Abstract
Hypoxic pulmonary vasoconstriction (HPV) is a homeostatic mechanism that is intrinsic to the pulmonary vasculature. Intrapulmonary arteries constrict in response to alveolar hypoxia, diverting blood to better-oxygenated lung segments, thereby optimizing ventilation/perfusion matching and systemic oxygen delivery. In response to alveolar hypoxia, a mitochondrial sensor dynamically changes reactive oxygen species and redox couples in pulmonary artery smooth muscle cells (PASMC). This inhibits potassium channels, depolarizes PASMC, activates voltage-gated calcium channels, and increases cytosolic calcium, causing vasoconstriction. Sustained hypoxia activates rho kinase, reinforcing vasoconstriction, and hypoxia-inducible factor (HIF)-1α, leading to adverse pulmonary vascular remodeling and pulmonary hypertension (PH). In the nonventilated fetal lung, HPV diverts blood to the systemic vasculature. After birth, HPV commonly occurs as a localized homeostatic response to focal pneumonia or atelectasis, which optimizes systemic Po2 without altering pulmonary artery pressure (PAP). In single-lung anesthesia, HPV reduces blood flow to the nonventilated lung, thereby facilitating thoracic surgery. At altitude, global hypoxia causes diffuse HPV, increases PAP, and initiates PH. Exaggerated or heterogeneous HPV contributes to high-altitude pulmonary edema. Conversely, impaired HPV, whether due to disease (eg, COPD, sepsis) or vasodilator drugs, promotes systemic hypoxemia. Genetic and epigenetic abnormalities of this oxygen-sensing pathway can trigger normoxic activation of HIF-1α and can promote abnormal metabolism and cell proliferation. The resulting pseudohypoxic state underlies the Warburg metabolic shift and contributes to the neoplasia-like phenotype of PH. HPV and oxygen sensing are important in human health and disease.
Key Words: chuvash polycythemia, high altitude pulmonary edema, mitochondria, oxygen-sensitive potassium channels, single-lung anesthesia, ventilation/perfusion matching
Abbreviations: ATP, adenosine triphosphate; CaL, voltage-gated calcium channels; ETC, electron transport chain; FADH2, flavin adenine dinucleotide; HAPE, high-altitude pulmonary edema; HIF, hypoxia-inducible factor; HPH, hypoxic pulmonary hypertension; HPV, hypoxic pulmonary vasoconstriction; Kv, voltage-gated potassium channels; mPAP, mean pulmonary artery pressure; NADH, nicotinamide adenine dinucleotide; NO, nitric oxide; PA, pulmonary artery; PAP, pulmonary artery pressure; PASMC, pulmonary artery smooth muscle cell; PH, pulmonary hypertension; ROS, reactive oxygen species; SOD2, superoxide dismutase 2; , ventilation/perfusion
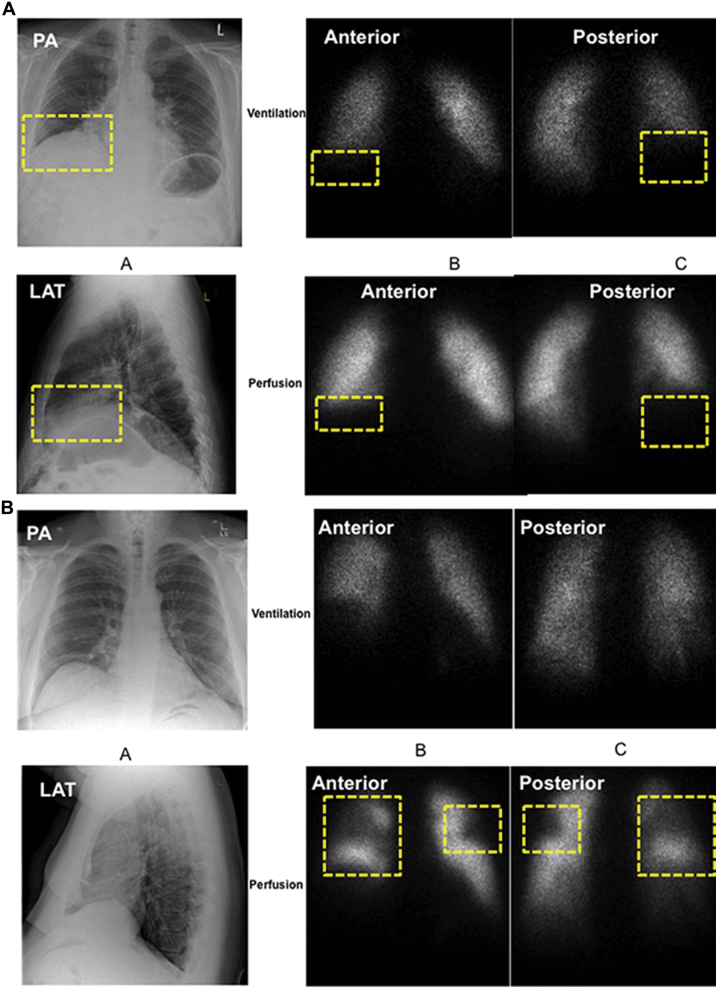
Hypoxic pulmonary vasoconstriction (HPV) was initially identified by Bradford and Dean1 and subsequently characterized by von Euler and Liljestrand.2 HPV is the lung’s intrinsic mechanism for matching perfusion to ventilation to optimize systemic oxygen delivery. HPV reflects the constriction of small intrapulmonary arteries in response to alveolar hypoxia.3 HPV can be global (in response to environmental hypoxia), in which case pulmonary artery pressure (PAP) rises; however, in most cases HPV is elicited by focal atelectasis or pneumonia, and both the alveolar hypoxia and the vasoconstriction are localized to a lung segment or lobe. In such cases, blood is diverted from the hypoxic lung segment to a better-oxygenated portion of the lung without elevation of PAP. Using the combination of a chest roentgenogram and a radioisotope ventilation/perfusion () scan, the matching defect due to parenchymal lung disease and HPV (Fig 1A) is readily distinguished from the mismatched defect caused by pulmonary embolism (Fig 1B).
Figure 1.
Matched vs mismatched ventilation/perfusion () defects. (A) Matched defect due to interstitial lung disease. An 83-year-old man with shortness of breath on exertion. Chest CT showed interstitial lung disease with a basal predominance consistent with usual interstitial pneumonitis. (B) Mismatched defect indicating pulmonary embolism. A 57-year-old man with obesity, hypertension, and OSA presenting with shortness of breath and desaturation with minimal exertion. PA = posteroanterior; LAT = lateral.
HPV has its onset within seconds of exposure to hypoxia and reaches a maximum intensity within minutes.4, 5 Although HPV can be sustained, it remains reversible on restoration of normal airway oxygen levels, unless pulmonary hypertension (PH) and adverse vascular remodeling has occurred. The reversibility of sustained lobar HPV is illustrated by a patient whose endobronchial adenoma caused longstanding left lung atelectasis and a matching abnormality, both of which reversed after resection of the adenoma.6 The vasoconstrictor response to hypoxia is unique to the resistance PAs7; the systemic vasculature (eg, renal, mesenteric, and cerebral arteries) dilates in response to hypoxia (which also serves to increase tissue oxygen delivery8, 9, 10).
Mechanism of HPV
The sensor and effector mechanisms of HPV have been the subject of considerable research.11, 12, 13, 14, 15 The sensor mechanism of HPV is hypothesized to reside within the mitochondria.16 The classically recognized function of the mitochondria is the generation of energy (adenosine triphosphate [ATP]). Electron transport from the electron donors, nicotinamide adenine dinucleotide (NADH) and flavin adenine dinucleotide (FADH2) occurs along the electron transport chain (ETC). Electron flux between ETC components is mediated by lipid-soluble semiquinones within the cristae. As electrons flow down a redox gradient to the terminal electron receptor (molecular oxygen), protons are transported across the inner mitochondrial membrane. According to Mitchell’s chemiosmotic theory,17 the buildup of hydrogen ions across this membrane generates the electrochemical gradient that powers an H+ transporter, the F1FO adenosine triphosphatase complex, and promotes ATP synthesis.18
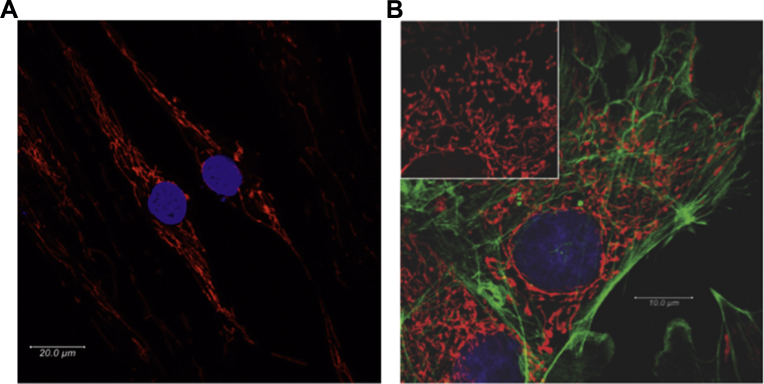
Mitochondria are prokaryotic endosymbionts that evolved within eukaryotic cells to support the energy demands of multicellular organisms.19 It is increasingly recognized that they have many noncanonical functions including oxygen sensing.20 Mitochondria form dynamic networks shaped by fission, fusion, and translocation. Live-cell imaging of the mitochondrial network suggests an analogy to the electrical wiring in a building (Fig 2, Video 1). Unlike the static two-dimensional electron microscopic images in fixed-tissue preparations, which make these organelles appear isolated, the use of mitochondria-targeted fluorophores in living pulmonary artery smooth muscle cells (PASMC) reveals that mitochondria form a pervasive and dynamic reticulum that reaches all areas of the cell and dynamically contacts other organelles, such as the sarcoplasmic reticulum. An appreciation of mitochondrial form aids understanding of the sensor function.
Figure 2.
Pulmonary artery (PA) mitochondrial network. (A) Mitochondria in pulmonary artery smooth muscle cells from a normal patient were stained with 20 nM tetramethylrhodamine and imaged using confocal microscopy. Nuclei stained with NucBlue Live Cell Stain. (B) Bovine pulmonary artery (PA) endothelial cells stained for mitochondria (inset: red, MitoTracker Red CMXRos), phalloidin (green, Alexa Fluor 488), and nucleus (blue, 4′,6-diamidino-2-phenylindole) and imaged using confocal microscopy. All dyes/stains from Life Technologies (Carlsbad, CA).
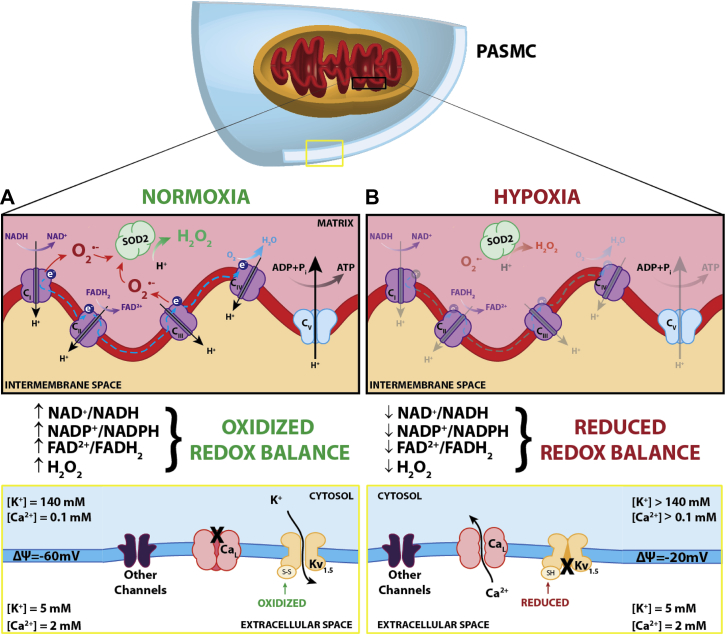
The sensor function of mitochondria is a byproduct of physiological electron flux, as illustrated in Figure 3. Briefly, during electron transfer some uncoupled electrons generate reactive oxygen species (ROS), specifically superoxide, which is rapidly converted by superoxide dismutase 2 (SOD2) to hydrogen peroxide, a diffusible redox mediator, which modulates the activity of redox-sensitive ion channels and enzymes and serves as a signaling molecule.21, 22 Teleologically, an oxygen sensor that relies on a parameter linked to Po2, (namely, ROS production that occurs as a byproduct of electron flux) but that resides proximal to ATP synthesis, permits a homeostatic response to counter hypoxia, without risking bioenergetic compromise. Oxygen sensing within the mammalian homeostatic oxygen sensing system relies on redox chemistry controlled by mitochondria and other redox sensors.23
Figure 3.
Mitochondrial redox oxygen sensing. The sensor-effector mechanism of hypoxic pulmonary vasoconstriction (HPV). (A) Under normoxic conditions, generation of reactive oxygen species (ROS) occurs at mitochondrial electron transport chain (ETC) complexes I and III, producing superoxide (O2–), which is converted to hydrogen peroxide (H2O2) by superoxide dismutase 2 (SOD2). Hydrogen peroxide, along with the oxidized redox couples (eg, nicotinamide adenine dinucleotide [NAD+], nicotinamide adenine dinucleotide phosphate [NADP+], and flavin adenine dinucleotide [FAD2+]) maintain Kv1.5 sulfhydryl group oxidation and channel open state, resulting in tonic egress of K+. This efflux of K+ sustains the resting membrane potential (ΔΨ) of the cell at –60 mV and inhibits voltage-gated calcium channel [CaL]-mediated Ca2+ influx into the cell. (B) During hypoxia, the limited presence of oxygen (1) prevents generation of hydrogen peroxide, (2) decreases the ratio of oxidized/reduced redox couples, and (3) reduces sulfhydryl groups on Kv1.5 channels, causing them to close. The subsequent buildup of K+ increases the resting membrane potential of the cell to –20 mV. This stimulates the opening of CaL, influx of Ca2+, and subsequent activation of the contractile apparatus (ie, vasoconstriction). ADP = adenosine diphosphate; FADH2 = flavin adenine dinucleotide; NADH = nicotinamide adenine dinucleotide.
Although increased by endothelium-derived vasoconstrictors (eg, endothelin and thromboxane) and inhibited by endothelium-derived vasodilators (eg, nitric oxide and prostacyclin), the core effector mechanism of HPV lies within the PASMC. Here HPV is triggered by a mitochondrial redox signal that involves the coordinated response of voltage- and redox-sensitive potassium and calcium channels (Fig 3). Briefly, voltage-gated potassium channels (Kv) maintain a resting membrane potential of about –60 mV (reflective of tonic egress of K+ from the PASMC). This negative membrane potential decreases the opening of voltage-gated, L-type calcium channels. Outward potassium current is inhibited during hypoxia, depolarizing the membrane and increasing the open-state probability of calcium channels, causing an influx of Ca2+ into the cell down a 20,000:1 extracellular/intracellular Ca2+ gradient. This rise in cytosolic calcium and a subsequent rho kinase-mediated calcium sensitization causes PA constriction.24, 25
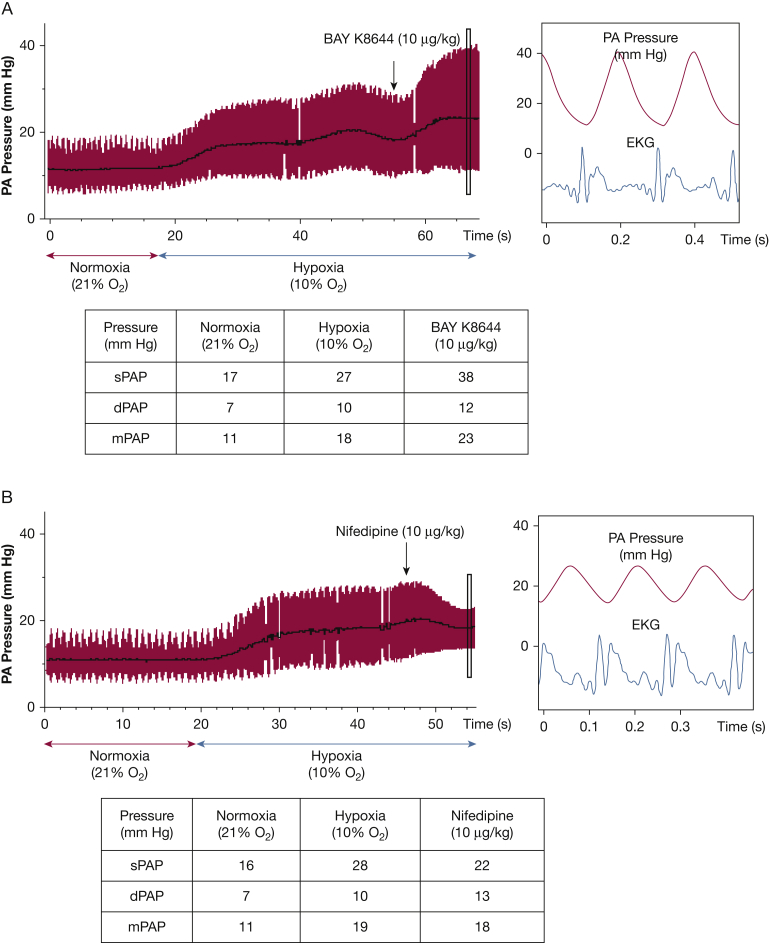
Pharmacologic and electrophysiological experimentation using the patch clamp technique to study isolated PASMC from resistance PAs identified Kv as well as large conductance, voltage-gated calcium channels (CaL) in the effector mechanism of HPV.25, 26 4-Aminopyridine is a Kv channel blocker and elicits pulmonary vasoconstriction in isolated perfused rodent lungs.27 Additionally, CaL blockers nifedipine and verapamil inhibit the HPV response, whereas BAY K8644, a Ca2+-channel agonist, enhances the vasoconstrictive response to hypoxia24, 28 (Fig 4). However, these effector components of HPV do not differ substantially between pulmonary and systemic arteries, both of which constrict in response to K+ channel inhibitors and relax in response to calcium channel blockers25. Rather, the tissue heterogeneity in response to hypoxia results primarily from differences in oxygen sensor (mitochondrial) function between smooth muscle cells in pulmonary vs systemic arteries.
Figure 4.
Voltage-gated calcium channels [CaL] and hypoxic pulmonary vasoconstriction (HPV). CaL agonist BAY K8644 and antagonist nifedipine exert opposing effects on HPV in the anesthetized hypoxic rat ventilated with 10% oxygen. This model of global hypoxia mimics changes that occur with ascent to altitude and results in a rapid increase in pulmonary artery pressure (PAP). (A) BAY K8644 enhances PA vasoconstriction during hypoxia. (B) Nifedipine administered during HPV inhibits CaL and reduces PAP. dPAP = diastolic PAP; ECG = electrocardiography; mPAP = mean PAP; sPAP = systolic PAP.
Molecular identification of the ion channels involved in HPV has revealed central roles for Kv1.5, a Shaker channel, and Kv2.1.29, 30 The Kv1.5 knockout mouse, for example, has markedly reduced HPV.31 However, there is evidence for the involvement of other classes of K+ and Ca2+ channels in the mechanism of HPV,32 including two-pore K+ channels,33, 34 which are active at very negative membrane potentials and thus are attractive targets for initiation of HPV. Although pharmacologic inhibition of these channels does not result in pulmonary vasoconstriction,34 some likely contribute to the PASMC’s electrophysiologic response to hypoxia. Likewise, in addition to the L-type Ca2+ channel, Ca2+ that contributes to HPV also derives from Ca2+-induced Ca2+ release. HPV also reflects calcium sensitization.35
Although there is agreement that the mitochondria act as oxygen sensors and that ETC-derived ROS alter effector mechanisms that mediate vasoconstriction,36, 37, 38 controversy remains regarding whether hypoxia elicits a rise or a fall in ROS/hydrogen peroxide levels.32, 39, 40, 41, 42, 43 Briefly, it has been proposed that hypoxia increases ROS and reflects autoxidation of the ETC due to distal inhibition of the ETC.41, 42 In contrast, our findings suggest that ROS production in PASMC increases as Po2 rises and decreases as Po2 falls. For example, we observe a rise in ROS levels in ductus arteriosus smooth muscle cells at birth as Po2 increases.44, 45 Likewise, ROS levels are low in cardiac myocytes during ischemia and increase with reoxygenation during the reperfusion phase of myocardial ischemia/reperfusion injury.46 We observe that ROS (including hydrogen peroxide) decrease in PASMC mitochondria during physiological hypoxia, reflecting a reduced rate of electron flux caused by reduced availability of the terminal electron acceptor (molecular oxygen).23
The basis for the discrepancies between those who find hypoxia to be an oxidized state with high ROS41, 42 vs those who find it to be a reduced state with low ROS23, 43 is unclear. Experimental discrepancies may relate to variation among groups in (1) the use of freshly isolated PASMC from resistance arteries, (2) the severity of hypoxia used, (3) attention to pH and Pco2, and (4) challenges in dynamic and accurate ROS measurement in subcellular compartments. Supporting the notion that hypoxia is a state of reduction (not oxidation), the opposing effects of hypoxia on the PA vs systemic vascular tone and cellular electrophysiology are mimicked by ETC inhibitors (eg, rotenone and antimycin A) and reducing agents (eg, dithiothreitol), rather than by ROS and oxidants.9, 47 Proposed mechanisms should be judged by the degree to which they explain core properties of HPV, which is rapid, reversible, evoked by mild hypoxia, and does not induce edema. Moreover, a unifying mechanism should account for the opposing effects of hypoxia on tone in the pulmonary circulation (constriction) vs the ductus arteriosus and systemic vasculature (vasodilatation).
Clinical Relevance of HPV
Although HPV is active in normal human physiology and patients with common lung diseases, it remains underappreciated by clinicians. In addition to optimizing matching and systemic oxygen delivery, HPV is exploited surgically to enhance oxygenation.
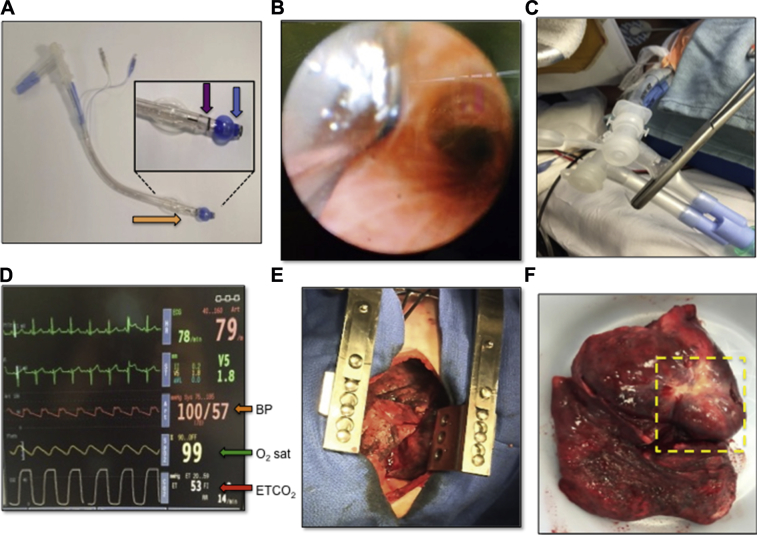
HPV is critical to single-lung anesthesia for patients undergoing thoracic surgery such as lung tumor resections.48 During these procedures, the patient is positioned on the side to facilitate surgical dissection, and single-lung ventilation is initiated using a double-lumen endobronchial tube (Fig 5A). During tube placement, the proximal and distal lumen tips are positioned above the carina and in the primary bronchus of the target lung, respectively (Fig 5B). Cuffs around each lumen are inflated to prevent air leakage and allow for selective ventilation of the nonoperative lung and collapse of the operative lung (Fig 5C). HPV within the nonventilated lung reduces perfusion by minimizing shunting that would otherwise lead to intraoperative systemic hypoxemia (Fig 5D) and excessive bleeding in the operative field (Fig 5E).
Figure 5.
Clinical application of hypoxic pulmonary vasoconstriction (HPV). Maintenance of Po2 during thoracic surgery through single-lung ventilation. (A) A Broncho-Cath double-lumen endotracheal tube (Covidien, Saint-Laurent, QC). The distal end (orange arrow) is inserted into the trachea until the bronchial lumen (inset, blue arrow) has entered either the right or left mainstem bronchus, whereas the tracheal lumen (inset, purple arrow) remains above the carina. (B) A bronchoscopic view of the carina from inside the tracheal lumen. The distal tip of the double-lumen endotracheal tube is seen entering the left mainstem bronchus to enable single-lung ventilation. (C) A clamp is placed on one limb of the ventilation circuit, allowing selective ventilation of the opposite lung. (D) Cardiorespiratory monitor during single-lung anesthesia/ventilation. The end tidal CO2 is elevated to 53 mm Hg because of the decreased ventilation (red arrow), whereas oxygen (O2) saturation is maintained at 99% (green arrow) and blood pressure (BP) remains stable (orange arrow) despite single-lung ventilation. (E) The nonventilated deflated lung undergoing surgery can be seen through an incision. Minimal bleeding is seen during surgery as a result of the HPV response. (F) Pneumonectomy specimen showing the complete lung removed with a large tumor (yellow box).
The benefits of HPV during thoracic surgery may be attenuated by drugs or patient physiology. Volatile anesthetics, vasodilators, and hypothermia can inhibit HPV. Thus, anesthetic choices and temperature can be adjusted to optimize HPV in patients during single-lung ventilation. Historically, HPV has been enhanced pharmacologically using low doses of the respiratory stimulant almitrine. During single-lung ventilation, adding almitrine (4 μg/kg/min) to inhaled nitric oxide (NO) increases HPV and improves systemic oxygenation.49 Although almitrine was withdrawn from clinical use because of its potential to cause peripheral neuropathy, this drug illustrates the value of enhancing HPV as a means of improving matching and systemic oxygenation.
HPV also optimizes systemic Po2 in patients with atelectasis, pneumonia, COPD, and asthma by reducing mismatch and shunting (Table 1). During pneumonia or atelectasis, HPV optimizes systemic oxygen delivery by reducing perfusion of the hypoxic segment. HPV rapidly reverses on relief of atelectasis (eg, as occurs on removal of a mucous plug or with the use of incentive spirometry) or on resolution of pneumonia. The intensity of HPV varies between individuals and can diminish with disease or medication (Table 1).50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64 For example, calcium channel blockers, which are commonly prescribed for systemic hypertension or coronary artery disease (comorbidities of COPD) can reduce HPV.65 Illustrative of this point, patients with cor pulmonale (pulmonary hypertension secondary to COPD) given nifedipine (20 mg) exhibited a decrease in arterial Po2 (from 52 to 47 mm Hg) and displayed a concomitant deterioration of matching.65 The route of administration determines the effects of PA vasodilators on HPV. Inhaled vasodilators (eg, inhaled NO) may enhance matching, because they only reach well-ventilated lung segments (and thus do not impair HPV); conversely, IV vasodilators worsen matching by relaxing arterioles serving poorly ventilated lung segments.66 For example, after inducing PH in an isolated perfused rabbit lung model, Walmrath et al66 demonstrated that although IV prostacyclin vasodilator treatment reduced mean pulmonary artery pressure (mPAP) (from 35 to 25 mm Hg), it also increased shunt fraction (to about 60%). In contrast, inhaled NO similarly decreased mPAP but maintained shunt fraction at about 25%.66
Table 1.
The Role of HPV in Respiratory Disease
| Disease | Role of HPV | Treatment | References |
|---|---|---|---|
| HAPE | Exaggerated HPV; overperfusion of nonconstricted pulmonary vasculature | Calcium channel blocker nifedipine reduces the incidence of HAPE from 63% to 10% | 50, 51, 52, 53 |
| Asthma | HPV maintains balance during acute bronchoconstriction | IV isoprenaline decreases mPAP in an isolated perfused rat lung model of asthma (by 3-5 mm Hg) | 53, 54, 55, 56 |
| COPD | HPV is reduced; however, the residual response improves balance and systemic oxygenation | In COPD, almitrine (100 mg/d), a drug that enhances HPV, increases Pao2 from 52 to 59 mm Hg | 53, 57, 58, 59 |
| Pneumonia | Diversion of blood flow away from the diseased lobes of lung optimizes systemic Po2 | Pneumonia impairs HPV in experimental models; in COPD with hypoxic exacerbations, IV prostaglandins increase systemic hypoxemia, consistent with the adverse effects of suppressing HPV | 53, 57, 60, 61, 62 |
| ALI/ARDS | HPV is reduced in ALI; however, the residual HPV improves balance and reduces shunting | In an ovine model of ARDS, inhibition of inducible NOS restores HPV, increases Pao2/Fio2, and decreases shunt fraction | 53, 57, 63, 64 |
ALI = acute lung injury; HAPE = high-altitude pulmonary edema; HPV = hypoxic pulmonary vasoconstriction; mPAP = mean pulmonary artery pressure; NOS = nitric oxide synthase; = ventilation/perfusion.
Suppression of HPV with Prolonged Exposure to Altitude
Suppression of HPV is a common adaptation to life at high altitude. The yak (Bos grunniens), native to the Himalayan region of Central Asia (altitudes > 3,500 m) has blunted HPV and thus maintains low PAP.67 Conversely, domestic cattle (Bos taurus) are native to lowlands and exhibit substantial HPV at altitude, resulting in severe PH, edema, and right-sided heart failure in about 20% of cattle.68 The sustained HPV and adverse pulmonary vascular remodeling causes right-sided heart failure, which leads to peripheral edema. Because of the cow’s anatomy, this dependent edema collects in the neck (the brisket area), rather than in the legs, as would occur in humans. This neck swelling gave the name brisket disease to this lethal syndrome of excessive HPV and right-sided heart failure.69 Cattle native to high altitudes are less prone to exaggerated HPV and brisket disease.67, 70 The crossbreeding of yak and cattle results in an animal that exhibits intermediate HPV. Anand et al71 found that the dzo (a cross between a cow and a yak) had pulmonary hemodynamics similar to those of the yak. In contrast, crossbreeding a dzo to a bull results in a stol. Half of the stols had pulmonary hemodynamics similar to the yak, whereas the other half resembled the cow. This variation indicates a genetic basis for “altitude resistance” in these animals.70, 72 Newman et al68 assessed the genetics of exaggerated HPV and high-altitude PH by comparing susceptible and resistant cattle (PAP at altitude, 86 ± 13 mm Hg vs 35 ± 1 mm Hg, respectively). In altitude-susceptible cattle, the expression of 46 genes was upregulated, whereas the expression of 14 genes was downregulated. Microarray analysis identified respiratory diseases and inflammatory disease pathways as being disordered in susceptible cattle. The susceptible phenotype was associated with abnormal interleukin-6, triggering receptor expressed on myeloid cells, peroxisome proliferator-activated receptor, and nuclear factor κB signaling.68 Whole-exome sequencing has implicated a mutation in the HIF-2α-encoding gene (endothelial PAS domain-containing protein 1 [EPAS1]) in the pathogenesis of high-altitude PH in cattle. A double variant in EPAS1 causing two nonsynonymous amino acid substitutions in the oxygen-dependent degradation domain of HIF-2α was found in 75% of cattle with elevated mPAP (> 50 mm Hg) and in all high-altitude cattle with PH (mPAP > 94 mm Hg) studied. It is hypothesized that this double variant is a gain of function mutation causing upregulation of multiple HIF-2α targets such as vascular endothelial growth factor and transforming growth factor-α.73
Human populations also display genetic variation in PAP at altitude. After millennia of living at altitudes > 4,000 m, native Tibetans exhibit minimal hypoxic PH (HPH) or polycythemia.74 In contrast, HPH is prevalent in native Quechua Indians living in the Andean highlands of Ecuador and Bolivia at altitudes of approximately 3,500 to 4,000 m.71, 75 Similarly, children in Leadville, Colorado (the highest city in North America at about 3,100 m) commonly exhibit HPH. The severity of HPH in Leadville residents is similar to that of the Quechua, although the Coloradans live at much lower altitudes. Grover70 showed that many healthy athletic children in Leadville exhibited HPH (mPAP, 28 mm Hg and 61 mm Hg at rest and during exercise, respectively). This indicates a high prevalence of moderate asymptomatic PH in a population of recent high-altitude residents.50 It is thus hypothesized that the magnitude of HPV and HPH are inversely proportional to the duration of evolution at high altitude (25,000 years for Tibetans vs about 13,000 years for Andean Quechua71 vs less than a century for populations in Colorado).70 These studies in human and animal populations suggest genetic transmission of susceptibility or resistance to chronic hypoxia, and these variations likely relate to variations in the expression and function of components of the oxygen-sensing pathway.
Excessive HPV With Acute Exposure to Altitude Precipitates High Altitude Pulmonary Edema
Rapid ascent to high altitude without proper acclimation to the decreased oxygen tension can result in high-altitude pulmonary edema (HAPE), which is a noncardiogenic form of pulmonary edema that is characterized by cough, dyspnea, and reduced exercise performance, with usual onset within 2 to 5 days of ascension to altitudes > 2,500 m.76 Exaggerated and heterogeneous HPV is implicated in the pathophysiology of HAPE51, 52 (Table 1). The rapid rise in PAP stresses and distends the arterial walls. Within a short time, these changes exceed the load capacity of the resistance PA resulting in rupture of the basement membrane and the alveolar-capillary barrier,77 Interestingly, HAPE exhibits an individual susceptibility, being most common in people with exaggerated HPV. The 10% of whites that have exaggerated HPV are particularly susceptible to HAPE. Dehnert et al78 assessed 421 healthy whites who were naive to high altitude; subjects were exposed to normobaric hypoxia (simulating the hypoxia found at 4,500 m), and PAP was measured using Doppler echocardiography. Thirty-nine subjects displayed exaggerated HPV during a simulated ascent to 4,559 m over 24 hours (systolic PAP in hypoxia, 51 ± 6 mm Hg). Four (13%) of these volunteers experienced HAPE during a 48-hour exposure. In contrast, no subjects with normal intensity of HPV (systolic PAP in hypoxia, 33 ± 5 mm Hg) experienced HAPE.78 Moreover, in experimental models of ascent to altitude, suppression of HPV reduces HAPE. Isolated perfused rabbit lungs treated with acetazolamide (33 uM), which is an agent used clinically to prevent HAPE, exhibited less HPV (hypoxic mPAP about 15 mm Hg) than did control lungs (hypoxic mPAP about 20 mm Hg).79
In addition to rupture of the alveolar-capillary barrier, hypoxia also inhibits alveolar fluid clearance within the lung. Under normal conditions, reabsorption of sodium through sodium channels and exchangers generates an osmotic gradient within the lung, allowing the reabsorption of water. Hypoxia inhibits the activity of sodium exchangers, thereby decreasing sodium transport and ultimately reducing fluid reabsorption in the lung.77
HAPE is reversible provided that the patient receives adequate treatment in a timely manner. Improving oxygenation is the end goal, typically with supplemental oxygen and a rapid descent from altitude. Patients typically improve within hours of treatment with supplemental oxygen and rest. Complete clinical recovery is expected within days at low altitude.80 In emergencies, when immediate descent is not feasible, treatment of HAPE with agents that inhibit HPV, such as nifedipine or sildenafil, can relieve symptoms until descent to a lower altitude can safely occur.81
Pseudohypoxia and Chuvash Disease
Another instance of pathologic HPV occurs in Chuvash disease. Named for the mid-Volga River region of Russia, patients with Chuvash disease have enhanced HPV, polycythemia, and PH, despite normal inspired oxygen concentrations.82 Patients with Chuvash disease have a missense mutation within the von Hippel-Lindau gene (VHL). This mutation impairs the VHL’s ability to interact with the α-subunits of HIF-1α and HIF-2α. Under normoxic conditions, HIF proteins are hydroxylated by prolyl hydroxylases, which causes them to be ubiquitinated by VHL, thereby targeting HIF for proteasomal degradation. During hypoxia, HIF proteins are not hydroxylated nor are they bound by VHL, and thus degradation is impaired. The Chuvash VHL mutation impairs the ability of VHL-HIF interaction, resulting in normoxic HIF stabilization and paradoxical normoxic transcription of HIF-regulated genes, such as erythropoietin.82, 83, 84 Thus, patients with Chuvash disease function as if they were exposed to chronic hypoxia due to derangement of normal oxygen sensing.
Fawn hooded rats, like patients with Chuvash disease, spontaneously develop PH and mild polycythemia with age. They too have normoxic activation of HIF-1α, although in fawn hooded rats this reflects epigenetic silencing of mitochondrial SOD2.85 HIF is also a redox-regulated, oxygen-sensing mechanism86; however, its protean transcription-mediated effects have a slower onset than do the ion-channel-initiated vascular response to hypoxia. Conditions in which oxygen sensing is disordered remind us that pseudohypoxic signaling can contribute to human diseases such as HPH. Indeed, the Warburg paradigm in cancer is another example of impaired oxygen sensing leading to abnormalities of metabolism, cell proliferation, and apoptosis.87
Conclusions
The ability of PASMC in small resistance PAs to sense changes in oxygen and trigger HPV optimizes oxygen uptake and systemic oxygen delivery. HPV reflects an elegant interaction between a mitochondrial-redox sensor and an effector, which comprises redox-sensitive ion channels, enzymes, and transcription factors. HPV is important in the fetal circulation and is active in optimizing oxygenation in normal humans. HPV optimizes systemic oxygen delivery in various lung diseases, including atelectasis, pneumonia, and COPD. Clinicians should be mindful that inadvertent suppression of HPV by vasodilator drugs can lead to mismatch and systemic hypoxemia; conversely, HPV can be inhibited to prevent or treat HAPE. HPV can also be exploited during single-lung ventilation to facilitate lung surgery.
Acknowledgments
Financial/nonfinancial disclosures: None declared.
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Additional information: The Video can be found in the Supplemental Materials section of the online article.
Footnotes
FUNDING/SUPPORT: This work is supported by the National Institutes of Health [R01-HL071115 and RC1HL099462], Canadian Institutes for Health Research (CIHR) Foundation Award 143261, the Canada Foundation for Innovation (CFI) 33012 and 229252, The W.J. Henderson Foundation and a Canada Research Chair 950-229252. K. D. S. and D. W. are funded by Scholar Awards from the Canadian Vascular Network.
Supplementary Data
Mitochondrial dynamics and motility. Mitochondria undergo continuous fission and fusion, resulting in a dynamic network. Mitochondria in PASMC from a normal patient stained with 20 nM tetramethylrhodamine. Images were acquired using confocal microscopy, 10 z-steps per frame, in 15-s intervals for 10 min.
References
- 1.Bradford J.R., Dean H.P. The pulmonary circulation. J Physiol. 1894;16(1-2):34–110. doi: 10.1113/jphysiol.1894.sp000493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Euler U.S.V., Liljestrand G. Observations on the pulmonary arterial blood pressure in the cat. Acta Physiol Scand. 1946;12(4):301–320. [Google Scholar]
- 3.Madden J.A., Dawson C.A., Harder D.R. Hypoxia-induced activation in small isolated pulmonary arteries from the cat. J Appl Physiol (1985) 1985;59(1):113–118. doi: 10.1152/jappl.1985.59.1.113. [DOI] [PubMed] [Google Scholar]
- 4.Baraka A.S., Taha S.K., Yaacoub C.I. Alarming hypoxemia during one-lung ventilation in a patient with respiratory bronchiolitis-associated interstitial lung disease. Can J Cardiol. 2003;50(4):411–414. doi: 10.1007/BF03021041. [DOI] [PubMed] [Google Scholar]
- 5.Jensen K.S., Micco A.J., Czartolomna J., Latham L., Voelkel N.F. Rapid onset of hypoxic vasoconstriction in isolated lungs. J Appl Physiol (1985) 1992;72(5):2018–2023. doi: 10.1152/jappl.1992.72.5.2018. [DOI] [PubMed] [Google Scholar]
- 6.Grant J.L., Naylor R.W., Crandell W.B. Bronchial adenoma resection with relief of hypoxic pulmonary vasoconstriction. Chest. 1980;77(3):446–449. doi: 10.1378/chest.77.3.446. [DOI] [PubMed] [Google Scholar]
- 7.Shirai M., Ninomiya I., Sada K. Constrictor response of small pulmonary arteries to acute pulmonary hypertension during left atrial pressure elevation. Jpn J Physiol. 1991;41(1):129–142. doi: 10.2170/jjphysiol.41.129. [DOI] [PubMed] [Google Scholar]
- 8.Madden J.A., Vadula M.S., Kurup V.P. Effects of hypoxia and other vasoactive agents on pulmonary and cerebral artery smooth muscle cells. Am J Physiol. 1992;263(3 Pt 1):L384–L393. doi: 10.1152/ajplung.1992.263.3.L384. [DOI] [PubMed] [Google Scholar]
- 9.Michelakis E.D., Hampl V., Nsair A. Diversity in mitochondrial function explains differences in vascular oxygen sensing. Circ Res. 2002;90(12):1307–1315. doi: 10.1161/01.res.0000024689.07590.c2. [DOI] [PubMed] [Google Scholar]
- 10.Yuan X.J., Tod M.L., Rubin L.J., Blaustein M.P. Contrasting effects of hypoxia on tension in rat pulmonary and mesenteric arteries. Am J Physiol. 1990;259(2 Pt 2):H281–H289. doi: 10.1152/ajpheart.1990.259.2.H281. [DOI] [PubMed] [Google Scholar]
- 11.Archer S.L., Weir E.K., Wilkins M.R. Basic science of pulmonary arterial hypertension for clinicians: new concepts and experimental therapies. Circulation. 2010;121(18):2045–2066. doi: 10.1161/CIRCULATIONAHA.108.847707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Archer S.L., Will J.A., Weir E.K. Redox status in the control of pulmonary vascular tone. Herz. 1986;11(3):127–141. [PubMed] [Google Scholar]
- 13.Moudgil R., Michelakis E.D., Archer S.L. Hypoxic pulmonary vasoconstriction. J Appl Physiol (1985) 2005;98(1):390–403. doi: 10.1152/japplphysiol.00733.2004. [DOI] [PubMed] [Google Scholar]
- 14.Rounds S., McMurtry I.F. Inhibitors of oxidative ATP production cause transient vasoconstriction and block subsequent pressor responses in rat lungs. Circ Res. 1981;48(3):393–400. doi: 10.1161/01.res.48.3.393. [DOI] [PubMed] [Google Scholar]
- 15.Weir E.K., Archer S.L. The mechanism of acute hypoxic pulmonary vasoconstriction: the tale of two channels. FASEB J. 1995;9(2):183–189. doi: 10.1096/fasebj.9.2.7781921. [DOI] [PubMed] [Google Scholar]
- 16.Hong Z., Kutty S., Toth P.T. Role of dynamin-related protein 1 (Drp1)-mediated mitochondrial fission in oxygen sensing and constriction of the ductus arteriosus. Circ Res. 2013;112(5):802–815. doi: 10.1161/CIRCRESAHA.111.300285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mitchell P. Aspects of the chemiosmotic hypothesis. Biochem J. 1970;116(4):5P–6P. doi: 10.1042/bj1160005p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jonckheere A.I., Smeitink J.A., Rodenburg R.J. Mitochondrial ATP synthase: architecture, function and pathology. J Inherit Metab Dis. 2012;35(2):211–225. doi: 10.1007/s10545-011-9382-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Margulis L. Genetic and evolutionary consequences of symbiosis. Exp Parasitol. 1976;39(2):277–349. doi: 10.1016/0014-4894(76)90127-2. [DOI] [PubMed] [Google Scholar]
- 20.Archer S.L. Mitochondrial dynamics—mitochondrial fission and fusion in human diseases. N Engl J Med. 2013;369(23):2236–2251. doi: 10.1056/NEJMra1215233. [DOI] [PubMed] [Google Scholar]
- 21.Michelakis E.D., Rebeyka I., Wu X. Oxygen sensing in the human ductus arteriosus: regulation of voltage-gated K+ channels in smooth muscle cells by a mitochondrial redox sensor. Circ Res. 2002;91(6):478–486. doi: 10.1161/01.res.0000035057.63303.d1. [DOI] [PubMed] [Google Scholar]
- 22.Michelakis E.D., Thebaud B., Weir E.K., Archer S.L. Hypoxic pulmonary vasoconstriction: redox regulation of oxygen-sensitive K+ channels by a mitochondrial oxygen-sensor in resistance artery smooth muscle cells. J Mol Cell Cardiol. 2004;37(6):1119–1136. doi: 10.1016/j.yjmcc.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 23.Weir E.K., Lopez-Barneo J., Buckler K.J., Archer S.L. Acute oxygen-sensing mechanisms. N Engl J Med. 2005;353(19):2042–2055. doi: 10.1056/NEJMra050002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McMurtry I.F., Davidson A.B., Reeves J.T., Grover R.F. Inhibition of hypoxic pulmonary vasoconstriction by calcium antagonists in isolated rat lungs. Circ Res. 1976;38(2):99–104. doi: 10.1161/01.res.38.2.99. [DOI] [PubMed] [Google Scholar]
- 25.Post J.M., Hume J.R., Archer S.L., Weir E.K. Direct role for potassium channel inhibition in hypoxic pulmonary vasoconstriction. Am J Physiol. 1992;262(4 Pt 1):C882–C890. doi: 10.1152/ajpcell.1992.262.4.C882. [DOI] [PubMed] [Google Scholar]
- 26.Archer S.L., Wu X.C., Thebaud B. Preferential expression and function of voltage-gated, oxygen-sensitive K+ channels in resistance pulmonary arteries explains regional heterogeneity in hypoxic pulmonary vasoconstriction: ionic diversity in smooth muscle cells. Circ Res. 2004;95(3):308–318. doi: 10.1161/01.RES.0000137173.42723.fb. [DOI] [PubMed] [Google Scholar]
- 27.Hasunuma K., Rodman D.M., McMurtry I.F. Effects of K+ channel blockers on vascular tone in the perfused rat lung. Am Rev Respir Dis. 1991;144(4):884–887. doi: 10.1164/ajrccm/144.4.884. [DOI] [PubMed] [Google Scholar]
- 28.McMurtry I.F. BAY K 8644 potentiates and A23187 inhibits hypoxic vasoconstriction in rat lungs. Am J Physiol. 1985;249(4 Pt 2):H741–H746. doi: 10.1152/ajpheart.1985.249.4.H741. [DOI] [PubMed] [Google Scholar]
- 29.Archer S.L., Souil E., Dinh-Xuan A.T. Molecular identification of the role of voltage-gated K+ channels, Kv1.5 and Kv2.1, in hypoxic pulmonary vasoconstriction and control of resting membrane potential in rat pulmonary artery myocytes. J Clin Invest. 1998;101(11):2319–2330. doi: 10.1172/JCI333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Firth A.L., Remillard C.V., Platoshyn O., Fantozzi I., Ko E.A., Yuan J.X. Functional ion channels in human pulmonary artery smooth muscle cells: Voltage-dependent cation channels. Pulm Circ. 2011;1(1):48–71. doi: 10.4103/2045-8932.78103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Archer S.L., London B., Hampl V. Impairment of hypoxic pulmonary vasoconstriction in mice lacking the voltage-gated potassium channel Kv1.5. FASEB J. 2001;15(10):1801–1803. doi: 10.1096/fj.00-0649fje. [DOI] [PubMed] [Google Scholar]
- 32.Ward J.P. Point: Hypoxic pulmonary vasoconstriction is mediated by increased production of reactive oxygen species. J Appl Physiol (1985) 2006;101(3):993–995. doi: 10.1152/japplphysiol.00480.2006. discussion 999. [DOI] [PubMed] [Google Scholar]
- 33.Duprat F., Guillemare E., Romey G. Susceptibility of cloned K+ channels to reactive oxygen species. Proc Natl Acad Sci U S A. 1995;92(25):11796–11800. doi: 10.1073/pnas.92.25.11796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gardener M.J., Johnson I.T., Burnham M.P., Edwards G., Heagerty A.M., Weston A.H. Functional evidence of a role for two-pore domain potassium channels in rat mesenteric and pulmonary arteries. Br J Pharmacol. 2004;142(1):192–202. doi: 10.1038/sj.bjp.0705691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ward J.P., McMurtry I.F. Mechanisms of hypoxic pulmonary vasoconstriction and their roles in pulmonary hypertension: new findings for an old problem. Curr Opin Pharmacol. 2009;9(3):287–296. doi: 10.1016/j.coph.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Archer S.L., Huang J., Henry T., Peterson D., Weir E.K. A redox-based oxygen sensor in rat pulmonary vasculature. Circ Res. 1993;73(6):1100–1112. doi: 10.1161/01.res.73.6.1100. [DOI] [PubMed] [Google Scholar]
- 37.Archer S.L., Nelson D.P., Weir E.K. Simultaneous measurement of oxygen radicals and pulmonary vascular reactivity in rat lung. J Appl Physiol (1985) 1989;67(5):1903–1911. doi: 10.1152/jappl.1989.67.5.1903. [DOI] [PubMed] [Google Scholar]
- 38.Paky A., Michael J.R., Burke-Wolin T.M., Wolin M.S., Gurtner G.H. Endogenous production of superoxide by rabbit lungs: effects of hypoxia or metabolic inhibitors. J Appl Physiol (1985) 1993;74(6):2868–2874. doi: 10.1152/jappl.1993.74.6.2868. [DOI] [PubMed] [Google Scholar]
- 39.Chandel N.S., McClintock D.S., Feliciano C.E. Reactive oxygen species generated at mitochondrial complex III stabilize hypoxia-inducible factor-1alpha during hypoxia: a mechanism of oxygen sensing. J Biol Chem. 2000;275(33):25130–25138. doi: 10.1074/jbc.M001914200. [DOI] [PubMed] [Google Scholar]
- 40.Guzy R.D., Hoyos B., Robin E. Mitochondrial complex III is required for hypoxia-induced ROS production and cellular oxygen sensing. Cell Metab. 2005;1(6):401–408. doi: 10.1016/j.cmet.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 41.Waypa G.B., Chandel N.S., Schumacker P.T. Model for hypoxic pulmonary vasoconstriction involving mitochondrial oxygen sensing. Circ Res. 2001;88(12):1259–1266. doi: 10.1161/hh1201.091960. [DOI] [PubMed] [Google Scholar]
- 42.Waypa G.B., Marks J.D., Mack M.M., Boriboun C., Mungai P.T., Schumacker P.T. Mitochondrial reactive oxygen species trigger calcium increases during hypoxia in pulmonary arterial myocytes. Circ Res. 2002;91(8):719–726. doi: 10.1161/01.res.0000036751.04896.f1. [DOI] [PubMed] [Google Scholar]
- 43.Weir E.K., Archer S.L. Counterpoint: hypoxic pulmonary vasoconstriction is not mediated by increased production of reactive oxygen species. J Appl Physiol (1985) 2006;101(3):995–998. doi: 10.1152/japplphysiol.00480a.2006. discussion 998. [DOI] [PubMed] [Google Scholar]
- 44.Thebaud B., Wu X.C., Kajimoto H. Developmental absence of the oxygen sensitivity of L-type calcium channels in preterm ductus arteriosus smooth muscle cells impairs oxygen constriction contributing to patent ductus arteriosus. Pediatr Res. 2008;63(2):176–181. doi: 10.1203/PDR.0b013e31815ed059. [DOI] [PubMed] [Google Scholar]
- 45.Archer S.L., Wu X.C., Thebaud B., Moudgil R., Hashimoto K., Michelakis E.D. O2 sensing in the human ductus arteriosus: redox-sensitive K+ channels are regulated by mitochondria-derived hydrogen peroxide. Biol Chem. 2004;385(3-4):205–216. doi: 10.1515/BC.2004.014. [DOI] [PubMed] [Google Scholar]
- 46.Henry T.D., Archer S.L., Nelson D., Weir E.K., From A.H. Postischemic oxygen radical production varies with duration of ischemia. Am J Physiol. 1993;264(5 Pt 2):H1478–H1484. doi: 10.1152/ajpheart.1993.264.5.H1478. [DOI] [PubMed] [Google Scholar]
- 47.Reeve H.L., Michelakis E., Nelson D.P., Weir E.K., Archer S.L. Alterations in a redox oxygen sensing mechanism in chronic hypoxia. J Appl Physiol (1985) 2001;90(6):2249–2256. doi: 10.1152/jappl.2001.90.6.2249. [DOI] [PubMed] [Google Scholar]
- 48.Nagendran J., Stewart K., Hoskinson M., Archer S.L. An anesthesiologist's guide to hypoxic pulmonary vasoconstriction: implications for managing single-lung anesthesia and atelectasis. Curr Opin Anaesthesiol. 2006;19(1):34–43. doi: 10.1097/01.aco.0000192777.09527.9e. [DOI] [PubMed] [Google Scholar]
- 49.Silva-Costa-Gomes T., Gallart L., Valles J., Trillo L., Minguella J., Puig M.M. Low- vs high-dose almitrine combined with nitric oxide to prevent hypoxia during open-chest one-lung ventilation. Br J Anaesth. 2005;95(3):410–416. doi: 10.1093/bja/aei194. [DOI] [PubMed] [Google Scholar]
- 50.Bartsch P., Maggiorini M., Ritter M., Noti C., Vock P., Oelz O. Prevention of high-altitude pulmonary edema by nifedipine. N Engl J Med. 1991;325(18):1284–1289. doi: 10.1056/NEJM199110313251805. [DOI] [PubMed] [Google Scholar]
- 51.Hackett P.H., Creagh C.E., Grover R.F. High-altitude pulmonary edema in persons without the right pulmonary artery. N Engl J Med. 1980;302(19):1070–1073. doi: 10.1056/NEJM198005083021907. [DOI] [PubMed] [Google Scholar]
- 52.Hultgren H.N. High-altitude pulmonary edema: current concepts. Ann Rev Med. 1996;47:267–284. doi: 10.1146/annurev.med.47.1.267. [DOI] [PubMed] [Google Scholar]
- 53.Lumb A.B., Slinger P. Hypoxic pulmonary vasoconstriction: physiology and anesthetic implications. Anesthesiology. 2015;122(4):932–946. doi: 10.1097/ALN.0000000000000569. [DOI] [PubMed] [Google Scholar]
- 54.Rodriguez-Roisin R., Ballester E., Roca J., Torres A., Wagner P.D. Mechanisms of hypoxemia in patients with status asthmaticus requiring mechanical ventilation. Am Rev Respir Dis. 1989;139(3):732–739. doi: 10.1164/ajrccm/139.3.732. [DOI] [PubMed] [Google Scholar]
- 55.Piercy V., Smith H., Arch J.R. Effects of isoprenaline, adrenaline and selective alpha 1- and alpha 2- adrenoceptor stimulation on hypoxic pulmonary vasoconstriction in rat isolated perfused lungs. Pulmon Pharmacol. 1990;3(2):59–63. doi: 10.1016/0952-0600(90)90033-f. [DOI] [PubMed] [Google Scholar]
- 56.Knudson R.J., Constantine H.P. An effect of isoproterenol on ventilation-perfusion in asthmatic versus normal subjects. J Appl Physiol. 1967;22(3):402–406. doi: 10.1152/jappl.1967.22.3.402. [DOI] [PubMed] [Google Scholar]
- 57.Sylvester J.T., Shimoda L.A., Aaronson P.I., Ward J.P. Hypoxic pulmonary vasoconstriction. Physiol Rev. 2012;92(1):367–520. doi: 10.1152/physrev.00041.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Weitzenblum E., Kessler R., Oswald M., Fraisse P. Medical treatment of pulmonary hypertension in chronic lung disease. Eur Respir J. 1994;7(1):148–152. doi: 10.1183/09031936.94.07010148. [DOI] [PubMed] [Google Scholar]
- 59.Melot C., Naeije R., Rothschild T., Mertens P., Mols P., Hallemans R. Improvement in ventilation-perfusion matching by almitrine in COPD. Chest. 1983;83(3):528–533. doi: 10.1378/chest.83.3.528. [DOI] [PubMed] [Google Scholar]
- 60.Light R.B. Indomethacin and acetylsalicylic acid reduce intrapulmonary shunt in experimental pneumococcal pneumonia. Am Rev Respir Dis. 1986;134(3):520–525. doi: 10.1164/arrd.1986.134.3.520. [DOI] [PubMed] [Google Scholar]
- 61.Archer S.L., Mike D., Crow J., Long W., Weir E.K. A placebo-controlled trial of prostacyclin in acute respiratory failure in COPD. Chest. 1996;109(3):750–755. doi: 10.1378/chest.109.3.750. [DOI] [PubMed] [Google Scholar]
- 62.McCormack D.G., Paterson N.A. Loss of hypoxic pulmonary vasoconstriction in chronic pneumonia is not mediated by nitric oxide. Am J Physiol. 1993;265(5 Pt 2):H1523–H1528. doi: 10.1152/ajpheart.1993.265.5.H1523. [DOI] [PubMed] [Google Scholar]
- 63.Dantzker D.R., Brook C.J., Dehart P., Lynch J.P., Weg J.G. Ventilation-perfusion distributions in the adult respiratory distress syndrome. Am Rev Respir Dis. 1979;120(5):1039–1052. doi: 10.1164/arrd.1979.120.5.1039. [DOI] [PubMed] [Google Scholar]
- 64.Enkhbaatar P., Murakami K., Shimoda K. The inducible nitric oxide synthase inhibitor BBS-2 prevents acute lung injury in sheep after burn and smoke inhalation injury. Am J Respir Crit Med. 2003;167(7):1021–1026. doi: 10.1164/rccm.200209-1031PP. [DOI] [PubMed] [Google Scholar]
- 65.Melot C., Hallemans R., Naeije R., Mols P., Lejeune P. Deleterious effect of nifedipine on pulmonary gas exchange in chronic obstructive pulmonary disease. Am Rev Respir Dis. 1984;130(4):612–616. doi: 10.1164/arrd.1984.130.4.612. [DOI] [PubMed] [Google Scholar]
- 66.Walmrath D., Schermuly R., Pilch J., Grimminger F., Seeger W. Effects of inhaled versus intravenous vasodilators in experimental pulmonary hypertension. Eur Respir J. 1997;10(5):1084–1092. doi: 10.1183/09031936.97.10051084. [DOI] [PubMed] [Google Scholar]
- 67.Durmowicz A.G., Hofmeister S., Kadyraliev T.K., Aldashev A.A., Stenmark K.R. Functional and structural adaptation of the yak pulmonary circulation to residence at high altitude. J Appl Physiol (1985) 1993;74(5):2276–2285. doi: 10.1152/jappl.1993.74.5.2276. [DOI] [PubMed] [Google Scholar]
- 68.Newman J.H., Holt T.N., Hedges L.K. High-altitude pulmonary hypertension in cattle (brisket disease): candidate genes and gene expression profiling of peripheral blood mononuclear cells. Pulm Circ. 2011;1(4):462–469. doi: 10.4103/2045-8932.93545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Glover G.H., Newsou L. Further studies on brisket disease. J Agricultural Res. 1918;15(0):409. [Google Scholar]
- 70.Grover R.F. Pulmonary circulation in animals and man at high altitude. Ann NY Acad Sci. 1965;127(1):632–639. doi: 10.1111/j.1749-6632.1965.tb49429.x. [DOI] [PubMed] [Google Scholar]
- 71.Anand I.S., Harris E., Ferrari R., Pearce P., Harris P. Pulmonary haemodynamics of the yak, cattle, and cross breeds at high altitude. Thorax. 1986;41:696–700. doi: 10.1136/thx.41.9.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Will D.H., Hicks J.L., Card C.S., Alexander A.F. Inherited susceptibility of cattle to high-altitude pulmonary hypertension. J Appl Physiol. 1975;38(3):491–494. doi: 10.1152/jappl.1975.38.3.491. [DOI] [PubMed] [Google Scholar]
- 73.Newman J.H., Holt T.N., Cogan J.D. Increased prevalence of EPAS1 variant in cattle with high-altitude pulmonary hypertension. Nature Commun. 2015;6:6863. doi: 10.1038/ncomms7863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Groves B.M., Droma T., Sutton J.R. Minimal hypoxic pulmonary hypertension in normal Tibetans at 3,658 m. J Appl Physiol (1985) 1993;74(1):312–318. doi: 10.1152/jappl.1993.74.1.312. [DOI] [PubMed] [Google Scholar]
- 75.Peñaloza D., Sime F., Banchero N., Gamboa R., Cruz J., Marticorena E. Pulmonary hypertension in healthy men born and living at high altitudes. Am J Cardiol. 1963;11(2):150–157. doi: 10.1016/0002-9149(63)90054-7. [DOI] [PubMed] [Google Scholar]
- 76.Bhagi S., Srivastava S., Singh S.B. High-altitude pulmonary edema: review. J Occup Health. 2014;56(4):235–243. doi: 10.1539/joh.13-0256-ra. [DOI] [PubMed] [Google Scholar]
- 77.Bartsch P., Mairbaurl H., Swenson E.R., Maggiorini M. High altitude pulmonary oedema. Swiss Med Wkly. 2003;133(27-28):377–384. doi: 10.4414/smw.2003.09657. [DOI] [PubMed] [Google Scholar]
- 78.Dehnert C., Mereles D., Greiner S. Exaggerated hypoxic pulmonary vasoconstriction without susceptibility to high altitude pulmonary edema. High Alt Med Biol. 2015;16(1):11–17. doi: 10.1089/ham.2014.1117. [DOI] [PubMed] [Google Scholar]
- 79.Deem S., Hedges R.G., Kerr M.E., Swenson E.R. Acetazolamide reduces hypoxic pulmonary vasoconstriction in isolated perfused rabbit lungs. Respir Physiol. 2000;123(1-2):109–119. doi: 10.1016/s0034-5687(00)00148-1. [DOI] [PubMed] [Google Scholar]
- 80.Maggiorini M. High altitude-induced pulmonary oedema. Cardiovasc Res. 2006;72(1):41–50. doi: 10.1016/j.cardiores.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 81.Oelz O., Maggiorini M., Ritter M. Nifedipine for high altitude pulmonary oedema. Lancet. 1989;2(8674):1241–1244. doi: 10.1016/s0140-6736(89)91851-5. [DOI] [PubMed] [Google Scholar]
- 82.Ang S.O., Chen H., Hirota K. Disruption of oxygen homeostasis underlies congenital Chuvash polycythemia. Nat Genet. 2002;32(4):614–621. doi: 10.1038/ng1019. [DOI] [PubMed] [Google Scholar]
- 83.Hickey M.M., Richardson T., Wang T. The von Hippel-Lindau Chuvash mutation promotes pulmonary hypertension and fibrosis in mice. J Clin Invest. 2010;120(3):827–839. doi: 10.1172/JCI36362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Smith T.G., Brooks J.T., Balanos G.M. Mutation of von Hippel-Lindau tumour suppressor and human cardiopulmonary physiology. PLoS Med. 2006;3(7):e290. doi: 10.1371/journal.pmed.0030290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Archer S.L., Marsboom G., Kim G.H. Epigenetic attenuation of mitochondrial superoxide dismutase 2 in pulmonary arterial hypertension: a basis for excessive cell proliferation and a new therapeutic target. Circulation. 2010;121(24):2661–2671. doi: 10.1161/CIRCULATIONAHA.109.916098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jin W.S., Kong Z.L., Shen Z.F., Jin Y.Z., Zhang W.K., Chen G.F. Regulation of hypoxia inducible factor-1alpha expression by the alteration of redox status in HepG2 cells. J Exp Cancer Res. 2011;30:61. doi: 10.1186/1756-9966-30-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Archer S.L., Gomberg-Maitland M., Maitland M.L., Rich S., Garcia J.G., Weir E.K. Mitochondrial metabolism, redox signaling, and fusion: a mitochondria-ROS-HIF-1alpha-Kv1.5 oxygen-sensing pathway at the intersection of pulmonary hypertension and cancer. Am J Physiol Heart Circ Physiol. 2008;294(2):H570–H578. doi: 10.1152/ajpheart.01324.2007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Mitochondrial dynamics and motility. Mitochondria undergo continuous fission and fusion, resulting in a dynamic network. Mitochondria in PASMC from a normal patient stained with 20 nM tetramethylrhodamine. Images were acquired using confocal microscopy, 10 z-steps per frame, in 15-s intervals for 10 min.