Abstract
Background
Surgical lung biopsy (SLB) is invasive and not possible in all patients with undiagnosed interstitial lung disease (ILD). We hypothesized that transbronchial biopsy (TBB) findings combined with clinical and high-resolution CT (HRCT) data leads to a confident diagnosis congruent to SLB and therefore avoids the need for SLB in some patients.
Methods
We evaluated 33 patients being investigated for suspected ILD who underwent HRCT, TBB, and SLB. First, clinicians, radiologists, and a pathologist reviewed the clinical information and HRCT and TBB findings. Clinicians were asked to provide a diagnosis and were also asked if SLB was needed for a more confident diagnosis. Subsequently, the clinical, HRCT, and SLB data were reviewed, and the same participants were asked to provide a final diagnosis. Clinician consensus and overall agreement between TBB- and SLB-based diagnoses were calculated.
Results
Four patients had definite usual interstitial pneumonia (UIP) on HRCT and would not be considered for biopsy using current guidelines. Of the 29 patients without a definitive HRCT diagnosis, the clinicians felt confident of the diagnosis (ie, would not recommend SLB) in six cases. In these cases, there was 100% agreement between TBB and SLB diagnoses. UIP was the most common diagnosis (n = 3) and was associated with an HRCT diagnosis of possible UIP/nonspecific interstitial pneumonia-like. Agreement was poor (33%) between TBB and SLB diagnoses when confidence in the TBB diagnosis was low.
Conclusions
Information from TBB, when combined with clinical and HRCT data, may provide enough information to make a confident and accurate diagnosis in approximately 20% to 30% of patients with ILD.
Key Words: idiopathic interstitial pneumonia, idiopathic pulmonary fibrosis, interstitial lung disease, lung biopsy
Abbreviations: DIP, desquamative interstitial pneumonia; HP, hypersensitivity pneumonitis; HRCT, high-resolution CT; IIP, idiopathic interstitial pneumonia; ILD, interstitial lung disease; IPF, idiopathic pulmonary fibrosis; NSIP, nonspecific interstitial pneumonia; SLB, surgical lung biopsy; TBB, transbronchial biopsy; UIP, usual interstitial pneumonia
Idiopathic interstitial pneumonias (IIPs) are a heterogeneous group of nonneoplastic disorders resulting from damage to lung parenchyma that manifests various patterns of inflammation and fibrosis. Different types of IIP exhibit different prognoses.1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11 For example, idiopathic pulmonary fibrosis (IPF) is a chronic, slowly progressive, and typically fatal disease characterized by the histopathologic pattern of usual interstitial pneumonia (UIP).9 Therapy for IPF is different than that for other IIPs, with previous data showing a positive impact for treatment with pirfenidone and nintedanib, whereas antiinflammatory therapy worsened outcomes.12, 13, 14, 15, 16, 17, 18, 19 Thus, an accurate diagnosis is critical to the management of patients with IIP.
The American Thoracic Society, European Respiratory Society, Japanese Respiratory Society, and the Latin American Thoracic Association international consensus statement outlined the importance of a dynamic approach to the diagnosis of patients with suspected IIP involving clinicians, radiologists, and pathologists experienced in the diagnosis of interstitial lung disease (ILD).9 In general, histopathologic information has the greatest impact on delineation of the various IIP entities; however, given HRCT specificity for recognition of the histopathologic UIP pattern, surgical lung biopsy (SLB) is not uniformly required.9, 20, 21, 22, 23, 24 In the absence of contraindications, SLB is advised in patients with suspected IIP who do not show lower lung and subpleural predominant septal lines, traction bronchiectasis with honeycombing, and minimal ground glass opacity, which are the diagnostic HRCT features of UIP.9, 12 TBB data are largely absent from current IIP diagnostic guidelines, with the exception of diffuse alveolar damage, acute interstitial pneumonia, granulomatous disorders such as sarcoidosis, and occasionally organizing pneumonias.9, 11, 12
A previous registry reported the use of histopathologic data from SLB for the diagnosis of IPF in a minority of patients (34.1%).25 The low percentage of biopsy procedures likely results from some cases in which a diagnosis can be confidently reached on clinical and HRCT factors, as well as the fact that SLB is invasive and not feasible in all patients. Patients with comorbidities, the elderly, and those with atypical clinical and HRCT features of UIP have a higher mortality risk associated with SLB, as well as an increased risk of acute exacerbation after SLB.26, 27, 28, 29 Increased in-hospital mortality has also been linked to male sex, open rather than thorascopic surgery, and a suspected diagnosis of IPF.30 Transbronchial lung cryobiospy is an alternative less invasive method for obtaining larger biopsy samples of lung parenchyma and may represent an advancement in IPF diagnostics, with lower complication and mortality rates compared with SLB; however, it is not widely available and may produce significant morbidity.31, 32, 33, 34, 35 Previous data suggest that characteristic histologic features of UIP can be identified on TBB pathologic specimens more frequently than previously appreciated and therefore may have greater utility in establishing IIP diagnoses than used currently in practice.36 Subsequent studies have shown that TBB can detect a UIP pattern in 30% of cases, with high specificity and positive predictive value but low negative predictive value.37 We hypothesized that in some cases, the findings from TBB combined with clinical and HRCT data lead to a confident diagnosis that is congruent to the diagnosis achieved using SLB, thus allowing some patients to avoid undergoing SLB. Some of the results of this study have been previously reported in the form of an abstract.38
Methods
Patient Selection and Data Collection
Institutional records from the University of Michigan and University of California, Los Angeles were reviewed to identify patients with a suspected diagnosis of ILD who underwent a workup that included HRCT, TBB, and SLB. In each case, TBB was performed for the purpose of diagnosing possible IIP. A standard form was used to abstract clinical data regarding patient age; sex; presenting symptoms; comorbidities, including a history of collagen vascular disease, smoking, exposure, and a family history of ILD; pertinent physical examination findings; complete pulmonary function test results; and relevant serologic studies from the medical record. Patients without sufficient clinical data or HRCT and biopsy data outside of a 6-month time frame were excluded (n = 7). A total of 30 and three patients were identified at each institution, respectively. The institutional review boards at the University of Michigan (HUM00018281) and the University of California, Los Angeles (G07-09-080-01) approved this study.
Radiology and Pathology Interpretation
HRCT examinations were first interpreted by three expert cardiothoracic radiologists independently and without any clinical or pathologic data. Consensus HRCT diagnosis was achieved through agreement among two or three radiologists. Similarly, four pathologists evaluated TBB and SLB pathologic findings independently without any HRCT or clinical data. Individual parameters scored included the presence or absence of architectural distortion (ie, tissue-destructive scarring or honeycomb changes), alveolar septal fibrosis, a patchwork distribution of fibrosis, fibroblast foci, and intraluminal organization (ie, organizing pneumonia). Alveolar septal and bronchial wall inflammation was also graded from absent (0) to diffuse and severe (3+). TBB results showing patchwork interstitial fibrosis, architectural distortion, and fibroblast foci were considered to be UIP. Biopsy results showing two of those features were considered consistent with or suggestive of UIP. All other biopsy results were considered diagnostic of a condition other than UIP or nondiagnostic or inadequate (bronchial wall only with no diagnostic features). Consensus was defined as agreement among three of the four examiners; consensus was reached for each TBB and SLB without knowledge of which TBB and SLB came from the same patient.
Study Organizational Scheme
Multidisciplinary team meetings followed the radiology and pathology reviews, and consensus diagnosis was established for each. Case information was presented to a group of three expert pulmonologists, two radiologists, and one pathologist from the University of Michigan on two separate occasions. During each phase, the radiologists and pathologist present conveyed the consensus opinion of the other radiologists/pathologists to the group. First, the clinical, HRCT, and TBB data were reviewed. Clinicians were asked to provide a diagnosis based on clinical and HRCT data alone along with confidence level. TBB data were then introduced, and clinicians were asked to provide a final diagnosis and whether they would request an SLB to obtain a more confident diagnosis. Next, approximately 5 months later, the same participants reviewed, in a different case order, the clinical, HRCT, and SLB data and were again asked to provide a final diagnosis. Clinicians were not informed of what the diagnosis was from the TBB consensus exercise during the SLB exercise.
Statistical Analysis
Each observer’s diagnosis was coded into one of eight categories: IPF/UIP, nonspecific interstitial pneumonia (NSIP), hypersensitivity pneumonitis (HP), respiratory bronchiolitis-ILD/desquamative interstitial pneumonia (DIP), emphysema, bronchiolitis, other, and normal/nondiagnostic. “Other” diagnoses included organizing pneumonia, cryptogenic organizing pneumonia, ILD with suspected underlying collagen vascular disease, sarcoidosis, small airways disease, asthma, and infection. Clinician consensus was determined on agreement of at least two of the three participating pulmonologists. The overall agreement regarding diagnoses with TBB compared with SLB was calculated.
Results
Clinical Features
Baseline demographic data for the 33 patients reviewed are presented in Table 1. The mean age of patients who were believed to have an accurate diagnosis with TBB data alone was similar to that of the study group as a whole, although there was one notable outlier who was 20 years of age with a diagnosis of cryptogenic organizing pneumonia. The primary respiratory symptoms for the entire cohort were dyspnea and cough, and the predominant pertinent examination finding was pulmonary crackles (n = 22). Specific collagen vascular disease diagnoses included rheumatoid arthritis (n = 2), Sjögren’s syndrome (n = 1), polymyositis (n = 1), and scleroderma (n = 1). Five patients were found to have emphysema as an alternative diagnosis on HRCT or biopsy, or both. Relevant exposures seen in patients in whom a diagnosis of HP was considered included asbestos, birds, mold, drugs (methotrexate), and chemical warfare. No occupational lung diseases were identified in our cohort.
Table 1.
Baseline Demographics
| Variable | All Patients | No SLBa | Yes SLB or Undecided | P Value |
|---|---|---|---|---|
| No. of patients, No. (%) | 33 | 10 (30.3) | 23 (69.7) | |
| Mean age, y (SD) | 57.8 (12.0) | 53.4 (13.9) | 59.7 (10.9) | .2262 |
| Male sex, No. (%) | 15 (45.5) | 5 (50) | 10 (43.5) | 1.0000 |
| Tobacco use, No. (%) | .5138 | |||
| Never smoker | 14 (42.4) | 5 (50) | 9 (39.1) | |
| Former smoker | 15 (45.5) | 5 (50) | 10 (43.5) | |
| Current smoker | 4 (12.1) | 0 | 4 (17.4) | |
| History of emphysema, No. (%) | 7 (21.2) | 1 (10) | 6 (26.1) | .3968 |
| Respiratory symptoms, % | 100 | 100 | 100 | 1.000 |
| Lung examination findings, No. (%) | ||||
| Crackles | 22 (66.7) | 8 (80) | 14 (60.9) | .4300 |
| Wheeze | 2 (6.1) | 0 | 2 (8.7) | 1.0000 |
| None | 10 (30.3) | 2 (20) | 8 (34.8) | .6822 |
| Exposures, No. (%) | 6 (18.2) | 0 | 6 (26.1) | .1445 |
| Family history of ILD, No. (%) | 4 (12.1) | 2 (20) | 2 (8.7) | .5672 |
| Personal history of collagen vascular disease, No. (%) | 5 (15.2) | 1 (10) | 4 (17.4) | 1.0000 |
| FEV1, % predicted (SD) | 80.8 (18.1) | 80.3 (12.0) | 81.0 (20.5) | .9035 |
| FVC, % predicted (SD) | 72.2 (16.6) b1 unknown |
69.4 (11.1) b1 unknown |
73.3 (18.5) | .4774 |
| Ratio, % (SD) | 83.1 (13.6) | 87.9 (17.2) | 80.9 (12.1) | .2886 |
| Diffusion capacity, % predicted (SD) | 55.4 (19.0) b3 unknown |
49.9 (12.1) b1 unknown |
57.8 (21.1) b2 unknown |
.2105 |
HRCT = high-resolution CT; ILD = interstitial lung disease; SLB = surgical lung biopsy.
No SLB includes patients with definitive HRCT diagnosis (n = 4).
Missing patient data, number as specified.
Radiology and Pathology Diagnosis
Radiologist interpretation of HRCT data resulted in consensus diagnosis in 29 cases (Table 2). More specifically three of three radiologists agreed in 15 cases, two of three agreed in 14 cases, and there was lack of consensus in four cases. The most common HRCT diagnosis was possible UIP/NSIP-like (n = 15). The second most common diagnosis was HP (n = 5). In the cases in which an SLB was deemed unnecessary by the clinicians after review of clinical, HRCT, and TBB data, a radiographic pattern of UIP ranging from possible to definite was seen in seven of 10 cases. Four HRCT examinations showed honeycombing and an HRCT diagnosis of definite (n = 1) or probable UIP (n = 3) and would not be considered for biopsy based on current management guidelines.9
Table 2.
HRCT Consensus and Clinician Consensus on TBB and SLB Final Diagnoses with Perceived Need for SLB Based on TBB Evaluation Alone
| Case | HRCT Diagnosis Radiology Consensus |
Clinical-Radiologic Impressiona (Confidenceb) | TBB Pathologic Diagnosis | SLB Pathologic Diagnosis | TBB Final Diagnosisa (Confidenceb) | SLB Final Diagnosisa (Confidenceb) | Get SLB? | Agreement in TBB and SLB Final Diagnoses |
|---|---|---|---|---|---|---|---|---|
| 1 | Definite UIP | 1 (2) | UIP | UIP | 1 (1) | 1 (1) | No | Yes |
| 2 | Probable UIP | 1 (1) | Inadequate | UIP | 1 (1) | 1 (1) | No | Yes |
| 3 | Probable UIP | 1 (1) | UIP | HP vs UIP | 1 (1) | 3 (2) | No | No |
| 4 | Probable UIP | 1 (1) | UIP | UIP | 1 (1) | 1 (1) | No | Yes |
| 5 | Possible UIP/NSIP-like | 2 (2) | UIP | UIP | 1 (1) | 1 (1) | No | Yes |
| 6 | Possible UIP/NSIP-like | 1 (2) | UIP | UIP | 1 (1) | 1 (1) | No | Yes |
| 7 | Possible UIP/NSIP-like | 1 (3) | UIP | UIP | 1 (2) | 1 (2) | No | Yes |
| 8 | HP | 3 (3) | HP | HP | 3 (1) | 3 (1) | No | Yes |
| 9 | Normal | 8 (1) | Organizing pneumonia | Organizing pneumonia | 7 (1) | 7 (1) | No | Yes |
| 10 | Other, non-IIP | 7 (2) | Nondiagnostic | Nondiagnostic | 7 (2) | 7 (2) | No | Yes |
| 11 | Possible UIP/NSIP-like | 1 (2) | Nondiagnostic | UIP | 1 (2) | 1 (1) | Undecided | Yes |
| 12 | Possible UIP/NSIP-like | 1 (2) | Inadequate | UIP | 1 (2) | 1 (1) | Undecided | Yes |
| 13 | Possible UIP/NSIP-like | 2 (2) | Nondiagnostic | Probable HP | 2 (2) | 2 (3) | Yes | Yes |
| 14 | Possible UIP/NSIP-like | 3 (2) | Nondiagnostic | UIP | 1, 2, 3 (3) | 1 (2) | Yes | No |
| 15 | Possible UIP/NSIP-like | 7 (2) | UIP | Chronic bronchiolitis | 1 (2) | 6 (2) | Yes | No |
| 16 | Possible UIP/NSIP-like | 2 (2) | UIP | Eosinophilic pneumonia, NSIP | 1 (2) | 7 (1) | Yes | No |
| 17 | Possible UIP/NSIP-like | 3 (2) | Nondiagnostic | UIP | 3, 1 (3) | 1 (2) | Yes | No |
| 18 | Possible UIP/NSIP-like | 1 (2) | Inadequate | UIP | 1 (2) | 1 (1) | Yes | Yes |
| 19 | Possible UIP/NSIP-like | 2, 3 (3) | HP | HP | 3 (2) | 3 (1) | Yes | Yes |
| 20 | Possible UIP/NSIP-like | 1, 2 (3) | Nondiagnostic | UIP | 1, 2 (3) | 1 (1) | Yes | No |
| 21 | Possible UIP/NSIP-like | 1, 2 (3) | Inadequate | UIP | 1 (3) | 1 (1) | Yes | Yes |
| 22 | Possible UIP/NSIP-like | 1, 2, 3 (3) | Inadequate | HP | 3 (3) | 3 (2) | Yes | Yes |
| 23 | HP | 4, 7 (3) | Nondiagnostic | RB | 7 (3) | 4 (2) | Yes | No |
| 24 | HP | 1, 3 (3) | Nondiagnostic | RB | 3 (3) | 4 (1) | Yes | No |
| 25 | HP | 1, 2, 3 (3) | Inadequate | UIP | 1, 2, 7 (3) | 1 (1) | Yes | No |
| 26 | HP | 3, 4 (3) | Nondiagnostic | Peribronchiolar metaplasia | 3 (3) | 4 (2) | Yes | No |
| 27 | Cryptogenic organizing pneumonia | 7 (3) | HP | Organizing pneumonia | 3, 7 (3) | 7 (1) | Yes | No |
| 28 | Emphysema | 5 (1), 2 (3) | Nondiagnostic | HP | 2 (3) | 4 (1) | Yes | No |
| 29 | Other, non-IIP | 7 (2) | Nondiagnostic | Follicular bronchiolitis | 7 (2) | 6 (1) | Yes | No |
| 30 | No consensus HP, small airways disease, other |
4 (3) | Nondiagnostic | RB | 4 (3) | 4 (1) | Yes | Yes |
| 31 | No consensus DIP, probable UIP, other |
2 (2) | Nondiagnostic | NSIP | 2 (2) | 2 (1) | Yes | Yes |
| 32 | No consensus HP, definite UIP, possible UIP |
1 (2), 3 (3) | Nondiagnostic | UIP | 3 (2) | 1 (1) | Yes | No |
| 33 | No consensus HP, possible UIP/NSIP-like, sarcoid |
1 (3) | UIP | HP | 1 (2) | 3 (1) | Yes | No |
DIP = desquamative interstitial pneumonia; HP = hypersensitivity pneumonitis; IIP = idiopathic interstitial pneumonia; NSIP = nonspecific interstitial pneumonia; RB = respiratory bronchiolitis; SLB = surgical lung biopsy; TBB = transbronchial biopsy; UIP = usual interstitial pneumonia. See Table 1 legend for expansion of other abbreviations.
1 = UIP; 2 = NSIP; 3 = HP, 4 = RB ILD/DIP; 5 = emphysema; 6 = bronchiolitis; 7 = other; 8 = normal.
Confidence: 1 = definite; 2 = probable; 3 = possible.
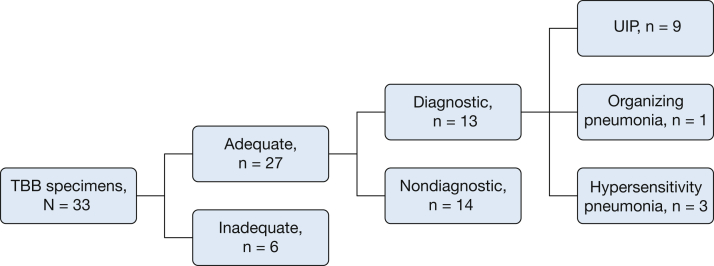
Of the 33 TBB pathologic specimens, six were inadequate, 14 were nondiagnostic, and 13 were diagnostic (Fig 1). Specimens were interpreted as diagnostic if the final pathologist reading was “consistent with” or “suggestive of” a specific diagnosis. Overall, 58% of TBB specimens were inadequate or nondiagnostic. The inadequate specimens had insufficient tissue for diagnosis and were primarily bronchial wall, pleura, or debris. Specimens were adequate if they contained at least one fragment of alveolated lung parenchyma for review. The nondiagnostic specimens showed nonspecific interstitial thickening, chronic interstitial pneumonia without further specification, patchy fibrosis without specific features, or a combination. The average number of pieces of alveolated parenchyma in all TBB specimens was 2.8 (SD, 1.5; median, 3) (Table 3). This average remained consistent when calculated for diagnostic specimens only (2.8; SD, 1.0; median, 3) and when further limited to TBB specimens diagnostic for UIP (2.8; SD, 1.1; median, 3). The average number of pieces of alveolated parenchyma when the TBB pathologic and SLB pathologic diagnoses were equivalent was again 2.8 (SD, 0.9; median, 3).
Figure 1.
Adequacy of TBB specimens. TBB = transbronchial biopsy; UIP = usual interstitial pneumonia.
Table 3.
Characteristics of TBB Specimens
| Variable | Total N = 33 |
UIP on SLB n = 15 |
Non-UIP on SLB n = 18 |
|---|---|---|---|
| No. of alveolated pieces, median (range) | 3 (0-7) | 3 (0-7) | 3 (0-6) |
| Adequate biopsy results, No. (%) | 27 (81.8) | 10 (66.7) | 17 (94.4) |
| Diagnostic biopsy results, No. (%) | 13 (39.4) | 5 (33.3) | 8 (47.1) |
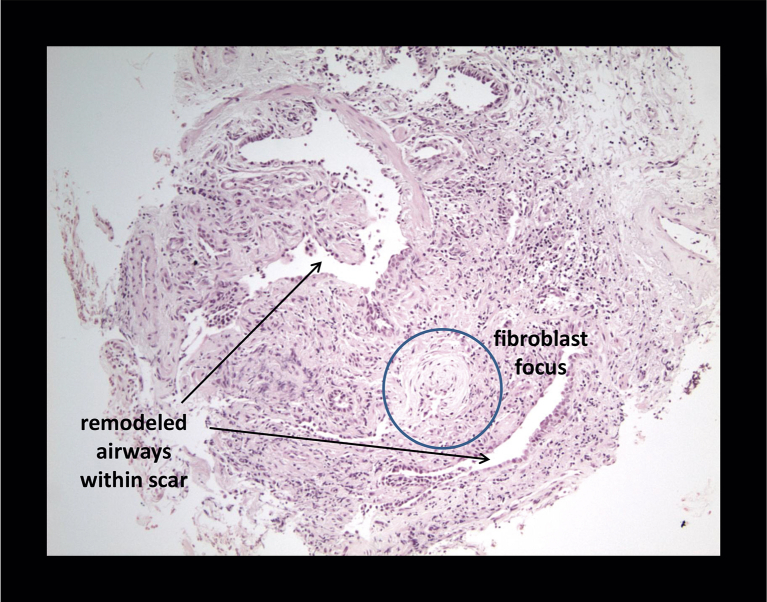
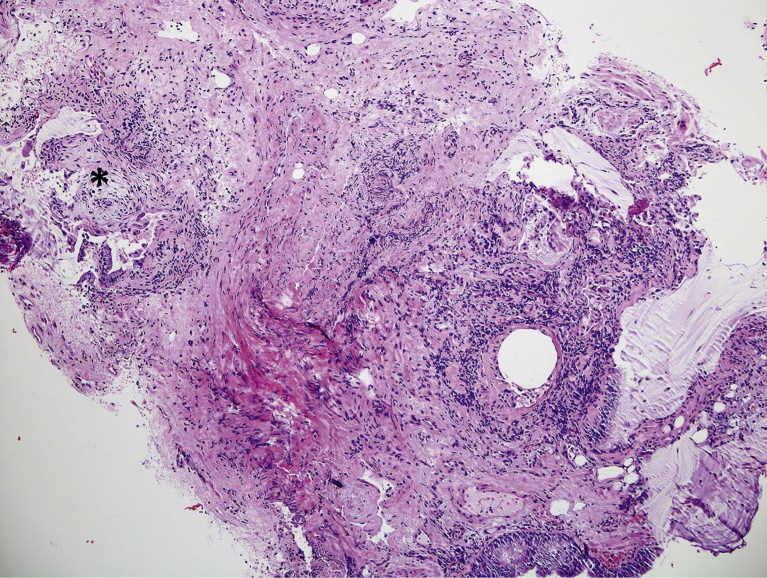
Of the 13 diagnostic TBB specimens, nine were consistent with UIP (Figs 2, 3), three with HP, and one with organizing pneumonia. Five of these diagnostic TBB specimens (for any diagnosis) were found among patients in whom SLB was deemed unnecessary and HRCT was not definitively diagnostic of UIP (n = 6). The case without adequate TBB was ultimately diagnosed as small airways disease based on clinical and HRCT data. Three TBB specimens were diagnostic for UIP in patients with HRCT showing probable or definite UIP. When the TBB was thought to be diagnostic, there was agreement between TBB and SLB pathologic diagnoses in 61.5% of cases (eight of 13).
Figure 2.
Transbronchial biopsy with honeycomb changes.
Figure 3.
Usual interstitial pneumonia on transbronchial biopsy with fibrosis, honeycomb changes, and fibroblast foci. The asterisk indicates a fibroblast focus.
Clinician Diagnosis and Decision About the Need for SLB
Clinician consensus diagnosis was reached for all patients presented during both sections of the study. Clinician consensus regarding the need for SLB was achieved in 31 of 33 cases (94%). In the two undecided cases, only two of the three participating pulmonologists provided decisions regarding the need for SLB that were discordant. Clinician consensus on the initial clinical/radiologic impression, TBB final diagnosis, and SLB final diagnosis, along with reported confidence level (1 = definite, 2 = probable, 3 = possible) are presented in Table 2.
Of the 33 patients presented, SLB was deemed unnecessary in 10 (30%), necessary in 21 (64%), and undecided in two (6%). However, radiology review identified four patients with honeycombing and an HRCT diagnosis of definite (n = 1) or probable (n = 3) UIP. Based on current management guidelines, HRCT would be diagnostic, and these patients would not be considered for SLB.9 Excluding these patients, of the remaining 29 cases, SLB was deemed unnecessary in six (21%), necessary in 21 (72%), and undecided in two (7%). There was agreement between TBB and SLB diagnoses in all cases in which SLB was deemed unnecessary after initial review with TBB pathologic findings only. In five of these six cases, there was either an increase in clinician-reported confidence level or a change in the final diagnosis with the addition of the TBB specimen. In the sixth case, TBB and SLB were both nondiagnostic, and patient was given a final diagnosis of small airways disease. Even after excluding cases of definite UIP by HRCT, IPF remained the most common clinical diagnosis (n = 3 [50%]). The other diagnoses were HP, small airways disease, and cryptogenic organizing pneumonia.
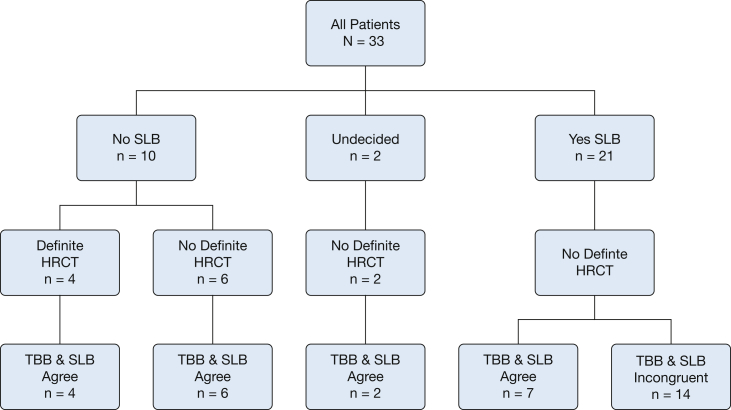
In 21 cases, the clinicians felt that SLB was necessary to confirm the diagnosis reached using TBB. In these cases, the clinician-reported confidence level was not increased by the addition of TBB data. Interestingly, the pre-SLB and post-SLB diagnoses were the same in seven of these cases (33%), whereas the ultimate diagnosis was incongruent in 14 cases (67%). The two undecided cases both had a diagnosis of UIP with TBB and SLB (Fig 4).
Figure 4.
Flow diagram of agreement between TBB and SLB final diagnoses. HRCT = high-resolution CT; SLB = surgical lung biopsy. See Figure 1 legend for expansion of other abbreviations.
Discussion
Prior research has demonstrated the potential use of TBB as a diagnostic technique in patients proven to have IPF.36, 37 Our investigation sought to evaluate the diagnostic utility of TBB combined with clinical and HRCT data as it applies to IIP. We demonstrated that information from TBB combined with clinical and HRCT data may provide enough information to make a confident and accurate diagnosis in approximately 20% to 30% of patients with suspected ILD. In our case series, TBB appears to have greatest diagnostic value in identifying UIP. When a TBB specimen showed appropriate UIP histopathologic findings in the context of an appropriate clinical history and HRCT, SLB was not recommended, and reported clinician confidence was increased. More specifically, our data suggest that the addition of a diagnostic TBB specimen to a possible UIP/NSIP-like HRCT pattern can result in a confident IPF diagnosis and preclude the need for SLB, which is the current recommendation based on most recent guidelines.
The overall utility of TBB is limited by the adequacy and size of the pathologic specimen. On review of our pathologic consensus data, only 18% of TBB specimens were inadequate, consisting of bronchial wall, pleura, or debris, which is a similar percentage as in older TBB studies.36 Even with an adequate specimen, the small sample size of the TBB specimen limits diagnostic capability, which has been considered a major impediment to the successful use of TBB for diagnosing ILD.12 However, of our adequate specimens with at least one fragment of alveolated lung parenchyma, 48% were felt to be diagnostic. Additionally, the average and median number of pieces of alveolated parenchyma did not change when we isolated only diagnostic or diagnostically accurate TBB specimens (average, 2.8; median, 3). When TBB was interpreted as diagnostic in the absence of a diagnostic HRCT, our expert clinicians agreed that further histopathologic evaluation was unnecessary in 50% of cases (five of 10). In the majority of these cases, the ultimate diagnosis was UIP/IPF (n = 3). In all cases in which SLB was not recommended, there was agreement between the final diagnoses reached using TBB and SLB. When TBB was diagnostic and SLB was still requested, agreement in final diagnoses was seen in only 20%. These data show that clinicians may be able to determine when clinical, HRCT, and TBB information is complete enough to preclude the need for SLB. It also shows that clinicians are able to sense when a pathologic diagnosis from TBB is not sufficient to reach a firm final diagnosis. A thorough assessment of the factors that contribute to clinician confidence in TBB pathologic diagnosis was not addressed is this study. Furthermore, our ability to make inferences on this topic is also limited by our small sample size. This represents an area for future investigation.
Interlobar and intralobar histologic variability in IIP is well known and guides recommendations for obtaining biopsy specimens from multiple lobes during a diagnostic evaluation for IIP.2 The presence of a UIP pattern in any biopsy sample confers a poor prognosis and when seen is generally classified as UIP.2 This principle, although originally applied to SLB, may also be extrapolated to TBB. Even with the small size of TBB samples, if definitive UIP characteristics are seen, the specimen may be considered diagnostic. However, absence of UIP on TBB does not rule out UIP/IPF. The need for and utility of transbronchial biopsy procedures in multiple lobes for IIP has not been investigated.
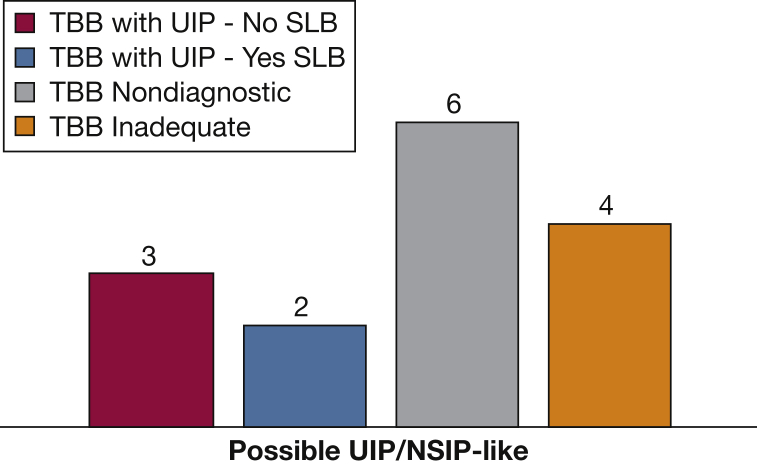
Although the ILDs reviewed in our retrospective analysis were not limited to IPF, this was the most common final diagnosis. The most common radiologic pattern seen in our study population was a possible UIP/NSIP-like pattern (n = 15). Adequate TBB specimens were seen in 11 of these patients (73%), 55% of which were thought to be diagnostic (Fig 5). The second most common HRCT pattern was HP (n = 5). Adequate TBB specimens were found in four (80%), 25% of which were thought to be diagnostic. Our limited data suggest that TBBs have the potential to provide most diagnostic benefit to cases in which HRCT shows a possible UIP/NSIP-like pattern. However, this may be skewed by overrepresentation of this radiographic pattern in our cohort.
Figure 5.
TBB results in patients with possible UIP/NSIP-like on HRCT. UIP/NSIP = usual interstitial pneumonia/nonspecific interstitial pneumonia. See Figure 1 and 4 legends for expansion of other abbreviations.
True diagnostic utility of TBB is achieved only in the context of suggestive clinical and HRCT data. It has been established that HRCT can accurately predict the presence of UIP at biopsy when features of honeycombing in a basilar peripheral distribution are present (without a predominance of ground glass or other features to suggest an alternative diagnosis).12, 21, 22 Further investigation has suggested that more modest amounts of fibrosis, without honeycombing, can be highly predictive of UIP/IPF when combined with patient age.39 In our study, the need for SLB was used as a surrogate for confidence level in diagnosis using clinical, HRCT, and TBB data, with a decision against SLB equating to a high level of confidence in the diagnosis. If TBB was suggestive of a UIP diagnosis when the HRCT showed a possible UIP/NSIP-like pattern, no further workup was recommended (n = 3). In the three cases in which TBB was suggestive of UIP and SLB was requested, possible confounding factors include lack of consensus on an HRCT diagnosis, atypical distribution of abnormalities on HRCT, and a concurrent alternative diagnosis suggested on the TBB specimen findings. The remaining three TBB specimens showing UIP were in patients with definite or probable UIP seen on HRCT who would not be considered for biopsy based on current management guidelines. Because of the small size of TBB specimens, the pathologic findings on TBB must be correlated with the clinical and radiologic data before making a diagnosis, and if the clinical or radiographic features (or both) do not fit, consideration should be given to obtaining a larger biopsy specimen.36
As with prior studies evaluating the utility of TBB in the diagnosis of UIP, our study was limited by a small sample size and retrospective sampling. A larger cohort would be necessary to more accurately determine the sensitivity and specificity of TBB for achieving UIP histopathologic diagnosis. Although prior studies addressing the utility of TBB have been limited to a population of patients with UIP/IPF only, our evaluation included other IIPs. However, the greatest utility of TBB was seen when the ultimate diagnosis was UIP/IPF. Other studies on a bronchoscopic sample such as cell count and differential or flow cytometry, which may be instructive and might improve diagnostic accuracy in alternative IIPs such as HP, were not explicitly evaluated in this study. As a retrospective evaluation, we have identified cases in which both TBB and SLB were performed and for which TBB was not felt to be informative in the initial clinical setting. We have applied new pathologic and diagnostic criteria to older specimens/cases, which is an inherent limitation to our evaluation. There has yet to be a trial investigating the prospective use of routine TBB in IIP. A prospective trial would need to address the number of samples taken at the time of biopsy and location of sampling to address concerns raised by consensus guidelines.9 The use of TBB is most appealing in cases in which SLB is deemed not to be feasible. Although TBB is less invasive than SLB, the overall safety of TBB in this likely at-risk patient population would also need to be addressed before its routine use.
Standard TBB has been used widely for obtaining lung samples for histologic evaluation. The diagnostic yield is variable and is influenced by factors such as the size of the samples harvested and the presence of crush artifacts left by conventional biopsy forceps.40, 41, 42 Methods of approaching TBB to best achieve an adequate diagnostic specimen have not been determined, particularly with respect to the diagnosis of UIP with its peripheral lung predominance.43 As previously mentioned, transbronchial lung cryobiospy may represent an alternative method to obtain larger tissue samples, with a reported diagnostic yield as high as 80% and lower complication and mortality rates than with SLB.35 However, further investigation is needed into how best to approach TBBs to achieve an accurate diagnosis. Our evaluation using multiple expert clinicians, pathologists, and a radiologist have identified a potential role for TBB in the multidisciplinary diagnostic pathway; however, our findings would need to be confirmed in a typical multidisciplinary team setting for TBB to be used in clinical practice.
In conclusion, when clinical and HRCT data are suggestive but not diagnostic of UIP, clinician confidence in IPF diagnosis is increased when a TBB specimen contains the characteristic histologic features of UIP.
Acknowledgments
Author contributions: K. R. F. conceived and designed the study. J. S. S. and K. R. F. conducted and coordinated the study, had full access to all the data, and take responsibility for the integrity of the data and the accuracy of the data analysis. J. S. S. analyzed the data with supervision and assistance from K. R. F. J. A. B. and D. Z. contributed to the study design and provided patients for analysis. M. C. F., A. L., J. L. M. provided pathologic data and interpretation. E. A. K. and B. S. provided radiographic interpretation. R. H. S., T. H. S., and E. S. W. participated as expert pulmonologists. J. S. S. and K. R. F. prepared the manuscript. All other authors revised the manuscript and approved the final draft. M. X. and S. M. performed additional analysis during the manuscript revision process.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: D. Z. is a member of the speaker bureaus and advisory boards for Genentech and Boehringer Ingelheim. None declared (J. S. S., J. A. B., M. C. F., E. A. K., A. L., S. M., J. L. M., R. H. S., T. H. S., B. S., E. S. W., M. X., K. R. F.).
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Footnotes
FUNDING/SUPPORT: This work was supported by National Institutes of Health NHLBI [grants R01 HL91743, T32 HL00749, and K24 HL111316 (K. R. F.)].
References
- 1.Flaherty K.R., Toews G.B., Travis W.D. Clinical significance of histological classification of idiopathic interstitial pneumonia. Eur Respir J. 2002;19(2):275–283. doi: 10.1183/09031936.02.00182002. [DOI] [PubMed] [Google Scholar]
- 2.Flaherty K.R., Travis W.D., Colby T.V. Histopathologic variability in usual and nonspecific interstitial pneumonias. Am J Respir Crit Care Med. 2001;164(9):1722–1727. doi: 10.1164/ajrccm.164.9.2103074. [DOI] [PubMed] [Google Scholar]
- 3.Katzenstein A.L., Fiorelli R.F. Nonspecific interstitial pneumonia/fibrosis. Histologic features and clinical significance. Am J Surg Pathol. 1994;18(2):136–147. [PubMed] [Google Scholar]
- 4.Nagai S., Kitaichi M., Itoh H., Nishimura K., Izumi T., Colby T.V. Idiopathic nonspecific interstitial pneumonia/fibrosis: comparison with idiopathic pulmonary fibrosis and BOOP. Eur Respir J. 1998;12(5):1010–1019. doi: 10.1183/09031936.98.12051010. [DOI] [PubMed] [Google Scholar]
- 5.Nicholson A.G., Colby T.V., du Bois R.M., Hansell D.M., Wells A.U. The prognostic significance of the histologic pattern of interstitial pneumonia in patients presenting with the clinical entity of cryptogenic fibrosing alveolitis. Am J Respir Crit Care Med. 2000;162(6):2213–2217. doi: 10.1164/ajrccm.162.6.2003049. [DOI] [PubMed] [Google Scholar]
- 6.Travis W.D., Matsui K., Moss J., Ferrans V.J. Idiopathic nonspecific interstitial pneumonia: prognostic significance of cellular and fibrosing patterns: survival comparison with usual interstitial pneumonia and desquamative interstitial pneumonia. Am J Surg Pathol. 2000;24(1):19–33. doi: 10.1097/00000478-200001000-00003. [DOI] [PubMed] [Google Scholar]
- 7.Bjoraker J.A., Ryu J.H., Edwin M.K. Prognostic significance of histopathologic subsets in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 1998;157(1):199–203. doi: 10.1164/ajrccm.157.1.9704130. [DOI] [PubMed] [Google Scholar]
- 8.Lama V.N., Flaherty K.R., Toews G.B. Prognostic value of desaturation during a 6-minute walk test in idiopathic interstitial pneumonia. Am J Respir Crit Care Med. 2003;168(9):1084–1090. doi: 10.1164/rccm.200302-219OC. [DOI] [PubMed] [Google Scholar]
- 9.Raghu G., Collard H.R., Egan J.J. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183(6):788–824. doi: 10.1164/rccm.2009-040GL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raghu G., Rochwerg B., Zhang Y. An official ATS/ERS/JRS/ALAT clinical practice guideline: treatment of s. An update of the 2011 clinical practice guideline. Am J Respir Crit Care Med. 2015;192(2):e3–e19. doi: 10.1164/rccm.201506-1063ST. [DOI] [PubMed] [Google Scholar]
- 11.Travis W.D., Costabel U., Hansell D.M. An official American Thoracic Society/European Respiratory Society statement: update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med. 2013;188(6):733–748. doi: 10.1164/rccm.201308-1483ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.American Thoracic Society, European Respiratory Society American Thoracic Society/European Respiratory Society international multidisciplinary consensus classification of the idiopathic interstitial pneumonias. This joint statement of the American Thoracic Society (ATS), and the European Respiratory Society (ERS) was adopted by the ATS board of directors, June 2001 and by the ERS Executive Committee, June 2001. Am J Respir Crit Care Med. 2002;165(2):277–304. doi: 10.1164/ajrccm.165.2.ats01. [DOI] [PubMed] [Google Scholar]
- 13.Katzenstein A.L., Myers J.L. Idiopathic pulmonary fibrosis: clinical relevance of pathologic classification. Am J Respir Crit Care Med. 1998;157(4 Pt 1):1301–1315. doi: 10.1164/ajrccm.157.4.9707039. [DOI] [PubMed] [Google Scholar]
- 14.Idiopathic Pulmonary Fibrosis Clinical Research Network. Raghu G., Anstrom K.J., King T.E., Jr. Prednisone, azathioprine, and N-acetylcysteine for pulmonary fibrosis. N Engl J Med. 2012;366(21):1968–1977. doi: 10.1056/NEJMoa1113354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Idiopathic Pulmonary Fibrosis Clinical Research Network. Martinez F.J., de Andrade J.A., Anstrom K.J. Randomized trial of acetylcysteine in idiopathic pulmonary fibrosis. N Engl J Med. 2014;370(22):2093–2101. doi: 10.1056/NEJMoa1401739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Noble P.W., Albera C., Bradford W.Z. Pirfenidone in patients with idiopathic pulmonary fibrosis (CAPACITY): two randomised trials. Lancet. 2011;377(9779):1760–1769. doi: 10.1016/S0140-6736(11)60405-4. [DOI] [PubMed] [Google Scholar]
- 17.King T.E., Jr., Bradford W.Z., Castro-Bernardini S. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med. 2014;370(22):2083–2092. doi: 10.1056/NEJMoa1402582. [DOI] [PubMed] [Google Scholar]
- 18.Richeldi L., du Bois R.M., Raghu G. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med. 2014;370(22):2071–2082. doi: 10.1056/NEJMoa1402584. [DOI] [PubMed] [Google Scholar]
- 19.Richeldi L., Costabel U., Selman M. Efficacy of a tyrosine kinase inhibitor in idiopathic pulmonary fibrosis. N Engl J Med. 2011;365(12):1079–1087. doi: 10.1056/NEJMoa1103690. [DOI] [PubMed] [Google Scholar]
- 20.Flaherty K.R., King T.E., Jr., Raghu G. Idiopathic interstitial pneumonia: what is the effect of a multidisciplinary approach to diagnosis? Am J Respir Crit Care Med. 2004;170(8):904–910. doi: 10.1164/rccm.200402-147OC. [DOI] [PubMed] [Google Scholar]
- 21.Hunninghake G.W., Zimmerman M.B., Schwartz D.A. Utility of a lung biopsy for the diagnosis of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2001;164(2):193–196. doi: 10.1164/ajrccm.164.2.2101090. [DOI] [PubMed] [Google Scholar]
- 22.Raghu G., Mageto Y.N., Lockhart D., Schmidt R.A., Wood D.E., Godwin J.D. The accuracy of the clinical diagnosis of new-onset idiopathic pulmonary fibrosis and other interstitial lung disease: a prospective study. Chest. 1999;116(5):1168–1174. doi: 10.1378/chest.116.5.1168. [DOI] [PubMed] [Google Scholar]
- 23.Flaherty K.R., Thwaite E.L., Kazerooni E.A. Radiological versus histological diagnosis in UIP and NSIP: survival implications. Thorax. 2003;58(2):143–148. doi: 10.1136/thorax.58.2.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Quadrelli S., Molinari L., Ciallella L., Spina J.C., Sobrino E., Chertcoff J. Radiological versus histopathological diagnosis of usual interstitial pneumonia in the clinical practice: does it have any survival difference? Respiration. 2010;79(1):32–37. doi: 10.1159/000225987. [DOI] [PubMed] [Google Scholar]
- 25.Behr J., Kreuter M., Hoeper M.M. Management of patients with idiopathic pulmonary fibrosis in clinical practice: the INSIGHTS-IPF registry. Eur Respir J. 2015;46(1):186–196. doi: 10.1183/09031936.00217614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tiitto L., Heiskanen U., Bloigu R., Paakko P., Kinnula V., Kaarteenaho-Wiik R. Thoracoscopic lung biopsy is a safe procedure in diagnosing usual interstitial pneumonia. Chest. 2005;128(4):2375–2380. doi: 10.1378/chest.128.4.2375. [DOI] [PubMed] [Google Scholar]
- 27.Utz J.P., Ryu J.H., Douglas W.W. High short-term mortality following lung biopsy for usual interstitial pneumonia. Eur Respir J. 2001;17(2):175–179. doi: 10.1183/09031936.01.17201750. [DOI] [PubMed] [Google Scholar]
- 28.Lettieri C.J., Veerappan G.R., Helman D.L., Mulligan C.R., Shorr A.F. Outcomes and safety of surgical lung biopsy for interstitial lung disease. Chest. 2005;127(5):1600–1605. doi: 10.1378/chest.127.5.1600. [DOI] [PubMed] [Google Scholar]
- 29.Kreider M.E., Hansen-Flaschen J., Ahmad N.N. Complications of video-assisted thoracoscopic lung biopsy in patients with interstitial lung disease. Ann Thorac Surg. 2007;83(3):1140–1144. doi: 10.1016/j.athoracsur.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 30.Hutchinson J.P., Fogarty A.W., McKeever T.M., Hubbard R.B. In-hospital mortality after surgical lung biopsy for interstitial lung disease in the United States. 2000 to 2011. Am J Respir Crit Care Med. 2016;193(10):1161–1167. doi: 10.1164/rccm.201508-1632OC. [DOI] [PubMed] [Google Scholar]
- 31.Casoni G.L., Tomassetti S., Cavazza A. Transbronchial lung cryobiopsy in the diagnosis of fibrotic interstitial lung diseases. PLoS One. 2014;9(2):e86716. doi: 10.1371/journal.pone.0086716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Poletti V., Hetzel J. Transbronchial cryobiopsy in diffuse parenchymal lung disease: need for procedural standardization. Respiration. 2015;90(4):275–278. doi: 10.1159/000439313. [DOI] [PubMed] [Google Scholar]
- 33.Gasparini S., Bonifazi M. Cryobiopsy for interstitial lung diseases. J Bronchology Interv Pulmonol. 2016;23(1):4–6. doi: 10.1097/LBR.0000000000000260. [DOI] [PubMed] [Google Scholar]
- 34.Tomassetti S., Wells A.U., Costabel U. Bronchoscopic lung cryobiopsy increases diagnostic confidence in the multidisciplinary diagnosis of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2016;193(7):745–752. doi: 10.1164/rccm.201504-0711OC. [DOI] [PubMed] [Google Scholar]
- 35.Ravaglia C., Bonifazi M., Wells A.U. Safety and diagnostic yield of transbronchial lung cryobiopsy in diffuse parenchymal lung diseases: a comparative study versus video-assisted thoracoscopic lung biopsy and a systematic review of the literature. Respiration. 2016;91(3):215–227. doi: 10.1159/000444089. [DOI] [PubMed] [Google Scholar]
- 36.Berbescu E.A., Katzenstein A.L., Snow J.L., Zisman D.A. Transbronchial biopsy in usual interstitial pneumonia. Chest. 2006;129(5):1126–1131. doi: 10.1378/chest.129.5.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tomassetti S., Cavazza A., Colby T.V. Transbronchial biopsy is useful in predicting UIP pattern. Respir Res. 2012;13(1):96. doi: 10.1186/1465-9921-13-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Votava J, Belperio JA, Kazerooni EA, et al. Utility of transbronchial biopsy versus surgical lung biopsy in the diagnosis of patients with suspected idiopathic interstitial pneumonia. A22 Interstitial Lung Disease: Evaluation and Pathobiology. Respir Crit Care Med. Abstract issue. 2013; A1089-A1089.
- 39.Fell C.D., Martinez F.J., Liu L.X. Clinical predictors of a diagnosis of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2010;181(8):832–837. doi: 10.1164/rccm.200906-0959OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fraire A.E., Cooper S.P., Greenberg S.D., Rowland L.P., Langston C. Transbronchial lung biopsy. Histopathologic and morphometric assessment of diagnostic utility. Chest. 1992;102(3):748–752. doi: 10.1378/chest.102.3.748. [DOI] [PubMed] [Google Scholar]
- 41.Kendall D.M., Gal A.A. Interpretation of tissue artifacts in transbronchial lung biopsy specimens. Ann Diagn Pathol. 2003;7(1):20–24. doi: 10.1053/adpa.2003.50003. [DOI] [PubMed] [Google Scholar]
- 42.Pajares V., Puzo C., Castillo D. Diagnostic yield of transbronchial cryobiopsy in interstitial lung disease:a randomized trial. Respirology. 2014;19(6):900–906. doi: 10.1111/resp.12322. [DOI] [PubMed] [Google Scholar]
- 43.Leslie K.O., Gruden J.F., Parish J.M., Scholand M.B. Transbronchial biopsy interpretation in the patient with diffuse parenchymal lung disease. Arch Pathol Lab Med. 2007;131(3):407–423. doi: 10.5858/2007-131-407-TBIITP. [DOI] [PubMed] [Google Scholar]