Abstract
Nanovesicles (NVs) represent a novel transporter for cell signals to modify functions of target cells. Therefore, NVs play many roles in both physiological and pathological processes. This report highlights biogenesis, composition and biological roles of erythrocytes derived nanovesicles (EDNVs). Furthermore, we address utilization of EDNVs as novel drug delivery cargo as well as therapeutic target. EDNVs are lipid bilayer vesicles rich in phospholipids, proteins, lipid raft, and hemoglobin. In vivo EDNVs biogenesis is triggered by an increase of intracellular calcium levels, ATP depletion and under effect of oxidative stress conditions. However, in vitro production of EDNVs can be achieved via hypotonic treatment and extrusion of erythrocyte. NVs can be used as biomarkers for diagnosis, monitoring of therapy and drug delivery system. Many therapeutic agents are suggested to decrease NVs biogenesis.
Keywords: Erythrocytes, Extracellular vesicles, Nanovesicles, Drug delivery
1. Introduction
Cells continuously secrete a large number of small molecules, macromolecules and nanovesicles into the extracellular space (Vlassov et al., 2012). Nanovesicles (NVs) are submicron membrane-coated vesicles of diameter up to 1000 nm, in which they are released from all cell types and contain cellular components of their parent’s cell (Vlassov et al., 2012). Exosomes and microvesicles (MVs) are collectively known as extracellular vesicles (EVs), and they have an aqueous core surrounded by a lipid bilayer membrane (Kastelowitz and Yin, 2014). EVs are considered as a new class of signal mediators, which allow the transport of nucleic acids, proteins, lipids and second messengers (Vlassov et al., 2012, Kastelowitz and Yin, 2014). EVs play significant roles in genetic transfer, cytokine release, angiogenesis, transfer of cell receptors, and proteinase release (Kastelowitz and Yin, 2014). Under activation, growth, apoptosis, senescence, shearing stress, oxidative stress and injury, cells release excessive amount of EVs (Antwi-Baffour et al., 2013A, Antwi-Baffour et al., 2013B).
Most of cells including endothelial cells, immune cells, cancer cells, hematopoietic cells, platelets, and erythrocytes are able to secret EVs (Fais et al., 2013). EVs are formed by budding of the plasma membrane through the dynamic redistribution of phospholipids (Lutz and Bogdanova, 2013). MVs and exosomes are differing in the biogenesis, size, surface markers, and biological roles (Fais et al., 2013). MVs are plasma membrane-derived vesicles with size range between 100 nm and 1000 nm. During their formation, MVs retain surface molecules from parent’s cell as well as part of their cytosolic content (Loyer et al., 2014).
On the contrary, exosomes have size up to 150 nm and are derived from a cascade of the fusion between early and late endosomes, lysosomes, and others depending on their cellular source (Fais et al., 2013, Loyer et al., 2014). The fusion of multivesicular bodies with the plasma membrane allows the release of exosomes into the extracellular space. Exosomes are membranous structures with a lipid bilayer rich in phospholipids, proteins, cholesterol, ceramide, and sphingolipids (Vlassov et al., 2012). EVs can circulate in the vascular network, where they can evade phagocytosis as well as they can participate in autocrine, paracrine and endocrine signaling (Prati et al., 2010).
In healthy humans, circulating EVs are mainly derived from platelets and to a lesser extent leukocyte and endothelial cells. An increase of EVs biogenesis has been demonstrated in physiological and pathological conditions (Lovren and Verma, 2013). The vascular endothelium is one of the primary targets of circulating EVs; they contribute to the regulation of endothelial cell functions, coagulation and inflammation (Lovren and Verma, 2013). However, abnormal biogenesis of EVs leads to endothelial dysfunction and development of cardiovascular diseases (Lovren and Verma, 2013).
The biogenesis of EVs is an inherent property of the erythrocyte plasma membrane. In healthy individuals, erythrocytes derived nanovesicles (EDNVs) are present at basal levels (Jank and Salzer, 2011). However, aging, high intracellular calcium levels and oxidative stress are triggers of erythrocytes vesiculation and release of EVs. Oxidized erythrocytes (Oxi-Ery) released EVs rich in oxidized proteins, lipid peroxides, cholesterol and other oxidized substances. Oxi-Ery and EDNVs are engulfed by macrophages and smooth muscle cells resulted in foam cells formation, and this induced endothelial dysfunction and enhanced atherosclerotic process (Blum, 2009, Tziakas et al., 2010). An increase in EVs release was observed in hypertension, thrombocytopenia, multiple sclerosis, sickle cell anemia, diabetes, and atherosclerosis (Antwi-Baffour et al., 2013A, Antwi-Baffour et al., 2013B).
Biogenesis, secretion mechanisms as well as biological roles of EVs have not been fully reported. Furthermore, their physiological and pathological roles are still a matter for research. Therefore, this report overviews biogenesis, beneficial and detrimental effects of EVs focus on EDNVs as new modulators in vascular disease. We hope that the present review will increase the understanding of EV biology.
2. EVs biogenesis
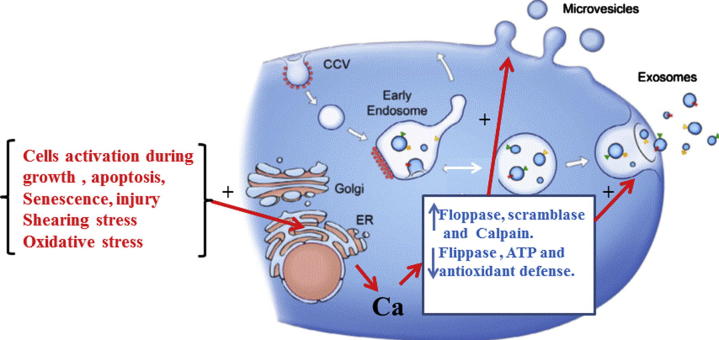
Under resting cellular conditions, phospholipids in the bilayer of plasma membrane are asymmetrically distributed. Phosphatidylcholine (PC) and sphingomyelin are mostly present in the outer leaflet (Larson et al., 2012). However, phosphatidylserine (PS), phosphatidylethanolamine and phosphatidylinositides are mainly present in the inner layer. This dynamic distribution of phospholipids is maintained by enzymes such as ATPases, scramblases, and floppases (Larson et al., 2012). Transient or irreversible redistribution of phospholipid(s) across the membrane bilayer has been shown to play important roles in cellular events (Larson et al., 2012). For example exposure of cells to oxidative stress, infection, cell signals, apoptosis and other stimuli resulted in EVs biogenesis (Prati et al., 2010). Calcium ions are key players in EVs biogenesis due to inactivation of flippase. However calcium ions activate floppase, scramblase, calpain and gelsolin. Calpain hydrolyzes actin binding proteins that decrease the association of actin with membranes glycoproteins. However, gelsolin is involving in the cleavage of the actin capping proteins. These events are inducing loss of phospholipid asymmetry and protein anchorage to the cytoskeleton is disrupted, resultant in membrane budding and EVs release (Burnier et al., 2009). EVs isolated from blood are rich in phosphatidylcholine, sphingomyelin and minor quantities of other phospholipids (Burnier et al., 2009). EVs are characterized by PS exposed on their outer surface and other component of the parent cell. Fig. 1 shows biogenesis and release of exosome and MVs; it clears that MVs bud directly from the plasma membrane, whereas exosomes are formed by budding of early endosomes. Calcium ions are key player in EVs biogenesis.
Figure 1.
General mechanism of biogenesis and release of extracellular vesicles (EVs), MVs bud directly from the plasma membrane, whereas exosomes are formed by budding of early endosomes.
3. Biological roles of EVs
In many biological systems, EVs are considered as important mediators of cell–cell communication to underpin physiological function (Lee et al., 2012). Under physiological conditions, EVs are released from various cell types to act as cellular messenger (Prati et al., 2010, Lamichhane et al., 2015). EVs transfer the bioactive molecules to target cells; therefore, EVs can modulate various biological processes such as angiogenesis, growth, cell differentiation, immune functions, stress response, and senescence (Prati et al., 2010, Lamichhane et al., 2015).
The cellular uptake machinery of EV may depend on proteins and glycoproteins found on the surface of both the vesicle and the target cell (Mulcahy et al., 2014). Moreover, the presence of external PS is a key determinant of the interaction of EVs with target cells; also the oxidized lipids may play the same role (Loyer et al., 2014). Cellular entrance of EVs is mediated by clathrin-dependent endocytosis, caveolin-mediated uptake, lipid raft-mediated internalization and phagocytosis (Mulcahy et al., 2014).
EVs are involved in inflammation and homeostasis, blood coagulation and apoptosis (Herring et al., 2013). In the nervous system, EVs transmit the information in the form of proteins to facilitate neural circuit function.
On contrary, EVs have several detrimental effects such as enhanced viral infection, neurodegeneration and tumorigenesis (Lee et al., 2012). Abnormal level of circulating EVs was documented in varieties of human diseases such as atherosclerosis, acute myocardial infarction, diabetes, hypertension, acute ischemic stroke, hyperlipidemia, and metabolic syndrome (Herring et al., 2013, Loyer et al., 2014).
4. Erythrocytes-derived vesicles(EDNVs)
Normal erythrocytes have flexible biconcave shape with a cell diameter microsize of 5–7 μm and a thickness of 2 μm. The main functions of erythrocytes are transport of oxygen and carbon dioxide, and release of adenosine triphosphate (ATP) and nitric oxide (NO). Moreover, erythrocytes carry significant enzymes and molecules involved in L-arginine/NO metabolic pathway (Porro et al., 2014).
Erythrocytes are the major vesicle-secreting cells in the circulating blood (Donadee et al., 2011), during their life span, and erythrocytes lose approximately 20 % of their hemoglobin and membrane through vesiculation (Alaarg et al., 2013). The erythrocyte vesicles are known as microvesicles, exovesicles, ectosomes, nanovesicles and microparticles (Jank and Salzer, 2011). Erythrocytes-derived nanovesicles (EDNVs) have average size approximately 100–200 nm with a lipid bilayer rich in phospholipids, proteins, cholesterol, lipid raft, hemoglobin and acetylcholinesterase (Jank and Salzer, 2011, Lutz and Bogdanova, 2013).
EDNVs biogenesis has been described as a part of erythrocytes senescence and also proposed as a part of an apoptosis-like process (eryptosis) in these cells. The release of EDNVs plays a protective role that allows erythrocytes to clear away dangerous molecules and prevent their early removal from circulation (Tissot et al., 2013). Therefore, EDNVs act as a self-protective mechanism for removal of dangerous molecules formed during erythrocytes life span (Willekens et al., 2008). EDNVs were demonstrated in ischemia, shearing stress, cardiovascular diseases, hematological disorders and diabetes (Antwi-Baffour et al., 2013B).
An increase of intracellular calcium levels leads to disruption membrane asymmetry with concomitant biogenesis of EDNVs (Jank and Salzer, 2011). Additionally, depletion of ATP depletion and exposure to membranotropic and hemolytic agents trigger NVs release from erythrocytes (Donadee et al., 2011, Lutz and Bogdanova, 2013). As well, morphological alterations, storage conditions, arachidonic acid, lysophosphatidic acid, and loss of deformability propagate erythrocytes vesiculation (Chung et al., 2007).
Oxidative stress and heavy metals exposure potentiate NVs shedding from erythrocytes due to depletion of antioxidant defense (Jank and Salzer, 2011, Noh et al., 2010). The inhibition of EDNVs biogenesis by antioxidants confirmed role of oxidative stress in stimulation of EVs production (Herring et al., 2013). Erythrocytes vesiculation is associated with protein oxidation, and lipid peroxidation leading to a loss of deformability (Herring et al., 2013). Similarly in many cells, EDNVs biogenesis triggered calcium concentrations that activate calpain, scramblase, and flippase (Larson et al., 2012).
4.1. Mechanisms of EDNVs biogenesis
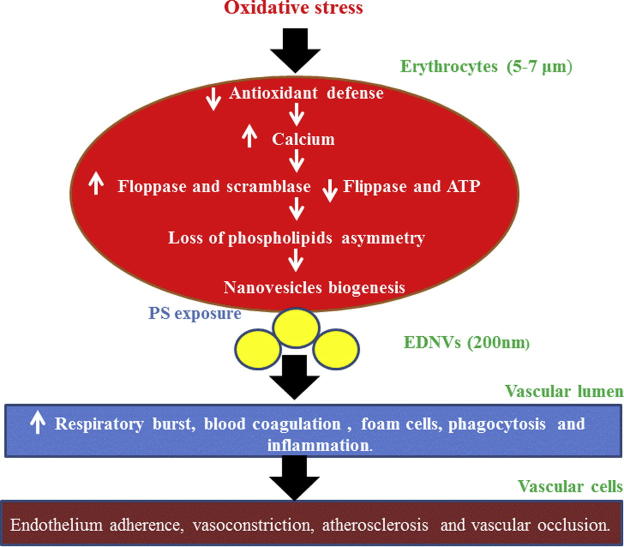
Three mechanisms have been proposed to explain EDNVs biogenesis, the eryptosis model, the band 3 clustering and oxidative stress. The eryptosis mechanism is similar to apoptosis of nucleated cells in response to various stresses but applied to erythrocytes (Tissot et al., 2013). Thus, a rise of ionic calcium influx through alteration of nonspecific cation channels leads to activation of calpain, scramblase and floppase; however, flippase was inactivated (Burnier et al., 2009). This resulted in disruption of phospholipid asymmetry and release of NVs. In band 3 clustering mechanism hemoglobin oxidation leads to hemichrome formation, followed by aggregation of band 3 multimers, degradation of cytoskeletal proteins and modification of band 3 phosphorylation (Tissot et al., 2013). In oxidative stress hypothesis, reactive species induce binding of hemoglobin to band 3, activation of calcium channels, and phosphorylation of proteins and aggregation of band 3 (Tissot et al., 2013). This hypothesis of erythrocytes vesiculation has been reinforced by the postulation of a study demonstrated that antioxidants addition inhibits NVs biogenesis (Stowell et al., 2013). Fig. 2 shows proposed mechanism of oxidative stress induced erythrocyte vesiculation.
Figure 2.
Proposed mechanism of oxidative stress induced erythrocyte vesiculation. EDNVs biogenesis triggered calcium concentrations that activate calpain, scramblase, and flippase.
5. Biological roles of EDNVs
In the past, EVs were considered as a garbage basket for removing unwanted materials as an integrated machinery of the lysosomal system. However, more recent data support the role of NVs in cellular homeostasis (Fais et al., 2013). Various studies have suggested the importance of NVs in numerous cellular processes (Jank and Salzer, 2011). EDNVs are produced as a machinery to prevent premature erythrocyte removal by reticuloendothelial system. Therefore, these NVs protect erythrocytes via removal of complement attack complex, band 3 antigens, nonfunctional hemoglobin and other unwanted membrane-associated elements (Herring et al., 2013).
5.1. EDNVs and oxidative stress
Normally, erythrocytes are provided with antioxidant molecules such as glutathione, thioredoxin, ascorbic acid and vitamin E. Furthermore, they contain superoxide dismutase, catalase, glutathione peroxidase, thioredoxin reductase, glutathione reductase, and methemoglobin reductase (Kennett and Kuchel, 2006, Minetti et al., 2007). Therefore, functional erythrocytes act as vascular guardian by scavenging reactive oxygen species (ROS) generated in itself, circulated blood, and vascular cells (Minetti et al., 2007). The decrease of erythrocytes antioxidant capacity resulted in oxidative stress and NO inactivation. In such cases, erythrocytes are labeled as key player in many diseases particularly cardiovascular disease (Eligini et al., 2013).
It has been reported that EDNVs are one of the priming causes of respiratory burst (Jank and Salzer, 2011), which is characterized by neutrophils activation with production of excessive amount of ROS. This is attributed to NVs having NADP oxidase subunits (Loyer et al., 2014). Moreover, Gaceb et al. (2014), reported that EVs upregulate NADPH-oxidase expression. Respiratory burst causes plethora of changes in cytoskeleton and cell membrane asymmetry resulted in formation of Oxi-ERY (Minetti et al., 2007). Oxi-ERY is rigid and undergoes hemolysis releasing cholesterol, lipids peroxidation product, aggregated protein and iron. All of them are potent stimulator of vascular cellular damage (Willekens et al., 2008). Therefore, Oxi-ERY and EDNVs act as dangerous bullets to vascular cells and trigger the damaging process (Minetti et al., 2007).
5.2. EDNVs and inflammation
EVs releases stimulate cell–cell interaction, adhesion molecule expression, cytokine release and increased macrophage differentiation (Loyer et al., 2014). The main components of erythrocyte membrane are phospholipids and cholesterol (Tziakas et al., 2010). Erythrocyte membranes are richer in cholesterol than other cell in the body; subsequently, EDNVs are enriched in cholesterol, lipid raft, membrane proteins and hemoproteins (Lutz and Bogdanova, 2013). The crystallization of cholesterol obtained from erythrocyte membrane may induce inflammatory reactions; furthermore, iron can act as a catalyst for ROS production (Minetti et al., 2007), that produce tissue damage and initiate extra inflammatory reactions (Moreno et al., 2006). Moreover, hemoglobin released from erythrocyte’s hemolysis activates the pro-inflammatory transcription factor (Moreno et al., 2006). Phospholipase A2 hydrolyzes EDNVs phospholipids resulted in production of inflammatory mediators (Alaarg et al., 2013). Also, NVs activate neutrophils that release myeloperoxidase as main source of ROS (Buesing et al., 2011). Altogether propagate vascular inflammation that accelerates the development of coronary heart diseases.
5.3. EDNVs and NO homeostasis
NVs might exert beneficial or deleterious effects for the vascular wall depending on their cellular origin (Puddu et al., 2010). Additionally, Gaceb et al. (2014), reported that EVs have contradictory effects on NO biology, and EVs decrease NO bioavailability by reducing the activity of endothelial NO synthase. However, EVs induced up-regulation of inducible NO synthase resulting in an over production of NO. Therefore, EVs can induce endothelial dysfunction by enhancement of ROS production that reduces NO bioavailability (Herring et al., 2013, França et al., 2015, Burnier et al., 2009). Similarly, it has been demonstrated that NVs disrupt NO homeostasis by oxidative stress mediated mechanism. Moreover, EDNVs are hemoglobin containing vesicles or hemoglobin devoid of vesicles, both vesicles enhanced NO inactivation (Donadee et al., 2011). It has been reported that, EDNVs scavenge NO faster than intact erythrocytes (Herring et al., 2013). Therefore, EDNVs are vasoconstrictors, increased erythrocyte adhesion, enhanced endothelial damage and caused vaso-occlusions (Camus et al., 2012).
Erythrocytes vesiculation was associated with releasing of arginase-1 and hemoglobin into blood stream (Donadee et al., 2011, Yang et al., 2013). Release of erythrocyte’s arginase was associated with lowered blood NO levels that induced erythrocyte dysfunction and endothelial damage (Kim et al., 2013). Beside erythrocyte’s arginase, their dimethylarginines and cationic amino transporter are important regulators of NO (Yokoro et al., 2012). Asymmetric dimethylarginine competes with L-arginine transport; however, symmetric dimethylarginine interferes with L-arginine into the cells (Davids et al., 2012, Eligini et al., 2013).
5.4. EDNVs and thrombosis
EDNVs have procoagulant activity by participation in blood clotting process and enhancing thrombus formation (Chung et al., 2007). These vesicles accelerate the coagulation cascade via conversion of prothrombin into thrombin (Martinez et al., 2005). The presence of PS on the surface of NVs provides a site for the assembly of the prothrombinase leading to formation of thrombin clot. Additionally, NVs can enhance clotting by inducing expression of clotting factors (Distler et al., 2005). Adhesion of PS-expressing EDNVs to endothelial cells induced vaso-occlusion. Moreover, PS-exposed NVs provide sites for adhesion of platelets and neutrophils localized at the subendothelium. Taken together, PS-exposed EDNVs are new players in the development of thrombosis that is a risk factor in cardiovascular disease progression (Noh et al., 2010).
5.5. EDNVs and foam cell formation
Erythrocyte life span is limited by senescence with subsequent clearance of the aged erythrocytes, prior to senescence; they undergo suicidal death or eryptosis (Lang et al., 2012). Phospholipid asymmetry is maintained throughout the life span of erythrocytes. Once the cell enters into eryptotic stage, PS was exposed on their outer surface and acted as a marker for macrophages to engulf aged erythrocytes; therefore, PS acts as signal for eryptosis and erythrophagocytosis (Kleinegris et al., 2012). Eryptosis shares other hallmarks of apoptosis, such as cell shrinkage, cell membrane blebbing and scrambling leading to PS exposure at the cell surface (Lang et al., 2012). EDNVs-PS acts as an “eat me” marker for macrophages for removing damaged erythrocytes (Willekens et al., 2008). Therefore, reticuloendothelial system removes the PS-exposing nanovesicles instead of their parent’s erythrocyte (Willekens et al., 2008).
Under oxidative stress erythrocytes are prone to subsequent vesiculation and production of NVs (Blum, 2009). The surface of Oxi-Ery is flagged by PS exposure on the outer leaflets. Therefore, they are recognized by macrophage scavenger receptors (Tziakas et al., 2010). This evokes foam cell formation (Schrijvers et al., 2007) and stimulates smooth muscle cell migration, which contributes to the progression of atherosclerosis (Schrijvers et al., 2007). The erythrocyte membrane is richer in cholesterol than other cells; therefore, intracellular free cholesterol accumulation is increased that triggers foam cells formation (Tziakas et al., 2010). Moreover, NVs stimulate inflammatory responses and growth of necrotic core (Loyer et al., 2014). Consequently, NVs contribute to the progression of atherosclerosis (Herring et al., 2013).
6. Isolation and detection of EVs
EVs separation is a major focus in the field of EVs research. The isolation processes of EVs include numerous sequential centrifugation steps with increasing centrifugal force. Thus size and density properties of EVs are used for the separation of EVs from other blood components. Initially, low-speed steps (300–500g) are applied to remove cells (Revenfeld et al., 2014). This is followed by higher speeds in the range of 10,000–20,000g, to remove cellular debris and to isolate larger EVs > 100 nm. Finally, one or more ultracentrifugation steps are applied to pellet the smallest EVs, using centrifugal forces in the range of 100,000–200,000g (Revenfeld et al., 2014).
It is also possible to combine the ultracentrifugation with a density gradient, or affinity purification. The choice of the isolation procedure may have a considerable effect on the downstream analyses of EVs. In addition to the isolation, the pre-analytical procedures such as choice of anticoagulant, processing temperature and storage before analysis may affect detection and characterization of NVs (Revenfeld et al., 2014).
Characterization of NVs with regard to size, morphology, concentration, biochemical composition and cellular origin has been documented (Herring et al., 2013). NVs surface markers allow identification and quantitation, although marker density must be sufficient to allow detection. Moreover, biochemical determinations of molecular components can allow enumeration in isolated EVs preparations (Pisetsky et al., 2012).
Assays of functional activity have also been used for certain NVs subpopulations. The presence of nucleic acids provides another means to measure NVs using dyes that interact with DNA and RNA. The use of nucleic acid-binding dyes can provide information on the cell of origin of EVs (Pisetsky et al., 2012). Furthermore, EVs can be characterized by the detection of the different cell surface antigens. These antigens reflect their origin and activation method (Burnier et al., 2009). Also, characterization of EVs requires complementary biochemical, mass spectrometry, and imaging techniques (Raposo and Stoorvogel, 2013). Isolation methods, centrifugation protocols, time and temperature between phlebotomy and initial centrifugation, thaw and freezing, reagent compositions, instrument settings and calibration affect EVs analysis (Jayachandran et al., 2012).
The common technologies widely applied for the detection of EVs include electron microscopy, flow cytometry, dynamic light scattering, and nanoparticle tracking analysis (Kastelowitz and Yin, 2014). The frequently used techniques in EVs detection are flow cytometry analysis, and enzyme-linked immunoassays. As well, fluorescence-based antibody array system can rapidly identify the cell origin of EVs. Despite several techniques are used in EVS characterization, the measurement of NVs still has standardization problems (Puddu et al., 2010).
7. Application of NVs
NVs are normally released from the organs into the blood stream and can be detected in the peripheral blood. Moreover, they can be detected in the urine and other body fluids (Fais et al., 2013). The circulating basal levels of EVs can be found in the blood of healthy individuals. However, an increase in their release may be considered as biomarker of biological changes; hence, they have possible diagnostic values (Antwi-Baffour et al., 2013A). Although some of these NVs populations occur in the blood of healthy individuals and patients, there are obvious changes in number, cellular origin, and composition in various disease states (Fais et al., 2013).
7.1. NVs as biomarkers for diseases
The release of NVs has been shown from endothelial cells, vascular smooth muscle cells, platelets, white blood cells, erythrocytes, neurons and cancer cells (Vanwijk et al., 2003). The concentration and composition of NVs depend upon their cellular origin and the stimuli that trigger their production (Jayachandran et al., 2012). Therefore, circulating NVs can be used as sensitive and specific biomarkers for diseases. NVs have been investigated for prognosis in cardiovascular diseases, paroxysmal nocturnal hemoglobinuria, heparin induced thrombocytopenia, sickle cell disease and sepsis (Jayachandran et al., 2012). Particularly, an increase of circulating NVs originating from blood cells was demonstrated as marker for atherosclerosis exacerbation (Cocucci et al., 2009). Furthermore, NVs were used as biomarker for neurodegenerative disorders, diabetes, rheumatic diseases, cancer and other diseases (Pap et al., 2009, Cocucci et al., 2009). NVs contain cytokines that can be used as indicator for inflammatory response (Distler et al., 2005). Increased level of EVs was documented in patients with neurological diseases (Colombo et al., 2012).
7.2. NVs as smart drug delivery tool
7.2.1. NVs as drug delivery cargo
EVs are promising biological drug delivery nano-carriers, and they could overcome restrictions of synthetic nanocarriers (Lai et al., 2013). They are well tolerated and could be delivering hydrophilic, hydrophobic drugs as well as biopharmaceuticals; therefore, EVs are suitable for the development of drug delivery cargo (Lai et al., 2013). EVs have ability to permeate plasma membrane of target cells, have an intrinsic ability to home target tissues, and their membrane can be amenable to improve specific cell targeting (Lai et al., 2013). Consequently, NVs can deliver various drugs to specific tissues, thereby reducing therapeutic doses and decreasing side effects (Sun et al., 2010). EVs are vectors which transfer therapeutic agents that maintain cell homeostasis, cell repair, and cardioprotective drugs. Hence, EVs have beneficial effects in the field of cardiovascular medicine and regenerative therapy (Fleury et al., 2014). Additionally, drug loaded NVs can be across blood–brain barrier to deliver their cargo to neurons (Lakhal and Wood, 2011). These biological carriers overcoming immunogenicity were associated with liposomes, nanoparticles and viral vectors (Fleury et al., 2014).
By mimicking EVs, artificial NVs are constructed from phospholipid precursors to form phospholipid bilayer membranous nanovesicles. They have amphipathic and biocompatibility properties. Artificial NVs are member of smart drug delivery systems, improve solubility, prolonged action, and reduced drug toxicity and increase cellular accumulation of drugs (Fleury et al., 2014). Despite, artificial NVs are attractive tools for drug delivery; they still have some biocompatibility problems such opsonization and recognition by immune system as foreign bodies (Lai et al., 2013, Elbialy and Mady, 2015). Therefore, they could be coated with hydrophilic polymers to reduce their recognition by opsonins and clearance by recto-endothelial system (Lai et al., 2013). This approach is partially solving the problem of nanoparticle opsonization. The production of biomimics of nanoparticle stealth is novel challenge in the field of drug delivery. EDNVs-coated nanoparticles for long-circulating cargo delivery may resolve the problem of nanoparticles opsonization (Hu et al., 2011).
7.2.2. EDNVs as nanoparticles stealth
In vivo administration of nanoparticles larger than 100 nm is opsonized and quickly eliminated from the circulation via the reticuloendothelial system (Cole et al., 2011). Therefore, production of camouflaged nanoparticles for long-circulating cargo delivery may resolve the problem of nanoparticles opsonization (Hu et al., 2011).
Erythrosomes are EDNVs, and they are biodegradable vesicles and non-immunogenic in autologous administration (Hu et al., 2011, Gupta et al., 2014). Nanoerythrosomes are prepared by extrusion of erythrocyte ghosts through filters of defined pore size. They are used as a novel drug delivery system and used as nanoparticles stealth to minimize nanoparticles clearance (Hu et al., 2011, Gupta et al., 2014).
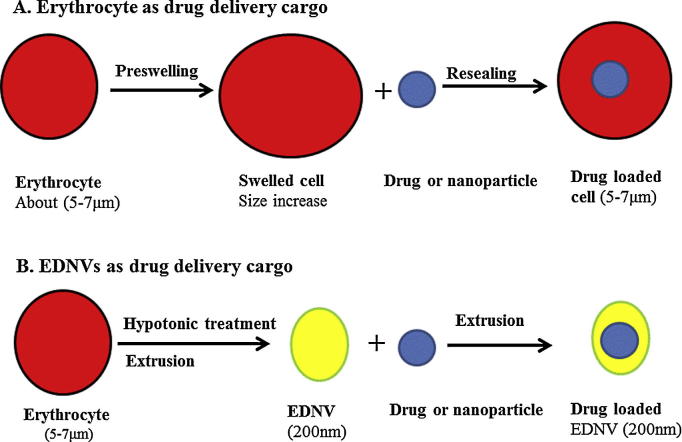
Nanoparticles stealth can be achieved using bioinspired nanoparticles with biomimetic camouflage coating such erythrocytes, erythrocyte ghosts and EDNVs (Balmert and Little, 2012, Noble et al., 2014). These biomimetic cargos have tremendous potential in drug delivery applications, as they provide the opportunity to actively inhibit the rapid immune clearance of their therapeutic cargo, thereby improving drug pharmacokinetics and therapeutic efficacy (Hu et al., 2013). Fig. 3 represents role of erythrocytes and EDNVs as drug delivery system and nanoparticle stealth.
Figure 3.
Erythrocyte and erythrocyte derived nanovesicles (EDNV) as drug delivery systems and as stealth for nanoparticles. As drug delivery system erythrocyte is used for sustained release, targeting, bioreactor and biomimics stealth. However, EDNV is mainly used as targeting and EDNV stealth vehicle. Preswelling is performed using hypotonic NaCl solution, whereas resealing step is achieved using hypertonic NaCl solution.
Also, these cargos can be delivered to specific tissues such as intravascular drug delivery, liver, spleen, lungs and bone marrow as well as selective tumor targeting (Muzykantov, 2010, Noble et al., 2014).
7.3. NVs as target for therapeutic agents
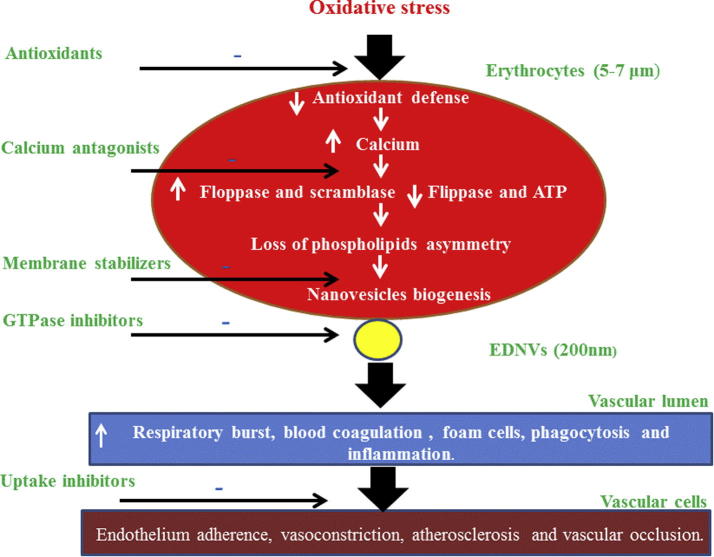
EVs are double weapon that has both valuable and unfavorable effects on the biological systems. Disruption of NVs biogenesis may elicit deleterious effects on cellular components. Consequently, approaches that minimize NVs biogenesis could represent novel therapeutic approach (Lovren and Verma, 2013). The design of NVs modulating therapy is based on inhibiting EVs biogenesis, inhibiting their cellular uptake, or inhibiting their active components (EL Andaloussi et al., 2013). Consequently, inhibition of ceramide biosynthesis decreases EVs biogenesis as well as GTPases inhibitors may inhibit EVs release. However, NVs cellular entrance is prohibited by blocking PS receptors and protein receptors on the receipt cells (EL Andaloussi et al., 2013, Mulcahy et al., 2014). Moreover, Mulcahy et al. (2014), listed several agents that inhibit EVs cellular entry such as heparin, chlorpromazine, amiloride, annexin-V, proton pump inhibitor and other agents (Mulcahy et al., 2014).
Statins have hypocholesterolemic effect and pleiotropic effects such as antioxidant, anti-proliferative, anti-thrombotic effects, inhibition of NADPH oxidase activity and inhibition of G-protein lipidation (Tramontano et al., 2004). Inhibition of G-protein signaling by statins was proposed as EVs mechanism (Loirand et al., 2013). As well statins inhibit lipid raft mediated endocytosis (Mulcahy et al., 2014).
It is well demonstrated that an increases of intracellular calcium, ROS, and apoptotic events are common causes in NVs production by most cells (Prati et al., 2010, Burnier et al., 2009). In this regard, calcium antagonists and ROS scavengers may elicit inhibitory effect on cellular vesiculation. Calcium channel blockers are an example of agent that attenuates EVs biogenesis (Lovren and Verma, 2013). Moreover, therapy by renin angiotensin inhibitor led to a decrease in EVs level (França et al., 2015). As well, lowering of oxidative stress lowered EVs level (Lovren and Verma, 2013). In this regard, Herring et al. (2013) reported that antioxidant therapy inhibits the NVs formation. Furthermore, reduction in oxidative stress resulted in decline of NVs biogenesis (França et al., 2015). Likewise, Stowell et al. (2013) demonstrated a significant decrease in EVs production by the addition of antioxidants to erythrocytes. Fig. 4 displays the possible effect of therapeutic agents on NVs biogenesis.
Figure 4.
Therapeutic interventions that modulate NVs biogenesis from erythrocytes.
It is important to emphasize that inhibition of EVs biogenesis could result in undesirable effects because EVs and their active components are important for the regulation of normal biological processes and other core cellular functions (EL Andaloussi et al., 2013).
8. Conclusion
EVs transmit biological signals that can implicate in numerous physiological and pathological processes. EVs are released in body fluids and can be used as potential biomarkers for diagnosis, prognosis and monitoring of the therapeutic efficacy. EDNVs can mediate harmful effects such as thrombosis, inflammation, endothelial dysfunction atherosclerosis and vaso-occlusion. Calcium antagonist, antioxidant and statins are proposed agents that may modulate EVs biogenesis. Despite EDNVs appear as new culprit that affects vascular functions, they can be used as smart drug delivery tool.
Acknowledgments
This Project was funded by the National Plan for Science, Technology and Innovation (MAARIFAH), King Abdulaziz City for Science and Technology, Kingdom of Saudi Arabia, Award Number (12-MED2563-02).
Footnotes
Peer review under responsibility of King Saud University.
References
- Alaarg A., Schiffelers R.M., van Solinge W.W., van Wijk R. Red blood cell vesiculation in hereditary hemolytic anemia. Front Physiol. 2013;4:365. doi: 10.3389/fphys.2013.00365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EL Andaloussi S., Mäger I., Mäger X.O., Wood M.J. Extracellular vesicles: biology and emerging therapeutic opportunities. Nat. Rev. Drug Discov. 2013;12(5):347–357. doi: 10.1038/nrd3978. [DOI] [PubMed] [Google Scholar]
- Antwi-Baffour S., Nyarkoah A.W., Kyeremeh R., Abdulai S.M. Plasma membrane-derived vesicles in sickle cell disease: a possible indicator of the continuous endothelial stimulation and/or injury to blood cells. Amer. J. Biomed. Life Sci. 2013;1(4):99–102. [Google Scholar]
- Antwi-Baffour S., Boafo A.O., Kyeremeh R., Mahmood S.A. Plasma Membrane-derived Vesicles (PMVs) in G6PD Deficient Patients. SOJ Immunol. 2013;1(1):4. [Google Scholar]
- Balmert S.C., Little S.R. Biomimetic delivery with micro- and nanoparticles. Adv. Mater. 2012;24(28):3757–3767. doi: 10.1002/adma.201200224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum A. The possible role of red blood cell microvesicles in atherosclerosis. Eur. J. Intern. Med. 2009;20(2):101–105. doi: 10.1016/j.ejim.2008.06.001. [DOI] [PubMed] [Google Scholar]
- Buesing K.L., Densmore J.C., Kaul S., Pritchard K.A., Jr, Jarzembowski J.A., Gourlay D.M., Oldham K.T. Endothelial microparticles induce inflammation in acute lung injury. J. Surg. Res. 2011;166(1):32–39. doi: 10.1016/j.jss.2010.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnier L., Fontana P., Kwak B., Angelillo-Scherrer A. Cell-derived microparticles in haemostasis and vascular medicine. Thromb. Haemost. 2009;101(3):439–451. [PubMed] [Google Scholar]
- Camus S.M., Gausserès B., Bonnin P., Loufrani L., Grimaud L. Erythrocyte microparticles can induce kidney vaso-occlusions in a murine model of sickle cell disease. Blood. 2012;120(25):5050–5058. doi: 10.1182/blood-2012-02-413138. [DOI] [PubMed] [Google Scholar]
- Chung S.M., Bae O.N., Lim K.M., Noh J.Y., Lee M.Y. Lysophosphatidic acid induces thrombogenic activity through phosphatidylserine exposure and procoagulant microvesicle generation in human erythrocytes. Arterioscler. Thromb. Vasc. Biol. 2007;27:414–421. doi: 10.1161/01.ATV.0000252898.48084.6a. [DOI] [PubMed] [Google Scholar]
- Cocucci E., Racchetti G., Meldolesi J. Shedding microvesicles: artefacts no more. Trends. Cell Biol. 2009;19(2):43–51. doi: 10.1016/j.tcb.2008.11.003. [DOI] [PubMed] [Google Scholar]
- Cole A.J., Yang V.C., David A.E. Cancer theranostics: the rise of targeted magnetic nanoparticles. Trends Biotechnol. 2011;29(7):323–332. doi: 10.1016/j.tibtech.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo E., Borgiani B., Verderio C., Furlan R. Microvesicles: novel biomarkers for neurological disorders. Front. Physiol. 2012;3:63. doi: 10.3389/fphys.2012.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davids M., van Hell A.J., Visser M., Nijveldt R.J., van Leeuwen P.A. Role of the human erythrocyte in generation and storage of asymmetric dimethylarginine. Am. J. Physiol. Heart Circ. Physiol. 2012;302:H1762–1770. doi: 10.1152/ajpheart.01205.2011. [DOI] [PubMed] [Google Scholar]
- Distler J.H., Pisetsky D.S., Huber L.C., Kalden J.R., Gay S., Distler O. Microparticles as regulators of inflammation: novel players of cellular crosstalk in the rheumatic diseases. Arthritis Rheum. 2005;52(11):3337–3348. doi: 10.1002/art.21350. [DOI] [PubMed] [Google Scholar]
- Donadee C., Raat N.J., Kanias T., Tejero J., Lee J.S. Nitric oxide scavenging by red blood cell microparticles and cell-free hemoglobin as a mechanism for the red cell storage lesion. Circulation. 2011;124(4):465–476. doi: 10.1161/CIRCULATIONAHA.110.008698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbialy N.S., Mady M.M. Ehrlich tumor inhibition using doxorubicin containing liposomes. Saudi. Pharm. J. 2015;23:182–187. doi: 10.1016/j.jsps.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eligini S., Porro B., Lualdi A., Squellerio I., Veglia F. Nitric oxide synthetic pathway in red blood cells is impaired in coronary artery disease. PLoS ONE. 2013;8(8):e66945. doi: 10.1371/journal.pone.0066945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fais S., Logozzi M., Lugini L., Federici C., Azzarito T. Exosomes: the ideal nanovectors for biodelivery. Biol. Chem. 2013;394(1):1–15. doi: 10.1515/hsz-2012-0236. [DOI] [PubMed] [Google Scholar]
- Fleury A., Martinez M.C., Le Lay S. Extracellular vesicles as therapeutic tools in cardiovascular diseases. Front. Immunol. 2014;5:370. doi: 10.3389/fimmu.2014.00370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- França, C.N., Izar, M.C., Amaral, J.B., Tegani, D.M., Fonseca, F.A., 2015. Microparticles as Potential Biomarkers of Cardiovascular Disease. Arq Bras Cardiol. pii: S0066-782X2015005040210. [DOI] [PMC free article] [PubMed]
- Gaceb A., Martinez M.C., Andriantsitohaina R. Extracellular vesicles: new players in cardiovascular diseases. Int. J. Biochem. Cell Biol. 2014;50:24–28. doi: 10.1016/j.biocel.2014.01.018. [DOI] [PubMed] [Google Scholar]
- Gupta N., Patel B., Ahsan F. Nano-engineered erythrocyte ghosts as inhalational carriers for delivery of fasudil: preparation and characterization. Pharm. Res. 2014;31(6):1553–1565. doi: 10.1007/s11095-013-1261-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herring J.M., McMichael M.A., Smith S.A. Microparticles in health and disease. J. Vet. Intern. Med. 2013;27(5):1020–1033. doi: 10.1111/jvim.12128. [DOI] [PubMed] [Google Scholar]
- Hu C.M., Zhang L., Aryal S., Cheung C., Fang R.H., Zhang L. Erythrocyte membrane-camouflaged polymeric nanoparticles as a biomimetic delivery platform. Proc. Natl. Acad. Sci. U.S.A. 2011;108(27) doi: 10.1073/pnas.1106634108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu C.M., Fang R.H., Luk B.T., Chen K.N., Carpenter C. Marker-of-self’ functionalization of nanoscale particles through a top-down cellular membrane coating approach. Nanoscale. 2013;5(7):2664–2668. doi: 10.1039/c3nr00015j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jank H., Salzer U. Vesicles generated during storage of red blood cells enhance the generation of radical oxygen species in activated neutrophils. Sci. World J. 2011;11:173–185. doi: 10.1100/tsw.2011.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayachandran M., Miller V.M., Heit J.A., Owen W.G. Methodology for isolation, identification and characterization of microvesicles in peripheral blood. J. Immunol. Methods. 2012;375(1–2):207–214. doi: 10.1016/j.jim.2011.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastelowitz N., Yin H. Exosomes and microvesicles: identification and targeting by particle size and lipid chemical probes. ChemBioChem. 2014;15(7):923–928. doi: 10.1002/cbic.201400043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennett E.C., Kuchel P.W. Plasma membrane oxidoreductases: effects on erythrocyte metabolism and redox homeostasis. Antioxid. Redox Signal. 2006;8:1241–1247. doi: 10.1089/ars.2006.8.1241. [DOI] [PubMed] [Google Scholar]
- Kim S.W., Cuong T.D., Hung T.M., Ryoo S., Lee J.H., Min B.S. Arginase II inhibitory activity of flavonoid compounds from Scutellaria indica. Arch. Pharm. Res. 2013;36(8):922–926. doi: 10.1007/s12272-013-0125-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinegris M.C., Koek G.H., Mast K., Mestrom E.H., Wolfs J.L., Bevers E.M. Ribavirin-induced externalization of phosphatidylserine in erythrocytes is predominantly caused by inhibition of aminophospholipid translocase activity. Eur. J. Pharmacol. 2012;693(1–3):1–6. doi: 10.1016/j.ejphar.2012.07.041. [DOI] [PubMed] [Google Scholar]
- Lai R.C., Yeo R.W., Tan K.H., Lim S.K. Exosomes for drug delivery – a novel application for the mesenchymal stem cell. Biotechnol. Adv. 2013;31(5):543–551. doi: 10.1016/j.biotechadv.2012.08.008. [DOI] [PubMed] [Google Scholar]
- Lakhal S., Wood M.J. Exosome nanotechnology: an emerging paradigm shift in drug delivery: exploitation of exosome nanovesicles for systemic in vivo delivery of RNAi heralds new horizons for drug delivery across biological barriers. BioEssays. 2011;33(10):737–741. doi: 10.1002/bies.201100076. [DOI] [PubMed] [Google Scholar]
- Lamichhane T.N., Sokic S., Schardt J.S., Raiker R.S., Lin J.W., Jay M. Emerging roles for extracellular vesicles in tissue engineering and regenerative medicine. Tissue Eng. Part B Rev. 2015;21(1):45–54. doi: 10.1089/ten.teb.2014.0300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang E., Qadri S.M., Lang F. Killing me softly – suicidal erythrocyte death. Int. J. Biochem. Cell Biol. 2012;44(8):1236–1243. doi: 10.1016/j.biocel.2012.04.019. [DOI] [PubMed] [Google Scholar]
- Larson M.C., Woodliff J.E., Hillery C.A., Kearl T.J., Zhao M. Phosphatidylethanolamine is externalized at the surface of microparticles. Biochim. Biophys. Acta. 2012;1821(12):1501–1507. doi: 10.1016/j.bbalip.2012.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y., El Andaloussi S., Wood M.J. Exosomes and microvesicles: extracellular vesicles for genetic information transfer and gene therapy. Hum. Mol. Genet. 2012;21(R1):R125–134. doi: 10.1093/hmg/dds317. [DOI] [PubMed] [Google Scholar]
- Loirand G., Sauzeau V., Pacaud P. Small G proteins in the cardiovascular system: physiological and pathological aspects. Physiol. Rev. 2013;93(4):1659–1720. doi: 10.1152/physrev.00021.2012. [DOI] [PubMed] [Google Scholar]
- Lovren F., Verma S. Evolving role of microparticles in the pathophysiology of endothelial dysfunction. Clin. Chem. 2013;59(8):1166–1174. doi: 10.1373/clinchem.2012.199711. [DOI] [PubMed] [Google Scholar]
- Loyer X., Vion A.C., Tedgui A., Boulanger C.M. Microvesicles as cell-cell messengers in cardiovascular diseases. Circ. Res. 2014;114(2):345–353. doi: 10.1161/CIRCRESAHA.113.300858. [DOI] [PubMed] [Google Scholar]
- Lutz H.U., Bogdanova A. Mechanisms tagging senescent red blood cells for clearance in healthy humans. Front Physiol. 2013;4:387. doi: 10.3389/fphys.2013.00387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez M.C., Tesse A., Zobairi F., Andriantsitohaina R. Shed membrane microparticles from circulating and vascular cells in regulating vascular function. Am. J. Physiol. Heart Circ. Physiol. 2005;288:H1004–H1009. doi: 10.1152/ajpheart.00842.2004. [DOI] [PubMed] [Google Scholar]
- Minetti M., Agati L., Malorni W. The microenvironment can shift erythrocytes from a friendly to a harmful behavior: pathogenetic implications for vascular diseases. Cardiovasc. Res. 2007;75(1):21–28. doi: 10.1016/j.cardiores.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Moreno P., Purushothaman K.R., Sirol M., Levy A.P., Fuster V. Neovascularization in human atherosclerosis. Circulation. 2006;2006(113):2245–2252. doi: 10.1161/CIRCULATIONAHA.105.578955. [DOI] [PubMed] [Google Scholar]
- Mulcahy L.A., Pink R.C., Carter R. Routes and mechanisms of extracellular vesicle uptake. J Extracell Vesicles. 2014;4:3. doi: 10.3402/jev.v3.24641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzykantov V.R. Drug delivery by red blood cells: vascular carriers designed by Mother Nature. Expert Opin. Drug Deliv. 2010;7(4):403–427. doi: 10.1517/17425241003610633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble G.T., Stefanick J.F., Ashley J.D., Kiziltepe T., Bilgicer B. Ligand-targeted liposome design: challenges and fundamental considerations. Trends Biotechnol. 2014;32(1):32–45. doi: 10.1016/j.tibtech.2013.09.007. [DOI] [PubMed] [Google Scholar]
- Noh J.Y., Lim K.M., Bae O.N., Chung S.M., Lee S.W. Procoagulant and prothrombotic activation of human erythrocytes by phosphatidic acid. Am. J. Physiol. Heart Circ. Physiol. 2010;299(2):H347–55. doi: 10.1152/ajpheart.01144.2009. [DOI] [PubMed] [Google Scholar]
- Pap E., Pállinger É., Pásztói M., Falus A. Highlights of a new type of intercellular communication: microvesicle-based information transfer. Inflammation Res. 2009;58(1):1–8. doi: 10.1007/s00011-008-8210-7. [DOI] [PubMed] [Google Scholar]
- Pisetsky D.S., Ullal A.J., Gauley J., Ning T.C. Microparticles as mediators and biomarkers of rheumatic disease. Rheumatology (Oxford) 2012;51(10):1737–1746. doi: 10.1093/rheumatology/kes028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porro B., Eligini S., Squellerio I., Tremoli E., Cavalca V. The red blood cell: a new key player in cardiovascular homoeostasis? Focus on the nitric oxide pathway. Biochem. Soc. Trans. 2014;42(4):996–1000. doi: 10.1042/BST20140122. [DOI] [PubMed] [Google Scholar]
- Prati C., Racadot E., Wendling D. Microparticles and inflammatory joint disease. Joint Bone Spine. 2010;77(6):496–508. doi: 10.1016/j.jbspin.2010.07.011. [DOI] [PubMed] [Google Scholar]
- Puddu P., Puddu G.M., Cravero E., Muscari S., Muscari A. The involvement of circulating microparticles in inflammation, coagulation and cardiovascular diseases. Can. J. Cardiol. 2010;26(4):140–145. doi: 10.1016/s0828-282x(10)70371-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raposo G., Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J. Cell Biol. 2013;200(4):373–383. doi: 10.1083/jcb.201211138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revenfeld A.L., Bæk R., Nielsen M.H., Stensballe A., Varming K., Jørgensen M. Diagnostic and prognostic potential of extracellular vesicles in peripheral blood. Clin. Ther. 2014;36(6):830–846. doi: 10.1016/j.clinthera.2014.05.008. [DOI] [PubMed] [Google Scholar]
- Schrijvers D.M., De Meyer G.R., Herman A.G., Martinet W. Phagocytosis in atherosclerosis: Molecular mechanisms and implications for plaque progression and stability. Cardiovasc. Res. 2007;73(3):470–480. doi: 10.1016/j.cardiores.2006.09.005. [DOI] [PubMed] [Google Scholar]
- Stowell S.R., Smith N.H., Zimring J.C., Fu X., Palmer A.F. Addition of ascorbic acid solution to stored murine red blood cells increases posttransfusion recovery and decreases microparticles and alloimmunization. Transfusion. 2013;53(10):2248–2257. doi: 10.1111/trf.12106. [DOI] [PubMed] [Google Scholar]
- Sun D., Zhuang X., Xiang X., Liu Y., Zhang S. A novel nanoparticle drug delivery system: the anti-inflammatory activity of curcumin is enhanced when encapsulated in exosomes. Mol. Ther. 2010;18(9):1606–1614. doi: 10.1038/mt.2010.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tissot J.-D., Canellini G., Rubin O., Angelillo-Scherrer A., Delobel J. Blood microvesicles: from proteomics to physiology. Translational Proteomic. 2013;1:38–52. [Google Scholar]
- Tramontano A.F., O’Leary J., Black A.D., Muniyappa R., Cutaia M.V., El-Sherif N. Statin decreases endothelial microparticle release from human coronary artery endothelial cells: implication for the Rhokinase pathway. Biochem. Biophys. Res. Commun. 2004;320:34–38. doi: 10.1016/j.bbrc.2004.05.127. [DOI] [PubMed] [Google Scholar]
- Tziakas D.N., Chalikias G.K., Stakos D., Boudoulas H. The role of red blood cells in the progression and instability of atherosclerotic plaque. Int. J. Cardiol. 2010;142(1):2–7. doi: 10.1016/j.ijcard.2009.10.031. [DOI] [PubMed] [Google Scholar]
- Vanwijk M., Vanbavel E., Sturk A., Nieuwland R. Microparticles in cardiovascular diseases. Cardiovasc. Res. 2003;59(2):277–287. doi: 10.1016/s0008-6363(03)00367-5. [DOI] [PubMed] [Google Scholar]
- Vlassov A.V., Magdaleno S., Setterquist R., Conrad R. Exosomes: current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials. Biochim. Biophys. Acta. 2012;1820(7):940–948. doi: 10.1016/j.bbagen.2012.03.017. [DOI] [PubMed] [Google Scholar]
- Willekens F.L., Were J.M., Groenen-Döpp Y.A., Roerdinkholder-Stoelwinder B., de Pauw B., Bosman G.J. Erythrocyte vesiculation: a self-protective mechanism? Br. J. Haematol. 2008;141(4):549–556. doi: 10.1111/j.1365-2141.2008.07055.x. [DOI] [PubMed] [Google Scholar]
- Yang J., Gonon A.T., Sjöquist P.O., Lundberg J.O., Pernow J. Arginase regulates red blood cell nitric oxide synthase and export of cardioprotective nitric oxide bioactivity. Proc. Natl. Acad. Sci. U.S.A. 2013;110(37):15049–15054. doi: 10.1073/pnas.1307058110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoro M., Suzuki M., Murota K., Otsuka C., Yamashita H. Asymmetric dimethylarginine, an endogenous NOS inhibitor, is actively metabolized in rat erythrocytes. Biosci. Biotechnol. Biochem. 2012;76:1334–1342. doi: 10.1271/bbb.120086. [DOI] [PubMed] [Google Scholar]