Abstract
Paracetamol (PAR), phenylephrine hydrochloride (PHE) and chlorpheniramine maleate (CPM) are commonly used in clinical practice as antipyretic and analgesic drugs to ameliorate pain and fever in cold and flu conditions. The present work describes the use of thermal analysis for the characterization of the physicochemical compatibility between drugs and excipients during the development of solid dosage forms. Thermogravimetric analysis (TGA) and Differential Scanning Calorimetry (DSC) were used to study the thermal stability of the drug and of the physical mixture (drug/excipients) in solid binary mixtures (1:1). DSC thermograms demonstrated reproducible melting event of the prepared physical mixture. Starch, mannitol, lactose and magnesium stearate influence thermal parameters. Information recorded from the derivative thermogravimetric (DTG) and TGA curves demonstrated the decomposition of drugs in well-defined thermal events, translating the suitability of these techniques for the characterization of the drug/excipients interactions.
Keywords: Differential Scanning Calorimetry (DSC), Thermogravimetric analysis (TGA), Paracetamol, Chlorpheniramine maleate and phenylephrine hydrochloride
1. Introduction
The development of a new pharmaceutical dosage form involves preliminary pre-formulation studies for which information about the physical, chemical and mechanical properties of the formulation constituents is necessary. Mixture of drug/excipient can affect the long-term stability of the solid dosage form, as well as the drug bioavailability, therapeutic efficiency and safety profile (de Oliveira et al., 2013b, de Oliveira et al., 2011). In addition, the interactions between drug and excipients can affect the quality of the mixture, including the polymorphic form and crystallization profile of the drug, but also the formulation properties such as the solubility of the mixture, color, odor, and taste (Wu et al., 2011).
Thermoanalytical techniques are useful for the analysis of drug/excipient interactions during the development of new formulations based on classical solid dosage forms (e.g. powders, tablets, capsules). The physical properties, stability, compatibility and interactions between drugs and drugs/excipients can be assessed by the study of the changes occurring in the onset and endset temperatures, melting point and enthalpy (Mazurek-Wadołkowska et al., 2012). The advantages of Thermogravimetric Analysis (TGA) and Differential Scanning Calorimetry (DSC) rely on the fast sample processing, small amount of sample required, and easy detection of physical interactions (Chadha and Bhandari, 2014, Severino et al., 2011). The development of solid dosage forms (e.g. capsules and tablets) for oral administration of drugs for the treatment of flu is an usual practice in commercially available medicines (de Oliveira et al., 2013a, de Oliveira et al., 2011). Examples of drugs are paracetamol (PAR), phenylephrine hydrochloride (PHE) and chlorpheniramine maleate (CPM) (Palabiyik and Onur, 2010, Samadi-Maybodi and Nejad-Darzi, 2010). These drugs are used in combination such as analgesic, decongestant and anti-histaminic (Samadi-Maybodi and Nejad-Darzi, 2010) to ameliorate cough, pain and fever.
It is important to evaluate the interaction of drugs with excipients. The presence of degradation products is not desired, as they may interfere with the formulation stability and cause toxicity. DSC and TGA are important tools in various stages of formulation development. Application in the study of compatibility between substances has gained great prominence because they allow predicting possible interactions and/or incompatibilities in the final product (Neto et al., 2009). These methods are described in the European Pharmacopoeia, United States Pharmacopoeia, Japanese Pharmacopoeia and the Brazilian Pharmacopoeia. The objective this work was the assessment by thermal analyses using TGA and DSC of free drugs (PAR, CPM and PHE) and their physical mixtures (drug/excipient).
2. Materials and methods
2.1. Materials
Paracetamol (PAR), phenylephrine hydrochloride (PHE), chlorpheniramine maleate (CPM), Plasdone®S-630, lactose, microcrystalline cellulose, croscarmellose, magnesium stearate, corn starch, Aerosil® (colloidal silica), and mannitol were purchased from Henrifarma (São Paulo, Brazil).
2.2. Methods
2.2.1. Binary mixtures
The binary mixtures were obtained by manual mixing of 1:1 ratios of drug/excipient using a pestle and mortar. For the preparation of the mixtures, selected excipients were Aerosil®, starch, lactose, Plasdone®S-630, microcrystalline cellulose, magnesium stearate, and mannitol.
2.2.2. Differential Scanning Calorimetry (DSC)
Thermal behavior of drugs and excipients was assessed by accurately weighting 3 mg of sample loaded into an aluminum pan and sealed hermetically, in inert atmosphere (N2). The analysis was performed from 25 to 300 °C at a heating rate of 10 °C/min. The assessment of drug purity and other thermodynamic parameters was determined by the program (TA Instruments, USA) by cryoscopic depression using the Van’t Hoff equation, as follows:
where Tm is the sample temperature (in K), T0 is the melting point of pure sample (in K), R is the gas constant (8.314 J/mol K), X is the mole fraction of impurity, ΔHf is the heat fusion (J/mol), and F is the fraction of total sample melted at Tm.
2.2.3. Thermogravimetric analysis (TGA)
The thermogravimetric curves were recorded employing a thermoanalytical balance (2950, TA Instruments, USA) in the temperature range 25–400 °C. The drugs were carefully weighted and transferred to a platinum crucible, sample mass of ∼10.0 mg, heating rate 10 °C/min under a dynamic atmosphere of nitrogen at a flow rate of 100 mL/min. All data were processed by the program software (TA Universal Analysis, USA).
3. Results and discussion
Respiratory tract infections are common clinical situations diagnosed worldwide, usually requiring the treatment of the symptoms (e.g. cough, pain, fever) by the use of classical drugs such as PAR, CPM, and PHE, either isolated or in combination (Tiţa et al., 2011).
PAR is the agent of first choice in the treatment of acute and chronic pain, associated or not with peripheral inflammatory reaction, being effective and having better safety profile compared to other analgesic drugs (Corvis et al., 2015). It is presented in the form of white crystalline powder, odorless and slightly bitter, with melting point recorded between 168 °C and 172 °C, being soluble in water, ethanol and sodium hydroxide and chloroform and slightly soluble in ether. It belongs to the Biopharmaceutical Classification System (BCS) Class I, i.e. it has high permeability and high solubility in aqueous medium. It is, therefore, possible to obtain the PAR in 3 polymorphic forms, from which two polymorphic forms can be isolated, i.e. I (monoclinic) and II (orthorhombic). Form I is more stable compared to form II, being ideal for the formulation in medicinal products (Mazurek-Wadołkowska et al., 2012). CPM is the first generation drugs used to prevent the symptoms of allergic conditions, such as rhinitis and urticaria. Its sedative effects are relatively weak, and has bitter taste when in contact with the oral mucosa (Jelvehgari et al., 2014). PHE is a decongestant agent used to relieve nasal discomfort caused by colds and flu (Picon et al., 2013).
The study of compatibility between the drug and the excipients provides information about the stability of drugs. These studies involve the assessment of the physical and chemical drug stability in the presence of excipients composing the final pharmaceutical dosage form. Table 1 shows DSC scans of PAR, CPM, and PHE. DSC shows that PAR and CPM have a sharp endothermic event at 169.55 °C and 135.66 °C, respectively. PHE depicted its melting temperatures at 144.95 °C with two thermal events. Both PAR and CPM depicted a single melting event at the same temperature observed for pure drugs, as commonly observed for pure drugs (Jelvehgari et al., 2014, Mazurek-Wadołkowska et al., 2012, Sacchetti, 2000).
Table 1.
Thermodynamic data of paracetamol, chlorpheniramine maleate and phenylephrine hydrochloride obtained from the DSC analyses.
| Parameters | Paracetamol | Chlorpheniramine | Phenylephrine |
|---|---|---|---|
| Purity (mol%) | 99.86 | 99.06 | 99.1 |
| Melting point (°C) | 169.55 | 135.66 | 144.95 |
| Onset temperature (°C) | 162.41 | 124.74 | 137.18 |
| Endset temperature (°C) | 185.52 | 150.33 | 162.06 |
| Depression (°C) | 0.06 | 0.35 | 0.46 |
| ΔH (kJ/mol) | 35.71 | 37.35 | 28.70 |
| Correction (%) | 20.00 | 10.25 | 20.00 |
| Molecular weight (g/mol) | 151.16 | 274.8 | 167.2 |
| Cell constant | 1.178 | 1.178 | 1.178 |
| Onset slope (mW/°C) | −17.54 | −17.54 | −17.54 |
| RMS deviation (°C) | 0.12 | 0.006 | 0.08 |
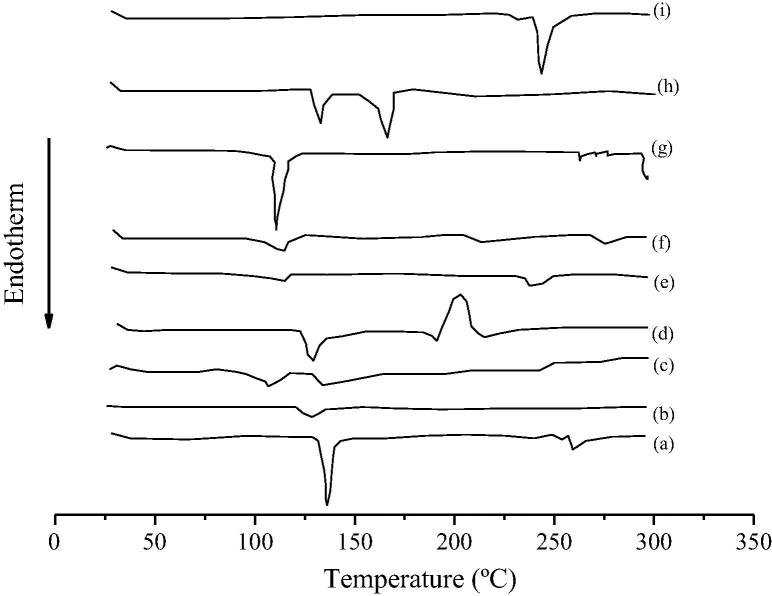
Figure 1, Figure 2, Figure 3 show the results of the DSC analyses of the free drugs and of the prepared physical mixtures (drug/excipients). Fig. 1 compares the thermal event of CPM (Fig. 1a) with those recorded for the physical mixtures with selected excipients (Fig. 1b–i), respectively, Aerosil®, starch, lactose, Plasdone®S-630, microcrystalline cellulose, magnesium stearate, and mannitol. A trend was observed, i.e. the addition of the excipient resulted in the decrease in the corresponding melting peak of the drug as expected. The ΔH values of the physical mixtures also decreased when compared to the free drug. The DSC curve of the CPM depicted an endothermic peak at 135.66 °C corresponding to the melting of the drug. For the physical mixture with lactose (Fig. 1d), a second event was recorded, attributed to the exothermic reaction of the loss of mass, whereas for the mixture with magnesium stearate (Fig. 1h) a second endothermic peak was also observed, also characterizing the decomposition of the sample. The mixture with corn starch (Fig. 1i) showed a dehydration effect which was attributed to the hydrophilic character of this polysaccharide. For the other tested excipients, only small shifts and lower peak intensities were recorded in comparison with the pure drug.
Figure 1.
DSC thermograms of pure chlorpheniramine (a) and its physical mixtures with (b), Aerosil® (c), corn starch (d), lactose (e), Plasdone®S-630 (f), microcrystalline cellulose (g), magnesium stearate (h), and mannitol (i).
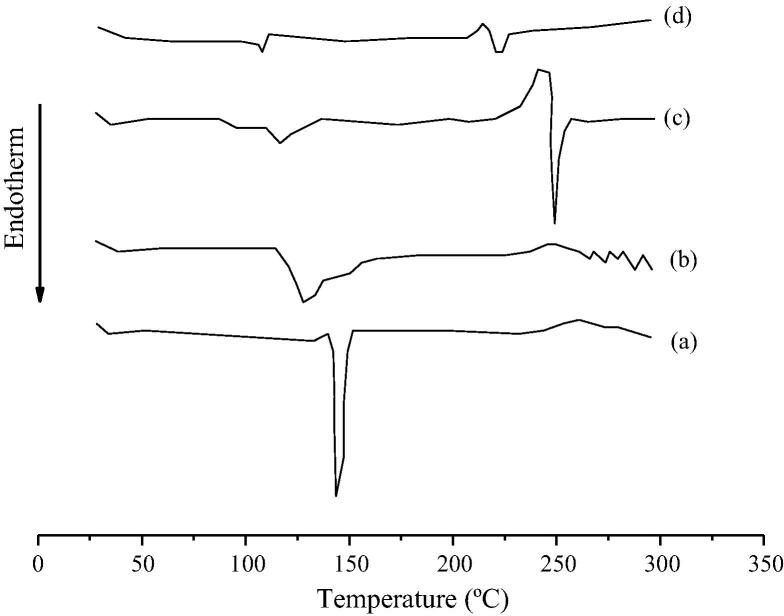
Figure 2.
DSC thermograms of pure phenylephrine (a) and its physical mixtures with mannitol (b), croscarmellose (c) and microcrystalline cellulose (d).
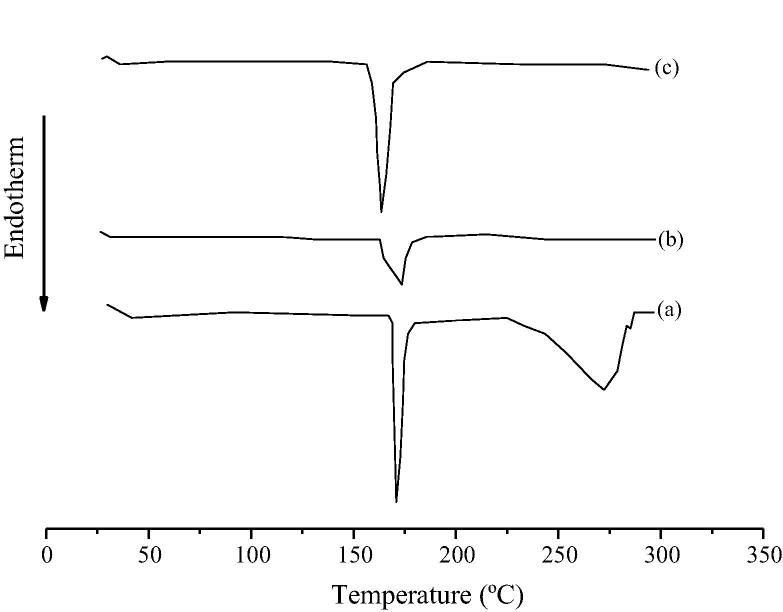
Figure 3.
DSC thermograms of pure paracetamol (a) and its physical mixtures with microcrystalline cellulose (b) and mannitol (c).
Fig. 2 shows the thermograms of pure PHE and its physical mixtures with mannitol, croscarmellose and microcrystalline cellulose. The thermal events showed a shift down to lower temperature values and lower peak intensities of the physical mixtures, compared to pure drug. Furthermore, a second event was observed for the mixtures of PHE with croscarmellose (Fig. 2c) and microcrystalline cellulose (Fig. 2d). These exothermic and endothermic events were attributed to the loss of mass and sample decomposition, respectively.
Fig. 3 shows the thermograms of PAR and its physical mixtures with microcrystalline cellulose (Fig. 3b) and mannitol (Fig. 3c). In comparison with the physical mixtures, the melting point of PAR was recorded in the same value (approximately 169 °C), but the ΔH of the mixtures decreased in comparison with the free drug powder. This effect was most evident in the mixture with microcrystalline cellulose (Fig. 3b). This suggests the reduction of drug crystallinity in the physical mixture. Fig. 3a shows a second endothermic peak suggesting decomposition of the sample, result that is in agreement with the DTG/TGA data obtained from DTG and TGA analyses.
TGA is a destructive technique, since it determines the thermal stability of the material by the quantification of the weight loss with the increase in the temperature. The variation of mass of a sample is therefore recorded as a function of temperature and time, carefully monitored in a temperature-controlled atmosphere. It assesses the loss or mass gain of the sample at various temperatures. The loss of mass or mass aggregation can be analyzed in the thermogravimetric analysis (TGA) and the derivative thermogravimetric (DTG) curves. While the TGA is an analytical technique that records the loss/gain of sample mass as a function of time and temperature, the DTG expresses the first derivative of weight change (m) versus time (dm/dt) without recorded as a function of time or temperature. The DTG curves show peaks whose areas are proportional to the weight variation of the sample.
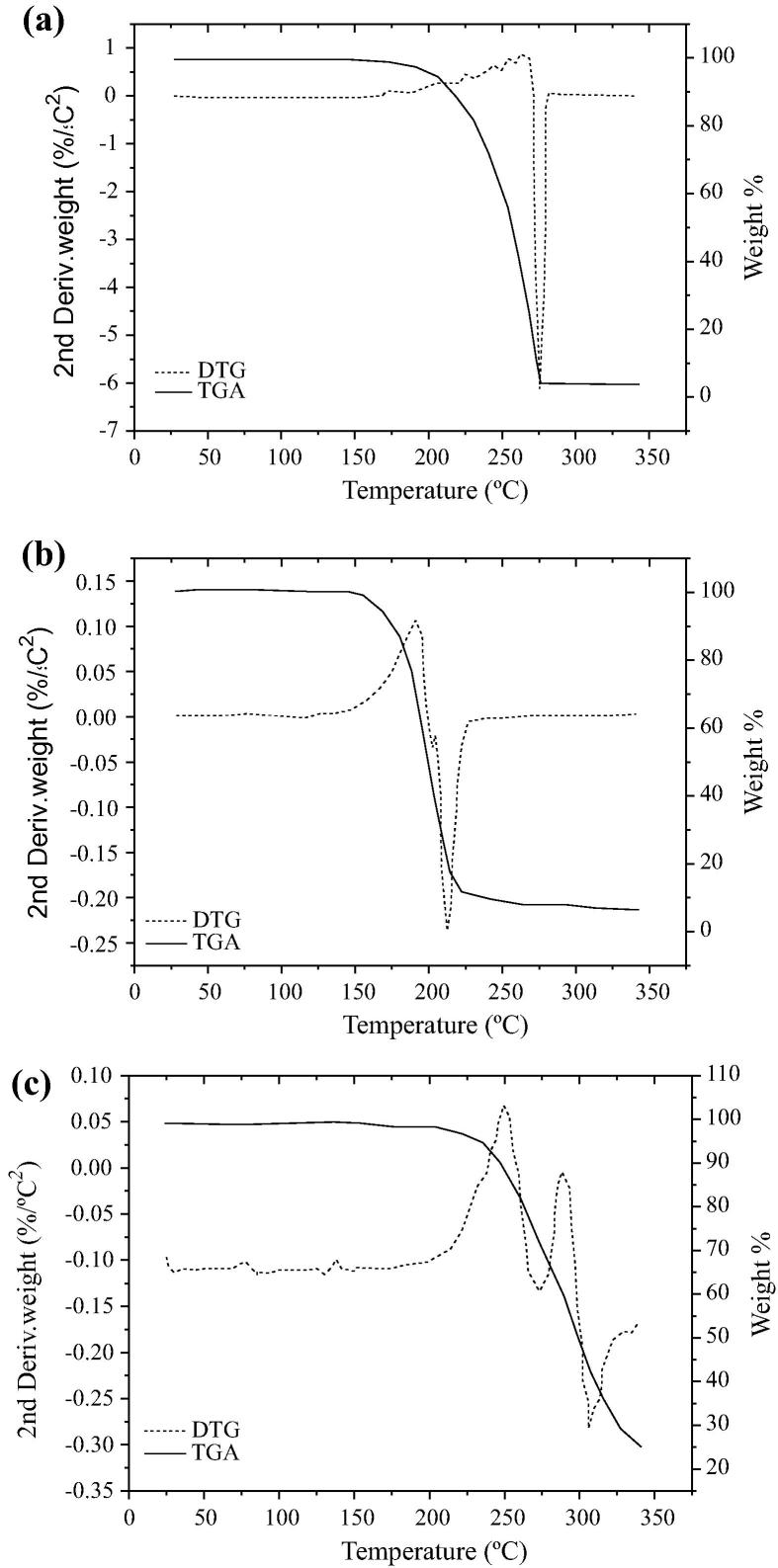
Fig. 4 shows the DTG/TGA curves of the tested pure drugs. In the TGA curves, the decomposition of drugs clearly depicted well-defined thermal events. The samples showed no weight loss and dehydration associated with the formation of residues, indicating that the thermal composition was complete. The DTG curves showed that PAR, PLC and PHE remained stable until 148.05 °C, 125.96 °C and 155.05 °C, respectively, followed by mass loss event being the maximum 254.07 °C (for paracetamol) and 197.08 °C (for chlorpheniramine). Phenylephrine hydrochloride depicted two events, at 248.00 °C and 276 °C. The data recorded by DTG/TGA confirm those shown in Figure 2, Figure 3.
Figure 4.
DTG/TGA curves of paracetamol (a), chlorpheniramine (b) and phenylephrine hydrochloride (c). Black line is the percentage of mass variation in the first run and dashed line is the 2nd derivative, mass/%/°C^2.
Similar results were published by Tomassetti et al. (2005), when studying by DSC the compatibility between paracetamol and several excipients used in solid dosage forms (e.g. polyvinylpyrrolidone, magnesium stearate, citric acid, aspartame, mannitol, cellulose and starch). The authors also analyzed binary mixtures in comparison with a commercial product. Results showed compatibility with excipients except for mannitol. Data for CPM and PHE were not found in the literature.
4. Conclusions
The analysis carried out by DSC allowed us to demonstrate the reproducible melting events of pure drugs and in physical binary mixtures in a series of excipients. The physical blends depicted thermal events translating a decrease or increase in the drug stability depending on the type of excipient used. DTG/TGA showed decomposition of drugs in a well-defined thermal event. Our preliminary results allow us the adequate selection of excipients to be combined with common drugs used in clinical practice, translating the advantages of DSC and DTG/TGA in the assessment of drug/excipients interactions.
Acknowledgments
The authors wish to acknowledge the sponsorship of the FAPESP (Fundação de Amparo à Pesquisa do Estado de São Paulo), CAPES (Coordenação Aperfeiçoamento de Pessoal de Nivel Superior) and CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico), Process #443238/2014-6, #470388/2014-5. This work was also financed through the project UID/QUI/50006/2013, receiving support from the Portuguese Science and Technology Foundation, Ministry of Science and Education (FCT/MEC) through national funds, and co-financed by FEDER, under the Partnership Agreement PT2020.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
E.B. Souto, Email: ebsouto@ff.uc.pt.
P. Severino, Email: patricia_severino@itp.org.br.
References
- Chadha R., Bhandari S. Drug–excipient compatibility screening—role of thermoanalytical and spectroscopic techniques. J. Pharm. Biomed. Anal. 2014;87:82–97. doi: 10.1016/j.jpba.2013.06.016. [DOI] [PubMed] [Google Scholar]
- Corvis Y., Menet M.-C., Espeau P. Incidence of the melting-degradation process of vitamin C on the determination of the phase diagram with acetaminophen enhanced by high performance liquid chromatography tools. New J. Chem. 2015;39(3):1938–1942. [Google Scholar]
- de Oliveira G., Ferraz H., Severino P., Souto E. Solid dosage forms for active antiretroviral therapy (HAART): dissolution profile study of nevirapine by experimental factorial design. Pharm. Dev. Technol. 2013;18(2):428–433. doi: 10.3109/10837450.2012.680597. [DOI] [PubMed] [Google Scholar]
- de Oliveira G.G., Ferraz H.G., Severino P., Souto E.B. Analysis of phase transition and dehydration processes of nevirapine. J. Therm. Anal. Calorim. 2011;108(1):53–57. [Google Scholar]
- de Oliveira G.G., Ferraz H.G., Severino P., Souto E.B. Compatibility studies of nevirapine in physical mixtures with excipients for oral HAART. Mater. Sci. Eng. C Mater. Biol. Appl. 2013;33(2):596–602. doi: 10.1016/j.msec.2012.09.030. [DOI] [PubMed] [Google Scholar]
- Jelvehgari M., Barghi L., Barghi F. Preparation of chlorpheniramine maleate-loaded alginate/chitosan particulate systems by the ionic gelation method for taste masking. Jundishapur J. Nat. Pharm. Prod. 2014;9(1):39. doi: 10.17795/jjnpp-12530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazurek-Wadołkowska E., Winnicka K., Czajkowska-Kośnik A., Czyzewska U., Miltyk W. Application of differential scanning calorimetry in evaluation of solid state interactions in tablets containing acetaminophen. Acta Pol. Pharm. 2012;70(5):787–793. [PubMed] [Google Scholar]
- Neto H., Novák C., Matos J. Thermal analysis and compatibility studies of prednicarbate with excipients used in semi solid pharmaceutical form. J. Therm. Anal. Calorim. 2009;97(1):367–374. [Google Scholar]
- Palabiyik I.M., Onur F. Multivariate optimization and validation of a capillary electrophoresis method for the simultaneous determination of dextromethorphan hydrobromur, phenylephrine hydrochloride, paracetamol and chlorpheniramine maleate in a pharmaceutical preparation using response surface methodology. Anal. Sci. 2010;26(8):853–859. doi: 10.2116/analsci.26.853. [DOI] [PubMed] [Google Scholar]
- Picon P.D., Costa M.B., da Veiga Picon R., Fendt L.C.C., Suksteris M.L., Saccilotto I.C., Dornelles A.D., Schmidt L.F.C. Symptomatic treatment of the common cold with a fixed-dose combination of paracetamol, chlorphenamine and phenylephrine: a randomized, placebo-controlled trial. BMC Infect. Dis. 2013;13(1):556. doi: 10.1186/1471-2334-13-556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacchetti M. Thermodynamic analysis of DSC data for acetaminophen polymorphs. J. Therm. Anal. Calorim. 2000;63(2):345–350. [Google Scholar]
- Samadi-Maybodi A., Nejad-Darzi S.K.H. Simultaneous determination of paracetamol, phenylephrine hydrochloride and chlorpheniramine maleate in pharmaceutical preparations using multivariate calibration 1. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2010;75(4):1270–1274. doi: 10.1016/j.saa.2009.12.048. [DOI] [PubMed] [Google Scholar]
- Severino P., Pinho S.C., Souto E.B., Santana M.H. Polymorphism, crystallinity and hydrophilic-lipophilic balance of stearic acid and stearic acid-capric/caprylic triglyceride matrices for production of stable nanoparticles. Colloids Surf. B Biointerfaces. 2011;86(1):125–130. doi: 10.1016/j.colsurfb.2011.03.029. [DOI] [PubMed] [Google Scholar]
- Tiţa B., Fuliaş A., Bandur G., Marian E., Tiţa D. Compatibility study between ketoprofen and pharmaceutical excipients used in solid dosage forms. J. Pharm. Biomed. Anal. 2011;56(2):221–227. doi: 10.1016/j.jpba.2011.05.017. [DOI] [PubMed] [Google Scholar]
- Tomassetti M., Catalani A., Rossi V., Vecchio S. Thermal analysis study of the interactions between acetaminophen and excipients in solid dosage forms and in some binary mixtures. J. Pharm. Biomed. Anal. 2005;37(5):949–955. doi: 10.1016/j.jpba.2004.10.008. [DOI] [PubMed] [Google Scholar]
- Wu Y., Levons J., Narang A.S., Raghavan K., Rao V.M. Reactive impurities in excipients: profiling, identification and mitigation of drug–excipient incompatibility. AAPS PharmSciTech. 2011;12(4):1248–1263. doi: 10.1208/s12249-011-9677-z. [DOI] [PMC free article] [PubMed] [Google Scholar]