Abstract
Breast cancer is a heterogeneous disease consisting of several subtypes. Among these subtypes, triple negative breast cancer is particularly difficult to treat. This is due to a lack of understanding of the mechanisms behind the disease, and consequently a lack of druggable targets. PAK4 plays critical roles in cell survival, proliferation, and morphology. PAK4 protein levels are high in breast cancer cells and breast tumors, and the gene is often amplified in basal like breast cancers, which are frequently triple negative. PAK4 is also overexpressed in other types of cancer, making it a promising drug target. However, its inhibition is complicated by the fact that PAK4 has both kinase-dependent and -independent functions. Here we investigate a new clinical compound KPT-9274, which has been shown to inhibit PAK4 and NAMPT. We find that KPT-9274 (and its analog, KPT-8752) can reduce the steady state level of PAK4 protein in triple negative breast cancer cells. These compounds also block the growth of the breast cancer cells in vitro, and stimulate apoptosis. Most importantly, oral administration of KPT-9274 reduces tumorigenesis in mouse models of human triple negative breast cancer. Our results indicate that KPT-9274 is a novel therapeutic option for triple negative breast cancer therapy.
Breast cancer is a heterogeneous disease and can be classified into at least 5 subtypes: (1) luminal A (usually ER and/or PR+, HER2−, low Ki67), (2) luminal B (usually ER+and/or PR+, HER2+, or HER2− with high Ki67), (3) HER2 positive, (4) basal like, and (5) normal breast like1,2. These 5 sub-divisions can be even further subdivided. Most basal like tumors are triple negative (lacking ER, PR, and HER2 expression)3, and most triple negative cancers have the basal like phenotype. Among the different types of breast cancer, triple negative breast cancer has a particularly poor prognosis. This is due in part to a lack of understanding of the mechanism behind the establishment and maintenance of this type of breast cancer, which consequently limits treatment options. The identification of new biomarkers for the disease is urgently needed to provide effective druggable targets and improve clinical therapy.
KPT-9274 and the closely related KPT-8752 were first identified on the basis of their ability to bind and reduce the steady state level of cellular PAK4 (P21 activated kinase 4) protein, and they were subsequently found to block the activity of NAMPT (nicotinamide phosphoribosyltransferase)4. PAK4 inhibition is significant because of the important links that have been found between PAK4 and many types of cancer, including breast cancer. We and others have found PAK4 protein and mRNA levels to be high in a number of breast cancer cells as well as in primary human breast cancer tumor samples5,6,7,8,9,10,11. Furthermore, in a study of 80 breast cancer patients with different stages of disease, PAK4 protein levels were shown to increase as the disease progressed, with the highest PAK4 levels being associated with the most advanced stage8. In another study of 93 invasive breast carcinoma patients, high PAK4 levels were associated with advanced stage cancer, large tumor size, lymph node metastasis, and poor survival11. In another panel of 300 human breast cancers, PAK4 protein was also highly expressed in the more severe grade invasive carcinomas10. Our group and others have found that PAK4 protein levels are high in breast cancer cell lines and primary breast cancer tissue5,6,7,8,9,10. In a study of basal like breast cancer, a subset that is frequently triple negative, DNA analysis revealed that the chromosomal region containing the gene for PAK4 was frequently amplified12. We found that PAK4 overexpression led to oncogenic transformation in mouse mammary epithelial cells while blocking PAK4 with siRNA inhibited tumor formation of a human breast cancer cell line6,13. These data suggest a significant role for PAK4 in breast cancer etiology and make it a potential therapeutic target.
The PAK family of protein kinases are important signaling molecules connected to many cellular functions including cell proliferation, migration, and cytoskeletal organization. Aberrant signaling in these pathways are often associated with cancer development and progression14. The PAK family consists of 6 members which fall into 2 groups, Group I (PAKs 1, 2, and 3) and Group II (PAKs 4, 5, and 6). Among the group II PAK genes, PAK4 is most frequently linked with cancer5,6,7,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30. In addition to breast cancer12, the PAK4 gene was shown to be amplified in a number of different cancer types, including pancreatic cancer17,24,25, squamous cell carcinomas26, esophageal squamous cell carcinoma (ESCC)31, endometrioid tumors, ovarian tumors and cell lines20, as well as prostate cancer28.
Our previous research has shown that when PAK4 is overexpressed in non-transformed immortalized mouse mammary epithelial cells (iMMECs), it results in improper formation of spherical acini in 3D culture. Specifically, elevated PAK4 protein levels lead to increased cell proliferation and survival, decreased apoptosis, filling of the luminal space with cells, increased acinar size, an increase in the outer layer of epithelial cells, and loss of cell polarity6. These changes are all hallmarks of precancerous conditions and early stage tumors such as atypical hyperplasias and Ductal Carcinoma in situ (DCIS). Even more importantly, the PAK4-expressing iMMECs formed tumors when implanted into the mammary fat pads of mice6, providing strong evidence that overexpression of the wild-type PAK4 protein is sufficient to lead to mammary tumorigenesis in mice.
In contrast to its role in carcinogenesis when overexpressed, PAK4 silencing using RNAi in the human breast cancer cell line MDA-MB-231 results in a dramatic reduction in cell proliferation and migration13. While cancer cells are generally less susceptible to cell death, PAK4 knockdown dramatically induces apoptosis in these cells. Most strikingly, when these siRNA PAK4 knockdown breast cancer cells are implanted into the mammary fat pads of athymic mice, tumor formation is dramatically disrupted13. Additionally, the microRNA, mir-199a.b-3p, which is down-regulated in several types of aggressive cancer, was found to directly target PAK4. mir-199a.b-3p can function as a tumor suppressor and specifically suppresses cell proliferation in breast cancer cells. It also alters the cell cycle while reducing the migratory and invasive activity of breast cancer cells, most likely due to its role in down-regulating PAK432. These data indicate that inhibiting PAK4 can restore many aspects of normal growth in breast cancer cells suggesting a central role for PAK4 in mammary cell transformation.
Because of the link between PAK proteins and cancer8,31,33,34,35,36,37, there has been considerable interest in developing PAK inhibitors. KPT-9274, along with the structural analog KPT-8752 (both developed by Karyopharm Therapeutics), function differently from other PAK4 inhibitors in that they reduce the steady state level of PAK4 protein in cells. This reduction is important because PAK4, like other PAK family members, has been found to have several kinase-independent functions10,11,38,39,40,41. For this reason, inhibitors that can reduce PAK4 protein and not just the kinase activity are needed in order to more efficiently block PAK4 in cancer.
In this study we show that KPT-9274 and KPT-8752 are highly effective at blocking the viability of several different breast cancer cell lines, especially three different triple negative cell lines. Most importantly, oral administration of KPT-9274 greatly reduced tumorigenesis in mouse xenograft models of human triple negative breast cancer cell lines. Since KPT-9274 is currently in a phase 1 human clinical trial of patients with advanced solid malignancies (NCT02702492), our data has practical applications to the breast cancer patient population.
Materials and Methods
Reagents and Cell culture
KPT-9274 and KPT-8752 from Karyopharm Therapeutics Inc (Newton, MA) were dissolved in dimethyl sulfoxide (DMSO). MCF7, MDA-MB-231 and SkBr-3 cells were maintained in DMEM/F-12 medium supplemented with 10% FBS serum and 1% penicillin/streptomycin. SUM159 cells were maintained in Ham’s F12 medium supplemented with 5% FBS; MDA-MB-468 cells were maintained in RPMI medium supplemented with 10% FBS serum and 1% penicillin/streptomycin. BT-474 were maintained in DMEM medium supplemented with 10% FBS serum, 1% penicillin/streptomycin and 1% glutamine. iMMECs were maintained in Hams F-12 medium supplemented by 10% FBS, 1% penicillin/streptomycin and other supplements. NIH3T3 cells were maintained in DMEM medium supplemented with 10% Bovine Calf Serum, 1% penicillin/streptomycin and 1% glutamine. All cells were maintained at 37 °C and 5% CO2.
Western Blot analysis
Cell lysates (25 μg) were resolved by SDS-PAGE and transferred to PVDF membrane. The membrane was blocked in TBS/T containing 0.1% Tween-20 (TBS/T) and 5% non-fat milk for 1 h. After washing with TBS/T, the membrane was incubated with primary antibody in TBS/T containing 0.1% Tween-20 (TBS/T) and 5% BSA overnight. After washing three times with TBS/T, the membrane was probed with HRP conjugated secondary antibody for 1 h. After washing three times with TBS/T, the part of membrane corresponding in size to the bands of interested protein was excised, and the immunocomplexes were visualized by Luminata Western HRP substrates from Millipore (Billerica, MA). Primary antibodies against PAK4, Cofilin, Phospho-cofilin (Ser3), β-Catenin and Phospho-β-Catenin (Ser675) and β-actin (Rabbit) and HRP-conjugated anti-rabbit antibodies were obtained from Cell Signaling Technologies (Danvers, MA, USA). Primary antibodies were diluted into TBS/T containing 5% bovine serum albumin at 1:1000. Secondary antibody was diluted into TBS/T containing 5% non-fat dry milk at 1:5000. The blots were analyzed either exposing the blots to X-Ray film, or by using the GeneGnome XRQ-NPC bioimaging system from SYNGENE (Cambridge, UK). This system utilizes a software GeneSys (Version 1.5) which automatically selects the right imaging conditions for each blot, backgrounds are adjusted as necessary, and the results are displayed digitally, without the use of X-Ray film. Quantitation of western blots were carried out using image J software. Protein is normalized to β-actin and results are plotted as percent of control, where the band intensity for control is set as 100% for each protein.
MTT assays
MDA-MB-231, MDA-MB-468, SUM159, MCF7, SkBr-3, BT-474, WT iMMEC and NIH3T3 were seeded into 96-well plates at 2000 cells/well. Cells were treated with KPT-9274 or KPT-8752 from Day 0 to Day 4. At each time point, 10 μl of MTT-I solution (thiazolyl blue tetrazolium bromide, M2128, Sigma-Aldrich, St, Louis, MO) was added into each well and incubated for 5 h, followed by addition of 100 μl of MTT-II solution (distilled water with 10% SDS and 0.01 M HCl). The plate was then incubated overnight and the absorbance was measured with a spectrophotometer (Tecan US, Durham NC) at 560 nm.
Apoptosis assay
MDA-MB-231, MDA-MB-468, SUM159, MCF7, BT-474, SkBr-3 and WT iMMECs were incubated with 15 μM of DMSO or 3 μM KPT-8752 or 1 μM KPT-9274 for 72 h. Apoptosis was assessed by staining with Annexin V and propidium iodide. Annexin V is a membrane phosphatidylserine (PS) binding protein. It binds to the cells early in apoptosis, which is characterized by PS being flipped to face the outer membrane of the cells. Propidium iodide can enter the cell and bind to nucleic acid, but only after the membrane has begun to rupture, a characteristic of more advanced apoptosis. To assess binding by Annexin V and propidium iodide, cells were trypsinized into single cell suspension, counted, washed with 1X Annexin V binding buffer and stained with Annexin V and Propidium Iodide (BD Pharmingen FITC Annexin V Apoptosis Detection Kit II, BD Biosciences, Franklin Lakes, NJ). The cells (1 × 105) were incubated with Annexin V and Propidium iodide for 15 minutes in the dark at room temperature, then washed with 1X Annexin V binding buffer and analyzed by flow cytometry using a Gallios Cytometer (Applied Biosystems, Foster City, CA).
Animal Studies
All animals were approved by the Institutional Review Board for the Animal Care and Facilities Committee of Rutgers University. All methods were approved by the guidelines at Rutgers University, and methods were carried out according to the guidelines and regulations of the animal care and facilities committee at Rutgers. Female nude mice (5–6 weeks old, weighing 20–25 grams) were purchased from Charles River Laboratories (Wilmington, MA). They were allowed to acclimatize to the facilities for two weeks following which MDA-MB-231, MDA-MB-468 and SUM159 cells were injected subcutaneously in both the flanks of the mice, at 106 cells per site, in a 100 μl mixture containing Matrigel (BD Biosciences) and Hank’s Buffer (Gibco) at a 1:1 ratio. Seven days post injection, mice were treated with placebo or KPT-9274 (100 mg/kg or 150 mg/kg) orally twice a day/four days per week. Tumor size and total body weights were measured twice weekly. Tumors were measured with a vernier caliper, and tumor volume (V; mm3) was calculated using the equation V = D ∗ d2/2 where D (mm) and d (mm) are the largest and smallest perpendicular diameters. After sacrificing the animals, tumors were excised, weighed, and snap frozen in liquid nitrogen for western blot analysis.
Statistical Analyses
Statistical analysis was done using a two-tailed t-test assuming unequal variance with error bars representing SD. *Represents a P value of < 0.001 and is considered significant.
Results
PAK4 protein levels are high in multiple breast cancer cell lines
We assessed the steady state level of PAK4 protein in several breast cancer cell lines by western blot analysis. As observed in Fig. 1, PAK4 levels are high in MDA-MB-468, SUM159, BT-549 and MDA-MB-231 (all triple negative), MCF7 (ER+/PR+), SkBr-3 (HER2+), and BT-474 (PR+/HER2+) breast cancer cell lines. These results are consistent with previous results where the PAK4 levels were high in primary breast cancer tissue5,8,10.
Figure 1. PAK4 is highly expressed in breast cancer cell lines.
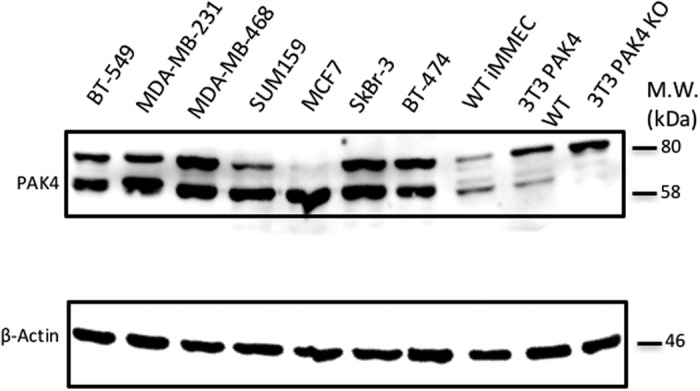
PAK4 protein levels in seven breast cancer cell lines were assessed by western blot analysis. β-actin was used as a loading control. 3T3 PAK4 WT and 3T3 PAK4 KO are 3T3 cells isolated from wild-type and PAK4 knockout mice, respectively. Knockout cells are used here only for the accurate identification of the PAK4 band. (The membranes were cut prior to exposure so that only the portion of gel containing bands in the size range of PAK4 or β-actin would be visualized, as described in materials and methods).
KPT-8752 and KPT-9274; a novel series of small molecules that can reduce cellular PAK4 protein levels
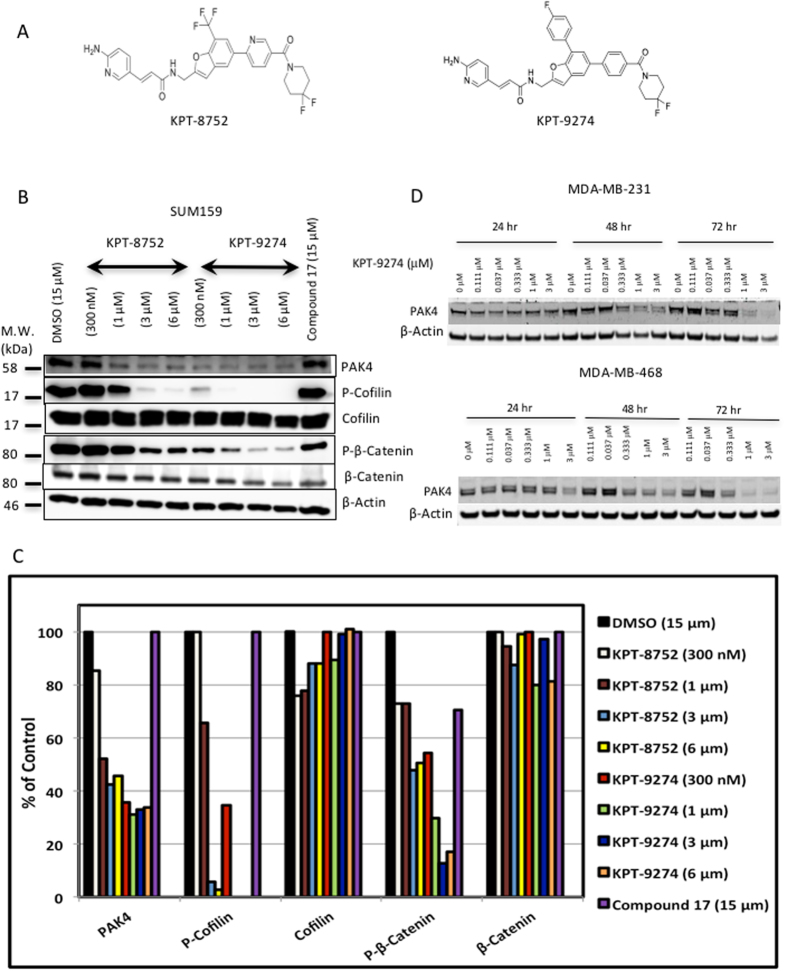
KPT-8752 and KPT-9274 (as well as other analogs in the series) were designed and created by Karyopharm Therapeutics. The structures of KPT-8752 and KPT-9274 are shown in Fig. 2A. KPT-8752 or KPT-9274 treatment of SUM159 cells, a triple negative breast cancer cell line, reduced PAK4 protein substantially after 72 h of treatment (see Fig. 2B,C). In contrast, a previously published PAK4 inhibitor, compound 17 42, which blocks PAK4 kinase activity, does not reduce PAK4 protein. In addition to PAK4, we also analyzed the level and phosphorylation status of several PAK4 downstream targets. Serine 675 of β-catenin was shown to be a direct phosphorylation substrate of PAK443. We found that phospho-S675-β-catenin was sharply reduced in response to either KPT-8752 or KPT-9274. The phosphorylation of Cofilin also occurs downstream to PAK4 signaling44. We found that KPT-8752 and KPT-9274 treatment of breast cancer cells reduced Phospho-Cofilin as expected. In addition to SUM159 cells, KPT-9274 also reduced PAK4 protein in two other triple negative breast cancer cell lines; MDA-MB-231 and MDA-MB-468 cells, which was most noticeable after 48–72 hours of treatment (Fig. 2D). KPT-8752 had a similar effect in these cells (data not shown).
Figure 2. KPT-8752 and KPT-9274 reduce PAK4 protein levels and reduce the phosphorylation of PAK4 downstream targets.
(A) Structures of KPT-8752 and KPT-9274. (B) Western blot analysis of SUM159 cells treated with either KPT-8752, KPT-9274, or compound 17 (72 hr). Western blots were probed with anti PAK4 and anti PAK1 antibodies, and with antibodies against the PAK4 pathway targets β-catenin and Cofilin. β-actin was used as a loading control. (C) The intensity of the bands in the western blot in panel B were quantitated using Image J software, and the bands were normalized to the β-actin control. Results are plotted as a percent of control, where the control represents the band intensity for DMSO, and set as 100% for each protein (PAK4, PAK1, Phospho-Cofilin, Cofilin, Phospho-β-Catenin and β-Catenin). This data was from a single experiment, where multiple doses of the inhibitor are represented. (D) Western blot analysis of PAK4 levels in MDA-MB-231 cells and MDA-MB-468 cells treated with KPT-9274. (The membranes were cut prior to exposure so that only the portion of gel containing bands in the size range of proteins under investigation would be detected, as described in materials and methods. In the top panel of (B) an upper band that represents a non-specific band that reacts with the PAK4 antibody, is spliced out of the figure, in order to focus on the PAK4 band).
KPT-8752 and KPT-9274 block cell growth in several human breast cancer cell lines
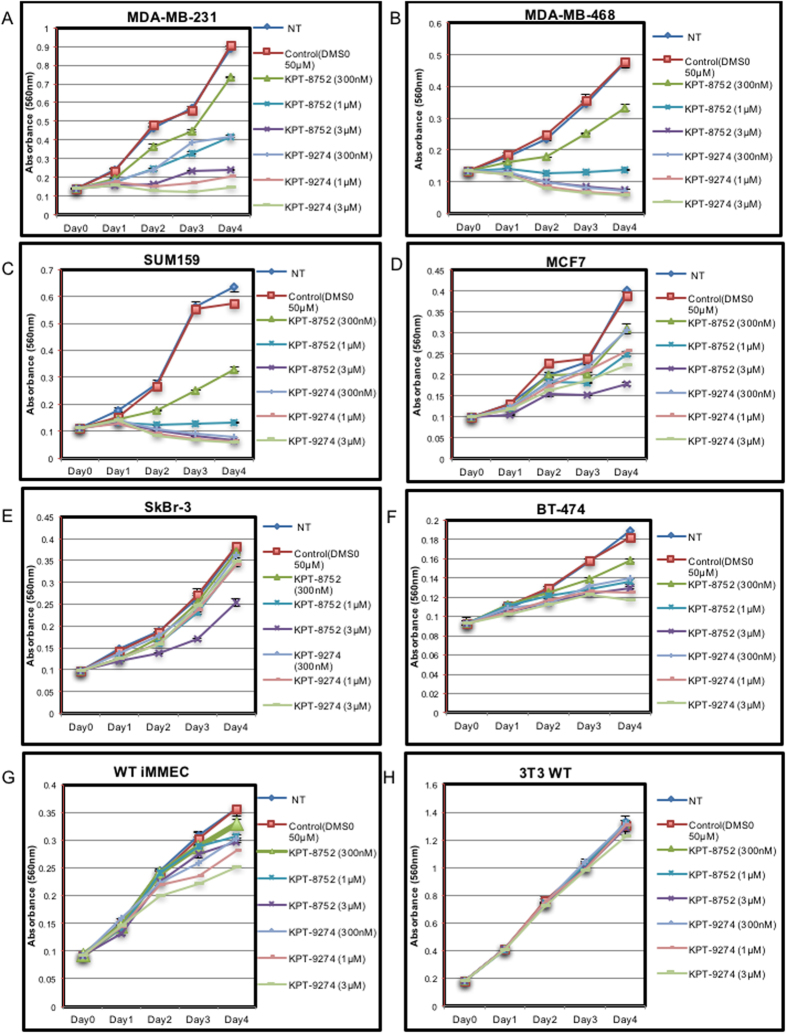
The effects of KPT-8752 and KPT-9274 on cell growth was analyzed by using a variation of an MTT cell proliferation assay. The assay is colorimetric and measures the change in the number of metabolically active cells over time as an indicator of cell proliferation (see Fig. 3). Several breast cancer cell lines with high levels of PAK4 protein were plated in tissue culture cluster plates. They were treated with different concentrations of KPT-8752 or KPT-9274 and incubated with MTT solution, and absorbance was measured at different time points as described in materials and methods. After KPT-8752 and KPT-9274 treatment, three of the cell lines, MDA-MB-231 (Fig. 3A), MDA-MB-468 (Fig. 3B), and SUM159 (Fig. 3C), displayed significantly reduced colorimetric change, or viability, over time. For MDA-MB-231 cells, growth was almost completely inhibited in the presence of 3 μm KPT-8752, or as little as 1 μm KPT-9274. For MDA-MB-468 and SUM159 cells proliferation was completely inhibited with 1 μm KPT-8752 or 300 nM KPT-9274. In contrast, the decrease in viability was less pronounced in MCF7 cells (an ER+/PR+cell line, Fig. 3D), and the inhibitory effect was even lower for SkBr-3 cells (HER2+, Fig. 3E) and BT-474 cells (PR+/HER2+, Fig. 3F). Treatment with KPT-8752 or KPT-9274 had no significant effect on cell viability of WT iMMEC or wild-type NIH-3T3 WT cells, but it did inhibit the growth of iMMECs that stably expressed PAK4, suggesting that inhibition of cell proliferation by the compounds is in fact related to PAK4 inhibition (see Supplemental Fig. 1). It is of interest that the triple negative breast cancer cells (MDA-MB-468, MBA-MB-231, and SUM159) were the most responsive to treatment with these compounds, responding to even the lowest concentrations. These results are consistent with previous studies from our lab indicating that PAK4 knockdown with siRNA reduces proliferation of MDA-MB-231 cells13.
Figure 3. Treatment with KPT-8752 and KPT-9274 leads to a decrease in cell proliferation in several breast cancer cell lines.
Cells were plated overnight and treated with DMSO (control), KPT-8752 or KPT-9274 from Day 0 to Day 4. The MTT colorimetric assay was then conducted where the cells were incubated with MTT solutions at different time points. MTT absorbance curves were then analyzed in MDA-MB-231 (A) MDA-MB-468 (B) SUM159 (C) MCF7 (D) Sk-Br3 (E) BT-474 (F) WT iMMEC (control) (G) and NIH3T3 (control) cells (H). The results, presented as change in absorbance over time, correlate with the number of viable cells over time and can be considered as an indicator of cell proliferation. (Note, the amount of MTT-I absorbed by each cell type varies, and therefore the overall growth rates cannot be compared from one cell type to another). Error bars represent SEM. Data shown is representative of three separate repeat experiments.
KPT-8752 and KPT-9274 induce apoptosis in human breast cancer cell lines
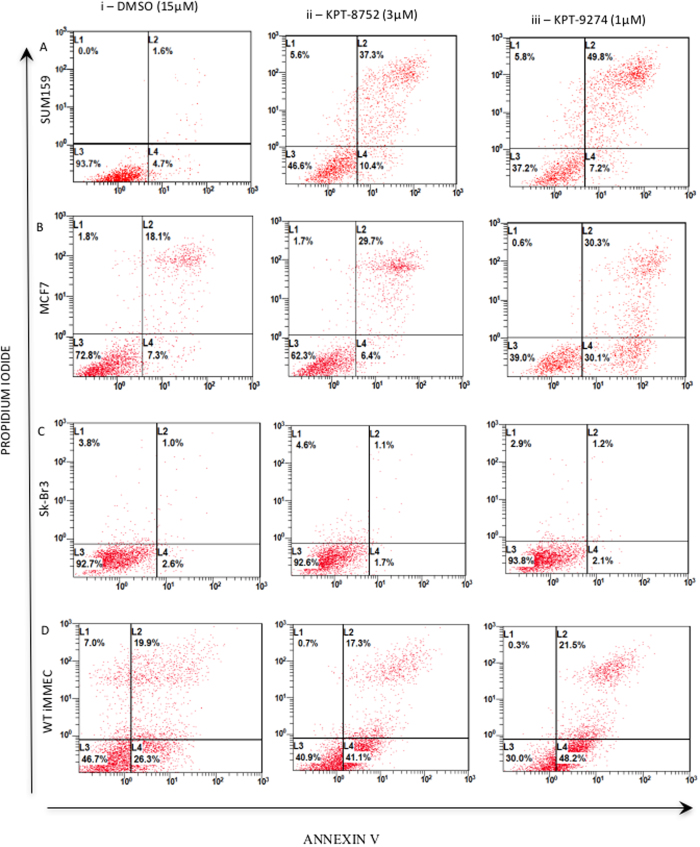
Since previous studies indicate that PAK4 siRNA knockdown induces apoptosis in MDA-MB-231 cells13, we next studied whether KPT-8752 or KPT-9274 could also affect the survival of additional breast cancer cell lines with various etiologies. The cells were treated with either vehicle control (DMSO), KPT-8752, or KPT-9274 for 72 hours, and apoptosis was measured by staining with Annexin V and propidium iodide (see Fig. 4). The proportion of apoptotic cells corresponds to the number of Annexin V positive and propidium iodide positive cells (L2), and drug induced change in apoptosis is assessed by quantitating the change in L2 in the treated versus untreated cells. SUM159 (Fig. 4A), MDA-MB-231, and MDA-MB-468 cells (Supplemental Fig. 2), (all triple negative), showed a strong increase in apoptosis after treatment with the inhibitors as indicated by high Annexin V and propidium iodide staining (L2). In MCF7 (ER+/PR+; Fig. 4B). although some basal level of apoptosis was seen in the DMSO treated cells, the increase in apoptosis after treatment with KPT-8752 or KPT-9274 was lower than what was observed for the SUM159 cells. For SkBr-3 (HER2+; Fig. 4C) and BT-474 cells (PR+/HER2+; Supplemental Fig. 2), treatment with either compound resulted in almost no increase in apoptosis. WT iMMECs (Fig. 4D) had a basal level of apoptosis, but no increase in apoptosis was observed following KPT-8752 or KPT-9274 treatment. These results are consistent with the MTT assay results demonstrating that the triple negative cells showed the greatest response to inhibitor treatment.
Figure 4. KPT-8752 and KPT-9274 cause an increase in cellular apoptosis in breast cancer cells.
SUM159 (triple negative) (A), MCF7 (ER+/PR+) (B), SkBr-3 (HER2+) (C) and WT iMMEC (control) cells (D) were treated with either (i) DMSO (15 μM), (ii) KPT-8752 (3 μM), or (iii) KPT-9274 (1 μM) for 72 hours followed by staining for Annexin V/Propidium Iodide (PI). For all cell types, L3 represents the proportion of cells that have low intensity of Annexin V and PI staining and hence have low apoptotic activity, L4 represents the proportion of cells that stain more intensely for Annexin V indicating the early stages of apoptosis, and L2 represents cells that have high levels of Annexin V and PI representing highly apoptotic cells.
Oral administration of KPT-9274 blocks tumorigenesis in mouse xenograft studies
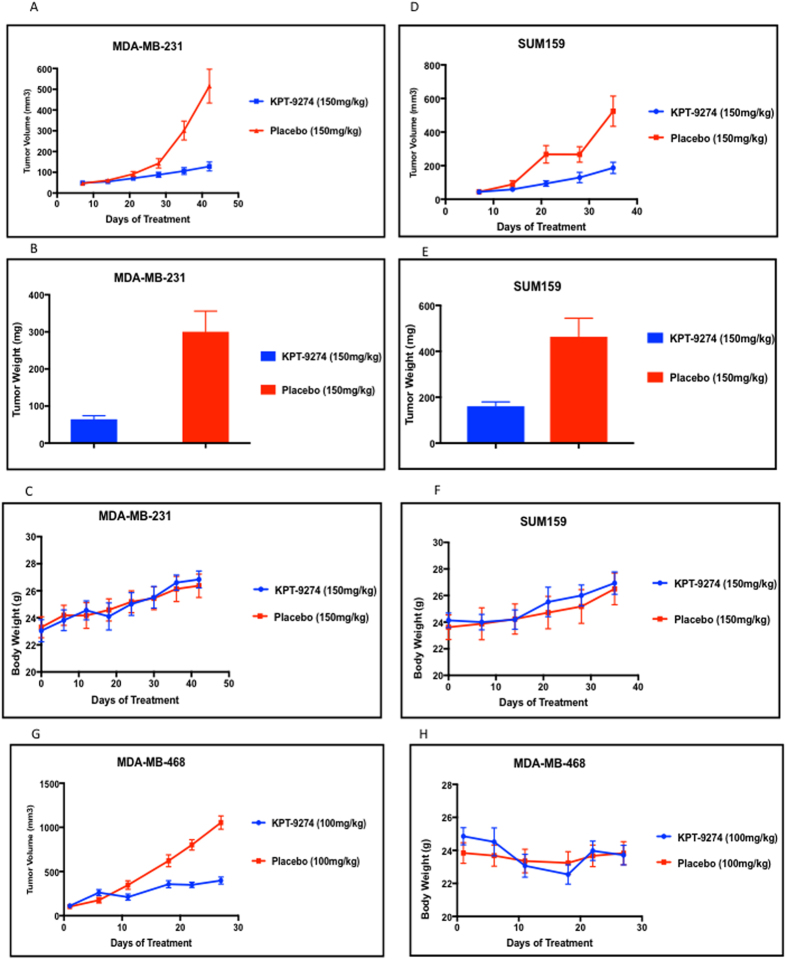
The triple negative breast cancer cells MDA-MB-231, SUM159 and MDA-MB-468 were the most responsive to treatment with KPT-9274 in vitro and represent a subtype of breast cancer that is in particular need of novel therapies. We therefore used these cell lines as a model to test the in vivo efficacy of the orally bioavailable clinical candidate KPT-9274 (Fig. 5). MDA-MB-231, SUM159 and MDA-MB-468 cells were injected into the flanks of female nude mice. Seven days following tumor cell injection (when tumors were approximately 50–100 mm3) mice were administered KPT-9274 or placebo. Treatment continued twice per day for four days per week. Treatment with orally administered KPT-9274 resulted in a significant reduction in the tumor volumes in all three models of the treatment groups as compared to the control groups, and in the tumor weights, which were measured for MDA-MB-231 and SUM159 (see Fig. 5 and Table 1). Treatment did not significantly affect the body weights of the mice (Fig. 5C,F and H).
Figure 5. Oral administration of KPT-9274 blocks tumor growth in mice.
106 cells (MDA-MB-231 (A,B,C), SUM159 (D,E,F), or MDA-MB-468 (G,H), all of which are triple negative breast cancer cell lines, were injected into both flanks of female nude mice. (MDA-MB-231: n = 8, treatment group; n = 10, control group; MDA-MB-468: n = 8, treatment group; n = 7, control group; SUM159: n = 5 treatment group; n = 5, control group). Seven days following injection, mice were treated with orally administered KPT-9274 or Placebo (150 mg/kg PO bidx4 for the MDA-MB-231 and SUM159 cells, or 100 mg/kg PO bidx4 for the MDA-MB-468 cells). Tumor volume (V; mm3) was calculated for each cell line (see A,D, and G). Tumor weight was assessed for the MDA-MB-231 cells and the SUM159 cells (see B and E). Body weight of mice (see C,F,H) was monitored throughout the course of dose administration.
Table 1. Summary of In-vivo data from Fig. 5.
| Cell line | No. of tumor (n) | Dosing Regimen | Average Tumor Volume (mm3) (Endpoint) | Average Tumor Weight (mg) | |||
|---|---|---|---|---|---|---|---|
| KPT-9274 | Placebo | KPT-9274 | Placebo | KPT-9274 | Placebo | ||
| MDA-MB-231 | 16 | 20 | 150 mg/kg PO bidx4 | 128 + 21.7 | 515.1 + 81.6 | 64.2 + 9.6 | 300.2 + 55.8 |
| MDA-MB-468 | 8 | 7 | 100 mg/kg PO bidx4 | 398.7 + 41 | 1053.8 + 76 | — | — |
| SUM159 | 10 | 10 | 150 mg/kg PO bidx4 | 187 + 33.7 | 524.2 + 90.2 | 160.8 + 19 | 463.1 + 81.1 |
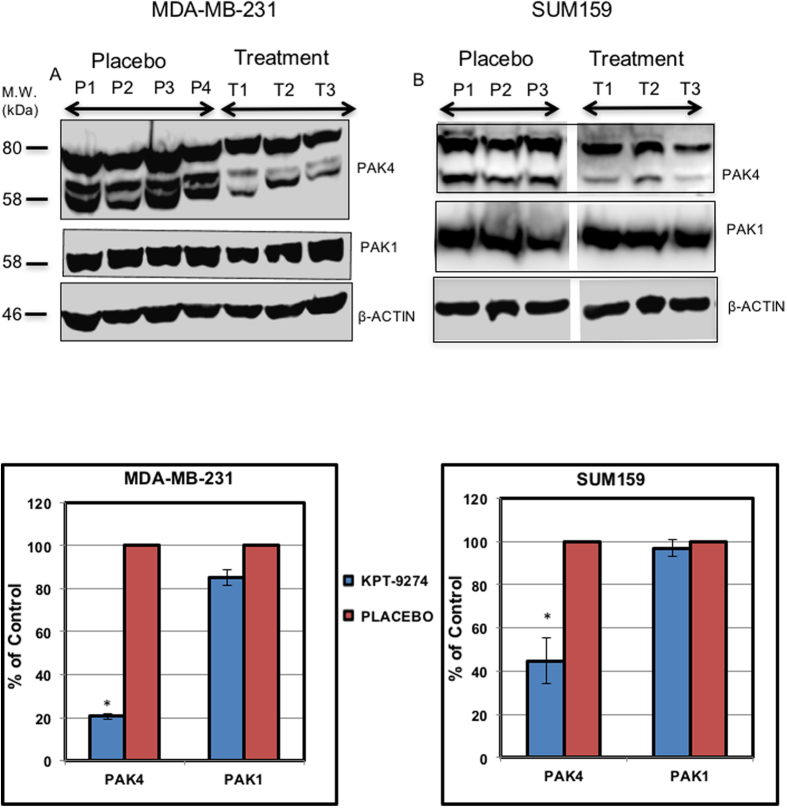
After treatment concluded, the MDA-MB-231 and SUM159 tumors were excised and immunoblotting was performed to measure PAK4 protein levels (Fig. 6). We observed a significant decrease in PAK4 levels in excised tumors from the treatment group, when compared to those from the control (placebo) group. Compared to PAK4, the levels of an off-target protein, PAK1, were not significantly changed after treatment (Fig. 6). Thus, our results indicate that orally administered KPT-9274 reduces the steady state level of PAK4 protein and is capable of reducing growth of the triple negative breast cancer cells MDA-MB-231, MDA-MB-468 and SUM159. These results are also consistent with previous studies indicating that PAK4 knockdown with siRNA blocks tumorigenesis caused by MDA-MB-231 cells13.
Figure 6. Orally administered KPT-9274 reduces PAK4 protein levels.
As the treatment concluded, MDA-MB-231 and SUM159 xenograft tumors were excised and analyzed by Western Blot (A). For the MDA-MB-231 mice, tumors from three independent mice that were treated with the KPT-9274 (T1, T2, T3), and from four independent mice that were treated with placebo (P1, P2, P3, P4) were analyzed by western blot, while for the SUM159 cells, tumors from three independent mice treated with KPT-9274 (T1, T2, T3) and three independent mice treated with placebo (P1, P2, P3) were analyzed by western blot. Blots were probed with anti PAK4, anti PAK1, or anti β-actin antibody as a loading control. The intensity of the bands in the blot in panel A were quantitated using Image J software, and the bands were normalized to the β-actin control (B). Results are plotted as a percent of control, where the control represents the band intensity for placebo, and set as 100% for each protein (PAK4 or PAK1). Data shown is representative of three separate repeat experiments. (The membranes were cut prior to exposure so that only the portion of gel containing bands in the size range of PAK4, PAK1, or β-actin would be visualized, as described in materials and methods. The top band in the PAK4 panel is a nonspecific band that appears in response to our PAK4 antibody, regardless of the presence of PAK4).
Discussion
In this study, we have found that KPT-8752 and KPT-9274 reduce cell proliferation and survival in several triple negative breast cancer cell lines in vitro. The most exciting outcome from this study is that KPT-9274 can inhibit breast cancer tumorigenicity in vivo, in three independent mouse xenograft models using human triple negative breast cancer cell lines. We examined several subtypes of breast cancer cell lines in the current study and were encouraged to find that treatment with these inhibitors blocks the growth of three triple negative breast cancer cell lines. Because this subtype is generally less responsive to many of the current therapies the data presented here provides a path forward to address the urgent need for novel treatment options for triple negative breast cancer patients.
KPT-8752 and KPT-9274 were identified as small molecules that bind to and reduce the steady state level of PAK4 protein in cells, and they were subsequently shown to inhibit the activity of NAMPT4. PAK4 has been found to be elevated in both breast cancer cells and primary breast tumors5,6,7,8,9,10. Furthermore, in basal like breast cancer (a type that is usually triple negative) the chromosomal region containing the PAK4 gene is frequently amplified12. However, not all of the cancer cell lines described here were responsive to KPT-8752 or KPT-9274 despite their having high levels of PAK4. Specifically, in contrast to the triple negative cells, treatment with the compounds had only moderate effects on MCF7 cells (ER+/PR+), BT-474 (PR+/HER2+) and SkBr-3 cells (HER2+) in vitro, even though all of these cell lines have high levels of PAK4. Investigation of additional breast cancer cells will help to determine whether the different responses we observed were in fact related to the backgrounds of the different cell types, and whether or not they are related to PAK4 status. From a patient population perspective, it will be vital to understand the reason why KPT-9274 was unable to reduce the viability of HER2+ and ER+ cells. One possibility is that KPT-9274 may operate through an additional target(s), in addition to, or possibly even instead of, PAK4, which may play a critical role in the biology of the triple negative cells. Alternatively, the results may indicate that HER2 and ER may promote tumorigenesis by mechanisms that are independent of PAK4. If this is the case, the use of KPT-9274 in combination with HER2 inhibitors or estrogen blockers may be warranted.
PAK4 has several cellular functions that are frequently linked to cancer such as promoting cell survival and proliferation38,39,45, prolonging activation of the ERK/MAP Kinase pathway6, and regulating cytoskeletal changes46. Because of this link, there has been considerable interest in generating PAK inhibitors. One of the first PAK4 inhibitors identified was PF-3758309, which was broadly active against both group I and group II PAKs as well as several other kinases47,48. This compound inhibited the growth of a number of tumor cell lines both in vitro and in vivo48,49. However, human clinical trials were terminated due to undesirable PK characteristics of the drug (i.e. low bioavailability), adverse side effects, and consequent lack of tumor responses50,51. A second PAK4 kinase inhibitor, LCH-7749944 52,53, reduced proliferation and invasion of gastric cancer cells in vitro, and reduced filopodia formation and cell elongation, but it has not been tested in vivo. A third inhibitor, Compound 17 (also called GNE-2861), is potent against Group II PAKs. It reduced the viability and motility of breast cancer cell lines42, and it enhanced tamoxifen sensitivity in MCF7 cells9, but has poor oral bioavailability54. GL-1196 is another small molecule that inhibits PAK4 kinase activity, and suppresses the invasive capability of gastric cancer cells55. While clinical studies involving several of the compounds described above have been terminated due to poor bioavailability, improved second generation derivatives of some of these compounds may hold more promise. KPT-9274 is a unique type of inhibitor in that it reduces the steady state level of the PAK4 protein, although the exact mechanism by which it reduces PAK4 levels is not completely understood. Reduction in PAK4 protein is important because like other PAK family members, PAK4 has been shown to have certain functions that are independent of its kinase activity10,38,39,40,41,45. Therefore, reduction of PAK4 protein advantageously reduces or inhibits any oncogenic process that requires the presence of the protein.
While our results indicate that KPT-9274 is a promising agent for triple negative breast cancer treatment, new data is emerging that it may also be effective against other types of cancers4,31. As a result, KPT-9274 is currently under phase I clinical trials to evaluate its safety, tolerability, and efficacy (NCT02702492), in patients with solid tumors and lymphomas. Among the other available PAK4 inhibitors, we have found that compound 1742 can also block the growth of breast cancer cell lines in our system, however it was less effective than KPT-9274 (Rane and Minden, unpublished results). In the future, it would be important to determine whether inhibiting PAK4 simultaneously with different types of inhibitors, could result in even stronger inhibition of PAK4 and inhibition of cancer cell growth. In addition to PAK4, other PAK family members such as PAK1 and PAK2 are often linked to breast cancer53–58. Therefore, in future studies it will be interesting to determine whether combinations of inhibitors against the different PAK isoforms may be even more effective, particularly in cells that are unresponsive to single isoform inhibition.
Although KPT-9274 and its analogs inhibit PAK4, other PAK isoforms could still be affected. In this study, we found that the reduction in PAK4 levels by KPT-9274 is significantly stronger than the reduction in PAK1, suggesting specificity for the group II PAKs. However, we have not tested the effects of KPT-9274 on the other group II PAK family members, PAK5 and PAK6, in breast cancer cells. Since PAK5 and PAK6 proteins are less frequently associated with breast cancer, our focus for this study was PAK4, but further investigation is warranted.
It is important to consider that in addition to PAK4, KPT-9274 and KPT-8752 also reduces the synthesis of NAD (nicotinamide adenine dinucleotide), by blocking the activity of the enzyme NAMPT (nicotinamide phosphoribosyltransferase)4. NAD is involved in a wide range of cellular processes, including DNA repair, and cell signaling, which are also thought to be important in cancer56. A direct link between PAK4 inhibition and NAD has not been established, but NAMPT has been reported to activate Cdc42, a known activator of PAK4, during cytoskeletal organization57. It is important to consider that many of the effects that we have seen in response to KPT-9274 could also be attributed to NAMPT inhibition, and more work will be required to determine which effects of the compound can be attributed to PAK4 and which can be attributed to NAMPT or possibly even other targets. It should be noted, however, that in breast cancer cell lines, we have seen that KPT-8752 and KPT-9274 do block NAMPT activity, but that inhibition of NAMPT did not correlate with the ability of the compounds to block cell proliferation (Minden lab, unpublished results). While it is important to consider the possibility that KPT-9274 has pleotropic effects and impacts multiple signaling pathways, it is interesting to note that nearly all of the effects observed with the inhibitor are consistent with what has been reported with PAK4 knockdown via siRNA. In particular, our previous work indicated that siRNA knockdown of PAK4 in the triple negative breast cancer cell line MBA-MB-231 reversed many aspects of tumorigenesis. The effects included inhibition of cell proliferation, increased apoptosis, and most importantly, decreased tumorigenesis in mice13. These studies strongly support the idea that blocking PAK4 correlates with inhibition of tumorigenesis in triple negative breast cancer. Although more work will be required to determine the exact mechanism by which KPT-9274 operates, our results provide support for the use of this promising clinical candidate in triple negative breast cancer, a disease that is refractory to many of the treatment options currently available.
Additional Information
How to cite this article: Rane, C. et al. A novel orally bioavailable compound KPT-9274 inhibits PAK4, and blocks triple negative breast cancer tumor growth. Sci. Rep. 7, 42555; doi: 10.1038/srep42555 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Acknowledgments
We would like to thank Dr. Philip Furmanski for his valuable advice, Dr. Suzie Chen for helpful discussions and generous provision of cell lines, Dr. Suh for helpful discussions, and Joseph Wahler for providing technical help. We are grateful to Raj Shah and Jeong Eun Park for their help. We would also like to thank Anna Lee and Pranjal Patel, for technical help. We thank Christian Argueta for critical review of the paper.
Footnotes
William Senapedis, Erkan Baloglu, Yosef Landesman and Marsha Crochiere are employees of Karyopharm Therapeutics Inc. and have financial interests in this company.
Author Contributions Chetan Rane and Audrey Minden prepared the main manuscript text. William Senapedis, Yosef Landesman, Erkan Baloglu and Marsha Crochiere provided data for Figures 2D and 5G,H. Soumyasri-Das Gupta helped in collecting MDA-MB-231 tumor samples.
References
- Perou C. M. et al. Molecular portraits of human breast tumors. Nature 406, 747–752 (2000). [DOI] [PubMed] [Google Scholar]
- Gazinska P. et al. Comparison of basal-like triple-negative breast cancer defined by morphology, immunohistochemistry and transcriptional profiles. Mod Pathol 26, 955–966 (2013). [DOI] [PubMed] [Google Scholar]
- Badve S. et al. Basal-like and triple negative breast cancers: a critical review with an emphasis on the implications for pathologists and oncologists. Mod Pathol 24, 157–167 (2011). [DOI] [PubMed] [Google Scholar]
- Abu Aboud A. et al. Dual and specific inhibition of NAMPT and PAK4 by KPT-9274 decreases kidney cancer growth. Mol Cancer Ther 15, 2119–2129 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y. et al. The Pak4 protein kinase plays a key role in cell survival and tumorigenesis in athymic mice. Molecular Cancer Research 6, 1215–1224 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y. et al. The protein kinase Pak4 disrupts mammary acinar architecture and promotes mammary tumorigenesis Oncogene 29, 5883–5894 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callow M. G. et al. Requirement for PAK4 in the anchorage-independent growth of human cancer cell lines. J Biol Chem 277, 550–558 (2002). [DOI] [PubMed] [Google Scholar]
- Bi Y. et al. Study on the expression of PAK4 and P54 protein in breast cancer. World J Surg Oncol 14, 160–164 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang T. et al. p21-activated kinase group II small compound inhibitor GNE-2861 perturbs estrogen receptor alpha signaling and restores tamoxifen-sensitivity in breast cancer cells. Oncotarget 6, 43853–43868 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dart A. E. et al. PAK4 promotes kinase-independent stabilization of RhoU to modulate cell adhesion. J Biol Chem 211, 863–879 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- He L.-F. et al. Activated-PAK4 predicts worse prognosis in breast cancer and promotes tumorigenesis through activation of PI3K/AKT signaling. Oncotarget Advance Online Publication (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W., Kanaan Y., Bae Y. K. & Gabrielson E. Chromosomal changes in aggressive breast cancers with basal-like features. Cancer Genet Cytogenet 193, 29–37 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong L. E., Chen N., Karantza V. & Minden A. The Pak4 protein kinase is required for oncogenic transformation of MDA-MB-231 breast cancer cells. Oncogenesis 2, 1–6 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eswaran J., Soundararajan M., Kumar R. & Knapp S. UnPAKing the class differences among p21-activated kinases. Trends Biochem Sci 33, 394–403 (2008). [DOI] [PubMed] [Google Scholar]
- Wells C. M. & Jones G. E. The emerging importance of group II PAKs. Biochem J 425, 465–473 (2010). [DOI] [PubMed] [Google Scholar]
- Ahmed T., Shea K., Masters J. R., Jones G. E. & Wells C. M. A Pak4-LIMK1 pathway drives prostate cancer cell migration downstream of HGF. Cell Signal 20, 1320–1328 (2008). [DOI] [PubMed] [Google Scholar]
- Chen S. et al. Copy number alterations in pancreatic cancer identify recurrent PAK4 amplification. Cancer Biol Ther 7 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siu M. K. Y. et al. p21-activated kinase 4 regulates ovarian cancer cell proliferation, migration, and invasion and contributes to poor prognosis in patients. Proc Natl Acad Sci USA 107, 18622–18627 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanivan S. et al. In vivo SILAC-Based Proteomics Reveals Phosphoproteome Changes during Mouse Skin Carcinogenesis. Cell Rep 3, 552–586 (2013). [DOI] [PubMed] [Google Scholar]
- Davis S. J. et al. Functional analysis of genes in regions commonly amplified in high-grade serous and endometrioid ovarian cancer. Clin Cancer Res 19, 1411–1421 (2013). [DOI] [PubMed] [Google Scholar]
- Minden A. PAK4-6 in cancer and neuronal development. Cell Logist 2, 95–104 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minden A. The pak4 protein kinase in breast cancer. ISRN Oncol 2012 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn H. K. et al. P21-activated kinase 4 overexpression in metastatic gastric cancer patients. Transl Oncol 4, 345–349 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmelman A. C. et al. Genomic alterations link Rho family of GTPases to the highly invasive phenotype of pancreas cancer. Proc Natl Acad Sci USA 105, 19372–19377 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahlamaki E. H. et al. High-resolution genomic and expression profiling reveals 105 putative amplification target genes in pancreatic cancer. Neoplasia 6, 432–439 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begum A. et al. Identification of PAK4 as a putative target gene for amplification within 19q13.12-q13.2 in oral squamous-cell carcinoma. Cancer Sci 100, 1908–1916 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafn B. et al. ErbB-driven breast cancer cell invasion depends on a complex signaling network activating myeloid zinc finger-1 dependent cathepsin B expression. Mol Cell 45, 764–776 (2012). [DOI] [PubMed] [Google Scholar]
- Park M. H. et al. p21-Activated kinase 4 promotes prostate cancer progression through CREB. Oncogene 32, 2475–2482 (2013). [DOI] [PubMed] [Google Scholar]
- Tabusa H., Brooks T. & Massey A. J. Knockdown of PAK4 or PAK1 inhibits proliferation of mutant KRAS colon cancer cells independently of RAF/MEK/ERK and PI3K/AKT signaling. Mol Cancer Res 11, 109–121 (2013). [DOI] [PubMed] [Google Scholar]
- Whale A. D., Dart A. E., Holt M., Jones G. E. & Wells C. M. PAK4 kinase activity and somatic mutation promote carcinoma cell motility and influence inhibitor sensitivity. Oncogene 32 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y. Y. et al. Targeted super-enhancer associated oncogenes in oesophageal squamous cell carcinoma. Gut (2016). [DOI] [PMC free article] [PubMed]
- Li S. Q., Wang Z. H., Mi X. G., Liu L. & Tan Y. Mir-199a/b-3p suppresses migration and invasion of breast cancer cells by downregulating PAK4/MEK/ERK signaling pathway. IUBMB Life 67, 768–777 (2015). [DOI] [PubMed] [Google Scholar]
- Kumar R. & Li D.-Q. PAK s in human cancer progression: from inception to cancer therapeutic to future oncobiology. Advances in Cancer Research 130, 137–209 (2016). [DOI] [PubMed] [Google Scholar]
- King H., Nicholas N. S. & Wells C. M. Role of p-21-activated kinases in cancer progression. Rev Cell Mol Biol 309, 347–387 (2014). [DOI] [PubMed] [Google Scholar]
- Shao Y. G., Ning K. & Li F. Group II p21-activated kinases as therapeutic targets in gastrointestinal cancer. World J Gastroenterol 22, 1224–1235 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo D., He H., Baldwin G. S. & Nikfarjam M. The role of p21-activated kinases in pancreatic cancer. Pancreas 44, 363–369 (2015). [DOI] [PubMed] [Google Scholar]
- Radu M., Semenova G., Kosoff R. & Chernoff J. PAK signalling during development and progression of cancer. Nature Reviews 14, 13–25 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnesutta N. & Minden A. Death Receptor Induced Activation of Initiator Caspase-8 is Antagonized by the Serine/Threonine Kinase PAK4. Mol. Cell. Biol. 23, 7838–7848 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnesutta N., Qu J. & Minden A. The Serine/Threonine Kinase PAK4 prevents caspase activation and protects cells from apoptosis. J Biol Chem 276, 14414–14419 (2001). [DOI] [PubMed] [Google Scholar]
- Daniels R. H., Hall P. S. & Bokoch G. M. Membrane targeting of p21-activated kinase 1 (PAK1) induces neurite outgrowth from PC12 cells. EMBO J 17, 754–764 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sells M. A. et al. Human p21-activated kinase (Pak1) regulates actin organization in mammalian cells. Curr Biol 7, 202–210 (1997). [DOI] [PubMed] [Google Scholar]
- Staben S. T. et al. Back pocket flexibility provides group II PAK selectivity for type 1-1/2 kinase inhibitors. J Medicinal Chem 57 (2014). [DOI] [PubMed] [Google Scholar]
- Li Y. et al. Nucleo-cytoplasmic shuttling of PAK4 modulates beta-catenin intracellular translocation and signaling. Biochem Biophys Acta 1823, 465–475 (2012). [DOI] [PubMed] [Google Scholar]
- Dan C., Kelly A., Bernard O. & Minden A. Cytoskeletal changes regulated by the PAK4 serine/threonine kinase are mediated by LIM kinase 1 and cofilin. J Biol Chem 276, 32115–32121 (2001). [DOI] [PubMed] [Google Scholar]
- Li X. & Minden A. PAK4 functions in tumor necrosis factor (TNF) alpha-induced survival pathways by facilitating TRADD binding to the TNF receptor. J Biol Chem 280, 41192–41200 (2005). [DOI] [PubMed] [Google Scholar]
- Abo A. et al. PAK4, a novel effector for Cdc42Hs, is implicated in the reorganization of the actin cytoskeleton and in the formation of filopodia. EMBO J 17, 6527–6540 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z. S. & Manser E. Do PAKs make good drug targets? F1000 Biol Rep 2, 70–73 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray B. W. et al. Small-molecule p21-activated kinase inhibitor PF-3758309 is a potent inhibitor of oncogenic signaling and tumor growth. Proc Natl Acad Sci USA 107, 9446–9451 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raja R. et al. Chronic exposure to cigarette smoke leads to activation of p21 (RAC1)-activated kinase 6 (PAK6) in non-small cell lung cancer cells. Oncotarget 7, 61229–61245 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen L. S. et al. Phase 1, dose-escalateion, safety, pharmacokinetic and pharmacodynamic study of single agent PF-03758309, an oral PAK inhibitor, in patients with advanced solid tumors. AACR-NCI-EORTC International Conference: Molecular Targets and Cancer Therapeutics 10, ABSTRACT A177 (2011). [Google Scholar]
- Crawford J. J., Hoeflich K. P. & Rudolph J. p21-Activated kinase inhibitors: a patent review. Expert Opin Ther Pat 22, 293–310 (2012). [DOI] [PubMed] [Google Scholar]
- Zhang J. et al. LCH-7749944, a novel and potent p21-activated kinase 4 inhibitor, suppresses proliferation and invasion in human gastric cancer cells. Cancer Lett 317, 24–32 (2012). [DOI] [PubMed] [Google Scholar]
- Dummler B. et al. Pak protein kinases and their role in cancer. Cancer Metastasis Rev 28(1–2), 51–63 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedland J. C. et al. Alpha6beta4 integrin activates Rac-dependent p21-activated kinase 1 to drive NF-kappaB-dependent resistance to apoptosis in 3D mammary acini. J Cell Sci 120(Pt 20), 3700–12 (2007). [DOI] [PubMed] [Google Scholar]
- Wang R. A. et al. PAK1 hyperactivation is sufficient for mammary gland tumor formation. Oncogene 25(20), 2931–6 (2006). [DOI] [PubMed] [Google Scholar]
- Arias-Romero L. E. et al. A Rac-Pak signaling pathway is essential for ErbB2-mediated transformation of human breast epithelial cancer cells. Oncogene 29(43), 5839–5849 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias-Romero L. E. & Chernoff J. p21-activated kinases in Erbb-positive breast cancer: A new therapeutic target? Small GTPases 1(2), 124–128 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X. et al. Phosphorylation of caspase-7 by p21-activated protein kinase (PAK) 2 inhibits chemotherapeutic drug-induced apoptosis of breast cancer cell lines. J Biol Chem 286(25), 22291–9 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.