mitogen-activated protein kinase inhibitor SB239063 is neuroprotective, decreases the number of activated microglia and facilitates neurogenesis in oxygen-glucose-deprived hippocampal slice cultures. Eur J Pharmacol 2008; 592: 55–61. [DOI] [PubMed] [Google Scholar]
- Baur JA, Ungvari Z, Minor RK, Le Couteur DG, de Cabo R. Are sirtuins viable targets for improving healthspan and lifespan? Nat Rev Drug Discov 2012; 11: 443–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavu S, Boss O, Elliott PJ, Lambert PD. Sirtuins—novel therapeutic targets to treat age-associated diseases. NAT Rev Drug Discov 2008; 7: 841–53. [DOI] [PubMed] [Google Scholar]
- Haigis MC, Sinclair DA. Mammalian sirtuins: biological insights and disease relevance. Annu Rev Pathol 2010; 5: 253–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramadori G, Lee CE, Bookout AL, Lee S, Williams KW. Anderson J, et al. Brain SIRT1: anatomical distribution and regulation by energy availability. J Neurosci 2008; 28: 9989–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvanese V, Lara E, Suarez-Alvarez B, Abu DR, Vazquez-Chantada M, Martinez-Chantar ML, et al. Sirtuin 1 regulation of developmental genes during differentiation of stem cells. Proc Natl Acad Sci U S A 2010; 107: 13736–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SI, Min KS, Bae WJ, Lee YM, Lee SY, Lee ES, et al. Role of SIRT1 in heat stress- and lipopolysaccharide-induced immune and defense gene expression in human dental pulp cells. J Endod 2011; 37: 1525–30. [DOI] [PubMed] [Google Scholar]
- Park GJ, Kim YS, Kang KL, Bae SJ, Baek HS, Auh QS, et al. Effects of sirtuin 1 activation on nicotine and lipopolysaccharide-induced cytotoxicity and inflammatory cytokine production in human gingival fibroblasts. J Periodontal Res 2013; 48: 483–92. [DOI] [PubMed] [Google Scholar]
- Wang L, Zhang L, Chen ZB, Wu JY, Zhang X, Xu Y. Icariin enhances neuronal survival after oxygen and glucose deprivation by increasing SIRT1. Eur J Pharmacol 2009; 609: 40–4. [DOI] [PubMed] [Google Scholar]