Abstract
Background: Novel oral anticoagulants (NOACs) are considered to be at least as effective and safe as warfarin with several advantages such as predictable pharmacokinetics, allowing for standardized dosing without monitoring, a lack of food interactions and fewer drug interactions; however, their misuse could potentially result in patient harm. Objective: To evaluate the appropriate use of the NOACs within a community teaching hospital. Setting: A community teaching hospital in the United States. Method: A retrospective chart review of patients that were prescribed dabigatran, rivaroxaban, or apixaban at our institution from October 2012 through November 2014 was conducted. Main outcome measure: The primary objective was to determine the percentage of patients that were appropriately prescribed NOACs. Secondary objectives were to determine the number of patients who were inappropriately transitioned from warfarin or parenteral anticoagulants to a NOAC or vice versa, the number of incidents when a NOAC was held or discontinued inappropriately before a procedure and the number of bleeding or thrombotic events while taking a NOAC. Results: Of the 113 patients receiving therapy with an NOAC, appropriate prescribing was observed in 79.7%. Dabigatran, rivaroxaban, and apixaban were appropriately prescribed in 73.8%, 88.3%, and 85.8% of patients respectively. Lack of renal dose-adjustment in patients with reduced renal function was the most common reason for inappropriate use (8.8%). Ten out of 38 patients (26%) were inappropriately transitioned from/to other anticoagulants. Two out of six patients underwent a procedure without holding NOACs as recommended prior to surgery. Of all patients receiving NOACs, a total of 3 bleeding incidents were observed, one with each NOAC. Conclusion: The NOACs were appropriately prescribed for the majority of patients within our institution. Future efforts however should focus on ensuring appropriate dose adjustments for renal impairment, procedures for transitioning between NOACs and parenteral anticoagulants, and adequate withholding times for NOACs prior to surgery in order to optimize the management of NOACs usage within our institution.
Keywords: Novel oral anticoagulants, Dabigatran, Rivaroxaban, Apixaban, New oral anticoagulants use assessment
1. Introduction
Until recently warfarin has been the sole oral anticoagulant for the treatment and prevention of venous thromboembolism (VTE) and stroke prevention in atrial fibrillation (AF). Despite its efficacy, warfarin use is complicated by drug and food interactions, a narrow therapeutic index, and required monitoring to maintain an international normalized ratio (INR) within the target therapeutic range (Coumadin, 2011). The novel oral anticoagulants (NOACs), dabigatran, rivaroxaban, and apixaban, are currently approved for the prevention of stroke or systemic embolism in patients with nonvalvular AF and the prevention and treatment of VTE (Pradaxa, 2013, Xarelto, 2014, Eliquis, 2014). Apixaban and rivaroxaban are approved for VTE prevention post hip or knee replacement, and for VTE treatment or prevention of recurrence (Xarelto, 2014, Eliquis, 2014). Dabigatran is approved for treatment of VTE in patients who have been treated with a parenteral anticoagulant for 5–10 days, and to reduce the recurrence of VTE in patients who have been previously treated (Pradaxa, 2013). In general these agents are considered to be at least as effective and safe as warfarin with several advantages such as predictable pharmacokinetics, allowing for standardized dosing without monitoring, a lack of food interactions and fewer drug interactions (Connolly et al., 2009, Schulman et al., 2009, Patel et al., 2011, Turpie et al., 2011, Lopes et al., 2012, Bauersachs et al., 2010, Buller et al., 2012, Agnelli et al., 2013, Lassen et al., 2010a, Lassen et al., 2010b).
Despite their advantages, these agents are nevertheless anticoagulants; therefore, their misuse could potentially result in patient harm (NSW, 2014). Dosing of the NOACs is based on indication and may require adjustments based on age, weight, concurrent medications and/or renal function. At our institution, initiation of the NOACs is performed by physicians while pharmacists are responsible for daily monitoring and usage. When initiating therapy, prescribers should take into consideration the patient’s indication, renal function, age, and body weight in addition to concomitant medications. Changes in renal function and medication profile may necessitate either a dose reduction or the discontinuation of these agents during therapy. Furthermore, the transition between warfarin or parenteral anticoagulants and NOACs should be performed appropriately to avoid complications in addition to avoiding inappropriate duplication of therapy.
The purpose of this study was to evaluate the appropriate use of the NOACs (rivaroxaban, dabigatran, and apixaban) within a community teaching hospital.
2. Methodology
A retrospective chart review of patients that were prescribed dabigatran, rivaroxaban, or apixaban from October 2012 through November 2014 was conducted. Patients initiated on a NOAC as well as those on a NOAC continued as a home medication were included. Patients were excluded if there was insufficient history or laboratory data to determine the appropriateness of therapy or if they were seen in the emergency department without an admission.
The primary objectives of the trial were to determine (1) percentage of patients that were appropriate prescribed NOACs (rivaroxaban, dabigatran, and apixaban), and (2) the percentage of patients who received the correct dose of each agent based on the specific indication, renal function, age and/or body weight and concomitant medications. The use of each NOAC was considered appropriate based on the criteria listed in Appendix A.
The secondary objectives were to determine (1) the number of patients who were inappropriately transitioned from warfarin or parenteral anticoagulants to a NOAC or vice versa, (2) the number of incidents when a NOAC was held or discontinued inappropriately before a procedure, (3) the number of orders for prothrombin time, aPTT, or INR obtained for the purpose of assessing NOAC therapy, and (4) the number of bleeding and thrombotic events in patients receiving a NOAC.
Data collected included the following: demographic data, NOAC received, indication, dose, serum creatinine, any known medications or concurrent disease states that may affect the pharmacokinetics of NOACs and consideration for dosing, coagulation laboratory results including INR values for appropriate transition between NOACs and warfarin or parenteral anticoagulants, any incidence of bleeding or thromboembolism, and any held, discontinued, or adjusted doses in the NOAC during therapy.
3. Results
Of the 133 patients who received a NOAC from October 2012 through November 2014, a total of 113 patients were included in the study. Twenty patients were excluded: fifteen due to insufficient data available to evaluate the appropriateness of use and five that were seen in the emergency department without an admission. Sixty-five patients received dabigatran, 34 rivaroxaban and 14 apixaban. The majority received NOACs for stoke prevention in atrial fibrillation. The mean age of patients was 70 years. For dabigatran, the majority of patients were receiving therapy as a continuation of therapy upon admission. For apixaban, more patients were initiated on therapy during hospitalization and the number of patients on rivaroxaban was more evenly split between the continuation of home therapy and initiation during hospitalization. A small portion of patients received antiplatelet therapy concurrently with NOACs for post-cardiac stent placement, post-myocardial infraction, or cerebral vascular accident (Table 1).
Table 1.
Baseline characteristics.
| Dabigatran (n = 65) | Rivaroxaban (n = 34) | Apixaban (n = 14) | |
|---|---|---|---|
| Mean ± SD age, yr | 71 ± 11 | 70 ± 15 | 70 ± 14 |
| Gender, n | |||
| Male | 28 | 16 | 6 |
| Female | 37 | 18 | 8 |
| Indication, n | |||
| Stroke prevention | 64 | 21 | 9 |
| VTE prophylaxis/treatment | 1 | 13 | 5 |
| Status of NOACs, n | |||
| Home medication | 51 | 18 | 4 |
| New start | 14 | 16 | 10 |
| Cancer, n | 2 | 4 | 1 |
| Concurrent use of antiplatelets, n | |||
| Aspirin 325 mg | 3 | 0 | 0 |
| Aspirin 81 mg | 18 | 9 | 4 |
| Clopidogrel 75 mg | 1 | 1 | 1 |
VTE = venous thromboembolism, NOACs = novel oral anticoagulants.
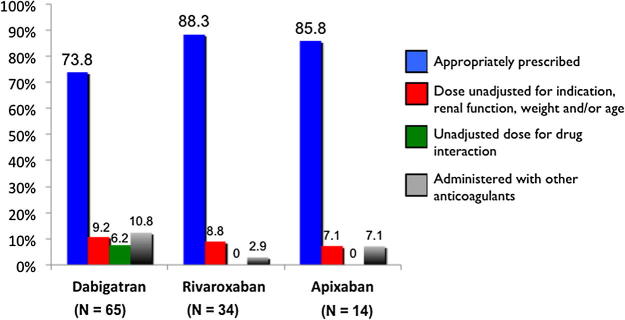
Collectively the NOACs were prescribed appropriately in 79.7% (n = 90) of the patients (Fig. 1). Dabigatran, rivaroxaban, and apixaban were prescribed appropriately in 73.8% (48 of 65), 88.3% (30 of 34), and 85.8% (12 of 14) respectively (Fig. 2). Regarding inappropriate use, the dose of NOACs was unadjusted (for specific indication, renal function, age and/or weight) in 8.8% (n = 10) of patients collectively. All cases were due to unadjusted doses in patients with renal impairment and occurred in 9.2% (n = 6) of patients receiving dabigatran, 8.8% (n = 3) of patients receiving rivaroxaban, and 7.1% (n = 1) of patients receiving apixaban. NOAC drug interactions without considering dose adjustment or medication discontinuation were identified in 3.5% (n = 4) of patients. All drug interactions were identified in the dabigatran group (6.2%). Concurrent administration of NOACs with other anticoagulants was identified in 8% (n = 9) of patients collectively; 10.8% (n = 7) in the dabigatran group, 2.9% (n = 1) in the rivaroxaban group, and 7.1% (n = 1) in the apixaban group. Most were given concurrently with enoxaparin (n = 6). Two patients were started on a NOAC while receiving warfarin, and one patient received two NOACs concurrently.
Figure 1.
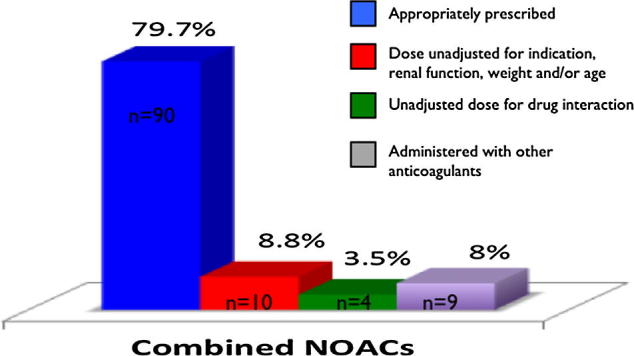
Appropriate use of combined novel oral anticoagulants (NOACs).
Figure 2.
Appropriate use of individual novel oral anticoagulants (NOACs).
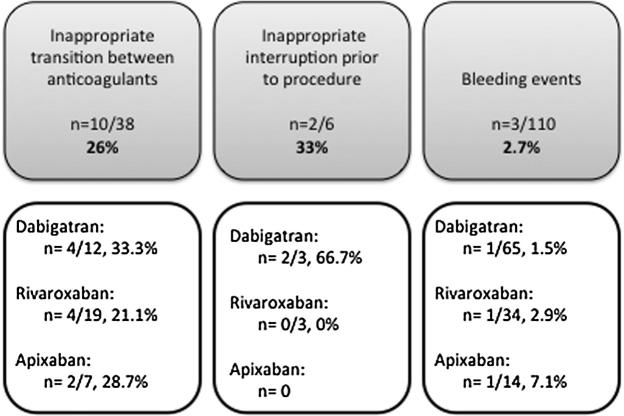
Thirty-eight patients were transitioned from a NOAC to another anticoagulant or vice versa (Fig. 3). Ten of the 38 patients (26%) were inappropriately transitioned. The majority of inappropriate transition was associated with the transition from enoxaparin to a NOAC (n = 7). The NOACs were administered sooner than recommended after the discontinuation of enoxaparin. Two were transitioned from a heparin infusion and another from argatroban. In each situation, administration of the NOAC was delayed for several hours past the recommended time of transition. Of all patients included in the study, six patients underwent a procedure while they were on a NOAC, 3 patients on dabigatran and 3 on rivaroxaban. In 2 of the dabigatran patients, surgery was initiated prior to dabigatran being held for the recommended duration. None of the surgeries were urgent in nature. Of all patients who received NOACs, a total of 3 bleeding incidents were observed: one bleeding incident with each NOAC. None of these bleeding incidents appear to have been associated with inappropriate use of the NOACs.
Figure 3.
Secondary outcomes.
4. Discussion
The majority of patients were appropriately prescribed dabigatran, rivaroxaban, and apixaban within our community teaching hospital. The most common reason for inappropriate use identified in this study was the absence of dose-adjustment in patients with reduced renal function. There have been several case reports of epistaxis, gastric hemorrhage, or other bleeding events with dabigatran use in patients with impaired renal function (Feinberg et al., 2014, Cano and Miyares, 2012, Felows et al., 2013, Freshour et al., 2012, Maddry et al., 2013, Schaeffer and Conway, 2013). Due to this concern and from the results of our study, the addition of the NOACs to the renal dose adjustment protocol at our institution will be recommended. This will allow the pharmacist to automatically adjust the dose of these agents based on the patient’s renal function.
Although there are fewer drug interactions with the NOACs, they are not obsolete. Interactions between the NOACs and CYP3A4 inducers/inhibitors or P-glycoprotein inducers/inhibitors have been reported. Cases resulting in patient death, gastrointestinal bleeding, and pulmonary embolism with rivaroxaban have been reported when given concurrently with rifampin, protease inhibitors and carbamazepine respectively (Atlena et al., 2014, Lakatos et al., 2014, Risselada et al., 2013). In our trial, concomitant administration of dabigatran with P-glycoprotein inducers was seen in two cases with carbamazepine, one case with both phenytoin and phenobarbital, and one case with phenytoin. In addition, the NOACs are contraindicated in patients with prosthetic heart valves due to an increased risk of thrombosis seen with dabigatran in clinical trials. Despite this contraindication, patients with mechanical valves have received dabigatran with resultant valve thrombosis (Atar et al., 2013, Kuwauchi et al., 2013, Price et al., 2012). None of the patients in our study had a prosthetic heart valve. Coagulation tests such as prothrombin time or activated partial thromboplastin time are often obtained as an indicator of anticoagulation response or used to guide dose adjustments with the NOACs; however, this practice is both inappropriate and costly (Deremer et al., 2011). A total of 21 coagulation tests were ordered for our patients in the study to assess therapy with the NOACs.
It is essential to understand the importance of discontinuing these agents prior to surgery and when to do so. A resultant patient death due to stroke has been reported in the literature when dabigatran was ceased inappropriately prior to a procedure (NSW, 2014). Although two patients in our study underwent surgical procedures without the NOAC being held for the recommendation duration, neither of these cases resulted in bleeding. There have also been cases in which patients received an additional anticoagulant such as warfarin or heparin while receiving one of the NOACs, resulting in significant bleeding. The concurrent administration of NOACs with other anticoagulants was observed in 9 cases in our study, most received enoxaparin along with the NOAC. None of these cases resulted in a bleeding incidence during hospitalization. The inappropriate transition between NOACs and other anticoagulants may also put a patient at risk of bleeding and/or thrombosis. We found that most patients being transitioned were being transitioned from another anticoagulant to a NOAC and there was a delay or advance in the administration of the NOAC once the other anticoagulant was discontinued. Therefore, developing a formalized guide for transitioning between NOACs and other anticoagulants, and providing additional education to physicians, nurses, and pharmacists appear to be warranted.
There were several limitations to our study. This was a retrospective review conducted at only one institution. All data were collected from patients’ electronic medical records; therefore, there was the potential for missing information. Other limitations include a limited follow-up period, and the majority of patients were receiving dabigatran rather than the newer NOACs, rivaroxaban and apixaban.
5. Conclusion
The novel oral anticoagulants were appropriately prescribed for the vast majority of patients in our retrospective review of patients receiving therapy at a community teaching hospital. Efforts appear to be warranted to address areas such as dose adjustment for renal impairment, transitioning between NOACs and parenteral anticoagulants, and withholding NOACs prior to surgery to optimize the management of NOACs usage at our institution.
Acknowledgments
This work was presented at Southeastern Residency Conference in Athens, Georgia, and as grand rounds presentation in Columbus, Georgia.
Footnotes
Peer review under responsibility of King Saud University.
Research was conducted at: Midtown Medical Center – Columbus Regional Health in Columbus, GA.
Contributor Information
Sultan Alghadeer, Email: salghadeer@ksu.edu.sa.
Lori Hornsby, Email: lori.hornsby@columbusregional.com, hornslb@auburn.edu.
Appendix A. Criteria of appropriate use of NOACs
| Apixaban |
| The use of apixaban was considered appropriate if |
|
| Dabigatran |
| The use of Dabigatran was considered appropriate if |
|
| Rivaroxaban |
| The use of rivaroxaban was considered appropriate if |
|
AF = atrial fibrillation; SCr = serum creatinine; VTE = venous thromboembolism; DVT = deep vein thrombosis; PE = pulmonary embolism; CrCl = creatinine clearance; INR = International normalized ratio.
References
- Agnelli G., Buller H.R., Cohen A., Curto M., Gallus A.S., Johnson M. Oral apixaban for the treatment of acute venous thromboembolism (AMPLIFY) New Engl. J. Med. 2013;369(9):799–808. doi: 10.1056/NEJMoa1302507. [DOI] [PubMed] [Google Scholar]
- Atar S., Wishniak A., Shturman A., Shtiwi S., Brezins M. Fatal association of mechanical valve thrombosis with dabigatran: a report of two cases. Chest. 2013;144(1):327–328. doi: 10.1378/chest.12-2486. [DOI] [PubMed] [Google Scholar]
- Atlena R., van Roon E., Folkeringa R., de Wit H., Hoogendoorn M. Clinical challenges related to novel oral anticoagulants: drug–drug interactions and monitoring. Haematologica. 2014;99(2) doi: 10.3324/haematol.2013.097287. e26-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauersachs R., Berkowitz S.D., Brenner B., Buller H.R., Decousus H., Gallus A.S. Oral rivaroxaban for symptomatic venous thromboembolism (EINSTEIN) New Engl. J. Med. 2010;363(26):2499–2510. doi: 10.1056/NEJMoa1007903. [DOI] [PubMed] [Google Scholar]
- Buller H.R., Prins M.H., Lensing A.W., Decousus H., Jacobson B.F., Minar E. Oral rivaroxaban for the treatment of symptomatic pulmonary embolism (EINSTEIN PE) New Engl. J. Med. 2012;366(14):1287–1297. doi: 10.1056/NEJMoa1113572. [DOI] [PubMed] [Google Scholar]
- Cano E.L., Miyares M.A. Clinical challenges in a patient with dabigatran-induced fatal hemorrhage. Am. J. Geriatr. Pharmacother. 2012;10(2):160–163. doi: 10.1016/j.amjopharm.2012.02.004. [DOI] [PubMed] [Google Scholar]
- Connolly S.J., Ezekowitz M.D., Yusuf S., Eikelboom J., Oldgren J., Parekh A. Dabigatran versus warfarin in patients with atrial fibrillation (RE-LY) New Engl. J. Med. 2009;361(12):1139–1151. doi: 10.1056/NEJMoa0905561. Sep. [DOI] [PubMed] [Google Scholar]
- Coumadin (warfarin Sodium), 2011. Prescribing Information. Bristol-Myers Squibb, Inc., Princeton (NJ).
- Deremer C.E., Gujral S.J., Thornton J.W., Sorrentino R.A. Dabigatran falsely elevates point of care international normalized ratio results. Am. J. Med. 2011;124(9):e5–e6. doi: 10.1016/j.amjmed.2011.02.009. [DOI] [PubMed] [Google Scholar]
- Eliquis (apixaban), 2014. Prescribing Information. Bristol-Myers Squibb Inc., Princeton (NJ) and Pfizer Inc., New York (NY).
- Feinberg J., Grabowitz L., Rotman-Pikielny P., Berla M., Levy Y. Dabigatran etexilate linked to fatal gastrointestinal hemorrhage. Isr Med. Assoc. J. 2014;16(6):388–389. [PubMed] [Google Scholar]
- Felows S.E., Rosini J.M., Curtis J.A., Volz E.G. Hemorrhagic gastritis with dabigatran in a patient with renal insufficiency. J. Emerg. Med. 2013;44(2):e221–e225. doi: 10.1016/j.jemermed.2012.02.042. [DOI] [PubMed] [Google Scholar]
- Freshour J.E., Hudson J.Q., Stevens A.B., Franks A.S. Epistaxis associated with dabigatran in an elderly patient with reduced creatinine clearance. Am. J. Health Syst. Pharm. 2012;69(14):1184–1186. doi: 10.2146/ajhp110644. [DOI] [PubMed] [Google Scholar]
- Health Department of New South Wales (NSW) Government, Safety Alerts for Professionals [Internet]. North Sydney NSW (Australia): the NSW Ministry of Health [updated 2014 Jun 10, cited 2014 Sep 30]. Available from: <http://www.health.nsw.gov.au/sabs/Documents/2014-sn-002.pdf>.
- Kuwauchi S., Watanabe S., Abe K., Yamasaki M., Ito J., Kawazoe K. Thromboembolism in a patient with a mechanical mitral valve during anticoagulation with dabigatran etexilate. Ann. Thorac. Surg. 2013;96(5):1863–1864. doi: 10.1016/j.athoracsur.2013.03.043. [DOI] [PubMed] [Google Scholar]
- Lakatos B., Stoeckle M., Elzi L., Battegay M., Marzolini C. Gastrointestinal bleeding associated with rivaroxaban administration in a treated patient infected with human immunodeficiency virus. Swiss Med Wkly. 2014;144:w13906. doi: 10.4414/smw.2014.13906. [DOI] [PubMed] [Google Scholar]
- Lassen M.R., Raskob G.E., Gallus A., Pineo G., Chen D., Hornick P. Apixaban versus enoxaparin for thromboprophylaxis after knee replacement (ADVANCE-2): a randomized double blind trial. Lancet. 2010;375(9717):807–815. doi: 10.1016/S0140-6736(09)62125-5. [DOI] [PubMed] [Google Scholar]
- Lassen M.R., Gallus A., Raskob G.E., Pineo G., Chen D., Ramirez L.M. Apixaban versus enoxaparin for thromboprophylaxis after hip replacement. (ADVANCE-3) New Engl. J. Med. 2010;363(26):2487–2498. doi: 10.1056/NEJMoa1006885. [DOI] [PubMed] [Google Scholar]
- Lopes R.D., Al-Khatib S.M., Wallentin L., Yang H., Ansell J., Bahit M.C. Efficacy and safety of apixaban compared with warfarin according to patient risk of stroke and of bleeding in atrial fibrillation: a secondary analysis of a randomised controlled trial. Lancet. 2012;380(9855):1749–1758. doi: 10.1016/S0140-6736(12)60986-6. [DOI] [PubMed] [Google Scholar]
- Maddry J.K., Amir M.K., Session D., Heard K. Fatal dabigatran toxicity secondary to acute renal failure. Am. J. Emerg. Med. 2013;31(2) doi: 10.1016/j.ajem.2012.08.015. 462.e1-2. [DOI] [PubMed] [Google Scholar]
- Patel M.R., Mahaffey K.W., Garg J., Pan G., Singer D.E., Hacke W. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation (ROCKET AF) New Engl. J. Med. 2011;365(10):883–891. doi: 10.1056/NEJMoa1009638. [DOI] [PubMed] [Google Scholar]
- Pradaxa (dabigatran etexilate), 2013. Prescribing Information. BoehringerIngelheim Pharmaceuticals, Inc., Ridgefield (CT).
- Price J., Hynes M., Labinaz M., Ruel M., Boodhwani M. Mechanical valve thrombosis with dabigatran. J. Am. Coll. Cardiol. 2012;60(17):1710–1711. doi: 10.1016/j.jacc.2012.06.039. [DOI] [PubMed] [Google Scholar]
- Risselada A.J., Visser M.J., van Roon E. Pulmonary embolism due to interaction between rivaroxaban and carbamazepine. Nederlands Tijdschr Geneeskd. 2013;157(52):A6568. [PubMed] [Google Scholar]
- Schaeffer S., Conway S.E. Fatal bleeding associated with dabigatran. Am. J. Health Syst. Pharm. 2013;70(19):1651–1652. doi: 10.2146/ajhp120518. [DOI] [PubMed] [Google Scholar]
- Schulman S., Kearon C., Kakkar A.K., Mismetti P., Schellong S., Eriksson H. Dabigatran versus warfarin in the treatment of acute venous thromboembolism (RE-COVER) New Engl. J. Med. 2009;361(24):2342–2352. doi: 10.1056/NEJMoa0906598. [DOI] [PubMed] [Google Scholar]
- Turpie A.G., Lassen M.R., Erikson B.I., Gent M., Berkowitz F., Bandel T.J. Rivaroxaban for the prevention of venous thromboembolism after hip or knee arthroplasty: pooled analysis of four studies (RECORD 1–4) ThrombHaemost. 2011;105(3):444–453. doi: 10.1160/TH10-09-0601. [DOI] [PubMed] [Google Scholar]
- Xarelto (rivaroxaban), 2014. Prescribing Information. Janssen Pharmaceuticals, Inc., Titusville (NJ).