Abstract
The present study emphasizes on synthesis of bimetallic silver–gold nanoparticles from cell free supernatant of Pseudomonas veronii strain AS41G inhabiting Annona squamosa L. The synthesized nanoparticles were characterized using hyphenated techniques with UV–Visible spectra ascertained absorbance peak between 400 and 800 nm. Possible interaction of biomolecules in mediating and stabilization of nanoparticles was depicted with Fourier transform infrared spectroscopy (FTIR). X-ray diffraction (XRD) displayed Bragg’s peak conferring the 1 0 0, 1 1 1, 2 0 0, and 2 2 0 facets of the face centered cubic symmetry of nanoparticles suggesting that these nanoparticles were crystalline in nature. Size and shape of the nanoparticles were determined using Transmission electron microscopy (TEM) microgram with size ranging from 5 to 50 nm forming myriad shapes. Antibacterial activity of nanoparticles against significant human pathogens was conferred with well diffusion assay and its synergistic effect with standard antibiotics revealed 87.5% fold increased activity with antibiotic “bacitracin” against bacitracin resistant strains Bacillus subtilis, Escherichia coli and Klebsiella pneumoniae followed by kanamycin with 18.5%, gentamicin with 11.15%, streptomycin with 10%, erythromycin with 9.7% and chloramphenicol with 9.4%. Thus the study concludes with biogenic and ecofriendly route for synthesizing nanoparticles with antibacterial activity against drug resistant pathogens and attributes growing interest on endophytes as an emerging source for synthesis of nanoparticles.
Keywords: Biogenic, Bimetallic silver–gold nanoparticles, Pseudomonas veronii AS41G, Synergistic activity, Broad spectrum antibiotics
1. Introduction
Nanoparticles have burgeoning interest in recent years owing to their innumerable applications in interdisciplinary area of science. Noble metal nanoparticles are widely used in biosensing, biocatalysts, drug delivery, semiconductor, fuel cells, fluorescent probe, and antimicrobial agents (Baker et al., 2013). One of the important aspects of nanoparticles relies on synthesis process. Nanoparticles can be synthesized via physical and chemical methods and these processes are bound with various limitations such as use of toxic elements, generation of environmental pollutant and high energy which restricts the use of nanoparticles in biomedical applications (Li et al., 2011). Hence as an alternative approach biogenic principles are employed for safe and ecofriendly processes to synthesize nanoparticles which involve use of biological entities. In recent years nanoparticles are synthesized by using prokaryotic bacteria and eukaryotic organisms such as fungi and plants or their products (Baker et al., 2013).
However use of plant species may pose a risk and imbalance to plant diversity especially when it comes to endangered species; hence, microorganisms have better advantage over plants as they are inexhaustible resource. Microorganisms inhabiting unique biological niches are of great interest as they are capable of secreting diverse bioactive compounds bearing biological activities, and one such clique of microorganisms are endophytes (Baker and Satish, 2012b). Endophytes are microorganisms colonizing healthy internal tissues of plants without causing any immediate, overt negative effects. These endophytes are reported to perform myriad biological activities which influence its host for survival during extreme conditions. Research on endophytes has yielded potential drug lead compounds with antibacterial, antiviral, antioxidant, insulin mimetic, anti-neurodegenerative and immunosuppressant properties (Strobel, 2003).
But interface between endophytes and nanomaterials is a relatively new and unexplored area which may open avenues in future to push forward the frontiers in coming decades (Baker and Satish, 2012a). Exploiting endophytic flora in synthesis of nanoparticles can lead to significant advances due to the fact that they secrete secondary metabolites bearing structurally diverse chemical compounds capable of reducing the metal salts and stabilize the nanoparticles as reported in various literatures (Sunkar and Nachiyar, 2013). The present investigation offers a valuable contribution to antimicrobial resistance area with synthesized nanoparticles bearing antibacterial activity against the drug resistant bacteria. Biosynthesis of nanoparticles is a growing area in bionanotechnology to unleash the ecofriendly approach as an alternative over conventional methods. The results emphasizes emerging role of endophytes toward synthesizing stable nanoparticles but reports of endophytic bacteria for nanoparticles synthesis are few which prompted us to isolate bacterial endophyte capable of nanoparticle synthesis which resulted in isolation of novel endophyte Pseudomonas veronii AS41G from Annona squamosa L. and employed for the synthesis of bimetallic nanoparticles and their antibacterial activity against significant human pathogens with synergistic effect using standard antibiotics conjugated with nanoparticles. Bioconjugation offers tremendous interest in biology, and biomolecules can be tailored with various other molecules but in recent years conjugation of nanoparticles with biomolecules has significant impact. The choice of the bioconjugation procedure depends strictly on physicochemical and biochemical properties of nanomaterials and bioactive molecules. The interaction between nanoparticles and biomolecules based on the electrostatic forces forms an organization resulting in functionalized nanoparticles in a reversible manner based on the opposite charges. As nanoparticles posses unique properties which form a base toward developing hybridization with the biomolecules resulting in a specific activity (Bagwea et al., 2003). Therefore, such interactions are promising enough toward developing bioconjugated nanoparticles as antimicrobial agents.
2. Materials and method
2.1. Materials
All the chemicals employed in the present investigation were procured from reputed international firms such as M/s Hi media and Sigma Aldrich. Silver nitrate and Chloroauric acid were obtained from Sigma Aldrich. Nutrient media and other chemicals were obtained from Hi media.
2.2. Surface sterilization
P. veronii strain AS 41G was isolated from surface sterilized leaf segment of Annona squamosa L. Surface sterilization was carried out with sequential steps by immersing plant material in 3.15% of sodium hypochlorite for five minutes and then followed by ethanol 70% for thirty seconds. After successive surface sterilization the stem and leaves tissues were rinsed three times in sterilized distilled water and aseptically cut into small pads (0.5 × 0.5 cm2) and placed nutrient agar supplemented with 250 μg/ml of cycloheximide and incubated till bacterial endophytic colonies are visible (Baker et al., 2015).
2.3. Synthesis of bimetallic silver–gold nanoparticles
Pure colony of actively growing P. veronii AS 41G was inoculated into nutrient broth and incubated for 72 h. Later the culture broth was centrifuged at 8000 rpm at 4 °C for 20 min to obtain cell free supernatant. 90 mL of stock solution with 1:1 of 1 mM AgNO3 and HAuCl4 was prepared. 10 ml of cell free supernatant was added to 90 ml of stock solution and incubated at different temperatures ranging from 30 to 80 °C. Samples were drawn periodically and change in color of the solution was monitored and synthesized nanoparticles were characterized.
2.4. Characterization of bimetallic silver–gold nanoparticles
Samples drawn periodically were monitored with UV–Visible spectroscopy by recording the spectra between 200 and 800 nm using Shimadzu double beam spectrophotometer. FTIR spectroscopy analysis was carried out to reveal the functional group of biomolecules responsible to reduce the metal salts and stabilization of bimetallic nanoparticles by using the instrument JASCO FT-IR 4100 at room temperature with a resolution of 4 cm−1. Crystalline nature of the bimetallic nanoparticles was studied with XRD by coating the dried sample on XRD grid and spectra were recorded by Rigaku Miniflex-II Desktop X-ray diffractometer instrument operating at a voltage of 30 kV. Size and morphology of nanoparticles were analyzed by using Transmission Electron Microscopy, and an aliquot of nanoparticles was transferred on to a carbon-coated copper TEM grids. The films on the TEM grids were allowed to stand for 2 min, then extra solution was removed and the grid was allowed to dry prior to measurement and scanned using a TECHNAI-T12 JEOL JEM-2100. Transmission electron microscope was operated at a voltage of 120 kV with Bioten objective lens. Subsequently, the particle size was ascertained using a Gatan ccd Camera and histogram was constructed by counting 200 bimetallic nanoparticles (Baker et al., 2015).
2.5. Test pathogens
All the test bacteria were procured from Microbial type culture collection (MTCC), Chandigarh, India, and stored at Department of Studies in Microbiology, University of Mysore until further use. Bacillus subtilis (MTCC 121), Escherichia coli (MTCC 7410), Klebsiella pneumoniae (MTCC 7407) and Staphylococcus aureus (MTCC 7443).
2.6. Selected antibiotics
Standard antibiotics were procured from Hi-media, in the present study six different commonly used antibiotics were selected viz., bacitracin, chloramphenicol, erythromycin, gentamicin, kanamycin, streptomycin.
2.7. Antibacterial activity
Antibacterial activity was carried via well diffusion assay to evaluate the synergistic effect of selected antibiotics with biologically synthesized bimetallic nanoparticles against important test pathogens. Pre-warmed MHA (Mueller-Hinton agar) plates were seeded with 106 CFU (colony forming unit) suspensions of test bacteria and standard antibiotics disc was placed onto the agar media. Simultaneously 10 μL of nanoparticles with 10 μg concentration was impregnated on sterile disc and placed onto the media and finally standard antibiotic discs were impregnated with 10 μL of nanoparticles (10 μg concentration) and dried completely and placed onto the media. All the plates were incubated at 37 °C for 24 h and zone of inhibition was measured. The experiment was carried out in triplicate. The increase fold in the antibacterial activity and synergistic effect was measured using the formula: b − a/a × 100 (Fayaz et al., 2010).
3. Results and discussion
The present investigation attributes toward emerging scientific knowledge of endophytes toward synthesizing bimetallic nanoparticles and study forms the first report of endophyte P. veronii strain AS 41G toward synthesizing bimetallic nanoparticles. Even though there are sporadic literatures being constantly reported pertaining to biogenic principle based nanoparticles synthesis, very scanty reports are available on bimetallic nanoparticles and their applications. The work is important in the frame of developing alternatives to combat wide spread of multidrug resistant pathogens which are ineffective with the available standard antibiotics. Isolation of endophyte was successful with the implementation of surface sterilization protocol using sodium hypochlorite and ethanol which suppressed the growth of epiphytes which was confirmed with no colonies observed on control plate and use of cycloheximide in surface sterilization protocol resulted in inhibition of fungal endophytes. Identification of endophyte mediating synthesis was characterized as previously characterized and published (Baker et al., 2015). The sequence was deposited at Genbank to avail accession number KC 480604. Synthesis of bimetallic nanoparticles was achieved by growing the endophyte P. veronii strain AS 41G at large scale and cell free supernatant was separated and treated with metal salts which resulted in reduction of metal salts to synthesize nanoparticles. Scientific literatures pertaining to endophytes suggest that bioactive compounds secreted from the endophytes reduce and stabilize nanoparticles. Accordingly endophyte Colletotrichum sp isolated from leaves of Pelargonium graveolens was capable of rapid reduction of the metal ions to synthesize stable gold nanoparticles of variable size extra-cellularly (Shankar et al., 2003). Similarly Pestalotia sp. isolated from healthy leaves of Syzygium cumini L. reported extracellular synthesis of spherical and polydispersed silver nanoparticles in the range of 10–40 nm (Raheman et al., 2011). Reports on P. veronii a novel species are few and are rarely isolated and reported earlier the present study envisions its new role in synthesizing bimetallic nanoparticles. Earlier studies on this bacterium reports the biotransformation of pentachlorophenol by P. veronii PH-05 isolated from enriched soil of timber yard (Nam et al., 2003). Similarly biodegradation of 4-n-butylphenol by P. veronii nBPS isolated from rhizosphere of giant duckweed, Spirodela polyrrhiza could completely degrade 4-n-butylphenol up to 1.0 mM (Hoang et al., 2009).
3.1. Biophysical characterization of bimetallic nanoparticles
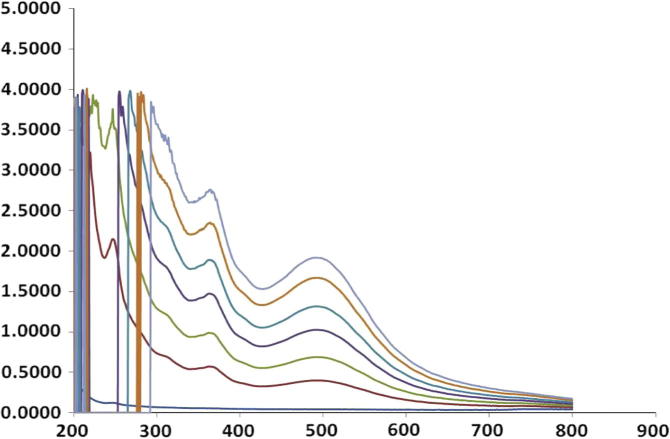
The synthesis of bimetallic silver–gold nanoparticles was initially confirmed with change in color of the solution from pale yellow color to purple color. Further confirmation of bimetallic nanoparticles was carried out by UV–Visible spectroscopic analysis with absorbance peak emerging between 400 and 600 nm wherein different spectral lines indicate synthesis of bimetallic nanoparticles at different time intervals and synthesis was rapid which was completed at 12 min and there was no further synthesis (Fig. 1). During the synthesis it was observed that various factors influenced synthesis of nanoparticles and it was noted that synthesis was maximum at elevated temperature at 80 °C and alkaline pH.
Figure 1.
UV spectra of bimetallic nanoparticles.
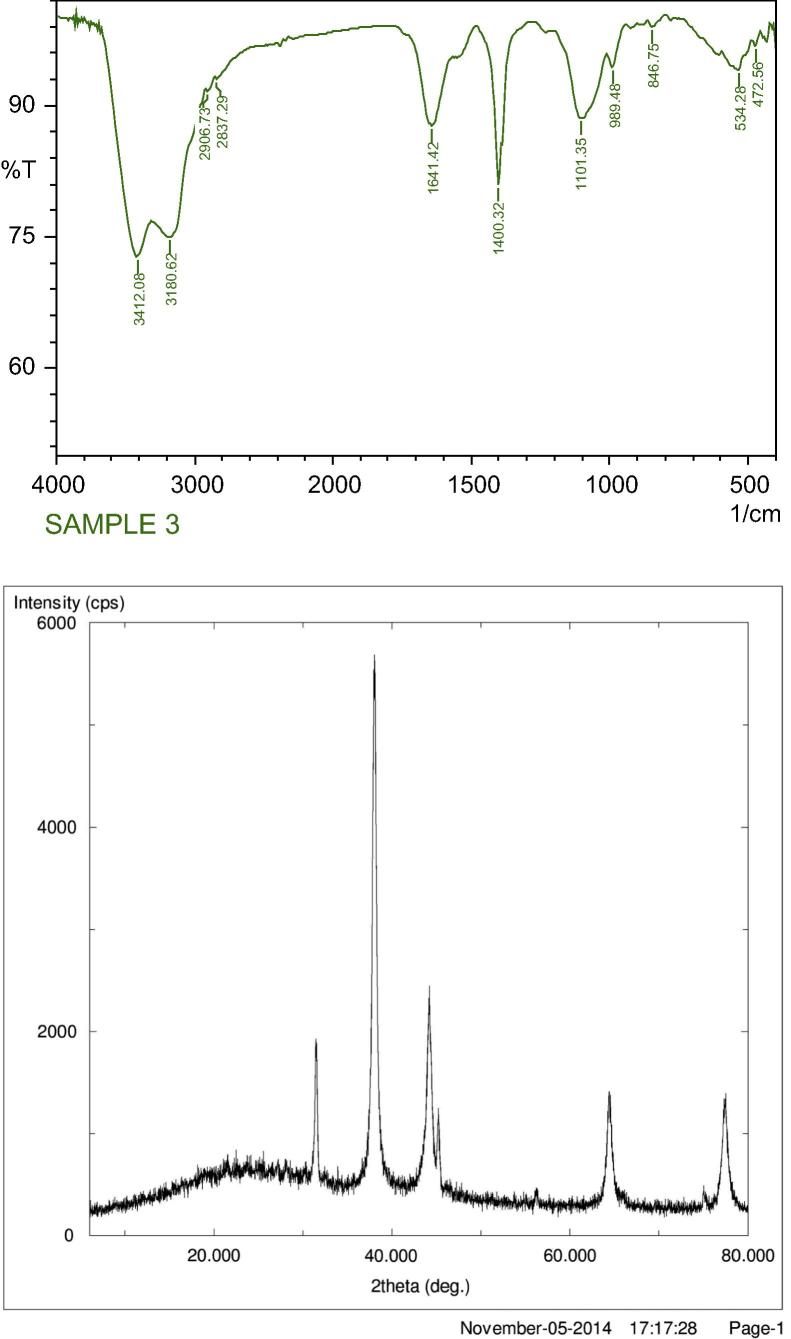
FTIR analysis depicted the possible biomolecules in the supernatant responsible for the reduction of metal salts to form nanoparticles and their stabilization. FTIR spectra of bimetallic nanoparticles (Fig. 2) showed major peaks at 3412, 3180, 2906, 2837, 1641, 1400, 1101 cm−1 corresponding to various functional groups as represented in Table 1. The XRD patterns of bimetallic nanoparticles represent the diffraction pattern appearing at 2θ values reflecting the lattice plane of face centered cubic (fcc) thus confirming the crystalline nature of the bimetallic nanoparticles (Fig. 2). Similar shift has been reported earlier for bimetallic nanoparticles synthesis (Tamulya et al., 2013). The XRD patterns reflect the lattice plane of face centered cubic (fcc) (Castro-Longoria et al., 2011). Synthesis was optimal at elevated temperature and this is due to the fact that at elevated temperature activation energy in the reaction mixture increases which influences nanoparticles formation (Oza et al., 2012).
Figure 2.
FTIR and XRD pattern of bimetallic nanoparticles.
Table 1.
Representative of predicted functional groups.
| Peaks | Possible functional group | References |
|---|---|---|
| 3412 | Hydroxyl (–OH) | Gao et al. (2014) |
| 3180 | Hydroxyl (–OH) | Bhuiyan and Rahman (2014) |
| 2906 | Methyl (–CH3) primary group | Gordin et al. (2009) |
| 2837 | Methylene (–CH2) secondary group | Bhat et al. (2003) |
| 1641 | Amide I | Ahmad et al. (2014) |
| 1400 | C–H bonding | Ferrari et al. (2003) |
| 1101 | C–O–C bonding | Tajau et al. (2013) |
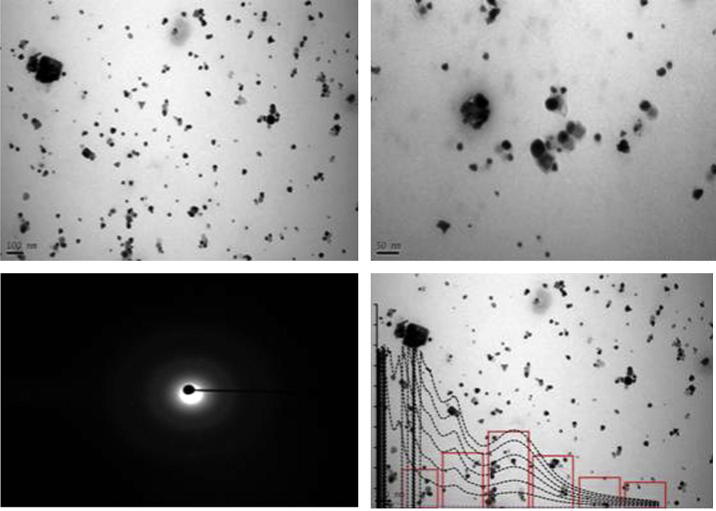
3.2. TEM microgram
Size and shape of bimetallic nanoparticles were studied by TEM (Fig. 3). TEM micrographs revealed nanoparticles were of spherical, triangular, pentagonal, and hexagonal planar. The particles were not well separated from each other which indicated the formation of gold nanoparticles was comparatively faster followed by the formation of silver nanoparticles due to differences in the reduction potential of the two metal ions. Histogram was constructed by counting the nanoparticles based on their size to reveal 30 nm as average size of the nanoparticles.
Figure 3.
TEM microgram of bimetallic nanoparticles.
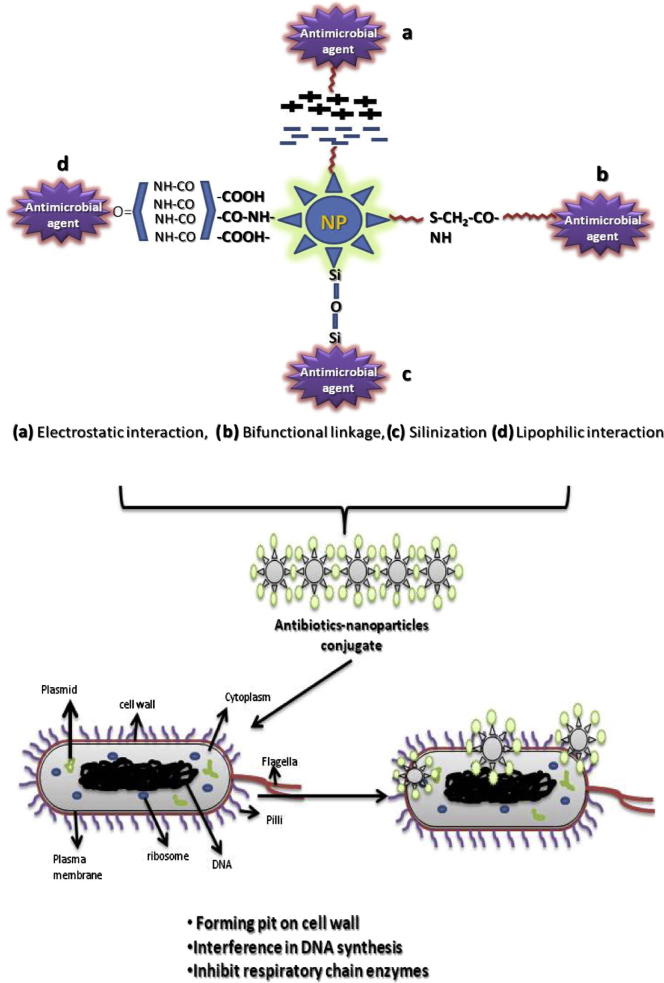
3.3. Probable mechanism of bimetallic nanoparticle synthesis and their bioconjugation with antibiotics
Mechanism behind biogenic principle based nanoparticles synthesis is yet to be completely elucidated. Based on the earlier scientific literatures, it can be suggested that endophyte secretes value added secondary metabolites bearing various biological activities such as bioreduction of metal salts into desired synthesis of nanoparticles even in the present investigation, biological components present in cell free extract facilitate synthesis by forming the scaffold and reducing the metal ions and the optimized parameters such as elevated temperature and alkaline pH influenced and catalyzed the nucleation followed by crystal growth and Ostwald ripening. During the ripening process, small crystals tend to dissolve and transfer their mass to aggregate toward forming bimetallic nanoparticles. It can be worth noting in the present investigation based on the results obtained, alkaline pH and elevated temperature influenced the synthesis of nanoparticles and similar findings were also observed in our previous study wherein same endophyte was capable of synthesizing silver nanoparticles (Baker et al., 2015). Similarly the mechanism in the formation of conjugates between antibiotics with bimetallic nanoparticles is in its infant stage with this preliminary investigation wherein bioconjugation might be due to electrostatic interactions between a negatively charged particle and positively charged region of antibiotics apart from these hydrophobic interactions and covalent bonding of the nanoparticle to sulfhydryl groups (–SH) present in antibiotics (Fig. 5) might be the possible mode of binding based on the earlier literatures (Baker and Satish, 2012a).
Figure 5.
Proposed mechanistic approach of antibiotic conjugation with nanoparticles.
3.4. Antibacterial activity of bimetallic nanoparticles and its synergistic effect
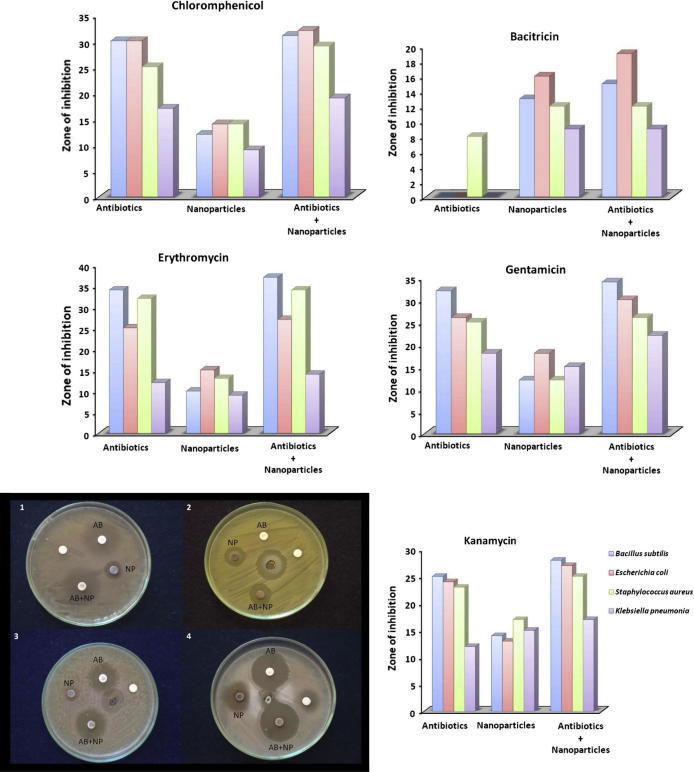
The antibacterial activity of selected standard antibiotics viz., bacitracin, chloramphenicol, erythromycin, gentamicin, kanamycin and streptomycin displayed activity against the test pathogens but increased fold activity was observed when antibiotics was evaluated in combination with bimetallic nanoparticles against test pathogens (Fig. 4). Different bar graphs represent the highest percentage of fold increase was observed with bacitracin in combination with nanoparticles against the test pathogens B. subtilis (MTCC 121), E. coli (MTCC 7410) and K. pneumoniae (MTCC 7407) which are resistant to bacitracin and significant activity with fold increase activity by 87.5% was observed with bacitracin followed by kanamycin with 18.5%, gentamicin with 11.15%, streptomycin with 10%, erythromycin with 9.7% and chloramphenicol 9.4% as shown in Table 2. The percentage of inhibition and synergistic effect was calculated using formula b − a/a × 100. Similar study carried out by Fayaz et al. (2010) suggests that the combination of silver nanoparticles and antibiotics resulted in increased activity against human pathogens.
Figure 4.
Zone of inhibition in mm with antibiotics, nanoparticles and their synergistic activity. Note: Different bar graphs represent zone of inhibition against test pathogens. AB: Antibiotics, NP: Nanoparticles, AB+NP: Antibiotics in combination with nanoparticles
Table 2.
Mean zone of inhibition (mm) of different antibiotics, silver nanoparticles and combined antibiotics with silver nanoparticles.
| Pathogens | Antibiotics (a) | SNP | Antibiotics + SNP (b) | Fold increase % [(b − a)/a] × 100 |
|---|---|---|---|---|
| Kanamycin | ||||
| B. subtilis | 25 | 14 | 28 | 12 |
| E. coli | 24 | 13 | 27 | 12.5 |
| S. aureus | 23 | 17 | 25 | 8.6 |
| K. pneumonae | 12 | 11 | 15 | 25 |
| Overall synergistic antibacterial effect: 16% | ||||
| Gentamicin | ||||
| B. subtilis | 32 | 12 | 33 | 3.1 |
| E. coli | 26 | 18 | 30 | 15.3 |
| S. aureus | 25 | 12 | 26 | 4.0 |
| K. pneumonae | 18 | 10 | 22 | 22 |
| Overall synergistic antibacterial effect: 11.15% | ||||
| Streptomycin | ||||
| B. subtilis | 26 | 12 | 27 | 3.8 |
| E. coli | 25 | 13 | 30 | 20 |
| S. aureus | 21 | 15 | 23 | 9.5 |
| K. pneumonae | 28 | 10 | 30 | 7.1 |
| Overall synergistic antibacterial effect: 10% | ||||
| Bacitracin | ||||
| B. subtilis | – | 13 | 15 | 100 |
| E. coli | – | 16 | 19 | 100 |
| S. aureus | 08 | 12 | 12 | 50 |
| K. pneumonae | – | 09 | 09 | 100 |
| Overall synergistic antibacterial effect: 87% | ||||
| Chloromphenicol | ||||
| B. subtilis | 30 | 12 | 31 | 3.3 |
| E. coli | 30 | 14 | 32 | 6.6 |
| S. aureus | 25 | 14 | 29 | 16 |
| K. pneumonae | 17 | 09 | 19 | 11.7 |
| Overall synergistic antibacterial effect: 9.4% | ||||
| Erythromycin | ||||
| B. subtilis | 34 | 10 | 37 | 8.8 |
| E. coli | 25 | 15 | 27 | 8.0 |
| S. aureus | 32 | 13 | 34 | 6.25 |
| K. pneumonae | 12 | 09 | 14 | 16.0 |
| Overall synergistic antibacterial effect: 9.7% | ||||
4. Conclusion
Thus the present investigation forms a preliminary study which envisions the emerging role of endophytic bacteria for synthesis of bimetallic nanoparticles by employing a novel endophyte P. veronii AS41G. The study highlights synergistic effect of broad spectrum antibiotics with nanoparticles resulting in increase fold activity against drug resistant pathogens conferring the emerging strategy to combat multidrug resistant microorganisms. Antibacterial activity of synthesized nanoparticles in conjugation with antibiotics resulted increase fold activity against the pathogens which were resistant to bacitracin attributing the significant role of nanoparticles in combating drug resistant pathogens.
Acknowledgments
Authors have no conflict of interest and are pleased to thank DST-SERB and CIMO for financial assistance. Further we thank C-CAMP, NCBS for TEM facility and Prof. Somashekar R., Nandaprakash M.B. and Thejas Urs, Department of Physics, University of Mysore, for their assistance for XRD analysis and finally we would like to thank Department of Studies in Microbiology and University of Mysore for providing facility and infrastructure.
Footnotes
Peer review under responsibility of King Saud University.
References
- Ahmad S.I., Syed I.A., Prasad R.P., Ahmad A. Quantitation of urea in urine by Fourier transforms infrared spectroscopy. Der. Pharma. Chemica. 2014;6:90–96. [Google Scholar]
- Bagwea R.P., Xiaojun Z., Weihong T. Bioconjugated luminescent nanoparticles for biological applications. J. Dispersion Sci. Technol. 2003;24:453–464. [Google Scholar]
- Baker S., Satish S. Endophytes: toward a vision in synthesis of nanoparticle for future therapeutic agents. Int. J. Bio-Inorg. Hybd. Nanomater. 2012;2:67–77. [Google Scholar]
- Baker S., Satish S. Screening of bacterial endophytes inhabiting Mimosa pudica L. Sci. J. Microbiol. 2012;5:136–140. [Google Scholar]
- Baker S., Rakshith D., Kavitha K.S., Santosh P., Kavitha H.U., Rao Y., Satish S. Plants emerging as nanofactories towards facile route in synthesis of nanoparticles. BioImpacts. 2013;3:111–117. doi: 10.5681/bi.2013.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker, S., Kumar, K.M., Santosh, P., Rakshith, D., Satish, S., 2015. Extracellular synthesis of silver nanoparticles by novel Pseudomonas veronii AS 41G inhabiting Annona squamosa L. and their bactericidal activity. Spectrochim. Acta Part A: Mol. Biomol. Spectrosc. (in press). doi: http://dx.doi.org/10.1016/j.saa.2014.10.033. [DOI] [PubMed]
- Bhat N.V., Upadhyay D.J., Deshmukh R.R., Gupta S.K. Investigation of plasma-induced photochemical reaction on a polypropylene surface. J. Phys. Chem. B. 2003;107:4550–4559. [Google Scholar]
- Bhuiyan M.R.A., Rahman M.K. Synthesis and characterization of Ni doped Zno nanoparticles. Int. J. Res. 2014;3:67–73. [Google Scholar]
- Castro-Longoria E., Vilchis-Nestor A.R., Avalos-Borja M. Biosynthesis of silver, gold and bimetallic nanoparticles using the filamentous fungus Neurospora Crassa. Colloids Surf. B. 2011;83:42–48. doi: 10.1016/j.colsurfb.2010.10.035. [DOI] [PubMed] [Google Scholar]
- Fayaz A.M., Balaji K., Girilal M., Yadav R., Kalaichelvan P.T., Venketesan R. Biogenic synthesis of silver nanoparticles and their synergistic effect with antibiotics: a study against gram positive and gram-negative bacteria. Nanomedicine. 2010;6:103–109. doi: 10.1016/j.nano.2009.04.006. [DOI] [PubMed] [Google Scholar]
- Ferrari A.C., Rodil S.E., Robertson J. Interpretation of infrared and Raman spectra of amorphous carbon nitrides. Phys. Rev. B. 2003;67:155306–155326. [Google Scholar]
- Gao S., Chen Y., Fan H., Wei X., Hu C., Wang L., Qu L. A green one-arrow-two-hawks strategy for nitrogen-doped carbon dots as fluorescent ink and oxygen reduction electrocatalyst. J. Mater. Chem. A. 2014;2:6320–6325. [Google Scholar]
- Gordin C., Rusu M., Delaite C., Salhi S., Elzein T., Brogly M. Crystalinity behaviour in poly(ε-caprolactone)-b-poly(dimethylsiloxane) diblock and triblock copolymers through FTIR and DSC. Mater. Plast. 2009;46:37–42. [Google Scholar]
- Hoang H., Inoue D., Momotani N., Yu N., Toyama T., Se K. Characterization of novel 4-n-butylphenol degrading Pseudomonas veronii strains isolated from rhizosphere of giant duckweed, Spirodela polyrhiza. Jpn. J. Water Treat. Biol. 2009;45:83–92. [Google Scholar]
- Li X., Xu H., Chen Z., Chen G. Biosynthesis of nanoparticles by microorganisms and their applications. J. Nanomater. 2011;2011:1–16. [Google Scholar]
- Nam I.H., Chang Y.S., Hong H.B., Lee Y.E. A novel catabolic activity of Pseudomonas veronii in biotransformation of pentachlorophenol. Appl. Microbiol. Biotechnol. 2003;62:284–290. doi: 10.1007/s00253-003-1255-1. [DOI] [PubMed] [Google Scholar]
- Oza G., Pandey S., Mewada A., Kalita G., Sharon M. Facile biosynthesis of gold nanoparticles exploiting optimum pH and temperature of fresh water algae Chlorella pyrenoidusa. Adv. Appl. Sci. Res. 2012;3:1405–1412. [Google Scholar]
- Raheman F., Deshmukh S., Ingle A., Gade A., Rai M. Silver nanoparticles: novel antimicrobial agent synthesized from an endophytic fungus Pestalotia sp. Isolated from leaves of Syzygium cumini. Nano Biomed. Eng. 2011;3:174–178. [Google Scholar]
- Shankar S., Ahmad A., Pasricha R., Sastry M. Bioreduction of chloroaurate ions by geranium leaves and its endophytic fungus yields gold nanoparticles of different shapes. J. Mater. Chem. 2003;13:1822–1826. [Google Scholar]
- Strobel G.A. Endophytes as sources of bioactive products. Microbes Infect. 2003;5:535–544. doi: 10.1016/s1286-4579(03)00073-x. [DOI] [PubMed] [Google Scholar]
- Sunkar S., Nachiyar V. Endophytes as potential nanofactories. Int. J. Chem. Environ. Biol. Sci. 2013;1:488–491. [Google Scholar]
- Tajau R., Wan Yunus W., Mohd Dahlan M.Z., Mahmood K.Z., Hashim M.H., Ismail K., Salleh M.M., Ismail C.R. Radiation-induced formation of acrylated palm oil nanoparticle using Pluronic F-127 microemulsion system. Pertanika J. Sci .Technol. 2013;21:127–134. [Google Scholar]
- Tamulya C., Hazarika M., Borah S., Das M.R., Boruah M.P. In situ biosynthesis of Ag, Au and bimetallic nanoparticles using Piper pedicellatum C.DC: green chemistry approach. Colloids Surf. B. 2013;102:627–634. doi: 10.1016/j.colsurfb.2012.09.007. [DOI] [PubMed] [Google Scholar]