Abstract
Changes in fifteen urine, blood and exhaled breath BoEs of HPHCs representing classes of compounds reported by FDA to be significant contributors to smoking-associated disease risks were measured in 105 clinical-confined subjects following randomization and a five-day forced-switch from usual brand conventional combustible cigarettes to: (i) exclusive commercial e-cigarette use; (ii) dual-use of commercial e-cigarettes and the subject’s usual cigarette brand; or (iii) discontinued use of all tobacco or nicotine products. Levels of urinary biomarkers in subjects that completely substituted their usual cigarette with e-cigarettes were significantly lower (29–95%) after 5 days. Percent reductions in eight of nine urinary BoEs were indistinguishable to smokers who had quit smoking, except for nicotine equivalents, which declined by 25–40%. Dual users who halved self-reported daily cigarette consumption with e-cigarettes exhibited reductions (7–38%) in eight of nine urinary biomarkers, but had increase (1–20%) in nicotine equivalents. Reductions were broadly proportional to the reduced numbers of cigarettes smoked. Dual user urinary nicotine equivalents were slightly higher, but not statistically significant. After 5 days, blood nicotine biomarker levels were lower in the cessation (75–96%) and exclusive use groups (11–83%); with dual users experiencing no significant reductions. All subjects experienced significant decreases in exhaled CO. Decreases in the cessation and exclusive groups ranged from 88–89% and 27–32% in dual users. Exhaled NO increased in the cessation and exclusive groups (46–63% respectively), whereas the dual users experienced minimal changes. Overall, smokers who completely or partially substituted conventional cigarettes with e-cigarettes over five days, experienced reductions in HPHCs.
Keywords: Biomarkers of exposure, cessation, e-cigarettes, exclusive and dual use, harm reduction
Introduction
Electronic cigarettes (e-cigarettes) represent a rapidly-emerging product category that holds promise as a conventional tobacco cigarette alternative as they simulate some of the familiar behavioral and sensorial aspects of the smoking experience which conventional nicotine replacement therapy products do not. E-cigarettes do not contain tobacco, do not require combustion, or generate side-stream smoke. E-cigarettes are battery-powered devices that deliver an aerosol (popularly referred to as “vapor”) to users from an e-liquid of known chemical composition. E-liquids typically contain glycerol and propylene glycol in varying proportions from which the aerosol is generated and may contain nicotine and various flavors. In contrast, tobacco smoke has been reported to contain many thousands of chemicals including HPHCs associated with the combustion process, as identified by the FDA (USFDA, 2012). The types and concentrations of potential toxicants associated with e-cigarette aerosols is a topic of current research reported in literature. However, the limited number of speculated constituents are ten to thousand times lower than in conventional tobacco cigarette smoke with many of the toxicants in tobacco products simply not present in e-cigarette aerosol at detectable levels when assessed following machine-based aerosol generation (e.g. Goniewicz et al., 2014; Tayyarah & Long, 2014) or are at levels equivalent to the tolerances allowed in medical products. As a result, e-cigarette aerosols elicit minimal biological responses in conventional regulatory in vitro toxicology assays compared with conventional tobacco cigarettes (e.g. Misra et al., 2014). Nevertheless, there is relatively little information available on actual adult smoker’s potential exposure to HPHCs resulting from the use of e-cigarettes compared to conventional tobacco cigarettes (Burstyn, 2014; Cahn & Siegel, 2011; Hajek et al., 2014; Polosa et al., 2013; Tayyarah & Long, 2014).
Recent scientific surveys and studies examining the habits and practices of e-cigarette users indicate that the dual use of cigarettes and e-cigarettes is common practice and that dual users report using e-cigarettes to reduce, replace or help stop smoking conventional tobacco cigarettes as well as reduce family member exposure to secondhand cigarette smoke (i.e. the mixture of the smoke that comes from the burning end of a cigarette and the smoke breathed out by the smoker) (Farsalinos et al., 2014; Farsalinos & Polosa, 2014; McRobbie et al., 2014; Pepper & Brewer, 2014). Exposures to HPHCs that result from dual use may be anticipated to depend on both the extent of daily uses of the respective products, as well as any conscious or subconscious changes in puffing topography consequent to any nicotine or sensory deficits that may derive from the partial substitution of conventional tobacco cigarettes by e-cigarettes. Previously, it had been reported that reductions in daily conventional tobacco cigarettes smoked resulted in reduced urinary excretion of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL) (Hecht et al., 2004), but those reductions fell short of those anticipated from the reduced number of conventional tobacco cigarettes consumed. However, a more recent study of adult smokers who switched to using only e-cigarettes and to dual use of e-cigarettes and conventional tobacco cigarettes showed significant reductions in exposure to carbon monoxide (CO) and the toxicant acrolein over a four-week period (McRobbie et al., 2015).
The primary objective of this study was to compare changes in selected urine, blood, and exhaled breath biomarkers of exposure to HPHCs among different user groups following a five-day forced-switch from usual brand conventional tobacco cigarettes to: (i) exclusive use of commercial e-cigarettes; (ii) dual-use of commercial e-cigarettes and the subject’s usual conventional tobacco cigarette brand; or (iii) discontinued use of all tobacco or nicotine products. The biomarkers of exposure to the selected HPHCs included a number of cigarette smoke constituents representing major classes of compounds believed to be the most significant contributors to smoking-associated disease risks as reported by the FDA.
Methods
Participants
The study protocol and the informed consent forms were approved by Chesapeake IRB, Columbia, MD. The clinical trial was registered on 6 February 2015 at: http://ClinicalTrials.gov identifier: NCT02385227. Two hundred and fourteen potential smokers were recruited from the Lincoln, NE (USA) area using standard advertising methods (i.e., print and radio advertisements) and from a database of subjects who had previously participated in a clinical research study or who had expressed interest in participating in a study. All potential subjects were provided details regarding the study and written informed consent was obtained prior to initiation of any study procedures. One hundred and two subjects were excluded for not satisfying the predefined inclusion/exclusion criteria; 15 subjects declined to participate prior to enrollment; and two subjects were excluded because the study had reached the randomization target of 105 eligible subjects. The 105 subjects meeting the eligibility criteria were enrolled into the study and randomized into one of six study groups. Two subjects withdrew consent from the study following randomization for personal reasons unrelated to study participation. All subjects participating in the study were paid a fee for their participation.
The main criteria for inclusion in the study were as follows: healthy adult male and female smokers, 21–65 years of age inclusive; a smoker for at least 12 months and currently smoked an average of 10 or more conventional manufactured tobacco cigarettes per day (any brand, flavor or style); consistent use of their current usual brand style for 14 days prior to check-in; positive urine cotinine at screening (≥ 500 ng/mL); and exhaled carbon monoxide CO >12 ppm at screening. Prior use of an e-cigarette was not an exclusion criterion, provided all other criteria were met; however, none of the subjects reported previous use of e-cigarettes. Exclusion criteria included: history or presence of clinically significant mental or physical health conditions; females who were pregnant or breastfeeding; high blood pressure; body mass index <18 kg/m2 or >40 kg/m2; acute illnesses (e.g., upper respiratory infection, viral infection) requiring treatment within 2 weeks prior to check-in; use of prescription smoking cessation treatments, anti-diabetic or insulin drugs or medications known to interact with Cytochrome P450 2A6; positive urine screen for alcohol or drugs of abuse; and self-reported mouth-hold smokers (i.e., smokers who draw smoke from the conventional tobacco cigarette into the mouth and throat but do not inhale). Subjects who had used any tobacco- or nicotine-containing products other than manufactured tobacco cigarettes or e-cigarettes within 28 days of in-clinic product use were also excluded.
Products tested
Three commercially available closed system blu™ e-cigarette products (manufacturer, Fontem Ventures B.V., The Netherlands) were evaluated during this study: rechargeable tobacco flavor, rechargeable cherry flavor, and disposable cherry flavor. The rechargeable e-cigarettes consist of a battery segment and a cartomizer segment comprising the heating unit and a liquid reservoir which can be separated from the battery for recharging or replaced when the e-liquid is depleted. The disposable e-cigarette was similar in form with the exception that the battery and cartomizer segments are included as a single, non-separable unit. Both units operated at a voltage of 3.7 volts (nominal). The resistance of the heating element was ∼3 ohms for the disposable unit and about 3.5 ohms for the rechargeable unit. The maximum operating temperature of each unit was dependent on the charge level of the battery, the state of reservoir fluid fill and on the manner of use and was not recorded in this study.
All e-cigarette products contained 24 mg/mL (2.4%) USP grade nicotine, USP grade vegetable glycerol (∼50% in cherry flavor and ∼80% in tobacco flavor), USP grade propylene glycol (∼45% in cherry flavor and ∼10% in tobacco flavor), distilled water, and flavorings. Each e-cigarette contained ∼1 mL of e-liquid by volume.
Subjects were provided unopened packs of their reported usual brand of conventional tobacco cigarettes for use during the study.
Study design
This was a randomized, open-label, forced-switch parallel arm study conducted at a single independent research center (Celerion, Lincoln, NE) to assess biomarkers of exposure to HPHCs (Gregg et al., 2013; Hecht et al., 2010) following short-term ad libitum use of e-cigarettes by established adult smokers. This proof-of-concept study evaluated the hypothesis that use of e-cigarettes, either exclusively or with dual use of conventional tobacco cigarettes (with a 50% reduction in self-reported conventional tobacco cigarettes per day [CPD]), can significantly reduce exposure to many of the HPHCs commonly associated with use of combustible tobacco cigarettes. A cessation arm served as a maximum effect control group comparator.
Following successful screening and study qualification, subjects checked into the clinic on Day −2 and continued to smoke their usual conventional tobacco cigarette brand ad libitum through the evening of Day −1. Subjects were confined in the research clinic for the entire duration of the study. During enrollment, subjects were also trained on how to use the e-cigarettes and were also informed of how to notify the clinical staff of situations involving non-operating e-cigarettes. A Fagerström Test for Cigarette Dependance (FTCD) (Fagerström, 2012; Heatherton et al., 1991) was also administered to all subjects upon enrollment. Baseline assessments occurred from the morning of Day −1 through the morning of Day 1 prior to the start of randomized product use and post-baseline assessments on the morning of Day 1 through the morning of Day 6.
On the morning of Day 1, subjects were randomized into one of six groups (N = 15 each):
Exclusive E-Cigarette Use Groups
Group A1 – Tobacco flavor rechargeable blu™ e-cigarette
Group A2 – Cherry flavor rechargeable blu™ e-cigarette
Group A3 – Cherry flavor disposable blu™ e-cigarette
Dual Use Groups
Group B1 – Tobacco flavor rechargeable blu™ e-cigarette + usual brand combustible tobacco cigarette
Group B2 – Cherry flavor rechargeable blu™ e-cigarette + usual brand combustible tobacco cigarette
Group B3 – Cherry flavor disposable blu™ e-cigarette + usual brand combustible tobacco cigarette
Cessation Group
Group C – Complete tobacco and nicotine product cessation
Product use
Use of the assigned products was documented daily and subjects were monitored during clinical confinement to ensure that no illicit nicotine or tobacco products were used. Subjects randomized to the cessation group were housed in an area of the clinic separate from the other groups. With limited exceptions, all product use was ad libitum from 07:30 to 23:00 on Days 2 to 5. These exceptions included meals, 15 min prior to blood sampling, and 30 min prior to exhaled CO and nitric oxide (NO) measurements.
Subjects randomized to receive the e-cigarette products were allowed to carry them throughout the day in designated sections of the clinic. New e-cigarettes were supplied to the subjects each morning and throughout the day if the e-liquid solution was fully consumed or the product failed to work properly. All e-cigarettes were weighed before and after use.
Subjects randomized to the dual use group were required to request a conventional tobacco cigarette product from the clinic staff and smoke only in specified sections of the clinic away from nonsmoking subjects. In order to standardize cigarette consumption during the study, subjects in the dual use groups were required to reduce their daily cigarette consumption on Days 1–5 by ∼50% from that reported at screening.
Determination of sample size
The sample size estimation was based on total NNAL because the group difference in percent change-from-baseline was expected to be smaller than the other biomarkers due to a longer half-life for elimination (approximately 45 days, Hecht et al., 1999). In a previous study, adult smokers who replaced conventional tobacco cigarettes with a snus product or discontinued use of all tobacco products completely for 5 days excreted ∼60–70% less NNAL, while subjects who reduced conventional tobacco cigarette use by half excreted ∼30% less total NNAL over the same timeframe (Sarkar et al., 2010). Based on these results, a sample size of 12 was estimated to detect a 70% reduction from baseline in the groups that stopped smoking and to be able to detect the differences between groups with at least 80% power using two-sided testing. Up to 15 subjects were assigned to each group to maximize the likelihood of a minimum of 12 subjects completing the study in each group.
Biomarker analysis
The urine and blood biomarkers of exposure evaluated in this study (Table 1) were chosen to represent major classes of HPHCs that have previously been reported for conventional tobacco cigarette smokers (Carmella et al., 2009; Gregg et al., 2013; Hecht, 2002; Hecht et al., 2010; USDHHS, 2014). All urine voided by each subject was collected in 24-h intervals from 07:30 on Day −1 through 07:30 on Day 1, and from 07:30 on Day 5 through 07:30 on Day 6, and aliquots were prepared from the 24-h collections. Blood samples were collected on Days −1 and 5 in the evening following dinner to assess exposure to CO and nicotine. Each biomarker was measured using validated methods based on: FDA’s Guidance to Industry for Bioanalytical Method Validation (2001); Good Laboratory Practices per 21 CFR Part 58; and the EMEA Guideline on Bioanalytical Method Validation (EMEA/CHMP/EWP/192217/2009 Rev. 1 Corr.2).
Table 1.
Urine, blood and inhalation biomarkers of tobacco smoke exposure.
| URINE: Biomarkers of exposure analyzed | ||||||||
|---|---|---|---|---|---|---|---|---|
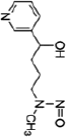
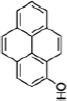
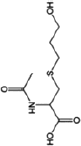
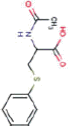
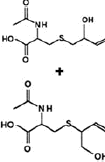
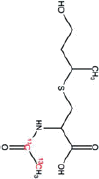
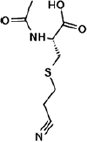
| Nicotine equivalentsa | NNN | NNAL | 1-OHP | 3-HPMA | S-PMA | MHBMA | HMPMA | CEMA |
Nicotine +5 major metabolites
|
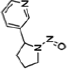 |
 |
 |
 |
 |
 |
 |
 |
|
aNicotine equivalents measured: included nicotine and five major nicotine metabolites: nicotine gluc; cotinine; cotinine-gluc; trans-3’-hydroxycotinine; and trans-3’-hydroxycotinine-gluc. NNAL: 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol; 1-HOP: 1-hydroxypyrene; 3-HPMA: 3-hydroxypropylmercapturic acid; S-PMA: S-phenylmercapturic acid; MHBMA: Monohydroxy-3-butenyl mercapturic acid; HMPMA: 3-hydroxy-1-ethylpropylmercapturic acid; CEMA: 2-cyanoethylmercapturic acid. | ||||||||
| HPHCs-associated with urine biomarkers | ||||||||
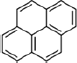
| Nicotine | Tobacco Specific Nitrosamines (TSNA) | PAH | Volatile Organic Compounds (VOC’s) | |||||
| Nicotine |
NNN |
NNK |
Pyrene |
Acrolein |
Benzene |
1,3-Butadiene |
Crotonaldehyde |
Acrylonitrile |
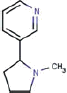 |
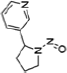 |
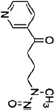 |
 |
|||||
| PAH: Polycyclic Aromatic Hydrocarbons; NNN: N-Nitrosonornicotine; NNK: 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone. | ||||||||
| Clinical endpoints of urine HPHCs | ||||||||
| Nicotine Exposure | Cancer | Cancer | Cancer | Cancer | Cancer | Cancer | Cancer | Cancer |
| Analysis method and Lower Limit of Quantification (LLOQ) | ||||||||
| LC-MS-MS 50–200 ng/mLb | LC-MS-MS 0.2 pg/mL | LC-MS-MS 5 pg/mL | LC-MS-MS 10 pg/mL | LC-MS-MS 20 ng/mL | LC-MS-MS 25 pg/mL | LC-MS-MS 0.1 ng/mL | LC-MS-MS 20 ng/mL | LC-MS-MS 0.275 ng/mL |
| aNicotine equivalents: calculated as the molar sum of nicotine and five major nicotine metabolites excreted in urine over 24 h (mg nicotine equivalents/24 h). bLLOQ for Nicotine equivalents: 50 ng/mL for Nicotine, Nicotine gluc, Cotinine and Trans-3’-hydroxycotinine; 200 ng/mL for Cotinine-gluc and Trans-3’-hydroxycotinine-gluc. | ||||||||
| BLOOD: Biomarkers of exposure analyzed | ||||||||
| Blood CarboxyHemoglobin (COHb) | Plasma Nicotine | Plasma Cotinine | Plasma Trans-3’hydroxycotinine | |||||
| Chemical constituents associated with blood biomarkers | ||||||||
| Carbon Oxide | Nicotine | |||||||
| Clinical endpoints associated with blood biomarkers | ||||||||
| Carbon Monoxide (CO) exposure | Nicotine exposure | Nicotine exposure | Nicotine exposure | |||||
| Analysis method and Lower Limit of Quantification (LLOQ) | ||||||||
| Spectrophotometric 0.50% | LC-MS-MS 0.2 ng/mL | LC-MS-MS 1.0 ng/mL | LC-MS-MS 1.0 ng/mL | |||||
| INHALATION: Biomarkers of exposure analyzed | ||||||||
| Exhaled Carbon Monoxide (CO) | Exhaled Nitric Oxide (NO) | |||||||
| Analysis method and Lower Limit of Quantification (LLOQ) | ||||||||
| Bedfont Micro + Smokerlyzer: 1 ppm (0–500 ppm) | Niox Mino: 5 ppb (5–300 ppb) | |||||||
Exhaled breath biomarkers
Exhaled CO and NO are measures of acute carbon monoxide exposure and nitric oxide synthase activity, respectively (Taylor et al., 2006). Smokers characteristically exhale higher CO (Deveci et al., 2004) and lower NO (Kharitonov et al., 1995) than nonsmokers. Exhaled CO and NO were measured during the study in the afternoon on Days 1 and 5 using a Bedfont Micro + Smokerlyzer and Niox Mino, respectively. Sampling was preceded by a 30-min (minimum) abstention from study product use.
Data analyzes
Statistical analyzes were performed using SAS procedures in SAS® Version 9.3 (SAS Institute Inc., Cary, NC). A paired t-test was used to make within-group comparisons between study days and a linear mixed model was used to assess between-group differences. Baseline values were included in the statistical models for the between-group comparisons as a covariate. Differences were considered statistically significant at an alpha level of 5% and no adjustments were made for multiple comparisons.
In addition, regression analyzes were performed for the Day −1 to Day 5 % change in urine biomarker concentrations against the Day −1 to Day 5 % change in CPD for the dual use groups and to evaluate the relationship between urine nicotine equivalents and the estimated amount of nicotine delivered by the e-cigarette products (Day 5 exclusive and dual use groups) and the number of cigarettes smoked (Day 5 in dual use groups, Day −1 in all groups).
Results
Participant characteristics
A summary of the subjects’ demographics, tobacco product use history, and FTCD scores for all study participants by study product sequence and overall is presented in Table 2.
Table 2.
Summary of study demographics and FTCD scores by product use groups and overall.
| Exclusive E-Cigarette use groups |
Dual use groups |
|||||||
|---|---|---|---|---|---|---|---|---|
| Trait/Test | Tobacco rechargeable N = 15 | Cherry rechargeable N = 15 | Cherry disposable N = 15 | Tobacco rechargeable N = 15 | Cherry rechargeable N = 15 | Cherry disposable N = 15 | Nicotine cessation N = 15 | Overall N = 105 |
| Gender | ||||||||
| Female | 6 (40%) | 3 (20%) | 9 (60%) | 6 (40%) | 3 (20%) | 7 (47%) | 3 (20%) | 37 (35%) |
| Male | 9 (60%) | 12 (80%) | 6 (40%) | 9 (60%) | 12 (80%) | 8 (53%) | 12 (80%) | 68 (65%) |
| Race | ||||||||
| American Indian/Alaska Native | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (7%) | 0 (0%) | 1 (1%) |
| Black or African American | 2 (13%) | 6 (40%) | 1 (7%) | 2 (13%) | 4 (27%) | 1 (7%) | 1 (7%) | 17 (16%) |
| Black or African American, American Indian/Alaska | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (7%) | 1 (1%) |
| White | 13 (87%) | 9 (60%) | 14 (93%) | 13 (87%) | 11 (73%) | 13 (87%) | 13 (87%) | 86 (82%) |
| Age (years) | ||||||||
| Mean | 37.1 | 40.1 | 33.9 | 36.6 | 36.8 | 39.3 | 41.1 | 37.8 |
| SD | 11.4 | 10.6 | 11.8 | 10.8 | 11.6 | 10.6 | 11.2 | 11.1 |
| BMI (kg/m2) | ||||||||
| Mean | 28.2 | 26.2 | 28.7 | 28.9 | 27.2 | 27.8 | 27.8 | 27.8 |
| SD | 5.5 | 6.5 | 5.7 | 5.5 | 4.7 | 5.1 | 4.9 | 5.4 |
| Cigarettes per Day | ||||||||
| Mean | 18.4 | 17.3 | 15.4 | 18.7 | 20.5 | 21.1 | 20.4 | 18.8 |
| SD | 7.1 | 6.2 | 3.3 | 6.6 | 7.3 | 5.8 | 7.5 | 6.5 |
| Years Smoked | ||||||||
| Mean | 19.2 | 20.3 | 15.0 | 19.3 | 14.6 | 21.7 | 21.3 | 18.8 |
| SD | 12.9 | 10.5 | 10.9 | 10.1 | 11.6 | 8.7 | 10.6 | 10.8 |
| Usual brand cigarette flavor | ||||||||
| Menthol | 6 (40%) | 7 (47%) | 8 (53%) | 3 (20%) | 7 (47%) | 5 (33%) | 3 (20%) | 39 (37%) |
| Non-Menthol | 9 (60%) | 8 (53%) | 7 (47%) | 12 (80%) | 8 (53%) | 10 (67%) | 12 (80%) | 66 (63%) |
| FTCD Score | ||||||||
| Mean | 5.3 | 5.1 | 5.3 | 5.5 | 5.7 | 5.2 | 5.6 | 5.4 |
| SD | 1.5 | 2.0 | 1.5 | 2.0 | 1.1 | 1.7 | 2.0 | 1.7 |
Urine and blood biomarker of exposure comparisons
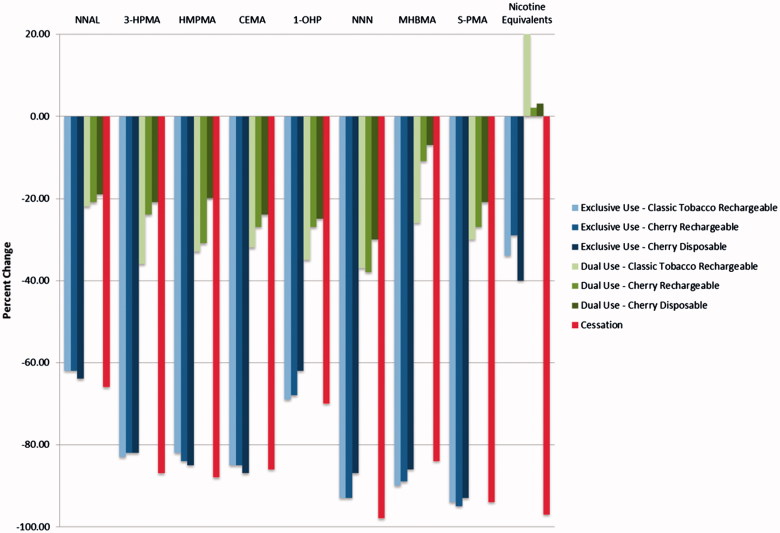
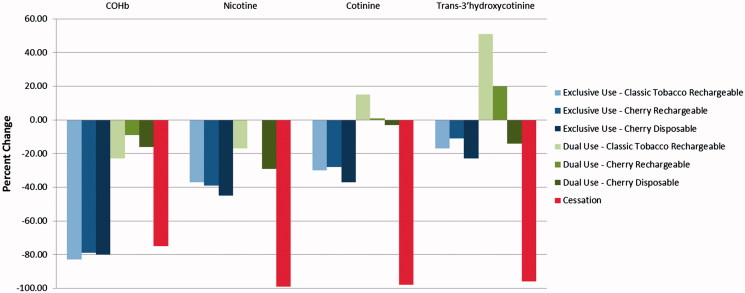
Reducing consumption of conventional tobacco cigarettes over 5 days according to the requirements of the study tended to result in sizeable reductions in exposure to a number of HPHCs as measured by BoE (Tables 3 and 4, Figures 1 and 2). Smoking cessation lead to a 66–98% decrease in excretion of the urine BoE evaluated in this study. The smallest reduction was observed for NNAL, which has the longest half-life of the individual biomarkers listed (Hecht et al., 1999). Predictably, significant decreases were also observed in the carboxyhemoglobin (COHb), nicotine, and the nicotine metabolites as the cessation subjects had no exposure to CO or nicotine.
Table 3.
Urine biomarker concentration summary and statistical comparisons.
| Exclusive E-Cigarette use groups |
Dual use groups |
||||||
|---|---|---|---|---|---|---|---|
| Biomarker | Classic tobacco rechargeable N = 15 | Cherry rechargeable N = 13 | TCherry disposable N = 13 | Classic tobacco rechargeable N = 14 | Cherry rechargeable N = 15 | Cherry disposable N = 13 | Nicotine cessation N = 13 |
| NNAL (ng/24 h) | |||||||
| Day −1 | 427.6 ± 218.8 | 383.7 ± 178.8 | 299.1 ± 165.0 | 430.8 ± 217.1 | 422.0 ± 257.5 | 343.3 ± 123.3 | 481.6 ± 377.5 |
| Day 5 | 174.3 ± 144.6 | 149.2 ± 80.3 | 111.1 ± 68.9 | 328.6 ± 178.9 | 321.1 ± 177.3 | 269.2 ± 96.3 | 175.1 ± 140.8 |
| p value Day −1 vs Day 5 | <0.0001 | <0.0001 | <0.0001 | 0.0063 | 0.0042 | 0.0028 | 0.0004 |
| p value Day 5 vs Cessation | 0.1940 | 0.2456 | 0.2593 | <0.0001 | <0.0001 | <0.0001 | NA |
| 3-HPMA (μg/24 h) | |||||||
| Day −1 | 1521.7 ± 820.0 | 1903.0 ± 1132.7 | 1353.7 ± 598.8 | 1644.1 ± 501.3 | 1474.6 ± 519.9 | 1489.5 ± 567.1 | 2004.1 ± 1137.8 |
| Day 5 | 214.4 ± 94.3 | 263.1 ± 64.7 | 246.7 ± 101.5 | 1046.2 ± 360.6 | 1070.7 ± 342.2 | 1155.4 ± 368.5 | 228.8 ± 84.2 |
| p value Day −1 vs Day 5 | <0.0001 | 0.0001 | <0.0001 | <0.0001 | 0.0009 | 0.0062 | <.0001 |
| p value Day 5 vs Cessation | 0.5137 | 0.6099 | 0.3194 | <0.0001 | <0.0001 | <0.0001 | NA |
| HMPMA (μg/24 h) | |||||||
| Day −1 | 523.8 ± 225.3 | 657.2 ± 328.9 | 533.4 ± 208.3 | 590.7 ± 178.7 | 597.5 ± 198.0 | 504.5 ± 167.1 | 797.7 ± 429.4 |
| Day 5 | 71.3 ± 33.1 | 83.2 ± 32.3 | 78.0 ± 20.7 | 391.8 ± 151.2 | 394.6 ± 119.3 | 386.8 ± 94.1 | 78.1 ± 18.6 |
| p-value Day −1 vs Day 5 | <0.0001 | <0.0001 | <0.0001 | 0.0001 | 0.0001 | 0.0094 | <.0001 |
| p-value Day 5 vs Cessation | 0.4785 | 0.5206 | 0.4211 | <0.0001 | <0.0001 | <0.0001 | NA |
| CEMA (μg/24 h) | |||||||
| Day −1 | 219.7 ± 98.5 | 266.1 ± 140.9 | 201.0 ± 72.8 | 256.0 ± 97.9 | 246.2 ± 109.8 | 223.5 ± 61.6 | 289.7 ± 132.2 |
| Day 5 | 33.4 ± 21.8 | 41.3 ± 30.4 | 25.9 ± 11.2 | 172.8 ± 72.1 | 168.3 ± 50.9 | 173.0 ± 63.7 | 41.0 ± 19.7 |
| p value Day −1 vs Day 5 | <0.0001 | <0.0001 | <0.0001 | 0.0002 | 0.0010 | 0.0002 | <.0001 |
| p value Day 5 vs Cessation | 0.2902 | 0.6357 | 0.4549 | <0.0001 | <0.0001 | <0.0001 | NA |
| 1-OHP (ng/24 h) | |||||||
| Day −1 | 317.4 ± 138.7 | 302.9 ± 171.5 | 260.9 ± 166.8 | 363.6 ± 174.1 | 294.5 ± 145.5 | 304.1 ± 122.7 | 364.0 ± 200.7 |
| Day 5 | 93.7 ± 52.9 | 85.9 ± 32.2 | 90.6 ± 38.4 | 235.1 ± 121.1 | 206.3 ± 90.9 | 224.1 ± 89.5 | 108.2 ± 55.0 |
| p value Day −1 vs Day 5 | <.0001 | 0.0002 | 0.0007 | 0.0006 | 0.0004 | 0.0007 | <.0001 |
| p value Day 5 vs Cessation | 0.8331 | 0.7524 | 0.4115 | <0.0001 | <0.0001 | <0.0001 | NA |
| NNN (ng/24 h) | |||||||
| Day −1 | 18.6 ± 12.1 | 13.7 ± 11.5 | 13.9 ± 12.5 | 14.3 ± 8.6 | 12.3 ± 8.1 | 11.3 ± 5.4 | 16.2 ± 12.1 |
| Day 5 | 1.2 ± 2.4 | 0.7 ± 0.9 | 1.2 ± 3.3 | 8.9 ± 7.7 | 7.6 ± 5.4 | 7.1 ± 3.1 | 0.2 ± 0.1 |
| p value Day −1 vs Day 5 | <.0001 | 0.0011 | 0.0045 | 0.0001 | 0.0019 | 0.0032 | 0.0005 |
| p value Day 5 vs Cessation | 0.6402 | 0.6223 | 0.3974 | <0.0001 | <0.0001 | <0.0001 | NA |
| MHBMA (μg/24 h) | |||||||
| Day −1 | 4.9 ± 3.5 | 5.9 ± 3.8 | 4.6 ± 3.2 | 5.0 ± 2.9 | 3.4 ± 2.5 | 4.5 ± 2.8 | 5.6 ± 3.6 |
| Day 5 | 0.3 ± 0.1 | 0.3 ± 0.1 | 0.3 ± 0.1 | 3.5 ± 2.1 | 2.8 ± 1.8 | 4.3 ± 2.3 | 0.4 ± 0.1 |
| p value Day −1 vs Day 5 | 0.0002 | 0.0002 | 0.0006 | 0.0024 | 0.0320 | 0.1539 | 0.0002 |
| p value Day 5 vs Cessation | 0.8548 | 0.8106 | 0.7313 | <0.0001 | <0.0001 | <0.0001 | NA |
| S-PMA (μg/24 h) | |||||||
| Day −1 | 6.3 ± 3.7 | 8.1 ± 4.9 | 6.3 ± 3.9 | 7.0 ± 4.4 | 4.9 ± 3.5 | 6.9 ± 5.5 | 7.6 ± 4.3 |
| Day 5 | 0.3 ± 0.1 | 0.3 ± 0.3 | 0.4 ± 0.2 | 4.9 ± 3.3 | 3.6 ± 2.3 | 6.0 ± 4.6 | 0.3 ± 0.2 |
| p value Day −1 vs Day 5 | <.0001 | <.0001 | 0.0001 | 0.0031 | 0.0055 | 0.0072 | <0.0001 |
| p value Day 5 vs Cessation | 0.5274 | 0.7602 | 0.4430 | <0.0001 | <0.0001 | <0.0001 | NA |
| Nicotine equivalents a (mg/24 h) | |||||||
| Day −1 | 17.0 ± 6.6 | 17.6 ± 8.7 | 14.5 ± 4.4 | 16.6 ± 5.5 | 16.0 ± 6.2 | 15.7 ± 3.9 | 20.0 ± 8.9 |
| Day 5 | 10.7 ± 9.1 | 12.7 ± 9.7 | 10.5 ± 9.6 | 18.4 ± 7.2 | 15.9 ± 5.5 | 15.8 ± 5.0 | 0.5 ± 0.2 |
| p value Day −1 vs Day 5 | 0.0115 | 0.0415 | 0.0033 | 0.4188 | 0.9103 | 0.8519 | <.0001 |
| p value Day 5 vs Cessation | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | NA |
Values are presented as mean ± SD.
Nicotine equivalents: calculated as the molar sum of nicotine and five major nicotine metabolites (nicotine gluc, cotinine, cotinine-gluc, trans-3’-hydroxycotinine; trans-3’-hydroxycotinine-gluc) excreted in urine over 24 h and reported as nicotine equivalents (mg/24 h). Nicotine equivalents (mg/24 h) = [nicotine (mg/162.23 (mg/mmol) + nicotine-gluc (mg/338.36 (mg/mmol) + cotinine (mg/176.22 (mg/mmol) + cotinine-gluc (mg/352.34 (mg/mmol) + trans-3'-hydroxycotinine (mg/192.22 (mg/mmol) + trans-3'-hydroxycotinine-gluc (mg/368.34 (mg/mmol)] × 162.23 (mg/mmol)].
Table 4.
Blood biomarker concentration summary and statistical comparisons.
| Exclusive E-Cigarette use groups |
Dual use groups |
||||||
|---|---|---|---|---|---|---|---|
| Biomarker | Classic tobacco rechargeable N = 15 | Cherry rechargeable N = 13 | Cherry disposable N = 13 | Classic tobacco rechargeable N = 14 | Cherry rechargeable N = 15 | Cherry disposable N = 13 | Nicotine cessation N = 13 |
| Blood COHb (%) | |||||||
| Day −1 | 6.3 ± 2.0 | 6.3 ± 2.0 | 6.0 ± 1.5 | 5.2 ± 2.0 | 4.8 ± 1.4 | 5.6 ± 1.9 | 7.4 ± 2.3 |
| Day 5 | 1.2 ± 0.6 | 1.2 ± 0.5 | 1.0 ± 0.6 | 4.0 ± 1.5 | 3.8 ± 0.9 | 4.3 ± 1.3 | 1.6 ± 0.4 |
| p value Day −1 vs Day 5 | 0.0001 | <0.0001 | <0.0001 | 0.0179 | 0.0775 | 0.0170 | 0.0001 |
| p value Day 5 vs Cessation | 0.5011 | 0.6009 | 0.4794 | <0.0001 | <0.0001 | <0.0001 | NA |
| Plasma Nicotine (ng/mL) | |||||||
| Day −1 | 13.0 ± 6.1 | 14.7 ± 5.2 | 13.3 ± 6.5 | 12.5 ± 5.9 | 8.8 ± 3.2 | 11.1 ± 4.1 | 16.0 ± 7.0 |
| Day 5 | 6.9 ± 6.3 | 8.4 ± 5.9 | 6.6 ± 5.6 | 9.5 ± 6.2 | 8.1 ± 5.2 | 7.9 ± 4.4 | 0.1 ± 0.0 |
| p value Day −1 vs Day 5 | 0.0033 | 0.0035 | 0.0053 | 0.0518 | 0.6197 | 0.0112 | <0.0001 |
| p value Day 5 vs Cessation | <0.0001 | <0.0001 | 0.0002 | <0.0001 | <0.0001 | <0.0001 | NA |
| Plasma Cotinine (ng/mL) | |||||||
| Day −1 | 260.1 ± 128.1 | 299.9 ± 93.7 | 250.1 ± 92.4 | 247.6 ± 99.0 | 213.6 ± 62.8 | 218.4 ± 58.3 | 282.2 ± 135.9 |
| Day 5 | 164.5 ± 167.4 | 202.1 ± 103.2 | 149.2 ± 116.1 | 261.5 ± 119.4 | 211.5 ± 70.2 | 212.9 ± 89.3 | 5.49 ± 6.7 |
| p value Day −1 vs Day 5 | 0.0438 | 0.0160 | 0.0112 | 0.6554 | 0.8935 | 0.7474 | <0.0001 |
| p value Day 5 vs Cessation | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | NA |
| Plasma Trans-3’ hydroxycotinine (ng/mL) | |||||||
| Day −1 | 164.5 ± 167.4 | 202.1 ± 103.2 | 149.2 ± 116.1 | 261.5 ± 119.4 | 211.5 ± 70.2 | 212.9 ± 89.3 | 5.49 ± 6.7 |
| Day 5 | 70.4 ± 59.0 | 85.0 ± 55.7 | 69.4 ± 56.5 | 102.2 ± 46.8 | 107.8 ± 50.7 | 98.5 ± 29.3 | 3.8 ± 2.7 |
| p value Day −1 vs Day 5 | 0.1626 | 0.3316 | 0.2073 | 0.0821 | 0.0051 | 0.1082 | <0.0001 |
| p value Day 5 vs Cessation | <0.0001 | <0.0001 | 0.0002 | <0.0001 | <0.0001 | <0.0001 | NA |
Values are presented as mean ± SD.
Figure 1.
Urine biomarkers – Day 5 percent change from Day −1.
Figure 2.
Blood biomarkers – Day 5 percent change from Day −1.
The changes from Day −1 observed in the exclusive use groups were mostly comparable to those seen in the cessation group, with the notable exceptions of the nicotine and nicotine metabolite urine and blood biomarkers as these subjects continued to consume nicotine from the e-cigarettes.
Dual users who had substituted half of their self-reported daily conventional tobacco cigarette consumption with e-cigarettes exhibited reduced biomarkers levels that were broadly proportional to the reduced numbers of cigarettes smoked. Reductions in the urine BoE for these groups ranged from ∼7% to 38% (Table 3).
Observed reductions in HPHCs
Measurable nicotine metabolites were present in the samples from e-cigarette users, but levels of biomarkers for HPHCs were significantly lower, and many were indistinguishable from those of subjects who had quit smoking entirely. Levels of polycyclic aromatic hydrocarbons (PAHs) such as pyrene (as measured by the BoE: 1-OHP) were reduced by 70% in users that had ceased smoking or using any nicotine product and by 62–69% in the exclusive use group. Similarly, levels of Tobacco Specific Nitrosamines (TSNAs) such as NNAL and NNN (as measured by the BoE: NNAL and NNN) were reduced by 66% to 98%, respectively in the cessation group and by 62–64% and 87–93%, respectively in the exclusive use group. Moreover, levels of Volatile Organic Compounds (VOCs) such as acrolein, benzene, 1-3-butadiene, crotonaldehyde and acyrlonitrile (as measured by the BoE: 3-HPMA, S-PMA, MHBMA, HMPMA and CEMA) were reduced by 87%, 94%, 84%, 88% and 86%, respectively, in the cessation group and by 82–83%, 93–94%, 86–90%, 82–85% and 85–87%, respectively, in the exclusive use group.
As might be expected, the excretion and concentration of all BoE evaluated in this study were significantly higher in the dual use groups at Day 5 compared with the cessation group. Levels of PAHs such as pyrene (as measured by the BoE: 1-OHP) were reduced by 25–35% in dual users versus 70% in the cessation group. Levels of TSNAs such as NNN and NNN (as measured by the BoE: NNAL and NNN) were reduced by 19–22% and 30–37%, respectively, in the dual use group versus 66% and 98%, respectively, in the cessation group. Levels of VOCs such as Acrolein, Benzene, 1-3-Butadiene, Crotonaldehyde and Acyrlonitrile (as measured by the BoE: 3-HPMA, S-PMA, MHBMA, HMPMA and CEMA) were reduced by 20–33%, 21–30%, 7–26%, 20–33%, 24–32%, respectively, in the dual use group versus 87%, 94%, 84%, 88% and 86%, respectively, in the cessation group.
Relationship between product use and urine biomarker Excretion
Statistically significant, positive linear relationships were observed between percent change in biomarker excretion and the percent change in CPD smoked for all urine BoE (p = < 0.0001–0.0018) except nicotine equivalents (p = 0.9316) (Tables 5 and 6). These results may indicate that smokers who reduce their conventional tobacco cigarette consumption may predictably expect to see reduced exposure to a number of HPHCs while replacing conventional tobacco cigarettes with nicotine-containing e-cigarettes.
Table 5.
Regression analyzes of the Day −1 to Day 5% change in the amount of urine biomarker amount excreted against the % change in cigarettes per day (CPD).
| Urine biomarker | Slope | R-square | p Value |
|---|---|---|---|
| NNAL | 0.4154 | 0.1518 | 0.0108 |
| 3-HPMA | 0.6940 | 0.4105 | <0.0001 |
| HMPMA | 0.7878 | 0.4289 | <0.0001 |
| CEMA | 0.7096 | 0.4891 | <0.0001 |
| 1-OHP | 0.6297 | 0.4227 | <0.0001 |
| NNN | 0.7766 | 0.3181 | 0.0001 |
| MHBMA | 0.7469 | 0.2874 | 0.0003 |
| S-PMA | 0.7259 | 0.4636 | <0.0001 |
| Nicotine Equivalents | −0.0296 | 0.0002 | 0.9316 |
Table 6.
Regression analyzes of nicotine equivalents excretion and Day −1 and Day 5 product use.
| Relationship assessed | Slope | R-square | p Value |
|---|---|---|---|
| Day −1 nicotine equivalents amount excreted against cigarettes smoked (all Groups) | 0.7485 | 0.2137 | <0.0001 |
| Day 5 nicotine equivalents amount excreted against cigarettes smoked (dual use Groups) | 0.3598 | 0.0289 | 0.2814 |
| Day 5 nicotine equivalents amount excreted against estimated nicotine from e-cigarettes (dual use Groups) | 0.3970 | 0.4502 | <0.0001 |
| Day 5 nicotine equivalents amount excreted against estimated nicotine from e-cigarettes (exclusive use Groups) | 0.4794 | 0.8538 | <0.0001 |
Furthermore, a statistically significant, positive linear relationship (p < 0.0001) was observed between nicotine equivalents excretion and the number of conventional tobacco cigarettes smoked on Day −1 when all groups were included, but not on Day 5 (p = 0.2814) when the dual use groups were included (Table 7). Within the exclusive and dual use groups, the Day 5 relationship between nicotine equivalents excreted and the estimated nicotine from the e-cigarettes were statistically significant (p < 0.0001). This finding may be due to the relatively consistent use and constant nicotine content in the e-cigarettes and the number of usual conventional tobacco cigarettes smoked, coupled with differences in individual smoking behaviors.
Table 7.
Summary of regression analyzes of nicotine equivalents excretion and Day −1 and Day 5 product use.
| Relationship assessed | Slope | p Value | R-square |
|---|---|---|---|
| Day −1 nicotine equivalents amount excreted against cigarettes smoked (All Cohorts) | 0.7485 | <0.0001 | 0.2137 |
| Day 5 nicotine equivalents amount excreted against cigarettes smoked (Dual Use Cohorts) | 0.3598 | 0.2814 | 0.0289 |
| Day 5 nicotine equivalents amount excreted against estimated nicotine from blu products (Dual use Cohorts) | 0.3970 | <0.0001 | 0.4502 |
| Day 5 nicotine equivalents amount excreted against estimated nicotine from blu products (Exclusive Use Cohorts) | 0.4794 | <0.0001 | 0.8538 |
Exhaled Breath
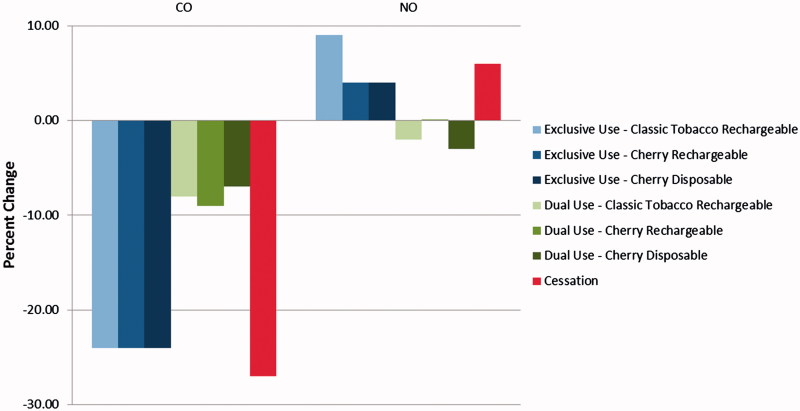
Physiological changes associated with smoking reduction were noted in the exhaled CO and NO endpoints. All groups experienced statistically significant decreases in exhaled CO at Day 5 compared to Day −1, with decreases in the cessation and exclusive use groups ranging from ∼88% to ∼89% and in the dual use groups by ∼26–32% (Table 8 and Figure 3). Further, there were no differences between the cessation and exclusive use group’s measurements on Day 5 whereas the dual use groups had significantly higher exhaled CO compared to cessation; this may be expected as this group continued to smoke conventional tobacco cigarettes.
Table 8.
Exhaled breath biomarker summary and statistical comparisons.
| Exclusive E-Cigarette use groups |
Dual use groups |
||||||
|---|---|---|---|---|---|---|---|
| Biomarker | Classic tobacco rechargeable N = 15 | Cherry rechargeable N = 13 | Cherry disposable N = 13 | Classic tobacco rechargeable N = 14 | Cherry rechargeable N = 15 | Cherry disposable N = 13 | Nicotine cessation N = 13 |
| CO (ppm) | |||||||
| Day −1 | 27.2 ± 10.5 | 27.3 ± 6.9 | 26.9 ± 6.4 | 25.1 ± 7.3 | 25.4 ± 7.7 | 24.7 ± 5.5 | 29.3 ± 10.4 |
| Day 5 | 2.9 ± 0.8 | 2.9 ± 0.8 | 2.7 ± 0.9 | 17.3 ± 5.7 | 16.1 ± 3.3 | 18.2 ± 5.7 | 2.8 ± 0.7 |
| p value Day −1 vs Day 5 | <0.0001 | <0.0001 | <0.0001 | 0.0021 | <0.0001 | 0.0002 | <0.0001 |
| p value Day 5 vs Cessation | 0.7990 | 0.8033 | 0.9109 | <0.0001 | <0.0001 | <0.0001 | NA |
| NO (ppb) | |||||||
| Day −1 | 14.8 ± 12.8 | 11.5 ± 4.8 | 10.0 ± 4.0 | 14.9 ± 11.1 | 10.6 ± 4.6 | 14.3 ± 13.5 | 11.3 ± 4.0 |
| Day 5 | 23.3 ± 21.6 | 15.5 ± 9.0 | 14.3 ± 6.5 | 12.9 ± 6.3 | 10.7 ± 4.4 | 11.4 ± 6.0 | 16.8 ± 10.1 |
| p value Day −1 vs Day 5 | 0.0075 | 0.1325 | 0.0053 | 0.3118 | 0.9415 | 0.3287 | 0.0321 |
| p value Day 5 vs Cessation | 0.2370 | 0.6031 | 0.5674 | 0.0313 | 0.0615 | 0.0119 | NA |
Values are presented as mean ± SD. A paired t-test was used to make the Day −1 vs Day 5 within group comparisons, a linear mixed model was used to make the Day 5 comparisons to the Cessation group.
Figure 3.
Exhaled breath biomarkers – Day 5 percent change from Day −1.
Nitric oxide can be detected in expired breath and has been identified in prior studies as a noninvasive biomarker of inflammation (Birrell et al., 2006; Zhou et al., 2012). Conventional tobacco cigarette has been reported to decrease exhaled NO, possibly by the inhibition of the enzyme NO synthase (Kharitonov et al., 1995), but the mechanism remains incompletely understood. Exhaled NO was observed to increase from Day −1 to Day 5 in the cessation and exclusive use groups (∼46–63%) whereas the dual use groups experienced minimal changes. On Day 5, exhaled NO in the cessation and exclusive use groups was similar, but tended to be lower in the dual use groups, though not all comparisons were statistically significant (Table 7 and Figure 3).
Discussion
Dual use of electronic cigarettes and conventional cigarettes has been cited as a potential public health concern because of a possibility that it may expose smokers to greater health risks than those encountered by smoking conventional tobacco cigarettes alone (Grana et al., 2014). A more recent study, however, reported that the dual use of e-cigarettes while continuing to smoke did not result in reduced exposure to known carcinogens and toxicants (Shahab et al., 2015). This study enforced a reduction in daily cigarettes smoked on a dual use group as an initial examination of the responsiveness of the measured smoke exposure biomarkers to moderately-reduced smoking combined with unlimited ad libitum usage of e-cigarettes. Under these conditions, the study showed that dual users’ experienced significant reductions in most of the biomarkers assessed (∼20–35% reduction in urine biomarkers) and that the magnitude of reduction in exposure to biomarkers of exposure in the dual use subject was broadly proportional to the reduction of conventional tobacco cigarettes smoked. As such, the study findings are consistent with an expectation of significantly reduced exposures to harmful smoke constituents in smokers who completely replace their conventional tobacco cigarettes with e-cigarettes.
The study also showed that subjects who switched to dual use also experienced significantly reduced exposure to HPHCs after partially replacing conventional tobacco cigarettes with an e-cigarette product. As expected, the excretion and concentration of all biomarkers evaluated in this study were significantly higher in the dual use group at Day 5 compared to the cessation group. However, reductions of 25–35% in the levels of PAHs such as pyrene were observed in dual users (versus 70% in the cessation group); levels of TSNAs such as NNAL and NNN were reduced by 19–22% and 30–37%, respectively, in the dual use group versus 66% and 98%, respectively in the cessation group; and levels of VOCs such as acrolein, benzene, 1-3-butadiene, crotonaldehyde and acyrlonitrile were also observed to be reduced by 20–33%, 21–30%, 7–26%, 20–33%, 24–32%, respectively, in the dual use group versus 87%, 94%, 84%, 88% and 86%, respectively, in the cessation group.
The results of this study also demonstrated that smokers who completely substitute conventional tobacco cigarettes with e-cigarettes over a short period of time (5-days) experience reductions in exposure to a number of HPHCs and toxicants as measured by urine, blood and exhaled breath BoE. Reductions in the HPHCs (PAHs, TSNAs VOCs and nicotine) analyzed showed that measurable nicotine metabolites were present in the samples from e-cigarette users, which was expected as subjects continued to consume nicotine in the e-cigarettes. However, levels of biomarkers for HPHCs were significantly lower, and many were similar to those of subjects who had quit smoking entirely. For example, levels of PAHs such as pyrene were reduced by 70% in users that had quit smoking or using nicotine products altogether and by 62–69% in the group that used e-cigarettes exclusively. Levels of TSNAs such as NNAL and NNN were also reduced by 66–98%, respectively, in the cessation group and by 62–64% and 87–93%, respectively, in the exclusive use group. Moreover, levels of VOCs such as acrolein, benzene, 1-3-butadiene, crotonaldehyde and acyrlonitrile were also reduced by 87%, 94%, 84%, 88% and 86%, respectively, in the cessation group and by 82–83%, 93–94%, 86–90%, 82–85% and 85–87%, respectively, in the exclusive use group.
Moreover, the study findings associated with exhaled breath biomarkers in the cessation and exclusive use groups were consistent with other research findings associated with reductions in exhaled CO and increases in NO following smoking cessation (Chambers et al., 1998; Hogman et al., 2002; Jarvis et al., 1980; Malinovschi et al., 2006; Ripoll et al., 2012; Robbins et al., 1997; West et al., 2005; Yates et al., 2001); both of which may be indicative of immediate and future physiological benefits to the smoker.
The results of this study also support the findings of other investigations which have demonstrated that e-cigarette use results in a different aerosol exposure, including a decrease in certain biomarkers typical for combusted tobacco cigarette consumption (Caponnetto et al., 2013; McRobbie et al., 2015; Polosa et al., 2011). Furthermore, a recently-published study on e-cigarette emissions from the products evaluated in this study, found the e-cigarette aerosol contained levels of HPHCs such as carbonyl compounds, tobacco-specific nitrosamines, polycyclic aromatic hydrocarbons and other constituents that were on the order of 1500 times lower than those found in the smoke of conventional tobacco cigarettes (<2 μg/puff vs. ∼3,000 μg/puff) (Tayyarah & Long, 2014). This study extends those findings with the observation that the e-cigarette produced markedly lower levels of BoE when used by smokers in lieu of their preferred conventional tobacco cigarette brand style for a period of 5 days.
Furthermore, this investigation also further confirms and extends the findings of several prior, smaller studies that have compared levels of BoE in users of e-cigarettes to those of conventional tobacco cigarette smokers. Vansickel et al. (2010), Farsalinos et al. (2014) and Walele et al. (2016) reported moderate plasma nicotine values in users of first generation e-cigarette devices similar to those used in this study. Typical use of later-generation, tank-style e-cigarettes or intensive use of the first-generation cigarette-like devices has been reported to produce plasma nicotine values similar to those from conventional tobacco cigarettes (D’Ruiz et al., 2015; Yan & D’Ruiz, 2015). Hecht et al. (2015) also have recently reported combined findings from three independent studies of smokers whose biomarkers levels were compared to those of 28 self-reported users of a variety of commercial cartridge- and tank-based e-cigarettes under uncontrolled ad libitum conditions. These authors concluded, with respect to the biomarkers analyzed, that the e-cigarettes had a more favorable toxicity profile than conventional tobacco cigarettes.
BoE to tobacco toxicants are well established and numerous studies exist that have utilized biomarkers of exposure to assess exposure to tobacco smoke constituents in humans and have generated meaningful measures of tobacco toxicant exposure. This has been useful for evaluating the individual potential risks associated with different classes of tobacco products (Mattes et al., 2014). Whether the reductions in exposure to toxicants such as those observed in this study may have the potential to reduce risks for chronic, smoking-caused diseases for long-term e-cigarette users who have partially or completely discontinued cigarette smoking warrants further investigation. It has been previously shown that for those Swedish smokers who completely switch from cigarettes to a noncombustible form of tobacco (snus, which delivers nicotine without smoke) there appears to be an association with lower relative risks for major smoking-related disease, including cancer (Round et al., 2015).
The study’s main limitation was that it was only a short-term (5-day) trial looking at select BoE associated with a single product type (i.e., closed system e-cigarette). Nevertheless, the study was able to provide data to address a deficit in scientific knowledge with regard to HPHC levels in different types of e-cigarette users by showing that reducing conventional cigarette smoking leads to reductions in HPHC exposure in individuals who exclusively use electronic cigarettes or are dual users (electronic cigarettes and conventional cigarette smokers) under short-term use conditions. Longer-term BoE or biomarker of effect studies may be informative for assessing the long-term implications and physiological relevance of reduced exposure to HPHCs in individuals who exclusively use e-cigarettes or are dual users. Information from longer-term e-cigarette product tolerability and adverse event surveillance studies may also be informative.
Data associated with several other secondary objectives were also collected during the course of this study but are not reported in this paper. These include estimates of daily nicotine delivery following exclusive e-cigarette and dual use of e-cigarettes and the subject’s usual brand combustible cigarette over a 5-day period; subjective effects related to urge to smoke and satisfaction; changes in selected physiological endpoints such as blood pressure and pulse; and tolerability and adverse events. Given the importance of our findings in these areas, the results will be reported under separate publications. Furthermore, participant biofluids were collected and frozen as part of this study in anticipation of further research related to targeted and untargeted biomarkers of effect. The results of our future investigations in this area will also be published when available.
Conclusions
This study provides data to address a deficit in scientific knowledge in regards to HPHC levels in different types of electronic cigarette users. The study showed that reducing conventional tobacco cigarette consumption over 5 days resulted in significant reductions in exposure to known biomarkers of HPHCs reported in tobacco smoke, with greater reductions observed in subjects who ceased consumption of all nicotine product use or switched to e-cigarette products compared to subjects who switched to dual use. The magnitude of reduction in exposure to biomarkers in dual use group was broadly proportional to reduction in conventional tobacco cigarettes smoked. Physiological effects of reducing cigarette consumption were also observed in the exhaled breath endpoints. This study also illustrates that biomarkers of exposure may play a role in assessing and comparing exposure to HPHCs across different product categories and exemplifies their potential for informing product regulatory reviews and tobacco product regulation. Overall, this data indicate the great potential that e-cigarettes may provide for smokers seeking an alternative to tobacco products and supports the case for regulating e-cigarettes differently from combustible tobacco-containing products.
Acknowledgements
We gratefully acknowledge the support and ideas from our current and past colleagues from the A.W. Spears Research Center and the study investigators at Celerion in Lincoln, Nebraska. We also thank the science team at Imperial Brands plc for critical review of the manuscript.
Disclosure statement
CDD is consultant for Fontem Ventures U.S. Inc.; GOC is an employee of Fontem Ventures, B.V.; DWG is an employee of Celerion, the contract research organization (CRO) that conducted the study.
Funding
The work in this manuscript was supported by Fontem Ventures B.V., a fully owned subsidiary of Imperial Brands plc, and the manufacturer of the e-cigarette products used in this study.
References
- Birrell MA, McCluskie K, Hardaker E, et al. Utility of exhaled nitric oxide as a noninvasive biomarker of lung inflammation in a disease model. Eur Respir J. 2006;28:1236–44. doi: 10.1183/09031936.00048506. [DOI] [PubMed] [Google Scholar]
- Burstyn I. Peering through the mist: systematic review of what the chemistry of contaminants in electronic cigarettes tells us about health risks. BMC Public Health. 2014;14:18. doi: 10.1186/1471-2458-14-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahn Z, Siegel M. Electronic cigarettes as a harm reduction strategy for tobacco control: a step forward or a repeat of past mistakes. J Public Health Policy. 2011;32:16–31. doi: 10.1057/jphp.2010.41. [DOI] [PubMed] [Google Scholar]
- Caponnetto P, Auditore R, Russo C, et al. Impact of an electronic cigarette on smoking reduction and cessation in schizophrenic smokers: a prospective 12-month pilot study. Int J Environ Res Public Health. 2013;10:446–61. doi: 10.3390/ijerph10020446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmella SG, Chen M, Han S, et al. Effects of smoking cessation on eight urinary tobacco carcinogen and toxicant biomarkers. Chem Res Toxicol. 2009;22:734–41. doi: 10.1021/tx800479s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers DC, Tunnicliffe WS, Ayres JG. Acute inhalation of cigarette smoke increases lower respiratory tract nitric oxide concentrations. Thorax. 1998;53:677–9. doi: 10.1136/thx.53.8.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deveci SE, Deveci F, Açik Y, Ozan AT. The measurement of exhaled carbon monoxide in healthy smokers and non-smokers. Respir Med. 2004;98:551–6. doi: 10.1016/j.rmed.2003.11.018. [DOI] [PubMed] [Google Scholar]
- D’Ruiz CD, Graff DW, Yan XS. Nicotine delivery, tolerability and reduction of smoking urge in smokers following short-term use of one brand of electronic cigarettes. BMC Public Health. 2015;15:991. doi: 10.1186/s12889-015-2349-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagerström K. Determinants of tobacco use and renaming the FTND to the Fagerstrom test for cigarette dependence. Nicotine Tob Res. 2012;14:75–8. doi: 10.1093/ntr/ntr137. [DOI] [PubMed] [Google Scholar]
- Farsalinos KE, Polosa R. Safety evaluation and risk assessment of electronic cigarettes as tobacco cigarette substitutes: a systematic review. Ther Adv Drug Saf. 2014;5:67–86. doi: 10.1177/2042098614524430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farsalinos KE, Romagna G, Tsiapras D, et al. Characteristics, perceived side effects and benefits of electronic cigarette use: a worldwide survey of more than 19,000 consumers. Int J Environ Res Public Health. 2014;11:4356–73. doi: 10.3390/ijerph110404356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farsalinos KE, Spyrou A, Tsimopoulou K, et al. Nicotine absorption from electronic cigarette use: comparison between first and new-generation devices. Sci Rep. 2014;4:4133. doi: 10.1038/srep04133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goniewicz ML, Knysak J, Gawron M, et al. Levels of selected carcinogens and toxicants in vapour from electronic cigarettes. Tob Control. 2014;23:133–9. doi: 10.1136/tobaccocontrol-2012-050859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grana R, Benowitz N, Glantz SA. E-cigarettes: a scientific review. Circulation. 2014;129:1972–86. doi: 10.1161/CIRCULATIONAHA.114.007667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregg EO, Minet E, McEwan M. Urinary biomarkers of smokers' exposure to tobacco smoke constituents in tobacco products assessment: a fit for purpose approach. Biomarkers. 2013;18:467–86. doi: 10.3109/1354750X.2013.821523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajek P, Etter J-F, Benowitz N, et al. Electronic cigarettes: review of use, content, safety, effects on smokers and potential for harm and benefit. Addiction. 2014;109:1801–10. doi: 10.1111/add.12659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström test for nicotine dependence: a revision of the Fagerström tolerance questionnaire. Br J Addict. 1991;86:1119–27. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hecht SS, Carmella SG, Kotandeniya D, et al. Evaluation of toxicant and carcinogen metabolites in the urine of E-cigarette users versus cigarette smokers. Nicotine Tob Res. 2015;17:704–9. doi: 10.1093/ntr/ntu218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht SS, Yuan J-M, Hatsukami D. Applying tobacco carcinogen and toxicant biomarkers in product regulation and cancer prevention. Chem Res Toxicol. 2010;23:1001–8. doi: 10.1021/tx100056m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht SS, Carmella SG, Chen M, et al. Quantitation of urinary metabolites of a tobacco-specific lung carcinogen after smoking cessation. Cancer Res. 1999;59:590–6. [PubMed] [Google Scholar]
- Hecht SS, Murphy SE, Carmella SG, et al. Effects of reduced cigarette smoking on the uptake of a tobacco-specific lung carcinogen. J Natl Cancer Inst. 2004;96:107–15. doi: 10.1093/jnci/djh016. [DOI] [PubMed] [Google Scholar]
- Hecht SS. Human urinary carcinogen metabolites: biomarkers for investigating tobacco and cancer. Carcinogenesis. 2002;23:907–22. doi: 10.1093/carcin/23.6.907. [DOI] [PubMed] [Google Scholar]
- Hogman M, Holmkvist T, Walinder R, et al. Increased nitric oxide elimination from the airways after smoking cessation. Clin Sci (Lond) 2002;103:15–19. doi: 10.1042/cs1030015. [DOI] [PubMed] [Google Scholar]
- Jarvis JM, Rusell MAH, Saloojee Y. Expired air carbon monoxide: a simple breath test of tobacco smoke intake. BMJ. 1980;281:484–5. doi: 10.1136/bmj.281.6238.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharitonov SA, Robbins RA, Yates D, et al. Acute and chronic effects of cigarette smoking on exhaled nitric oxide . Am J Respir Crit Care Med. 1995;152:609–12. doi: 10.1164/ajrccm.152.2.7543345. [DOI] [PubMed] [Google Scholar]
- Malinovschi A, Janson C, Holmkvist T, et al. Effect of smoking on exhaled nitric oxide and flow-independent nitric oxide exchange parameters. Eur Respir J. 2006;28:339–45. doi: 10.1183/09031936.06.00113705. [DOI] [PubMed] [Google Scholar]
- Mattes W, Yang X, Orr MS, et al. Biomarkers of tobacco smoke exposure. Adv Clin Chem. 2014;67:1–45. doi: 10.1016/bs.acc.2014.09.001. [DOI] [PubMed] [Google Scholar]
- McRobbie H, Phillips A, Goniewicz ML, et al. Effects of switching to electronic cigarettes with and without concurrent smoking on exposure to nicotine, carbon monoxide, and acrolein. Cancer Prev Res (Phila) 2015;8:873–8. doi: 10.1158/1940-6207.CAPR-15-0058. [DOI] [PubMed] [Google Scholar]
- McRobbie H, Bullen C, Hartmann-Boyce J, Hajek P. Electronic cigarettes for smoking cessation and reduction. Cochrane Database Syst Rev. 2014;12:CD010216. doi: 10.1002/14651858.CD010216.pub2. [DOI] [PubMed] [Google Scholar]
- Misra M, Leverette RD, Cooper BT, et al. E Comparative in vitro toxicity profile of electronic and tobacco cigarettes, smokeless tobacco and nicotine replacement therapy products: e-liquids, extracts and collected aerosols. Int J Environ Res Public Health. 2014;11:11325–47. doi: 10.3390/ijerph111111325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepper JK, Brewer NT. Electronic nicotine delivery system (electronic cigarette) awareness, use, reactions and beliefs: a systematic review. Tob Control. 2014;23:375–84. doi: 10.1136/tobaccocontrol-2013-051122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polosa R, Rodu B, Caponnetto P, et al. A fresh look at tobacco harm reduction: the case for the electronic cigarette. Harm Reduct J. 2013;10:19. doi: 10.1186/1477-7517-10-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polosa R, Caponnetto P, Morjaria JB, et al. Effect of an electronic nicotine delivery device (e-cigarette) on smoking reduction and cessation: a prospective 6-month pilot study. BMC Public Health. 2011;11:786. doi: 10.1186/1471-2458-11-786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ripoll J, Girauta H, Ramos M, et al. Clinical trial on the efficacy of exhaled carbon monoxide measurement in smoking cessation in primary health care. BMC Public Health. 2012;12:322. doi: 10.1186/1471-2458-12-322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins RA, Millatmal T, Lassi K, et al. Smoking cessation is associated with an increase in exhaled nitric oxide. Chest. 1997;112:313–18. doi: 10.1378/chest.112.2.313. [DOI] [PubMed] [Google Scholar]
- Round EK, Campbell LR, Stiles MF, et al. Changes in biomarkers of exposure and subjective effects when smokers switch to dual use of cigarettes and either snus or a dissolvable tobacco product: a summary of three clinical studies. Beitr Tabakforsch Int. 2015;26:242–60. [Google Scholar]
- Sarkar M, Liu J, Koval T, et al. Evaluation of biomarkers of exposure in adult cigarette smokers using Marlboro Snus. Nicotine Tob Res. 2010;12:105–16. doi: 10.1093/ntr/ntp183. [DOI] [PubMed] [Google Scholar]
- Shahab L, Goniewicz M, Alwis U, et al. Exposure to selected toxicants and carcinogens as a function of smoking status and long-term use of nicotine replacement therapy or electronic cigarettes. Abstract presented at the 21st annual meeting of the Society for Research on Nicotine and Tobacco; Philadelphia, PA: 2015. [Google Scholar]
- Taylor DR, Pijnenburg MW, Smith AD. Exhaled nitric oxide measurements: clinical application and interpretation. Thorax. 2006;61:817–27. doi: 10.1136/thx.2005.056093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tayyarah R, Long GA. Comparison of select analytes in aerosol from e-cigarettes with smoke from conventional cigarettes and with ambient air. Reg Toxicol Pharmacol. 2014;70:704–10. doi: 10.1016/j.yrtph.2014.10.010. [DOI] [PubMed] [Google Scholar]
- USFDA . 2012. http://www.fda.gov/TobaccoProducts/GuidanceComplianceRegulatoryInformation/ucm297786.htm [Google Scholar]
- USDHHS . 2014. [Google Scholar]
- Vansickel AR, Cobb CO, Weaver MF, Eissenberg TE. A clinical laboratory model for evaluating the acute effects of electronic “cigarettes”: nicotine delivery profile and cardiovascular and subjective effects. Cancer Epidem Biomar. 2010;19:1945–53. doi: 10.1158/1055-9965.EPI-10-0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walele T, Sharma G, Savioz R, et al. A randomised, crossover study on an electronic vapour product, a nicotine inhalator and a conventional cigarette. Part B: safety and subjective effects. Reg Toxicol Pharmacol. 2016;74:187–92. doi: 10.1016/j.yrtph.2015.12.003. [DOI] [PubMed] [Google Scholar]
- West R, Hajek P, Stead L, Stapleton J. Outcome criteria in smoking cessation trials: proposal for a common standard. Addiction. 2005;100:299–303. doi: 10.1111/j.1360-0443.2004.00995.x. [DOI] [PubMed] [Google Scholar]
- Yan XS, D’Ruiz CD. Effects of using electronic cigarettes on nicotine delivery and cardiovascular function in comparison with regular cigarettes . Regul Toxicol Pharmacol. 2015;71:24–34. doi: 10.1016/j.yrtph.2014.11.004. [DOI] [PubMed] [Google Scholar]
- Yates DH, Breen H, Thomas PS. Passive smoke inhalation decreases exhaled nitric oxide in normal subjects. Am J Respir Crit Care Med. 2001;164:1043–6. doi: 10.1164/ajrccm.164.6.2005043. [DOI] [PubMed] [Google Scholar]
- Zhou M, Liu Y, Duan Y. Breath biomarkers in diagnosis of pulmonary diseases. Clin Chim Acta. 2012;413:1770–80. doi: 10.1016/j.cca.2012.07.006. [DOI] [PubMed] [Google Scholar]