Abstract
Background
Obesity is prevalent in the United States; however, the impact of obesity on COPD morbidity is unclear. We hypothesized that obesity is associated with worse outcomes in COPD.
Methods
We examined 3,631 participants from the multicenter prospective cohort study Genetic Epidemiology of COPD (COPDGene) who had spirometry-confirmed COPD, a postbronchodilator FEV1 < 80% predicted, and a BMI ≥ 18.5 kg/m2. We conducted logistic and linear regression analyses to determine the association between COPD outcomes and obesity class, adjusting for relevant confounders. The referent for obesity classes included normal/overweight individuals (BMI range, 18.5-29.9 kg/m2).
Results
Overall, 35% of participants were obese, with 21% class I (BMI range, 30-34.9 kg/m2), 9% class II (BMI range, 35-39.9 kg/m2), and 5% class III (BMI ≥ 40 kg/m2). The number of comorbidities increased with increasing obesity class (P < .001). Increasing obesity class was independently associated with worse respiratory-specific and general quality of life (QOL) (St. George’s Respiratory Questionnaire score and Short Form-36 score version 2, respectively), reduced 6-min walk distance (6MWD), increased dyspnea (Modified Medical Research Council score ≥ 2), and greater odds of severe acute exacerbation of COPD (AECOPD). The associations between obesity and worse outcomes were independent of the presence of comorbidities, except in the case of SF-36 and severe exacerbations.
Conclusions
Obesity is prevalent among individuals with COPD and associated with worse COPD-related outcomes, ranging from QOL and dyspnea to 6MWD and severe AECOPD. These associations were strengthened when obesity was analyzed as a dose-dependent response. Obesity in patients with COPD may contribute to a worse COPD-related course.
Key Words: COPD, dose response, exacerbation, morbidity, obesity
Abbreviations: 6MWD, 6-min walk distance; AECOPD, acute exacerbation of COPD; COPDGene, Genetic Epidemiology of COPD; GOLD, Global Initiative for Chronic Obstructive Lung Disease; MCID, minimum clinically important difference; mMRC, Modified Medical Research Council; NHW, non-Hispanic White; QOL, quality of life; SF-36, Short Form-36; SGRQ, St. George’s Respiratory Questionnaire
In the United States, 6% of adults have COPD1 and 35% are obese2, 3, 4; however, the prevalence of obesity among patients with COPD is unclear, with estimates ranging from 6% to 54%.5, 6, 7, 8, 9 Much attention has been given to the association between COPD and low BMI,10, 11, 12, 13 with studies suggesting a U-shaped relationship between weight and general health outcomes in individuals with COPD.14, 15, 16, 17 Obesity may be linked to adverse health consequences in patients with COPD18, 19, 20; however, these consequences are not well delineated.
Obesity is associated with myriad pulmonary decrements, including an increased prevalence and severity of asthma, rate of FEV1 decline, prevalence of sleep-disordered breathing, and risk for perioperative complications.19, 21, 22 Among those with COPD, obesity has demonstrated mixed effects, including worse quality of life (QOL), dyspnea, and exercise tolerance (6-min walk distance [6MWD]),23 but also reduced mortality14, 24, 25 and less severe airflow obstruction.23, 26 Outcomes associated with risk for morbidity and mortality, such as COPD exacerbations, have not been consistently reported. Whether subpopulations of obese individuals with COPD, based upon sex and race for example, may be at greatest risk for worse outcomes is unknown. Despite emerging information regarding racial differences in the effect of weight on outcomes in chronic diseases,27, 28 representation of minorities in clinical COPD cohorts is lacking. Because women comprise a growing proportion of incident COPD cases and blacks incur greater mortality than whites,29 the impact of race and sex on risk factors, such as obesity for COPD-related outcomes, needs investigation.
We examined a large well-characterized cohort of subjects with Global Initiative for Chronic Obstructive Lung Disease (GOLD) stages 2 through 4 COPD from the multicenter Genetic Epidemiology of COPD (COPDGene) study to determine the impact of obesity on COPD morbidity.
Methods
Study Population
The COPDGene study details have been reported.30 Briefly, the COPDGene study is a multicenter observational study including current and former smokers designed to identify genetic factors associated with COPD. Between January 2008 and April 2011, 10,192 non-Hispanic White (NHW) and black adults aged 45 to 80 years of age with a minimum 10 pack-year smoking history were enrolled. Participants were phenotyped by completing questionnaires, blood tests, imaging, and spirometry measurements. This study was conducted in accordance with the amended Declaration of Helsinki and approved by the institutional review board at each study center; all participants provided written, informed consent.
From the full cohort, 3,753 participants had spirometry-confirmed COPD at baseline, with an FEV1 to FVC ratio < 0.70 and a postbronchodilator FEV1 < 80% predicted. We excluded participants with a low BMI (BMI < 18.5 kg/m2; n = 122) because these participants may represent a unique phenotype compared with normal or overweight individuals.23, 31
Measurements
Weight and height were measured; BMI was calculated as mass in kilograms divided by height in meters squared. Demographic characteristics and comorbidities were self-reported through standardized study questionnaires. Level of education was defined as less than high school, high school ± some college, or college and beyond. Comorbidity count represented the number of the following conditions present: allergies, asthma, cancer, coronary heart disease, congestive heart failure, cerebrovascular attack/transient ischemic attack, diabetes, gastroesophageal reflux disease, hypertension, hyperlipidemia, OSA, osteoarthritis, osteoporosis, peptic ulcer disease, peripheral arterial disease, and rheumatoid arthritis.32, 33
Continuous outcomes were analyzed using linear regression, including the St. George’s Respiratory Questionnaire (SGRQ),34, 35 the Short Form-36 (SF-36) total score,36, 37 and the 6MWD.38, 39 Binary outcomes were analyzed using logistic regression, including Modified Medical Research Council (mMRC)40, 41 score ≥ 2 vs < 2, aligned with the GOLD42 definition of more (vs less) symptoms, moderate acute exacerbation of COPD (AECOPD) defined as a self-reported exacerbation treated with antibiotics and/or oral corticosteroids in the last year, and severe AECOPD requiring ED visitation or hospital admission.43 Accounting for FEV1 percent predicted, mMRC score, and exacerbation history, GOLD 2013 ABCD1 categories were defined as previously done by Han and colleagues.44
Statistical Analyses
Cohort characteristics are presented as frequencies, means (SDs), and medians (interquartile ranges). Comparisons were conducted using the t test for continuous variables or the χ2 test for categorical variables. A two-sided P value ≤ .05 defined statistical significance. The exposure of interest was obesity class. Exploratory data analyses examined BMI as a continuous vs categorical variable and explored univariable associations with obesity class. Histograms, locally weighted scatterplot smoothing plots, and univariate regressions demonstrated similar behavior for normal and overweight individuals. Therefore, the reference group included normal and overweight individuals (BMI range, 18.5-29.9 kg/m2). Obesity was categorized into three classes: class I (BMI range, 30-34.9 kg/m2), class II (BMI range, 35-39.9 kg/m2), and class III (BMI ≥ 40 kg/m2).45, 46
Adjusted models accounted for age, sex, race, level of education, FEV1 to height squared ratio,47 smoking status (current/former), and smoking pack-years. Additional models also adjusted for comorbidity count. When FEV1 was the outcome of interest, the FEV1 to height squared ratio was removed from the covariates. Interaction terms were constructed between obesity status and race, sex, and lung function separately to determine if effect modification was present. Stratified analyses were conducted by race and sex to illustrate the impact of covariates on obesity class and the outcomes of interest.
Results
Participant Characteristics
Demographic and clinical characteristics are presented in Table 1, stratified by obesity class. Overall, 34% of participants were obese, with 21% in class I, 9% in class II, and 5% in class III. Age, sex, race, education, and smoking pack-years were similar across weight categories. Active tobacco use (current smoking) was most prevalent among the normal/overweight individuals (43%) and those with class III obesity (40%). Absolute and percent predicted FEV1, and the FEV1 to FVC ratio, were lowest among those who were normal/overweight. As obesity class increased, the proportion of GOLD A and C participants decreased and the proportion of GOLD B increased (P < .001 for trend across obesity class for each GOLD category). The proportion of GOLD D was not statistically different across obesity class (P = .452). Comorbidity count increased with increasing obesity class (P < .001), ranging from a mean of 2.6 comorbid conditions among normal/overweight individuals to a mean of 4.0 comorbid conditions among the most obese (Table 2). Across all obesity classes, gastroesophageal reflux disease, hypertension, and hyperlipidemia were the most prevalent comorbidities.
Table 1.
Clinical and Demographic Characteristics of Study Participants
| Characteristic | Normal/Overweight (18.5-29.9 kg/m2) | Obesity Class I (30-34.9 kg/m2) | Obesity Class II (35-39.9 kg/m2) | Obesity Class III (≥ 40 kg/m2) | P Value for Trend |
|---|---|---|---|---|---|
| Participants | 2,383 (66) | 748 (21) | 316 (9) | 184 (5) | |
| Age, y | 63 ± 9 | 64 ± 9 | 62 ± 8 | 61 ± 9 | .078 |
| Male | 1,365 (57) | 423 (57) | 133 (42) | 70 (38) | .073 |
| NHW race | 1,850 ± 78 | 567 ± 76 | 240 ± 76 | 135 ± 73 | .144 |
| Education | .359 | ||||
| Less than high school | 334 (14) | 116 (16) | 46 (15) | 30 (16) | |
| High school ± some college | 1,313 (55) | 390 (52) | 185 (59) | 105 (57) | |
| College and beyond | 735 (31) | 242 (32) | 85 (27) | 49 (27) | |
| Current smoker | 1,034 (43) | 274 (37) | 106 (34) | 73 (40) | < .001 |
| Smoking, pack-y | 53 ± 28 | 53 ± 27 | 56 ± 28 | 51 ± 27 | .246 |
| FEV1 | |||||
| Absolute, L | 1.4 ± 0.7 | 1.5 ± 0.6 | 1.5 ± 0.6 | 1.5 ± 0.5 | .001 |
| Percent predicted | 49 ± 19 | 53 ± 17 | 53 ± 16 | 53 ± 14 | < .001 |
| FEV1 to FVC ratio | |||||
| Absolute | 0.48 ± 0.1 | 0.53 ± 0.1 | 0.54 ± 0.1 | 0.57 ± 0.1 | < .001 |
| GOLD category | |||||
| A | 644 (27) | 189 (25) | 60 (19) | 21 (11) | < .001 |
| B | 434 (18) | 186 (25) | 100 (32) | 61 (33) | < .001 |
| C | 239 (10) | 59 (8) | 18 (6) | 6 (3) | < .001 |
| D | 1,066 (45) | 314 (42) | 138 (44) | 96 (52) | .452 |
Values are presented as mean ± SD, No. (%), or as otherwise indicated. GOLD = Global Initiative for Chronic Obstructive Lung Disease; NHW = non-Hispanic white.
Table 2.
Self-Reported Comorbidities of Study Participants
| Characteristic | Normal/Overweight (18.5-29.9 kg/m2) | Obesity Class I (30-34.9 kg/m2) | Obesity Class II (35-39.9 kg/m2) | Obesity Class III (≥ 40 kg/m2) | P Value for Trend |
|---|---|---|---|---|---|
| Participants | 2,383 (66) | 748 (21) | 316 (9) | 184 (5) | |
| Comorbidity counta | 2.6 ± 2 | 3.4 ± 2 | 3.9 ± 2 | 4.0 ± 2 | < .001 |
| Comorbidity | |||||
| Allergies | 555 (23) | 187 (25) | 95 (30) | 50 (27) | .027 |
| Asthma | 502 (21) | 207 (28) | 96 (30) | 59 (32) | < .005 |
| Cancer | 150 (6) | 63 (8) | 20 (6) | 9 (5) | .218 |
| CHD | 373 (16) | 131 (18) | 71 (22) | 30 (16) | .024 |
| CHF | 90 (4) | 46 (6) | 35 (11) | 23 (13) | < .001 |
| CVA/TIA | 138 (6) | 48 (6) | 19 (6) | 7 (4) | .864 |
| Diabetes mellitus | 198 (8) | 145 (19) | 92 (29) | 50 (27) | < .001 |
| GERD | 657 (28) | 269 (36) | 110 (35) | 65 (35) | < .001 |
| Hypertension | 1,080 (45) | 432 (58) | 210 (66) | 125 (68) | < .001 |
| Hyperlipidemia | 918 (39) | 352 (47) | 162 (51) | 91 (49) | < .001 |
| OSA | 243 (10) | 182 (24) | 113 (36) | 93 (51) | < .001 |
| Osteoarthritis | 414 (17) | 190 (25) | 94 (30) | 56 (30) | < .001 |
| Osteoporosis | 431 (18) | 111 (15) | 41 (13) | 26 (14) | .004 |
| Peptic ulcer disease | 237 (10) | 78 (10) | 31 (10) | 19 (10) | .764 |
| Peripheral arterial disease | 74 (3) | 30 (4) | 14 (4) | 6 (3) | .157 |
| Rheumatoid arthritis | 187 (8) | 81 (11) | 35 (11) | 22 (12) | .001 |
Values are presented as mean ± SD, No. (%), or as otherwise indicated. CHD = coronary heart disease; CHF = congestive heart failure; CVA = cerebrovascular attack; GERD = gastroesophageal reflux disease; TIA = transient ischemic attack.
Count of the comorbidities listed in the table.
Association of Obesity Class With COPD Outcomes
In univariable analyses, increasing obesity class was associated with worse QOL, lower 6MWD, and higher odds of dyspnea, in a dose-dependent fashion (e-Table 1). In addition, class III obesity was associated with higher odds of severe AECOPD (OR, 1.57; P = .007).
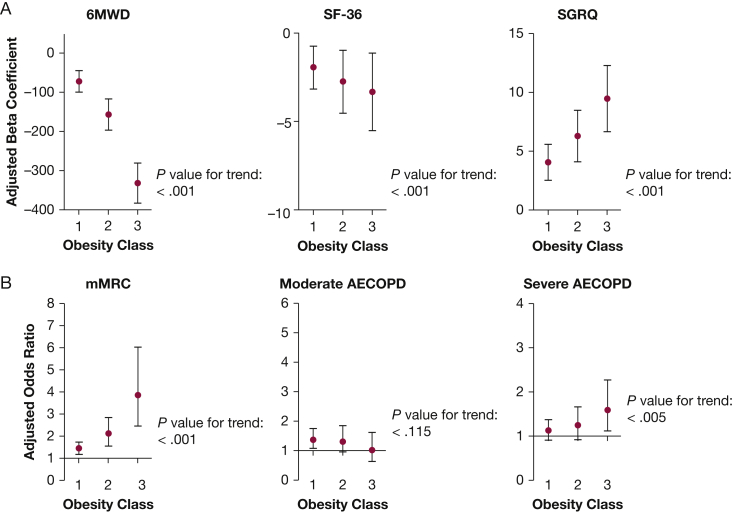
In adjusted models, increasing severity of obesity remained statistically significantly associated with higher SGRQ score and lower SF-36 score (worse respiratory-specific and general QOL, respectively), reduced 6MWD, and higher odds of an mMRC score ≥ 2 (increased dyspnea) (Fig 1, Table 3). These associations increased in magnitude with increasing obesity class. For example, class I obesity was associated with a 1.4-fold increased odds of dyspnea, and class III obesity was associated with nearly a fourfold increased odds of dyspnea. Obesity was associated with increased odds of reporting severe exacerbations, and odds of severe AECOPD increased with increasing obesity class (P = .005). The link between obesity and risk of moderate exacerbations was apparent, but less consistent. Obesity class I was significantly associated with increased odds of moderate AECOPD (OR, 1.38; 95% CI, 1.08-1.75; P = .009); however, the trend across increasing obesity classes was not statistically significant (P = .115). Obesity was not associated with worse lung function (data not shown). On the contrary, those who were obese had slightly higher FEV1 percent predicted compared with normal/overweight participants (obesity class I: β = 4.05; 95% CI, 2.64-5.46; obesity class II: β = 4.92; 95% CI, 2.89-6.96; obesity class III: β = 3.76; 95% CI, 1.15-6.38).
Figure 1.
A, B, Dose-dependent response to higher obesity class. Adjusted for age, sex, race, education, FEV1 to height squared ratio, smoking status, and smoking pack-y. Referent in all models includes normal/overweight individuals (BMI range, 18.5-29.9 kg/m2). Markers represent the adjusted β coefficients (A) or adjusted ORs (B) for the association between each obesity class and the specified outcome. Bars represent the 95% CIs for each β coefficient (A) or OR (Panel B). The P values for trend represent the statistical significance of the increase or decrease in the outcome across obesity classes. 6MWD = 6-min walk distance; AECOPD = acute exacerbation of COPD; mMRC = Modified Medical Research Council; SF-36, Short Form-36; SGRQ, St. George’s Respiratory Questionnaire.
Table 3.
Health Outcome Multivariable Models
| Outcome | Base Models |
Base Model + Comorbidity Count |
||||
|---|---|---|---|---|---|---|
| β | 95% CI | P Value | β | 95% CI | P Value | |
| SGRQ total score | ||||||
| Obese class I | 4.06 | 2.54 to 5.58 | < .001 | 2.37 | 0.87 to 3.86 | .002 |
| Obese Class II | 6.28 | 4.09 to 8.48 | < .001 | 3.24 | 1.07 to 5.40 | .003 |
| Obese Class III | 9.45 | 6.64 to 12.27 | < .001 | 6.09 | 3.33 to 8.85 | < .001 |
| P value for trend | < .001 | < .001 | ||||
| SF-36 total score | ||||||
| Obese class I | −1.95 | −3.17 to −0.73 | .002 | −0.90 | −2.09 to 0.28 | .135 |
| Obese class II | −2.76 | −4.54 to −0.97 | .003 | −0.74 | −2.50 to 1.02 | .412 |
| Obese class III | −3.32 | −5.51 to −1.12 | .003 | −1.03 | −3.19 to 1.12 | .347 |
| P value for trend | < .001 | .146 | ||||
| 6MWD, ft | ||||||
| Obese class I | −71.66 | −99.25 to −44.07 | < .001 | −55.65 | −83.39 to −27.90 | < .001 |
| Obese class II | −156.28 | −196.08 to −116.47 | < .001 | −128.93 | −169.11 to −88.76 | < .001 |
| Obese class III | −330.82 | −382.21 to −279.43 | < .001 | −299.75 | −351.43 to −248.07 | < .001 |
| P value for trend | < .001 | < .001 | ||||
| OR | 95% CI | P value | OR | 95% CI | P value | |
|---|---|---|---|---|---|---|
| mMRC score ≥ 2 | ||||||
| Obese class I | 1.42 | 1.16 to 1.72 | .001 | 1.22 | 1.00 to 1.50 | .052 |
| Obese class II | 2.09 | 1.54 to 2.83 | < .001 | 1.66 | 1.21 to 2.27 | .001 |
| Obese class III | 3.84 | 2.44 to 6.04 | < .001 | 2.95 | 1.86 to 4.69 | < .001 |
| P value for trend | < .001 | < .001 | ||||
| Moderate AECOPD | ||||||
| Obese class I | 1.38 | 1.08 to 1.75 | .009 | 1.24 | 0.97 to 1.59 | .082 |
| Obese class II | 1.31 | 0.93 to 1.85 | .120 | 1.08 | 0.76 to 1.54 | .669 |
| Obese class III | 1.02 | 0.64 to 1.62 | .944 | 0.83 | 0.52 to 1.33 | .433 |
| P value for trend | .115 | .916 | ||||
| Severe AECOPD | ||||||
| Obese class I | 1.12 | 0.91 to 1.38 | .286 | 1.03 | 0.83 to 1.28 | .772 |
| Obese class II | 1.24 | 0.92 to 1.66 | .152 | 1.05 | 0.78 to 1.42 | .734 |
| Obese class III | 1.59 | 1.12 to 2.27 | .010 | 1.35 | 0.94 to 1.93 | .102 |
| P value for trend | .005 | .172 |
Base models are adjusted for age, sex, race, education, FEV1 to height squared ratio, smoking status, and smoking pack-years. Referent in all models includes normal/overweight individuals (BMI range, 18.5-29.9 kg/m2). 6MWD = 6-min walk distance; AECOPD = acute exacerbation of COPD; mMRC = Modified Medical Research Council; SF-36 = Short Form-36; SGRQ = St. George’s Respiratory Questionnaire.
When also adjusting for comorbidity count, increasing obesity class remained significantly associated with higher SGRQ score, lower 6MWD, and increased odds of an mMRC score ≥ 2; however, effect sizes were attenuated. Again, the dose-dependent response was observed, with increasing obesity class associated with greater decrements in outcomes. Obesity was no longer associated with SF-36 score and exacerbations, suggesting that the effect of obesity on these outcomes may be mediated by comorbidity burden among obese individuals (Table 3). Adjusted models including individual comorbidities as covariates, rather than comorbidity count, demonstrated similar findings, with the exception of the moderate AECOPD-obesity class I association, which was attenuated (e-Table 2). These models demonstrated the consistent negative impact of osteoporosis and cerebrovascular attack/transient ischemic attack. Separately examining the impact of metabolic (hypertension, diabetes, and hyperlipidemia) vs nonmetabolic comorbidities (remaining 13 conditions) did not alter our findings or suggest that metabolic disorders more strongly mediate the effect of BMI on outcomes compared with nonmetabolic comorbidities.
Race, Sex, and Lung Function Interactions With Obesity Class
A statistically significant interaction between obesity class and sex was observed for SGRQ score (interaction P = .043 for trend) (Table 4). Obesity was more strongly associated with worsening SGRQ score among women compared with men. The interaction between obesity and race was statistically significant for 6MWD (interaction P = .011 for overall trend) (Table 4). Increasing obesity class was associated with greater reduction in 6MWD among NHW compared with black participants. Finally, the interaction between obesity and lung function was statistically significant for both SGRQ score and severe AECOPD (Table 4). Increasing obesity class was associated with worse SGRQ scores and greater odds of severe AECOPD among those with healthier lung function (≥ 50% predicted) compared with those with lower lung function (< 50% predicted). The association between obesity and other outcomes did not vary significantly by race, sex, or lung function.
Table 4.
Interactions by Sex, Race, and Lung Function With Obesity Categories
| Health Outcome | Stratified Models |
Interaction Model | |||||
|---|---|---|---|---|---|---|---|
| Men | Women | ||||||
| SGRQ total score | β | 95% CI | P Value | β | 95% CI | P Value | P Value |
| Obese class I | 2.78 | 0.77 to 4.80 | .007 | 5.71 | 3.37 to 8.05 | < .001 | |
| Obese class II | 4.21 | 1.33 to 7.08 | .004 | 9.00 | 5.56 to 12.44 | < .001 | |
| Obese class III | 8.17 | 3.74 to 12.60 | < .001 | 10.65 | 6.96 to 14.33 | < .001 | |
| P value for trend | < .001 | < .001 | .043 | ||||
| NHW Race | Black Race | ||||||
|---|---|---|---|---|---|---|---|
| 6MWD, ft | OR | 95% CI | P Value | OR | 95% CI | P Value | |
| Obese class I | −83.84 | −114.66 to −53.02 | < .001 | −29.95 | −91.33 to 31.42 | .338 | |
| Obese class II | −175.89 | −220.20 to −131.57 | < .001 | −80.67 | −170.49 to 9.14 | .078 | |
| Obese class III | −359.45 | −417.53 to −301.37 | < .001 | −245.55 | −355.34 to −135.76 | < .001 | |
| P value for tend | < .001 | < .001 | .011 | ||||
| FEV1 ≥ 50% Predicted | FEV1 < 50% Predicted | ||||||
|---|---|---|---|---|---|---|---|
| SGRQ total score | β | 95% CI | P Value | β | 95% CI | P Value | P Value |
| Obese class I | 4.84 | 2.61 to 7.06 | < .001 | 2.37 | 0.12 to 4.62 | .039 | |
| Obese class II | 8.53 | 5.36 to 11.70 | < .001 | 2.03 | −1.21 to 5.27 | .218 | |
| Obese class III | 11.62 | 7.54 to 15.71 | < .001 | 5.19 | 1.08 to 9.30 | .013 | |
| P value for tend | < .001 | .003 | .001 | ||||
| Severe AECOPD | OR | 95% CI | P Value | OR | 95% CI | P Value | P Value |
| Obese class I | 1.35 | 1.00 to 1.83 | .049 | 0.90 | 0.67 to 1.20 | .470 | |
| Obese class II | 1.43 | 0.95 to 2.15 | .089 | 0.95 | 0.63 to 1.42 | .803 | |
| Obese class III | 1.81 | 1.11 to 2.95 | .017 | 1.20 | 0.73 to 1.97 | .474 | |
| P value for tend | .004 | .886 | .050 | ||||
Sex models are adjusted for age, race, obesity class, education, baseline FEV1 to height squared ratio, smoking status, and smoking pack-y. Race models are adjusted for age, sex, obesity class, education, baseline FEV1 to height squared ratio, smoking status, and smoking pack-y. Lung function models are adjusted for age, sex, race, obesity class, education, smoking status, and smoking pack-y. See Table 1 and 3 legends for expansion of other abbreviations.
Discussion
In this large, well-characterized cohort of individuals with COPD and GOLD stages 2 through 4 severity of airflow obstruction, obesity was prevalent, affecting approximately one-third of the population. Increasing severity of obesity was associated with worse COPD morbidity. We show that obesity is not only linked to subjective outcomes, such as worse QOL and dyspnea, but also to increased risk of severe AECOPD. The link between obesity and exacerbations may be partly explained by increased prevalence of comorbidities among obese individuals compared with normal/overweight individuals. We also show that increasing severity of obesity adversely impacts outcomes in a dose-dependent fashion, including those with class I obesity, but most dramatically affecting those with class III obesity. Furthermore, obesity is more likely to be associated with worse QOL among women compared with men, and more likely to lead to decreased functional status among NHW compared with black participants; however, generally, the link between obesity and worse COPD outcomes was consistent regardless of sex or race.
Our findings add to the existing understanding of the obesity to COPD relationship by reporting the dose-dependent impact of obesity on markers of QOL, symptom burden, and disease severity of COPD. The clinical characteristics and size of our study population allow the reporting of prevalence and morbidity associated with obesity within a well-defined cohort of patients with COPD. Estimates of obesity prevalence among individuals with COPD vary widely from 5% to 55%.5, 6, 7, 8, 9 The prevalence of obesity may be higher in those with preserved ratio impaired spirometry compared with those with COPD.48 The prevalence of obesity (34%) and severe obesity (BMI ≥ 40 kg/m2; 5.1%) in our cohort was similar to the general US population.2, 3, 49 Obese individuals reported worse COPD outcomes when compared with normal/overweight individuals, including respiratory-specific and general QOL, exercise tolerance, and dyspnea. These results are consistent with the literature linking obesity to reduced QOL,50 impaired exercise tolerance,50, 51 and dyspnea.50, 52 Our findings extend prior reports through the observation of dose-dependent detriments in adverse effects in response to increasing obesity class, with significant impact occurring even at the lowest obesity class. The strength of obesity-COPD association is further supported by the magnitude of our results, which exceeded the minimum clinically important difference (MCID)53 for several outcomes. For example, although individuals with class I obesity had a 4-point increase or worsening, in respiratory-specific QOL (SGRQ) compared with normal/overweight individuals (the SGRQ MCID is 4), those with class III obesity demonstrated a 9.5-point increase. Similarly, although those with class I obesity walked 72 fewer feet than their normal/overweight counterparts on the 6MWD (the 6MWD MCID in COPD is 98 ft), those with class III obesity exhibited remarkably reduced exercise capacity, walking 330 fewer feet than their normal/overweight counterparts, which is > 3 times the MCID for 6MWD.54 Similarly, those with class I obesity were 1.4 times as likely to report greater levels of dyspnea (mMRC score ≥ 2, consistent with GOLD guidelines of symptomatic disease),42 whereas those with class III obesity were nearly 4 times as likely to report greater levels of dyspnea.
Our results also expand previous research by highlighting that obesity was linked to severe COPD exacerbations—a novel finding to our knowledge. Severe obesity has been shown to increase health-care utilization in other populations, by increasing risk for hospitalization, death from respiratory infections,55 and prolonging length of hospital stay.56 Obese individuals had increased comorbidity burden, and accounting for comorbidities did not eliminate the impact of obesity on respiratory symptoms and QOL; however, the association between obesity and severe AECOPD was attenuated after adjusting for this comorbidity count. Therefore, the link between obesity and severe AECOPD may be driven by the complexity of overlapping conditions among obese patients. Obesity is increasingly recognized to correlate with increased comorbidity burden,23, 32, 50 leading to complex management of multifactorial symptom expression and disease burden. Therefore, diagnosing comorbidities and understanding their role in the expression of COPD among obese individuals is critical to both their symptomatic and preventative management.
Overall, the contribution of obesity to worse outcomes was consistent across sex and race; however, the impact of obesity was greater in women compared with men regarding respiratory QOL and was greater among NHW compared with black participants regarding reduced functional status. Our findings support known sex imbalances in the experience of COPD.57, 58 A combination of smaller airway caliber and increased hyperresponsiveness,59 along with greater anxiety and depression,60 has been hypothesized to cause the disproportionate symptom burden experienced by women. Women also demonstrate altered leptin metabolism with higher secreted leptin levels per BMI strata.61 These increases in leptin secretion may contribute to sex differences in COPD pathogenesis through a pathway of chronic systemic inflammation.62, 63, 64 Race may further moderate the role of obesity in COPD. Variations in BMI and body fat distribution may explain the moderation by race/ethnicity65; however, there is a paucity of information regarding the role of obesity in COPD among black populations. Generally, obesity prevalence and severity were similar among black and NHW participants, and the impact of obesity on COPD morbidity was similarly impacted across race. The impact of race and sex on COPD-related morbidity among obese individuals requires further investigation of these understudied populations. In addition, we found that increasing obesity class was more strongly associated with worse respiratory-specific QOL and greater odds of severe AECOPD among those with healthier lung function. The reason that the negative impact of obesity on both symptomatic and severe exacerbation risk is greater among those with better lung function is unclear but provides a targeted population for more aggressive risk modification and further study.
This study has limitations. Participants were enrolled in an observational cohort; therefore, our findings may not be generalizable to the general clinical population. However, our recruitment from 21 centers did allow for a diverse sampling of the US population. Our cohort has relatively equal sex distribution and approximately 30% black participants, not typical of research cohorts often predominantly composed of NHW men, and allowed for the assessment of the impact of obesity by sex and race. Our results are cross sectional and cannot determine causality; however, biological mechanisms support the plausibility of our findings. Obesity alters lung mechanics and therefore may directly contribute to pulmonary symptoms and impairment.19 In addition, increased adipose may cause local tissue hypoxia and lead to chronic inflammation,20 which have been hypothesized to advance disease progression and worsen outcomes. BMI has been criticized as a crude marker of both weight and body habitus. The COPDGene study did not collect anthropometric measurements, such as waist-to-hip ratio, to allow a more in-depth examination of the role of body habitus; however, BMI is a readily available measurement routinely collected in the clinical arena; therefore, our findings may more easily be translated into practice.
Conclusions
Comorbid obesity and COPD is prevalent, and increasing obesity is associated with increased comorbidity, reduced QOL, impaired functional status, and increased risk for severe AECOPD. Importantly, even class I obesity adversely impacted COPD outcomes, with increasing severity of obesity associated with greater magnitude of deficits in a dose-dependent fashion. Patients with COPD should be assessed for comorbid obesity and closely monitored for COPD outcomes. Determination of the impact of weight loss on these outcomes in obese patients warrants investigation.
Acknowledgments
Author contributions: A. A. L. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. A. A. L., N. P., M. C. M., and N. N. H. contributed to the analysis plan, interpretation, and writing and editing of the report. M. B. D., A. M. B., N. A. H., V. K., G. L. K., M.-L. N. M., E. P. B., and R. A. W. contributed to the data interpretation and writing and editing of the report.
Financial/nonfinancial disclosures: None declared.
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Other contributions: We thank the Genetic Epidemiology of COPD (COPDGene) study participants and staff. A list of the COPDGene Investigators can be found in e-Appendix 1.
Additional information: The e-Appendix and e-Tables can be found in the Supplemental Materials section of the online article.
Footnotes
FUNDING/SUPPORT: This study was funded by the National Institutes of Health Genetic Epidemiology of COPD [Grant R01 HL089856] to Dr Silverman (PI) and [Grant R01 HL089897] to Dr Crapo (PI); the National Institutes of Health [Grant KL2 TR001077] to A. A. L., [Grant K23 HL123594] to N. P., [Grant K23 HL094696] to V. K., [Grants P50MD010431 and R01ES022607] to N. N. H. and M. C. M., and [Grant R01ES023500] to N. N. H.; the National Institutes of Health, National Heart, Lung, and Blood Institute [Grant 1K99HL121087-01A1] to M.-L. N. M.; the National Institutes of Health, National Center for Advancing Translational Sciences [Grant 4KL2TR001077-04] to E. P. B.; and the Environmental Protection Agency [Grant RD-83615001] to N. N. H. and M. C. M.
Supplementary Data
References
- 1.Centers for Disease Control and Prevention Chronic obstructive pulmonary disease among adults–United States, 2011. MMWR Morb Mortal Wkly Rep. 2012;61(46):938–943. [PubMed] [Google Scholar]
- 2.Mozaffarian D., Benjamin E.J., Go A.S. Heart disease and stroke statistics–2015 update: a report from the American Heart Association. Circulation. 2015;131(4):e29–e322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 3.Flegal K.M., Carroll M.D., Kit B.K., Ogden C.L. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999-2010. JAMA. 2012;307(5):491–497. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 4.Flegal K.M., Carroll M.D., Ogden C.L., Curtin L.R. Prevalence and trends in obesity among US adults, 1999-2008. JAMA. 2010;303(3):235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 5.Steuten L.M., Creutzberg E.C., Vrijhoef H.J., Wouters E.F. COPD as a multicomponent disease: inventory of dyspnoea, underweight, obesity and fat free mass depletion in primary care. Prim Care Respir J. 2006;15(2):84–91. doi: 10.1016/j.pcrj.2005.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eisner M.D., Blanc P.D., Sidney S. Body composition and functional limitation in COPD. Respir Res. 2007;8:7. doi: 10.1186/1465-9921-8-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Montes de Oca M., Talamo C., Perez-Padilla R. Chronic obstructive pulmonary disease and body mass index in five Latin America cities: the PLATINO study. Respir Med. 2008;102(5):642–650. doi: 10.1016/j.rmed.2007.12.025. [DOI] [PubMed] [Google Scholar]
- 8.O'Donnell D.E., Deesomchok A., Lam Y.M. Effects of BMI on static lung volumes in patients with airway obstruction. Chest. 2011;140(2):461–468. doi: 10.1378/chest.10-2582. [DOI] [PubMed] [Google Scholar]
- 9.Vozoris N.T., O'Donnell D.E. Prevalence, risk factors, activity limitation and health care utilization of an obese, population-based sample with chronic obstructive pulmonary disease. Can Respir J. 2012;19(3):e18–e24. doi: 10.1155/2012/732618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wagner P.D. Possible mechanisms underlying the development of cachexia in COPD. Eur Respir J. 2008;31(3):492–501. doi: 10.1183/09031936.00074807. [DOI] [PubMed] [Google Scholar]
- 11.Lainscak M., von Haehling S., Doehner W. Body mass index and prognosis in patients hospitalized with acute exacerbation of chronic obstructive pulmonary disease. J Cachexia Sarcopenia Muscle. 2011;2(2):81–86. doi: 10.1007/s13539-011-0023-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schols A.M., Slangen J., Volovics L., Wouters E.F. Weight loss is a reversible factor in the prognosis of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;157(6 Pt 1):1791–1797. doi: 10.1164/ajrccm.157.6.9705017. [DOI] [PubMed] [Google Scholar]
- 13.Katsura H., Yamada K., Kida K. Both generic and disease specific health-related quality of life are deteriorated in patients with underweight COPD. Respir Med. 2005;99(5):624–630. doi: 10.1016/j.rmed.2004.09.017. [DOI] [PubMed] [Google Scholar]
- 14.Landbo C., Prescott E., Lange P., Vestbo J., Almdal T.P. Prognostic value of nutritional status in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999;160(6):1856–1861. doi: 10.1164/ajrccm.160.6.9902115. [DOI] [PubMed] [Google Scholar]
- 15.Kastorini C.M., Panagiotakos D.B. The obesity paradox: methodological considerations based on epidemiological and clinical evidence–new insights. Maturitas. 2012;72(3):220–224. doi: 10.1016/j.maturitas.2012.04.012. [DOI] [PubMed] [Google Scholar]
- 16.Prescott E., Almdal T., Mikkelsen K.L., Tofteng C.L., Vestbo J., Lange P. Prognostic value of weight change in chronic obstructive pulmonary disease: results from the Copenhagen City Heart Study. Eur Respir J. 2002;20(3):539–544. doi: 10.1183/09031936.02.00532002. [DOI] [PubMed] [Google Scholar]
- 17.Rutten E.P., Calverley P.M., Casaburi R. Changes in body composition in patients with chronic obstructive pulmonary disease: Do they influence patient-related outcomes? Ann Nutr Metab. 2013;63(3):239–247. doi: 10.1159/000353211. [DOI] [PubMed] [Google Scholar]
- 18.O'Donnell D.E., Ciavaglia C.E., Neder J.A. When obesity and chronic obstructive pulmonary disease collide: physiological and clinical consequences. Ann Am Thorac Soc. 2014;11(4):635–644. doi: 10.1513/AnnalsATS.201312-438FR. [DOI] [PubMed] [Google Scholar]
- 19.Koenig S.M. Pulmonary complications of obesity. Am J Med Sci. 2001;321(4):249–279. doi: 10.1097/00000441-200104000-00006. [DOI] [PubMed] [Google Scholar]
- 20.Franssen F.M., O'Donnell D.E., Goossens G.H., Blaak E.E., Schols A.M. Obesity and the lung: 5. Obesity and COPD. Thorax. 2008;63(12):1110–1117. doi: 10.1136/thx.2007.086827. [DOI] [PubMed] [Google Scholar]
- 21.McClean K.M., Kee F., Young I.S., Elborn J.S. Obesity and the lung: 1. Epidemiology. Thorax. 2008;63(7):649–654. doi: 10.1136/thx.2007.086801. [DOI] [PubMed] [Google Scholar]
- 22.Katz P., Iribarren C., Sanchez G., Blanc P.D. Obesity and functioning among individuals with chronic obstructive pulmonary disease (COPD) COPD. 2016;13(3):352–359. doi: 10.3109/15412555.2015.1087991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Divo M.J., Cabrera C., Casanova C. Comorbidity distribution, clinical expression and survival in COPD patients with different body mass index. Chronic Obstr Pulm Dis. 2014;1(2):229–238. doi: 10.15326/jcopdf.1.2.2014.0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blum A., Simsolo C., Sirchan R., Haiek S. “Obesity paradox” in chronic obstructive pulmonary disease. Isr Med Assoc J. 2011;13(11):672–675. [PubMed] [Google Scholar]
- 25.Vestbo J., Prescott E., Almdal T. Body mass, fat-free body mass, and prognosis in patients with chronic obstructive pulmonary disease from a random population sample: findings from the Copenhagen City Heart Study. Am J Respir Crit Care Med. 2006;173(1):79–83. doi: 10.1164/rccm.200506-969OC. [DOI] [PubMed] [Google Scholar]
- 26.Cecere L.M., Littman A.J., Slatore C.G. Obesity and COPD: associated symptoms, health-related quality of life, and medication use. COPD. 2011;8(4):275–284. doi: 10.3109/15412555.2011.586660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haldar S., Chia S.C., Henry C.J. Body composition in Asians and Caucasians: comparative analyses and influences on cardiometabolic outcomes. Adv Food Nutr Res. 2015;75:97–154. doi: 10.1016/bs.afnr.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 28.Katz D.A., McHorney C.A., Atkinson R.L. Impact of obesity on health-related quality of life in patients with chronic illness. J Gen Intern Med. 2000;15(11):789–796. doi: 10.1046/j.1525-1497.2000.90906.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mannino D.M., Homa D.M., Akinbami L.J., Ford E.S., Redd S.C. Chronic obstructive pulmonary disease surveillance–United States, 1971-2000. MMWR Surveill Summ. 2002;51(6):1–16. [PubMed] [Google Scholar]
- 30.Regan E.A., Hokanson J.E., Murphy J.R. Genetic epidemiology of COPD (COPDGene) study design. COPD. 2010;7(1):32–43. doi: 10.3109/15412550903499522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martinez C.H., Mannino D.M., Divo M.J. Defining COPD-related comorbidities, 2004-2014. Chronic Obstr Pulm Dis. 2014;1(1):51–63. doi: 10.15326/jcopdf.1.1.2014.0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Putcha N., Han M.K., Martinez C.H. Comorbidities of COPD have a major impact on clinical outcomes, particularly in African Americans. Chronic Obstr Pulm Dis. 2014;1(1):105–114. doi: 10.15326/jcopdf.1.1.2014.0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Putcha N., Puhan M.A., Drummond M.B. A simplified score to quantify comorbidity in COPD. PloS One. 2014;9(12):e114438. doi: 10.1371/journal.pone.0114438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jones P.W., Quirk F.H., Baveystock C.M. The St George's Respiratory Questionnaire. Respir Med. 1991;85(suppl B):25–31. doi: 10.1016/s0954-6111(06)80166-6. [DOI] [PubMed] [Google Scholar]
- 35.Barr J.T., Schumacher G.E., Freeman S., LeMoine M., Bakst A.W., Jones P.W. American translation, modification, and validation of the St. George's Respiratory Questionnaire. Clin Ther. 2000;22(9):1121–1145. doi: 10.1016/S0149-2918(00)80089-2. [DOI] [PubMed] [Google Scholar]
- 36.Ware J.E., Jr., Sherbourne C.D. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30(6):473–483. [PubMed] [Google Scholar]
- 37.McHorney C.A., Ware J.E., Jr., Raczek A.E. The MOS 36-Item Short-Form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care. 1993;31(3):247–263. doi: 10.1097/00005650-199303000-00006. [DOI] [PubMed] [Google Scholar]
- 38.Butland R.J., Pang J., Gross E.R., Woodcock A.A., Geddes D.M. Two-, six-, and 12-minute walking tests in respiratory disease. BMJ. 1982;284(6329):1607–1608. doi: 10.1136/bmj.284.6329.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Solway S., Brooks D., Lacasse Y., Thomas S. A qualitative systematic overview of the measurement properties of functional walk tests used in the cardiorespiratory domain. Chest. 2001;119(1):256–270. doi: 10.1378/chest.119.1.256. [DOI] [PubMed] [Google Scholar]
- 40.Fletcher C.M. The clinical diagnosis of pulmonary emphysema; an experimental study. Proc R Soc Med. 1952;45(9):577–584. [PubMed] [Google Scholar]
- 41.Bestall J.C., Paul E.A., Garrod R., Garnham R., Jones P.W., Wedzicha J.A. Usefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax. 1999;54(7):581–586. doi: 10.1136/thx.54.7.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Global Initiative for Chronic Obstructive Lung Disease. Global Strategy for Diagnosis, Management, and Prevention of COPD – 2016. http://goldcopd.org/global-strategy-diagnosis-management-prevention-copd-2016/. Accessed September 19, 2016.
- 43.Celli B.R., MacNee W., Force A.E. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J. 2004;23(6):932–946. doi: 10.1183/09031936.04.00014304. [DOI] [PubMed] [Google Scholar]
- 44.Han M.K., Muellerova H., Curran-Everett D. GOLD 2011 disease severity classification in COPDGene: a prospective cohort study. Lancet Respir Med. 2013;1(1):43–50. doi: 10.1016/S2213-2600(12)70044-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.World Health Organization. BMI classification. http://apps.who.int/bmi/index.jsp?introPage=intro_3.html. Accessed May 5, 2015.
- 46.Executive summary of the clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults. Arch Intern Med. 1998;158(17):1855–1867. doi: 10.1001/archinte.158.17.1855. [DOI] [PubMed] [Google Scholar]
- 47.Checkley W., Foreman M.G., Bhatt S.P. Differences between absolute and predicted values of forced expiratory volumes to classify ventilatory impairment in chronic obstructive pulmonary disease. Respir Med. 2016;111:30–38. doi: 10.1016/j.rmed.2015.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wan E.S., Castaldi P.J., Cho M.H. Epidemiology, genetics, and subtyping of preserved ratio impaired spirometry (PRISm) in COPDGene. Respir Res. 2014;15:89. doi: 10.1186/s12931-014-0089-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ogden C.L., Carroll M.D., Curtin L.R., McDowell M.A., Tabak C.J., Flegal K.M. Prevalence of overweight and obesity in the United States, 1999-2004. JAMA. 2006;295(13):1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 50.Garcia-Rio F., Soriano J.B., Miravitlles M. Impact of obesity on the clinical profile of a population-based sample with chronic obstructive pulmonary disease. PloS One. 2014;9(8):e105220. doi: 10.1371/journal.pone.0105220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rodriguez D.A., Garcia-Aymerich J., Valera J.L. Determinants of exercise capacity in obese and non-obese COPD patients. Respir Med. 2014;108(5):745–751. doi: 10.1016/j.rmed.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 52.Launois C., Barbe C., Bertin E. The modified Medical Research Council scale for the assessment of dyspnea in daily living in obesity: a pilot study. BMC Pulm Med. 2012;12:61. doi: 10.1186/1471-2466-12-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jones P.W. St. George's Respiratory Questionnaire: MCID. COPD. 2005;2(1):75–79. doi: 10.1081/copd-200050513. [DOI] [PubMed] [Google Scholar]
- 54.Polkey M.I., Spruit M.A., Edwards L.D. Six-minute-walk test in chronic obstructive pulmonary disease: minimal clinically important difference for death or hospitalization. Am J Respir Crit Care Med. 2013;187(4):382–386. doi: 10.1164/rccm.201209-1596OC. [DOI] [PubMed] [Google Scholar]
- 55.Morgan O.W., Bramley A., Fowlkes A. Morbid obesity as a risk factor for hospitalization and death due to 2009 pandemic influenza A(H1N1) disease. PloS One. 2010;5(3):e9694. doi: 10.1371/journal.pone.0009694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hauck K., Hollingsworth B. The impact of severe obesity on hospital length of stay. Med Care. 2010;48(4):335–340. doi: 10.1097/MLR.0b013e3181ca3d85. [DOI] [PubMed] [Google Scholar]
- 57.de Torres J.P., Casanova C., Hernandez C., Abreu J., Aguirre-Jaime A., Celli B.R. Gender and COPD in patients attending a pulmonary clinic. Chest. 2005;128(4):2012–2016. doi: 10.1378/chest.128.4.2012. [DOI] [PubMed] [Google Scholar]
- 58.Cote C.G., Chapman K.R. Diagnosis and treatment considerations for women with COPD. Int J Clin Pract. 2009;63(3):486–493. doi: 10.1111/j.1742-1241.2008.01987.x. [DOI] [PubMed] [Google Scholar]
- 59.Kanner R.E., Connett J.E., Altose M.D. Gender difference in airway hyperresponsiveness in smokers with mild COPD. The Lung Health Study. Am J Respir Crit Care Med. 1994;150(4):956–961. doi: 10.1164/ajrccm.150.4.7921469. [DOI] [PubMed] [Google Scholar]
- 60.Di Marco F., Verga M., Reggente M. Anxiety and depression in COPD patients: the roles of gender and disease severity. Respir Med. 2006;100(10):1767–1774. doi: 10.1016/j.rmed.2006.01.026. [DOI] [PubMed] [Google Scholar]
- 61.Breyer M.K., Rutten E.P., Vernooy J.H. Gender differences in the adipose secretome system in chronic obstructive pulmonary disease (COPD): a pivotal role of leptin. Respir Med. 2011;105(7):1046–1053. doi: 10.1016/j.rmed.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 62.Bastard J.P., Maachi M., Lagathu C. Recent advances in the relationship between obesity, inflammation, and insulin resistance. Eur Cytokine Netw. 2006;17(1):4–12. [PubMed] [Google Scholar]
- 63.Sood A. Obesity, adipokines, and lung disease. J Appl Physiol. 2010;108(3):744–753. doi: 10.1152/japplphysiol.00838.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Broekhuizen R., Vernooy J.H., Schols A.M., Dentener M.A., Wouters E.F. Leptin as local inflammatory marker in COPD. Respir Med. 2005;99(1):70–74. doi: 10.1016/j.rmed.2004.03.029. [DOI] [PubMed] [Google Scholar]
- 65.Carroll J.F., Chiapa A.L., Rodriquez M. Visceral fat, waist circumference, and BMI: impact of race/ethnicity. Obesity. 2008;16(3):600–607. doi: 10.1038/oby.2007.92. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.