Abstract
Unintentional and uncontrollable processing of threat has been suggested to contribute to the pathology of social anxiety disorder (SAD). The present study investigated the neural correlates of processing task-irrelevant, highly ecologically valid, disorder-related stimuli as a function of symptom severity in SAD. Twenty-four SAD patients and 24 healthy controls (HC) performed a feature-based comparison task during functional magnetic resonance imaging, while task-irrelevant, disorder-related or neutral scenes were presented simultaneously at a different spatial position. SAD patients showed greater activity than HC in response to disorder-related versus neutral scenes in brain regions associated with self-referential processing (e.g. insula, precuneus, dorsomedial prefrontal cortex) and emotion regulation (e.g. dorsolateral prefrontal cortex (dlPFC), inferior frontal gyrus). Symptom severity was positively associated with amygdala activity, and negatively with activation in dorsal anterior cingulate cortex and dlPFC in SAD patients. Additional correlation analysis revealed that amygdala-prefrontal coupling was positively associated with symptom severity. A network of brain regions is thus involved in SAD patients' processing of task-irrelevant, complex, ecologically valid, disorder-related scenes. Furthermore, increasing symptom severity in SAD patients seems to reflect a growing imbalance between neural mechanisms related to stimulus-driven bottom-up and regulatory top-down processes resulting in dysfunctional regulation strategies.
Keywords: Social phobia, Bottom-up, Top-down, Correlation analysis, Amygdala
Highlights
-
•
SAD patients process task-irrelevant disorder-related scenes automatically.
-
•
Automatic threat processing in SAD involves a broad emotion network of brain regions.
-
•
Symptom severity has a great impact on automatic disorder-related processing.
-
•
Amygdala activity increases with higher levels of symptom severity.
1. Introduction
Social anxiety disorder (SAD) describes the pathological fear of negative evaluation by other people. Patients suffering from SAD are characterized by anxiety in social interactions (e.g. small talk on parties, discussions) and performance situations (e.g. giving a speech, job interview) (American Psychiatric Association, 2013). With a prevalence rate of 7–13% in Western countries (Furmark, 2002) and 12.1% in the USA (Kessler et al., 2005), SAD is one of the most frequent anxiety disorders.
Automatic threat processing, that is, the attentional capture by, and the detection and processing of, threat stimuli that are outside the current attentional focus and/or task-irrelevant (Carretié, 2014, Moors and De Houwer, 2006), is considered a critical factor for the development and maintenance of SAD and other anxiety disorders (Bar-Haim et al., 2007, Morrison and Heimberg, 2013, Öhman and Mineka, 2001). Automatic processing as defined here is often operationalized by engaging participants in a main task with neutral stimuli, while threat stimuli are presented simultaneously, but remain task-irrelevant (Carretié, 2014). According to biased-competition models, the extent to which task-irrelevant stimuli are processed is strongly mediated by both top-down control and stimulus-driven bottom-up mechanisms (Beck and Kastner, 2009). Thus, unintentional processing of task-irrelevant threat stimuli may be caused by their strong exogenous influence on attention and enhanced sensory processing (bottom-up), which seems to be associated with increased amygdala activity in anxiety. Additionally, attentional control (top-down) may be reduced, due to altered prefrontal functioning (Bishop, 2008, Connor et al., 2004, Eysenck and Derakshan, 2011, Ochsner and Gross, 2005, Öhman, 2005). This imbalance may well be aggravated with increasing anxiety (Bishop, 2009, Bishop et al., 2004b, Cisler and Koster, 2010, Eysenck and Derakshan, 2011), rendering the processing of threat more unintentional and uncontrollable, which represent two important indicators of automatic information processing (Bargh, 1994, Teachman et al., 2012).
Most functional imaging studies on brain responses during automatic processing of task-irrelevant threat stimuli in SAD presented emotional faces, which were judged with respect to emotion-irrelevant aspects such as gender discrimination (Blair et al., 2008a, Campbell et al., 2007, Gentili et al., 2008, Stein et al., 2002, Straube et al., 2004). Other studies either used gender judgment on stimuli with emotional prosody (Quadflieg et al., 2008), disorder-related words in grammatical decision (Schmidt et al., 2010) or in an emotional Stroop task (Boehme et al., 2015). These studies particularly reported amygdala hyperactivation and less consistent hyperactivations in the insula, prefrontal regions (e.g. orbitofrontal cortex, dorsolateral prefrontal cortex (dlPFC)), anterior cingulate cortex (ACC), and superior temporal sulcus (STS) in SAD patients, suggesting emotional encoding even when the task does not focus on stimulus valence. These findings are in large parts compatible to those obtained when attention is not focused elsewhere (e.g. Heitmann et al., 2016, Klumpp et al., 2012, Straube et al., 2005). These studies without attentional restrictions present a neural network including amygdala, thalamus, insula, globus pallidus, ACC, mid-cingulate cortex (MCC), posterior cingulate cortex (PCC), precuneus, STS, cuneus, medial prefrontal cortex (mPFC), and lateral prefrontal cortex (lPFC), associated with increased threat detection, abnormal self-referential processing and interoception in SAD patients (Brühl et al., 2014, Etkin and Wager, 2007, Freitas-Ferrari et al., 2010, Miskovic and Schmidt, 2012).
However, previous studies on automatic processing in SAD did not use a visually separated feature-based attention task with emotionally neutral stimuli (in the presence of task-irrelevant emotional distractor stimuli), or used emotional stimuli that were only partially relevant for SAD, such as faces (Schulz et al., 2013) or words, which are limited in their ecological validity. Thus, the question arises how patients with SAD process highly ecologically valid disorder-related stimuli, when these are task-irrelevant and presented spatially separate from the task stimuli. This situation, often encountered in real life outside the laboratory, is implemented in concurrent but distinct target-distractor (CDTD) tasks (Carretié, 2014). Previous studies in healthy participants (HC) could show that processing of task-irrelevant stimuli, although presented at a central position, is significantly affected by a spatially non-overlapping main task (e.g. Mocaiber et al., 2010, Nordström and Wiens, 2012, Sand and Wiens, 2011, Wiens et al., 2012, Wiens et al., 2011). This task configuration allows to investigate to which degree task-irrelevant emotional stimuli capture attention, and are processed even at task-irrelevant locations (Wiens et al., 2012).
The present study investigated neural correlates of such automatic, disorder-related scene processing in SAD patients and HC. We used visually complex, disorder-related scenes that depict situations SAD patients are afraid of (and neutral control scenes). We used such scenes as task-irrelevant stimuli in an attention-demanding CDTD task. The task-irrelevant scenes were presented at the center of the screen and the emotionally neutral task-stimuli above and below the scene.
Additionally, the influence of symptom severity was examined with correlation analysis. We expected increased automatic threat processing in SAD patients, reflected by hyperactivation in the regions related to affective processing in SAD (amygdala, insula, thalamus, globus pallidus, cingulate cortex, precuneus, STS and prefrontal cortex) (Brühl et al., 2014, Etkin and Wager, 2007, Freitas-Ferrari et al., 2010, Miskovic and Schmidt, 2012), relative to HC (interaction of Scene Type by Group: SAD patients > HC, disorder-related scenes > neutral scenes). Furthermore, we expected hyperactivations in SAD patients to increase with increasing symptom severity. Finally, based on biased-competition models suggesting diminished attentional control depending on interindividual differences in anxiety vulnerability (Bishop, 2008), we expected increasing symptom severity in SAD patients to be accompanied by reduced activation in prefrontal regions.
2. Materials and methods
2.1. Subjects
SAD patients were recruited via public notices, local paper ads and from a collaborating outpatient clinic. HC were selected from a volunteer database of the Collaborative Research Center “Fear, Anxiety, Anxiety Disorders” (TRR SFB 58; http://sfbtrr58.uni-muenster.de/) or were recruited by means of flyers and newspaper ads. All participants had normal or corrected-to-normal vision, were right-handed (Oldfield, 1971), met the general MRI-requirements, had no history of neurological diseases or psychotic disorders, did not currently take psychotropic medication, and were screened by a psychologist using the standardized clinical interview (SCID; Wittchen et al., 1997). SAD patients fulfilled the criteria for current generalized social anxiety disorder according to DSM-IV as main diagnosis. HC were free of any diagnosis. All participants completed the clinician-administered Liebowitz-Social-Anxiety-Scale (LSAS; Stangier and Heidenreich, 2005), Social Phobia Scale (SPS; Stangier et al., 1999b), Social Interaction Anxiety Scale (SIAS; Stangier et al., 1999a), Fragebogen zur Selbstbeschreibung in sozialen Situationen (FSSS; Kolbeck, 2008), Social Phobia Anxiety Inventory (SPAI; Fydrich, 2003), and Fear of Negative Evaluation Scale (FNE; Vormbrock and Neuser, 1983). To address depressive symptomatology all participants filled in the Beck Depression Inventory (BDI; Hautzinger et al., 1995).
Out of 33 SAD patients, nine were excluded from statistical analysis due to a BDI score > 30 (n = 2), missing behavioral responses or technical problems (n = 2), misunderstanding of the behavioral task (n = 1), or < 90% correct answers in the behavioral task (n = 4). Matched to the 24 SAD patients (17 female), a control group consisting of 24 HC (16 female), who had ≥ 90% correct answers in the behavioral task, was chosen. Patients and HC groups did not differ in gender, mean age, and educational attainment (see Table 1 for sample details).
Table 1.
Mean age, mean educational attainment (years), and mean scores (± standard deviation) for social anxiety-related questionnaires (LSAS, SPS, SIAS, FSSS, SPAI, FNE) and Beck Depression Inventory (BDI) for patients suffering from social anxiety disorder (SAD) and healthy controls (HC).
| SAD (M ± SD) |
HC (M ± SD) |
t-value |
P-value (2-tailed) |
|
|---|---|---|---|---|
| Age | 27.29 ± 7.69 | 27.38 ± 5.77 | − 0.042 | 0.966 |
| Education | 12.88 ± 1.30 | 13.38 ± 1.14 | − 1.422 | 0.162 |
| LSAS | 64.13 ± 16.32 | 9.67 ± 6.93 | 15.050 | ≤ 0.001 |
| SPS | 31.38 ± 9.90 | 2.17 ± 2.94 | 13.850 | ≤ 0.001 |
| SIAS | 45.88 ± 14.03 | 10.13 ± 6.77 | 11.243 | ≤ 0.001 |
| FSSS | 1.80 ± 0.39 | 0.37 ± 0.27 | 14.841 | ≤ 0.001 |
| SPAI | 3.72 ± 0.76 | 0.58 ± 0.57 | 16.200 | ≤ 0.001 |
| FNE | 62.00 ± 8.72 | 31.83 ± 6.47 | 13.617 | ≤ 0.001 |
| BDI | 10.54 ± 7.32 | 1.50 ± 2.99 | 5.602 | ≤ 0.001 |
Note: M = Mean; SD = standard deviation; LSAS = Liebowitz Social Anxiety Scale; SPS = Social Phobia Scale; SIAS = Social Interaction Anxiety Scale; FSSS = Fragebogen zur Selbstbeschreibung in sozialen Situationen; SPAI = Social Phobia and Anxiety Inventory; FNE = Fear of Negative Evaluation Scale; BDI = Beck Depression Inventory.
Comorbid diagnoses in SAD patients (n = 9, multiple entries possible) were current Major Depression Episode (n = 2), specific phobia (n = 7), Obsessive Compulsive Disorder (n = 1), and General Anxiety Disorder (n = 1). As expected, SAD patients scored higher than HC in all social anxiety-sensitive questionnaires (Table 1). BDI scores were also significantly increased in SAD patients, but remained under the clinical significance level, and were comparable to scores from other studies (e.g. Straube et al., 2004).
The study conforms to the Declaration of Helsinki and was approved by the ethics committee of the University of Muenster, Germany. Written informed consent was obtained from each participant prior to the experiment. Participants received monetary compensation for participation.
2.2. Experimental design
Fifty disorder-related scenes and 50 matched neutral scenes from the Social Anxiety Picture Set Muenster (SAPS-M; see Heitmann et al. (2016) for a detailed description of the properties of the stimulus set and the rating procedure) were used in this study. Disorder-related scenes depict situations SAD patients are afraid of, for example giving a speech, a job interview, a discussion scene, or show persons with bodily symptoms due to anxiety. As in Heitmann et al. (2016), SAD patients in the current study rated the disorder-related versus neutral scenes as more unpleasant, arousing, and anxiety-inducing compared to HC (all P < 0.001, see Supplement ST1 and SF1).
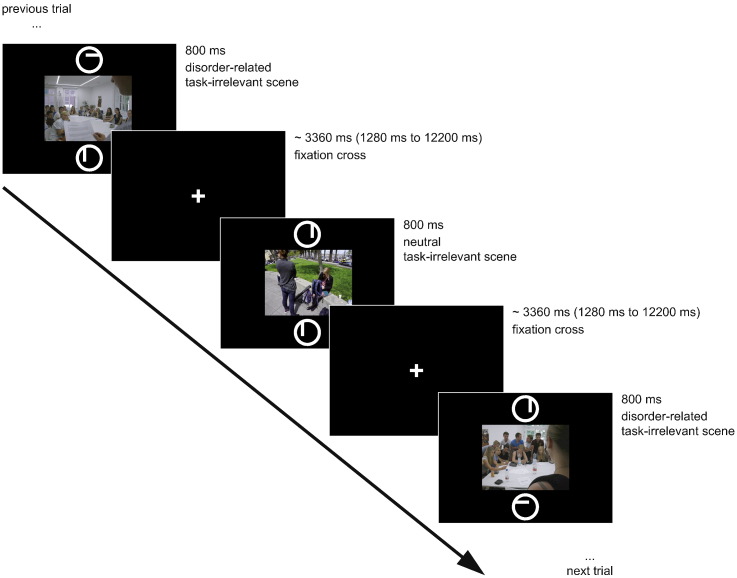
The 50 disorder-related and 50 neutral scenes (with a resolution of 600 dpi, degree of visual angle: 6.3° × 4.73°) were each shown once in the center of a black screen for 800 ms in randomized order. Concurrently, two circles were presented, one above and one below the picture (distance between centers of circles: 7.79°), each with a horizontal or vertical bar inside (see Fig. 1). Participants had to solve a feature-based comparison task (in the style of CDTD-task; Carretié, 2014) and to push the left button on a response box when the bars in the circles had the same orientation, and the right button for bars with different orientation. The presented scenes were irrelevant for the task.
Fig. 1.
Example of an experimental trial.
Stimulus presentation and response recording was controlled by Presentation software (version 17.2, Neurobehavioral Systems, Albany, CA, USA). Between stimulus presentations, a white fixation cross was shown for an average time period of 3360 ms (jittered between 1280 ms and 12,200 ms; see Fig. 1). To increase signal discriminability (Dale, 1999), the random stimulus sequence was optimized using the “optimal sequencing” (optseq) algorithm (http://www.surfer.nmr.mgh.harvard.edu/optseq/). The duration of the experiment was approximately 7 min.
2.3. Behavioral data
To assess potential differences between SAD patients and HC in task performance, errors and reaction times (RT) were analyzed. Incorrect answers, missing responses, trials with contradictory responses, outliers in RT (RT > mean + 2 SD; RT < 300 ms) were counted as errors. Only RTs of correct trials were included and log-transformed to compensate for skewness. Error and RT data were analyzed by means of repeated measures analyses of variance (ANOVAs) with emotion (disorder-related or neutral) as within-subjects factor and group (SAD or HC) as between-subjects factor. For the ANOVAs, a probability level of P ≤ 0.05 was considered statistically significant. Post-hoc t-tests used to resolve interactions were Bonferroni-corrected for multiple comparisons (corrected significance level P < 0.0125). Furthermore, correlational analyses between symptom severity and number of errors as well as RTs were conducted. To account for the extensive and comprehensive symptomatology of SAD, a composite symptom severity score was calculated by averaging the standardized (z-transformed) scores in the six social anxiety-sensitive questionnaires used.
2.4. Functional MRI data
Anatomical and functional MRI data were acquired with a 3 T magnetic resonance scanner (“Magnetom PRISMA”, Siemens, Erlangen; GER) using a 20 channel head-neck coil. Functional data were measured using a T2*-weighted echo-planar sequence (TE = 30 ms, flip angle = 90°, matrix = 92 × 92 voxels, FOV = 208 mm2, TR = 2080 ms). 215 volumes of 36 axial slices (thickness = 3 mm, 0.3 mm gap, in plane resolution = 2.26 mm × 2.26 mm) were acquired. To minimize susceptibility artifacts in inferior parts of anterior brain areas, the volumes were tilted approximately 20° from the AC/PC line. A shimming field was applied before functional imaging, to reduce external magnetic-field inhomogeneities. A high-resolution T1-weighted anatomical volume with 192 slices was also recorded.
Pre-processing and analysis of functional data were performed using Brain Voyager QX software (version 2.4, Brain Innovation, Maastricht, NL). The first ten volumes were discarded from analysis to secure steady-state tissue magnetization. Volumes were realigned to the first volume, to minimize effects of head movements on data analysis. No participant showed excessive head movement (> 1 voxel). Further data preprocessing comprised spatial (6 mm full-width half-maximum isotropic Gaussian kernel, FWHMK) as well as temporal (low pass filter: 2.8 s; high pass filter: 10 cycles in time course) smoothing. Anatomical and functional images were co-registered and normalized to Talairach space (Talairach and Tournoux, 1988). Finally, volumes were resampled to voxels of 2 × 2 × 2 mm and slice time correction was applied.
Multiple linear regression of the signal time course at each voxel was calculated. The expected blood oxygenation level-dependent (BOLD) signal change for each predictor was modeled by a canonical double-gamma hemodynamic response function (HRF). In accordance with the behavioral analysis, only trials without errors (see above for definition of errors) were included in the functional analysis. The two predictors of interest were disorder-related scene and neutral scene (factor Scene Type). First, voxel-wise statistical maps were generated and predictor estimates (beta weights) were computed for each individual. Second, a random-effect group analysis of the individual contrasts was calculated.
Amygdala (dilated 1 mm in radius), insula (dilated 1 mm in radius), thalamus, globus pallidus, cingulate cortex, PFC, STS, precuneus, and cuneus served as Regions of Interest (ROIs), defined a priori according to Automated Anatomical Labeling atlas (Maldjian et al., 2003, Tzourio-Mazoyer et al., 2002) and transformed into Talairach space according to Lancaster et al. (2007) using ICBM2TAL in Matlab (version 8.2, The MathWorks Inc., Natick, MA). Masks of all ROIs were combined into a single mask. In addition, we conducted an exploratory whole brain analysis to investigate reliable task-related activations outside the ROIs. Furthermore, a correlation between the composite symptom severity score and brain activity in the defined ROIs during processing of disorder-related versus neutral scenes was calculated in SAD patients. To resolve the relationship between amygdala and prefrontal cortex, amygdala-prefrontal functional connectivity was correlated with symptom severity in SAD patients. Psychophysiological interaction (PPI) analysis was performed to explore emotion-dependent connectivity patterns between amygdala and prefrontal cortex in SAD patients for the contrast disorder-related > neutral scenes. The anatomical ROI for left amygdala for this contrast was defined as seed region. PPI analysis was conducted with an interaction regressor that is the product of the HRF-convolved task regressor (psychological factor) and the seed region time course (physiological factor). In a second step, the relationship between amygdala-prefrontal cortex functional connectivity and symptom severity was analyzed by means of correlation analysis. Brodmann areas (BA) for all significant activation clusters were identified with the Talairach client (Lancaster et al., 1997, Lancaster et al., 2000).
Statistical parametric maps resulting from voxel-wise analyses were considered significant for clusters that survived cluster-based correction for multiple comparisons. For small-volume corrected analysis, a voxel-level threshold was initially set to P ≤ 0.005 (uncorrected, Lieberman and Cunningham, 2009), for whole-brain analysis to P ≤ 0.001. Cluster thresholds were calculated using a cluster-level statistical threshold plugin in Brain Voyager (Goebel et al., 2006) and carried out across the ROI mask (small-volume correction) and the whole-brain mask. Correction criteria were always based on the estimate of the maps' spatial smoothness and on an iterative procedure (Monte Carlo simulation) used to estimate cluster-level false-positive rates (Forman et al., 1995). After 1000 iterations, the minimum cluster size threshold that yielded a cluster-level false-positive rate of 5% was applied to the statistical maps.
3. Results
3.1. Behavioral data: Scene Type and Group
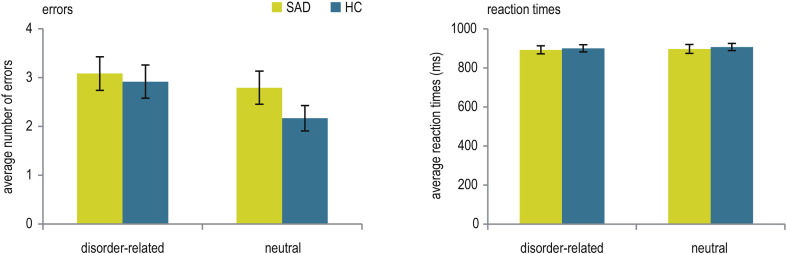
Analysis of errors yielded neither significant main effects nor a significant interaction (all P ≥ 0.118). The average number of errors was 5.88 ± 2.071 in SAD patients, and 5.08 ± 1.283 in HC (see Fig. 2). Thus, for both groups, ≥ 94% of all trials were included in fMRI data analysis. Similarly, analysis of RT in correct trials of the bar comparison task yielded neither significant main effects nor a significant interaction (all P ≥ 0.144; see Fig. 2). Correlational analyses in patients between the composite symptom-severity scores and number of errors (P ≥ 0.342) or RT (P ≥ 0.403) yielded no significant effects.
Fig. 2.
Mean (± standard error) number of errors and mean reaction times in correct trials during the feature-based geometric comparison task in patients suffering from social anxiety disorder (SAD) and healthy controls (HC).
3.2. fMRI data: interaction Scene Type by Group
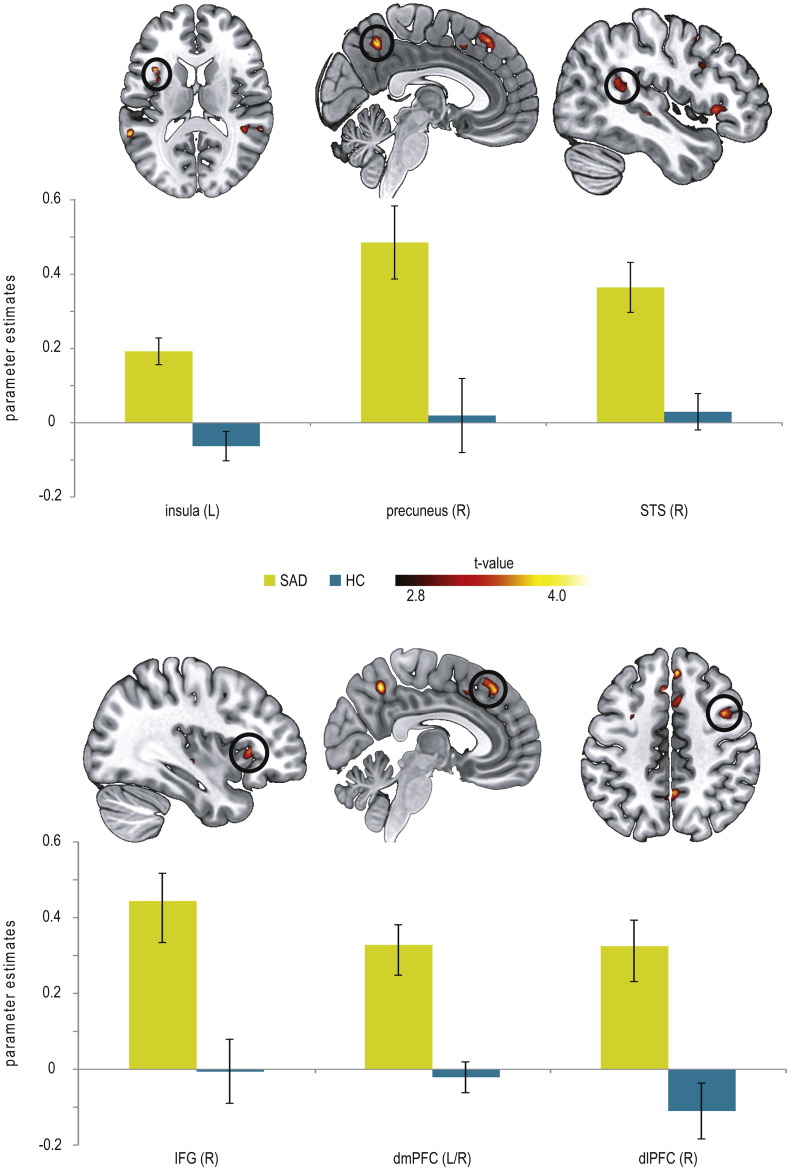
Small-volume corrected analysis for the contrast disorder-related versus neutral scenes showed increased brain activation in SAD patients as compared to HC in the insula (BA13), STS (BA13, BA22), precuneus (BA7), and several frontal regions (BA6, BA8, BA9, BA32, BA45; see Table 2; Fig. 3).
Table 2.
Significant hyperactivations for disorder-related versus neutral scenes in patients suffering from social anxiety disorder (SAD) relative to healthy controls (HC) as revealed by small-volume corrected analysis (P ≤ 0.005 uncorrected, and P ≤ 0.05 corrected).
| Region | Lateralization | Talairach coordinates of peak voxel |
Cluster size (mm3) | Maximum t-value | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Insula (BA13) | L | − 37 | 12 | 15 | 403 | 4.275 |
| Precuneus (BA7) | R | 4 | − 53 | 48 | 663 | 4.107 |
| STS (BA13) | R | 46 | − 46 | 19 | 721 | 3.389 |
| STS (BA22) | L | − 61 | − 46 | 15 | 480 | 4.103 |
| STS (BA13) | L | 43 | − 26 | − 5 | 103 | 3.835 |
| IFG (BA45) | R | 36 | 24 | 3 | 799 | 3.526 |
| dmPFC (BA8) | L/R | 5 | 36 | 46 | 1270 | 4.012 |
| dmPFC (BA32) | R | 8 | 20 | 42 | 315 | 3.681 |
| dlPFC (BA9) | R | 46 | 12 | 29 | 97 | 3.053 |
| dlPFC (BA6) | R | 39 | 6 | 46 | 837 | 3.695 |
| dlPFC (BA6) | R | 17 | 3 | 55 | 196 | 3.125 |
Note: STS = superior temporal sulcus; IFG = inferior frontal gyrus; dmPFC = dorsomedial prefrontal gyrus; dlPFC = dorsolateral prefrontal cortex.
Fig. 3.
Differential brain activations during automatic disorder-related versus neutral scene processing in patients suffering from social anxiety disorder (SAD) as compared to healthy controls (HC) yielded by small-volume corrected analysis (P < 0.005 uncorrected, P < 0.05 corrected, L = left; R = right). SAD patients display enhanced activation in the insula (z = 14), precuneus (x = 4), superior temporal gyrus (STS; x = 46), inferior frontal gyrus (IFG; x = 36), dorsomedial prefrontal cortex (dmPFC; x = 5), and dorsolateral prefrontal cortex (dlPFC; z = 46) compared with HC. Diagrams show contrasts of parameter estimates (disorder-related versus neutral; mean ± s.e.).
Results of the exploratory whole-brain analysis are provided in Table 3. Activation patterns revealed by whole-brain analysis were largely consistent with activation clusters yielded by small-volume corrected analysis. Whole-brain analysis revealed additional activations in temporal lobe (BA13) and parahippocampal gyrus.
Table 3.
Significant hyperactivations for disorder-related versus neutral scenes in patients suffering from social anxiety disorder (SAD) relative to healthy controls (HC) as revealed by whole-brain analysis (P ≤ 0.001 uncorrected, and P ≤ 0.05 corrected).
| Region | Lateralization | Talairach coordinates of peak voxel |
Cluster size (mm3) | Maximum t-value | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Insula (BA13) | L | − 37 | 12 | 15 | 90 | 4.275 |
| STS (BA22) | L | − 61 | − 46 | 15 | 185 | 4.103 |
| Precuneus (BA7) | R | 4 | − 53 | 48 | 182 | 4.107 |
| Temporal lobe (BA13) | R | 41 | − 21 | − 9 | 295 | 4.608 |
| Parahippocampal gyrus | R | 24 | − 28 | − 2 | 53 | 3.798 |
| IFG (BA45) | R | 36 | 24 | 3 | 48 | 3.526 |
| IFG (BA47) | R | 44 | 21 | 0 | 52 | 3.458 |
| dmPFC (BA32) | R | 8 | 20 | 42 | 51 | 3.681 |
| dmPFC (BA8) | R | 5 | 36 | 46 | 186 | 4.012 |
| dlPFC (BA6) | R | 39 | 6 | 46 | 153 | 3.695 |
Note: IFG = inferior frontal gyrus; STS = superior temporal sulcus; IFG = inferior frontal gyrus; dmPFC = dorsomedial prefrontal gyrus; dlPFC = dorsolateral prefrontal cortex.
3.3. fMRI data: correlation analysis
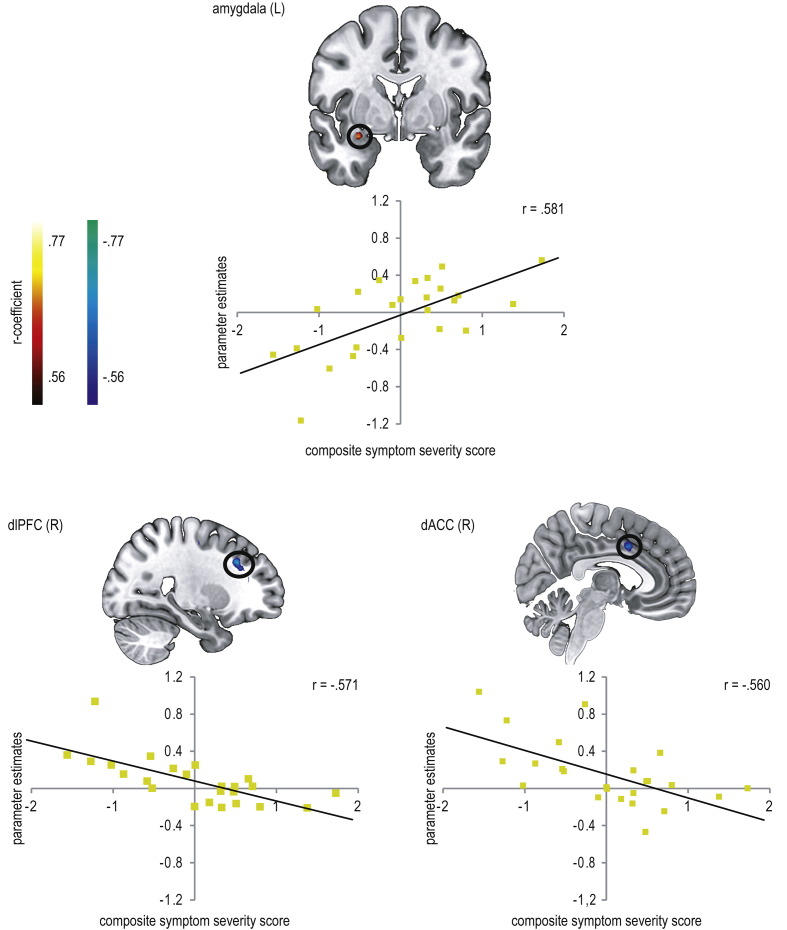
Correlation analysis of brain activity within ROIs were calculated with a composite symptom severity score in patients, yielding higher activity in the amygdala and decreasing activity in the dorsolateral PFC (dlPFC; BA8, BA9, BA10), dorsal anterior cingulate cortex (dACC; BA32), posterior midcingulate cortex (pMCC; BA31), and STS (BA13) with increasing symptom severity (see Table 4, Fig. 4).
Table 4.
Significant correlation of beta-weights (disorder-related versus neutral scenes) with a composite symptom severity score of six social anxiety-sensitive questionnaires in patients suffering from social anxiety disorder (SAD) as revealed by small-volume corrected analysis (P ≤ 0.005 uncorrected, and P ≤ 0.05 corrected).
| Region | Lateralization | Talairach coordinates of peak voxel |
Cluster size (mm3) | Average r-value | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| amygdala | L | − 30 | − 1 | − 16 | 131 | 0.58 |
| dACC (BA32) | R | 5 | 8 | 38 | 305 | − 0.56 |
| pMCC (BA31) | L | − 16 | − 21 | 37 | 118 | − 0.585 |
| STS (BA13) | R | 44 | − 45 | 16 | 213 | − 0.557 |
| dlPFC (BA9) | R | 46 | 3 | 26 | 384 | − 0.559 |
| dlPFC (BA10) | R | 32 | 35 | 11 | 133 | − 0.548 |
| dlPFC (BA8) | R | 25 | 25 | 34 | 403 | − 0.571 |
| dlPFC (BA9) | L | − 23 | 33 | 21 | 167 | − 0.578 |
Note: dACC = dorsal anterior cingulate cortex; pMCC = posterior midcingulate cortex, STS = superior temporal sulcus; dlPFC = dorsolateral prefrontal cortex.
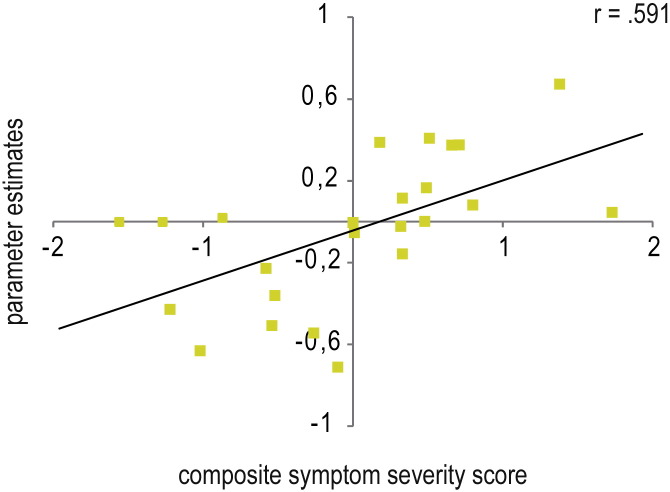
Fig. 4.
Correlation between parameter estimates (disorder-related versus neutral) and composite symptom severity scores in patients suffering from social anxiety disorder (SAD) (P < 0.005 uncorrected, P < 0.05 corrected, L = left; R = right). SAD patients showed a positive correlation between symptom severity and brain activation in the amygdala (y = − 1) and negative correlations between symptom severity and brain activation in the dorsolateral prefrontal cortex (dlPFC; x = 25) and dorsal anterior cingulate cortex (dACC; x = 5). Diagrams display the average correlation.
To clarify the relationship between symptom severity and brain activation in SAD patients, amygdala-prefrontal functional connectivity (seed: left amygdala) was correlated with symptom severity (see Table 5). This analysis yielded a positive correlation between symptom severity and amygdala-prefrontal coupling, with less affected SAD patients yielding more negative coupling, and thus an inverse relation between prefrontal activation and amygdala activation, and more severely affected SAD patients showing rather more positive coupling between these brain regions (see Fig. 5).
Table 5.
Significant correlation of beta weights (disorder-related versus neutral scenes) resulting from PPI analysis (with left amygdala as seed region) and symptom severity in patients suffering from social anxiety disorder (SAD) (P ≤ 0.005 uncorrected, and P ≤ 0.05 corrected).
| Region | Lateralization | Talairach coordinates of peak voxel |
Cluster size (mm3) | Average r-value | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| vlPFC (BA44) | R | 49 | 17 | 6 | 120 | 0.562 |
| dlPFC (BA9) | R | 41 | 15 | 30 | 784 | 0.591 |
| dlPFC (BA6) | R | 29 | 6 | 60 | 96 | 0.551 |
| dlPFC (BA6) | R | 24 | 5 | 43 | 120 | 0.560 |
| dlPFC (BA6) | L | − 37 | 1 | 34 | 256 | 0.591 |
| dlPFC/dmPFC (BA8) | R | 18 | 21 | 47 | 120 | 0.549 |
| dlPFC/vlPFC (BA13) | L | − 36 | 5 | 16 | 80 | 0.537 |
Note: BA = Brodmann area; vlPFC = ventrolateral prefrontal cortex; dlPFC = dorsolateral prefrontal cortex; dmPFC = dorsomedial prefrontal cortex.
Fig. 5.
Average correlation of beta weights (disorder-related versus neutral scenes) resulting from PPI analysis (with left amygdala as seed region) in a dorsolateral prefrontal cluster (x = 41, y = 15, z = 30, 784 voxel) and symptom severity in patients suffering from social anxiety disorder (SAD).
4. Discussion
The present study examined the neural correlates of unintentional and uncontrollable processing of disorder-related scenes and the influence of symptom severity in SAD patients by means of fMRI. For this purpose, SAD patients and HC had to solve a CDTD task (Carretié, 2014), in which the spatial orientation of bars at different screen positions had to be judged. At the same time, task-irrelevant disorder-related or neutral control scenes were presented. SAD patients and HC did not differ in task performance on the behavioral level, so behavioral differences cannot account for differences in brain activation. However, relative to HC, SAD patients exhibited increased activation in the insula, precuneus, STS and in several prefrontal regions during presentation of disorder-related versus neutral, task-irrelevant scenes. Moreover, correlation analysis in SAD patients showed that increasing symptom severity goes along with greater amygdala activity and decreased brain activity in prefrontal regions paralleled by a positive correlation between symptom severity and amygdala-prefrontal coupling. These results suggest enhanced automatic emotional processing of disorder-related scenes in SAD in a broad neural network. Furthermore, differences in bottom-up and top-down emotional processes seem to be determined by dysfunctions in down-regulatory mechanisms associated with varying levels of symptom severity. In the following, the results will be discussed in detail.
Task-irrelevant disorder-related versus neutral scenes evoked hyperactivations in the insula, precuneus, STS, dmPFC, IFG, and dlPFC in SAD patients compared with HC, as shown by small-volume corrected analysis. These brain regions are considered to be part of the neural network involved in affective processing in SAD (Brühl et al., 2014, Etkin and Wager, 2007, Freitas-Ferrari et al., 2010, Miskovic and Schmidt, 2012). Their activation was observed especially under conditions without attentional restriction during emotional stimulus encoding (Heitmann et al., 2016, Klumpp et al., 2012, Straube et al., 2005), but some regions (insula, STS) also during automatic processing, such as with facial affect in gender discrimination tasks (Blair et al., 2008a, Boehme et al., 2015, Gentili et al., 2008, Straube et al., 2004).
Our results particularly indicate heightened self-referential processing in SAD patients in response to disorder-related versus neutral scenes, although the scenes were task-irrelevant and outside the focus of attention. Insula hyperactivation in particular was often reported in SAD studies (e.g. Amir et al., 2005, Gentili et al., 2009, Straube et al., 2004). It is associated with increased interoceptive processing and awareness of the subjective feeling of anxiety (Critchley et al., 2004, Menon and Uddin, 2010, Paulus and Stein, 2006), both assumed to be key elements in the pathophysiology of SAD (Clark and Wells, 1995, Rapee and Heimberg, 1997). Next to the insula, precuneus hyperactivation in SAD patients can also be interpreted as indicator of increased internal self-presentation and self-referential processing, involving episodic memory retrieval (Cavanna and Trimble, 2006). Note that precuneus activation was reported in SAD patients before (e.g. Gentili et al., 2015a, Gentili et al., 2015b, Gentili et al., 2009, Heitmann et al., 2016), but, to our knowledge, not yet under conditions of automatic processing. Together with the precuneus, the dmPFC is assumed to represent a critical structure of the Default-Mode Network (DMN) that plays an important role in self-referential processing as well as in emotion regulation (Raichle et al., 2001, Schneider et al., 2008). Accordingly, mPFC hyperactivation is a common outcome in studies on affective processing in SAD, and is specifically attributed to enhanced self-focus and self-relevant memory retrieval (Blair et al., 2008b, Gusnard et al., 2001, Heitmann et al., 2016, Moran et al., 2009, Northoff et al., 2006).
In addition to the structures described, which are mainly associated with self-referential processing, the present study also yielded hyperactivation of the STS in SAD patients during automatic disorder-related processing. STS hyperactivation in SAD patients was reported for emotional face processing (Gentili et al., 2015a, Gentili et al., 2008, Straube et al., 2005). STS activation is associated with social perception of visual cues, s in face perception and motion processing (Allison et al., 2000, Hein and Knight, 2008), and in the present study probably indicate the automatic processing of social attributes of disorder-related scenes. However, in addition to attentional capture by social stimulus attributes, STS activation is also taken to reflect top-down attentional control during attentional tasks, e.g. during irrelevant emotional face processing (Corbetta et al., 1998, Hopfinger et al., 2000, Narumoto et al., 2001, Tseng et al., 2014). Similarly, activations in the PFC, in particular dlPFC and IFG, may indicate that SAD patients engaged in top-down regulation and control of emotion concerning the distracting stimuli, to ensure appropriate task performance. First, the IFG is involved in a wide range of inhibitory cognitive control mechanisms, as required in CDTD tasks (Aron, 2007, Ochsner and Gross, 2005, Tabibnia et al., 2011). Next, quite a few studies demonstrated dlPFC activation for emotion regulation and top-down attentional control (Cohen et al., 2000, MacDonald et al., 2000, Ochsner and Gross, 2005). In line with these observations, altered activation in prefrontal regions was reported in SAD, for example during emotion regulation (Brühl et al., 2013, Goldin et al., 2009, Ziv et al., 2013).
Whole-brain analysis supported the results of small-volume corrected analysis, and yielded additional hyperactivations in temporal lobe and parahippocampal gyrus in SAD patients. The often reported amygdala hyperactivation as correlate of initial threat detection during automatic processing (Bishop, 2008, Schulz et al., 2013) could not be confirmed by differential analysis in SAD patients in our study, but emerged in correlation analysis.
Correlation analysis yielded a strong positive correlation between symptom severity and brain activation in the left amygdala. In contrast, symptom severity correlated inversely, thus negatively, with activation in dlPFC, dACC, pMCC, and STS. The amygdala is described as a threat detector of incoming information (Bishop, 2008, Sergerie et al., 2008). It is assumed that amygdala activity contributes to facilitated attention and vigilance potentiation towards the salient stimulus, and to a subsequent initiation of autonomic and behavioral responses (Janak and Tye, 2015, LeDoux, 2007, Öhman, 2005, Phelps and LeDoux, 2005). These functions of the amygdala explain its role in bottom-up, stimulus-driven processing during uncontrollable and unintentional stimulus processing and studies reporting heightened amygdala activity during automatic processing in SAD patients confirm this (e.g. Boehme et al., 2015, Campbell et al., 2007, Gentili et al., 2008, Schmidt et al., 2010, Straube et al., 2004). Bottom-up emotional processing is stimulus-focused and depends on stimulus features such as personal significance (McRae et al., 2012, Öhman and Mineka, 2001). When differences in bottom-up processing strength are reflected by amygdala activation, individual relevance of stimuli should matter. The personal relevance of the disorder-related scenes used here is probably more pronounced in more severely affected patients, who accordingly exhibit stronger bottom-up processing. This interpretation is supported by studies in SAD patients reporting amygdala activity (Blair et al., 2008) or behavioral indices of attentional bias in dot-probe tasks (e.g. Pishyar et al., 2008, Stevens et al., 2009) to correlate with symptom severity. However, our behavioral data do not support this, since there was no correlation with symptom severity in reaction times, error or rating data. But note also that the reported studies used a different kind of experimental design to investigate attentional bias in SAD. Furthermore, effects of automatic processing are not necessarily observable on the behavioral level (Schulz et al., 2013, Teachman et al., 2012) and the rating procedure unlike the fMRI paradigm required an explicit emotion evaluation.
Biased competition models of attention suggest automatic stimulus processing to be mediated by bottom-up mechanisms as well as top-down attentional control (Beck and Kastner, 2009). Prefrontal regions such as dlPFC, IFG, and ACC are considered highly relevant for attentional control (Aron, 2007, Bishop, 2008, Bishop et al., 2004a, Ochsner and Gross, 2005). In the present study, differential analysis showed hyperactivations of dlPFC and IFG in SAD patients versus HC during disorder-related, task-irrelevant processing, which were interpreted as indicators of enhanced regulatory processing for disorder-related versus neutral scenes. Correlation analysis revealed that for disorder-related versus neutral scenes, prefrontal activation (dlPFC, dACC) decreased with increasing symptom severity in SAD patients. It should be noted that the same regions, which are involved in several executive control functions, also show increased activation in explicitly anxiety-inducing designs (Boehme et al., 2014, Crottaz-Herbette and Menon, 2006, Etkin et al., 2011, Straube et al., 2006, Straube et al., 2007), the role of activations seems to differ depending on explicit and implicit emotional experimental designs. While explicit tasks induce increased top-down processing of highly salient emotional stimuli, more or less adaptive anxiety responses and general behavioral activation, implicit tasks require stronger executive control and inhibition of emotional distractor processing. Prefrontal activation, including activation in dACC helps to amplify task-relevant activation and to diminish task-irrelevant activation (Bishop, 2008, Crottaz-Herbette and Menon, 2006).
The present correlational results might be interpreted in terms of Bishop's (2008) model, in which decreased prefrontal activation is taken to reflect impoverished attentional control of task-irrelevant emotional information. In addition, more severely affected patients might exhibit low abilities to cope with emotional distress, for example by means of attentional control. Both impairments might explain the correlational results, and fit with studies reporting decreasing attentional control with increasing anxiety in HC (Bishop, 2009, Bishop et al., 2004a, Bishop et al., 2007).
Furthermore, prefrontal regions are suggested to mediate emotional processing due to down-regulation of the amygdala (Kim et al., 2011a, Ochsner and Gross, 2005, Quirk and Beer, 2006), which might explain the inverse association between brain activity in these brain regions and symptom severity in SAD patients. This assumption is confirmed by the correlation analysis, showing amygdala-prefrontal coupling to be positively correlated with symptom severity, so that less affected SAD patients exhibited more negative connectivity, and more severely affected SAD patients more positive connectivity. Negative connectivity means a negative correlation in activation strength depending on stimulus condition, which might be related to suppression of amygdala activation by prefrontal cortex to disorder-related scenes (Gee et al., 2013). In line with this, less affected SAD patients exhibited even greater amygdala activity for neutral than for disorder-related scenes, which might be a result of down-regulation within the process of effective attentional control. In contrast, positive connectivity would relate to positively correlated activation increases in both regions depending on stimulus condition, and might indicate unsuccessful attempts to (down)-regulate amygdala responses and thus impaired regulatory functioning (e.g. Gee et al., 2013, Kim et al., 2011b).
Some limitations of the current studies need to be mentioned. Performance data did not yield an interference effect in SAD patients, suggesting a dissociation between behavioral and neural data. This finding is not uncommon, and brain-activation data can inform on processing of distractors, even if these distractors do not overtly interfere with the main task. Nevertheless, future studies could investigate designs that are associated with measurable attentional biases in more detail, even though it should be noted that the reliability of attentional bias scores is low (Schulz et al., 2013).
On the basis of the current fMRI data, it is not possible to decide whether our stimulus arrangement induced one automatic fixation of the scene, or whether participants engaged in several short fixations, for example when switching between the task stimuli. Thus, further investigations are needed to shed light on the specific mechanisms underlying task-irrelevant threat processing. Eye-tracking would allow to determine whether and how task-irrelevant stimuli are fixated, and what impact fixation has on disorder-related scene processing in a CDTD task design as used here.
Finally, our disorder-related stimuli contain more scenes in which the observer perspective was self-related (i.e. looking over someone's shoulder or being in the situation) than the neutral scenes, which was an intended manipulation. Future studies might investigate whether self-relatedness is a driving factor that is differentially processed in SAD as compared to HC.
5. Conclusions
In sum, results indicate that SAD patients process ecologically valid, disorder-related stimuli automatically, even when these are task-irrelevant and presented at a different spatial location than the task. The higher activations observed in SAD patients point to emotional processing mechanisms associated with particular higher cognitive functions (e.g. self-referential processing, emotion regulation) although the disorder-related stimuli were task-irrelevant. Furthermore, results suggest that increasing symptom severity is characterized by a growing imbalance between neural mechanisms related to stimulus-driven bottom-up and regulatory top-down processes that is mediated by an impaired regulatory amygdala-prefrontal coupling.
The following are the supplementary data related to this article.
Mean valence, arousal and anxiety ratings for disorder-related and neutral scenes in patients suffering from social anxiety disorder (SAD) and healthy controls (HC). Asterisks mark significant differences (P ≤ 0.05).
Supplementary figure.
Funding
The present study was supported by research grants awarded by German Research Society (Deutsche Forschungsgemeinschaft, DFG; SFB/TRR 58: C06, C07).
Acknowledgement
The authors report no financial relationships with commercial interests.
References
- Allison T., Puce A., McCarthy G. Social perception from visual cues: role of the STS region. Trends Cogn. Sci. 2000;4(7):267–278. doi: 10.1016/s1364-6613(00)01501-1. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . fifth ed. American Psychiatric Publishing; Arlington, VA: 2013. Diagnostic and Statistical Manual of Mental Disorders. [Google Scholar]
- Amir N., Klumpp H., Elias J., Bedwell J.S., Yanasak N., Miller L.S. Increased activation of the anterior cingulate cortex during processing of disgust faces in individuals with social phobia. Biol. Psychiatry. 2005;57(9):975–981. doi: 10.1016/j.biopsych.2005.01.044. [DOI] [PubMed] [Google Scholar]
- Aron A.R. The neural basis of inhibition in cognitive control. Neuroscientist. 2007;13(3):214–228. doi: 10.1177/1073858407299288. [DOI] [PubMed] [Google Scholar]
- Bargh J.A. The four horsemen of automaticity: awareness, intention, efficiency, and control in social cognition. In: Wyer R.S., Srull T.K., editors. Handbook of Social Cognition. Lawrence Erlbaum Associates, Inc.; Hillsdale, NJ, England: 1994. pp. 1–40. [Google Scholar]
- Bar-Haim Y., Lamy D., Pergamin L., Bakermans-Kranenburg M.J., van IJzendoorn M.H. Threat-related attentional bias in anxious and nonanxious individuals: a meta-analytic study. Psychol. Bull. 2007;133(1):1–24. doi: 10.1037/0033-2909.133.1.1. [DOI] [PubMed] [Google Scholar]
- Beck D.M., Kastner S. Top-down and bottom-up mechanisms in biasing competition in the human brain. Vis. Res. 2009;49(10):1154–1165. doi: 10.1016/j.visres.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop S.J. Neural mechanisms underlying selective attention to threat. Ann. N. Y. Acad. Sci. 2008;1129(1):141–152. doi: 10.1196/annals.1417.016. [DOI] [PubMed] [Google Scholar]
- Bishop S.J. Trait anxiety and impoverished prefrontal control of attention. Nat. Neurosci. 2009;12(1):92–98. doi: 10.1038/nn.2242. [DOI] [PubMed] [Google Scholar]
- Bishop S.J., Duncan J., Brett M., Lawrence A.D. Prefrontal cortical function and anxiety: controlling attention to threat-related stimuli. Nat. Neurosci. 2004;7(2):184–188. doi: 10.1038/nn1173. [DOI] [PubMed] [Google Scholar]
- Bishop S.J., Duncan J., Lawrence A.D. State anxiety modulation of the amygdala response to unattended threat-related stimuli. J. Neurosci. Off. J. Soc. Neurosci. 2004;24(46):10364–10368. doi: 10.1523/JNEUROSCI.2550-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop S.J., Jenkins R., Lawrence A.D. Neural processing of fearful faces: effects of anxiety are gated by perceptual capacity limitations. Cereb. Cortex. 2007;17(7):1595–1603. doi: 10.1093/cercor/bhl070. [DOI] [PubMed] [Google Scholar]
- Blair K., Geraci M., Devido J., McCaffrey D., Chen G., Vythilingam M.…Pine D.S. Neural response to self- and other referential praise and criticism in generalized social phobia. Arch. Gen. Psychiatry. 2008;65(10):1176–1184. doi: 10.1001/archpsyc.65.10.1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair K., Shaywitz J., Smith B.W., Rhodes R., Geraci M., Jones M.…Pine D.S. Response to emotional expressions in generalized social phobia and generalized anxiety disorder: evidence for separate disorders. Am. J. Psychiatry. 2008;165(9):1193–1202. doi: 10.1176/appi.ajp.2008.07071060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehme S., Mohr A., Becker M.P., Miltner W.H., Straube T. Area-dependent time courses of brain activation during video-induced symptom provocation in social anxiety disorder. Biol. Mood Anxiety Disord. 2014;4:6. doi: 10.1186/2045-5380-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehme S., Ritter V., Tefikow S., Stangier U., Strauss B., Miltner W.H.R., Straube T. Neural correlates of emotional interference in social anxiety disorder. PLoS ONE. 2015;10(6) doi: 10.1371/journal.pone.0128608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brühl A.B., Delsignore A., Komossa K., Weidt S. Neuroimaging in social anxiety disorder—a meta-analytic review resulting in a new neurofunctional model. Neurosci. Biobehav. Rev. 2014;47:260–280. doi: 10.1016/j.neubiorev.2014.08.003. [DOI] [PubMed] [Google Scholar]
- Brühl A.B., Herwig U., Delsignore A., Jäncke L., Rufer M. General emotion processing in social anxiety disorder: neural issues of cognitive control. Psychiatry Res. 2013;212(2):108–115. doi: 10.1016/j.pscychresns.2012.05.006. [DOI] [PubMed] [Google Scholar]
- Campbell D.W., Sareen J., Paulus M.P., Goldin P.R., Stein M.B., Reiss J.P. Time-varying amygdala response to emotional faces in generalized social phobia. Biol. Psychiatry. 2007;62(5):455–463. doi: 10.1016/j.biopsych.2006.09.017. [DOI] [PubMed] [Google Scholar]
- Carretié L. Exogenous (automatic) attention to emotional stimuli: a review. Cogn. Affect. Behav. Neurosci. 2014;14(4):1228–1258. doi: 10.3758/s13415-014-0270-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanna A.E., Trimble M.R. The precuneus: a review of its functional anatomy and behavioural correlates. Brain J. Neurol. 2006;129(3):564–583. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- Cisler J.M., Koster E.H.W. Mechanisms of attentional biases towards threat in the anxiety disorders: an integrative review. Clin. Psychol. Rev. 2010;30(2):203. doi: 10.1016/j.cpr.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark D.M., Wells A. A cognitive model of social phobia. In: Heimberg R., Liebowitz M., Hope D.A., Schneier F.R., editors. Social Phobia: Diagnosis, Assessment and Treatment. Guilford Press; New York: 1995. pp. 69–93. [Google Scholar]
- Cohen J.D., Botvinick M., Carter C.S. Anterior cingulate and prefrontal cortex: who's in control? Nat. Neurosci. 2000;3(5):421–423. doi: 10.1038/74783. [DOI] [PubMed] [Google Scholar]
- Connor C.E., Egeth H.E., Yantis S. Visual attention: bottom-up versus top-down. Curr. Biol. 2004;14(19):R850–R852. doi: 10.1016/j.cub.2004.09.041. [DOI] [PubMed] [Google Scholar]
- Corbetta M., Akbudak E., Conturo T.E., Snyder A.Z., Ollinger J.M., Drury H.A.…Shulman G.L. A common network of functional areas for attention and eye movements. Neuron. 1998;21(4):761–773. doi: 10.1016/s0896-6273(00)80593-0. [DOI] [PubMed] [Google Scholar]
- Critchley H.D., Wiens S., Rotshtein P., Ohman A., Dolan R.J. Neural systems supporting interoceptive awareness. Nat. Neurosci. 2004;7(2):189–195. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- Crottaz-Herbette S., Menon V. Where and when the anterior cingulate cortex modulates attentional response: combined fMRI and ERP evidence. J. Cogn. Neurosci. 2006;18(5):766–780. doi: 10.1162/jocn.2006.18.5.766. [DOI] [PubMed] [Google Scholar]
- Dale A.M. Optimal experimental design for event-related fMRI. Hum. Brain Mapp. 1999;8(2–3):109–114. doi: 10.1002/(SICI)1097-0193(1999)8:2/3<109::AID-HBM7>3.0.CO;2-W. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A., Egner T., Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn. Sci. 2011;15(2):85–93. doi: 10.1016/j.tics.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A., Wager T.D. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am. J. Psychiatr. 2007;164(10):1476–1488. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eysenck M.W., Derakshan N. New perspectives in attentional control theory. Personal. Individ. Differ. 2011;50(7):955–960. [Google Scholar]
- Forman S.D., Cohen J.D., Fitzgerald M., Eddy W.F., Mintun M.A., Noll D.C. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn. Reson. Med. 1995;33(5):636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Freitas-Ferrari M.C., Hallak J.E.C., Trzesniak C., Filho A.S., Machado-de-Sousa J.P., Chagas M.H.N.…Crippa J.A.S. Neuroimaging in social anxiety disorder: a systematic review of the literature. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2010;34(4):565–580. doi: 10.1016/j.pnpbp.2010.02.028. [DOI] [PubMed] [Google Scholar]
- Furmark T. Social phobia: overview of community surveys. Acta Psychiatr. Scand. 2002;105(2):84–93. doi: 10.1034/j.1600-0447.2002.1r103.x. [DOI] [PubMed] [Google Scholar]
- Fydrich T. Hogrefe; Göttingen: 2003. Soziale Phobie und Angstinventar (SPAI). Deutschsprachige Adaptation des “Social Phobia and Anxiety Inventory” von Turner und Beidel. [Google Scholar]
- Gee D.G., Humphreys K.L., Flannery J., Goff B., Telzer E.H., Shapiro M.…Tottenham N. A developmental shift from positive to negative connectivity in human amygdala-prefrontal circuitry. J. Neurosci. Off. J. Soc. Neurosci. 2013;33(10):4584–4593. doi: 10.1523/JNEUROSCI.3446-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentili C., Cristea I.A., Angstadt M., Klumpp H., Tozzi L., Phan K.L., Pietrini P. Beyond emotions: a meta-analysis of neural response within face processing system in social anxiety. Exp. Biol. Med. 2015 doi: 10.1177/1535370215603514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentili C., Gobbini M.I., Ricciardi E., Vanello N., Pietrini P., Haxby J.V., Guazzelli M. Differential modulation of neural activity throughout the distributed neural system for face perception in patients with Social Phobia and healthy subjects. Brain Res. Bull. 2008;77(5):286–292. doi: 10.1016/j.brainresbull.2008.08.003. [DOI] [PubMed] [Google Scholar]
- Gentili C., Ricciardi E., Gobbini M.I., Santarelli M.F., Haxby J.V., Pietrini P., Guazzelli M. Beyond amygdala: Default Mode Network activity differs between patients with Social Phobia and healthy controls. Brain Res. Bull. 2009;79(6):409–413. doi: 10.1016/j.brainresbull.2009.02.002. [DOI] [PubMed] [Google Scholar]
- Gentili C., Vanello N., Cristea I., David D., Ricciardi E., Pietrini P. Proneness to social anxiety modulates neural complexity in the absence of exposure: a resting state fMRI study using Hurst exponent. Psychiatry Res. 2015;232(2):135–144. doi: 10.1016/j.pscychresns.2015.03.005. [DOI] [PubMed] [Google Scholar]
- Goebel R., Esposito F., Formisano E. Analysis of functional image analysis contest (FIAC) data with brainvoyager QX: from single-subject to cortically aligned group general linear model analysis and self-organizing group independent component analysis. Hum. Brain Mapp. 2006;27(5):392–401. doi: 10.1002/hbm.20249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldin P.R., Manber T., Hakimi S., Canli T., Gross J.J. Neural bases of social anxiety disorder: emotional reactivity and cognitive regulation during social and physical threat. Arch. Gen. Psychiatry. 2009;66(2):170–180. doi: 10.1001/archgenpsychiatry.2008.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusnard D.A., Akbudak E., Shulman G.L., Raichle M.E. Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proc. Natl. Acad. Sci. 2001;98(7):4259–4264. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hautzinger M., Bailer M., Worall H., Keller F. 2. Auflage. Hans Huber; Bern: 1995. Beck-Depressions-Inventar (BDI). Testhandbuch. [Google Scholar]
- Hein G., Knight R.T. Superior temporal sulcus — it's my area: or is it? J. Cogn. Neurosci. 2008;20(12):2125–2136. doi: 10.1162/jocn.2008.20148. [DOI] [PubMed] [Google Scholar]
- Heitmann C.Y., Feldker K., Neumeister P., Zepp B.M., Peterburs J., Zwitserlood P., Straube T. Abnormal brain activation and connectivity to standardized disorder-related visual scenes in social anxiety disorder. Hum. Brain Mapp. 2016;37(4):1559–1572. doi: 10.1002/hbm.23120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopfinger J.B., Buonocore M.H., Mangun G.R. The neural mechanisms of top-down attentional control. Nat. Neurosci. 2000;3(3):284–291. doi: 10.1038/72999. [DOI] [PubMed] [Google Scholar]
- Janak P.H., Tye K.M. From circuits to behaviour in the amygdala. Nature. 2015;517(7534):284–292. doi: 10.1038/nature14188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler R.C., Berglund P., Demler O., Jin R., Merikangas K.R., Walters E.E. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch. Gen. Psychiatry. 2005;62(6):593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Kim M.J., Gee D.G., Loucks R.A., Davis F.C., Whalen P.J. Anxiety dissociates dorsal and ventral medial prefrontal cortex functional connectivity with the amygdala at rest. Cereb. Cortex. 2011;21(7):1667–1673. doi: 10.1093/cercor/bhq237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M.J., Loucks R.A., Palmer A.L., Brown A.C., Solomon K.M., Marchante A.N., Whalen P.J. The structural and functional connectivity of the amygdala: from normal emotion to pathological anxiety. Behav. Brain Res. 2011;223(2):403–410. doi: 10.1016/j.bbr.2011.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klumpp H., Angstadt M., Phan K.L. Insula reactivity and connectivity to anterior cingulate cortex when processing threat in generalized social anxiety disorder. Biol. Psychol. 2012;89(1):273–276. doi: 10.1016/j.biopsycho.2011.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolbeck S. University of Hamburg; Eine empirische Studie an nichtklinischen und klinischen Stichproben: 2008. Zur psychometrischen Differenzierbarkeit von sozialen Ängsten und sozialen Defiziten.http://ediss.sub.uni-hamburg.de/volltexte/2008/3642/ Retrieved from. [Google Scholar]
- Lancaster J.L., Rainey L.H., Summerlin J.L., Freitas C.S., Fox P.T., Evans A.C.…Mazziotta J.C. Automated labeling of the human brain. Hum. Brain Mapp. 1997;5(4):238–242. doi: 10.1002/(SICI)1097-0193(1997)5:4<238::AID-HBM6>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster J.L., Woldorff M.G., Parsons L.M., Liotti M., Freitas C.S., Rainey L.…Fox P.T. Automated Talairach atlas labels for functional brain mapping. Hum. Brain Mapp. 2000;10(3):120–131. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster J.L., Tordesillas-Gutiérrez D., Martinez M., Salinas F., Evans A., Zilles K.…Fox P.T. Bias between MNI and Talairach coordinates analyzed using the ICBM-152 brain template. Hum. Brain Mapp. 2007;28(11):1194–1205. doi: 10.1002/hbm.20345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux J. The amygdala. Curr. Biol. 2007;17(20):R868–R874. doi: 10.1016/j.cub.2007.08.005. [DOI] [PubMed] [Google Scholar]
- Lieberman M.D., Cunningham W.A. Type I and Type II error concerns in fMRI research: re-balancing the scale. Soc. Cogn. Affect. Neurosci. 2009;4(4):423–428. doi: 10.1093/scan/nsp052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald A.W., Cohen J.D., Stenger V.A., Carter C.S. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science. 2000;288(5472):1835–1838. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- Maldjian J.A., Laurienti P.J., Kraft R.A., Burdette J.H. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. NeuroImage. 2003;19(3):1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- McRae K., Misra S., Prasad A.K., Pereira S.C., Gross J.J. Bottom-up and top-down emotion generation: implications for emotion regulation. Soc. Cogn. Affect. Neurosci. 2012;7(3):253–262. doi: 10.1093/scan/nsq103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V., Uddin L.Q. Saliency, switching, attention and control: a network model of insula function. Brain Struct. Funct. 2010;214(5–6):655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miskovic V., Schmidt L.A. Social fearfulness in the human brain. Neurosci. Biobehav. Rev. 2012;36(1):459–478. doi: 10.1016/j.neubiorev.2011.08.002. [DOI] [PubMed] [Google Scholar]
- Mocaiber I., Pereira M.G., Erthal F.S., Machado-Pinheiro W., David I.A., Cagy M.…de Oliveira L. Fact or fiction? An event-related potential study of implicit emotion regulation. Neurosci. Lett. 2010;476(2):84–88. doi: 10.1016/j.neulet.2010.04.008. [DOI] [PubMed] [Google Scholar]
- Moors A., De Houwer J. Automaticity: a theoretical and conceptual analysis. Psychol. Bull. 2006;132(2):297–326. doi: 10.1037/0033-2909.132.2.297. [DOI] [PubMed] [Google Scholar]
- Moran J.M., Heatherton T.F., Kelley W.M. Modulation of cortical midline structures by implicit and explicit self-relevance evaluation. Soc. Neurosci. 2009;4(3):197–211. doi: 10.1080/17470910802250519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison A.S., Heimberg R.G. Social anxiety and social anxiety disorder. Annu. Rev. Clin. Psychol. 2013;9:249–274. doi: 10.1146/annurev-clinpsy-050212-185631. [DOI] [PubMed] [Google Scholar]
- Narumoto J., Okada T., Sadato N., Fukui K., Yonekura Y. Attention to emotion modulates fMRI activity in human right superior temporal sulcus. Cogn. Brain Res. 2001;12(2):225–231. doi: 10.1016/s0926-6410(01)00053-2. [DOI] [PubMed] [Google Scholar]
- Nordström H., Wiens S. Emotional event-related potentials are larger to figures than scenes but are similarly reduced by inattention. BMC Neurosci. 2012;13:49. doi: 10.1186/1471-2202-13-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northoff G., Heinzel A., de Greck M., Bermpohl F., Dobrowolny H., Panksepp J. Self-referential processing in our brain—a meta-analysis of imaging studies on the self. NeuroImage. 2006;31(1):440–457. doi: 10.1016/j.neuroimage.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Ochsner K.N., Gross J.J. The cognitive control of emotion. Trends Cogn. Sci. 2005;9(5):242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Öhman A. The role of the amygdala in human fear: automatic detection of threat. Psychoneuroendocrinology. 2005;30(10):953–958. doi: 10.1016/j.psyneuen.2005.03.019. [DOI] [PubMed] [Google Scholar]
- Öhman A., Mineka S. Fears, phobias, and preparedness: toward an evolved module of fear and fear learning. Psychol. Rev. 2001;108(3):483–522. doi: 10.1037/0033-295x.108.3.483. [DOI] [PubMed] [Google Scholar]
- Oldfield R. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Paulus M.P., Stein M.B. An insular view of anxiety. Biol. Psychiatry. 2006;60(4):383–387. doi: 10.1016/j.biopsych.2006.03.042. [DOI] [PubMed] [Google Scholar]
- Phelps E.A., LeDoux J. Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron. 2005;48(2):175–187. doi: 10.1016/j.neuron.2005.09.025. [DOI] [PubMed] [Google Scholar]
- Pishyar R., Harris L.M., Menzies R.G. Responsiveness of measures of attentional bias to clinical change in social phobia. Cognit. Emot. 2008;22(7):1209–1227. [Google Scholar]
- Quadflieg S., Mohr A., Mentzel H.-J., Miltner W.H.R., Straube T. Modulation of the neural network involved in the processing of anger prosody: the role of task-relevance and social phobia. Biol. Psychol. 2008;78(2):129–137. doi: 10.1016/j.biopsycho.2008.01.014. [DOI] [PubMed] [Google Scholar]
- Quirk G.J., Beer J.S. Prefrontal involvement in the regulation of emotion: convergence of rat and human studies. Curr. Opin. Neurobiol. 2006;16(6):723–727. doi: 10.1016/j.conb.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Raichle M.E., MacLeod A.M., Snyder A.Z., Powers W.J., Gusnard D.A., Shulman G.L. A default mode of brain function. Proc. Natl. Acad. Sci. 2001;98(2):676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapee R.M., Heimberg R.G. A cognitive-behavioral model of anxiety in social phobia. Behav. Res. Ther. 1997;35(8):741–756. doi: 10.1016/s0005-7967(97)00022-3. [DOI] [PubMed] [Google Scholar]
- Sand A., Wiens S. Processing of unattended, simple negative pictures resists perceptual load. Neuroreport. 2011;22(7):348–352. doi: 10.1097/WNR.0b013e3283463cb1. [DOI] [PubMed] [Google Scholar]
- Schmidt S., Mohr A., Miltner W.H.R., Straube T. Task-dependent neural correlates of the processing of verbal threat-related stimuli in social phobia. Biol. Psychol. 2010;84(2):304–312. doi: 10.1016/j.biopsycho.2010.03.005. [DOI] [PubMed] [Google Scholar]
- Schneider F., Bermpohl F., Heinzel A., Rotte M., Walter M., Tempelmann C.…Northoff G. The resting brain and our self: self-relatedness modulates resting state neural activity in cortical midline structures. Neuroscience. 2008;157(1):120–131. doi: 10.1016/j.neuroscience.2008.08.014. [DOI] [PubMed] [Google Scholar]
- Schulz C., Mothes-Lasch M., Straube T. Automatic neural processing of disorder-related stimuli in social anxiety disorder: faces and more. Front. Psychol. 2013:4. doi: 10.3389/fpsyg.2013.00282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergerie K., Chochol C., Armony J.L. The role of the amygdala in emotional processing: A quantitative meta-analysis of functional neuroimaging studies. Neurosci. Biobehav. Rev. 2008;32(4):811–830. doi: 10.1016/j.neubiorev.2007.12.002. [DOI] [PubMed] [Google Scholar]
- Stangier U., Heidenreich T. Liebowitz Social Anxiety Scale (LSAS) [Liebowitz Social Anxiety Scale] In: Scalarum Collegium Internationale Psychiatriae., editor. Internationale Skalen für Psychiatrie [International Psychiatry Scales] Weinheim; Beltz: 2005. [Google Scholar]
- Stangier U., Heidenreich T., Berardi A., Golbs U., Hoyer J. Deutsche Fassung; 1999. Social Interaction Anxiety Scale. [Google Scholar]
- Stangier U., Heidenreich T., Berardi A., Golbs U., Hoyer J. Deutsche Fassung; 1999. Social Phobia Scale. [Google Scholar]
- Stein M.B., Goldin P.R., Sareen J., Zorrilla L.T.E., Brown G.G. Increased amygdala activation to angry and contemptuous faces in generalized social phobia. Arch. Gen. Psychiatry. 2002;59(11):1027–1034. doi: 10.1001/archpsyc.59.11.1027. [DOI] [PubMed] [Google Scholar]
- Stevens S., Rist F., Gerlach A.L. Influence of alcohol on the processing of emotional facial expressions in individuals with social phobia. Brit. J. Clin. Psychol. 2009;48(2):125–140. doi: 10.1348/014466508X368856. [DOI] [PubMed] [Google Scholar]
- Straube T., Kolassa I.-T., Glauer M., Mentzel H.-J., Miltner W.H.R. Effect of task conditions on brain responses to threatening faces in social phobics: an event-related functional magnetic resonance imaging study. Biol. Psychiatry. 2004;56(12):921–930. doi: 10.1016/j.biopsych.2004.09.024. [DOI] [PubMed] [Google Scholar]
- Straube T., Mentzel H.-J., Miltner W.H.R. Common and distinct brain activation to threat and safety signals in social phobia. Neuropsychobiology. 2005;52(3):163–168. doi: 10.1159/000087987. [DOI] [PubMed] [Google Scholar]
- Straube T., Mentzel H.-J., Miltner W.H.R. Neural mechanisms of automatic and direct processing of phobogenic stimuli in specific phobia. Biol. Psychiatry. 2006;59(2):162–170. doi: 10.1016/j.biopsych.2005.06.013. [DOI] [PubMed] [Google Scholar]
- Straube T., Mentzel H.-J., Miltner W.H.R. Waiting for spiders: brain activation during anticipatory anxiety in spider phobics. NeuroImage. 2007;37(4):1427–1436. doi: 10.1016/j.neuroimage.2007.06.023. [DOI] [PubMed] [Google Scholar]
- Tabibnia G., Monterosso J.R., Baicy K., Aron A.R., Poldrack R.A., Chakrapani S.…London E.D. Different forms of self-control share a neurocognitive substrate. J. Neurosci. Off. J. Soc. Neurosci. 2011;31(13):4805–4810. doi: 10.1523/JNEUROSCI.2859-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J., Tournoux P. Thieme. 1988. Co-planar stereotaxic atlas of the human brain. 3-Dimensional proportional system: an approach to cerebral imaging. [Google Scholar]
- Teachman B.A., Joormann J., Steinman S., Gotlib I.H. Automaticity in anxiety disorders and major depressive disorder. Clin. Psychol. Rev. 2012;32(6):575–603. doi: 10.1016/j.cpr.2012.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng L.-Y., Tseng P., Liang W.-K., Hung D.L., Tzeng O.J.L., Muggleton N.G., Juan C.-H. The role of superior temporal sulcus in the control of irrelevant emotional face processing: a transcranial direct current stimulation study. Neuropsychologia. 2014;64:124–133. doi: 10.1016/j.neuropsychologia.2014.09.015. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N., Landeau B., Papathanassiou D., Crivello F., Etard O., Delcroix N.…Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage. 2002;15(1):273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Vormbrock F., Neuser J. Konstruktion zweier spezifischer Trait-Fragebogen zur Erfassung von Angst in sozialen Situationen (SANB und SVSS) Diagnostica. 1983;29:165–182. [Google Scholar]
- Wiens S., Molapour T., Overfeld J., Sand A. High negative valence does not protect emotional event-related potentials from spatial inattention and perceptual load. Cogn. Affect. Behav. Neurosci. 2012;12(1):151–160. doi: 10.3758/s13415-011-0072-8. [DOI] [PubMed] [Google Scholar]
- Wiens S., Sand A., Norberg J., Andersson P. Emotional event-related potentials are reduced if negative pictures presented at fixation are unattended. Neurosci. Lett. 2011;495(3):178–182. doi: 10.1016/j.neulet.2011.03.042. [DOI] [PubMed] [Google Scholar]
- Wittchen H.-U., Zaudig M., Fydrich T. SKID Strukturiertes Klinisches Interview für DSM-IV. Achse I und II. Göttingen: Hogrefe. Z. Klin. Psychol. Psychother. 1997;28(1):68–70. [Google Scholar]
- Ziv M., Goldin P.R., Jazaieri H., Hahn K.S., Gross J.J. Emotion regulation in social anxiety disorder: behavioral and neural responses to three socio-emotional tasks. Biol. Mood Anxiety Disord. 2013;3:20. doi: 10.1186/2045-5380-3-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Mean valence, arousal and anxiety ratings for disorder-related and neutral scenes in patients suffering from social anxiety disorder (SAD) and healthy controls (HC). Asterisks mark significant differences (P ≤ 0.05).
Supplementary figure.