Abstract
Background
We conducted a systematic review and network meta-analysis to examine comparative efficacy and tolerability of pharmacologic interventions for pulmonary arterial hypertension (PAH).
Methods
MEDLINE, the Cochrane Register, EMBASE, CINAHL, and clinicaltrials.gov were searched (January 1, 1990 to March 3, 2016). Randomized controlled trials (RCTs) studying the approved pharmacologic agents endothelin receptor antagonists (ERA), phosphodiesterase inhibitors (PDE5i), the oral/inhaled (PO/INH) and IV/subcutaneous (SC) prostanoids, and riociguat and selexipag, alone or in combination, for pulmonary arterial hypertension (PAH) and reporting at least one efficacy outcome were selected.
Results
Thirty-one RCTs with 6,565 patients were selected. In network meta-analysis, when compared with a median placebo rate of 14.5%, clinical worsening was estimated at 2.8% with riociguat (risk ratio [RR], 0.19; 95% CI, 0.05-0.76); at 3.9% with ERA + PDE5i (RR, 0.27; 95% CI, 0.14-0.52), and at 5.7% with PDE5i (RR, 0.39; 95% CI, 0.24-0.62). For improvement in functional status, when compared with 16.2% in the placebo group, improvement in at least one New York Heart Association/World Health Organization (NYHA/WHO) functional class was estimated at 81.8% with IV/SC prostanoids (RR, 5.06; 95% CI, 2.3211.04), at 28.3% with ERA + PDE5i (RR, 1.75; 95% CI, 1.05-2.92), and at 25.2% with ERA (RR, 1.56; 95% CI, 1.22-2.00). Differences in mortality were not significant. Adverse events leading to discontinuation of therapy were highest with the PO/INH prostanoids (RR, 2.92; 95% CI, 1.68-5.06) and selexipag (RR, 2.06; 95% CI, 1.04-3.88) compared with placebo.
Conclusions
Currently approved pharmacologic agents have varying effects on morbidity and functional status in patients with PAH. Future comparative effectiveness trials are warranted with a focus on a patient-centered approach to therapy.
Registration
PROSPERO CRD42016036803
Key Words: comparative efficacy, network meta-analysis, pulmonary arterial hypertension
Abbreviations: 6MWD, 6-min walk distance; ERA, endothelin receptor antagonist; FDA, Food and Drug Administration; GRADE, Grading of Recommendations Assessment, Development, and Education; NYHA, New York Heart Association; PAH, pulmonary arterial hypertension; PDE5i, phosphodiesterase-5 inhibitor; RCT, randomized controlled trial; RR, risk ratio; SUCRA, surface under the cumulative ranking area; WHO, World Health Organization; WMD, weighted mean difference
Pulmonary arterial hypertension (PAH) or World Health Organization (WHO) group 1 pulmonary hypertension is a progressive disease associated with significant morbidity and a 5% to 15% annual mortality rate.1, 2, 3 In recent years, a number of drug classes to treat PAH have been approved for clinical use. These include endothelin receptor antagonists (ERA), phosphodiesterase-5 inhibitors (PDE5i), parenteral and nonparenteral prostacyclins, a soluble guanylate cyclase stimulator, and a prostacyclin-receptor agonist. Although randomized controlled trials (RCTs) have compared individual drugs to conventional therapy or placebo, head-to-head comparisons of different pharmacologic agents are limited. Conventional meta-analyses are limited by estimates between two interventions compared directly with each other, precluding assessment of comparative efficacy and safety of all available interventions.4, 5, 6, 7 Hence, evidence regarding the best treatment, either alone or in combination, is limited, leaving such decisions to individual clinical judgment.8, 9 A network meta-analysis approach can bridge this gap and guide both clinical decision-making and future research.10, 11
Therefore, we performed a network meta-analysis combining direct and indirect evidence to evaluate comparative efficacy and safety of all US Food and Drug Administration (FDA)-approved pharmacologic interventions, alone or in combination, in patients with PAH.
Methods
This systematic review is reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement for network meta-analysis and was conducted following a priori established protocol (PROSPERO-CRD42016036803).12, 13 We followed the International Society for Pharmacoeconomics and Outcomes Research approach on interpreting network meta-analyses for health-care decision-making.14, 15 We used Grading of Recommendations Assessment, Development and Evaluation (GRADE) to appraise quality of evidence.16
Selection Criteria
We included phase II or phase III RCTs with a minimum of 8 weeks of follow-up, meeting the following criteria: (1) Patients were primarily adults with symptomatic PAH (group 1 pulmonary hypertension). Some trials studied subjects 12 years of age and older and were included; however, trials restricted to pediatric or neonatal patients were excluded. (2) Interventions included all FDA-approved drugs specifically for PAH, including ERA (bosentan, ambrisentan, macitentan), PDE5i (sildenafil, tadalafil), oral/inhaled (PO/INH) prostanoids (treprostinil, iloprost), IV/subcutaneous (SC) prostanoids (epoprostenol, treprostinil), the soluble guanylate cyclase simulator riociguat, and the selective prostacyclin-receptor agonist selexipag, alone or in combination, administered for 8 weeks or longer. (3) The comparator consisted of another active agent, placebo, or conventional therapy. (4) Outcomes included trials reporting any of the efficacy outcomes (clinical worsening, hospitalization, mortality, and improvement in functional class or 6-min walk distance [6MWD]). As in prior studies,4, 7 RCTs in which a PAH therapy was initiated on the background of another PAH-specific cointervention were included as trials of active agents against placebo, and nature and rates of background therapy in each arm were examined narratively. Detailed exclusion criteria are presented in e-Appendix 1, Methods.
Search Strategy
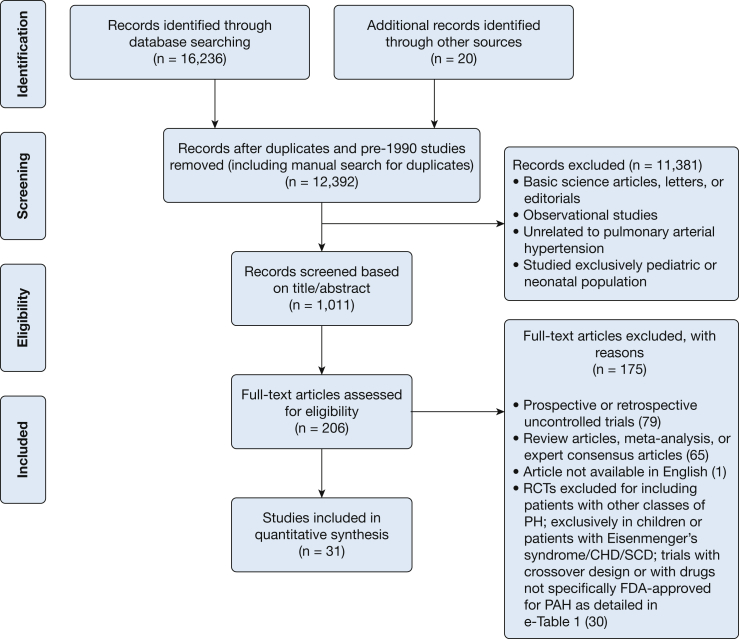
The search strategy was designed and conducted by an experienced medical librarian with input from study investigators. Multiple databases were searched for RCTs of pharmacologic therapy for PAH until March 3, 2016 (details in e-Appendix 1, Methods). Figure 1 shows study selection and e-Table 1 details the reasons for exclusion of randomized trials.
Figure 1.
Flowsheet for study selection. CHD = congenital heart disease; FDA = Food and Drug Administration; PAH = pulmonary arterial hypertension; PH = pulmonary hypertension; RCT = randomized controlled trials; SCD = sickle cell disease.
Data Abstraction and Quality Assessment
Data were abstracted independently by two reviewers using a standardized data abstraction form, and discrepancies were resolved after mutual agreement and discussion with a third reviewer. The risk of bias for individual studies was assessed using the Cochrane Risk of Bias assessment tool.17
Outcomes Assessed
We defined five major efficacy outcomes and one safety outcome. The efficacy outcomes were selected to reflect two aspects of PAH therapy. First, improvements in patient morbidity and mortality were assessed by reduction in (1) study-defined clinical worsening, representing a composite of death, PAH-related hospitalization, lung transplantation, atrial septostomy, initiation of rescue therapy and deterioration of functional class or worsening of 6MWD, varying across studies (e-Table 2) (primary efficacy outcome); (2) PAH-related hospitalization; and (3) all-cause mortality. Second, improvement in functional status was assessed by two outcomes: (1) improvement by ≥ 1 functional class from baseline (New York Heart Association [NYHA] or WHO) and (2) change in 6MWD (from baseline). For 6MWD, the a priori minimal clinically important difference was an increase of ≥ 33 meters from baseline, associated with lower mortality and improved functional status.18 Tolerability was assessed by medication-related adverse events leading to drug discontinuation. For studies reporting outcomes at multiple time points, outcomes were preferentially assessed at 16 ± 4 weeks (e-Appendix 1, Methods).
Statistical Analysis
First, we performed direct meta-analysis for all treatment comparisons using the DerSimonian-Laird random-effects approach, incorporating within-study and between-study heterogeneity.19 We assessed statistical heterogeneity using the I2 statistic, with values > 50% indicating substantial heterogeneity.20 To assess for publication bias, we examined the network funnel plot for evidence of small study effects.21, 22 Second, we conducted network meta-analysis using a multivariate random-effects meta-regression.23, 24 Categorical outcomes were reported as risk ratio (RR), and continuous outcome (6MWD) was reported as weighted mean difference (WMD), with their corresponding 95% CIs. For categorical outcomes, an estimate for the absolute effect size was additionally obtained by multiplying the RR for each agent with the median placebo response rate for that outcome.25 Differences between direct and indirect evidence were assessed using tests of model consistency by including trial design as an additional covariate in the model.26 Third, we ranked drugs in order of their efficacy and tolerability using the surface under the cumulative ranking (SUCRA).27 Finally, to address between-study heterogeneity, we performed multiple sensitivity analyses that were restricted to trials (1) with a minimum follow-up duration ≥ 12 weeks, (2) published after the year 2000, and (3) with no or < 20% of study participants receiving background therapy. Further details are presented in e-Appendix 1, Methods.
Quality of Evidence
Using the GRADE framework, we rated the quality of evidence of estimates derived from network meta-analysis from high quality to very low quality (e-Table 3) for efficacy outcomes, ie, clinical worsening and improvement in functional class.16 For this, evidence was rated down for risk of bias, indirectness of evidence, heterogeneity, imprecision, and publication bias. Further details are presented in e-Appendix 1, Methods.
Results
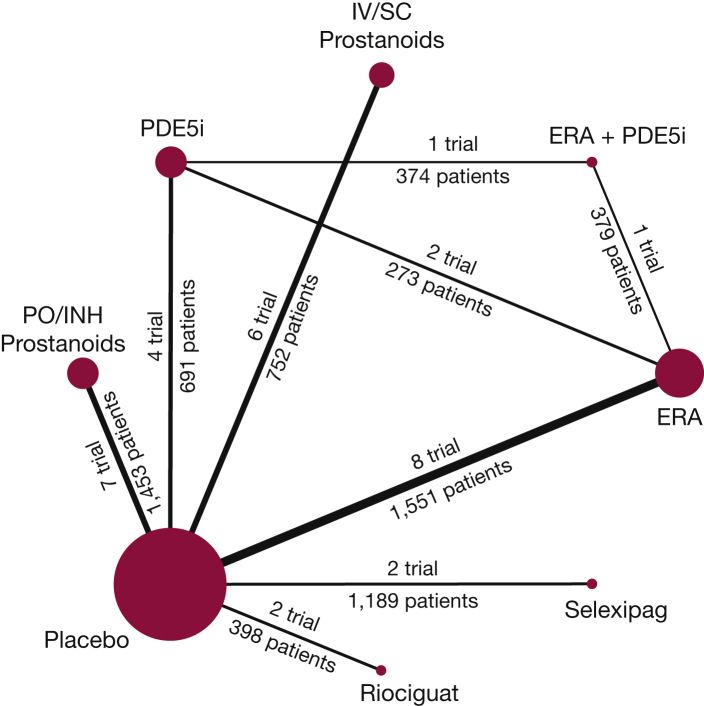
From 16,236 articles identified in the search, 31 RCTs were included in the network meta-analysis. These included 29 two-arm trials comparing active intervention to placebo,28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55 one two-arm trial comparing active agents against each other,56 and one three-arm trial comparing combination therapy of two active agents against each of the agents as monotherapy.57 Figure 1 shows study selection. Figure 2 demonstrates all available direct comparisons across outcomes (outcome-specific networks) (e-Fig 1, A-F).
Figure 2.
Network diagram of all available direct comparisons. e-Figures 1, A-F include network diagrams for each individual outcome. ERA = endothelin receptor antagonist; INH = inhaled; PDE5i = phosphodiesterase-5 inhibitor; PO = orally.
Characteristics and Quality of Included Studies
Overall, trials included 6,565 participants (range, 18-1,156 participants). Table 1 summarizes the trial characteristics. Median duration of outcome assessment for 6MWD and functional class, as well as for clinical worsening and mortality assessment, was 12 weeks (range, 8-26 weeks and 8-165 weeks, respectively). Table 2 summarizes baseline patient characteristics. The median age of subjects across trials was 51 years (range, 30-61 years), and a median 79% were women (range, 55%-100%). Twenty-six trials included PAH from different causes; among these, a median 65% had idiopathic PAH. Five RCTs studied idiopathic PAH exclusively,43, 45, 47, 50, 51 whereas one trial studied only connective tissue disease-associated PAH.49 Across studies, a majority of patients were in NYHA/WHO functional classes III (median, 70%; range, 33%-100%) and II (median, 24%; range, 0%-67%). Background therapy varied across trials (Table 2); however, such therapy was not reported to be significantly different between study arms within these trials.
Table 1.
Characteristics of Included Randomized Controlled Trials Comparing Pharmacologic Agents for Treatment of Pulmonary Arterial Hypertension
| First Author/Year (Trial Name) | Study Design | Study Location | Study Period | Timing of Outcome Assessment of Functional Status/Morbidity/Mortality | Intervention (n) | Comparator (n) | PAH- Specific Concomitant Therapy | Outcomes Assessed |
||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CW | Hosp | Mortality | FC | 6MWD | ||||||||
| ERA vs placebo | ||||||||||||
| McLaughlin28/2015 (COMPASS-2) | MC, DB, PC | USA, Europe, Brazil, Saudi Arabia | 2006-2012 | 16 wk/165 wk | Bosentan (n = 175) | Placebo (n = 159) | Sildenafil | ✓ | ✓ | ✓ | ✓ | ✓ |
| Galie29/2008 (EARLY) | MC, DB, PC | USA, Europe, Brazil | NR | 26 wk/26 wk | Bosentan (n = 93) | Placebo (n = 92) | Sildenafil | ✓ | ✓ | ✓ | ✓ | ✓ |
| Humbert30/2004 (BREATHE-2) | MC, DB, PC | USA, Europe | NR | 16 wk/16 wk | Bosentan (n = 22) | Placebo (n = 11) | Epoprostenol | – | – | ✓ | ✓ | – |
| Rubin31/2002 (BREATHE-1) | MC, DB, PC | USA, Mexico, Europe, Israel, Australia | NR | 16 wk/28 wk | Bosentan (n = 144) | Placebo (n = 69) | None | ✓ | ✓ | ✓ | ✓ | ✓ |
| Channick32/2001 | MC, DB, PC | USA, France | NR | 12 wk/12 wk | Bosentan (n = 21) | Placebo (n = 11) | None | ✓ | – | ✓ | ✓ | ✓ |
| Galie33/2008 (ARIES 1) | MC, DB, PC | USA, Australia South America, Europe, Mexico | 2003-2006 | 12 wk/12 wk | Ambrisentan (n = 67) | Placebo (n = 67) | None | ✓ | ✓ | ✓ | ✓ | ✓ |
| Galie33/2008 (ARIES 2) | MC, DB, PC | Europe, Israel, South America | 2003-2006 | 12 wk/12 wk | Ambrisentan (n = 63) | Placebo (n = 65) | None | ✓ | ✓ | ✓ | ✓ | ✓ |
| Pulido34/2013 (SERAPHIN) | MC, DB, PC | USA, Canada, Europe, Asia, South America, Australia | 2008-2012 | 26 wk/115 wk | Macitentan (n = 242) | Placebo (n = 250) | PDE5i PO or inhaled PCA |
✓ | ✓ | ✓ | ✓ | ✓ |
| PDE5i vs Placebo | ||||||||||||
| Galie35/2009 (PHIRST) | MC, DB, PC | USA, Canada, Europe, Japan | 2005-2007 | 16 wk/16 wk | Tadalafil (n = 79) | Placebo (n = 82) | Bosentan | ✓ | ✓ | ✓ | ✓ | ✓ |
| Galie36/2005 (SUPER) | MC, DB, PC | USA, Mexico, South America, Europe, Asia, South Africa, Australia | 2002-2003 | 12 wk/12 wk | Sildenafil (n = 69) | Placebo (n = 70) | None | ✓ | ✓ | ✓ | ✓ | ✓ |
| Zhuang37/2014 | Single center, DB, PC | China | 2011-2013 | 16 wk/16 wk | Tadalafil (n = 60) | Placebo (n = 64) | Ambrisentan | ✓ | ✓ | ✓ | ✓ | ✓ |
| Simonneau38/2008 (PACES) | MC, DB, PC | USA, Canada, Europe, Israel | 2003-2006 | 16 wk/16 wk | Sildenafil (n = 134) | Placebo (n = 133) | Epoprostenol | ✓ | ✓ | ✓ | – | ✓ |
| ERA vs PDE5i | ||||||||||||
| Galie57/2015 (AMBITION) | MC, DB, PC | USA, Canada, Europe, Japan, Australia | 2010-2014 | 24 wk/74 wk | Ambrisentan (n = 126) | Tadalafil (n = 121) | None | ✓ | ✓ | ✓ | ✓ | ✓ |
| Wilkins56/2005 (SERAPH) | Single center, DB, PC | UK | 2002-2003 | 16 wk/16 wk | Sildenafil (n = 14) | Bosentan (n = 12) | None | – | – | ✓ | – | ✓ |
| ERA + PDE5i vs PDE5i | ||||||||||||
| Galie57/2015 (AMBITION) | MC, DB, PC | USA, Canada, Europe, Japan, Australia | 2010-2014 | 24 wk/74 wk | Ambrisentan (n = 253) | Placebo (n = 121) | Tadalafil | ✓ | ✓ | ✓ | ✓ | ✓ |
| ERA + PDE5i vs ERA | ||||||||||||
| Galie57/2015 (AMBITION) | MC, DB, PC | USA, Canada, Europe, Japan, Australia | 2010-2014 | 24 wk/74 wk | Tadalafil (n = 253) | Placebo (n = 126) | Ambrisentan | ✓ | ✓ | ✓ | ✓ | ✓ |
| PO or INH PCA vs placebo | ||||||||||||
| Jing39/2013 (FREEDOM-M) | MC, DB, PC | USA, Canada, Europe, China, India, Mexico | 2006-2011 | 12 wk/12 wk | Treprostinil PO (n = 233) | Placebo (n = 116) | None | ✓ | ✓ | ✓ | – | – |
| Tapson40/2013 (FREEDOM-C2) | MC, DB, PC | USA, Europe, China, Australia | 2009-2011 | 16 wk/16 wk | Treprostinil PO (n = 157) | Placebo (n = 153) | ERA PDE5i |
✓ | ✓ | ✓ | – | – |
| Tapson41/2012 (FREEDOM-C) | MC, DB, PC | USA, China, Australia | 2005-2008 | 16 wk/16 wk | Treprostinil PO (n = 174) | Placebo (n = 176) | ERA PDE5i |
✓ | – | ✓ | ✓ | ✓ |
| McLaughlin42/2010 (TRIUMPH-1) | MC, DB, PC | USA, Europe | 2005-2007 | 12 wk/12 wk | Treprostinil INH (n = 115) | Placebo (n = 120) | ERA PDE5i |
✓ | ✓ | ✓ | – | ✓ |
| Olschewski43/2002 (AIR) | MC, DB, PC | USA, Europe | 1998-2001 | 12 wk/12 wk | Iloprost INH (n = 51) | Placebo (n = 51) | None | – | – | ✓ | ✓ | – |
| McLaughlin44/2006 (STEP) | MC, DB, PC | USA | 2004 | 12 wk/12 wk | Iloprost INH (n = 34) | Placebo (n = 33) | Bosentan | ✓ | ✓ | ✓ | ✓ | ✓ |
| Hoeper45/2006 (COMBI) | MC, OL, PC | Germany | 2004 | 12 wk/12 wk | Iloprost INH (n = 19) | Placebo (n = 21) | Bosentan | ✓ | ✓ | ✓ | – | ✓ |
| IV or SC PCA vs placebo | ||||||||||||
| Hiremath46/2010 (TRUST-1) | MC, DB, PC | India | 2005 | 12 wk/12 wk | Treprostinil IV (n = 30) | Placebo (n = 14) | None | – | – | ✓ | ✓ | ✓ |
| McLaughlin47/2003 | MC, DB, PC | USA | NR | 8 wk/8 wk | Treprostinil SC (n = 17) | Placebo (n = 9) | None | – | – | ✓ | – | ✓ |
| Simonneau48/2002 | MC, DB, PC | USA, Canada, Mexico, Europe, Israel, Australia | 1998-1999 | 12 wk/12 wk | Treprostinil SC (n = 233) | Placebo (n = 236) | None | – | – | ✓ | – | ✓ |
| Badesch49/2000 | MC, OL | USA, Canada | NR | 12 wk/12 wk | Epoprostenol IV (n = 56) | Conventional therapy (n = 55) | None | – | – | ✓ | ✓ | ✓ |
| Barst50/1996 | MC, OL | USA | NR | 12 wk/12 wk | Epoprostenol IV (n = 41) | Conventional therapy (n = 40) | None | – | – | ✓ | ✓ | ✓ |
| Rubin51/1990 | MC, OL | USA | NR | 8 wk/8 wk | Epoprostenol IV (n = 11) | Conventional therapy (n = 12) | None | – | – | ✓ | ✓ | ✓ |
| Riociguat vs placebo | ||||||||||||
| Ghofrani52/2013 (PATENT-1) | MC, DB, PC | USA, Canada, Mexico, Asia Europe, South America, Australia | 2008-2012 | 12 wk/12 wk | Riociguat (n = 254) | Placebo (n = 126) | ERA PO, INH, or SC PCA |
✓ | ✓ | ✓ | ✓ | ✓ |
| Galie53/2015 (PATENT-PLUS) | MC, DB, PC | Europe | 2010-2012 | 12 wk/12 wk | Riociguat (n = 12) | Placebo (n = 6) | Sildenafil | – | – | ✓ | ✓ | ✓ |
| Selexipag vs placebo | ||||||||||||
| Sitbon54/2015 (GRIPHON) | MC, DB, PC | North America, Europe, Asia, Latin America, Australia | 2009-2013 | 26 wk/70 wk | Selexipag (n = 574) | Placebo (n = 582) | ERA, PDE5i, ERA + PDE5i | ✓ | ✓ | ✓ | ✓ | ✓ |
| Simonneau55/2012 | MC, DB, PC | Europe | 2008-2009 | 17 wk/17 wk | Selexipag (n = 33) | Placebo (n = 10) | ERA, PDE5i, ERA + PDE5i | – | – | ✓ | ✓ | ✓ |
6MWD = 6-min walk distance; AIR = Aerosolized Iloprost Randomized; AMBITION = Ambrisentan and Tadalafil in Patients with Pulmonary Arterial Hypertension; ARIES-1 and ARIES-2 = Ambrisentan in PAH - A Phase III, Randomized, Double-blind, Placebo-controlled, Multicenter, Efficacy Study of Ambrisentan in Subjects With Pulmonary Arterial Hypertension; BREATHE-1 = Bosentan Randomized trial of Endothelin Antagonist Therapy for PAH -1; BREATHE-2 = Bosentan Randomized trial of Endothelin Antagonist Therapy for PAH - 2; COMBI = Combination Therapy of Bosentan and aerosolised Iloprost in Idiopathic Pulmonary Arterial Hypertension; COMPASS-2 = Effects of the Combination of Bosentan and Sildenafil vs Sildenafil Monotherapy on Pulmonary Arterial Hypertension; CW = clinical worsening; DB = double blind; EARLY = Efficacy and Safety of Oral Bosentan in Pulmonary Arterial Hypertension Class II; ERA = endothelin receptor antagonist; FC = functional class (World Health Organization or New York Heart Association); FREEDOM-C = Oral Treprostinil in Combination With an ERA and/or a PDE-5I for the Treatment of PAH; FREEDOM-C2 = Oral Treprostinil for the Treatment of Pulmonary Arterial Hypertension in Patients Receiving Background Endothelin Receptor Antagonist and Phosphodiesterase Type 5 Inhibitor Therapy; FREEDOM-M = Oral Treprostinil as Monotherapy for the Treatment of PAH; GRIPHON = Prostacyclin (PGI2) Receptor Agonist In Pulmonary Arterial Hypertension; Hosp = PAH-related hospitalization; INH = inhaled; MC = multicenter; NR = not reported; OL = open label; PACES = Pulmonary Arterial Hypertension Combination Study of Epoprostenol and Sildenafil; PATENT-1 = Pulmonary Arterial Hypertension Soluble Guanylate Cyclase Stimulator Trial 1; PATENT-PLUS = Evaluation of the Pharmacodynamic Effect of the Combination of Sildenafil and Riociguat on Blood Pressure and Other Safety Parameters; PC = placebo controlled; PCA = prostacyclin; PDE5i = phosphodiesterase-5 inhibitor; PHIRST = Pulmonary Arterial Hypertension and Response to Tadalafil; PO = orally; SB = single blind; SC = subcutaneous; SERAPH = Sildenafil vs Endothelin Receptor Antagonist for Pulmonary Hypertension; SERAPHIN = Study with an Endothelin Receptor Antagonist in Pulmonary Arterial Hypertension to Improve Clinical Outcome; STEP = Safety and pilot efficacy Trial in combination with bosentan for Evaluation in Pulmonary arterial hypertension; SUPER = Sildenafil Use in Pulmonary Arterial Hypertension; TRIUMPH-1 = TReprostinil sodium Inhalation Used in the Management of Pulmonary Arterial Hypertension; TRIUMPH-1 = TReprostinil sodium Inhalation Used in the Management of Pulmonary Arterial Hypertension; TRUST-1 = Treprostinil for Untreated Symptomatic PAH Trial; ✓= yes; – = no.
Table 2.
Patient Characteristics of Subjects Included in the Selected Randomized Controlled Trials
| Study/Year | Age, y, mean (SD) |
Sex, % women |
Cause of PAH, % subjects |
Baseline 6MWD, m |
NYHA/WHO Functional Class |
Background PAH Therapy | % Receiving Background Therapy |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Int | Ctrl | Int | Ctrl | Int |
Ctrl |
Int | Ctrl | Int |
Ctrl |
Int | Ctrl | ||||||||||
| IPAH | APAH | IPAH | APAH | I | II | III | IV | I | II | III | IV | ||||||||||
| McLaughlin28/2015 (COMPASS-2) | 52.9 (15.4) | 54.7 (15.7) | 78.6 | 73.1 | 62.3 | 27.0 | 65.1 | 25.7 | 363.1 (78.5) | 357.6 (73.1) | 0 | 0 | 44.7 | 55.3 | 0 | 39.4 | 59.4 | 1.1 | Sildenafil | 100 | 100 |
| Galie29/2008 (EARLY) | 45.2 (17.9) | 44.2 (16.5) | 76 | 63 | 58 | 41 | 63 | 35 | 438 (86) | 431 (91) | – | – | – | – | – | – | – | – | Sildenafil | 15 | 16 |
| Humbert30/2004 (BREATHE-2) | 45 (17) | 47 (19) | 77 | 55 | 77 | 9 | 91 | 23 | NR | NR | 0 | 0 | 77 | 23 | 0 | 0 | 73 | 27 | Epoprostenol | 100 | 100 |
| Rubin31/2002 (BREATHE-1) | 48.7 (15.8) | 47.2 (16.2) | 79 | 78 | 71 | 29 | 70 | 30 | 330 (74) | 344 (76) | 0 | 0 | 90 | 10 | 0 | 0 | 94 | 6 | None | NA | NA |
| Channick32/2001 | 52.2 (12.2) | 47.4 (14.0) | 81 | 100 | 81 | 19 | 91 | 9 | 360 (86) | 355 (82) | 0 | 0 | 100 | 0 | 0 | 0 | 100 | 0 | None | NA | NA |
| Galie57/2015 (AMBITION) | 54.5 (14.3) | 54.5 (15.2) | 74 | 83 | 53 | 47 | 58 | 42 | 353.5 (87.9) | 349.2 (91.6) | 0 | 30 | 70 | 0 | 0 | 34 | 66 | 0 | Tadalafil | 100 | 100 |
| Galie33/2008 (ARIES 1) | 53 (14) | 48 (16) | 84 | 88 | 63 | 36 | 64 | 36 | 340 (77) | 342 (73) | 2 | 30 | 60 | 9 | 3 | 34 | 61 | 2 | None | NA | NA |
| Galie33/2008 (ARIES 2) | 50 (16) | 51 (14) | 81 | 68 | 65 | 35 | 65 | 36 | 355 (84) | 343 (86) | 2 | 44 | 52 | 2 | 3 | 37 | 57 | 3 | None | NA | NA |
| Pulido34/2013 (SERAPHIN) | 45.5 (15) | 46.7 (17) | 80 | 74 | 55.6 | 43.6 | 51.0 | 47.7 | 363 (93.2) | 352 (110.6) | 0 | 50 | 48 | 2 | 0 | 52 | 47 | 1 | PDE5i Oral or inhaled prostanoids |
62.0 6.2 |
60.2 2.8 |
| Galie57/2015 (AMBITION) | 54.5 (14.3) | 53.9 (14.7) | 74 | 79 | 53 | 47 | 59 | 41 | 353.5 (87.9) | 354.2 (92.3) | 0 | 30 | 70 | 0 | 0 | 30 | 70 | 0 | Ambrisentan | 100 | 100 |
| Zhuang37/2014 | 52 (12) | 51 (14) | 76.7 | 81.3 | 68.3 | 31.7 | 57.8 | 42.1 | 356 (87) | 343 (71) | 0 | 60 | 35 | 5 | 0 | 57.7 | 42.2 | 3.1 | Ambrisentan | 100 | 100 |
| Galie5/2009 (PHIRST) | 53 (15) | 55 (15) | 75 | 79 | 58 | 42 | 66 | 34 | 352 (78) | 343 (84) | 3 | 33 | 65 | 0 | 1 | 28 | 68 | 2 | Bosentan | 53 | 55 |
| Simonneau38/2008 (PACES) | 47.8 (12.9) | 47.5 (13.2) | 82 | 77 | 80 | 20 | 79 | 21 | 348.9 (71.4) | 341.6 (77.3) | 0.7 | 25.4 | 65.7 | 7.5 | 1.5 | 25.6 | 65.4 | 4.5 | Epoprostenol | 100 | 100 |
| Galie36/2005 (SUPER) | 47 (14) | 49 (17) | 71 | 81 | 64 | 36 | 60 | 40 | 347 (90) | 344 (79) | 0 | 35 | 58 | 7 | 1 | 46 | 49 | 4 | None | NA | NA |
| Galie57/2015 (AMBITION) | 53.9 (14.7) | 54.5 (15.2) | 79 | 83 | 58 | 42 | 59 | 41 | 354.2 (92.3) | 349.2 (91.6) | 0 | 30 | 70 | 0 | 0 | 34 | 66 | 0 | None | NA | NA |
| Wilkins56/2005 (SERAPH) | 44.4 (8.5) | 41.1 (7) | 83.3 | 78.6 | 91.7 | 8.3 | 85.7 | 14.3 | 304.6 (74.1) | 290 (88.5) | – | – | – | – | – | – | – | – | None | NA | NA |
| Jing39/2013 (FREEDOM-M) | 40.6 (15.2) | 42.5 (12.5) | 74 | 78 | 73 | 26 | 76 | 24 | 332.3 (71.6) | 325.2 (77.1) | – | – | – | – | – | – | – | – | None | NA | NA |
| Tapson40/2013 (FREEDOM-C2) | 51.5 (14.5) | 50.4 (13.7) | 76 | 80 | 66 | 34 | 65 | 35 | 329.4 (69.2) | 336.8 (63.5) | 0 | 27 | 71 | 2 | 0 | 24 | 76 | 0 | PDE5i + ERA PDE5i ERA |
41 43 16 |
39 42 18 |
| Tapson41/2012 (FREEDOM-C) | 51 (14.5) | 50 (14) | 85 | 80 | 65 | 35 | 68 | 32 | 346.1 (71.4) | 345.4 (75.5) | 1 | 24 | 73 | 2 | 1 | 18 | 79 | 3 | PDE5i + ERA PDE5i ERA |
43 26 32 |
42 24 29 |
| Hiremath46/2010 (TRUST-1) | 30 (12.5) | 36 (11.2) | 63 | 57 | 97 | 3 | 93 | 7 | 259.2 (11.9) | 231.4 (19.7) | 0 | 0 | 97 | 3 | 0 | 0 | 93 | 7 | None | NA | NA |
| McLaughlin42/2010 (TRIUMPH-1) | 55 (13.7) | 52 (14.2) | 80.9 | 81.7 | 56 | 35 | 56 | 31 | 346 (63) | 351 (69) | 0 | 0 | 97 | 3 | 0 | 0 | 98 | 2 | Bosentan Sildenafil |
67 33 |
73 27 |
| McLaughlin47/2003a | 37 (17) | 37 (17) | 81 | 81 | 100 | 0 | 100 | 0 | NR | NR | 0 | 0 | 96 | 4 | 0 | 0 | 96 | 4 | None | NA | NA |
| Simonneau48/2002 | 44.6 (1.0) | 44.4 (0.9) | 85 | 78 | 58 | 42 | 58 | 42 | 326 (5) | 327 (6) | 0 | 11 | 82 | 8 | 0 | 12 | 81 | 7 | None | NA | NA |
| McLaughlin44/2006 (STEP) | 51 (14) | 49 (15) | 79 | 79 | 50 | 50 | 61 | 39 | 331 (64) | 340 (73) | 0 | 0 | 97 | 3 | 0 | 3 | 91 | 6 | Bosentan | 100 | 100 |
| Hoeper45/2006 (COMBI) | 48 (14) | 56 (13) | 76.2 | 78.9 | 100 | 0 | 100 | 0 | 317 (74) | 296 (79) | 0 | 0 | 100 | 0 | 0 | 0 | 100 | 0 | Bosentan | 100 | 100 |
| Olschewski43/2002b | 51.2 (13.2) | 52.8 (12) | 68.3 | 66.7 | 100 | 0 | 100 | 0 | 332 (93) | 315 (96) | 0 | 0 | 59 | 41 | 0 | 0 | 58 | 42 | None | NA | NA |
| Badesch49/2000 | 53.0 (13.1) | 57.3 (10.3) | 91 | 82 | 0 | 100 | 0 | 100 | 271.5 | 240 | 0 | 2 | 75 | 23 | 0 | 7 | 82 | 11 | None | NA | NA |
| Barst50/1996 | 40 (3) | 40 (2) | 76 | 70 | 100 | 0 | 100 | 0 | 316 (18) | 272 (23) | 0 | 0 | 76 | 24 | 0 | 0 | 73 | 28 | None | NA | NA |
| Rubin51/1990 | 37.4 (12.6) | 35 (15.5) | 63.6 | 75 | 100 | 0 | 100 | 0 | 246 | 205 | – | – | – | – | – | – | – | – | None | NA | NA |
| Galie53/2015 (PATENT PLUS) | 58 (11) | 61 (10) | 67 | 67 | 42 | 58 | 67 | 33 | NR | NR | 8 | 50 | 33 | 8 | 0 | 67 | 33 | 0 | Sildenafil | 100 | 100 |
| Ghofrani52/2013 (PATENT 1) | 51 (17) | 51 (17) | 80 | 78 | 59 | 41 | 67 | 33 | 361 (68) | 368 (75) | 2 | 43 | 55 | 0 | 3 | 48 | 46 | 2 | ERA oral, SC, or inhaled PCAs |
44 5 |
43 7 |
| Sitbon54/2015 (GRIPHON) | 48.2 (15.2) | 47.9 (15.6) | 79.6 | 80.1 | 54.4 | 29.1 | 57.9 | 28.7 | 358.5 (76.3) | 348.0 (83.2) | 0.7 | 47.7 | 51.0 | 0.5 | 0.9 | 43.8 | 54.0 | 1.4 | ERA + PDE5i PDE5i ERA |
31.2 32.9 16.4 |
33.8 31.8 13.1 |
| Simonneau55/2012 | 54.8 (16.8) | 53.8 (16.3) | 81.8 | 80 | 72.7 | 12.1 | 70 | 20 | 396.2 (71.4) | 350.3 (123.5) | 0 | 45.5 | 54.5 | 0 | 0 | 20 | 80 | 0 | ERA + PDE5i PDE5i ERA |
36.4 27.2 36.4 |
30.0 30.0 40.0 |
APAH = associated pulmonary arterial hypertension; Ctrl = control; Int = intervention; IPAH = idiopathic pulmonary arterial hypertension; NA = not available; PAH = pulmonary arterial hypertension; – = data not reported for these variables. See Table 1 legend for expansion of other abbreviations.
Data reported for intervention and control groups combined.
Data reported for all groups of pulmonary hypertension combined. Outcomes data are used only for group 1 pulmonary arterial hypertension.
e-Figures 2A and 2B present overall and study-level quality of trials. Most studies reported adequate randomization and allocation concealment. Six RCTs reported inadequate blinding of participants and personnel or outcome assessment. Overall, most studies had a low to moderate risk of bias.
Efficacy Outcomes: Clinical Worsening, Hospitalization, and Mortality
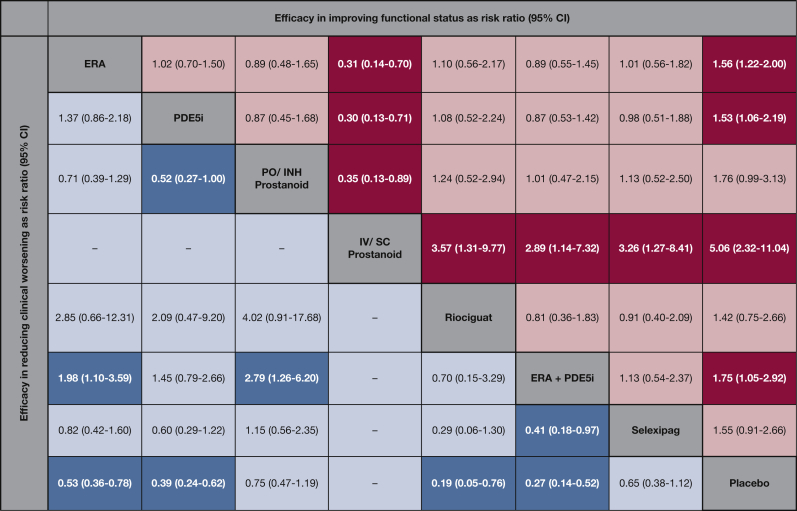
The primary outcome, clinical worsening, was available in 20 RCTs with 22 direct comparisons (e-Fig 3). Direct meta-analysis for these outcomes is presented in e-Figures 3-5 and in e-Appendix 1, Results. In network meta-analysis, when compared with a median 14.5% in the placebo group, ERA was associated with clinical worsening in an estimated 7.7% of patients (RR, 0.53; 95% CI, 0.36-0.78), PDE5i in 5.7% (RR, 0.39; 95% CI, 0.24-0.62), the combination of ERA + PDE5i in 3.9% (RR, 0.27; 95% CI, 0.14-0.52), and riociguat in 2.8% (RR, 0.19; 95% CI, 0.05-0.76) (Fig 3). Here, riociguat and ERA + PDE5i were ranked highest (SUCRA, 0.89 and 0.86, respectively), followed by PDE5i (SUCRA, 0.68) and ERA (SUCRA, 0.46) in reducing clinical worsening (e-Table 4). For the hospitalization outcome, only the ERA + PDE5i combination was associated with improvement compared with placebo (RR, 0.19; 95% CI, 0.06-0.64). For all-cause mortality, events rates were low across trials, and between-group differences were not significant (Table 3).
Figure 3.
Comparative efficacy of pharmacologic agents in improving functional class by at least one class and reducing the risk of clinical worsening. Comparisons should be read from left (active agent) to right (comparator agent or placebo). Columns in pink/red represent improvement in functional class, with risk ratio > 1 consistent with higher improvement. Columns in blue represent efficacy in reducing clinical worsening, with risk ratio < 1 favoring active agent. Bold numbers with darker backgrounds are statistically significant. Numbers in parentheses indicate 95% CIs. See Figure 2 legend for expansion of abbreviations.
Table 3.
Pooled Risk Ratio for Discrete Outcomes and Weighted Mean Difference for Continuous Outcomes Based on Combined Direct and Indirect Evidence from Network Meta-Analysis
| Pharmacologic Intervention | Mortality RR (95% CI) |
6MWD WMD (95% CI) |
PAH-Related Hospitalization RR (95% CI) |
Adverse Events Leading to Discontinuation RR (95% CI) |
|---|---|---|---|---|
| Compared against placebo | ||||
| ERA | 0.70 (0.45-1.07) | 33.19 (21.23-45.14) | 0.72 (0.44-1.18) | 1.05 (0.70-1.58) |
| PDE5i | 0.68 (0.24-1.95) | 26.80 (13.28-40.32) | 0.52 (0.27-1.03) | 0.77 (0.44-1.35) |
| PO/INH PCA | 0.88 (0.45-1.73) | 13.95 (–1.24-29.13) | 0.88 (0.40-1.94) | 2.92 (1.68-5.06) |
| IV/SC PCA | 0.55 (0.30-1.03) | 35.80 (19.11-52.49) | 0.49 (0.04-5.61) | 2.66 (0.86-8.23) |
| Riociguat | 0.36 (0.07-1.81) | 24.28 (–1.78-50.34) | 0.12 (0.01-1.20) | 0.49 (0.18-1.37) |
| ERA + PDE5i | 0.65 (0.19-2.25) | 54.14 (29.78-78.49) | 0.20 (0.07-0.55) | 0.97 (0.44-2.13) |
| Selexipag | 1.52 (0.86-2.70) | 16.32 (–5.46-38.10) | 0.73 (0.37-1.43) | 2.01 (1.04-3.88) |
| Compared against ERA | ||||
| PDE5i | 0.98 (0.35-2.79) | –6.39 (–22.04-9.26) | 0.73 (0.37-1.43) | 0.73 (0.40-1.33) |
| PO/INH PCA | 1.27 (0.57-2.83) | –19.24 (–38.61-0.13) | 1.23 (0.49-3.07) | 2.78 (1.41-5.50) |
| IV/SC PCA | 0.80 (0.37-1.70) | 2.61 (–17.25-22.48) | 0.68 (0.06-8.18) | 2.53 (0.76-8.41) |
| Riociguat | 0.52 (0.10-2.75) | -8.91 (-38.07-20.26) | 0.17 (0.02-1.76) | 0.47 (0.16-1.41) |
| ERA + PDE5i | 0.93 (0.28-3.17) | 20.95 (–2.58-44.47) | 0.28 (0.11-0.72) | 0.93 (0.44-1.94) |
| Selexipag | 2.19 (1.07-4.49) | –16.87 (–41.58-7.84) | 1.01 (0.43-2.34) | 1.91 (0.88-4.15) |
| Compared against PDE5i | ||||
| PO/INH PCA | 1.29 (0.37-4.49) | –12.85 (–33.19-7.49) | 1.68 (0.60-4.71) | 3.79 (1.72-8.34) |
| IV/SC PCA | 0.81 (0.24-2.74) | 9.01 (–12.41-30.42) | 0.93 (0.07-11.71) | 3.45 (0.97-12.22) |
| Riociguat | 0.53 (0.08-3.61) | –2.51 (–31.93-26.90) | 0.24 (0.02-2.52) | 0.64 (0.20-2.01) |
| ERA + PDE5i | 0.95 (0.36-2.50) | 27.34 (3.84-50.84) | 0.39 (0.15-1.01) | 1.26 (0.60-2.65) |
| Selexipag | 2.23 (0.68-7.37) | –10.48 (–36.10-15.14) | 1.38 (0.53-3.60) | 2.61 (1.10-6.21) |
| Compared against PO/INH PCA | ||||
| IV/SC PCA | 0.63 (0.25-1.58) | 21.86 (–0.79-44.50) | 0.55 (0.04-7.18) | 0.91 (0.26-3.20) |
| Riociguat | 0.41 (0.07-2.35) | 10.34 (–19.76-40.43) | 0.14 (0.01-1.55) | 0.17 (0.05-0.54) |
| ERA + PDE5i | 0.74 (0.18-3.04) | 40.19 (11.47-68.91) | 0.23 (0.06-0.82) | 0.33 (0.13-0.87) |
| Selexipag | 1.73 (0.71-4.20) | 2.37 (–24.19-28.94) | 0.82 (0.29-2.32) | 0.69 (0.29-1.62) |
| Compared against IV/SC PCA | ||||
| Riociguat | 0.65 (0.12-3.66) | –11.52 (–43.40-20.36) | 0.25 (0.01-7.13) | 0.19 (0.04-0.85) |
| ERA + PDE5i | 1.17 (0.29-4.71) | 18.33 (–10.94-47.61) | 0.42 (0.03-5.84) | 0.37 (0.09-1.45) |
| Selexipag | 2.75 (1.18-6.40) | –19.48 (–46.67-7.71) | 1.49 (0.12-18.76) | 0.76 (0.20-2.80) |
| Compared against | ||||
| ERA + PDE5i riociguat | 1.80 (0.24-13.77) | 29.85 (–6.04-65.75) | 1.64 (0.14-19.59) | 1.97 (0.55-7.05) |
| Selexipag | 4.22 (0.76-23.33) | –7.96 (–42.14-26.22) | 5.85 (0.55-62.49) | 4.07 (1.21-13.75) |
| Compared against ERA + PDE5i | ||||
| Selexipag | 2.35 (0.60-9.23) | –37.82 (–70.43-–5.20) | 3.56 (1.06-11.95) | 2.07 (0.74-5.75) |
The column treatment is compared with the row treatment (ie, row treatment is reference for each comparison). Numbers in bold represent statistically significant results. RR = risk ratio, WMD = weighted mean difference. See Table 1 legend for expansion of other abbreviations.
Efficacy Outcomes: Functional Status
Improvement in NYHA/WHO functional class was reported in 23 RCTs with 25 comparisons (e-Fig 6). Direct meta-analysis is presented in e-Figures 6 and 7 and in e-Appendix 1, Results. In network meta-analysis, when compared with a median 16.2% for placebo, improvement in functional class was estimated in 25.2% of patients with ERA (RR, 1.56; 95% CI, 1.22-2.00), 24.8% of patients with PDE5i (RR, 1.53; 95% CI, 1.06-2.19), 82.8% of patients with IV/SC prostanoids (RR, 5.06; 95% CI, 2.32-11.04), and 28.3% of patients with ERA + PDE5i (RR, 1.75; 95% CI, 1.05-2.92) (Fig 3). Here IV/SC prostanoids were associated with the highest rank for improvement in functional class over all other active agents (SUCRA, 0.99). For improving 6MWD, when compared with placebo, the combination of ERA+ PDE5i was ranked highest (SUCRA, 0.96; WMD, 54.1 m; 95% CI, 29.8-8.5), followed by IV/SC prostanoids (SUCRA, 0.74; WMD, 35.8 m; 95% CI, 19.1-52.5 m), ERA (SUCRA, 0.69; WMD, 33.2 m; 95% CI, 21.2-45.1 m), and PDE5i (SUCRA, 0.53; WMD, 26.8 m; 95% CI, 13.3-40.3) (Table 3), but no agent consistently achieved the a priori defined minimal clinically important difference of 33m (all CIs crossed this value).
Adverse Events Leading to Discontinuation
Low adverse-event-related medication discontinuation was examined as a marker for higher tolerability. Twenty-six RCTs reported data on this outcome. Direct meta-analysis is presented in e-Figure 8 and e-Appendix 1, Results. In network meta-analysis for this outcome, the highest SUCRA ranking was achieved by riociguat (0.92), followed by PDE5i (0.80), ERA + PDE5i (0.62), and ERA (0.56) (higher SUCRA corresponds to lower adverse events) (e-Table 4). Risk of discontinuation for PO/INH prostanoids was significantly higher compared with placebo (RR, 2.92; 95% CI, 1.68-5.06) but was also higher than ERA (RR, 2.78; 95% CI, 1.41-5.50), PDE5i (RR, 3.79; 95% CI, 1.72-8.34), riociguat (RR, 5.92; 95% CI, 1.85-18.94), and ERA + PDE5i (RR, 3.00; 95% CI, 1.16-7.81) (Table 3).
The results of our sensitivity analyses restricted to trials with follow-up ≥ 12 weeks, recent publication (after 2000), or those with < 20% patients receiving background therapy did not differ substantially from our primary analysis (e-Table 5). There was no evidence for small study effects based on funnel plot asymmetry, suggesting absence of publication bias (e-Fig 9). There was no evidence of network inconsistency (P > .05 for all comparisons).
Quality of Evidence
The GRADE quality of evidence for the primary efficacy outcomes of clinical worsening and improvement in functional class is summarized in e-Table 6. Placebo comparisons were rated down for indirectness due to differences in study population (background therapy and PAH subtypes) as well as the definition of outcomes (for clinical worsening). Head-to-head comparisons were further downgraded for indirectness and imprecision due to limited head-to-head trials and wide CIs, respectively. Moderate-quality evidence supported the use of ERA, PDE5i, their combination, riociguat, and selexipag for reducing clinical worsening in PAH. The combination of ERA + PDE5i was supported by high-quality and moderate-quality evidence in the comparison against monotherapy with ERA and PDE5i, respectively. Other head-to-head comparisons were supported by low- to very low-quality evidence. For the functional class outcome, moderate-quality evidence supported ERA, ERA + PDE5i, IV/SC prostanoids, and selexipag in improving functional class over placebo, whereas low-quality evidence supported the use of PDE5i and riociguat. In head-to-head comparisons, most agents were supported by only low-quality evidence.
Discussion
In this network meta-analysis combining evidence from 31 RCTs including 6,565 patients with PAH and using the GRADE framework to appraise the quality of evidence, we made several important observations. First, treatment with riociguat, ERA, PDE5i, and the combination of ERA and PDE5i compared with placebo was associated with significant reduction in risk of clinical worsening, supported by a low to moderate quality of evidence. Although riociguat had the strongest effect, the point estimate was based on a single study. Second, parenteral (IV/SC) prostanoids, ERA, PDE5i, and the ERA + PDE5i combination were associated with significant improvement in WHO/NYHA functional class compared with placebo, with most placebo comparisons supported by moderate-quality evidence. The same agents led to significant improvements in 6MWD, another marker of exercise capacity with prognostic implications.58 Third, only the combination of ERA + PDE5i was associated with a lower likelihood of PAH-related hospitalization, but the data were derived from a single trial. Fourth, none of the studied agents was associated with reduced mortality. Finally, nonparenteral (PO/INH) prostanoids and selexipag were more likely to be discontinued secondary to adverse events.
There is limited evidence to guide choice of therapy between different agents, with only two head-to-head trials comparing different drug classes against each other.56, 57 The current clinical guidelines derive data from individual drug studies and single-agent meta-analyses.58, 59 However, the direct meta-analyses in the published literature synthesized evidence informing about either the pooled effect of drug therapy on relevant outcomes4, 7 or the effect of combination vs monotherapy as a whole without addressing the role of individual agents.60, 61 They do not provide composite evidence incorporating all available data to assess the comparative effectiveness of different drug therapies in improving specific outcomes. By synthesizing evidence from existing trials to analyze both direct and indirect treatment comparisons, our network meta-analysis provides comprehensive evidence in which therapy-specific relative risks are comparable across classes.
Clinical worsening, defined differently across trials to include a combination of hospitalization, mortality, and a need for invasive therapy, has increasingly been reported as a primary outcome in recent randomized trials. In our study, we found that ERA, PDE5i, their combination, and riociguat are associated with reduced clinical worsening. Although riociguat was associated with the highest probability of reducing clinical worsening, this effect was supported by low-quality evidence. The combination of ERA + PDE5i had the second highest probability in improving this outcome. PAH-related hospitalization, a component of clinical worsening, was also studied as a stand-alone outcome, and these two outcomes may therefore be correlated. Although the clinical worsening outcome for most individual studies followed the direction of the hospitalization outcome (e-Figs 3, 4), it cannot be confirmed if reported rates of clinical worsening were driven by hospitalization events given the limited reporting of other individual components of clinical worsening. The impact of parenteral prostanoids on clinical worsening remains to be studied, since none of the trials evaluated this outcome.
PAH-related hospitalization and mortality were not significant in most placebo-drug and drug-drug comparisons, other than a reduction in hospitalizations with the combination of ERA + PDE5i. For the mortality outcome, although prior studies have suggested a reduction in mortality with overall pharmacologic therapy7 and with some prostacyclin analogues,4 mortality events across trials were low, and we did not find a significant difference for these comparisons. The included studies were likely underpowered for assessing mortality, particularly given their short duration of follow-up and the use of time-to-first-event of clinical worsening as a primary end point in recent trials that potentially led to censoring of patients earlier in the course of disease. Long-term follow-up in registries may allow further future analyses of this important end point.
Since current guidelines emphasize initiation of therapy with worsening functional capacity (defined by functional class II or worse), measures of improving exercise capacity were commonly reported. For these outcomes, we found that parenteral prostanoids were associated with the highest probability of achieving improvement in functional class compared with placebo, as well as with individual agents, including ERA, PDE5i, riociguat, selexipag, and the combination of ERA + PDE5i. Compared with placebo, ERA, PDE5i, and their combination improved functional class significantly, supported by moderate-quality evidence. The combination of ERA + PDE5i was also associated with the highest probability of improving 6MWD, an outcome that has been suggested to be associated with patient prognosis. Individual components of this combination, as well as parenteral prostanoids, were associated with significant improvement in 6MWD; however, the previously suggested minimal clinically significant improvement for 6MWD was not consistently achieved for this outcome. On the contrary, nonparenteral prostanoids did not improve functional status or measures of morbidity. Furthermore, medication safety and tolerability, as assessed by adverse events leading to discontinuation, were least favorable for nonparenteral prostanoids, consistent with the results of a recent meta-analysis.60 Hence, this provides evidence against their role as primary therapy in the management of PAH. Their suggested role as “ancillary therapy” in clinical guidelines would need to be further investigated. Next, although riociguat was associated with improved rates of clinical worsening, it did not have a significant effect on either functional class or 6MWD and may not be sufficient therapy in patients with decompensated disease with poor functional status.
Our findings must be interpreted in light of the following limitations. First, most results in our analysis are supported by low- to moderate-quality evidence, which, by definition, suggests that future studies are likely to affect our confidence in these estimates (e-Table 3). Differences in participant characteristics, cointerventions, and outcome assessment downgraded the quality of evidence. Although such conceptual heterogeneity is a limitation inherent to any meta-analysis, in PAH, variability in the characteristics of enrolled patients and definitions for important outcomes (clinical worsening) makes interpretation of evidence challenging for clinicians and investigators. Moreover, temporal evolution of PAH trials over time further adds to this between-study variation. In our study, we attempted to minimize heterogeneity by establishing strict inclusion criteria for the trials and assessing outcomes at prespecified time points. We also performed sensitivity analyses in more homogeneous subgroups, specifically including only contemporary trials and those with limited or no background PAH therapy. Although these sensitivity analyses were consistent with our primary findings, uniform criteria for inclusion and outcome assessment for future trials are necessary to generate high-quality evidence. Second, because of the limited number of head-to-head trials, comparisons between active agents were mainly derived from lower-quality indirect evidence and should therefore be interpreted with caution. Future comparative efficacy trials are, therefore, warranted to make definitive conclusions. Third, similar to previous PAH meta-analyses,4, 7, 60, 61 our analysis is somewhat limited by the small number of RCTs available for each drug; we had to combine the effect of multiple agents in a drug class. It is possible that the efficacy of agents is drug specific rather than class specific. Next, the readership should be careful about “ecologic fallacy,” since conclusions derived from group-level data may not be applicable to individual patients. Fifth, multiple ORs produced for the various pairwise comparisons in network meta-analysis may be considered multiple testing and therefore may be at risk for type 1 error and represent chance findings. There are no established techniques to address this; however, the consistency among significant findings would suggest that such effects may be limited. Finally, since most contemporary trials were conducted with patients receiving one or more agents as background therapy, it was not possible to perform indirect comparisons among all direct classes with agents only as monotherapy.
Conclusions
Among oral agents, ERA, PDE5i, and their combination are associated with improvement in patient morbidity (both clinical worsening and hospitalization) and functional status. Other approved agents are associated with improvement in different measures of efficacy, and selection of an agent may be guided by the most desired outcome for each particular patient. Our findings are limited by few head-to-head trials and differences in reporting across trials. We therefore emphasize the need for future studies focusing on head-to-head comparisons with uniform enrollment and outcome assessment to improve comparability and produce higher-quality evidence that informs clinical decision-making.
Acknowledgments
Author contributions: S. J., R. K., and A. G. had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis, including and especially any adverse effects. They also assume full responsibility for the integrity of the submission as a whole, from inception to published article. S. J., R. K., S. S., and A. G. were responsible for study concept and design. S. J., R. K., A. B., and A. G. were responsible for acquisition of data. S. J., R. K., S. G., Z. W., M. H. M., S. S., and A. G. were responsible for analysis and interpretation of data. S. J. was responsible for drafting the manuscript. R. K., S. G., Z., M. H. M., A. B., D. B., G. A. S., S. S., and A. G. were responsible for critical revision of the manuscript for important intellectual content. S. J., R. K., S. G., Z. W., M. H. M., A. B., D. B., G. S., S. S., and A. G. were responsible for approval of the final manuscript.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: D. B. serves as a consultant, advisory board member, and steering committee member for Actelion, CoTherix, Gilead, Pfizer, United Therapeutics/Lung Rx, Bayer, Arena, Ikaria, and Belleraphon Therapeutics. None declared (S. J., R. K., S. S., A. B., M. H. M., Z. W., G. S., A. G.).
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Additional information: The e-Appendix, e-Figures, and e-Tables can be found in the Supplemental Materials section of the online article.
Footnotes
Drs Jain and Khera contributed equally as co-first authors.
FUNDING/SUPPORT: A. K. G. and S. G. are supported by career development awards from the National Heart, Lung, and Blood Institute [grants K23HL114640 and K08HL122527]. S. S. is supported by the National Library of Medicine/National Institute of Health [training grant T15LM011271]. R. K. received support from the National Center for Advancing Translational Sciences of the National Institutes of Health [UL1TR001105]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Supplementary Data
References
- 1.McLaughlin V.V., Archer S.L., Badesch D.B. ACCF/AHA 2009 expert consensus document on pulmonary hypertension a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association developed in collaboration with the American College of Chest Physicians; American Thoracic Society, Inc.; and the Pulmonary Hypertension Association. J Am Coll Cardiol. 2009;53(17):1573–1619. doi: 10.1016/j.jacc.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 2.Rich S., Dantzker D.R., Ayres S.M. Primary pulmonary hypertension. A national prospective study. Ann Intern Med. 1987;107(2):216–223. doi: 10.7326/0003-4819-107-2-216. [DOI] [PubMed] [Google Scholar]
- 3.Rubin L.J. Primary pulmonary hypertension. N Engl J Med. 1997;336(2):111–117. doi: 10.1056/NEJM199701093360207. [DOI] [PubMed] [Google Scholar]
- 4.Coeytaux R.R., Schmit K.M., Kraft B.D. Comparative effectiveness and safety of drug therapy for pulmonary arterial hypertension: a systematic review and meta-analysis. Chest. 2014;145(5):1055–1063. doi: 10.1378/chest.13-1864. [DOI] [PubMed] [Google Scholar]
- 5.Macchia A., Marchioli R., Marfisi R. A meta-analysis of trials of pulmonary hypertension: a clinical condition looking for drugs and research methodology. Am Heart J. 2007;153(6):1037–1047. doi: 10.1016/j.ahj.2007.02.037. [DOI] [PubMed] [Google Scholar]
- 6.He B., Zhang F., Li X. Meta-analysis of randomized controlled trials on treatment of pulmonary arterial hypertension. Circ J. 2010;74(7):1458–1464. doi: 10.1253/circj.cj-09-0971. [DOI] [PubMed] [Google Scholar]
- 7.Galie N., Manes A., Negro L., Palazzini M., Bacchi-Reggiani M.L., Branzi A. A meta-analysis of randomized controlled trials in pulmonary arterial hypertension. Eur Heart J. 2009;30(4):394–403. doi: 10.1093/eurheartj/ehp022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Galie N., Corris P.A., Frost A. Updated treatment algorithm of pulmonary arterial hypertension. J Am Coll Cardiol. 2013;62(25 suppl):D60–D72. doi: 10.1016/j.jacc.2013.10.031. [DOI] [PubMed] [Google Scholar]
- 9.McLaughlin V.V., Shah S.J., Souza R., Humbert M. Management of pulmonary arterial hypertension. J Am Coll Cardiol. 2015;65(18):1976–1997. doi: 10.1016/j.jacc.2015.03.540. [DOI] [PubMed] [Google Scholar]
- 10.Mills E.J., Thorlund K., Ioannidis J.P. Demystifying trial networks and network meta-analysis. BMJ. 2013;346:f2914. doi: 10.1136/bmj.f2914. [DOI] [PubMed] [Google Scholar]
- 11.Lu G., Ades A.E. Combination of direct and indirect evidence in mixed treatment comparisons. Stat Med. 2004;23(20):3105–3124. doi: 10.1002/sim.1875. [DOI] [PubMed] [Google Scholar]
- 12.Hutton B., Salanti G., Caldwell D.M. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162(11):777–784. doi: 10.7326/M14-2385. [DOI] [PubMed] [Google Scholar]
- 13.Jain S, Khera R, Girotra S, et al. Comparative effectiveness of pharmacologic interventions for pulmonary arterial hypertension: a systematic review and network meta-analysis. http://www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42016036803. Accessed May 4, 2016. [DOI] [PMC free article] [PubMed]
- 14.Jansen J.P., Fleurence R., Devine B. Interpreting indirect treatment comparisons and network meta-analysis for health-care decision making: report of the ISPOR Task Force on Indirect Treatment Comparisons Good Research Practices: part 1. Value Health. 2011;14(4):417–428. doi: 10.1016/j.jval.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 15.Hoaglin D.C., Hawkins N., Jansen J.P. Conducting indirect-treatment-comparison and network-meta-analysis studies: report of the ISPOR Task Force on Indirect Treatment Comparisons Good Research Practices: part 2. Value Health. 2011;14(4):429–437. doi: 10.1016/j.jval.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 16.Puhan M.A., Schunemann H.J., Murad M.H. A GRADE Working Group approach for rating the quality of treatment effect estimates from network meta-analysis. BMJ. 2014;349:g5630. doi: 10.1136/bmj.g5630. [DOI] [PubMed] [Google Scholar]
- 17.Higgins JPT, Greene S. Cochrane handbook for systematic reviews of interventions. www.handbook.cochrane.org. Accessed November 5, 2015.
- 18.Mathai S.C., Puhan M.A., Lam D., Wise R.A. The minimal important difference in the 6-minute walk test for patients with pulmonary arterial hypertension. Am J Respir Crit Care Med. 2012;186(5):428–433. doi: 10.1164/rccm.201203-0480OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DerSimonian R., Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 20.Higgins J.P., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Egger M., Davey Smith G., Schneider M., Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chaimani A., Higgins J.P., Mavridis D., Spyridonos P., Salanti G. Graphical tools for network meta-analysis in STATA. PLoS One. 2013;8(10):e76654. doi: 10.1371/journal.pone.0076654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.White I.R. Network meta-analysis. Stata J. 2015;15(4):951–985. [Google Scholar]
- 24.White I.R., Barrett J.K., Jackson D., Higgins J. Consistency and inconsistency in network meta-analysis: model estimation using multivariate meta-regression. Res Synth Methods. 2012;3(2):111–125. doi: 10.1002/jrsm.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saggar R., Khanna D., Vaidya A. Changes in right heart haemodynamics and echocardiographic function in an advanced phenotype of pulmonary hypertension and right heart dysfunction associated with pulmonary fibrosis. Thorax. 2014;69(2):123–129. doi: 10.1136/thoraxjnl-2013-204150. [DOI] [PubMed] [Google Scholar]
- 26.Higgins J.P., Jackson D., Barrett J.K., Lu G., Ades A.E., White I.R. Consistency and inconsistency in network meta-analysis: concepts and models for multi-arm studies. Res Synth Methods. 2012;3(2):98–110. doi: 10.1002/jrsm.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Salanti G., Ades A.E., Ioannidis J.P. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J Clin Epidemiol. 2011;64(2):163–171. doi: 10.1016/j.jclinepi.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 28.McLaughlin V., Channick R.N., Ghofrani H.A. Bosentan added to sildenafil therapy in patients with pulmonary arterial hypertension. Eur Respir J. 2015;46(2):405–413. doi: 10.1183/13993003.02044-2014. [DOI] [PubMed] [Google Scholar]
- 29.Galie N., Rubin L., Hoeper M. Treatment of patients with mildly symptomatic pulmonary arterial hypertension with bosentan (EARLY study): a double-blind, randomised controlled trial. Lancet. 2008;371(9630):2093–2100. doi: 10.1016/S0140-6736(08)60919-8. [DOI] [PubMed] [Google Scholar]
- 30.Humbert M., Barst R.J., Robbins I.M. Combination of bosentan with epoprostenol in pulmonary arterial hypertension: BREATHE-2. Eur Respir J. 2004;24(3):353–359. doi: 10.1183/09031936.04.00028404. [DOI] [PubMed] [Google Scholar]
- 31.Rubin L.J., Badesch D.B., Barst R.J. Bosentan therapy for pulmonary arterial hypertension. N Engl J Med. 2002;346(12):896–903. doi: 10.1056/NEJMoa012212. [DOI] [PubMed] [Google Scholar]
- 32.Channick R.N., Simonneau G., Sitbon O. Effects of the dual endothelin-receptor antagonist bosentan in patients with pulmonary hypertension: a randomised placebo-controlled study. Lancet. 2001;358(9288):1119–1123. doi: 10.1016/S0140-6736(01)06250-X. [DOI] [PubMed] [Google Scholar]
- 33.Galie N., Olschewski H., Oudiz R.J. Ambrisentan for the treatment of pulmonary arterial hypertension: results of the ambrisentan in pulmonary arterial hypertension, randomized, double-blind, placebo-controlled, multicenter, efficacy (ARIES) study 1 and 2. Circulation. 2008;117(23):3010–3019. doi: 10.1161/CIRCULATIONAHA.107.742510. [DOI] [PubMed] [Google Scholar]
- 34.Pulido T., Adzerikho I., Channick R.N. Macitentan and morbidity and mortality in pulmonary arterial hypertension. N Engl J Med. 2013;369(9):809–818. doi: 10.1056/NEJMoa1213917. [DOI] [PubMed] [Google Scholar]
- 35.Galie N., Brundage B.H., Ghofrani H.A. Tadalafil therapy for pulmonary arterial hypertension. Circulation. 2009;119(22):2894–2903. doi: 10.1161/CIRCULATIONAHA.108.839274. [DOI] [PubMed] [Google Scholar]
- 36.Galie N., Ghofrani H.A., Torbicki A. Sildenafil citrate therapy for pulmonary arterial hypertension. N Engl J Med. 2005;353(20):2148–2157. doi: 10.1056/NEJMoa050010. [DOI] [PubMed] [Google Scholar]
- 37.Zhuang Y., Jiang B., Gao H., Zhao W. Randomized study of adding tadalafil to existing ambrisentan in pulmonary arterial hypertension. Hypertens Res. 2014;37(6):507–512. doi: 10.1038/hr.2014.28. [DOI] [PubMed] [Google Scholar]
- 38.Simonneau G., Rubin L.J., Galie N. Addition of sildenafil to long-term intravenous epoprostenol therapy in patients with pulmonary arterial hypertension: a randomized trial. Ann Intern Med. 2008;149(8):521–530. doi: 10.7326/0003-4819-149-8-200810210-00004. [DOI] [PubMed] [Google Scholar]
- 39.Jing Z.C., Parikh K., Pulido T. Efficacy and safety of oral treprostinil monotherapy for the treatment of pulmonary arterial hypertension: a randomized, controlled trial. Circulation. 2013;127(5):624–633. doi: 10.1161/CIRCULATIONAHA.112.124388. [DOI] [PubMed] [Google Scholar]
- 40.Tapson V.F., Jing Z.C., Xu K.F. Oral treprostinil for the treatment of pulmonary arterial hypertension in patients receiving background endothelin receptor antagonist and phosphodiesterase type 5 inhibitor therapy (the FREEDOM-C2 study): a randomized controlled trial. Chest. 2013;144(3):952–958. doi: 10.1378/chest.12-2875. [DOI] [PubMed] [Google Scholar]
- 41.Tapson V.F., Torres F., Kermeen F. Oral treprostinil for the treatment of pulmonary arterial hypertension in patients on background endothelin receptor antagonist and/or phosphodiesterase type 5 inhibitor therapy (the FREEDOM-C study): a randomized controlled trial. Chest. 2012;142(6):1383–1390. doi: 10.1378/chest.11-2212. [DOI] [PubMed] [Google Scholar]
- 42.McLaughlin V.V., Benza R.L., Rubin L.J. Addition of inhaled treprostinil to oral therapy for pulmonary arterial hypertension: a randomized controlled clinical trial. J Am Coll Cardiol. 2010;55(18):1915–1922. doi: 10.1016/j.jacc.2010.01.027. [DOI] [PubMed] [Google Scholar]
- 43.Olschewski H., Simonneau G., Galie N. Inhaled iloprost for severe pulmonary hypertension. N Engl J Med. 2002;347(5):322–329. doi: 10.1056/NEJMoa020204. [DOI] [PubMed] [Google Scholar]
- 44.McLaughlin V.V., Oudiz R.J., Frost A. Randomized study of adding inhaled iloprost to existing bosentan in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2006;174(11):1257–1263. doi: 10.1164/rccm.200603-358OC. [DOI] [PubMed] [Google Scholar]
- 45.Hoeper M.M., Leuchte H., Halank M. Combining inhaled iloprost with bosentan in patients with idiopathic pulmonary arterial hypertension. Eur Respir J. 2006;28(4):691–694. doi: 10.1183/09031936.06.00057906. [DOI] [PubMed] [Google Scholar]
- 46.Hiremath J., Thanikachalam S., Parikh K. Exercise improvement and plasma biomarker changes with intravenous treprostinil therapy for pulmonary arterial hypertension: a placebo-controlled trial. J Heart Lung Transplant. 2010;29(2):137–149. doi: 10.1016/j.healun.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 47.McLaughlin V.V., Gaine S.P., Barst R.J. Efficacy and safety of treprostinil: an epoprostenol analog for primary pulmonary hypertension. J Cardiovasc Pharmacol. 2003;41(2):293–299. doi: 10.1097/00005344-200302000-00019. [DOI] [PubMed] [Google Scholar]
- 48.Simonneau G., Barst R.J., Galie N. Continuous subcutaneous infusion of treprostinil, a prostacyclin analogue, in patients with pulmonary arterial hypertension: a double-blind, randomized, placebo-controlled trial. Am J Respir Crit Care Med. 2002;165(6):800–804. doi: 10.1164/ajrccm.165.6.2106079. [DOI] [PubMed] [Google Scholar]
- 49.Badesch D.B., Tapson V.F., McGoon M.D. Continuous intravenous epoprostenol for pulmonary hypertension due to the scleroderma spectrum of disease. A randomized, controlled trial. Ann Intern Med. 2000;132(6):425–434. doi: 10.7326/0003-4819-132-6-200003210-00002. [DOI] [PubMed] [Google Scholar]
- 50.Barst R.J., Rubin L.J., Long W.A. A comparison of continuous intravenous epoprostenol (prostacyclin) with conventional therapy for primary pulmonary hypertension. N Engl J Med. 1996;334(5):296–301. doi: 10.1056/NEJM199602013340504. [DOI] [PubMed] [Google Scholar]
- 51.Rubin L.J., Mendoza J., Hood M. Treatment of primary pulmonary hypertension with continuous intravenous prostacyclin (epoprostenol). Results of a randomized trial. Ann Intern Med. 1990;112(7):485–491. doi: 10.7326/0003-4819-112-7-485. [DOI] [PubMed] [Google Scholar]
- 52.Ghofrani H.A., Galie N., Grimminger F. Riociguat for the treatment of pulmonary arterial hypertension. N Engl J Med. 2013;369(4):330–340. doi: 10.1056/NEJMoa1209655. [DOI] [PubMed] [Google Scholar]
- 53.Galie N., Muller K., Scalise A.V., Grunig E. PATENT PLUS: a blinded, randomised and extension study of riociguat plus sildenafil in pulmonary arterial hypertension. Eur Respir J. 2015;45(5):1314–1322. doi: 10.1183/09031936.00105914. [DOI] [PubMed] [Google Scholar]
- 54.Sitbon O., Channick R., Chin K.M. Selexipag for the treatment of pulmonary arterial hypertension. N Engl J Med. 2015;373(26):2522–2533. doi: 10.1056/NEJMoa1503184. [DOI] [PubMed] [Google Scholar]
- 55.Simonneau G., Torbicki A., Hoeper M.M. Selexipag: an oral, selective prostacyclin receptor agonist for the treatment of pulmonary arterial hypertension. Eur Respir J. 2012;40(4):874–880. doi: 10.1183/09031936.00137511. [DOI] [PubMed] [Google Scholar]
- 56.Wilkins M.R., Paul G.A., Strange J.W. Sildenafil versus Endothelin Receptor Antagonist for Pulmonary Hypertension (SERAPH) study. Am J Respir Crit Care Med. 2005;171(11):1292–1297. doi: 10.1164/rccm.200410-1411OC. [DOI] [PubMed] [Google Scholar]
- 57.Galie N., Barbera J.A., Frost A.E. Initial use of ambrisentan plus tadalafil in pulmonary arterial hypertension. N Engl J Med. 2015;373(9):834–844. doi: 10.1056/NEJMoa1413687. [DOI] [PubMed] [Google Scholar]
- 58.Galie N., Humbert M., Vachiery J.L. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Rev Esp Cardiol (Engl Ed) 2016;69(2):177. doi: 10.1016/j.rec.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 59.Taichman D.B., Ornelas J., Chung L. Pharmacologic therapy for pulmonary arterial hypertension in adults: CHEST guideline and expert panel report. Chest. 2014;146(2):449–475. doi: 10.1378/chest.14-0793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lajoie A.C., Lauziere G., Lega J.C. Combination therapy versus monotherapy for pulmonary arterial hypertension: a meta-analysis. Lancet Respir Med. 2016;4(4):291–305. doi: 10.1016/S2213-2600(16)00027-8. [DOI] [PubMed] [Google Scholar]
- 61.Liu H.L., Chen X.Y., Li J.R. Efficacy and safety of pulmonary arterial hypertension-specific therapy in pulmonary arterial hypertension: a meta-analysis of randomized controlled trials. Chest. 2016;150(2):353–366. doi: 10.1016/j.chest.2016.03.031. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.