Abstract
Background
Mortality after smoke inhalation–associated acute lung injury (SI-ALI) remains substantial. Age and burn surface area are risk factors of mortality, whereas the impact of patient- and center-level variables and treatments on survival are unknown.
Methods
We performed a retrospective cohort study of burn and non-burn centers at 68 US academic medical centers between 2011 and 2014. Adult inpatients with SI-ALI were identified using an algorithm based on a billing code for respiratory conditions from smoke inhalation who were mechanically ventilated by hospital day 4, with either a length-of-stay ≥ 5 days or death within 4 days of hospitalization. Predictors of in-hospital mortality were identified using logistic regression. The primary outcome was the odds ratio for in-hospital mortality.
Results
A total of 769 patients (52.9 ± 18.1 years) with SI-ALI were analyzed. In-hospital mortality was 26% in the SI-ALI cohort and 50% in patients with ≥ 20% surface burns. In addition to age > 60 years (OR 5.1, 95% CI 2.53-10.26) and ≥ 20% burns (OR 8.7, 95% CI 4.55-16.75), additional risk factors of in-hospital mortality included initial vasopressor use (OR 5.0, 95% CI 3.16-7.91), higher diagnostic-related group–based risk-of-mortality assignment and lower hospital bed capacity (OR 2.3, 95% CI 1.23-4.15). Initial empiric antibiotics (OR 0.93, 95% CI 0.58-1.49) did not impact survival. These new risk factors improved mortality prediction by 9.9% (P < .001).
Conclusions
In addition to older age and major surface burns, mortality in SI-ALI is predicted by initial vasopressor use, higher diagnostic-related group–based risk-of-mortality assignment, and care at centers with < 500 beds, but not by initial antibiotic therapy.
Key Words: adult respiratory distress syndrome, burns, epidemiology, risk factors, smoke inhalation
Abbreviations: ABA, American Burn Association; APR-DRG, All Patient Refined Diagnosis-Related Group Classification System; AUC, area under the curve; CDB/RM, clinical database/resource manager; ICD-9, International Classification of Diseases, version 9; SI-ALI, smoke inhalation-associated acute lung injury; TBSA, total burn surface area; UHC, University Health System Consortium
Each year, burn injuries account for nearly 265,000 deaths globally and 3,500 deaths in the United States.1, 2 Ninety percent of burn-related mortality is partially attributable to smoke inhalation–associated acute lung injury (SI-ALI).3 After smoke inhalation, direct cellular injury, airway obstruction, regional blood flow changes, toxin- and cytokine-mediated inflammation, and bacterial infection contribute to ALI.4, 5 In contrast to cutaneous burns, few evidence-based guidelines exist for managing SI-ALI because of a lack of consensus on defining criteria resulting in wide variations in clinical practice.6 Despite advances in burn care and rehabilitation, SI-ALI–related mortality, 21.3% in 2015, remains substantial.7
Improving SI-ALI survival may be feasible by individualizing supportive care and prioritizing innovative therapies for high-risk victims.8 Identifying risk factors of mortality in SI-ALI may facilitate risk stratification, enhance inter-institutional comparisons, and enable benchmarking of quality care metrics. The best-characterized risk factors of mortality in SI-ALI are increasing age and burn size.7, 9 The effects of other patient and center-level determinants of survival remain underexplored.
Several health-care data repositories are available to investigate risk factors of mortality in SI-ALI.3, 7, 10 The National Burn Repository of the American Burn Association (ABA) provides comprehensive data on burn victims nationally at burn centers, but is limited by missing data and duplicate records.7, 11 The Nationwide Inpatient Sample, an administrative data source covering one in five US nonfederal hospital discharges lacks details on some key variables.3 Recent quality improvement–targeted enhancements to some administrative databases, such as encounter-level, date-stamped interventions, have facilitated benchmarking, surveillance, and comparative-effectiveness research. Characterizing these interventions in patients with SI-ALI enables evaluation of unique processes of burn care otherwise not available. We developed an operational algorithm to identify patients with putative SI-ALI in an enhanced administrative database and investigated clinical risk factors of mortality.
Methods
Study Design
We performed a retrospective cohort study to identify inpatient encounters for SI-ALI using a prespecified algorithm to query the clinical database/resource manager (CDB/RM) of the University Health System Consortium (UHC; Chicago, IL), which is a collaborative of 117 academic medical centers and 300 affiliates, whose CDB/RM contains inpatient billing records and charges for drugs, laboratory, and other services by mapping charge masters of participating institutions. It has been a data source for several epidemiologic publications.12 The study was exempted from institutional board review by the National Institutes of Health Office of Human Subjects Research Protections (Bethesda, MD).
SI-ALI Operational Algorithm
Following a trend analysis of International Classification of Diseases, version-9 (ICD-9), respiratory system burn injury diagnosis codes (e-Table 1; Fig 1), adult (age ≥ 18 years) inpatient encounters discharged between October 2011 and March 2014, and associated with diagnosis 508.2 (respiratory conditions from smoke inhalation) were identified. Outside hospital transfers were analyzed separately. Length of stay ≥ 5 days (or < 5 days if death occurred) with mechanical ventilation within 4 days of hospital admission were added as inclusion criteria to enhance capture of SI-ALI (Fig 2). Patients with upper airway edema/obstruction following smoke exposure are often intubated but may not develop ALI and most are extubated within 1 to 2 days.13 Patients intubated but discharged alive within 4 days were excluded. We limited the analysis to centers providing charge data to ensure availability of date-stamped medication administrations. For patients with multiple encounters with diagnosis 508.2, only the initial encounter was selected.
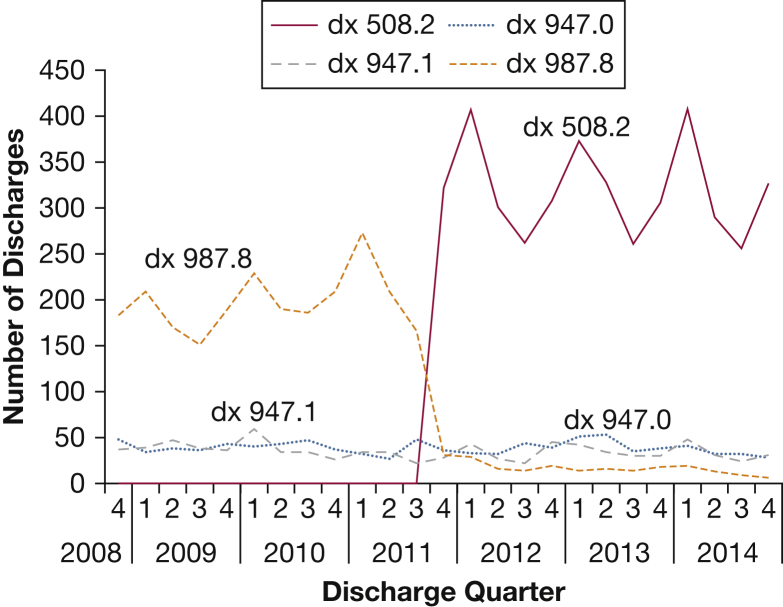
Figure 1.
Trends in four respiratory system burn injury diagnosis codes among discharges from 154 academic medical centers and affiliates between October 2008 and October 2014. A trend analysis of the most common International Classification of Diseases, version 9, diagnosis codes representing respiratory system burn injury in discharge abstracts at 154 centers in the University System Health Consortium was performed for the period between October 2008 and October 2014. The diagnosis code diagnosis (dx) 508.2 (respiratory conditions from smoke inhalation), which was introduced in October 2011, nearly replaced dx 987.8 (toxic effect of unspecified gas, fume, or vapor) as being the most frequently assigned. Diagnosis 947.0 (burn of mouth and pharynx) and dx 947.1 (burn of larynx, trachea and lung) remained relatively unchanged over time.
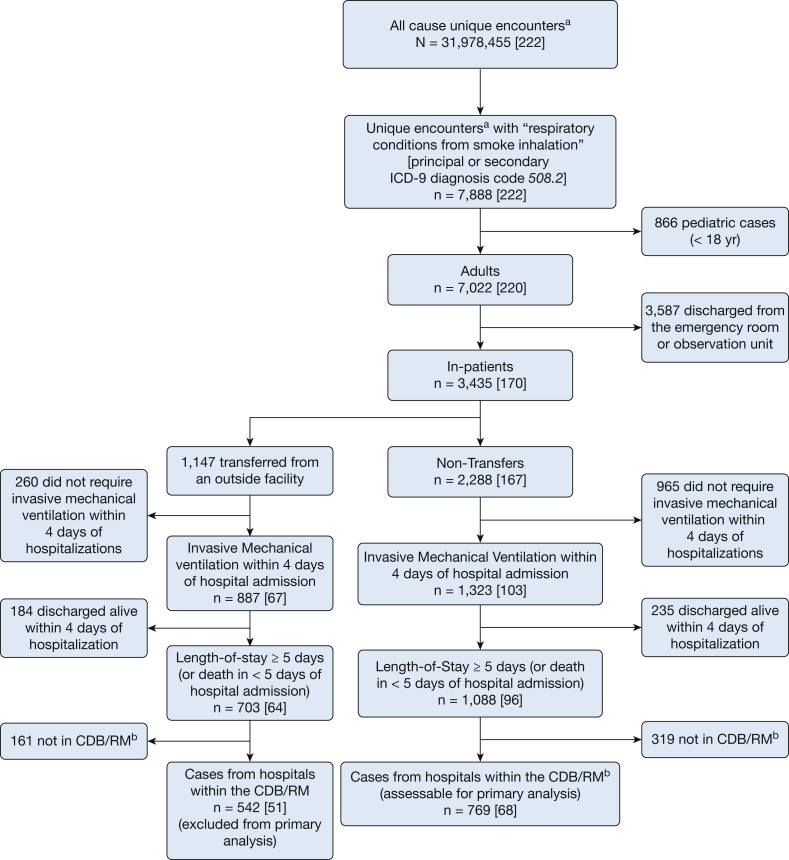
Figure 2.
Flowchart describing the selection of patients with smoke inhalation-associated acute lung injury based on a prespecified algorithm. aDischarged between October 2011 and March 2014. bContains encounter-level date-stamped charges on medications administered and services rendered. CDB/RM = clinical database/resource manager of the University Health System Consortium; ICD-9 = International Classification of Diseases, version 9.
Demographic (ie, age and sex) information was recorded and burn surface area and Charlson Comorbidity Index calculated using ICD-9 codes.14 The 3M All Patient Refined (APR) Diagnosis-Related Group (DRG) Classification System was used for stratification of acute illness severity based on projected risk of in-hospital mortality at admission. It has been used for risk adjustment of hospitalized patients in performance measurements and research.15 Continuous invasive mechanical ventilation was identified by ICD-9 procedure codes: 96.71 or 96.72. Labeling charges for antibiotics and vasopressors within the first 2 days of hospitalization were considered “initial” use. Hospital characteristics were obtained from UHC hospital profile data and the ABA website regarding burn center designation.2
Statistical Analysis
All patient baseline characteristics were treated as categorical variables and reported as frequencies and percentages. The exposures of interest were candidate risk factors of mortality in SI-ALI (as defined previously); the outcome of interest was in-hospital mortality. We performed a forward stepwise variable selection (with an entry criterion of P < .05) in a multivariable logistic regression model to investigate the association of potential risk factors with in-hospital mortality. All variables with P < .20 in univariate analysis were selected in the stepwise variable selection. Variables for which the effect on mortality was specifically sought but that failed to be selected were added in the final model. The absence of a gold-standard definition for or prospective studies in patients with SI-ALI precluded estimations of effect size and, hence, sample size calculation. Subgroup analyses were performed on three subsets: (1) mechanical ventilation ≥ 96 h, (2) burns involving ≥ 20% total burn surface area (TBSA), and (3) any initial vasopressor requirement. A secondary analysis was performed on patients with SI-ALI who were transferred from other facilities. Receiver-operator characteristic curves assessed the discrimination of mortality in the primary and secondary analyses. The increase in the area under the receiver-operator characteristic curve (ΔAUC) represented the prediction increment offered by newly identified risk factors over age and burn surface area. This metric was chosen over the net reclassification index because the index may overestimate the improvement from uninformative risk factors.16 All analyses were performed using SAS, version 9.3. A two-sided P value of < .05 was considered statistically significant.
Results
Derivation of the SI-ALI Cohort Using the Operational Algorithm
Between October 2011 and March 2014, 1,088 cases at 96 hospitals fulfilled the predetermined criteria for putative SI-ALI. Among these, 769 cases treated at 68 hospitals providing data on medication administration were included in the primary analysis. Figure 2 shows the case selection algorithm.
Baseline Characteristics
Patient- and center-level characteristics are detailed in Table 1. The mean age was 52.8 ± 18.1 and men comprised 61% of the cases. Forty-eight percent had no comorbidities (Charlson Comorbidity Index = 0) and > 75% had a major or extreme admission risk-of-mortality assignment. Nearly 30% of SI-ALI cases received initial vasopressor therapy and 31% received initial systemic antibacterial therapy, with at least one of 31 agents (e-Figs 1, 2). Vancomycin was the most commonly prescribed initial antibiotic, followed by cefazolin and piperacillin-tazobactam.
Table 1.
Baseline Characteristics of the SI-ALI Cohort: Overall and by TBSA Category
| Variable | All SI-ALI N = 769 (%) |
SI-ALI Subset With 0% TBSA Burns n = 200 (%) |
SI-ALI Subset With 1%-19% TBSA Burns n = 280 (%) |
SI-ALI Subset With ≥20% TBSA Burns n = 289 (%) |
|---|---|---|---|---|
| Age, y | ||||
| 18-40 | 196 (25.5) | 36 (18.0) | 69 (24.6) | 91 (31.5) |
| 41-50 | 130 (16.9) | 38 (19.0) | 39 (13.9) | 53 (18.3) |
| 51-60 | 179 (23.3) | 58 (29.0) | 63 (22.5) | 58 (20.1) |
| 61-70 | 143 (18.6) | 30 (15.0) | 65 (23.2) | 48 (16.6) |
| >70 | 121 (15.7) | 38 (19.0) | 44 (15.7) | 39 (13.5) |
| Sex | ||||
| Male | 468 (60.9) | 104 (52.0) | 171 (61.1) | 193 (66.8) |
| Female | 301 (39.1) | 96 (48.0) | 109 (38.9) | 96 (33.2) |
| Patient region | ||||
| Midwest | 224 (29.1) | 65 (32.5) | 85 (30.4) | 74 (25.6) |
| Northeast | 141 (18.3) | 44 (22.0) | 58 (20.7) | 39 (13.5) |
| South | 242 (31.5) | 47 (23.5) | 83 (29.6) | 112 (38.8) |
| West | 162 (21.1) | 44 (22.0) | 54 (19.3) | 64 (22.1) |
| 3M APR-DRG Admission ROM Classification Scale | ||||
| Minor | 49 (6.4) | 22 (11.0) | 27 (9.6) | 0 (0.0) |
| Moderate | 135 (17.6) | 46 (23.0) | 65 (23.2) | 24 (8.3) |
| Major | 306 (39.8) | 78 (39.0) | 126 (45.0) | 102 (35.3) |
| Extreme | 279 (36.3) | 54 (27.0) | 62 (22.1) | 163 (56.4) |
| Charlson Comorbidity Index | ||||
| 0 | 368 (47.9) | 81 (40.5) | 123 (43.9) | 164 (56.7) |
| 1-2 | 304 (39.5) | 95 (47.5) | 109 (38.9) | 100 (34.6) |
| >2 | 97 (12.6) | 24 (12.0) | 48 (17.1) | 25 (8.7) |
| Initial vasopressor usea | 231 (30.0) | 42 (21.0) | 59 (21.1) | 130 (45.0) |
| Initial empiric systemic antibacterial therapya | 241 (31.3) | 63 (31.5) | 92 (32.9) | 86 (29.8) |
| Hospital bed capacity | ||||
| < 500 beds | 233 (30.3) | 76 (38.0) | 82 (29.3) | 75 (9.8) |
| ≥ 500 beds | 536 (69.7) | 124 (62.0) | 198 (70.7) | 214 (74.1) |
| Burn center status (no. of centers) | ||||
| ABA-verified burn center (27) | 468 (60.9) | 90 (45.0) | 182 (65.0) | 196 (67.8) |
| Self-reported burn center (17) | 234 (30.4) | 70 (35.0) | 87 (31.1) | 77 (26.6) |
| Non-burn center (24) | 67 (8.7) | 40 (20.0) | 11 (3.9) | 16 (5.5) |
APR-DRG = All Patient Refined Diagnoses-Related Group Classification System; ROM = risk of mortality; SI-ALI = smoke inhalation-associated acute lung injury; TBSA = total burn surface area.
Within 2 days of hospital admission.
Centers were well-distributed by region, and 70% of cases were admitted to hospitals with ≥ 500 beds. Sixty (88%) of the 68 hospitals included in the primary analysis were academic medical centers, whereas eight (12%) were affiliated community hospitals. Forty-four of the 68 hospitals were burn centers, in which 91% (702) of SI-ALI cases were initially admitted. Sixty-seven percent (468) were cared for at ABA-verified burn centers and the remaining (234) at self-described burn centers.
Assessment of Risk Factors
In-hospital mortality was 26% in the SI-ALI cohort (199/769) and 50% among patients with ≥ 20% surface burns (144/289). In-hospital mortality among adult inpatients with diagnosis 508.2 without mechanical ventilation was 2.26% (10/443). Five of the 10 variables were statistically significant; age and TBSA categories, risk-of-mortality assignment, initial vasopressor therapy, and hospital bed size (Table 2).
Table 2.
Multivariate Logistic Regression Identifying Risk Factors of In-Hospital Mortality in 769 Patients With SI-ALI at 68 Centers
| Variable | OR | 95% CI | P Value |
|---|---|---|---|
| Age, y (reference: 18-40) | |||
| 41-50 | 1.34 | 0.66-2.74 | .414 |
| 51-60 | 1.31 | 0.65-2.62 | .453 |
| 61-70 | 5.10 | 2.53-10.25 | < .001 |
| >70 | 6.61 | 3.22-13.59 | < .001 |
| Sex (reference: female) | 0.88 | 0.56-1.39 | .585 |
| Patient region (reference: West) | |||
| Midwest | 0.75 | 0.40-1.43 | .384 |
| Northeast | 1.42 | 0.66-3.04 | .373 |
| South | 1.05 | 0.54-2.06 | .882 |
| 3M APR-DRG Admission Risk of Mortality Scale (reference: extreme) | |||
| Minor or moderate | 0.15 | 0.07-0.34 | < .001 |
| Major | 0.29 | 0.18-0.47 | < .001 |
| Charlson Comorbidity Index (reference: 0) | |||
| 1-2 | 0.85 | 0.52-1.40 | .529 |
| >2 | 0.74 | 0.36-1.54 | .421 |
| TBSA category (reference: 0%) | |||
| 1%-19% | 1.15 | 0.58-2.29 | .690 |
| ≥20% | 8.73 | 4.55-16.75 | < .001 |
| Initial vasopressor use (reference: none)a | 5.00 | 3.16-7.91 | < .001 |
| Initial empiric systemic antibacterial therapy (reference: none)a | 0.93 | 0.58-1.49 | .773 |
| Hospital bed capacity (reference: ≥ 500 beds) | |||
| < 500 beds | 2.26 | 1.23-4.15 | .008 |
| Burn center status (reference: ABA burn center) | |||
| Self-reported burn center | 0.83 | 0.50-1.38 | .479 |
| Non-burn center | 0.84 | 0.36-1.95 | .684 |
ABA = American Burn Association. See the Table 1 legend for expansion of abbreviations.
Within 2 days of hospitalization.
Older patients (age ranges 61-70 and > 70) had 5.1- and 6.6-fold odds of death, respectively, compared with 18- to 40-year-old patients (OR 5.10 [95% CI 2.53-10.25] and 6.60 [95% CI 3.22-13.59]). Charlson comorbidity index, sex, and hospital region did not significantly influence mortality.
The odds of death were similar comparing SI-ALI cases of isolated inhalation injury to those involving < 20% TBSA (OR 1.15 [95% CI 0.58-2.29]). However, the odds of death rose more than 8 fold when concomitant burns involved ≥ 20% TBSA (OR 8.73 [95% CI 4.55-16.75]). With the extreme risk-of-mortality assignment as the reference, the ORs for corresponding minor or moderate as well as major categories remained low at 0.15 (0.07-0.34) and 0.29 (0.18-0.47), respectively. Cases requiring initial vasopressor therapy carried a 5 fold odds of death (OR 5.00 [3.16-7.91]) and the odds rose 29 fold in the absence of concomitant burns (OR 28.64 [4.44-184.82]) (e-Table 2). The odds of in-hospital mortality following care at centers with < 500 beds were higher compared with care at ≥ 500-bed hospitals (OR 2.26 [1.23-4.15]). However, the same were not significantly different at self-reported (OR 0.83 [0.50-1.38]) and non-burn centers (OR 0.84 [0.36-1.95]) when compared with ABA-verified burn centers. The OR of mortality for combined ABA and self-reported burn centers compared with non-burn center cases continued to lack statistical significance in a reanalysis (OR 1.121 [0.491-2.558]). The prescribing pattern of initial antibiotic therapy widely varied among centers (e-Figs 1, 2). Receipt of initial systemic antibacterial therapy had no effect on in-hospital mortality across all SI-ALI cases (OR 0.93 [0.58-1.49]) as well as across all sensitivity analyses (e-Tables 2-6).
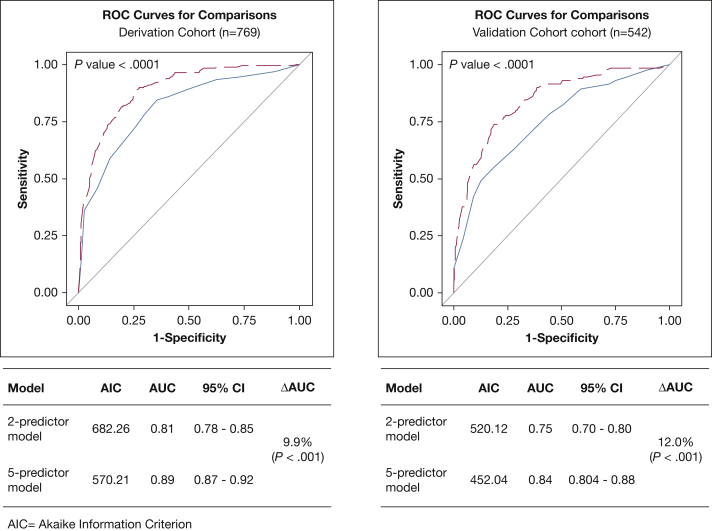
The secondary analysis was performed on 542 patients identified by algorithm filters at 51 hospitals providing medication data (Fig 2, e-Tables 7-8). In the primary analysis (n = 769), the AUC for the two-variable model (ie, age and TBSA) was 0.81 (95% CI 0.76-0.85) vs 0.89 (95% CI 0.87-0.92) for the five-variable model (ie, age, TBSA, risk-of-mortality assignment, initial vasopressor use, and hospital size) demonstrating the mortality risk-prediction increment (ΔAUC) of 9.9% (P < .001; Fig 3A). In the secondary analysis (n = 542), the AUC for the two-variable model was 0.75 (95% CI 0.70-0.80) vs 0.84 (95% CI 0.804-0.88) for the five-variable model (ΔAUC = 12.0%; P < .001) (Fig 3B).
Figure 3.
Receiver-operator characteristic (ROC) curves showing improved discrimination using a afive-risk factor model compared with a btwo-risk factor model. A, Primary analysis of all patients with SI-ALI (n = 769): ΔAUC = 9.9% (P < .001). B, Secondary analysis of patients transferred from other facilities otherwise meeting SI-ALI criteria (n = 542): ΔAUC = 12.0% (P < .001). aAge, TBSA, 3M; all patients refined DRG admission ROM assignment, hospital size, and initial vasopressor use (IV norepinephrine, epinephrine, dopamine, vasopressin, and phenylephrine). bAge and TBSA. 3M = 3M All Patient Refined Diagnosis-Related Group Classification System; AUC = area under receiver-operator characteristic curve; ΔAUC = mortality risk-prediction increment; ROM = risk of mortality; SI-ALI = smoke inhalation-associated acute lung injury; TBSA = total burn surface area.
Discussion
We describe the largest study to date exploring systems of care and risk factors of mortality in SI-ALI in a cohort of burn and non-burn centers. In patients with SI-ALI, greater acute illness severity manifested by initial vasopressor use and/or higher APR-DRG risk-of-mortality assignment and care at < 500-bed hospitals predicted an increased risk of mortality. These three additional risk factors improved the predictability of in-hospital mortality in SI-ALI offered by age and burn size by 10%. Sex, Charlson Comorbidity Index, and initial systemic antibiotic use are not significant predictors of mortality. These data will enhance the identification and risk stratification of patients with SI-ALI.
Airway obstruction from soot, edema, sloughing, casts, and bronchoconstriction and greater fluid resuscitation needs make SI-ALI unique from ALI from other causes. Without standard diagnostic criteria of SI-ALI, we used an operational algorithm to identify cases in a large database based on clinical logic, identifying patients with a billing code for respiratory conditions from smoke inhalation that required intubation within 4 days of arrival. This filter will miss late-onset SI-ALI (> 5 days after exposure). We found only 3% of eligible cases being initially intubated on or after day 4. Furthermore, later mechanical ventilation may have been initiated for other reasons (eg, postsurgical recovery, health-care associated pneumonia).
The requirement of mechanical ventilation enhanced the likelihood of capturing SI-ALI. Mean mortality among patients with SI-ALI in our study (26%) resembles previous reports (27.6%)17 and is significantly higher than the excluded nonventilated cohort (2.26%). A Nationwide Inpatient Sample study reported mean mortality from inhalation injury, including nonventilated patients at 16%.3 Burn and non-burn centers were not differentiated in this report.3 Contrary to other studies,3, 18 sex was not associated with outcome in our study.
Higher center volume has been associated with lower mortality among mechanically ventilated patients and overall burn victims.7, 19 We found a similar hospital bed capacity–outcome relationship in SI-ALI, suggesting that triage to larger centers may improve survival possibly related to better infrastructure for organ support and rescue therapies for refractory hypoxemia, and more subspecialty services. Although the impact of burn center status was itself underpowered to infer any effect of this factor, 95% of patients with SI-ALI cared for at > 500-bed centers were at centers with burn units, suggesting that these patients are likely to have a better outcome if transferred to > 500-bed burn centers. However, if transfer to such large-volume burn centers is unavailable because of logistical reasons or distance, then transfer to large-volume non-burn centers within reach should be considered.
Initial systemic antibiotics were administered to one-third of patients with SI-ALI, including one-third with isolated inhalation injury but the specific indications could not be determined without date-stamped diagnosis codes. Animal models and a prospective observational study have associated bronchial bacterial infection with poor outcomes in SI-ALI.5, 20, 21 However, we found no survival benefit from initial systemic antibiotics in the overall SI-ALI cohort in those with isolated inhalation injury or > 96 h of mechanical ventilation. Those given initial antibiotics may have been sicker and included those with vasopressor requirements.22 However, the lack of benefit persisted even among those not requiring initial vasopressor therapy. A study of antibiotics given to burn victims within 2 days of admission improved 28-day in-hospital survival only among those requiring mechanical ventilation although the proportion of patients with SI-ALI was not specified.23
Vasopressors may have been given for different reasons; distributive shock from major burns, sepsis, or vascular collapse from carbon monoxide poisoning.24 Fluid requirements in patients with SI-ALI are 50% greater than predicted by standard formulae.25 Although fluid restriction is desirable in ARDS, it is detrimental to the integrity of pulmonary microvasculature in SI-ALI.26, 27 We were unable to identify shock etiology or quantify administered fluid volume. Notwithstanding, vasopressor use raised the odds of death 5 fold in those with SI-ALI and 29 fold in those with isolated inhalation injury.
Comorbidity burden did not impact in-hospital mortality in our study; however, a previous study associated Charlson Comorbidity Index with a higher 1-year mortality, which was attributed to poorly managed comorbidities and new lower baseline health status postdischarge.28
Burn victims are often transferred to tertiary centers for specialized care. In our analysis of transferred patients, higher TBSA did not predict mortality. This contradicts both previous evidence17 as well as our primary analysis results. Possible causes include survival bias among transfers, a selection bias introduced by transfer to centers with greater experience and resources, or transfers dictated by hospital or third-party payer policy.
We acknowledge limitations in our analysis. The available data precluded studying factors that predicted SI-ALI development, as in ALI.29 Billing coding practices are subject to variability that can impact case classification by the algorithm. The large multicenter study sample as well as sensitivity analyses partially mitigate the bias in conclusions drawn from administrative data. Some of our sensitivity analysis contained low events per variable (eg, e-Table 2), which may bias associations.30 However, their direction was similar to that of the primary analysis. Our analysis misses patients with less severe forms of inhalation injury, and our findings may not be generalizable to burn victims that do not require mechanical ventilation for smoke inhalation injury. Nonavailability of historical and examination findings as well as comprehensive results of laboratory, radiologic testing and endoscopy in the database precluded assessments of type, severity, and circumstances of inhalation injury or concurrent trauma as well as reason for intubation. However, it can be assumed that patients without surface burns were more likely intubated because of smoke inhalation than those with concomitant burns, where other reasons for mechanical ventilation may have existed. With time-varying exposures (ie, vasopressors and antibiotics), there is the potential for differential likelihood of exposure. The predominance of academic medical centers, 6% of US hospitals, limits generalizability yet they include 78% of the US burn units.31 Some adjunctive therapies could not be evaluated. Systemic corticosteroids were given to 8.8% and inhaled steroids to none of the patients within 2 days of hospitalization; further, inability to glean administered dose and specific indication in the database precluded investigations on the impact of steroids on outcome. Their effect on mortality in SI-ALI remains unknown.32 None received hyperbaric oxygen therapy. No mortality benefit from early excision of burns in the presence of inhalation injury has been found.33
Diagnosis codes other than 508.2 (Fig 1) have been used in previous studies to indicate inhalation injury.3 However, our trend analysis (Fig 1) confirmed the amalgamation of myriad respiratory burn codes over time into one code (ICD 9 diagnosis 508.2 and now ICD-10 code J70.5), lending uniformity to the SI-ALI algorithm and enhancing its value as a surveillance tool. The algorithm could not be validated against clinical cases, and sampling bias remains a concern. Retrospective validation against true cases identified by a stratified clinical definition (e-Fig 3) in our pilot chart review at three of the 68 centers revealed considerable variability in bronchoscopic, laboratory and radiologic testing to diagnose SI-ALI (data not shown), precluding a uniform estimation of true cases and the algorithm’s positive predictive value. Our suggested stratified clinical definition is included in the online supplement (e-Fig 3).
Conclusions
Our findings suggest that in addition to older age and concomitant surface burns, initial vasopressor requirements, higher DRG-based risk-of-mortality assignment, and care at centers with < 500 beds predict increased mortality in SI-ALI. These data advocate for early recognition of concomitant shock in inhalation injury victims and suggest that these patients may benefit from preferential triage to larger volume centers.
Acknowledgments
Author contributions: Conception of the study and its design: S. S. K., A. C. M., and A. F. S. Acquisition, analysis, and interpretation of data and drafting of manuscript: S. S. K. Overall supervision and contribution to design of the study and critical revision of manuscript for intellectual content: A. F. S. and B. A. C. Primary data collection, contribution to analysis, and critical revision of the manuscript for intellectual content: S. H. Data collection from charts and assistance with analysis: C. N., C. W., C. G., and S. B. Statistical analysis and contribution to the manuscript: J. S. and R. C. Contribution to study design and critical revision of the manuscript for intellectual content: S. A. Q., S. B., B. D. F., P. E. M., and J. H. H. Guarantor: S. S. K.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: S. A. Q. received consulting fees from Lungpacer, Inc. and Travena, Inc. J. H. H. has equity positions in ABT, ABBV, LLY, Permeaderm, Inc., and RegenMed Therapeutics. None declared (S. S. K., A. C. M., S. H., S. B., C. N., C. W., C. G., J. S., R. C., P. E. M., B. D. F., B. A. C.).
Other contributions: We thank informationist Judith Welsh, National Institutes of Health Library, for performing the literature search for this manuscript and Kelly Byrne for assistance with editing and formatting the manuscript. We acknowledge the members and leadership of the United States Critical Illness and Injury Trials Group for facilitating inter-institutional collaborations and providing critical study feedback.
Additional information: The e-Tables and e-Figures can be found in the Supplemental Materials section of the online article.
Footnotes
Drs Cairns and Suffredini contributed equally to this manuscript.
FUNDING/SUPPORT: This study was funded by the Intramural Research Program, National Institutes of Health.
Supplementary Data
References
- 1.World Health Organization. WHO: Burns-Key Facts. 2014. http://www.who.int/mediacentre/factsheets/fs365/en/. Accessed June 23, 2016.
- 2.American Burn Association. Burn center verification. http://www.ameriburn.org/verification_verifiedcenters.php. Accessed June 23, 2016.
- 3.Veeravagu A., Yoon B.C., Jiang B. National trends in burn and inhalation injury in burn patients: results of analysis of the nationwide inpatient sample database. J Burn Care Res. 2015;36(2):258–265. doi: 10.1097/BCR.0000000000000064. [DOI] [PubMed] [Google Scholar]
- 4.Rehberg S., Maybauer M.O., Enkhbaatar P., Maybauer D.M., Yamamoto Y., Traber D.L. Pathophysiology, management and treatment of smoke inhalation injury. Expert Rev Respir Med. 2009;3(3):283–297. doi: 10.1586/ERS.09.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jones S.W., Zhou H., Ortiz-Pujols S.M. Bronchoscopy-derived correlates of lung injury following inhalational injuries: a prospective observational study. PLoS One. 2013;8(5):e64250. doi: 10.1371/journal.pone.0064250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Palmieri T.L. Inhalation injury consensus conference: conclusions. J Burn Care Res. 2009;30(1):209–210. doi: 10.1097/BCR.0b013e3181923f5c. [DOI] [PubMed] [Google Scholar]
- 7.American Burn Association. National Burn Repository - Report of data from 2005-2014. http://www.ameriburn.org/2015NBRAnnualReport.pdf. Accessed June 23, 2016.
- 8.Dries D.J., Endorf F.W. Inhalation injury: epidemiology, pathology, treatment strategies. Scand J Trauma Resusc Emerg Med. 2013;21:31. doi: 10.1186/1757-7241-21-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Waters J.A., Lundy J.B., Aden J.K. A comparison of acute respiratory distress syndrome outcomes between military and civilian burn patients. Mil Med. 2015;180(3 Suppl):56–59. doi: 10.7205/MILMED-D-14-00390. [DOI] [PubMed] [Google Scholar]
- 10.Fantus R.J., Rivera E.A. NTDB datapoints: all smoke, no fire. Bull Am Coll Surg. 2015;100(7):70–72. [PubMed] [Google Scholar]
- 11.Taylor S.L., Lee D., Nagler T., Lawless M.B., Curri T., Palmieri T.L. A validity review of the National Burn Repository. J Burn Care Res. 2013;34(2):274–280. doi: 10.1097/BCR.0b013e3182642b46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kadri S.S., Hohmann S.F., Orav E.J. Tracking colistin-treated patients to monitor the incidence and outcome of carbapenem-resistant Gram-negative infections. Clin Infect Dis. 2015;60(1):79–87. doi: 10.1093/cid/ciu741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.You K., Yang H.T., Kym D. Inhalation injury in burn patients: establishing the link between diagnosis and prognosis. Burns. 2014;40(8):1470–1475. doi: 10.1016/j.burns.2014.09.015. [DOI] [PubMed] [Google Scholar]
- 14.Deyo R.A., Cherkin D.C., Ciol M.A. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 15.Iezzoni L.I., Ash A.S., Shwartz M., Daley J., Hughes J.S., Mackiernan Y.D. Judging hospitals by severity-adjusted mortality rates: the influence of the severity-adjustment method. Am J Public Health. 1996;86(10):1379–1387. doi: 10.2105/ajph.86.10.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hilden J., Gerds T.A. A note on the evaluation of novel biomarkers: do not rely on integrated discrimination improvement and net reclassification index. Stat Med. 2014;33(19):3405–3414. doi: 10.1002/sim.5804. [DOI] [PubMed] [Google Scholar]
- 17.Colohan S.M. Predicting prognosis in thermal burns with associated inhalational injury: a systematic review of prognostic factors in adult burn victims. J Burn Care Res. 2010;31(4):529–539. doi: 10.1097/BCR.0b013e3181e4d680. [DOI] [PubMed] [Google Scholar]
- 18.McGwin G., Jr., George R.L., Cross J.M., Reiff D.A., Chaudry I.H., Rue L.W., III Gender differences in mortality following burn injury. Shock. 2002;18(4):311–315. doi: 10.1097/00024382-200210000-00004. [DOI] [PubMed] [Google Scholar]
- 19.Kahn J.M., Goss C.H., Heagerty P.J., Kramer A.A., O'Brien C.R., Rubenfeld G.D. Hospital volume and the outcomes of mechanical ventilation. N Engl J Med. 2006;355(1):41–50. doi: 10.1056/NEJMsa053993. [DOI] [PubMed] [Google Scholar]
- 20.Lange M., Hamahata A., Traber D.L. A murine model of sepsis following smoke inhalation injury. Biochem Biophys Res Commun. 2010;391(3):1555–1560. doi: 10.1016/j.bbrc.2009.12.124. [DOI] [PubMed] [Google Scholar]
- 21.D'Avignon L.C., Hogan B.K., Murray C.K. Contribution of bacterial and viral infections to attributable mortality in patients with severe burns: an autopsy series. Burns. 2010;36(6):773–779. doi: 10.1016/j.burns.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 22.Kumar A., Roberts D., Wood K.E. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med. 2006;34(6):1589–1596. doi: 10.1097/01.CCM.0000217961.75225.E9. [DOI] [PubMed] [Google Scholar]
- 23.Tagami T., Matsui H., Fushimi K., Yasunaga H. Prophylactic antibiotics may improve outcome in patients with severe burns requiring mechanical ventilation: propensity score analysis of a Japanese nationwide database. Clin Infect Dis. 2016;62(1):60–66. doi: 10.1093/cid/civ763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garg J, Krishnamoorthy P, Palaniswamy C, et al. Cardiovascular abnormalities in carbon monoxide poisoning [published online ahead of print February 10, 2016]. Am J Ther. Epub ahead of publication. [DOI] [PubMed]
- 25.Navar P.D., Saffle J.R., Warden G.D. Effect of inhalation injury on fluid resuscitation requirements after thermal injury. Am J Surg. 1985;150(6):716–720. doi: 10.1016/0002-9610(85)90415-5. [DOI] [PubMed] [Google Scholar]
- 26.Herndon D.N., Traber D.L., Traber L.D. The effect of resuscitation on inhalation injury. Surgery. 1986;100(2):248–251. [PubMed] [Google Scholar]
- 27.National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network. Wiedemann H.P. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med. 2006;354(24):2564–2575. doi: 10.1056/NEJMoa062200. [DOI] [PubMed] [Google Scholar]
- 28.Lundgren R.S., Kramer C.B., Rivara F.P. Influence of comorbidities and age on outcome following burn injury in older adults. J Burn Care Res. 2009;30(2):307–314. doi: 10.1097/BCR.0b013e318198a416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gajic O., Dabbagh O., Park P.K. Early identification of patients at risk of acute lung injury: evaluation of lung injury prediction score in a multicenter cohort study. Am J Respir Crit Care Med. 2011;183(4):462–470. doi: 10.1164/rccm.201004-0549OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peduzzi P., Concato J., Kemper E., Holford T.R., Feinstein A.R. A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol. 1996;49(12):1373–1379. doi: 10.1016/s0895-4356(96)00236-3. [DOI] [PubMed] [Google Scholar]
- 31.Association of American Medical Colleges. Patient care at AAMC-member teaching hospitals. https://www.aamc.org/download/379180/data/patientcareone-pager. Accessed June 23, 2016.
- 32.Greenhalgh D.G. Steroids in the treatment of smoke inhalation injury. J Burn Care Res. 2009;30(1):165–169. doi: 10.1097/BCR.0b013e3181923c08. [DOI] [PubMed] [Google Scholar]
- 33.Ong Y.S., Samuel M., Song C. Meta-analysis of early excision of burns. Burns. 2006;32(2):145–150. doi: 10.1016/j.burns.2005.09.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.