Abstract
Elaborate ornamental traits are often under directional selection for greater elaboration, which in theory should deplete underlying genetic variation. Despite this, many ornamental traits appear to remain highly variable and how this essential variation is maintained is a key question in evolutionary biology. One way to address this question is to compare differences in intraspecific variability across different types of traits to determine whether high levels of variation are associated with specific trait characteristics. Here we assess intraspecific variation in more than 100 plumage colours across 55 bird species to test whether colour variability is linked to their level of elaboration (indicated by degree of sexual dichromatism and conspicuousness) or their condition dependence (indicated by mechanism of colour production). Conspicuous colours had the highest levels of variation and conspicuousness was the strongest predictor of variability, with high explanatory power. After accounting for this, there were no significant effects of sexual dichromatism or mechanisms of colour production. Conspicuous colours may entail higher production costs or may be more sensitive to disruptions during production. Alternatively, high variability could also be related to increased perceptual difficulties inherent to discriminating highly elaborate colours. Such psychophysical effects may constrain the exaggeration of animal colours.
Keywords: variation, melanin, carotenoid, structural, sexual selection, discriminability
1. Introduction
Highly elaborate and conspicuous ornamental traits are often used for signalling by animals, for example, to attract mates or deter rivals. Such traits are frequently highly variable within species [1]. High variability is important because these differences between individuals may convey information about the quality of the signallers. At the same time, however, signalling traits are generally under strong directional selection which should lead to the erosion of underlying genetic variation, reduced phenotypic variability and thus limited use as signals [2,3]. Variability in sexually selected and signalling traits, and how it is maintained, are thus contentious issues [4,5]. Variability may be maintained by temporally or spatially variable patterns of selection [6] or frequency-dependent selection, for example, if variation is used to signal individual identity [7]. However, the best supported and most general hypothesis suggests that signalling traits have high levels of variability because their expression is contingent on the condition of the individual: genetic variation that underlies variation in condition is unlikely to be depleted as it probably depends on multiple and pleotropic loci dispersed throughout the genome [5].
Several empirical studies have found considerable support for heightened variability of signalling traits, showing that signalling traits are more variable than similar non-signalling traits within the same species [8–13]. However, not all signalling or sexually selected traits show high or heightened variability. For example, calls in frogs and insects that are under strong selection through female choice are less variable [14], while tail ornaments of polygynous species can be less variable than those of monogamous ones [15], consistent with the idea that genetic variation can be depleted under strong directional selection. Hence whether signalling traits should show higher levels of variability may depend on the type of trait studied. Moreover, most studies have done rather broad comparisons, for example, putative sexual signals versus putative naturally selected traits [16], or comparing male and female traits [17]. Most of the studies that have confirmed the higher variability of ornamental or sexually selected traits have been based on metric traits, in particular, tail length in birds [8,9,13,15,17,18]. Testing for the generality and applicability of these patterns requires broadening the scope, including greater diversity of types of traits studied in larger numbers of species. The existence of considerable differences in how intraspecific variability levels are distributed across traits and species suggest that we have only a rudimentary understanding of the drivers of intraspecific variation. Understanding the causes of intraspecific levels of variation is a central question today as it has become clear that this variation underpins the ability of populations to adapt to change [19,20] or to diverge and diversify [21]. Here we take advantage of recent methodological advances to determine the main potential correlates of intraspecific variability for one of the most widespread communication types, animal coloration.
Animal colours are often used to communicate between individuals. Such visual signals are generally exaggerated, conspicuous, convey information on the quality of the signaller [22], and therefore, should also be highly variable. Measuring variability in coloration is, however, challenging as variability does not scale with the mean and hence coefficients of variation cannot be used [7]. This complicates comparing variability across very different colours (e.g. blue versus red). Recently, this problem was overcome by quantifying colour variation using psychophysical models of avian colour vision [23] that allow us to assess variation closer to the sensory world of the intended receivers. Using this approach, Delhey & Peters [10] compared plumage colour variability for six species of European songbirds and concluded that colours which have been shown to be sexually selected or were correlated with individual quality were more variable than other colours within the same species. While these results suggest higher variability in a handful of ornamental colours, the generality of this pattern requires comprehensive comparative studies. Here we take advantage of newly developed meta-analytical methods to study variation [24] to assess the correlates of intraspecific variation in plumage coloration in a sample of more than 100 male plumage colours across 55 species of Australian passerines and parrots. Specifically, we test the prediction that those colours that are likely to fulfil a signalling function, namely: (i) more elaborate colours and (ii) more condition-dependent colours, will show higher levels of variability than other colours. We quantify the degree of elaboration using two proxies: the level of conspicuousness against natural backgrounds and the level of sexual dichromatism [25]. We infer the level of condition dependence of each plumage colour based on the mechanism of colour production responsible for it.
Plumage colours can be produced by different mechanisms: the deposition of pigments, the microstructure of the feather or a combination thereof. The most common bird colours are those based on the deposition of melanin or carotenoid pigments (melanin- and carotenoid-based colours) [26–28]. Carotenoid-based colours (which largely vary from red to yellow) are often assumed to be highly condition-dependent as carotenoids are plant pigments that cannot be produced by the birds, but need to be ingested with the food [27,29]. Moreover, carotenoids fulfil other important functions in the body, such as free-radical scavenging and immunostimulation, which may trade off against deposition in the plumage [27] and they can play key roles in cellular processes such as vitamin A metabolism [30]. Melanins, on the other hand, which produce a range of more subdued hues from black to grey and brown to rufous, are endogenously produced and their levels of condition dependence have been assumed to be lower [26,29]. This generalization might not be entirely appropriate as recent studies suggest that the expression of melanin-based colours can correlate with aspects of individual quality [31,32]. Also endogenously produced are psittacofulvins, pigments exclusive to parrots and cockatoos that, like carotenoids, produce red to yellow hues [28,33]. Given their endogenous nature psittacofulvin-based colours are assumed to be less condition-dependent [26,29], but detailed studies are lacking (but see [34]). Finally, structural colours are produced by the physical interaction between light and the microstructure of the feather. While it has been argued that the production of a regular and ordered microstructure may entail costs [35], these have not been universally demonstrated [36]: some studies support this idea [37] and others do not [38]. The diversity of mechanisms of colour production thus provides an opportunity to evaluate how intraspecific variability in colours is distributed across them and whether the predicted links with condition dependence are found. Based on current understanding, we predict that if condition dependence is an important component of colour variation, carotenoid-based colours should have highest and melanin-based colours the lowest levels of variation.
2. Material and methods
(a). Study species
To obtain a representative sample of bird colours, we chose Australian passerines (order Passeriformes), and parrots and cockatoos (order Psittaciformes) because these speciose radiations within Australia cover the vast majority of colours and include all major mechanisms of colour production [28]. To select the subset of study species, all species were first randomly ranked (based on [28]). We then selected species in order from this list, provided that at least 20 adult males of the same species and subspecies were listed as available in the online databases of the ornithological collections of the Melbourne Museum and the Australian National Wildlife Collection in Canberra. For all species (n = 55), we selected male specimens from the same subspecies to minimize the effects of geographical variation [20]. Some colour-producing mechanisms are more common than others (melanins are, by far, the most common colour-producing mechanism; [28]). Thus, to get appropriate representation of mechanisms in the sample, extra species with structural and carotenoid-based colours had to be selected by skipping higher ranked species without these colours. For each species, we measured up to three (average = 1.89, mode = 2) different plumage patches produced by different mechanisms of colour production.
Mechanisms of colour production were identified based on the shape of reflectance spectra as described in [28] and classified as: structural, melanin-based, carotenoid-based, psittacofulvin-based and those based on the deposition of yellow psittacofulvins on blue structural plumage (psittacofulvin + structures, parrot green). We measured 19 carotenoid-based colours, 43 melanin-based colours, 20 structural colours, 14 psittacofulvin-based colours and eight colour patches caused by psittacofulvins and structures. Reflectance spectra for each colour patch can be found in the electronic supplementary material, figures S1–S5. A full description of the spectral characteristics used to identify these mechanisms and an assessment of it can be found in [28].
For some of the selected species not all specimens listed online were suitable for measuring (juveniles, damaged plumage, moulting, etc.) and this reduced the sample size in some cases. On average, we measured 20 specimens per species (s.e. = 0.53, range = 6–54). In total, we measured 104 plumage patches for 2079 specimens belonging to 37 species of passerines and 18 species of parrots and cockatoos. A full list of species and plumage patches measured can be found in the electronic supplementary material table S1.
(b). Reflectance spectrometry and visual models
Plumage reflectance was measured using an AvaSpec 2048 spectrometer connected to a Xenon flash (Avalight-Xe; Avantes, Eerbek, The Netherlands) light source using a bifurcated fibre-optic cable fitted at the end with a cylindrical probe to standardize measuring distance and exclude ambient light. The probe was held perpendicular to the feather's surface and we collected five reflectance spectra per plumage patch per specimen. Reflectance spectra between 300 and 700 nm (the visual sensitivity range of birds, [39]) were calculated relative to a WS-2 white standard using the program AvaSoft 7.5.3 (Avantes) and exported into spreadsheets. Reflectance spectra for each plumage patch are depicted in the electronic supplementary material, figures S1–S5.
To compute intraspecific variability, contrast against natural backgrounds, and sexual dichromatism we used psychophysical models of avian colour vision [23] with the formulae of Cassey et al. [40] as implemented in [41]. We acknowledge that there are other valid approaches, for example, one could measure variation directly using normalized reflectance spectra, quantifying variation in spectral shape [25]. We chose visual modelling because it provides a robust approach to model colour variation closer to the visual world of the intended receivers (conspecifics), it has been used before for this purpose [10] and allows comparing variation between highly different types of colours in the same currency [42]. Visual models require knowledge on the visual sensitivity functions of the four types of cones used by birds in colour vision, the noise-to-signal ratios of these cones and the spectrum of illuminating light.
Colour vision in birds is mediated by four types of single cones sensitive to very short (VS), short (S), medium (M) and long (L) wavelengths of light [23]. Variation in visual sensitivity between species is mainly restricted to the VS and S cones and birds can be generally classified in two groups: ultraviolet-sensitive (U-type) and violet-sensitive (V-type) species. While both types have some sensitivity to ultraviolet (UV) light, U-type species have VS cones with peak sensitivity shifted towards shorter wavelengths [43]. In our sample, both types of visual sensitivity functions are represented [44] and we model both to see whether it affects our results. Visual sensitivity functions for U- and V-type species were obtained from the study of Endler & Mielke [45].
The noise-to-signal ratio of each cone type is assumed to be a function of their relative abundance in the retina. In general, birds have more S cones than VS, more M cones than S cones and similar numbers of M and L cones [46] and these proportions do not differ consistently between U- and V-type species [46]. We used average cone proportions as obtained from the study of Hart [46] 0.38 : 0.68 : 1.13 : 1 (VS:S:M:L, respectively) and combined these with behavioural estimates of the Weber fraction (0.1, [23,47]) using formula 10 in [23] to obtain the noise-to-signal ratios for each cone type (ωVS: 0.162, ωS: 0.12, ωM: 0.094, ωL: 0.1). Note that this is a necessary simplification and that between species variation exists in the abundance of the different cone types in the retina as measured by microscopy [46] and gene expression patterns [48]. Such variation can introduce noise [49], but its relevance is hard to assess as for most (87%) species included this data is not available. Finally, we used the spectrum of standard daylight (d65, [23]) as illuminant.
Visual models yield a set of quantum catches in four cones (how much each cone type is stimulated by a specific combination of reflectance spectrum and irradiance) that can be transformed into three coordinates which define the position of each spectrum in the visual space of birds (figure 1). This visual space takes the shape of a tetrahedron where each apex represents the sole stimulation of one cone type [45]. Using the formulae in [40], distances between points in visual space are measured in just notable differences (JND), whereby distances > 1 JND are considered to be discriminable by birds.
Figure 1.
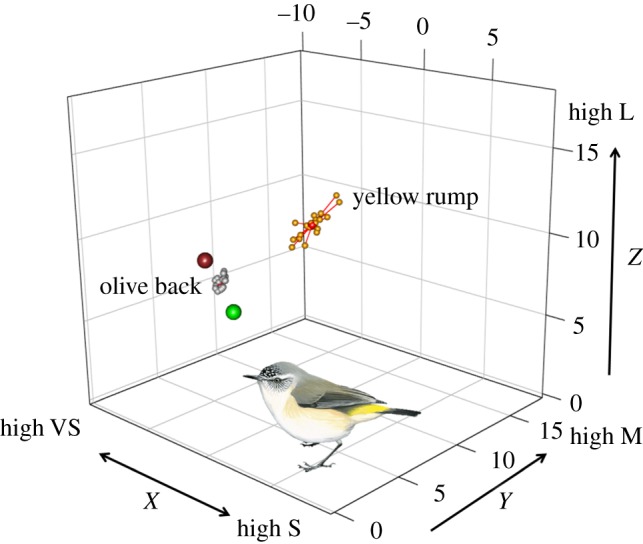
Graphic representation of the methods used to compute chromatic variability in the avian visual space using colour variation of the yellow rump (yellow spheres) and olive back (grey spheres) plumage of the yellow-rumped thornbill (Acanthiza chrysorrhoa) as an example. Chromatic variability was computed as the (loge transformed) average distance (red lines) to the patch-specific centroid (red symbols inside each cloud of points). Note how the dispersion around the centroid is larger for the rump than for the back, indicative of the higher chromatic variability of the former. The large green and brown spheres represent the location of green (green leaves) and brown (bark, leaf litter, soil) natural background colours in avian visual space. The distance from the centroid of each plumage patch to these natural backgrounds represents the average conspicuousness of each plumage patch. In this case, the olive back, by being closer to the natural backgrounds, is less conspicuous than the yellow rump. In this representation, the x-axis represents stimulation of the VS cone relative to the S cone, higher values of the y-axis represents higher stimulation of the M cone relative to VS and S cones, while the z-axis represents higher relative stimulation of the L cone compared with the other three. Bird image reproduced with permission from Gregory [50]. (Retrieved from http://www.hbw.com/node/59838 on 16 September 2016.)
Chromatic variability for each plumage colour was computed as the average natural logarithm of the distance to the centroid of that plumage patch (the joint XYZ average, figure 1) [10]. More variable colours have higher average distances to the centroid (figure 1). We took the natural logarithm of the distances to reduce heterogeneity of variance in the statistical analysis. We also computed the standard error (s.e.) of variability to incorporate in the analysis as sampling error [24]. Contrast against natural backgrounds was computed as the distance from the centroid of each plumage colour to two representative types of natural backgrounds from the study of Delhey et al. [49]: brown backgrounds (typical of soil, bark and leaf litter) and green backgrounds (typical of green leaves). Contrast against brown and green backgrounds are strongly correlated (Pearsons's r = 0.918, t104 = 23.63, p < 0.001) and hence we computed the average contrast against these two natural backgrounds. Finally, sexual dichromatism for each plumage patch was computed as the chromatic distance between male and female centroids for that patch. For this, we used the data from the study of Delhey & Peters [51] as here we only measured male colours. We used patch-specific sexual dichromatism values rather than species-specific ones [51] because we are interested in explaining variability for different colours and in most species we measured more than one type of colour/plumage patch.
(c). Statistical analysis
We used a newly developed meta-analytical approach to the study of variation [24], which accounts for sampling error, multiple measurements per species and phylogenetic relatedness, implemented using the R package MCMCglmm [52,53]. The dependent variable was always chromatic variability (average loge-transformed distances to the centroid as indicated above) and independent variables were: contrast against the background, sexual dichromatism and the mechanism of colour production as a factor. We also included in all models estimates of spatial and temporal variability of each sample as it can be expected that samples which span a larger geographical area or a longer time frame may be more variable. Spatial and temporal variability were computed in the same way as colour variability, as the average distance between each sample and the geographical or temporal centroid, based on geographical coordinates or year of collection, respectively. All covariates were scaled by subtracting the mean and dividing by the standard deviation.
Random factors in the model included phylogenetic relatedness (as the inverse of the phylogenetic covariance matrix, [52]) and species identity, the latter because in some species more than one plumage patch was measured. Phylogenetic uncertainty was accounted for by sampling over a sample of a posterior distribution of phylogenetic trees from the study of Jetz et al. [54] following the approach outlined in [55]. For each tree, we ran the model over 1000 iterations saving the last MCMC sample. Latent variable values and variance components from this last iteration were used as a starting point for the next tree. This process was done over 1300 trees, whereby results from the first 300 trees were discarded as burn-in, resulting in a posterior sample of 1000 for each model. Sampling error variances (squared s.e. of each variability estimate) and covariances (between different plumage patches within a species) were also accounted for with a sampling error covariance matrix, where the diagonal represents sampling error variances and off-diagonal elements the error covariances between plumage patches within the same species. We used inverse gamma priors for the residuals and random effects and normal distributions centred on zero with large variances as fixed effects priors. Model convergence was assessed using trace graphs and autocorrelation plots. For each model, we computed marginal (fixed effects) and conditional (fixed + random effects) R2 values [56]. Different models were compared using the deviance information criterion (DIC) the MCMCglmm equivalent of the Akaike information criterion.
3. Results
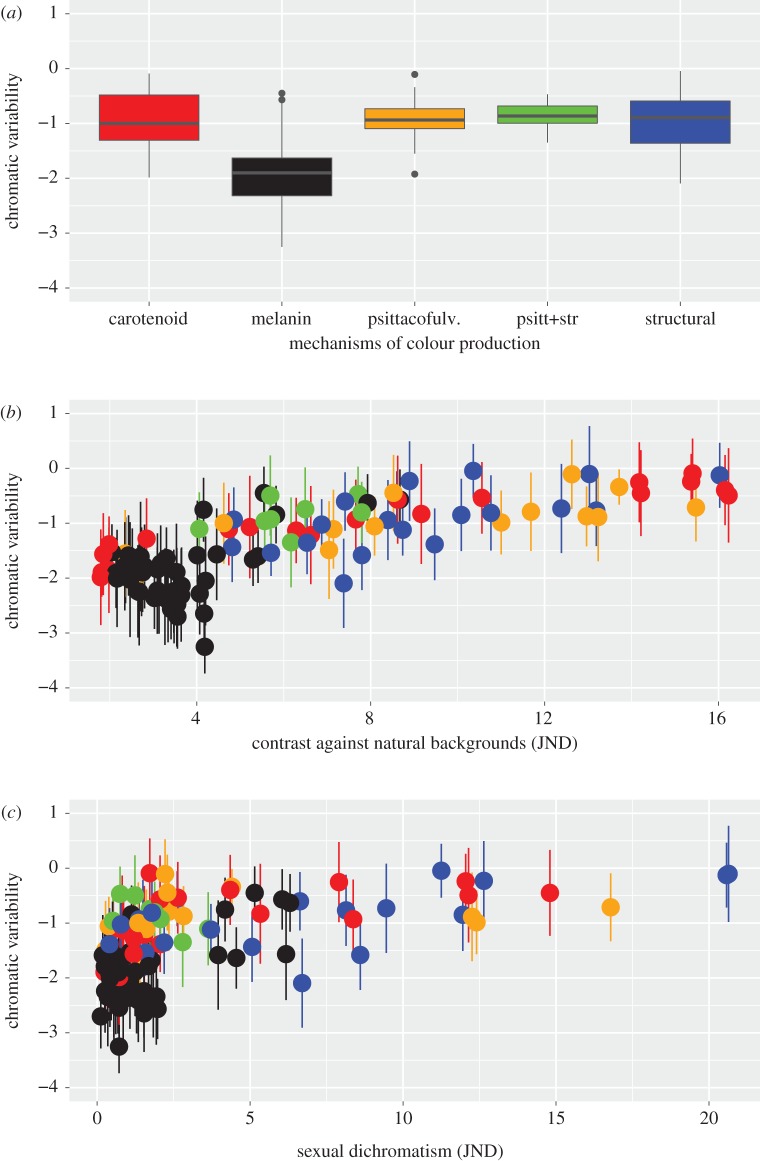
We report results for variability as computed using U-type visual sensitivity functions, results using V-type functions are very similar and are reported in the electronic supplementary material, tables S2–S7. First, we tested the separate effects of each of explanatory variable of interest, mechanisms of colour production, sexual dichromatism and contrast against natural backgrounds (but always including the covariates spatial and temporal variation to account for them, although they never showed significant effects, table 1). All three variables explained sizeable amounts of variability on their own (table 1). Across mechanisms of colour production, melanin-based colours showed the lowest levels of variability being significantly lower than any of the other mechanisms (table 1, figure 2a), while both sexual dichromatism and contrast against the background correlated positively with colour variability (table 1, figure 2a,b). Based on DIC values the model with contrast against the background was the best of the three models with single focal explanatory variables (table 1).
Table 1.
Bayesian phylogenetic mixed model results depicting effects of different explanatory variables on chromatic variability of plumage colours of Australian passerine and parrots computed using U-type visual sensitivities. (Depicted for each model are their deviance information criteria (DIC) and the posterior means and their 95% credible intervals (CI) for: fixed effect coefficients, marginal R2 values (fixed effects), conditional R2 values (fixed + random effects) and λ (phylogenetic signal). Note that the reference category for mechanisms of colour production is carotenoids (the effects of the other mechanisms are expressed as the difference with carotenoids).)
| model |
|||||||
|---|---|---|---|---|---|---|---|
| mechanisms DIC = −269.3 |
contrast DIC = −314.3 |
sexual dich. DIC = −283.8 |
mechanisms + contrast DIC = −335.2 |
mechanisms + sexual dich. DIC = −298.2 |
contrast + sexual dich. DIC = −242.9 |
full model DIC = −247.9 |
|
| post. mean (95%CI) | post. mean (95%CI) | post. mean (95%CI) | post. mean (95%CI) | post. mean (95%CI) | post. mean (95%CI) | post. mean (95%CI) | |
| intercept | −0.86** (−1.31; −0.37) | −1.07** (−1.28; −0.86) | −1.07** (−1.44;−0.73) | −0.94** (−1.31; −0.61) | −0.87** (−1.28; −0.40) | −1.06** (−1.28; −0.88) | −0.93** (−1.28; −0.56) |
| temp. var. | 0.07 (−0.08; 0.21) | 0.09 (−0.03; 0.21) | 0.08 (−0.06; 0.23) | 0.07 (−0.05; 0.19) | 0.08 (−0.05; 0.23) | 0.08 (−0.05; 0.21) | 0.07 (−0.05; 0.21) |
| spatial var. | 0.05 (−0.09; 0.19) | 0.05 (−0.06; 0.17) | 0.02 (−0.11; 0.15) | 0.06 (−0.07; 0.18) | 0.05 (−0.07; 0.18) | 0.05 (−0.08; 0.17) | 0.06 (−0.07; 0.18) |
| contrast | 0.45** (0.32; 0.56) | 0.38** (0.22; 0.56) | 0.42** (0.25; 0.58) | 0.34** (0.14; 0.55) | |||
| mechanisms | |||||||
| melanins | −0.69** (−1.09; −0.36) | −0.32† (−0.70; 0.02) | −0.57** (−0.93; −0.22) | −0.33† (−0.70; 0.02) | |||
| psittacofulvins | 0.24 (−0.25; 0.81) | 0.04 (−0.43; 0.57) | 0.18 (−0.40; 0.68) | 0.037 (−0.49; 0.50) | |||
| psittacofulv.+struct. | −0.02 (−0.56; 0.61) | 0.20 (−0.39; 0.72) | 0.05 (−0.56; 0.62) | 0.20 (−0.33; 0.76) | |||
| structural | −0.07 (−0.53; 0.40) | −0.11 (−0.5; 0.34) | −0.07 (−0.56; 0.32) | −0.12 (−0.48; 0.32) | |||
| sexual dich. | 0.31** (0.16; 0.45) | 0.20* (0.05; 0.36) | 0.04 (−0.14; 0.21) | 0.05 (−0.12; 0.25) | |||
| marginal R2 | 0.54 (0.25; 0.82) | 0.86 (0.61; 0.99) | 0.51 (0.17; 0.88) | 0.86 (0.64; 0.99) | 0.71 (0.43; 0.99) | 0.86 (0.63; 0.99) | 0.86 (0.65; 0.99) |
| conditional R2 | 0.97 (0.91; 0.99) | 0.97 (0.89; 0.99) | 0.96 (0.84; 0.99) | 0.97 (0.91; 0.99) | 0.97 (0.88; 0.99) | 0.97 (0.88; 0.99) | 0.97 (0.91; 0.99) |
| λ | 0.93 (0.76; 0.99) | 0.68 (0.12; 0.99) | 0.89 (0.53; 0.99) | 0.75 (0.16; 0.99) | 0.83 (0.23; 0.99) | 0.65 (0.09; 0.99) | 0.73 (0.16; 0.99) |
Figure 2.
Chromatic variability estimates (loge(average distance to centroid), see text and figure 1 for more details) vary with (a) mechanisms of colour production, (b) contrast against natural backgrounds (conspicuousness), and (c) sexual dichromatism. Colours represent different colour production mechanisms as indicated in (a). Boxplots in (a) depict median (dark line), 50% quantiles (box), 2.5% and 97.5% quantiles (whiskers) and outliers (black dots), same lowercase letters denote colour-producing mechanisms with non-significant (p > 0.05) pairwise differences in variability (based on first model in table 1). Error bars in (b) and (c) represent sampling error for each variability estimate (s.e.).
Given that all three variables correlated with colour variability, we tested which combination of them provided the best explanatory model (table 1). The best model, based on comparing values of DIC, was the model that included mechanisms of colour production and contrast against the background (table 1). The fixed effects in this model explained 86% of the variation in variability. While the effect of contrast against the background was highly significant, the effect of mechanisms of colour production was marginally non-significant (table 1). The full model including all three explanatory variables explained a similar amount of variation but had higher DIC owing to the extra parameter (table 1). In this model, contrast against the background had again a highly significant effect while the effect of mechanisms of colour production was marginally non-significant and that of sexual dichromatism non-significant. Partitioning the unique and common effects of each focal predictor on the response variable using commonality analyses ([57]; electronic supplementary material, table S8) confirms that contrast against the background has the strongest effects on chromatic variability. On the other hand, the unique effects of mechanisms of colour production and sexual dichromatism were negligible, and their only sizeable contributions were through their common effects with contrast (electronic supplementary material, table S8).
4. Discussion
Intraspecific levels of chromatic variability were best explained by variation in conspicuousness (estimated here as contrast against natural backgrounds): more conspicuous colours displayed higher variability. Although both mechanisms of colour production and sexual dichromatism were correlated with colour variability on their own (figure 2), after statistically controlling for contrast against the background their effects became weaker and non-significant (table 1), and the commonality analysis confirmed their negligible independent contributions (electronic supplementary material, table S8).
(a). Condition dependence
The best supported conceptual framework for the evolutionary maintenance of variability in elaborate traits under direction selection is heightened condition dependence [5]. Our observation that variability was in general lower for melanin-based colours than for colours produced by other mechanisms of colour production (figure 2a) appears to support this hypothesis, as melanin-based colours have been linked to lower levels of condition dependence [29]. However, the effect of mechanisms of colour production was not particularly strong. Furthermore, mechanisms of colour production also differ in their conspicuousness (melanin-based colours in particular being less conspicuous, figure 2b). After accounting for this, the effect of mechanisms of colour production disappears and clearly the strongest predictor of variability was contrast against the background, that is, their level of conspicuousness. Hence, our data do not support the hypothesis that melanin-based colours are necessarily less variable owing to their lower hypothesized levels of condition-dependent expression.
A similarly strong correlation with signal variability exists for duration of vocalizations of anurans and insects [58]. Long calls, simply owing to their duration, can be affected by more variables, leading to higher variability [58]. Following this argument, one possibility could be that sensitivity to developmental disturbances during colour production increases with colour conspicuousness. The most conspicuous colours are the most extreme expressions of that colour type, for each mechanism of colour production [59] more conspicuous colours are more saturated colours, which require higher concentrations of pigments or more regular or elaborate feather microstructure [26,27,36]. Assuming that these take greater investment of time or resources, such an interpretation would be consistent with the hypothesized link between condition dependence and variability [5], and heightened condition dependence of signalling traits [59,60]. A major caveat here is that we currently do not know how costs scale with intraspecific variation in colour elaboration. It is plausible (and highly likely) that a similar increase in conspicuousness across different mechanisms of colour production could entail widely different increases in costs.
(b). Psychophysical constraints
Our data show that two of the most important attributes of signal efficacy [61], detectability (conspicuousness) and discriminability (variability) are strongly linked across plumage colours. Possibly, only colours that maintain high levels of variability can become successful, elaborate signals and receivers may only pay attention to those signals with enough variation to be informative [62]. The need for high levels of variability might be further exacerbated by psychophysical constraints that become more marked as trait elaboration increases. Such constraints may have strong consequences on signal elaboration and variability [63]. To be discriminable, differences between individuals need to be larger for more elaborate traits. This is because discrimination is based on proportional rather than absolute differences, a phenomenon known as Weber–Fechner's Law [64]. For example, the fact that birds with longer tails have also more variable tail lengths has been interpreted as being consistent with Weber–Fechner's Law [13], as the same size difference would be harder to discriminate between individuals in species with long, compared with species with short tails. Weber–Fechner's Law, however, cannot account for our results as discriminability thresholds between colours are already based on proportional differences (i.e. visual models take this law into account, [23]). However, there are other psychophysical constraints related to detection thresholds that could be at play.
The consistent increase in variability with conspicuousness could be explained by the fact that chromatic discrimination thresholds increase for those colours that are very different from the backgrounds to which vision is adapted [65,66]. Previously only known from humans, this effect has been recently confirmed [66] in zebra finches (Taeniopygia guttata), where colour discrimination was considerably poorer when colours had to be discriminated against highly different (contrasting) backgrounds. This is exactly the case with conspicuous colours, which are very different from natural backgrounds that fill most of the field of vision, and to which animal eyes are most likely adapted. As a result of this psychophysical constraint, only colours with a certain degree of variability can convey information for a given level of conspicuousness. More elaborate, conspicuous colours need to be more different to be discriminable, and hence require higher intraspecific variability. This then raises the question whether the observed increase in variability with conspicuousness would be enough to counter the higher discrimination thresholds encountered among conspicuous colours or whether, in some cases, there might not be enough discriminable variation to assess differences in coloration (as shown for acoustic traits [67]). If this were the case, if the increase in variability is at least in part a compensation for more difficult discrimination, this would imply that realized levels of discriminable variation increase less strongly and less steeply across the range of plumage colours than depicted in figure 2. The potential of such psychophysical constraints to affect discriminable variation of colours can only be confirmed with extensive behavioural data.
5. Conclusion
We have found an exceptionally strong correlation between intraspecific levels of chromatic variability and plumage colour conspicuousness. We suggest two alternative, but not mutually exclusive, explanations: (i) that more conspicuous, elaborate, colours are more sensitive to availability of resources or disturbances during development or more costly to maintain than less conspicuous ones, leading to higher levels of intraspecific variation; and (ii) that high intraspecific variation in more conspicuous colours may be owing to perceptual discrimination constraints that apply to colours which are very different from natural backgrounds. These ideas make clear predictions that can be tested by suitable experiments. For the first, conditions during moult should be experimentally manipulated and the effects of this manipulation assessed on colours with different levels of conspicuousness, ideally across colours produced by the same and different mechanisms of colour production [59,60]. The second hypothesis, that behavioural discrimination abilities decrease with colour conspicuousness, could be tested with behavioural discrimination experiments that compare actual discrimination abilities of conspicuous versus cryptic colours in a set of different species [66]. Note that while these explanations have been developed mainly with conspecific receivers in mind they could also apply to heterospecifics. For example, if colours are used as pursuit-deterrents in predator–prey communication [68] high variability linked to differences in quality may enable high quality, unprofitable prey to signal this to predators. Levels of discriminability between individuals would be expected to be higher for conspicuous colours given their potential to increase the risk of predation. The fact that we find similar effects if we model variability using the V-type visual system (electronic supplementary material, tables S2–S7)—typical of birds of prey, the most sophisticated visual predators of birds—suggests that this option is plausible.
Supplementary Material
Supplementary Material
Acknowledgements
We thank K. Roberts, K. Smith, B. Bird and W. Longmore at the Melbourne Museum, and L. Joseph, and R. Palmer at the Australian National Wildlife Collection, Canberra for granting access to specimens under their care and to O. Lind, D. Osorio and two anonymous reviewers for comments on the manuscript. We are grateful to Lynx Edicions for allowing reproduction of the bird image in figure 1.
Data accessibility
Data are available in the electronic supplementary material.
Authors' contributions
Conceived study: K.D., A.P.; collected data: B.S., K.D.; analysed data: K.D., S.N.; wrote manuscript K.D., A.P. with contributions from S.N. and B.S.
Competing interests
We declare we have no competing interests.
Funding
This work was supported by the Australian Research Council (DE120102323 to K.D.; FT110100505 to A.P. and FT130100268 to S.N.) and Monash University.
References
- 1.Darwin C. 1871. The descent of man and selection in relation to sex. London, UK: John Murray. [Google Scholar]
- 2.Taylor PD, Williams GC. 1982. The lek paradox is not resolved. Theor. Popul. Biol. 22, 392–409. ( 10.1016/0040-5809(82)90052-1) [DOI] [Google Scholar]
- 3.Merilä J, Sheldon BC. 1999. Genetic architecture of fitness and nonfitness traits: empirical patterns and development of ideas. Heredity 83, 103–109. ( 10.1046/j.1365-2540.1999.00585.x) [DOI] [PubMed] [Google Scholar]
- 4.Pomiankowski A, Møller AP. 1995. A resolution of the lek paradox. Proc. R. Soc. Lond. B 260, 21–29. ( 10.1098/rspb.1995.0054) [DOI] [Google Scholar]
- 5.Rowe L, Houle D. 1996. The lek paradox and the capture of genetic variance by condition dependent traits. Proc. R. Soc. Lond. B 263, 1415–1421. ( 10.1098/rspb.1996.0207) [DOI] [Google Scholar]
- 6.Chaine AS, Lyon BE. 2008. Adaptive plasticity in female mate choice dampens sexual selection on male ornaments in the lark bunting. Science 319, 459–462. ( 10.1126/science.1149167) [DOI] [PubMed] [Google Scholar]
- 7.Dale J. 2006. Intraspecific variation in bird coloration. In Bird coloration (eds Hill GE, McGraw K), pp. 36–86. Cambridge, MA: Harvard University Press. [Google Scholar]
- 8.Alatalo RV, Hoglund J, Lundberg A. 1988. Patterns of variation in tail ornament size in birds. Biol. J. Linn. Soc. 34, 363–374. ( 10.1111/j.1095-8312.1988.tb01969.x) [DOI] [Google Scholar]
- 9.Cuervo JJ, Moller AP. 1999. Phenotypic variation and fluctuating asymmetry in sexually dimorphic feather ornaments in relation to sex and mating system. Biol. J. Linn. Soc. 68, 505–529. ( 10.1111/j.1095-8312.1999.tb01186.x) [DOI] [Google Scholar]
- 10.Delhey K, Peters A. 2008. Quantifying variability of avian colours: are signalling traits more variable? PLoS ONE 3, e1689 ( 10.1371/journal.pone.0001689) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grégoire A, Mcfarlane ML, Faivre B, Matthew R, Cherry MI. 2007. Patterns of morphological variation in two sexually dimorphic bird species with different tail shapes. Biol. J. Linn. Soc. 91, 437–443. ( 10.1111/j.1095-8312.2007.00808.x) [DOI] [Google Scholar]
- 12.Barnard P. 1991. Ornament and body size variation and their measurement in natural populations. Biol. J. Linn. Soc. 42, 379–388. ( 10.1111/j.1095-8312.1991.tb00570.x) [DOI] [Google Scholar]
- 13.Fitzpatrick S. 1997. Patterns of morphometric variation in birds’ tails: length, shape and variability. Biol. J. Linn. Soc. 62, 145–162. ( 10.1111/j.1095-8312.1997.tb01619.x) [DOI] [Google Scholar]
- 14.Reinhold K. 2011. Variation in acoustic signalling traits exhibits footprints of sexual selection. Evolution (NY) 65, 738–745. ( 10.1111/j.1558-5646.2010.01130.x) [DOI] [PubMed] [Google Scholar]
- 15.Evans MR, Barnard P. 1995. Variable sexual ornaments in scarlet-tufted malachite sunbirds (Nectarinia johnstoni) on Mount Kenya. Biol. J. Linn. Soc. 54, 371–381. ( 10.1111/j.1095-8312.1995.tb01043.x) [DOI] [Google Scholar]
- 16.Cuervo JJ, Moller AP, Møller AP. 2001. Components of phenotypic variation in avian ornamental and non-ornamental feathers. Evol. Ecol. 15, 53–72. ( 10.1023/A:1011913804309) [DOI] [Google Scholar]
- 17.Bonduriansky R, Day T. 2003. The evolution of static allometry in sexually selected traits. Evolution 57, 2450–2458. ( 10.1111/j.0014-3820.2003.tb01490.x) [DOI] [PubMed] [Google Scholar]
- 18.Barnard P. 1995. Timing of ornament growth, phenotypic variation, and size dimorphism in two promiscuous African whydahs (Ploceidae, Vidua). Biol. J. Linn. Soc. 55, 129–141. ( 10.1111/j.1095-8312.1995.tb01055.x) [DOI] [Google Scholar]
- 19.Forsman A, Ahnesjo J, Caesar S, Karlsson M. 2008. A model of ecological and evolutionary consequences of color polymorphism. Ecology 89, 34–40. ( 10.1890/07-0572.1) [DOI] [PubMed] [Google Scholar]
- 20.Delhey K, Smith J, Peters A. 2013. Colour-variable birds have broader ranges, wider niches and are less likely to be threatened. J. Evol. Biol. 26, 1559–1568. ( 10.1111/jeb.12157) [DOI] [PubMed] [Google Scholar]
- 21.Hugall AF, Stuart-Fox D. 2012. Accelerated speciation in colour-polymorphic birds. Nature 485, 631–634. ( 10.1038/nature11050) [DOI] [PubMed] [Google Scholar]
- 22.Hill GE, McGraw K. 2006. Bird coloration. Cambridge, MA: Harvard University Press. [Google Scholar]
- 23.Vorobyev M, Osorio D, Bennett ATD, Marshall NJ, Cuthill IC. 1998. Tetrachromacy, oil droplets and bird plumage colours. J. Comp. Physiol. A 183, 621–633. ( 10.1007/s003590050286) [DOI] [PubMed] [Google Scholar]
- 24.Nakagawa S, Poulin R, Mengersen K, Reinhold K, Engqvist L, Lagisz M, Senior AM. 2015. Meta-analysis of variation: ecological and evolutionary applications and beyond. Methods Ecol. Evol. 6, 143–152. ( 10.1111/2041-210X.12309) [DOI] [Google Scholar]
- 25.Dale J, Dey C, Delhey K, Kempenaers B, Valcu M. 2015. The effects of life-history and social selection on male and female plumage coloration. Nature 527, 367–370. ( 10.1038/nature15509) [DOI] [PubMed] [Google Scholar]
- 26.McGraw KJ. 2006. Mechanics of melanin coloration in birds. In Bird coloration. Vol 1. Mechanisms and measurements (eds Hill GE, McGraw KJ), pp. 243–294. Cambridge, MA: Harvard Univeristy Press. [Google Scholar]
- 27.McGraw KJ. 2006. Mechanics of carotenoid-based coloration. In Bird coloration. Vol. 1. Mechanisms and measurements (eds GE Hill, KJ McGraw), pp. 177–242. Cambridge, MA: Harvard University Press. [Google Scholar]
- 28.Delhey K. 2015. The colour of an avifauna: a quantitative analysis of the colour of Australian birds. Sci. Rep. 5, 18514 ( 10.1038/srep18514) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hill GE. 2006. Environmental regulation of ornamental coloration. In Bird coloration. vol. 1. Mechanisms and measurements (eds GE Hill, KJ McGraw), pp. 507–560. Cambridge, MA: Harvard University Press. [Google Scholar]
- 30.Hill GE, Johnson JD. 2012. The vitamin A-redox hypothesis: a biochemical basis for honest signaling via carotenoid pigmentation. Am. Nat. 180, E127–E150. ( 10.1086/667861) [DOI] [PubMed] [Google Scholar]
- 31.Guindre-Parker S, Love OP. 2013. Revisiting the condition-dependence of melanin-based plumage. J. Avian Biol. 45, 29–33. ( 10.1111/j.1600-048X.2013.00190.x) [DOI] [Google Scholar]
- 32.Roulin A. 2016. Condition-dependence, pleiotropy and the handicap principle of sexual selection in melanin-based colouration. Biol. Rev. 91, 328–348. ( 10.1111/brv.12171) [DOI] [PubMed] [Google Scholar]
- 33.Berg ML, Bennett ATD. 2010. The evolution of plumage colouration in parrots: a review. Emu 110, 10–20. ( 10.1071/MU09076) [DOI] [Google Scholar]
- 34.Masello JF, Lubjuhn T, Quillfeldt P. 2008. Is the structural and psittacofulvin-based coloration of wild burrowing parrots Cyanoliseus patagonus condition dependent? J. Avian Biol. 39, 653–662. ( 10.1111/j.1600-048X.2008.04417.x) [DOI] [Google Scholar]
- 35.Andersson S. 1999. Morphology of UV reflectance in a whistling-thrush: implications for the study of structural colour signalling in birds. J. Avian Biol. 30, 193–204. ( 10.2307/3677129) [DOI] [Google Scholar]
- 36.Prum RO. 2006. Anatomy, physics, and evolution of structural colours. In Bird coloration (eds Hill GE, McGraw K), pp. 295–353. Cambridge, MA: Harvard University Press. [Google Scholar]
- 37.McGraw KJ, Mackillop EA, Dale J, Hauber ME. 2002. Different colors reveal different information: how nutritional stress affects the expression of melanin- and structurally based ornamental plumage. J. Exp. Biol. 205, 3747–3755. [DOI] [PubMed] [Google Scholar]
- 38.Peters A, Kurvers RHJM, Roberts ML, Delhey K. 2011. No evidence for general condition-dependence of structural plumage colour in blue tits: an experiment. J. Evol. Biol. 24, 976–987. ( 10.1111/j.1420-9101.2011.02229.x) [DOI] [PubMed] [Google Scholar]
- 39.Cuthill IC. 2006. Color perception. In Bird coloration (eds Hill GE, McGraw K), pp. 3–40. Cambridge, MA: Harvard University Press. [Google Scholar]
- 40.Cassey P, Ewen J, Blackburn T, Hauber M, Vorobyev M, Marshall N. 2008. Eggshell colour does not predict measures of maternal investment in eggs of Turdus thrushes. Naturwissenschaften 95, 713–721. ( 10.1007/s00114-008-0376-x) [DOI] [PubMed] [Google Scholar]
- 41.Delhey K, Delhey V, Kempenaers B, Peters A. 2015. A practical framework to analyze variation in animal colors using visual models. Behav. Ecol. 26, 367–375. ( 10.1093/beheco/aru198) [DOI] [Google Scholar]
- 42.Delhey K, Burger C, Fiedler W, Peters A. 2010. Seasonal changes in colour: a comparison of structural, melanin- and carotenoid-based plumage colours. PLoS ONE 5, e11582 ( 10.1371/journal.pone.0011582) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hart NS, Hunt DM. 2007. Avian visual pigments: characteristics, spectral tuning, and evolution. Am. Nat. 169, 7–26. ( 10.1086/510141) [DOI] [PubMed] [Google Scholar]
- 44.Ödeen A, Håstad O. 2013. The phylogenetic distribution of ultraviolet sensitivity in birds. BMC Evol. Biol. 13, 36 ( 10.1186/1471-2148-13-36) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Endler JA, Mielke PW. 2005. Comparing entire colour patterns as birds see them. Biol. J. Linn. Soc. 86, 405–431. ( 10.1111/j.1095-8312.2005.00540.x) [DOI] [Google Scholar]
- 46.Hart N. 2001. Variations in cone photoreceptor abundance and the visual ecology of birds. J. Comp. Physiol. A 187, 685–697. ( 10.1007/s00359-001-0240-3) [DOI] [PubMed] [Google Scholar]
- 47.Lind O, Chavez J, Kelber A. 2014. The contribution of single and double cones to spectral sensitivity in budgerigars during changing light conditions. J. Comp. Physiol. A 200, 197–207. ( 10.1007/s00359-013-0878-7) [DOI] [PubMed] [Google Scholar]
- 48.Bloch NI. 2015. Evolution of opsin expression in birds driven by sexual selection and habitat. Proc. R. Soc. B 282, 20142321 ( 10.1098/rspb.2014.2321) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Delhey K, Hall M, Kingma SA, Peters A. 2013. Increased conspicuousness can explain the match between visual sensitivities and blue plumage colours in fairy-wrens. Proc. R. Soc. B 280, 20121771 ( 10.1098/rspb.2012.1771) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gregory P. 2016. Yellow-rumped thornbill (Acanthiza chrysorrhoa). In Handbook of the birds of the world alive (eds del Hoyo J, Elliott A, Sargatal J, Christie DA, de Juana E). Barcelona, Spain: Lynx Edicions. [Google Scholar]
- 51.Delhey K, Peters A. In press The effect of colour producing mechanisms on plumage sexual dichromatism in passerines and parrots. Funct. Ecol. ( 10.1111/1365-2435.12796) [DOI] [Google Scholar]
- 52.Hadfield JD, Nakagawa S. 2010. General quantitative genetic methods for comparative biology: phylogenies, taxonomies and multi-trait models for continuous and categorical characters. J. Evol. Biol. 23, 494–508. ( 10.1111/j.1420-9101.2009.01915.x) [DOI] [PubMed] [Google Scholar]
- 53.Hadfield JD. 2010. MCMC methods for multi-response generalised linear mixed models: the MCMCglmm R package. J. Stat. Softw. 33, 1–22. ( 10.18637/jss.v033.i02)20808728 [DOI] [Google Scholar]
- 54.Jetz W, Thomas GH, Joy JB, Hartmann K, Mooers AO. 2012. The global diversity of birds in space and time. Nature 491, 444–448. ( 10.1038/nature11631) [DOI] [PubMed] [Google Scholar]
- 55.Ross L, Gardner A, Hardy N, West SA. 2013. Ecology, not the genetics of sex determination, determines who helps in eusocial populations. Curr. Biol. 23, 2383–2387. ( 10.1016/j.cub.2013.10.013) [DOI] [PubMed] [Google Scholar]
- 56.Nakagawa S, Schielzeth H. 2010. Repeatability for Gaussian and non-Gaussian data: a practical guide for biologists. Biol. Rev. 85, 935–956. ( 10.1111/j.1469-185X.2010.00141.x) [DOI] [PubMed] [Google Scholar]
- 57.Ray-Mukherjee J, Nimon K, Mukherjee S, Morris DW, Slotow R, Hamer M. 2014. Using commonality analysis in multiple regressions: a tool to decompose regression effects in the face of multicollinearity. Methods Ecol. Evol. 5, 320–328. ( 10.1111/2041-210X.12166) [DOI] [Google Scholar]
- 58.Reinhold K. 2009. Variation of acoustic courtship signals in insects and amphibians: no evidence for bimodality, but identical dependence on duration. Ethology 115, 134–140. ( 10.1111/j.1439-0310.2008.01587.x) [DOI] [Google Scholar]
- 59.Peters A, Delhey K, Andersson S, van Noordwijk H, Förschler MI. 2008. Condition-dependence of multiple carotenoid-based plumage traits: an experimental study. Funct. Ecol. 22, 831–839. ( 10.1111/j.1365-2435.2008.01437.x) [DOI] [Google Scholar]
- 60.Cotton S, Fowler K, Pomiankowski A. 2004. Do sexual ornaments demonstrate heightened condition-dependent expression as predicted by the handicap hypothesis? Proc. Biol. Sci. 271, 771–783. ( 10.1098/rspb.2004.2688) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Guilford T, Dawkins MS. 1991. Receiver psychology and the evolution of animal signals. Anim. Behav. 42, 1–14. ( 10.1016/S0003-3472(05)80600-1) [DOI] [Google Scholar]
- 62.Reid ML, Weatherhead PJ. 1990. Mate-choice criteria of Ipswich sparrows: the importance of variability. Anim. Behav. 40, 538–544. ( 10.1016/S0003-3472(05)80534-2) [DOI] [Google Scholar]
- 63.Cohen A. 1984. Sexual selection and the psychophysics of female choice. J. Comp. Ethol. 64, 1–8. ( 10.1111/j.1439-0310.1984.tb00348.x) [DOI] [Google Scholar]
- 64.Akre KL, Johnsen S. 2014. Psychophysics and the evolution of behavior. Trends Ecol. Evol. 29, 291–300. ( 10.1016/j.tree.2014.03.007) [DOI] [PubMed] [Google Scholar]
- 65.Krauskopf J, Gegenfurtner K. 1992. Color discrimination and adaptation. Vision Res. 32, 2165–2175. ( 10.1016/0042-6989(92)90077-V) [DOI] [PubMed] [Google Scholar]
- 66.Lind O. 2016. Colour vision and background adaptation in a passerine bird, the zebra finch (Taeniopygia guttata). R. Soc. open sci. 3, 160383 ( 10.1098/rsos.160383) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Höbel G. 2016. Female discrimination thresholds frequently exceed local male display variation: implications for mate choice dynamics and sexual selection. J. Evol. Biol. 29, 572–582. ( 10.1111/jeb.12806) [DOI] [PubMed] [Google Scholar]
- 68.Cresswell W. 1994. Song as a pursuit-deterrent signal, and its occurrence relative to other anti-predation behaviours of skylark (Alauda arvensis) on attack by merlins (Falco columbarius). Behav. Ecol. Sociobiol. 34, 217–223. ( 10.1007/BF00167747) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available in the electronic supplementary material.