ABSTRACT
Adenovirus serotype 5 (Ad5) is one of the most widely used viral vectors and is known to generate potent T cell responses. While many previous studies have characterized Ad5-induced CD8 T cell responses, there is a relative lack of detailed studies that have analyzed CD4 T cells elicited by Ad5 vaccination. Here, we immunized mice with Ad5 vectors encoding lymphocytic choriomeningitis virus (LCMV) glycoprotein (GP) and examined GP-specific CD4 T cell responses elicited by Ad5 vectors and compared them to those induced by an acute LCMV infection. In contrast to LCMV infection, where balanced CD4 T helper 1 (Th1) and T follicular helper (Tfh) responses were induced, Ad5 immunization resulted in a significantly reduced frequency of Th1 cells. CD4 T cells elicited by Ad5 vectors expressed decreased levels of Th1 markers, such as Tim3, SLAM, T-bet, and Ly6C, had smaller amounts of cytotoxic molecules like granzyme B, and produced less interferon gamma than CD4 T cells induced by LCMV infection. This defective CD4 Th1 response appeared to be intrinsic for Ad5 vectors and not a reflection of comparing a nonreplicating vector to a live viral infection, since immunization with a DNA vector expressing LCMV-GP generated efficient CD4 Th1 responses. Analysis at early time points (day 3 or 4) after immunization with Ad5 vectors revealed a defect in the expression of CD25 (interleukin-2 [IL-2] receptor alpha chain) on Ad5-elicited CD4 T cells, and administration of exogenous IL-2 following Ad5 immunization partially restored CD4 Th1 responses. These results suggest that impairment of Th1 commitment after Ad5 immunization could be due to reduced IL-2-mediated signaling.
IMPORTANCE During viral infection, generating balanced responses of Th1 and Tfh cells is important to induce effective cell-mediated responses and provide optimal help for antibody responses. In this study, to investigate vaccine-induced CD4 T cell responses, we characterized CD4 T cells after immunization with Ad5 vectors expressing LCMV-GP in mice. Ad5 vectors led to altered effector differentiation of LCMV GP-specific CD4 T cells compared to that during LCMV infection. CD4 T cells following Ad5 immunization exhibited impaired Th1 lineage commitment, generating significantly decreased Th1 responses than those induced by LCMV infection. Our results suggest that suboptimal IL-2 signaling possibly plays a role in reduced Th1 development following Ad5 immunization.
KEYWORDS: CD4 T cell responses, T helper 1 (Th1), T follicular helper (Tfh), adenovirus serotype 5, vaccination
INTRODUCTION
Recombinant adenovirus (Ad) vectors are one of the most favorable vaccine platforms as they have certain potential advantages, such as strong immunogenicity, large transgene capacity, and good safety profile. Ad-based vaccine vectors can induce strong T cell-mediated and humoral immune responses to the encoded transgenes. Currently, Ad vectors from multiple species and serotypes are being explored, either alone or as a prime-boost strategy, for a number of infectious diseases, including human immunodeficiency virus (HIV), tuberculosis, malaria, Ebola, hepatitis C, and influenza, as well as cancer. Preclinical and clinical studies in mice, nonhuman primates, and humans have shown promising results (1, 2). Human adenovirus serotype 5 (Ad5) is the best-studied and the most commonly used adenovirus vector in vaccine development because of its superior immunogenicity compared to other Ad serotype vectors. Although preexisting antivector immunity in the human population and the lack of protection against HIV infection have raised concerns over the clinical application of Ad5 vectors (3, 4), Ad5 is still one of the most frequently used viral vectors in clinical studies, as significant efficacy has been shown against a broad range of pathogens and cancers (1, 2).
Ad5 vectors have been shown to induce high-frequency CD8 T cell responses in preclinical and clinical studies. More detailed studies on the characteristics of CD8 T cells have demonstrated that Ad5 immunization induces sustained CD8 T cell responses with more effector-like phenotypes (5), probably due to prolonged transgene expression (6). Assessment of Ad5-induced T cell responses has been more focused on CD8 T cells, while relatively less is known about CD4 T cell responses elicited by Ad5 immunization. Previous studies have shown that CD4 T cells are critical for transgene-specific CD8 T cell and antibody responses elicited by Ad vector immunization (7–9). However, the phenotypic and functional properties of CD4 T cells induced by Ad vectors remain less well investigated.
CD4 T cells orchestrate immune responses against various types of pathogens by differentiating to diverse effector subsets with unique effector functions (10, 11). During viral infection, for example, lymphocytic choriomeningitis virus (LCMV) infection in mice, both T helper 1 (Th1) and T follicular helper (Tfh) cells are generated (12). Th1 cells produce their signature cytokine, interferon-gamma (IFN-γ) and play a critical role in cell-mediated immunity and the host defense against intracellular pathogens (10, 11). Tfh cells are specialized in providing help to cognate B cells and essential for the initiation and maintenance of germinal center reactions and the generation of high-affinity antibodies, long-lived plasma cells and memory B cells (13). Given the critical roles of CD4 T cells in immune protection, it is crucial to better understand vaccine-induced CD4 T cell responses.
In this study, we examined CD4 T cell responses following immunization of mice with Ad5 vectors encoding full-length LCMV glycoprotein (GP). We characterized LCMV GP-specific CD4 T cells elicited by Ad5 vectors in comparison to those induced by infection with the original virus, LCMV. Ad5-elicited CD4 T cells undergo a distinct differentiation program which leads to suboptimal Th1 responses, at least partly due to reduced interleukin-2 (IL-2) signaling.
RESULTS
Ad5 immunization results in suboptimal CD4 Th1 responses.
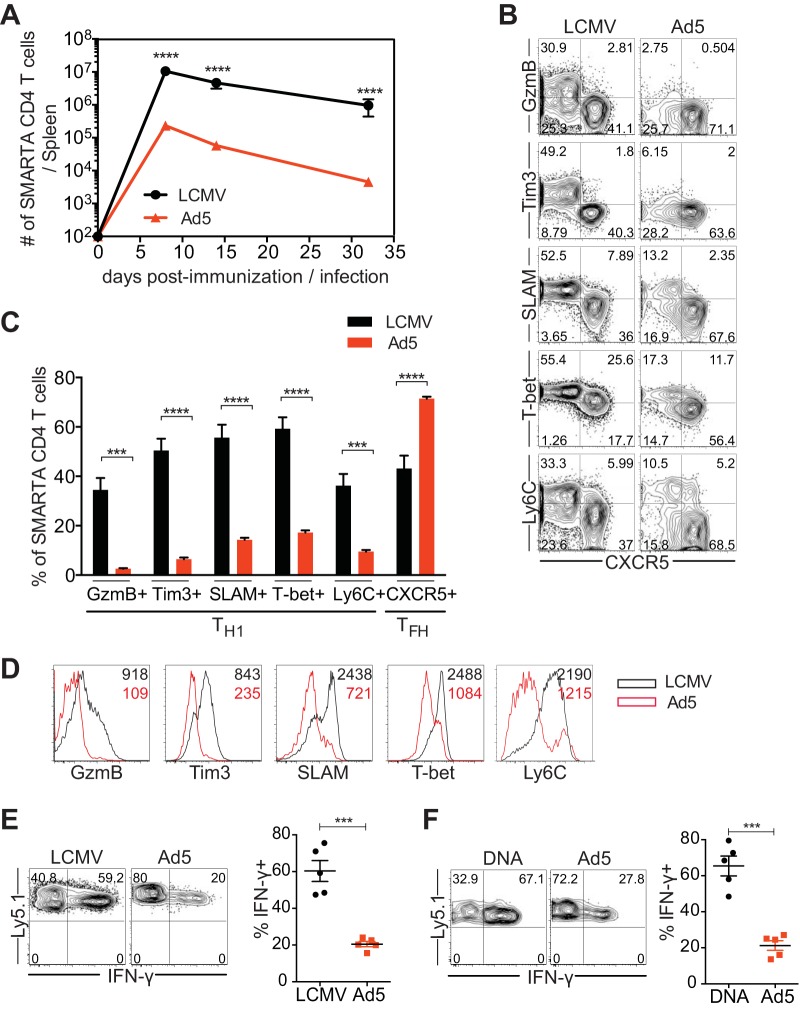
To examine Ad5-elicited CD4 T cell responses, we utilized SMARTA T cell receptor (TCR) transgenic cells specific for the major histocompatibility complex (MHC) class II-restricted epitope of LCMV GP66-77 and Ad5 vectors encoding full-length LCMV GP. SMARTA CD4 T cells (CD45.1+) were transferred into naive C57BL/6 mice (CD45.2+) that were subsequently immunized with Ad5-LCMV GP vectors or infected with LCMV Armstrong that causes an acute infection. Following Ad5 immunization or LCMV infection, congenically marked donor cells were analyzed in the spleen. Both Ad5 immunization and LCMV infection led to similar expansion and contraction of SMARTA CD4 T cells, with the peak response at day 8. However, the magnitude of CD4 T cell responses elicited by Ad5 was significantly low compared to that induced by LCMV, and the difference was maintained throughout the course of immunization/infection (Fig. 1A). To access differentiation of CD4 T cells following Ad5 immunization, the phenotypes of SMARTA CD4 T cells were analyzed at the peak of the response (day 8). Consistent with the previous report, balanced responses of two CD4 effector T cell subsets, Th1 and Tfh, were generated during LCMV infection (12). Approximately 45 to 50% of SMARTA CD4 T cells in the spleen were differentiated into Tfh cells that expressed CXCR5 and downregulated Th1-associated molecules in LCMV infection (Fig. 1B and C). The other half of SMARTA CD4 T cells (CXCR5−) upregulated granzyme B and Ly6C, and the majority of them expressed high levels of Tim3, SLAM, and T-bet, thus representing Th1 cells. In comparison, dramatically reduced Th1 cells were found following Ad5 immunization; granzyme B and Tim3 expression levels were minimal, and approximately 10 to 15% of SMARTA CD4 T cells expressed SLAM, T-bet, and Ly6C. Mean fluorescence intensity (MFI) of Th1-associated molecules was also significantly lower in Ad5-elicited SMARTA CD4 T cells than those induced by LCMV infection (Fig. 1D). On the other hand, a high proportion of SMARTA CD4 T cells differentiated into CXCR5+ Tfh cells following Ad5 immunization (Fig. 1B and C). To further characterize CD4 T cells elicited by Ad5 vectors, cytokine production was measured after ex vivo stimulation with cognate peptide. SMARTA CD4 T cells produced substantially less IFN-γ after Ad5 immunization than after LCMV infection (Fig. 1E). These results demonstrated that immunization with Ad5 vectors resulted in significantly reduced Th1 differentiation.
FIG 1.
Ad5 immunization leads to suboptimal Th1 differentiation. CD45.1+ SMARTA transgenic CD4 T cells specific for the LCMV GP66-77 epitope were transferred into C57BL/6 mice (CD45.2+) that were subsequently immunized with Ad5 vectors expressing full-length LCMV GP or infected with LCMV Armstrong strain. Congenically marked (CD45.1+) donor cells were analyzed in the spleen. (A) Kinetics of SMARTA CD4 T cells. (B to F) Analysis was performed at day 8 postimmunization or postinfection. (B) Representative fluorescence-activated cell sorting (FACS) plots, showing the phenotype of SMARTA CD4 T cells. (C) The frequency of SMARTA CD4 T cells expressing Th1 markers (granzyme B [GzmB], Tim3, SLAM, T-bet, Ly6C) or a Tfh marker (CXCR5). (D) Representative histograms of the indicated molecules expressed by SMARTA CD4 T cells. The numbers indicate the MFI of the indicated molecules. (E) Cytokine production of SMARTA CD4 T cells after ex vivo stimulation with GP61-80 peptide. (Left) Representative FACS plots show IFN-γ production of SMARTA CD4 T cells. (Right) The frequency of IFN-γ+ cells in SMARTA CD4 T cells. (F) SMARTA chimeric mice were generated and immunized intramuscularly with Ad5 or DNA vectors expressing full-length LCMV GP. Analysis was performed at day 8 postimmunization. Cytokine production was assessed after ex vivo stimulation with GP61-80 peptide. (Left) Representative FACS plots show IFN-γ production of SMARTA CD4 T cells. (Right) Frequency of IFN-γ+ cells in SMARTA CD4 T cells. Data are representative of 2 independent experiments with 4 to 5 mice per group per experiment. Error bars indicate standard errors of means. ***P < 0.001; ****P < 0.0001.
We asked whether the reduced Th1 responses following Ad5 immunization, compared to those after LCMV infection, are due to the differences between nonreplicating vaccine vectors and live virus infection. To address this, we compared CD4 T cell responses induced by Ad5 vectors and DNA vectors expressing the same antigen, LCMV GP, at day 8 postimmunization. In contrast to Ad5 vectors, DNA immunization generated more balanced Th1 and Tfh responses. DNA vectors induced SMARTA CD4 T cells that produced significantly more IFN-γ than Ad5 vectors, and this was comparable to the levels after LCMV infection (Fig. 1F). Expression levels of granzyme B, SLAM, and T-bet were also significantly elevated in SMARTA CD4 T cells following DNA immunization compared to levels after Ad5 immunization (data not shown). Therefore, impaired Th1 development seems to be an intrinsic property of Ad5 vectors, rather than an outcome of using nonreplicating vectors.
Next, to determine whether similar CD4 T cell responses were observed in other tissues, we examined SMARTA CD4 T cells in inguinal lymph nodes (LNs), liver, and blood after immunization with Ad5 vectors. Following Ad5 immunization, expansion of SMARTA CD4 T cells was remarkably reduced in all tissues examined compared to that after LCMV infection (Fig. 2A). After acute viral infection, Tfh cells are predominantly found in secondary lymphoid tissues and blood, whereas a very small population of CXCR5+ cells is found in the nonlymphoid tissues where, instead, Th1 responses are dominant (12). Consistent with these localization patterns, both SLAM+ Th1 and CXCR5+ Tfh effector cells were found in LNs and blood, while the majority of CD4 T cells were Th1 in the liver at day 8 post-LCMV infection (Fig. 2B and C). In Ad5-immunized mice, development of Th1 cells was greatly reduced in the LNs, where the majority of CD4 T cells were Tfh cells. There was also a significantly lower frequency of Th1 cells in liver and blood compared to the frequencies in LCMV-infected animals. Taken together, these data indicate that Ad5 immunization led to substantially reduced Th1 responses in all tissues examined, including lymphoid and nonlymphoid organs.
FIG 2.
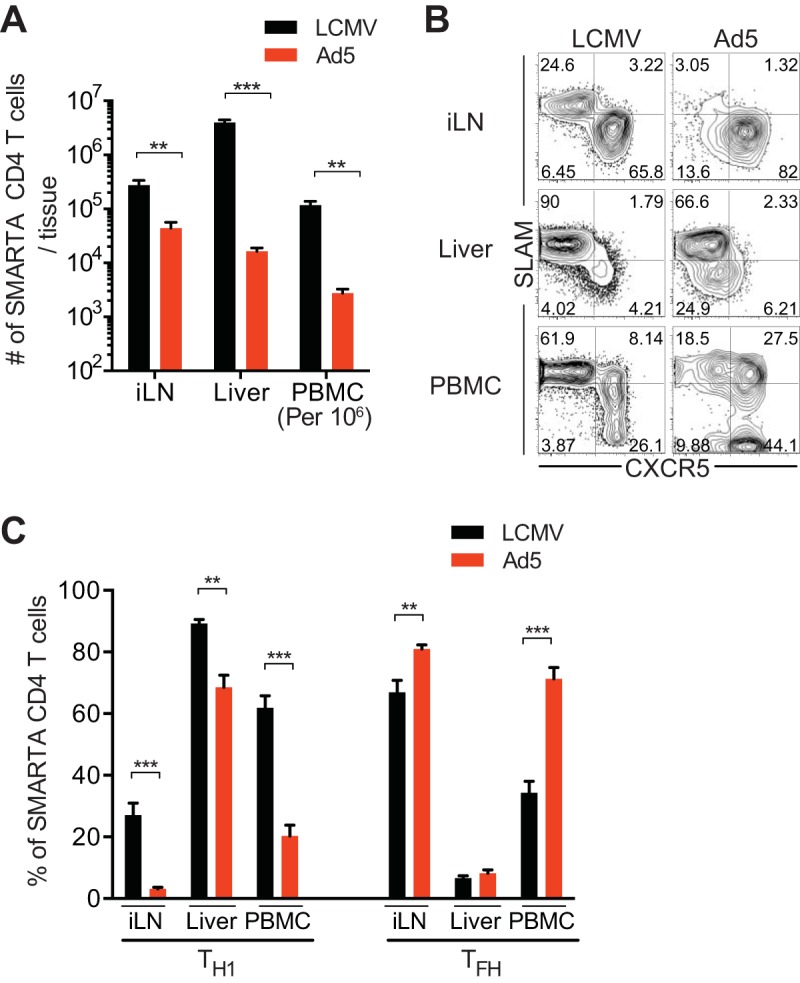
Suboptimal Th1 responses are found in tissues, including lymphoid and nonlymphoid organs, after Ad5 immunization. Data are from the same experimental setup as in Fig. 1. SMARTA CD4 T cells were analyzed in inguinal lymph nodes (iLN), liver, and peripheral blood mononuclear cells (PBMC) 8 days after Ad5 immunization or LCMV infection. (A) Numbers of SMARTA CD4 T cells in the indicated tissues. Numbers of SMARTA CD4 T cells in blood were calculated per 1 million PBMC. (B) Representative FACS plots showing the phenotype of SMARTA CD4 T cells. (C) Percentages of SLAM+ CXCR5− Th1 or CXCR5+ Tfh SMARTA CD4 T cells in the indicated tissues. Data are representative of 2 independent experiments with 4 to 5 mice per group per experiment. Error bars indicate standard errors of the means. **, P < 0.01; ***, P < 0.001.
Immunization with Ad5 vectors does not generate CD4 Th2 or Th17 cells.
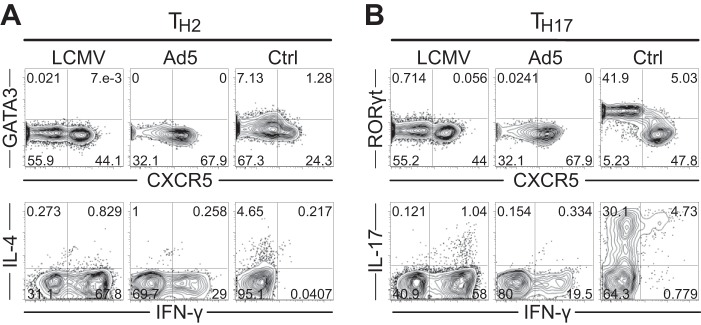
In LCMV infection, virus-specific CD4 T cells predominantly develop into Th1 and Tfh cells with minimal generation of other CD4 T cell subsets, such as Th2 or Th17. Given that Ad5 immunization possibly induces different environmental conditions, such as the cytokine milieu during CD4 T cell activation, we wanted to determine whether other CD4 T cell subsets besides Th1 and Tfh were generated following Ad5 immunization. We assessed Th2- and Th17-associated transcription factors and cytokines at day 8 postimmunization. SMARTA CD4 T cells elicited by Ad5 vectors did not express GATA3 or RORγt and did not produce IL-4 or IL-17 upon cognate peptide stimulation (Fig. 3A and B), indicating that no Th2 and Th17 differentiation occurred.
FIG 3.
Immunization with Ad5 vectors does not generate Th2 or Th17 cells. SMARTA chimeric mice were generated and immunized with Ad5-LCMV GP vectors or infected with LCMV. At day 8 postimmunization or infection, SMARTA CD4 T cells were analyzed for Th2-associated (A) or Th17-associated (B) transcription factors and cytokines. (A) Representative FACS plots show GATA3 and IL-4 expression levels. (B) Representative FACS plots show RORγt and IL-17 expression levels. Th2 positive-control cells expressing GATA3 and IL-4 and Th17 positive-control cells expressing RORγt and IL-17 were analyzed together. Cytokine production was measured after ex vivo stimulation with GP61-80 peptide.
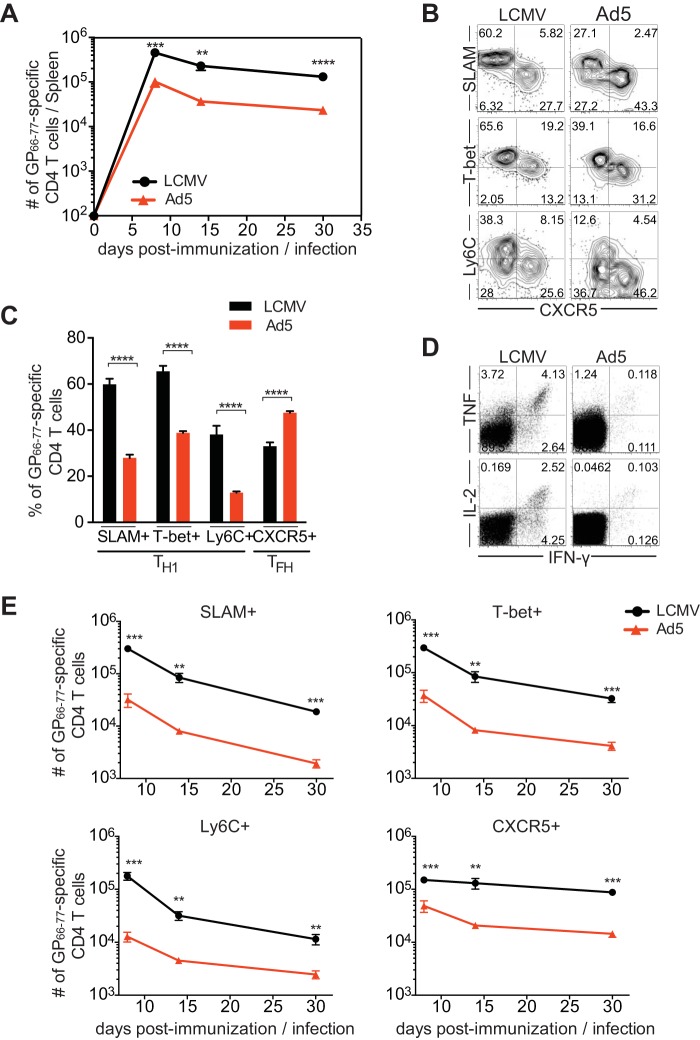
Similar to SMARTA CD4 T cells, Ad5-elicited endogenous GP66-77-specific CD4 T cells display significantly decreased Th1 responses.
To confirm whether endogenous LCMV GP-specific CD4 T cells behave similarly to the transgenic CD4 T cells, we analyzed endogenous polyclonal LCMV GP66-77-specific CD4 T cells by tetramer staining after immunization with Ad5-LCMV GP vectors. Endogenous GP66-77-specific CD4 T cell responses were analogous to the responses of SMARTA transgenic CD4 T cells and displayed similar kinetics (Fig. 4A). Similar to the responses of SMARTA CD4 T cells, Ad5 induced significantly lower endogenous GP66-77-specific CD4 T cell responses than did LCMV, generating approximately 4.5-fold fewer GP66-77-specific CD4 T cells at the peak of the expansion (day 8). Consistently, endogenous GP66-77-specific CD4 T cells following Ad5 immunization exhibited a significantly lower frequency of Th1 cells than those generated by LCMV infection (Fig. 4B and C). Ad5 vector-induced CD4 T cells also resulted in decreased production of cytokines such as IFN-γ, tumor necrosis factor (TNF), and IL-2 (Fig. 4D). Therefore, endogenous polyclonal LCMV GP-specific CD4 T cell responses were similar to those of transgenic monoclonal CD4 T cells and resulted in suboptimal Th1 development after Ad5 immunization.
FIG 4.
Ad5-elicited endogenous GP66-77-specific CD4 T cells exhibit significantly reduced Th1 responses. Endogenous polyclonal GP66-77-specific CD4 T cells were analyzed in the spleen after immunizing C57BL/6 mice with Ad5-LCMV GP vectors or infecting mice with LCMV. (A) Kinetics of GP66-77-specific (MHC class II tetramer+) CD4 T cells. (B to D) Analysis was performed 8 days after Ad5 immunization or LCMV infection. (B) Representative FACS plots showing the phenotype of GP66-77-specific CD4 T cells. (C) The frequency of GP66-77-specific Th1 cells (CXCR5− cells expressing T-bet, SLAM, or Ly6C) or Tfh cells (CXCR5+ cells). (D) Cytokine production of CD4 T cells after ex vivo stimulation with GP61-80 peptide. Representative FACS plots show IFN-γ, TNF, and IL-2 production, with gating on total CD4 T cells. (E) The number of Th1 cells (CXCR5− cells expressing T-bet, SLAM, or Ly6C) and Tfh cells (CXCR5+ cells) in the spleen by day 30 postimmunization or infection. Data are representative of 2 independent experiments with 4 to 5 mice per group per experiment. Error bars indicate standard errors of the means. **, P < 0.01; ***, P < 0.001; ****, P < 0.0001.
Next, to examine the kinetics of Th1 and Tfh cells following Ad5 immunization, we tracked GP66-77-specific CD4 T cells by day 30 postimmunization. With Ad5 immunization, after the peak response at day 8, Th1 cells, identified by their high expression of SLAM, T-bet, or Ly6C, decreased over time, whereas CXCR5+ Tfh cells were relatively stably maintained (Fig. 4E). Consequently, at day 30 post-Ad5 immunization, transgene-specific CD4 T cells exhibited a further decline in Th1 responses, with responses skewing toward Tfh cells. The kinetics of Th1 and Tfh responses following Ad5 immunization was similar to that observed in LCMV infection. This analysis showed suboptimal Th1 responses at the memory phase following Ad5 immunization.
Ad5 vectors generate GP-specific antibody responses.
Immunization with Ad5 vectors generated a high frequency of Tfh cells. Since the major function of Tfh cells is to provide help to B cells in generating optimal antibody responses, we sought to determine Ad5-elicited GP-specific antibody responses. Following immunization with Ad5 vectors, the GP-specific serum antibody titer was very low at day 8 postimmunization compared to that after LCMV infection (Fig. 5). However, a greater increase in the antibody titer was detected between days 8 and 15 post-Ad5 immunization, and the difference in the titers between Ad5 and LCMV became smaller by day 30. The differences in antibody titers and kinetics between Ad5 immunization and LCMV infection could be attributed to the significantly low magnitude of Ad5-elicited CD4 T cell responses and/or different conditions of antigen load and persistence during Ad5 immunization versus LCMV infection.
FIG 5.
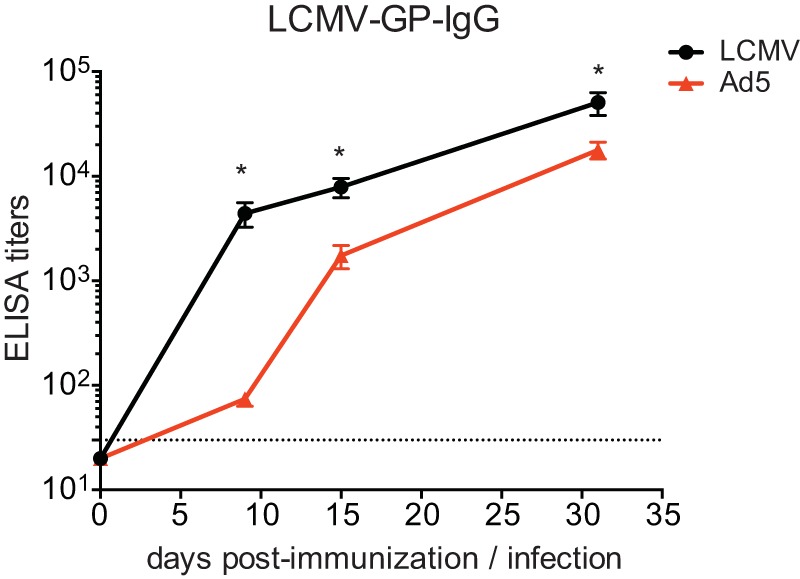
GP-specific antibody responses elicited by Ad5 vectors. Serum GP-specific antibody titers were measured by ELSIA at day 8, 15, and 30 following immunization with Ad5 vectors or LCMV infection. The dotted line indicates the lower limit of detection. Data are representative of 2 independent experiments with 4 to 5 mice per group per experiment. Error bars indicate standard errors of the means. *, P < 0.05.
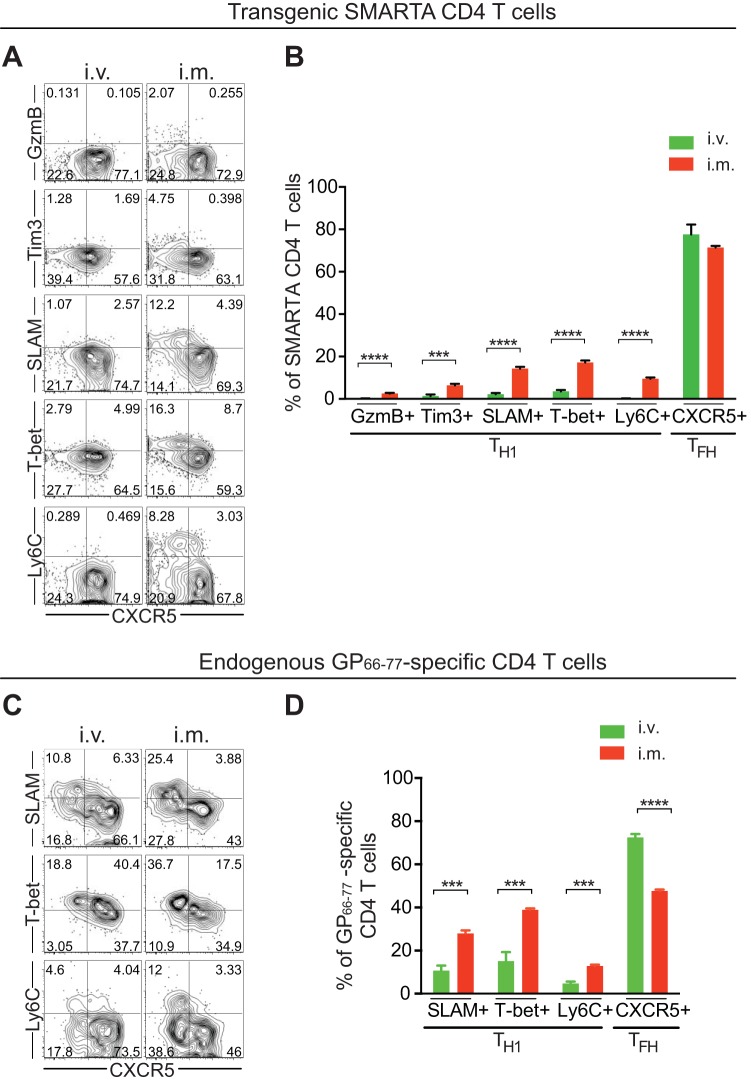
Ad5 vectors administered via the intravenous (i.v.) route result in a greater decrease in CD4 Th1 responses than intramuscular (i.m.) immunization.
The route of immunization and the subsequent delivery of antigens to different sites can impact on the phenotypes of vaccine-induced T cell responses. In the experiments above, we administered Ad5 vectors through i.m. injections, a standard route of vaccination. Alternatively, in this experiment we immunized mice with Ad5 vectors intravenously and examined the impacts of the route of administration on vaccine-induced CD4 T cell responses. SMARTA CD4 T cells were transferred into C57BL/6 mice that were subsequently given Ad5 vectors via the i.m or i.v. route. Eight days later, Ad5-elicited CD4 T cells were analyzed in the spleen. There was a trend for slightly higher numbers of SMARTA CD4 T cells following i.v. administration of Ad5 vectors than after i.m. immunization (data not shown). Compared to i.m. immunization, i.v. administration of Ad5 vectors generated even lower Th1 responses (almost absent) in the spleen, and the majority of SMARTA CD4 T cells were differentiated into Tfh cells (Fig. 6A and B). We also compared endogenous GP66-77-specific CD4 T cell responses following i.m. and i.v. immunization of Ad5 vectors. Besides further decreasing endogenous Th1 cells, i.v. immunization of Ad5 vectors also increased Tfh cells compared to i.m. immunization (Fig. 6C and D). Therefore, Ad5 immunization via either the i.m. or i.v. route led to significantly reduced Th1 responses compared to those induced by LCMV infection. Interestingly, Th1 development was further impaired following i.v. immunization compared to i.m. immunization of Ad5 vectors.
FIG 6.
Ad5 vectors administered via the i.v. route impair Th1 development further than via i.m. immunization. (A and B) SMARTA chimeric mice were generated and immunized with Ad5 vectors via the i.m. or i.v. route. (A) Representative FACS plots showing the phenotype of SMARTA CD4 T cells in the spleen at day 8 postimmunization. (B) The frequency of Th1 or Tfh SMARTA CD4 T cells. (C and D) Following i.m. or i.v. administration of Ad5 vectors, endogenous GP66-77-specific (MHC class II tetramer+) CD4 T cells were analyzed in the spleen at day 8 postimmunization. (C) Representative FACS plots showing the phenotype of GP66-77-specific CD4 T cells. (D) The frequency of GP66-77-specific Th1 or Tfh cells. Data are representative of 2 independent experiments with 3 to 5 mice per group per experiment. Error bars indicate standard errors of the means. ***, P < 0.001; ****, P < 0.0001.
Regardless of the vector dose administered, Ad5 immunization generates suboptimal Th1 responses compared to those induced in LCMV infection.
The dose of vector administered can also influence vaccine-induced immune responses. All experiments so far were performed with administration of 1010 virus particles (vp) of Ad5 vectors. To examine the effects of the vector dose on the quantity and quality of Ad5-elicited CD4 T cells, mice were given 108, 109, or 1010 vp of Ad5 vectors, and CD4 T cell responses were assessed at day 8 postimmunization. Interestingly, there were no significant dose-dependent effects on the magnitude of transgene-specific CD4 T cell responses. (Fig. 7A). However, various doses of Ad5 vectors generated CD4 T cells with somewhat different phenotypes. Ad5 vectors at the lower doses tended to generate more Th1 cells and fewer Tfh cells (Fig. 7B and C). Still, the lower doses of Ad5 did not raise effective Th1 responses above those induced in LCMV infection. Although the increased frequency of T-bethi CXCR5− or IFN-γ-producing SMARTA CD4 cells at 108 vp of Ad5 vectors was similar to that in LCMV infection, SLAM and Ly6C expression of Ad5-elicited CD4 T cells was lower than that in LCMV infection. In particular, granzyme B and Tim3 expression levels on Ad5-elicited SMARTA CD4 T cells were significantly lower than that in LCMV infection, irrespective of the dose administered. Similar results were obtained when endogenous GP66-77-specific CD4 T cells were analyzed following administration of the lower doses (108 or 109 vp) of Ad5 vectors. More GP66-77-specific Th1 cells and fewer Tfh cells were observed with 109 vp as well as 108 vp of Ad5 vectors compared to those at 1010 vp (data not shown). Therefore, the dose of Ad5 vectors impacted Th1/Tfh differentiation to some degree; however, regardless of the dose administered, immunization with Ad5 vectors resulted in suboptimal Th1 responses compared to those in LCMV infection.
FIG 7.
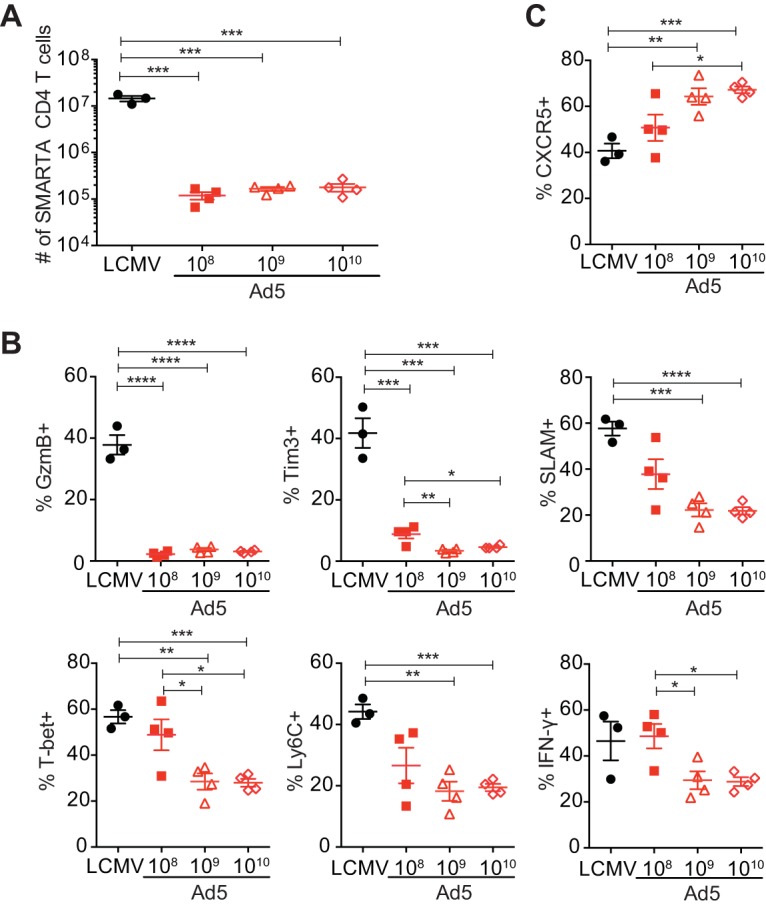
Regardless of the dose delivered, Ad5 vectors do not raise effective Th1 responses compared to those induced with LCMV infection. SMARTA chimeric mice were generated and immunized with 108, 109, or 1010 vp of Ad5 vector or infected with LCMV Armstrong. SMARTA CD4 T cells were analyzed in the spleen at day 8 postimmunization or infection. (A) The total number of SMARTA CD4 T cells. (B) The frequency of Th1 cells (CXCR5− SMARTA CD4 T cells expressing granzyme B [GzmB], Tim3, SLAM, T-bet, and Ly6C or SMARTA CD4 T cells producing IFN-γ after ex vivo stimulation with GP61-80 peptide). (C) The frequency of CXCR5+ Tfh cells. Data are representative of 2 independent experiments with 4 mice per group per experiment. Error bars indicate standard errors of the means. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001.
Impaired CD4 Th1 commitment following Ad5 immunization.
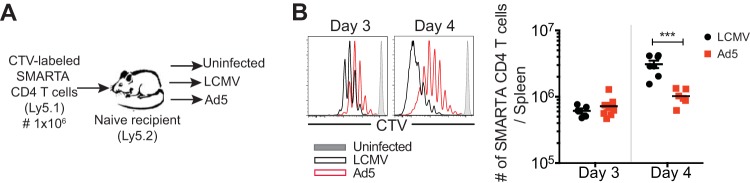
We observed that Ad5-elicited CD4 T cell counts were substantially lower in magnitude than LCMV-induced CD4 T cell counts. Consequently, we sought to determine whether the decreased expansion of Ad5-elicited CD4 T cells was due to slow proliferation. Cell trace violet-labeled SMARTA CD4 T cells were transferred into naive recipients that were subsequently immunized with Ad5-LCMV GP vectors, infected with LCMV, or remained uninfected (Fig. 8A). At 3 and 4 days later, early proliferation of SMARTA CD4 T cells was examined. Ad5-induced SMARTA CD4 T cells were less proliferative than those in LCMV infection, and this difference was more significant at day 4 postimmunization, as indicated by slow decay of cell trace violet intensity (Fig. 8B, left). As a result, significantly fewer SMARTA CD4 T cells were detected in spleens at day 4 post-Ad5 immunization (Fig. 8B, right).
FIG 8.
Ad5-elicited CD4 T cells are less proliferative than those induced by LCMV infection. Cell trace violet (CTV)-labeled SMARTA CD4 T cells (CD45.1+) were transferred into C57BL/6 mice (CD45.2+) that were subsequently immunized with Ad5-LCMV GP vectors, infected with LCMV, or not immunized or infected. Three and 4 days later, proliferation of SMARTA CD4 T cells from the spleen was analyzed. (A) Experimental setup. (B, left) Proliferation of SMARTA CD4 T cells. Representative histograms gated on SMARTA CD4 T cells show the cell trace violet intensity distribution. (Right) The absolute number of SMARTA CD4 T cells. Data are representative of 2 independent experiments with 2 to 4 mice per group per experiment. Error bars indicate standard errors of the means. ***, P < 0.001.
Fate decisions of naive CD4 T cells occur within the first few rounds of cell division (14, 15). In LCMV infection, it has been shown that CD4 T cells rapidly bifurcate into a Th1 versus Tfh differentiation program by day 3 postinfection. To confirm whether early commitment of CD4 T cells marks their effector phenotypes, we analyzed differentiation of SMARTA CD4 T cells 3 and 4 days after Ad5 immunization. At these early time points, SMARTA CD4 T cells elicited by Ad5 immunization or LCMV infection displayed remarkably different phenotypes, which reflected their effector cell differentiation (Fig. 9A). In LCMV infection, both Th1-like and Tfh-like SMARTA CD4 T cells were found in the spleen at days 3 and 4 postinfection. Strikingly, however, following Ad5 immunization, SMARTA CD4 T cells did not upregulate Th1-associated molecules, such as Tim3, SLAM, and Ly6C, and expressed significantly lower levels of T-bet than SMARTA CD4 T cells after LCMV infection, both at days 3 and 4 postimmunization. On the other hand, both Ad5 immunization and LCMV infection induced a similar frequency of SMARTA CD4 T cells expressing CXCR5. Following Ad5 immunization, the majority of SMARTA CD4 T cells expressed a high level of folate receptor 4 (FR4), which is also a Tfh marker (16). Expression of the cytotoxic molecule granzyme B was minimal in SMARTA CD4 T cells following Ad5 immunization (Fig. 9B). Ad5-induced SMARTA CD4 T cells also produced less IFN-γ and TNF. IL-2 production was not significantly different between Ad5-elicited and LCMV-elicited SMARTA CD4 T cells. Together, while antigen-specific CD4 T cells exhibited distinguishable Th1 and Tfh populations early after LCMV infection, Ad5-elicited CD4 T cells showed impaired Th1 commitment.
FIG 9.
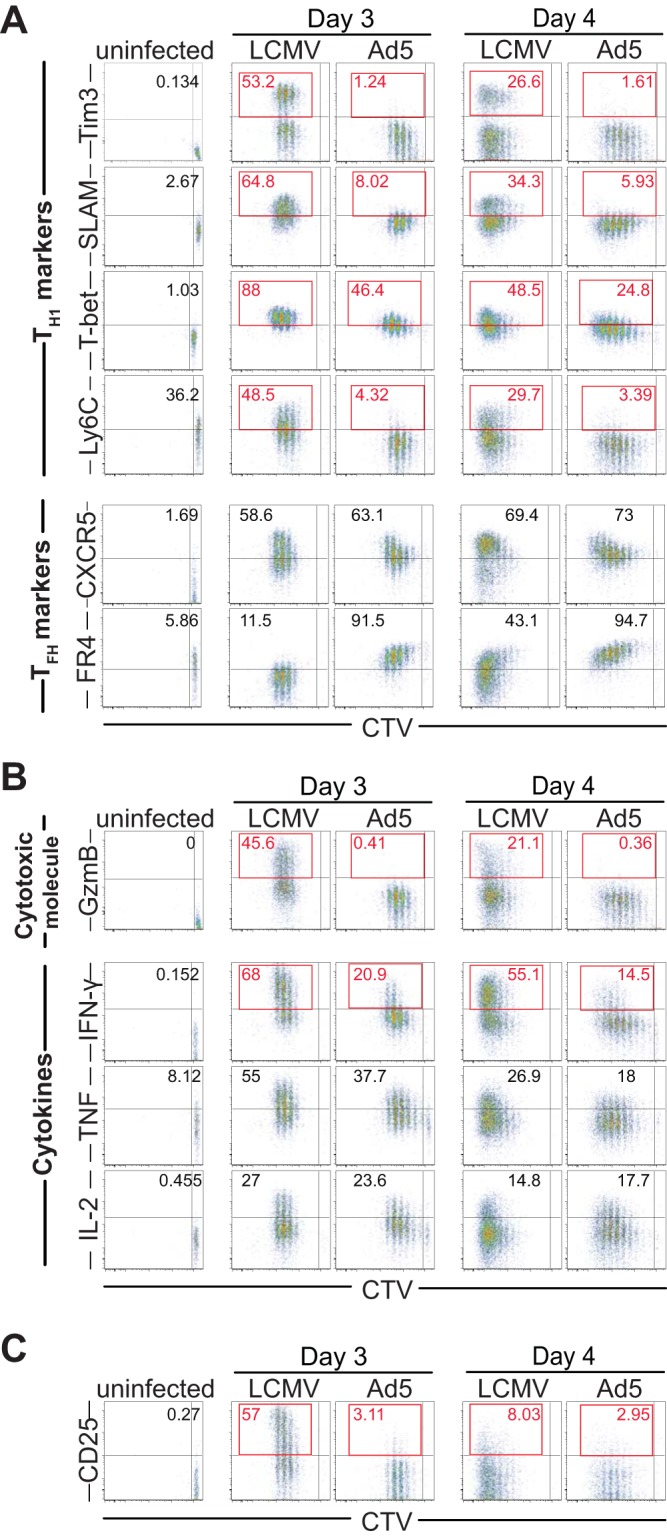
Impaired Th1 commitment following Ad5 immunization. The same experimental setup was used as shown in Fig. 8. At 3 or 4 days after Ad5 immunization or LCMV infection, differentiation of SMARTA CD4 T cells was analyzed in the spleen. Representative FACS plots gated on SMARTA CD4 T cells show the expression of Th1 and Tfh markers (A), cytotoxic molecule and cytokines (B), and CD25 (C), together with cell trace violet (CTV) dilution. The numbers in the flow plots indicate the percentages of cells corresponding to the upper right quadrant (uninfected) or the upper left quadrant (day 3 and 4 response). Data are representative of 2 independent experiments with 2 to 4 mice per group per experiment.
Notably, expression of CD25, a high-affinity IL-2 receptor alpha chain (IL-2Rα), was minimal on SMARTA CD4 T cells after Ad5 immunization, whereas SMARTA CD4 T cells markedly upregulated CD25 following LCMV infection (Fig. 9C). Given that IL-2 receptor (IL-2R)-mediated signaling is known to be required for inducing Th1 differentiation while negatively regulating Tfh development (17, 18), a reduction in IL-2 signals could contribute to suboptimal Th1 responses and skew responses toward Tfh differentiation after Ad5 immunization.
IL-2 administration following Ad5 immunization restores CD4 Th1 differentiation.
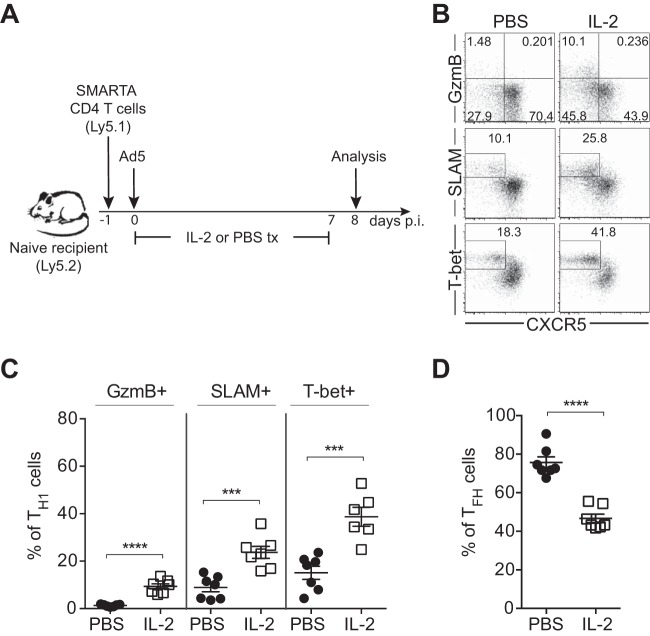
To determine whether IL-2/IL-2R signaling actually plays a role in regulating Ad5-elicited Th1 and Tfh differentiation, IL-2 was administered following immunization with Ad5 vectors. Mice were given 15,000 IU of recombinant IL-2 or PBS twice daily from the day of immunization until day 7 postimmunization before sacrifice at day 8 (Fig. 10A). IL-2 administration did not significantly affect expansion of SMARTA CD4 T cells; the numbers of SMARTA CD4 T cells in IL-2-treated and PBS-treated groups were similar at day 8 postimmunization (data not shown). However, administration of IL-2 following Ad5 immunization significantly promoted Th1 differentiation, as shown by the increased frequency of SMARTA CD4 T cells expressing granzyme B, SLAM, and T-bet, whereas the frequency of Tfh cells was decreased by IL-2 treatment (Fig. 10B, C, and D). Increased generation of Th1 cells by exogenous IL-2 indicated that attenuated IL-2 signaling in Ad5 immunization possibly plays a role in reduced Th1 responses.
FIG 10.
IL-2 administration following Ad5 immunization restores Th1 differentiation. (A) Experimental setup. SMARTA CD4 T cells were transferred into C57BL/6 mice that were subsequently immunized with Ad5 vectors. A dose of 15,000 IU of recombinant IL-2 or PBS was injected intraperitoneally (i.p.) into the mice every 12 h from the day of immunization and until day 7 postimmunization, before sacrifice at day 8. (B) Representative FACS plots showing the phenotype of SMARTA CD4 T cells in the spleen. (C and D) The frequency of Th1 (C) or Tfh (D) SMARTA CD4 T cells. Data are representative of 2 independent experiments with 3 to 4 mice per group per experiment. Error bars indicate standard errors of the means. ***, P < 0.001; ****, P < 0.0001.
DISCUSSION
Despite the crucial role of CD4 T cells in protective immunity, differentiation of transgene-specific CD4 T cells following Ad5 immunization has not been well described. In this study, we characterized Ad5-elicited CD4 T cell responses after immunizing mice with Ad5 vectors encoding LCMV GP. Immunization with Ad5 vectors generated significantly lower Th1 responses than did LCMV infection. These distinct differentiation phenotypes were also observed at early time points, indicating that commitment to Th1 cells was impaired after Ad5 immunization. Our results suggest that this impaired Th1 development is, at least partly, mediated by the attenuation of IL-2 signaling in Ad5 immunization.
CD25 (IL-2Rα) is rapidly and transiently upregulated on antigen-specific T cells following TCR activation and required for the responsiveness to IL-2 by forming high-affinity IL-2Rs along with CD122 (IL-2Rβ) and γc (the common cytokine receptor γ-chain, CD132) (17, 18). Expression of CD25 is not only regulated by TCR stimulation but also highly dependent on IL-2. IL-2 signaling through STAT5 can directly upregulate CD25, whose expression is thus enhanced via a positive feedback loop. IL-2 signaling has a decisive influence on regulating Th1 versus Tfh differentiation. IL-2-induced activation of signal transducer and activator of transcription 5 (STAT5) upregulates IL-12Rβ, increasing responsiveness to the Th1-driving cytokine IL-12 and T-bet, the Th1 master regulator (19). On the other hand, IL-2 signaling via STAT5 and the phosphatidylinositol 3-kinase (PI3K) pathway inhibits expression of B cell lymphoma 6 (Bcl6), the transcription factor directing Tfh generation (20), through several mechanisms, including induction of B lymphocyte-induced maturation protein-1 (Blimp-1) (21, 22), an antagonist of Bcl6 (23). Therefore, IL-2 signaling promotes development of Th1 cells while suppressing Tfh differentiation. In acute infection, it has been shown that expression of CD25 during CD4 T cell priming strongly correlates with Blimp-1 expression but inversely correlates with Bcl6 expression (14, 15). Following Ad5 immunization, we found that CD25 expression was markedly low on the majority of transgene-specific CD4 T cells, consistent with reduced effector Th1 differentiation. Increased Th1 responses after exogenous IL-2 administration following Ad5 immunization further confirmed that suboptimal Th1 responses were attributable, at least in part, to the decrease in IL-2 signaling.
An important question for this study was what initially causes the attenuation of IL-2 signaling and reduced CD25 expression in Ad5-elcitied CD4 T cells. Of note, significantly more FoxP3+ regulatory T cells (Tregs) were found at early time points (day 2 to day 4) and day 8 following Ad5 immunization than after LCMV infection. One of the suppressive functions of Tregs is to consume IL-2 secreted by other cells, which can limit IL-2 availability to effector T cells. Tregs constitutively express high levels of CD25, which renders them highly accessible to IL-2. A relatively low proportion of effector CD4 T cells and a high proportion of Tregs with Ad5 immunization could reduce local IL-2 concentrations and lead to attenuated IL-2 signaling during CD4 T cell priming. A recent report showed that transforming growth factor beta (TGF-β) acts to suppress CD25 expression on virus-specific CD4 T cells, thereby restricting IL-2 signaling and resulting in CD4 T cell differentiation toward Tfh from Th1 cells (24). Tregs also produce TGF-β and therefore possibly have a negative impact on IL-2 signaling. In a pilot study to determine the role of Tregs in Ad5 immunziation, we depleted Tregs in FoxP3DTR knock-in mice, in which FoxP3+ Treg cells were specifically depleted by diphtheria toxin (DT) administration. Elimination of Tregs following Ad5 immunization partly restored Th1 responses, suggesting that Tregs contribute to suboptimal Th1 responses elicited by Ad5 vectors. We also observed that CD25 expression was upregulated on Ad5-elcitied CD4 T cells in DT-treated mice, indicating that those cells were receiving more IL-2 signals upon removal of Tregs (data not shown).
The innate immune environment, including inflammatory cytokines and antigen presentation by dendritic cells (DCs), can influence CD4 T cell differentiation. Type I IFNs have been shown to promote Th1 differentiation by enhancing CD25 expression and STAT5 activation while inhibiting Tfh development (25). CD4 T cell fate decisions occur during DC priming (14). Antigen display by DCs and the duration of T-DC interactions can impact the strength of TCR signaling and CD4 T cell lineage determination (26–28). Consistent with this concept, the lower doses of Ad5 vectors tended to generate more Th1 cells and fewer Tfh cells. Further studies will be needed to determine other mechanisms, such as whether type I IFNs or DC priming could contribute to differentiation of CD4 T cells following Ad5 immunization.
Similar CD4 T cell responses were observed when SMARTA transgenic CD4 T cells and endogenous GP66-77-specific CD4 T cells were analyzed, but certain differences were also found between these two types of cells. In terms of the magnitude of CD4 T cell responses, at the peak of the response, 45-fold-fewer SMARTA CD4 T cells were detected after Ad5 immunization than after LCMV infection, whereas the number of endogenous GP66-77-specific CD4 T cells was 4.5-fold lower following Ad5 immunization compared to that after LCMV infection. In term of phenotypes, SMARTA CD4 T cells displayed relatively lower percentages of Th1 cells and higher percentages of Tfh cells compared to endogenous cells. This could be explained by different properties of TCRs on SMARTA CD4 T cells and endogenous GP66-77-specific CD4 T cells (such as different TCR affinities/avidities), since SMARTA CD4 T cells bear monoclonal TCRs whereas endogenous GP66-77-specific CD4 T cells are polyclonal. For example, SMARTA CD4 T cells exhibit a mean affinity which is ∼10-fold higher than that of endogenous GP66-77-specific cells (29). The clonal differences in TCRs can lead to various degree of proliferative capacity or lineage commitment following immunization or infection.
In this study, we investigated CD4 T cell responses induced by adenovirus vectors. Ad5 immunization resulted in suboptimal Th1 differentiation due to impaired commitment to Th1 cells. Our results demonstrated that Ad5 vectors can mediate altered effector differentiation of transgene-specific CD4 T cells compared to the original pathogen. We suggest reduced IL-2 signaling as one of the potential mechanisms that result in suboptimal Th1 responses following Ad5 immunization. IL-2 signaling has been shown to play a critical role in regulating Th1 versus Tfh differentiation in acute viral infections. Our study also demonstrated the importance of IL-2 signals in vaccine-induced CD4 T cell responses, implicating the potential of manipulating IL-2 signaling to drive favorable vaccine-induced CD4 T cell responses. Additionally, it will be interesting to examine CD4 T cell responses after immunization with alternative serotype Ad vectors in comparison to Ad5, as the vectors have different biological properties and have been shown to elicit distinct immune responses (30, 31).
MATERIALS AND METHODS
Mice and immunization/infection.
Six- to 8-week-old female C57BL/6 mice were purchased from the Jackson Laboratory (Bar Harbor, ME). SMARTA mice bearing the transgenic TCR specific to the GP66-77 epitope of LCMV (32) were bred in-house on a C57BL/6 background. For Ad5 immunization, C57BL/6 mice were immunized intramuscularly (i.m.) with 1010 vp of replication-incompetent (E1/E3 deleted) Ad5 vectors expressing full-length LCMV GP (Ad5-LCMV GP). For the experiments on alternative routes of immunization, mice were given 1010 vp of Ad5 vectors intravenously (i.v.). For the dose experiments, the lower doses (108 or 109 vp) of Ad5 vectors were administered to mice. Ad5 vectors were produced in the Fred Hutchinson Cancer Research Center and verified by restriction analysis, sequencing, and immunostaining. In parallel, mice were infected with 2 × 105 PFU of LCMV Armstrong i.p. For analysis at early time points (days 3 and 4), mice were immunized with 1010 vp of Ad5 vectors i.v. or infected with 2 × 106 PFU of LCMV Armstrong i.v. to facilitate synchronization of the activation of CD4 T cells. For DNA immunization, 200 μg of DNA vectors expressing full-length LCMV GP was administered i.m. All experiments were conducted in accordance with the Emory University Institutional Animal Care and Use committee guidelines.
Cell transfer.
To generate SMARTA chimeric mice, SMARTA CD4 T cells were isolated from the spleens of naive SMARTA mice by using a CD4+ T cell isolation kit (Miltenyi Biotech, San Diego, CA). For the analysis at day 8 and later time points, 1 × 105 purified SMARTA CD4 T cells were transferred i.v. into C57BL/6 mice 1 day before Ad5 immunization or LCMV infection. For early proliferation experiments, 1 × 106 purified SMARTA CD4 T cells were transferred to C57BL/6 mice after labeling with cell trace violet (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions.
Antibodies and flow cytometry.
All antibodies were purchased from BD Biosciences (San Jose, CA), except for CD45.1 (Biolegend, San Diego, CA), granzyme B (Invitrogen), T-bet (eBiosciences, San Diego, CA), and Tim3 (R&D Systems, Minneapolis, MN). CXCR5 staining was performed using a three-step staining protocol described previously (23). Transcription factors were stained using the FoxP3/transcription factor staining buffer set (eBiosciences). Intracellular cytokine staining was performed after 5 h of stimulation with GP61-80 peptide as described previously (33). Endogenous LCMV GP66-77-specific CD4 T cell responses were measured by staining with I-Ab GP66-77 tetramers (DIYKGVYQFKSV; National Institutes of Health [NIH] Tetramer Core Facility, Emory University, Atlanta, GA) at 37°C for 2 h. Dead cells were excluded by using Live/Dead fixable dead cell stain kits (Invitrogen). Samples were acquired using a FACSCanto II or LSR II flow cytometers (BD Biosciences). Flow cytometry data were analyzed using FlowJo software (Tree Star, Ashland, OR).
IL-2 administration.
A dose of 15,000 IU of recombinant human IL-2 (Amgen, Thousand Oaks, CA) diluted in PBS containing 0.1% normal mouse serum was administered i.p. to the mice, twice daily (every 12 h) from the day of Ad5 immunization (day 0), for 8 consecutive days until day 7 postimmunization.
ELISA.
LCMV glycoprotein-specific antibodies were measured via an enzyme-linked immunosorbent assay (ELISA). The plates were coated with LCMV glycoprotein and incubated at 4°C overnight. Serially diluted serum was added to the plates and incubated for 1.5 h. Bound serum antibodies were detected with horseradish peroxidase (HRP)-conjugated goat anti-mouse immunoglobulin G (IgG; SouthernBiotech, Birmingham, AL). The antibody titers were determined by endpoint titration.
Statistical analysis.
Data were analyzed using Prism 6 software (GraphPad, La Jolla, CA). Statistical significance was determined by using two-tailed unpaired Student's t tests. P values of less than 0.05 were considered statistically significant.
ACKNOWLEDGMENTS
We thank H. Wu for technical assistance. We acknowledge the NIH Tetramer Core Facility (contract HHSN272201300006C) for provision of MHC class II tetramers.
This work was supported by NIH grants AI030048 to R.A. and AI091493 to R.A. and M.J.M.
REFERENCES
- 1.Afkhami S, Yao Y, Xing Z. 2016. Methods and clinical development of adenovirus-vectored vaccines against mucosal pathogens. Mol Ther Methods Clin Dev 3:16030. doi: 10.1038/mtm.2016.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Majhen D, Calderon H, Chandra N, Fajardo CA, Rajan A, Alemany R, Custers J. 2014. Adenovirus-based vaccines for fighting infectious diseases and cancer: progress in the field. Hum Gene Ther 25:301–317. doi: 10.1089/hum.2013.235. [DOI] [PubMed] [Google Scholar]
- 3.Buchbinder SP, Mehrotra DV, Duerr A, Fitzgerald DW, Mogg R, Li D, Gilbert PB, Lama JR, Marmor M, Del Rio C, McElrath MJ, Casimiro DR, Gottesdiener KM, Chodakewitz JA, Corey L, Robertson MN, Step Study Protocol Team. 2008. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet 372:1881–1893. doi: 10.1016/S0140-6736(08)61591-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McElrath MJ, De Rosa SC, Moodie Z, Dubey S, Kierstead L, Janes H, Defawe OD, Carter DK, Hural J, Akondy R, Buchbinder SP, Robertson MN, Mehrotra DV, Self SG, Corey L, Shiver JW, Casimiro DR, Step Study Protocol Team. 2008. HIV-1 vaccine-induced immunity in the test-of-concept Step Study: a case-cohort analysis. Lancet 372:1894–1905. doi: 10.1016/S0140-6736(08)61592-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bassett JD, Swift SL, Bramson JL. 2011. Optimizing vaccine-induced CD8+ T-cell immunity: focus on recombinant adenovirus vectors. Expert Rev Vaccines 10:1307–1319. doi: 10.1586/erv.11.88. [DOI] [PubMed] [Google Scholar]
- 6.Tatsis N, Fitzgerald JC, Reyes-Sandoval A, Harris-McCoy KC, Hensley SE, Zhou D, Lin SW, Bian A, Xiang ZQ, Iparraguirre A, Lopez-Camacho C, Wherry EJ, Ertl HC. 2007. Adenoviral vectors persist in vivo and maintain activated CD8+ T cells: implications for their use as vaccines. Blood 110:1916–1923. doi: 10.1182/blood-2007-02-062117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang TC, Millar J, Groves T, Zhou W, Grinshtein N, Parsons R, Evelegh C, Xing Z, Wan Y, Bramson J. 2007. On the role of CD4+ T cells in the CD8+ T-cell response elicited by recombinant adenovirus vaccines. Mol Ther 15:997–1006. doi: 10.1038/sj.mt.6300130. [DOI] [PubMed] [Google Scholar]
- 8.Provine NM, Larocca RA, Penaloza-MacMaster P, Borducchi EN, McNally A, Parenteau LR, Kaufman DR, Barouch DH. 2014. Longitudinal requirement for CD4+ T cell help for adenovirus vector-elicited CD8+ T cell responses. J Immunol 192:5214–5225. doi: 10.4049/jimmunol.1302806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Provine NM, Badamchi-Zadeh A, Bricault CA, Penaloza-MacMaster P, Larocca RA, Borducchi EN, Seaman MS, Barouch DH. 2016. Transient CD4+ T cell depletion results in delayed development of functional vaccine-elicited antibody responses. J Virol 90:4278–4288. doi: 10.1128/JVI.00039-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Swain SL, McKinstry KK, Strutt TM. 2012. Expanding roles for CD4+ T cells in immunity to viruses. Nat Rev Immunol 12:136–148. doi: 10.1038/nri3152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kara EE, Comerford I, Fenix KA, Bastow CR, Gregor CE, McKenzie DR, McColl SR. 2014. Tailored immune responses: novel effector helper T cell subsets in protective immunity. PLoS Pathog 10:e1003905. doi: 10.1371/journal.ppat.1003905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hale JS, Youngblood B, Latner DR, Mohammed AU, Ye L, Akondy RS, Wu T, Iyer SS, Ahmed R. 2013. Distinct memory CD4+ T cells with commitment to T follicular helper- and T helper 1-cell lineages are generated after acute viral infection. Immunity 38:805–817. doi: 10.1016/j.immuni.2013.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crotty S. 2014. T follicular helper cell differentiation, function, and roles in disease. Immunity 41:529–542. doi: 10.1016/j.immuni.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Choi YS, Kageyama R, Eto D, Escobar TC, Johnston RJ, Monticelli L, Lao C, Crotty S. 2011. ICOS receptor instructs T follicular helper cell versus effector cell differentiation via induction of the transcriptional repressor Bcl6. Immunity 34:932–946. doi: 10.1016/j.immuni.2011.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pepper M, Pagan AJ, Igyarto BZ, Taylor JJ, Jenkins MK. 2011. Opposing signals from the Bcl6 transcription factor and the interleukin-2 receptor generate T helper 1 central and effector memory cells. Immunity 35:583–595. doi: 10.1016/j.immuni.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iyer SS, Latner DR, Zilliox MJ, McCausland M, Akondy RS, Penaloza-Macmaster P, Hale JS, Ye L, Mohammed AU, Yamaguchi T, Sakaguchi S, Amara RR, Ahmed R. 2013. Identification of novel markers for mouse CD4+ T follicular helper cells. Eur J Immunol 43:3219–3232. doi: 10.1002/eji.201343469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boyman O, Sprent J. 2012. The role of interleukin-2 during homeostasis and activation of the immune system. Nat Rev Immunol 12:180–190. doi: 10.1038/nri3156. [DOI] [PubMed] [Google Scholar]
- 18.Liao W, Lin JX, Leonard WJ. 2013. Interleukin-2 at the crossroads of effector responses, tolerance, and immunotherapy. Immunity 38:13–25. doi: 10.1016/j.immuni.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liao W, Lin JX, Wang L, Li P, Leonard WJ. 2011. Modulation of cytokine receptors by IL-2 broadly regulates differentiation into helper T cell lineages. Nat Immunol 12:551–559. doi: 10.1038/ni.2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nurieva RI, Chung Y, Martinez GJ, Yang XO, Tanaka S, Matskevitch TD, Wang YH, Dong C. 2009. Bcl6 mediates the development of T follicular helper cells. Science 325:1001–1005. doi: 10.1126/science.1176676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnston RJ, Choi YS, Diamond JA, Yang JA, Crotty S. 2012. STAT5 is a potent negative regulator of TFH cell differentiation. J Exp Med 209:243–250. doi: 10.1084/jem.20111174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ray JP, Staron MM, Shyer JA, Ho PC, Marshall HD, Gray SM, Laidlaw BJ, Araki K, Ahmed R, Kaech SM, Craft J. 2015. The interleukin-2-mTORc1 kinase axis defines the signaling, differentiation, and metabolism of T helper 1 and follicular B helper T cells. Immunity 43:690–702. doi: 10.1016/j.immuni.2015.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnston RJ, Poholek AC, DiToro D, Yusuf I, Eto D, Barnett B, Dent AL, Craft J, Crotty S. 2009. Bcl6 and Blimp-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation. Science 325:1006–1010. doi: 10.1126/science.1175870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marshall HD, Ray JP, Laidlaw BJ, Zhang N, Gawande D, Staron MM, Craft J, Kaech SM. 2015. The transforming growth factor beta signaling pathway is critical for the formation of CD4 T follicular helper cells and isotype-switched antibody responses in the lung mucosa. eLife 4:e04851. doi: 10.7554/eLife.04851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ray JP, Marshall HD, Laidlaw BJ, Staron MM, Kaech SM, Craft J. 2014. Transcription factor STAT3 and type I interferons are corepressive insulators for differentiation of follicular helper and T helper 1 cells. Immunity 40:367–377. doi: 10.1016/j.immuni.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Panhuys N. 2016. TCR signal strength alters T-DC activation and interaction times and directs the outcome of differentiation. Front Immunol 7:6. doi: 10.3389/fimmu.2016.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fazilleau N, McHeyzer-Williams LJ, Rosen H, McHeyzer-Williams MG. 2009. The function of follicular helper T cells is regulated by the strength of T cell antigen receptor binding. Nat Immunol 10:375–384. doi: 10.1038/ni.1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tubo NJ, Pagan AJ, Taylor JJ, Nelson RW, Linehan JL, Ertelt JM, Huseby ES, Way SS, Jenkins MK. 2013. Single naive CD4+ T cells from a diverse repertoire produce different effector cell types during infection. Cell 153:785–796. doi: 10.1016/j.cell.2013.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blanchfield JL, Shorter SK, Evavold BD. 2013. Monitoring the dynamics of T cell clonal diversity using recombinant peptide:MHC technology. Front Immunol 4:170. doi: 10.3389/fimmu.2013.00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tan WG, Jin HT, West EE, Penaloza-MacMaster P, Wieland A, Zilliox MJ, McElrath MJ, Barouch DH, Ahmed R. 2013. Comparative analysis of simian immunodeficiency virus gag-specific effector and memory CD8+ T cells induced by different adenovirus vectors. J Virol 87:1359–1372. doi: 10.1128/JVI.02055-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Penaloza-MacMaster P, Provine NM, Ra J, Borducchi EN, McNally A, Simmons NL, Iampietro MJ, Barouch DH. 2013. Alternative serotype adenovirus vaccine vectors elicit memory T cells with enhanced anamnestic capacity compared to Ad5 vectors. J Virol 87:1373–1384. doi: 10.1128/JVI.02058-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oxenius A, Bachmann MF, Zinkernagel RM, Hengartner H. 1998. Virus-specific MHC-class II-restricted TCR-transgenic mice: effects on humoral and cellular immune responses after viral infection. Eur J Immunol 28:390–400. doi:. [DOI] [PubMed] [Google Scholar]
- 33.Wherry EJ, Blattman JN, Murali-Krishna K, van der Most R, Ahmed R. 2003. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J Virol 77:4911–4927. doi: 10.1128/JVI.77.8.4911-4927.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]