Abstract
There are many human oral antimicrobial peptides responsible for playing important roles including maintenance, repairing of oral tissues (hard or soft) and defense against oral microbes. In this review we have highlighted the biochemistry, physiology and proteomics of human oral histatin peptides, secreted from parotid and submandibular salivary glands in human. The significance of these peptides includes capability for ionic binding that can kill fungal Candida albicans. They have histidine rich amino acid sequences (7–12 family members; corresponding to residues 12–24, 13–24, 12–25, 13–25, 5–11, and 5–12, respectively) for Histatin-3. However, Histatin-3 can be synthesized proteolytically from histatin 5 or 6. Due to their fungicidal response and high biocompatibility (little or no toxicity), these peptides can be considered as therapeutic agents with most probable applications for example, artificial saliva for denture wearers and salivary gland dysfunction conditions. The objectives of current article are to explore the human histatin peptides for its types, chemical and biological aspects. In addition, the potential for therapeutic bio-dental applications has been elaborated.
Keywords: Saliva, Oral cavity, Histatin, Antifungal activity
1. Introduction
The significance and beneficial roles of organic macromolecules isolated from human body secretions are well known. Human peptides are secreted physiologically by certain organs such as salivary glands (Trindade et al., 2015, Kotál et al., 2015) or by pathologically by diseased tissues such as ameloblastoma (Jhamb and Kramer, 2014, Vered et al., 2003, Garg et al., 2015). In last few decades, a number of major developments such as better understanding and advent of proteomic tools started helping in isolation of protein based material from human secretions for example lysozyme (Sabatini et al., 1990), α-defensin, β-defensin, cathelicidins (Selsted et al., 1985), histatin family (Oppenheim et al., 1988), statherin, granulysin, thrombocidin-1, chemokine CCL20, psoriasin S100A7, neuropeptide (Dawidson et al., 1996), substance P and dermcidin (Zasloff, 2002). Reported secretions that have been mainly utilized included blood, lymph, saliva, glands secretion (endocrine and exocrine glands), urine, and body fluids (Schrader and Schulz-Knappe, 2001, Hu et al., 2006). Proteomics is a discovery science technology that helped categorizing genomics sequencing, physiology, microarray analysis and metabolite profiling of proteins and peptides (Cellulaire, 2002). Commonly used proteomic instruments are two-dimensional (2D) gel electrophoresis, liquid chromatography (LC), mass spectrometry with electrospray ionization (ESI), and matrix-assisted laser desorption ionization (MALDI) coupled with time of flight (TOF) (Gorg et al., 2004, Wittmann-Liebold et al., 2006).
These techniques and instruments can be used for the analysis of body fluids. For example, in case of oral and dental health, human saliva can be analyzed to differentiate between diseased and healthy patients without any surgical interventions (De Smet and Contreras, 2005). Saliva contains a vast number of protein species. The salivary peptidome (low molecular weight) comprises approximately 40–50% of the total secreted proteins in addition to peptides generated by proteolysis of proteins from different sources (Vitorino et al., 2004, Hu et al., 2005, Loo et al., 2010, Thomadaki et al., 2011). The timeline of human salivary proteins and peptides identification is listed in Table 1 (Hu et al., 2005, Piludu et al., 2006, Wong, 2006, Rudney et al., 2009). Up till now, more than 1165 types of salivary proteins have been identified and reported in human salivary proteome central depository (http://www.skb.ucla.edu/cgi-bin/hspmscgi-bin/welcome_c.cgi) supported by National Institute of Dental and Craniofacial Research (NIDCR). The objectives of current article are to explore the human histatin peptides for its types, chemical and biological aspects. In addition, the potential for therapeutic bio-dental applications has been elaborated.
Table 1.
Timeline of human salivary protein (http://aps.unmc.edu/AP/).
| Proteins and peptides | Year | Amino acids | Functions |
|---|---|---|---|
| Statherin | 1977 | 43 | Potent inhibitor of calcium phosphate precipitation |
| α-Defensins (HNP-1-4) | 1985 | <50 | Antibacterial, antifungal and antiviral activities because of six cysteine disulfide bonds |
| Histatin-1 | 1988 | 38 | Responsible for maintenance of oral hemostasis, role in forming acquired tooth pellicle and help in bonding of some metal ions |
| Histatin-3 | 1988 | 32 | Responsible for maintenance of oral hemostasis, role in forming acquired tooth pellicle and help in bonding of some metal ions |
| Histatin-5 | 1988 | 24 | Responsible for maintenance of oral hemostasis, role in forming acquired tooth pellicle and help in bonding of some metal ions |
| Adrenomedullin | 1993 | 52 | Mitogenic, inducible, vasodilator and antibacterial peptide |
| β-Defensins (hBD-1) | 1995 | 36–47 | Poor antibacterial activity, antiviral and antifungal activity, Also help in tissue repair |
| Cathelicidins (LL-37) | 1995 | 37 | Chemo-attractant to immune cells and act as “alarmin” by inducing immune response |
| β-Defensins (hBD-2) | 1997 | 41 | Antibacterial against Gram-negative and Gram-positive, antiviral and antifungal activity against HIV |
| β-Defensins (hBD-3) | 2001 | 45 | Antibacterial against Gram-negative and Gram-positive, also drug resistant microbes, chemo-attractant antiviral and antifungal activity |
| C-C motif Chemokine 28 | 2008 | 128 | Act as chemokine, salt sensitive and broad spectral antibacterial |
| Azurocidin | 251 | Strong antibacterial activity against Gram-negative bacteria | |
| Neuropeptides | 1997 | – | They have five types; Substance P (SP), Neurokinin A (NKA), Calcitonin Gene |
| Related Peptide (CGRP), Neuropeptide Y (NPY) and Vasoactive Intestinal | |||
| Polypeptide (VIP), effective against Candida albicans and bacteria | |||
| Calprotectin | 1999 | 93–114 | Act as calcium sensors and potent zinc binder, also play role in tissue repair |
| Mucins | 1990 | 3750 | Aggregate oral flora bacteria and prevent dental caries |
2. Histatin peptides
Histatin peptides belong to a family of antimicrobial peptides that are rich in histidine amino acids. The first ever histatin was isolated from human parotid salivary gland secretions in 1988 (Oppenheim et al., 1988). Histidine rich polypeptides have been proven to have antimicrobial and antifungal properties (VAN et al., 1997). They are secreted by major salivary glands including parotid and submandibular glands. The concentration of histatin peptides in saliva ranges from 50 to 425 μg/ml (VAN et al., 1997). Based on chemistry and sequence of amino acids, there are variety of histatin peptides. The common variants of natural histatins found in saliva are Histatin-1 (38 amino acids; Mw ∼ 4929 Da), histatin-3 (32 amino acids; Mw ∼ 4063 Da) and Histatin-5 (24 amino acids; Mw ∼ 3037 Da) (Sabatini and Azen, 1989, Raj et al., 1990, Troxler et al., 1990). Histatin-1 and Histatin-3 are derived from the available genes HTN1 and HTN3 present in humans (Table 2) (VAN et al., 1997). Histatin-5 is originated from parent peptide i.e. histatin-3 and contains N-terminal that is thought to be highly reactive and highly affinitive to bond with metals. The chemical nature leads to precipitate reactive oxygen species (Nikawa et al., 2002, Cabras et al., 2007).
Table 2.
Representing the gene encoding series of human histatin family.
| Allele | Gene | Protein |
|---|---|---|
| HIS1 | HTN1 | Histatin-1 |
| HIS2 | HTN3 | Histatin-3 – histatin-4 to -6 |
The analysis of Amino acid sequence of 12 histatin peptides (Table 3) suggested that histatin-2 is primarily degradable product of histatin-1. On the other hand, remaining histatins are proteolytic product of histatin-3. Considering the fact that histatins are humans own defense peptides, these peptides have gained popularity in the field of therapeutic and biodental medicine. In addition, the antimicrobial drugs containing natural peptides prevent resistance development against pathogens such as bacteria, fungi, viruses and parasites (De Smet and Contreras, 2005, Ryley, 2001, Wang, 2014).
Table 3.
Histatin Family with their proteolytic fragments (Khurshid et al., 2016b).
| Human histatins | Sequences |
|---|---|
| Histatin 1 | DSpHEKRHHGYRRKFHEKHHSHREFPFYGDYGSNYLYDN |
| Histatin 3 | DSHAKRHHGYKRKFHEKHHSHRGYRSNYLYDN |
| Histatin 5 | DSHAKRHHGYKRKFHEKHHSHRGY |
| Proteolytic fragments in saliva | |
| Histatin 2 | RKFHEKHHSHREFPFYGDYGSNYLYDN |
| Histatin 4 | KFHEKHHSHRGYRSNYLYDN |
| Histatin 6 | DSHAKRHHGYKRKFHEKHHSHRGYR |
| Histatin 7 | RKFHEKHHSHRGY |
| Histatin 8 | KFHEKHHSHRGY |
| Histatin 9 | RKFHEKHHSHRGYR |
| Histatin 10 | KFHEKHHSHRGYR |
| Histatin 11 | KRHHGYKR |
| Histatin 12 | KRHHGYK |
3. Role of histatin peptides in human oral cavity
We are very well aware of the protective role of saliva that aids in digestion, lubrication, protection, and host defense immunization of the oral cavity (Dawes et al., 2015). These processes are made possible due to its unique composition and chemistry. Human saliva bio-fluid instead of blood can be used as disease biomarkers for diagnosis and therapeutic targets. In addition, it has major benefits of being cost effective and non-invasive not causing any pain or discomforts (Thomadaki et al., 2011, Halgand et al., 2012). Human saliva contains various forms of anti-microbial polypeptides that play few vital roles such as innate immunity and combating against invading foreign pathogens (Lamkin and Oppenheim, 1993), wound healing (Oudhoff et al., 2008), and apoptosis (Rudney et al., 2009). Among various peptides, histatin has been targeted and gained quite interests in field of biodental research utilizing proteomics approach (Khurshid et al., 2016a, Siqueira et al., 2012). The eminent forms of human histatins are Histatin-1, Histatin-3 and Histatin-5 with individual contribution of around 20–30% to the total histatin pool. The theorized functions of histatin are buffering (Amado, 2005), wound healing (Kavanagh and Dowd, 2004), anti-candidal (Tsai and Bobek, 1998), antiviral (Nikawa et al., 2002), antibacterial (Cabras et al., 2007, Ryley, 2001) and balance of minerals formation (Wang, 2014).
In 2011 McDonald et al. worked on Histatin-1 and studied the composition, structure and function of acquired enamel pellicle. The key role of Histatin-1 absorbed by hydroxyapatite crystals was to protect from proteolytic degradation and demineralization (McDonald et al., 2011). Histatin 1 comprises phosphorylated serine at number 2 residue that increases affinity of the peptide to bind with HA crystals (Yin et al., 2003). One of the distinctive features of human oral mucosa is that, it has a high turnover rate. Hence, this characteristic is considered due to the presence of many factors such as presence of Histatin-1 and Histatin-2. These peptides indirectly induced wound healing by stimulating epithelial migration (Oudhoff et al., 2008). In comparison, the epithelial growth factor (EGF) and nerve growth factor (NGF) have been reported less stable in terms of wound healing compared to histatins (Oudhoff et al., 2009, Sun et al., 2009).
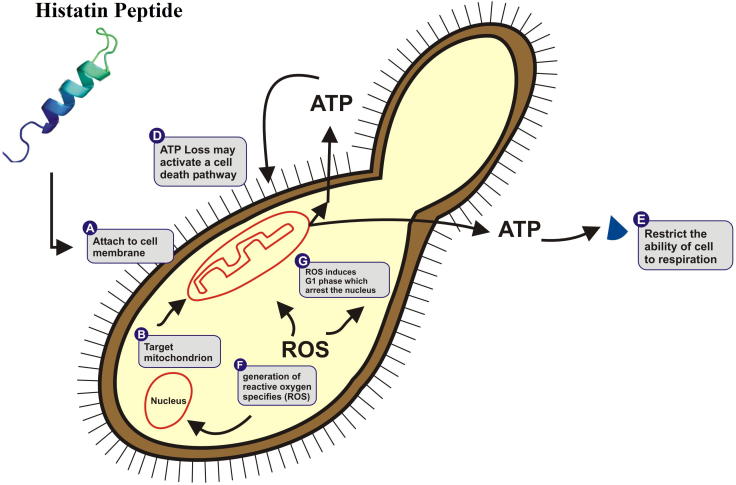
In recent years, certain valuable researches concerning the effects of histatin on Candida albicans have been performed and reported (Tsai et al., 1996, Tsai and Bobek, 1997a, Edgerton et al., 1998). It has been investigated and found that all three major types of histatin peptides (Histatin-1, Histatin-3 and Histatin-5) have the capability to inhibit and kill candidal organisms however, varies in their abilities to kill blastoconidia and germinated cells. In comparison, Histatin-5 has been reported as most active, Histatin-3 being moderately active and His-1 being least active (Xu et al., 1991). Due to this pharmacological nature, histatin is attractive candidate for therapeutic products. The reported mechanism by which histatin acts as fungistatic and fungicidal agent includes disruption of plasma membrane, which leads to the loss of intracellular components (Oppenheim et al., 1988). It was also found that histatins were also effective in killing of yeast by damaging its membrane (due to release of potassium) by binding to the Trk1 potassium transporter and hence loss of intracellular, and azole-resistant fungal species (Fig. 1) (Tsai and Bobek, 1997b, Swidergall and Ernst, 2014). In acquired immuno deficiency syndrome (AIDS), the amount of histatin was found to be decreased and hence it is suggested that they can be used as a biomarker for diagnosis (Khan et al., 2013).
Figure 1.
Mechanism of action of human histatin peptides against Candida albicans.
Antimicrobial potential of histatin peptides has been discussed above. This can better be explained by knowing its structure. His-3 and His-5 have capacity to bind to metals, due to the presence of reactive N-terminal (Tay et al., 2009). Herein, this N-terminal has high potential to bind with the metals especially copper and nickel, thus generating reactive oxygen species which damages membranes of cell organelles, also may damage the DNA and hence leads to the fungal and bacterial cell death (Harford and Sarkar, 1997, Grogan et al., 2001). Bacteriodes gingivalis, one of the important micro floras of the oral cavity is associated with the destruction of the periodontium as it produces proteases (Gusman et al., 2001). In addition, effects of histatin peptides on secreted proteases and clostripain have been investigated (Imatani et al., 2000). It was concluded that Histatin-5 inhibited the activity of proteases and clostripain (clostridiopeptidase B, Clostridium histolytium proteinase B). It binds to the protease active sites making non-covalent complexes, therefore playing a key role in preventing the periodontitis (Nishikata et al., 1991). The antiviral property of histatins was also investigated; however, little is known about it. There is need of further research to better understand the three dimensional structure of histatin and to rule out which salivary polypeptide is responsible for antiviral activity (Hardestam et al., 2008).
4. Histatin and advances in bio-dental research
The ability of histatin in inhibiting fungal infections can be observed in patients suffering from xerostomia, since they are most susceptible of acquiring infections. Therefore histatins can be used in medicines to help in preventing such infections (Tati et al., 2014). In denture wearers where their immunity is compromised it was observed that they were prone to opportunistic pathogens such as C. albicans (Farah et al., 2000, Samaranayake and Samaranayake, 2001). Experimentation was done to see the effect of growth of candidal species on polymethyl methacrylate (PMMA) and it was demonstrated that histatins inhibited the growth of these microorganisms (Vukosavljevic et al., 2012). Thus, this can be utilized as a powerful tool in prevention and as a therapeutic agent in minimizing the possibilities of getting fungal infections (Peters et al., 2010, Iqbal and Zafar, 2016).
There are a number of potential clinical applications for using histatin peptides as a therapeutic agent. As previously mentioned, histatin peptides are playing an effective role in binding to hydroxyapatite crystals. Familiar with this phenomenon, it can be used as a reagent in materials to prevent abrasion and wearing off enamel (Yin et al., 2003, Ullah and Zafar, 2015). Recently, the prime functions of histatins in the field of peptidomics as demonstrated were applied in dental implants for the prevention of implantitis that is considered as one of the important causes of failure of dental implants (Holmberg et al., 2013). The surface of titanium implant was modified by conjoining antimicrobial with titanium-binding peptides, which resulted in decreased formation of biofilm (Yoshinari et al., 2010) and reduced adherence of microorganisms such as Pseudomonas gingivalis on the sub gingival and supra gingival surfaces of implants (Yoshinari et al., 2010, Yeo et al., 2012).
5. Conclusion and recommendations
Histatin peptides are the key elements of antimicrobial peptides that have bactericidal and anti-inflammatory activities (Helmerhorst et al., 2001). One of the drawbacks of histatin to be used in drugs as a therapeutic agent was that they were not stable against proteolysis (Sabatini et al., 1989). After studying metallo-peptide nature of histatin and its morphology, we are very well aware of the fact that they have potential to form bond with metal ions especially with copper and nickel at a physiological pH (Cabras et al., 2007). This property can greatly be used in terms of histatin binding to these metals. In addition, histatin becomes more stable and resistant to proteolysis hence, can be used in drugs to combat against oral pathogens (Zawisza et al., 2014).
Footnotes
Peer review under responsibility of King Saud University.
References
- Amado F.M. The role of salivary peptides in dental caries. Biomed. Chromatogr. 2005;19:214–222. doi: 10.1002/bmc.438. [DOI] [PubMed] [Google Scholar]
- Cabras T., Patamia M., Melino S., Inzitari R., Messana I., Castagnola M., Petruzzelli R. Pro-oxidant activity of histatin 5 related Cu (II)-model peptide probed by mass spectrometry. Biochem. Biophys. Res. Commun. 2007;358:277–284. doi: 10.1016/j.bbrc.2007.04.121. [DOI] [PubMed] [Google Scholar]
- Cellulaire B. Two-dimensional gel electrophoresis in proteomics: old, old fashioned, but it still climbs up the mountains. Proteomics. 2002;2:3–10. [PubMed] [Google Scholar]
- Dawes C., Pedersen A.M.L., Villa A., Ekström J., Proctor G.B., Vissink A., Aframian D., McGowan R., Aliko A., Narayana N., Sia Y.W., Joshi R.K., Jensen S.B., Kerr A.R., Wolff A. The functions of human saliva: a review sponsored by the World Workshop on Oral Medicine VI. Arch. Oral Biol. 2015;60:863–874. doi: 10.1016/j.archoralbio.2015.03.004. [DOI] [PubMed] [Google Scholar]
- Dawidson I., Blom M., Lundeberg T., Theodorsson E., Angmar-Månsson B. Neuropepties in the saliva of healthy subjects. Life Sci. 1996;60:269–278. doi: 10.1016/s0024-3205(96)00627-3. [DOI] [PubMed] [Google Scholar]
- De Smet K., Contreras R. Human antimicrobial peptides: defensins, cathelicidins and histatins. Biotechnol. Lett. 2005;27:1337–1347. doi: 10.1007/s10529-005-0936-5. [DOI] [PubMed] [Google Scholar]
- Edgerton M., Koshlukova S.E., Lo T.E., Chrzan B.G., Straubinger R.M., Raj P.A. Candidacidal activity of salivary histatins identification of a histatin 5-binding protein on Candida albicans. J. Biol. Chem. 1998;273:20438–20447. doi: 10.1074/jbc.273.32.20438. [DOI] [PubMed] [Google Scholar]
- Farah C.S., Ashman R.B., Challacombe S.J. Oral candidosis. Clin. Dermatol. 2000;18:553–562. doi: 10.1016/s0738-081x(00)00145-0. [DOI] [PubMed] [Google Scholar]
- Garg K., Chandra S., Raj V., Fareed W., Zafar M. Molecular and genetic aspects of odontogenic tumors: a review. Iran. J. Basic Med. Sci. 2015;18:529–536. [PMC free article] [PubMed] [Google Scholar]
- Gorg A., Weiss W., Dunn M.J. Current two-dimensional electrophoresis technology for proteomics. Proteomics. 2004;4:3665–3685. doi: 10.1002/pmic.200401031. [DOI] [PubMed] [Google Scholar]
- Grogan J., McKnight C.J., Troxler R.F., Oppenheim F.G. Zinc and copper bind to unique sites of histatin 5. FEBS Lett. 2001;491:76–80. doi: 10.1016/s0014-5793(01)02157-3. [DOI] [PubMed] [Google Scholar]
- Gusman H., Travis J., Helmerhorst E.J., Potempa J., Troxler R.F., Oppenheim F.G. Salivary histatin 5 is an inhibitor of both host and bacterial enzymes implicated in periodontal disease. Infect. Immun. 2001;69:1402–1408. doi: 10.1128/IAI.69.3.1402-1408.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halgand F., Zabrouskov V., Bassilian S., Souda P., Loo J., Faull K., Wong D., Whitelegge J. Defining intact protein primary structures from saliva: a step toward the human proteome project. Anal. Chem. 2012;84:4383–4395. doi: 10.1021/ac203337s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardestam J., Petterson L., Ahlm C., Evander M., Lundkvist Å., Klingström J. Antiviral effect of human saliva against hantavirus. J. Med. Virol. 2008;80:2122–2126. doi: 10.1002/jmv.21332. [DOI] [PubMed] [Google Scholar]
- Harford C., Sarkar B. Amino terminal Cu (II)-and Ni (II)-binding (ATCUN) motif of proteins and peptides: metal binding, DNA cleavage, and other properties. Acc. Chem. Res. 1997;30:123–130. [Google Scholar]
- Helmerhorst E.J., van’t Hof W., Breeuwer P., Veerman E.C., Abee T., Troxler R.F., Amerongen A.V., Oppenheim F.G. Characterization of histatin 5 with respect to amphipathicity, hydrophobicity, and effects on cell and mitochondrial membrane integrity excludes a candidacidal mechanism of pore formation. J. Biol. Chem. 2001;276:5643–5649. doi: 10.1074/jbc.M008229200. [DOI] [PubMed] [Google Scholar]
- Holmberg K.V., Abdolhosseini M., Li Y., Chen X., Gorr S., Aparicio C. Bio-inspired stable antimicrobial peptide coatings for dental applications. Acta Biomater. 2013;9:8224–8231. doi: 10.1016/j.actbio.2013.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S., Loo J.A., Wong D.T. Human body fluid proteome analysis. Proteomics. 2006;6:6326–6353. doi: 10.1002/pmic.200600284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S., Xie Y., Ramachandran P., Ogorzalek Loo R.R., Li Y., Loo J.A., Wong D.T. Large-scale identification of proteins in human salivary proteome by liquid chromatography/mass spectrometry and two-dimensional gel electrophoresis-mass spectrometry. Proteomics. 2005;5:1714–1728. doi: 10.1002/pmic.200401037. [DOI] [PubMed] [Google Scholar]
- Imatani T., Kato T., Minaguchi K., Okuda K. Histatin 5 inhibits inflammatory cytokine induction from human gingival fibroblasts by Porphyromonas gingivalis. Oral Microbiol. Immunol. 2000;15:378–382. doi: 10.1034/j.1399-302x.2000.150607.x. [DOI] [PubMed] [Google Scholar]
- Iqbal Z., Zafar M.S. Role of antifungal medicaments added to tissue conditioners: a systematic review. J. Prosthodont. Res. 2016;60 doi: 10.1016/j.jpor.2016.03.006. (E-pub ahead of print) [DOI] [PubMed] [Google Scholar]
- Jhamb T., Kramer J.M. Molecular concepts in the pathogenesis of ameloblastoma: implications for therapeutics. Exp. Mol. Pathol. 2014;97:345–353. doi: 10.1016/j.yexmp.2014.09.001. [DOI] [PubMed] [Google Scholar]
- Kavanagh K., Dowd S. Histatins: antimicrobial peptides with therapeutic potential. J. Pharm. Pharmacol. 2004;56 doi: 10.1211/0022357022971. [DOI] [PubMed] [Google Scholar]
- Khan S.A., Fidel P.L., Jr., Al Thunayyan A., Varlotta S., Meiller T.F., Jabra-Rizk M.A. Impaired histatin-5 levels and salivary antimicrobial activity against C. albicans in HIV infected individuals. J. AIDS Clin. Res. 2013;4 doi: 10.4172/2155-6113.1000193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khurshid Z., Zafar M.S., Zohaib S., Najeeb S., Naseem M. Green tea (Camellia sinensis) chemistry and oral health. Open Dent. J. 2016 doi: 10.2174/1874210601610010166. (E-pub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khurshid Z., Naseem M., Sheikh Z., Najeeb S., Shahab S., Zafar M.S. Oral antimicrobial peptides: types and role in the oral cavity. Saudi Pharm. J. 2016;24(5):515–524. doi: 10.1016/j.jsps.2015.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotál J., Langhansová H., Lieskovská J., Andersen J.F., Francischetti I.M.B., Chavakis T., Kopecký J., Pedra J.H.F., Kotsyfakis M., Chmelař J. Modulation of host immunity by tick saliva. J. Proteom. 2015;128:58–68. doi: 10.1016/j.jprot.2015.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamkin M.S., Oppenheim F.G. Structural features of salivary function. Crit. Rev. Oral Biol. Med. 1993;4:251–259. doi: 10.1177/10454411930040030101. [DOI] [PubMed] [Google Scholar]
- Loo J.A., Yan W., Ramachandran P., Wong D.T. Comparative human salivary and plasma proteomes. J. Dent. Res. 2010;89:1016–1023. doi: 10.1177/0022034510380414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald E.E., Goldberg H.A., Tabbara N., Mendes F.M., Siqueira W.L. Histatin 1 resists proteolytic degradation when adsorbed to hydroxyapatite. J. Dent. Res. 2011;90:268–272. doi: 10.1177/0022034510388653. [DOI] [PubMed] [Google Scholar]
- Nikawa H., Jin C., Makihira S., Hamada T., Samaranayake L.P. Susceptibility of Candida albicans isolates from the oral cavities of HIV-positive patients to histatin-5. J. Prosthet. Dent. 2002;88:263–267. doi: 10.1067/mpr.2002.127907. [DOI] [PubMed] [Google Scholar]
- Nishikata M., Kanehira T., Oh H., Tani H., Tazaki M., Kuboki Y. Salivary histatin as an inhibitor of a protease produced by the oral bacterium Bacteroides gingivalis. Biochem. Biophys. Res. Commun. 1991;174:625–630. doi: 10.1016/0006-291x(91)91463-m. [DOI] [PubMed] [Google Scholar]
- Oppenheim F.G., Xu T., McMillian F.M., Levitz S.M., Diamond R.D., Offner G.D., Troxler R.F. Histatins, a novel family of histidine-rich proteins in human parotid secretion. Isolation, characterization, primary structure, and fungistatic effects on Candida albicans. J. Biol. Chem. 1988;263:7472–7477. [PubMed] [Google Scholar]
- Oudhoff M.J., Bolscher J.G., Nazmi K., Kalay H., van ’t Hof W., Amerongen A.V., Veerman E.C. Histatins are the major wound-closure stimulating factors in human saliva as identified in a cell culture assay. FASEB J. 2008;22:3805–3812. doi: 10.1096/fj.08-112003. [DOI] [PubMed] [Google Scholar]
- Oudhoff M.J., van den Keijbus P.A., Kroeze K.L., Nazmi K., Gibbs S., Bolscher J.G., Veerman E.C. Histatins enhance wound closure with oral and non-oral cells. J. Dent. Res. 2009;88:846–850. doi: 10.1177/0022034509342951. [DOI] [PubMed] [Google Scholar]
- Peters B.M., Shirtliff M.E., Jabra-Rizk M.A. Antimicrobial peptides: primeval molecules or future drugs. PLoS Pathog. 2010;6:e1001067. doi: 10.1371/journal.ppat.1001067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piludu M., Lantini M.S., Cossu M., Piras M., Oppenheim F.G., Helmerhorst E.J., Siqueira W., Hand A.R. Salivary histatins in human deep posterior lingual glands (of von Ebner) Arch. Oral Biol. 2006;51:967–973. doi: 10.1016/j.archoralbio.2006.05.011. [DOI] [PubMed] [Google Scholar]
- Raj P.A., Edgerton M., Levine M.J. Salivary histatin 5: dependence of sequence, chain length, and helical conformation for candidacidal activity. J. Biol. Chem. 1990;265:3898–3905. [PubMed] [Google Scholar]
- Rudney J., Staikov R., Johnson J. Potential biomarkers of human salivary function: a modified proteomic approach. Arch. Oral Biol. 2009;54:91–100. doi: 10.1016/j.archoralbio.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryley H.C. Human antimicrobial peptides. Rev. Med. Microbiol. 2001;12:177–186. [Google Scholar]
- Sabatini L.M., He Y., Azen E.A. Structure and sequence determination of the gene encoding human salivary statherin. Gene. 1990;89:245–251. doi: 10.1016/0378-1119(90)90012-g. [DOI] [PubMed] [Google Scholar]
- Sabatini L., Azen E. Histatins, a family of salivary histidine-rich proteins, are encoded by at least two loci (HIS1 and HIS2) Biochem. Biophys. Res. Commun. 1989;160:495–502. doi: 10.1016/0006-291x(89)92460-1. [DOI] [PubMed] [Google Scholar]
- Sabatini L.M., Warner T.F., Saitoh E., Azen E.A. Tissue distribution of RNAs for cystatins, histatins, statherin, and proline-rich salivary proteins in humans and macaques. J. Dent. Res. 1989;68:1138–1145. doi: 10.1177/00220345890680070101. [DOI] [PubMed] [Google Scholar]
- Samaranayake Y.H., Samaranayake L.P. Experimental oral candidiasis in animal models. Clin. Microbiol. Rev. 2001;14:398–429. doi: 10.1128/CMR.14.2.398-429.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrader M., Schulz-Knappe P. Peptidomics technologies for human body fluids. Trends Biotechnol. 2001;19:S55–S60. doi: 10.1016/S0167-7799(01)01800-5. [DOI] [PubMed] [Google Scholar]
- Selsted M.E., Harwig S.S., Ganz T., Schilling J.W., Lehrer R.I. Primary structures of three human neutrophil defensins. J. Clin. Invest. 1985;76:1436–1439. doi: 10.1172/JCI112121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siqueira W.L., Lee Y.H., Xiao Y., Held K., Wong W. Identification and characterization of histatin 1 salivary complexes by using mass spectrometry. Proteomics. 2012;12:3426–3435. doi: 10.1002/pmic.201100665. [DOI] [PubMed] [Google Scholar]
- Sun X., Salih E., Oppenheim F.G., Helmerhorst E.J. Kinetics of histatin proteolysis in whole saliva and the effect on bioactive domains with metal-binding, antifungal, and wound-healing properties. FASEB J. 2009;23:2691–2701. doi: 10.1096/fj.09-131045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swidergall M., Ernst J.F. Interplay between Candida albicans and the antimicrobial peptide armory. Eukaryot. Cell. 2014;13:950–957. doi: 10.1128/EC.00093-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tati S., Li R., Puri S., Kumar R., Davidow P., Edgerton M. Histatin 5-spermidine conjugates have enhanced fungicidal activity and efficacy as a topical therapeutic for oral candidiasis. Antimicrob. Agents Chemother. 2014;58:756–766. doi: 10.1128/AAC.01851-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tay W.M., Hanafy A.I., Angerhofer A., Ming L. A plausible role of salivary copper in antimicrobial activity of histatin-5—metal binding and oxidative activity of its copper complex. Bioorg. Med. Chem. Lett. 2009;19:6709–6712. doi: 10.1016/j.bmcl.2009.09.119. [DOI] [PubMed] [Google Scholar]
- Thomadaki K., Helmerhorst E.J., Tian N., Sun X., Siqueira W.L., Walt D.R., Oppenheim F.G. Whole-saliva proteolysis and its impact on salivary diagnostics. J. Dent. Res. 2011;90:1325–1330. doi: 10.1177/0022034511420721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trindade F., Amado F., Pinto da Costa J., Ferreira R., Maia C., Henriques I., Colaço B., Vitorino R. Salivary peptidomic as a tool to disclose new potential antimicrobial peptides. J. Proteom. 2015;115:49–57. doi: 10.1016/j.jprot.2014.12.004. [DOI] [PubMed] [Google Scholar]
- Troxler R.F., Offner G.D., Xu T., Vanderspek J.C., Oppenheim F.G. Structural relationship between human salivary histatins. J. Dent. Res. 1990;69:2–6. doi: 10.1177/00220345900690010101. [DOI] [PubMed] [Google Scholar]
- Tsai H., Bobek L.A. Human salivary histatin-5 exerts potent fungicidal activity against Cryptococcus neoformans. Biochimica et Biophysica Acta (BBA) – Gen. Subjects. 1997;1336:367–369. doi: 10.1016/s0304-4165(97)00076-7. [DOI] [PubMed] [Google Scholar]
- Tsai H., Bobek L.A. Studies of the mechanism of human salivary histatin-5 candidacidal activity with histatin-5 variants and azole-sensitive and -resistant Candida species. Antimicrob. Agents Chemother. 1997;41:2224–2228. doi: 10.1128/aac.41.10.2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai H., Bobek L.A. Human salivary histatins: promising anti-fungal therapeutic agents. Crit. Rev. Oral Biol. Med. 1998;9:480–497. doi: 10.1177/10454411980090040601. [DOI] [PubMed] [Google Scholar]
- Tsai H., Raj P.A., Bobek L.A. Candidacidal activity of recombinant human salivary histatin-5 and variants. Infect. Immun. 1996;64:5000–5007. doi: 10.1128/iai.64.12.5000-5007.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullah R., Zafar M.S. Oral and dental delivery of fluoride: a review. Fluoride. 2015;48:195–204. [Google Scholar]
- Van T.H., Simoons-Smit I., NIEUW A. Synthetic histatin analogues with broad-spectrum antimicrobial activity. Biochem. J. 1997;326:39–45. doi: 10.1042/bj3260039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vered M., Shohat I., Buchner A. Epidermal growth factor receptor expression in ameloblastoma. Oral Oncol. 2003;39:138–143. doi: 10.1016/s1368-8375(02)00034-9. [DOI] [PubMed] [Google Scholar]
- Vitorino R., Lobo M.J., Ferrer-Correira A.J., Dubin J.R., Tomer K.B., Domingues P.M., Amado F.M. Identification of human whole saliva protein components using proteomics. Proteomics. 2004;4:1109–1115. doi: 10.1002/pmic.200300638. [DOI] [PubMed] [Google Scholar]
- Vukosavljevic D., Custodio W., Del Bel Cury A., Siqueira W. The effect of histatin 5, adsorbed on PMMA and hydroxyapatite, on Candida albicans colonization. Yeast. 2012;29:459–466. doi: 10.1002/yea.2925. [DOI] [PubMed] [Google Scholar]
- Wang G. Human antimicrobial peptides and proteins. Pharmaceuticals. 2014;7:545–594. doi: 10.3390/ph7050545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittmann-Liebold B., Graack H., Pohl T. Two-dimensional gel electrophoresis as tool for proteomics studies in combination with protein identification by mass spectrometry. Proteomics. 2006;6:4688–4703. doi: 10.1002/pmic.200500874. [DOI] [PubMed] [Google Scholar]
- Wong D.T. Salivary diagnostics powered by nanotechnologies, proteomics and genomics. J. Am. Dent. Assoc. 2006;137:313–321. doi: 10.14219/jada.archive.2006.0180. [DOI] [PubMed] [Google Scholar]
- Xu T., Levitz S.M., Diamond R.D., Oppenheim F.G. Anticandidal activity of major human salivary histatins. Infect. Immun. 1991;59:2549–2554. doi: 10.1128/iai.59.8.2549-2554.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo I., Kim H., Lim K.S., Han J. Implant surface factors and bacterial adhesion: a review of the literature. Int. J. Artif. Organs. 2012;35:762–772. doi: 10.5301/ijao.5000154. [DOI] [PubMed] [Google Scholar]
- Yin A., Margolis H., Grogan J., Yao Y., Troxler R., Oppenheim F. Physical parameters of hydroxyapatite adsorption and effect on candidacidal activity of histatins. Arch. Oral Biol. 2003;48:361–368. doi: 10.1016/s0003-9969(03)00012-8. [DOI] [PubMed] [Google Scholar]
- Yoshinari M., Kato T., Matsuzaka K., Hayakawa T., Shiba K. Prevention of biofilm formation on titanium surfaces modified with conjugated molecules comprised of antimicrobial and titanium-binding peptides. Biofouling. 2010;26:103–110. doi: 10.1080/08927010903216572. [DOI] [PubMed] [Google Scholar]
- Zasloff M. Antimicrobial peptides of multicellular organisms. Nature. 2002;415:389–395. doi: 10.1038/415389a. [DOI] [PubMed] [Google Scholar]
- Zawisza I., Mital M., Polkowska-Nowakowska A., Bonna A., Bal W. The impact of synthetic analogs of histidine on copper (II) and nickel (II) coordination properties to an albumin-like peptide. Possible leads towards new metallodrugs. J. Inorg. Biochem. 2014;139:1–8. doi: 10.1016/j.jinorgbio.2014.05.011. [DOI] [PubMed] [Google Scholar]