Abstract
Fatigue-induced reductions in saccade velocity have been reported following acute, prolonged exercise. Interestingly, the detrimental impact of fatigue on oculomotor control can be prevented by a moderate dose of caffeine. This effect may be related to central catecholamine upregulation via caffeine’s action as an adenosine antagonist. To test this hypothesis, we compared the protective effect of caffeine on oculomotor control post-exercise to that of a norepinephrine-dopamine reuptake inhibitor. Within a placebo-controlled crossover design, 12 cyclists consumed placebo, caffeine or a norepinephrine-dopamine reuptake inhibitor (bupropion) during 180 minutes of stationary cycling. Saccades, smooth pursuit and optokinetic nystagmus were measured using infrared oculography. Exercise fatigue was associated with an 8 ± 11% reduction in the peak velocity of prosaccades, and a 10 ± 11% decrement in antisaccade peak velocity. Optokinetic nystagmus quick phases decreased in velocity by 15 ± 17%. These differences were statistically significant (p < 0.05). Norepinephrine-dopamine reuptake inhibition and caffeine prevented fatigue-related decrements in eye movement velocity. Pursuit eye movements and visual attention were unaffected. These findings show that norepinephrine-dopamine reuptake inhibition protects oculomotor function during exercise fatigue. Caffeine’s fatigue-reversing effects on eye movements appear to be mediated, at least in part, via modulation of central catecholamines.
Saccades are rapid changes in fixation that align the foveae with salient targets in the visual field1. In a recent study, we reported that saccade velocity is reduced following prolonged exercise and that this effect can be prevented by the administration of caffeine2. This suggests that caffeine exerts a protective effect on oculomotor control during exercise-induced fatigue2. Furthermore, the detrimental effect of a corticospinal motor system fatigue on the oculomotor system suggests that aspects of exercise-induced central fatigue are ubiquitous within the brain.
The mechanisms responsible for central fatigue are not well understood; though research involving exercising rodents suggests that prolonged physical activity is associated with widespread alterations in the activity of neurotransmitters such as serotonin, dopamine and noradrenaline3,4,5. As such, it has been proposed that these monoamines are involved in the development of fatigue during exercise6. Contrary to early hypotheses regarding the involvement of serotonin in the development of central fatigue7, it appears that serotonin does not act as a key factor8. Instead, the central catecholamines dopamine and norepinephrine are thought to play a principal role in central fatigue.
Studies exploring the influence of central catecholamines on human performance report positive effects on exercise capacity (time to exhaustion), time trial performance and subjective effort sense following the enhancement of central catecholaminergic neurotransmission via targeted pharmacological treatments9,10,11,12,13. Additionally, caffeine, which indirectly upregulates dopamine and accelerates the synthesis and turnover of norepinephrine via adenosine antagonism14, is a common ergogenic aid in prolonged exercise15,16,17,18. Overall, these findings provide indirect evidence for an involvement of central catecholamines in the development of central fatigue during whole-body, prolonged exercise. However, measures of exercise performance may be susceptible to confounds from peripheral factors, subject motivation and learning effects. Furthermore, while the benefits of caffeine on exercise performance are largely attributed to its effects on the central nervous system, caffeine also has several actions in the periphery that influence the contractile properties of skeletal muscle, such as increased mobilization of intracellular calcium, and inhibition of phosphodiesterases19, that may contribute to certain performance enhancements.
Our previous findings in the oculomotor system demonstrated direct, fatigue-related impairments on motor control and showed that these impairments can be prevented by a moderate dose of caffeine2. We propose that the protective effects of caffeine on oculomotor control during fatiguing exercise are the result of indirect upregulation of central catecholaminergic neurotransmission. In this study the effects of caffeine were compared to bupropion, a drug that modulates noradrenergic and dopaminergic reuptake inhibition, increasing the concentration of dopamine and norepinephrine in several brain regions20,21,22,23. Acute therapeutic doses of bupropion have previously been reported to confer an ergogenic effect during exercise9,10.
Oculomotor control was investigated by assessing saccades, smooth pursuit eye movements and optokinetic nystagmus (OKN). Like saccades, smooth pursuit eye movements are constantly utilised during normal visual activity, stabilising visual images on the retina as objects of interest move through the visual field24,25. OKN is an involuntary oculomotor response elicited by large-scale movement of the visual field, resulting in alternating slow tracking movements (slow phase) followed by rapid, resetting eye movements (quick phase), similar to smooth pursuit and saccades, respectively26,27. The oculomotor control of eye movements is related to the spatial orienting of attention28. To quantify any effects of fatigue on attention, covert spatial attention, or the orienting of attention that occurs without an eye movement toward the attended location, was measured using a cueing paradigm29.
In this study a three-hour cycling exercise protocol was used to provide a physiological challenge capable of inducing central fatigue. Prolonged cycling has long-lasting effects on the central nervous system, reducing voluntary activation of the knee extensors for up to 45 minutes following exercise cessation30 and causing significant perturbations to cerebral energetics31,32. Saccades, smooth pursuit, optokinetic nystagmus and covert spatial attention were assessed to investigate the influence of caffeine and norepinephrine-dopamine reuptake inhibition (NDRI) on oculomotor control in the context of prolonged, fatiguing exercise. We predicted that caffeine’s ability to preserve oculomotor control following fatiguing exercise is related to its actions on central catecholamines, therefore a similar influence will be exerted by NDRI.
Experimental Procedures
Participants
Twelve healthy volunteers (7 females, maximal aerobic capacity 56 ± 6 ml · kg · min−1) with a mean age of 25 (20–48) years and body mass of 74 ± 16 kg, participated in the study. Participants provided written informed consent and visited the laboratory on four occasions to take part in a protocol conducted in accordance with the Declaration of Helsinki and approved by the University of Auckland Human Ethics Committee. Based on responses to a health screening questionnaire, participants were free of medical contraindications to exercise, medication influencing central nervous system function, and had normal or corrected to normal vision.
Experimental design
Caffeine (7.5 mg · kg−1 body mass), bupropion (Zyban, GlaxoSmithKline, Auckland, NZ; maximum therapeutic dose: 300 mg/day) or placebo (maltodextrin) treatments were administered by capsule within a double-blind, placebo-controlled, repeated measures, randomised cross-over design. Participants completed three experimental trials involving 180 minutes of continuous cycling with a self-selected cadence at a work rate equivalent to 60% of maximal aerobic capacity. A minimum of 5 days separated cross-over phases. Caffeine was administered in two doses – a 2.5 mg · kg−1 body mass dose 1 hour before the exercise protocol, and a 5 mg · kg−1 body mass at 90 minutes into exercise. A 300 mg dose of a NDRI (bupropion) was administered before the exercise protocol, and a placebo pill was given 90 minutes into exercise. In the placebo treatment, a capsule filled with maltodextrin was administered before the exercise protocol and at the 90 minute time point. During exercise, participants were given a carbohydrate solution to maintain hydration and euglycaemia (see Experimental protocol below). In all experimental trials, participants completed a battery of visual tasks (see below, visual performance measures) before exercise (pre-exercise), and immediately after the exercise protocol (post-exercise). A schematic of the experiment workflow and design is depicted in Fig. 1.
Figure 1. Experimental workflow.
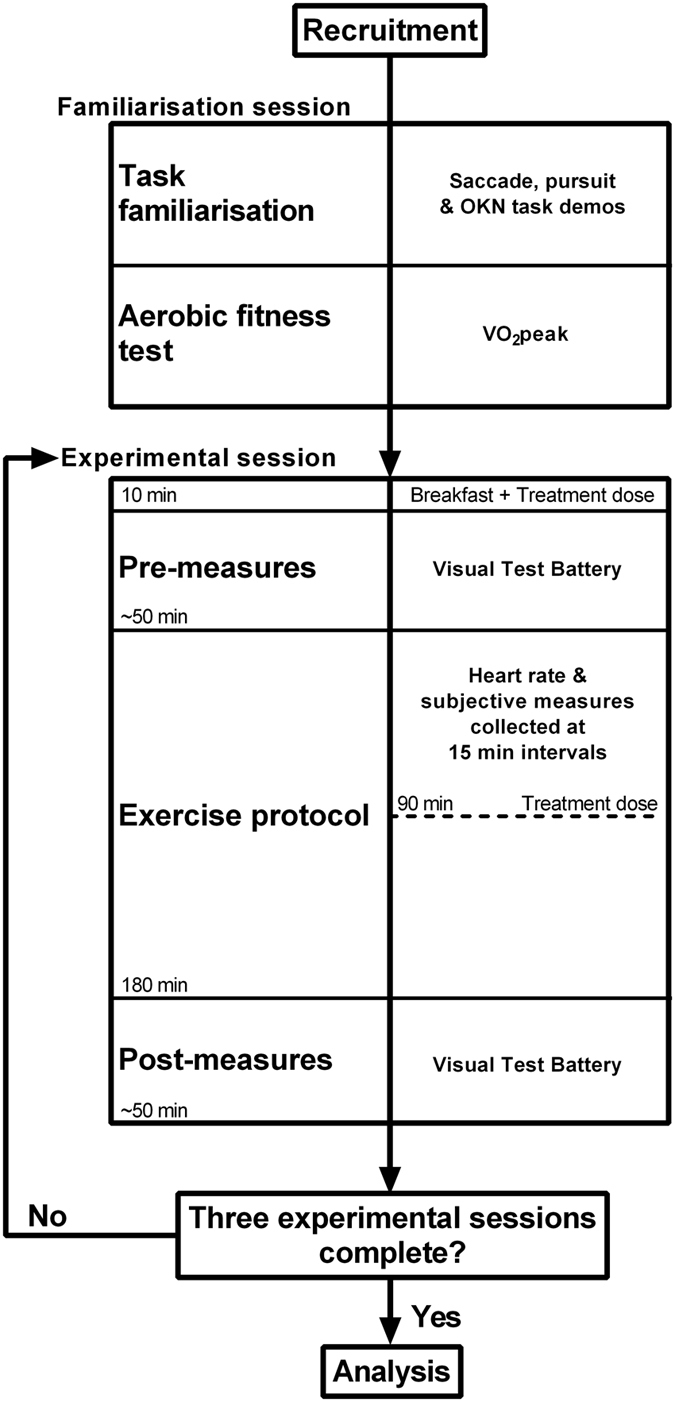
Twelve participants were recruited. A familiarisation session accustomed participants with the visual test battery. The three experimental sessions involved completion of the visual test battery at two time points, separated by prolonged cycling exercise lasting 180 minutes. Treatment doses were administered with breakfast, immediately before initial performance of the visual test battery (pre measures), and at 90 minutes through exercise.
Preliminary tests
A minimum of one week before the first experimental trial, participants visited the lab for familiarisation with study protocols and the visual test battery tasks (see below, Visual test battery). A maximal cardiopulmonary exercise test on an electromagnetically braked cycle ergometer (Velotron Dynafit Pro, Seattle, WA, USA) with respiratory gas analysis equipment (pneumotachometer, MLT1000L, ADInstruments; paramagnetic oxygen analyser, S-3A/I, AEI Technologies; infrared carbon dioxide analyser, CD-3A, AEI Technologies) was performed to measure peak oxygen uptake. Maximal aerobic capacity (VO2 max) was estimated and used to prescribe a power output requiring 60% VO2 max for the experimental trials.
Experimental protocol
Participants arrived at the laboratory following a 12 hour overnight fast. Participants were instructed to abstain from caffeine-containing items for 24 hours before each experimental session. Body mass was measured following voiding of the bladder. After this, the first treatment dose was consumed with a breakfast cereal. The quantity of breakfast was self-selected on the first visit and repeated for the remaining trials. Immediately following breakfast, participants completed the visual test battery (~50 minutes). Participants then began 180 minutes of continuous cycling performed in a temperature-controlled exercise chamber maintained at 18° Celsius. At 90 minutes into exercise, participants received a second treatment dose. A carbohydrate solution (0.7 g carbohydrate·kg−1 · h−1) was ingested every 15-minutes throughout exercise. Mean rates of fluid and carbohydrate ingestion were 645 ± 141 ml · h−1 and 52 ± 11 g · h−1, respectively. Heart rate was recorded at 15 minute intervals using a heart rate monitor (FS1, Polar Electro, Kempele, Finland). Coincident with heart rate recording, participants’ perceived exertion and felt arousal was self-rated on visual analogue scales. After the exercise protocol, participants completed the visual task battery. A post-exercise measurement of body mass was obtained to assess fluid loss or gain as a result of exercise.
Visual test battery
The visual test battery consisted of five discrete tasks. Participants were comfortably seated in a quiet, darkened room for the duration of the visual test battery. Stimuli were presented on a cathode ray tube monitor (Philips 109S2; 1280 × 1024 pixel resolution; 85 Hz refresh rate) at a viewing distance of 66 cm. Head movements were minimized using a chin and forehead rest. In four of the tasks, eye movements were tracked with infrared cameras sampling at a rate of 400 Hz (ViewPoint Eye Tracker, Arrington Research Systems, Scottsdale, USA). A 16-point calibration procedure was carried out before the beginning of each task. All participants completed visual tasks in the same order for all experimental sessions. This reduced variance caused by task sequencing effects at the expense of causing the time from exercise cessation to vary across the different tasks.
Antisaccade
Each trial began with the presentation of a black, centrally-located circular fixation point subtending a visual angle of 0.5°. A black, circular peripheral target stimulus (0.5° diameter) appeared ± 10° to the left or right of the central fixation point and was visible for a duration of 1000 ms. The peripheral target was presented in gap or overlap conditions33. In the gap condition, the central fixation point was presented for 800 ms and extinguished 200 ms before peripheral target presentation. In the overlap condition, the central fixation point remained visible throughout the trial. Participants were instructed to “look away from the peripheral target stimulus to a mirror opposite position on the screen, and to move your eyes to that position as quickly and accurately as possible”. A total of 100 trials, 50 trials per condition, were presented. The sequence of gap, overlap, right target location and left target location was randomised.
Prosaccade
Prosaccade and antisaccade tasks had identical stimulus presentation. However, in the prosaccade task, participants were instead instructed to move their eyes to look at the target stimulus as quickly and accurately as possible.
Smooth pursuit
A triangular target waveform was presented at three velocities (5° · s−1, 10° · s−1 and 30° · s−1). The target, a circular black dot (0.8° diameter), was presented in the centre of the screen (0°) for 1000 ms at the start of each trial. Following fixation, the target moved horizontally until it reached ±15°, where it reversed direction abruptly and moved to the opposite side. The direction of the initial ramp was randomised. One trial consisted of 5.5 passes of the target across the display screen. 21 total trials were performed, with each target velocity presented 7 times. The sequence of target velocity and initial ramp direction were randomised. Participants were instructed to follow the target with their eyes as accurately as possible.
Optokinetic nystagmus (OKN)
To evoke an OKN response, participants were presented with a stimulus screen consisting of 100% contrast square wave gratings with a fundamental spatial frequency of 0.833 cycles per degree. Gratings were shown for a duration of 20 seconds per trial at two velocities (5° · s−1 and 10° · s−1) and two stimulus directions (right to left, or left to right) for two trials each, resulting in 8 total trials. A blank, black screen was presented between trials for 4 seconds. Participants were instructed to watch the screen, keeping the stimulus in focus. The objective of these instructions was to encourage a “stare” OKN.
Covert spatial attention
A Posner cueing task29 with endogenous and exogenous cueing conditions assessed covert attentional orienting. This task has been employed previously to assess the influence of caffeine on covert spatial attention34. The start of each trial was marked with a display (1000 ms duration) consisting of a central fixation cross and two peripheral boxes located 10° laterally to the fixation cross. A visual cue was then presented for 200 ms and extinguished. After a further 200 ms, a peripheral target (black circle, radius of 0.5°) appeared in the right or left peripheral box for 150 ms. Participants were instructed to maintain fixation on the central cross and respond as quickly as possible with a key press (left arrow key or right arrow key) when they detected a peripheral target. Two types of cues were presented. Endogenous cues consisted of a centrally presented arrow pointing to the left or right peripheral box. Arrows were centred 0.2° above the visual fixation cross and subtended 0.5°. Exogenous cues consisted of an increase in the line width of the peripheral box from 0.15° to 0.20°. Cues were presented in valid, invalid or neutral trials. In valid trials the target appeared in the cued location, whilst on invalid trials the peripheral target appeared opposite to the cued location. Neutral trials provided no information regarding the target location. Neutral cues were a doubled-headed arrow, or a change in line width of both peripheral boxes, for endogenous and exogenous conditions, respectively. 90 trials for each condition were presented. In the exogenous condition, valid, invalid and neutral trials occurred with equal probability (30 trials per cue), while for the endogenous condition, the valid cues correctly predicted target location 80 percent of the time. Thus, neutral cues were presented in 30 trials, valid cues in 48 trials and invalid cues in 12 trials. The cue-target contingency for exogenous and endogenous conditions was based on previous literature29,35,36. In both conditions, targets appeared in the right or left peripheral box with equal probability. Cue type was randomised within endogenous and exogenous blocks.
In the preliminary testing session, participants completed abbreviated versions of all visual tasks to ensure they were familiar with stimulus presentation, experimental setup and task requirements.
Data treatment and analysis
Stimulus presentation, data collection and data analysis were performed using custom software written in Matlab (MathWorks R2010b, Massachusetts, USA). The experimenter visually inspected all eye movement traces. Final sample size satisfied a priori power analyses. Sample size was estimated using an expected effect size of 0.4. This effect size was derived from a previous study investigating eye movement kinematics within a similar experimental design2. Previous research suggests a high correlation (0.62–0.97) across repeated measures for saccades and smooth pursuit37. Thus, with power set to 0.95, p < 0.05, and correlation among repeated measures of 0.62, a sample size of 12 participants was estimated to provide appropriate statistical power for eye movement kinematic measures.
Prosaccade and antisaccades
Initiation of a saccade was identified using an amplitude deviation (deviation of >1° from fixation) and a velocity criterion (≥30° · s−1). The end of the saccade was detected by a drop in the saccade velocity below 30° · s−1 38,39. Anticipatory saccades (latency <70 ms) were excluded from analysis40. Latency (ms), amplitude (°), peak velocity (° · s−1) and task performance (percentage of saccades in the correct direction) were the dependent variables derived from the saccade tasks. For kinematic measures, only saccades performed in the correct direction were included in statistical analysis.
Smooth pursuits
Saccades and blinks during pursuit were identified by computing a velocity signal from the horizontal eye position during each trial and applying a velocity criterion (≥64° · s−1). Sections of pursuit containing saccades and blinks were removed before applying a linear interpolation to the remaining data to calculate eye velocity. Eye velocity was used to derive pursuit gain (eye velocity/target velocity), the dependent measure of the smooth pursuit task. The initial ramp in which the target only completed a pass across half of the screen was not included in analysis.
Optokinetic nystagmus
Quick phases were also identified with a velocity criterion (≥64° · s−1). Amplitude (°) and peak velocity (° · s−1) were derived from the quick phases. Quick phase identification permitted slow phase determination, as slow phases were assumed to occur between quick phases. Slow phase eye velocity was calculated by applying a linear interpolation to the slow phase, thereby allowing the calculation of slow phase gain.
Covert spatial attention
Keyboard responses (response time and left/right key) were collected using custom software written in Matlab (Mathworks, R2010b, Massachusetts, USA). A “validity effect” in endogenous and exogenous task conditions for each participant was derived from response times to valid and invalid cues. This measure, which was calculated by subtracting valid response time from invalid response time, reflects the time required to disengage covert attention from an invalidly cued location, and shift attention to the target location. Eye movements were monitored throughout the task. Trials in which the eyes deviated >1° from fixation, or if response time was >1000 ms were rejected from analysis.
Statistical analyses
Repeated measures analyses of variance (ANOVA) with the factor treatment (Caffeine/NDRI/Placebo) was used to explore the effect of exercise on fluid loss. The validity effect in endogenous and exogenous conditions within the covert spatial attention task was explored by adding the factor timepoint (pre-exercise/post-exercise) to the repeated measures ANOVA. timepoint (pre-exercise/post-exercise) and condition (gap/overlap) were added as factors to statistical analyses for measures from the prosaccade and antisaccade tasks. Similarly, stimulus speed was added as a factor to statistical analyses for smooth pursuit gain (5° · s−1, 10° · s−1 and 30° · s−1) and OKN slow and quick phases (5° · s−1, and 10° · s−1). There was no influence of stimulus direction, so this was not included as a factor in the statistical analysis. To explore the influence of treatment on subjective experiences and heart rate, the number of levels within timepoint was extended to eleven.
The repeated measures design of the experiment increases possibility of task learning influencing our dependent variables. To identify possible learning effects repeated measures ANOVAs with the factor trial (first vs. second) used in place of treatment. The presence of a task learning effect is commented on where relevant.
Where necessary, interaction effects were explored using within-subject paired comparisons. The multiple comparison type I error rate was controlled using a false discovery rate criterion procedure41. Violations of sphericity were controlled with the Greenhouse-Geisser correction. Statistical significance was set at α = 0.05. Results are reported as mean ± standard deviation (SD) unless otherwise stated.
Results
Visual performance measures
Prosaccades
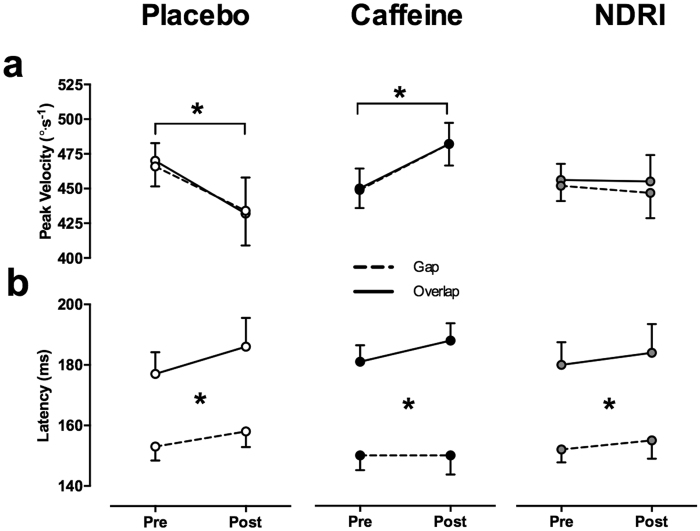
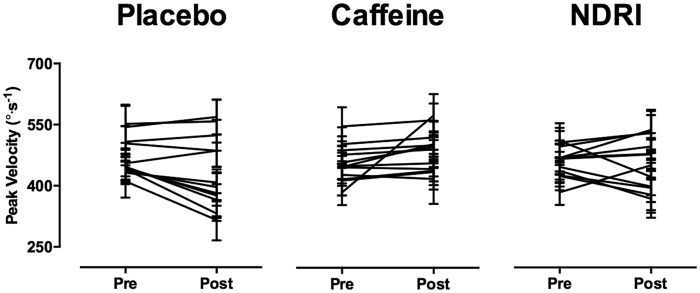
There was an effect of exercise on peak prosaccade velocity that was modulated by treatment (interaction TREATMENT × TIMEPOINT, F2,22 = 7.67, p < 0.01). Changes in prosaccade velocity after exercise were similar in magnitude for gap and overlap conditions (Fig. 2, panel a). Post-hoc analysis of peak velocity, collapsed across gap and overlap conditions, revealed that with placebo, exercise-induced fatigue significantly reduced peak velocity compared to pre-exercise by 8 ± 11% (t23 = 3.51, p < 0.05). In the majority of participants, prosaccade peak velocity was influenced by exercise-induced fatigue in the placebo trial, with eight of twelve participants exhibiting a lower post-exercise peak prosaccade velocity compared to pre-exercise. To explore the magnitude of this change while accounting for within-subject variability, an effect size comparing pre-exercise prosaccade velocity to post exercise was calculated for each participant. The average effect size of the eight participants that displayed a drop in peak prosaccade velocity with placebo was 0.77 ± 0.41. The remaining participants displayed an increase in peak velocity post-exercise with an average effect size of 0.19 ± 0.13. Conversely, caffeine prevented this exercise-induced decrement in prosaccade velocity and increased velocity significantly above pre-exercise levels (6 ± 9%, t23 = −2.96, p < 0.05)(see Fig. 2, panel a). Ten of twelve participants exhibited an increase in prosaccade velocity above pre-exercise levels. However, a large increase was evident in one participant (effect size >1.0), while the remaining nine participants exhibited only a small to moderate increase in velocity post exercise (average effect size, 0.24 ± 0.17). Two participants displayed a drop in prosaccade peak velocity following exercise, however, this was only of a small magnitude (average effect size, 0.09 ± 0.06). NDRI also prevented post exercise decrements in peak prosaccade velocity, maintaining velocity at pre-exercise levels (−1 ± 12%, t23 = 0.33, p = 0.74). Seven participants displayed a slight increase in prosaccade velocity post-exercise, with an average effect size of 0.40 ± 0.32, while five participants exhibited a moderate decrease post-exercise (average effect size, 0.66 ± 0.2.4). Figure 3 depicts average peak prosaccade velocity (collapsed across gap and overlap conditions) pre and post-exercise for placebo, caffeine and NDRI treatments for each participant.
Figure 2. Prosaccade peak velocity and latency for placebo, caffeine and bupropion (NDRI) treatments.
(Panel a) – Prosaccade velocity in gap (dashed line) and overlap (solid line) task conditions pre and post exercise. (Panel b) – Prosaccade latency for gap (dashed line) and overlap (solid line) conditions for pre and post exercise categories in placebo, caffeine and NDRI. Here and in Fig. 3, the connecting lines are shown for illustrative purposes only and do not infer the assumption of a linear change from pre to post exercise. Significance labelling between gap and overlap conditions indicates a main effect of condition. Data represent mean ± SE. *<0.05.
Figure 3. Prosaccade velocity in placebo, caffeine and bupropion (NDRI) treatments for each participant.
Peak prosaccade velocity collapsed across task condition for pre and post exercise categories in placebo, caffeine and NDRI treatments. Each point represents mean peak prosaccade velocity ± 95% confidence interval for each participant.
A robust main effect of condition was observed for prosaccade latency (F1,11 = 45.27, p < 0.01) whereby prosaccades in the overlap condition were performed with slower latencies compared to the gap condition. The influence of condition on prosaccade latency pre and post exercise for each treatment is illustrated in panel b of Fig. 2. No significant changes were observed across time point or between treatments for prosaccade latency. Similarly, condition also influenced task performance, with higher performance in the overlap condition (F1,11 = 14.12, p < 0.05). However, task performance in both conditions was modulated by an interaction between treatment and time point (F2,22 = 4.11, p < 0.05). Post-hoc analyses revealed a significant drop in task performance post exercise with NDRI (t23 = 2.93, p < 0.05), while there was no change in task performance following exercise with caffeine or placebo treatments. There were no alterations in prosaccade amplitude as a result of condition, time point or treatment. These data are reported in Table 1.
Table 1. Measures of oculomotor control.
| Placebo |
Caffeine |
Bupropion |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre exercise |
Post exercise |
Pre exercise |
Post exercise |
Pre exercise |
Post exercise |
|||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Prosaccade | ||||||||||||
| Peak velocity(° · s−1) | 468 | 47 | 433 | 88 | 454 | 44 | 485 | 50 | 458 | 37 | 455 | 62 |
| Gap condition | 466 | 50 | 434 | 87 | 453 | 44 | 485 | 52 | 456 | 37 | 451 | 61 |
| Overlap condition | 470 | 44 | 432 | 90 | 454 | 48 | 485 | 51 | 460 | 39 | 459 | 64 |
| Latency(ms) | 167 | 18 | 174 | 26 | 166 | 17 | 171 | 16 | 167 | 18 | 170 | 26 |
| Gap condition | 155 | 14 | 160 | 18 | 151 | 16 | 153 | 18 | 154 | 14 | 155 | 20 |
| Overlap condition | 179 | 26 | 187 | 34 | 182 | 20 | 189 | 19 | 181 | 25 | 185 | 33 |
| Amplitude(°) | 9 | 0.5 | 9 | 0.9 | 9 | 0.4 | 9 | 0.6 | 9 | 0.6 | 9 | 0.9 |
| Gap condition | 9 | 0.6 | 9 | 0.5 | 9 | 0.6 | 9 | 0.7 | 9 | 0.6 | 9 | 0.9 |
| Overlap condition | 9 | 0.4 | 9 | 1.5 | 9 | 0.4 | 9 | 0.6 | 9 | 0.7 | 9 | 0.9 |
| Task performance(%correct) | 97 | 4 | 96 | 2 | 96 | 5 | 97 | 5 | 98 | 3 | 95 | 5 |
| Gap condition | 95 | 6 | 96 | 4 | 92 | 9 | 95 | 8 | 95 | 5 | 92 | 8 |
| Overlap condition | 99 | 3 | 100 | 1 | 99 | 1 | 99 | 2 | 100 | 1 | 98 | 3 |
| Antisaccade | ||||||||||||
| Peak velocity(° · s−1) | 444 | 62 | 413 | 63 | 441 | 73 | 462 | 82 | 432 | 40 | 413 | 62 |
| Gap condition | 457 | 61 | 411 | 70 | 443 | 76 | 465 | 84 | 433 | 41 | 421 | 66 |
| Overlap condition | 432 | 66 | 416 | 58 | 439 | 70 | 458 | 81 | 431 | 43 | 404 | 61 |
| Latency(ms) | 231 | 61 | 247 | 43 | 238 | 38 | 233 | 37 | 242 | 45 | 242 | 52 |
| Gap condition | 228 | 63 | 239 | 47 | 232 | 41 | 225 | 43 | 240 | 49 | 236 | 56 |
| Overlap condition | 233 | 60 | 254 | 40 | 243 | 35 | 241 | 32 | 244 | 42 | 248 | 50 |
| Amplitude(°) | 9 | 2.1 | 9 | 1 | 9 | 1.8 | 9 | 1.6 | 9 | 1.8 | 9 | 1.5 |
| Gap condition | 9 | 2.1 | 9 | 1 | 9 | 1.8 | 9 | 1.6 | 9 | 1.7 | 9 | 1.4 |
| Overlap condition | 9 | 2.1 | 9 | 1 | 9 | 1.9 | 9 | 1.6 | 9 | 2 | 9 | 1.7 |
| Task performance(% correct) | 82 | 17 | 87 | 8 | 87 | 7 | 87 | 8 | 88 | 10 | 85 | 11 |
| Gap condition | 76 | 18 | 82 | 12 | 83 | 11 | 80 | 13 | 82 | 15 | 78 | 14 |
| Overlap condition | 87 | 18 | 91 | 5 | 92 | 4 | 94 | 4 | 93 | 7 | 92 | 8 |
| Smooth pursuit | ||||||||||||
| Gain | 0.91 | 0.26 | 0.87 | 0.29 | 0.89 | 0.26 | 0.87 | 0.3 | 0.87 | 0.23 | 0.9 | 0.31 |
| 5° · s−1 stimulus | 0.97 | 0.27 | 0.96 | 0.26 | 0.96 | 0.25 | 0.94 | 0.33 | 0.93 | 0.23 | 0.96 | 0.29 |
| 10° · s−1 stimulus | 0.91 | 0.23 | 0.91 | 0.27 | 0.9 | 0.25 | 0.86 | 0.26 | 0.89 | 0.23 | 0.94 | 0.32 |
| 30° · s−1 stimulus | 0.75 | 0.19 | 0.66 | 0.25 | 0.73 | 0.22 | 0.7 | 0.22 | 0.74 | 0.17 | 0.67 | 0.23 |
| Optokinetic Nystagmus | ||||||||||||
| Slow phase gain | 0.67 | 0.11 | 0.64 | 0.11 | 0.74 | 0.08 | 0.72 | 0.07 | 0.7 | 0.1 | 0.73 | 0.1 |
| 5° · s−1 stimulus | 0.72 | 0.1 | 0.65 | 0.06 | 0.71 | 0.06 | 0.7 | 0.07 | 0.67 | 0.07 | 0.7 | 0.09 |
| 10° · s−1 stimulus | 0.64 | 0.21 | 0.65 | 0.16 | 0.76 | 0.14 | 0.74 | 0.1 | 0.72 | 0.16 | 0.75 | 0.14 |
| Quick phase amplitude(°) | 5 | 1.4 | 4 | 1.5 | 5 | 1.2 | 5 | 1.9 | 5 | 1.5 | 5 | 1.8 |
| 5° · s−1 stimulus | 4 | 1.7 | 4 | 1.2 | 4 | 1.3 | 5 | 2.2 | 4 | 1.8 | 4 | 1.6 |
| 10° · s−1 stimulus | 5 | 1.4 | 5 | 1.8 | 5 | 1.3 | 6 | 1.9 | 6 | 1.4 | 6 | 2 |
| Quick phase peak velocity(° · s−1) | 245 | 54 | 203 | 54 | 235 | 52 | 245 | 58 | 250 | 47 | 237 | 62 |
| 5° · s−1 stimulus | 223 | 59 | 182 | 51 | 213 | 52 | 232 | 71 | 218 | 23 | 208 | 57 |
| 10° · s−1 stimulus | 258 | 59 | 216 | 63 | 248 | 53 | 254 | 54 | 266 | 44 | 254 | 66 |
Kinematic and task performance measures derived from prosaccade, antisaccade, smooth pursuit and OKN visual tasks, pre and post exercise for placebo, caffeine and NDRI treatments.
Antisaccades
There was an effect of exercise-induced fatigue on antisaccade peak velocity that was modulated by treatment and condition (3-way interaction TREATMENT × TIMEPOINT × CONDITION, F2, 22 = 7.98, p < 0.01). Post-hoc analysis revealed that exercise-induced fatigue significantly reduced the peak velocity of antisaccades in the gap condition compared to pre-exercise by 10 ± 11%. Nine participants displayed a moderate decrease in antisaccade peak velocity (average effect size, 0.58 ± 0.58) with the placebo. The remaining three participants exhibited a small to moderate increase in peak velocity following exercise after placebo (average effect size, 0.29 ± 0.10). Conversely, peak antisaccade velocity did not differ across time point in the placebo treatment for the overlap condition, nor were any significant alterations in antisaccade peak velocity detected across time and condition for caffeine and NDRI treatments.
A main effect of condition was observed for antisaccade latency (F1,11 = 8.39, p < 0.05) whereby antisaccades in the gap condition had faster latencies than the overlap condition. An interaction effect between condition and time point also modulated antisaccade latency (F1,11 = 6.11, p < 0.05). This appeared to stem from longer latencies post exercise in the overlap condition, whereas gap latencies remain stable across time. However, the increase in overlap latencies post exercise did not reach statistical significance after correcting for multiple comparisons (t35 = 1.71, p = 0.05, critical p < 0.025). A robust effect of condition was detected for task performance, with a higher percentage of antisaccades performed in the correct direction in the overlap condition (91 ± 9%) compared to the gap condition (80 ± 14%)(F1, 11 = 26.09, p < 0.01). There were no alterations in antisaccade amplitude as a result of condition, time point or treatment.
Smooth pursuit
Pursuit gain differed depending on the stimulus speed presented (F2,18 = 95.40, p < 0.01). For the slowest stimulus speed (5° · s−1), pursuit gain was closest to unity and progressively declined for faster stimulus speeds (10° · s−1 and 30° · s−1). An interaction between stimulus speed and time point was detected for pursuit gain (F2,18 = 7.78, p < 0.01). Post-hoc tests revealed that this effect stems from a decrease in smooth pursuit gain post-exercise, irrespective of treatment, for the 30° · s−1 stimulus speed (t29 = 4.69, p < 0.01). These data are reported in Table 1.
Optokinetic nystagmus
Slow phase gain of OKN was modulated by an interaction between stimulus speed and treatment (F2,20 = 4.53, p < 0.05). Post-hoc tests revealed significantly reduced slow phase gain in response to the 10° · s−1 stimulus speeds with placebo compared to caffeine (t21 = 2.79 p < 0.01) and NDRI (t21 = 2.87, p < 0.01) respectively.
Alterations in amplitude and peak velocity of OKN quick phases were detected. Amplitude differed depending on stimulus speed presented (F1,10 = 28.93, p < 0.01), with slightly higher quick phase amplitudes in response to the 10° · s−1 speed (5.3 ± 1.6°) compared to the 5° · s−1 speed (4.3 ± 1.68). Amplitude was also modulated by an interaction between treatment and time point (F2,20 = 3.88, p < 0.05). This stemed from a slight increase in amplitude post-exercise with caffeine (pre-exercise, 4.7 ± 1.4°; post-exercise, 5.3 ± 2.0°). However, this difference was not statistically significant after correcting for multiple comparisons.
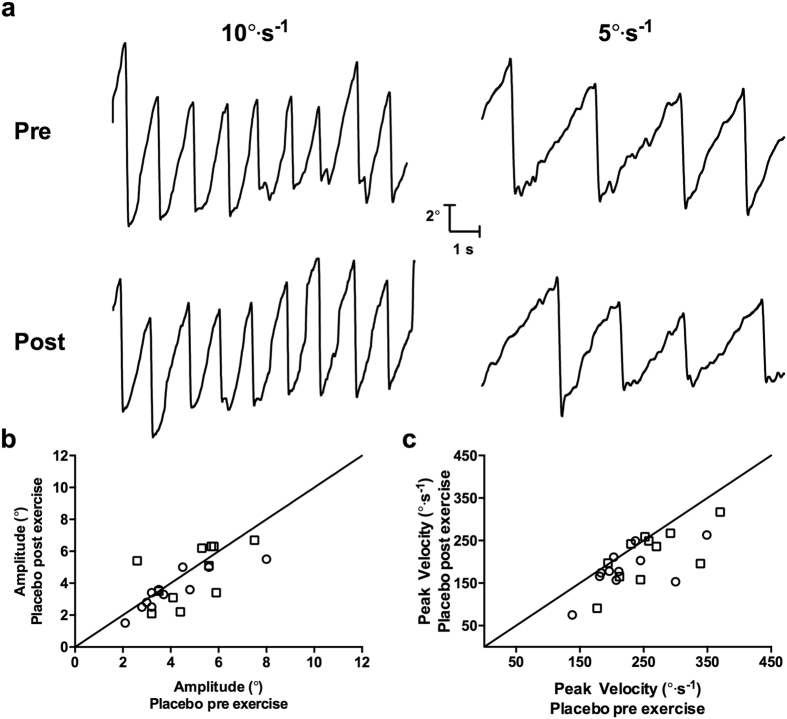
Peak velocity of quick phases also differed between stimulus speeds (F1,10 = 23.74, p < 0.01), with higher velocities to the 10° · s−1 (249 ± 57° · s−1) compared to the 5° · s−1 per second stimulus speed (134 ± 28° · s−1). An interaction between treatment and time point was also evident (F2,20 = 7.51, p < 0.01). Post-hoc tests revealed a significant decrease in peak velocity post-exercise with placebo (t21 = 3.80, p < 0.01), while there were no differences pre to post-exercise in peak velocity with caffeine and NDRI treatments. These data, along with example OKN traces pre and post-exercise with placebo are illustrated in Fig. 4.
Figure 4. Kinematic characteristics of OKN quick phases in placebo treatment.
(Panel a) – Sample eye movement traces of optokinetic nystagmus pre exercise (top traces) and post exercise (bottom traces) in response to 10° · s−1 (left traces) and 5° · s−1 (right traces) stimulus speeds. (Panel b) – Mean amplitude of OKN quick phases pre and post exercise categories with placebo. Each symbol represents one participant, while the shape of the symbol corresponds to one stimulus velocity: 5° · s−1 (circle) and 10° · s−1 (square). The diagonal unity line represents no difference between pre exercise and post exercise. (Panel c) – Mean peak velocity of OKN quick phases in placebo and caffeine.
Covert spatial attention
The number of trials rejected due to deviations from fixation did not differ as a consequence of treatment or time point. The validity effect in endogenous and exogenous cueing conditions was equivalent between treatments and across time points. Table 2 reports the validity effect for both task conditions across time for each treatment.
Table 2. Measures of covert spatial attention.
| Placebo |
Caffeine |
NDRI |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre exercise |
Post exercise |
Pre exercise |
Post exercise |
Pre exercise |
Post exercise |
|||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Validity effect | ||||||||||||
| Endogenous (ms) | 48 | 33 | 58 | 46 | 42 | 36 | 58 | 45 | 42 | 35 | 61 | 51 |
| Exogenous (ms) | 25 | 29 | 28 | 37 | 18 | 25 | 37 | 30 | 18 | 27 | 13 | 22 |
Validity effect for endogenous and exogenous conditions of the covert spatial attention task across placebo, caffeine and NDRI treatments.
Heart rate, fluid balance and subjective measures
Heart rate and fluid loss were equivalent between treatments. For placebo, caffeine and NDRI treatments average heart rate (beats per minute) was 147 ± 19, 150 ± 18, and 153 ± 20 respectively. Figure 5 (panel a) illustrates heart rate over time for each treatment. Mean change in body mass was −0.2 ± 0.7%, −0.1 ± 0.8% and −0.3% ± 0.9% of pre-exercise body mass, for placebo, caffeine and NDRI treatments respectively. There was no influence of treatment on change in body mass (F2,22 = 1.00, p = 0.40).
Figure 5. Heart rate, felt arousal and perceived exertion during exercise for placebo (white fill) caffeine (black fill) and NDRI (gray fill).
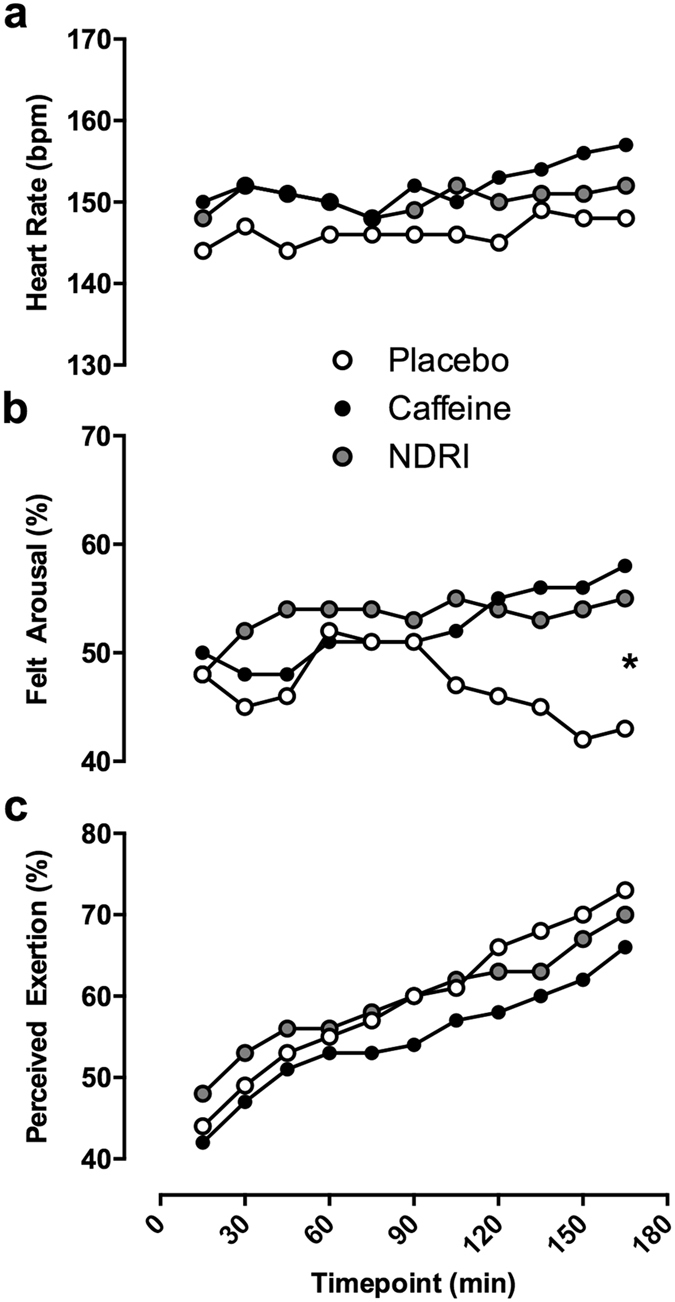
(Panel a) – Heart rate across the 180 minute exercise protocol in experimental treatments. (Panel b) – Felt arousal across the 180 minute exercise protocol. Significance labelling represents a significant posthoc comparison between placebo versus caffeine and NDRI at the 165 minute time point through exercise. (Panel c) – Perceived exertion across the 180 minute exercise protocol. Standard error for each point is not depicted here to improve the clarity of the figure. *<0.05.
Figure 5 (panels b and c) shows average ratings of perceived exertion and felt arousal for placebo, caffeine and NDRI treatments. A main effect of time (F10,110 = 20.01, p < 0.05) was detected for ratings of perceived exertion. Perceived exertion steadily increased over the duration of the exercise protocol, to a similar extent for all treatments.
An interaction between treatment and time (F20,220 = 2.50, p < 0.05) was found for felt arousal. Felt arousal with NDRI appears relatively stable across the duration of the exercise protocol, and was higher than caffeine and placebo for the first 90 minutes of exercise. After 90 minutes, felt arousal with caffeine started to increase, reaching a maximum by 165 minutes into exercise. Conversely, after 90 minutes, felt arousal in placebo steadily declined. Post-hoc comparisons revealed a significant difference in felt arousal at the 165 minutes between caffeine and placebo (t11 = 3.60, p < 0.05), and NDRI and placebo (t11 = 2.20, p < 0.05), while there was no difference between caffeine and NDRI (t11 = 1.01, p = 0.33).
Discussion
This study provides evidence for a role of noradrenergic and dopaminergic neurotransmission in fatigue-related impairments in the oculomotor control of high-velocity eye movements. The findings suggest that caffeine exerts at least part of its protective effects through these neurochemicals. Furthermore, the detrimental effects of fatigue on eye movement kinematics were restricted to velocity, while eye movement amplitude, tracking performance of pursuit eye movements and visual attention were robust to fatigue and psychotropic drugs.
In a previous study2, we reported a significant decrease in average prosaccade velocity following prolonged exercise that could not be accounted for by changes in saccade amplitude. This effect was proposed to result from central fatigue. In the present study, using measures of peak eye movement velocity, we extended these findings by observing that prolonged exercise exerts a similar effect on the velocities of pro- and antisaccades as well as the quick phase of OKN. This suggests that the detrimental influence of fatigue on saccade velocity is stable across differing task conditions and even extends to reflexive eye movements. Whether the velocity reductions we measured (8–10% reduction in peak velocity) have a discernible influence on visual function is yet to be determined. However, it is plausible that fatigue-related reductions in saccade velocity lengthen the time required to gather information from the visual field.
The detrimental effect of exercise-induced fatigue did not extend to smooth pursuit or the slow phase of OKN, as gain was not modulated as a result of exercise or treatment. The slow phase gain of OKN for the 10° · s−1 stimulus was significantly lower in the placebo condition than the treatment conditions. This may reflect a pre-exercise influence of caffeine and norepinephrine-dopamine reuptake inhibition as participants received treatment doses before starting the pre-exercise visual test battery.
Caffeine and norepinephrine-dopamine reuptake inhibition exerted a similar effect on oculomotor control, protecting the peak velocity of saccades and the quick phases of OKN following exercise. Both treatments increase levels of norepinephrine and dopamine release20,42. Therefore, we propose that prolonged exercise impaired the synthesis and metabolism of central catecholamines resulting in inhibitory tone that disrupted oculomotor function. This effect appeared to influence both subcortical and cortical components of the oculomotor control system. In particular, exercise fatigue affected reflexive eye movements (prosaccades, OKN quick-phase) that involve the paramedian pontine formation (PPRF), superior colliculus and basal ganglia43,44,45 as well as antisaccades that also involve frontal cortical oculomotor regions such as the frontal eye fields, supplementary eye fields and dorsolateral prefrontal cortex45. The effects may have been most pronounced for regions within the oculomotor network, such as the PPRF burst neurons, that generate the neural drive for high-velocity eye movements and that utilize dopamine and norepinephrine46, 47, 48.
The measures of visual attention derived from the gap-overlap paradigm within the saccade tasks and the covert spatial attention task assessed the influence of exercise-induced fatigue on attentional factors involved in oculomotor control. A clear effect of gap and overlap conditions, similar in magnitude, was observed for saccade latency and task performance across all experimental treatments. In prosaccades and antisaccades, latencies in the gap condition were significantly shorter than those in the overlap condition (Fig. 2 and Table 1, respectively). Primate research has shown that gap trials are associated with higher preparatory neural activity in the frontal eye fields, the superior colliculus and the paramedian pontine reticular formation49,50,51, and inhibition of fixation-related activity in the superior colliculus50. This pattern of neural activity is thought to promote a reduction in saccadic latency and lower task performance rates in gap trials52,53. Conversely, in the overlap trials, persistence of the fixation point during onset of the peripheral target promotes fixation-related neural activity after target appearance, causing slower saccade latencies54. Thus, it appears that exercise-induced fatigue does not selectively influence the release or maintenance of visual fixation, nor does it exert a general effect on the mechanisms underlying the disengagement and re-engagement of visual attention. Further, the orienting of covert spatial attention was unaffected by exercise-fatigue. Overall, these findings suggest that components of visual attention relevant to oculomotor control are not vulnerable to the central effects of fatigue, and are unlikely to have contributed to the alterations in eye movement kinematics that were observed following exercise.
To our knowledge, this is the first study to compare the actions of caffeine and norepinephrine-dopamine reuptake inhibition during fatigue. Both treatments prevented fatigue-related alterations in oculomotor control with caffeine exerting a slightly larger effect. It is likely that the more pronounced effect of caffeine was a consequence of a dose discrepancy between the two treatments as it was not possible to possible to titrate caffeine and bupropion doses to equivalent influence on catecholaminergic neurotransmission.
Because we imposed equivalent physiological stress between trials, within a protocol that elicits perturbations in cerebral energetics55 we are able to contrast the influence of treatments on arousal during exercise, which has not been reported previously with these drugs. Caffeine and NDRI exerted a similar effect on subjective ratings of arousal, promoting significantly higher levels of arousal for 165 minutes of exercise compared to placebo. The observed effects on arousal confirm adequate dosing as this response is consistent with previous reports of the influence of caffeine during exercise56.
One of the strengths of this study is that it averted some of the difficulties of investigating alterations in central nervous system function following whole-body exercise by measuring motor output from the relatively well understood neural circuitry of the human oculomotor system25,45,57,58. However, the interpretation of our findings is somewhat limited to prolonged steady-state exercise. Further evaluation is required to establish the manner in which whole-body exercise of differing modes, intensities and durations influences the development of central fatigue detectable by changes in oculomotor control.
Overall, our findings suggest that caffeine’s protective effect on oculomotor control following exercise-induced fatigue is mediated by its action on central catecholaminergic neurotransmission, as targeted manipulation of these neurotransmitter systems via norepinephrine-dopamine reuptake inhibition also prevented fatigue-related impairments to the peak velocity of quick phases and saccades. These observations cannot be explained by direct stress to the brain circuitry controlling the extra-ocular muscles as the oculomotor system is functionally independent of the locomotion, and was not challenged during cycling exercise.
Additional Information
How to cite this article: Connell, C. J. W. et al. Fatigue-related impairments in oculomotor control are prevented by norepinephrine-dopamine reuptake inhibition. Sci. Rep. 7, 42726; doi: 10.1038/srep42726 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Acknowledgments
We thank the participants who volunteered their time to take part in the study. We are grateful to Hayden Green for his assistance in data collection. This study was funded by the University of Auckland.
Footnotes
The authors declare no competing financial interests.
Author Contributions Conceptualization, C.J.W.C., B.T. and N.G.; Methodology, C.J.W.C., B.T. and N.G.; Software, C.J.W.C. and J.T.; Formal Analysis, C.J.W.C.; Investigation, C.J.W.C.; Resources, N.G.; Writing, C.J.W.C., B.T. and N.G.; Medical Supervision, A.S.; Supervision, N.G.
References
- Leigh R. J. & Kennard C. Using saccades as a research tool in the clinical neurosciences. Brain 127, 460–477 (2004). [DOI] [PubMed] [Google Scholar]
- Connell C. J. W. et al. Fatigue related impairments in oculomotor control are prevented by caffeine. Sci. Rep. 6, 26614 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley T. E. & Fleshner M. Neuroplasticity of dopamine circuits after exercise: implications for central fatigue. Neuromolecular Med. 10, 67–80 (2008). [DOI] [PubMed] [Google Scholar]
- Meeusen R. et al. Effects of tryptophan and/or acute running on extracellular 5-HT and 5-HIAA levels in the hippocampus of food-deprived rats. Brain Res. 740, 245–252 (1996). [DOI] [PubMed] [Google Scholar]
- Meeusen R. et al. Endurance training effects on neurotransmitter release in rat striatum: an in vivo microdialysis study. Acta Physiol. Scand. 159, 335–341 (1997). [DOI] [PubMed] [Google Scholar]
- Meeusen R., Watson P., Hasegawa H., Roelands B. & Piacentini M. F. Central fatigue: the serotonin hypothesis and beyond. Sports Med. 36, 881–909 (2006). [DOI] [PubMed] [Google Scholar]
- Newsholme E., Acworth I. & Blomstrand E. Amino acids, brain neurotransmitters and a functional link between muscle and brain that is important in sustained exercise. Adv. Myochem. 1, 127–133 (1987). [Google Scholar]
- Taylor J. L., Amann M., Duchateau J., Meeusen R. & Rice C. L. Neural contributions to muscle fatigue: from the brain to the muscle and back again. Med. Sci. Sports Exerc (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson P. et al. Acute dopamine/noradrenaline reuptake inhibition enhances human exercise performance in warm, but not temperate conditions. J. Physiol. 565, 873–883 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roelands B. et al. A dopamine/noradrenaline reuptake inhibitor improves performance in the heat, but only at the maximum therapeutic dose. Scand. J. Med. Sci. Sports 22, e93–e98 (2012). [DOI] [PubMed] [Google Scholar]
- Piacentini M. F., Meeusen R., Buyse L., De Schutter G. & De Meirleir K. Hormonal responses during prolonged exercise are influenced by a selective DA/NA reuptake inhibitor. Br. J. Sports Med. 38, 129–133 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs I. & Bell D. G. Effects of acute modafinil ingestion on exercise time to exhaustion. Med. Sci. Sports Exerc. 36, 1078–1082 (2004). [DOI] [PubMed] [Google Scholar]
- Swart J. et al. Exercising with reserve: evidence that the central nervous system regulates prolonged exercise performance. Br. J. Sports Med. 43, 782–788 (2009). [DOI] [PubMed] [Google Scholar]
- Kalmar J. M. & Cafarelli E. Caffeine: a valuable tool to study central fatigue in humans? Exerc. Sport Sci. Rev. 32, 143–147 (2004). [DOI] [PubMed] [Google Scholar]
- Cox G. R. et al. Effect of different protocols of caffeine intake on metabolism and endurance performance. J. Appl. Physiol. 93, 990–999 (2002). [DOI] [PubMed] [Google Scholar]
- Pasman W. J., van Baak M. A., Jeukendrup A. E. & de Haan A. The effect of different dosages of caffeine on endurance performance time. Int. J. Sports Med. 16, 225–230 (1995). [DOI] [PubMed] [Google Scholar]
- Bell D. G. & McLellan T. M. Exercise endurance 1, 3, and 6 h after caffeine ingestion in caffeine users and nonusers. J. Appl. Physiol. 93, 1227–1234 (2002). [DOI] [PubMed] [Google Scholar]
- Anderson M. E. et al. Improved 2000-meter rowing performance in competitive oarswomen after caffeine ingestion. Int. J. Sport Nutr. Exerc. Metab. 10, 464–475 (2000). [DOI] [PubMed] [Google Scholar]
- Nehlig A., Daval J. L. & Debry G. Caffeine and the central nervous system: mechanisms of action, biochemical, metabolic and psychostimulant effects. Brain Res. Rev. 17, 139–170 (1992). [DOI] [PubMed] [Google Scholar]
- Hasegawa H. et al. Acute dopamine/norepinephrine reuptake inhibition increases brain and core temperature in rats. J. Appl. Physiol. 99, 1397–1401 (2005). [DOI] [PubMed] [Google Scholar]
- Sidhpura N., Redfern P., Rowley H., Heal D. & Wonnacott S. Comparison of the effects of bupropion and nicotine on locomotor activation and dopamine release in vivo. Biochem. Pharmacol. 74, 1292–1298 (2007). [DOI] [PubMed] [Google Scholar]
- Piacentini M. F. et al. Effect of bupropion on hippocampal neurotransmitters and on peripheral hormonal concentrations in the rat. J. Appl. Physiol. 95, 652–656 (2003). [DOI] [PubMed] [Google Scholar]
- Bredeloux P., Dubuc I. & Costentin J. Comparisons between bupropion and dexamphetamine in a range of in vivo tests exploring dopaminergic transmission. Br. J. Pharmacol. 150, 711–719 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobre A. C., Gitelman D. R., Dias E. C. & Mesulam M. M. Covert visual spatial orienting and saccades: overlapping neural systems. Neuroimage 11, 210–216 (2000). [DOI] [PubMed] [Google Scholar]
- Posner M. I. Orienting of attention. Q. J. Exp. Psychol. 32, 3–25 (1980). [DOI] [PubMed] [Google Scholar]
- Bense S. et al. Brainstem and cerebellar fMRI-activation during horizontal and vertical optokinetic stimulation. Exp. Brain Res. 174, 312–323 (2006). [DOI] [PubMed] [Google Scholar]
- Garbutt S., Harwood M. & Harris C. Comparison of the main sequence of reflexive saccades and the quick phases of optokinetic nystagmus. Br. J. Ophthalmol. 85, 1477–1483 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidhu S. K., Bentley D. J. & Carroll T. J. Locomotor exercise induces long-lasting impairments in the capacity of the human motor cortex to voluntarily activate knee extensor muscles. J. Appl. Physiol. 106, 556–565 (2009). [DOI] [PubMed] [Google Scholar]
- Nybo L., Nielsen B., Blomstrand E., Møller K. & Secher N. Neurohumoral responses during prolonged exercise in humans. J. Appl. Physiol. 95, 1125–1131 (2003). [DOI] [PubMed] [Google Scholar]
- Nybo L., Dalsgaard M. K., Steensberg A., Møller K. & Secher N. H. Cerebral ammonia uptake and accumulation during prolonged exercise in humans. J. Physiol. 563, 285–290 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer B., Gezeck S. & Hartnegg K. The analysis of saccadic eye movements from gap and overlap paradigms. Brain Res. Protoc. 2, 47–52 (1997). [DOI] [PubMed] [Google Scholar]
- Connell C. J. W., Thompson B., Kuhn G. & Gant N. Exercise-induced fatigue and caffeine supplementation affect psychomotor performance but not covert visuo-spatial attention. PLoS ONE 11, e0165318, doi: 10.1371/journal.pone.0165318 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peelen M. V., Heslenfeld D. J. & Theeuwes J. Endogenous and exogenous attention shifts are mediated by the same large-scale neural network. Neuroimage 22, 822–830 (2004). [DOI] [PubMed] [Google Scholar]
- Thiel C. M., Zilles K. & Fink G. R. Nicotine modulates reorienting of visuospatial attention and neural activity in human parietal cortex. Neuropsychopharmacology 30, 810–820 (2005). [DOI] [PubMed] [Google Scholar]
- Roy-Byrne P., Radant A., Wingerson D. & Cowley D. S. Human oculomotor function: reliability and diurnal variation. Biol. Psychiatry 38, 92–97 (1995). [DOI] [PubMed] [Google Scholar]
- Hutton S. B. et al. Smooth pursuit and saccadic abnormalities in first-episode schizophrenia. Psychol. Med. 28, 685–692 (1998). [DOI] [PubMed] [Google Scholar]
- Ettinger U. et al. Reliability of smooth pursuit, fixation, and saccadic eye movements. Psychophysiology 40, 620–628 (2003). [DOI] [PubMed] [Google Scholar]
- Allman A.-A., Ettinger U., Joober R. & O’Driscoll G. A. Effects of methylphenidate on basic and higher-order oculomotor functions. J. Psychopharmacol. 26, 1471–1479 (2012). [DOI] [PubMed] [Google Scholar]
- Benjamini Y., Drai D., Elmer G., Kafkafi N. & Golani I. Controlling the false discovery rate in behavior genetics research. Behav. Brain Res. 125, 279–284 (2001). [DOI] [PubMed] [Google Scholar]
- Fisone G., Borgkvist A. & Usiello A. Caffeine as a psychomotor stimulant: mechanism of action. Cell. Mol. Life Sci. 61, 857–872 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curthoys I. S. Generation of the quick phase of horizontal vestibular nystagmus. Exp. Brain Res. 143, 397–405 (2002). [DOI] [PubMed] [Google Scholar]
- Kashou N. H. et al. Instruction dependent activation during optokinetic nystagmus (OKN) stimulation: an fMRI study at 3T. Brain Res. 1336, 10–21 (2010). [DOI] [PubMed] [Google Scholar]
- McDowell J. E., Dyckman K. A., Austin B. P. & Clementz B. A. Neurophysiology and neuroanatomy of reflexive and volitional saccades: evidence from studies of humans. Brain Cogn. 68, 255–270 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K. & Hikosaka O. Role of dopamine in the primate caudate nucleus in reward modulation of saccades. J. Neurosci. 26, 5360–5369 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kori A. et al. Eye movements in monkeys with local dopamine depletion in the caudate nucleus. II. Deficits in voluntary saccades. J. Neurosci. 15, 928–941 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman S. E. & Holtzman D. M. Molecular neuropharmacology: a foundation for clinical neuroscience. Third edn, (New York: The McGraw-Hill Companies, Inc. 2015, 2014). [Google Scholar]
- Dorris M. C., Pare M. & Munoz D. P. Neuronal activity in monkey superior colliculus related to the initiation of saccadic eye movements. J. Neurosci. 17, 8566–8579 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorris M. C. & Munoz D. P. A neural correlate for the gap effect on saccadic reaction times in monkey. J. Neurophysiol. 73, 2558–2562 (1995). [DOI] [PubMed] [Google Scholar]
- Everling S. & Munoz D. P. Neuronal correlates for preparatory set associated with pro-saccades and anti-saccades in the primate frontal eye field. J. Neurosci. 20, 387–400 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell A. H., Everling S. & Munoz D. P. Influence of stimulus eccentricity and direction on characteristics of pro- and antisaccades in non-human primates. J. Neurophysiol. 84, 2595–2604 (2000). [DOI] [PubMed] [Google Scholar]
- Reuter-Lorenz P. A., Hughes H. C. & Fendrich R. The reduction of saccadic latency by prior offset of the fixation point: an analysis of the gap effect. Percept. Psychophys. 49, 167–175 (1991). [DOI] [PubMed] [Google Scholar]
- Hutton S. B. Cognitive control of saccadic eye movements. Brain Cogn. 68, 327–340 (2008). [DOI] [PubMed] [Google Scholar]
- Nybo L. & Secher N. H. Cerebral perturbations provoked by prolonged exercise. Prog. Neurobiol. 72, 223–261 (2004). [DOI] [PubMed] [Google Scholar]
- Backhouse S. H., Biddle S. J., Bishop N. C. & Williams C. Caffeine ingestion, affect and perceived exertion during prolonged cycling. Appetite 57, 247–252 (2011). [DOI] [PubMed] [Google Scholar]
- Johnston K. & Everling S. Neurophysiology and neuroanatomy of reflexive and voluntary saccades in non-human primates. Brain Cogn. 68, 271–283 (2008). [DOI] [PubMed] [Google Scholar]
- Thier P. & Ilg U. J. The neural basis of smooth-pursuit eye movements. Curr. Opin. Neurobiol. 15, 645–652 (2005). [DOI] [PubMed] [Google Scholar]
- Lencer R. & Trillenberg P. Neurophysiology and neuroanatomy of smooth pursuit in humans. Brain Cogn. 68, 219–228 (2008). [DOI] [PubMed] [Google Scholar]
- Rucker J. C. in Handbook of Clinical Neurophysiology Vol. 9 (eds Scott Eggers, D. Z. & David Zee, S.) 18–42 (Elsevier, 2010).