ABSTRACT
Porcine reproductive and respiratory syndrome virus (PRRSV) is the causative agent of PRRS, which has important impacts on the pig industry. PRRSV infection results in disruption of the swine leukocyte antigen class I (SLA-I) antigen presentation pathway. In this study, highly pathogenic PRRSV (HP-PRRSV) infection inhibited transcription of the β2-microglobulin (β2M) gene (B2M) and reduced cellular levels of β2M, which forms a heterotrimeric complex with the SLA-I heavy chain and a variable peptide and plays a critical role in SLA-I antigen presentation. HP-PRRSV nonstructural protein 4 (Nsp4) was involved in the downregulation of β2M expression. Exogenous expression of Nsp4 downregulated β2M expression at both the mRNA and the protein level and reduced SLA-I expression on the cell surface. Nsp4 bound to the porcine B2M promoter and inhibited its transcriptional activity. Domain III of Nsp4 and the enhancer PAM element of the porcine B2M promoter were identified as essential for the interaction between Nsp4 and B2M. These findings demonstrate a novel mechanism whereby HP-PRRSV may modulate the SLA-I antigen presentation pathway and provide new insights into the functions of HP-PRRSV Nsp4.
IMPORTANCE PRRSV modulates the host response by disrupting the SLA-I antigen presentation pathway. We show that HP-PRRSV downregulates SLA-I expression on the cell surface via transcriptional inhibition of B2M expression by viral Nsp4. The interaction between domain III of Nsp4 and the enhancer PAM element of the porcine B2M promoter is essential for inhibiting B2M transcription. These observations reveal a novel mechanism whereby HP-PRRSV may modulate SLA-I antigen presentation and provide new insights into the functions of viral Nsp4.
KEYWORDS: β2-microglobulin, Nsp4, porcine reproductive and respiratory syndrome virus, PRRSV, SLA-I, porcine B2M promoter
INTRODUCTION
Major histocompatibility complex class I (MHC-I) antigens in pigs are termed swine leukocyte antigen class I (SLA-I), which comprises a heterotrimeric complex consisting of the SLA-I heavy chain (SLA-I HC), β2-microglobulin (β2M), and a variable peptide. In pigs, SLA-I is expressed on the surfaces of all nucleated cells to display peptide fragments derived from invading viruses for T cell recognition. This antigen presentation pathway is essential for the immune system to recognize viral peptides and initiate appropriate immune responses against invading viruses (1, 2).
The SLA-I antigen presentation pathway is tightly regulated. Typically, the SLA-I HC and β2M form a heterodimeric complex in the endoplasmic reticulum (ER). A viral peptide generated by proteasomal degradation within the virus-infected cells then binds to a groove in the SLA-I HC–β2M complex to form a heterotrimeric SLA-I complex, which is subsequently expressed on the cell surface, where it becomes available for immune surveillance by T cells (3). Because of its critical role in initiating an immune response to virus infection, the SLA-I antigen presentation pathway is targeted by viruses at all essential stages. For example, viruses may inhibit SLA-I synthesis, block the peptide-binding groove, target SLA-I for proteasomal and lysosomal degradation, or retain SLA-I in the ER (2). Targeting of the SLA-I antigen presentation pathway by viruses leads to impaired expression of SLA-I on the cell surface and subsequent suppression of the host antiviral immune response.
β2M is an approximately 13-kDa protein encoded by the B2M gene, which associates with the SLA-I HC and plays an essential role in SLA-I cell surface expression. β2M-deficient mice showed limited SLA-I expression on the cell surface, no mature CD4−8+ T cells, and defective CD4−8+ T cell-mediated cytotoxicity (4). Its critical role in the SLA-I antigen presentation pathway means that β2M is also targeted by invading pathogens. For example, the UL18 protein of human cytomegalovirus binds to β2M and affects SLA-I expression on the cell surface (5), while ESAT-6 protein of Mycobacterium tuberculosis sequesters β2M in the ER to impair SLA-I expression on the cell surface, resulting in downregulation of the SLA-I antigen presentation pathway (6).
Porcine reproductive and respiratory syndrome virus (PRRSV), also called classical PRRSV, is the causative agent of PRRS and first emerged in the United States in 1987 (7). A highly pathogenic PRRSV (HP-PRRSV) strain characterized by high and continuous fever and high morbidity and mortality emerged in China in 2006 (8). The genetic difference between HP-PRRSV and classical PRRSV strains is a unique discontinuous deletion of 30 amino acids in nonstructural protein 2 (Nsp2) of HP-PRRSV, which is a genetic marker of the HP-PRRSV strain differentiating it from the classical PRRSV strain. A PRRSV stain with the unique discontinuous deletion of 30 amino acids in Nsp2 is considered an HP-PRRSV strain (8, 9). Both classical PRRSV and HP-PRRSV strains circulate in pig herds and have important impacts on the pig industry. PRRSV (family Arteriviridae, order Nidovirales) is an enveloped RNA virus with a positive-sense single-stranded RNA genome that encodes eight structural proteins (GP2a, E, GP3, GP4, GP5, GP5a, M, and N) and at least 16 Nsps (Nsp1α, Nsp1β, Nsp2 to -6, Nsp2TF, Nsp2N, Nsp7α, Nsp7β, and Nsp8 to -12) (10). PRRSV infection results in immune suppression, including delayed, low-level induction of cytotoxic T lymphocyte responses in pigs (11), which is thought to relate to disruption of the SLA-I antigen presentation pathway (12). PRRSV infection has been shown to downregulate SLA-I expression on the cell surface (13, 14). Proteasomal degradation of SLA-I HC by HP-PRRSV Nsp1α is responsible for the downregulation of SLA-I expression on the cell surface (12). In this study, we investigated the mechanisms whereby HP-PRRSV impairs SLA-I expression and show that HP-PRRSV Nsp4 binds to the porcine B2M promoter to inhibit β2M transcription, thereby impairing SLA-I expression on the cell surface.
RESULTS
HP-PRRSV reduces SLA-I expression on the cell surface.
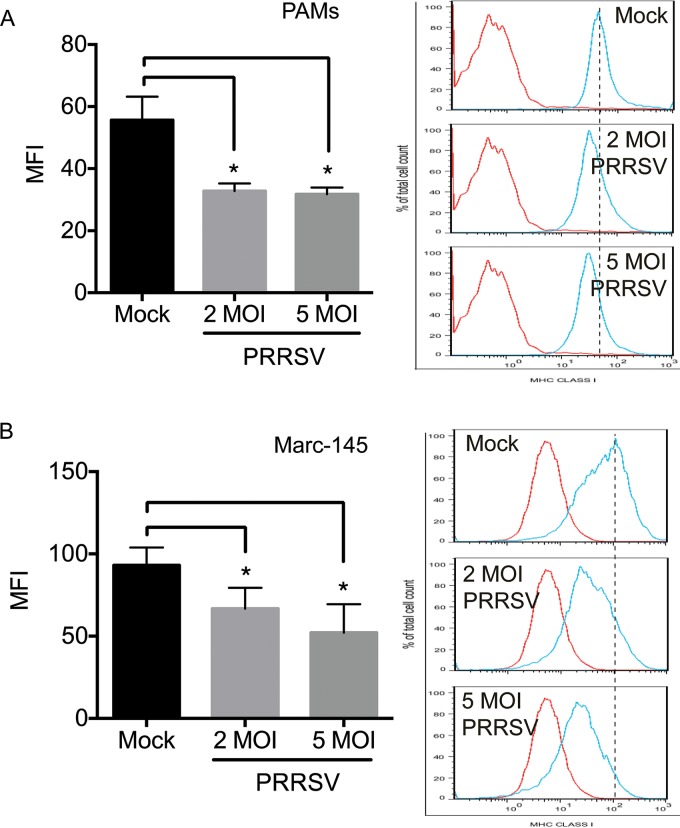
HP-PPRSV has a stronger inhibitory effect on the host antiviral immune response than classical PRRSV (15). We therefore used a strain of HP-PRRSV in the current study. Although different PRRSV strains, including HP-PPRSV and classical PRRSV strains, have been shown to downregulate SLA-I expression on the cell surface (11–14), the inhibitory effects on host antiviral immune responses vary among different PRRSV strains (15), and we therefore confirmed that the HP-PRRSV strain used in this study also downregulated SLA-I expression on the cell surface. We infected porcine alveolar macrophages (PAMs), which are the major target cells of PRRSV infection (16), with HP-PRRSV at multiplicities of infection (MOI) of 2 and 5, harvested them at 24 h postinfection (hpi), and detected SLA-I expression on the cell surface by flow cytometry using antibody against the SLA-I HC. Following HP-PRRSV infection, SLA-I expression on the surfaces of PRRSV-infected PAMs was significantly reduced compared with that of mock-infected PAMs (Fig. 1A). HP-PRRSV also inhibited SLA-I expression on the cell surfaces of Marc-145 cells, which are susceptible to PRRSV infection (17) (Fig. 1B). These observations indicate that the HP-PRRSV strain used in this study was able to downregulate SLA-I expression on the cell surface, in agreement with previous observations (11–14).
FIG 1.
Downregulation of SLA-I expression on the cell surface by HP-PRRSV infection. PAMs (A) and Marc-145 cells (B) were mock infected (Mock) or infected with HP-PRRSV at MOI of 2 and 5 and incubated for 24 h. The expression levels of SLA-I on the cell surface were measured by flow cytometry with anti-SLA-I HC monoclonal antibodies and are presented as mean fluorescence intensities (MFI). Data are presented as means ± SD from three independent experiments. *, P < 0.05 between HP-PRRSV- and mock-infected cells as determined by Student's t test.
Proteasomal degradation is partially responsible for downregulation of cellular β2M protein levels.
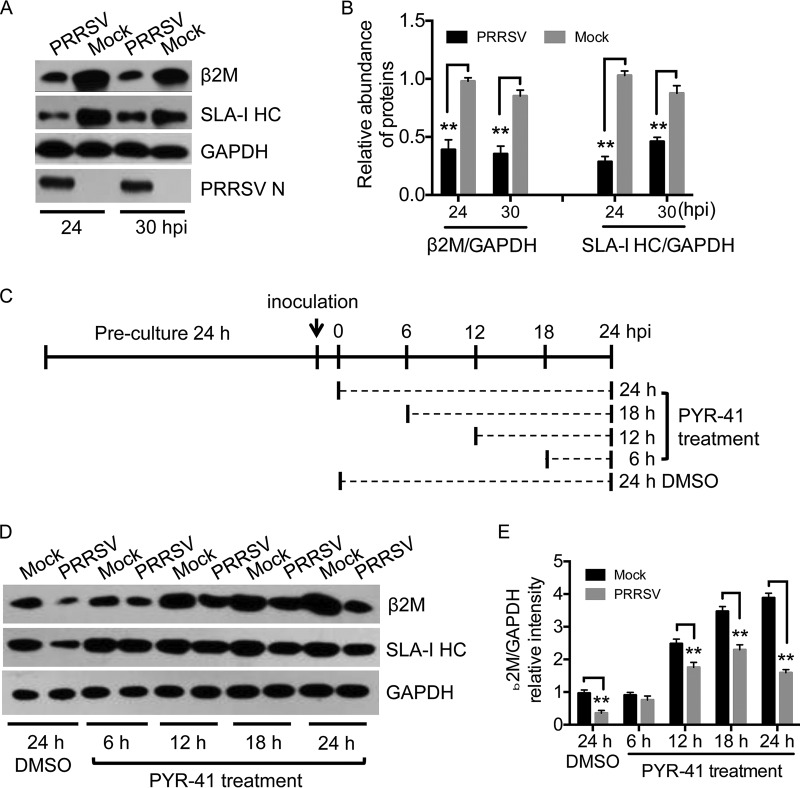
Both β2M and SLA-I HC are targeted by viruses to modulate SLA-I expression on the cell surface (3, 5). We explored the molecular basis of HP-PRRSV-induced downregulation of SLA-I expression on the cell surface by determining changes in the cellular levels of β2M and SLA-I HC proteins by Western blotting in Marc-145 cells in response to HP-PRRSV infection. HP-PRRSV replication was indicated by detection of viral N protein. Both β2M and SLA-HC expression levels were significantly reduced in HP-PRRSV-infected cells compared with those in mock-infected cells (Fig. 2A and B), demonstrating that HP-PRRSV infection reduced the cellular abundance of β2M and SLA-I HC proteins.
FIG 2.
Cellular levels of β2M protein in HP-PRRSV-infected cells. Marc-145 cells were mock infected (Mock) or infected with HP-PRRSV at an MOI of 2 and incubated at 37°C for the indicated times. Cellular levels of β2M, the SLA-I HC, GAPDH, and PRRSV N were detected by Western blotting. (A) Infected Marc-145 cells were harvested at 24 and 30 h postinfection (hpi) and subjected to Western blot analysis. (B) Intensities of protein bands were determined by densitometric analysis. Relative cellular levels of β2M and the SLA-I HC were normalized to GAPDH levels and are presented relative to the level in mock-infected cells at 24 hpi (set as 1). (C) Schematic representation of PYR-41 treatment. Infected Marc-145 cells were treated with PYR-41 at 125 μM or mock treated with DMSO and incubated at 37°C for the indicated times. (D) Cellular levels of the indicated proteins in PYR-41-treated cells were determined by Western blotting. (E) Intensities of protein bands were determined by densitometric analysis. Relative cellular levels of β2M were normalized to GAPDH levels and are presented relative to the levels in mock-infected and DMSO-treated cells (set as 1). Data are presented as means ± SD from three independent experiments. **, P < 0.01 between the indicated groups analyzed by Student's t test.
Targeting of the SLA-I complex for proteasomal degradation is a common strategy employed by viruses to modulate the SLA-I antigen presentation pathway (3). We determined if proteasomal degradation contributed to the downregulation of cellular β2M and SLA-I HC proteins in HP-PRRSV-infected cells by treating HP-PRRSV- and mock-infected Marc-145 cells with PYR-41 (18) to block downstream ubiquitination and ubiquitination-dependent proteasomal degradation (Fig. 2C), followed by Western blot analysis. HP-PRRSV infection reduced the cellular levels of β2M and SLA-I HC proteins at 24 h with dimethyl sulfoxide (DMSO) (Fig. 2D), consistent with the results in Fig. 2A. PYR-41 treatment blocked the downregulation of SLA-I HC by HP-PRRSV and restored it to mock-infection levels (Fig. 2D), suggesting that proteasomal degradation was responsible for SLA-I HC downregulation, in accordance with previous observations (11). However, although β2M protein levels in HP-PRRSV-infected cells treated with PYR-41 for 12, 18, and 24 h were higher than in HP-PRRSV-infected cells treated with DMSO for 24 h (Fig. 2D), levels were still significantly lower than in mock-infected cells treated with PYR-41 (Fig. 2D and E). These results indicate that proteasomal degradation is only partially responsible for the downregulation of β2M by PRRSV and that additional mechanisms may be involved in this process.
HP-PRRSV downregulates β2M expression at the transcriptional level.
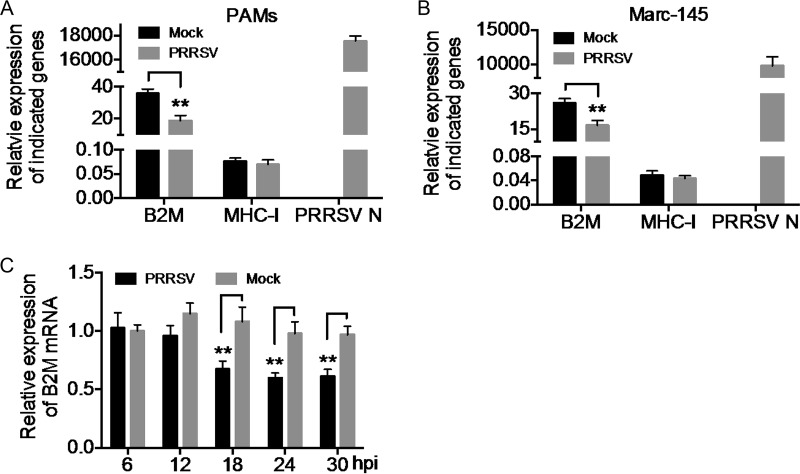
We explored the additional mechanisms responsible for the downregulation of β2M by analyzing the effect of HP-PRRSV infection on β2M expression at the transcriptional level by quantitative real-time reverse transcription-PCR (qRT-PCR). The mRNA abundance of PRRSV N protein was probed to indicate PRRSV replication. mRNA levels of SLA-I, encoding the SLA-I HC, were similar in HP-PRRSV- and mock-infected PAMs (Fig. 3A), suggesting that HP-PRRSV infection had no effect on the transcription of the SLA-I gene. However, B2M mRNA levels were significantly reduced in HP-PRRSV-infected PAMs compared with levels in mock-infected PAMs (Fig. 3A). B2M gene expression was also reduced in Marc-145 cells infected with HP-PRRSV (Fig. 3B). We also determined the dynamics of B2M transcription in Marc-145 cells during HP-PRRSV infection. B2M gene expression was significantly reduced at 18, 24, and 30 hpi with HP-PRRSV compared with that in mock-infected cells (Fig. 3C). These results indicate that HP-PRRSV infection inhibited the transcription of the B2M gene. To the best of our knowledge, this is the first evidence of the downregulation of β2M expression by HP-PRRSV at the transcriptional level.
FIG 3.
Downregulation of β2M transcription by HP-PRRSV infection. PAMs (A) and Marc-145 cells (B) were mock infected (Mock) or infected with HP-PRRSV at an MOI of 2 and incubated at 37°C for 24 h. mRNA levels of B2M, SLA-I, and PRRSV N were determined by qRT-PCR. Relative mRNA levels of each gene were normalized to levels of the GAPDH housekeeping gene and plotted. (C) Marc-145 cells were mock infected (Mock) or infected with HP-PRRSV at an MOI of 2 and incubated at 37°C for the indicated times. mRNA levels of B2M were determined by qRT-PCR. Relative mRNA levels of B2M were normalized to GAPDH levels and are expressed relative to the level in mock-infected cells at 6 h postinfection (hpi) (set as 1). Data are presented as means ± SD from three independent experiments. **, P < 0.01 between the indicated groups analyzed by Student's t test.
Nsp4 reduces cellular levels of β2M protein and impairs SLA-I expression on the cell surface.
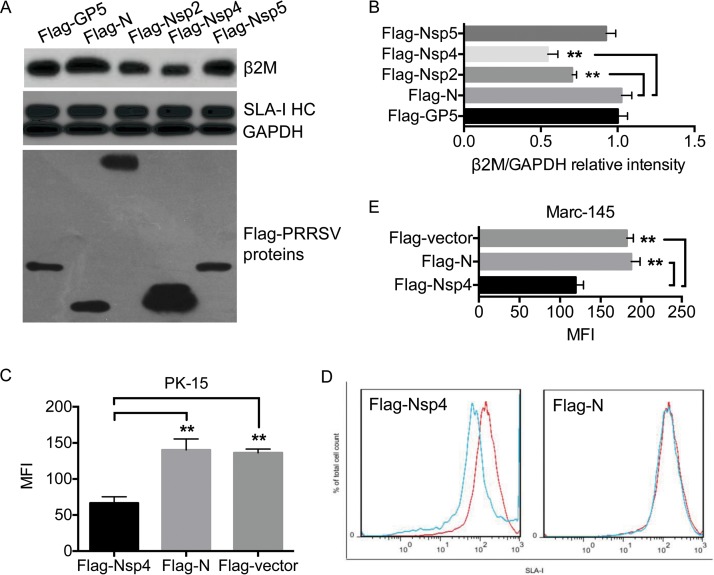
Given that the Tat protein of human immunodeficiency virus downregulates β2M expression (19), we screened for HP-PRRSV proteins responsible for downregulating β2M expression. Five Flag-tagged HP-PRRSV proteins (Flag-GP5, Flag-N, Flag-Nsp2, Flag-Nsp4, and Flag-Nsp5) were transiently expressed in Marc-145 cells, and their effects on β2M and SLA-I HC protein levels were determined by Western blotting. None of the expressed viral proteins had any significant effect on the cellular abundance of SLA-I HC protein, but Flag-Nsp2 and Flag-Nsp4 significantly reduced the cellular abundance of β2M protein (Fig. 4A and B), with Flag-Nsp4 having a greater effect than Flag-Nsp2. Furthermore, Nsp4 is a 3C-like serine protease responsible for most nonstructural protein processing and for antagonizing interferon (IFN) expression (20, 21), playing an essential role in PRRSV infection, and we therefore selected Nsp4 for further studies.
FIG 4.
Nsp4 reduces the cellular level of β2M protein. (A) Marc-145 cells were transfected with the indicated plasmids and incubated at 37°C for 24 h. The expression of Flag-tagged viral proteins in the transfectants was probed by Western blotting with anti-Flag antibody. Cellular levels of β2M, the SLA-I HC, and GAPDH were detected by Western blotting. (B) Intensities of protein bands were determined by densitometric analysis. Relative cellular levels of β2M in the transfectants were normalized to GAPDH levels and are presented relative to the level in cells expressing Flag-N (set as 1). PK-15 (C and D) and Marc-145 (E) cells were transfected with plasmids expressing Flag-Nsp4, Flag-N, or the Flag-vector and incubated at 37°C for 24 h. Expression levels of SLA-I on the cell surface were measured by flow cytometry with anti-SLA-I monoclonal antibodies and are presented as mean fluorescence intensities (MFI). Data are presented as means ± SD from three independent experiments. **, P < 0.01 between the indicated groups analyzed by Student's t test.
Nsp4 reduced β2M protein levels, and we therefore determined whether it inhibited SLA-I expression on the cell surface. We transiently expressed Flag-Nsp4 in PK-15 cells (a cell line used to analyze the effect of PRRSV on SLA-I expression [11, 12]) and determined its effect on SLA-I expression on the cell surface by flow cytometry. Flag-N was expressed as a negative control. Flag-Nsp4 significantly reduced SLA-I expression on the cell surface compared with Flag-N and the Flag-labeled vector (Flag-vector) (Fig. 4C and D). Flag-Nsp4 also significantly reduced SLA-I expression on the surfaces of Marc-145 cells (Fig. 4E). These results indicate that PRRSV Nsp4 was responsible for reducing the cellular abundance of β2M protein and impairing SLA-I expression on the cell surface.
Nsp4 binds to the porcine B2M promoter and represses its transcription.
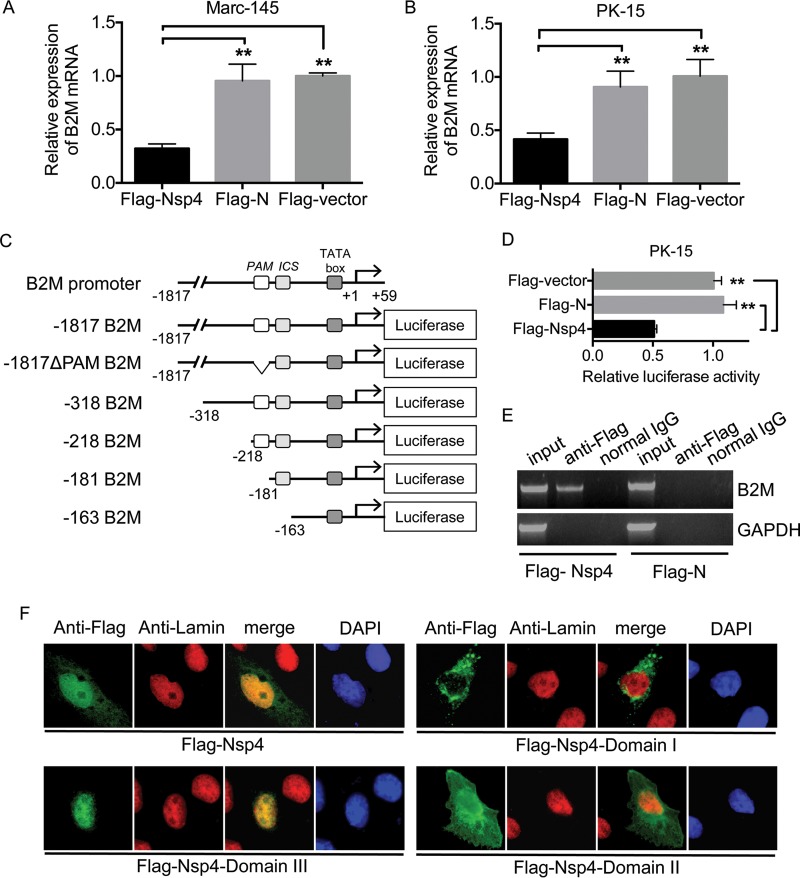
We explored the mechanisms whereby Nsp4 reduced β2M expression by examining the effect of exogenous Flag-Nsp4 expression on B2M transcription in Marc-145 and PK-15 cells. As expected, the abundance of B2M mRNA was significantly reduced in cells expressing Flag-Nsp4 compared with that in cells expressing Flag-N or the Flag-vector (Fig. 5A and B). Because of a lack of information on the porcine B2M promoter, we cloned the upstream region of the porcine B2M gene based on that of the swine reference genome (Sus scrofa Sscrofa10.2) and derived the porcine B2M promoter region from the murine B2M promoter region containing the basal promoter sequence elements and the upstream enhancers, including PAM, and the IFN consensus sequence (ICS) (22) (Fig. 5C). The cloned porcine B2M promoter from nucleotides −1817 to +59 was inserted into a pGL3 luciferase reporter vector to generate the luciferase reporter plasmid (−1817 B2M) (Fig. 5C). The inhibitory effect of Nsp4 on the porcine B2M promoter was determined by coexpression of Flag-Nsp4 and the luciferase reporter plasmid −1817 B2M in PK-15 cells. Expression of Flag-N had no significant effect on luciferase activity, but expression of Flag-Nsp4 significantly reduced the luciferase activity of −1817 B2M compared with the level resulting after expression of Flag-N or the Flag-vector (Fig. 5D), suggesting that Nsp4 represses porcine B2M promoter activity. Binding of a viral protein to a host gene promoter is a mechanism employed by viruses to repress transcription (23). We determined whether Nsp4 bound to the porcine B2M promoter by a chromatin immunoprecipitation (ChIP) assay using PK-15 cells transfected with Flag-Nsp4. Anti-Flag antibodies immunoprecipitated a DNA fragment containing part of the porcine B2M promoter ranging from nucleotides −218 to −181, as confirmed by DNA sequencing (data not shown), in cells expressing Flag-Nsp4 but not in cells expressing Flag-N (Fig. 5E). Nsp4 is known to localize in the nucleolus to inhibit IFN-β transcription (24), and we therefore detected the subcellular localization of Flag-Nsp4 expressed in PK-15 cells by immunofluorescence assay. Flag-Nsp4 was distributed mainly in the nucleolus, as demonstrated by its colocalization with the nuclear marker lamins (Fig. 5F). These results indicate that Nsp4 binds to the porcine B2M promoter and represses its transcription.
FIG 5.
Nsp4 inhibits transcription of B2M. Marc-145 (A) and PK-15 (B) cells were transfected with plasmids expressing Flag-Nsp4, Flag-N, or the Flag-vector and incubated at 37°C for 24 h. Levels of B2M mRNA in the transfectants were determined by qRT-PCR. Relative levels of B2M mRNA were normalized to levels of the GAPDH housekeeping gene and are expressed relative to the level in cells transfected with the Flag-vector (set as 1). (C) Schematic representation of the porcine B2M promoter region and luciferase reporter plasmids. (D) PK-15 cells were cotransfected with the luciferase reporter plasmid −1817 B2M and a plasmid expressing Flag-Nsp4, Flag-N, or the Flag-vector. Luciferase activities of the transfectants were analyzed at 24 h posttransfection. Luciferase activity was normalized to Renilla luciferase activity and is presented relative to the activity in cells transfected with the Flag-vector (set as 1). (E) PK-15 cells were transfected with a plasmid expressing Flag-Nsp4 or Flag-N and incubated at 37°C for 24 h. Transfectants were harvested 24 h posttransfection and subjected to ChIP assay with anti-Flag antibody. The bound DNA fractions were detected by PCR with appropriate primers (Table 1). PCR products were analyzed by 4% agarose gel electrophoresis. (F) PK-15 cells were transfected with a plasmid expressing Flag-Nsp4 or Flag-Nsp4 domains and incubated at 37°C for 24 h. The subcellular localization of the expressed Nsp4 proteins (green) was visualized by immunofluorescence assay with anti-Flag antibody. Nuclear marker lamins (red) were probed with anti-lamin A/C antibody to indicate the nuclei. Nuclei were counterstained with 4′,6-diamidino-2-phenylindole (DAPI) (blue). Data are presented as means ± SD from three independent experiments. **, P < 0.01 between the indicated groups analyzed by Student's t test.
Domain III of Nsp4 downregulates β2M protein and represses B2M promoter activity.
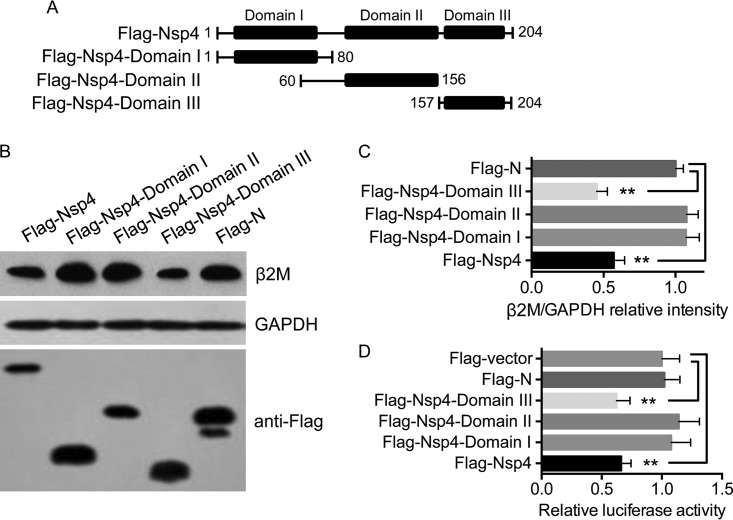
Nsp4 is folded into three domains (20) (Fig. 6A). We generated three plasmids expressing Flag-tagged mutant domains of Nsp4 to map the domain responsible for inhibiting B2M expression (Fig. 6A). We transfected Marc-145 cells with these plasmids and analyzed the cellular abundance of β2M in the transfectants by Western blotting. Flag-Nsp4 expression was associated with a significant decrease in β2M abundance compared with that produced with Flag-N expression (Fig. 6B and C), consistent with the results shown in Fig. 4A. β2M expression was significantly inhibited by expression of Flag-Nsp4-domain III, but not Flag-Nsp4-domain I or Flag-Nsp4-domain II (Fig. 6B and C), indicating that domain III of Nsp4 was responsible for the downregulation of β2M. We investigated the effect of Flag-Nsp4-domain III on porcine B2M promoter activity further and found that Flag-Nsp4-domain III, but not Flag-Nsp4-domain I or Flag-Nsp4-domain II, significantly reduced luciferase activities (Fig. 6D), suggesting that domain III of Nsp4 repressed B2M transcription. Nsp4 was localized mainly in the nucleolus, as shown in Fig. 5F. We determined whether domain III of Nsp4 was localized in the nucleolus by immunofluorescence assay. In contrast to Flag-Nsp4-domain I and Flag-Nsp4-domain II, Flag-Nsp4-domain III was distributed in the nucleolus (Fig. 5F). These observations indicate that Nsp4 domain III is responsible for the downregulation of β2M abundance and B2M promoter activity.
FIG 6.
Domain III of Nsp4 inhibits β2M expression. (A) Schematic representation of Nsp4 domains and truncated mutant Nsp4 domains. (B) Marc-145 cells were transfected with the indicated plasmids and incubated at 37°C for 24 h. Expression levels of β2M, GAPDH, and Flag-tagged viral proteins were determined by Western blotting. (C) Intensities of protein bands were determined by densitometric analysis. Relative cellular levels of β2M in the transfectants were normalized to GAPDH levels and are presented relative to the level in cells expressing Flag-N (set as 1). (D) PK-15 cells were cotransfected with the luciferase reporter plasmid −1817 B2M and the plasmid expressing Flag-Nsp4, the Flag-Nsp4 domains, Flag-N, or the Flag-vector. Luciferase activities of the transfectants were analyzed at 24 h posttransfection. Luciferase activity was normalized to Renilla luciferase activity and is presented relative to the activity in cells transfected with the Flag-vector (set as 1). Data are presented as means ± SD from three independent experiments. **, P < 0.01 between the indicated groups analyzed by Student's t test.
Domain III of Nsp4 binds to the PAM element of the B2M promoter to repress B2M transcriptional activity.
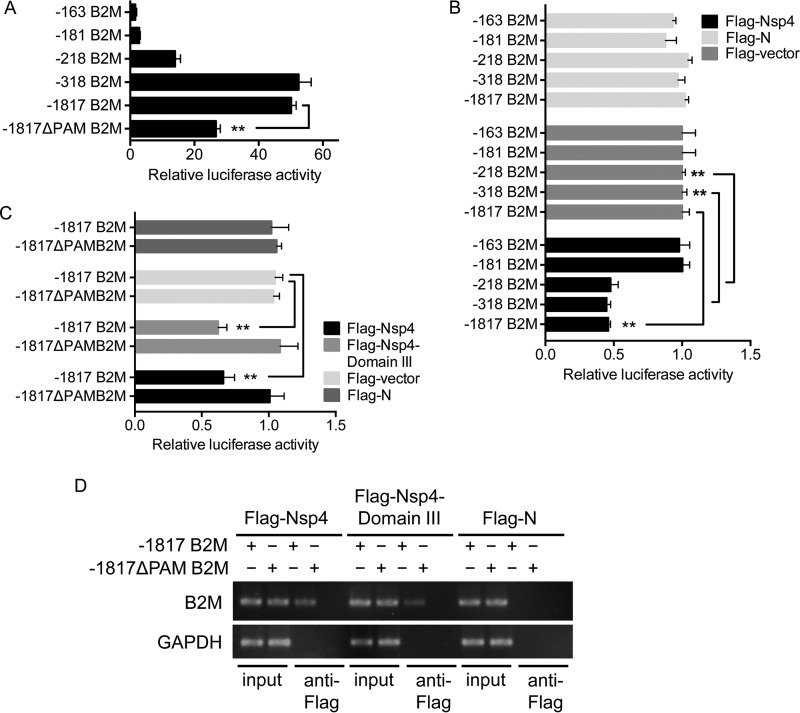
We mapped the region of the porcine B2M promoter targeted by Nsp4 by inserting a series of truncated B2M promoters into a pGL3 luciferase reporter vector to generate luciferase reporter plasmids (Fig. 5C). The transcriptional activity of each truncated B2M promoter was determined in PK-15 cells by luciferase assay. Truncation of the B2M promoter resulted in reduced luciferase activities (Fig. 7A), suggesting essential roles for the enhancer elements, including PAM and ICS (Fig. 5C). We determined the inhibitory effect of Nsp4 on the transcriptional activity of each truncated B2M promoter by coexpression of the luciferase reporter plasmids with Flag-Nsp4 in PK-15 cells, followed by determination of the luciferase activities. Cotransfection of luciferase reporter plasmids with Flag-N or the Flag-vector was used as a control. Flag-Nsp4 had no significant effect on the luciferase activities of the −163 B2M and −181 B2M truncation B2M promoters but significantly reduced the luciferase activities of the −218 B2M, −318 B2M, and −1817 B2M truncation promoters (Fig. 7B). This implies that the Nsp4-targeted region might be located between nucleotides −181 and −218, in accordance with the result of the ChIP assay (Fig. 5E). Analysis of the sequence between nucleotides −181 and −218 showed a putative PAM element (ATCATA) within this region (Fig. 5C). The PAM element is an enhancer identified in the murine B2M promoter (22) and targeted by the Tat protein of human immunodeficiency virus to repress B2M expression (19). We therefore determined whether the PAM element was a target of Nsp4. We deleted the PAM element from the porcine B2M promoter and determined the promoter activity of this deletion mutant (−1817ΔPAM B2M) (Fig. 5C) using the luciferase assay. Deletion of the PAM element resulted in a significant decrease in B2M promoter activity (Fig. 7A), suggesting that this element was required for porcine B2M promoter activity. We compared the inhibitory effect of Flag-Nsp4 on the transcriptional activities of the truncated B2M promoter −1817 B2M and the deletion mutant −1817ΔPAM B2M. Flag-Nsp4 significantly reduced the promoter activity of −1817 B2M but showed no significant inhibitory effect on the promoter activity of −1817ΔPAM B2M (Fig. 7C). Given that Nsp4 domain III inhibited B2M promoter activity (Fig. 6D), we also compared the inhibitory effects of Flag-Nsp4-domain III on the transcriptional activities of the truncated B2M promoter −1817 B2M and the deletion mutant −1817ΔPAM B2M. As expected, Flag-Nsp4-domain III did not significantly inhibit the promoter activity of −1817ΔPAM B2M (Fig. 7C). These results indicate that the PAM element is the target of Nsp4. To determine whether Nsp4-domain III bound to the PAM element, we cotransfected the truncated B2M promoter −1817 B2M or the deletion mutant −1817ΔPAM B2M into Vero cells with Flag-Nsp4, Flag-Nsp4-domain III, or Flag-N for ChIP assays. Both Flag-Nsp4 and Flag-Nsp4-domain III immunoprecipitated a DNA fragment that contained part of the porcine B2M promoter in cells transfected with the truncated B2M promoter −1817 B2M. However, no DNA fragment was immunoprecipitated by Flag-Nsp4 or Flag-Nsp4-domain III in cells transfected with the deletion mutant −1817ΔPAM B2M (Fig. 7D). These data suggest that the PAM element is essential for Nsp4-domain III binding to the B2M promoter and therefore for repressing B2M promoter activity.
FIG 7.
The PAM element is necessary for Nsp4-domain III binding to B2M promoter. (A) PK-15 cells were transfected with the indicated luciferase reporter plasmids containing a series of truncated B2M promoters. The transcriptional activity of each truncated B2M promoter was determined at 24 h posttransfection using luciferase assay and normalized to Renilla luciferase activity. (B) PK-15 cells were cotransfected with the indicated luciferase reporter plasmids and a plasmid expressing Flag-Nsp4, Flag-N, or the Flag-vector. The transcriptional activity of each truncated B2M promoter was determined at 24 h posttransfection using a luciferase assay. The luciferase activity of each transfectant was normalized to Renilla luciferase activity and is presented relative to the activity in cells cotransfected with each truncated B2M promoter and the Flag-vector (set as 1). (C) PK-15 cells were cotransfected with a luciferase reporter plasmid containing −1817 B2M or −1817ΔPAM B2M and a plasmid expressing Flag-Nsp4, Flag-Nsp4-domain III, Flag-N, or the Flag-vector. The transcriptional activity of each truncated B2M promoter was determined at 24 h posttransfection by a luciferase assay. The luciferase activity of each transfectant was normalized to Renilla luciferase activity and is presented relative to the activity in cells cotransfected with each truncated B2M promoter and the Flag-vector (set as 1). (D) Vero cells were cotransfected with a luciferase reporter plasmid containing −1817 B2M or −1817ΔPAM B2M and a plasmid expressing Flag-Nsp4, Flag-Nsp4-domain III, or Flag-N. The transfectants were harvested 24 h posttransfection and subjected to a ChIP assay with anti-Flag antibody. The bound DNA fractions were detected by PCR with appropriate primers (Table 1). The PCR products were analyzed by 4% agarose gel electrophoresis. Data are presented as means ± SD from three independent experiments. **, P < 0.01 between the indicated groups analyzed by Student's t test.
DISCUSSION
Disruption of the SLA-I antigen presentation pathway is thought to be employed by PRRSV as a strategy for modulating the host immune response (11–14), although the molecular basis of this process is unclear. PRRSV was shown previously to downregulate SLA-I expression on the cell surface via viral Nsp1α-mediated proteasomal degradation of the SLA-I HC (11). In this study, we found that HP-PRRSV downregulated SLA-I expression on the cell surface via transcriptional inhibition of B2M expression by viral Nsp4, representing a novel mechanism employed by HP-PRRSV to modulate the SLA-I antigen presentation pathway.
We confirmed that the HP-PRRSV strain used in this study was able to downregulate SLA-I expression on the cell surface and showed that cellular levels of β2M and SLA-I HC proteins were significantly decreased in response to HP-PRRSV infection. Proteasomal degradation was responsible for the downregulation of the SLA-I HC in HP-PRRSV-infected cells. These observations were consistent with those of previous studies (11). However, proteasomal degradation was only partially responsible for the downregulation of β2M, given that inhibition of the ubiquitin-proteasome pathway by the chemical inhibitor PYR-41 (18) did not restore β2M to mock-infection levels. We further showed that HP-PRRSV infection inhibited transcription of the B2M gene, encoding β2M, by targeting the enhancer PAM element of the porcine B2M promoter. To the best of our knowledge, this is the first evidence for targeting of the porcine B2M promoter by PRRSV and thereby of its downregulation of B2M transcription as well as β2M protein levels to modulate the SLA-I antigen presentation pathway.
A previous study described by Du et al. (11) showed that proteasomal degradation was responsible for the downregulation of β2M during HP-PRRSV infection. However, we observed that the proteasomal degradation was only partially responsible for the downregulation of β2M. This apparent discrepancy may be attributable to differences in the durations of chemical inhibitor treatment between our and Du et al.'s experiments. Du et al. treated virus-infected cells with the inhibitor MG132 for 8 h and observed a restoration of β2M to mock-infection levels (11). We also observed the restoration of β2M in virus-infected cells to mock-infection levels at 6 h posttreatment (hpt), but not at 12, 18, and 24 hpt. In addition, inhibition of the ubiquitin-proteasome pathway results in an accumulation of nonubiquitinated protein levels (25). However, no significant accumulation of the nonubiquitinated β2M level was observed in MG132-treated cells in Du et al.'s study (11), implying that the ubiquitin-proteasome pathway might not be completely blocked by MG132 treatment at this time point. We observed a significant accumulation of the nonubiquitinated β2M level at 12, 18, and 24 hpt but not at 6 hpt in PYR-41-treated cells. These data suggested that the treatment duration of ubiquitin-proteasome pathway inhibitors might be crucial for analysis of the proteasomal degradation of β2M during HP-PRRSV infection.
We identified that PRRSV Nsp4 was involved in the inhibition of B2M transcription. Exogenous expression of Nsp4 downregulated β2M expression at both the mRNA and protein levels, thereby impairing SLA-I expression on the cell surface. Nsp4 bound to the porcine B2M promoter to interfere with its transcription, and the interaction between Nsp4 domain III and the enhancer PAM element of the porcine B2M promoter was identified as essential for this inhibitory effect. Nsp4 is a 3C-like serine protease responsible for most nonstructural protein processing of PRRSV and plays an essential role in PRRSV infection (20). Nsp4 also impairs type I IFN expression by targeting the NF-κB essential modulator (NEMO) and virus-induced signaling adaptor (VISA) (10, 21), supporting Nsp4's critical role in modulating the host antiviral response. In this study, Nsp4 inhibited B2M transcription and impaired SLA-I expression on the cell surface, indicating its role in interfering with the SLA-I antigen presentation pathway and providing new insights into the functions of HP-PRRSV Nsp4.
Nsp4 consists of three domains located at amino acids 12 to 69, 89 to 153, and 157 to 199. The active site of the 3C-like serine protease of Nsp4 is located between domains I and II and harbors a canonical catalytic triad comprising Ser118, His39, and Asp64 (20). In the current study, we observed that domain III of Nsp4 bound to the enhancer PAM element of the porcine B2M promoter and contributed to inhibition of B2M transcription, suggesting that its 3C-like serine protease activity was not involved in this inhibitory activity. The function of Nsp4 domain III is currently unknown. Analysis of the crystal structure of the Nsp4 crystal indicates the presence of two patches of solvent-exposed hydrophobic residues in domain III, implying possible interactions with other molecules (20), thus reinforcing our observation of the binding of Nsp4 domain III to the B2M promoter.
A virus evolves multiple strategies to target the SLA-I antigen presentation pathway because of its critical role in initiating the host antiviral immune response (2). For example, mouse cytomegalovirus utilizes viral gp34, gp40, and gp48 to block SLA-I at the cell surface, cause its retention in the ER, and target SLA-I to lysosomes, respectively (26–28). We therefore speculated that multiple PRRSV proteins might be involved in its modulation of the SLA-I antigen presentation pathway, and Nsp1α, Nsp2TF, and GP3 have been shown to downregulate SLA-I expression on the cell surface (11, 12). In the current study, we identified Nsp4 as being involved in the downregulation of SLA-I expression on the cell surface, though Cao et al. (12) previously reported that Nsp4 had no significant effect on SLA-I expression on the cell surface. Rapid molecular evolution is known to result in diverse PRRSV isolates with multifarious pathogenicities (29), and the apparent discrepancy between our and Cao et al.'s results may be attributable to differences in the roles of Nsp4 between the PRRSV strains used. The PRRSV strain used in this study was a highly pathogenic strain, whereas the PRRSV VR2385 strain used in Cao et al.'s study (12) was a classical PRRSV strain isolated in 1994 (30).
Previous studies showed distinct effects of Nsp4 from different pathogenic PPRSV strains on the inhibition of IFN-β induction. Nsp4 in classical PRRSV has a less inhibitory effect on the promoter activities of IFN-β, IFN regulatory factors, and NF-κB than Nsp4 in HP-PRRSV (10, 21). Furthermore, compared with Nsp4 in HP-PRRSV, Nsp4 in classical PRRSV was unable to mediate the cleavage of VISA, a unique adaptor molecule essential for retinoic acid-induced gene I and melanoma differentiation-associated gene 5 signal transduction (10). These observations, together with our results, suggest that HP-PRRSV Nsp4 might play broader roles in modulating the host antiviral immune response than Nsp4 in classical PPRSV.
In conclusion, our results show that HP-PRRSV Nsp4 is involved in the downregulation of β2M expression, thereby impairing SLA-I expression on the cell surface. Nsp4 binds to the porcine B2M promoter to inhibit its transcription via interactions between domain III of Nsp4 and the enhancer PAM element of the porcine B2M promoter. These findings reveal a novel mechanism employed by HP-PRRSV to modulate the SLA-I antigen presentation pathway and provide new insights into the functions of HP-PRRSV Nsp4.
MATERIALS AND METHODS
Cells and virus infection.
Porcine alveolar macrophages (PAMs) were isolated from 5-week-old clinically healthy crossbred pigs raised in the animal facility of Shanghai Academy of Agricultural Sciences, Shanghai, China. The pigs tested negative for PRRSV antibody using a reproductive and respiratory syndrome virus antibody test kit (HerdChek PRRS 2XR; IDEXX Laboratories, Westbrook, ME, USA), as well as for PRRSV, porcine circovirus type 2, classical swine fever virus, and swine influenza virus by real-time PCR. Isolation of PAMs was performed as described previously (31). All animal experiments were performed in compliance with the Guidelines on the Humane Treatment of Laboratory Animals (Ministry of Science and Technology of the People's Republic of China, policy no. 2006 398) and were approved by the Institutional Animal Care and Use Committee at the Shanghai Veterinary Research Institute (IACUC no. Shvri-Pi-0124).
PAMs isolated from at least five pigs were pooled and cultured in RPMI 1640 medium (Thermo Fisher Scientific, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (FBS) (Thermo Fisher Scientific), penicillin (100 U/ml), and streptomycin (100 mg/ml) at 37°C in an atmosphere containing 5% CO2. Marc-145, PK-15, and Vero cell lines were cultured in Dulbecco's modified Eagle's medium (Thermo Fisher Scientific) supplemented with 10% FBS, penicillin (100 U/ml), and streptomycin (100 mg/ml) at 37°C in an atmosphere containing 5% CO2. The HP-PRRSV strain SH-PRRS01 isolated in our laboratory was used in this study. For HP-PRRSV infection, precultured cells were inoculated with HP-PRRSV at the multiplicities of infection (MOI) indicated in the figures and incubated at 37°C for 1 to 2 h. After inoculation, the inocula were removed and the cells were cultured in media containing 5% FBS for PAMs or 2% FBS for Marc-145 cells at 37°C for the indicated times. Mock-infected cells were generated using culture medium as the control inoculum.
Flow cytometry.
Flow cytometry was performed as described previously (11) to determine SLA-I expression on the cell surface. Briefly, cells precultured on six-well plates were infected with HP-PRRSV at MOI of 2 or 5 and incubated at 37°C for 24 h. The infected cells were washed gently three times with cold phosphate-buffered saline (PBS), dissociated from the plates with 0.1% EDTA, and rewashed with cold PBS. The washed cells were stained with anti-SLA-I monoclonal antibody (1:100) (Kingfisher Biotech, Saint Paul, MN, USA) for PAMs or anti-MHC-I monoclonal antibody (1:100) (W6/32; Santa Cruz Biotechnology, Santa Cruz, CA, USA) for Marc-145 cells, followed by incubation with fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse IgG antibody (1:400) (Thermo Fisher Scientific). The fluorescence intensity of the cells was assessed, and the expression level of SLA-I on the cell surface is presented as the mean fluorescence intensity.
Drug administration.
Marc-145 cells precultured on six-well plates were inoculated with HP-PRRSV at an MOI of 2 and incubated at 37°C for 24 h. Ubiquitination and ubiquitination-dependent proteasomal degradation was blocked using the chemical inhibitor of ubiquitin-activating enzyme E1, PYR-41 (Selleck Chemicals, Houston, TX, USA). PYR-41 was dissolved in culture-grade dimethyl sulfoxide (DMSO) at a concentration of 100 mM and then added at a final concentration of 125 μM into the culture media at 6, 12, 18, and 24 h before cells were harvested for Western blot analysis. DMSO was added into the media at 24 h before harvest as a control.
Plasmids and transfection.
Plasmids engineered to express Flag-tagged viral proteins, including Flag-GP5, Flag-N, Flag-Nsp2, Flag-Nsp5, Flag-Nsp4, and the Flag-Nsp4 domains, were constructed by inserting HP-PRRSV cDNA into p3xFlag-CMV-14 (Sigma, St. Louis, MO, USA). The porcine B2M promoter was cloned using total DNA extracted from PK-15 cells. DNA fragments containing the porcine B2M promoter ranging from nucleotides −1817 to +59 were amplified by PCR using appropriate primers (Table 1), designed according to the upstream sequence of the porcine B2M gene (NCBI gene ID 397033). The fragments were inserted into a pGL3 luciferase reporter vector (Promega, Madison, WI, USA) to generate a series of luciferase reporter plasmids, including −1817 B2M, −318 B2M, −218 B2M, −181 B2M, and −163 B2M. A deletion mutant of the luciferase reporter plasmid −1817ΔPAM B2M, from which the enhancer PAM element of the porcine B2M promoter was deleted, was generated by a modified PCR-based site-directed mutagenesis (32). All the above-named plasmids were verified by DNA sequence analysis. For transfection, cells precultured with appropriate media were transfected using Lipofectamine 2000 (Thermo Fisher Scientific), according to the manufacturer's protocol.
TABLE 1.
Sequences of primers used
| Name | Primer | Primer sequence (5′ to 3′) |
|---|---|---|
| B2M | Forward | CCCGAAGGTTCAGGTTTA |
| Reverse | CCAGATACATAGCAGTTCAG | |
| SLA-I HC | Forward | AGCCATCTTTCCAGTCTAC |
| Reverse | CAGTGACCACAGTTCCAA | |
| PRRSV N | Forward | ATCCAGACTGCCTTCAAT |
| Reverse | AACTCCACAGTGTAACTTATC | |
| ChIP | Forward | TCCAACCTTTGCCTTCCTA |
| Reverse | CCGTCACCCGTCTCTTAG | |
| GAPDH | Forward | TCTGGCAAAGTGGACATT |
| Reverse | GGTGGAATCATACTGGAACA | |
| MHC-I HC | Forward | GATGTGGAGGAGGAAGAG |
| Reverse | TTTACAAGCCGTGAGAGA | |
| −1817 B2M | Forward | TAGGTAGAGCCCGTAG |
| Reverse | AGGCCAGACAGTGAGA | |
| −318 B2M | Forward | CGTTGGTGCAAACTGC |
| Reverse | AGGCCAGACAGTGAGA | |
| −218 B2M | Forward | CAGTCCAACCTTTGCC |
| Reverse | AGGCCAGACAGTGAGA | |
| −181 B2M | Forward | AGACTTAGAAAATGAA |
| Reverse | AGGCCAGACAGTGAGA | |
| −163 B2M | Forward | TGAAACTGAAAACGGG |
| Reverse | AGGCCAGACAGTGAGA |
ChIP assay.
A chromatin immunoprecipitation (ChIP) assay was performed using an EZ-ChIP chromatin immunoprecipitation kit (EMD Millipore, Billerica, MA, USA) according to the manufacturer's instructions. Briefly, PK-15 or Vero cells precultured on 150-mm dishes were transfected with the plasmids indicated in Fig. 5 and incubated at 37°C for 24 h. Cells were fixed by the addition of 275 μl of 37% formaldehyde into 10 ml of medium, followed by incubation at room temperature for 10 min. Glycine was added at a concentration of 125 mM and incubated at room temperature for 5 min to quench unreacted formaldehyde. The fixed cells were washed twice with cold PBS containing the protease inhibitor cocktail supplied with the kit. The cell pellet was collected by centrifugation and resuspended in 1 ml of lysis buffer (50 mM Tris [pH 8.0], 1% sodium dodecyl sulfate, 10 mM EDTA, and 5 μg/ml sonicated salmon sperm DNA). The cell lysates were sonicated on ice to shear cross-linked DNA to fragments of about 200 to 1,000 bp in length and centrifuged at 12,000 × g for 10 min. The supernatants were diluted with the ChIP dilution buffer containing protease inhibitor cocktail and incubated with protein G agarose beads for 1 h. After a brief centrifugation, the supernatants were incubated with anti-Flag monoclonal antibody (M2; Sigma) or normal mouse IgG overnight at 4°C with rotation. The protein G agarose beads were collected by brief centrifugation and washed sequentially with low-salt immune complex wash buffer, high-salt immune complex wash buffer, LiCl immune complex wash buffer, and the Tris-EDTA (TE) buffer supplied with the kit. The washed protein G agarose beads were incubated with 200 μl of elution buffer at room temperature for 15 min and centrifuged at 4,000 × g for 1 min. The supernatants were incubated with 5 μl of 5 M NaCl at 65°C for 4 h, followed by incubation with 1 μl RNase A at 37°C for 30 min to free the DNA from the protein-DNA complexes. The supernatants were further mixed with 4 μl of 0.5 M EDTA, 8 μl of 1 M Tris-HCl, and 1 μl of proteinase K and incubated at 45°C for 2 h. The DNA was finally purified using the spin columns supplied with the kit. The bound target DNA fractions were detected by PCR with appropriate primers (Table 1). The PCR products were analyzed by 4% agarose gel electrophoresis.
Luciferase assay.
PK-15 cells precultured on 24-well plates were transfected with a combination of the luciferase reporter plasmids containing the porcine B2M promoter, control plasmid Renilla luciferase pRL-TK (Promega), and a plasmid expressing Flag-tagged viral proteins and incubated at 37°C. The transfectants were collected 24 h posttransfection for analysis of firefly luciferase activity using a dual-luciferase reporter assay system (Promega) according to the manufacturer's protocol and normalized to Renilla luciferase activity.
qRT-PCR.
Total RNA was isolated from cells using TRIzol reagent (Thermo Fisher Scientific) according to the manufacturer's instructions and transcribed into cDNA using a PrimeScript RT reagent kit with gDNA Eraser (TaKaRa, Kyoto, Japan). PCR was performed using SYBR Premix Ex Taq (TaKaRa) according to the manufacturer's protocol. The housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an internal control. Primer sequences are shown in Table 1. Relative mRNA levels of each gene were normalized to GAPDH levels. Fold change in gene expression was calculated using the 2–ΔΔCT method (33), where CT is the cycle threshold. Data are presented as fold changes in gene expression normalized to the GAPDH expression level and relative to levels in mock-infected/treated controls.
Immunofluorescence and Western blotting.
Immunofluorescence and Western blotting were performed as described previously (34). The antibodies used were anti-MHC-I monoclonal antibody (1:1,000, W6/32; Santa Cruz), anti-β2M monoclonal antibody (1:1,000; Abcam, Shanghai, China), anti-GAPDH monoclonal antibody (1:2,000; Proteintech, Chicago, IL, USA), anti-Flag monoclonal antibody (1:1,000, M2; Sigma), rabbit anti-lamin A/C antibody (1:200; Santa Cruz), goat anti-mouse IgG-FITC antibody (1:400; Santa Cruz), and Alexa Fluor 594-conjugated anti-rabbit IgG antibody (1:400; Thermo Fisher Scientific).
Statistical analysis.
All experiments were carried out in triplicate. The measured values are expressed as the means with standard deviations (SD). Significance was analyzed using Student's t tests. A P value of <0.05 was considered significant.
ACKNOWLEDGMENTS
This study was supported by the National Basic Research Program (973 Program grant 2014CB542702), the National Natural Science Foundation of China (grant 31502046), the Chinese Academy of Agricultural Sciences (grant 2015ZL065), and the LinkTADs Project (grant FP7-613804).
REFERENCES
- 1.Lunney JK, Ho CS, Wysocki M, Smith DM. 2009. Molecular genetics of the swine major histocompatibility complex, the SLA complex. Dev Comp Immunol 33:362–374. doi: 10.1016/j.dci.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 2.van de Weijer ML, Luteijn RD, Wiertz EJ. 2015. Viral immune evasion: lessons in MHC class I antigen presentation. Semin Immunol 27:125–137. doi: 10.1016/j.smim.2015.03.010. [DOI] [PubMed] [Google Scholar]
- 3.Raghavan M, Del Cid N, Rizvi SM, Peters LR. 2008. MHC class I assembly: out and about. Trends Immunol 29:436–443. doi: 10.1016/j.it.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zijlstra M, Bix M, Simister NE, Loring JM, Raulet DH, Jaenisch R. 1990. Beta 2-microglobulin deficient mice lack CD4−8+ cytolytic T cells. Nature 344:742–746. doi: 10.1038/344742a0. [DOI] [PubMed] [Google Scholar]
- 5.Browne H, Smith G, Beck S, Minson T. 1990. A complex between the MHC class I homologue encoded by human cytomegalovirus and beta 2 microglobulin. Nature 347:770–772. doi: 10.1038/347770a0. [DOI] [PubMed] [Google Scholar]
- 6.Sreejit G, Ahmed A, Parveen N, Jha V, Valluri VL, Ghosh S, Mukhopadhyay S. 2014. The ESAT-6 protein of Mycobacterium tuberculosis interacts with beta-2-microglobulin (β2M) affecting antigen presentation function of macrophage. PLoS Pathog 10:e1004446. doi: 10.1371/journal.ppat.1004446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cho JG, Dee SA. 2006. Porcine reproductive and respiratory syndrome virus. Theriogenology 66:655–662. doi: 10.1016/j.theriogenology.2006.04.024. [DOI] [PubMed] [Google Scholar]
- 8.Tian K, Yu X, Zhao T, Feng Y, Cao Z, Wang C, Hu Y, Chen X, Hu D, Tian X, Liu D, Zhang S, Deng X, Ding Y, Yang L, Zhang Y, Xiao H, Qiao M, Wang B, Hou L, Wang X, Yang X, Kang L, Sun M, Jin P, Wang S, Kitamura Y, Yan J, Gao GF. 2007. Emergence of fatal PRRSV variants: unparalleled outbreaks of atypical PRRS in China and molecular dissection of the unique hallmark. PLoS One 2:e526. doi: 10.1371/journal.pone.0000526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu X, Chen N, Wang L, Wu J, Zhou Z, Ni J, Li X, Zhai X, Shi J, Tian K. 2012. New genomic characteristics of highly pathogenic porcine reproductive and respiratory syndrome viruses do not lead to significant changes in pathogenicity. Vet Microbiol 158:291–299. doi: 10.1016/j.vetmic.2012.02.036. [DOI] [PubMed] [Google Scholar]
- 10.Huang C, Du Y, Yu Z, Zhang Q, Liu Y, Tang J, Shi J, Feng WH. 2016. Highly pathogenic porcine reproductive and respiratory syndrome virus Nsp4 cleaves VISA to impair antiviral responses mediated by RIG-I-like receptors. Sci Rep 6:28497. doi: 10.1038/srep28497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Du J, Ge X, Liu Y, Jiang P, Wang Z, Zhang R, Zhou L, Guo X, Han J, Yang H. 2015. Targeting swine leukocyte antigen class I molecules for proteasomal degradation by the nsp1α replicase protein of the Chinese highly pathogenic porcine reproductive and respiratory syndrome virus strain JXwn06. J Virol 90:682–693. doi: 10.1128/JVI.02307-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cao QM, Subramaniam S, Ni YY, Cao D, Meng XJ. 2016. The non-structural protein Nsp2TF of porcine reproductive and respiratory syndrome virus down-regulates the expression of swine leukocyte antigen class I. Virology 491:115–124. doi: 10.1016/j.virol.2016.01.021. [DOI] [PubMed] [Google Scholar]
- 13.Wang X, Eaton M, Mayer M, Li H, He D, Nelson E, Christopher-Hennings J. 2007. Porcine reproductive and respiratory syndrome virus productively infects monocyte-derived dendritic cells and compromises their antigen-presenting ability. Arch Virol 152:289–303. doi: 10.1007/s00705-006-0857-1. [DOI] [PubMed] [Google Scholar]
- 14.Park JY, Kim HS, Seo SH. 2008. Characterization of interaction between porcine reproductive and respiratory syndrome virus and porcine dendritic cells. J Microbiol Biotechnol 18:1709–1716. [PubMed] [Google Scholar]
- 15.Wang R, Zhang YJ. 2014. Antagonizing interferon-mediated immune response by porcine reproductive and respiratory syndrome virus. Biomed Res Int 2014:315470. doi: 10.1155/2014/315470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kimman TG, Cornelissen LA, Moormann RJ, Rebel JM, Stockhofe-Zurwieden N. 2009. Challenges for porcine reproductive and respiratory syndrome virus (PRRSV) vaccinology. Vaccine 27:3704–3718. doi: 10.1016/j.vaccine.2009.04.022. [DOI] [PubMed] [Google Scholar]
- 17.Kim HS, Kwang J, Yoon IJ, Joo HS, Frey ML. 1993. Enhanced replication of porcine reproductive and respiratory syndrome (PRRS) virus in a homogeneous subpopulation of MA-104 cell line. Arch Virol 133:477–483. doi: 10.1007/BF01313785. [DOI] [PubMed] [Google Scholar]
- 18.Yang Y, Kitagaki J, Dai RM, Tsai YC, Lorick KL, Ludwig RL, Pierre SA, Jensen JP, Davydov IV, Oberoi P, Li CC, Kenten JH, Beutler JA, Vousden KH, Weissman AM. 2007. Inhibitors of ubiquitin-activating enzyme (E1), a new class of potential cancer therapeutics. Cancer Res 67:9472–9481. doi: 10.1158/0008-5472.CAN-07-0568. [DOI] [PubMed] [Google Scholar]
- 19.Carroll IR, Wang J, Howcroft TK, Singer DS. 1998. HIV Tat represses transcription of the beta 2-microglobulin promoter. Mol Immunol 35:1171–1178. doi: 10.1016/S0161-5890(98)00107-2. [DOI] [PubMed] [Google Scholar]
- 20.Tian X, Lu G, Gao F, Peng H, Feng Y, Ma G, Bartlam M, Tian K, Yan J, Hilgenfeld R, Gao GF. 2009. Structure and cleavage specificity of the chymotrypsin-like serine protease (3CLSP/nsp4) of porcine reproductive and respiratory syndrome virus (PRRSV). J Mol Biol 392:977–993. doi: 10.1016/j.jmb.2009.07.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang C, Zhang Q, Guo XK, Yu ZB, Xu AT, Tang J, Feng WH. 2014. Porcine reproductive and respiratory syndrome virus nonstructural protein 4 antagonizes beta interferon expression by targeting the NF-κB essential modulator. J Virol 88:10934–10945. doi: 10.1128/JVI.01396-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lonergan M, Dey A, Becker KG, Drew PD, Ozato K. 1993. A regulatory element in the beta 2-microglobulin promoter identified by in vivo footprinting. Mol Cell Biol 13:6629–6639. doi: 10.1128/MCB.13.11.6629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jiang S, Willox B, Zhou H, Holthaus AM, Wang A, Shi TT, Maruo S, Kharchenko PV, Johannsen EC, Kieff E, Zhao B. 2014. Epstein-Barr virus nuclear antigen 3C binds to BATF/IRF4 or SPI1/IRF4 composite sites and recruits Sin3A to repress CDKN2A. Proc Natl Acad Sci U S A 111:421–426. doi: 10.1073/pnas.1321704111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rascón-Castelo E, Burgara-Estrella A, Mateu E, Hernández J. 2015. Immunological features of the non-structural proteins of porcine reproductive and respiratory syndrome virus. Viruses 7:873–886. doi: 10.3390/v7030873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Myung J, Kim KB, Crews CM. 2001. The ubiquitin-proteasome pathway and proteasome inhibitors. Med Res Rev 21:245–273. doi: 10.1002/med.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kleijnen MF, Huppa JB, Lucin P, Mukherjee S, Farrell H, Campbell AE, Koszinowski UH, Hill AB, Ploegh HL. 1997. A mouse cytomegalovirus glycoprotein, gp34, forms a complex with folded class I MHC molecules in the ER which is not retained but is transported to the cell surface. EMBO J 16:685–694. doi: 10.1093/emboj/16.4.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ziegler H, Thale R, Lucin P, Muranyi W, Flohr T, Hengel H, Farrell H, Rawlinson W, Koszinowski UH. 1997. A mouse cytomegalovirus glycoprotein retains MHC class I complexes in the ERGIC/cis-Golgi compartments. Immunity 6:57–66. doi: 10.1016/S1074-7613(00)80242-3. [DOI] [PubMed] [Google Scholar]
- 28.Reusch U, Muranyi W, Lucin P, Burgert HG, Hengel H, Koszinowski UH. 1999. A cytomegalovirus glycoprotein re-routes MHC class I complexes to lysosomes for degradation. EMBO J 18:1081–1091. doi: 10.1093/emboj/18.4.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kappes MA, Faaberg KS. 2015. PRRSV structure, replication and recombination: origin of phenotype and genotype diversity. Virology 479–480:475–486. doi: 10.1016/j.virol.2015.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meng XJ, Paul PS, Halbur PG. 1994. Molecular cloning and nucleotide sequencing of the 3′-terminal genomic RNA of the porcine reproductive and respiratory syndrome virus. J Gen Virol 75(Part 7):1795–1801. doi: 10.1099/0022-1317-75-7-1795. [DOI] [PubMed] [Google Scholar]
- 31.Delputte PL, Nauwynck HJ. 2004. Porcine arterivirus infection of alveolar macrophages is mediated by sialic acid on the virus. J Virol 78:8094–8101. doi: 10.1128/JVI.78.15.8094-8101.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li X, Qiu Y, Shen Y, Ding C, Liu P, Zhou J, Ma Z. 2008. Splicing together different regions of a gene by modified polymerase chain reaction-based site-directed mutagenesis. Anal Biochem 373:398–400. doi: 10.1016/j.ab.2007.10.021. [DOI] [PubMed] [Google Scholar]
- 33.Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔC(T) method. Methods 25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 34.Zhu Z, Shi Z, Yan W, Wei J, Shao D, Deng X, Wang S, Li B, Tong G, Ma Z. 2013. Nonstructural protein 1 of influenza A virus interacts with human guanylate-binding protein 1 to antagonize antiviral activity. PLoS One 8:e55920. doi: 10.1371/journal.pone.0055920. [DOI] [PMC free article] [PubMed] [Google Scholar]