Abstract
This is a case description of two patients with bipolar affective disorder, who presented complications, possibly due to underlying, undiagnosed obstructive sleep apnea syndrome (OSAS), during anesthesia for electroconvulsive therapy (ECT).
The first patient, just after receiving the second ECT, developed tachypnea with spasm of the upper airways and severe oxygen desaturation He was intubated and transferred to the medical intensive care unit where he was extubated 15 h later. The second patient, just after the eighth ECT, developed tachycardia and severe hypertension. He was transferred to the recovery room where he received oxygen therapy via nasal cannula and amlodipine. Both patients in the diagnostic polysomnographic tests which followed revealed a moderate to high apnea – hypopnea index (AHI) and distortion of sleep architecture. These cases highlight the need to assess for OSAS patients who receive ECT, especially if they exhibit peri-anesthesia complications.
Keywords: Obstructive sleep apnea syndrome (OSAS), Apnea – hypopnea index (AHI), Bipolar affective disorder, Electroconvulsive therapy (ECT), Anesthesia
1. Introduction
OSAS is a common, chronic condition characterized by repeated episodes of complete or partial obstruction of the upper airway during sleep, associated with increased respiratory efforts, intermittent arterial oxygen desaturation, systemic and pulmonary arterial blood pressure alterations and sleep fragmentation [1].
The HypnoLaus study reported a high prevalence of moderate-to-severe sleep-disordered breathing (≥15 events per hour), of 23,4% in women and 49,7% in men, in a population-based sample using full polysomnography, [2]. Another similar study from Brazil also reported a high prevalence of sleep-disordered breathing (apnoea-hypopnoea index >5 events per hour) of 46,6% in men and 30,5% in women [3]. Few studies examine the prevalence of OSAS in individuals with clinically diagnosed psychiatric disorders [4]. According to a recent review, it appears that there is an elevated prevalence of the disease in psychiatric patients with major depressive disorder (MDD) and posttraumatic stress disorder (PTSD) [4]. OSAS is associated with greater mortality, as well as significant adverse cardiovascular, gastrointestinal, genitourinary, endocrine, psychiatric, and social consequences [1]. Moreover, patients with OSAS are particularly vulnerable during anesthesia and sedation [5].
Electroconvulsive therapy is generally considered a safe procedure, in which electricity is used to create a seizure in a patient who has received general anesthesia. Although there is no survey examining the prevalence of OSAS among patients receiving anesthesia for ECT, failure to recognize and manage the risk for OSAS in patients receiving this treatment might expose them to anesthetic difficulties [6]. As spontaneous breath returns, a sleep apneic patient can express difficulty in supporting ventilation. Oxygen saturation can rapidly deteriorate due to the supine position and the diminished pulmonary reserve, particularly in obese patients.
In this paper, we describe two cases with cardiopulmonary complications during the ECT procedure; in both patients an underlying OSAS was diagnosed.
2. Patients and methods
2.1. Patient 1
The first patient was a 52 year old man with a Body Mass Index (BMI) 28.4kgr/m2, suffering from bipolar affective disorder (BPAD). He had no other significant, known medical history. Because of a depressive episode, he was hospitalized at the 1st Department of Psychiatry of Eginition Hospital. He was under treatment with mirtazapine, quetiapine, venlafaxine, olanzapine, lorazepam and he was planned to follow a series of 12 ECTs.
The patient received intravenous diazepam and propofol, as anesthetic drugs, and immediately after the 2nd ECT was completed, while still in the period of semiconsciousness, he presented paradoxical motion of his chest and the upper airway collapsed. Because of respiratory failure - arterial blood gases, pH: 7.49, PaO2: 69 mmHg, PaCO2: 30 mmHg, HCO3-: 22mmol/lt, while breathing oxygen 10lt/min via a nasal cannula - he was intubated and transferred to the intensive care unit (ICU). After 15 hours in the ICU, the patient was medically stabilized and transferred back to the psychiatry unit. Hematologic and biochemical examinations were normal. Both, a cardiologic evaluation, including echocardiography and stress test and a pulmonary evaluation, including chest X-ray and spirometry, were unremarkable.
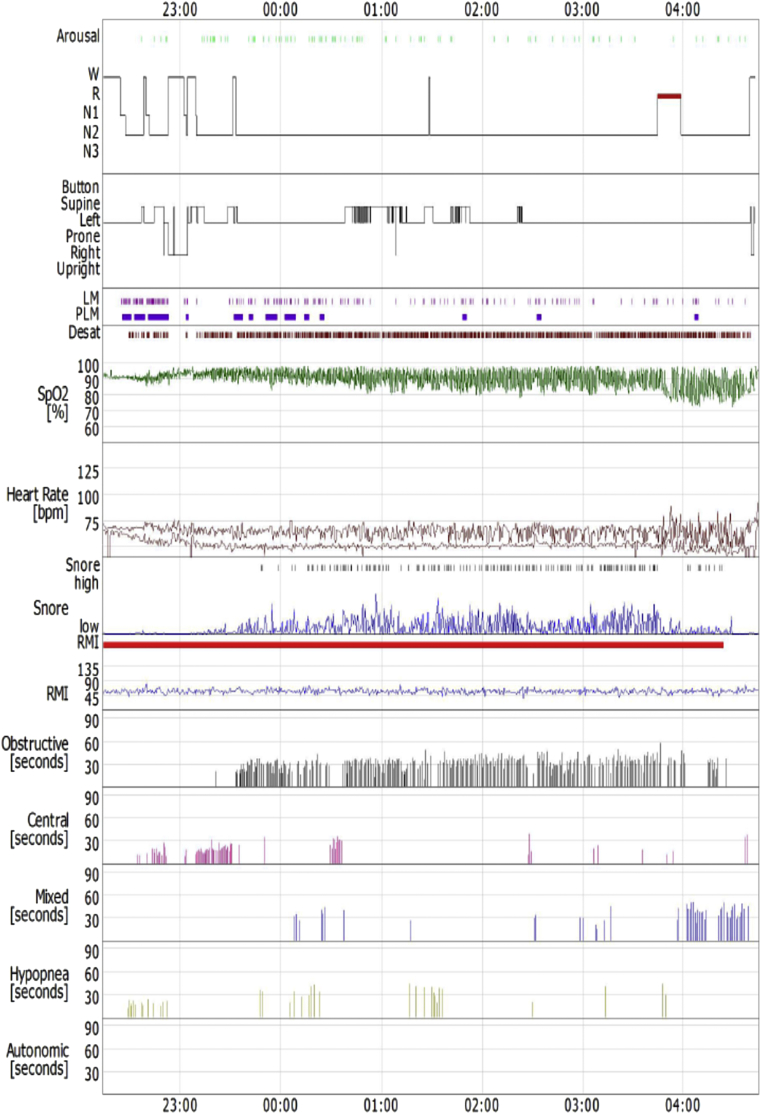
As the patient was overweight and reported snoring during sleep, he underwent standard full-night polysomnography performed in a quiet, sound and light proof room, with stable humidity and temperature. Polysomnographic findings - according to standard scoring criteria [7] - were as follows: time in bed 392min, sleep latency (SL) 13.2min, total sleep time (TST) 357min, sleep efficiency (SE) 91.1%; stage 1, 2min (7%), stage 2, 336min (94.1%) and rapid eye movement sleep (REM), 14min (3.9%) No slow wave sleep (SWS) was detected. The absolute number of respiratory events during sleep was 444 (73 central, 288 obstructive and 48mixed apneas, and 35 hypopneas) and the apnea hypopnea index (AHI) was 74.6 (severe OSAS), with a mean SaO289.4%, a minimum SaO272% and percentage of sleep time with SaO2<90% (t < 90%) 48.8% (Fig. 1). Positive Airways Pressure (PAP) titration was carried out on a second night, normalizing the AHIat an inspiratory pressure (IPAP)17cmH2O and an expiratory pressure (EPAP) 11cmH2O (bi-level mode, BiPAP).
Fig. 1.
Sleep study of the 1st patient.
2.2. Patient 2
The second patient was a 55 year old man with a Body Mass Index (BMI) 31.5kgr/m2, suffering also from BPAD and OCD. He had no other significant, known medical history. Because of a depressive episode and obsessive thoughts, he was hospitalized at the 1st Department of Psychiatry of Eginition Hospital, under venlafaxine, olanzapine, sertraline, alprazolam and a plan to receive 10 ECTs.
The patient received intravenous diazepam and propofol, as anesthetic drugs, and 15min later, when the 8th ECT was completed, he developed severe hypertension (210/120 mmHg) and tachycardia (130 beats per minute) with mild desaturation. He was admitted to the recovery room and received oxygen (3lt/min via a nasal cannula) and amlodipine. After 1.5 hours in the recovery room that the patient was medically stabilized, he transferred back to the psychiatry unit. Hematologic and biochemical examinations were normal. A cardiologic evaluation, including echocardiography, stress test and 24-h ambulatory blood pressure monitoring revealed mild nocturnal hypertension. The pulmonary evaluation, including chest X-ray and spirometry, was normal.
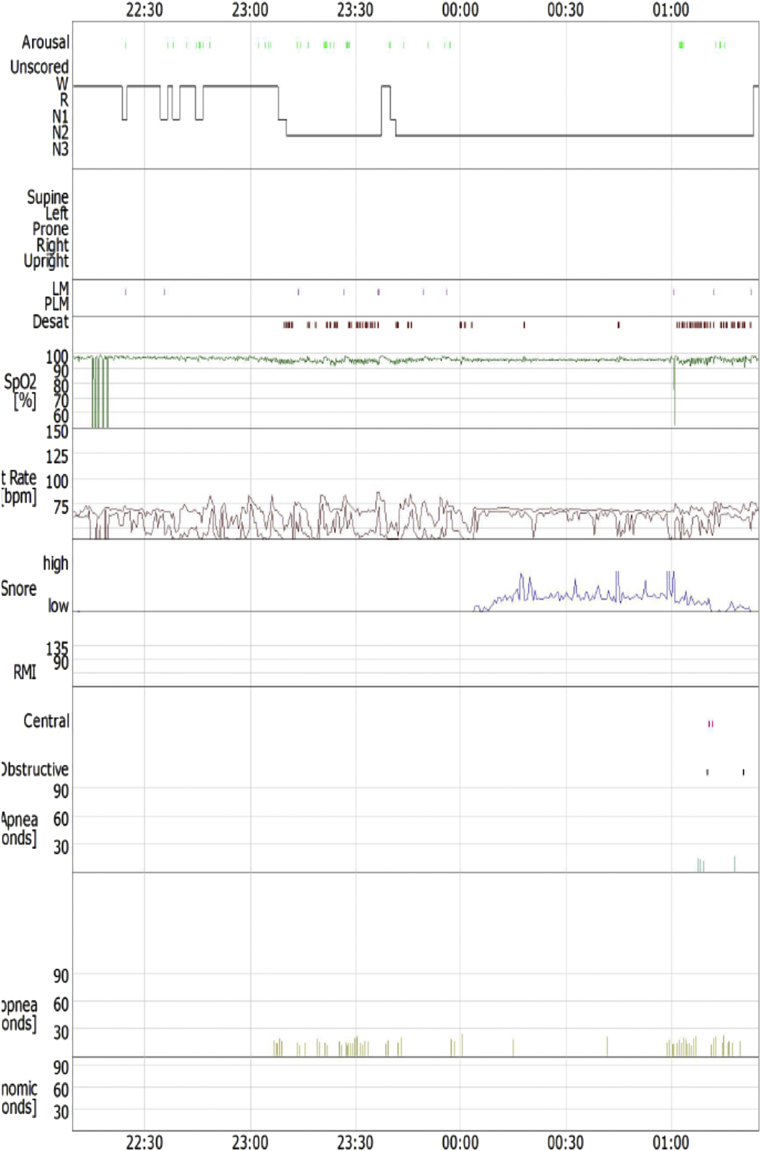
As the patient was obese and had nocturnal hypertension, he underwent standard polysomnography performed in a quiet, sound and light proof room, with stable humidity and temperature. Polysomnographic findings - according to standard scoring criteria [7] - were as follows: time in bed 195.9min, SL 14.2min, TST 140.5min, SE 91.1%; stage 1, 11.5min (8.2%) and stage 2, 129min (91.8%). No SWS and REM sleep were detected. The absolute number of respiratory events during sleep was 61 (2 central and2 obstructive apneas, and 57 hypopneas) and theAHI was 26 (moderate OSAS), with a mean SaO295.4% and a minimum SaO2 <90% (Fig. 2). Positive Airways Pressure (PAP) titration was carried out on a second night, normalizing the AHI at a range of pressures 5-10cmH2O (auto mode, auto-CPAP).
Fig. 2.
Sleep study of the 2nd patient.
3. Discussion
OSAS is being increasingly recognized as an important cause of medical morbidity and mortality. Polysomnography is the gold standard to diagnose sleep-disordered breathing [1].
Recent studies in general unselected populations, based on polysomnography data, assess a very high prevalence of the disease, ranging from 46,6 to 49,7% in men and 23,4 to 30,5% in women [2], [3]. Although it is a relatively common sleep disorder, up to 80% of individuals with moderate to severe OSAS may remain undiagnosed and, more alarmingly, untreated [8]. Apneas and hypopneas occur during sleep and most patients are unaware of these events. A high degree of suspicion is mandatory to detect OSAS in patients with risk factors, symptoms, and typical physical findings.
People with serious mental illness (SMI), particularly MDD, have a high prevalence of OSAS, ranging from 13.9 to 42.4% in different clinical studies [9]. Higher frequencies of OSAS were seen in MDD (36.3%), than in BPAD (24.5%) and schizophrenia (15.4%) [9]. It is mandatory to recognize OSAS in patients with BPAD because disruption of the sleep-wake cycle caused by OSAS could possibly lead to mood instability and relapse of BPAD [10]. Furthermore, the treatments for BPAD (benzodiazepines and antipsychotics) may trigger respiratory failure in patients with an underlying, undiagnosed OSAS [4], [5], [10]. Unfortunately, existing data are characterized by considerable heterogeneity of the study population and a high risk of bias.
Although ECT procedure is quite safe, there is a need for sedation. Failure to diagnose and treat OSAS in ECT patients may expose them in anesthetic complications. Propofol affects breathing and upper airway (UA) patency by direct inhibition of the dilator muscle activity [11]. Also, supine position with decreased pulmonary reserve and loss of consciousness with reduced arousal during ECT, further increase the risk of UA collapse. In addition, benzodiazepines relax muscular tone, causing an increased risk of upper airway obstruction, and suppress the ventilation response to hypercapnia and may even reduce the hypoxic response [4], [5]. Taken together, all these parameters can potentially lead to apnea in an already unstable UA.
We believe that, regarding our first patient, a serious, sustained UA collapse was the reason of his inability to breathe.
The second patient seems to have had an episode of Negative Pressure Pulmonary Edema (NUPPE), which develops when a patient struggles to breathe against an obstructed airway during an UA collapse. In that case, very negative intrathoracic pressures are generated, which, can potentially lead to NUPPE or post-obstructive pulmonary edema, with severe hypertension, tachycardia and oxygen desaturation [12]. Resolution of this pulmonary edema is usually rapid, because alveolar fluid clearance mechanisms are intact.
Given that all other examination were unremarkable, we strongly hypothesize that in both our patients, the underlying pathophysiological mechanism for the serious complications during ECT was undiagnosed and untreated OSAS.
Although apnea subsequent to anesthetic drugs administration has been rarely observed in patients receiving ECT (3 cases per 73,440 treatments) [6], we believe that every institution should establish a specific plan for the identification, diagnosis and care of psychiatric patients suffering from OSAS.
Patients should be examined for risk factors and symptoms associated with sleep apnea before ECT. Symptoms which may reveal OSAS are snoring, waking with dry mouth and/or headache, sleepiness during the day, fatigue, low energy, concentration problems, and insomnia; unfortunately many of these symptoms are also frequent in psychiatric patient without OSAS [13]. Obesity, male sex and comorbidities like arterial hypertension, atrial fibrillation, diabetes mellitus and dyslipidemia are the principal risk factors of OSAS. Standardized questionnaires for the identification of OSAS, such as the widely used Epworth Sleepiness Scale and STOP-Bang questionnaire, are not validated in psychiatric patients. Future studies should validate reliable, and easy-to-use screening questionnaires, for the identification of high risk OSAS patients, in psychiatric population.
A peri-ECT care plan could include conduction of the procedure in an operating room, ventilation with 100% oxygen before anesthesia, insertion of a nasal-pharyngeal airway, semiupright position and close monitoring of vital signs and SaO2 after.
Ideally, each patient at high suspicion for OSAS should be referred to a sleep specialist and perform standard, overnight polysomnography for a prompt diagnosis. However, a sleep study requires the expertise of sleep medicine specialists, who may not be available at many hospitals, is time-consuming and costly. Moreover, sometimes psychiatric urgency makes this impossible. The first line of therapy is continuous positive airways pressure (CPAP) which improves the safety of OSAS patients undergoing anesthesia [4], [5].
4. Conclusion
Failure to diagnose and treat OSAS in ECT patients may expose them in peri-anesthesia complications. Future studies should validate reliable, and easy-to-use screening tools, like Epworth Sleepiness Scale and STOP-Bang questionnaire, for the identification of high risk OSAS patients, in psychiatric population.
References
- 1.American Academy of Sleep Medicine . third ed. American Academy of Sleep Medicine; Darien, IL: 2014. International Classification of Sleep Disorders. [Google Scholar]
- 2.Heinzer R., Vat S., Marques-Vidal P. Prevalence of sleep-disordered breathing in the general population: the HypnoLaus study. Lancet Respir. Med. 2015;3(4):310–318. doi: 10.1016/S2213-2600(15)00043-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tufi kS., Santos-Silva R., Taddei J.A. Obstructive sleep apnea syndrome in the Sao Paulo epidemiologic sleep study. Sleep. Med. 2010;11:441–446. doi: 10.1016/j.sleep.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 4.Gupta M.A., Simpson F.C. Obstructive sleep apnea and psychiatric disorders: a systematic review. J. Clin. Sleep. Med. 2015;11(2):165–175. doi: 10.5664/jcsm.4466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Practice guidelines for the perioperative management of patients with obstructive sleep apnea: an updated report by the American Society of Anesthesiologists Task Force on Perioperative Management of patients with obstructive sleep apnea. Anesthesiology. 2014;120(2):268–286. doi: 10.1097/ALN.0000000000000053. American society of anesthesiologists task force on perioperative management of patients with obstructive sleep apnea. [DOI] [PubMed] [Google Scholar]
- 6.Watts B.V., Groft A., Bagian J.P., Mills P.D. An examination of mortality and other adverse events related to electroconvulsive therapy using a national adverse event report system. J. ECT. 2011;27:105–108. doi: 10.1097/YCT.0b013e3181f6d17f. [DOI] [PubMed] [Google Scholar]
- 7.Berry R.B., Budhiraja R., Gottlieb D.J., Gozal D., Iber C. The AASM manual for the scoring of sleep and associated events: rules terminology and technical specifications, version 2.0. Darien, IL: American academy of sleep medicine. J. Clin. Sleep. Med. 2012;8(5):597–619. doi: 10.5664/jcsm.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Young T., Evans L., Finn L., Palta M. Estimation of the clinically diagnosed proportion of sleep apnea syndrome in middle-aged men and women. Sleep. 1997;9:705–706. doi: 10.1093/sleep/20.9.705. [DOI] [PubMed] [Google Scholar]
- 9.Stubbs B., Vancampfort D., Veronese N., Solmi M., Gaughran F. The prevalence and predictors of obstructive sleep apnea in major depressive disorder, bipolar disorder and schizophrenia: a systematic review and meta-analysis. J. Affect Disord. 2016;197:259–267. doi: 10.1016/j.jad.2016.02.060. [DOI] [PubMed] [Google Scholar]
- 10.Trakada G., Steiropoulos P., Bouros D. Continuous positive airways pressure treatment in a patient with sleep apnea-hypopnea syndrome and coexisting bipolar disorder. Psychopharmacol. Bull. 2008;41(2):89–92. [PubMed] [Google Scholar]
- 11.Evans R.G., Crawford M.W., Noseworthy M.D., Yoo S.J. Effect of increasing depth of propofol anesthesia on upper airway configuration in children. Anesthesiology. 2003;99(3):596–602. doi: 10.1097/00000542-200309000-00014. [DOI] [PubMed] [Google Scholar]
- 12.Bhattacharya M., Kallet R.H., Ware L.B., Matthay M.A. Negative pressure pulmonary edema. Chest. 2016 doi: 10.1016/j.chest.2016.03.043. [DOI] [PubMed] [Google Scholar]
- 13.Vaughn McCall W., Arias L., Onafuye R., Rosenquist P.B. What the electroconvulsive therapy practitioner needs to Know about obstructive sleep apnea. J. ECT. 2009;25:50–53. doi: 10.1097/YCT.0b013e31817144a6. [DOI] [PubMed] [Google Scholar]