Abstract
Recent studies confirm that chronic low-grade inflammation is closely associated with metabolic syndromes, and anti-inflammatory therapy is a potential approach for treating cardiovascular diseases and type 2 diabetes. Accumulating evidence suggests that GPR120 activation is a feasible solution to ameliorating chronic inflammation and improving glucose metabolism. In this study we investigated whether ginsenoside Rb2 (Rb2), which exhibited regulatory activities in glucose and lipid metabolism, affected GPR120 expression in lipopolysaccharide (LPS)-activated mouse macrophage RAW264.7 cells, and examined the contribution of GPR120 activation to reducing the LPS-induced inflammatory response. LPS (100 ng/mL) activated the macrophages, resulting in dramatic increases in TNF-α, IL-6, IL-1β and NO production. Treatment with a ω-3 fatty acid α-linolenic acid (ALA, 50 μmol/L) produced moderate reduction in LPS-stimulated inflammatory cytokines and NO production (TNF-α and IL-6 were decreased by 46% and 42%, respectively). Pre-incubation with Rb2 (1 or 10 μmol/L) for 12 h before ALA treatment dramatically amplified the inhibitory effects of ALA (TNF-α and IL-6 were decreased by 74% and 86%, respectively). Compared to the treatment with ALA alone, pre-incubation with Rb2 resulted in a more prominent reduction in LPS-stimulated expression of iNOS and COX-2 and LPS-stimulated IKK/NF-κB phosphorylation and MAPK pathway activation. Rb2 (0.1–100 μmol/L) dose- and time-dependently increased both mRNA and protein expression of GPR120 in RAW264.7 cells, but treatment with Rb2 alone did not exert anti-inflammatory effect in LPS-activated RAW264.7 cells. In RAW264.7 cells transfected with GPR120 shRNA, the ameliorating effects of Rb2 on LPS-induced inflammation were abolished. In conclusion, Rb2 exerts anti-inflammatory effect in LPS-stimulated mouse macrophage RAW264.7 cells in vitro by increasing GPR120 expression and subsequently enhancing ω-3 fatty acid-induced GPR120 activation.
Keywords: ginsenoside Rb2, LPS, α-linolenic acid, RAW264.7 cells, GPR120, inflammatory cytokines, iNOS, COX-2, IKK/NF-κB, MAPK pathway, diabetes, chronic inflammation
Introduction
Inflammation is a well-controlled physiological response evoked by injury and infection. However, prolonged inflammation is often harmful to health. Upon activation, macrophages, a key component of the immune system, release various inflammation-promoting factors. Long-term macrophage activation in tissues increases the abnormal production of inflammatory mediators such as tumor necrosis factor (TNF)-α, interleukin (IL)-6, IL-1β, nitric oxide and prostaglandin E2, stimulates a network of inflammatory signaling pathways and eventually leads to chronic low-grade inflammation1,2. As recent studies have confirmed that chronic low-grade inflammation is closely associated with metabolic syndromes3,4, anti-inflammatory therapy is a potential approach for treating cardiovascular disease and type 2 diabetes.
GPR120, an ω-3 fatty acid receptor, is abundantly expressed in monocytes, adipocytes and enteroendocrine cells5. Research has shown that GPR120 interacts with the Gαq family of heterotrimeric G proteins to elevate intracellular calcium. In turn, the calcium influx triggered by stimulated GPR120 increases the release of GLP-1, GIP and CCK in enteroendocrine L cells6,7. Ligand-activated GPR120 also recruits β-arrestin2, leading to internalization of the GPR120-β-arrestin2-TAB1 complex. It has been reported that an increase in the level of GPR120-β-arrestin2-TAB-1 complex induces inactivation of TAK1 in macrophages and represses activation of IKKβ/NF-κB and the JNK/AP1 pathway8. As a result, GPR120 activation inhibits lipopolysaccharide (LPS)-induced release of pro-inflammatory cytokines and nitric oxide from macrophages8. An animal study showed that ω-3 fatty acids exert their anti-inflammatory effects through GPR120 expressed on liver Kupffer cells9. In addition, a selective GPR120 agonist was recently reported to improve insulin resistance by attenuating tissue chronic inflammation in obese mice, suggesting its potential in treating metabolic diseases10.
Ginsenosides are known to be responsible for the major health-promoting properties of ginseng, a widely used traditional Chinese medicine. More than a hundred types of ginsenosides have been identified in the roots of different ginsengs, representing various pharmacological activities, including immune regulation and anti-tumor and anti-diabetes effects11,12,13. Ginsenoside Rg3 and Re are reported to exert anti-inflammatory effects by directly inhibiting iNOS and TLR14,15,16. As one of the main bioactive components of ginseng extracts, Rb2 improves glucose metabolism in hepatocytes by activating AMPK17 and reduces cholesterol and triacylglycerol levels in 3T3-L1 cells by reducing oxidative damage18. Most recently, researchers have found that Rb2 can upregulate GPR120 gene expression and exert anti-apoptosis effects in murine bone marrow-derived mesenchymal stem cells (BMMSCs)19. Although its regulatory activity on glucose and lipid metabolism has been demonstrated, the role of Rb2 in inflammation processes remains unclear. Here, we investigate the effect of Rb2 on GPR120 expression in murine macrophages (RAW264.7 cells) and test its contribution to reducing the LPS-induced inflammatory response. In addition, experiments were designed to identify the novel anti-inflammatory mechanism of Rb2, which involves improving the efficacy of ω-3 fatty acid-induced GPR120 activation in LPS-stimulated RAW264.7 cells.
Methods and materials
Reagents
Ginsenoside Rb2 (purity >98.0%) was purchased from Tauto Biotech Co, Ltd (Shanghai, China). Dulbecco's Modified Eagle's medium (DMEM), Fluo 4-AM and fetal bovine serum (FBS) for cell culture were obtained from Invitrogen (Carlsbad, USA). 5-Diphenyltetrazoliumbromide (MTT), lipopolysaccharide (LPS) from Escherichia coli, probenecid, α-linolenic acid (ALA) and 3-(4,5-dimethylthiazol-2-yl)-2 were obtained from Sigma-Aldrich (St Louis, USA). Primary antibody dilutions and sources were as follows: rabbit anti-iNOS (1:500) and goat anti-GPR120 (1:1000) were obtained from Santa Cruz Biotechnology (CA, USA); rabbit anti-COX-2, rabbit anti-IKKβ, rabbit anti-NF-κB, rabbit anti-p38, rabbit anti-JNK, rabbit anti-ERK, rabbit anti-phospho-IKKβ (Ser176/180), rabbit anti-phospho-NF-κB (Ser536), rabbit anti-phospho-p38 (Thr180/Tyr182), rabbit anti-phospho-JNK (Thr183/Tyr185), and rabbit anti-phospho-ERK (Thr202/Tyr204), all at 1:1000 dilution, were obtained from Cell Signaling Technology (MA, USA); mouse anti-β-actin (1:10000) was obtained from Sigma-Aldrich (MO, USA). The secondary antibodies anti-mouse IgG, anti-goat IgG and anti-rabbit IgG (1:10000) were purchased from Jackson Laboratory.
Cell culture
RAW264.7 cells, a murine macrophage cell line, were purchased from American Type Culture Collection (Manassas, USA) and maintained in DMEM supplemented with 10% FBS in a humidified incubator (5% CO2) at 37 °C.
MTT assay for cell viability
Cell viability was determined using the MTT assay. RAW264.7 cells (2×104 cells/well) were plated in 96-well plates and cultured overnight in growth medium. The cells were then incubated with Rb2 at the indicated concentrations in the absence or presence of ALA for 48 h before addition of the MTT reagent (0.5 mg/mL). After incubation for 4 h, the medium was removed, and the formazan crystals formed were dissolved with 100 μL dimethylsulfoxide (DMSO). Absorbance at a wavelength of 492 nm was measured using a FlexStation 3 (Molecular Devices, USA).
Calcium mobilization assay
A CHO cell line stably expressing GPR120 was seeded in 96-well cell culture plates (Corning) and incubated overnight in 5% CO2 at 37 °C. The cells were then incubated in Hank's Balanced Salt Solution containing 3 μmol/L Fluo 4-AM (a calcium-sensitive dye) and 2.5 mmol/L probenecid for 90 min. The cells were then washed three times with Hank's Balanced Salt Solution and subjected to equilibration for 10 min in Hank's Balanced Salt Solution containing probenecid prior to the assay. Intracellular calcium concentrations were measured as the difference between 585/525 ratios before and after addition of the test compounds using a Flexstation 3.0 plate reader (Molecular Devices). EC50 and Emax values for each curve were calculated using Prism 5.0 (GraphPad Software).
Construction and transfection of short hairpin RNA (shRNA)
GPR120-specific shRNA (target sequence: 5′-GATCCCCGAAATGACTTGTCTGTTATTCTCGAGAATAACAGACAAGTCATTTCGTTTTTGGAT-3′) or negative control shRNA (target sequence: 5′-TTCTCCGAACGTGTCACGT-3′) was inserted into the GV102 expression vector (Genechem, China). Plasmids were transiently transfected into RWA264.7 cells using X-tremeGENE HP DNA Transfection Reagent (Roche Diagnostic Systems, USA). After 12 h, the medium was replaced with fresh complete culture medium before additional drug treatment.
RNA isolation and quantitative RT-PCR
Total RNA was extracted from RAW264.7 cells using the TRIzol reagent (Invitrogen, USA) according to the manufacturer's instructions. Reverse-transcription reactions were performed using PrimeScript™ II 1st Strand cDNA Synthesis Kit (Takara, Japan) to obtain complementary DNA (cDNA). mRNA levels were quantified by real-time PCR using SYBR Green qPCR Master Mix (Biotool, China) and an ABI 7500 Fast Real-Time PCR machine (Applied Biosystems, USA). The primers used for PCR amplification are shown in Table 1. The relative expression levels of target genes were normalized using ribosomal 18s RNA.
Table 1. The primers used in RT-PCR.
| Gene name | Primer sequence |
|---|---|
| GPR120 | Forward: CAACCGCATAGGAGAAATCT Reverse: GGACTCCACATGATGAAGAA |
| 18s | Forward: TGTGCCGCTAGAGGTGAAATT Reverse: TGGCAAATGCTTTCGCTTT |
| IL-1β | Forward: GGTCAAAGGTTTGGAAGCAG Reverse: TGTGAAATGCCACCTTTTGA |
| IL-6 | Forward: TAGTCCTTCCTACCCCAATTTCC Reverse: TTGGTCCTTAGCCACTCCTTC |
| TNF-α | Forward: ATGGGAAGGGAATGAATCCACC Reverse: GTCCACATCCTGTAGGGCGTCT |
| iNOS | Forward: GAGCGAGTTGTGGATTGTC Reverse: CTCCTTTGAGCCCTTTGT |
| COX-2 | Forward: TGCCTGGTCTGATGATGTATG Reverse: AGTAGTCGCACACTCTGTTGT |
Enzyme-linked immunosorbent assay (ELISA)
ELISA was performed to assess cytokine production by RAW264.7 cells. Cells were plated in 24-well plates (105 cells/well). After overnight incubation, the cells were pretreated with Rb2 (1 or 10 μmol/L) for 12 h prior to incubation with ALA (50 μmol/L) for 1 h. The cells were then subjected to induction with LPS (100 ng/mL) for another 6 h. The culture supernatants were collected and stored at -20 °C for cytokine measurement. The amounts of TNF-α and IL-6 were measured using ELISA kits according to the manufacturer's instructions.
Western blotting analysis
RAW264.7 cells (2×105 cells/mL) were plated in growth medium in 12-well plates. After overnight incubation, the cells were pretreated with Rb2 (1 or 10 μmol/L) for 12 h prior to incubation with ALA (50 μmol/L) for 1 h. Following LPS (100 ng/mL) induction for another 15 min (for MAPKs and IKK/NF-κB) or 24 h (for iNOS and COX-2), the cells were harvested and lysed in RIPA buffer (Beyotime, China). The levels of protein expression were determined using Western blot analysis as previously described20 and then quantified using ImageJ software (National Institutes of Health, USA). The relative density of the target protein bands were normalized with the housekeeping protein β-actin (Actin).
Measurement of NO production
NO levels were assessed by measuring the nitrite concentration of the cell culture medium. Briefly, RAW264.7 cells (2×105 cells/mL) were plated in 12-well plate and cultured overnight in growth medium. The cells were then pretreated with Rb2 (1 or 10 μmol/L) for 12 h prior to incubation with ALA (50 μmol/L) for 1 h; the cells were then subjected to LPS (100 ng/mL) induction for another 12 h. The nitrite levels in the medium were immediately detected using Total NO Assay Kit (Beyotime, China) according to the manufacturer's instructions.
Statistical analysis
All data are expressed as the mean±standard deviation (SD) of at least 3 independent experiments. The significance of differences among groups was assessed by one-way ANOVA analysis followed by Dunnett's test or Student's t-test. Differences were considered statistically significant at a level of P<0.05.
Results
Rb2 dose- and time-dependently upregulated GPR120 gene expression in RAW264.7 macrophages
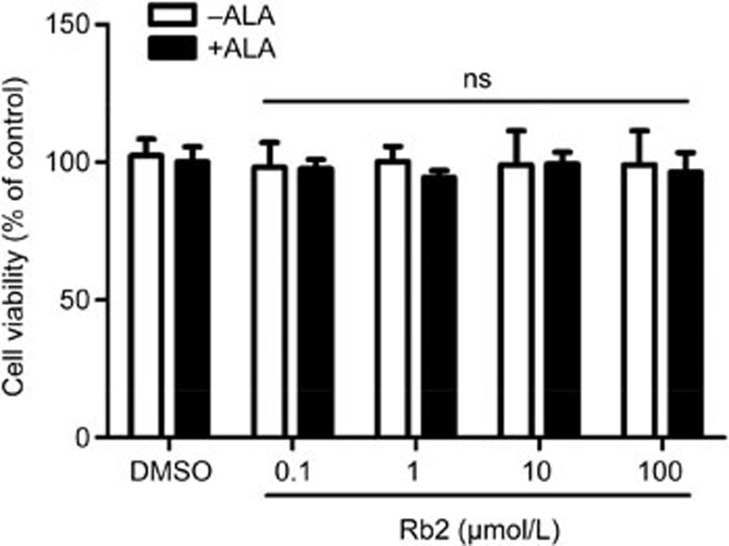
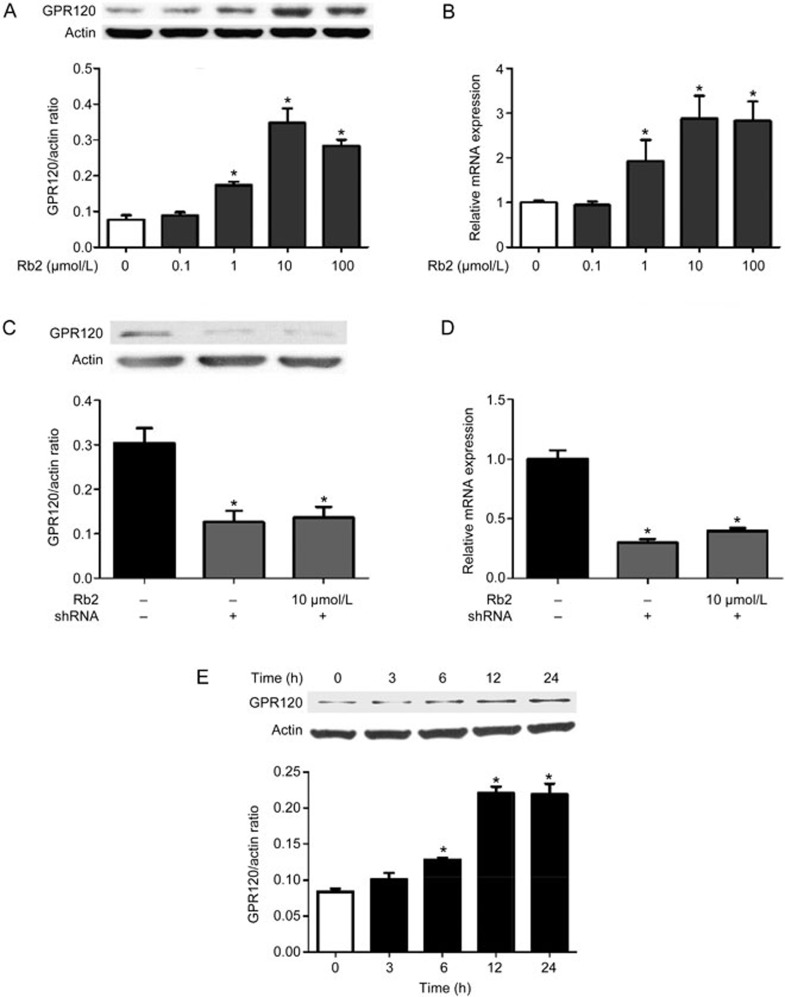
MTT assay results showed no obvious cytotoxicity of Rb2 (up to 100 μmol/L) toward RAW264.7 cells in the absence or presence of ALA (Figure 1). We next investigated the influence of Rb2 on GPR120 expression in RAW264.7 macrophages by treating the cells with Rb2 (0.1–100 μmol/L) for 12 h followed by harvesting and lysis. Subsequent Western blot analysis showed that expression of GPR120 was dose-dependently upregulated by Rb2 (Figure 2A). Furthermore, real-time PCR results indicated that incubation of RAW264.7 macrophages with Rb2 (10 μmol/L) for 12 h led to a 2.8-fold increase in GPR120 mRNA expression (Figure 2B). In addition, this increase in GPR120 expression stimulated by Rb2 was time dependent and began as early as 6 h (Figure 2E). These results indicated that Rb2 upregulates GPR120 expression in a dose- and time-dependent manner in RAW264.7 macrophages.
Figure 1.
Effects of Rb2 on the viability of RAW 264.7 macrophages. RAW264.7 cells were incubated with various concentrations of Rb2 in the absence or presence of ALA (50 μmol/L) for 48 h. Cell viability was determined by MTT assay. The data are presented as mean±SD of three independent experiments. 'ns' (no significant difference) means P>=0.05 vs vehicle (DMSO) group which acts as control.
Figure 2.
Effect of Rb2 on GPR120 expression in RAW264.7 macrophages. RAW264.7 cells were incubated with Rb2 (0.1–100 μmol/L) or vehicle (DMSO) for 24 h. The expression levels of GPR120 protein (A) in cell lysates were detected by Western blot analysis and mRNA (B) in cells were detected by quantitative RT-PCR. The expression levels of GPR120 protein (C) and mRNA (D) in cells transfected with GPR120 shRNA or control shRNA were detected by Western blot analysis and quantitative RT-PCR, respectively. (E) The induction of GPR120 by Rb2 behaved in a time-dependent manner as determined by Western blot analysis. The data are presented as mean±SD of three independent experiments. *P<0.05 vs vehicle (DMSO) group, control shRNA or '0 h' group which acts as control.
Rb2 enhanced the inhibitory effect of ALA on LPS-induced production of pro-inflammatory cytokines and NO in a GPR120-dependent manner
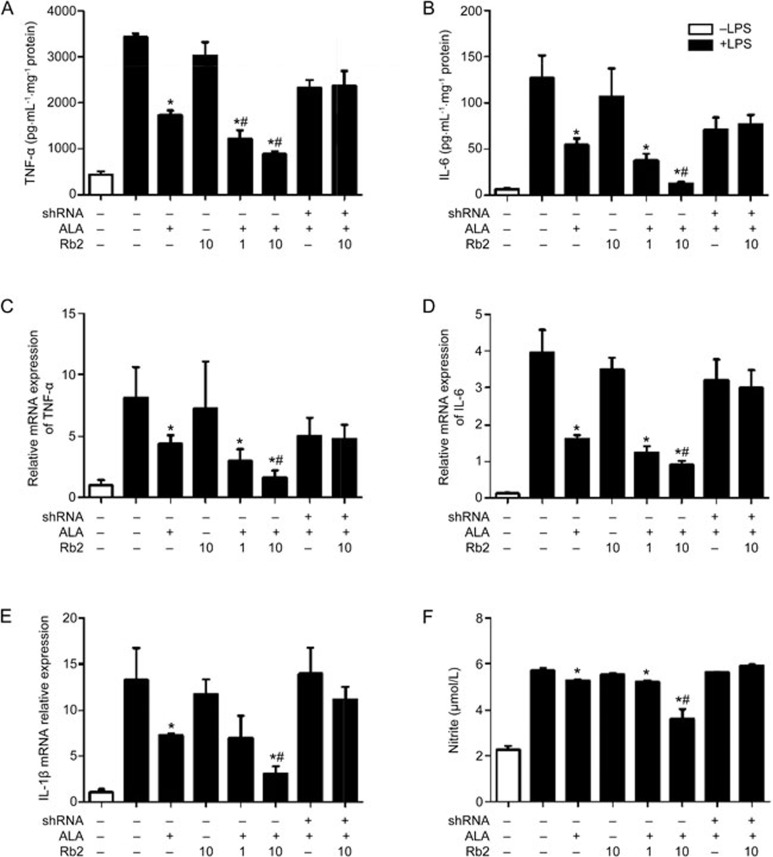
LPS-induced macrophage activation leads to dramatic increases in TNF-α, IL-6 and IL-1β, which are important pro-inflammatory cytokines21. ALA, a plant-derived ω-3 FFA, acts as an endogenous ligand for GPR120 and exerts an anti-inflammatory effect. Treatment of RAW264.7 cells with ALA for 1 h resulted in less LPS-induced production of TNF-α (46% decrease) and IL-6 (42% decrease). Given the above observations, we sought to evaluate whether upregulation of GPR120 induced by Rb2 would negatively influence the LPS-induced inflammation process in macrophages. We therefore incubated RAW264.7 cells with Rb2 for 12 h prior to ALA treatment and then stimulated the cells with LPS. In accordance with the expectation, pre-incubation with Rb2 at the higher dose (10 μmol/L) significantly enhanced the reductions in TNF-α (74% decrease) and IL-6 (86% decrease) (Figure 3A, 3B). Accordingly, mRNA expression of TNF-α, IL-6 and IL-1β also reached low levels in the Rb2 and ALA co-treated group (Figure 3C–3E). In addition, LPS-induced production of NO, another important pro-inflammatory molecule, was decreased by 34% in the Rb2 (10 μmol/L) and ALA-co-treated group, which was significantly lower than that in the group treated with ALA alone (10% decrease) (Figure 3F). However, Rb2 treatment alone had no anti-inflammatory effect. Furthermore, we used a calcium mobilization assay to assess the agonist response of Rb2 in CHO cells stably expressing GPR120. The data showed that ALA notably activated GPR120 (EC50=1.24 μmol/L), though Rb2 exhibited no significant agonist activity even at a high dose (Supplementary Figure 1).
Figure 3.
Rb2 enhanced reduction effect of ALA on inflammatory cytokines and NO production induced by LPS in RAW264.7 macrophages. RAW264.7 cells were transfected with either GPR120 shRNA or negative control shRNA for 12 h, then pretreated with Rb2 (1 and 10 μmol/L) or DMSO for 12 h prior to incubation of ALA (50 μmol/L) or DMSO for 1 h and finally stimulated with LPS (100 ng/mL) or DMSO for 24 h. The production of cytokines TNF-α (A) and IL-6 (B) in culture supernatants were measured by ELISA, NO (F) in supernatant was measured by Total NO Assay Kit. The mRNA expression levels of TNF-α (C), IL-6 (D) and IL-1β (E) in cells were detected by quantitative RT-PCR. The data are presented as mean±SD of three independent experiments. *P<0.05 vs 'LPS alone' group. #P<0.05 vs 'LPS plus ALA' group.
To examine whether the increased expression of GPR120 is responsible for this enhancing effect of Rb2, we knocked down GPR120 expression in RAW264.7 cells using shRNA interference. The levels of GPR120 mRNA and protein were markedly decreased in RAW264.7 cells transfected with GPR120-specific shRNA (Figure 2C, 2D). As a result, the cytokine-reducing effect was completely abrogated in the GPR120 shRNA transfection group (Figure 3), demonstrating a key role for GPR120 in enhancing the anti-inflammatory effect of Rb2 and ALA.
Rb2 enhanced the effect of ALA in repressing LPS-induced expression of iNOS and COX-2 in a GPR120-dependent manner
LPS-induced expression of iNOS and COX-2 results in increased production of NO and PEG2, respectively, which characterizes the inflammatory response in macrophages22.
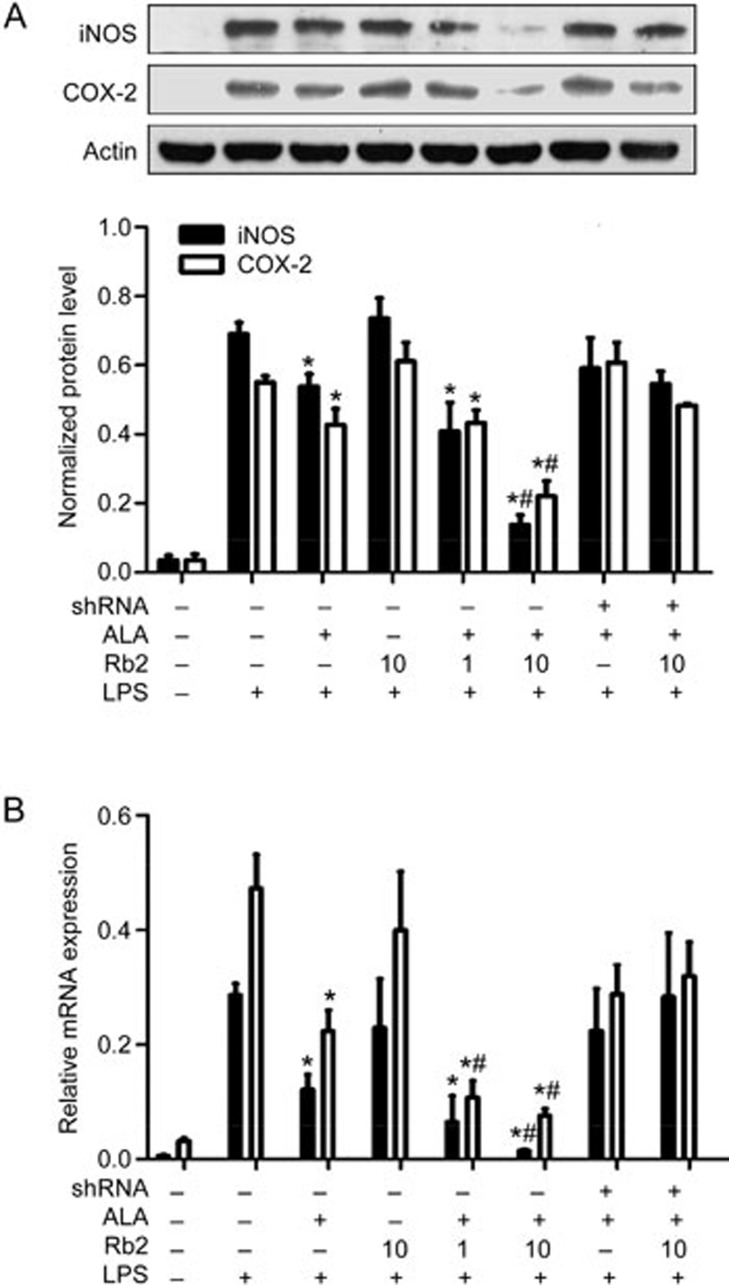
To further evaluate the contribution of Rb2 to inhibition of the LPS-induced inflammatory response in macrophages, protein and mRNA expression of iNOS and COX-2, two key pro-inflammatory enzymes, was examined by Western blotting analysis (Figure 4A) and quantitative real-time PCR (Figure 4B). The expression levels of iNOS and COX-2 were strongly upregulated in response to LPS (100 ng/mL), and ALA (50 μmol/L) treatment (1 h) alone slightly decreased the LPS-induced expression of the two enzymes. Pre-incubation with Rb2 (10 μmol/L) dramatically lowered the level of iNOS and COX-2 expression compared to ALA treatment alone. However, the enhancement effect of Rb2 was completely abolished in GPR120-knocked down RAW264.7 cells.
Figure 4.
Rb2 enhanced the reduction effect of ALA on expression of iNOS and COX-2 induced by LPS. RAW264.7 cells were transfected with either GPR120-specific shRNA or negative control shRNA for 12 h, then pretreated with Rb2 (1 and 10 μmol/L) or DMSO for 12 h prior to incubation of ALA (50 μmol/L) or DMSO and finally stimulated with LPS (100 ng/mL) or DMSO for 6 h (for RT-PCR) or 12 h (for Western blot). The expression levels of iNOS and COX-2 protein (A) were detected by Western blot and relative density was normalized by Actin. Relative mRNA expression levels (B) of iNOS and COX-2 were detected by quantitative RT-PCR and were normalized by 18s. The data are presented as mean±SD of three independent experiments. *P<0.05 vs 'LPS alone' group. #P<0.05 vs 'LPS plus ALA' group.
The Rb2-mediated enhancement of ALA repression of LPS-induced IKKβ/NF-κB pathway activation depends on GPR120
GPR120 activation can inhibit LPS-induced IKKβ/NF-κB pathway activation. To confirm that the anti-inflammatory activity of Rb2 occurs through GPR120, activation of IKKβ and NF-κB was determined by Western analysis. ALA (50 μmol/L) treatment slightly reduced the levels of phosphorylated IKKβ and NF-κB induced by LPS, and ALA-mediated suppression of IKKβ (Figure 5A)/NF-κB (Figure 5B) signaling in RAW264.7 macrophages was apparently enhanced by pre-incubation with Rb2 for 12 h. However, Rb2 pre-incubation did not reduce the phosphorylation of IKKβ and NF-κB induced by LPS in RAW264.7 cells transfected with GPR120-specific shRNA. Taken together, the results suggested that the enhancing effect on inflammation suppression by Rb2 is dependent on increased GPR120 expression.
Figure 5.
The Inhibitory effect of Rb2 on LPS-induced IKKβ/NF-κB signal pathway activation is GPR120 activation-dependent. RAW264.7 cells were transfected with either GPR120 shRNA or negative control shRNA for 12 h, then pretreated with Rb2 (1 and 10 μmol/L) or DMSO for 12 h prior to incubation of ALA (50 μmol/L) or DMSO for 1 h and finally stimulated with LPS (100 ng/mL) or DMSO for 15 min. (A, B) Protein levels of phosphorylated IKKβ (p-IKKβ) and phosphorylated NF-κB (p-NF-κB) were detected by Western blotting and relative density was normalized by total IKKβ (t-IKKβ) and total NF-κB (t-NF-κB), respectively. The data are presented as mean±SD of three independent experiments. *P<0.05 vs 'LPS alone' group. #P<0.05 vs 'LPS plus ALA' group.
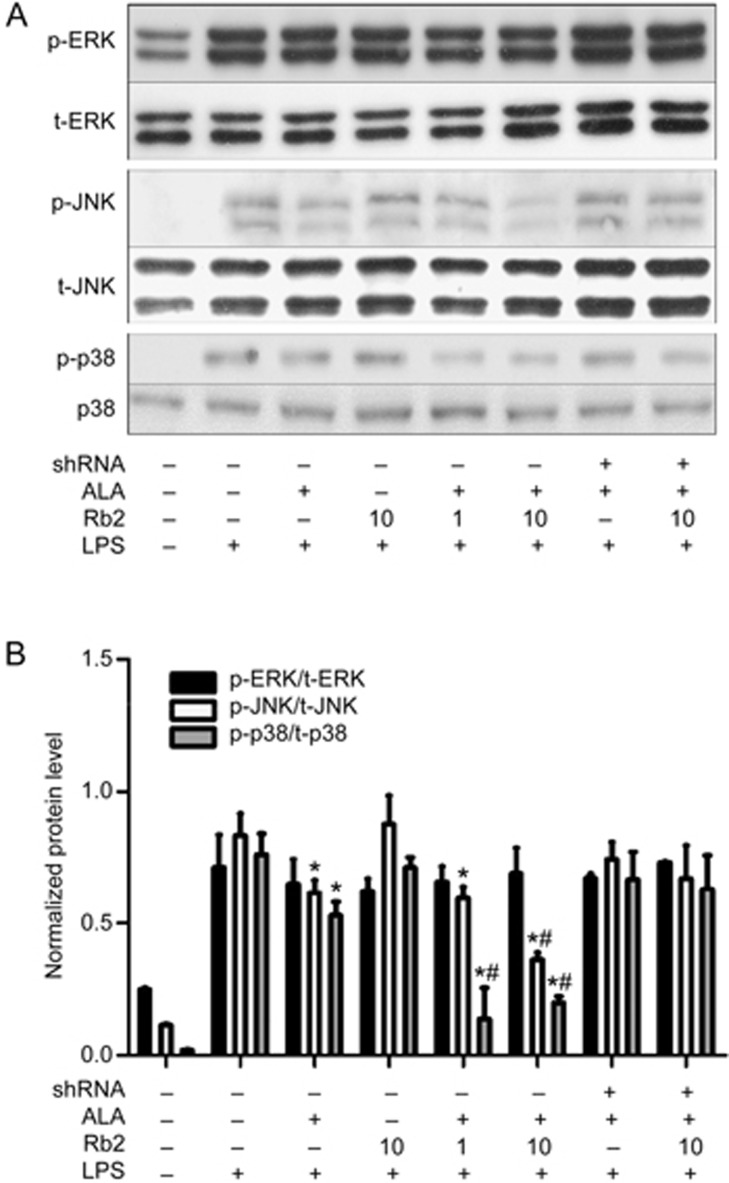
JNK and p38, but not the ERK/MAPK pathway, are involved in the anti-inflammatory properties of Rb2
LPS-induced activation of Toll-like receptor (TLR) 4 in macrophages also triggers signaling cascades that phosphorylate MAPKs (ERK, JNK and p38). MAPKs then activate downstream effector caspases, resulting in increased production of various inflammatory factors. It has been reported that GPR120 activation blocks part of the MAPK pathways by inactivating transforming growth factor β-activated kinase 1 (TAK1)23. Therefore, we investigated the enhancing effect of Rb2 on the ALA-mediated reduction of LPS-induced phosphorylation of MAPKs in RAW264.7 macrophages. Western blotting results showed that ALA incubation for 1 h tended to decrease the high levels of MAPK (p-ERK, p-JNK and p-p38) phosphorylation stimulated by LPS. Pre-treatment with Rb2 for 12 h dose-dependently amplified the reducing effect of ALA on JNK and p38 activation, leading to significantly greater attenuation of inflammation induced by LPS than ALA treatment alone (Figure 6). However, neither ALA alone nor ALA with Rb2 pre-treatment could prevent LPS-induced phosphorylation of ERK in RAW264.7 macrophages.
Figure 6.
Different role of MAPKs in the anti-inflammatory process of Rb2. RAW264.7 cells were transfected with either GPR120 shRNA or negative control shRNA for 12 h, then pretreated with Rb2 (1 and 10 μmol/L) or DMSO for 12 h prior to incubation of ALA (50 μmol/L) or DMSO for 1 h and finally stimulated with LPS (100 ng/mL) or DMSO for 15 min. Protein levels of phosphorylated ERK (p-ERK), phosphorylated JNK (p-JNK) and phosphorylated p38 (p-p38) were detected by Western blot (A) and relative density was normalized by total ERK (t-ERK), total JNK (t-JNK) and total p38 (t-p38) (B), respectively. The data are presented as mean±SD of three independent experiments. *P<0.05 vs LPS alone group. #P<0.05 vs 'LPS plus ALA' group.
Discussion
In this study, we demonstrated that Rb2 could enhance the anti-inflammatory effect of ω-3 fatty acid in LPS-stimulated RAW264.7 cells by upregulating GPR120 expression. We further explored the GPR120 downstream signaling pathways involved in this enhancing effect of Rb2 and demonstrated that Rb2 pre-treatment enhanced the anti-inflammatory effect of ALA and that the enhancing effect was strictly dependent on GPR120 activation.
Omega-3 fatty acids exert anti-inflammatory actions in macrophages by activating GPR120 and improve insulin sensitivity in mice fed a high-fat diet. However, desensitization and degradation of the receptor following ligand binding limits sustained activation of GPR120 and consequently weakens the expected anti-inflammatory effects, which presents a critical challenge for the application of GPR120 agonists in long-term treatment24.
Accumulating evidence suggests that GPR120 activation is a feasible solution to ameliorating chronic inflammation and improving glucose metabolism. Indeed, induction of GPR120 is expected to protect BMMSCs against dexamethasone-induced apoptosis19 and increase glucose intake by myocytes25. These results indicate that modulation of gene transcription is another way to regulate GPR120 signaling and augment its anti-inflammatory effect. We found that Rb2 markedly upregulated GPR120 expression in murine RAW264.7 macrophages in a time- and concentration-dependent manner. ALA is considered to perform this anti-inflammatory function in a more specific way, relying on GPR120 activation26, whereas DHA may exert anti-inflammatory effects through additional pharmacological activities toward PPARγ27,28, resolvin, protectin and maresin through its derivatives29,30. Our preliminary results showed that ALA slightly, but significantly, reduced LPS-induced phosphorylation of JNK in RAW264.7 cells at concentrations up to 50 μmol/L (data not shown). Thus, to examine the enhancing effect of Rb2 with regard to inhibiting the LPS-stimulated inflammation response via GPR120 induction, we employed ALA at the concentration of 50 μmol/L.
Next, experiments were designed to test the assumption that Rb2 has an anti-inflammatory effect due to increases in GPR120. RAW264.7 cells, a murine macrophage cell line, were stimulated with LPS to establish an in vitro inflammatory model, which is characterized as having an excessive inflammatory response and abnormally high levels of pro-inflammatory cytokines31. Macrophages directly participate in the process of obesity-induced chronic low-grade inflammation, which is tightly associated with macrophage infiltration into peripheral tissues and the pathogenesis of insulin resistance32,33. Recent research has indicated that GPR120 plays an important role in energy homeostasis, chronic inflammation and insulin resistance and represents a promising target for obesity and type 2 diabetes (T2DM) therapy34. In the present study, LPS-induced production of NO, TNF-α and IL-6 as well as expression of iNOS and COX-2 tended to be reduced by ALA. Incubation with Rb2 prior to ALA treatment dramatically amplified the inhibitory effect of ALA, leading to statistically significantly lower levels of these cytokines and pro-inflammatory enzymes than with ALA treatment alone. The results showed that Rb2 treatment might amplify the inhibitory effect of ALA on chronic inflammation in a GPR120 activation-dependent manner.
The IKKβ/NF-κB pathway plays a critical role in TLR-mediated inflammation, contributing to iNOS and COX-2 expression and cytokine production in macrophages35. GPR120 activation leads to inactivation of TAK1, which subsequently represses the IKKβ/NF-κB and MAPKs/AP pathways stimulated by LPS via TLR48,36. To test whether Rb2 exerts anti-inflammatory activity through a GPR120-mediated signaling pathway, we examined the IKKβ/NF-κB pathway by Western blotting. The results showed that compared with ALA treatment alone, pre-incubation of RAW264.7 cells with Rb2 dose-dependently amplified the inhibitory effect of ALA on LPS-induced phosphorylation of IKK/NF-κB, JNK and p38. Interestingly, the LPS-induced high level of phosphorylated ERK was not affected by ALA or Rb2. However, Rb2 alone had no influence on any of the inflammatory pathways activated by LPS. We next studied whether Rb2 reduced the release of inflammatory factors by inducing GPR120 expression. As expected, knocking down GPR120 abolished the anti-inflammatory effect of Rb2 and ALA. These results together highlight the fact that Rb2 exerts its anti-inflammatory property largely by increasing the expression of GPR120 and subsequently enhancing ALA-stimulated GPR120 activation.
The underlying mechanism of ginsenosides with regard to inflammatory regulation remains unclear. As an important bioactive ingredient, the regulatory effect of ginsenoside Rb2 on inflammation in macrophages has not yet been reported. For the first time, we demonstrated that Rb2 could enhance the inhibitory effect of ω-3 fatty acid on the LPS-induced inflammatory process in RAW264.7 macrophages by increasing GPR120 expression. Furthermore, we proved that this enhancing effect of Rb2 was strictly dependent on GPR120 activation. Our study provides a new mechanism to explain the suppressing effect of ginsenosides on inflammation. In addition, our results also suggest that the decreased response efficacy caused by receptor desensitization can be overcome by increasing GPR120 expression, indicating that GPR120 remains a promising target for treating chronic inflammation and T2D.
Author contribution
He-yao WANG designed the research. Qi HUANG and Ting WANG performed the research. Qi HUANG and He-yao WANG analyzed the data and wrote the paper.
Acknowledgments
This work was supported by a grant from the National Natural Science Foundation (No 81473262, 81503124), the Science and Technology Commission of Shanghai Municipality Project (No 15431901000, 14ZR1447700) and Grant for Drug Discovery and Development, Chinese Academy of Sciences (No CASIMM0120162025, CASIMM0120164014).
Footnotes
Supplementary information is available at the website of Acta Pharmacologica Sinica.
Supplementary Information
References
- Dilshara MG, Jayasooriya RG, Kang CH, Lee S, Park SR, Jeong JW, et al. Downregulation of pro-inflammatory mediators by a water extract of Schisandra chinensis (Turcz) Baill fruit in lipopolysaccharide-stimulated RAW 264.7 macrophage cells. Environ Toxicol Pharmacol 2013; 36: 256–64. [DOI] [PubMed] [Google Scholar]
- Gordon S. Alternative activation of macrophages. Nat Rev Immunol 2003; 3: 23–35. [DOI] [PubMed] [Google Scholar]
- Osborn O, Olefsky JM. The cellular and signaling networks linking the immune system and metabolism in disease. Nat Med 2012; 18: 363–74. [DOI] [PubMed] [Google Scholar]
- Lackey DE, Olefsky JM. Regulation of metabolism by the innate immune system. Nat Rev Endocrinol 2016; 12: 15–28. [DOI] [PubMed] [Google Scholar]
- Hirasawa A, Tsumaya K, Awaji T, Katsuma S, Adachi T, Yamada M, et al. Free fatty acids regulate gut incretin glucagon-like peptide-1 secretion through GPR120. Nat Med 2005; 11: 90–4. [DOI] [PubMed] [Google Scholar]
- Iwasaki K, Harada N, Sasaki K, Yamane S, Iida K, Suzuki K, et al. Free fatty acid receptor GPR120 is highly expressed in enteroendocrine K cells of the upper small intestine and has a critical role in GIP secretion after fat ingestion. Endocrinology 2015; 156: 837–46. [DOI] [PubMed] [Google Scholar]
- Tanaka T, Katsuma S, Adachi T, Koshimizu TA, Hirasawa A, Tsujimoto G. Free fatty acids induce cholecystokinin secretion through GPR120. Naunyn Schmiedebergs Arch Pharmacol 2008; 377: 523–7. [DOI] [PubMed] [Google Scholar]
- Oh DY, Talukdar S, Bae EJ, Imamura T, Morinaga H, Fan W, et al. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell 2010; 142: 687–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raptis DA, Limani P, Jang JH, Ungethum U, Tschuor C, Graf R, et al. GPR120 on Kupffer cells mediates hepatoprotective effects of omega3-fatty acids. J Hepatol 2014; 60: 625–32. [DOI] [PubMed] [Google Scholar]
- Oh da Y, Walenta E, Akiyama TE, Lagakos WS, Lackey D, Pessentheiner AR, et al. A Gpr120-selective agonist improves insulin resistance and chronic inflammation in obese mice. Nat Med 2014; 20: 942–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi KT. Botanical characteristics, pharmacological effects and medicinal components of Korean Panax ginseng C A Meyer. Acta Pharmacol Sin 2008; 29: 1109–18. [DOI] [PubMed] [Google Scholar]
- Im DS, Nah SY. Yin and Yang of ginseng pharmacology: ginsenosides vs gintonin. Acta Pharmacol Sin 2013; 34: 1367–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto J, Sakaguchi H, Aoki I, Toyoki H, Khatun S, Tamaya T. Inhibitory effect of ginsenoside-Rb2 on invasiveness of uterine endometrial cancer cells to the basement membrane. Eur J Gynaecol Oncol 2001; 22: 339–41. [PubMed] [Google Scholar]
- Yoon SJ, Park JY, Choi S, Lee JB, Jung H, Kim TD, et al. Ginsenoside Rg3 regulates S-nitrosylation of the NLRP3 inflammasome via suppression of iNOS. Biochem Biophys Res Commun 2015; 463: 1184–9. [DOI] [PubMed] [Google Scholar]
- Lee IA, Hyam SR, Jang SE, Han MJ, Kim DH. Ginsenoside Re ameliorates inflammation by inhibiting the binding of lipopolysaccharide to TLR4 on macrophages. J Agric Food Chem 2012; 60: 9595–602. [DOI] [PubMed] [Google Scholar]
- Song Y, Zhao F, Zhang L, Du Y, Wang T, Fu F. Ginsenoside Rg1 exerts synergistic anti-inflammatory effects with low doses of glucocorticoids in vitro. Fitoterapia 2013; 91: 173–9. [DOI] [PubMed] [Google Scholar]
- Lee KT, Jung TW, Lee HJ, Kim SG, Shin YS, Whang WK. The antidiabetic effect of ginsenoside Rb2 via activation of AMPK. Arch Pharm Res 2011; 34: 1201–8. [DOI] [PubMed] [Google Scholar]
- Huang Q, Gao B, Jie Q, Wei BY, Fan J, Zhang HY, et al. Ginsenoside-Rb2 displays anti-osteoporosis effects through reducing oxidative damage and bone-resorbing cytokines during osteogenesis. Bone 2014; 66: 306–14. [DOI] [PubMed] [Google Scholar]
- Gao B, Huang Q, Jie Q, Zhang HY, Wang L, Guo YS, et al. Ginsenoside-Rb2 inhibits dexamethasone-induced apoptosis through promotion of GPR120 induction in bone marrow-derived mesenchymal stem cells. Stem Cells Dev 2015; 24: 781–90. [DOI] [PubMed] [Google Scholar]
- Wang T, Sun P, Chen L, Huang Q, Chen K, Jia Q, et al. Cinnamtannin D-1 protects pancreatic beta-cells from palmitic acid-induced apoptosis by attenuating oxidative stress. J Agric Food Chem 2014; 62: 5038–45. [DOI] [PubMed] [Google Scholar]
- Martinez FO, Sica A, Mantovani A, Locati M. Macrophage activation and polarization. Front Biosci 2008; 13: 453–61. [DOI] [PubMed] [Google Scholar]
- Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol 2004; 25: 677–86. [DOI] [PubMed] [Google Scholar]
- Chen IT, Hsu PH, Hsu WC, Chen NJ, Tseng PH. Polyubiquitination of transforming growth factor beta-activated kinase 1 (TAK1) at lysine 562 residue regulates TLR4-mediated JNK and p38 MAPK activation. Sci Rep 2015; 5: 12300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson BD, Shimpukade B, Mackenzie AE, Butcher AJ, Pediani JD, Christiansen E, et al. The pharmacology of TUG-891, a potent and selective agonist of the free fatty acid receptor 4 (FFA4/GPR120), demonstrates both potential opportunity and possible challenges to therapeutic agonism. Mol Pharmacol 2013; 84: 710–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim N, Lee JO, Lee HJ, Kim HI, Kim JK, Lee YW, et al. Endogenous ligand for GPR120, docosahexaenoic acid, exerts benign metabolic effects on the skeletal muscles via AMP-activated protein kinase pathway. J Biol Chem 2015; 290: 20438–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im DS. Functions of omega-3 fatty acids and FFA4 (GPR120) in macrophages. Eur J Pharmacol 2016; 785: 36–43. [DOI] [PubMed] [Google Scholar]
- Groeger AL, Cipollina C, Cole MP, Woodcock SR, Bonacci G, Rudolph TK, et al. Cyclooxygenase-2 generates anti-inflammatory mediators from omega-3 fatty acids. Nat Chem Biol 2010; 6: 433–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Ruan XZ, Powis SH, Fernando R, Mon WY, Wheeler DC, et al. EPA and DHA reduce LPS-induced inflammation responses in HK-2 cells: evidence for a PPAR-gamma-dependent mechanism. Kidney Int 2005; 67: 867–74. [DOI] [PubMed] [Google Scholar]
- Calder PC. Marine omega-3 fatty acids and inflammatory processes: effects, mechanisms and clinical relevance. Biochim Biophys Acta 2015; 1851: 469–84. [DOI] [PubMed] [Google Scholar]
- Im DS. Omega-3 fatty acids in anti-inflammation (pro-resolution) and GPCRs. Prog Lipid Res 2012; 51: 232–7. [DOI] [PubMed] [Google Scholar]
- Mantovani A, Sica A, Locati M. Macrophage polarization comes of age. Immunity 2005; 23: 344–6. [DOI] [PubMed] [Google Scholar]
- Lee BC, Lee J. Cellular and molecular players in adipose tissue inflammation in the development of obesity-induced insulin resistance. Biochim Biophys Acta 2014; 1842: 446–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanda H, Tateya S, Tamori Y, Kotani K, Hiasa K, Kitazawa R, et al. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin Invest 2006; 116: 1494–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulven T, Christiansen E. Dietary fatty acids and their potential for controlling metabolic diseases through activation of FFA4/GPR120. Annu Rev Nutr 2015; 35: 239–63. [DOI] [PubMed] [Google Scholar]
- Massaro M, Habib A, Lubrano L, Del Turco S, Lazzerini G, Bourcier T, et al. The omega-3 fatty acid docosahexaenoate attenuates endothelial cyclooxygenase-2 induction through both NADP(H) oxidase and PKC epsilon inhibition. Proc Natl Acad Sci U S A 2006; 103: 15184–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Yu Y, Funk CD. Cyclooxygenase-2 induction in macrophages is modulated by docosahexaenoic acid via interactions with free fatty acid receptor 4 (FFA4). FASEB J 2013; 27: 4987–97. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.