Abstract
Objective.
Whether patients with type 2 diabetes change their lifestyle in response to their diagnosis and maintain behavior changes is unclear. This study aimed to 1) compare changes in lifestyle behaviors among participants who were newly diagnosed with type 2 diabetes and those never diagnosed with type 2 diabetes and 2) investigate changes in lifestyle behaviors in relation to the duration of newly diagnosed type 2 diabetes.
Methods.
We used self-reported information from the New South Wales 45 and Up Study and a follow-up study. Changes in body weight; amount of walking, moderate to vigorous physical activity (MVPA), and sitting; fruit and vegetable consumption; and smoking status and number of cigarettes smoked were used as measures of health behavior change. These variables were compared between participants in a “new type 2 diabetes” group and a “no type 2 diabetes” group.
Results.
The new type 2 diabetes group had a smaller decrease in vegetable consumption, lost more weight, and were more likely to quit smoking than the no type 2 diabetes group. MVPA, fruit consumption, and number of cigarettes smoked did not change significantly for either group. Although no significant changes were found in any of the health behaviors based on time since diagnosis, the magnitude of changes in weight and walking increased as duration of diagnosis increased, whereas changes in MVPA, number of cigarettes smoked, and proportion of participants who quit smoking decreased.
Conclusion.
In this population-based study, participants with incident type 2 diabetes reported only minimal changes in their lifestyle factors after receiving their diagnosis.
Type 2 diabetes is a lifelong disease and can lead to severe complications (1) and increased risk for mortality (2). Strict glucose control can delay or prevent the progression of complications associated with diabetes (1,3,4), and there is also substantial evidence that leading a healthy lifestyle, including following a healthy diet, achieving modest weight loss, and performing regular physical activity can maintain healthy blood glucose levels and reduce the risk of complications of type 2 diabetes (5,6).
Long-term diabetes management involves key health behaviors such as physical activity, healthy eating, weight management, and smoking cessation (7,8). Regular physical activity can help prevent the onset of type 2 diabetes, reduce the risk of complications (9), and improve blood pressure control in people with type 2 diabetes (10). Moderate to vigorous physical activity (MVPA) reduces the risk of all-cause and cardiovascular mortality among those with type 2 diabetes, independent of their BMI, blood pressure, total cholesterol level, and smoking status (2). Weight management (11) and dietary modification such as adoption of a Mediterranean or low–glycemic index eating pattern (12,13) have been shown to be effective in improving markers of cardiovascular disease (CVD) risk. Smoking is a well-established risk factor for CVD, and people with type 2 diabetes should be advised to quit smoking (14).
In Australia, it is recommended that all people diagnosed with type 2 diabetes be referred to structured diabetes patient education programs that are evidence-based, culturally sensitive, and delivered by trained educators either individually or in a group setting (15). There is good evidence that structured diabetes patient education improves patients’ knowledge and understanding of their condition and has a positive effect on changing dietary habits (14). Patient education also may increase the frequency of physical activity and is effective in helping patients quit smoking, but these effects may only be short term (14). Overall, whether patients with type 2 diabetes change their lifestyle in response to their diagnosis and maintain behavior changes is unclear. A Canadian study (16) reported that 60% of people aged 35–64 years with diabetes (type 1 or type 2) were not achieving the recommended amount of physical activity.
One other important factor that may also affect the sustainability of lifestyle behavioral changes is the time since diagnosis (i.e., duration of living with diabetes). Receiving a diabetes diagnosis from a health professional may increase a person’s awareness of the need for lifestyle changes (17) and can be a motivating factor for making them (18). However, in patients diagnosed with CVD who were initially motivated to undertake physical exercise, the motivation faded with time (19). Thus, maintaining such lifestyle changes may be difficult.
Using data from a large cohort study in New South Wales (NSW), Australia, we aimed to 1) compare changes in lifestyle behaviors among participants who were newly diagnosed with type 2 diabetes to those who had no diagnosis of type 2 diabetes and 2) investigate changes in lifestyle behaviors in relation to duration of newly diagnosed type 2 diabetes.
Methods
Study Population
Participants were drawn from the baseline 45 and Up Study and the Social, Economic and Environmental Factors (SEEF) Study. The 45 and Up Study is a population-based cohort survey of NSW residents ≥45 years of age. Recruitment was undertaken between 2006 and 2009. Potential participants were randomly selected from the database of Medicare Australia, the country’s universal public health insurance system. Participants joined the study by completing a mailed self-administered questionnaire and providing consent for long-term follow-up, including linkage to personal health records. The full study cohort consists of 267,153 people ≥45 years of age at the time of recruitment. The response rate was 18%, and participants comprised 11% of the NSW population aged ≥45 years (20).
In 2010, the SEEF Study questionnaire was distributed to the first 100,000 participants in the 45 and Up Study, of whom 60,404 returned the completed questionnaire (response rate 60.4%). The average follow-up period was 3.3 ± 0.9 years (median 2.8 years, range 1.7–5.1 years, interquartile range 2.6–4.6 years). Questionnaires for both the 45 and Up Study and the SEEF Study are available from the Sax Institute website (21).
The baseline 45 and Up Study and the SEEF Study were approved by the University of New South Wales Human Research Ethics Committee and the University of Sydney Human Research Ethics Committee, respectively.
Measures
Outcome Variables
Body weight, walking (min/week), MVPA (min/week), sitting (h/week), fruit and vegetable consumption (servings/day), smoking status (smoker or nonsmoker), and number of cigarettes smoked per day were measured both at baseline and in the follow-up survey. Physical activity was assessed using the Active Australia Survey (22), which has acceptable reliability (23) and validity (24). In this instrument, walking is defined as walking for recreation or exercise or to get to or from places. Vigorous physical activity refers to any activity that causes a participant to breathe harder or puff and pant. Moderate physical activity refers to gentle exercise such as gentle swimming, social tennis, vigorous gardening, or work around the house. Total minutes of MVPA per week is calculated by adding minutes of walking, minutes of moderate physical activity, and twice the minutes of vigorous physical activity (22). Reported time spent on walking and MVPA >14 h/day was recoded to 14 h to avoid measurement error due to over-reporting (25).
Independent Variables
The main independent variables were incident or new cases of type 2 diabetes and time since the incident diagnosis of type 2 diabetes. New cases were defined as those participants who did not report type 2 diabetes at the baseline survey but reported it in the follow-up survey (new type 2 diabetes group). The comparator group was participants who did not report type 2 diabetes at baseline or in the follow-up survey (no type 2 diabetes group). The questions asked to determine a diagnosis of type 2 diabetes at baseline were “Has a doctor ever told you that you have diabetes?” and “Have you taken Diabex, Diaformin, or Metformin for most of the last 4 weeks?”
Participants who reported that they had been told by a doctor that they had diabetes were then also asked their age at diagnosis. For new type 2 diabetes participants, the duration of time since diagnosis to completion of the SEEF Study questionnaire was also calculated (age at type 2 diabetes diagnosis minus age at time of completion of SEEF Study questionnaire). Self-reported diagnosis of type 2 diabetes in the 45 and Up Study has high sensitivity (83.7%) and specificity (97.7%) compared to administrative hospitalization data (26).
Covariates
Participant-reported sociodemographic characteristics, including age, sex, educational attainment (university/technical and further education [TAFE], high school, or <10 years of schooling), family history of type 2 diabetes (yes or no), and country of birth (English-speaking countries, Europe, Middle East, Asia, or other), which were included as covariates in the regression models. Also included as covariates in the model were physical functioning as measured using the Medical Outcomes Study Physical Functioning Scale (scores ranging from 0 to 100 and categorized as no limitation [100], minor limitation [95–99], moderate limitation [85–94], or severe limitation [0–84]) (27); psychological distress as measured by the Kessler-10 (K10; a K10 score of ≥22 reflects high or very high psychological distress [28]); and area-level deprivation as measured by the 2006 Index of Relative Socio-Economic Disadvantage (IRSED) quintiles at the postcode level. (The IRSED was created by the Australian Bureau of Statistics to compare social and economic disadvantage across geographical areas in Australia. The index is derived from 2006 Census variables such as low income and educational attainment, high unemployment, and employment in unskilled occupations [29]).
Exclusion Criteria
A total of 1,333 participants were excluded because of inconsistencies in reporting. Of these, 440 participants reported type 2 diabetes at baseline but not at the follow-up study, 139 did not report a diagnosis of type 2 diabetes at baseline but reported the use of diabetes medication for the most of the past 4 weeks, and 754 reported type 2 diabetes in the follow-up study but the age at diagnosis was either before the year they completed the baseline study (n=501) or not reported at all (n=253). In addition, 4,213 participants who reported type 2 diabetes in both surveys (prevalent cases) were also excluded. This study included 54,858 participants. Because type 2 diabetes may lead to physical disability, participants with physical functioning scores between 0 and 84 (n = 13,707) were identified as having a severe level of physical functional limitation and were excluded from examinations of changes in weight, walking, MVPA, and sitting.
Statistical Analysis
We initially used analysis of covariance, with baseline values as covariates, to examine differences in baseline–to–follow-up changes in outcome variables between groups (new type 2 diabetes vs. no type 2 diabetes). Separate multivariate linear regression models were used to examine the relationship between type 2 diabetes status and change in outcome variables from baseline to follow-up, except for the smoking cessation outcome (yes or no), for which we used logistic regression. Similar multivariate regression models were used to examine the relationship between the duration of newly diagnosis type 2 diabetes and change in outcome variables. Actual change values (i.e., marginal means), prevalence estimates (i.e., predicted population margins), and associated 95% CIs are reported, where appropriate.
All regression models were adjusted for age, sex, educational attainment, country of birth, physical functioning, psychological distress, IRSED score, duration of time since type 2 diabetes diagnosis, and baseline lifestyle variables. Baseline lifestyle variables included were weight (kg), walking (min/week), MVPA (min/week), sitting (h/week), fruit and vegetable consumption (servings/day), and number of cigarettes smoked per day at baseline. Statistical analyses were conducted using SAS version 9.4 (SAS Institute, Cary, N.C.).
Results
Of the 54,858 participants, 53,970 (98.4%) did not report type 2 diabetes at baseline or follow-up (no type 2 diabetes group). A total of 888 reported type 2 diabetes in the follow-up survey but not in the baseline survey (new type 2 diabetes group). The average duration of time since diagnosis was 1.8 ± 1.1 years (median 1.7 years, interquartile range 0.0–5.1 years).
More than half (54.5%) of all the participants were female, and the average age of participants was 61.8 ± 10.5 years. The majority of participants were born in an English-speaking country, and about one-third had not completed a high school education (Table 1).
TABLE 1.
Baseline Characteristics of the Study Population (n = 54,858)
| Total (n [%]) | Type 2 Diabetes Status |
P* | ||
|---|---|---|---|---|
| No(median [range]) | New†(median [range]) | |||
| Age (years) | 60.3 (45.1–101.4) | 62.5 (45.6–90.0) | <0.0001 | |
| No (%) | New† (%) | |||
| Sex | <0.0001 | |||
| Male | 24,980 (45.5) | 98.0 | 2.0 | |
| Female | 29,878 (54.5) | 98.7 | 1.3 | |
| Country of birth (missing = 499) | 0.088 | |||
| English-speaking country | 49,349 (90.8) | 98.4 | 1.6 | |
| Europe | 2,707 (5.0) | 98.0 | 2.0 | |
| Asia | 1,243 (2.3) | 98.1 | 1.9 | |
| Other | 783 (1.4) | 97.4 | 2.4 | |
| Highest education (missing = 696) | <0.0001 | |||
| University/TAFE | 27,097 (50.0) | 98.7 | 1.3 | |
| High school | 10,710 (19.8) | 98.1 | 1.9 | |
| Less than 10 years of schooling | 16,355 (30.2) | 98.0 | 2.0 | |
| Family history of type 2 diabetes (missing = 5) | <0.0001 | |||
| Yes | 11,040 (20.1) | 97.2 | 2.8 | |
| No | 43,813 (79.9) | 98.7 | 1.3 | |
| IRSED (missing = 52) | 0.001 | |||
| Most disadvantaged group | 10,748 (19.6) | 98.1 | 1.9 | |
| 2nd disadvantaged group | 11,069 (20.2) | 98.3 | 1.7 | |
| 3rd disadvantaged group | 10,778 (19.7) | 98.3 | 1.7 | |
| 4th disadvantaged group | 10,845 (19.8) | 98.2 | 1.8 | |
| Least disadvantaged group | 11,366 (20.7) | 98.9 | 1.1 | |
| Moderate/severe psychological distress (missing = 1,214) | <0.0001 | |||
| Yes | 1,923 (3.6) | 97.1 | 2.9 | |
| No | 51,721 (96.4) | 98.4 | 1.6 | |
| Physical functional limitation (missing = 4,709) | <0.0001 | |||
| Severe | 13,707 (27.3) | 97.6 | 2.4 | |
| Moderate | 9,802 (19.6) | 98.1 | 1.9 | |
| Minor | 9,364 (18.7) | 98.7 | 1.3 | |
| None | 17,276 (34.5) | 99.1 | 0.9 | |
Incident or new cases (participants who did not report type 2 diabetes at baseline but did report type 2 diabetes at the follow-up survey).
P values determined through χ2 tests except for age, for which a Kruskal-Wallis test was used.
Participants in the new type 2 diabetes group were more likely to be male and older and to have lower educational attainment; they were less likely to be born in an English-speaking country and more likely to live in more disadvantaged areas, reported more psychological distress, and had more severe physical functional limitation compared to participants in the no type 2 diabetes group (Table 1). After excluding participants with severe physical functional limitation (n = 16,256), the participants in the new type 2 diabetes group still had similar characteristics.
Table 2 presents differences in behavioral outcomes between the baseline and follow-up surveys after adjusting for the baseline values of behavioral outcomes and covariates. The second column shows the mean change in outcome variables for the no type 2 diabetes group. The third column shows the mean change in outcome variables for new type 2 diabetes group. The fourth column shows the mean difference in changes in outcome variables between the two groups.
TABLE 2.
| Type 2 Diabetes |
New vs. No Diabetes Groups(mean change [95% CI]; P) | ||
|---|---|---|---|
| No (mean change [95% CI]; P) | New†(mean change [95% CI]; P) | ||
| Body weight (kg) | 0.69 (0.43–0.96); <0.0001 | –0.67 (–1.21 to –0.14); 0.014 | –1.38 (–1.85 to –0.89); <0.0001 |
| Sitting (h/day) | –0.55 (–0.70 to –0.42); <0.0001 | –0.36 (–0.61 to –0.10); 0.006 | 0.19 (–0.03 to 0.42); 0.101 |
| Walking (min/week) | 2.08 (–8.63 to 12.79); 0.703 | 12.87 (–8.14 to 33.88); 0.228 | 10.79 (–7.82 to 29.39); 0.256 |
| MVPA (min/week) | 10.44 (–20.56 to 41.44); 0.509 | –2.34 (–64.01 to 59.33); 0.941 | –11.51 (–66.36 to 43.34); 0.680 |
| Fruit consumption (servings/day) | –0.14 (–0.18 to –0.10); <0.0001 | –0.08 (–0.16 to –0.003); 0.043 | 0.05 (–0.02 to 0.12); 0.148 |
| Vegetable consumption (servings/day) | –0.45 (–0.54 to –0.36); <0.0001 | –0.24 (–0.42 to –0.09); 0.011 | 0.21 (0.05–0.38); 0.011 |
| Cigarettes smoked (n/day)‡ | –0.41 (–1.41 to 0.61); 0.469 | –2.23 (–4.62 to 0.17); 0.069 | –1.82 (–4.03 to 0.39); 0.107 |
| No (%) | New (%) | New vs. No Diabetes Groups (odds of quitting [95% CI]; P) | |
| Cessation of smoking§ | 30.8 | 50.0 | 2.71 (1.59–4.63); 0.0002 |
Mean adjusted for baseline and demographic characteristics.
Incident or new cases (participants who did not report type 2 diabetes at the baseline survey but did report type 2 diabetes at the follow-up survey).
Among participants who reported smoking at both the baseline and follow-up surveys.
Among participants who reported smoking at the baseline survey.
Both the no type 2 diabetes group and the new type 2 diabetes group showed significant decreases in the number of hours spent sitting and in fruit and vegetable consumption at follow-up. However, the difference between the two groups was only significant for vegetable consumption, for which the no group had a larger decrease than the new group. During the follow-up period, participants in the no group gained ∼0.7 kg of body weight, whereas those in the new group lost a similar amount of weight. The between-group difference in weight change was significant. Physical activity did not change significantly for either group. Participants in the new type 2 diabetes group who were smokers at baseline had almost three times the odds of quitting at follow-up compared to those in the no type 2 diabetes group who smoked at baseline, although the daily number of cigarettes smoked did not change significantly among those who did not quit (Table 2).
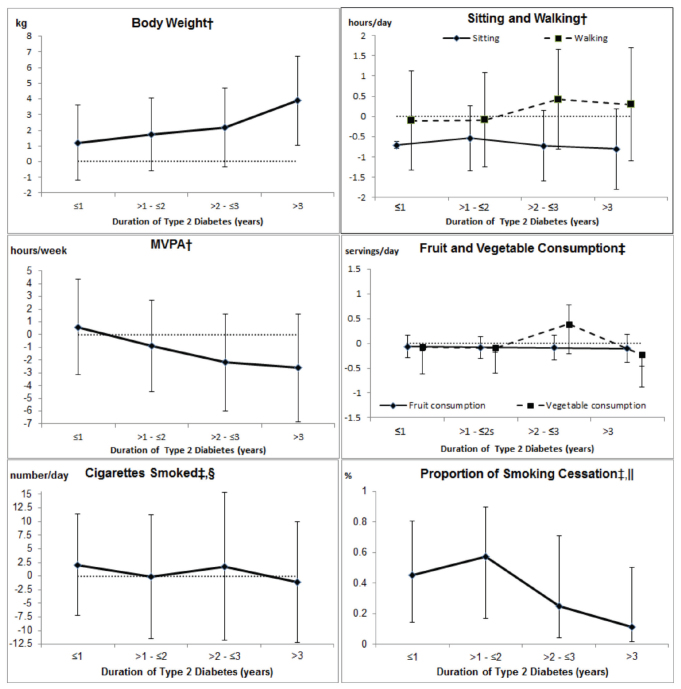
For the new type 2 diabetes group, Figure 1 presents the marginal means, prevalence, and associated 95% CIs for body weight, number of hours spent sitting, amounts of walking and MVPA, fruit and vegetable consumption, and number of cigarettes smoked daily by duration of type 2 diabetes diagnosis. There were no significant changes in weight (except at a duration of type 2 diabetes >3 years), number of hours spent sitting, amount of walking and MVPA, fruit and vegetable consumption, or daily cigarettes smoked by duration of type 2 diabetes diagnosis. For example, there were no significant changes in weight at follow-up at each category of type 2 diabetes duration, except for weight when the duration of type 2 diabetes was >3 years. Participants who had a diagnosis of type 2 diabetes for >3 years had significant weight gain at follow-up. Participants in the new type 2 diabetes group were significantly more likely to report quitting smoking at every time point, although the proportion of quitters decreased with increasing duration of type 2 diabetes.
FIGURE 1.
Change (with 95% CI) in outcomes by duration of type 2 diabetes. *Among incident or new cases (participants who did not report type 2 diabetes at the baseline survey but did report type 2 diabetes at the follow-up survey). †Adjusted for age, sex, educational attainment, country of birth, level of physical functional limitation, psychological distress, IRSED, duration of type 2 diabetes diagnosis, and baseline lifestyle variables. ‡Adjusted for age, sex, educational attainment, country of birth, psychological distress, IRSED, duration of type 2 diabetes diagnosis, and baseline lifestyle variables. §Among participants who reported smoking at both the baseline and follow-up surveys. ||Among participants who reported smoking at the baseline survey.
Discussion
The primary aim of our study was to compare changes in lifestyle behaviors between participants with newly diagnosed type 2 diabetes (new type 2 diabetes group; incident cases) and those never diagnosed with type 2 diabetes (no type 2 diabetes group). When compared to participants without a diagnosis of type 2 diabetes, those with type 2 diabetes reported significantly more weight loss and a smaller decrease in vegetable consumption and, importantly, were more likely to quit smoking. There were no significant differences in the amount of MVPA, walking, or sitting between the two groups.
In Australia, it is recommended that all people with new diagnosis of type 2 diabetes be referred for structured diabetes patient education because there is good evidence that such education improves knowledge and understanding of type 2 diabetes and has a positive effect on changing dietary habits, increasing physical activity, and quitting smoking (14). There have also been initiatives based on financial incentives to general practitioners and other health professionals to encourage implementation of care processes that include an annual cycle of diabetes care comprising at least twice yearly weight, height, and blood pressure measurements and feet examinations; at least yearly lipid, A1C, and renal function assessments; annual medication, self-care education, and risk factor review; and eye examination every 2 years. These incentives, supported by education and system support through primary care organizations, aim to encourage implementation of best-practice standards of care (30).
Despite these nationwide initiatives to improve type 2 diabetes outcomes, the prevalence of inadequate physical activity, smoking, and inadequate consumption of fruits and vegetables in people with type 2 diabetes remains high. In our study, any significant differences between the new type 2 diabetes group and the no type 2 diabetes group were small, despite existing guidelines for optimal type 2 diabetes management. This may be the result of considerable variation in the implementation of processes of diabetes care in clinical practice (31).
We also examined the relationship between the duration of type 2 diabetes and change in lifestyle behaviors among newly diagnosed participants. Previous studies have provided conflicting evidence on the onset and sustainability of behavior changes. Receiving a diagnosis of diabetes or impaired glucose tolerance from a health professional is a strong motivation for lifestyle change (17,18). However, it is unclear how successfully these changes are sustained. It is likely that positive lifestyle changes are more likely to occur early in the diagnosis of type 2 diabetes.
In our study, there was a gradual small increase in weight with increasing duration of type 2 diabetes and a notable increased chance for smoking cessation in those who had a diagnosis of type 2 diabetes for 1–2 years compared to those with a duration of diabetes ≤1 year. However, this increased chance of quitting smoking was no longer evident in participants who had a diagnosis of type 2 diabetes for >3 years, implying that smoking cessation generally occurs mainly within the first 2 years after diabetes diagnosis. This is congruent with a study showing that, in patients initially diagnosed with cardiac disease, the motivation for undertaking physical activity decreased with time (19). The salient implication is that positive behavioral changes are difficult to sustain in the long term, which highlights the need for clinicians to provide support for patients in setting long-term goals to achieve a lasting healthy lifestyle. Frequent and long-lasting contact with multidisciplinary clinicians is a strong motivation for sustained behavior change (18).
We found decreasing MVPA, increasing weight, and minimal changes in fruit and vegetable consumption with increasing time since diagnosis. The reasons for these relationships are complex. Psychological factors may be one possible explanation for our negative findings (32). The benefits of physical activity are mainly long term rather than immediate (33). Furthermore, campaigns primarily aimed at providing knowledge about the health benefits of fruit and vegetable consumption have failed to increase vegetable and fruit intake at a population level, suggesting that knowledge alone may not be sufficient to trigger behavioral change at a population level (34). Continuing individual professional support is important to target patients’ psychological barriers, especially with regard to perseverance with physical activity regimens and healthy eating patterns. Continuing support to develop self-motivational strategies is also important so that healthy lifestyle changes can become lifelong behaviors (18).
The strength of this study is that we had a large population-based sample with a comprehensive list of lifestyle factors and potential confounders at the individual level. A limitation is our reliance on self-reported type 2 diabetes status, physical activity, and sedentary behavior. Diagnostic or clinical information was not available to confirm participants’ diagnoses of type 2 diabetes. However, we are confident that the self-reporting of type 2 diabetes was accurate because, in this population, self-reported diagnosis of type 2 diabetes has been shown to have high sensitivity and specificity compared to hospital administrative data collections (26). Another limitation to this study is that we only had measurements at two time points over 2–5 years, which limited our ability to track changes in lifestyle behaviors over longer periods of time.
Conclusion
In participants with incident type 2 diabetes, there were positive changes in some lifestyle factors at the follow-up study (e.g., decreased duration of sitting and increased incidence of smoking cessation). However, there were no changes in some other important lifestyle factors (e.g., body weight, walking, MVPA, and fruit and vegetable consumption). No positive lifestyle changes were associated with increasing time since diagnosis; rather, body weight was found to increase, MVPA to decrease, and smoking cessation to become less likely with longer duration of diabetes. Providing intensive long-term diabetes management support, including tools for monitoring lifestyle factors, goal-setting, and frequent contact, for people with newly diagnosed type 2 diabetes may help them achieve and maintain positive lifestyle changes.
Duality of Interest
No potential conflicts of interest relevant to this article were reported.
References
- 1.American Diabetes Association Standards of Medical Care in Diabetes—2014. Diabetes Care 2014;37(Suppl. 1):S1–S80 [DOI] [PubMed] [Google Scholar]
- 2.Hu G, Jousllahti P, Barengo NC. Physical activity, cardiovascular risk factors, and mortality among Finnish adults with diabetes. Diabetes Care 2005;28:799–805 [DOI] [PubMed] [Google Scholar]
- 3.Miller KM, Beck RW, Bergenstral RM, et al.; T1D Exchange Clinic Network . Evidence of a strong association between frequency of self-monitoring of blood glucose and hemoglobin A1c levels in T1D Exchange clinic registry participants. Diabetes Care 2013;36;2009–2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008;59:1577–1589 [DOI] [PubMed] [Google Scholar]
- 5.Norris S, Zhang X, Anvenell A, et al. . Long-term effectiveness of weight-loss interventions in adults with pre-diabetes: a review. Am J Prev Med 2005;28:126–139 [DOI] [PubMed] [Google Scholar]
- 6.Klein S, Sheard N, Pi-Sunyer X, et al. . Weight management through lifestyle modification for the prevention and management of type 2 diabetes: rationale and strategies. A statement of the American Diabetes Association, the North American Assocation for the Study of Obesity, and the American Society for Clinial Nutrition. Am J Clin Nutr 2004;80:257–263 [DOI] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention National diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the United States, 2011. Atlanta, Ga, U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, 2011 [Google Scholar]
- 8.U.S. Department of Health and Human Services How tobacco smoke causes disease: the biology and behavioral basis for smoking-attributable disease. A report of the Surgeon General. Atlanta, Ga, Centers for Disease Control and Prevention, 2010 [PubMed] [Google Scholar]
- 9.Tuomileho J, Lindostrom J, Eriksson JG, Valle TT, Hamalainen H, Ilanne-Parikka P. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Eng J Med 2001;344:1343–1350 [DOI] [PubMed] [Google Scholar]
- 10.Castaneda C, Layne JE, Munoz-Orians L. A randomised controlled trial of resistance exercise training to improve glycemic control in older adults with type 2 diabetes. Diabetes Care 2002;25:2335–2341 [DOI] [PubMed] [Google Scholar]
- 11.Look AHEAD Research Group Long term effects of a lifestyle intervention on weight and cardiovascular risk factors in individuals with type 2 diabetes: four year results of the Look AHEAD Trial. Arch Intern Med 2010;170:1566–1575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ajala O, English P, Pinkney J. Systematic review and meta-analysis of different dietary approaches to the management of type 2 diabetes. Am J Clin Nutr 2013;97:505–516 [DOI] [PubMed] [Google Scholar]
- 13.Esposito K, Maiorino MI, Bellastella G, Chiodini P, Panagiotakos D, Giugliano D. A journey into a Mediterranean diet and type 2 diabetes: a systematic review with meta-analyses. BMJ Open 2015;5:e008222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Colagiuri R, Girgis S, Eigenmann C, Gomez M, Griffiths R. National evidence based guideline for patient education in type 2 diabetes: Diabetes Australia and the National Health and Medical Research Council, 2009. Available from http://static.diabetesaustralia.com.au/s/fileassets/diabetes-australia/b9b8789d-c7ba-473d-bd49-0b7d793a0835.pdf. Accessed 9 September 2016
- 15.Royal Australian College of General Practitioners, Diabetes Australia General practice management of type 2 diabetes: 2014–15. Available from https://static.diabetesaustralia.com.au/s/fileassets/diabetes-australia/5ed214a6-4cff-490f-a283-bc8279fe3b2f.pdf. Accessed 9 September 2016
- 16.Public Health Agency of Canada Diabetes in Canada: national statistics and opportunities for improved surveillance, prevention and control. Available from http://www.phac-aspc.gc.ca/cd-mc/diabetes-diabete/dic-dac-99/index-eng.php. Accessed 9 September 2016
- 17.Schneider KL, Andrews C, Hovey KM, et al. . Change in physical activity after a diabetes diagnosis: opportunity for intervention. Med Sci Sports Exerc 2014;46:84–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Penn L, Moffatt SM, White M. Participants’ perspective on maintaining behaviour change: a qualitative study within the European Diabetes Prevention Study. BMC Public Health 2008;8:235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Plotnikoff RC, Higginbotham N. Protection motivation theory and the prediction of exercise and low-fat diet behaviours among Australian cardiac patients. Psychol Health 1998;13:411–429 [Google Scholar]
- 20.Sax Institute Guidelines for authors regarding technical review of 45 and Up Study papers 2015. Available from http://saxinstitute.org.au/wp-content/uploads/Guidelines-for-authors-regarding-technical-review-of-45-and-Up-Study-papers.pdf. Accessed 11 January 2016
- 21.Sax Institute Questionnaires. Available from https://www.saxinstitute.org.au/our-work/45-up-study/questionnaires. Accessed 11 January 2016
- 22.Australian Institute of Health and Welfare The Active Australia survey: a guide and manual for implementation, analysis and reporting. Canberra, Australia, Australian Institute of Health and Welfare, 2003 [Google Scholar]
- 23.Brown WJ, Trost SG, Bauman A, Mummery K, Owen N. Test-retest reliability of four physical activity measures used in population surveys. J Sci Med Sport 2004;7:205–215 [DOI] [PubMed] [Google Scholar]
- 24.Heesch HC, Hill RL, Van Uffelen JG, Brown WJ. Are Active Australia physical activity questions valid for older adults? J Sci Med Sport 2011;14:233–237 [DOI] [PubMed] [Google Scholar]
- 25.Bauman A. Trends in exercise prevalence in Australia. Community Health Stud 1987;11:190–196 [DOI] [PubMed] [Google Scholar]
- 26.Comino EJ, Tran DT, Haas M, et al. . Validating self-report of diabetes use by participants in the 45 and Up study: a record linkage study. BMC Health Serv Res 2013;13:481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stewart A, Kamberg CJ. Physical functioniong measures. In Measuring Functioning and Well-Being: The Medical Outcomes Study Approach. Stewart A, Ware JE, Eds. Durham, N.C., Duke University Press, 1992, p. 86–101 [Google Scholar]
- 28.Brooks RT, Beard J. Factor structure and interpretation of the K10. Psychol Assess 2006;18:62–70 [DOI] [PubMed] [Google Scholar]
- 29.Australian Bureau of Statistics National health survey: summary of results, Australia 2004–05. Canberra, Australia, Australian Bureau of Statistics, 2006 [Google Scholar]
- 30.Brand D, Wright D. Chapter 11: quality and outcomes. In General Practice in Australia: 2004. Rea H, Ed. Canberra, Australia, Australian Department of Health and Ageing, 2005, p. 467–501 [Google Scholar]
- 31.Comino EJ, Islam MF, Tran DT, et al. . Association of process of primary care and hospitalisation of people with diabetes: a record linkage study. Diabetes Res Clin Pract 2015;108:296–305 [DOI] [PubMed] [Google Scholar]
- 32.Lin SP, Wang MJ. Strategic management of behavioural change in type 2 diabetic patients. Public Health 2012;126:18–24 [DOI] [PubMed] [Google Scholar]
- 33.Krug LM, Haire-Joshu D, Heady SA. Exercise habits and exercise relapse in persons with non-insulin-dependent diabetes mellitus. Diabetes Educ 1991;17:185–188 [DOI] [PubMed] [Google Scholar]
- 34.Whitehead RD, Ozakinci G, Stephen ID, Perrett DI. Appealing to vanity: could potential appearance improvement motivate fruit and vegetable consumption? Am J Public Health 2012;102:207–211 [DOI] [PMC free article] [PubMed] [Google Scholar]