Participants of expert group on CPG for Sleep Disorders
Ravi Gupta, Kishore Gujar, K K Mishra, Navendu Gaur, Abdul Majid, Gautam Saha, Amrit Pattojoshi, RK Solanki
INTRODUCTION
Sleep disorders are common, still we have limited data regarding the prevalence and management of sleep disorders from India. Although the systematic research is limited from our country, still case reports from Indian population suggest that we see all kinds of sleep disorders.
Prevalence of various sleep disorders is shown in Table 1.
Table 1.
Prevalence of various sleep disorders in general population

Among all, we have population data for two disorders from Indian adult population- one is Obstructive Sleep Apnea (OSA) and second is Restless Legs Syndrome (RLS). Sleep problems have been investigated among Indian children more frequently through variety of approaches, most common through the questionnaire based screening in school-based cohorts.
Knowledge of sleep disorders is essential for psychiatrists for two reasons. First, some of the sleep disorders are common in Psychiatric patients. They may be related directly to the pathophysiology of psychiatric illness, or may be the consequence of the treatment modalities offered. Insomnia is a common complaint with a number of Psychiatric disorders e.g., depression, anxiety, and withdrawal from the substances that depress cerebral functioning. In addition, we now have evidence that link the depression, bipolar disorder and schizophrenia with the disordered circadian rhythms and many of these patients show delayed sleep wake phase cycle. Similarly, antidepressants are known to induce a number of sleep disorders including NREM parasomnias (sleep talking, sleep walking) as well as REM parasomnias (REM sleep behavior disorder) and restless legs syndrome. Antipsychotics may cause weight gain and thus they may lead to obstructive sleep apnea in a number of patients. Similarly, opioid users suffer from central sleep apnea and during withdrawal many of them develop RLS. Both these conditions may worsen the quality of the sleep.
Secondly, daytime manifestations of a number of sleep disorders e.g., insomnia, hypersomnia, restless legs syndrome, sleep apnea mimic that of Psychiatric disorders e.g., depression, fibromyalgia, chronic fatigue syndrome and somatoform disorders.
Considering the recent evidences and changes in the management of sleep disorders, Indian Psychiatric Society has decided to update the existing guidelines. However, few points must be kept in mind while you consider these guidelines for your practice:
These are consensus statements
Original research in this area from India is limited. Most of the literature reviewed has been generated from the studies involving Caucasian and European population. They are culturally, phenotypically and genetically different from Indian population. All three factors-culture, phenotype and genotype influence the sleep patterns, pathophysiology of sleep disorders and their management- both pharmacological as well as non-pharmacological.
With this background, we will discuss the guidelines regarding management of individual sleep disorders.
INSOMNIA
ICD-10 defines insomnia as a condition where there is a problem in initiating the sleep, staying asleep or waking up early in the morning at least for 3 nights/week for at least 1 month. It should be associated with significant distress and persistent preoccupation with the deficiency of sleep.
DSM-5 defines insomnia as a condition where a problem has been reported in initiating, maintaining the sleep or there is an early morning awakening. This problem should occur despite adequate opportunities to fall asleep and must occur at least 3 nights a week. It should be associated with significant distress in the personal, social or occupational life. If it persists for at least 1 month but less than 3 months, it is considered as episodic; if it persists for at least 3 months, it is considered as persistent insomnia.
Our understanding regarding insomnia has changed over the years. Earlier we used to differentiate between primary and secondary insomnia, however, the recent research has challenged this belief. Current literature suggests that insomnia cannot be considered merely as a symptom of psychiatric disorders. It is rather co-morbid with the psychiatric and other medical conditions, and if not treated early, through the process of kindling it becomes chronic which has multiple health and economic implications.
For this reason, in the third edition of International Classification of Sleep Disorder (ICSD-3), which appeared in 2014, insomnia has been divided into two categories: short term insomnia disorder and chronic insomnia disorder. In addition, subtyping of the primary insomnia into adjustment, psychophysiological, paradoxical and idiopathic that prevailed till ICSD-2 has been omitted. This has happened for multiple reasons. First, all the insomnia sufferers have in common one issue i.e., hyperarousal and second, change in the sleep related behavior and compensatory mechanisms were found similar across different insomnia subtypes. Hence, all the modalities that are used for the treatment of insomnia are directed towards reducing the hyperarousal.
Assessment and evaluation
Management of the insomnia case starts with the history taking and general physical examination. It is of paramount importance as a number of sleep disorders may mimic insomnia. Hence, having knowledge regarding these mimics will help the clinician to reach to an accurate diagnosis. Through a careful history and clinical examination, these conditions can be ruled out (Table 2).
Table 2.
Sleep Disorders that may mimic insomnia
While taking the history of a patient with insomnia, special focus should be provided to the initiation of symptoms, its course and progression. Information from the bed-partner/ room-partner who had seen the patient while asleep should be sought and incorporated.(Table 3).
Table 3.
Information regarding a typical night

He should be asked for the daytime symptoms of insomnia, as in their absence, insomnia can’t be diagnosed. Frequency of symptoms must be asked along with the duration and frequency of symptoms per week. It must be ensured that the patient has adequate opportunities to fall asleep. Excessive daytime sleepiness must be ruled out. Clues regarding the predisposing, precipitating and perpetuating factors of insomnia should be assessed in detail.
It is essential to ask for the sleep pattern while the patient was asymptomatic and compare it with the sleep schedule during symptomatic period. Sleep related behavior and rituals must be asked before and after the symptoms onset as they may provide a good idea about the possible interventions.
Many of the patients with insomnia start worrying while they are not able to fall asleep in the bed. Dysfunctional thoughts before the bedtime or while in bed lead to hyperarousal and they may be assessed using Dysfunctional Beliefs and Attitudes about Sleep, which is available in English as well as Hindi Language.
Many of the medical conditions may induce symptoms that may mimic insomnia. Hence, the disorders provided in Table 4 must be ruled out.
Table 4.
Other medical disorders that may induce sleep disturbance

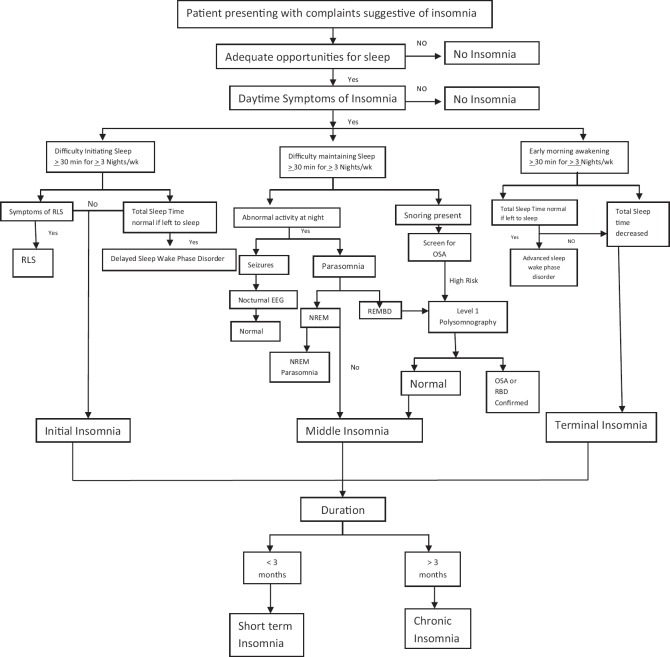
Algorithm for the diagnosis of insomnia is depicted in Fig 1.
Figure 1.
Algorithm for the diagnosis of Insomnia
Effect of the insomnia on the daytime functioning must be assessed. Special care should be taken to differentiate between fatigue and sleepiness. Mood during the day must be assessed. Depression and other psychiatric disorders that may mimic daytime symptoms of insomnia may be differentiated by asking “how do you feel during the day which is followed by a good night sleep?”. If the patient reports a remarkable improvement, diagnosis of psychiatric disorder shall be deferred till the insomnia resolves.
If the patient is undergoing any treatment for other medical disorders (pharmacological as well as non-pharmacological), its’ effect on the sleep should be examined.
Severity of insomnia may be assessed using a brief questionnaire- Insomnia severity index, available in English as well as Hindi.
This should be followed by a thorough general physical examination and if any system appears dysfunctional, that should be examined in detail.
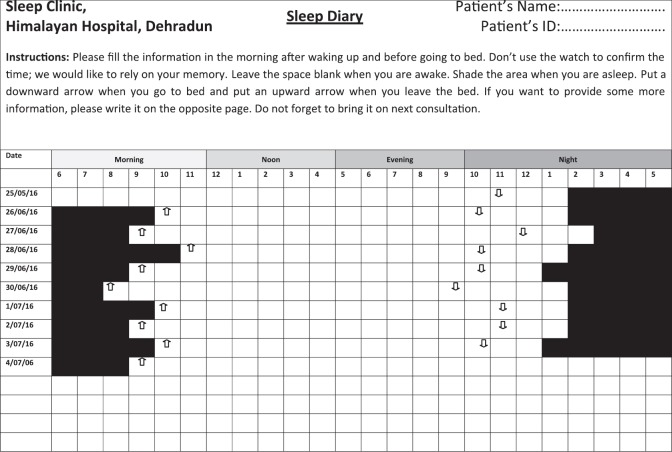
Sleep diary provides a good opportunity to assess the sleep-pattern and helps in differentiating insomnia from circadian rhythm sleep disorders. (Figure 2).
Figure 2.
Sleep diary depicting long sleep onset latency with normal total sleep time when the patient is following natural pattern of sleep. However, the patient gets the sleep late in the night and wakes up late in the morning. This data suggests delayed sleep wake phase disorder
At times, objective data is necessary to reach to a diagnosis and in those cases actigraphy may be performed. In cases of chronic insomnia that is not responding to treatment, video-synchronized 24-channel polysomnography is desirable.
Formulating a treatment plan
Treatment of insomnia is individualized and tailor-made. For the short term insomnia, pharmacotherapy is indicated, while for the chronic insomnia, cognitive behavior therapy for insomnia (CBT-I) is preferred.
Various hypnotic agents are described below. Importance of behavioral intervention should not be underestimated and it is better that they should be started even in cases with short term insomnia. For example, a person might be having genetic predisposition to insomnia and a recent stress might have precipitated the insomnia, which could be perpetuated by the dysfunctional beliefs about sleep or maladalptive strategies to control it. Common maladaptive strategies that we see include, but not limited to- spending excessive time in bed, start smoking or start drinking caffeine while awake, spending time on screen while awake or spending majority of the time during the day in bed.
Wherever indicated, opinion from a relevant specialist may be sought. If there is evidence of sleep disorders that mimic insomnia, management should be directed to those disorders, rather than the insomnia. In some cases, insomnia is co-morbid with these sleep disorders, and in these cases, both should be treated.
Goals of the therapy
Improve the sleep onset latency, total sleep time and reduce awakenings, thus improving sleep efficiency
Improving quality of sleep
Ameliorate or significantly reduce the daytime symptoms of insomnia
Sustain the effect of treatment and reduce the chances of the relapse
Choice of treatment settings
Treatment of insomnia is usually offered on an outpatient basis. Hospitalization may rather worsen the condition by enhancing hyperarousal. However, in some patients it may improve sleep by removing environmental factors and may provide a clue to the underlying pathophysiology.
Pharmacological treatment
Pharmacological treatment for the insomnia is limited to short-term insomnia and they are not routinely recommended for the management of chronic insomnia. A wide variety of drugs is available that may induce sleep e.g., benzodiazepines, Benzodiazepine receptor agonists, sedating antidepressants, second generation antipsychotics, antihistaminics, melatonin and its agonists and orexin receptor antagonists. Table 5 shows the factors that influence the choice of a particular drug.
Table 5.
How to choose a drug from the available molecules?

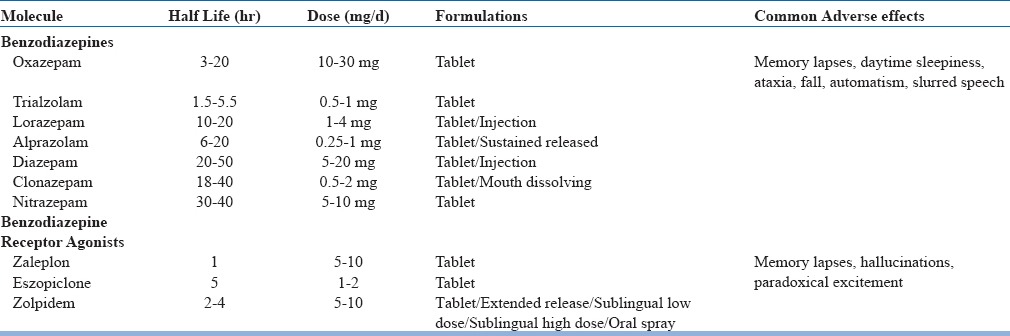
Benzodiazepinesand benzodiazepine receptor agonists (BzRA) are usually divided into short, intermediate and long acting and one of them may be chosen based upon the case. In general, short acting are preferred when the patient is having difficulty in sleep initiation, intermediate acting when the patient has difficulty in maintaining the sleep and long acting when the patients complain of early morning awakening. However, intermediate and long acting agents may have residual daytime effects and may produce the somnolence during the day (Table 6).
Table 6.
Benzodiazepines and Benzodiazepine Receptor Agonists
Melatonin and its agonists include the melatonin itself and the molecules that act on MT1 and MT2 receptors. Melationin is available as 3 mg tablet formulation. This may be used to induce sleep, however, data do not support its efficacy as a hypnotic agent. It is rather used as a chronobiotic. Melatonin receptor agonists- ramelteon (8-24 mg/day) and agomelatine (25-50 mg/day) are available in India. Remelteon is a short-acting-drug with half-life of around 3-4 hours. It shows an improvement in the time to fall asleep with minimal adverse effects. Agomelatine is another molecule that in addition to having an agonist action on melatonin receptors, also has antagonist action on 5-HT2C receptors. It has been found effective in improving both sleep and mood in clinical trials, and because of it's short half life (1-2 h) is free from daytime somnolence.
Orexin-receptors antagonists have recently been discovered for the management of insomnia. Controlled trial data is available for one of the molecule i.e., Survorexant. It has been found to improve total sleep time with variable findings on the nocturnal arousals and time spent awake after sleep onset. Usual prescribing dose varies between 10-40 mg/day. Common adverse effects include nausea abnormal dreams.
Sedating antidepressants e.g., tricyclics, trazodone and mirtazapine may be used if there is comorbid psychiatric disorder that warrants their use. In addition, they may also be preferred when adverse effects of the benzodiazepines and BzRAs are not tolerable. However, there are insufficient evidences for their efficacy in insomnia.
Antihistaminicdrugs are available as over-the-counter drugs, and they are commonly used for self medication. However, data regarding their efficacy in insomnia is limited. However, a tricyclic drug Doxeipin has been approved for the treatment of insomnia in low doses (10 mg). At this dose, it primarily acts on the H1 receptors and work as an antihistaminic.
Antipsychotics are used off-label for the treatment of insomnia, particularly chlorpromazine, clozapine, olanzapine and qutiapine. However, we do not have data regarding their efficacy as hypnotic agents. In addition, while prescribing them, it is essential to consider potential adverse effects e.g., metabolic syndrome and extrapyramidal symptoms.
Long term pharmacotherapy
Although the pharmacotherapy is not routinely recommended for the long term treatment in view of availablibility of non-pharmacological therapies, still, data is available for treatement with Z drugs for as long as 6 months without any major adverse effects. Considering the limited availability of trained CBT-I therapist, consensus was reached that in certain circunstances where CBT-I is not possible for any reason, pharmacothepray may be institutedfor long term.
Non-Pharmaclogical therapies
Cognitive behaviour therapy for Insomnia (CBT-I) is the mainstay of therapy for chronic insomnia. It is a multicomponent therapy that includes education regarding sleep physiology, sleep hygiene, addressing dysfunctional beliefs, stimulus control therapy, sleep restriction and relaxation training. Each of these components may be used as a primary focus in a given patient which makes this therapy highly individualized. In general, goal of the therapy is to reduce the hyperarousal, hence, educating the patient, cognitive restructuring (to address dysfunctional belief) and relaxation are necessary in almost all patients. CBT-I has been found effective for long term management of insomnia in the randomized controlled trials comparing it with hypnotic agents. It has been repeatedly shown that though, it takes some time to show its effects, once they appear, they are longer lasting as compared to pharmacotherapy and also reduce the chances of relapse. One of the major advantages of the CBT-I is the fact that it has also been found useful in cases of insomnia co-occurring in context of medical disorders.
Since the CBT-I is time consuming and requires the expertise, it has been tried to be delivered through internet, computer and in groups. Though the computerized CBT-I has been found superior to the placebo and pharmacotherapy, still it's efficacy has been found low when compared to the face to face CBT-I. Administration of CBT-I in a group has also been tried, with the results similar to the computerized CBT-I.
Results of sleep education and sleep hygiene alone have been found to have limited value unless they are associated with some other component of the CBT-I.
Exercise, especially the aerobic exercise along with the sleep hygeine has been found to be beneficial for the sleep and daytime activity. It has been found to reduce the time taken to fall asleep and improve the sleep efficiency in randomized control trials. Thus, patients should not be advised not to engage into any exercise before bed-time, as conventionally thought. Rather they should be allowed to analyse their sleep reactivity towards the exercise and if is found to interfere with sleep, then they should be advised not to get engaged into it before bedtime.
One of the focus of the therapy is to reduce the total time spent in bed in an effort to fall asleep by sleep restriction. Evidence is less robust regarding the utility of the sleep restriction therapy. However, a recent review suggested that sleep restriction therapy is one of the most effective components of the CBT-I. This must not be used in cases of bipolar disorders and epilepsy as it may worsen the comorbid medical condition.
In Indian context, since patients have been found focussing on the daytime worries, rather than on the sleep, problem solving technique may be added. Recently, mindfulness based relaxation therapy has been found to improve the sleep. Mindfulness based stress relaxation (MBSR) has been found equally efficacious to the face to face CBT-I, pharmacotherapy and better than sleep hygiene alone. This has been found more efficacious when it has been included as a component of CBT-I.
Management as per the different phases of illness
On of the major issues with the patients with chronic insomnia is that they desperately want to sleep soon after the initiation of treatment. However, CBT-I takes longer time to improve the situation because it is delivered in sessions. And each session has a focus which is guided by prevailing sleep problems since last session. During CBT-I patient is expected to maintain a sleep diary and change his cognition and behavior. Many of the patients are not able to do that. In those cases, the treatment is started with pharmacotherapy along with the CBT-I. Gradually, the pharmacotherpay is tapered and patient remains only on the CBT-I. Thus, patient benefits from the immediate response of hypnotics that reduces the burden and hyperarousal in addition to long term benefits of CBT-I. Though many sleep specialists are practicing it, still it has not been thoroughly investigated.
When to stop treatment
Though we do not have any literature on this issue, still, for the short term insomnia, patient should be requested to give drug holidays intermittently and to restart the treatment when the symptoms appear again. CBT-I may be discontinued once the sleep of the patient is stabilized for at least 4 weeks, though this number has been chosen arbitrarily.
Special population
Pharmacotherapy should be cautiously advised to the elderly because of the drug interactions and adverse effect profile. This should also be cautiously used in children and patients with other medical disorders for the same reasons. CBT-I is not possible in patients with neurocognitive deficits and non-compliant patients, hence they should not be started it without assessing their motivation.
Pregnancy: Duing first trimester it is best to avoid all medications. In disabling cases, sedating antihistaminitcsmay be used for the shortest possible period.
Lactation: Amount of psychotropicdrugs that is excreted in milk varies from molecule to molecule. Reader is advised to consult the pharmacology book for details regarding individual molecule.
HYPEROSMNIA
Alertness is an integral necessity for learning, performance and safety. Excessive daytime sleepiness impairs productivity and exponentially increases the risk of accidents, particularly in occupations like transportation, military, healthcare, factory workers etc. excessive sleepiness is reported by 10-25% of the general population in different parts of the world. However, as of now there are no prevalence studies from India on hypersomnia/ excessive sleepiness.
International Classification of Sleep Disorder, 3rd edition (ICSD 3) defines daytime sleepiness as: “the inability to stay awake and alert during the major waking episodes of the day, resulting in periods of irrepressible need for sleep or unintended lapses into drowsiness or sleep”. Sleepiness varies in severity and is more common during sedentary, boring, and monotonous situations that require little active participation. Some patients are aware of increasing sleepiness before falling asleep, whereas others can fall asleep with little or no prodromal symptoms (“sleep attacks”).
The most common cause of excessive daytime sleepiness (EDS) in modern day world is the combination of suboptimal duration of sleep, poor sleep hygiene, and changing work schedules. In addition, various sleep disorders like obstructive sleep apnea, circadian rhythm sleep disorders and periodic limb movement disorder may be associated with excessive daytime sleepiness.
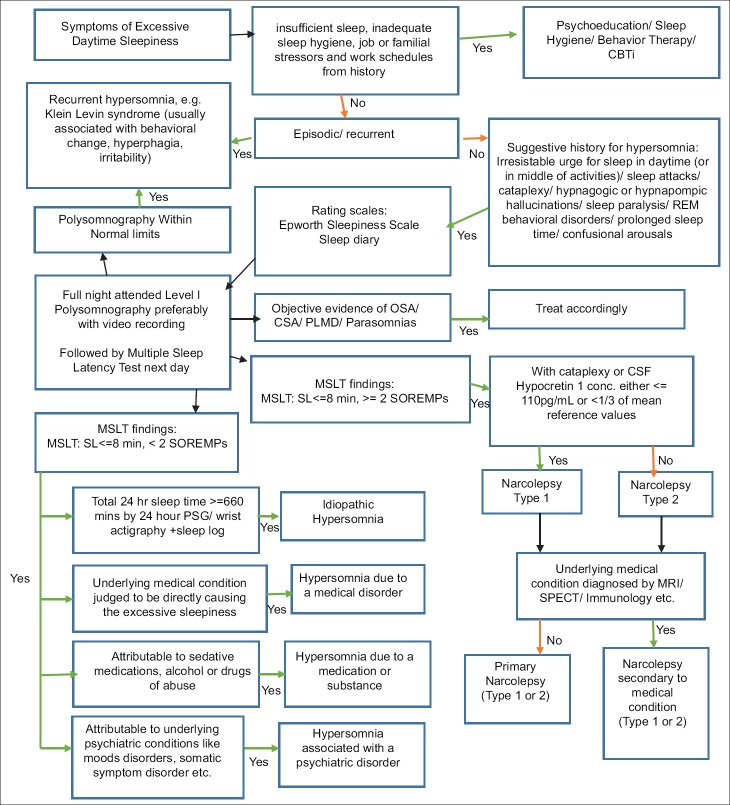
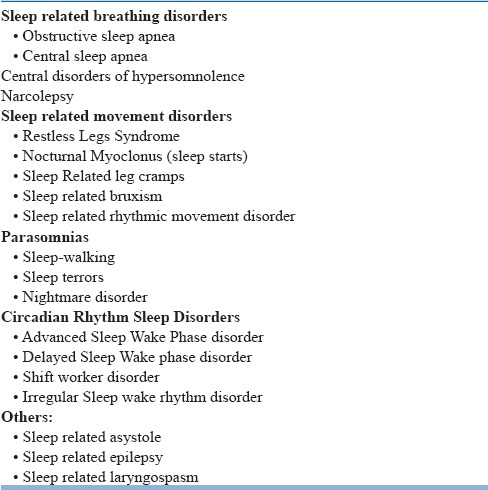
Primary causes of hypersomnolence have been classified under the heading of “Central disorders of hypersomnolence” in ICSD 3. The various central causes of hypersomnolence according to different classification systems is given in the table 7 below and Algorithm to approach a case of hypersomnia is given in Fig 3.
Table 7.
Comparative terminologies of causes of hypersomnolence between ICD-10 and ICSD-3
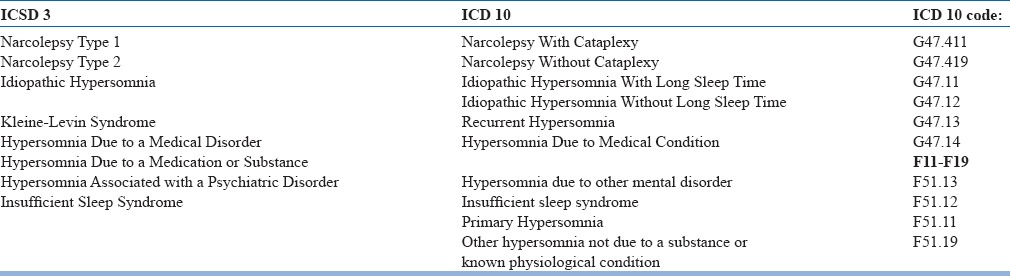
Figure 3.
Algorithm for the diagnosis of Hypersomnia
Hypersomnias of central origin
Narcolepsy type 1: caused by a deficiency of hypothalamic hypocretin (orexin) signaling. Excessive daytime sleepiness and signs of REM-sleep dissociation are the characteristic features of narcolepsy, however, the most specific feature is cataplexy (defined as more than one episode of generally brief (< 2 minutes), usually bilaterally symmetrical sudden loss of muscle tone with retained consciousness). There are repeated daily episodes of an irrepressible need to sleep or lapses into sleep (sleep attacks). Most patients awaken refreshed after a sleep episode but begin to feel sleepy again after variable times. Many narcolepsy patients have lapses in vigilance, sometimes in combination with automatic behavior, such as writing gibberish or interrupting a conversation with a completely different topic. Apart from cataplexy and sleep attacks, 33% to 80% of narcolepsy patients have hypnagogic hallucinations and/or sleep paralysis. Hypnagogic hallucinations are defined as vivid dreamlike experiences occurring at the transition from wake to sleep. Typically, hypnagogic hallucinations have a multimodal or “holistic” character, often combining visual, auditory, and tactile phenomena. Hypnopompic hallucinations are similar but occur at sleep to wake transitions. Sleep paralysis describes the disturbing temporary inability to move voluntary muscles at sleep-wake transitions. Despite being awake and conscious of the sleeping environment, it is impossible for subjects to move their limbs or even open their eyes. The experience may last for several minutes and can be very distressing. Other symptoms may include ptosis, blurred vision, and diplopia, presumably as a result of sleepiness. Obesity is another common symptom of narcolepsy.
Narcolepsy type 1 due to a medical condition: This condition is primarily associated with central nervous system (CNS) disorders, including autoimmune or paraneoplastic disorders associated with anti-Ma2 or antiaquaporin4 antibodies, and tumors or other lesions of the hypothalamus or severe head trauma.
Narcolepsy type 2: is characterized by excessive daytime sleepiness and abnormal manifestations of REM sleep on polysomnography/MSLT [mean sleep latency less than or equal to eight minutes and two or more sleep onset REM periods (SOREMPs) on an MSLT (or one SOREMP on an MSLT and one on the preceding nocturnal polysomnogram)]. Cataplexy is absent, although some atypical sensations of weakness triggered by unusual emotions such as stress and anger may be reported. Refreshing daytime naps are characteristic. Sleep paralysis, hypnagogic hallucinations, or automatic behavior, memory lapses, automatic behavior, ptosis, blurred vision, and diplopia may be present.
Narcolepsy type 2 due to a medical condition: Neurologic disorders associated with narcolepsy type 2 include tumors or sarcoidosis of the hypothalamus, autoimmune or paraneoplastic disorders associated with anti-Ma-2 or anti-aquaporin-4 antibodies, multiple sclerosis, myotonic dystrophy, Prader-Willi syndrome, Parkinson disease, and head trauma.
Idiopathic hypersomnia: is characterized by excessive daytime sleepiness that occurs in the absence of cataplexy, is accompanied by no more than one SOREMP on MSLT and preceding polysomnogram combined, and is not adequately explained by another disorder. Sleep drunkenness, consisting of prolonged difficulty waking up with repeated returns to sleep, irritability, automatic behavior, and confusion may be present. Subjects typically do not easily awaken to alarm clocks and frequently use special devices or procedures to wake up. Naps are generally long, often more than 60 minutes, and described as unrefreshing by 46% to 78% of patients. Associated symptoms which suggest a dysfunction of the autonomic nervous system may be present. These symptoms include headache, orthostatic disturbance, perception of temperature dysregulation, and peripheral vascular complaints (Raynaud-type phenomena with cold hands and feet).
Recurrent Hypersomnia: Kleine-Levin syndrome is characterized by recurrent episodes of excessive sleepiness, however, associated cognitiveand behavioral disturbances are not uncommon. A typical episode lasts a median 10 days (range, 2.5–80 days), with rare episodes lasting several weeks to months. Usual reported triggering factors for the first episodes are infection or alcohol intake, with further episodes recurring every 1–12 months (median three months) for years. During episodes, patients may spend as long as 16 to 20 hours per day in sleep, and they usually wake-up only for the natural calls (incontinence is not observed). They remain arousable, but are irritable if prevented from sleeping. When they are awake during episodes, most patients appear exhausted, indifferent, confused with psychomotor retardation. Anterograde amnesia is typical and most of the patients report derealization. A larger chunk of patients eat ravenously (66%, although one third eat less), nearly half of them turn hypersexual (53%, principally men), may show childish behavior and nearly half appear depressed (53%, predominantly women). They often become anxious when left alone and after seeing strangers, and nearly third of them experience hallucinations and delusions (30%). Importantly, patients are completely asymptomatic in between episodes.
Hypersomnia Due to a Medical Disorder: Patients with this disorder have excessive nocturnal sleep, daytime sleepiness, or excessive napping that is attributable to a coexisting medical or neurological disorder. Daytime sleepiness may be of variable severity and may resemble that of narcolepsy (i.e., refreshing naps) or idiopathic hypersomnia (i.e., long periods of unrefreshing sleep). Other symptoms of narcolepsy e.g., sleep paralysis, hypnagogic hallucinations and automatic behavior may be present. Hypersomnia due to a medical disorder is only diagnosed if the medical condition is judged to be directly causing the excessive sleepiness. Hypersomnolence has been described in association with a number of conditions e.g., metabolic encephalopathy, head trauma, stroke, brain tumors, encephalitis, systemic inflammation (e.g., chronic infections, rheumatologic disorders, cancer), genetic disorders, and neurodegenerative diseases.
Hypersomnia due to a medication or substance: Patients with this disorder have excessive nocturnal sleep, daytime sleepiness, or excessive napping that is attributable to sedating medications, alcohol, or drugs of abuse. This diagnosis also includes hypersomnolence associated with withdrawal from amphetamines and other stimulant drugs.
Subtypes
Hypersomnia due to sedating medications:
Hypersomnia due to substance abuse:
Hypersomnia due to stimulant withdrawal:
Hypersomnia associated with a psychiatric disorder: Patient may report increased duration of nocturnal sleep associated with daytime sleepiness or excessive napping. In addition, they often complain of poor quality and nonrestorative sleep. Patients are often intensely focused on their hypersomnolence, and psychiatric symptoms may become apparent only after prolonged interviews or psychometric testing. Associated psychiatric conditions include mood disorders, conversion or undifferentiated somatoform disorder, and less frequently other mental disorders such as schizoaffective disorder, adjustment disorder, or personality disorders.
Subtypes:
Hypersomnia associated with mood disorder
Hypersomnia associated with a conversion disorder or somatic symptom disorder
Insufficient sleep syndrome: Many people curtail a small portion from their normal sleep duration for prolonged periods owing to societal or professional demands. Since sleep deprivation has cumulative effect, they remain in a state of chronic partial sleep deprivation that is insufficient to maintain normal levels of alertness and wakefulness. Physical examination reveals no medical explanation for the patient's sleepiness. A detailed history of the sleep pattern reveals that patient is not spending adequate time in sleep and that he has curtailed his sleep in past few days/months. Sleep time that is markedly extended on weekend nights or during holidays compared to weekday nights is also suggestive of this disorder. Individuals with this condition may show cognitive and behavioral symptoms e.g., irritability, attention deficits, distractibility, reduced drive, anergia, dysphoria, fatigue, impatience, incoordination, and tiredness.
Management
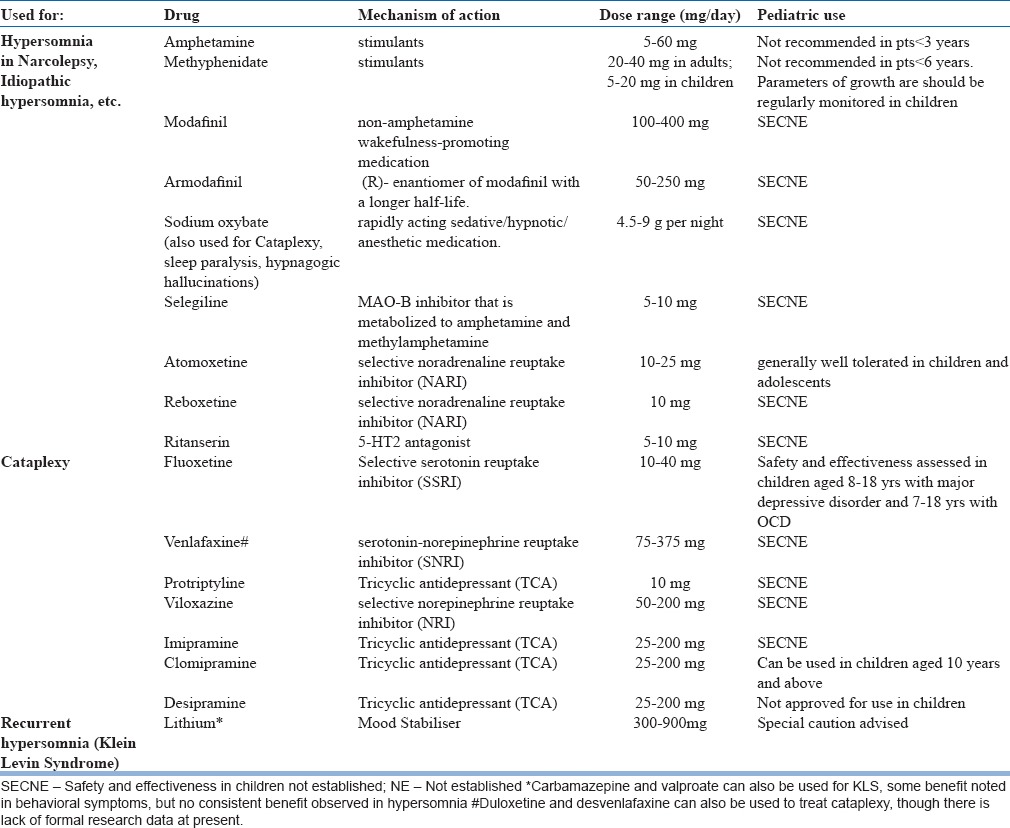
A. Pharmacotherapy: Pharmacotherapeutic options for the management of hypersomnia are depicted in Table 8.
Table 8.
Pharmacotherapy for Hypersomnia
B. Behavioral Treatment:
Education about type of hypersomnia, course, prognosis and management principles
Regular frequent nap times of 10-20 mins at 2-4 hour intervals during the day
Emphasize need for a regular nocturnal sleep schedule
Try to obtain 9 hours of nocturnal sleep
CIRCADIAN RHYTHM SLEEP DISORDERS
The circadian rhythm sleep disorders comprise of following disorders having major feature is a misalignment between the patient's sleep pattern and the sleep pattern that is desired or regarded as the societal norm ie. share a common underlying chronophysiologic basis.
Time Zone Change (Jet Lag) Syndrome
Shift Work Sleep Disorder
Irregular Sleep-Wake Pattern
Delayed Sleep-Phase Syndrome
Advanced Sleep-Phase Syndrome
Non-24-Hour Sleep-Wake Disorder
Circadian Rhythm Sleep Disorder Not Otherwise Specified
Diagnostic subtypes can be specified with the diagnosis of intrinsic type (due to neurologic disease) or extrinsic type (due to environmental or social circumstances) Severity Criteria: Mild: Moderate: Severe:
Duration Criteria: Acute: Subacute: Chronic:
Major problem among all CRSDs is the inability to fall asleep or staying awake when desired or expected. Since sleep episodes occurring at inappropriate times, the corresponding wake periods also seen at undesired times. Therefore, the patient complains of insomnia or sleepiness but importantly both of them are occurring at inappropriate times. For most of the CRSDs, once sleep is initiated, the major sleep episode is of normal duration with normal architecture. However, if any other sleep disorder appears to influence the sleep timing, diagnosis of CRSD should be deferred. For example patients with inadequate sleep hygiene may show some degree of shift in sleep and wake timing. Hence, CRSD should be diagnosed only when the symptoms can be ascribed to the major shift in timing of sleep.
1. Time Zone Change (Jet Lag) Syndrome: Synonyms and Key Words: Jet lag, transmeridian flight desynchronosis, air travel, transmeridiandyschronism.
Essential Features: Time zone change (jet lag) syndrome consists of varying degrees of difficulties in initiating or maintaining sleep, excessive sleepiness, decrements in subjective daytime alertness and performance, and somatic symptoms (largely related to gastrointestinal function) following rapid travel across multiple time zones.
2. Shift Work Sleep Disorder: Symptoms of insomnia or excessive sleepiness are seen in association of work shifts. These patients are often having work shifts that defy the natural sleep-wake cycle and thus they feel sleepy or unable to fall asleep at inappropriate time, e.g., while on work or after reaching home, respectively.
3. Irregular Sleep-Wake Pattern: These patients have inconsistent timings of sleep and wakefuleness, although the total duration of sleep remains normal in 24 hours. When their circadian rhythm is examined through sleep diary or actigraphy, it appears constantly changing.
4. Delayed Sleep-Phase Syndrome: In this condition, sleep time and wake time both are delayed in relation to the environmental timing, however, total sleep time remains adequate (Fig 1). These patients can’t fall asleep till late in the night and wake up late in the morning having optimal duration of sleep. If they go to bed early in the evening, they have a long sleep onset latency and if they are made to wake up early in morning, they have reduced awareness and feel sleep.
5. Advanced Sleep-Phase Syndrome: This is opposite of the delayed sleep phase disorder. These patients starts feeling sleepy early in the evening, spent optimal time in sleep but wake up early. If they are made to stay awake till late in night, they show reduced vigilance.
6. Non-24-Hour Sleep-Wake Syndrome
When seen through sleep diary and Actigraphy, these patients have optimal duration of sleep, although the timing of sleep appears delayed by 1-2 hours each day.
In January of 2014, the FDA approved the melatonin agonist Hetlioz™ (tasimelteon) for the treatment of N24SWD among the blind. This is the first FDA-approved drug for any CRSWD.
Diagnosis of CRSD
Polysomnography and Morningness-Eveningness Questionnaire is not routinely indicated for any of the CRSDs. Actigraphy is recommended for the delayed sleep wake phase disorder and advanced sleep wake phase disorder but may also be used to diagnose other CRSDs. Sleep diary must be maintained to diagnose all CRSDs. Actigraphy is a good tool to measure the progress.
Management
Planned naps are recommended for Shift worker disorder. Timed bright light therapy is useful for shift worker disorder and delayed sleep wake phase disorder. Jet lag disorder can be managed by timed melatonin administration. Hypnotics may be used to induce sleep in Shift worker disorder, however, stimulants are usually not recommended for the management of CRSDs.
RESTLESS LEG SYNDROME
Restless legs syndrome/Willis-Ekbom disease (RLS/WED) is characterized by an urge to move legs that is often accompanied by dysesthesias in the muscles. The urge improves with the movement of the legs or application of a counterirritant. Symptoms are seen only in the evening and rest worsens the symptoms. A number of conditions like nocturnal leg cramps, habitual leg movement, varicose, arthralgia, positional leg discomfort and leg edema must be excluded before making the diagnosis of RLS. This condition interferes with the sleep significantly. In some patients, significant sleep fatigue or poor concentration is reported in absence of RLS, but with polysomnographic evidence of periodic limb movements during sleep (PLMS), in all such cases the term periodic limb movement disorder (PLMD) is used. It is an autosomal dominant, sensorimotor disorder in which patient complains of a peculiar creepy or crawling sensation in the extremities Prevalence varies between 2-11% across different studies depending upon the geographical locations and populations included.
Assessment & evaluation in case of RLS
The diagnosis of restless leg syndrome is primarily based on the symptoms reported by the patient or the observer. Nocturnal polysomnography may help in the diagnosis by having PLMs in the recording. Serum iron level estimation may be helpful as iron is a co-factor for tyrosine hydroxylase which is essential for synthesis of dopamine. The Cambridge Hopkins RLS diagnostic questionnaire(CHRLSQ) and its Hindi version can be used during surveys and epidemiological studies. Looking for risk factors viz - elderly population, low iron level, Parkinsonism disorder, end stage renal disease is important.
Management
The selection of therapy depends upon a number of factors, including disease severity, patient age, co morbidities (e.g., pain, depression, anxiety and history of impulse control behaviours), drug side effects and patient preferences.
1. Non – pharmacological
Proper maintenance of sleep hygiene, exercise, restriction of coffee beverages, pneumatic compression stocking help in the symptom reduction
Behavioral strategies — Use of the following interventions is supported primarily on the basis of clinical experience in some cases, and small randomized trials
Avoidance of aggravating factors, including consideration of withdrawal of possibly predisposing medications
Moderate regular exercise
Reduced caffeine intake
For symptomatic relief - walking, bicycling, soaking the affected limbs, and leg massage, including pneumatic compression
Short daily hemodialysis for patients with end-stage renal disease.
Avoidance of aggravating factors — Sleep deprivation is known to aggravate symptoms of restless legs syndrome/Willis-Ekbom disease (RLS/WED) in many patients, and general principles of sleep hygiene should be reviewed.
Psychotropic drugs e.g., antidepressants, antipsychotics and other dopamine-blocking antiemetics such as metoclopramide, and sedating antihistamines (including those found in nonprescription medications) may lead to emergence of RLS/WED or worsening of prior symptoms. Most antidepressant classes have been associated withRLS/WED, including tricyclics, selective serotonin reuptake inhibitors, and serotonin-norepinephrine reuptake inhibitors.
Discontinuation of antidepressantsmay not be possible among all patients. In all such cases, symptoms of secondary RLS/WED should be treated in the same way as primary RLS/WED. Bupropion is an alternative antidepressant that may be less likely to induce or worsenRLS/WED.
Iron replacement
Serum ferritin concentration lower than 45 to 50 mcg/L (ng/mL) has been associated with an increased severity of restless legs syndrome. If the serum ferritin level is lower than 75 ng/ml, iron replacement is suggested. However, care must be taken not to induce iron overlaod.
Both oral iron therapy as well as intravenous iron have been found effective in treatment of RLS.
2. Pharmacological
The major classes of drugs used include dopaminergic agents, alpha-2-delta calcium channel ligands, opioids, and benzodiazepines
Chronic persistent symptoms
Chronic persistent restless legs syndrome is defined as RLS that is frequent and troublesome enough to require daily treatment, with symptoms usually occurring at least twice a week on average and resulting in moderate or severe distress.
Choice of therapy — Patients who do not respond to non pharmacologic therapy and correction of iron deficiency, pharmacologic treatment with a dopamine agonist or an alpha-2-delta calcium channel ligand is recommended.
For patients with very severe RLS/WED, co morbid depression, or obesity / metabolic syndrome, a dopamine agonist is preferred over other drugs as initial therapy.
For patients with comorbid pain, anxiety, or insomnia or a history of impulse control disorder or addiction associated with use of a dopamine agonist an alpha-2-delta calcium channel ligand. Is preffered
Most other patients, initial trial an alpha-2-delta calcium channel ligand because of the of augmentation with dopamine agonists, but other potential side effects of the various drugs should also be considered. In general, older patients are more prone to side effects of alpha-2-delta ligands. If the first drug chosen is ineffective or poorly tolerated, then a drug of the other class should be tried, including levodopa, benzodiazepines, and opioids, but generally these are reserved for intermittent use or in patients with more refractory symptoms.
Dopamine agonists — A number of dopamine agonsits are available in market e.g., cabergoline, lisuride, pergolide, pramipexole, ropinirole, rotigotine, and sumanirole. All except sumanirolehave been found superior to placebo. In two trials, cabergoline and pramipexole were superior to levodopa for improvement in disease severity as measured by the International Restless Legs Syndrome Study Group (IRLS) rating scale.
Pramipexole and Ropinirole — Action of pramipexole and ropinirole usually starts 90 to 120 minutes after intake. Therefore, these medications should be started two hours before RLS/WED symptoms start. The recommended doses are as follows:
Pramipexole 0.125 mg once daily. The dose may be increased by 0.125 mg every two to three days until relief is obtained. In a clinical trial, all three doses of pramipexole (0.25, 0.50, and 0.75 mg daily) were equally effective, and some patients responded to the initial dose of 0.125 mg daily. However, side effects were more common with the 0.50 mg and 0.75 mg daily doses. Therefore, it is expected that 0.25 mg daily has the best therapeutic margin. Most patients require 0.5 mg or less, but doses up to 1 mg may be needed.
Ropinirole 0.25 mg once daily. The dose may be increased by 0.25 mg every two to three days until relief is obtained. Most patients require at least 2 mg, and doses up to 4 mg may be needed. The maximum recommended dose is 3 mg in patients with end-stage renal disease on hemodialysis.
Rotigotine — Rotigotine is a non-ergot dopamine agonist that is formulated as a 24-hour transdermal patch. Transdermal rotigotine is a once-daily patch that is typically started at 1 mg/24 hours and titrated upwards to a maximum dose of 3 mg/24 hours. Application site reaction is the most common adverse effect of rotigotine, reported by 40 to 50 percent of patients.
Alpha-2-delta calcium channel ligands — Gabapentin enacarbil, gabapentin, and pregabalin are alternative choices for patients with chronic persistent RLS.
Gabapentin enacarbil — Several randomized, placebo-controlled studies have demonstrated that gabapentin enacarbil is effective in reducing RLS/WEDsymptom severity. The recommended dose of gabapentin enacarbil for RLS/WED is 600 mg, taken in the early evening
Gabapentin —Limited data suggest gabapentin may be effective in RLS/WED.
Pregabalin — Pregabalin in the doses of 75-300 mg/day found effective in treatment of RLS.
Duration of therapy
RLS/WED is often a lifelong disease, but the optimal and safe duration of pharmacologic therapy has not been well established. Most of the supporting data are based on relatively short (≤12-week) randomized trials, with fewer long-term extension studies supporting efficacy for 6 to 12 months of therapy with either a dopamine agonist or gabapentin enacarbil.
Special populations
Pregnancy and lactation — Management of restless legs syndrome during pregnancy should be individualized based on symptom severity, comorbidities such as depression or anxiety, and patient preferences. Many patients can be managed successfully with education, reassurance, iron supplementation if indicated, and nonpharmacologic strategies. Pharmacologic therapies such as clonazepam or carbidopa-levodopa may be considered for severe symptoms.
End-stage renal disease — The management of RLS/WED in patients with end-stage renal disease is similar to that in patients with normal renal function. However, medication doses may need to be adjusted, especially if the patient is not yet receiving dialysis, as dopamine agonists and alpha-2-delta ligands are all excreted by the kidneys. Careful attention to iron status is especially important in this group..
REM-SLEEP BEHAVIOUR DISORDER
Most parasomnias are benign unwelcome phenomenon observed in and around sleep that seldom present alarmingly. It is a common observation that more often than not, they are a cause of least concern both to patient and clinician. One such sleep-related phenomenon is REM-sleep behaviour disorder (RBD) that was first systematically defined 30 years back. It is a kind of parasomnia characterized by dream enactment behaviour (DEB) that emerges during rapid eye movement sleep and may lead to injury or disturbance of sleep. Behaviours include excessive abnormal and/or purposeful motor activities such as vocalization and simple limb twitching to flailing and punching of arms, sitting up, and kicking. Occasionally, patients climb out of bed, something akin to sleep walking. More complex and violent behaviours may occur rarely or many a times during the same night, most action occurring in bed. Not keeping with their waking personality, the vocalizations may be loud and full of abuse and obscenities. The routine REM-sleep atonia is typically absent. It manifests as sustained muscle activity or transient phasic muscle twitches in either the chin or limb electromyography (EMG) on polysomnography (PSG). Most cases arise after the age of 50. Its usual presentation in a speciality clinic is like a 65-year old, male whose partner expresses concern about some unusual sleep behaviour or who had injured himself or assaulted his partner during sleep while having vivid, action-filled dreams of self-defence. The reported prevalence rates are 0.38%-0.5% in general population and, probably higher (≥6%) in 70-90 year olds. Even more interesting is the equally higher prevalence of RBD in psychiatric population (5.8%). The early-onset (<50y) variety (EORBD) has more atypical clinical presentation showing female preponderance, less violent behaviour, may occur early during the night, and may get out of bed more frequently. There is preponderance of idiopathic cases and more association is seen with narcolepsy, depression, anti-depressants and parasomnia overlap disorder (POD).
Indian demographics regarding this illness are largely unknown and the published evidence is limited to half a dozen case reports, retrospective chart reviews, prospective questionnaire-based study and a case control study. All discuss RBD as part of PD except one (table 9).
Table 9.
Evidence base on RBD- Indian studies

The aetiology of RBD is still speculative. RBD may be either primary idiopathic type (iRBD) or secondary (table 10) to other disorders. It is unclear whether the idiopathic variety is an independent entity or it is merely a precursor ‘cryptogenic’ prodromal syndrome anti-dating degenerative brain disorders predominantly of α-synucleinopathy type ((Parkinson's disease (PD), dementia of Lewybody(DLB), Multiple system atrophy (MSA)). RBD patients are at higher risk of having cognitive, motor and autonomic impairments at baseline. It may be that spontaneous iRBD precede a parkinsonian neurodegenerative illness by several decades and thus have a prognostic and therapeutic relevance. The risk of iRBD converting to parkinsonian disorder varies between 40-80% over 5-15 years. There is now a growing body of evidence that suggests that depression may also be a preclinical marker of PD in iRBD cases and anti-depressants simply unmask the underlying pathology. Following are the environmental and behavioural risk factors associated with RBD (in common with PD):
Table 10.
Secondary causes of RBD
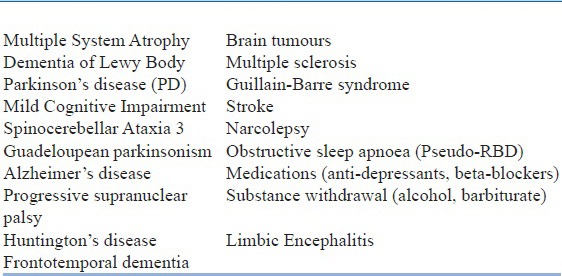
traumatic brain injury,
farming,
pesticide exposure, and
low education.
Studies on animal model suggest involvement of brainstem REM-sleep regulating nuclei, namely dorsal pontinesublateral dorsal nucleus and/or magnocellular reticular formation. The role of several genetic links is under study at present.
The risk of sleep-related injury (SRI) is markedly increased in RBD cases and their bed partners (33%-95%). DEB vary from harmless actions like knitting, singing, etc., to injurious ones and common injuries include bruises, abrasions, lacerations, fractures and dislocations, attempt at strangulation of partner and occasionally, subdural hematomas. Lifetime incidence of head injury is around 20%. Risk factors for SRI are:
Idiopathic RBD
Severe limb movements during DEB
Dream recall
Falling out of bed during DEB
It is to be noted that as against the common wisdom, DEB frequency has not been found to be associated with the frequency and severity of SRI. Cases of PD-RBD are less likely to injure themselves compared to idiopathic RBD and injuries and falls are more common in PD-RBD than PD alone. RBD is a progressive disorder and spontaneous remissions are witnessed very seldom. There can be a gradual reduction of RBD symptoms over the years in approximately 30% of the patients and they may remit spontaneously in 14-30% of RBD-PD patients per year. These data are probably reflective of progressive neurodegeneration. DEB is absent in up to 30% of reported cases of RBD.
Assessment and evaluation
Despite the presence of RBD in a higher number of patients with PD, less than 1% complain about it at the time of presentation. The reasons can be
RBD being considered not severe enough or infrequent to consult a physician
Symptoms being perceived not worthy of discussion
Unaware of their presence
Waiting for them to resolve with time
Feel shy to discuss.
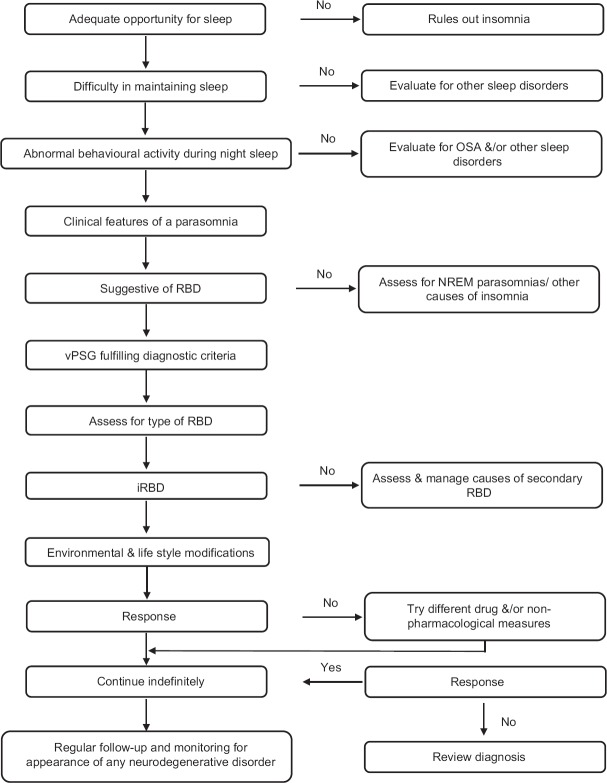
Even if the above hiccups get surmounted, the required support may still elude the patient either because the physician is unaware of RBD or the case may be misdiagnosed a more common ailment such as epilepsy or sleep-walking. Thus, an elaborate history with direct questions to confirm the presence or absence of the symptoms of some parasomnia is a must [Figure 4].
Figure 4.
Diagnosis and management of REM-sleep behaviour disorder
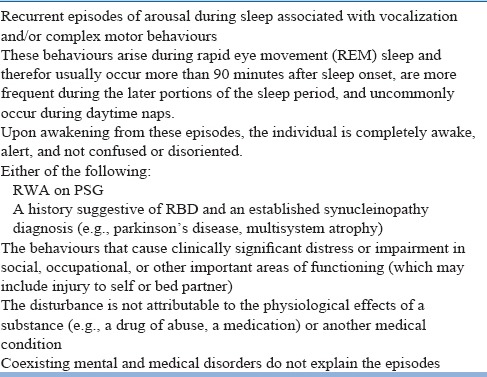
In RBD, a careful interviewing of bed-/room-partner will help in eliciting of a history of brief recurrent DEB that occur chiefly in the latter half of the night with patient confined to bed distinguishing it from non-REM sleep parasomnias that predominantly occur during first half without any dream mentation. Two major classificatory systems define the criteria for RBD diagnosis- International classification of sleep disorders- third edition (ICSD-3) criteria and DSM-5 (Table 11 & 12). The DSM-5 now classifies RBD as an independent disorder. ICD-10 is yet to come up with its own criteria of RBD though ICD-10 CM does include RBD (G47.52) as a billable code for the purpose of reimbursement claims in America.
Table 11.
ICSD-3 criteria

Table 12.
DSM-5 criteria of RBD (327.42)
There are several disorders that can present in a fashion similar to RBDs that one needs to rule out. It should be also kept in mind that these conditions may co-exist with RBD (Table 13). On such example is POD, a younger-onset variant of RBD.
Table 13.
Differential diagnosis of RBD

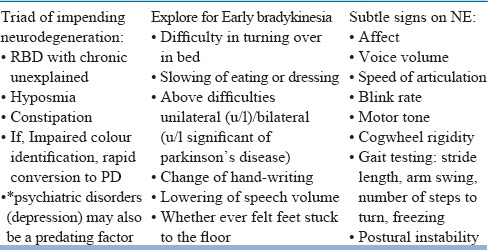
A baseline neurological examination (NE) that involves specific evaluation of cognition and screening for extrapyramidal symptoms should be done once a diagnosis of RBD is established (Table 14). RBD precedes parkinsonian neurodegenerative disorders and is fairly prevalent (50-90%) in α-synuclein disorders, while in rest of the neurodegenerative disorders RBD follows (except spinocerebellar ataxia 3) other neurological deficits and is uncommon.
Table 14.
Neurological history and examination-salient features
Following investigations will aid to the diagnosis of RBD:
Specific
PSG with time-synchronized video (vPSG) is the single most important investigation.
Electro-oculogram (EOG) monitoring
EMG with multiple channels for chin, bilateral extensor digitorum, and tibialis anterior muscles.
Electrocardiogram (ECG)
Nasal air flow
Arterial blood oxygen saturation.
The revised scoring of PSG features of RBD has been detailed in AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications, Version 2.2. RBD is the only parasomnia that requires vPSG for confirmation of diagnosis.
Others
To aid in diagnosis or monitor:
Routine lab investigations
Neuroimaging – currently used as research tools to identify pre-clinical disease markers
Transcranialsonography (TCS)- substantianigra (SN) echogenicity, a disease marker
Positron-emission tomography (PET)- to assess severity and extent of neurodegeneration
Single photon emission computed tomography (SPECT)- to study the SN dopaminergic neuronal status
Post-mortem brain autopsy
Scales/Questionnaires/Inventories
Since access to vPSG is limited, clinical interview and questionnaires may come in handy in early screening of RBD cases and related morbidities. Number of tools are available for this purpose and to choose the right tool will depend on whether it is required for rapid screening of RBD in general population, or in specific specialized population such as psychiatric cases and patients suspected of neurodegenerative illness, or to monitor progress of illness or effects of some intervention, and who is the source of information. It is to be noted that despite their ease of availability and applicability, screening tools are not a replacement for vPSG. RBD scales help us point out probable RBD cases and thus to plan out RBD specific assessment and targeted intervention services without causing a resource crunch.
For RBD screening
RBD Questionnaire-Hong Kong (RBDQ-HK)
RBD screening questionnaire (RBDSQ),
Mayo sleep questionnaire (MSQ),
RBD1 questionnaire,
Innsbruk RBD inventory,
For RBD severity
RBD severity index (in Japanese),
Minnesota parasomnia injury scale,
For sleep
Epsworth sleepiness scale,
Pittsburgh sleep quality index
For baseline cognitive screening
Modified mini-mental state examination
Montreal cognitive assessment
For parkinsonian features
Unified Parkinson disease rating scale
Formulation of a treatment plan & Choice of treatment setting [Figure 4]
Any treatment plan concerning RBD will involve reduction of DEB and prevention of injury, irrespective of frequency and severity of DEB. The choice of treatment setting will be largely determined by the presenting complaints. Hospitalization may be required for assessment of RBD, treatment of SRI and/or current medical condition. SRIs in RBD can be life-threatening and may have medico-legal consequences.
While formulating a treatment plan of RBD following should be included:
-
Non-pharmacological measures
- Safety measures
- Removal or minimization of aggravating factors
- Counselling of patient and care-givers
- Others
Pharmacotherapy
Non-pharmacological meassures
The primary goal of RBD treatment is SRI prevention. Among the non-pharmacologic measures, modification of sleep environment should be initiated as the first step.
-
Environmental modification may include:
-
Patient safety. e.g.,
- lowering of bed
- putting a mattress on the floor alongside bed
- sleeping on the floor mattress itself
- shift bed/ bedroom to the lowest floor of the house
- barricading with pillows around the bed
- using a bed with padded rails
- minimize furniture around the bed
- maximize the distance from ungrilled windows
- soft-pad the sharp edges of furniture around the house
- remove fire-arms or knives from within reach (preferably lock away)
- safety locking of windows and doors
- physical restraint. e.g., sleeping bags, padded belts, padded waterbeds
-
Partner safety
- Bed partner should sleep separate from the patient till the problem symptoms of RBD are brought under control with treatment.
It is a matter of investigation whether environmental manipulation is enough in itself for the management of RBD or it has only an adjunct role to pharmacotherapy.
-
Patient or caregiver should be asked to maintain a sleep diary
Avoid sleep deprivation- maintain adequate total sleep time with a fixed waking time.
Avoid alcohol and/or other recreational drugs.
Treat co-morbid sleep disorders, if any.
Reassure and counsel patient and family about need of treatment, regular follow-ups, and the possibility of onset of a neurodegenerative disorder.
Review ongoing medication charts. Remove or reduce the dose of aggravating agent wherever possible. Antidepressants can trigger RBD or expose RBD in up to 6% cases of depression. Tapering off might result in symptom reduction but not complete remission in such cases. Bupropion is the only anti-depressant not reported to be associated with RBD till date. Thus, it might be considered for use in those where pharmacotherapy is indicated for depression.
Along with these measures, pharmacological treatment should be instituted. As no large randomized double-blind or head-to-head controlled trials are available till date to assess efficacy of medications, the current treatment guidelines are largely expert consensus, based on evidence of large case series or small clinical trials.
Pharmacological treatment
The only REM-sleep parasomnia where pharmacotherapy is clearly indicated is RBD. Many pharmacological agents have been tried for this purpose, but there is marked variation in their efficacy and there is lack of standardization of the dose of medication and the duration of its continuation (Table 15). Among all the medications utilized till date, the most robust support is for clonazepam and melatonin. A medication that improves sleep quality with minimum day time effects and results in improvement of objective measures of RBD such as RWA on PSG should be chosen.
Table 15.
Drugs used in treatment of RBD
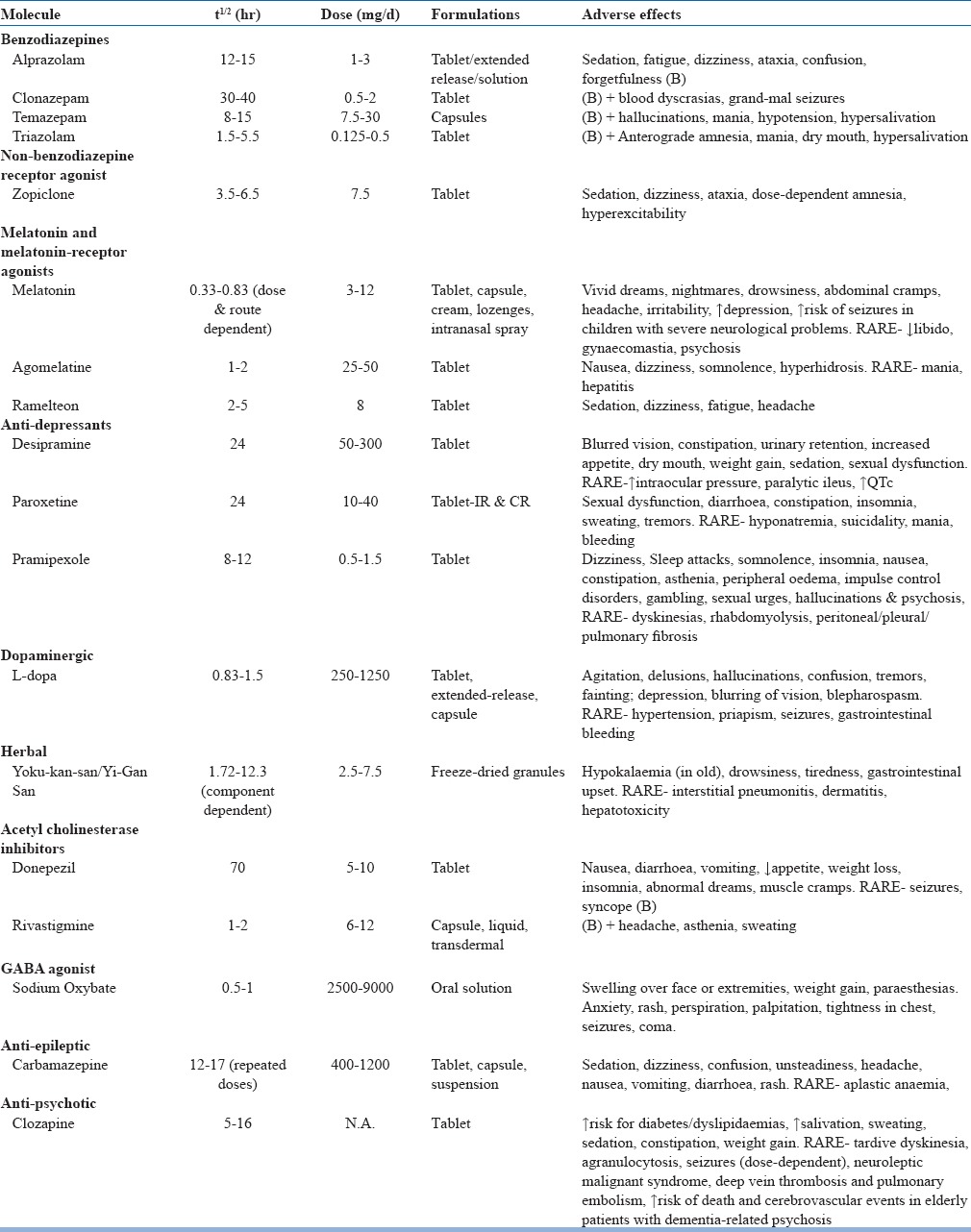
Clonazepam
Recommended as first-line medication. The evidence base is almost exclusively in terms of retrospective case reports and case series in over 300 patients. Initial response to low dose clonazepam (0.5-2mg) is seen in upto 90% of patients but later tolerance and treatment failures are reported. Because of its long half-life, it may become a cause of concern if causing impaired alertness, morning sedation and gait impairment in parkinsonian disorders and elderly patients and can worsen concomitant obstructive sleep apnoea (OSA). It vanquishes DEB but do not re-establish REM sleep atonia. It helps markedly in reduction of SRI, one study reporting a reduction from 80.8% pre-treatment events to 5.6% post-treatment levels. Patients on clonazepam should be regularly monitored for development of newer symptoms of dementia or gait disorders. Clonazepam is metabolized by cytochrome p450 enzyme system (cyp2C19 & cyp3A4). Thus, the possibility of drug-drug interactions should be kept in mind especially in elderly population where polypharmacy is a rule rather than exception.
Melatonin
The pineal gland secretion, melatonin when used exogenously is said to be effective in RBD in few case reports, case series and a small randomized controlled trial of eight patients. Recent studies find melatonin as efficacious as clonazepam in RBD with a better adverse effect profile and re-establishment of REM atonia especially in those with dementia or sleep apnoea. Efficacy is not known in cases where RBD co-exists with both PD/dementia and depression that requires treatment with anti-depressants. Most patients respond to 3mg dose with minimal or no side effects. The mechanism of action of melatonin in RBD is not known, though calmodulin antagonism is suggested as a potential mechanism and the effects probably go beyond simple hypnosis.
Others
In cases where clonazepam and melatonin are not indicated or are refractory to these medications, other medications may be tried. But so far, the evidence in their favour is inadequate or at times, contradictory. e.g., there are just two case reports of successful RBD treatment with sodium oxybate. Acetylcholinesterase inhibitors may be instituted in patients with dementia or synuleinopathies.
Among all the tried medications, the only preparation that is claimed to have some neuroprotective effects in in-vitro and in-vivo animal studies is Yi-Gan San/Yoku-kan san. It is a Chinese/ Japanese traditional kampo preparation that consists of seven different herbs- Japanese Angelica root (3g), Atractylodeslancea rhizome (4g), Bupleurum root (2g), Poriasclerotium (4g), Glycyrrhiza root (1.5g), Cnidium rhizome (3g), and Uncaria hook (3g). Its neuropharmacodynamic actions include serotonergic, glutamatergic, cholinergic, dopaminergic, adrenergic, GABAergic along with anti-inflammatory, anti-stress, neuroplasticity, and neuroprotective effects. It is through these mechanisms it is supposed to exert its effects in its primary indication for behavioural and psychological symptoms of dementia including alzheimer's disease, dementia of Lewy body, and PD. There is one case report of successful management of three RBD patients with Yi-Gan San.
Other Pharmacological Treatment Modalities
It is very ironic that currently there are no cognitive or cognitive-behaviour therapies available for REM-sleep “behaviour” disorder probably highlighting the ‘non-functional’ organic nature of the disorder. Two therapies- one behaviour and one somatic- does require a mention though.
Bed Alarm Relying on the brain's ability to process complex auditory stimuli during REM sleep similar to wake state and low arousal threshold for external stimulus Howell et al. at university of Minnesota developed a pressurized bed alarm customized with a pacifying pre-recorded message in a familiar person's reassuring voice to calm down the patient at the onset of dream enactment behaviour. This prevents bed-exiting and averts potential injuries. It can be used in patients who are refractory to pharmacotherapy or do not tolerate it.
Deep brain stimulation of subthalamic nucleus is ineffective in RBD though it does improve the subjective sleep quality.
Management as per different phases of illness & when to stop
RBD is a progressive neurological disorder. Though clonazepam is highly effective and works in almost 70-90% of the cases, relapse rates are high on discontinuation so pharmacologic treatment should be continued indefinitely. Regular monitoring to assess risk for neurodegenerative disorders should be taken up keeping in mind the strong relationship of RBD as a prodrome of such illnesses.
At present, there are no treatment strategies available to prevent or delay the development of PD in iRBD cases. It should be noted that most patients who ultimately developed neurodegenerative illness were on clonazepam indicating clonazepam's ineffectiveness as a preventive agent. It is recommended that an annual neurological examination should be done for the earliest detection and management of PD.
Special populations
The basic tenets of diagnosis, assessment and management of medicine are equally applicable to the special populations of RBD. The following few things should be kept in mind while dealing with them:
Children
Assessment of children will require a careful approach depending on their ability to communicate which can be limited given their age or co-morbidity. Idiopathic RBD in this population is rare and is usually seen in context with narcolepsy, epilepsy, brain tumours, or, medication effects.
Elderly
After the onset of idiopathic RBD almost half of the patients will develop a parkinsonian disorder within a decade, and nearly 80-90% will develop some neurodegenerative disorder in their lifetimes. Therefore, middle-aged or elderly people presenting with RBD symptoms should be counselled and monitored about these future possibilities. An opportunity to enrol them in any ongoing research clinical drug trials for developing and testing disease-modifying drugs can also be explored.
Women
RBD is infrequently reported in females. This may be because firstly, they might be having less injurious and less dramatic behaviours during dream enactment; and secondly, they may outlive their partner (gender difference in life expectancy) thus having less likelihood of coming to the notice of family members and receive the required medical attention. All medications require caution for use during pregnancy and lactation and should be taken only under expert guidance.
The paramount importance of recognizing RBD as a treatable parasomnia lies in the fact that it can prevent serious life-threatening injuries. Psychiatrists should actively screen for the presence of RBD as patients usually do not come forward to report these symptoms for a variety of reasons. The population of India grew at 17.7% between 2001-11. During the same period there was a quantum jump in the population of those above 60 years- a staggering 35.5%. With an all-time high elderly population of 8.6%, it is high time we brace-up for the identification of RBD and appreciate its importance as a pre-clinical marker of neurodegenerative illnesses. The success of any future neuroprotective and preventive interventions will, thus, rely on early and reliable identification of illness in its nascent stage.
OBSTRUCTIVE SLEEP APNOEA (OSA)
Sleep apnoea is charcaterized by recurrent pauses (at least 5/hour) in breath, each lasting at least 10 seconds and is important for the Psychiatric practice as it can mimic or exacerbate symptoms of psychiatric disorders such as depression, anxiety and panic disorder.
Three types of apnoeas have been described in literature. In obstructive sleep apnoea (OSA), cessation of breathing occurs despite persistent respiratory efforts. In central apnoea, there is no respiratory effort. Mixed apnoea has initial part similar to central apnea but in the later part, effort to breath is seen with absence of airflow throughout the period. Excessive daytime sleepiness, one of the major symptoms of OSA is seen in one-third of the patients and many report mid-nocturnal awakenings due to chocking which may be mistaken for panic. On the other hand, central sleep apnea has subtle clinical manifestations and often these patients present with chronic non-restorative sleep.
Obstructive sleep apnoea (OSA) is a common disorder with an estimated prevalence in the general population of 2-5% (Table 16). Apnoea during sleep leads to decrease in blood oxygen level which leads to disturbance in sleep and frequent arousals. Sleep apnoea severity is assessed with apnoea-hypopnoea index (AHI), which is the number of apnoeas and hypopnoeas per hour of sleep.
Table 16.
Symptoms of Obstructive sleep apnoea (OSA)
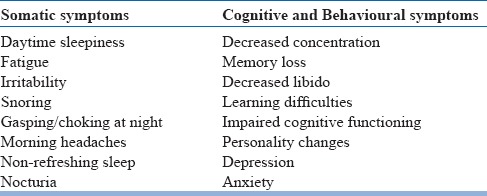
Obesity is a major risk factor for OSA. Untreated OSA leads to significant clinical problems including cardiovascular and cerebrovascular disorders, diabetes mellitus, pulmonary hypertension, arrhythmias, systemic hypertension, memory disturbances, cognitive dysfunction and sexual problems.
In addition, psychiatric disorders and OSA are also frequently comorbid, especially depression. Mood disturbance may represent a consequence of sleep apnoea. Psychiatric disorders associated with weight gain may also contribute to and promote the development of sleep apneas. Medications with depressent effect on CNS can worsen or exacerbate the symptoms of OSA. Patients with sleep apnea often have fregmatned sleep and thus they may have daytime symptoms of poor quality sleep e.g., fatigue, lethary, poor appetite, poor concentration, memory lapses, headache, distressed mood that may be mistaken for depression. Fregmented sleep often presents as multiple somatic symptoms during the daytime suggesting the diagnosis of somatoform disorder.
The symptoms are the direct consequences of OSA and occur due to the repetitive collapse of upper air ways.
Thin and lean individuals with significant apnoea are likely to show upper airway abnormalities. These include, e.g., hypertrophic tonsils and adenoids, a low-set palate or palatal webbing, a large uvula, a large tongue or a small mandible.
Risk factors, Screening, Examination and Diagnosis of OSA
The prevalence of OSA is higher in patients who have a combination of the following risk factors: obesity, neck larger than 17 inches for men or 16 inches for women, male gender, middle age, large tonsils, or recessed chin. Besides anatomical factors, physiological factors that influence the tone of the upper airway muslces play equally important role in generation of OSA. It is prudent to screen all patents who are at risk since untreated OSA is an independent risk factor for mortality. In addition, information regrading conditions as described in Table 17 must be gathered.
Table 17.
Medical history to be taken in patients with OSA

Physical Examination
Examination is very important to exclude other causes for the patient's symptoms mentioned below:
Following points should be kept in mind during physical examination
Weight and height should be documented.
Mandibular and tongue abnormality.
Assessment of Nasopharyngeal abnormality.
Measurement of Blood Pressure.
Perform routine respiratory, cardiovascular and neurological examination to detect any coexisting disease.
Diagnosis
Certain questionnaires e.g., STOP-Bang, Berlin Questionnaire have been developed to screen the OSA. However, the gold standard for the diagnosis of OSA is polysomnography. Ideally, in-lab attended video-synchronized full polysomnography (level 1) is indicated for the diagnosis of OSA, however, among high risk cases, limited channel level 3 polysomnography may also be used. This is also known as Home Sleep testing.
Treatment
Depending upon the severity the various treatment options is most appropriate for the management of OSA.
Treatment options can be broadly divided into:
Patient education
Behavioural interventions
Non-surgical options
Surgical options.
Treatment for comorbidities.
Patient Education
Patients and their attendants should be educated about the pathophysiology, risk factors, clinical consequences and the treatment of OSA.
Education should include behavioural modification like weight loss, sleep position, alcohol avoidance, risk factor modification, and medication effects.
Behavioural Interventions
Lifestyle changes can be very effective in mitigating the symptoms of sleep apnoea. However, large control trials are not available in this regard.
Weight loss is most important in all those who are overweight. Weight reduction improves symptoms in OSA
Exercise
Avoidance/Reduction of smoking and alcohol is beneficial.
Sedatives or sleeping tablets should be avoided.
Snorers should be discouraged from sleeping on their backs.
Non-Surgical Options
Positive Air Pressure (CPAP). This is the “gold standard” treatment for OSA. Optimal pressure of the PAP should be titrated as per the standard guidelines so as to ameliorate all kinds of sleep related breathing events- apnea/hypopnea, RERA and snoring. Automatic PAP has limited application in these cases.
-
Oral Appliances: These are effective only in mild to moderate severe cases of OSA.
- I. Mandibular Advancement Splints (MAS). This works by moving the mandible forward along with the tongue and increasing the caliber of upper airway.
Surgical Options
The treatment of choice for moderate to severe OSAS is continuous positive airway pressure (CPAP) devices, but patient adherence to these devices has been a limiting factor. In ‘selected cases’, surgical treatment on the upper airway (UA) or on the facial skeleton may be beneficial in alleviating this disease or improving the use of CPAP. Following surgical procedures can be beneficial to patients with OSA.
Any of the single staged procedure has not been found effective in management of OSA in large controlled trials. Patient should be educated regarding possible recurrence of OSA after 1-2 years of surgery, especially when it is done on the soft tissues.
Treatment for comorbidities
Hypothyroidism, depression, etc, are more prevalent. Obstructive sleep apnea (OSA) and hypothyroidism are relatively common disorders that have similar clinical features and are thought to be causally linked. The mechanisms proposed to explain how hypothyroidism might cause OSA include mucoprotein deposition in the upper airway, decreased neural output to the upper airway musculature, obesity, and abnormalities in ventilatory control.
Treatment of hypothyroidism in the presence of sleep apnea is potentially hazardous and may lead to cardiovascular complications. Management by a combination of Nasal continuous positive airways pressure(CPAP) and low-dose thyroxine is helpful in this situation.
OSA patients may have comorbid depression or any other psychiatric illness. In such cases bothe the disorders must be adequately and optimally treated.
Obstructive sleep apnea (OSA) leads to frequent arousals, which are characterized by fragmented sleep. Persistent sleep loss can lead to depressive symptoms. Patients may be treated for depression, but if the underlying symptoms are caused by OSA and the apnoea is not treated, depressive symptoms can remain. We may be treating someone with antidepressants when what we really should be doing is treating their sleep disorder, which could in turn restore their normal mood.
SUGGESTED READINGS
- 1.Sharma S, Trivedi JK. Guidelines for the treatment of Sleep disorders. [Last accessed 10 Oct 2016]. Available at www.indianjpsychiatry.org/cpg/cpg2006/cpg-mgmt_16.pdf .
- 2.Sharma SK, Kumpawat S, Banga A, Goel A. Prevalence and risk factors of obstructive sleep apnea syndrome in a population of Delhi, India. Chest. 2006;130:149–56. doi: 10.1378/chest.130.1.149. [DOI] [PubMed] [Google Scholar]
- 3.Rangarajan S, Rangarajan S, D’Souza GA. Restless legs syndrome in an Indian urban population. Sleep Med. 2007;9:88–93. doi: 10.1016/j.sleep.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 4.American Academy of Sleep Medicine. International Classification of Sleep Disorders. 3rd ed. Darian, IL: American Academy of Sleep Medicine; 2014. [Google Scholar]
- 5.Gupta R, Zalai D, Spence DW, BaHammam AS, Ramasubramanian C, Monti JM, et al. When insomnia is not just insomnia: the deeper correlates of disturbed sleep with reference to DSM-5. Asian J Psychiatr. 2014;12:23–30. doi: 10.1016/j.ajp.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 6.Gupta R. Presleep thoughts and dysfunctional beliefs in subjects of insomnia with or without depression: Implications for cognitive behavior therapy for insomnia in Indian context. Indian J Psychiatry. 2016;58:77–82. doi: 10.4103/0019-5545.174385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dhyani M, Rajput R, Gupta R. Hindi translation and validation of dysfunctional beliefs and attitudes about sleep (DBAS - 16) Ind Psychiatry J. 2013;22:80–5. doi: 10.4103/0972-6748.123639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morin CM, Vallières A, Ivers H. Dysfunctional beliefs and attitudes about sleep (DBAS): validation of a brief version (DBAS-16) Sleep. 2007;30:1547–54. doi: 10.1093/sleep/30.11.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morin CM, Belleville G, Bélanger L, Ivers H. The Insomnia Severity Index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep. 2011;34:601–8. doi: 10.1093/sleep/34.5.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lahan V, Gupta R. Translation and validation of the insomnia severity index in hindi language. Indian J Psychol Med. 2011;33:172–6. doi: 10.4103/0253-7176.92060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thompson W, Quay TAW, Rojas-Fernandez C, Farrell B, Bjerre LM, Pringsheim T, et al. Atypical antipsychotics for insomnia: a systematic review. Sleep Med. 2016;22:13–7. doi: 10.1016/j.sleep.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 12.Laudon M, Frydman-Marom A. Therapeutic effects of melatonin receptor agonists on sleep and comorbid disorders. Int J Mol Sci. 2014;15:15924–50. doi: 10.3390/ijms150915924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mitchell MD, Gehrman P, Perlis M, Umscheid CA. Comparative effectiveness of cognitive behavioral therapy for insomnia: a systematic review. BMC Fam Pract. 2012;13:40. doi: 10.1186/1471-2296-13-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morin CM, Edinger JD, Krystal AD, Buysse DJ, Beaulieu-Bonneau S, Ivers H. Sequential psychological and pharmacological therapies for comorbid and primary insomnia: study protocol for a randomized controlled trial. Trials. 2016;17:118. doi: 10.1186/s13063-016-1242-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Allen RP, Burchell BJ, MacDonald B, Hening WA, Earley CJ. Validation of the self-completed Cambridge-Hopkins questionnaire (CH-RLSq) for ascertainment of restless legs syndrome (RLS) in a population survey. Sleep Med. 2009;10:1097–100. doi: 10.1016/j.sleep.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 16.Gupta R, Allan RP, Pundeer A, Das S, Dhyani M, Geol D. Hindi translation and validation of Cambridge-Hopkins diagnostic questionnaire for RLS (CHRLSq) Annals of Indian Academy Of Neurology. 2015 Jul;18(3):303. doi: 10.4103/0972-2327.162290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scholz H, Trenkwalder C, Kohnen R, et al. Dopamine agonists for restless legs syndrome. Cochrane Database Syst Rev. 2011:CD006009. doi: 10.1002/14651858.CD006009.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wilt TJ, MacDonald R, Ouellette J, et al. Pharmacologic therapy for primary restless legs syndrome: a systematic review and meta-analysis. JAMA Intern Med. 2013;173:496. doi: 10.1001/jamainternmed.2013.3733. [DOI] [PubMed] [Google Scholar]
- 19.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington (VA): American Psychiatric Association; 2013. [Google Scholar]
- 20.Aurora RN, Zak RS, Maganti RK, Auerbach SH, Casey KR, Chowdhuri S, et al. Standards of Practice Committee, American Academy of Sleep Medicine. Best practice guide for the treatment of REM sleep behavior disorder (RBD) J Clin Sleep Med. 2010;6:85–95. [PMC free article] [PubMed] [Google Scholar]
- 21.Berry RB, Brooks R, Gamaldo CE, Harding SM, Lloyd RM, Marcus CL, et al. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications, Version 2.2. Darien (IL): American Academy of Sleep Medicine; 2015. [Google Scholar]
- 22.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14(6):540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 23.Chung F, Yegneswaran B, Liao P, et al. STOP questionnaire: a tool to screen patients for obstructive sleep apnea. Anesthesiology. 2008;108:812. doi: 10.1097/ALN.0b013e31816d83e4. [DOI] [PubMed] [Google Scholar]
- 24.Oğuztürk Ö, Ekici M, Çimen D, Ekici A, Senturk E. Attention deficit/hyperactivity disorder in adults with sleep apnea. J ClinPsychol Med Settings. 2013;20(2):234–239. doi: 10.1007/s10880-012-9331-2. [DOI] [PMC free article] [PubMed] [Google Scholar]