Abstract
From 1735 to 1940, maize-based diets led to the death of hundreds of thousands of people from pellagra, a complex disease caused by tryptophan and vitamin B3 deficiencies. The current cereal monoculture trend restricts farmland animals to similarly monotonous diets. However, few studies have distinguished the effects of crop nutritional properties on the reproduction of these species from those of other detrimental factors such as pesticide toxicity or agricultural ploughing. This study shows that maize-based diets cause high rates of maternal infanticides in the European hamster, a farmland species on the verge of extinction in Western Europe. Vitamin B3 supplementation is shown to effectively restore reproductive success in maize-fed females. This study pinpoints how nutritional deficiencies caused by maize monoculture could affect farmland animal reproduction and hence their fitness.
Keywords: conservation, corn, feeding ecology, fitness, niacin, pellagra
1. Introduction
In recent decades, there have been major changes in agricultural practices, directly associated with the increasing demand for food, plastics and biofuels [1–3]. This increased demand is currently satisfied by intensively managed monoculture of cereal crops [2,3]. Cereal monoculture has been associated with an increase in the mortality of farmland species because of pesticide toxicity, agricultural mechanization or higher predation rates [4–9]. The resulting decline in biodiversity has led to a drastic reduction in ‘ecosystem services’, which are currently threatening human safety and nutrition worldwide [1,10–12]. These nutritional threats notably occur through a reduction in access to micronutrients (minerals, amino acids and vitamins) by pollinators [13].
Intensive monoculture has also been linked to the increase in the size of plots, the loss of edge zones and of green corridors associated with marked environmental stochasticity in agroecosystems [1,2]. In parallel, the intensive use of inputs is reducing the diversity and abundance of adventive species, soil fauna and microbial communities [10,14,15]. Taken together, these phenomena are making food availability unpredictable and poorly diversified for farmland wildlife, which are thus restricted to a more monotonous and stochastic diet [16–18]. Goulson et al. [16] recently stated that ‘it seems certain that bees inhabiting intensive farmland have a more monotonous diet than they would have experienced in their evolutionary past, but how this impacts upon their fitness remains unclear’. Indeed, the lack of flower diversity in intensively managed farmland with the predominance of flowers in the form of mass-flowering crops such as wheat, maize or canola strongly constrain pollinators in their diet [16,19]. More generally, all species with small home ranges that live in agricultural landscapes appear to be constrained in their diet by thousands of hectares dominated by one or two intensively cultivated crops. However, studies are still lacking on how crop-based diets with varying macronutrient, mineral or vitamin contents and amino acid composition influence the physiology, behaviour or key life-history traits—such as reproduction—of farmland animals. This is especially true for species with small home ranges, a marked seasonal cycle or for rare or endangered species that are complex to monitor in the wild without threatening their survival. Indeed, studies including the effects of crop-based diets on wildlife in agricultural landscapes are still scarce and limited to invertebrates [16,19] or mammals with large home ranges [20].
The European hamster (Cricetus cricetus)—critically endangered in Europe [21–23]—is particularly threatened by the expansion of wheat and maize monocultures in Western Europe [22,24]. The highly fluctuating food availability (with crop rotation and harvest) associated with the strong seasonality of the species (which hibernates from October–April and reproduces from April–September) put it at high risk of facing periods of food scarcity, even during the reproductive period (e.g. after harvest). The European hamster belongs to the food-hoarding hibernators [25–27], known to hoard very large amounts of food in their burrow to feed during winter arousals but also during the active period (for up to 11 months in some species [27,28]) when above-ground foraging is not possible [27]. Given that species at high risk of facing periods of food scarcity are those that rely the most on their hoarded food [27], the survival and reproductive success of wild hamsters might greatly depend on the nutritional value of their hoards. However, up-to-date data on the link between nutrition and fitness of wild hamsters in the agroecosystems of Western Europe are severely lacking, most current studies on this aspect involve hamsters living in urban habitats [29–32]. The most recent data on the nutrition of the species in agroecosystems date back to the 1970s–1980s [26,33] and revealed that hamsters mostly feed on cereal crops (wheat, corn, rape), tubers and invertebrates. We therefore designed this experimental study specifically to analyse the impact of natural-based diets composed of cereals supplemented with natural protein-rich items on the reproductive investment and ultimately on the reproductive success of captive female hamsters. This design is based on the study of Gorecki & Grygielska [33], and on the postulate of Nechay [34] which states that wild female hamsters do not emerge from their burrow before their first gestation. They thus rely on the food they hoarded the previous summer (mainly seeds) until gestation occurs, then supplement their diet with fresh food items, including plants available in spring, such as clover or invertebrates such as earthworms during gestation and lactation.
Maize appears to be slightly energy richer than wheat but contains marginally less proteins [35], whereas earthworms are significantly richer in proteins than clover [35]. Consequently, and given the importance of protein and energy supplies during reproduction in vertebrates [36–41], we were expecting to observe (i) slightly larger litters or larger pups at parturition by females fed maize-based diets, (ii) slightly higher growth rates of pups of females fed wheat-based diets compared with maize-based diets, and (iii) a significantly greater growth rate in pups of females fed with diets supplemented with earthworms than those fed with diets supplemented with clover. Following the results of a study dating from 1945 [42] reporting that the vitamin B3 deficiency (i.e. niacin or nicotinamide) in maize was responsible for delayed growth in rats, we designed a second experiment in which hamsters were fed on maize-earthworms diets, one of which included a vitamin B3 supplement. Given the positive effects of this vitamin on growth parameters in livestock and rats, we were expecting (iv) a significantly greater growth rate and body mass in pups at weaning in the group fed with the vitamin B3-supplemented diet. By focusing on the direct impact of crop-based diets on reproduction, rather than the indirect impact of monoculture on mortality, we provide new insights into the impact of food on the life-history traits of farmland animals. This could thus help explain how the expansion of intensive monoculture is affecting wildlife fitness in agroecosystems.
2. Material and methods
(a). Animal care and breeding protocol
Hamsters were maintained in controlled environmental conditions (temperature 20°C–23°C; 35–55% humidity; summer photoperiod, 16 L : 8 D) and housed individually (W × H × D: 265 × 237 × 420 mm) until breeding (i.e. two weeks after the beginning of the experiment). The first experiment lasted from April to July 2014 and used 29 one-year-old primiparous females born in 2013 in our captive breeding unit (CNRS, IPHC-DEPE, Strasbourg, France). The second experiment lasted from April to July 2015 and used 14 one-year-old primiparous females from our captive breeding unit. Prior to the experiments, the females in both experiments were fed a conventional diet (pellets 105, from Safe, Augy, France, composed of 19.3% protein, 54.9% carbohydrates, 5.1% lipids, 4.2% cellulose, 5.0% minerals and 11.5% water). During the experiments, they were bred with 1-year-old males from our breeding unit (29 males in 2014 and 14 in 2015), which were fed the conventional diet until being paired with the females. Breeding pairs were placed in large cages (W × H × D: 380 × 257 × 590 mm) equipped with a shelter box (W × H × D: 140 × 230 × 230 mm) for two weeks.
(b). First experiment: effect of natural-based diet on the maternal investment in reproduction
(i). Experimental protocol: diets and food intake
In the first experiment (2014), at emergence from hibernation, the 29 females were fed ad libitum with either wheat (Triticum spp.) or maize (Zea maize) grains and supplemented with either clover (Trifolium pretense) or earthworms (Lumbricus terrestris) after mating. Before parturition, the complement consisted of 5 g female−1 d−1 of either earthworm or clover. It was then increased by 1 g young−1 d−1 between parturition and weaning (30 days after parturition). This led to four different diets: wheat-worm (WW, n = 7), wheat-clover (WC, n = 8), maize-worm (MW, n = 7) and maize-clover (MC, n = 7). Water was provided ad libitum throughout the experiment.
Females' daily intake of maize and wheat grains was recorded along with the total protein, lipid and energy content of each diet. Grains were freeze-dried to constant mass and ground under liquid nitrogen to obtain a homogeneous powder for analysis. Just before analysis, the powder was lyophilized for 48 h to eliminate any remaining traces of water. Nitrogen content was determined in triplicate using 150–200 mg aliquots according to the Kjeldahl method [43]. Protein content was calculated as nitrogen content × 6.25 [44]. Lipid content was determined in duplicate using 1 g aliquots according to a procedure adapted from the Folch method [45] with a chloroform/methanol (2/1, v/v) solution as extraction solvents. Ash content was determined gravimetrically in duplicate from 1 to 2 g samples ignited in a muffle furnace at 400°C for 24 h. Total body water was then calculated by subtracting total dry body mass from fresh body mass. Finally, energy content was determined on dry 0.7–1.4 g aliquots by using an isoperibol bomb calorimeter Parr 6200 with benzoic acid as standard. The carbohydrate ration was equal to 100% of energy value minus the lipid and protein percentages.
(ii). Litter size, reproductive success and body mass
Twice a day (at 08.00 and 19.00), we monitored the number of females that initiated parturition. Females that did not give birth or did not raise their litter were subjected to a second mating event. Their body mass (±0.01 g) was recorded 1 day before pairing, 8 days after parturition and at the end of the experiment (i.e. 30 days after parturition if they initiated parturition and raised their litter or 30 days after the second reproductive attempt if they did not). These three periods are hereafter referred to as ‘before’, ‘middle’ and ‘end’ of the experiment. We monitored the size of the litters every day to obtain the early survival rate of pups (from birth to weaning). The body mass of the pups (±0.01 g) was recorded at 8, 14 and 30 days of age.
(iii). Oxytocin plasma levels of mothers
Blood sampling: approximately 200 µl of blood was sampled from the sublingual vein under 2% isoflurane anaesthesia before pairing, 8 days after parturition and at weaning. Plasma levels of oxytocin (i.e. an important maternal hormone [46,47]) were measured, using commercially available ELISA kits (Enzo Life Sciences, ADI-900-153A). This ELISA kit was formulated for the measurement of rat plasma. All the assays were validated with serial dilutions of hamster plasma showing linear changes in sample values that were parallel with standard curves produced according to the manufacturer's standards. All measurements were made in duplicate. The average intra-assay variation coefficient was 2.5%.
(c). Second experiment: effects of vitamin B3 supplement on maternal investment in reproduction
In the second experiment (2015), the 14 females were fed ad libitum with maize at emergence from hibernation and supplemented with earthworms after mating. Before parturition, the supplement consisted of 5 g of earthworm per female per day. It was then increased by 1 g young−1 d−1 between parturition and weaning.
The vitamin B3 solution was prepared by dissolving 3 g of nicotinamide powder (greater than or equal to 99.5% (HPLC), 72340 Sigma) in 1 l of saline solution (NaCl, 9 g l−1), leading to a concentration of 3 g l−1. Earthworms were injected with 100 µl of vitamin B3 solution and immediately given to the females (‘maize-worm-B3’ group, n = 7). The non-supplemented females (‘maize-worm’ group, n = 7) were given earthworms injected with 100 µl of NaCl. Each female in the ‘maize-worm-B3’ group was thus supplemented with 0.3 mg of vitamin B3 per day, meeting the estimated levels in the wheat-earthworm diet from the first experiment. It was the increased by 0.05 mg per pup at parturition and until weaning.
Twice a day, we checked parturition and litter size. Females were weighed (±0.01 g) 1 day prior to pairing and at the end of the experiment (see §2b). Pups were weighed (± 0.01 g) at 8 and 30 days old.
(d). Data analyses
Data presented are means ± s.e.m. Normality was tested, using a Kolmogorov–Smirnov test and variance homogeneity was checked using a Levene test. We first looked at the effect of the diet on the average number of pups per female at parturition and at weaning, including females that did not give birth. This variable was analysed using a GLM (probability distribution: Poisson, link function: log) with diet as a fixed factor. Data concerning the two reproductive attempts for each female were grouped in this first analysis and tested separately for 2014 (four diets) and 2015 (two diets, one supplemented with vitamin B3). We performed a Cox regression to check for an effect of the diet on the early survival of the pups. The body mass of the pups was analysed, using linear mixed models. The diet, the age, the sex of the pups and the interactions between these variables were included as fixed factors, and the litter size was included as a covariate in this LMM model. The identity of the pups nested in the identity of the litter was included as a random factor for repeated measurements on the same individuals and the same litter. Data on pups' body mass collected in 2014 and 2015 were analysed separately. Regarding mothers, body mass and plasma levels of oxytocin in 2014 were also analysed using an LMM. The diet, the period and the diet × period interaction were included as fixed factors, and the identity of the females as the random factor for repeated measurements on the same individual (for body mass analysis). Finally, the number of pups was included as a covariate. Multiple comparisons were analysed via post hoc least significant difference testing. Final model selection was based on the best Akaike information criterion for small samples value. Analyses were conducted using IBM SPSS software (IBM SPSS Statistics for Windows v. 21.0. Armonk, NY: IBM Corp.), and the significance threshold was set at p < 0.05. Figures were prepared, using GraphPad Prism software (v. 5, La Jolla, CA, USA).
3. Results
(a). Macronutrient content of the diets, average number of pups per female at parturition and early survival of the pups
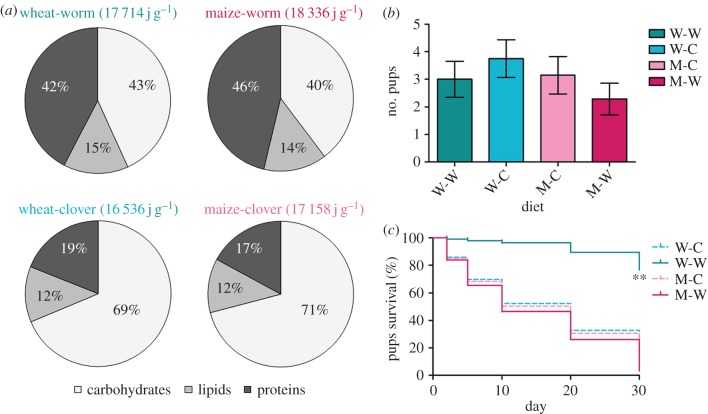
Females ingested on average 14.5 ± 1.2 and 15.8 ± 4.8 g d−1 of grains in the maize- and wheat-based diets, respectively. The whole 5 g of supplements (both of earthworm and clover) was entirely consumed each day. Both the earthworm diets, and both the clover diets, had similar macronutrient and energy contents (figure 1a), respectively. The wheat-worm diet was composed of 42% proteins, 43% carbohydrates and 15% lipids with a dry food energy content of 17.7 kJ g−1, whereas the maize-worm diet was composed of 46% proteins, 40% carbohydrates and 14% lipids with a dry food energy content of 18.3 kJ g−1. The wheat-clover diet was composed of 19% proteins, 69% carbohydrates and 12% lipids with a dry food energy content of 16.5 kJ g−1, whereas the maize-clover diet was composed of 17% proteins, 71% carbohydrates and 12% lipids with a dry food energy content of 17.2 kJ g−1.
Figure 1.
Gross energy and macronutrient content of the four diets, average number of pups per female at parturition and survival of the pups according to diet. (a) Protein, lipid and carbohydrate contents are represented as a percentage (%) of the energy content (in j g−1 of DM). (b) Average number of pups per female at parturition (i.e. including females that did not gave birth) according to the diet and (c) early survival (%) of the pups (i.e. from birth to 30 days old = weaning) according to the diet. Solid lines correspond to diets supplemented with earthworms and the dashed lines correspond to diets supplemented with clover. (b,c) W-W, wheat-worm; W-C, wheat-clover, M-C, maize-clover and M-W, maize-worm. The asterisk indicates a significant difference in survival between the wheat-worm and the three other diets (p = 0.003). See methods section for details. (Online version in colour.)
We found no effect of the diet on the average number of pups per female at parturition (figure 1b; Wald χ2 = 2.60, p = 0.46). However, we found a strong effect of the diet on the early survival of the pups (Wald χ2 = 43.77, p = 1.7 × 10−9). Pups born to females fed the wheat-worm diet had a survival rate of about 80% at weaning, which was significantly higher than pups born to females fed the other diets (survival rate lower than 12%, figure 1c, p = 7.3 × 10−11). We found an effect of diet (F3,23.6 = 21.4, p = 6.5 × 10−7) and age (i.e. 8, 14 and 30 days, F2,49.9 = 135.11, p = 7.2 × 10−21) on the body mass of the surviving pups, as well as an effect of the age × diet interaction (electronic supplementary material, figure S1, F6,47.4 = 13.2, p = 1 × 10−8). Post hoc analyses indicated that at 8 days (electronic supplementary material, figure S1a), the body mass of the pups born to females fed the wheat-based diets was significantly higher than the body mass of the pups born to females fed both maize-based diets (p < 0.009). At 14 days (electronic supplementary material, figure S1b), the pups born to females fed the wheat-worm diet weighed significantly more than the pups born to females fed the three other diets (p = 2.9 × 10−5), and the pups born to females fed the wheat-clover diet weighed more than the pups born to females fed the maize-worm diet (p = 0.037), but did not significantly differ from the pups born to females fed the maize-clover diet (p > 0.2).
(b). Maternal body mass and plasma levels of oxytocin
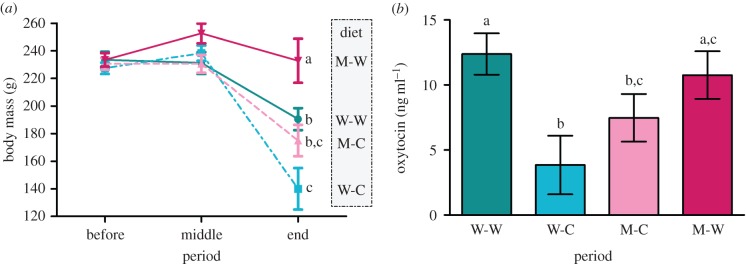
Concerning variations in body mass of the mothers during reproduction, we found an effect of period (F2,64 = 26.98, p = 3.2 × 10−9), diet (F3,64 = 7.107, p = 3.4 × 10−4) and the diet × period interaction (F6,64 = 2.97, p = 0.013). Post hoc analyses revealed that females fed the maize-worm diet lost significantly less body mass than females fed the three other diets (figure 2a, p < 0.001), whereas females fed the wheat-clover diet lost more body mass than females fed the wheat- and maize-worm diets (figure 2a, p < 0.03). We found no effect of the reproductive attempt (first or second) on the body mass of females (p > 0.1). We found no effects of the diet or of the period on maternal plasma levels of oxytocin (p > 0.27). However, we found an effect of the diet × period interaction (F5,47 = 2.99, p = 0.020) and post hoc analyses indicated that, 8 days post-parturition, females fed the wheat-worm and the maize-worm diets had significantly higher oxytocin plasma levels than females fed the wheat-clover diets (p = 0.014 and p = 0.044, respectively), whereas females fed the maize-clover diet were between the two (figure 2b).
Figure 2.
Variations in body mass and oxytocin plasma levels of mothers. (a) Changes in body mass during the first experiment according to the diet: the three periods ‘before’, ‘middle’ and ‘end’ correspond respectively to 1 day prior to pairing, 8 days after parturition and 30 days after parturition (or 30 days after the second reproductive attempt for females that did not give birth). The solid lines represent diets supplemented with earthworms and the dashed lines represent diets supplemented with clover. (b) Oxytocin plasma levels of mothers 8 days post-parturition, according to the diet. Different letters indicate significant differences between the diets (p < 0.05). (Online version in colour.)
(c). Average number of pups per female at weaning, pups' body mass and effects of the vitamin B3 supplement
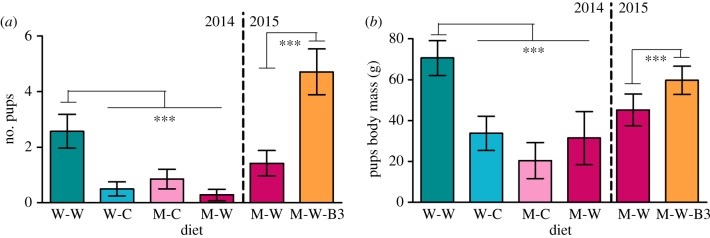
In the first experiment (2014) and as a consequence of the differences in pups' survival rates between the diets, the number of pups at weaning was significantly higher in the wheat-worm diet than in the three other diets (figure 3a, 2014, Wald χ2 = 17.37, p < 0.001). The body mass of the pups at weaning was also significantly higher in pups born to females fed the wheat-worm diet than in pups born to females fed the three other diets (figure 3b, 2014, F = 15.16, p = 2 × 10−5). In the second experiment (2015), the vitamin B3 supplement significantly increased the average number of pups per female in the group fed the maize-worm-B3 diet compared with the group fed the maize-worm diet (figure 3a, 2015, Wald χ2 = 10.94, p < 0.001). Vitamin B3 supplementation also significantly affected pups body mass at weaning (F = 15.157, p < 0.001): pups in the group with the vitamin B3 supplement were heavier than pups in the group with the maize-worm diet (figure 3b, 2015, F = 10.795, p = 0.041).
Figure 3.
Effects of the diet and the vitamin B3 supplement on the number of pups and on pups' phenotype. Average number of pups per female (a) and body mass of the pups (b) at weaning depending on the diet. Data represent the first experiment (2014) and the vitamin-B3-supplement experiment (2015). W-C , wheat-clover; W-W, wheat-worm; M-C, maize-clover, M-W , maize-worm and M-W-B3, maize-worm + vitamin B3 supplementation. Stars indicate significant differences between the diets (p < 0.001). See methods section for details. (Online version in colour.)
4. Discussion
Contrary to our original expectations, maize caused reproductive failure in captive European hamsters. Females were not affected in their capacity to give birth, but in their ability to wean their litters. Indeed, the pups' survival was severely reduced in both groups fed the maize-based diets, caused by litter suppression the first day after parturition (in 95% of the cases). In the group fed the wheat-based diets, significant reductions in litter size were observed in the group fed the wheat-clover diet but not in the group fed the wheat-worm diet, where the survival rate of the pups at weaning was 80%. When comparing the wheat-worm and maize-worm diets—similar in macronutrient and energy content—it appeared that the litter suppressions observed in the maize-worm diet could not be explained by looking at these factors. In the second experiment, we demonstrated that adding a vitamin B3 supplement restored reproductive success similar to that recorded for the group fed the wheat-worm diet in the first experiment.
(a). Macronutrient and energy content of the diets and maternal investment in reproduction
The two clover-supplemented diets had lower protein and energy content than the two earthworm-supplemented diets. Lack of proteins or energy could explain the low survival rates of pups observed in the group fed the clover-supplemented diets [36,37]. This hypothesis was supported by the fact that the oxytocin plasma levels of females fed the maize- and wheat-clover diets were lower at 8 days post-parturition, which could explain the abandonment of their litters [46,47]. Moreover, females fed on these two diets also displayed the highest body mass loss throughout reproduction. However, despite similar results in terms of body mass loss and oxytocin plasma levels between females of these two diets, we observed discrepancies in maternal behaviours. Females fed the wheat-clover diet gave birth in the nest and remained with their pups during the first 7 days. We then observed a gradual reduction in the litter, to 3, 2 and then 1 or ultimately zero pups and the pups' growth gradually decreased from 7 to 30 days. In contrast, most of the mothers in the group fed the maize-clover diet did not display maternal behaviour: they did not gave birth in the nest (pups were spread out in the cage) and then placed their pups on top of their hoard of maize grains before eating them. This litter suppression always occurred on the first day after parturition. Only one female weaned a litter (in her second reproductive attempt), composed of four pups. In this litter, two cases of siblicides were observed at the age of 34 days (the two males cannibalized their female siblings while they were still alive), echoing the dementia found in humans fed on maize [48,49].
Regarding the maize-worm and wheat-worm diets, we found they had very similar macronutrient and energy contents. However, the pups' survival rate and body mass were respectively 75% and 45% lower in the group fed the maize-worm diet compared with the group fed the wheat-worm diet. The extremely low survival rate of the pups in the group fed the maize-worm diet was associated with the same ‘abnormal’ behaviours of mothers as in the group fed the maize-clover diet (see above). These observations suggest a suppression of maternal behaviours and association of the pups with food items. Only one female weaned a litter (of two pups) on the maize-worm diet (also during her second reproductive attempt). However, females on the maize-worm diet—which displayed suppression of maternal behaviours—lost significantly less body mass than females fed the three other diets. Surprisingly, this lower investment in reproduction was not associated with reduced oxytocin plasma levels 8 days post-parturition in females fed the maize-worm diet, as we might have expected, given the high rate of infanticide [50]. This suggests that the modifications in maternal behaviour in maize-fed females are not a consequence of reduced maternal hormones but rather of a modification of the neural system, inducing dementia-like behaviours like that recorded in humans feeding intensively on maize [48].
(b). Vitamin B3/tryptophan deficiencies in maize, related pathologies in animals, including humans and effects of the supplementation
Maize is known to be lacking of several micronutrients: calcium, tryptophan (trp), lysine, riboflavin and bioavailable vitamin B3 (i.e. niacin, nicotinamide or vitamin PP) [51,52]. Most of these micronutrients were expected to be complemented by clover or earthworms, which are rich in minerals [35] but contain low levels of trp and vitamin B3 [35,53]. Daily supplementation with 0.3 mg of vitamin B3 in females fed the maize-worm diet restored reproductive success, with an 85% increase in the number of weaned pups compared with the group fed the maize-worm diet. This highlights the fundamental role of vitamin B3 in European hamsters' reproductive success.
Deficiencies in trp and vitamin B3 have been linked to growth retardation in rats [42], the ‘black-tongue’ syndrome in dogs [54] and pellagra in humans (i.e. three-Ds disease: diarrhoea, dementia and dermatitis) [48,49]. Trp is an essential amino acid, precursor of vitamin B3 (i.e. nicotinamide, involved in the synthesis of NAD and NADP, crucial for cell functioning [49]) and serotonin (involved in aggressiveness and depression [55]). The only way for animals to access trp and vitamin B3 is through their diet, and while trp levels in maize are particularly low [52], vitamin B3 is present in a tightly bound form, not bioavailable for animals [52]. Tryptophan metabolism in vivo is extremely complex [49,55] and therefore, the detrimental effects of a predominance of maize in the diet are extremely difficult to counteract with other food items [55]. This is why improperly cooked [56,57] maize-based diets have been associated with higher rates of homicide, suicide and cannibalism in humans [55,58] and have caused pellagra [48,49], which decimated three million people in North America and Europe from 1735 to 1940. The high propensity of maize in our experimental diets caused ‘abnormal’ maternal behaviour (pups stored with maize stores), infanticide and siblicide associated with diarrhoea and skin/fur problems in pups; these symptoms resemble those found in humans affected by pellagra [48] (see electronic supplementary material, table S1 for details and frequencies of the observed symptoms in our experiment). Although there is a vast—though ancient—literature on the effects of maize on human, livestock or rats [42,55,56,59–62], this is the first study revealing such a strong negative effect (i.e. 95% reduction in reproductive success) of maize-based diets and vitamin B3 deficiency on such an important fitness-related life-history trait as reproduction.
(c). Maize, vitamin deficiencies and farmland wildlife
Only a few studies have investigated how maize monoculture could influence the diet of wildlife or how vitamin deficiencies could harm farmland animals [9,20,63]. Black bears living in agricultural landscapes consume corn (i.e. maize), sunflower and oats but females with cubs appear to exhibit a risk-aversion towards these crops and rather feed on other food items during reproductive periods [20]. Regarding invertebrates, wild bees are known to be threatened by monotonous diets imposed by mass-flowering crops [16] and some species rely intensively on maize pollen [64]. Given that proper trp and vitamin B3 dietary intakes appear to be crucial for bees [65,66] and that both pollen quality and diversity influence their longevity, physiology and resistance/tolerance to disease [16], we argue that maize monoculture is probably strongly impairing the fitness of these endangered pollinators because of nutritional deficiencies. However, data are still lacking and experimental studies are needed to confirm this hypothesis. Finally, regarding the European hamster, given that wild populations of this species are surrounded by 55–80% of intensively managed maize monoculture in Alsace (France), with sized field of 1.4 ha that corresponds to seven times the home range of a female, extremely low crop rotations (i.e. sometimes more than seven successive years of maize cultivated in the same plot) and high use of herbicides—dramatically reducing the proportion of adventive species—wild hamsters are undoubtedly constrained in their diet. Indeed, a hamster would need to ingest 22–45 g of weeds to obtain 0.3 mg of vitamin B3 per day (see electronic supplementary material, table S2 for details of the calculation). These numbers equal or surpass the daily food intake of female hamsters, which was of 14 ± 5 grs d−1 in our experiment.
In a previous study [24], we revealed the detrimental effects of the intensification of maize monoculture on the body mass of wild hamsters in France, which has decreased by 21% since 1937. We reported that years with high maize production and high acreage allocated to this cereal led to lower hamsters' body mass upon emergence from hibernation. We were not able to conclude on whether this negative effect was caused by monoculture (i.e. a lack of food diversity/availability or a particular microclimate) or by maize itself (i.e. its composition), but we hypothesized that maize was not an adequate food resource for this species. In this study, we found that maize is nutritionally inadequate for this species during reproduction. However, regarding the maize-worm diet, the slight differences in pups' body mass and reproductive success between 2014 and 2015 is explained by two females having successfully raised a litter of four and six pups, respectively, in 2015. This is likely revealing an interindividual difference of sensitivity and symptom expression to the trp and vitamin B3 deficiencies, previously recorded in humans [58,61], and potentially reflecting differences of individual quality [67].
Taken together, our results therefore strongly suggest that an over-abundance of maize in the diet of the European hamster could be particularly detrimental for reproduction in the wild and support the hypothesis of a decline in the reproductive success of wild hamsters in France [68]. The average number of pups per female (2.4 ± 1.2 pups per litter) and number of litters per female (0.93 litter female−1) in our experiment echoes data recorded in the wild in France in 2014 (2.5 ± 1.4 pups per litter and 0.76 litter female−1 [69]). Knowing that we observed a 94% decrease in French populations of the species in recent decades [70], it is now crucial to determine how the fitness of wild hamsters could be affected by maize monoculture, and whether it is possible to improve their reproductive success through better management of agriculture and the inclusion of feeding patches.
5. Conclusion
Here we demonstrated that—independently of macronutrient and energy content—a vitamin B3 deficiency in maize grains is responsible for reproductive failure and abnormal maternal behaviour in captive-fed European hamsters. More ecophysiological studies are needed to better understand how monoculture can influence the fitness of farmland species by modifying their diet, and particularly how vitamin deficiencies can affect the reproduction, longevity, and hence the evolution of farmland wildlife. However, given the intensification of maize monoculture across the globe—associated with a reduction in both the diversity and the abundance of other plants, soil fauna and microbial communities—we argue that an over-abundance of maize compared with other food items in the diet of farmland animals could be particularly detrimental for their fitness. Knowing that these species already face many threats and that most of them are in danger of extinction, it is urgent to restore a diverse range of plants in agricultural schemes, to ensure that farmland animals have access to a more diversified diet.
Supplementary Material
Supplementary Material
Acknowledgements
We are grateful to Rita Fragueira and Thibaut Boehly for helping in oxytocin analyses. We also thank Daphne Goodfellow for the copy-editing. Many thanks to Martine Bergaentzlé for her guidance in preparing the vitamin B3 solution. Finally, we thank Emilio R. Rojas (Wildstat) for his statistical advice.
Ethics
The experimental protocols followed EU Directive 2010/63/EU guidelines for animal experiments and the care and use of laboratory animals, and was approved by the Ethical Committee (CREMEAS) under agreement no. 00624-01.
Authors' contributions
M.L.T. and C.H. conceived the study and M.L.T., C.H. and Y.H. designed the study. M.L.T. collected the data with the help of O.D. in 2014, performed data analyses and wrote the first draft of the manuscript. C.H. made the first corrections and Y.H., O.D. and J.P.R. contributed substantially to revisions.
Competing interests
The authors declare no competing interests.
Funding
This work was supported by the LIFE + Biodiversity grant no. LIFE12 BIO/FR/000979 and the Ministère de l'Ecologie, du Développement durable et de l'Energie. The funders did not participate in the study design, data collection and analysis, decision to publish or preparation of the manuscript.
References
- 1.Foley JA, et al. 2005. Global consequences of land use. Science 309, 570–574. ( 10.1126/science.1111772) [DOI] [PubMed] [Google Scholar]
- 2.Fargione JE, et al. 2009. Bioenergy and wildlife: threats and opportunities for grassland conservation. Bioscience 59, 767–777. ( 10.1525/bio.2009.59.9.8) [DOI] [Google Scholar]
- 3.Williams N. 2007. Questions on biofuels. Curr. Biol. 17, 617 ( 10.1016/j.cub.2007.07.054) [DOI] [PubMed] [Google Scholar]
- 4.Kayser A, Weinhold U, Stubbe M. 2003. Mortality factors of the common hamster Cricetus cricetus at two sites in Germany. Acta Theriol. (Warsz). 48, 47–57. ( 10.1007/BF03194265) [DOI] [Google Scholar]
- 5.Villemey A, Besnard A, Grandadam J, Eidenschenck J. 2013. Testing restocking methods for an endangered species: effects of predator exclusion and vegetation cover on common hamster (Cricetus cricetus) survival and reproduction. Biol. Conserv. 158, 147–154. ( 10.1016/j.biocon.2012.08.007) [DOI] [Google Scholar]
- 6.Jacob J. 2003. Short-term effects of farming practices on populations of common voles. Agric. Ecosyst. Environ. 95, 321–325. ( 10.1016/S0167-8809(02)00084-1) [DOI] [Google Scholar]
- 7.Rands MRW, Sotherton NW. 1986. Pesticide use on cereal crops and changes in the abundance of butterflies on arable farmland in England. Biol. Conserv. 36, 71–82. ( 10.1016/0006-3207(86)90102-3) [DOI] [Google Scholar]
- 8.Wolansky MJ, Harrill JA. 2008. Neurobehavioral toxicology of pyrethroid insecticides in adult animals: a critical review. Neurotoxicol. Teratol. 30, 55–78. ( 10.1016/j.ntt.2007.10.005) [DOI] [PubMed] [Google Scholar]
- 9.Gill RJ, Raine NE. 2014. Chronic impairment of bumblebee natural foraging behaviour induced by sublethal pesticide exposure. Funct. Ecol. 28, 1459–1471. ( 10.1111/1365-2435.12292) [DOI] [Google Scholar]
- 10.Björklund J, Limburg KE, Rydberg T. 1999. Impact of production intensity on the ability of the agricultural landscape to generate ecosystem services: an example from Sweden. Ecol. Econ. 29, 269–291. ( 10.1016/S0921-8009(99)00014-2) [DOI] [Google Scholar]
- 11.Allan E, et al. 2015. Land use intensification alters ecosystem multifunctionality via loss of biodiversity and changes to functional composition. Ecol. Lett. 18, 834–843. ( 10.1111/ele.12469) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Díaz S, Fargione J, Chapin FS, Tilman D. 2006. Biodiversity loss threatens human well-being. PLoS Biol. 4, 1300–1305. ( 10.1371/journal.pbio.0040277) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chaplin-Kramer R, Dombeck E, Gerber J, Knuth KA, Mueller ND, Mueller M, Ziv G, Klein A-M. 2014. Global malnutrition overlaps with pollinator-dependent micronutrient production. Proc. R. Soc. B 281, 20141799 ( 10.1098/rspb.2014.1799) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsiafouli MA, et al. 2015. Intensive agriculture reduces soil biodiversity across Europe. Glob. Change Biol. 21, 973–985. ( 10.1111/gcb.12752) [DOI] [PubMed] [Google Scholar]
- 15.Altieri MA. 1999. The ecological role of biodiversity in agroecosystems. Agric. Ecosyst. Environ. 74, 19–31. ( 10.1016/S0167-8809(99)00028-6) [DOI] [Google Scholar]
- 16.Goulson D, Nicholls E, Botías C, Rotheray EL. 2015. Bee declines driven by combined stress from parasites, pesticides, and lack of flowers. Science 347, 1–16. ( 10.1126/science.1255957) [DOI] [PubMed] [Google Scholar]
- 17.Medan D, Torretta JP, Hodara K, de la Fuente EB, Montaldo NH. 2011. Effects of agriculture expansion and intensification on the vertebrate and invertebrate diversity in the Pampas of Argentina. Biodivers. Conserv. 20, 3077–3100. ( 10.1007/s10531-011-0118-9) [DOI] [Google Scholar]
- 18.Butler SJ, Vickery JA, Norris K. 2007. Farmland biodiversity and the footprint of agriculture. Science. 315, 825–828. ( 10.1126/science.1136607) [DOI] [PubMed] [Google Scholar]
- 19.Lebeau J, Wesselingh RA, Van Dyck H. 2016. Nectar resource limitation affects butterfly flight performance and metabolism differently in intensive and extensive agricultural landscapes. Proc. R. Soc. B 278, 3465–3473. ( 10.1098/rspb.2016.0455) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ditmer MA, Garshelis DL, Noyce KV, Laske TG, Iaizzo PA, Burk TE, Forester JD, Fieberg JR. 2015. Behavioral and physiological responses of American black bears to landscape features within an agricultural region. Ecosphere 6, part28. ( 10.1890/ES14-00199.1) [DOI] [Google Scholar]
- 21.Monecke S. 2013. All things considered? Alternative reasons for hamster extinction. Zool. Pol. 58, 41–57. ( 10.2478/zoop-2013-0004) [DOI] [Google Scholar]
- 22.O'Brien J. 2015. Saving the common hamster (Cricetus cricetus) from extinction in Alsace (France): potential flagship conservation or an exercise in futility? Hystrix, Ital. J. Mammal. 26, 89–94. ( 10.4404/hystrix-26.2-11230) [DOI] [Google Scholar]
- 23.Franceschini-Zink C, Millesi E. 2008. Population development and life expectancy in common hamsters. In The common hamster: perspectives on an endangered species (eds Millesi E, Winkler H, Hengsberger R), pp. 45–59. Vienna, Austria: Austrian Academy of Sciences Press. [Google Scholar]
- 24.Tissier ML, Handrich Y, Robin J-P, Weitten M, Pevet P, Kourkgy C, Habold C. 2016. How maize monoculture and increasing winter rainfall have brought the hibernating European hamster to the verge of extinction. Sci. Rep. 6, 25531 ( 10.1038/srep25531) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Humphries MM, Thomas DW, Kramer DL. 2003. The role of energy availability in mammalian hibernation: a cost–benefit approach. Physiol. Biochem. Zool. 76, 165–179. ( 10.1086/367950) [DOI] [PubMed] [Google Scholar]
- 26.Nechay G, Hamar M, Grulich I. 1977. The common hamster (Cricetus cricetus [L.]); a review. EPPO Bull. 7, 255–276. ( 10.1111/j.1365-2338.1977.tb02727.x) [DOI] [Google Scholar]
- 27.Wall SB. 1990. Food hoarding in animals. Chicago, IL: University of Chicago Press. [Google Scholar]
- 28.Munro D, Thomas DW, Humphries MM. 2008. Extreme suppression of aboveground activity by a food-storing hibernator, the eastern chipmunk (Tamias striatus). Can. J. Zool. 86, 364–370. ( 10.1139/Z08-008) [DOI] [Google Scholar]
- 29.Hufnagl S, Siutz C, Millesi E. 2010. Diet composition of common hamsters (Cricetus cricetus) living in an urban environment. Säugetierkundl. Inf. 7, 57–66. [Google Scholar]
- 30.Franceschini C, Millesi E. 2005. Reproductive timing and success in common hamsters. In Int. Hamsterworkgroup, Strasbourg, pp. 63–66. [Google Scholar]
- 31.Hufnagl S, Franceschini-Zink C, Millesi E. 2011. Seasonal constraints and reproductive performance in female common hamsters (Cricetus cricetus). Mamm. Biol. - Zeitschrift für Säugetierkd. 76, 124–128. ( 10.1016/j.mambio.2010.07.004) [DOI] [Google Scholar]
- 32.Franceschini-Zink C, Millesi E. 2008. Reproductive performance in female common hamsters. Zoology 111, 76–83. ( 10.1016/j.zool.2007.05.001) [DOI] [PubMed] [Google Scholar]
- 33.Gorecki A, Grygielska M. 1975. Consumption and utilization of natural foods by the common hamster. Acta Theriol. (Warsz). 20, 237–246. ( 10.4098/AT.arch.75-20) [DOI] [Google Scholar]
- 34.Nechay G. 2000. Status of hamsters Cricetus cricetus, Cricetus migratorius, Mesocricetus newtoni, and other hamster species in Europe, vol. 106. Strasbourg, France: Council of Europe.
- 35.AFZ, INRA, CIRAD, FAO. Feedipedia, Animal Feed Resources Information System. 2011 http://www.feedipedia.org/. (accessed 5 September 2016)
- 36.Speakman JR. 2008. The physiological costs of reproduction in small mammals. Phil. Trans. R. Soc. B 363, 375–398. ( 10.1098/rstb.2007.2145) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Koskela E, Jonsson P, Hartikainen T, Mappes T. 1998. Limitation of reproductive success by food availability and litter size in the bank vole, Clethrionomys glareolus. Proc. R. Soc. Lond. B 265, 1129–1134. ( 10.1098/rspb.1998.0408) [DOI] [Google Scholar]
- 38.Skibiel AL, Hood WR. 2015. Milk matters: offspring survival in Columbian ground squirrels is affected by nutrient composition of mother's milk. Front. Ecol. Evol. 3, 1–10. ( 10.3389/fevo.2015.00111) [DOI] [Google Scholar]
- 39.Schneider J, Wade G. 1989. Effects of maternal diet, body weight and body composition on infanticide in Syrian hamsters. Physiol. Behav. 46, 815–821. ( 10.1016/0031-9384(89)90042-5) [DOI] [PubMed] [Google Scholar]
- 40.Criscuolo F, Monaghan P, Nasir L, Metcalfe NB. 2008. Early nutrition and phenotypic development: ‘catch-up’ growth leads to elevated metabolic rate in adulthood. Proc. R. Soc. B 275, 1565–1570. ( 10.1098/rspb.2008.0148) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zambrano E, Rodríguez-González GL, Guzmán C, García-Becerra R, Boeck L, Díaz L, Menjivar M, Larrea F, Nathanielsz PW. 2005. A maternal low protein diet during pregnancy and lactation in the rat impairs male reproductive development. J. Physiol. 563, 275–284. ( 10.1113/jphysiol.2004.078543) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Krehl WA, Teply LJ, Sarma PS, Elvehjem CA. 1945. Growth-retarding effect of corn in nicotinic acid-low rations and its counteraction by tryptophane. Science 101, 489–490. ( 10.1126/science.101.2628.489) [DOI] [PubMed] [Google Scholar]
- 43.Kjeldahl J. 1883. A new method for the determination of nitrogen in organic matter. Z. Anal. Chem. 22, 366–382. ( 10.1007/BF01338151) [DOI] [Google Scholar]
- 44.Campbell RR, Leatherland JF. 1980. Estimating body protein and fat from water content in lesser snow geese. J. Wildl. Manage. 44, 438 ( 10.2307/3807975) [DOI] [Google Scholar]
- 45.Folch J, Lees M, Sloane Stanley GH. 1957. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem 226, 497–509. [PubMed] [Google Scholar]
- 46.McCarthy MM. 1990. Oxytocin inhibits infanticide in female house mice (Mus domesticus). Horm. Behav. 24, 365–375. ( 10.1016/0018-506X(90)90015-P) [DOI] [PubMed] [Google Scholar]
- 47.Witt DM, Carter CS, Walton DM. 1990. Central and peripheral effects of oxytocin administration in prairie voles (Microtus ochrogaster). Pharmacol. Biochem. Behav. 37, 63–69. ( 10.1016/0091-3057(90)90042-G) [DOI] [PubMed] [Google Scholar]
- 48.Hegyi J, Schwartz RA, Hegyi V. 2004. Pellagra: dermatitis, dementia, and diarrhea. Int. J. Dermatol. 43, 1–5. ( 10.1111/j.1365-4632.2004.01959.x) [DOI] [PubMed] [Google Scholar]
- 49.Wan P, Moat S, Anstey A. 2011. Pellagra: a review with emphasis on photosensitivity. Br. J. Dermatol. 164, 1188–1200. ( 10.1111/j.1365-2133.2010.10163.x) [DOI] [PubMed] [Google Scholar]
- 50.Harmon AC, Huhman KL, Moore TO, Albers HE. 2002. Oxytocin inhibits aggression in female Syrian hamsters. J. Neuroendocrinol. 14, 963–969. ( 10.1046/j.1365-2826.2002.00863.x) [DOI] [PubMed] [Google Scholar]
- 51.Nuss ET, Tanumihardjo SA. 2010. Maize: a paramount staple crop in the context of global nutrition. Compr. Rev. Food Sci. Food Saf. 9, 417–436. ( 10.1111/j.1541-4337.2010.00117.x) [DOI] [PubMed] [Google Scholar]
- 52.Burkholder PR, Mc Veigh I, Moyer D. 1944. Niacin in maize. Yale J. Biol. Med. 16, 659–663. [PMC free article] [PubMed] [Google Scholar]
- 53.Warne MA, Lenz EM, Osborn D, Weeks JM, Nicholson JK. 2001. Comparative biochemistry and short-term starvation effects on the earthworms Eisenia veneta and Lumbricus terrestris studied by 1H NMR spectroscopy and pattern recognition. Soil Biol. 33, 1171–1180. ( 10.1016/S0038-0717(01)00021-9) [DOI] [Google Scholar]
- 54.Ammerman CB, Baker DH, Lewis AJ. 1995. Bioavailability of nutrients for animals: amino acids, minerals and vitamins. London, UK: Academic Press Limited. [Google Scholar]
- 55.Ernandes M, Guardia M, Giammanco S. 1996. Maize based diets and possible neurobehavioural after-effects among some populations in the world. Hum. Evol. 11, 67–77. ( 10.1007/BF02456990) [DOI] [Google Scholar]
- 56.Katz SH, Hediger ML, Valleroy LA. 1974. Traditional maize processing techniques in the new world. Science. 184, 765–773. ( 10.1126/science.184.4138.765) [DOI] [PubMed] [Google Scholar]
- 57.Sefa-Dedeh S, Cornelius B, Sakyi-Dawson E, Afoakwa EO. 2004. Effect of nixtamalization on the chemical and functional properties of maize. Food Chem. 86, 317–324. ( 10.1016/j.foodchem.2003.08.033) [DOI] [Google Scholar]
- 58.Mawson AR, Jacobs KW. 1978. Corn consumption, tryptophan, and cross-national homicide rates. J. Orthomol. Psychiatry 7, 227–230. [Google Scholar]
- 59.Carter EGA, Carpenter AJ. 1982. The available niacin values of foods for rats and their relation to analytical values. J. Nutr. 112, 2091–2103. [DOI] [PubMed] [Google Scholar]
- 60.Carpenter KJ, Schelstraete M, Wall S, Vilicch VC. 1988. Immature corn as a source of niacin for rats. J. Nutr. 118, 165–169. [DOI] [PubMed] [Google Scholar]
- 61.Goldsmith GA, Sarett HP, Register UD, Gibbens J. 1952. Studies of niacin requirement in man. I. Experimental pellagra in subjects on corn diets low in niacin and tryptophan. J. Clin. Invest. 31, 533–542. ( 10.1172/JCI102638) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hundley JM. 1947. Production of niacin deficiency in rats. J. Nutr. 34, 253–262. [DOI] [PubMed] [Google Scholar]
- 63.Vanderplanck M, Moerman R, Rasmont P, Lognay G, Wathelet B, Wattiez R, Michez D. 2014. How does pollen chemistry impact development and feeding behaviour of polylectic bees? PLoS ONE 9, e86209 ( 10.1371/journal.pone.0086209) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Danner N, Härtel S, Steffan-Dewenter I. 2014. Maize pollen foraging by honey bees in relation to crop area and landscape context. Basic Appl. Ecol. 15, 677–684. ( 10.1016/j.baae.2014.08.010) [DOI] [Google Scholar]
- 65.de Arruda VAS, Pereira AAS, de Freitas AS, Barth OM, de Almeida-Muradian LB. 2013. Dried bee pollen: B complex vitamins, physicochemical and botanical composition. J. Food Compos. Anal. 29, 100–105. ( 10.1016/j.jfca.2012.11.004) [DOI] [Google Scholar]
- 66.Fengkui Z, Baohua X, Ge Z, Hongfang W. 2015. The appropriate supplementary level of tryptophan in the diet of Apis mellifera (Hymenoptera: Apidae) worker bees. J. Insect Sci. 15, 161 ( 10.1093/jisesa/iev142) [DOI] [Google Scholar]
- 67.Hamel S, Côté SD, Gaillard JM, Festa-Bianchet M. 2009. Individual variation in reproductive costs of reproduction: high-quality females always do better. J. Anim. Ecol. 78, 143–151. ( 10.1111/j.1365-2656.2007.0) [DOI] [PubMed] [Google Scholar]
- 68.Surov A, Banaszek A, Bogomolov P, Feoktistova N, Monecke S. 2016. Dramatic global decrease in the range and reproduction rate of the European hamster Cricetus cricetus. Endanger. Species Res. 31, 119–145. ( 10.3354/esr00749) [DOI] [Google Scholar]
- 69.Kourkgy C, Eidenschenck J. 2015. Délivrable Action D1. Rapport annuel de présentation des données collectées et premières analyses, année 2014.
- 70.Reiners TE, Eidenschenk J, Neumann K, Nowak C. 2014. Preservation of genetic diversity in a wild and captive population of a rapidly declining mammal, the common hamster of the French Alsace region. Mamm. Biol. 79, 240–246. ( 10.1016/j.mambio.2013.10.004) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.