Abstract
Background
Systemic lupus erythematosus (SLE) patients are at high risk for depression and anxiety. However, the estimated prevalence of these disorders varies substantially between studies. This systematic review aimed to establish pooled prevalence levels of depression and anxiety among adult SLE patients.
Methods
We systematically reviewed databases including PubMed, Embase, PsycINFO, and the Cochrane database library from their inception to August 2016. Studies presenting data on depression and/or anxiety in adult SLE patients and having a sample size of at least 60 patients were included. A random-effect meta-analysis was conducted on all eligible data.
Results
A total of 59 identified studies matched the inclusion criteria, reporting on a total of 10828 adult SLE patients. Thirty five and thirteen methods of defining depression and anxiety were reported, respectively. Meta-analyses revealed that the prevalence of major depression and anxiety were 24% (95% CI, 16%-31%, I2 = 95.2%) and 37% (95% CI, 12%–63%, I2 = 98.3%) according to clinical interviews. Prevalence estimates of depression were 30% (95% CI, 22%–38%, I2 = 91.6%) for the Hospital Anxiety and Depression Scale with thresholds of 8 and 39% (95% CI, 29%–49%, I2 = 88.2%) for the 21-Item Beck Depression Inventory with thresholds of 14, respectively. The main influence on depression prevalence was the publication years of the studies. In addition, the corresponding pooled prevalence was 40% (95% CI, 30%–49%, I2 = 93.0%) for anxiety according to the Hospital Anxiety and Depression Scale with a cutoff of 8 or more.
Conclusions
The prevalence of depression and anxiety was high in adult SLE patients. It indicated that rheumatologists should screen for depression and anxiety in their patients, and referred them to mental health providers in order to identify effective strategies for preventing and treating depression and anxiety among adult SLE patients.
Trial registration
Current Meta-analysis PROSPERO Registration Number: CRD 42016044125. Registered 4 August 2016.
Electronic supplementary material
The online version of this article (doi:10.1186/s12888-017-1234-1) contains supplementary material, which is available to authorized users.
Keywords: Depression, Anxiety, Meta-analysis, Systematic review
Background
Systemic lupus erythematosus (SLE) is a multisystem, autoimmune, connective-tissue disorder with frequent psychological comorbidities, of which depression and anxiety are two common manifestations [1, 2]. It has been reported that there were 2 times higher prevalence of depression in SLE patients compared to the general population [3]. In addition, previous study has reported that the anxiety disorders were twice as prevalent among SLE patients as compared to the controls [4]. Depression and anxiety often have profound impacts on SLE patients’ health and well-being including increased incidence of cardiovascular diseases [5], myocardial infarction [6], suicidal ideation [7], physical disability [8], decreased quality of life [9, 10], and a higher risk of premature mortality [11]. Therefore, depression and anxiety may be useful targets for interventions aimed at improving subjective health and quality of life in individuals with SLE. However, current epidemiological evidence found that the prevalence of depression and/or anxiety in SLE patients ranged widely from 2% to 91.7% in different studies [12, 13]. This vast inter-study difference was previously attributed to multiple factors, including study quality, unclear definition of depression or anxiety, diverse screening strategies used across studies [14]. Reliable estimates of depression and anxiety prevalence are important for informing efforts to prevent, treat, and identify causes of depression and anxiety among SLE patients. Recent meta-analyses have estimated the overall prevalence of depression and/or anxiety in rheumatoid arthritis and osteoarthritis patients [14, 15]. There has only been one previous systematic review of psychiatric symptoms in SLE [16]; however, no systematic review was conducted to quantify the prevalence of depression and anxiety in SLE using meta-analysis techniques. Our goal was to address this limitation. The objectives of this systematic review were (i) to establish pooled prevalence levels of depression and anxiety among adult SLE patients; (ii) to provide a summary of the methods used to define depression and anxiety in SLE; and (iii) to explore the impacts of study characteristics on prevalence estimates.
Methods
This systematic review was conducted within the Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) statement [17] and followed a predetermined registered protocol (PROSPERO: CRD42016044125).
Search strategy
A systematic review of published literature in scientific journals that reported on the prevalence of depression and/or anxiety among SLE patients was conducted by two independent reviewers using the following databases from their inception to August 2016: PubMed, Embase, PsycINFO, and the Cochrane database library. The computer-based searches combined terms related to SLE patients and study design with those related to depression or anxiety (see Additional file 1). We conducted citation chasing search strategy with all reference lists of included articles and relevant review papers were considered to identify potentially omitted articles. Finally, we corresponded with the authors for further information if we encountered articles just provided the mean and standard deviation of the depression and/or anxiety assessment scale.
Inclusion and exclusion criteria
Studies were included if they met the following criteria: (i) cross-sectional design, baseline cross-sectional data from a longitudinal study or baseline cross-sectional data from a trial, before group allocation; (ii) used validated methods (clinical interviews or self-report instruments) to assess depression or anxiety; and (iii) the sample size was no less than 60.
Case reports, review articles, animal studies, studies investigating neuropsychiatric syndromes, studies in languages other than English and papers not dealing with SLE patients were excluded. For this meta-analysis, studies using pediatrics sample or screening tools without stating the cut-off thresholds used to detect depression or anxiety were also excluded. Table 2 and Table 3 presented a full list of the eligible methods of detecting depression and anxiety, alongside the numbers of articles utilizing each method and the number of participants assessed.
Table 2.
Methods of detecting depression and summary of prevalence and heterogeneity findings
| Tool | Definition/cutoff | No. of studies | No. of participants | Prevalence, % (95% CI) | Heterogeneity I2, % |
|---|---|---|---|---|---|
| DSM and/or ICD | |||||
| Major depressive disorder | 10 | 2960 | 24 (16, 31) | 95.2 | |
| Dysthymic disorder | 6 | 922 | 12 (5, 18) | 93.4 | |
| Adjustment disorder | 2 | 280 | 20 (15, 24) | 0.0 | |
| Minor depression | 1 | 150 | 6 (2, 10) | - | |
| HADS | ≥8 | 12 | 1474 | 30 (22, 38) | 91.6 |
| CES-D | >10 | 1 | 344 | 55 (49, 60) | - |
| ≥16 | 8 | 1640 | 38 (32, 44) | 81.3 | |
| >16.7 | 1 | 80 | 44 (33, 55) | - | |
| ≥17 | 1 | 343 | 47 (42, 52) | - | |
| ≥24 | 1 | 716 | 25 (22, 28) | - | |
| >27 | 1 | 93 | 16 (9, 24) | - | |
| 21 Item-BDI | ≥5 | 2 | 451 | 61 (56, 66) | 17.7 |
| ≥10 | 1 | 167 | 21 (15, 27) | - | |
| ≥11 | 1 | 81 | 35 (24, 45) | - | |
| ≥13 | 1 | 63 | 24 (13, 34) | - | |
| ≥14 | 6 | 781 | 39 (29, 49) | 88.2 | |
| ≥16 | 2 | 131 | 76 (45, 107) | 95.4 | |
| ≥17 | 1 | 103 | 40 (30, 49) | - | |
| ≥18 | 1 | 127 | 42 (33, 50) | - | |
| ≥19 | 1 | 100 | 21 (13, 29) | - | |
| ≥20 | 2 | 213 | 50 (12, 89) | 96.8 | |
| ≥21 | 3 | 545 | 34 (2, 65) | 98.8 | |
| ≥29 | 1 | 153 | 19 (13, 25) | - | |
| ≥30 | 3 | 326 | 5 (0, 9) | 72.1 | |
| ≥31 | 1 | 160 | 39 (31, 46) | - | |
| ≥32 | 1 | 71 | 9 (3, 16) | - | |
| >40 | 1 | 160 | 21 (14, 27) | - | |
| HDS | ≥8 | 1 | 126 | 41 (32, 49) | - |
| ≥11 | 1 | 62 | 45 (33, 58) | - | |
| ≥16 | 1 | 126 | 2 (0, 5) | - | |
| >17 | 1 | 71 | 20 (10, 29) | - | |
| PHQ-9 | ≥10 | 1 | 75 | 29 (19, 40) | - |
| PHQ-2 | ≥3 | 1 | 612 | 28 (25, 23) | - |
| SCL-90-R | 1 | 97 | 5 (1, 10) | - | |
| Zung SDS | ≥53 | 1 | 156 | 33 (26, 41) | - |
DSM Diagnostic and Statistical Manual of Mental Disorders, ICD International Classification of Diseases, HADS Hospital Anxiety and Depression Scale, CES-D Centre for Epidemiological Studies Depression Scale, BDI Beck Depression Inventory, HDS Hamilton Depression Scale, PHQ Patient Health Questionnaire, SCL-90-R Symptoms Checklist-90-Revised, Zung SDS Zung Self-rating Depression Scale
Table 3.
Methods of detecting anxiety and summary of prevalence and heterogeneity findings
| Tool | Definition/cutoff | No. of studies | No. of participants | Prevalence, % (95% CI) | Heterogeneity I2, % |
|---|---|---|---|---|---|
| DSM and/or ICD for anxiety disorder | 5 | 663 | 37 (12, 63) | 98.3 | |
| HADS | ≥8 | 10 | 1332 | 40 (30, 49) | 93.0 |
| 21 Item-BAI | ≥8 | 2 | 313 | 71 (51, 91) | 94 |
| ≥16 | 2 | 313 | 48 (39, 56) | 59.2 | |
| ≥26 | 2 | 313 | 18 (14, 22) | 0 | |
| HAS | ≥6 | 1 | 126 | 75 (67, 82) | - |
| ≥14 | 1 | 62 | 37 (25, 49) | - | |
| ≥15 | 1 | 126 | 27 (19, 35) | - | |
| >17 | 1 | 71 | 24 (14, 34) | - | |
| Cattell questionnaire | ≥21 | 1 | 166 | 85 (79, 90) | - |
| SCL-90-R | 1 | 97 | 4 (0, 8) | - | |
| Zung SAS | >44 | 1 | 81 | 17 (9, 26) | - |
| ≥50 | 1 | 156 | 21 (14, 27) | - |
DSM Diagnostic and Statistical Manual of Mental Disorders, ICD International Classification of Diseases, HADS Hospital Anxiety and Depression Scale, BAI Beck Anxiety Inventory, HAS Hamilton Anxiety Scale, SCL-90-R Symptoms Checklist-90-Revised, Zung SAS Zung Self-rating Anxiety Scale
Data extraction and quality assessment
Two researchers read the relative studies independently by the titles and abstracts to exclude the references which did not met the inclusion criteria. Then, they read full texts in the remaining studies as mentioned above, and determined whether these references included were final studies or not. When multiple publications spanned the years of longitudinal studies, baseline prevalence levels were reported. The following information was independently extracted from each article by other two trained investigators using a standardized form: year, country, mean disease duration, percentage of female participants, sample size, average age of participants, criteria for detection of depression and anxiety, and reported prevalence of depression and/or anxiety. If we encountered multiple publications from the same cohort, we used the data from the most recent or the paper reporting data from the largest number of participants. The methodological quality of each study included in the present meta-analysis was assessed using a modified version of the Newcastle-Ottawa Scale [18]. Studies were judged to be at low risk of bias (≥3 points) or high risk of bias (<3 points). Any disagreements in data extraction and quality assessment were resolved through discussion between the two reviewers or adjudication with a third reviewer.
Outcome measures
The outcomes were major/minor depression and affective/dysthymic/adjustment/anxiety disorder diagnosed with a structured clinical assessment [e.g., Diagnostic and Statistical Manual of Mental Disorders (DSM)-IV or International Classification of Diseases (ICD)-10] or depression and/or anxiety assessed with validated assessment tools [e.g., the Hospital Anxiety and Depression Scale (HADS), the Centre for Epidemiologic Studies Depression Scale (CES-D)] (see Additional file 2).
Statistical analyses
Because random-effects models tended to provide wider confidence intervals (CI) and were preferable in the presence of between-study heterogeneity, we used a random-effects meta-analysis to pool studies reporting the prevalence of depression and/or anxiety in SLE patients [19]. Between-study heterogeneity was assessed by the I2 with thresholds of ≥25%, ≥50% and ≥75% indicating low, moderate and high heterogeneity, respectively [20]. The influence of individual study on the overall prevalence estimate was explored by serially excluding each study in sensitivity analyses. Wherever possible, subgroup analyses were planned by overall study quality, sample size, country of origin and publication year, if there was more than one study in the subgroup. Pearson’s and Spearman’s correlation analyses were used to assess the association between variables and prevalence of depression and anxiety in people with SLE. Funnel plots and Egger’s test were combined to explore the potential publication bias in this meta-analysis [21, 22]. Statistical analyses were performed with STATA version 12.0. Statistical tests were 2-sided and used a significance threshold of P < 0.05.
Results
Search results
Fig. 1 provided the details of the study selection process. The initial search identified a total of 3347 potentially relevant articles. After removal of duplicates, titles and then abstracts were screened for potential eligibility. From this, 121 were considered in the full-text review, of which 59 articles met the inclusion criteria, and a full reference list was presented in Additional file 3. Inter-rater reliability of reviewers regarding study relevancy was high (Kappa = 0.87).
Fig. 1.
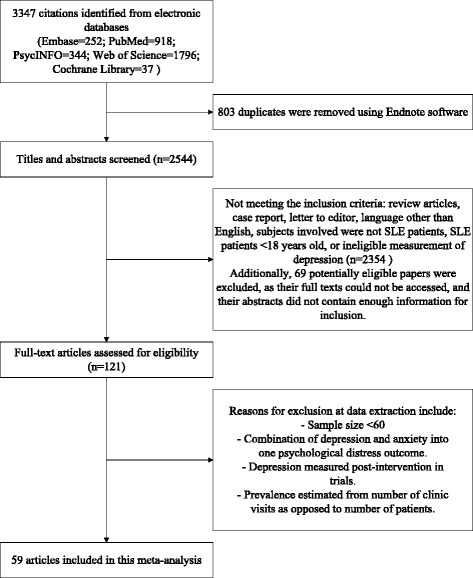
Search results and study selection
Study characteristics
A summary of the included study characteristics was shown in Table 1. A total of 59 identified studies matched the inclusion criteria, reporting on a total of 10828 adult SLE patients. Twenty took place in North America, 18 in Asia, 12 in Europe, 6 in South America, 1 in Oceania, and 1 in Africa. The median of mean ages was 39 years (range, 30.0-50.1), and the median percentage of females represented in the sample was 93% (range, 75%–100%). In addition, the median number of participants per study was 100 (range, 60–1827), and the median of mean disease duration was 9 years (range, 0.22–16.3). Depression was defined in 35 different ways (Table 2). Seventeen studies assessed for depression using the 21 Item-Beck Depression Inventory (BDI), with sixteen different thresholds were presented in the articles. Thirteen articles used the CES-D; six different cut-off points were presented, and the most commonly used being 16. Twelve used the HADS with a cutoff of 8 or more, and 6 used other screening tools. Ten studies assessed for major depression using diagnostic criteria (DSM or ICD). The most commonly used screening questionnaire to assess anxiety was the HADS, with 10 studies using this screening tool with thresholds of 8. The methods employed to assess depression and anxiety and the frequency of their use were presented in Table 2 and Table 3. When evaluated by Newcastle-Ottawa quality assessment criteria, out of 5 possible points, 2 studies received 5 points, 7 received 4 points, 13 received 3 points, 36 received 2 points, and 1 received 1 point. The details of the assessment of individual studies were shown in Additional file 4.
Table 1.
Overview of prevalence studies of mood in SLE patients (N ≥ 60)
| Study ID | Country | Disease duration, mean ± SD/median (range) | Women, % | Sample size | Age, mean ± SD/median (range), years | Criteria for detection of anxiety (cutoff) | Anxiety prevalence, % | Criteria for detection of depression (cutoff) | Depression prevalence, % | NOS |
|---|---|---|---|---|---|---|---|---|---|---|
| Abdul-Sattar 2015 | Egypt | 10.0 ± 4.6 years | 95% | 80 | 30.9 ± 11.7 | CES-D (>16.7) | 43.75 | 2 | ||
| Appenzeller 2009 | Brazil | 64.5 ± 48.5 months | 94.6% | 167 | 32.1 ± 11.0 | 21 Item-BDI (≥10) | 20.9 | 2 | ||
| Bachen 2009 | USA | 15.4 ± 9.7 years | 100% | 326 | 47.9 ± 11.3 | DSM-IV | 64 | DSM-IV | Major depressive disorder: 42.4, dysthymic disorder: 2.9 | 5 |
| Bogdanovic 2015 | Serbia | 6.8 ± 2.9 years | 100% | 60 | 43.4 ± 12.8 | 21 Item-BDI (≥16/≥20/≥30) | 91.7/70/3.3 | 2 | ||
| Calderon 2014 | Chile | Median: 32.0 (0–243.0) months | 100% | 82 | Median: 36.0 (17.0–64.0) | HADS (≥8) | 37 | 2 | ||
| Cho 2014 | South Korea | NS | 90.1% | 201 | 41.3 ± 13.2 | CES-D (≥16) | 39.3 | 3 | ||
| Chin 1993 | Malaysia | 4.1 ± 3.5 years | 95% | 79 | 31.1 ± 9.1 | ICD-9 and DSM-III | 7.6 | ICD-9 and DSM-III | Major depressive disorder: 6.3, dysthymic disorder: 32.9 | 2 |
| Da Costa 2005 | Canada | 13.8 ± 10.1 years | 100% | 100 | 45.4 ± 14.0 | CES-D (≥16) | 31 | 3 | ||
| Doria 2004 | Italy | 9.9 ± 6.3 years | 87.3% | 126 | 38.9 ± 11.9 | HAS (≥6/≥15) | 74.6/27 | HDS (≥8/≥16) | 40.5/2.4 | 2 |
| Duvdevany 2011 | Israel | 11.4 ± 9.1 years | 88% | 100 | 37.0 ± 11.8 | HADS (≥8) | 20 | HADS (≥8) | 37 | 4 |
| García-Carrasco 2011 | Mexico | 106.5 ± 85.5 months | 100% | 106 | 40.5 ± 12.0 | CES-D (≥16) | 38.8 | 2 | ||
| García-Carrasco 2013 | Mexico | 10.5 ± 7.4 years | 100% | 105 | 43.6 ± 11.3 | CES-D (≥16) | 33 | 2 | ||
| Greco 2009 | USA | 16.3 ± 7.0 years | 100% | 161 | 50.1 ± 10.0 | CES-D (≥16) | 27 | 2 | ||
| Hanly 2015 | Canada | 5.6 ± 4.8 years | 88.9% | 1827 | 35.1 ± 13.3 | DSM-IV | 12.7 | 4 | ||
| Harrison 2006 | USA | 15.3 ± 3.2 years | 100% | 93 | 43.3 ± 13.7 | CES-D (>27) | 16.1 | 2 | ||
| Huang 2007 | China | 7.5 ± 6.9 years | 91.5% | 129 | 37.4 ± 10.7 | HADS (≥8) | 32 | HADS (≥8) | 20 | 2 |
| Iverson 2002 | Canada | NS | NS | 103 | NS | 21 Item-BDI (≥17) | 39.8 | 1 | ||
| Jarpa 2011 | Chile | Median: 5.0 (0.1–40.0) years | 90.8% | 87 | Median: 39.0 (16.0–27.0) | DSM-IV | 18.1 | DSM-IV | Major depressive disorder: 21.7, dysthymic disorder: 4.8 | 2 |
| Julian 2011 | USA | 15.8 ± 9.3 years | 93% | 150 | 48.8 ± 12.3 | ICD-10 and DSM-IV | Major depressive disorder: 17, dysthymic disorder: 4, minor depression: 6 | 3 | ||
| Jung 2015 | Korea | 6.8 ± 4.4 years | 93% | 100 | 40.6 ± 10.3 | 21 Item-BDI (≥21) | 13 | 2 | ||
| Katz 2011 | USA | 13.6 ± 8.5 years | 100% | 716 | 48.1 ± 12.6 | CES-D (≥24) | 25 | 3 | ||
| Karol 2013 | USA | NS | 93% | 127 | 38.1 ± 12.3 | 21 Item-BDI (≥18) | 41.7 | 2 | ||
| Karimifar 2013 | Iran | 4.1 ± 0.5 years | 80% | 100 | 34.8 ± 10.9 | 21 Item-BDI (≥14) | 60 | 2 | ||
| Kheirandish 2015 | Iran | 9.0 ± 7.7 years | 92.2% | 166 | 33.1 ± 11.1 | Cattell questionnaire (≥21) | 84.9 | 21 Item-BDI (≥5/≥30) | 64.5/9 | 2 |
| Kotsis 2014 | Greece | 13.2 ± 9.1 years | 84% | 75 | 44.1 ± 13.3 | PHQ-9 (≥10) | 29.3 | 2 | ||
| Kim 2015 | USA | 12.0 ± 8.0 years | 93% | 89 | 39.0 ± 15.0 | CES-D (≥16) | 63 | 3 | ||
| Lapteva 2006 | USA | 13.8 ± 10.2 years | 75% | 60 | 41.0 ± 13.0 | DSM-IV | Major depressive disorder: 16.6 | 2 | ||
| Lisitsyna 2014 | NS | 134.9 ± 8.8 months | 85.6% | 180 | 34.6 ± 0.93 | ICD-10 | Major depressive disorder: 24.4, dysthymic disorder: 25.6, adjustment disorders: 18.9 | 2 | ||
| Mak 2011 | Singapore | 54.9 ± 70.7 months | 88% | 60 | 40.5 ± 12.9 | HADS (≥8) | 38 | HADS (≥8) | 22 | 2 |
| Maneeton 2013 | Thailand | 6.1 ± 4.8 years | 98% | 62 | 31.8 ± 9.0 | HAS (≥14) | 37.1 | HDS (≥11) | 45.2 | 2 |
| Mirbagher 2016 | Iran | 8.3 ± 3.8 years | 100% | 77 | 36.5 ± 10.1 | HADS (≥8) | 71.4 | HADS (≥8) | 46.1 | 3 |
| Monaghan 2007 | Australia | 10.2 ± 8.7 years | 97% | 60 | 44.4 ± 12.2 | HADS (≥8) | 44 | HADS (≥8) | 36 | 3 |
| Montero-Lo’pez 2016 | Spain | 0.2 ± 0.7 years | 100% | 97 | 38.6 ± 9.3 | SCL-90-R | 4.1 | SCL-90-R | 5.2 | 2 |
| Nery 2008 | Brazil | 9.8 ± 6.5 years | 100% | 71 | 34.8 ± 10.1 | SCID for DSM-IV | 46.5 | SCID for DSM-IV | Major depressive disorder: 40.8 | 2 |
| Neville 2014 | Canada | 10.2 ± 9.5 years | 92.4% | 612 | 46.8 ± 16.7 | PHQ-2 (≥3) | 28.1 | 4 | ||
| Palagini 2014 | Italy | 15.0 ± 8.0 years | 100% | 81 | 43.6 ± 11.2 | SAS (>44) | 17.3 | 21 Item-BDI (≥11) | 34.6 | 3 |
| Panopalis 2010 | USA | 13.8 ± 8.9 years | 91% | 807 | 47.6 ± 13.1 | CES-D (≥16) | 38.5 | 5 | ||
| Pettersson 2015 | Sweden | Median: 12.0 years | 92% | 305 | Median: 48 | HADS (≥8) | 34 | HADS (≥8) | 51 | 4 |
| Postal 2016 | Brazil | Median: 9.0 (0–33.0) years | 96.7% | 153 | Median: 30.0 (10.0–62.0) | 21 Item-BAI (≥8/≥16/≥26) | 60.7/43.1/18.3 | 21 Item-BDI (≥14/≥20/≥29) | 45.7/30.7/18.9 | 2 |
| Radhakrishan 2011 | India | NS | 100% | 100 | 18-60 | SCID for DSM-IV | 51 | SCID for DSM-IV | Major depressive disorder: 46, adjustment disorder: 21, dysthymic disorder: 9 | 2 |
| Roebuck-Spencer 2006 | USA | 13.8 ± 10.2 years | 80% | 60 | 41.3 ± 12.8 | 21 Item-BDI (≥14) | 20 | 2 | ||
| Segal 2012 | USA | 12.0 ± 2.3 years | 93% | 71 | 41.7 ± 1.5 | CES-D (≥16) | 39 | 2 | ||
| Sehlo 2013 | Saudi Arabia | 6.9 ± 4.2 years | 100% | 80 | 34.8 ± 11.2 | SCID for DSM-IV | Major depressive disorder: 11.25 | 2 | ||
| Sfikakis 1998 | Greece | 7.8 ± 6.4 years | 91.5% | 71 | 37.0 ± 13.0 | HAS (>17) | 23.9 | HDS (>17) | 19.7 | 2 |
| Shakeri 2015 | Iran | NS | 92.5% | 160 | 30.1 ± 6.2 | 21 Item-BAI (≥8/≥16/≥26) | 81.2/51.9/18.1 | 21 Item-BDI (≥21/≥31/>40) | 69.3/38.7/20.6 | 2 |
| Shen 2015 | China | NS | 91.2% | 156 | 32.9 ± 10.2 | Zung SAS (≥50) | 20.51 | Zung SDS (≥53) | 33.33 | 3 |
| Skare 2014 | Brazil | 8.2 ± 6.9 years | 93% | 100 | 39.2 ± 12.5 | 21 Item-BDI (≥19/≥ 30) |
21/2 | 2 | ||
| Shorta1l 1995 | England | 11.0 ± 7.1 years | 95% | 80 | 41.0 ± 11.2 | HADS (≥8) | 39 | HADS (≥8) | 26 | 2 |
| Stoll 2001 | Switzerland | 11.4 ± 9.0 years | 90% | 60 | 44.5 ± 15.4 | HADS (≥8) | 16 | 3 | ||
| Tam 2008 | China | 9.7 years | 95.9% | 291 | 42.0 ± 12.0 | HADS (≥8) | 22 | HADS (≥8) | 18.2 | 3 |
| Tay 2015 | Singapore | 72.3 ± 81.1 months | 86.4% | 110 | 38.7 ± 12.6 | HADS (≥8) | 40.9 | HADS (≥8) | 15.5 | 2 |
| Tench 2000 | England | Median: 36.0 (12.0–79.5) months | 100% | 120 | Median: 38.0 (32.0–45.0) | HADS (≥8) | 60 | HADS (≥8) | 37 | 2 |
| Tjensvoll 2010 | Norway | 12.3 ± 8.6 years | 87% | 63 | 43.4 ± 13.3 | 21 Item-BDI (≥13) |
23.8 | 2 | ||
| Utset 2014 | USA | Median: 9 years | 95% | 344 | >18 | CES-D (>10) | 54.5 | 4 | ||
| van Exel 2013 | Netherlands | 7.8 ± 7.0 years | 88.2% | 102 | 44.4 ± 12.5 | 21 Item-BDI (≥14) |
27 | 3 | ||
| Vina 2015 | USA | 143.2 ± 117.8 months | 93% | 343 | 44.4 ± 12.9 | CES-D (≥17) | 47.2 | 4 | ||
| Weder-Cisneros 2004 | USA | Mean: 97.0 (6–348) months | 91.4% | 81 | 31.2 ± 9.7 | 21 Item-BDI (≥14) |
40.7 | 3 | ||
| Xie 2012 | China | Median: 1.3 years | 93.7% | 285 | 34.0 ± 13.0 | 21 Item-BDI (≥5/14/≥21) |
59.3/40.7/19.3 | 4 | ||
| Zakeri 2012 | Iran | NS | 90.5% | 71 | >18 | 21 Item-BDI (≥16/≥32) |
60/9.4 | 2 |
NS not stated, CES-D Centre for Epidemiological Studies Depression Scale, BDI Beck Depression Inventory, BAI Beck Anxiety Inventory, DSM-III/IV Diagnostic and Statistical Manual of Mental Disorders, Third/Fourth Edition, HADS Hospital Anxiety and Depression Scale, ICD International Classification of Diseases, HAS the Hamilton Anxiety Scale, HDS the Hamilton Depression Scale, PHQ Patient Health Questionnaire, SCID Structured Clinical Interview for Diagnostic and Statistical Manual, SCL-90-R Symptoms Checklist-90-Revised, Zung SAS Zung Self-rating Anxiety Scale, Zung SDS Zung Self-rating Depression Scale
Prevalence of depression among SLE patients
Prevalence estimates of depression ranged from 2% to 91.7% in individual studies (Table 1). Table 2 indicated the summary of meta-analyses and heterogeneity assessments. Meta-analyses revealed the prevalence of major depressive disorder to be 24% (95% CI, 16%–31%) according to the DSM and/or ICD diagnostic criteria, with high heterogeneity (I2 = 95.2%). Prevalence estimates of depression were 30% (95% CI, 22%–38%, I2 = 91.6%) for the HADS with thresholds of 8 and 38% (95% CI, 32%–44%, I2 = 81.3%) for the CES-D with thresholds of 16, respectively. Prevalence of depression according to the 21 Item-BDI with a cutoff of 14 or more was 39% (95% CI, 29%–49%), with high heterogeneity (I2 = 88.2%) (Fig. 2).
Fig. 2.
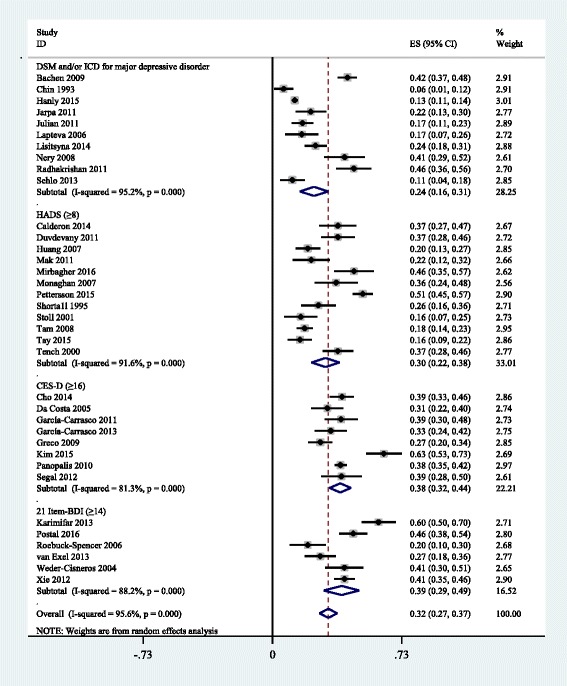
Prevalence of depressive disorder in SLE
Prevalence of anxiety among SLE patients
Prevalence of anxiety alone ranged between 4% and 85% in individual studies (Table 1). Table 3 presented the summary of meta-analyses and heterogeneity assessments. Meta-analyses pooled the prevalence of anxiety to be 40% (95% CI, 30%–49%, I2 = 93.0%) and 37% (95% CI, 12%–63%, I2 = 98.3%) according to the HADS with thresholds of 8 and the DSM and/or ICD diagnostic criteria, respectively (Fig. 3).
Fig. 3.
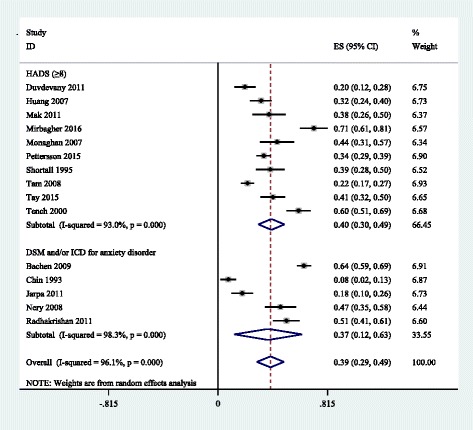
Prevalence of anxiety in SLE
Sensitivity and subgroup analyses
Table 4 suggested depression and anxiety prevalence estimates according to each sensitivity and subgroup analysis, in comparison with the primary analysis. Sensitivity analyses revealed that the exclusion of studies with less sample representativeness tended to decrease dysthymic disorder prevalence estimates according to DSM and/or ICD. The removal of studies with less comparable respondent and non-respondent comparability tended to increase depression prevalence estimates according to the HADS with a cutoff of 8 or more. According to DSM and/or ICD, anxiety prevalence estimates had a trend to decrease by exclusion of studies only using female sample. The subgroup analyses were conducted according to sample size, overall quality, publication year, and country of origin. The results showed that studies with sample size <200 had higher anxiety estimates [43% (95% CI, 31%–55%) vs 28% (95% CI, 16%–40%)] according to the HADS with a cutoff of 8 or more. When evaluated by Newcastle-Ottawa criteria, studies with lower total overall quality scores yielded higher dysthymic disorder estimates [18% (95% CI, 6%–29%) vs 3% (95% CI, 2%–25%)] according to DSM and/or ICD. In contrast with clinical interviews (DSM and/or ICD), more recent publications tended to yield higher depression and anxiety prevalence estimates according to self-report instruments. The subgroup analyses for country of origin showed no clear patterns. There was no particular trend or pattern in any other sensitivity analyses or subgroup analyses.
Table 4.
Impact of study characteristics on prevalence estimates for depression and anxiety in SLE: sensitivity and subgroup analyses
| Depression definition (cutoff) | Anxiety definition (cutoff) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Major depressive disorder (DSM and/or ICD) | Dysthymic disorder (DSM and/or ICD) | HADS (≥8) | CES-D (≥16) | 21 Item-BDI (≥14) | 21 Item-BDI (≥21) | 21 Item-BDI (≥30) | HADS (≥8) | Anxiety disorder (DSM and/or ICD) |
|
| Primary analysis | 24 (16, 31) I2 = 95.2% 10 studies 2960 patients |
12 (5, 18) I2 = 93.4% 6 studies 922 patients |
30 (22, 38) I2 = 91.6% 12 studies 1474 patients |
38 (32, 44) I2 = 81.3% 8 studies 1640 patients |
39 (29, 49) I2 = 88.2% 6 studies 781 patients |
34 (2, 65) I2 = 98.8% 3 studies 545 patients |
5 (0, 9) I2 = 72.1% 3 studies 326 patients |
40 (30, 49) I2 = 93.0% 10 studies 1332 patients |
37 (12, 63) I2 = 98.3% 5 studies 663 patients |
| Sensitivity analyses | |||||||||
| Excluding studies with less sample representativeness | 24 (6, 42) I2 = 98.2% 3 studies 2303 patients |
3 (2, 5) I2 = 0% 2 studies 476 patients |
29 (15, 44) I2 = 82.7% 3 studies 220 patients |
- | 36 (27, 45) I2 = 72.4% 3 studies 468 patients |
- | - | 31 (8, 55) I2 = 90.1% 2 studies 160 patients |
- |
| Excluding studies with less comparable respondent and non-respondent comparability | - | - | 45 (37, 54) I2 = 68.1% 3 studies 482 patients |
44 (29, 59) I2 = 91.9% 3 studies 996 patients |
- | - | - | 42 (17, 66) I2 = 96.9% 3 studies 482 patients |
- |
| Excluding studies only using female sample |
16 (11, 21) I2 = 79.8% 6 studies 2383 patients |
16 (4, 28) I2 = 95.0% 4 studies 496 patients |
27 (17, 36) I2 = 92.9% 9 studies 1195 patients |
44 (35, 54) I2 = 85.6% 4 studies 1168 patients |
39 (29, 49) I2 = 88.2% 6 studies 781 patients |
34 (2, 65) I2 = 98.8% 3 studies 545 patients |
5 (−2, 12) I2 = 85.9% 2 studies 266 patients |
33 (27, 39) I2 = 79.4% 8 studies 1135 patients |
12 (2, 23) I2 = 76.5% 2 studies 166 patients |
| Subgroup analyses | |||||||||
| Sample size | |||||||||
| <200 | 22 (14, 31) I2 = 90.5% 8 studies 807 patients |
14 (5, 23) I2 = 93.3% 5 studies 596 patients |
29 (22, 36) I2 = 81.1% 10 studies 878 patients |
38 (28, 48) I2 = 86.3% 6 studies 1008 patients |
39 (25, 52) I2 = 90.5% 5 studies 496 patients |
41 (−14, 96) I2 = 99.2% 2 studies 260 patients |
5 (0, 9) I2 = 72.1% 3 studies 326 patients |
43 (31, 55) I2 = 91.8% 8 studies 736 patients |
30 (9, 52) I2 = 96.0% 4 studies 337 patients |
| ≥200 | 27 (2, 57) I2 = 99.1% 2 studies 2153 patients |
- | 35 (2, 67) I2 = 98.8% 2 studies 596 patients |
39 (36, 42) I2 = 0.0% 2 studies 632 patients |
- | - | - | 28 (16, 40) I2 = 90.8% 2 studies 596 patients |
- |
| Overall quality | |||||||||
| <3 points (low quality) | 23 (13, 34) I2 = 91.8% 7 studies 657 patients |
18 (6, 29) I2 = 93.2% 4 studies 446 patients |
26 (18, 33) I2 = 77.5% 6 studies 581 patients |
34 (28, 40) I2 = 45.5% 4 studies 443 patients |
42 (21, 63) I2 = 93.8% 3 studies 313 patients |
41 (−14, 96) I2 = 99.2% 2 studies 260 patients |
5 (0, 9) I2 = 72.1% 3 studies 326 patients |
42 (32, 52) I2 = 82.5% 5 studies 499 patients |
30 (9, 52) I2 = 96.0% 4 studies 337 patients |
| ≥3 points (high quality) | 26 (6, 42) I2 = 98.2% 3 studies 2303 patients |
3 (2, 5) I2 = 0% 2 studies 476 patients |
34 (20, 48) I2 = 95.0% 6 studies 893 patients |
42 (33, 52) I2 = 87.9% 4 studies 1197 patients |
36 (27, 45) I2 = 72.4% 3 studies 468 patients |
- | - | 38 (23, 53) I2 = 95.5% 5 studies 833 patients |
- |
| Publication year | |||||||||
| 1990s | - | - | - | - | - | - | - | - | - |
| 2000s | 33 (17, 50) I2 = 91.0% 3 studies 457 patients |
- | 25 (17, 33) I2 = 81.3% 5 studies 660 patients |
28 (23, 34) I2 = 0.0% 2 studies 261 patients |
30 (10, 51) I2 = 86.8% 2 studies 141 patients |
- | - | 39 (22, 57) I2 = 95.0% 4 studies 600 patients |
56 (39, 73) I2 = 86.3% 2 studies 397 patients |
| 2010- | 21 (14, 29) I2 = 91.5% 6 studies 2424 patients |
11 (2, 19) I2 = 92.0% 4 studies 517 patients |
35 (22, 48) I2 = 93.1% 6 studies 734 patients |
42 (35, 48) I2 = 78.6% 6 studies 1379 patients |
43 (32, 55) I2 = 88.5% 4 studies 640 patients |
34 (2, 65) I2 = 98.8% 3 studies 545 patients |
5 (0, 9) I2 = 72.1% 3 studies 326 patients |
41 (26, 56) I2 = 93.8% 5 studies 652 patients |
34 (2, 67) I2 = 96.1% 2 studies 187 patients |
| Country of origin | |||||||||
| North America | 22 (8, 37) I2 = 97.3% 4 studies 2363 patients |
3 (2, 5) I2 = 0% 2 studies 476 patients |
- | 38 (31, 45) I2 = 83.9% 7 studies 1439 patients |
30 (10, 51) I2 = 86.8% 2 studies 141 patients |
- | - | - | - |
| Asia | 21 (0, 41) I2 = 96.0% 3 studies 259 patients |
21 (−3, 44) I2 = 93.7% 2 studies 179 patients |
26 (18, 34) I2 = 85.4% 6 studies 767 patients |
- | 50 (31, 69) I2 = 91.3% 2 studies 385 patients |
34 (2, 65) I2 = 98.8% 3 studies 545 patients |
- | 37 (23, 51) I2 = 94.4% 6 studies 767 patients |
29 (−13, 72) I2 = 98.2% 2 studies 179 patients |
| Europe | - | - | 33 (17, 49) I2 = 93.8% 4 studies 565 patients |
- | - | - | - | 44 (28, 61) I2 = 91.9% 3 studies 505 patients |
- |
| South America | 31 (12, 50) I2 = 85.3% 2 studies 158 patients |
- | - | - | - | - | - | - | 32 (4, 60) I2 = 93.5% 2 studies 158 patients |
The first line in each set of data is percentage prevalence (95% CI)
DSM Diagnostic and Statistical Manual of Mental Disorders, ICD International Classification of Diseases, HADS Hospital Anxiety and Depression Scale, CES-D Centre for Epidemiological Studies Depression Scale, BDI Beck Depression Inventory
Associated study variables
We used Pearson’s and Spearmen’s correlation analyses to assess the association between variables including mean/medium disease duration, proportion of female participants, mean/medium age, representativeness, sample size, comparability, overall quality, country of origin, publication year, and the prevalence of depression and anxiety. Table 5 indicated that more recent publications was significantly associated with increased depression prevalence (r = 0.26, P = 0.04). No study characteristics presented a significant association with anxiety prevalence estimate.
Table 5.
Pearson's and Spearmen’s correlation between study characteristics and prevalence estimates
| Study characteristic | Depression prevalence estimate | Anxiety prevalence estimate | ||||
|---|---|---|---|---|---|---|
| No. of studies | r | P | No. of studies | r | P | |
| Female, % | 59 | 0.03 | 0.84 | 24 | 0.07 | 0.76 |
| Mean/medium age, year | 55 | −0.13 | 0.35 | 23 | −0.18 | 0.94 |
| Mean/medium disease duration, year | 53 | −0.07 | 0.64 | 21 | 0.24 | 0.29 |
| Representativeness | 59 | 0.03 | 0.85 | 24 | 0.08 | 0.70 |
| Sample size | 59 | 0.12 | 0.38 | 24 | 0.01 | 0.97 |
| Comparability | 59 | 0.24 | 0.07 | 24 | −0.11 | 0.61 |
| Overall quality | 59 | 0.13 | 0.33 | 24 | −0.10 | 0.64 |
| Country of origin | 59 | 0.01 | 0.92 | 24 | −0.10 | 0.63 |
| Publication year | 59 | 0.26* | 0.04 | 24 | −0.04 | 0.84 |
*Significant at a P <0.05 level
Assessment of publication bias
Assessment of publication bias indicated significant publication bias, according to the Egger’s test, in studies reporting depression according to HADS with thresholds of 8 and CES-D with a cutoff of 16 or more [Egger: bias = 0.81 (95% CI: 0.04, 1.58), P = 0.04, and Egger: bias = 2.79 (95% CI: 0.61, 4.97), P = 0.02, respectively]. There was no significant evidence of publication bias in any other analyses (see Additional file 5).
Discussion
This systematic review and meta-analysis of 59 studies involving 10828 adult SLE patients demonstrated that a few studies using gold standard clinical interviews (DSM and/or ICD) reported that major depression and anxiety were presented in 24% and 37% among SLE patients, respectively. The majority of studies using screening tools found that significant depression were presented in 30% using the HADS a cutoff of 8 or more and 39% using the 21 Item-BDI with thresholds of 14. This study also found that more recent publications was significantly associated with increased depression prevalence among SLE patients. Furthermore, the prevalence of anxiety was 40% according to the HADS with thresholds of 8. These prevalence estimates are significantly higher than those observed in the general population [23, 24] and other rheumatic and connective tissue diseases [15, 25, 26]. Furthermore, these findings demonstrated that SLE patients tended to have a higher prevalence of anxiety than depression, which was in line with previous studies [27, 28]. Such discrepancy could be explained by the differences in time frames when these studies were performed, disease characteristics, social and cultural contexts of the lupus patients and tools used for assessing depression or anxiety. Because the development of depression and/or anxiety could result in increased incidence of cardiovascular diseases [5], decreased quality of life [9, 10], and a higher risk of premature mortality [11] among SLE patients, these findings highlighted an important issue in health education for this population.
Neuropsychiatric (NP) disorders appeared in about 70% of the patients diagnosed with SLE [29]. Previous meta-analyses have assessed the prevalence of the 19 NP syndromes defined by the American College of Rheumatology (ACR) in 1999 among SLE patients [30]. However, there were a wide variety of neurologic and psychiatric manifestations of SLE, which extended beyond those identified in the 1999 ACR classification criteria for SLE [31]. Several attempts have been made to devise a classification of NP-SLE manifestations because there were controversies regarding the inclusion of mood disorders in the 1999 ACR NP-SLE criteria [31, 32]. That’s why we excluded the studies investigating neuropsychiatric syndromes among SLE patients in this meta-analysis.
Although studies varied widely in terms of quality, our sensitivity analyses suggested that depression and/or anxiety prevalence estimates (except dysthymic disorder estimates) were reasonably stable. Variation in study sample size contributed importantly to the observed heterogeneity in the data. Studies with sample size <200 had higher anxiety estimates according to the HADS with thresholds of 8. Furthermore, studies with lower total overall quality scores yielded higher dysthymic disorder estimates according to DSM and/or ICD. Country, publication year, age, and gender also contributed to the heterogeneity between studies.
In this meta-analysis, many methods were used for data extraction and synthesis. The gold standard method was diagnostic interviews using DSM or ICD criteria, which were often time consuming and expensive. Therefore, it was not ideal for examining patients in a busy hospital environment [33]. Alternatively, self-report screening tools might be used, because they were quick and easy to complete and cheaper to use than diagnostic interviews. However, prevalence estimates using screening tools were often overestimated, because such tools tended to prioritize sensitivity over specificity [33]. Furthermore, there have not been validation studies to determine the best cut-point for screening tools in SLE patients, and several cut-off scores on self-report tools were often used in many studies. It indicated that the rheumatologists should always report prevalence at conventional cut-points, and screen for depression and anxiety among SLE patients according to the social and cultural contexts of the rheumatologists and SLE patients in clinical practice.
There are, however, additional important shortcomings in the evidence on prevalence of depression in SLE that need to be addressed. First, a substantial amount of the heterogeneity among the studies remained unexplained by the variables examined. Unexamined factors, such as gender, age, disease duration, might contribute to the risk for depression and/or anxiety symptom among SLE patients. Second, the data were derived from studies that used different designs and involved different groups of patients (e.g., from different countries), which might result in heterogeneity among the studies. Third, we did not look for healthy subjects in each study reporting the prevalence of depression or anxiety in SLE patients, which should be addressed in future research.
Conclusions
The prevalence of depression and anxiety was high in adult SLE patients. It indicated that rheumatologists should screen for depression and anxiety in their patients, and they should refer them to mental health providers in order to identify effective strategies for preventing and treating depression and anxiety among SLE patients.
Acknowledgments
We would like to thank Chenlin Zhang and Alick for their great assistance with this study.
Funding
This work was supported by the Natural Science Foundation of China (Grant no. 81401124); the Humanistic Nursing Care Foundation of China (Grant no. RW2016AM14); Preventive Medicine Projects from Bureau of Jiangsu Province (Y2012083); “Top Six Types of Talents” Financial Assistance of Jiangsu Province (Grant no. 10.WSN016); Jiangsu Provincial Commission of Health and Family Planning Foundation (Grant no. Z201622); Science Foundation of Nantong City (Grant no. MS22015003); the College graduate research and innovation of Jiangsu Province (KYZZ15_0353); and the Nantong University Graduate Innovation Program (YKC15075).
Availability of data and materials
The majority of data generated or analyzed during this study are included in this published article (and its Additional files). Remaining data not published here are available from the corresponding author on reasonable request.
Authors’ contributions
LZ and TF searched and checked the databases according to the inclusion and exclusion criteria, extracted the data and assessed their quality. LZ analyzed the data and wrote the draft of the paper. RY, QZ and BS gave advice on meta-analysis methodology and revised the paper. All authors contributed to reviewing or revising the paper. BS is the guarantor of this work and had full access to all the data in the study and takes responsibility for its integrity and the accuracy of the data analysis. All authors read and approved the final manuscript.
Competing interests
The authors declared that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Ethical approval and consent to participate are not required for this review.
Additional files
Search Terms. (DOCX 10 kb)
Summaries of symptom thresholds required for diagnosis of depression/anxiety. (DOCX 19 kb)
The list of 59 studies included in the meta-analysis. (DOCX 19 kb)
Quality Assessment. (DOCX 19 kb)
Assessment of Publication Bias. (DOCX 56 kb)
References
- 1.Yilmaz-Oner S, Oner C, Dogukan FM, Moses TF, Demir K, Tekayev N, et al. Anxiety and depression predict quality of life in Turkish patients with systemic lupus erythematosus. Clin Exp Rheumatol. 2015;33(3):360–5. [PubMed] [Google Scholar]
- 2.Kheirandish M, Faezi ST, Paragomi P, Akhlaghi M, Gharibdoost F, Shahali A, et al. Prevalence and severity of depression and anxiety in patients with systemic lupus erythematosus: An epidemiologic study in Iranian patients. Mod Rheumatol. 2015;25(3):405–9. doi: 10.3109/14397595.2014.962241. [DOI] [PubMed] [Google Scholar]
- 3.Bachen EA, Chesney MA, Criswell LA. Prevalence of mood and anxiety disorders in women with systemic lupus erythematosus. Arthritis Rheum. 2009;61(6):822–9. doi: 10.1002/art.24519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ainiala H, Loukkola J, Peltola J, Korpela M, Hietaharju A. The prevalence of neuropsychiatric syndromes in systemic lupus erythematosus. Neurology. 2001;57(3):496–500. doi: 10.1212/WNL.57.3.496. [DOI] [PubMed] [Google Scholar]
- 5.Greco CM, Li T, Sattar A, Kao AH, Danchenko N, Edmundowicz D, et al. Association between depression and vascular disease in systemic lupus erythematosus. J Rheumatol. 2012;39(2):262–8. doi: 10.3899/jrheum.110327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lisitsyna TA, Vel’tishchev D, Seravina OF, Kovalevskaia OB, Marchenko AS, Novikova DS, et al. Prevalence of mental disorders in SLE patients: correlations with the disease activity and comorbid chronic conditions. Ter Arkh. 2009;81(6):10–6. [PubMed] [Google Scholar]
- 7.Mok CC, Chan KL, Cheung EF, Yip PS. Suicidal ideation in patients with systemic lupus erythematosus: incidence and risk factors. Rheumatology (Oxford) 2014;53(4):714–21. doi: 10.1093/rheumatology/ket404. [DOI] [PubMed] [Google Scholar]
- 8.Ward MM, Lotstein DS, Bush TM, Lambert RE, van Vollenhoven R, Neuwelt CM. Psychosocial correlates of morbidity in women with systemic lupus erythematosus. J Rheumatol. 1999;26(10):2153–8. [PubMed] [Google Scholar]
- 9.Mak A, Tang CS, Ho RC. Serum tumour necrosis factor-alpha is associated with poor health-related quality of life and depressive symptoms in patients with systemic lupus erythematosus. Lupus. 2013;22(3):254–61. doi: 10.1177/0961203312471872. [DOI] [PubMed] [Google Scholar]
- 10.Mok CC, Chan KL, Ho LY. Association of depressive/anxiety symptoms with quality of life and work ability in patients with systemic lupus erythematosus. Clin Exp Rheumatol. 2016;34(3):389–95. [PubMed] [Google Scholar]
- 11.Xie LF, Chen PL, Pan HF, Tao JH, Li XP, Zhang YJ, et al. Prevalence and correlates of suicidal ideation in SLE inpatients: Chinese experience. Rheumatol Int. 2012;32(9):2707–14. doi: 10.1007/s00296-011-2043-3. [DOI] [PubMed] [Google Scholar]
- 12.Doria A, Rinaldi S, Ermani M, Salaffi F, Iaccarino L, Ghirardello A, et al. Health-related quality of life in Italian patients with systemic lupus erythematosus. II. Role of clinical, immunological and psychological determinants. Rheumatology (Oxford) 2004;43(12):1580–6. doi: 10.1093/rheumatology/keh392. [DOI] [PubMed] [Google Scholar]
- 13.Bogdanovic G, Stojanovich L, Djokovic A, Stanisavljevic N. Physical Activity Program Is Helpful for Improving Quality of Life in Patients with Systemic Lupus Erythematosus. Tohoku J Exp Med. 2015;237(3):193–9. doi: 10.1620/tjem.237.193. [DOI] [PubMed] [Google Scholar]
- 14.Matcham F, Rayner L, Steer S, Hotopf M. The prevalence of depression in rheumatoid arthritis: a systematic review and meta-analysis. Rheumatology (Oxford) 2013;52(12):2136–48. doi: 10.1093/rheumatology/ket169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stubbs B, Aluko Y, Myint PK, Smith TO. Prevalence of depressive symptoms and anxiety in osteoarthritis: a systematic review and meta-analysis. Age Ageing. 2016;45(2):228–35. doi: 10.1093/ageing/afw001. [DOI] [PubMed] [Google Scholar]
- 16.Meszaros ZS, Perl A, Faraone SV. Psychiatric symptoms in systemic lupus erythematosus: a systematic review. J Clin Psychiatry. 2012;73(7):993–1001. doi: 10.4088/JCP.11r07425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Panic N, Leoncini E, de Belvis G, Ricciardi W, Boccia S. Evaluation of the endorsement of the preferred reporting items for systematic reviews and meta-analysis (PRISMA) statement on the quality of published systematic review and meta-analyses. PLoS One. 2013;8(12):e83138. doi: 10.1371/journal.pone.0083138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–5. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 19.Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synth Methods. 2010;1(2):97–111. doi: 10.1002/jrsm.12. [DOI] [PubMed] [Google Scholar]
- 20.Higgins JPT, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sterne JA, Egger M. Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. J Clin Epidemiol. 2001;54(10):1046–55. doi: 10.1016/S0895-4356(01)00377-8. [DOI] [PubMed] [Google Scholar]
- 23.Greco CM, Kao AH, Sattar A, Danchenko N, Maksimowicz-McKinnon KM, Edmundowicz D, et al. Association between depression and coronary artery calcification in women with systemic lupus erythematosus. Rheumatology (Oxford) 2009;48(5):576–81. doi: 10.1093/rheumatology/kep020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shen B, Tan W, Feng G, He Y, Liu J, Chen W, et al. The correlations of disease activity, socioeconomic status, quality of life, and depression/anxiety in Chinese patients with systemic lupus erythematosus. Clin Dev Immunol. 2013;2013:270878. doi: 10.1155/2013/270878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang L, Xia Y, Zhang Q, Fu T, Yin R, Guo G, et al. The correlations of socioeconomic status, disease activity, quality of life, and depression/anxiety in Chinese patients with rheumatoid arthritis. Psychol Health Med. 2017;22(1):28–36. doi: 10.1080/13548506.2016.1198817. [DOI] [PubMed] [Google Scholar]
- 26.Westhoff G, Dörner T, Zink A. Fatigue and depression predict physician visits and work disability in women with primary Sjögren’s syndrome: results from a cohort study. Rheumatology (Oxford) 2012;51(2):262–9. doi: 10.1093/rheumatology/ker208. [DOI] [PubMed] [Google Scholar]
- 27.Mak A, Tang CS, Chan MF, Cheak AA, Ho RC. Damage accrual, cumulative glucocorticoid dose and depression predict anxiety in patients with systemic lupus erythematosus. Clin Rheumatol. 2011;30(6):795–803. doi: 10.1007/s10067-010-1651-8. [DOI] [PubMed] [Google Scholar]
- 28.Tay SH, Cheung PP, Mak A. Active disease is independently associated with more severe anxiety rather than depressive symptoms in patients with systemic lupus erythematosus. Lupus. 2015;24(13):1392–9. doi: 10.1177/0961203315591026. [DOI] [PubMed] [Google Scholar]
- 29.Buća A, Perković D, Martinović-Kaliterna D, Vlastelica M, Titlić M. Neuropsychiatric systemic lupus erythematosus: diagnostic and clinical features according to revised ACR criteria. Coll Antropol. 2009;33(1):281–8. [PubMed] [Google Scholar]
- 30.Unterman A, Nolte JE, Boaz M, Abady M, Shoenfeld Y, Zandman-Goddard G. Neuropsychiatric Syndromes in Systemic Lupus Erythematosus: A Meta-Analysis. Semin Arthritis Rheum. 2011;41(1):1–11. doi: 10.1016/j.semarthrit.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 31.Hanly JG. ACR classification criteria for systemic lupus erythematosus: limitations and revisions to neuropsychiatric variables. Lupus. 2004;13(11):861–4. doi: 10.1191/0961203304lu2024oa. [DOI] [PubMed] [Google Scholar]
- 32.Davey R, Bamford J, Emery P. The ACR classification criteria for headache disorders in SLE fail to classify certain prevalent headache types. Cephalalgia. 2008;28(3):296–9. doi: 10.1111/j.1468-2982.2007.01510.x. [DOI] [PubMed] [Google Scholar]
- 33.Hotopf M, Chidgey J, Addington-Hall J, Ly KL. Depression in advanced disease: a systematic review. Part 1. Prevalence and case finding. Palliat Med. 2002;16(2):81–97. doi: 10.1191/02169216302pm507oa. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The majority of data generated or analyzed during this study are included in this published article (and its Additional files). Remaining data not published here are available from the corresponding author on reasonable request.


