ABSTRACT
Dengue virus (DENV) is responsible for growing numbers of infections worldwide and has proven to be a significant challenge for vaccine development. We previously demonstrated that CD8+ T cell responses elicited by a dengue live attenuated virus (DLAV) vaccine resemble those observed after natural infection. In this study, we screened peripheral blood mononuclear cells (PBMCs) from donors vaccinated with a tetravalent DLAV vaccine (TV005) with pools of dengue virus-derived predicted major histocompatibility complex (MHC) class II binding peptides. The definition of CD4+ T cell responses after live vaccination is important because CD4+ T cells are known contributors to host immunity, including cytokine production, help for CD8+ T and B cells, and direct cytotoxicity against infected cells. While responses to all antigens were observed, DENV-specific CD4+ T cells were focused predominantly on the capsid and nonstructural NS3 and NS5 antigens. Importantly, CD4+ T cell responses in vaccinees were similar in magnitude and breadth to those after natural infection, recognized the same antigen hierarchy, and had similar profiles of HLA restriction. We conclude that TV005 vaccination has the capacity to elicit CD4+ cell responses closely mirroring those observed in a population associated with natural immunity.
IMPORTANCE The development of effective vaccination strategies against dengue virus infection is of high global public health interest. Here we study the CD4 T cell responses elicited by a tetravalent live attenuated dengue vaccine and show that they resemble responses seen in humans naturally exposed to dengue virus. This is an important issue, since it is likely that optimal immunity induced by a vaccine requires induction of CD4+ responses against the same antigens as those recognized as dominant in natural infection. Detailed knowledge of the T cell response may further contribute to the identification of robust correlates of protection against dengue virus.
KEYWORDS: dengue virus, live attenuated vaccine, TV005, CD4+ T cell, CD4, HLA, MHC, T cells, live attenuated, tetravalent, vaccines
INTRODUCTION
Four dengue virus serotypes (DENV1 to -4) are responsible for a growing number of infections worldwide, making DENV infection the most frequently mosquito-transmitted viral disease in humans and creating a unique challenge for vaccine development. Any of the four DENV serotypes can cause infection and associated severe disease, involving hospitalization with dengue fever (DF), dengue hemorrhagic fever (DHF), or dengue shock syndrome (DSS). Severe clinical manifestations are particularly frequent in the case of secondary infections caused by heterotypic serotypes (1).
While considerable debate exists regarding which immune mechanisms may confer protection, a hallmark of live attenuated vaccines is their ability to induce both humoral and cellular immune memory (2). In the case of another RNA virus, influenza virus, it has been shown that only a live attenuated influenza vaccine, not an inactivated influenza vaccine, was able to elicit virus-specific T cell responses while inducing comparable antibody responses (3). The development of broadly cross-reactive neutralizing antibodies follows secondary infections, but the antibodies resulting from a previous infection are generally not cross-neutralizing and can actually be linked to antibody-dependent enhancement (ADE) of infection and disease (1, 4). Cohort studies, human challenge studies, and phase III efficacy studies indicate that antibodies alone are not a perfect correlate of protection (5–8). CD8+ responses were previously regarded as a possible correlate of disease risk, but recent data suggest that they may instead be considered a potential correlate of protection, based on mouse model data showing decreased tissue viremia after challenge (9–11) and the association of certain HLA alleles with strong CD8+ responses and decreased disease susceptibility (12).
Major histocompatibility complex (MHC) class II-restricted CD4+ T cell responses are an additional potential correlate of protection. In general, CD4+ T cells are necessary for the development and maturation of both antibody and cytotoxic T lymphocyte (CTL) responses (13, 14). In animal models of DENV disease, depletion of CD4+ T cells does not exacerbate disease, but their induction with DENV-specific T cell epitopes can protect animals from DENV challenge (15). In humans, DENV-specific CD4+ T cells display unique phenotypes, and in several cases, MHC class II alleles associated with the most vigorous responses are also associated with decreased disease susceptibility (16). These CD4+ T cells dominantly recognize the capsid (C) and the nonstructural 3 (NS3) and NS5 antigens, while the envelope (E) protein appears to be a minor target (17, 18).
Several different experimental vaccines are being developed concurrently (19). The most advanced vaccine, CYD-TDV Dengvaxia, is based on the expression of the DENV prM and E proteins in a yellow fever virus (YF 17D) backbone. Recent phase III clinical studies of the CYD-TDV vaccine supported the limited licensure of this vaccine in some areas where DENV is endemic. However, the protection against DENV2 was weak despite the presence of neutralizing antibodies, and vaccine efficacy appeared to be dependent on previous exposure to DENV (5, 8).
In order to develop accurate correlates of protection that allow comparison and contrasting of different vaccination approaches, it is necessary to first establish assays that quantify the different potentially relevant immune responses induced by vaccination. Our goal here was to establish such assays for a particular vaccine construct based on a live attenuated tetravalent vaccine, TV005, developed by the National Institute of Allergy and Infectious Diseases (20, 21). TV005 is a mix of four recombinant dengue live attenuated virus (DLAV) vaccine candidates (rDEN1Δ30, rDEN2/4Δ30, rDEN3Δ30/31, and rDEN4Δ30) and was previously shown to induce seroconversion to four DENV serotypes in 90% of vaccine recipients (21). Furthermore, recent studies showed that high levels of neutralizing antibodies correlated with sterilizing immunity in a dengue human challenge model (DHCM) after vaccination with TV003 (7). Interestingly, patients who did show an antibody boost were also protected from the primary endpoint (viremia), suggesting a role for cell-mediated immunity. The interplay between low titers, cross-reactive neutralizing antibody titers, and cell-mediated immunity in subjects who are protected is currently unknown. In previous studies, we further showed that either a monovalent or tetravalent DLAV formulation elicited vigorous CD8+ responses comparable to those observed in areas of hyperendemicity and associated with high frequencies of secondary infections and natural immunity (16). However, it is unknown whether tetravalent DLAV vaccination results in the induction of effective CD4+ responses. In the present study, we addressed this issue by measuring TV005-induced CD4+ responses and comparing them to the responses observed in the context of natural immunity. Knowledge of the vaccine-induced T cell responses will help to dissect the role of cell-mediated immunity in protection.
RESULTS
DENV-specific CD4+ T cell responses after vaccination with a tetravalent live attenuated DENV vaccine (TV005).
To investigate CD4+ T cell responses induced by tetravalent DLAV vaccination, we examined responses from 10 recipients of a tetravalent DLAV vaccine (TV005). Peripheral blood mononuclear cell (PBMC) samples from study participants were screened in gamma interferon (IFN-γ)-specific enzyme-linked immunosorbent spot (ELISPOT) assays with pools of predicted MHC class II binding peptides (HLA matched) corresponding to pools of peptides previously utilized to characterize DENV-specific CD4+ T cell responses induced by natural infection (17). Any additional predicted binders specific to the DLAVs contained in the administered vaccine were subsequently added to ensure a comprehensive analysis of vaccine-induced responses. In the case of the DENV2 component (DEN2/4Δ30), DENV2-specific peptides were predicted for the prM and E proteins, while DENV4-specific peptides were predicted for the remaining proteins.
Sets of predicted binding peptides were available for 17 of 20 instances of a given DRB1 allele being expressed in the 10 donors analyzed (Table 1). The remaining three instances corresponded to the HLA DRB1*1302 allele, which was not analyzed due to the lack of a reliable predictive algorithm at the initiation of the study. Therefore, one of the donors who was homozygous for HLA DRB1*1302 was not considered further. For the remaining nine donors, two alleles were studied for eight of the subjects and one allele for the remaining subject. T cell reactivity was detected in nine of nine (100%) vaccine recipients analyzed for at least one HLA DRB1 allele. In total, responses to 280 unique CD4+ T cell epitopes were identified.
TABLE 1.
HLA phenotypes of donors used in this study
| Donor ID | HLA DRB1 phenotype |
|
|---|---|---|
| Allele 1 | Allele 2 | |
| GW0053 | DRB1*01:02 | DRB1*03:01 |
| GW0054 | DRB1*01:02 | DRB1*14:01 |
| GW0055 | DRB1*13:02 | DRB1*13:02 |
| GW0056 | DRB1*10:01 | DRB1*11:01 |
| GW0057 | DRB1*04:01 | DRB1*0701 |
| GW0058 | DRB1*03:01 | DRB1*11:02 |
| GW0059 | DRB1*03:01 | DRB1*11:01 |
| GW0060 | DRB1*03:01 | DRB1*08:04 |
| GW0061 | DRB1*08:04 | DRB1*13:02 |
| GW0062 | DRB1*07:01 | DRB1*09:01 |
For each HLA DRB1 allele, Table 2 lists the number of donors tested who expressed that specific allele, the average number of epitopes detected for each donor, the average magnitude of the response detected for each positive response restricted by the allele, and the sum total response of all positive responses restricted by that allele (averaged over the number of donors tested to normalize for the various numbers of donors expressing the HLA DRB1 alleles). Across the nine HLA alleles studied, the response magnitude was, on average, 25,969 spot-forming cells (SFC)/106 PBMCs, with an average repertoire breadth of 20 epitopes per HLA allele and an average magnitude of 941 SFC/epitope. As shown, the response varied among the different HLA alleles, with the highest response associated with HLA DRB1*0401 and the lowest response associated with HLA DRB1*1404, with no responses detected with this allele.
TABLE 2.
Comparison of CD4+ T cell immune responses between TV005 immunization and natural infection
| HLA allele | TV005 immunization |
Natural infectiona |
||||||
|---|---|---|---|---|---|---|---|---|
| No. of donors tested per HLA allele | Avg no. of epitopes per donor | Avg response per epitope (SFC/106 PBMCs) | Avg response per donor (SFC/106 PBMCs) | No. of donors tested per HLA allele | Avg no. of epitopes per donor | Avg response per epitope (SFC/106 PBMCs) | Avg response per donor (SFC/106 PBMCs) | |
| DRB1*0101 | 2 | 7 | 898 | 5,037 | 10 | 7 | 607 | 5,447 |
| DRB1*0301 | 4 | 11 | 784 | 9,670 | 10 | 3 | 657 | 1,858 |
| DRB1*0401 | 1 | 76 | 1,530 | 110,600 | 8 | 13 | 2,265 | 28,467 |
| DRB1*0701 | 2 | 35 | 1,463 | 51,253 | 9 | 10 | 1,043 | 11,159 |
| DRB1*0802 | 2 | 10 | 682 | 7,703 | 9 | 3 | 1,310 | 4,195 |
| DRB1*0901 | 1 | 2 | 672 | 2,322 | 2 | 13 | 929 | 11,417 |
| DRB1*1001 | 1 | 15 | 1,291 | 19,060 | 7 | 8 | 851 | 7,145 |
| DRB1*1101 | 3 | 26 | 1,153 | 28,078 | 8 | 2 | 744 | 1,421 |
| DRB1*1404 | 1 | 0 | 0 | 0 | 10 | 7 | 768 | 6,313 |
| Avg | 2 | 20 | 941 | 25,969 | 8 | 7 | 1,019 | 8,602 |
As previously reported (14).
The set of peptides tested in the present study was also previously tested in the context of natural infection with dengue virus (17). This allowed for a comparison of the magnitudes of responses observed for the various peptide sets for HLA-matched donors following either natural infection or experimental vaccination with dengue virus. Accordingly, the average number of epitopes detected for each donor, the average magnitude, and the sum total of responses restricted by each allele previously reported for natural infection are shown in Table 2. We observed that TV005-induced responses were associated with trends toward larger numbers of epitopes (20, on average, versus 7; P = 0.2478 [not significant] by the Mann-Whitney test), similar magnitudes of responses/epitopes (941 versus 1,019; P = 0.8427 [not significant] by the Mann-Whitney test), and higher overall magnitudes of responses (25,969 versus 8,602; P = 0.3799 [not significant] by the Mann-Whitney test).
Vaccine-induced CD4+ T cells dominantly recognize the capsid, NS2A, and NS5 proteins, highlighting distinct immunodominance patterns for CD4+ versus CD8+ responses.
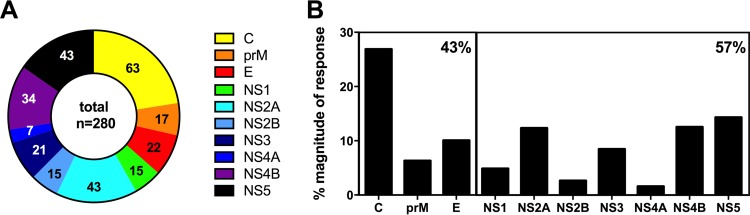
Figure 1A shows numbers of unique epitopes as a function of the proteins from which they are derived, while Fig. 1B shows the distribution of the relative magnitudes of responses for the three structural (C, prM, and E) and seven nonstructural (NS1 to NS5) proteins. The C, NS5, and NS2A proteins were the most dominantly recognized CD4+ antigens, accounting for slightly over 50% of either the number of epitopes or overall responses. In total, approximately 60% of the total response was associated with NS proteins and 40% with structural proteins. This is in contrast to what was previously reported for CD8+ responses, which were remarkably focused on the nonstructural proteins, as NS3 and NS5 together accounted for 97% of the response (16). That is, while the NS3 and NS5 antigens are dominant for both CD4+ and CD8+ responses, C and NS2A are dominant only for CD4+, not CD8+, responses (12). Finally, the prM and E proteins were found to be lesser targets for both CD4+ and CD8+ responses.
FIG 1.
Protein locations of identified epitopes. (A) Numbers of unique epitopes plotted as a function of the proteins from which they are derived. Yellow, capsid; orange, membrane precursor (prM); red, envelope (E); green, NS1; turquoise, NS2A; blue, NS2B; dark blue, NS3; purple, NS4A; pink, NS4B; black, NS5. Numbers represent the actual numbers of epitopes identified. (B) Distribution of the relative magnitudes of response for the three structural (C, prM, and E) and seven nonstructural (NS1 to NS5) proteins. The large boxes represent structural proteins and nonstructural proteins. The percentage in the upper right corner of each box reflects the relative response directed at either structural or nonstructural proteins.
CD4+ responses following TV005 immunization have an immunodominance pattern and magnitude similar to those observed in natural infection.
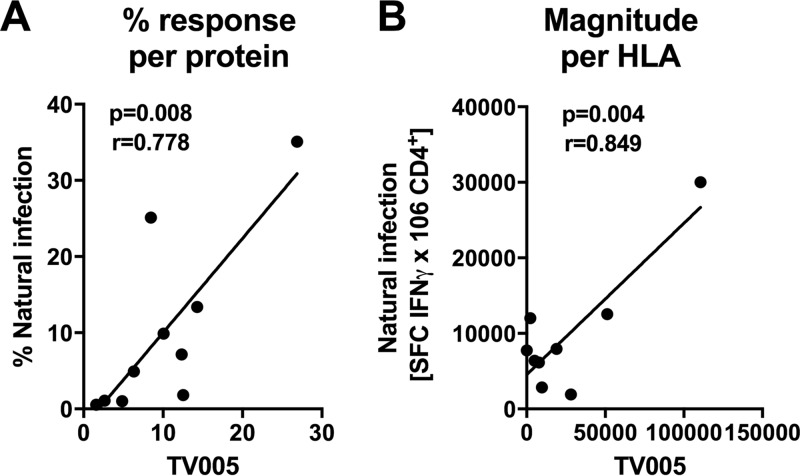
As mentioned above, previous studies characterized the pattern of immunodominance for CD4+ T cell epitopes observed as a result of natural dengue virus infection in the region of hyperendemicity of Colombo, Sri Lanka. To examine whether the pattern of immunodominance observed following TV005 immunization resembled the pattern observed in natural infection, we compared the percentages of the total response attributable to each antigen between TV005 immunization and natural infection, as previously reported (17) (Fig. 2A). We observed a striking correlation of the response patterns between natural infection and TV005 immunization (P = 0.003; r = 0.8585), which indicated strongly overlapping antigen specificities in the CD4+ responses to natural infection and tetravalent vaccination.
FIG 2.
Correlation of TV005-induced responses with responses induced by natural infection. (A) Relative magnitudes of responses directed against a given DENV protein observed in the TV005 vaccinees (x axis) versus relative magnitudes of responses directed against a given DENV protein observed in previous natural infection studies (17). Each symbol represents one of the 10 different DENV proteins (C, prM, E, NS1, NS2A, NS2B, NS3, NS4A, NS4B, and NS5). (B) Magnitudes of responses restricted by a given HLA allele observed in the TV005 vaccinees (x axis) versus those of the responses restricted by the same HLA allele observed in previous natural infection studies (17). Each symbol represents one of the HLA alleles analyzed in this study (DRB1*0101, -0301, -0401, -0701, -0802, -0901, -1001, -1101, and -1404). r and P values were calculated by standard linear regression methods.
As shown above, the CD4+ response to TV005 immunization varied among the different HLA alleles. This is in line with allele-specific variation that was noted for natural dengue virus infection (17). We plotted the total response observed for all tested donors for each HLA allele in natural infection versus TV005 immunization (Fig. 2B). A significant correlation was detected (P = 0.004; r = 0.849), underlining the resemblance of responses induced by TV005 immunization to those induced by natural infection.
TV005-induced responses recognize epitopes from all four DENV serotypes.
CD4+ T cell reactivity was next categorized in regard to how it was directed against sequences for a given serotype. Responses were further analyzed to determine if they were directed against conserved sequences (sequences found in two or more serotypes). The DENV2-specific prM and E proteins were considered separately in this analysis, since the other proteins in the rDEN2/4Δ30 component are contributed by the DENV4 backbone. In our experiments, we tested all the predicted binders previously tested in the Sri Lankan endemic population studies, regardless of serotype and protein of origin, and this afforded an opportunity to determine whether DENV2 sequences from proteins other than prM and E might be recognized in a cross-reactive fashion.
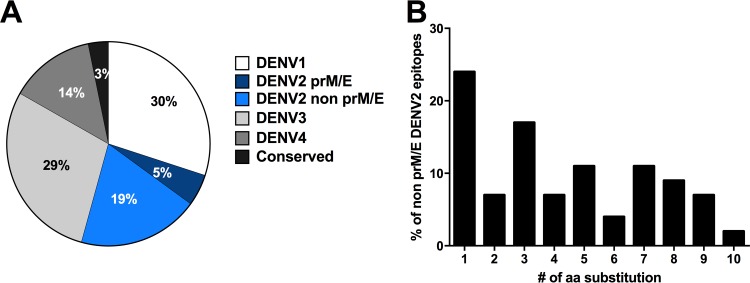
The results of this analysis are shown in Fig. 3A. Conserved sequences accounted for 3% of the overall response, and DENV2 prM and E accounted for an additional 5%. Sequences derived from DENV1, -3, and -4 each accounted for 14%, 29%, and 30% of the response, respectively. Surprisingly, DENV2 sequences from proteins other than prM and E, which by definition are recognized in a cross-reactive fashion, accounted for 19% of the total reactivity, or 54 epitopes in total.
FIG 3.
TV005-induced responses recognize epitopes of proteins from all four serotypes. (A) Distribution of CD4+ T cell epitopes recognized following TV005 immunization as a function of serotype. Epitopes derived from DENV1 (white), DENV2 (dark blue), DENV3 (light gray), DENV4 (medium gray), or regions conserved between serotypes (black) are shown. The DENV2 component of the vaccine consists of the DENV2 prM and E proteins in a DENV4 backbone. Recognition of non-prM/E DENV2 epitopes, which are not contained in TV005 and by definition relate to cross-reactive responses, is shown as a separate pie slice (light blue). (B) Sequence similarity of the cross-reactive non-prM/E DENV2 epitopes to the DENV1, -3, and -4 sequences. The proportions of epitopes carrying the indicated numbers of substitutions are shown.
These data suggest that cross-reactivity of CD4+ responses in the TV005 setting is extensive, perhaps reflective of the overall sequence similarity of different DENV serotypes. To investigate if some degree of sequence variability is allowed, we analyzed how many of the recognized non-prM/E DENV2 epitopes were conserved in other serotypes (Fig. 3B). Of the 54 non-prM/E DENV2 epitopes not conserved in other serotypes, 48% were conserved with sequences from other DENV serotypes at the 80% sequence homology level. Of the remaining epitopes, an additional 10 were conserved at the 67% homology level (up to 5 substitutions allowed). Overall, these data suggest that the CD4+ responses induced by the TV005 vaccine effectively recognize sequences derived from the four different serotypes in a fairly balanced manner.
Epitopes induced by TV005 largely overlap those detected in natural infection.
The results presented above highlight the close similarity of TV005-induced responses to those induced by natural infection. Here we examined whether this correspondence extended to the level of the actual epitope sequences recognized in the two different settings.
First we considered whether similar epitopes might be recognized following either TV005 immunization or infection with naturally occurring dengue viruses. As mentioned above, we synthesized and tested 45 potential HLA binding peptides present in the TV005 sequences but not found in the most frequent DENV isolates used to assess responses to natural DENV infection. We discovered that only 9 of these sequences were positive and accounted for less than 4% of the total response (data not shown).
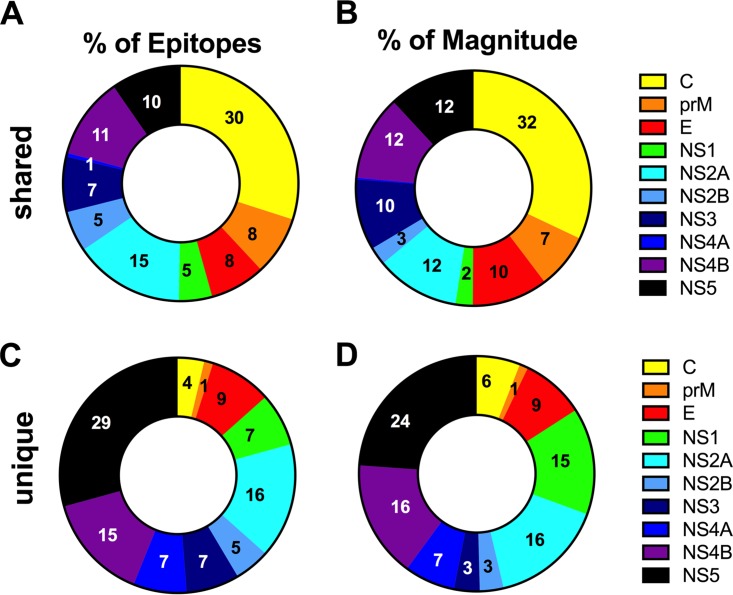
We also examined the overlap between epitopes recognized upon both natural infection and TV005 immunization. Using an 80% homology threshold, 197 of 280 peptides (70%) recognized in the TV005 response were also recognized in the response to natural infection, while 83 of 280 positive peptides (20%) were unique to TV005 immunization. Similarly, the magnitudes of responses to TV005 immunization and natural infection accounted for 80% of the total response, while the ones unique to TV005 immunization accounted for the remaining 20%. When the sources of the TV005 unique epitopes were compared to those of the epitopes shared with natural infection, we found that they were derived mainly from the NS5, NS4B, and NS2A antigens (Fig. 4). Overall, the results presented in this section demonstrate that the recognized epitopes have a large proportion of overlap.
FIG 4.
Antigen distribution of epitopes induced by TV005. The relative numbers and magnitudes of responses induced by TV005 and also recognized in natural infection (shared) (A and B) versus uniquely recognized in TV005 recipients (C and D) are shown. Panels A and C relate to the percentages of epitopes recognized, while panels B and D relate to the relative proportions of the total magnitude of responses for each protein. Yellow, capsid; orange, membrane precursor (prM); red, envelope (E); green, NS1; turquoise, NS2A; blue, NS2B; dark blue, NS3; purple, NS4A; pink, NS4B; black, NS5. The pie charts in panels A and C were obtained by counting the total number of epitopes detected in the study and subdividing them as a function of whether or not they were also detected in natural infection studies (17). Similarly, the pie charts in panels B and D were obtained by summing the total response for all detected epitopes derived from each protein and dividing it by the total response for all epitopes detected in the study.
CD4+ T cells induced by tetravalent DLAV recognize epitopes identified in natural infection.
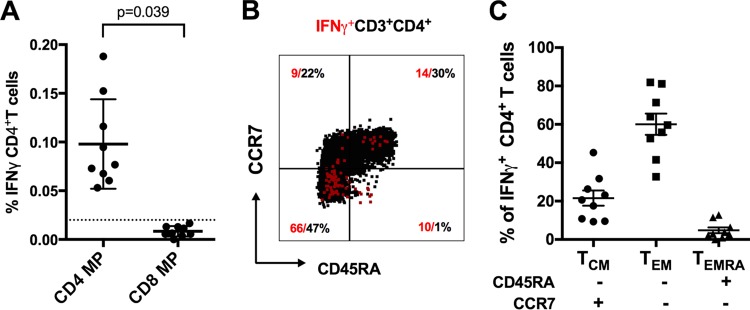
For our cohorts exposed to natural dengue virus infection, we previously designed dengue virus-specific CD4+ and CD8+ megapools (CD4+-MP and CD8+-MP) containing epitopes present in primarily and secondarily infected donors and restricted by a variety of MHC molecules and also covering serotype-specific epitopes derived from all four serotypes as well as a large fraction of epitopes conserved between serotypes (17, 22). We further showed that the CD4+-MP can be used to detect CD4+ responses ex vivo in samples from donors exposed naturally to dengue virus (17). To investigate if CD4+ epitopes identified in natural infection can be recognized in experimental vaccinees, the CD4+-MP was tested in intracellular cytokine staining (ICS) assays utilizing PBMCs from TV005 recipients (Fig. 5). The CD4+-MP induced a vigorous ex vivo IFN-γ response significantly higher than that for stimulation with the CD8+-MP as a control (Fig. 5A). The majority of responses elicited by the tetravalent vaccine were associated with an effector memory phenotype (CD45RA− CCR7−) (Fig. 5B and C).
FIG 5.
Reactivity of a DENV-specific CD4+ T cell megapool. (A) Percentages of CD4+ T cells that produced IFN-γ upon stimulation with the CD4+ megapool (CD4+-MP) for donors previously vaccinated with TV005 (n = 9). IFN-γ production of CD4+ T cells stimulated with a CD8+ megapool (CD8+-MP) was used as a control. The dotted line at 0.02% represents the cutoff for positivity. The average (± standard error of the mean [SEM]) response for all cohorts is shown. Statistical significance was determined using a two-tailed Wilcoxon test. (B) Staining of the memory CD4+ T cell subsets and IFN-γ-producing cells of a representative donor. Memory subsets were defined as naive T cells (CCR7+ CD45RA+), central memory T cells (TCM) (CCR7+ CD4+ 5RA−), and the two effector memory subsets (TEM and TEMRA) (CCR7− CD45RA− and CCR7− CD4+ 5RA+), according to their expression of the indicated markers. (C) The relative IFN-γ response for each memory T cell subset was measured by ICS after stimulation with the CD4+-MP (n = 9).
Overall, our results demonstrate that the CD4+ epitopes recognized after TV005 vaccination and natural dengue virus infection largely overlap, focusing on the C, NS2A, and NS5 proteins, and that the resultant DENV-specific CD4+ T cells induced by TV005 induce a robust effector phenotype typified by high IFN-γ production ex vivo.
DISCUSSION
The development of an effective vaccine for the prevention of DENV infection and associated disease remains a high priority (19). Significant progress has been made with the registration of the CYD-TDV vaccine (23). However, the protection afforded by this chimeric DENV/yellow fever virus vaccine remains suboptimal compared to that of established live attenuated vaccines, thus renewing interest in alternative vaccine constructs (5, 8).
Here we present the results of an analysis of the CD4+ responses elicited by tetravalent DLAV (TV005) vaccination. We demonstrated that vaccine-specific CD4+ T cell responses are similar in magnitude, frequency, and specificity to those observed in the context of naturally immune populations. Since the same experimental approach was previously utilized to assess responses in the context of natural dengue virus infection in a Sri Lankan area of hyperendemicity, the magnitudes of responses could be compared (17). We found that the overall magnitude of responses trended toward a higher level in the case of TV005, perhaps reflective of relatively recent immunization. The comparable frequencies of CD4+ dengue virus peptide recognition (in magnitude, frequency, and breadth) induced by TV005 immunization and natural infection are an important consideration and may suggest that this vaccine construct will generate a level of CD4+ response comparable to that detected in a population associated with at least partial natural immunity. The similarity of CD4+ responses extended to HLA restriction, since the same HLA alleles that were dominant in natural infection were also dominant in TV005 responses. Similar observations were previously made at the level of CD8+ responses, as it was found that responses in Sri Lanka and Nicaragua were associated with similar patterns of HLA immunodominance (24). These results underscore the key role of HLA molecules in determining T cell responsiveness in humans.
Recent data suggest that neutralizing antibody titers might be insufficient to predict vaccine efficacy and that CD8+ responses might be an important component of natural protection (12). In our previous study, we described CD8+ responses to the same live attenuated viruses given as individual monovalent vaccinations and in a tetravalent formulation (16). Beginning with the monovalent samples, we identified nonstructural proteins as the major CD8+ targets, and in vaccinees given the tetravalent formulation, we found that DENV-specific CD8+ responses were intensely focused on NS3 and NS5 (25). As a complement to the CD8+ study, here we also found that with the exception of C, the CD4+ response is directed mainly at the nonstructural DENV antigens NS2A, NS4B, and NS5. Together, these studies provide a more complete description of cellular immunity induced by DENV infection and vaccination.
CD4+ responses might contribute an additional potential correlate of protection. In animal models, CD4+ T cells are not necessary for viral clearance, but vaccination with synthetic peptides representing CD4+ epitopes confers protection from viral challenge in vivo (15). Furthermore, several reports indicate that MHC class II responses restricted by the DRB1 locus are a potential correlate of protection (26, 27). The TV005-induced responses were also similar to responses observed for natural dengue virus infection in terms of the relative immunodominance of the various DENV proteins targeted by CD4+ T cells.
Furthermore, the TV005 responses were focused largely on the same epitopes as those detected in natural infection, suggesting that the vaccine-induced responses target epitopes generated in the course of natural processing in vivo during natural infection. At the same time, a small fraction of epitope responses appeared to be unique to TV005. These unique responses were largely limited to the nonstructural proteins, possibly reflecting the lower replication of the attenuated virus, which would favor the generation and recognition of nonstructural proteins over structural proteins. In particular, the epitopes uniquely recognized following TV005 immunization were mostly derived from NS5, NS4B, and NS2A. While the significance of this observation is not clearly established, it is striking that these antigens are the ones located in the cytoplasm of infected cells, thus suggesting that cellular processing of these antigens by an endogenous pathway might be related to the effect. It is possible that the attenuation of the DENV sequences might result in differences in processing and presentation, since the lower viral replication associated with attenuation might result in less mature virus being produced and, consequently, fewer structural proteins accessing the exogenous presentation pathway.
It has been hypothesized that vaccines based on the mixing of several different components are potentially vulnerable to the phenomenon of vaccine interference, where the performance of one or more of the vaccine components is inhibited in the multivalent formulation. However, in the present study, we tested pools of predicted MHC class II binding peptides (HLA matched) corresponding to pools of peptides previously utilized to characterize DENV-specific responses in the region of hyperendemicity of Colombo (Sri Lanka) (17). In the case of CD4+ responses measured following TV005 immunization, we observed that the recognized epitopes were fairly evenly distributed among serotype-specific epitopes, indicating the lack of any apparent detrimental interference.
We previously found that 80% homology is required for cross-reactivity at the level of DENV CD8+ responses; the data suggest that cross-reactivity of DENV epitopes is far more extensive for CD4+ than for CD8+ responses (28). Secondary T cell responses to conserved/cross-reactive regions may contribute to the heightened protection and lower disease severity observed for tertiary heterotypic DENV infections (6, 29) and may thereby represent a desirable attribute of vaccine-induced cellular responses.
In conclusion, the present study, in conjunction with a previous study analyzing CD8+ responses, indicates that the cellular immune responses induced by tetravalent DLAV vaccination are similar and comparable in magnitude, antigen specificity, and capacity to simultaneously target all four different DENV serotypes to the cellular immune responses observed in the context of populations from areas of hyperendemicity and associated with significant levels of natural immunity.
MATERIALS AND METHODS
Ethics statement.
Clinical data and serum samples for this study were derived from separate phase I clinical trials performed at the University of Vermont (UVM) Vaccine Testing Center and the Center for Immunization Research at the Johns Hopkins Bloomberg School of Public Health (JHSPH). These clinical trials are registered at ClinicalTrials.gov under registration numbers NCT01506570 and NCT01436422. The study design and clinical protocols were approved by the Committees for Human Research Protection (UVM), the Western Institutional Review Board (JHSPH), and the Institutional Review Board of the La Jolla Institute for Allergy and Immunology.
Study populations.
Healthy adult male and nonpregnant female volunteers of 18 to 50 years of age were enrolled and vaccinated with a tetravalent vaccine formulation (TV005). All enrolled subjects were seronegative for all DENV serotypes, yellow fever virus, West Nile virus, St. Louis encephalitis virus, hepatitis B and C viruses, and human immunodeficiency virus. Eight of 10 subjects received two doses of the tetravalent vaccine 6 months apart. The remaining two received one dose of TV005. Study participants were recalled after vaccination (mean, 16 months; range, 10 to 26 months) to donate a unit of blood. Blood processing and HLA typing for both study populations were performed as previously described (12). The HLA phenotypes of the donors used in this study are provided in Table 1.
Vaccine.
Attenuation of the different dengue viruses was achieved by deleting one (rDEN1Δ30 and rDENV4Δ30) or two (DEN3Δ30/31) regions from the 3′ untranslated region (UTR) as previously described (30). DEN2/4Δ30 is a chimeric virus in which the DENV2 prM and E genes replace those of the DEN4Δ30 vaccine candidate (30). For the tetravalent vaccine used in this study (TV005), the four monovalent vaccines were combined into a tetravalent admixture prior to vaccination. Each dose of the tetravalent vaccine contained 103 PFU each of rDEN1Δ30, rDEN3Δ30/31, and rDEN4Δ30 and 104 PFU of rDEN2/4Δ30 (21).
HLA typing.
HLA typing of class II DRB1 alleles was performed by an ASHI-accredited laboratory at Murdoch University (Perth, Western Australia), using locus-specific PCR amplification of genomic DNA. Primers used for amplification were patient-specific barcoded primers. Amplified products were quantitated and pooled by subject, and products for up to 48 subjects were pooled. An unindexed (454 8-lane runs) or indexed (8 indexed MiSeq runs) library was then quantitated using Kappa universal QPCR library quantification kits. Sequencing was performed using either a Roche 454 FLX+ sequencer with titanium chemistry or an Illumina MiSeq sequencer (Illumina, San Diego, CA) using 2-by-300 paired-end chemistry. Reads were quality filtered and passed through a proprietary allele calling algorithm and analysis pipeline, using the latest ImMunoGeneTics (IMGT; http://www.ebi.ac.uk/ipd/imgt/hla/) HLA allele database as a reference.
MHC class II binding predictions and peptide selection.
To analyze DENV-specific HLA-restricted CD4+ responses, we utilized sets of DENV peptides predicted to bind nine HLA DRB1* allelic variants expressed in the donor population (DRB1*0101, -*0301, -*0401, -*0701, -*0802, -*0901, -*1001, -*1101, and -*1302). These sets of peptides included 15-mer peptides from all serotypes and were previously utilized to characterize CD4+ responses in the area of hyperendemicity of Colombo, Sri Lanka (17). Epitope predictions for MHC class II binding were performed for DENV1, -2, -3, and -4 for all isolates in the database, using the consensus prediction methods publicly available through the IEDB Analysis Resource (www.iedb.org) (31, 32). For each allele, any peptide predicted to bind with high affinity and present in 30% or more of the isolates was synthesized after removal of redundant peptides overlapping by 8 residues or more (2% consensus threshold; roughly corresponds to the top 10% of 15-mers overlapping by 10 residues). Any additional peptides predicted to bind to the specific DENVs composing the vaccine were also synthesized and used to supplement the various peptide sets. This resulted in the analysis of more than 360 HLA DRB1 predicted binding peptides (Mimotopes, Victoria, Australia) from general DENV sequences and an additional 45 “DLAV-specific” peptides. For screening studies, the class II peptides were combined into pools of approximately 20 individual peptides, according to their predicted HLA restriction, resulting in approximately 7 pools per HLA allele.
In vitro expansion of DENV-specific T cells.
PBMCs were thawed, and CD4+ T cells were isolated by magnetic bead negative selection and cultured separately in RPMI 1640 medium (Omega Scientific, Tarzana, CA) supplemented with 5% human serum (Cellgro, Manassas, VA) at a density of 2 × 106 cells per ml in 24-well plates (BD Biosciences, San Diego, CA). Cells were cocultured with autologous antigen-presenting cells (APCs) at a density of 1 × 106 cells/ml and stimulated with DENV-specific peptide pools (averaging 20 peptides per pool). Cells were kept at 37°C in 5% CO2, and additional interleukin-2 (IL-2) (10 U/ml; eBioscience, San Diego, CA) was added 4, 7, and 11 days after initial antigenic stimulation. On day 14, cells were harvested and screened for reactivity against individual DENV-specific peptides as previously described (17).
IFN-γ ELISPOT assay.
After 14 days of in vitro expansion, 5 × 104 PBMCs were incubated in triplicate with 0.1 ml complete RPMI 1640 medium in the presence of HLA-matched peptide pools and individual peptides (2 μg/ml). Following 20 h of incubation at 37°C, the cells were incubated with a biotinylated IFN-γ monoclonal antibody (MAb 7-B6-1; Mabtech, Stockholm, Sweden) for 2 h and developed as previously described (12).
Flow cytometry.
The following antibodies were used in this study: anti-CD3 (Alexa Fluor 700; UCHT1), anti-CD14 (V500; M5E2), and anti-CD19 (V500; HIB19), all from BD Biosciences; anti-CD4 (allophycocyanin-eFluor788; RPA-T4), anti-CD45RA (eFluor450; HI100), anti-IFN-γ (fluorescein isothiocyanate [FITC]; 4S.B3), and live/dead dye (ef506), purchased from eBioscience; and anti-CD8 (BV650; RPA-T8) and anti-CCR7 (peridinin chlorophyll protein [PerCP]-Cy5.5; G043H7), purchased from Biolegend. For intracellular cytokine staining (ICS), PBMCs were cultured in the presence of the CD4+-MP or CD8+-MP (1 μg/ml) and with GolgiPlug containing brefeldin A (BD Biosciences, San Diego, CA) for 6 h and subsequently permeabilized, stained, and analyzed as previously described (12).
Conservancy analysis.
Full-length DENV polyprotein sequences for each serotype were retrieved from the NCBI Protein database by using the following query: txid11053 AND 3000:5000[slen], with the corresponding NCBI taxonomy ID substituted for each serotype. To eliminate geographical bias, the number of isolates from any one country was limited to 10. Sequences were considered unique if they varied by at least 1 amino acid from all other sequences. As a result, 162 DENV1, 171 DENV2, 169 DENV3, and 53 DENV4 sequences (for a total of 555 sequences) were retrieved from the NCBI Protein database and utilized to evaluate the conservancy of the identified epitopes within the sequences of the respective serotypes (33).
ACKNOWLEDGMENTS
We thank all study participants and the University of Vermont Vaccine Testing Center, Center for Immunization Research at the Johns Hopkins School of Public Health, and General Clinical Research Center teams for their invaluable participation. Finally, we thank the National Blood Center, Ministry of Health, Colombo, Sri Lanka, for providing the buffy coat samples used in this study.
M.A.A. and P.H.O. performed experiments, reviewed data, and planned experimental strategy. A.G., J.S., S.P., and B.P. performed bioinformatics analyses. A.D.D.S. performed comparisons to natural infection data. E.P. and S.M. performed and coordinated HLA typing and related analysis. S.S.W. developed and provided the tetravalent DLAV and analyzed data from the clinical studies. B.D.K., A.P.D., and S.A.D. performed the clinical studies and obtained the PBMC donations analyzed. A.S. and D.W. designed, conducted, and supervised the overall study. All authors participated in the writing and editing of the manuscript.
We declare that we have no conflicts of interest.
This work was supported by National Institutes of Health contracts HHSN272200900042C and HHSN27220140045C (to A.S.), by BMGF grant OPP1104710 (to A.P.D.), and by the Intramural Research Program of the NIH National Institute of Allergy and Infectious Diseases.
REFERENCES
- 1.Guzman MG, Alvarez M, Halstead SB. 2013. Secondary infection as a risk factor for dengue hemorrhagic fever/dengue shock syndrome: an historical perspective and role of antibody-dependent enhancement of infection. Arch Virol 158:1445–1459. doi: 10.1007/s00705-013-1645-3. [DOI] [PubMed] [Google Scholar]
- 2.Srikiatkhachorn A, Yoon IK. 2016. Immune correlates for dengue vaccine development. Expert Rev Vaccines 15:455–465. doi: 10.1586/14760584.2016.1116949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoft DF, Babusis E, Worku S, Spencer CT, Lottenbach K, Truscott SM, Abate G, Sakala IG, Edwards KM, Creech CB, Gerber MA, Bernstein DI, Newman F, Graham I, Anderson EL, Belshe RB. 2011. Live and inactivated influenza vaccines induce similar humoral responses, but only live vaccines induce diverse T-cell responses in young children. J Infect Dis 204:845–853. doi: 10.1093/infdis/jir436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Endy TP, Nisalak A, Chunsuttitwat S, Vaughn DW, Green S, Ennis FA, Rothman AL, Libraty DH. 2004. Relationship of preexisting dengue virus (DV) neutralizing antibody levels to viremia and severity of disease in a prospective cohort study of DV infection in Thailand. J Infect Dis 189:990–1000. doi: 10.1086/382280. [DOI] [PubMed] [Google Scholar]
- 5.Capeding MR, Tran NH, Hadinegoro SR, Ismail HI, Chotpitayasunondh T, Chua MN, Luong CQ, Rusmil K, Wirawan DN, Nallusamy R, Pitisuttithum P, Thisyakorn U, Yoon IK, van der Vliet D, Langevin E, Laot T, Hutagalung Y, Frago C, Boaz M, Wartel TA, Tornieporth NG, Saville M, Bouckenooghe A, CYD14 Study Group. 2014. Clinical efficacy and safety of a novel tetravalent dengue vaccine in healthy children in Asia: a phase 3, randomised, observer-masked, placebo-controlled trial. Lancet 384:1358–1365. doi: 10.1016/S0140-6736(14)61060-6. [DOI] [PubMed] [Google Scholar]
- 6.Gordon A, Kuan G, Mercado JC, Gresh L, Aviles W, Balmaseda A, Harris E. 2013. The Nicaraguan pediatric dengue cohort study: incidence of inapparent and symptomatic dengue virus infections, 2004–2010. PLoS Negl Trop Dis 7:e2462. doi: 10.1371/journal.pntd.0002462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kirkpatrick BD, Whitehead SS, Pierce KK, Tibery CM, Grier PL, Hynes NA, Larsson CJ, Sabundayo BP, Talaat KR, Janiak A, Carmolli MP, Luke CJ, Diehl SA, Durbin AP. 2016. The live attenuated dengue vaccine TV003 elicits complete protection against dengue in a human challenge model. Sci Transl Med 8:330ra336. doi: 10.1126/scitranslmed.aaf1517. [DOI] [PubMed] [Google Scholar]
- 8.Villar L, Dayan GH, Arredondo-Garcia JL, Rivera DM, Cunha R, Deseda C, Reynales H, Costa MS, Morales-Ramirez JO, Carrasquilla G, Rey LC, Dietze R, Luz K, Rivas E, Miranda Montoya MC, Cortes Supelano M, Zambrano B, Langevin E, Boaz M, Tornieporth N, Saville M, Noriega F, CYD15 Study Group. 2015. Efficacy of a tetravalent dengue vaccine in children in Latin America. N Engl J Med 372:113–123. doi: 10.1056/NEJMoa1411037. [DOI] [PubMed] [Google Scholar]
- 9.Yauch LE, Zellweger RM, Kotturi MF, Qutubuddin A, Sidney J, Peters B, Prestwood TR, Sette A, Shresta S. 2009. A protective role for dengue virus-specific CD8+ T cells. J Immunol 182:4865–4873. doi: 10.4049/jimmunol.0801974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elong Ngono A, Chen HW, Tang WW, Joo Y, King K, Weiskopf D, Sidney J, Sette A, Shresta S. 2016. Protective role of cross-reactive CD8 T cells against dengue virus infection. EBioMedicine 13:284–293. doi: 10.1016/j.ebiom.2016.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zellweger RM, Tang WW, Eddy WE, King K, Sanchez MC, Shresta S. 2015. CD8+ T cells can mediate short-term protection against heterotypic dengue virus reinfection in mice. J Virol 89:6494–6505. doi: 10.1128/JVI.00036-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weiskopf D, Angelo MA, de Azeredo EL, Sidney J, Greenbaum JA, Fernando AN, Broadwater A, Kolla RV, De Silva AD, de Silva AM, Mattia KA, Doranz BJ, Grey HM, Shresta S, Peters B, Sette A. 2013. Comprehensive analysis of dengue virus-specific responses supports an HLA-linked protective role for CD8+ T cells. Proc Natl Acad Sci U S A 110:E2046–E2053. doi: 10.1073/pnas.1305227110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sallusto F. 2016. Heterogeneity of human CD4(+) T cells against microbes. Annu Rev Immunol 34:317–334. doi: 10.1146/annurev-immunol-032414-112056. [DOI] [PubMed] [Google Scholar]
- 14.Crotty S. 2011. Follicular helper CD4 T cells (TFH). Annu Rev Immunol 29:621–663. doi: 10.1146/annurev-immunol-031210-101400. [DOI] [PubMed] [Google Scholar]
- 15.Yauch LE, Prestwood TR, May MM, Morar MM, Zellweger RM, Peters B, Sette A, Shresta S. 2010. CD4+ T cells are not required for the induction of dengue virus-specific CD8+ T cell or antibody responses but contribute to protection after vaccination. J Immunol 185:5405–5416. doi: 10.4049/jimmunol.1001709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weiskopf D, Bangs DJ, Sidney J, Kolla RV, De Silva AD, de Silva AM, Crotty S, Peters B, Sette A. 2015. Dengue virus infection elicits highly polarized CX3CR1+ cytotoxic CD4+ T cells associated with protective immunity. Proc Natl Acad Sci U S A 112:E4256–E4263. doi: 10.1073/pnas.1505956112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weiskopf D, Angelo MA, Grifoni A, O'Rourke PH, Sidney J, Paul S, De Silva AD, Phillips E, Mallal S, Premawansa S, Premawansa G, Wijewickrama A, Peters B, Sette A. 2016. HLA DRB1 alleles are associated with different response magnitudes of dengue virus specific CD4+ T cell responses. J Infect Dis 214:1117–1124. doi: 10.1093/infdis/jiw309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rivino L, Kumaran EA, Jovanovic V, Nadua K, Teo EW, Pang SW, Teo GH, Gan VC, Lye DC, Leo YS, Hanson BJ, Smith KG, Bertoletti A, Kemeny DM, MacAry PA. 2013. Differential targeting of viral components by CD4+ versus CD8+ T lymphocytes in dengue virus infection. J Virol 87:2693–2706. doi: 10.1128/JVI.02675-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vannice KS, Durbin A, Hombach J. 2016. Status of vaccine research and development of vaccines for dengue. Vaccine 34:2934–2938. doi: 10.1016/j.vaccine.2015.12.073. [DOI] [PubMed] [Google Scholar]
- 20.Durbin AP, Kirkpatrick BD, Pierce KK, Elwood D, Larsson CJ, Lindow JC, Tibery C, Sabundayo BP, Shaffer D, Talaat KR, Hynes NA, Wanionek K, Carmolli MP, Luke CJ, Murphy BR, Subbarao K, Whitehead SS. 2013. A single dose of any of four different live attenuated tetravalent dengue vaccines is safe and immunogenic in flavivirus-naive adults: a randomized, double-blind clinical trial. J Infect Dis 207:957–965. doi: 10.1093/infdis/jis936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Whitehead SS. 2016. Development of TV003/TV005, a single dose, highly immunogenic live attenuated dengue vaccine; what makes this vaccine different from the Sanofi-Pasteur CYD vaccine? Expert Rev Vaccines 15:509–517. doi: 10.1586/14760584.2016.1115727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weiskopf D, Cerpas C, Angelo MA, Bangs DJ, Sidney J, Paul S, Peters B, Sanches FP, Silvera CG, Costa PR, Kallas EG, Gresh L, de Silva AD, Balmaseda A, Harris E, Sette A. 2015. Human CD8+ T-cell responses against the 4 dengue virus serotypes are associated with distinct patterns of protein targets. J Infect Dis 212:1743–1751. doi: 10.1093/infdis/jiv289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Durbin AP. 2016. A dengue vaccine. Cell 166:1. doi: 10.1016/j.cell.2016.06.036. [DOI] [PubMed] [Google Scholar]
- 24.de Alwis R, Bangs DJ, Angelo MA, Cerpas C, Fernando A, Sidney J, Peters B, Gresh L, Balmaseda A, de Silva AD, Harris E, Sette A, Weiskopf D. 2016. Immunodominant dengue virus-specific CD8+ T cell responses are associated with a memory PD-1+ phenotype. J Virol 90:4771–4779. doi: 10.1128/JVI.02892-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weiskopf D, Angelo MA, Bangs DJ, Sidney J, Paul S, Peters B, de Silva AD, Lindow JC, Diehl SA, Whitehead S, Durbin A, Kirkpatrick B, Sette A. 2015. The human CD8+ T cell responses induced by a live attenuated tetravalent dengue vaccine are directed against highly conserved epitopes. J Virol 89:120–128. doi: 10.1128/JVI.02129-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Malavige GN, Rostron T, Rohanachandra LT, Jayaratne SD, Fernando N, De Silva AD, Liyanage M, Ogg G. 2011. HLA class I and class II associations in dengue viral infections in a Sri Lankan population. PLoS One 6:e20581. doi: 10.1371/journal.pone.0020581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nguyen TP, Kikuchi M, Vu TQ, Do Q H, Tran TT, Vo DT, Ha MT, Vo VT, Cao TP, Tran VD, Oyama T, Morita K, Yasunami M, Hirayama K. 2008. Protective and enhancing HLA alleles, HLA-DRB1*0901 and HLA-A*24, for severe forms of dengue virus infection, dengue hemorrhagic fever and dengue shock syndrome. PLoS Negl Trop Dis 2:e304. doi: 10.1371/journal.pntd.0000304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weiskopf D, Angelo MA, Sidney J, Peters B, Shresta S, Sette A. 2014. Immunodominance changes as a function of the infecting dengue virus serotype and primary versus secondary infection. J Virol 88:11383–11394. doi: 10.1128/JVI.01108-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wikramaratna PS, Simmons CP, Gupta S, Recker M. 2010. The effects of tertiary and quaternary infections on the epidemiology of dengue. PLoS One 5:e12347. doi: 10.1371/journal.pone.0012347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lindow JC, Durbin AP, Whitehead SS, Pierce KK, Carmolli MP, Kirkpatrick BD. 2013. Vaccination of volunteers with low-dose, live-attenuated, dengue viruses leads to serotype-specific immunologic and virologic profiles. Vaccine 31:3347–3352. doi: 10.1016/j.vaccine.2013.05.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang P, Sidney J, Dow C, Mothe B, Sette A, Peters B. 2008. A systematic assessment of MHC class II peptide binding predictions and evaluation of a consensus approach. PLoS Comput Biol 4:e1000048. doi: 10.1371/journal.pcbi.1000048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang P, Sidney J, Kim Y, Sette A, Lund O, Nielsen M, Peters B. 2010. Peptide binding predictions for HLA DR, DP and DQ molecules. BMC Bioinformatics 11:568. doi: 10.1186/1471-2105-11-568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bui HH, Sidney J, Li W, Fusseder N, Sette A. 2007. Development of an epitope conservancy analysis tool to facilitate the design of epitope-based diagnostics and vaccines. BMC Bioinformatics 8:361. doi: 10.1186/1471-2105-8-361. [DOI] [PMC free article] [PubMed] [Google Scholar]