Abstract
Ghrelin produced in the gut stimulates GH and ACTH secretion from the pituitary and also stimulates appetite and gastric emptying. We have shown that ghrelin also induces insulin resistance via GH-independent mechanisms, but it is unknown if this effect depends on ambient fatty acid (FFA) levels. We investigated the impact of ghrelin and pharmacological antilipolysis (acipimox) on insulin sensitivity and substrate metabolism in 8 adult hypopituitary patients on stable replacement with GH and hydrocortisone using a 2 × 2 factorial design: Ghrelin infusion, saline infusion, ghrelin plus short-term acipimox, and acipimox alone. Peripheral and hepatic insulin sensitivity was determined with a hyperinsulinemic euglycemic clamp in combination with a glucose tracer infusion. Insulin signaling was assayed in muscle biopsies. Peripheral insulin sensitivity was reduced by ghrelin independently of ambient FFA concentrations and was increased by acipimox independently of ghrelin. Hepatic insulin sensitivity was increased by acipimox. Insulin signaling pathways in skeletal muscle were not consistently regulated by ghrelin. Our data demonstrate that ghrelin induces peripheral insulin resistance independently of GH, cortisol, and FFA. The molecular mechanisms remain elusive, but we speculate that ghrelin is a hitherto unrecognized direct regulator of substrate metabolism. We also suggest that acipimox per se improves hepatic insulin sensitivity.
Ghrelin is the endogenous ligand for the growth hormone (GH) secretagogue receptor (GHS-R)1 and potently stimulates the release of GH and - to a lesser degree - ACTH from the anterior pituitary gland2.
It is well documented that GH is lipolytic and induces insulin resistance in skeletal muscle and liver3. It is therefore not unexpected that ghrelin administration in healthy subjects is associated with hyperglycemia and increased lipolysis4,5. The presence of GHS-R in skeletal muscle, adipose tissue, and liver6,7 suggests that ghrelin also exerts direct tissue effects. In support of this, we have previously demonstrated that ghrelin acutely induces insulin resistance in skeletal muscle independently of GH and cortisol8. We also observed that free fatty acid (FFA) concentrations and lipolysis increased in response to ghrelin administration8, which is noteworthy since FFAs are known to induce insulin resistance also in the context of GH exposure9.
The aim of the present study was to further investigate the direct peripheral effects of ghrelin on insulin sensitivity and substrate metabolism in the presence and absence of concomitant suppression of lipolysis by means of acipimox administration, which suppresses lipolysis and lowers serum FFA levels via inhibition of the hormone sensitive lipase (HSL)10. We studied hypopituitary patients on stable replacement therapy with GH and hydrocortisone in order to control for the effects of ghrelin on GH and ACTH release.
Research Design and Methods
The study was conducted in accordance with the Helsinki Declaration and all subjects gave their oral and written informed consent to participate. The local Ethics Committee and the Danish Medicines Agency approved the study protocol and the protocol was registered at Clinicaltrials.gov NCT01209416 before the onset of enrolment.
Preparation of synthetic ghrelin
Synthetic human acyl ghrelin (GMP-grade human acyl ghrelin; Bachem, Weil am Rhein, Germany) was dissolved in isotonic saline immediately before infusion. The infusion solution was formulated by the hospital pharmaceutical services and complied with GDP and GCP guidelines.
Subjects
Eight hypopituitary men on stable replacement therapy with daily sc GH injections in the evening and oral hydrocortisone for >6 months participated in the study (Table 1). GH deficiency was documented by GH stimulation tests (mean ± SE peak GH levels of 0.57 ± 0.21 (range: 0 to 1.61) μg/l). HbA1c at screening was 5.5 ± 0.1% (37 ± 1 mmol/mol). None of the patients had diabetes or any other concomitant chronic disease. The participants were 53 ± 5 years of age and had a BMI of 30.3 ± 4.6 kg/m2.
Table 1. Characterization of the subjects.
| Patient | Age yr | BMI kg/m2 | Diagnosis | Diagnostic test | GH peak μg/l | GH dose mg daily | HbA1c |
Insufficient pituitary axes | |
|---|---|---|---|---|---|---|---|---|---|
| mmol/mol | % | ||||||||
| 1 | 68 | 32.8 | Pituitary apoplexy | Insulin tolerance test | 0.08 | 0.15 | 34 | 5.3 | GH, T, C, Gn |
| 2 | 67 | 26.4 | Pituitary apoplexy | Insulin tolerance test | 0.99 | 0.4 | 41 | 5.9 | GH, T, C, Gn |
| 3 | 26 | 26.0 | Congenital hypopituitaism | GHRH plus arginine stimulation test | 1.61 | 0.3 | 36 | 5.4 | GH, T, C |
| 4 | 48 | 31.7 | Pituitary apoplexy | Insulin tolerance test | 0 | 0.5 | 39 | 5.7 | GH, T, C, Gn |
| 5 | 67 | 27.0 | Pituitary adenoma, surgical treatment and radiotherapy | Insulin tolerance test | 0.41 | 0.2 | 41 | 5.9 | GH, T, C, Gn |
| 6 | 52 | 30.4 | Clinically nonfunctioning pituitary adenoma | Arginine stimulation test | 1.12 | 0.3 | 40 | 5.8 | GH, T, C, Gn |
| 7 | 50 | 25.8 | Pituitary cyst, surgical treatment | Insulin tolerance test | 0.20 | 0.3 | 33 | 5.2 | GH, T, C, Gn |
| 8 | 42 | 42.0 | Traumatic brain injury | Arginine stimulation test | 0.13 | 0.3 | 30 | 4.9 | GH, T, C, Gn |
BMI, body mass index; IGF-I levels at baseline during GH substition. GH, growth hormone; T, thyrotropin; C, corticotropin; Gn, gonadotropin.
Study protocol
All participants were examined on four occasions in a 2 × 2 factorial design separated by a minimum of two weeks. The studies were performed in a quiet, thermoneutral indoor environment. The subjects fasted during the trials, but were allowed oral water intake. The patients emptied their urinary bladder before starting the metabolic study day. All patients continued replacement therapy with GH and hydrocortisone during the study; GH was administered subcutaneously at 2200 hr before the metabolic study day and hydrocortisone was administered at 0800 hr on the metabolic study day using the individual subjects normal replacement doses.
In a double-blind and placebo-controlled crossover study each subject underwent four randomized interventions: Ghrelin infusion (1 pmol/min/kg i.v.) and placebo capsules [Ghr], saline infusion and placebo capsules [Control], Ghrelin infusion and acipimox capsules [Ghr + Aci], and saline infusion and acipimox capsules [Aci]. The ghrelin dose of 1 pmol/min/kg was based on our experience from a previous experiment, where that dose increased FFA levels11.
In protocol arms Ghr + Aci and Aci, the patients received four doses of acipimox 250 mg, p.o., with two doses administered at 2000 and 2300 hr. the evening before and two doses administered at 0600 and 1000 hr. on the day of the metabolic study. In protocol arms Ghr and Control, the patients received placebo capsules at the same time points. All metabolic studies were performed between 0800 and 1300 hr. (0–300 min) after an overnight fast. One i.v. cannula was inserted into an antecubital region for infusion, and one i.v. cannula was positioned in a dorsal hand vein for blood sampling. The hand was placed in a heat pad in order to arterialize venous blood samples. At t = 0, acyl ghrelin or placebo [isotonic saline (‘Sal’)] infusions as well as a primed (12 μCi) continuous (12 μCi/h) infusion of [3-3H] glucose were commenced. The subjects were studied in the basal postabsorptive state (referred to as ‘basal’) for 120 min followed by a hyperinsulinemic/euglycemic clamp (referred to as ‘clamp’) for 180 min, during which they received a constant infusion of insulin (0.6 mU/kg/min; Actrapid, Novo Nordisk, Gentofte, Denmark). Serum insulin was measured at t = 240, 270 and 300 min to document that steady state conditions were achieved. During the insulin infusion, plasma glucose was clamped at ≈5.0 mmol/l by adjusting the rate of infusion of 20% glucose according to plasma glucose measurements carried out every 10 min. Insulin sensitivity was estimated by the level of glucose infusion rate (GIR) during the terminal 30 min of the hyperinsulinemic, euglycemic clamp. Additional blood samples were drawn at the time points as indicated by Figs 1 and 2 and analyzed for acyl and desacyl ghrelin, GH, cortisol, insulin, C-peptide, glucagon, and FFA. Glucose metabolism and indirect calorimetry were assessed during the terminal 30 minutes of both the basal and the clamp period. Skeletal muscle biopsies were obtained t = 30 and 150 min from the lateral vastus muscle with a Bergström biopsy needle under local anesthesia with lidocaine (Xylocain 10 mg/ml; AstraZeneca, Albertslund, Denmark). A total amount of approximately 200 mg muscle was aspirated. Subcutaneous periumbilical adipose tissue biopsies were taken by liposuction technique at t = 30 and 150 min after applying lidocaine as local anesthesia. Biopsies were immediately cleaned for blood, snap-frozen in liquid nitrogen, and stored at −80 °C until analyzed.
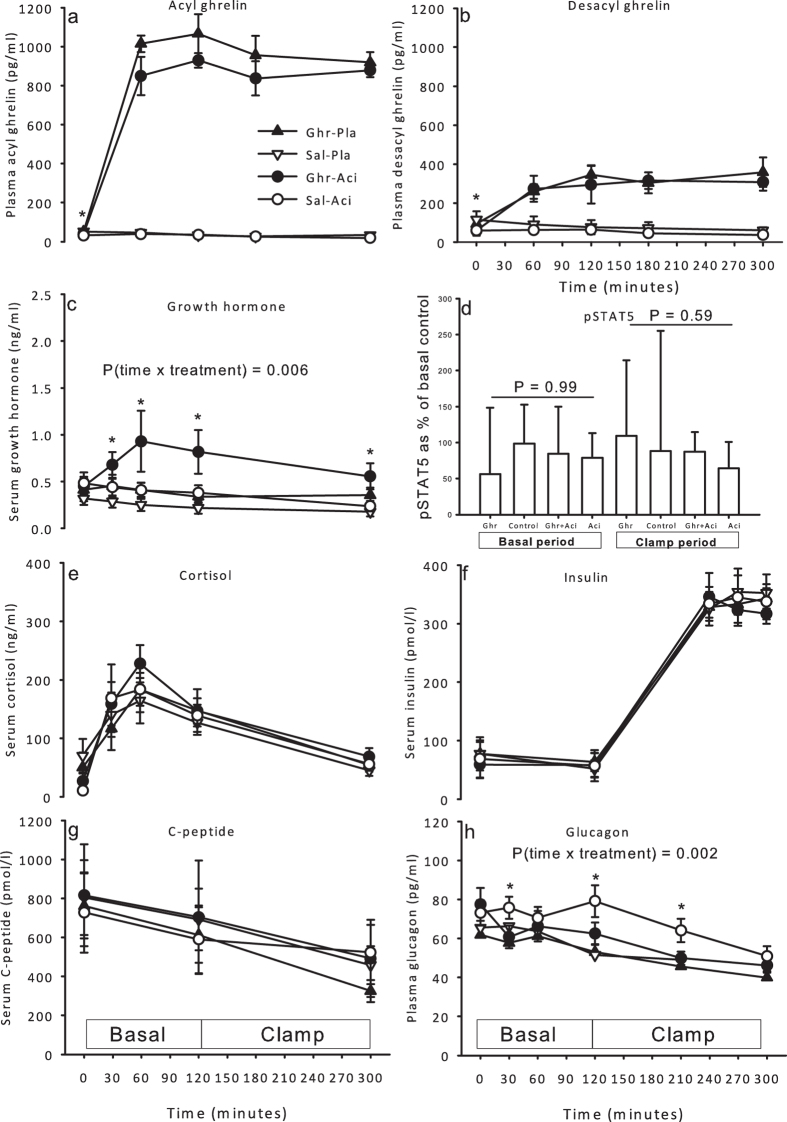
Figure 1. Hormones and phosphorylated STAT5 during ghrelin, saline, ghrelin and acipimox, and acipimox.
(a) Plasma levels of acyl ghrelin increased in response to ghrelin infusion. (b) Plasma levels of desacyl ghrelin at baseline were lower during acipimox treatment. Desacyl ghrelin concentrations increased in response to ghrelin infusion. (c) Serum levels of GH increased in response to ghrelin and acipimox treatment. (d) Relative levels of pSTAT5 content in skeletal muscle tissue in the basal and in the clamp period. pSTAT5 was similar during all conditions. (e) Serum levels of cortisol. (f) Serum levels of insulin. (g) Serum levels of C-peptide. (h) Plasma levels of glucagon increased initially during acipimox treatment and were normalized at the end of the clamp period. Printed P values refer to one-way ANOVA analyses or two-way analyses as indicated. *P < 0.05 at a given time point. All data are presented as mean ± SE.
Figure 2. Metabolites during ghrelin, saline, ghrelin and acipimox, and acipimox.
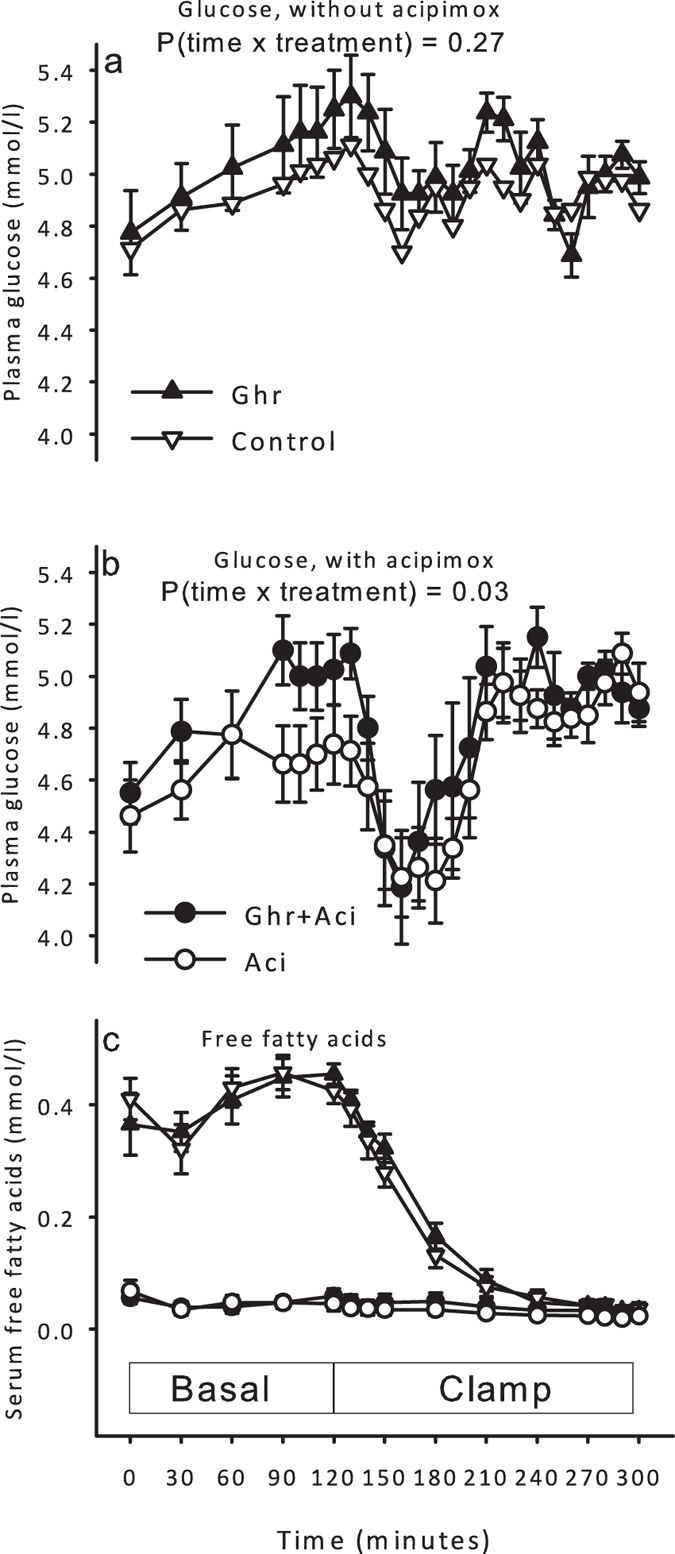
(a) Plasma glucose during ghrelin and saline infusion. Ghrelin did not impact on plasma glucose. (b) Plasma glucose increased during combined ghrelin and acipimox treatment as compared to acipimox-alone. (c) Serum free fatty acids. All data are presented as mean ± SE.
The patients voided at t = 120 and 300 min and the urine was measured by volume and a sample was stored at −20 °C for later analysis.
Biochemical analyses
Plasma glucose was analyzed bedside using the glucose oxidase method (YSI 2300 STAT Plus; YSI Life Sciences, Yellow Springs, OH). Serum and plasma samples were frozen and stored at −20 °C or at −80 °C (ghrelin and glucagon). Serum FFAs were analyzed by a commercial kit (Wako Chemicals, Neuss, Germany). Samples for plasma ghrelin measurements were drawn in 2 ml acidified EDTA prepared vacutainers with 20 μl 200 mg/ml AEBSF (Sigma-Aldrich Denmark A/S, Copenhagen, Denmark) and centrifuged immediately at 2,500 g for 10 minutes at 4 °C. AEBSF is a serine protease inhibitor, which is added to the collecting blood tubes to inhibit/reduce breakdown of acyl ghrelin. Plasma was then transferred to 1.8 ml tubes and stored at −80 °C until analysis. Plasma acyl and desacyl ghrelin were determined using ELISAs (Bertin Pharma, Montigny-le-Bretonneux, France; A05106 and A05119, respectively) using a modified protocol according to Delhanty et al.12 Serum GH was analyzed using chemiluminescence technology (IDS-iSYS Multi-Discipline Automated Analyzer, Immunodiagnostic Systems Nordic a/s Herlev, Denmark). Serum insulin was analyzed using time-resolved fluoroimmunoassay assay (AutoDELFIA Insulin kit, catalog no. B080–101, PerkinElmer, Turku, Finland). Serum cortisol was measured using a DRG ELISA kit (DRG Instruments GmbH, Marburg, Germany). The specific activity of [3-3H] glucose was determined as previously described13. Rates of glucose appearance (Ra) and disappearance (Rd) were calculated using Steele’s non-steady state equation using a pool fraction of 0.65. Endogenous glucose production (EGP) during the clamp, which is a measure of hepatic insulin sensitivity, was calculated by subtracting the glucose infusion rate from glucose Ra during the terminal 30 min of the clamp. Energy expenditure (EE) and respiratory exchange rate (RER) were calculated by indirect calorimetry using a computerized open circuit system (Deltatrac; Datex Instruments, Helsinki, Finland). Oxidation rates of glucose (GOX) and lipids were calculated from EE and RER after correction for protein oxidation, which was estimated from the urinary excretion of urea. Nonoxidative glucose disposal (NOGD) was calculated as whole body glucose disposal Rd minus the rate of GOX14.
Freeze dried muscle biopsies were homogenized at 4 °C in a buffer (pH 7.4) containing 50 mM HEPES, 137 mM NaCl, 10 mM Na4P2O7, 20 mM NaF, 5 mM EDTA, 1 mM MgCl2, 1 mM CaCl2, 2 mM sodium orthovanadate (NaOV), 5 mM nicotinamide (NAM), 10 μM trichostatin A (TSA), HALT Protease Inhibitor Cocktail, Nonidet P-40 (NP-40) 1%, and 10% glycerol. Samples were centrifuged at 14,000 g for 20 minutes.
Western blot analyses were used to measure phosphorylated and total levels of intracellular insulin and GH signaling proteins. The primary antibodies used were from Cell Signaling Technology, Danvers, MA (Akt, pAkt Ser473 and Thr308, pAS160 Thr642, STAT5, pSTAT5 Tyr694, Glycogen Synthase (GS), and pGS Ser641) and Merck Millipore, Darmstadt, Germany (AS160 and GLUT4). Control for equal loading was performed using the stain-free technology15. Proteins were visualized and quantified using Image Lab 5.0, Bio-Rad laboratories (BioRad, CA). Quantifications are expressed as the ratio between phosphorylated protein and the total protein measurement on the same membranes. Differences between interventions are expressed as the ratio change from the measurement made in the basal period of the placebo day for each subject.
Skeletal muscle glycogen content: Muscle samples were hydrolyzed in 2 M HCl at 100 °C for 2 h, followed by neutralization with 2 M NaOH16, and glucose content was measured by the hexokinase enzymatic method using a glucose hexokinase reagent (Eagle Diagnostics, Desoto, TX)17.
Statistical analysis
Results are expressed as mean ± standard error of the mean (mean ± SE) or median and 25–75 percentile. The statistical analyses were performed by using SigmaPlot 11.0 (©Systat Software, CA). A two way-ANOVA (time × treatment) for repeated measurements with Student-Newman-Keuls post-hoc analysis was used to test for significant differences in time series and the GIR. Concentrations at single time points were analyzed by a Student’s two-tailed paired t test or a one-way ANOVA depending on the number of variables or a Signed Rank Test, if data was not normally distributed. A P value <0.05 was considered significant.
Results
Hormones
Circulating hormone concentrations are shown in Fig. 1. Baseline plasma levels of ghrelin (pg/ml) were lower during acipimox: 49.6 ± 9.4 [Ghr], 51.9 ± 15.1 [Control], 39.4 ± 7.8 [Ghr + Aci], and 31.6 ± 8.2 [Aci] P = 0.045, and plasma ghrelin increased approximately 20 fold during ghrelin infusion (Fig. 1a). Baseline plasma levels of unacylated ghrelin (UAG, pg/ml) were also lower during acipimox: 93.4 ± 34.3 [Ghr], 115.4 ± 45.5 [Control], 58.8 ± 25.4 [Ghr + Aci], and 59.5 ± 20.6 [Aci] P = 0.02, and increased approximately 6 fold during ghrelin infusion (Fig. 1b). Serum GH levels increased slightly but significantly during Ghr + Aci (P = 0.006), but were not increased during Ghr alone (Fig. 1c). The small increase in GH levels during Ghr + Aci did not translate into detectable GH-signal transduction as measured by phosphorylation of STAT5 at Tyr694 in skeletal muscle tissue (Fig. 1d and Supplemental Figure S1). Serum cortisol levels were similar during all 4 conditions (P = 0.48, Fig. 1e). Serum insulin levels increased during the clamp, but were not affected by treatment (Fig. 1f), and serum C-peptide levels decreased during insulin infusion, but independently of treatment (Fig. 1g). Plasma glucagon levels interacted significantly with time and treatment (P = 0.002) and were increased by acipimox (Fig. 1h).
Metabolites
Plasma glucose levels were similar at baseline and in the basal period without acipimox exposure: [Ghr] vs. [Control] P = 0.27 (Fig. 2a), whereas concomitant acipimox exposure caused basal plasma glucose concentrations to increase in response to ghrelin infusion (P = 0.03, Fig. 2b).
Acipimox suppressed serum FFA levels (mmol/l): 0.37 ± 0.06 [Ghr], 0.41 ± 0.04 [Control], 0.06 ± 0.01 [Ghr + Aci], and 0.07 ± 0.02 [Aci] (P < 0.01), but ghrelin infusion did not impact on FFA levels ([Ghr] vs. [Control] P = 0.46, Fig. 2c).
Insulin sensitivity and glucose turnover
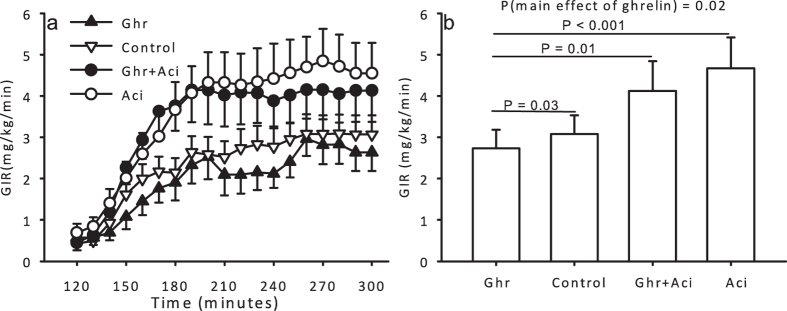
Ghrelin infusion induced peripheral insulin resistance, as assessed by the GIR, independently of acipimox (P = 0.02), whereas acipimox improved peripheral insulin sensitivity independently of ghrelin (P = 0.005, Fig. 3a and b). Post hoc pairwise comparison revealed an insulin antagonistic effect of ghrelin as compared to saline ([Ghr] vs. [Control], P = 0.03).
Figure 3.
(a) Glucose infusion rates during ghrelin, saline, ghrelin and acipimox, and acipimox. (b) GIR the four treatment conditions. Printed P values refer to paired t tests or one-way ANOVA where indicated. All data are presented as mean ± SE.
Ghrelin and acipimox did not affect EGP in the basal period (Fig. 4a). Hepatic insulin sensitivity, as determined by EGP during the clamp, was not affected by ghrelin alone (P = 0.25), whereas acipimox significantly decreased EGP independently of ghrelin (acipimox main effect P = 0.03, Fig. 4a).
Figure 4. Glucose metabolism during ghrelin, saline, ghrelin and acipimox, and acipimox-alone in the basal and in the clamp periods.
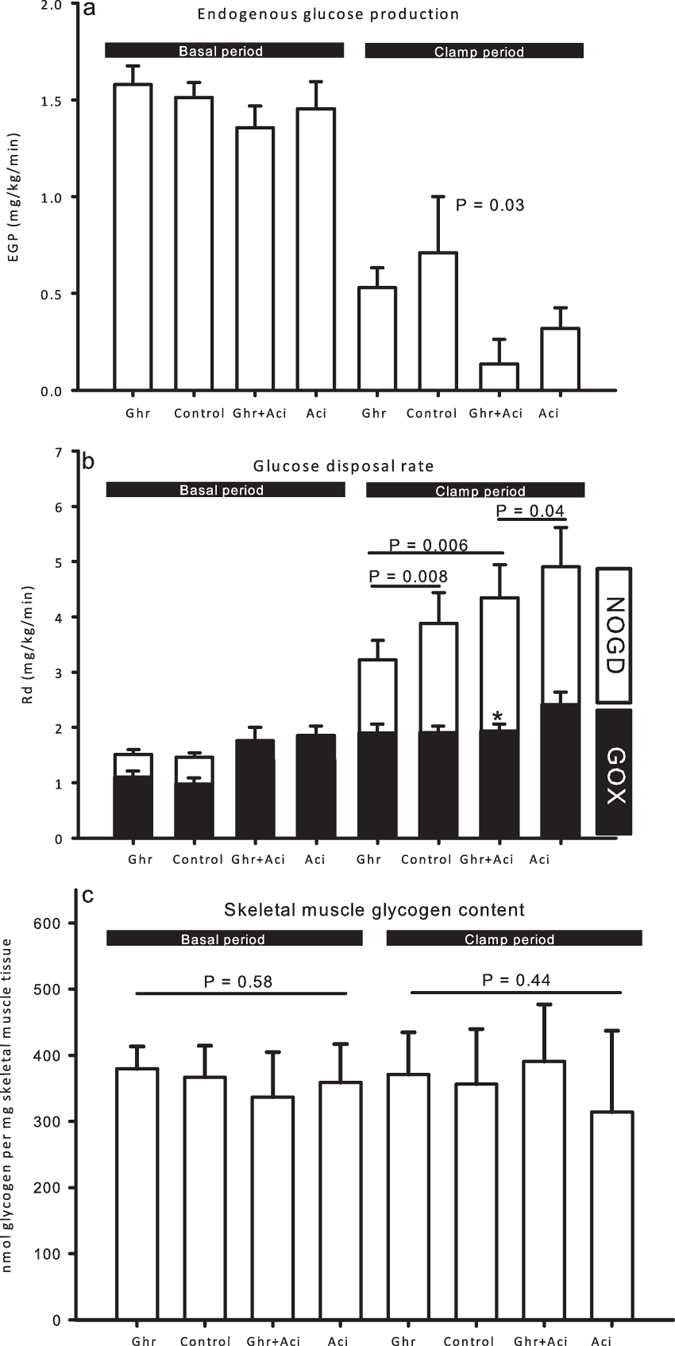
(a) EGP was equal in the basal period during all four conditions. During the clamp EGP was suppressed by acipimox. Printed P value refers to two-way ANOVA treatment effect of acipimox. (b) Glucose utilization during the terminal 30 min of basal and clamp periods. Glucose metabolism was similar in the basal period. During the clamp, ghrelin reduced glucose disposal both with and without acipimox treatment. Acipimox reversed the suppressive effect of ghrelin on glucose disposal during the clamp. Printed P values refer to paired t tests. (c) Skeletal muscle glycogen content. Printed P values refer to one-way ANOVA tests. All data are presented as mean ± SE.
Ghrelin did not significantly impact glucose turnover during the basal period (Fig. 4b). In the clamp period, ghrelin reduced glucose disposal (mg/kg/min) both in the absence of acipimox (3.22 ± 0.35 [Ghr] vs. 3.88 ± 0.56 [Control], P = 0.008) and in the presence of acipimox (4.34 ± 0.60 [Ghr + Aci] vs. 4.91 ± 0.71 [Aci], P = 0.04). However, acipimox significantly counteracted the suppressive effect of ghrelin on glucose disposal (Fig. 4b). Oxidative glucose disposal (GOX) was reduced during ghrelin plus acipimox as compared to acipimox alone (Fig. 4b). This was not associated with detectable changes in GLUT4 protein levels in skeletal muscle biopsies (data not shown). Post hoc analyses showed that the effects of ghrelin on glucose turnover were most pronounced with regards to suppression of non-oxidative glucose disposal (NOGD) (Fig. 4b), but this did not result in differences in skeletal muscle glycogen content (nmol glycogen/mg muscle) (399.9 ± 26.1 [Ghr] vs. 336.2 ± 51.1 [Control] vs. 394.9 ± 27.5 [Ghr + Aci] vs. 305.2 ± 52.4 [Aci], NS, Fig. 4c). In agreement with unaltered glycogen content, phosphorylation of glycogen synthase at the activity-regulating phosphorylation site on Ser641 was similar between groups (data not shown).
Insulin infusion induced a significant (P < 0.01) 8-fold increase in pAkt expression at Thr308 and Ser473 (Fig. 5a and b and Supplemental Figure S1). This was associated with an increased downstream signaling to AS160 in terms of a 4-fold increase in phosphorylation at Thr642 during insulin stimulation (P < 0.01) (data not shown). However, this effect of insulin was not modified by either ghrelin or acipimox.
Figure 5. Phosphorylated enzymes in the insulin signaling cascade and lipid oxidation.
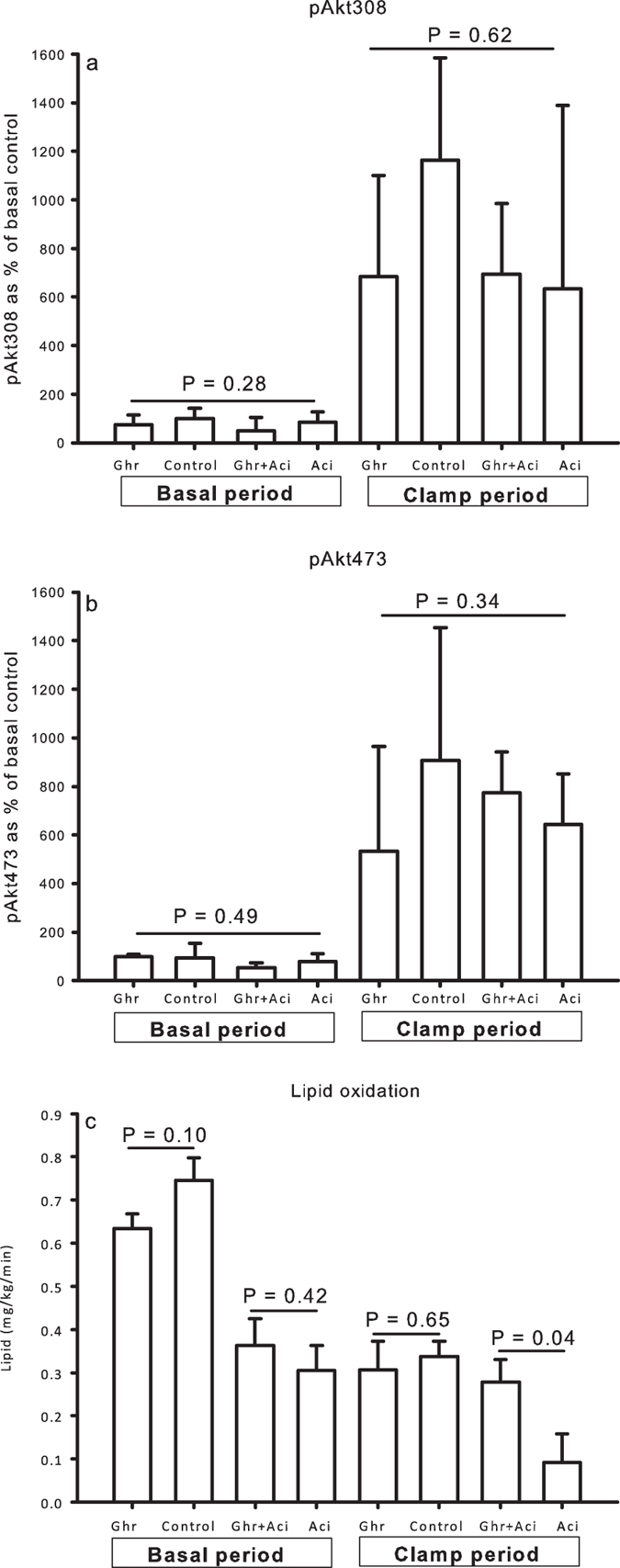
(a and b) Ghrelin did not impact on relative levels of pAkt308 and -473 content in skeletal muscle tissue in the basal and in the clamp period. (c) Ghrelin antagonized the suppressive effect of acipimox on lipid oxidation during the clamp. Printed P values refer to one-way ANOVA tests (a and b) or paired t tests (C). All data are presented as mean ± SE.
Resting energy expenditure and lipid oxidation
REE (kcal/24-h) was comparable in the basal period (1731 ± 138 [Ghr] vs. 1794 ± 111 [Control] vs. 1713 ± 120 [Ghr + Aci] vs. 1720 ± 131 [Aci], P = 0.13) and in the clamp period (1784 ± 124 [Ghr] vs. 1825 ± 106 [Control] vs. 1838 ± 122 [Ghr + Aci] vs. 1803 ± 91 [Aci], P = 0.64). Ghrelin did not significantly influence RER in the basal period ([Ghr] vs. [Control]: P = 0.40; [Ghr + Aci] vs. [Aci]: P = 0.40), whereas the RER increased during acipimox in the basal period (0.83 ± 0.01 [Ghr] vs. 0.81 ± 0.01 [Control] vs. 0.88 ± 0.02 [Ghr + Aci] vs. 0.90 ± 0.01 [Aci], P < 0.001). Ghrelin had no influence on the RER in the clamp period in the absence of acipimox ([Ghr] vs. [Control]: P = 0.77). The RER also increased during acipimox exposure in the clamp period (0.90 ± 0.01 [Ghr] vs. 0.89 ± 0.01 [Control] vs. 0.90 ± 0.01 [Ghr + Aci] vs. 0.94 ± 0.02 [Aci], P = 0.03); this effect was abrogated by ghrelin ([Ghr + Aci] vs. [Aci]: P = 0.03).
Ghrelin had no significant effects on lipid oxidation rates (mg/kg/min) in the absence of acipimox (0.31 ± 0.07 [Ghr] vs. 0.34 ± 0.07 [Control], P = 0.65), but ghrelin significantly antagonized the suppressive effect of acipimox on lipid oxidation (0.28 ± 0.05 [Ghr + Aci] vs. 0.09 ± 0.07 [Aci], P = 0.04, Fig. 5c).
Discussion
The present study was designed to investigate whether ghrelin-induced insulin resistance depends on lipolysis and ambient FFA levels after correction for ghrelin-induced stimulation of GH and cortisol release. Our data demonstrate that ghrelin per se induces peripheral insulin resistance independently of ambient FFA levels.
We have previously reported that ghrelin infusion increases serum FFA levels in hypopituitary patients which could imply a direct lipolytic effect8. This was not reproduced in the present study, where serum FFA levels during ghrelin infusion vs. saline were similar. It is possible that this discrepancy reflects a dose-dependent lipolytic effect of ghrelin, inasmuch as the dose employed in the present study was 5-fold lower and thus more physiological as compared to our previous studies5,8,18. It remains to be experimentally tested whether ghrelin stimulates lipolysis or FFA turnover in a dose-dependent manner, but regardless of that, our data reveals a direct FFA-independent effect of ghrelin on peripheral insulin sensitivity.
Acipimox is an antilipolytic nicotinic acid analogue, which binds to a G-protein coupled receptor – “protein upregulated in macrophages by interferon-γ” (PUMA-G/HM74) – in adipose tissue and thereby inhibits the hormone sensitive lipase (HSL)19,20 resulting in acute suppression of systemic FFA levels and improved peripheral insulin sensitivity21. The present study documents these effects and confirms that acipimox also improves hepatic insulin sensitivity22. The latter effect of acipimox is unmasked by our model with stabilized GH levels, inasmuch as acipimox is known to increase GH secretion23,24, which in turn induces hepatic (and peripheral) insulin resistance25,26. We therefore suggest that our model is well suited to study the direct effect of acipimox and also propose an explanation as to why hepatic and peripheral insulin sensitivity frequently fails to improve following acipimox administration to subjects with an intact anterior pituitary function23,27. In line with previous studies we recorded a stimulatory effect of acipimox on plasma glucagon concentrations9,23, which may represent a compensatory mechanism.
Skeletal muscle uptake accounts for approximately 80% of infused glucose during a hyperinsulinemic euglycemic clamp in humans28, but it has proven difficult to demonstrate a link between the diabetogenic effects of ghrelin to the insulin signaling pathway in human skeletal muscle biopsies8,11. Likewise, no evidence of impaired insulin signaling at the level of protein expression or phosphorylation could be recorded in the present study. It is possible that more frequent biopsies in combination with activity assays might provide additional sensitivity to our human in vivo model, but it is also possible that in vitro studies or transgenic mice models may be necessary to unveil the molecular mechanism.
Ghrelin infusion increased both acyl ghrelin and desacyl ghrelin levels to supraphysiologic concentrations in line with earlier reports11,29,30, which supports that acyl ghrelin is metabolized to desacyl ghrelin in vivo. Surprisingly, acipimox reduced both ghrelin and desacyl ghrelin concentrations at baseline, which is interesting since desacyl ghrelin exposure has been shown to improve insulin sensitivity31,32,33.
Both ghrelin and acipimox are recognized as potent GH secretagogues24. Our participants all had documented GH deficiency as a consequence of pituitary disease, yet we did record a minimal increase in serum GH levels when ghrelin and acipimox were co-administered. This observation indicates synergy between the GH secreting effects of ghrelin and acipimox and is in line with effects reported in healthy elderly men treated with acipimox and the GH-secretagogue GHRP-234. This response could theoretically have impacted on glucose and lipid metabolism, but it was not accompanied by detectable activation of GH signaling in skeletal muscle. Taken together, we find it unlikely that it could account for the metabolic effects of ghrelin observed in our study.
Baseline plasma glucose concentrations were not increased by ghrelin alone, which is in line with previous reports29 but plasma glucose levels during administration of ghrelin plus acipimox were elevated as compared to the levels recorded after acipimox alone, which appeared to increase plasma glucagon levels. This lack of effect of ghrelin on glucose concentrations contrasts with our earlier data5,8,18, but, again, in the present study we used a more physiologic ghrelin infusion rate and the present data are in line with other clinical trials using a similar ghrelin infusion rate11. Plasma insulin and ghrelin appear to correlate inversely. Physiological ghrelin concentrations inhibit insulin secretion35,36 and vice versa. It is speculated that lower ghrelin levels in obesity38,39 and type 2 diabetes40 may represent a counter-regulatory mechanism against hyperglycemia.
Some limitations apply to our study. The number of participants was relatively small, the age and BMI ranges were relatively large, and the study included male patients only. However, the crossover design minimizes inter-individual differences and ensures that the observed effects of ghrelin and acipimox are not attributable to differences in the study population.
In conclusion, our data show that ghrelin induces acute peripheral insulin resistance via mechanisms that are independent of GH, cortisol, and ambient serum FFA levels but does not impact on hepatic insulin sensitivity.
Additional Information
How to cite this article: Vestergaard, E. T. et al. Acyl Ghrelin Induces Insulin Resistance Independently of GH, Cortisol, and Free Fatty Acids. Sci. Rep. 7, 42706; doi: 10.1038/srep42706 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Acknowledgments
Mrs. E. Hornemann and Mrs. L. Buus are acknowledged for excellent technical assistance. The data were presented as an oral presentation at the Endocrine Society Annual Meeting 2015. Grants and fellowships: The study was supported by a postdoctoral research fellow grant (11-105283) from the Danish Council for Independent Research (Medical Sciences) and grants from the Riisfort Fonden and the A.P. Moller Foundation.
Footnotes
The authors declare no competing financial interests.
Author Contributions E.T.V. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. E.T.V.: wrote protocol, screened patients, performed the clinical study, collected data, analyzed data, interpreted results, wrote first manuscript draft, edited and revised manuscript, created figures and table, literature research, approved the final manuscript. N.J.: analyzed data, interpreted results, edited and revised manuscript, literature research, approved the final manuscript. N.M.: interpreted results, edited and revised manuscript, approved the final manuscript. J.O.L.J.: conceptualized study, study design, wrote protocol, analyzed data, interpreted results, edited and revised manuscript, literature research, approved the final manuscript.
References
- Kojima M. et al. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 402, 656–660 (1999). [DOI] [PubMed] [Google Scholar]
- Takaya K. et al. Ghrelin strongly stimulates growth hormone release in humans. Journal of Clinical Endocrinology and Metabolism 85, 4908–4911 (2000). [DOI] [PubMed] [Google Scholar]
- Moller N. & Jorgensen J. O. L. Effects of Growth Hormone on Glucose, Lipid and Protein Metabolism in Human Subjects. Endocrine Reviews, er (2009). [DOI] [PubMed] [Google Scholar]
- Broglio F. et al. Ghrelin, a natural GH secretagogue produced by the stomach, induces hyperglycemia and reduces insulin secretion in humans. J. Clin. Endocrinol. Metab 86, 5083–5086 (2001). [DOI] [PubMed] [Google Scholar]
- Vestergaard E. T. et al. Constant intravenous ghrelin infusion in healthy young men: Clinical pharmacokinetics and metabolic effects. Am. J Physiol Endocrinol Metab 292, E1829–E1836 (2007). [DOI] [PubMed] [Google Scholar]
- Gnanapavan S. et al. The tissue distribution of the mRNA of ghrelin and subtypes of its receptor, GHS-R, in humans. Journal of Clinical Endocrinology and Metabolism 87, 2988–2991 (2002). [DOI] [PubMed] [Google Scholar]
- Papotti M. et al. Growth hormone secretagogue binding sites in peripheral human tissues. Journal of Clinical Endocrinology and Metabolism 85, 3803–3807 (2000). [DOI] [PubMed] [Google Scholar]
- Vestergaard E. T. et al. Ghrelin infusion in humans induces acute insulin resistance and lipolysis independent of GH-signaling. Diabetes 57, 3205–3210 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen S., Moller N., Christiansen J. S. & Jorgensen J. O. Pharmacological antilipolysis restores insulin sensitivity during growth hormone exposure. Diabetes 50, 2301–2308 (2001). [DOI] [PubMed] [Google Scholar]
- Haemmerle G. et al. Hormone-sensitive lipase deficiency in mice causes diglyceride accumulation in adipose tissue, muscle, and testis. J Biol Chem 277, 4806–4815 (2002). [DOI] [PubMed] [Google Scholar]
- Vestergaard E. T. et al. Acute Peripheral Metabolic Effects of Intraarterial Ghrelin Infusion in Healthy Young Men. Journal of Clinical Endocrinology and Metabolism 468–477 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delhanty P. J. et al. The acylated (AG) to unacylated (UAG) ghrelin ratio in esterase inhibitor treated blood is higher than previously described. Clinical endocrinology, doi: 10.1111/cen.12489 (2014). [DOI] [PubMed] [Google Scholar]
- Moller N., Butler P. C., Antsiferov M. A. & Alberti K. G. Effects of growth hormone on insulin sensitivity and forearm metabolism in normal man. Diabetologia 32, 105–110 (1989). [DOI] [PubMed] [Google Scholar]
- Ferrannini E. The theoretical bases of indirect calorimetry: a review. Metabolism 37, 287–301 (1988). [DOI] [PubMed] [Google Scholar]
- Gurtler A. et al. Stain-Free technology as a normalization tool in Western blot analysis. Anal Biochem 433, 105–111 (2013). [DOI] [PubMed] [Google Scholar]
- Witczak C. A. et al. JNK1 deficiency does not enhance muscle glucose metabolism in lean mice. Biochem Biophys Res Commun 350, 1063–1068 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondar Rj Fau - Mead D. C. & Mead D. C. Evaluation of glucose-6-phosphate dehydrogenase from Leuconostoc mesenteroides in the hexokinase method for determining glucose in serum. Clin Chem 20, 586–590 (1974). [PubMed] [Google Scholar]
- Vestergaard E. T. et al. Acute Effects of Ghrelin Administration on Glucose and Lipid Metabolism. Journal of Clinical Endocrinology and Metabolism 93, 438–444 (2008). [DOI] [PubMed] [Google Scholar]
- Carlson L. A. Studies on the effect of nicotinic acid on catecholamine stimulated lipolysis in adipose tissue in vitro. Acta Med Scand 173, 719–722 (1963). [DOI] [PubMed] [Google Scholar]
- Tunaru S. et al. PUMA-G and HM74 are receptors for nicotinic acid and mediate its anti-lipolytic effect. Nature medicine 9, 352–355 (2003). [DOI] [PubMed] [Google Scholar]
- Santomauro A. T. et al. Overnight lowering of free fatty acids with Acipimox improves insulin resistance and glucose tolerance in obese diabetic and nondiabetic subjects. Diabetes 48, 1836–1841 (1999). [DOI] [PubMed] [Google Scholar]
- Saloranta C., Franssila-Kallunki A., Ekstrand A., Taskinen M. R. & Groop L. Modulation of hepatic glucose production by non-esterified fatty acids in type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia 34, 409–415 (1991). [DOI] [PubMed] [Google Scholar]
- Saloranta C. et al. Metabolic Consequences of Sustained Suppression of Free Fatty Acids by Acipimox in Patients With NIDDM. Diabetes 42, 1559–1566 (1993). [DOI] [PubMed] [Google Scholar]
- Peino R. et al. Acipimox-mediated plasma free fatty acid depression per se stimulates growth hormone (GH) secretion in normal subjects and potentiates the response to other GH-releasing stimuli. The Journal of Clinical Endocrinology & Metabolism 81, 909–913 (1996). [DOI] [PubMed] [Google Scholar]
- Rabinowitz D., Klassen G. A. & Zierler K. L. Effect of Human Growth Hormone on Muscle and Adipose Tissue Metabolism in the Forearm of Man. The Journal of Clinical Investigation 44, 51–61 (1965). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altszuler N Fau - Steele R., Steele R Fau - Wall J. S., Wall Js Fau - De Bodo R. C. & De Bodo R. C. Increased production and utilization of circulating glucose during growth hormone regimen. Proc Soc Exp Biol Med 94, 744–746 (1957). [DOI] [PubMed] [Google Scholar]
- van de Weijer T. et al. Evidence for a Direct Effect of the NAD+ Precursor Acipimox on Muscle Mitochondrial Function in Humans. Diabetes 64, 1193–1201 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiebaud D. et al. The Effect of Graded Doses of Insulin on Total Glucose Uptake, Glucose Oxidation, and Glucose Storage in Man. Diabetes 31, 957–963 (1982). [DOI] [PubMed] [Google Scholar]
- Tong J., Davis H. W., Gastaldelli A. & D’Alessio D. Ghrelin impairs prandial glucose tolerance and insulin secretion in healthy humans despite increasing GLP-1. JCEM (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong J. et al. Acute Administration of Unacylated Ghrelin Has No Effect on Basal or Stimulated Insulin Secretion in Healthy Humans. Diabetes, doi: 10.2337/db13-1598 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauna C. et al. Administration of acylated ghrelin reduces insulin sensitivity, whereas the combination of acylated plus unacylated ghrelin strongly improves insulin sensitivity. Journal of Clinical Endocrinology and Metabolism 89, 5035–5042 (2004). [DOI] [PubMed] [Google Scholar]
- Ozcan B. et al. Does des-acyl ghrelin improve glycemic control in obese diabetic subjects by decreasing acylated ghrelin levels? European journal of endocrinology/European Federation of Endocrine Societies 170, 799–807 (2014). [DOI] [PubMed] [Google Scholar]
- Benso A. et al. Metabolic effects of overnight continuous infusion of unacylated ghrelin in humans. European journal of endocrinology/European Federation of Endocrine Societies 166, 911–916 (2012). [DOI] [PubMed] [Google Scholar]
- Sytze van Dam P. et al. Reduction of Free Fatty Acids By Acipimox Enhances the Growth Hormone (GH) Responses to GH-Releasing Peptide 2 in Elderly Men. The Journal of Clinical Endocrinology & Metabolism 85, 4706–4711, doi: 10.1210/jcem.85.12.7087 (2000). [DOI] [PubMed] [Google Scholar]
- Tong J. et al. Physiologic concentrations of exogenously infused ghrelin reduces insulin secretion without affecting insulin sensitivity in healthy humans. J Clin Endocrinol Metab 98, 2536–2543, doi: 10.1210/jc.2012-4162 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong J. et al. Ghrelin Suppresses Glucose-stimulated Insulin Secretion and Deteriorates Glucose Tolerance in Healthy Humans. Diabetes 59, 2145–2151 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanagan D. E. et al. The influence of insulin on circulating ghrelin. Am. J Physiol Endocrinol Metab 284, E313–E316 (2003). [DOI] [PubMed] [Google Scholar]
- Hansen T. K. et al. Weight loss increases circulating levels of ghrelin in human obesity. Clin. Endocrinol. (Oxf) 56, 203–206 (2002). [DOI] [PubMed] [Google Scholar]
- Cummings D. E. et al. Plasma ghrelin levels after diet-induced weight loss or gastric bypass surgery. N. Engl. J Med. 346, 1623–1630 (2002). [DOI] [PubMed] [Google Scholar]
- Poykko S. M. et al. Low plasma ghrelin is associated with insulin resistance, hypertension, and the prevalence of type 2 diabetes. Diabetes 52, 2546–2553 (2003). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.